Abstract
Probenecid is used to treat gout and hyperuricemia as well as increase plasma levels of antiviral drugs and antibiotics. In vivo, probenecid mainly inhibits the renal SLC22 organic anion transporters OAT1 (SLC22A6), OAT3 (SLC22A8), and URAT1 (SLC22A12). To understand the endogenous role of these transporters in humans, we administered probenecid to 20 healthy participants and metabolically profiled the plasma and urine before and after dosage. Hundreds of metabolites were significantly altered, indicating numerous drug-metabolite interactions. We focused on potential OAT1 substrates by identifying 97 metabolites that were significantly elevated in the plasma and decreased in the urine, indicating OAT-mediated clearance. These included signaling molecules, antioxidants, and gut microbiome products. In contrast, urate was the only metabolite significantly decreased in the plasma and elevated in the urine, consistent with an effect on renal reuptake by URAT1. Additional support comes from metabolomics analyses of our Oat1 and Oat3 knockout mice, where over 50% of the metabolites that were likely OAT substrates in humans were elevated in the serum of the mice. Fifteen of these compounds were elevated in both knockout mice, while 6 were exclusive to the Oat1 knockout and 4 to the Oat3 knockout. These may be endogenous biomarkers of OAT function. We also propose a probenecid stress test to evaluate kidney proximal tubule organic anion transport function in kidney disease. Consistent with the Remote Sensing and Signaling Theory, the profound changes in metabolites following probenecid treatment support the view that SLC22 transporters are hubs in regulation of systemic human metabolism.
Keywords: drug-metabolite interactions, probenecid, transporters, SLC22, remote sensing and signaling theory, endogenous biomarkers, plasma, urine, metabolomics, kidney, proximal tubule, OAT1, OAT3, URAT1
INTRODUCTION
Probenecid is an FDA-approved drug that has historically been used to slow the clearance of drugs in short supply (1). In World War II, probenecid was co-administered with penicillin to increase the half-life of the antibiotic to treat infections in wounded soldiers (1). Probenecid has also been used with several other drugs in this manner, exploiting what are now referred to as drug-drug interactions (DDI) (2). Although probenecid is used to increase the half-life or drugs excreted by the kidney, it is also used in the treatment of gout and hyperuricemia, conditions associated with disordered urate homeostasis (3–5). Probenecid increases renal excretion of urate by inhibiting its reabsorption from the proximal tubule lumen back into the blood, exploiting a drug-metabolite interaction (6–9). In addition to clinical usage, probenecid is also used as an inhibitor of several transporters of organic anions for in vitro studies in research settings (1, 10).
The early uses of probenecid were discovered without specific knowledge of its molecular targets (11). Since then, many of the proteins that participate in the renal organic anion secretory system have been identified in mice and humans, and several have been shown to have direct interactions with probenecid (12). Probenecid has three widely accepted in vivo renal targets primarily expressed in the proximal tubule: organic anion transporters 1 and 3 (SLC22A6/OAT1, SLC22A8/OAT3) and uric acid transporter 1 (SLC22A12/URAT1). Following oral administration, probenecid rapidly enters the bloodstream, where it is highly bound to albumin (11). Protein-bound probenecid is carried through the blood and ultimately the peritubular capillaries, where it inhibits the function of OAT1 and OAT3 (Figure 1). These SLC22 uptake drug transporters are localized to the basolateral membrane of the proximal tubule and transport a wide array of substrates including drugs, endogenous metabolites, natural products, and toxins, as evidenced by the characterization of the Oat1 and Oat3 knockout mice, as well as numerous in vitro studies (13–20). Free, unbound probenecid is filtered by the glomerulus and enters the urinary filtrate, where it acts by inhibiting URAT1, an apical transporter that is involved in the reabsorption of urate from the urine into the cell (21, 22) (Figure 1).
Figure 1: Probenecid effect on the kidney.
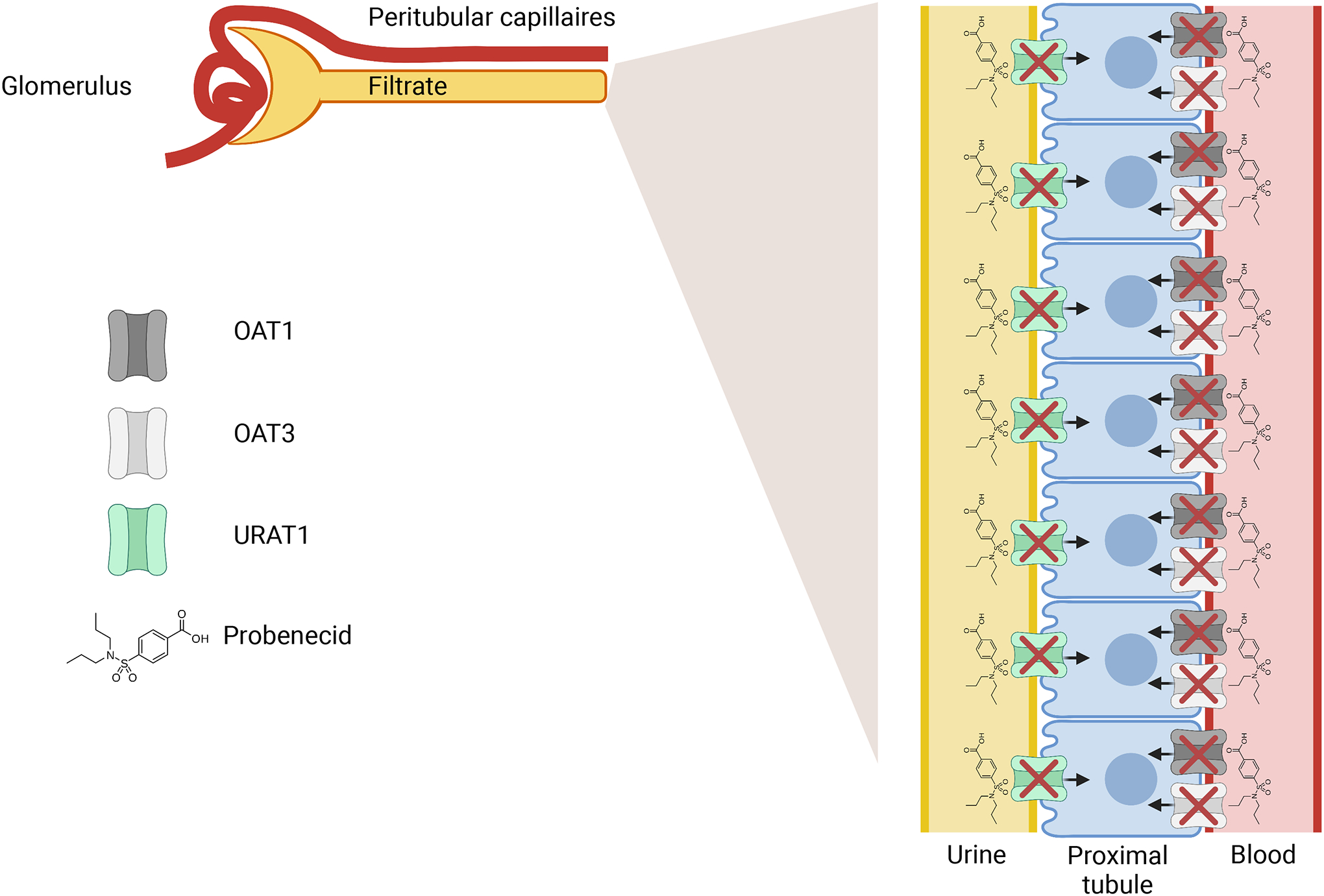
Probenecid inhibits the function of URAT1 on the apical membrane of the proximal tubule. Unfiltered probenecid goes through the peritubular capillaries and inhibits the function of OAT1/OAT3.
