Abstract
Biological sex is a fundamental source of phenotypic variability across species. Males and females have different nutritional needs and exhibit differences in nutrient digestion, utilization, leading to different health outcomes throughout life. With personalized nutrition gaining popularity in scientific research and clinical practice, it is important to understand the fundamentals of sex differences in nutrition research. Here, we review key studies that investigate sex dimorphism in nutrition research: sex differences in nutrient intake and metabolism; sex-dimorphic response in nutrient restricted conditions; and sex differences in diet and gut-microbiome interactions. Within each area above, factors from sex chromosomes, sex hormones, and sex-specific loci are highlighted.
Keywords: sex-dimorphism, nutrient, animal models, dietary restriction, gut-microbiome
1. The importance of understanding sex difference in nutrition research
Nutrition has important implications throughout lifespan, providing nourishment for a species at any given time with impact on growth, reproduction, and healthspan. Nutrition also appears to be a key area where biological sex leads to important differences in outcomes. Men and women have different energy and nutrient requirements, and exhibit differences in food intake and metabolism, which can in turn lead to different health outcomes (66).
Biological sex [distinct from gender, related to social experience] is a fundamental biological variable. Yet, sex differences are only beginning to be investigated in clinical and preclinical nutrition research. The National Institutes of Health (NIH) Revitalization Act first mandated the inclusion of women in clinical trials in 1993 (112). Aimed at addressing the overrepresentation of men and male animals in biomedical research and the lack of attention to sex-biased responses to medical treatments, the NIH policy for including sex as a biological variable (SABV) into research designs, analyses, and reporting went into effect in 2016 (116). However, as of 2020, only half of the U.S. scientists surveyed report analyzing study results by sex (116).
More work will be needed to ensure that the SABV framework is applied across different model organisms, so that both men and women receive high-quality benefit from research advancements (116). While immediate individual genetic testing may be challenging, understanding the sex-differences is likely to confer short-term invaluable insights in promoting personalized healthcare.
2. Dissecting sex differences in nutrition research: molecular drivers and study toolkit
To understand how sex differences may influence nutritional needs and specificities, a clear definition of the components that can shape sex differences is necessary. Though gender, defined as a social concept, may have a wide range of influence on one’s nutritional status and health outcome, its magnitude and mechanisms are difficult to assess in laboratory animals, which do not experience gender identity. Thus, the present review will focus on biological determinants of sex: gonadal hormones and sex-chromosome complement.
a. Gonadal hormones
Studies of sex differences have long emphasized the effect of gonadal differentiation, which is initiated by the male-determining gene Sry on the mammalian Y chromosome. Specifically, in mammals, the presence of Sry in an embryo leads to the development of testes, whereas the absence of Sry leads to the development of ovaries (7). Gonadal identity (testes vs. ovaries), in turn leads to life-long differences in the relative circulating levels of gonadal hormones (e.g. estrogens vs. androgens) (7). In humans, nonhuman primates and rats, 17β-estradiol [E2] is the most potent estrogen, and testosterone is the primary androgen (7). The differences in gonadal hormonal levels are likely to impact food behavior and nutrient metabolism. For instance, changes in E2 levels have been found to be associated with changes in the food intake in humans and laboratory animals (18). Importantly, the effects of gonadal hormones can be further subdivided into “organizational” (long-term, permanent) or “activational” (acute, reversible). Organizational effects are set in utero during perinatal period, leading to life-long masculinization or feminization of somatic phenotypes, whereas activational effects will take place after sexual maturity (2; 3). These effects of gonadal hormones have been examined in behavioral studies, including ones involving food intake (2).
b. Sex chromosome complement
Separate from gonadal hormones, the effect of sex chromosome complement (e.g. XX vs. XY karyotype) has recently come into focus. Double dosage of X chromosomes in female cells is partially compensated by X-chromosome inactivation [XCI], where one X chromosome is randomly inactivated early in embryogenesis (61). Approximately 15–25% of X chromosome genes escape XCI in humans, and some of these escapees overlap with mice (e.g. Eif2s3x, Kdm6a, Kdm5c, Ddx3x) (23). The higher expression of escapees in XX cells compared to XY cells, as well as the expression of Y-linked genes in XY cells, can broadly impact gene expression in a sex-specific manner (3).
c. Study toolkit for sex-dimorphism in nutrition research
The ability to recruit appropriate human subjects, as well as the identification of appropriate animal models, are both pivotal elements in the study of sex-dimorphic phenotypes driven by gonadal hormones and/or sex-chromosomes in nutrition research.
i. Human subjects
In humans, organizational effects of gonadal hormones may be assessed by comparing same-sex and opposite-sex fraternal twin pairs. Females from opposite-sex twin pairs are believed to have higher prenatal testosterone exposure, compared to females from same-sex twin pairs (31). Activational effects of gonadal hormones may be assessed by comparing females before and after menopause (age-related or surgically induced). Studies of these populations have revealed differences in dietary intake, body fat distribution and incidence of metabolic disorders (60). However, perinatal levels of gonadal hormones cannot be manipulated in humans for ethical reasons, making it impossible to dissect causal relationships of hormones with lifelong phenotypes in humans. Furthermore, studies on postmenopausal females are confounded by using different individuals in the pre- and postmenopausal groups, as longitudinal studies are often difficult to conduct and fund (60).
The effects of sex chromosomes ploidy in humans are even harder to assess. Though sex chromosome aneuploidies (e.g. Turner syndrome [45, XO] or Klinefelter syndrome [47, XXY]) may offer useful handles to examine the impact of sex chromosomes, they often co-occur with alterations in gonadal hormone status (60). Thus, with our current knowledge, the roles of sex chromosome karyotype and gonadal hormones cannot be distinguished in human studies, which makes the use of model organisms critical in deciphering the impact of biological sex on the response to dietary interventions or on nutritional needs.
ii. Animal models
Given the wide range of limitations in human studies for investigating sex-dimorphic hormonal or chromosomal effects, animal models are invaluable to elucidate the mechanisms underlying sex-dimorphism in biological phenomena (Figure 1, 2). In this section, we introduce relevant models that can be leveraged to determine sources of sex-differences in nutrition research.
Figure 1. Animal models for studying sex hormone effects.
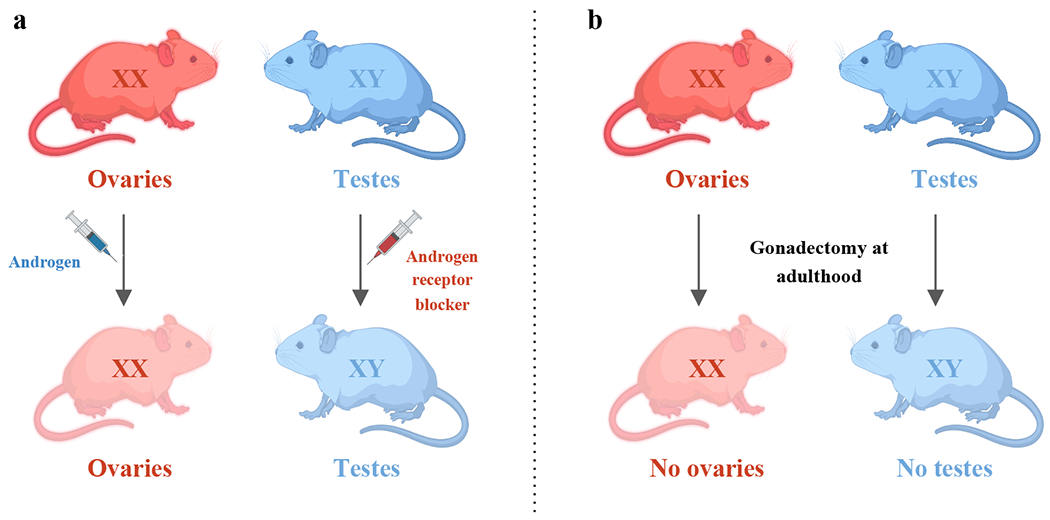
a) Administration of androgens (e.g. testosterone propionate) to females, and androgen receptor antagonist (e.g. flutamide) to males during perinatal period. This model allows for the investigation of organizational effects of gonadal hormones without affecting sex chromosomes or the gonadal status. b) Surgical removal of ovaries from XX females and testes from XY males. This model allows for the investigation of activational effects of gonadal hormones without altering the sex chromosomes or the organizational effects of gonadal hormones.
Figure 2. Animal models for studying sex chromosome effects.
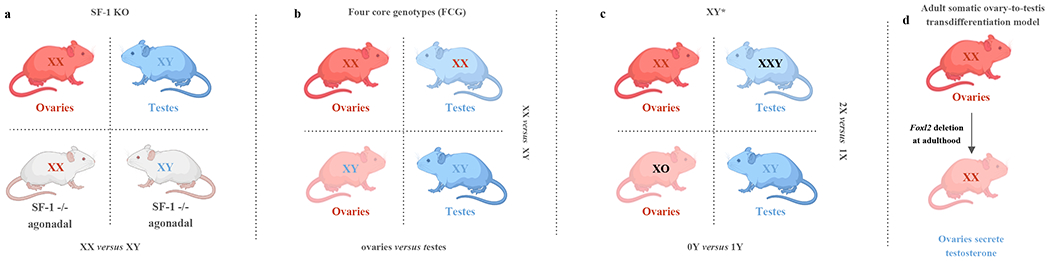
a) SF-1 KO mouse model. Comparing SF-1 KO XX mice with SF-1 KO XY mice allows for investigation of sex differences due to chromosomal effects (XY versus XX), independent of differences from gonadal steroid hormones. b) Four core genotype mouse models allow for the comparison of sex chromosomes (XX versus XY) with the same gonadal identity, and the comparison of gonadal status (ovaries versus testes) with the same sex chromosomes. c) After identifying the effects are due to sex chromosomes, XY* mouse model allows for further comparison of the number of X chromosomes (2X versus 1X), and the comparison of the presence or absence of Y chromosome (0Y versus 1Y). d) Adult somatic ovary-to-testis transdifferentiation model [aOTT] (inducible deletion of Foxl2 in XX females at adulthood). This model enables the ovaries in adult XX females to secrete testosterone, allowing the investigation of activational effects of gonadal hormones without altering the sex chromosomes or the organizational effects of gonadal hormones.
