Abstract
Objectives:
Nearly 40% of African children under five are stunted. We leveraged the Ghana Randomized Air Pollution and Health Study (GRAPHS) cohort to examine whether poorer growth was associated with worse childhood lung function.
Study Design:
GRAPHS measured infant weight and length at birth and three, six, nine and twelve months and four years of age. At age four years, n=567 children performed impulse oscillometry (IOS). We employed multivariable linear regression to estimate associations between birth and age anthropometry and lung function. Next, we employed Latent Class Growth Analysis (LCGA) to generate growth trajectories through age four years. We employed linear regression to examine associations between growth trajectory assignment and lung function.
Results:
Birth weight and age four weight-for-age and height-for-age z-scores were inversely associated with airway resistance [for example, R5, or total airway resistance: birth weight β = −0.90cmH2O/L/s, 95% CI −1.64, −0.16 per 1-kilogram increase; and R20, or large airway resistance: age four height-for-age β = −0.40 cmH2O/L/s, 95% CI −0.57, −0.22 per 1-unit z-score increase]. Impaired growth trajectories identified through LCGA were associated with higher airway resistance. For example, children assigned to a persistently stunted trajectory had higher R5 (β =2.71 cmH20/L/s, 95% CI 1.07, 4.34) and R20 (β=1.43 cmH20/L/s, 95% CI 0.51, 2.36) as compared to normal.
Conclusions:
Children with poorer anthropometrics through to age 4 years had higher airway resistance in early childhood. These findings have implications for lifelong lung health, including pneumonia risk in childhood and reduced maximally attainable lung function in adulthood.
Keywords: growth, stunting, impulse oscillometry, low- and middle-income country
Introduction
Lung function in early life is a critical determinant of lung health over the life course. Children with poorer lung function are at increased risk for pneumonia1; 2, a leading cause of morbidity and mortality in children under five3, and persistently low lung function over childhood1; 4. Despite the importance of early life lung function, there is a paucity of data from low- and middle-income countries (LMICs) on lung function in early childhood and an incomplete understanding of modifiable risk factors that are associated with poorer lung health, which may differ from those in high-income countries5. A better understanding of conditions that increase risk of poorer lung health is critical to inform LMIC-specific public health interventions to mitigate risk.
Stunting and wasting, defined as a height-for-age z-score (HAZ) or weight-for-height z-score (WHZ), respectively, less than −2, and fetal growth restriction result in 2.2 million deaths and 21% of the total disability adjusted life years (DALYs) in children under five6. Sub-Saharan African children are disproportionately affected -- an estimated 40% (56.9 million) are stunted, 3.9% are severely wasted (5.6 million) and 21% are underweight (defined as weight-for-age z-score (WAZ) less than −2; 31 million)6. Children with impaired growth who survive early childhood have far-reaching health consequences7. Emerging evidence suggests that childhood undernutrition may impair lung growth and increase risk for poorer lung health over the life course8–10.
Studies largely from high-income countries suggest that low birth weight and fetal growth restriction are associated with increased risk for poorer lung function suggestive of a restrictive impairment11. Supporting evidence from LMICs is sparse and with mixed results. Higher birth weight and larger infant size were associated with better lung function in South African infants aged 5–17 weeks12. Lower birth weight has been associated with lower lung function as measured by spirometry in Indian adults aged 38–59 years old. However, in Brazilian adult males aged 18 years, low birth weight and preterm birth were not associated with lung function13; 14.
Postnatal lung growth may be further impaired by poor nutrition and inadequate somatic growth in childhood, although evidence is limited to cross-sectional, unadjusted analyses15. A cross-sectional study of Ethiopian children aged 6–9 years found that children who were underweight or stunted had lower forced expiratory volume in 1-second (FEV1) than those who were not underweight or stunted16. A multicenter study from Angola, DR Congo and Madagascar found that children with body mass index (BMI) z-score of less than −2 had symmetrically reduced FEV1 and forced vital capacity (FVC), suggestive of a restrictive ventilatory defect17. Conversely, a study of lung function at age 9 in survivors of severe acute malnutrition events found no difference in lung function as compared to controls, although on average all groups had HAZ and WAZ less than −118.
