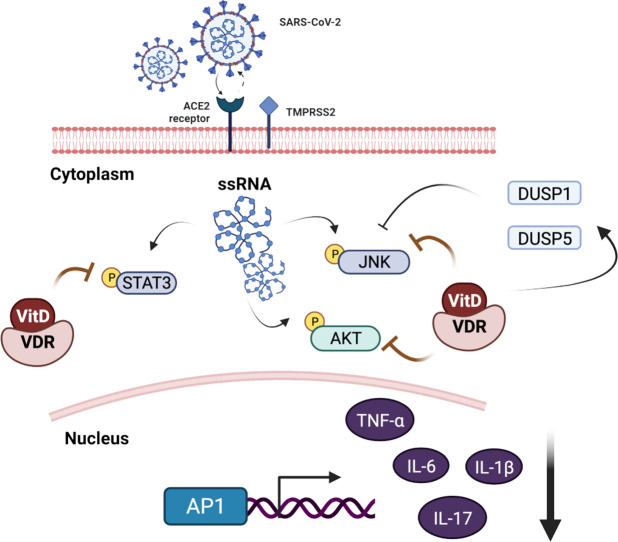
Abstract
Aims
The ability of vitamin D (VitD) to modulate immune responses in the clinical setting of COVID-19 infection is not well investigated. This study aimed to evaluate the ability of VitD to attenuate inflammatory responses in patients with severe COVID-19.
Materials and methods
Blood samples and nasopharyngeal swabs were obtained from patients with severe COVID-19 who had been treated (20 patients), or not (25 patients), with VitD, during their stay in the intensive care unit. Western blotting was used to evaluate the expressions of STAT3, JNK and AKT signaling pathways and ELISA was used to measure levels of IL-6, IL-17, and IL-1β in blood of these patients.
Key findings
Reduced levels of STAT3, JNK and AKT pathways and lower levels of proinflammatory cytokines such as IL-6, IL-17, and IL-1β were observed in VitD treated patients (50,000 IU of cholecalciferol weekly for 3 weeks), and in vitro following treatment of poly I:C stimulated PBMCs with VitD (50 nM of calcitriol). Moreover, lower circulatory levels of these proinflammatory cytokines following treatment with VitD were associated with lower serum levels of COVID-19-related severity markers such as D-dimer and C-reactive proteins (P < 0.001) which in overall resulted in shorter length of ICU stay for VitD treated compared to untreated patients (18 days for VitD treated vs. 28 days for VitD untreated; P = 0.01).
Significance
This study reveals that VitD plays immunomodulatory role during COVID-19 infection, which further emphasizes the importance of maintaining a normal level of this vitamin for the prevention of hyperinflammatory conditions associated with COVID-19.
Keywords: Vitamin D, Anti-inflammatory effects, COVID-19, STAT3, IL-6, IL-17
Graphical abstract
1. Introduction
As of the beginning of pandemic two years ago, SARS-CoV-2, the causative agent of COVID-19, has infected >500 million people worldwide. In some patients COVID-19 infection can be asymptomatic while in others it can progress to acute respiratory distress syndrome (ARDS), which can be fatal. Patient factors that contribute to COVID-19 severity include being male, obesity, diabetes, hypertension, as well as nutritional status [1], [2]. It has been well documented that disease severity can be due to dysregulated host immune response, resulting in a cytokine storm [3].
The hyperactive immune response characterized by “cytokine storm” has been defined as acute overproduction and uncontrolled release of many inflammatory cytokines including interleukin (IL)-6, IL-1β, IL-17 and tumor necrosis factor-alpha (TNFα) [4]. Notably, IL-6 and IL-17 are signal transducer and activator of transcription 3 (STAT3) target genes [5], [6], responsible for hyper activation of IL6/STAT3 inflammatory pathway that are central to pathological features of COVID-19 [7]. Vitamin D (VitD) has been shown to downregulate IL6/STAT3 in vitro in calcitriol treated cancer cell lines [8], [9], and calcitriol treated mice with experimental allergic encephalomyelitis (EAE), a model of multiple sclerosis [10]. Moreover, in the clinical setting of patients with multiple sclerosis high-dose vitamin D supplementation (10,400 IU per day of cholecalciferol) reduced IL-17 producing CD4+ T-cells and effector-memory CD4+ T-cells of these patients, an effect that was observed in those with 25-hydroxyvitamin D level of above 20 ng/mL [11]. Based on the established immunoregulatory properties of VitD, it is reasonable to speculate the role of VitD in attenuating hyperinflammation caused by COVID-19. Therefore, here, we have demonstrated that VitD can suppress COVID-19 hyper inflammation of hospitalized patients through attenuating STAT3 signaling and lowering the level of circulatory proinflammatory cytokines.