Pharmaceutical animal models
In animal models, organizational effects of gonadal hormones may be assessed by perinatal administration of (i) androgens (e.g. testosterone propionate) to females, or (ii) androgen receptor antagonists (e.g. flutamide) to males (5; 11). Resulting females are permanently masculinized, whereas resulting males are permanently feminized (5). The timing of these hormone manipulation is critical for producing permanent effects (5). For instance, in rats, the critical periods where brain masculinization and defeminization occur has been mapped from gestational day 18 through postnatal day 10 day (11). This technique has also been used successfully in rodents and guinea pigs to understand in utero effects of gonadal hormones (7).
Surgical animal models
In laboratory animals, activational effects of gonadal hormones can be assessed through gonadectomy combined with hormonal replacement treatment, to abolish or acutely rescue hormone levels. For instance, mice gonadectomized at 6 weeks of age experience complete loss of sex-steroid production (Figure 1)(86). Thus, if sex differences persist after gonadectomy, they can be attributed to either organizational effects or to sex chromosomes (60). Importantly, because gonads can produce other endocrine and paracrine factors in addition to sex-steroids, acute replacement is required to conclude that phenotypes are specifically driven by sex hormones. This type of model has been leveraged for dietary studies across models (7).
However, it is important to note that insights gained from gonadectomy do not always allow for distinguishing whether sex-dimorphic phenotypes result from organizational effects or from sex chromosome inputs.
SF-1 KO model
Elegant genetic models have been devised to study mechanistic underpinnings of sex-dimorphic phenotypes. Steroidogenic factor 1 knock-out (SF-1 KO) mice are an agonadal model that allows exploration of the direct contribution of sex chromosomes independent of gonadal hormones (43). Steroidogenic factor 1 (SF-1), encoded by Nr5a1 gene, is required for the development of gonads and adrenal glands (43). SF-1 heterozygous mice can be mated to produce homozygous SF-1 KO and wild-type offspring (43). SF-1 KO male and female mice are born without gonads nor adrenal glands, thus lacking endogenous gonadal steroids completely (43). To prevent death from adrenal deficiency, newborn SF-1 KO pups need to be injected daily with corticosteroid for 6-7 days, followed by adrenal transplantations at day 7-8 (42). By comparing SF-1 KO XX mice to SF-1 KO XY mice, sex-differences due to chromosomal effects can be evaluated independent of gonadal hormones (43). This model has been used to study sex-differences in behaviors due to disruptions in the brain region containing the ventromedial nucleus of the hypothalamus (VMH), involved in regulating behavior and energy balance (43).
Four-core genotypes (FCG) mice
The four-core genotype (FCG) model is another widely used genetic model in nutrition research. This model carries a deletion Sry from the Y chromosome combined to an insertion of an Sry transgene on chromosome 3 on a C57BL/6 background (3). In this model, animals are referred to by a shorthand including information about their sex chromosome karyotype and gonadal identity (e.g. gonadal females are mice with ovaries). This model yields four animal types: (i) XXF (XX females with ovaries; XXO), (ii) XYM (XY males with testes; XYT), (iii) XYF (XY females with ovaries; XYO) and (iv) XXM (XX males with testes; XXT) (3). Interestingly, FCG mice may also be gonadectomized to subtract acute gonadal hormone effects, revealing whether the hormonal effects are organizational or activational (3). This model has been used to examine the genetic interplay in food consumption, glucose homeostasis, and lipid metabolism (23; 60; 100).
XY* mice
Because XX mice differ genetically from XY mice in both the dose of the X chromosome and the absence of a Y chromosome, karyotype-driven mechanisms of phenotypic regulation remain unclear (4). Thus, with confirmed sex chromosome effects, whether differences are driven by X chromosome ploidy or the presence of a Y chromosome can be further explored by using the XY* mouse model (4). This model involves mating XY* males (with an aberrant Y chromosome pseudoautosomal region) with XX females, yielding 4 types of progenies: XX and XO females with ovaries, XY and XXY males with testes (17). Comparing XO females vs. XX females, or XY males vs. XXY males, allows to decipher the impact of X chromosome ploidy. Comparing XY vs. XO animals, and XXY vs. XX animals, can help reveal the impact of a Y chromosome. Once the chromosomal driver of sex-dimorphic phenotypes are detected, specific X or Y candidate genes can then be identified to assess their contribution for the sex chromosome effects (4).
Adult somatic ovary-to-testis transdifferentiation (Foxl2 inducible knock-out)
We believe that the mouse adult Foxl2 inducible knock-out model can be a fantastic model to shed light into sex-dimorphic phenotypes (108). Inducible deletion of Foxl2 in adult XX females leads to adult somatic ovary-to-testis transdifferentiation [aOTT] (108). Reprogramming of ovarian somatic cells into their testis counterparts leads to male-like levels of circulating testosterone in XX aOTT animals within 2 weeks of knock-out induction (108). Thus, activational effects of estrogens vs. androgen prevalence can be readily assessed in this model. Although it has not yet been used outside of the realm of gonadal biology, leveraging this model could provide important insights into the adult effects of sex-hormones, including as a model of transmasculine transition.
iii. Genome-Wide Association Studies [GWAS]
Genome-wide association studies (GWAS) have allowed unbiased exploration of genetic determinants of key complex phenotypes (107). However, males and females have commonly been analyzed together instead of separately to disentangle sex-dimorphic effects (56). Moreover, GWAS often exclude sex chromosomes due to the statistical complexity of comparing haploid males to diploid females (98). Analysis tools are starting to be developed to incorporate sex chromosomes, and they have yielded sex-specific candidate regulators of nutrient intake and metabolism. For instance, single nucleotide polymorphisms (SNPs), such as SNP rs9939609 on the fat mass and obesity-associated (FTO) genotype, have been identified to have sex-specific impact on food intake and lipid metabolism and will be further discussed below (103).
3. Sex differences in nutrient intake
In humans and most mammals, nutrient intake - food quantity (calorie amount) and food profile (types of nutrients) - set the stage for affecting body composition and health outcomes. While food choices may depend on a wide spectrum of socioeconomic factors, understanding the genetic differences on total energy, carbohydrate, and fat intake between sexes is crucial for developing appropriate dietary recommendations.
a. Total food intake
In humans, not only do females consume fewer total calories per day, they also consume fewer total calories per kilogram of lean mass (122), a trend conserved in animal studies. In laboratory rats, total daily energy intake of males exceeds that of females, even after accounting for larger lean body mass and metabolic rate (7). Importantly, sex-differences in eating behaviors are under the influence of gonadal hormones. For instance, at 6 weeks of age, female mice that were treated with neonatal testosterone treatment ate as much as intact males, which was more than untreated females (83).
i. Impact of estrogens
Regarding the influence of activational hormone on food intake, the effects of estrogens have been most widely studied. Particularly, the role of E2, the most abundant estrogen in women of reproductive age, is best documented for its effects on food intake through estrogen receptor signaling (18). In humans, elevated E2 levels during the peri-ovulatory period is associated with decreased food intake (6; 18). Similar observations have been made in rats, mice and monkeys (6; 18). For instance, adult female rodents eat less during the peri-ovulatory phase of the ovarian cycle than other phases (6). Experiments using ovariectomy and E2 replacement have provided further evidence for its inhibitory effects on food intake: female rats and rhesus monkeys had significant increase in food intake after ovariectomy, and exogenous E2 in turn led to decreased food intake (18; 104). The impact of estrogens on appetite regulation are likely mediated by estrogen receptor-α (ERα) and E2-sensitive signals, which include cholecystokinin (CCK), ghrelin, insulin and leptin (6). During high E2 peri-ovulatory period in rats, neurons expressing ERα have increased sensitivity to CCK-induced vagal afferent inputs, leading to increased satiety and decreased food intake (6). ERα and ERβ are nuclear receptors, encoded by ESR1 and ESR2 genes in humans (Esr1 and Esr2 in mice, respectively) (7). E2 treatment after ovariectomy reduced daily food intake upon ovariectomy in wild-type mice, but not in ERα knockout mice (41), indicating that ERα mediates the effects of estrogens on food intake. Importantly, E2 levels affect leptin levels, which in turn modulate hunger and food intake. For instance, low E2 levels reduce leptin sensitivity in ovariectomized rats, leading to increased food intake (29). Conversely, treating ovariectomized females with estradiol leads to increased leptin sensitivity, thus increasing inhibition of food intake by leptin (29). Thus, due to their clear role in the regulation of food intake, future nutrition research studies should more systematically control for E2 levels, which will be key to determine tailored dietary guidance at different stages of women’s life, including puberty, pregnancy or menopause.