These transporters, as well as several other multi-specific, oligo-specific, and mono-specific transporters and enzymes are important regulators of endogenous metabolism, as proposed in the Remote Sensing and Signaling Theory (23, 24). The Remote Sensing and Signaling Theory describes the combined role of drug transporters and drug metabolizing enzymes in inter-organ and intra-organ communication through the movement of small molecules (23–26). Many of these proteins are best known for their role in the absorption, distribution, metabolism, and excretion (ADME) of drugs, but their tissue expression patterns and shared substrate specificity allow them to collaboratively handle many other classes of small molecules, such as endogenous metabolites, natural products, toxins, gut microbiome products and nutrients. One of the key tenets of the Remote Sensing and Signaling Theory is that the primary function of these “drug” ADME proteins is to regulate endogenous metabolism, and that drugs are effectively probes for the endogenous Remote Sensing and Signaling system (24). This is supported by the evolutionary conservation of these gene families across several species, including worms, flies, and sea urchins (27, 28). The OATs and SLC22s in general, are important hubs in a proposed network of multi-specific proteins that aid in returning the body to homeostasis following perturbations, and there is now considerable experimental and human genetic support for this view (16, 29). Given that the primary expression of known in vivo probenecid targets are OAT1, OAT3, and URAT1 in the proximal tubule of the kidney, we hypothesized that the inhibition of these proteins by probenecid would markedly alter the levels of key metabolites and signaling molecules in the blood and urine.
Here, we show that the administration of a clinically prescribed drug, probenecid, has a major impact on the human plasma and urine metabolomes, likely through the direct inhibition of physiologically important transporters, OAT1, OAT3, and URAT1 expressed in the kidney. This broad effect on metabolism is consistent with predictions of the Remote Sensing and Signaling Theory. While DDIs receive the majority of drug transporter-related research attention, the wide substrate specificity of these OATs and other multi-specific transporters raises the possibility of several other competitive interactions, including drug-metabolite, drug-nutrient, and drug-toxin interactions (30–32). In brief, we performed global metabolomics on the plasma and urine of 20 healthy participants collected before and 5 hours after an oral dose of probenecid and analyzed numerous small molecules reflective of potential drug-metabolite interactions (DMI), drug-nutrient interactions (DNI), and other competitive interactions. Hundreds of metabolites were significantly altered in each medium, including 124 that were significantly elevated in the plasma and decreased in the urine, indicating likely OAT-mediated transport. We then compared the altered metabolites to those impacted in the serum of Oat1 and Oat3 knockout mice to identify potential biomarkers for drugs that are handled by the organic anion transport system, which is the main elimination pathway for protein-bound drugs in the kidney. The data support the value of the Oat knockouts for understanding human physiology. They also suggest that the body experiences a metabolic shift following administration of a drug that inhibits organic anion transporters, hubs in human systemic metabolism. We also propose the “probenecid stress test”, which can provide a functional readout on tubular function in healthy and diseased states.
METHODS
Participants
All experimental protocols were reviewed and approved by the Institutional Review Board and abide by the Declaration of Helsinki Ethical Principles. Blood and urine were collected from 20 healthy participants (14 female, 6 male) before and five hours after an oral dose of 1 gram of probenecid. Based on clinical settings (e.g., treatment of gonorrhea with antibiotics), one gram of probenecid was the dose used (33). Participants had an average age of 30.85±10.93 and an average body mass index of 24.18±3.52. The protocol was developed in consultation with clinical researchers at University of California San Diego Altman Clinical and Translational Research Institute (ACTRI). According to the protocol, all participants were asked to be on a diet of no meat, fish, or eggs for 3 days prior to their visit and the day of their visit. Participants were also asked to not take any medications, vitamin C tablets, nutritional supplements, caffeine, chocolates, or cruciferous vegetables the day before and the day of the visit. Participants were also asked to consume an extra liter of water the morning of the visit. There were no additional restrictions on diet between blood/urine collection. All participant data was deidentified. Samples were stored at −80 C until metabolomic analysis.
Metabolomic analysis
Samples were shipped on dry ice to Metabolon Inc. (Durham, NC, USA). Plasma and urine samples were processed separately. Protein was removed from samples, and samples were separated into fractions. These fractions were analyzed by either reverse phase UPLC-MS/MS with positive ion mode electrospray ionization, RP/UPLC-MS/MS with negative ion mode ESI, or HILIC/UPLC-MS/MS with negative ion mode ESI. Following quality control and accounting for instrument and process variability, data was extracted, peak-identified, and assessed for quality by Metabolon. Plasma data was normalized to volume, and urine data was normalized to volume and osmolality. Oat1 and Oat3 knockout plasma metabolomics data, which has been previously extensively analyzed by us, were collated from prior publications and other available data from our lab and followed previous protocols with respect to acquisition and analysis (14, 18, 20). Recent serum metabolomics data on newer platforms is generally consistent with previously published data, and relevant information is included in Supplemental Information.
Metabolic pathway analysis
Plasma and urine metabolomics data were analyzed separately. Missing values were replaced with the minimum observed value for each compound. Plasma fold changes were calculated using volume normalized data, and urine fold changes were calculated using volume and osmolality normalized data by Metabolon. For statistical comparisons, data were log transformed, and a pairwise t-test was used to calculate p-values in plasma and urine. Enrichment was calculated as previously described (18). Principal component analysis was performed using the sci-kit learn package in Python 3.8. Visualizations were performed using the Seaborn package in Python 3.8.
RESULTS
Short-term probenecid treatment alters the levels of hundreds of circulating metabolites
We first focused our attention on the plasma of the participants, as OAT1 and OAT3 have been shown to impact circulating levels of small molecules in human and rodent models due to their basolateral localization (blood-facing) and have been associated with far more small molecule compounds than URAT1 (34). Thus, the combined metabolic roles of OAT1 and OAT3 could best be determined by analyzing the changes in plasma levels of endogenous metabolites and other compounds. We measured the levels of 1,234 unique metabolites, including probenecid and multiple unidentified compounds, spanning numerous biochemical pathways in 20 healthy participants (Figure 2A, Table S1). The global metabolic profiles before and after treatment were largely separable by principal component analysis (PCA), showing that oral dosage of probenecid impacts the plasma metabolome in a consistent fashion (Figure 2B). Initially, we focused on the levels of metabolites that were elevated, as they are likely due to the inhibition of OAT1 and OAT3 in the kidney, and this can potentially be validated by considering metabolites elevated in the Oat1 and Oat3 KO mice previously described by our group (14, 15, 18, 20, 35). We found 354 metabolites that were significantly elevated (p < 0.05, fold change > 1) (Figure 2C) and that many of them were present in biochemical pathways implicated in rodent models, such as tryptophan, tyrosine, phenylalanine, and bile acid metabolism (Figure 2D) (14, 15, 17, 18, 35–37). In addition to determining the metabolic roles of OAT1 and OAT3, we were also interested in how URAT1 inhibition might impact endogenous metabolism. Since probenecid inhibits URAT1 and potentially other organic anion reuptake transporters in the kidney, it is possible that certain metabolites decreased in the plasma are a result of small molecules not being reabsorbed back into the blood from the urine. We identified 230 metabolites that were significantly decreased in the urine, suggesting that URAT1 may play an important role in the circulating levels of compounds in the blood (Figure S1). However, considering the relatively limited substrate specificity of URAT1, it is unlikely that many of these compounds are elevated due to URAT1 inhibition, and some of these changes may be due to temporal variations.
Figure 2: Probenecid treatment alters the plasma metabolome.