2. Materials and methods
2.1. COVID-19 patients' cohort
We obtained blood and nasopharyngeal samples of 45 patients with severe COVID-19-related pneumonia who were confirmed to be SARS-CoV-2 positive by polymerase chain reaction (PCR) test. These patients were hospitalized in the intensive care unit (ICU) at the Rashid Hospital in Dubai between September 2020, and January 2021. COVID-19 severity status was defined based on the National Institute of Health (NIH) guidelines as COVID-19 pneumonia requiring hospitalization and oxygen support as high-flow oxygen therapy or mechanical ventilation (non-invasive or by intubation) [12]. At the time of the study, 20 of these patients were treated with VitD (cholecalciferol) administered as 50,000 IU weekly for 3 weeks during their ICU stay. The rest of the patients (n = 25) did not receive VitD. The allocation of patients to VitD was based on national treatment protocols that recommended the addition of 50,000 units of VitD once weekly, and based on the physician's judgment [13]. Laboratory and clinical data at admission is listed in Table 1 , with the exception for serum 25-hydroxyvitamin D [25(OH)D] level as it was not routinely measured per national clinical management protocols of COVID-19 [13]. The selected patients were adjusted for all demographics, comorbidities, and common laboratory inflammation markers of COVID-19 severity, and the use of any kind of immunomodulatory medications (Table 1). All the specimens were collected as part of patient routine clinical care. The ethical approval for this study was obtained from the Dubai Scientific Research Ethics Committee (DSREC), Dubai Health Authority at Rashid Hospital (DSREC-12/2020_02). Written informed consent was obtained from all study participants. Precautions recommended by CDC for safe collection, handling and testing of biological fluids were strictly followed [14].
Table 1.
Clinical characteristics and outcomes of COVID-19 patients.
| Variables | VitD treated patients (n = 20) | Untreated patients (n = 25) |
P-value |
|---|---|---|---|
| Age, years | 59 (46–68) | 62 (53–79) | 0.19 |
| Male sex | 18 (90) | 21 (84) | 0.68 |
| BMI | 29 (25–32) | 28 (24–32) | 0.90 |
| Diabetes mellitus | 11 (58) | 9 (45) | 0.53 |
| Baseline laboratory data (normal range) | |||
| White cell count (3.9–11.10 × 109/L) | 6 (5–12) | 9 (7–13) | 0.63 |
| C-reactive protein (1.0–3.0 mg/L) | 107 (25–172) | 120 (50–201) | 0.13 |
| D-Dimer (0–0.5 μ/mL) | 2 (1–2) | 4 (2–7) | 0.01 |
| Ferritin (10–204 ng/mL) | 495 (352–1557) | 387 (181–1050) | 0.65 |
| COVID-19 supportive therapya | |||
| Tocilizumab | 1 (5) | 4 (16) | 0.36 |
| IFN-α/β | 3 (50) | 3 (50) | 0.77 |
| Outcome measuredb | |||
| Cytokines | |||
| Plasma IL-6 | 15 (12–22) | 419 (110–658) | <0.001 |
| Plasma IL-17 | 22 (18–31) | 329 (82–595) | 0.01 |
| Plasma IL-1β | 33 (30–44) | 48 (43–58) | 0.04 |
| Plasma TNFα | 174 (110–207) | 204 (122–282) | 0.30 |
| Length of ICU stay, median, days | 18 (12–28) | 28 (22–28) | 0.01 |
Data are n (%) or median (IQR). Abbreviation: BMI, body mass index; CRP, C-reactive protein, ICU, Intensive care unit.
COVID-19 supportive therapy during patient hospital stay.
P-values for plasma cytokine levels and length of stay were adjusted for patient's age, male sex, BMI, and diabetes mellitus.
2.2. PBMC in vitro treatment with calcitriol
Peripheral blood mononuclear cells (PBMCs) were isolated from the peripheral blood of 3 healthy controls using Ficoll-Paque, and were resuspended in complete RPMI-1640 media. Furthermore, the cells were treated, or not, with 50 nM calcitriol (Sigma-Aldrich, St. Louis, MO, USA) and/or 25 μg/mL of poly I:C (cat#P1530, Sigma-Aldrich, St. Louis, MO, USA) for 8 h. Then, the total RNA and proteins were isolated from these cells in order to perform reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blot assays, respectively.