ii. Impact of androgens
Although it has not been studied as extensively as the role of estrogens, there is emerging evidence that testosterone can also directly influence eating behaviors. For instance, in male rats, adult orchiectomy decreased food intake, while exogenous androgen treatment rescued food intake behaviors (22). A study using both intact and gonadectomized FCG mice found that intact males had greater total food intake and more meals than intact females during the dark phase, implicating male hormones as promotors of increased food take (24). Consistently, after exposure to high-fat diet (HFD) in intact FCG mice, food intake in gonadal males was increased at least in the light phase of the circadian cycle after 1-8 week of HFD (61). Together, these findings support the notion that male hormones promote increase food intake, at least in rodents. However, further mechanistic insights into androgen-mediated regulation of food intake are still missing (7), and will deserve further investigation.
iii. Impact of sex chromosomes
Studies leveraging the FCG model have offered valuable insights into the contribution of XX vs. XY chromosomes to eating behaviors. In a study of FCG mice where mice were gonadectomized at adulthood, Chen and colleagues revealed that XX mice, regardless of gonadal identity, showed greater food intake during daylight hours when compared to XY mice (23), revealing that the XX karyotype promotes increased food intake, independent of gonadal hormones. Consistently, another study in intact FCG mice fed HFD confirmed food intake was higher in XX mice at least during light time, regardless of gonadal identity (61). Subsequent studies with XY* mice revealed that chromosomally-driven differences could be attributed to X chromosome ploidy, rather than Y chromosome presence (23). Together, these studies support a mechanism where food intake can be regulated by genes that escape XCI, resulting in higher levels of expression in cells with >1 X chromosome. For instance, Kdm5c, which encodes histone demethylase that removes tri- and dimethyl marks from histone 3 lysine 4 (H3K4) to regulate chromatin access by transcription factors, is of the X chromosome genes that escape XCI in both mice and humans, serving as a candidate for underlying X chromosome ploidy effects (61). Future studies should focus on identifying relevant “XCI escapee genes” and their interaction with dietary manipulation, to elucidate how X chromosome ploidy contributes to the sex differences in food intake.
b. Food choices
Epidemiological studies have revealed differences in food choices of males and females. Throughout life, females consistently have higher fruit, vegetables intake and carbohydrate cravings, whereas males show higher intakes of protein rich foods (Reviewed in (66)). These differences are likely driven, at least in part, by societal norms. However, females are reported to eat more sweets during the luteal phase of the cycle than other phases, possibly due to simultaneous increases in E2 and progesterone (6), consistent with findings in females and males in the context of obesity, where females had higher intake proportions of high-sugar and high-fat foods than did males (65).
Animal studies are invaluable in disentangling societal from biological drivers in food choices. Interestingly, gonad-intact female rats start ingesting conditioned sweet solution sooner than males, and do so in greater quantities (Reviewed in (32)). Moreover, exogenous administration of testosterone increased the duration of conditioned sweet solution avoidance in both sexes, whereas exogenous E2 in ovariectomized female rats accelerated consumption of sweet solution (Reviewed in (32)). These observed activational effects of sex steroids on taste aversion conditioning using a sweet solution parallel human data where females show higher preference for high-sugar foods. Studies using FCG mice have also revealed a role for sex chromosomes in preference and consumption for highly palatable food. Seu and colleagues used sweetened condensed milk as a highly palatable fat- and sugar-rich food item to measure food seeking behaviors in gonadectomized FCG mice (100). XY mice consumed more sweetened condensed milk than their XX counterparts at all concentrations (100). However, the preference for highly palatable fat- and sugar-rich food in XY mice is not consistent with human data, where obese females reported increased preference for high-fat and high-sugar foods (65), although it is possible that complex interplay may exist between sex chromosomes and gonadal hormones on food choices. Thus, these studies underlie the importance of parsing out the relative (and potentially contradictory) impacts of sex chromosome complement and gonadal hormones when studying sex differences in food choice and exploring targeted dietary interventions. Studies with increased variety of food options may also be needed to better represent the human dietary environment and provide more directly translatable guidance.
c. Genetic polymorphisms and food intake
One of the most studied genes with regards to food intake is FTO, the first obesity risk gene identified by GWAS, which is strongly associated with increased body mass index (63). FTO is highly expressed in the hypothalamus where regulation of food intake and energy expenditure occurs (63). Studies indicate that the allele A of the FTO variant is associated with higher energy, fat and carbohydrates intake compared to TT homozygotes (103). Particularly, positive associations between obesity-risk alleles of SNP rs9939609 and fat intake were observed only in females (103), consistent with the female environment acting as a modifier. Both hormonal inputs and chromosomal inputs can generally influence gene expression regulation even on autosomes, and which mechanism is responsible for the sex-specificity of rs9939609 has not yet been elucidated. However, the sex-specific effects of rs0039609 highlight the importance of systematically segregating such GWAS studies by sex, so as to avoid missing important sex-dimorphic effects that may be leveraged to specifically help females or males prevent and/or manage obesity and other metabolic conditions.
4. Sex differences in nutrient metabolism
Downstream of nutrient intake, nutrient metabolism related to utilization or storage is equally as important in determining health outcomes. Sex has a significant impact on metabolism, for instance modulating insulin sensitivity in response to glucose intake (66), through mechanisms involving both gonadal hormones and sex chromosomes. Here, we highlight available evidence on the sex differences and underlying factors in the metabolism of glucose, protein, and fatty acid.
a. Glucose metabolism
Glucose homeostasis is primarily regulated by skeletal muscle through basal and insulin-stimulated glucose uptake, and may be evaluated by measuring fasting glucose and fasting insulin levels (73). Key metabolic disturbances include impaired fasting glucose and impaired glucose tolerance, both of which are central features in the pathogenesis of type 2 diabetes mellitus (T2DM) (60). Deciphering sex differences in glucose metabolism has important implications for personalized diabetes prevention and management.
i. Impact of gonadal hormones
Prior to menopause, males are more likely to have impaired fasting glucose, but females show higher prevalence of impaired glucose tolerance (111). However, incidence of insulin resistance and T2DM significantly increases after menopause in females, although estrogen replacement therapies may offer a degree of protection (73). Indeed, prospective case-cohort studies have found that early onset of natural menopause is associated with a greater risk of T2DM in postmenopausal females (80). Estrogen replacement therapy leads to decreased fasting glucose and insulin levels, improvement in insulin resistance and reduced fasting glucose in females with diabetes, a 30% reduction in diabetes incidence in post-menopausal females, and a 69 % reduction in diabetes incidence in females with premature menopause (88; 93). Together, these studies support the notion that estrogens are protective in maintaining glucose homeostasis, consistent with lower observed incidence of T2DM in premenopausal females and increased incidence of T2DM after menopause.
Consistent with human observations, animal studies have further supported a protective role of estrogens in glucose metabolism (73). For instance, studies on ovariectomized rodents and primates have shown that ovariectomy accelerates the development of insulin resistance and glucose intolerance (86). Specifically, significant sex differences were found for insulin resistance in mice fed on high-fat and high-carbohydrate diet (86). In addition, orchiectomized males had improved insulin sensitivity, whereas ovariectomized females had decreased insulin sensitivity, demonstrating that estrogens promote insulin sensitivity, while testosterone exacerbates insulin resistance (86). Finally, HFD in intact FCG mice led to high fasting glucose and insulin levels in gonadal males, and rapid clearance of glucose bolus in gonadal females, consistent with a protective effect of estrogen vs. testosterone (4). Together, these studies show that it would be unwise to consider the treatment or management of impaired glucose metabolism or insulin sensitivity without the context of gonadal hormones. Thus, the development of therapeutic strategies aimed at improving glucose metabolism and/or diabetes management, especially in older individuals, should account for hormonal status (and potentially include hormonal replacement therapies) to improve impact.
ii. Impact of sex chromosomes
Studies on FCG mice have also uncovered the existence of sex chromosome effects on glucose metabolism and insulin levels. Indeed, HFD on gonadectomized FCG mice caused a two-fold elevation in fasting insulin levels in XX mice, but not XY mice (23). Although HFD experiments in intact FCG mice supported a key role of gonadal hormones on glucose metabolism and insulin sensitivity (4), insulin levels values were higher in XX mice regardless of the presence or identity of gonads, indicating that the XX karyotype itself also contributes to elevated fasting glucose levels and increased risk of insulin resistance (23; 61). These findings reveal (i) that high fasting insulin levels in males can be driven by acute effects of androgens and (ii) that, in conditions where estrogen levels are reduced (e.g. post-menopause), the XX karyotype underlies increased risk of insulin resistance. Together, these observations indicate that that those complex interplays between sex hormones and sex chromosomes exist on glucose metabolism regulation. Thus, not only is biological sex should be considered a key element in informing therapeutic decisions, but hormonal status should also always be taken into account as an important element.
iii. Sex-specific impact of genetic variants
Accumulating studies have identified genetic variants with sex-specific association to glucose metabolism parameters. For instance, COL26A1 (EMID2) was reported to have female-specific association with fasting glucose (46). In a more recent GWAS meta-analysis, sex differences were also identified for fasting insulin levels at IRS1 and ZNF12 loci (56). Variant rs2943645 at the IRS1 locus had a greater effect on fasting insulin in males than females, consistent with previous reports of IRS1 male-specificity on percentage body fat and lipids (54; 56). The first intron of ZNF12 at rs7798471 had significant association to higher fasting insulin levels in females but not in males, consistent with higher female ZNF12 expression in whole blood (56). Finally, SNP rs1281962 on RGS17 had larger influence on increasing fasting glucose levels in females vs. males (56). Dissecting the molecular underpinning of the sex-dimorphic influence of these genetic variants will be an important steppingstone to harness the complexities of metabolic and glycemic health in humans as a function of sex.
b. Fat metabolism
Males and females also exhibit differences in fatty acid metabolism and fat storage (66). Females have higher body fat than males, and store more fat in the subcutaneous gluteal-femoral region, whereas males store more fat in the visceral region (12).
i. Adiposity
During the postprandial period, males oxidize greater percentages of ingested fat, whereas females tend to incorporate free fatty acids into triglycerides as precursors to stored fat (12; 72). Basal fat oxidation and postprandial free fatty acid release from adipose tissue are lower in females than males, contributing to higher postprandial fat storage in females (12; 92). In-vivo studies using meal fatty acid tracer and adipose tissue biopsies have shown that a higher proportion of dietary fatty acids was stored in the upper body and lower body subcutaneous fat regions in females than males (12). Estrogens are believed to be partly responsible for the low postprandial fatty acid oxidation in females. Indeed, oral estrogen supplementation in postmenopausal females was associated with reduction in postprandial fatty acid oxidation and increased fat mass (64).