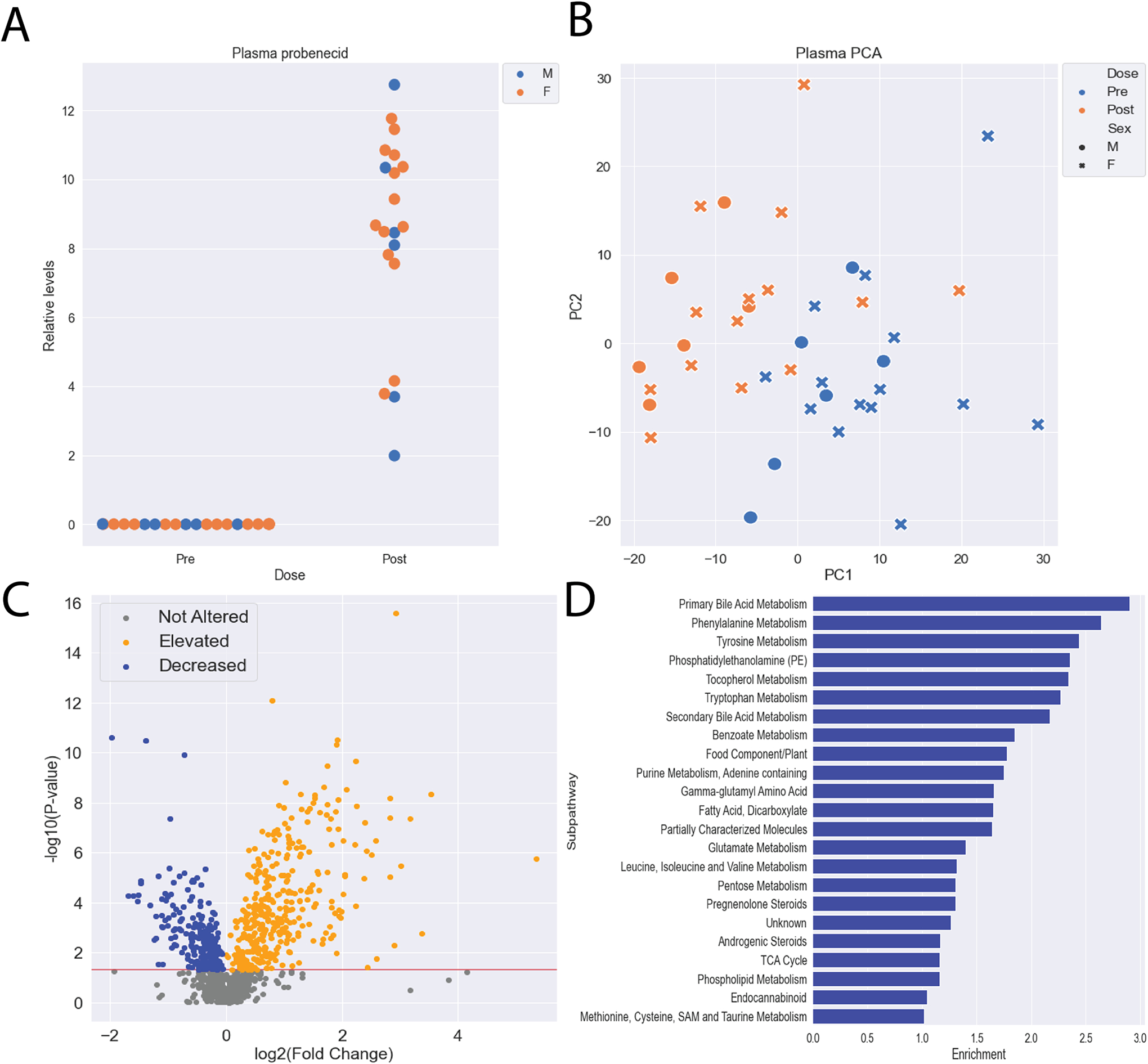
A) Probenecid levels in the plasma were significantly elevated 5 hours after oral dosage in all participants. B) Principal component analysis (PCA) reveals separation between pre and post treatment with probenecid plasma metabolomes. C) Hundreds of metabolites were significantly altered (elevated and decreased) following treatment with probenecid. D) Among the significantly elevated metabolites, 21 subpathways with at least 5 metabolites were enriched, including subpathways traditionally associated with OAT-mediated transport (Primary Bile Acid Metabolism, Phenylalanine Metabolism, Tyrosine Metabolism, Tryptophan Metabolism, etc.).
Metabolites in the urine are mainly decreased following probenecid treatment
Our initial focus was on the plasma of the participants because of the clear implication of OAT1/OAT3 function, but we were also interested in the urine, as OAT1/OAT3 uptake is often the rate limiting step for excretion into the urine. Hence, we assumed that OAT1/OAT3 substrates would have lower levels in the urine. In contrast, URAT1 acts by reabsorbing urate and other compounds from the urine into the proximal tubule, so we also analyzed the metabolites that were elevated in the urine. We measured 1,315 unique metabolites in the urine of the same 20 healthy participants before and after probenecid administration (Table S2). High levels of probenecid were detected in the urine 5 hours after the oral dosage in all the participants (Figure 3A). PCA revealed a separation between pre and post treatment, demonstrating the effect on excretion (Figure 3B). Due to the number of metabolites that were elevated in the plasma, we focused on the metabolites that were decreased in the urine under the assumption that probenecid also prevents these metabolites from passing through the tubular secretion system and entering the urine. 622 metabolites were significantly decreased in the urine (p < 0.05, FC < 1), including multiple metabolites that overlapped with those altered in the plasma. Indeed, some of the subpathways containing elevated metabolites in the plasma also had decreased metabolites in the urine, suggesting that their levels of these metabolites were mediated by probenecid targets (Figure 3C, 3D). While the exact metabolites and subpathways altered in the plasma and urine slightly differed, tryptophan, tyrosine, and bile acid pathways were all enriched for decreased metabolites, which reflected the changes observed in the plasma. In addition, 113 metabolites were elevated in the urine, some presumably due to the inhibition of URAT1 and other less well characterized organic anion reuptake transporters, which covered a different set of metabolic subpathways (Figure S2).
Figure 3: Probenecid treatment alters the urine metabolome.
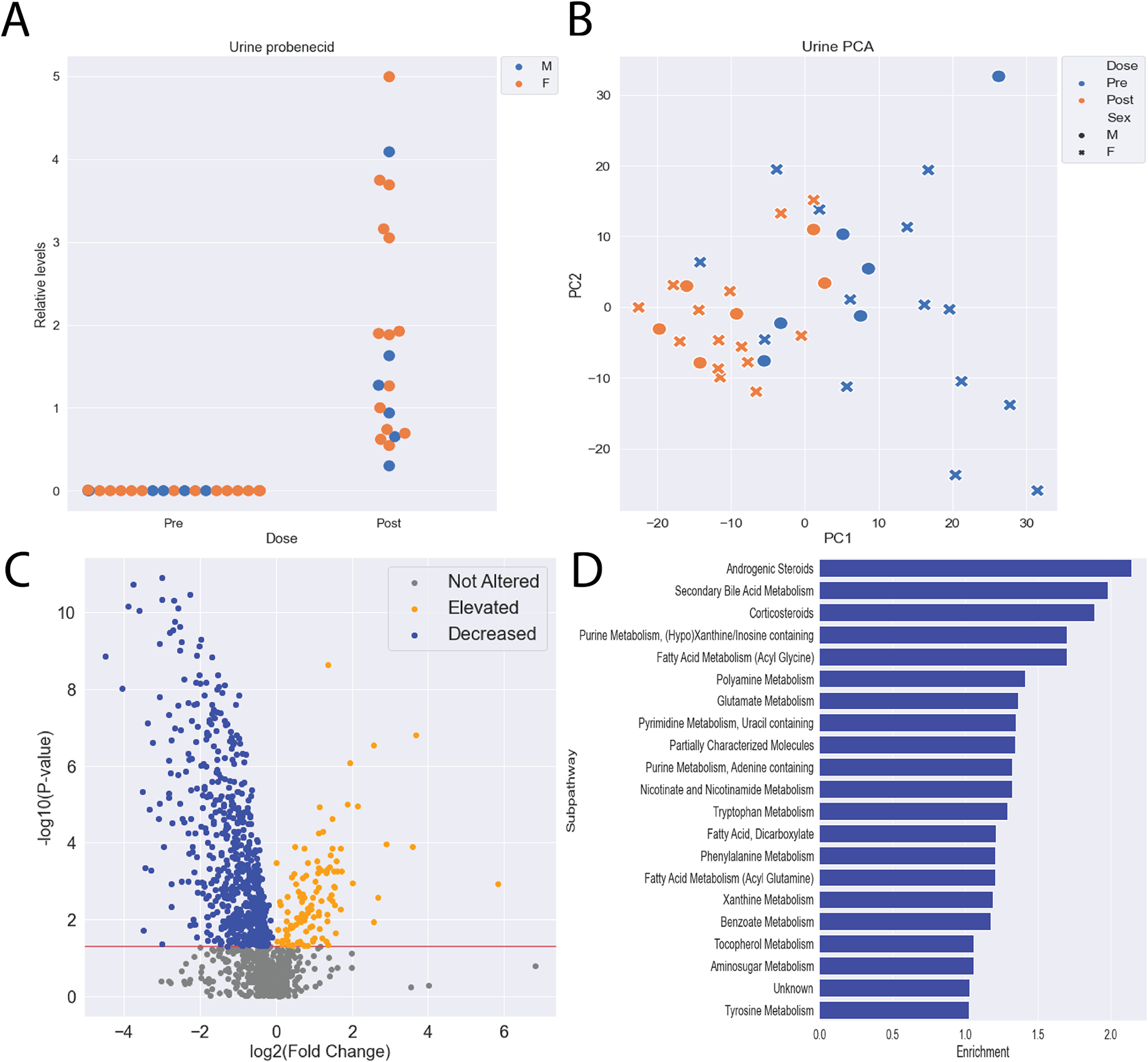
A) Probenecid levels in the urine were significantly elevated 5 hours after oral dosage in all participants. B) Principal component analysis reveals separation between pre and post treatment with probenecid urine metabolomes. C) Hundreds of metabolites were significantly altered following treatment with probenecid, with most being decreased. D) Among the significantly decreased metabolites, 23 subpathways with at least 5 metabolites were enriched, including subpathways traditionally associated with OAT-mediated transport (Secondary Bile Acid Metabolism, Tryptophan Metabolism, Phenylalanine Metabolism, etc.).