2.3. Analysis of protein expression by western blot
Whole blood and nasopharyngeal swab samples were pelleted by centrifugation at 14,000g for 20 min at 4 °C. Protein concentrations were measured using the Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL). Cells were lysed using 10× RIPA Buffer (Abcam, UK) after supplementation with 1× Protease Inhibitor Cocktail (cat#P8340, Sigma-Aldrich, St. Louis, MO, USA) and 1 mM phenylmethylsulfonyl fluoride (cat#78830, Sigma-Aldrich, St. Louis, MO, USA). Fifteen micrograms total proteins were separated using 10 % SDS-PAGE gels. The proteins were transferred onto a nitrocellulose membrane (Bio-Rad, Ca, USA), blocked in skimmed milk for 1 h at room temperature, incubated overnight at 4 °C with antibodies specific to VDR (cat#12550, Cell Signaling Technology, Beverly, MA, USA), p-STAT3 (cat# 9145, Cell Signaling Technology, Beverly, MA, USA), STAT3 (cat# 9132, Cell Signaling Technology, Beverly, MA, USA), p-JNK (cat# 9251, Cell Signaling Technology, Beverly, MA, USA), JNK (cat# 9252, Cell Signaling Technology, Beverly, MA, USA), p-AKT (cat# 9271, Cell Signaling Technology, Beverly, MA, USA), AKT (cat# 9272, Cell Signaling Technology, Beverly, MA, USA), DUSP1 (cat# 35217, Cell Signaling Technology, Beverly, MA, USA), DUSP5 (cat# 3483, Cell Signaling Technology, Beverly, MA, USA), MMP1 (cat#54376, Cell Signaling Technology, Beverly, MA, USA), and β-Actin (cat#8457, Cell Signaling Technology, Beverly, MA, USA) was used as loading controls. The blots were developed using the Clarity Western ECL Substrate (Bio-Rad, Ca, USA) in the ChemiDoc™ Touch Imaging System (Bio-Rad, Ca, USA) and the protein bands were detected and quantified using the Image Lab software (Bio-Rad, Ca, USA).
2.4. Detection of inflammatory cytokine by enzyme-linked immunosorbent assay (ELISA)
IL-17, IL-6, IL-1β, and TNFα cytokine concentrations were determined in plasma samples using commercially available human ELISA kit (human IL-17, DY317-05; human IL-6, DY206-05; human IL-1β, DY201-05 [R&D systems, MN, USA]; and human TNFα, ab181421; Abcam, Cambridge, MA, USA). Assays were preformed following the manufacturer's instructions. All samples were measured in duplicates.
2.5. Gene expression assay using qRT-PCR
Total RNA from PBMCs and whole blood samples was isolated using Trizol reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, CA) [15]. Complementary cDNA was synthesized from 1 μg of RNA using the High-Capacity cDNA Reverse Transcription Kit (cat# 4368814, Applied Biosystems, Foster City, CA, USA) according to the manufacturer's protocol. For cDNA amplification, 5× Hot FirePol EvaGreen qRT-PCR SuperMix (cat#08-36-00008, Solis Biodyne, Tartu, Estonia) was used, and qRT-PCR was performed in QuantStudio 3 Real-Time PCR System (Applied Biosystems, CA, USA) [16]. Primer sequences for IL-6, IL-17, IL-1β, TNFα, and 18s which were used in the qRT-PCR are deposited in Supplementary Table 1. Gene expression was analyzed using the Comparative Ct (ΔΔCt) method upon normalization to the reference gene 18s rRNA [17].
2.6. Statistical analysis
Plasma levels of IL-17, IL-6, IL-1β, and TNFα in VitD-treated and untreated patients was adjusted for patient's age, male sex, body mass index (BMI), and comorbidity of diabetes mellitus (DM) using a linear regression model. Moreover, association of plasma levels of IL-17, IL-6, IL-1β, and TNFα with serum markers of COVID-19 severity such as D-dimer and C-reactive protein (CRP) was analyzed using a linear regression model adjusted for patient's age, male sex, BMI, and DM. The length of ICU stay between these treatment groups were assessed using a generalized linear mixed model, which included fixed effect of treatment and random effect of age, male sex, BMI, and DM. Statistical analysis was performed using IBM SPSS version 26.0, R software version 3.6.1, and GraphPad Prism version 8.0. Furthermore, comparison between two groups was done using unpaired t-test or Mann-Whitney U test, depending on the skewness of the data. All tests were two-tailed and a P value of <0.05 was considered statistically significant.