Animal studies have not determined a specificity in the impact of estrogens vs. androgens on fat metabolism. Indeed, both sexes of SF-1 KO mice showed significantly elevated fat mass and decreased lean body mass in response to HFD compared with control counterparts (54). Although the results did not show sex differences in this model, they implied a potential role of SF-1 and/or of gonadal hormones deficiency in fat metabolism and body composition. In contrast, XX chromosomes may promote increased fat mass independently of gonadal hormones. Indeed, gonadectomized FCG XX mice accumulated nearly twice as much adipose tissue and developed fatty liver more frequently than XY counterparts (23). Further experiments with XY* mice demonstrated that the X chromosome ploidy contributes to sex differences in fat mass increase (23). These findings indicate that the expression of XCI escapee genes in XX animals can drive sex differences in fat storage, independently of sex hormones, and should thus be constant throughout life.
ii. Plasma lipid profile
There is also considerable sex-dimorphism in plasma lipid profiles, which has led to sex-dimorphic standards for hyperlipidemia diagnoses in males and females (59). Males tend to have higher low-density lipoprotein (LDL) and triglyceride levels, and lower high-density lipoprotein (HDL) levels (59). In intact FCG mice fed on a chow diet, plasma LDL and triglyceride levels were higher in mice with testes, regardless of sex chromosome complement (59). In contrast, HDL cholesterol levels were consistently higher in XX mice, regardless of gonads (59). Further investigations using the XY* mice revealed that the presence of two X chromosomes, not the absence of Y chromosome, led to elevated HDL cholesterol concentrations (59). Thus, the X-inactivation escapee genes in XX mice may drive aspects of observed sex differences in plasma lipid profile. Altogether, sex dimorphic plasma lipid profiles may be a result of interactions between gonadal hormones, sex chromosome complement and dietary intake.
In addition to circulating HDL, LDL, and triglyceride levels, sex-differences in circulating omega-3 fatty acids have also been observed, even after dietary intake adjustment (25). In both humans and rats, females had higher plasma omega-3 fatty acids levels than males (e.g. docosahexaenoic acid [DHA]) (26). High omega-3 levels positively correlate with circulating E2 and progesterone, and negatively with circulating testosterone (26). Use of oral contraceptives and hormone-replacement therapy also correlate with higher plasma concentrations of omega-3 fatty acids (26). The importance of sex hormones can also be gleaned from a study of transgender individuals, in which male-to-female transgender individuals who received a combination of oral ethynyl estradiol and cyproterone acetate had higher DHA levels compared to female-to-male transgender individuals receiving intramuscular testosterone (26). Collectively, these studies show that sex hormones play an important role in regulating plasma content of omega-3 fatty acids, with estrogens associating with higher plasma levels, and testosterone with lower plasma levels (25; 26).
c. Protein metabolism
Although studies of sex-differences in protein metabolism are still rare, emerging evidence suggests that protein metabolism is another area of physiology influenced by sex. For instance, a sex-dimorphic response to mixed meal ingestion has been observed, with a more pronounced anabolic response in males vs. females (66; 102). Amino acids are generally lower in the plasma of females vs. males, with the exception of citrulline, cysteine, aspartate, glycine, serine and taurine (89). Studies using stable isotopic tracers to examine leucine kinetics have identified some sex differences in whole body protein and amino acid metabolism (105). However, the direction of sex-dimorphism in leucine metabolism has not been robust across studies (105), although there may be more support to at least suggest that leucine oxidation may be generally greater in males than females (74; 113). While sex-differences in leucine oxidation may be confounded with differences in intake, McKenzie and colleagues calculated that protein intake accounted for only 12% of the difference in leucine oxidation between males and females (74). Volpi and colleagues also found that leucine oxidation was lower in females regardless of protein intake (113), suggesting that sex-differences in leucine oxidation should be independent of dietary protein intake. Because of contradictory evidence on the direction of sex-differences in leucine metabolism (105), independent replication studies will be required to settle the influence of sex on leucine metabolism.
However, trends on global protein metabolism, especially in skeletal muscle tissue, have been more robustly established. In males, higher testosterone levels are correlated with higher muscle protein synthesis and net muscle protein balance, with dramatic increases in muscle mass after puberty co-occurring with increasing testosterone (105). In ovariectomized rats, lean mass is increased compared to sham-operated rats and ovariectomized rats with hormone replacement (106). Although muscle breakdown was not measured, observed differences in lean mass suggest that ovarian hormones inhibit muscle protein synthesis (105). Collectively, these findings are consistent with the notion that testosterone promotes increased muscle protein synthesis in males, while ovarian hormones limit muscle protein synthesis in females. However, the study of sex-differences in protein metabolism is still in its infancy, with contradictory reports suggesting that more controlled studies, using well controlled human cohorts as well as mechanistic animal models, will be required to determine the extent and impact of sex-dimorphism in protein metabolism.
5. Sex-dimorphic response to dietary restriction
In addition to “normal” ad libitum conditions where feeding is not deliberately limited, the impact of nutrients and energy restriction has been studied in humans and animal models through dietary restriction (DR) in both sexes, revealing interesting divergences in physiological responses.
a. Types of DR regimens
Several DR paradigms have been studied in longitudinal studies to investigate the impact of nutrient sensing on overall health (8; 57). Caloric restriction (CR), an overall reduction in caloric intake (e.g 40%) in absence of malnutrition, is one of the most well studied forms of DR with health and longevity benefits (8). Low energy diet (LED) is similar to CR but involves a fixed energy formula (e.g. 825–853 kcal/day), rather than a percentage reduction in caloric intake (28). Fasting or abstaining from food for a period of time, has also been shown to be associated with positive physiological and health outcomes, as well as weight loss (71). Intermittent fasting (IF) refers to little or no energy intake for extended time periods (e.g. 16–48 h), alternating with periods of normal food intake (71). Time-restricted feeding (TRF) refers to consuming food within a short period of time (e.g. 8 h/day) (39). Short-daily fast (SDF) corresponds to daily 6-hour fasting periods (38). Finally, the fasting mimicking diet (FMD) mimics a fasting state by limiting calorie intake, but provides nourishment to decrease the physical burden of fasting (14). These DR regimens impact metabolism, weight loss, reproduction, and longevity of humans and other animal models, with varied effects across sexes (39; 71).
b. Sex-dimorphic metabolic responses to DR
In a study that explored the use of LED in individuals with pre-diabetes, weight loss was notably greater in males than in females (28). In addition to increased weight loss with LED and CR, men lost more fat from intra-abdominal compartments, while females lost more fat from subcutaneous compartments (28; 121). TRF has been explored in humans in the context of Ramadan and religious fasting, where females have been found to lose more subcutaneous fat than males (47). Similar patterns were also observed in rodents subjected to CR. Female rats conserved their body mass more effectively and lost more adipose depots than the males under CR (110). In mice exposed to DR, males waited until food was returned and then consumed more food, but female mice decreased their overall energy expenditure, and therefore their overall food intake (101). Unlike humans, female mice conserved subcutaneous fat, but lost visceral fat, while males lost both visceral and subcutaneous fat (101). This difference in fat loss is likely to be influenced by differences in gene expression: for example, Cpt1a (the gene encoding carnitine palmitoyltransferase 1a), which is involved in fat oxidation in the liver, is more highly expressed in the visceral fat of male mice and the subcutaneous fat of female mice during fasting (10). When subjected to IF, male mice show an increase in fat oxidation markers while female mice show a decrease in adipocyte differentiation and storage markers (120). A recent study using 6-hour SDF at night showed sex-differences in the magnitude and extent of responses relating hepatic lipid metabolism (38). Females had a significantly greater increase in hepatic triglyceride content and expression of de novo lipogenic proteins (38). This was accompanied by greater hepatic mRNA expression of Acyl-CoA thioesterase 2 (Acot2) and enoyl-CoA hydratase and 3-hydroxyacyl CoA dehydrogenase (Ehhadh), genes involved in fatty acid oxidation (38). Sex-dimorphic gene expression in somatic cells and tissues is prevalent, and thought to drive a number of key health-relevant sex-dimorphic organismal phenotypes throughout lifespan, with likely contributions of both gonadal hormones and sex chromosomes to the establishment of such patterns (94). Thus, analysis of gene regulation in response to dietary interventions with sex-dimorphic outcomes should help elucidate underlying mechanisms underlying sex-dimorphism and thus allow the design of more tailored and effective strategies as a function of sex.