97 compounds are likely human OAT substrates based on plasma and urine metabolomics
Of the nearly 2,000 metabolites measured across both experiments, 124 were elevated in the plasma and decreased in the urine, indicating physiologically relevant inhibition of OAT1/OAT3, as both uptake and excretion were altered in the expected manner. To better determine which metabolites may be relevant as endogenous biomarkers, we applied fold change criteria used by regulatory agencies, which generally indicate that for DDI studies the safety margins are between 80 and 125% (38). Hence, we limited our overlap to metabolites with fold changes over 1.25 in the plasma and under 0.8 in the urine, resulting in 97 metabolites, 40 of which have known chemical structures, including 14 with in vitro support (Figure 4, Table S3). Many of these metabolites come from a subset of biochemical pathways, with 7 from tryptophan metabolism, 6 from tyrosine metabolism, 9 from either primary or secondary bile acid metabolism, and 4 from androgenic steroids, which have all previously been associated with OAT function. Several of the altered compounds were characterized by ring structures and negative charges, and many are linked to Phase II drug metabolism. Sulfation and glucuronidation are among the most common conjugations that improve renal excretion, and they are well represented in our subset, with 27 sulfated compounds and 7 glucuronidated compounds, indicating the close association between the OATs and drug metabolizing enzymes. 30 unidentified metabolites were also present, but these have not been linked to any chemical structure or known biochemical role.
Figure 4: Metabolites elevated in the plasma and decreased in the urine are likely OAT1/3 substrates.
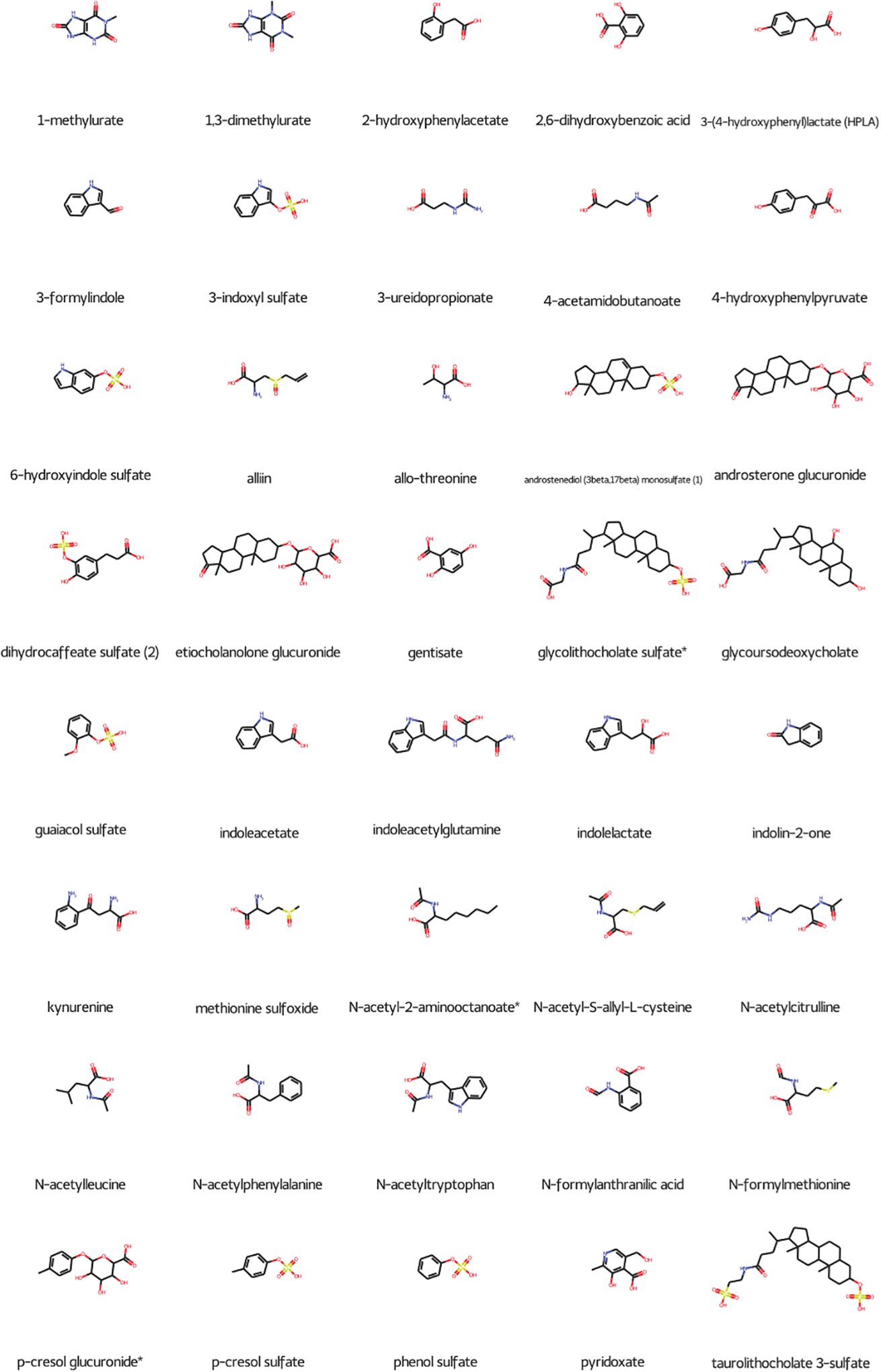
Metabolites were further filtered by fold change criteria, with only plasma metabolites with fold changes over 1.25 and urine metabolites with fold changes under 0.80 included. Overall, 97 metabolites fit these criteria, with 40 having known chemical structures. 34 of these 40 compounds had a total negative charge, and many were also supported by existing in vitro data (Table S3).
Probenecid has a specific drug-metabolite interaction with urate
While the nature of the compounds that were elevated in the plasma and decreased in the urine suggested inhibition of OAT1/OAT3, we also aimed to understand the potential effect of URAT1 inhibition. Along with OAT1 and OAT3, URAT1 is considered a primary in vivo target of probenecid, and its mechanism of action is blocking reabsorption of urate from the urine into the proximal tubule and back into the blood. We compared the metabolites decreased in the plasma and elevated in the urine and found that three compounds (urate, quinate, N-acetylglycine) satisfied both criteria. However, when the more stringent fold changes (FC < 0.8, FC >1.25) were applied only one compound, urate, satisfied both conditions (Figure 5). Since probenecid was highly elevated in the urine, it was able to exert its inhibitory effect, which led to a specific drug-metabolite interaction between probenecid and urate at URAT1. Again, we find it remarkable that of the hundreds of metabolites analyzed in both plasma and urine, only one was elevated in the urine and decreased in the plasma, as might be inferred from clinical, knockout, and in vitro data.
Figure 5: Presumed inhibition of urate reuptake transporters such as URAT1 led to a specific drug-metabolite interaction between probenecid and urate.