3. Results
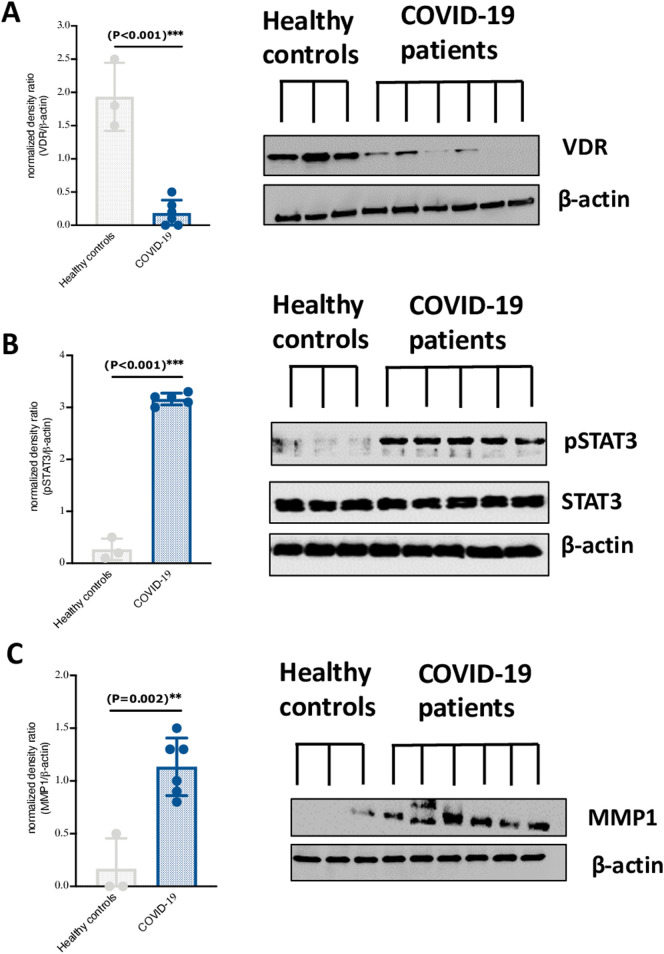
3.1. Downregulation of VDR expression and increased phosphorylation of STAT3 in nasopharyngeal swabs of COVID-19 patients
The relationship between VDR expression and proinflammatory markers with SARS-CoV-2 infection is not well understood. We found that level of VDR was significantly reduced in nasopharyngeal swabs of COVID-19 patients compared to those of healthy controls (Fig. 1A). Additionally, the lower level of VDR was in conjunction with elevated levels of STAT3 phosphorylation and matrix metallopeptidase 1 (MMP1), which is known to mediate expression of inflammatory cytokines regulated via STAT3 signaling [9] (Fig. 1B and C).
Fig. 1.
VDR is lower expressed in nasopharyngeal swabs of COVID-19 patients and associate with higher levels of STAT3 phosphorylation and MMP1 expression. Expression level of (A) VDR, (B) pSTAT3, (C) and MMP1 in nasopharyngeal swabs of COVID-19 patients (n = 5) and healthy controls (n = 3). Statistical tests; Comparison was done using unpaired t-test or Mann-Whitney U test, depending on the skewness of the data. P value of <0.05 was considered significant.
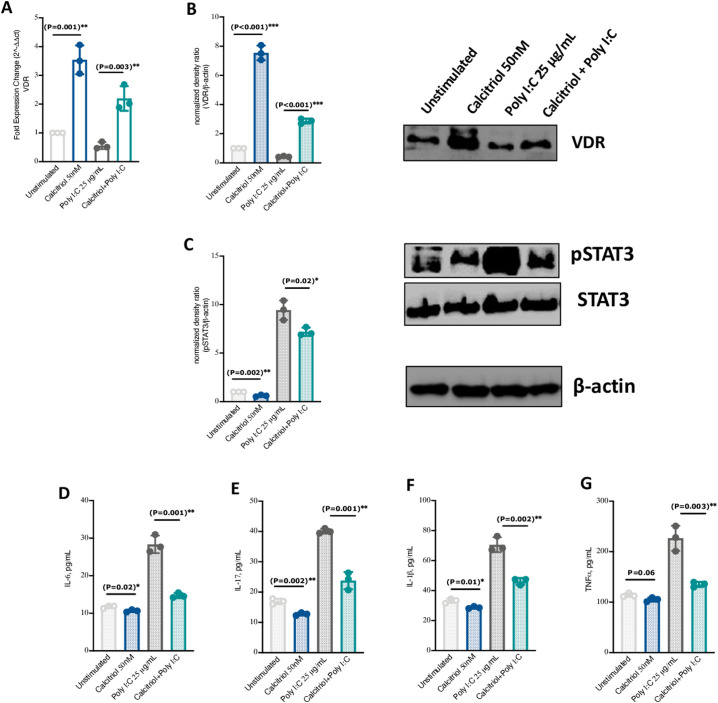
3.2. VitD treatment increased VDR expression while inhibiting STAT3 signaling in PBMCs
In PBMCs isolated from healthy subjects and treated with 50 nM VitD (calcitriol) and/or stimulated with 25 μg/mL poly I:C (mimicking viral infections), the mRNA and protein levels of VDR were significantly upregulated in VitD treated PBMCs stimulated or not with poly I:C (Fig. 2A and B, around 2 Fold Change (FC) increase of VDR mRNA levels; P = 0.003). This upregulation of VDR was also in conjunction with lower STAT3 phosphorylation (Fig. 2C). Additionally, the mRNA and protein levels of pro-inflammatory cytokines such as IL-17, IL-6, IL-1β, and TNFα were significantly lower in VitD treated PBMCs stimulated or not with poly I:C (Fig. 2D–G, and Supplementary Fig. 1).
Fig. 2.
VitD treatment increased VDR expression while attenuating STAT3 signaling in Poly I:C -stimulated PBMCs. (A and B) mRNA and protein expression levels of VDR in VitD treated PBMCs and VitD treated Poly I:C-stimulated PBMCs. (C) Expression levels of pSTAT3 in VitD treated PBMCs and VitD treated Poly I:C-stimulated PBMCs. (D–G) The protein levels of IL-6, IL-17, IL-1β, and TNFα proinflammatory cytokines in VitD treated PBMCs and VitD treated Poly I:C-stimulated PBMCs. PBMCs were isolated from blood of healthy donors (n = 3). Peripheral blood mononuclear cell; PBMC. Statistical tests; Comparison was done using unpaired t-test or Mann-Whitney U test, depending on the skewness of the data. P value of <0.05 was considered significant.