c. Sex-dimorphic impact of DR on reproduction
While the effects of DR on human reproduction have not been studied extensively, its effects on reproduction in animal models have been found to differ between sexes (69; 70; 109). In a study of CR in rats, limited daily energy intake led to smaller gonads in females, but not in males. CR and IF also caused irregularities in the cycling patterns of female rats (70). These changes in gonad size and cycle regularity were accompanied by changes in hormone levels. In response to CR, plasma E2 levels decreased in both males and females, while testosterone levels increased in both sexes (70). TRF improved reproductive outcomes when mice are subjected to a diet high in fat, by reducing placental inflammation and fetal developmental defects (109). CR in female rats after puberty also extends reproductive longevity and increases the number of reproductive cycles the rats go through in their lifetime. Mechanistically, CR was associated to increased levels of luteinizing hormone (LH), which could be responsible for the delay in reproductive aging, since LH levels decrease with age (75). Other hormones related to reproduction, including growth hormone (GH), were shown to increase with CR, with more pronounced effects in males in CF and IF (69). Fasting has also been linked to a decrease in gonadotropin releasing hormone (GnRH) gene expression in male rats (44). This leads to a decrease in GnRH secretion, a central regulator of the reproductive axis, and therefore leads to a decrease in the secretion of serum gonadotropins (44). Although more studies are required to determine how these results extend to humans, sex differences in the impact of DR on reproduction may be important to inform medical recommendations for DR regimens in women of reproductive age.
d. Sex-dimorphic impact of DR on longevity
DR has been extensively linked to increased longevity (51; 77; 81). However, sex-differences in Dr-induced longevity are not always consistent across studies, with observations of no sex dimorphism in the effect on longevity, larger increases in DR-driven longevity in females, or larger increases in DR-driven longevity in males (51). These variable observations on the impact of DR on longevity may be partly related to genetic backgrounds, as well as DR regimens. In C57BL/6 mice, 20% CR extended lifespan in both males and females, but 40% CR led to male-specific lifespan benefit (77). In contrast, 40% CR leads to increased lifespan in both males and females in DBA/2J mice (77). Although the reasons for sex dimorphism in responses to DR have not yet been elucidated, it has been hypothesized that the differences could be due to variations in gonadal hormones, mitochondrial control, body fat distribution, adiponectin and leptin hormones, or differences in investment in reproduction (81).
Collectively, studies up to date have revealed sex-dimorphic effects of DR in both human and animal studies, ranging from energy balance and metabolism, to reproduction, and to lifespan. These differing effects indicate that males and females exhibit fundamental differences in nutrient and energy preservation. Nonetheless, further studies that differentiate whether these effects are due to gonadal hormones or sex chromosomes are needed to decipher the underlying mechanisms.
6. Gastrointestinal and microbiome-relevant sex-dimorphism in nutrition
The interactions between food and consumers also include the interaction between food and gut microbiomes of the consumer, with important implications for nutrient metabolism and health. The adult human gut microbiota, consisting of approximately 1013 to 1314 microbes, plays important functions in metabolism, including food degradation, lipid storage, and vitamin synthesis (99). Perturbations of the gut microbiota have been linked to various metabolic conditions, including adiposity and insulin resistance. In a healthy human adult, the gut microbiome is composed of high proportions of bacteria from the Bacteroidetes and Firmicutes phyla, and low levels of Proteobacteria, Actinobacteria, Fusobacteria and Verrucomicrobia phyla. Bacteroidetes are involved in polysaccharide metabolism and calorie absorption; Firmicutes are required to ferment non-digestible polysaccharides ingested in the diet (e.g. cellulose) to produce short-chain fatty acids (SCFAs), including butyrate, acetate and propionate (15). Additionally, other commensal bacteria in the gut are essential for mediating bile acid synthesis, lipid absorption, amino acid metabolism, and vitamin synthesis (49; 68). In an “unhealthy gut,” or gut dysbiosis, reduced microbial diversity and overgrowth of pathogenic micro-organisms (e.g. Enterobacteriaceae, Bacteroides fragilis) are observed (84; 118). In support of these findings, germ-free mice are resistant to diet-induced metabolic diseases (9).
a. Lifelong sex-dimorphism in the gut microbiome
Sex-specific characteristics in the gut microbiome profile have been reported across host species (79; 85). Animal studies have reported higher abundances of Bacteroidetes, Firmicutes and Proteobacteria in the gut microbiome of male mice compared to female mice (50; 67). For example, in a European population study, both Bacteroidetes and Prevotella were higher in males compared to female subjects (79). Proteobacteria, Veillonella and Blautia are more abundant in females (45). In humans, microbiome profiles between sexes start to diverge only after puberty, which is indicative of a close functional relationship between the gut microbiome and sex hormones (36; 123). Although abundances of specific microbes in the gut differ among organisms, sex-dimorphic profile of the gut microbiome is a widespread phenomenon.
Cumulative studies have indicated causal effects of the gut microbiome on sex-specific characteristics of host, through sex hormone metabolism and sex-specific gene expression regulation. Human fecal micro-organisms have been shown to affect metabolism of androgens and estrogens (62). Specifically, the human gut microbiome is capable of hydrolyzing estrogen sulfate and glucuronide conjugates; thus, circulating estrogen levels can be manipulated through alterations of the gut microbial composition (35; 62). Interestingly, sex hormones are reported to affect the gut microbial composition of the host. For example, ovariectomized female mice acquire similar microbial profiles to males, and supplementation of ovariectomized female mice with E2 alters gut microbial composition (50). Additionally, administration of testosterone to female neonatal rats decreased gut microbial diversity during adulthood (78). Animal model studies have also shown that the gut microbiome is important for sex-relevant gene expression profiles in distal somatic tissues (119). Collectively, these findings support a close functional relationship between the gut microbiome and sex, mediated by both sex hormones and sex-specific gene expression networks of the host.
b. Sex-dimorphism in the gut microbiome, nutrition and metabolic diseases
Gut microbiome-relevant sex-dimorphic host response to diet and metabolism has been investigated through human and model organism studies (Table 1).
Table 1. Studies on sex-dimorphism in the gut microbiome, nutrition and metabolic diseases.
Summary of gut microbiome-relevant sex-dimorphic host response to diet and metabolism in human and model organism studies
| Condition | Organism | Sex-dimorphic effect | Reference |
|---|---|---|---|
| Diet – CR | Mouse | - CR altered age-related microbiome composition only in female mice | (30) |
| Diet – HFD | Mouse | - Male vs. female mice gained more weight on the same diet - Gut microbiota of females had higher abundance in Parabacteroides, Lactobacillus, Bacteroides and Bifidobacterium vs. males on the same diet |
(87) |
| Diet – high fat overfed | Zebrafish | - HFD regime significantly affected only males - Males overfed with HFD presented with higher abundances of Proteobacteria and Gammaproteobacteria vs. females on the same diet |
(82) |
| Diet – high fat, high sugar vs. low fat, low sugar | Mouse | - Male mice fed with high fat, high sugar diet gained more weight vs. females on the same diet - Male mice fed with high fat, high sugar diet had increased abundances of Allobaculum, whereas female fed had reduced levels of Allobaculum |
(33) |
| Diet – Methionine restriction | Mouse | - Methionine-restricted diet increased Bacteroidaceae and Verrucoccaceae, and decreased Rumminococcaceae in males, whereas it decreased Bacteroidaceae, Verrucoccaceae and Rumminococcaceae in females | (115) |
| Diet – vegetarian and inulin-supplemented | Mouse (Fecal microbiota transfer) | - Microbiota recipient male and female mice showed different microbial profiles after grafting - Parabacteroides distasonis and Blautia faecis overrepresented in male recipients; Clostridium and Escherichia fergusonii/Shigella sonnei overrepresented in female recipients |
(117) |
| Obesity - HFD | Mouse | - HFD decreased abundance of Bacteroidetes and increased levels of Firmicutes and Actinobacteria in ovariectomized female mice - Treatment with E2 slowed down the changes in the gut microbial profiles induced by HFD |
(1) |
| Obesity/fat distribution | Human | - Distinct microbial species between males and females contribute to sex-dimorphic fat distribution patterns | (76) |
| Obesity/Body mass index | Human | - Fusobacterium is enriched in obese males, whereas Bifidobacterium, Coprococcus and Dialister genera are enriched in obese females | (40) |
| Type 1 diabetes | Mouse | - Female nonobese diabetic mouse model of type 1 diabetes [T1D] are more prone to develop T1D - Female mice grafted with male fecal microbiota were less likely to develop T1D |
(67) |
| Metabolic syndrome predisposition | Human | - Females with metabolic syndrome shows high levels of Collinsella, Alistipes, Anaerotruncus and Phascolarctobacterium - Males with metabolic syndrome shows high levels of Faecalibacterium and Prevotella |
(95) |
Sex differences in diet-microbiome interactions
Diet, including DR regimens, has been shown to be a major factor that shapes gut microbiota composition (13; 114). Studies have reported gut microbiota-relevant sex-dimorphic responses to diet in different animal models, but further investigation is needed to understand the molecular mechanisms underlying such responses. For example, sex-based differences in host behavior and gut microbiota composition were observed in response to HFD and high sucrose diet in mice (16; 33). Another recently published study showed sex-specific association between the gut microbiome and HFD-induced metabolic disorders in mice (87). Additionally, overfeeding and HFD induced sex-specific alternations in the gut microbiota of zebrafish (82). In mice under high-fat/high-sucrose diet, the abundance of Ruminococcaceae was markedly different between orchiectomized males vs. controls (124). Tuna oil and algae oil supplementations were also found to module gut microbiome differently in males and females – a number of butyric acid producing microbes and Lactobacillus were higher in males, whereas Clostridium XlVa was higher in female mice (40). Concurrently, such sex-dimorphic gut microbiome profiles have been attributed to sex differences in outcomes of metabolic conditions, including obesity and diabetes.