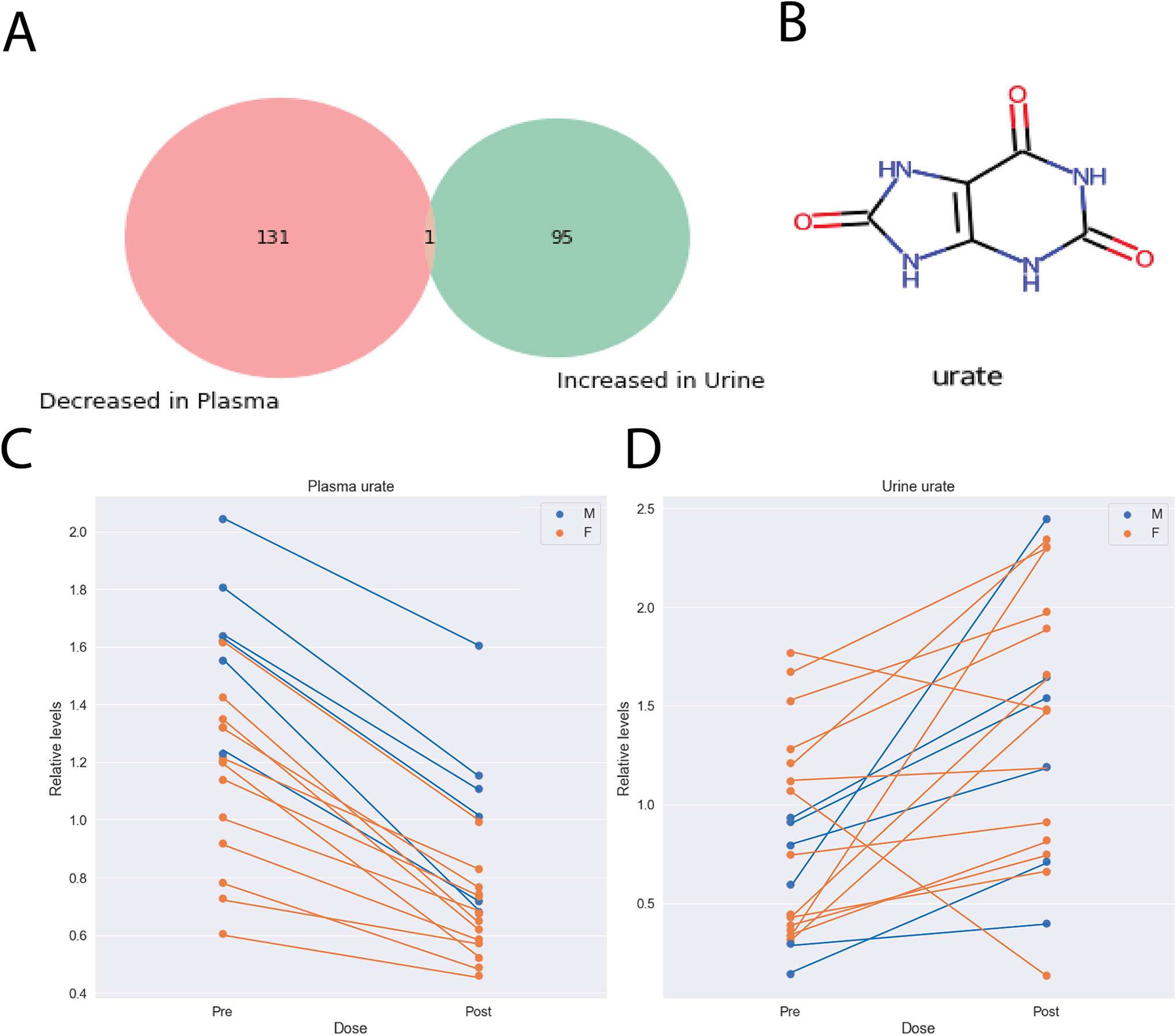
A) Urate was the only metabolite to be significantly decreased in the plasma (fold change < 0.8) and increased in the urine (fold change > 1.25) with more selective fold change criteria. B) The chemical structure for urate. C) Urate levels were significantly decreased in the plasma following treatment with probenecid (p-value: 1.20E-10, fold change: 0.606). D) Urate levels were significantly increased in the urine following treatment with probenecid (p-value: 0.008, fold change: 1.705).
Probenecid treated humans and Oat1/3 knockout mice metabolomics reveal candidate endogenous biomarkers
While OAT1, OAT3, and URAT1 are widely accepted as the main in vivo targets of probenecid, there is the possibility of effects on other transporters (SLC and ABC) which are inhibited in vitro by probenecid. That said, in vivo evidence is lacking for a major role for these other transporters in probenecid-sensitive organic anion transport. Thus, we focused on OAT1, OAT3, and URAT1, for which considerable in vitro, in vivo, and ex vivo support exists (12, 14, 15, 17, 18, 20, 35, 39). Previous work by our group has focused on the roles of these transporters in in vivo endogenous metabolism using genetically engineered mice. We analyzed global metabolic profiling data from the serum of Oat1 and Oat3 knockout mice from multiple studies by our group and found changes that support the important physiological role of the OATs (15, 17, 18, 20, 35, 40, 41). Though there are differences in general physiology, gene expression patterns, and microbiome composition between mice and humans, it was expected that there would be overlap between the knockout mice and humans treated with probenecid. Indeed, there were multiple metabolites that were elevated in the human plasma, the knockout mouse serum, and decreased in the urine (indicative of OAT-mediated transport).
When using the 124 metabolites elevated in the plasma and decreased in the urine, we found that 52 and 48 of these metabolites were measured in the Oat1 and Oat3 knockout mice, respectively. We focused on the metabolites that were elevated in each knockout mouse and 52% (27/52) for the Oat1 and 48% (23/48) for the Oat3. However, we mainly focused on the 97 metabolites that fit the fold change criteria previously described. For this subset, 55% (21/38) of the metabolites measured in humans and Oat1 mice had in vivo knockout mouse support. With respect to OAT3, 56% (19/34) had in vivo knockout mouse support. Fifteen metabolites were elevated in both knockout mice, while 6 were unique to OAT1, and 4 were unique to OAT3 (Figure 6). This work demonstrates that Oat knockout mice can be used to predict potential DMIs at the site of transporters and produce candidates for endogenous biomarkers. While these compounds would need to be further characterized (rate of synthesis, metabolic breakdown, other routes of clearance, etc.) to prioritize for usefulness as endogenous biomarkers, many of them have been shown to be impacted by OAT perturbation in vivo (humans and mice) and in vitro.
Figure 6: Multiple metabolites suggested to be OAT substrates are supported by in vivo Oat1 and Oat3 knockout mice.
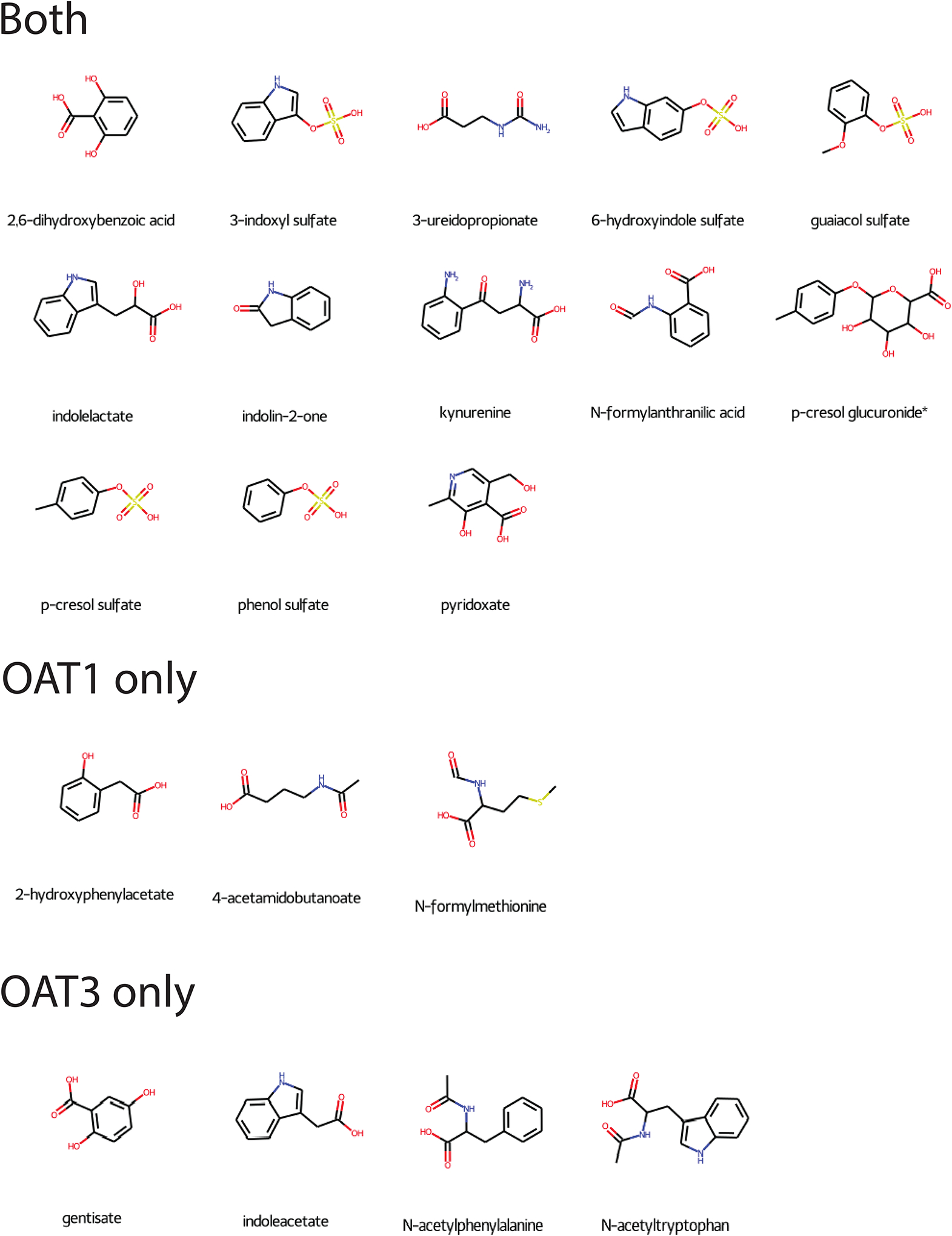
Twenty-five metabolites elevated in the probenecid-treated human plasma, decreased in the probenecid-treated urine, and elevated in one or both knockout mice. Twenty of these metabolites had associated chemical structures. Fifteen (13 with chemical structures) were common to both knockout mice, while 6 (3 with chemical structures) were unique to the Oat1 knockout mice, and 4 were unique to the Oat3 knockout mice.