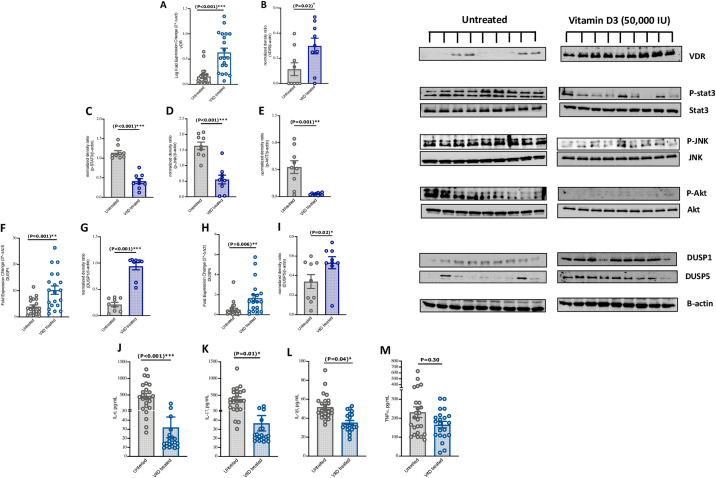
3.3. Lower inflammatory markers in blood of VitD treated patients with severe COVID-19
We then evaluated the effect of VitD on the blood level of inflammatory mediators in COVID-19 patients treated, or not, with VitD. Interestingly, higher mRNA and protein VDR levels were observed in blood of VitD treated compared to the untreated patients (Fig. 3A and B, 0.5 FC increase of VDR mRNA levels, P < 0.001). The phosphorylation level of STAT3 was also found to be reduced in the blood of VitD-treated compared to untreated patients (Fig. 3C, P < 0.001).
Fig. 3.
VitD treatment decreased COVID-19 hyperinflammatory responses through attenuating STAT3 signaling, as well as JNK and AKT of the MAPK cascades in blood of severe COVID-19 patients. (A and B) mRNA and protein expression levels of VDR in whole blood of VitD treated compared to the VitD untreated COVID-19 patients. (C-E) Expression levels of pSTAT3, pJNK, and pAKT in whole blood of VitD treated compared to the VitD untreated COVID-19 patients. (F–I) mRNA and protein expression levels of DUSP1 and DUSP5 in whole blood of VitD treated compared to the VitD untreated COVID-19 patients. (J–M) The protein levels of IL-6, IL-17, IL-1β, and TNFα proinflammatory cytokines in plasma of VitD treated compared to VitD untreated patients. Correlation of IL-6, IL-17, IL-1β plasma levels with serum levels of D-dimer and CRP of these patients. Statistical tests; Comparison was done using unpaired t-test or Mann-Whitney U test, depending on the skewness of the data. Linear regression models adjusted for patient's age, male sex, BMI, and DM. P value of <0.05 was considered significant.
Next, we measured the level of mitogen-activated kinase (MAPK) pathway signaling components in the blood of these patients. Interestingly, the phosphorylation levels of Jun N-terminal kinase (JNK) and phosphoinositide 3-kinase (PI3K)/AKT were lower in blood of VitD treated patients compared to those untreated (Fig. 3D and E, P = 0.001).
Additionally, as dual specificity phosphatases (DUSPs) modulate the magnitude of phospho-activation of JNK and AKT of the MAPK cascades [18], we then evaluated the levels of DUSP1 and DUSP5 in blood of these patients. We found that the mRNA and protein levels of DUSP1 and DUSP5 were elevated in blood of VitD-treated compared to untreated patients (Fig. 3F–I, around 6 FC increase of DUSP1 mRNA level; P = 0.001; and a FC increase of DUSP5 mRNA level; P = 0.006).
Furthermore, the effect of VitD on the levels of IL-17, IL-6, IL-1β, and TNFα in blood of VitD-treated compared to untreated patients was also determined. Of note, mRNA levels in whole blood and protein levels in plasma of IL-6, IL-17, and IL-1β, but not TNFα were significantly reduced in VitD-treated compared to untreated patients when adjusted for patient's age, male sex, BMI, and DM. (Table 1, Fig. 3J–M, and Supplementary Fig. 2).