Sex-differences in microbiota and obesity management
Emerging evidence has linked altered gut microbial composition to host obesity. A population study showed distinct signatures in microbial composition between obese and non-obese individuals (53). Transplantation of gut microbiota from obese human or mouse donors resulted in obese phenotype in recipient mice, demonstrating a causal link between the gut microbiome and obesity (91). Interestingly, depletion of gut microbiota through antibiotics treatment resulted in increased adiposity in mice (27). Additionally, changes in body weight have been associated with gut microbial diversity in humans and rodents (20). Obesity-relevant sex-specific microbiome signatures have been reported in humans. For example, in a US population study, lower microbial diversity and reduced abundance of Bacteroidetes were observed in obese females, but not in males (34). In another study, obese males presented with higher abundance of Fusobacterium, whereas obese females showed higher levels of Bifidobacterium, Coprococcus and Dialister (40). Sex-specific differences in body mass index were shown to be associated with the gut microbial composition (40). Interestingly, E2 and HFD were shown to be associated with changes in the gut microbiota in female obesity model mice (ob/ob mice) (1). The study showed that HFD induced decrease in Bacteroidetes and increase in Firmicutes and Actinobacteria in ovariectomized ob/ob females. Treatment of 17ß-estradiol slowed down the changes in the gut microbial profiles induced by HFD in these ob/ob females (1). In HFD-induced obesity, the gut microbiota was shown to be sufficient to drive sex-dimorphic epigenetic changes in colon epithelium (90). Transplantation of gut microbiota from obesity-induced donor mice resulted in epigenetic alterations in male recipient mice only. The observed epigenetic alterations were associated with changes in the expression of genes functionally relevant to intestinal cancers, which highlights the wide-range impact of the gut microbiome on host metabolism and health. Collectively, these findings demonstrate the existence of a complex interplay among the gut microbiota, sex hormones and host gene expression network in obesity management.
Microbiota and diabetes management
Gut dysbiosis also has been attributed to pathogenesis of the two main types of diabetes mellitus (55; 96). Although studies on sex differences in the onset and/or development of diabetes are limited, some sex-dimorphic impact of the gut microbiome has been reported in animal studies regarding type 1 diabetes (T1DM). For example, female Non-Obese Diabetes (NOD) model mice were shown to be more prone to spontaneously develop T1DM compared to NOD male mice (67). However, such differences disappeared when mice were raised in germ-free conditions (67). In the same study, when cecal contents from male NOD mice were transferred to female NOD mice prior to disease onset, recipient female mice were less likely to develop pancreatic islet inflammation, autoantibody production and development of diabetes. Additionally, increased levels of testosterone were observed in the recipient mice. An independent study showed that supplementation of testosterone to NOD female mice prevented disease onset, which further supports a functional association between the gut microbiome, diabetes and biological sex (37).
Sex-differences in response to prebiotics and probiotics
Rodent studies have demonstrated that metabolism and response to prebiotics and probiotics can be sex specific (52; 125). For example, IL-10-deficient male mice that received high-dose isomaltodextrin showed enhanced richness and evenness in microbial diversity and limited reduction in Coprococcus whereas females showed significant reduction in both diversity and Coprococcus abundance (125). The study showed that the observed microbial compositional changes in males were negatively associated with IL-12p70 levels, demonstrating a sex-dimorphic response to dietary fiber supplementation (125). In another study, administration of Lactobacillus animalis NP-51, a probiotic, to BALB/c mice resulted in sex-dimorphic response of the host in terms of cytokine reactions, including IL-1α/β, IL-17, IL-6, IL-10, and IL-12 secretions (52). Thus, sex is an important variable in assessing the potential efficacy of prebiotics and probiotics, although the relative role of sex hormones vs. chromosomes remains unclear. Further studies will be needed to elucidate the molecular drivers that cause gastrointestinal and microbiome-relevant sex-dimorphism in nutrition.
c. Possible impact of the gut-brain axis on sex-dimorphic metabolism
The “gut-brain axis” has called attention to investigations on how the gut microbiome affects brain function through production of bacterial metabolites and/or direct interaction with the enteric nervous system (21). Studies have shown that diet-induced microbiota alterations may influence host behavior, including appetite and eating behavior. For example, lean ground beef fed mice showed reduced activity and food-seeking behavior compared to standard rodent chow-fed mice (58). SCFAs produced by colonic microbiota affects appetite regulation and energy homeostasis (19). Additionally, the gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides (97). Studies have reported and characterized sex differences in the gut-brain axis and changes in such differences throughout lifespan (Reviewed in (48)). However, the impact of the gut-brain axis on host sex-dimorphic response to diet and metabolism has not been fully understood yet.
7. Conclusion and future perspectives
In the context of health and disease, sex has been found to play a differing role across life stages. As highlighted in this review, clinical and preclinical studies have begun to reveal sex-differences in nutrient intake, nutrient metabolism, nutrient restriction, and dietary interactions with the gut microbiome. Since observations of sex-dimorphic responses to dietary intervention and nutrient metabolism are accumulating, there is a clear research need to evaluate the relative contributions of sex hormones and sex chromosome complements in driving such sex differences if we are to harness them to ultimately guide tailored dietary recommendations for women and men’s health. A growing panoply of elegant genetic models (e.g. SF-1 KO, FCG, XY*, aOTT) now provides an exquisite toolkit to understand how sex shapes responses to dietary interventions and metabolic states.
More than a description of sex-differences in nutrition-related phenotypes, understanding the molecular underpinnings of sex-dimorphism in effectiveness of dietary interventions and metabolism will be crucial to open novel and previously inconceivable therapeutic avenues taking into account the biological sex of patients. Indeed, accounting for sex-differences should open exciting new areas for the future of personalized nutrition research, enabling concrete guidelines to optimize growth, reproduction, nutrient metabolism, and disease management across the lifespan. Ultimately, a thorough understanding of hormonal vs. genetics determinants of sex differences will set the stage for the design of optimal, personalized and effective medical and dietary guidelines to elicit maximal health benefits in humans of both sexes.
Acknowledgements
We thank members of the Benayoun laboratory for feedback on the manuscript. We apologize to authors whose work we could not cite due to space constraints.
Mouse image panels generated with Biorender.com. This work was supported by a GCRLE-2020 post-doctoral fellowship from the Global Consortium for Reproductive Longevity and Equality at the Buck Institute, made possible by the Bia-Echo Foundation (to M.K.), and NIA R00 AG049934, Pew Biomedical Scholar award #00034120, an innovator grant from the Rose Hills foundation, and the Kathleen Gilmore Biology of Aging research award (to B.A.B).
References
- 1.Acharya KD, Gao X, Bless EP, Chen J, Tetel MJ. 2019. Estradiol and high fat diet associate with changes in gut microbiota in female ob/ob mice. Sci Rep 9:20192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold AP. 2009. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav 55:570–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold AP. 2019. Rethinking sex determination of non-gonadal tissues. Curr Top Dev Biol 134:289–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold AP. 2020. Four Core Genotypes and XY* mouse models: Update on impact on SABV research. Neurosci Biobehav Rev 119:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold AP, Breedlove SM. 1985. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav 19:469–98 [DOI] [PubMed] [Google Scholar]
- 6.Asarian L, Geary N. 2006. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci 361:1251–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asarian L, Geary N. 2013. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol 305:R1215–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Astafev AA, Patel SA, Kondratov RV. 2017. Calorie restriction effects on circadian rhythms in gene expression are sex dependent. Sci Rep 7:9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. 2007. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A 104:979–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazhan N, Jakovleva T, Feofanova N, Denisova E, Dubinina A, et al. 2019. Sex Differences in Liver, Adipose Tissue, and Muscle Transcriptional Response to Fasting and Refeeding in Mice. Cells 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, et al. 2005. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146:1650–73 [DOI] [PubMed] [Google Scholar]
- 12.Blaak E 2001. Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care 4:499–502 [DOI] [PubMed] [Google Scholar]
- 13.Bolnick DI, Snowberg LK, Hirsch PE, Lauber CL, Org E, et al. 2014. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat Commun 5:4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, et al. 2015. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab 22:86–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brestoff JR, Artis D. 2013. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol 14:676–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridgewater LC, Zhang C, Wu Y, Hu W, Zhang Q, et al. 2017. Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Sci Rep 7:10776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgoyne PS, Arnold AP. 2016. A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues. Biol Sex Differ 7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butera PC. 2010. Estradiol and the control of food intake. Physiol Behav 99:175–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrne CS, Chambers ES, Morrison DJ, Frost G. 2015. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obes (Lond) 39:1331–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cani PD. 2018. Human gut microbiome: hopes, threats and promises. Gut 67:1716–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carabotti M, Scirocco A, Maselli MA, Severi C. 2015. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28:203–9 [PMC free article] [PubMed] [Google Scholar]