DISCUSSION
We found that probenecid, a drug used to treat gout and hyperuricemia and increase levels of OAT-transported drugs (e.g., antibiotics, antivirals), had a major impact on the levels of endogenous metabolites and diet-derived compounds in the plasma and urine. The altered pathways spanned several biochemical pathways and functional clusters based on chemical structures, such as bile acids and aromatic amino acids. These biochemical pathways were generally similar to those altered in Oat1 and Oat3 knockout mice (14, 15, 18, 42, 43). Among the surveyed metabolites in the plasma and urine, we noted that many metabolites were elevated in the plasma and decreased in the urine, indicating that inhibition of OAT1 and OAT3 by probenecid leads to a much more pronounced systemic impact than URAT1 inhibition. However, use of probenecid’s known mechanism of action (inhibition of urate reabsorption at URAT1) was also apparent, as urate alone was both elevated in the urine and decreased in the plasma.
Overall, the profound metabolite alterations support the view that OAT1 and OAT3 are “hubs” in a Remote Sensing and Signaling Network of transporters and enzymes regulating metabolism; in particular, the Remote Sensing and Signaling theory emphasizes the roles of these multi-specific “drug” transporters in inter-organ communication mediated by endogenous small organic compounds such as key metabolites, antioxidants, gut microbiome products and signaling molecules that activate GPCRs and nuclear receptors (16, 24, 26).
Many of the impacted metabolites were also altered in the serum of Oat1 and Oat3 knockout mice, supporting the view that the resulting changes are due to the inhibition of OATs. Thus, in the process of analyzing human data, we further validated the usefulness of previous mouse knockout models. In particular, the Oat knockout mice may be useful in predicting drug-metabolite interactions at the site of these transporters and identifying potential endogenous biomarkers of transporter function. Since probenecid is known to target both OAT1 and OAT3, the endogenous biomarkers implicated here would be for general OAT function rather than for one specific transporter. Nonetheless, by comparison with the knockouts, we identified metabolites that may be OAT1 or OAT3 selective, as previously described (17).
While probenecid is not known to have notable short or long term side effects, it is striking that within hours of taking probenecid so many metabolites are elevated in the plasma, such as bile acids and indole derivatives, which have important signaling roles in the body (44, 45). Their increases in circulation can lead to the activation of signaling cascades in organs that interface with the blood, as their bioavailability is elevated. For example, bile acids activate bile acid-specific receptors (i.e., TGR5) and the nuclear receptor FXR (expressed in liver and kidney), which govern many key processes in the gut-liver-kidney axis (46, 47). Consistent with the Remote Sensing and Signaling Theory, the elevated levels of tryptophan and tyrosine metabolites could also play be physiologically important, as they each have distinct signaling roles in different organs (48, 49).
It is important to point out that this increase in OAT-regulated metabolites may also occur with other drugs that inhibit the OATs at varying levels, which could lead to a wide range of metabolic side effects via drug-metabolite interactions at the level of the transporter. While the main targets of probenecid are the renal transporters (OAT1, OAT3, URAT1), several other proteins are known to interact with the drug in vitro, including multi-specific SLC (often SLC22) and ABC transporters (MRPs) and enzymes that also play important roles in key aspects of endogenous metabolism (50). Whether these other proteins are in vivo targets of probenecid in humans is far from clear, but it is conceivable that the inhibition of these proteins may lead to drug-metabolite interactions that are reported in this work.
In the kidney, many metabolites must be salvaged from the urinary filtrate and reabsorbed into the blood. Hundreds of measured compounds in the urine were decreased by probenecid, likely due to the lack of tubular secretion by the OATs, the rate-limiting step in urinary clearance for many organic anions. These metabolites, which include purine derivatives, aromatic amino acid derivatives, and others are primarily excreted through the urine. Steroids were also decreased in the urine, which is consistent with the use of probenecid to mask levels of androgenic steroids in urine samples (51). Like the plasma, it appears that the urine metabolome is more influenced by the inhibition of the OATs than URAT1. In the context of OAT1/3 (basolateral uptake transporters) vs URAT1 (apical uptake transporter), metabolites elevated in the plasma and decreased in the urine are linked to OAT1/3, while metabolites decreased in the plasma and elevated in the urine are linked to URAT1. However, we must also consider the possibility that probenecid may inhibit efflux and retro-transporters expressed on the apical membrane of the proximal tubule, although this remains to be established in vivo.
Recent work by our group and others has highlighted the endogenous role of OAT1 and OAT3 in regulating uremic toxins, amino acid derivatives, lipids, and several other classes of metabolites using genetically engineered knockout mice (14, 15, 17, 18, 20). We hypothesized that the inhibition of these physiologically important proteins with probenecid in humans would lead to similar changes and found that while not all metabolite alterations were reproduced, several classes of metabolites were consistently altered in both the plasma and the urine, namely the metabolites known to interact with OAT1 and OAT3. URAT1, on the other hand, is more specific, with only a few known unique interacting small molecules. While knockout mice appear to be useful models in determining potential drug-metabolite interactions, the inter-species differences in gene expression, diet, and gut microbiome should also be considered, particularly considering that many of the metabolites altered in the mice and humans originate from the gut microbiome. Nonetheless, some of these compounds are strong candidates for endogenous biomarkers used in predicting drug-drug interactions at the site of the OATs.
Of the 25 compounds altered across human and knockout mouse experiments, 6 of these (2-hydroxyphenylacetate, 3-acetylphenol sulfate, 4-acetamidobutanoate, 4-methylcatechol sulfate, N-formylmethionine, 4-methoxyphenol sulfate) are unique to OAT1 and they differ from the compounds currently being tested as endogenous biomarkers for OAT1 (52). As for OAT3, 3 of 4 unique compounds (gentisate, N-acetylphenylalanine, N-acetyltryptophan) altered in humans and Oat3 knockout mice are novel--except for indoleacetate, which has been shown to be a potential endogenous biomarker for OAT function (53). We also compared our results to other potential OAT biomarkers identified by other groups, and we found that our results supported the use of p-cresol sulfate and pyridoxate, in that both of these compounds were elevated in the plasma and decreased in the urine (53, 54). Further criteria, including consistent levels throughout the day, stable production independent of diet, minimal interactions with other proteins, and in vitro support for specificity to OAT1/3 would further support these compounds as strong endogenous biomarkers. The altered compounds could potentially be used as biomarkers for organic anion-related tubular transport, which is largely mediated by OATs and is gaining more attention in the context of renal diseases (55).