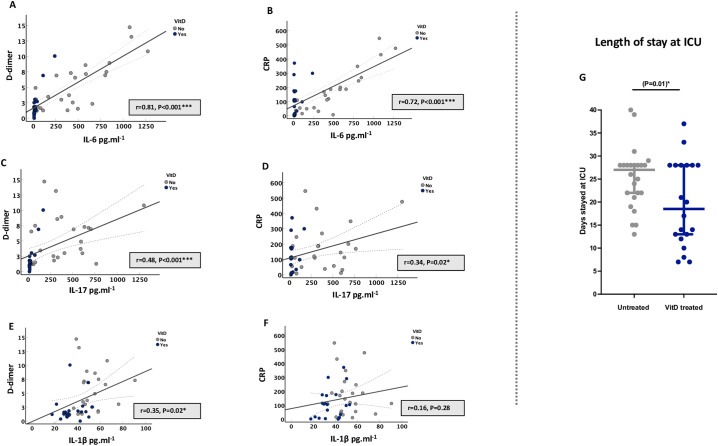
3.4. Lower circulatory pro-inflammatory cytokines of VitD treated patients were associated with less COVID-19 severity
We also determined the association of VitD-induced suppression of pro-inflammatory cytokines on markers of COVID-19 severity. VitD-mediated lower plasma levels of IL-6, IL-17, and IL-1β were associated with lower levels of markers of COVID-19 severity such as D-dimer and CRP; suggesting that VitD treatment could lower disease severity of these patients (Fig. 4A and B, for IL-6; Fig. 4C and D, for IL-17; and Fig. 4E and F, for IL-1β). Moreover, VitD treated patients had shorter length of ICU stay compared to untreated patients (median of 18 vs. 28 days of ICU stay for VitD treated vs. VitD untreated patients, P = 0.01; Table 1 and Fig. 4G).
Fig. 4.
Lower circulatory pro-inflammatory cytokines of VitD treated patients were associated with lower serum levels of COVID-19 severity markers and resulted in shorter length of ICU stay. (A–F) Correlation of IL-6, IL-17, and IL-1β plasma levels with serum levels of D-dimer and CRP of COVID-19 patients. (G) Length of stay at ICU. Statistical tests: Linear regression models adjusted for patient's age, male sex, BMI, and DM; and generalized linear mixed model, which included fixed effect of treatment and random effect of age, male sex, BMI, and DM. P value of <0.05 was considered significant.
4. Discussion
In this study, we have highlighted the immunomodulatory effect of VitD to control systemic inflammation in severe COVID-19 patients. We have shown that patients who received 50,000 units of cholecalciferol per week during their hospital stay had reduced levels of STAT3 signaling, as well as JNK and AKT pathways of MAPK in whole blood of VitD treated COVID-19 patients compared to untreated patients. This was also associated with reduced levels of proinflammatory cytokines such as IL-6, IL-17, and IL-1β; as well as COVID-19-related severity markers in the blood of VitD treated COVID-19 patients.
This immune modulatory effect of VitD can be fundamental in the context of SARS-CoV-2 infection. COVID-19 pathophysiology is initiated by SARS-CoV-2 gene products, the NSP1 and ORF6 proteins, as these viral components induce dysfunction of STAT1 pathway and compensatory hyperactivation of STAT3 [7]. CD4 T cells isolated from bronchoalveolar lavage fluid (BALF) of patients with severe COVID-19 have been shown to be associated with increased STAT3 signaling [19]. Accordingly, we found elevated STAT3 signaling in nasopharyngeal swabs of COVID-19 infected patients as well as blood of patients with severe COVID-19. Although there is evidence that VitD attenuates immune responses possibly by reducing the activity of nuclear factor kappa B (NF-κB) pathways [20]; our data suggest that VitD immune effects may extend to attenuate STAT3 pathways which could contribute to the control of the “cytokine storms” seen in patients with severe COVID-19.
Furthermore, we observed an upregulation of the negative regulator proteins, DUSP1 and DUSP5, with VitD treatment. Even though, transcriptional up-regulation of DUSP1 by VitD has been demonstrated in vitro in VitD-treated monocytes [21], we were able to demonstrate that VitD treatment upregulates DUSP1 and DUSP5 levels in the blood of patients with severe COVID-19. DUSP1 is a corticosteroid dependent gene and therefore, VitD can be beneficial in lowering steroid hyporesponsiveness in these patients [22].
Furthermore, our study has shown that VitD treated patients with severe COVID-19 had significantly lower levels of IL-6, IL-17, and IL-1β cytokines and lower levels of markers of COVID-19 severity such as D-dimer and CRP compared with untreated patients. As for immunomodulatory effect of VitD in treating COVID-19, there is limited evidence of its therapeutic benefit in COVID-19 apart from preliminary data from small, randomized control trials [23], [24], In one trial with VitD in patients with COVID-19 and serum 25(OH)D levels of <30 ng/mL, oral 25(OH)D3 (25 μg of Calcifediol, equivalent to approximately 3000 to 6000 IU per day of cholecalciferol) was associated with increased lymphocyte counts and a decrease in the blood neutrophil-to-lymphocyte ratio of patients. The lower neutrophil-to-lymphocyte ratio was significantly associated with shorter length of stay in ICU and lower mortality rate [8]. Moreover, the SHADE trial of short term, high dose VitD therapy in patients with asymptomatic or mildly symptomatic COVID-19 and serum 25(OH)D levels of <20 ng/mL, showed that a greater proportion of patients taking oral 60,000 IU of cholecalciferol per day for 7 days had a negative test result for SARS-CoV-2 RNA before 21-days, and a significant decrease in serum level of fibrinogen, an inflammatory COVID-19 serum biomarker [24]. Therefore, our results suggest that VitD could potentially be important for the hyper-inflammation in COVID-19 and might have implications for being a therapeutic option for severe COVID-19 cases.