- 22.Chai JK, Blaha V, Meguid MM, Laviano A, Yang ZJ, Varma M. 1999. Use of orchiectomy and testosterone replacement to explore meal number-to-meal size relationship in male rats. Am J Physiol 276:R1366–73 [DOI] [PubMed] [Google Scholar]
- 23.Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, et al. 2012. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet 8:e1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Wang L, Loh DH, Colwell CS, Tache Y, et al. 2015. Sex differences in diurnal rhythms of food intake in mice caused by gonadal hormones and complement of sex chromosomes. Horm Behav 75:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Childs CE. 2020. Sex hormones and n-3 fatty acid metabolism. Proc Nutr Soc 79:219–24 [DOI] [PubMed] [Google Scholar]
- 26.Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. 2008. Gender differences in the n-3 fatty acid content of tissues. Proc Nutr Soc 67:19–27 [DOI] [PubMed] [Google Scholar]
- 27.Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, et al. 2012. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488:621–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christensen P, Meinert Larsen T, Westerterp-Plantenga M, Macdonald I, Martinez JA, et al. 2018. Men and women respond differently to rapid weight loss: Metabolic outcomes of a multi-centre intervention study after a low-energy diet in 2500 overweight, individuals with pre-diabetes (PREVIEW). Diabetes Obes Metab 20:2840–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clegg DJ, Brown LM, Woods SC, Benoit SC. 2006. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 55:978–87 [DOI] [PubMed] [Google Scholar]
- 30.Cox LM, Schafer MJ, Sohn J, Vincentini J, Weiner HL, et al. 2019. Calorie restriction slows age-related microbiota changes in an Alzheimer’s disease model in female mice. Sci Rep 9:17904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Culbert KM, Sisk CL, Klump KL. 2021. A Narrative Review of Sex Differences in Eating Disorders: Is There a Biological Basis? Clin Ther 43:95–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalla C, Shors TJ. 2009. Sex differences in learning processes of classical and operant conditioning. Physiol Behav 97:229–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daly CM, Saxena J, Singh J, Bullard MR, Bondy EO, et al. 2020. Sex differences in response to a high fat, high sucrose diet in both the gut microbiome and hypothalamic astrocytes and microglia. Nutr Neurosci:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dominianni C, Sinha R, Goedert JJ, Pei Z, Yang L, et al. 2015. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One 10:e0124599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ervin SM, Li H, Lim L, Roberts LR, Liang X, et al. 2019. Gut microbial beta-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J Biol Chem 294:18586–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, et al. 2016. Population-level analysis of gut microbiome variation. Science 352:560–4 [DOI] [PubMed] [Google Scholar]
- 37.Fox HS. 1992. Androgen treatment prevents diabetes in nonobese diabetic mice. J Exp Med 175:1409–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freire T, Senior AM, Perks R, Pulpitel T, Clark X, et al. 2020. Sex-specific metabolic responses to 6 hours of fasting during the active phase in young mice. J Physiol 598:2081–92 [DOI] [PubMed] [Google Scholar]
- 39.Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, et al. 2018. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr Healthy Aging 4:345–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao X, Zhang M, Xue J, Huang J, Zhuang R, et al. 2018. Body Mass Index Differences in the Gut Microbiota Are Gender Specific. Front Microbiol 9:1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. 2001. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology 142:4751–7 [DOI] [PubMed] [Google Scholar]
- 42.Grgurevic N, Budefeld T, Rissman EF, Tobet SA, Majdic G. 2008. Aggressive behaviors in adult SF-1 knockout mice that are not exposed to gonadal steroids during development. Behav Neurosci 122:876–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grgurevic N, Budefeld T, Spanic T, Tobet SA, Majdic G. 2012. Evidence that sex chromosome genes affect sexual differentiation of female sexual behavior. Horm Behav 61:719–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gruenewald DA, Matsumoto AM. 1993. Reduced gonadotropin-releasing hormone gene expression with fasting in the male rat brain. Endocrinology 132:480–2 [DOI] [PubMed] [Google Scholar]
- 45.Haro C, Rangel-Zuniga OA, Alcala-Diaz JF, Gomez-Delgado F, Perez-Martinez P, et al. 2016. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS One 11:e0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horikoshi M, Mgi R, van de Bunt M, Surakka I, Sarin AP, et al. 2015. Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density Imputation. PLoS Genet 11:e1005230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Husain R, Duncan MT, Cheah SH, Ch’ng SL. 1987. Effects of fasting in Ramadan on tropical Asiatic Moslems. Br J Nutr 58:41–8 [DOI] [PubMed] [Google Scholar]
- 48.Jasarevic E, Morrison KE, Bale TL. 2016. Sex differences in the gut microbiome-brain axis across the lifespan. Philos Trans R Soc Lond B Biol Sci 371:20150122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Just S, Mondot S, Ecker J, Wegner K, Rath E, et al. 2018. The gut microbiota drives the impact of bile acids and fat source in diet on mouse metabolism. Microbiome 6:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaliannan K, Robertson RC, Murphy K, Stanton C, Kang C, et al. 2018. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 6:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kane AE, Sinclair DA, Mitchell JR, Mitchell SJ. 2018. Sex differences in the response to dietary restriction in rodents. Curr Opin Physiol 6:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karunasena E, McMahon KW, Chang D, Brashears MM. 2014. Host responses to the pathogen Mycobacterium avium subsp. paratuberculosis and beneficial microbes exhibit host sex specificity. Appl Environ Microbiol 80:4481–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, et al. 2015. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol 15:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kilpelainen TO, Zillikens MC, Stancakova A, Finucane FM, Ried JS, et al. 2011. Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nat Genet 43:753–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knip M, Siljander H. 2016. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol 12:154–67 [DOI] [PubMed] [Google Scholar]
- 56.Lagou V, Magi R, Hottenga JJ, Grallert H, Perry JRB, et al. 2021. Sex-dimorphic genetic effects and novel loci for fasting glucose and insulin variability. Nat Commun 12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lessan N, Ali T. 2019. Energy Metabolism and Intermittent Fasting: The Ramadan Perspective. Nutrients 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li W, Dowd SE, Scurlock B, Acosta-Martinez V, Lyte M. 2009. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol Behav 96:557–67 [DOI] [PubMed] [Google Scholar]
- 59.Link JC, Chen X, Prien C, Borja MS, Hammerson B, et al. 2015. Increased high-density lipoprotein cholesterol levels in mice with XX versus XY sex chromosomes. Arterioscler Thromb Vasc Biol 35:1778–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Link JC, Reue K. 2017. Genetic Basis for Sex Differences in Obesity and Lipid Metabolism. Annu Rev Nutr 37:225–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Link JC, Wiese CB, Chen X, Avetisyan R, Ronquillo E, et al. 2020. X chromosome dosage of histone demethylase KDM5C determines sex differences in adiposity. J Clin Invest 130:5688–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lombardi P, Goldin B, Boutin E, Gorbach SL. 1978. Metabolism of androgens and estrogens by human fecal microorganisms. J Steroid Biochem 9:795–801 [DOI] [PubMed] [Google Scholar]
- 63.Loos RJ, Yeo GS. 2014. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat Rev Endocrinol 10:51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lwin R, Darnell B, Oster R, Lawrence J, Foster J, et al. 2008. Effect of oral estrogen on substrate utilization in postmenopausal women. Fertil Steril 90:1275–8 [DOI] [PubMed] [Google Scholar]
- 65.Macdiarmid JI, Vail A, Cade JE, Blundell JE. 1998. The sugar-fat relationship revisited: differences in consumption between men and women of varying BMI. Int J Obes Relat Metab Disord 22:1053–61 [DOI] [PubMed] [Google Scholar]
- 66.Marino M, Masella R, Bulzomi P, Campesi I, Malorni W, Franconi F. 2011. Nutrition and human health from a sex-gender perspective. Mol Aspects Med 32:1–70 [DOI] [PubMed] [Google Scholar]
- 67.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, et al. 2013. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339:1084–8 [DOI] [PubMed] [Google Scholar]
- 68.Martens JH, Barg H, Warren MJ, Jahn D. 2002. Microbial production of vitamin B12. Appl Microbiol Biotechnol 58:275–85 [DOI] [PubMed] [Google Scholar]
- 69.Martin B, Golden E, Carlson OD, Egan JM, Mattson MP, Maudsley S. 2008. Caloric restriction: impact upon pituitary function and reproduction. Ageing Res Rev 7:209–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin B, Pearson M, Kebejian L, Golden E, Keselman A, et al. 2007. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology 148:4318–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mattson MP, Longo VD, Harvie M. 2017. Impact of intermittent fasting on health and disease processes. Ageing Res Rev 39:46–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mauvais-Jarvis F 2015. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ 6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mauvais-Jarvis F, Clegg DJ, Hevener AL. 2013. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 34:309–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McKenzie S, Phillips SM, Carter SL, Lowther S, Gibala MJ, Tarnopolsky MA. 2000. Endurance exercise training attenuates leucine oxidation and BCOAD activation during exercise in humans. Am J Physiol Endocrinol Metab 278:E580–7 [DOI] [PubMed] [Google Scholar]