Our results suggest that drugs handled by the OATs produce several simultaneous drug-metabolite interactions. The main mechanism of action of probenecid is as an inhibitor of key renal transporters; thus, its impact on the plasma and urine metabolomes is likely stronger than drugs with other targets (1, 4). However, the absorption, distribution, metabolism, and excretion (ADME) of nearly every drug and xenobiotic is handled by a subset of drug transporters and drug metabolizing enzymes (56). It is possible that other drugs cleared by OAT1 or OAT3 could lead to similar consequences in humans if they inhibit the transporter strongly enough. OAT1 and OAT3, in particular, are among the most multi-specific drug transporters, with each interacting with over 100 unique drugs, including NSAIDs, antivirals, antibiotics, and others (19). Chronic treatment with drugs that interfere with the function of transporters could lead to long term metabolic side effects. In HIV patients taking anti-retroviral therapy drugs (many of which are OAT1/3 substrates), it is common to see metabolic side effects relating to lipids after several months (57). Similar situations have been reported with NSAIDs and antibiotics, many of which are OAT substrates (58, 59).
Finally, we note again that tubular secretion and glomerular filtration both contribute to overall kidney function but in many contexts, only glomerular filtration rate is considered. In recent years, there has been a new emphasis on assessing tubular secretion, which is, however, complicated by the fact that renal disease state (CKD, AKI, etc.), genetics and other factors all influence tubular secretion. In light of this, and the data presented in this article, we propose a “probenecid stress test” to evaluate the transport capacity of the proximal tubule, as there is a need to assess organic anion-related tubular function, preferably without the administration of furosemide, a strong diuretic (60).
The probenecid stress test, as we currently envision it, is similar in design to the studies performed here. It would measure the plasma and urine levels of the ~100 metabolites identified here as potential human OAT substrates before and after a single dose of probenecid. With this, a quantitative measure of how compromised organic anion-related tubular function is can be calculated to influence drug dosing and help assess disease state. It can also be used to follow progression of disease. From the pharmaceutical perspective, the test can be used determine how similar a novel drug entity is to probenecid (a complete inhibitor of OATs). Patients may also need to avoid (or be differently dosed) drugs that are primarily secreted through OAT1 and OAT3. Indeed, many drugs that are prescribed in the setting of renal disease (e.g., antibiotics, antivirals, antihypertensives, diuretics) are substrates of OAT1 and OAT3. The use of probenecid in late stage renal disease to isolate tubular function has previously been demonstrated in animal models (40). From the disease perspective, the proposed OAT substrates can be used as biomarkers to assess renal OAT1 and OAT3 tubular function, which comprise the bulk of organic anion transport in the proximal tubule of the kidney and serve as indicatiors of interact drugs, toxins, endogenous metabolites, natural products, and several other classes of small molecule compounds. By focusing on endogenous metabolites, it is possible to assess the physiological function of the organic anion handling proteins in the proximal tubule without (or before) subjecting the tubular system to one or more drugs that may pose risks, especially in the context of declining renal function. The probenecid stress test can also be used as a novel clinical test to measure tubular secretion relative to glomerular filtration rate. Finally, administration of probenecid can also be useful for determining and following organic anion tubular function responsiveness as CKD progresses.
Supplementary Material
STUDY HIGHLIGHTS.
What is the current knowledge on the topic?
Probenecid is believed to inhibit three kidney proximal tubule transporters in vivo. URAT1 mainly transports urate from the urine into the cell, while the basolateral uptake transporters, OAT1 and OAT3, regulate endogenous metabolism as evidenced by alterations in Oat1 and Oat3 knockout mice.
What question did this study address?
What alterations in metabolism does probenecid cause, presumably by drug-metabolite interactions (DMI), and are they occurring at the level of OAT1, OAT3, or URAT1?
What does this study add to our knowledge?
We have identified in vivo DMI potential endogenous biomarkers for OAT1 and OAT3, as well as potential biomarkers for more general tubular function. We also propose a “probenecid stress test” to assess tubular function.
How might this change clinical pharmacology or translational science?
These findings could prove helpful in drug development, and in assessing a patient’s tubular function in acute kidney injury and chronic kidney disease. We have also shown that a drug that is considered quite safe nonetheless leads to hundreds of simultaneous drug-metabolite interactions and suspect this to be the case for other drugs, which could explain some drug side effects or adverse drug reactions.
ACKNOWLEDGEMENTS
Some figures were generated using Biorender. The authors would like to thank Dr. Pranav Garimella for his help in editing the manuscript. This article is dedicated to the memory of Vibha Bhatnagar, MD, MPH.
FUNDING
This work was supported by a grant from the National Institutes of Health (NIH) to S. K. N. from the National Institute of General Medical Sciences (NIGMS) (R01GM132938). The work was also supported by the Clinical and Translational Science Awards (CTSA) (UL1TR001442) from the NIH. Support for J. C. G. comes from a supplement to R01GM132938. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
SUPPORTING INFORMATION
Supplementary information accompanies this paper on the Clinical Pharmacology & Therapeutics website (www.cpt-journal.com).
REFERENCES
- (1).Robbins N, Koch SE, Tranter M & Rubinstein J The history and future of probenecid. Cardiovasc Toxicol 12, 1–9 (2012). [DOI] [PubMed] [Google Scholar]
- (2).Butler D Wartime tactic doubles power of scarce bird-flu drug. Nature 438, 6 (2005). [DOI] [PubMed] [Google Scholar]
- (3).Pea E Pharmacology of drugs for hyperuricemia - Mechanisms, kinetics and interactions. Hyperuricemic Syndromes: Pathophysiology and Therapy 147, 35–46 (2005). [DOI] [PubMed] [Google Scholar]
- (4).Pui K, Gow P & Dalbeth N Efficacy and Tolerability of Probenecid as Urate-Lowering Therapy in Gout; Clinical Experience in a High Prevalence Population. Internal Medicine Journal 42, 30- (2012). [DOI] [PubMed] [Google Scholar]
- (5).Nigam SK & Bhatnagar V The systems biology of uric acid transporters: the role of remote sensing and signaling. Current Opinion in Nephrology and Hypertension 27, 305–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).So A & Thorens B Uric acid transport and disease. J Clin Invest 120, 1791–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Capasso G, Jaeger P, Robertson WG & Unwin RJ Uric acid and the kidney: urate transport, stone disease and progressive renal failure. Curr Pharm Des 11, 4153–9 (2005). [DOI] [PubMed] [Google Scholar]
- (8).Sato Y et al. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat Rev Nephrol 15, 767–75 (2019). [DOI] [PubMed] [Google Scholar]
- (9).Pui K, Gow PJ & Dalbeth N Efficacy and Tolerability of Probenecid as Urate-lowering Therapy in Gout; Clinical Experience in High-prevalence Population. Journal of Rheumatology 40, 872–6 (2013). [DOI] [PubMed] [Google Scholar]
- (10).Ragab G, Elshahaly M & Bardin T Gout: An old disease in new perspective - A review. J Adv Res 8, 495–511 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Cunningham RF, Israili ZH & Dayton PG Clinical pharmacokinetics of probenecid. Clin Pharmacokinet 6, 135–51 (1981). [DOI] [PubMed] [Google Scholar]
- (12).Lopez-Nieto CE, You G, Bush KT, Barros EJ, Beier DR & Nigam SK Molecular cloning and characterization of NKT, a gene product related to the organic cation transporter family that is almost exclusively expressed in the kidney. J Biol Chem 272, 6471–8 (1997). [DOI] [PubMed] [Google Scholar]