This study has several limitations that should be acknowledged. Firstly, serum 25(OH)D levels on admission and during the hospital stay were not available for VitD treated or VitD untreated patients as it was not recommended by the national guidelines for COVID-19 treatment [13]. Secondly, VitD metabolism may be influenced by genetic factors, but this was not the primary objective of our study. However, despite these limitations, this study is the first to demonstrate the ability of VitD to attenuate major proinflammatory signaling pathways both in vitro, and in COVID-19 patients suffering from severe infection.
In conclusion, our data show that VitD therapy could ameliorate signaling of STAT3 as well as JNK and AKT pathways of MAPK pathway and the production of IL-6, IL-17, and IL-1β cytokines in the blood of patients with severe COVID-19. Such findings suggest the potential use of VitD as an adjunct treatment for COVID-19. Further mechanistic studies and RCTs are required to confirm the immunomodulatory role of VitD on COVID-19.
Abbreviations
- 25(OH)D
25-Hydroxyvitamin D
- AKT
Phosphoinositide 3-kinase (PI3K)/AKT
- ARDS
Acute respiratory distress syndrome
- BMI
Body mass index
- COVID-19
Coronavirus Disease 2019
- CRP
C-reactive protein
- DM
Diabetes mellitus
- DUSPs
Dual specificity phosphatases
- ELISA
Enzyme-linked immunosorbent assay
- ICU
Intensive care unit
- IL-17
Interleukin 17
- IL-1β
Interleukin 1beta
- IL-6
Interleukin 6
- JNK
Jun N-terminal kinase
- MMP1
Matrix metallopeptidase 1
- PBMCs
Peripheral blood mononuclear cells
- SARS-CoV-2
Severe Acute Respiratory Syndrome-Coronavirus-2
- STAT3
Signal transducer and activator of transcription 3
- TNFα
Tumor necrosis factor-alpha
- VDR
Vitamin D receptor
- VitD
Vitamin D
The following are the supplementary data related to this article.
List of primer sequences used in qRT-PCR.
Supplementary figures
Funding
The authors extend their appreciation to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs; Research Chair of Prince Abdullah Ben Khalid Celiac Disease research chair; Riyadh, Kingdom of Saudi Arabia.
CRediT authorship contribution statement
FSSA, RH, SH, and NSSA conceived and designed the experiments; SH and HAHA collected samples and/or performed experiments; FSSA analyzed the data. All authors contributed to writing and revision of the manuscript.
Declaration of competing interest
All authors declare that they don't have any conflict of interests.
Data availability
Data will be made available on request.
References
- 1.Li Y., Tong S., Hu X., Wang Y., Lv R., Ai S., et al. The relationship between nutritional status and the prognosis of COVID-19: a retrospective analysis of 63 patients. Medicine. 2021;100(14) doi: 10.1097/MD.0000000000025287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L., et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern. Med. 2020;180(11):1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cron R.Q., Caricchio R., Chatham W.W. Calming the cytokine storm in COVID-19. Nat. Med. 2021;27(10):1674–1675. doi: 10.1038/s41591-021-01500-9. [DOI] [PubMed] [Google Scholar]
- 4.Mahmudpour M., Roozbeh J., Keshavarz M., Farrokhi S., Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133 doi: 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gharibi T., Babaloo Z., Hosseini A., Abdollahpour-alitappeh M., Hashemi V., Marofi F., et al. Targeting STAT3 in cancer and autoimmune diseases. Eur. J. Pharmacol. 2020;878 doi: 10.1016/j.ejphar.2020.173107. [DOI] [PubMed] [Google Scholar]
- 6.Chang Z., Wang Y., Zhou X., Long J.-E. STAT3 roles in viral infection: antiviral or proviral? Futur. Virol. 2018;13(8):557–574. doi: 10.2217/fvl-2018-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuyama T., Kubli S.P., Yoshinaga S.K., Pfeffer K., Mak T.W. An aberrant STAT pathway is central to COVID-19. Cell Death Differ. 2020;27(12):3209–3225. doi: 10.1038/s41418-020-00633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen P.-T., Hsieh C.-C., Wu C.-T., Yen T.-C., Lin P.-Y., Chen W.-C., et al. 1α,25-dihydroxyvitamin D3 inhibits esophageal squamous cell carcinoma progression by reducing IL6 signaling. Mol. Cancer Ther. 2015;14(6):1365–1375. doi: 10.1158/1535-7163.MCT-14-0952. [DOI] [PubMed] [Google Scholar]