- 75.McShane TM, Wise PM. 1996. Life-long moderate caloric restriction prolongs reproductive life span in rats without interrupting estrous cyclicity: effects on the gonadotropin-releasing hormone/luteinizing hormone axis. Biol Reprod 54:70–5 [DOI] [PubMed] [Google Scholar]
- 76.Min Y, Ma X, Sankaran K, Ru Y, Chen L, et al. 2019. Sex-specific association between gut microbiome and fat distribution. Nat Commun 10:2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, et al. 2016. Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab 23:1093–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moreno-Indias I, Sanchez-Alcoholado L, Sanchez-Garrido MA, Martin-Nunez GM, Perez-Jimenez F, et al. 2016. Neonatal Androgen Exposure Causes Persistent Gut Microbiota Dysbiosis Related to Metabolic Disease in Adult Female Rats. Endocrinology 157:4888–98 [DOI] [PubMed] [Google Scholar]
- 79.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, et al. 2006. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol 72:1027–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muka T, Asllanaj E, Avazverdi N, Jaspers L, Stringa N, et al. 2017. Age at natural menopause and risk of type 2 diabetes: a prospective cohort study. Diabetologia 60:1951–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakagawa S, Lagisz M, Hector KL, Spencer HG. 2012. Comparative and meta-analytic insights into life extension via dietary restriction. Aging Cell 11:401–9 [DOI] [PubMed] [Google Scholar]
- 82.Navarro-Barron E, Hernandez C, Llera-Herrera R, Garcia-Gasca A, Gomez-Gil B. 2019. Overfeeding a High-Fat Diet Promotes Sex-Specific Alterations on the Gut Microbiota of the Zebrafish (Danio rerio). Zebrafish 16:268–79 [DOI] [PubMed] [Google Scholar]
- 83.Nohara K, Zhang Y, Waraich RS, Laque A, Tiano JP, et al. 2011. Early-life exposure to testosterone programs the hypothalamic melanocortin system. Endocrinology 152:1661–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.O’Toole PW, Jeffery IB. 2015. Gut microbiota and aging. Science 350:1214–5 [DOI] [PubMed] [Google Scholar]
- 85.Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, et al. 2016. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 7:313–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parks BW, Sallam T, Mehrabian M, Psychogios N, Hui ST, et al. 2015. Genetic architecture of insulin resistance in the mouse. Cell Metab 21:334–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peng C, Xu X, Li Y, Li X, Yang X, et al. 2020. Sex-specific association between the gut microbiome and high-fat diet-induced metabolic disorders in mice. Biol Sex Differ 11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pentti K, Tuppurainen MT, Honkanen R, Sandini L, Kroger H, et al. 2009. Hormone therapy protects from diabetes: the Kuopio osteoporosis risk factor and prevention study. Eur J Endocrinol 160:979–83 [DOI] [PubMed] [Google Scholar]
- 89.Pitkanen HT, Oja SS, Kemppainen K, Seppa JM, Mero AA. 2003. Serum amino acid concentrations in aging men and women. Amino Acids 24:413–21 [DOI] [PubMed] [Google Scholar]
- 90.Qin Y, Roberts JD, Grimm SA, Lih FB, Deterding LJ, et al. 2018. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome Biol 19:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, et al. 2013. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341:1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Romanski SA, Nelson RM, Jensen MD. 2000. Meal fatty acid uptake in adipose tissue: gender effects in nonobese humans. Am J Physiol Endocrinol Metab 279:E455–62 [DOI] [PubMed] [Google Scholar]
- 93.Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. 2006. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab 8:538–54 [DOI] [PubMed] [Google Scholar]
- 94.Sampathkumar NK, Bravo JI, Chen Y, Danthi PS, Donahue EK, et al. 2020. Widespread sex dimorphism in aging and age-related diseases. Hum Genet 139:333–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santos-Marcos JA, Haro C, Vega-Rojas A, Alcala-Diaz JF, Molina-Abril H, et al. 2019. Sex Differences in the Gut Microbiota as Potential Determinants of Gender Predisposition to Disease. Mol Nutr Food Res 63:e1800870. [DOI] [PubMed] [Google Scholar]
- 96.Sato J, Kanazawa A, Ikeda F, Yoshihara T, Goto H, et al. 2014. Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care 37:2343–50 [DOI] [PubMed] [Google Scholar]
- 97.Schele E, Grahnemo L, Anesten F, Hallen A, Backhed F, Jansson JO. 2013. The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous system. Endocrinology 154:3643–51 [DOI] [PubMed] [Google Scholar]
- 98.Schurz H, Salie M, Tromp G, Hoal EG, Kinnear CJ, Moller M. 2019. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics 13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sender R, Fuchs S, Milo R. 2016. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 164:337–40 [DOI] [PubMed] [Google Scholar]
- 100.Seu E, Groman SM, Arnold AP, Jentsch JD. 2014. Sex chromosome complement influences operant responding for a palatable food in mice. Genes Brain Behav 13:527–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi H, Strader AD, Woods SC, Seeley RJ. 2007. Sexually dimorphic responses to fat loss after caloric restriction or surgical lipectomy. Am J Physiol Endocrinol Metab 293:E316–26 [DOI] [PubMed] [Google Scholar]
- 102.Smith GI, Atherton P, Villareal DT, Frimel TN, Rankin D, et al. 2008. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65–80 year old men and women. PLoS One 3:e1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Steemburgo T, Azevedo MJ, Gross JL, Milagro FI, Campion J, Martinez JA. 2013. The rs9939609 polymorphism in the FTO gene is associated with fat and fiber intakes in patients with type 2 diabetes. J Nutrigenet Nutrigenomics 6:97–106 [DOI] [PubMed] [Google Scholar]
- 104.Sullivan EL, Daniels AJ, Koegler FH, Cameron JL. 2005. Evidence in female rhesus monkeys (Macaca mulatta) that nighttime caloric intake is not associated with weight gain. Obes Res 13:2072–80 [DOI] [PubMed] [Google Scholar]
- 105.Tipton KD. 2001. Gender differences in protein metabolism. Curr Opin Clin Nutr Metab Care 4:493–8 [DOI] [PubMed] [Google Scholar]
- 106.Toth MJ, Poehlman ET, Matthews DE, Tchernof A, MacCoss MJ. 2001. Effects of estradiol and progesterone on body composition, protein synthesis, and lipoprotein lipase in rats. Am J Physiol Endocrinol Metab 280:E496–501 [DOI] [PubMed] [Google Scholar]
- 107.Trace SE, Baker JH, Penas-Lledo E, Bulik CM. 2013. The genetics of eating disorders. Annu Rev Clin Psychol 9:589–620 [DOI] [PubMed] [Google Scholar]
- 108.Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, et al. 2009. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139:1130–42 [DOI] [PubMed] [Google Scholar]
- 109.Upadhyay A, Anjum B, Godbole NM, Rajak S, Shukla P, et al. 2019. Time-restricted feeding reduces high-fat diet associated placental inflammation and limits adverse effects on fetal organ development. Biochem Biophys Res Commun 514:415–21 [DOI] [PubMed] [Google Scholar]
- 110.Valle A, Catala-Niell A, Colom B, Garcia-Palmer FJ, Oliver J, Roca P. 2005. Sex-related differences in energy balance in response to caloric restriction. Am J Physiol Endocrinol Metab 289:E15–22 [DOI] [PubMed] [Google Scholar]
- 111.van Genugten RE, Utzschneider KM, Tong J, Gerchman F, Zraika S, et al. 2006. Effects of sex and hormone replacement therapy use on the prevalence of isolated impaired fasting glucose and isolated impaired glucose tolerance in subjects with a family history of type 2 diabetes. Diabetes 55:3529–35 [DOI] [PubMed] [Google Scholar]
- 112.Ventura-Clapier R, Dworatzek E, Seeland U, Kararigas G, Arnal JF, et al. 2017. Sex in basic research: concepts in the cardiovascular field. Cardiovasc Res 113:711–24 [DOI] [PubMed] [Google Scholar]
- 113.Volpi E, Lucidi P, Bolli GB, Santeusanio F, De Feo P. 1998. Gender differences in basal protein kinetics in young adults. J Clin Endocrinol Metab 83:4363–7 [DOI] [PubMed] [Google Scholar]
- 114.von Schwartzenberg RJ, Bisanz JE, Lyalina S, Spanogiannopoulos P, Ang QY, et al. 2021. Caloric restriction disrupts the microbiota and colonization resistance. Nature 595:272–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wallis KF, Melnyk SB, Miousse IR. 2020. Sex-Specific Effects of Dietary Methionine Restriction on the Intestinal Microbiome. Nutrients 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Waltz M, Saylor KW, Fisher JA, Walker RL. 2021. Biomedical Researchers’ Perceptions of the NIH’s Sex as a Biological Variable Policy for Animal Research: Results from a U.S. National Survey. J Womens Health (Larchmt) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang JJ, Wang J, Pang XY, Zhao LP, Tian L, Wang XP. 2016. Sex differences in colonization of gut microbiota from a man with short-term vegetarian and inulin-supplemented diet in germ-free mice. Sci Rep 6:36137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang T, Cai G, Qiu Y, Fei N, Zhang M, et al. 2012. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J 6:320–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weger BD, Gobet C, Yeung J, Martin E, Jimenez S, et al. 2019. The Mouse Microbiome Is Required for Sex-Specific Diurnal Rhythms of Gene Expression and Metabolism. Cell Metab 29:362–82 e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilson RA, Stathis CG, Hayes A, Cooke MB. 2020. Intermittent Fasting and High-Intensity Exercise Elicit Sexual-Dimorphic and Tissue-Specific Adaptations in Diet-Induced Obese Mice. Nutrients 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wirth A, Steinmetz B. 1998. Gender differences in changes in subcutaneous and intra-abdominal fat during weight reduction: an ultrasound study. Obes Res 6:393–9 [DOI] [PubMed] [Google Scholar]
- 122.Wu BN, O’Sullivan AJ. 2011. Sex differences in energy metabolism need to be considered with lifestyle modifications in humans. J Nutr Metab 2011:391809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, et al. 2012. Human gut microbiome viewed across age and geography. Nature 486:222–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoon K, Kim N. 2021. Roles of Sex Hormones and Gender in the Gut Microbiota. J Neurogastroenterol Motil 27:314–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Z, Hyun JE, Thiesen A, Park H, Hotte N, et al. 2020. Sex-Specific Differences in the Gut Microbiome in Response to Dietary Fiber Supplementation in IL-10-Deficient Mice. Nutrients 12 [DOI] [PMC free article] [PubMed] [Google Scholar]