- (13).Liu HC et al. An Organic Anion Transporter 1 (OAT1)-centered Metabolic Network. Journal of Biological Chemistry 291, 19474–86 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Bush KT, Wu W, Lun C & Nigam SK The drug transporter OAT3 (SLC22A8) and endogenous metabolite communication via the gut-liver-kidney axis. J Biol Chem 292, 15789–803 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Wu W, Bush KT & Nigam SK Key Role for the Organic Anion Transporters, OAT1 and OAT3, in the in vivo Handling of Uremic Toxins and Solutes. Sci Rep 7, 4939 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Nigam SK The SLC22 Transporter Family: A Paradigm for the Impact of Drug Transporters on Metabolic Pathways, Signaling, and Disease. Annual Review of Pharmacology and Toxicology, Vol 58 58, 663–87 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Nigam AK et al. Unique metabolite preferences of the drug transporters OAT1 and OAT3 analyzed by machine learning. J Biol Chem 295, 1829–42 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Granados JC et al. Coordinate regulation of systemic and kidney tryptophan metabolism by the drug transporters OAT1 and OAT3. J Biol Chem, 100575 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Burckhardt G Drug transport by Organic Anion Transporters (OATs). Pharmacology & Therapeutics 136, 106–30 (2012). [DOI] [PubMed] [Google Scholar]
- (20).Granados JC, Nigam AK, Bush KT, Jamshidi N & Nigam SK A key role for the transporter OAT1 in systemic lipid metabolism. J Biol Chem 296, 100603 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Tan PK, Ostertag TM & Miner JN Mechanism of high affinity inhibition of the human urate transporter URAT1. Sci Rep 6, 34995 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Shin HJ et al. Interactions of urate transporter URAT1 in human kidney with uricosuric drugs. Nephrology (Carlton) 16, 156–62 (2011). [DOI] [PubMed] [Google Scholar]
- (23).Ahn SY & Nigam SK Toward a Systems Level Understanding of Organic Anion and Other Multispecific Drug Transporters: A Remote Sensing and Signaling Hypothesis. Molecular Pharmacology 76, 481–90 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Nigam SK What do drug transporters really do? Nature Reviews Drug Discovery 14, 29–44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Wu W, Dnyanmote AV & Nigam SK Remote Communication through Solute Carriers and ATP Binding Cassette Drug Transporter Pathways: An Update on the Remote Sensing and Signaling Hypothesis. Molecular Pharmacology 79, 795–805 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Nigam SK, Bush KT, Bhatnagar V, Poloyac SM & Momper JD The Systems Biology of Drug Metabolizing Enzymes and Transporters: Relevance to Quantitative Systems Pharmacology. Clin Pharmacol Ther 108, 40–53 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Engelhart DC et al. Systems Biology Analysis Reveals Eight SLC22 Transporter Subgroups, Including OATs, OCTs, and OCTNs. Int J Mol Sci 21, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Zhang P, Azad P, Engelhart DC, Haddad GG & Nigam SK SLC22 Transporters in the Fly Renal System Regulate Response to Oxidative Stress In Vivo. Int J Mol Sci 22, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Rosenthal SB, Bush KT & Nigam SK A Network of SLC and ABC Transporter and DME Genes Involved in Remote Sensing and Signaling in the Gut-Liver-Kidney Axis. Scientific Reports 9, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Konig J, Muller F & Fromm MF Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol Rev 65, 944–66 (2013). [DOI] [PubMed] [Google Scholar]
- (31).Lepist EI & Ray AS Beyond drug-drug interactions: effects of transporter inhibition on endobiotics, nutrients and toxins. Expert Opinion on Drug Metabolism & Toxicology 13, 1075–87 (2017). [DOI] [PubMed] [Google Scholar]
- (32).Lai RE, Jay CE & Sweet DH Organic solute carrier 22 (SLC22) family: Potential for interactions with food, herbal/dietary supplements, endogenous compounds, and drugs. Journal of Food and Drug Analysis 26, S45–S60 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Meheus AZ, Mubiligi V & Vandenberghe G Treatment of gonorrhoea with a combination of probenecid and procaine penicillin in Rwanda. Afr J Med Med Sci 5, 209–12 (1976). [PubMed] [Google Scholar]
- (34).Nigam SK et al. The organic anion transporter (OAT) family: a systems biology perspective. Physiol Rev 95, 83–123 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Wikoff WR, Nagle MA, Kouznetsova VL, Tsigelny IF & Nigam SK Untargeted Metabolomics Identifies Enterobiome Metabolites and Putative Uremic Toxins as Substrates of Organic Anion Transporter 1 (Oat1). Journal of Proteome Research 10, 2842–51 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Bahn A et al. Murine renal organic anion transporters mOAT1 and mOAT3 facilitate the transport of neuroactive tryptophan metabolites. Am J Physiol Cell Physiol 289, C1075–84 (2005). [DOI] [PubMed] [Google Scholar]
- (37).Nigam SK, Wu W, Bush KT, Hoenig MP, Blantz RC & Bhatnagar V Handling of Drugs, Metabolites, and Uremic Toxins by Kidney Proximal Tubule Drug Transporters. Clin J Am Soc Nephrol 10, 2039–49 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Sudsakorn S, Bahadduri P, Fretland J & Lu C 2020 FDA Drug-drug Interaction Guidance: A Comparison Analysis and Action Plan by Pharmaceutical Industrial Scientists. Curr Drug Metab 21, 403–26 (2020). [DOI] [PubMed] [Google Scholar]
- (39).Eraly SA et al. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. Journal of Biological Chemistry 281, 5072–83 (2006). [DOI] [PubMed] [Google Scholar]
- (40).Bush KT, Singh P & Nigam SK Gut-derived uremic toxin handling in vivo requires OAT-mediated tubular secretion in chronic kidney disease. Jci Insight 5, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Vallon V et al. Organic anion transporter 3 contributes to the regulation of blood pressure. Journal of the American Society of Nephrology 19, 1732–40 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Ahn SY et al. Linkage of Organic Anion Transporter-1 to Metabolic Pathways through Integrated “Omics”-driven Network and Functional Analysis. Journal of Biological Chemistry 286, 31522–31 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Wu W et al. Multispecific drug transporter Slc22a8 (Oat3) regulates multiple metabolic and signaling pathways. Drug Metab Dispos 41, 1825–34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Mertens KL, Kalsbeek A, Soeters MR & Eggink HM Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System. Front Neurosci 11, 617 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Roager HM & Licht TR Microbial tryptophan catabolites in health and disease. Nature Communications 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Pols TW, Noriega LG, Nomura M, Auwerx J & Schoonjans K The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol 54, 1263–72 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Rizzo G, Renga B, Mencarelli A, Pellicciari R & Fiorucci S Role of FXR in regulating bile acid homeostasis and relevance for human diseases. Curr Drug Targets Immune Endocr Metabol Disord 5, 289–303 (2005). [DOI] [PubMed] [Google Scholar]
- (48).Deng H, Hu H & Fang Y Multiple tyrosine metabolites are GPR35 agonists. Sci Rep 2, 373 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Cervenka I, Agudelo LZ & Ruas JL Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 357, (2017). [DOI] [PubMed] [Google Scholar]
- (50).Zhang Y et al. Detection of Weak Organic Anion-Transporting Polypeptide 1B Inhibition by Probenecid with Plasma-Based Coproporphyrin in Humans. Drug Metab Dispos 48, 841–8 (2020). [DOI] [PubMed] [Google Scholar]
- (51).Hemmersbach P The Probenecid-story - A success in the fight against doping through out-of-competition testing. Drug Test Anal 12, 589–94 (2020). [DOI] [PubMed] [Google Scholar]
- (52).Willemin ME et al. Clinical Investigation on Endogenous Biomarkers to Predict Strong OAT-Mediated Drug-Drug Interactions. Clinical Pharmacokinetics 60, 1187–99 (2021). [DOI] [PubMed] [Google Scholar]
- (53).Tang J et al. Endogenous Plasma Kynurenic Acid in Human: A Newly Discovered Biomarker for Drug-Drug Interactions Involving Organic Anion Transporter 1 and 3 Inhibition. Drug Metab Dispos 49, 1063–9 (2021). [DOI] [PubMed] [Google Scholar]
- (54).Shen H et al. Evidence for the Validity of Pyridoxic Acid (PDA) as a Plasma-Based Endogenous Probe for OAT1 and OAT3 Function in Healthy Subjects. J Pharmacol Exp Ther 368, 136–45 (2019). [DOI] [PubMed] [Google Scholar]
- (55).Bullen AL & Garimella PS Beyond the Glomerulus-Kidney Tubule Markers and Diabetic Kidney Disease Progression. Kidney Int Rep 6, 1200–2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Shi SJ & Li YQ Interplay of Drug-Metabolizing Enzymes and Transporters in Drug Absorption and Disposition. Current Drug Metabolism 15, 915–41 (2014). [DOI] [PubMed] [Google Scholar]
- (57).Feeney ER & Mallon PW HIV and HAART-Associated Dyslipidemia. Open Cardiovasc Med J 5, 49–63 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Meropol SB et al. Adverse events associated with prolonged antibiotic use. Pharmacoepidemiol Drug Saf 17, 523–32 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Marcum ZA & Hanlon JT Recognizing the Risks of Chronic Nonsteroidal Anti-Inflammatory Drug Use in Older Adults. Ann Longterm Care 18, 24–7 (2010). [PMC free article] [PubMed] [Google Scholar]
- (60).Armenta A, Madero M & Rodriguez-Iturbe B Functional Reserve of the Kidney. Clin J Am Soc Nephrol 17, 458–66 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