- 9.Zugowski C., Lieder F., Müller A., Gasch J., Corvinus F.M., Moriggl R. STAT3 Controls Matrix Metalloproteinase-1 Expression in Colon Carcinoma Cells by Both Direct and AP-1-mediated Interaction With the MMP-1 Promoter. 392(5) 2011. pp. 449–459. [DOI] [PubMed] [Google Scholar]
- 10.Muthian G., Raikwar H.P., Rajasingh J., Bright J.J. 1,25 dihydroxyvitamin-D3 modulates JAK–STAT pathway in IL-12/IFNγ axis leading to Th1 response in experimental allergic encephalomyelitis. J. Neurosci. Res. 2006;83(7):1299–1309. doi: 10.1002/jnr.20826. [DOI] [PubMed] [Google Scholar]
- 11.Bhargava P., Sotirchos E., Eckstein C., Ntranos A., Gocke A., Mowry E., et al. High-dose vitamin D supplementation reduces IL-17-producing CD4+ T-cells and effector-memory CD4+ T-cells in multiple sclerosis patients (S38.001) Neurology. 2015;84(14 Supplement) S38.001. [Google Scholar]
- 12.COVID-19 treatment guidelines panel. https://www.covid19treatmentguidelines.nih.gov
- 13.Dubai Health Authority https://services.dha.gov.ae/sheryan/wps/portal/home/circular-details?circularRefNo=CIR-2020-00000259&isPublicCircular=true&fromHome=true
- 14.Interim guidelines for collecting and handling of clinical specimens for COVID-19 testing. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html
- 15.Muhammad J.S., Saheb Sharif-Askari N., Cui Z.-G., Hamad M., Halwani R. SARS-CoV-2 infection-induced promoter hypomethylation as an epigenetic modulator of heat shock protein A1L (HSPA1L) gene. Front. Genet. 2021;12(129) doi: 10.3389/fgene.2021.622271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goel S., Saheb Sharif-Askari F., Saheb Sharif-Askari N., Mdkhana B., Mahboub B., Zakri A.M., et al. SARS-CoV-2 switches ‘on’MAPK and NF B signaling via the reduction of nuclear DUSP1 and DUSP5 expression. Front. Pharmacol. 2021;12:404. doi: 10.3389/fphar.2021.631879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuchipudi S.V., Tellabati M., Nelli R.K., White G.A., Perez B.B., Sebastian S., et al. 18S rRNA is a reliable normalisation gene for real time PCR based on influenza virus infected cells. Virol. J. 2012;9(1):1–7. doi: 10.1186/1743-422X-9-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caunt C.J., Keyse S.M. Dual-specificity MAP kinase phosphatases (MKPs) FEBS J. 2013;280(2):489–504. doi: 10.1111/j.1742-4658.2012.08716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chauss D., Freiwald T., McGregor R., Yan B., Wang L., Nova-Lamperti E., et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of TH1 cells. Nat. Immunol. 2022;23(1):62–74. doi: 10.1038/s41590-021-01080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y.-H., Yu Z., Fu L., Wang H., Chen X., Zhang C., et al. Vitamin D3 inhibits lipopolysaccharide-induced placental inflammation through reinforcing interaction between vitamin D receptor and nuclear factor kappa B p65 subunit. Sci. Rep. 2015;5(1):10871. doi: 10.1038/srep10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dauletbaev N., Herscovitch K., Das M., Chen H., Bernier J., Matouk E., et al. Down-regulation of IL-8 by high-dose vitamin D is specific to hyperinflammatory macrophages and involves mechanisms beyond up-regulation of DUSP1. Br. J. Pharmacol. 2015;172(19):4757–4771. doi: 10.1111/bph.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saheb Sharif-Askari F., Saheb Sharif-Askari N., Goel S., Hafezi S., Assiri R., Al-Muhsen S., et al. SARS-CoV-2 attenuates corticosteroid sensitivity by suppressing DUSP1 expression and activating p38 MAPK pathway. Eur. J. Pharmacol. 2021;908 doi: 10.1016/j.ejphar.2021.174374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maghbooli Z., Sahraian M.A., Jamalimoghadamsiahkali S., Asadi A., Zarei A., Zendehdel A. Treatment with 25-hydroxyvitamin D<sub>3</sub> (Calcifediol) is associated with a reduction in the blood neutrophil-to-lymphocyte ratio marker of disease severity in hospitalized patients with COVID-19: a pilot multicenter, randomized, placebo-controlled, double-blinded clinical trial. Endocr. Pract. 2021;27(12):1242–1251. doi: 10.1016/j.eprac.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rastogi A., Bhansali A., Khare N., Suri V., Yaddanapudi N., Sachdeva N., et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study) Postgrad. Med. J. 2022;98(1156):87. doi: 10.1136/postgradmedj-2020-139065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of primer sequences used in qRT-PCR.
Supplementary figures
Data Availability Statement
Data will be made available on request.