We hypothesized that poorer anthropometry and growth over early childhood is associated with worse lung function. We used a rural Ghanaian pregnancy cohort, followed prospectively with repeated anthropometric measures over childhood and lung function [impulse oscillometry (IOS)] at age four years. IOS is an effort-independent modality that requires minimal patient cooperation and therefore is suitable for lung function evaluation in young children. First, we analyzed associations between birth weight and length and being born small-for-gestational age, considered separately, and age four lung function. Next, we analyzed cross-sectional associations between age four World Health Organization (WHO) WAZ, HAZ and WHZ, again considered separately, and lung function at age four. Finally, we employed latent class growth analyses (LCGA) to construct z-score growth trajectories over early childhood and assigned each child to a WAZ, HAZ, and WHZ trajectory. We then analyzed associations between z-score trajectory assignment and age four lung function.
Study Design and Methods
Study Participants
Participants were from the Ghana Randomized Air Pollution and Health Study (GRAPHS)19; 20. As has been described in detail elsewhere9; 20; 21, between August 2013 and March 2016, GRAPHS recruited n=1414 non-smoking women pregnant with a singleton fetus at gestational age (GA) less than 24 weeks from Kintampo North Municipality and Kintampo South District of Ghana. GA at enrollment was established by ultrasound22. The primary objective of GRAPHS was to understand associations between household air pollution exposure and 1) birth weight; and 2) pneumonia risk over the first year of life. GRAPHS follow-up concluded after the age one-year study visit. In July 2017, additional funding was obtained for lung function measurement in n=700 children beginning at age four years. The analyses presented herein include those children who attended the age four visit. All pregnant women and, subsequently, mothers provided written informed consent. The study was approved by the regional ethics committees at each participating institution and regulatory authorities in Ghana. The funding agencies played no role in study design nor data analysis.
Anthropometric Measures
As previously described, trained fieldworkers measured infant anthropometrics including weight and length, once within 24 hours of birth23 and at ages three, six, nine and twelve months of age9. Length was measured to the nearest 0.1 centimeter (Ayrton Infantometer Model M-200, Ayrton Corp, MN, USA) and weight was measured to the nearest 0.1 kilogram (Tanita digital scale model BD-590, Tanita Corp, Il, USA). We created Ghanaian-specific birth weight for gestational week curve following methodology described by the World Health Organization24. We considered an infant small for gestational age if the infant was born alive with a birth weight less than the 10th percentile for that specific week gestation.
At the age four lung phenotyping visit, we performed duplicate measures of weight (Seca 803 Clara Digital Floor Scale) to the nearest 0.1 kilogram and height (Seca 213 Portable Stadiometer) to the nearest 0.1 centimeter. We calculated HAZ, WAZ, WHZ at three, six, nine, and twelve months and four years of age using the 2006 WHO child growth standard reference for age and sex25. Stunting, underweight or wasting was defined as an infant or child with an HAZ, WAZ, or WHZ, respectively, less than two standard deviations below the WHO child growth standard reference median for age and sex.
Impulse Oscillometry (IOS)
IOS was performed at age four years by a trained study pediatrician in community clinics using the MasterScreen IOS with Jaeger pneumotach (Vyaire Medical) following American Thoracic Society/European Respiratory Society guidelines26. The IOS device was calibrated daily with a standard 3-liter syringe. Lung function parameters included resistance at 5 Hertz (Hz), R5, a measure of total airway resistance, and 20 Hertz (Hz), R20, a measure of large airway resistance; the difference in resistance between 5Hz and 20Hz, R5–20, a measure of small airway resistance; reactance at 5 Hz (X5), a measure of small airway elastance; resonant frequency (Fres) or the frequency at which the total reactance is null, and area of reactance (AX) or total reactance at all frequencies between 5Hz and Fres. Children were tested while standing, with a nose clip in place and cheeks firmly supported following standardized operating procedures. Three acceptable tests free from artifact and reproducible with within session coefficient of variability of Rrs of ≤15% were performed and IOS variables were averaged across these three tests27. All studies were overread by the study pulmonologist.
Covariates
Maternal ethnicity (categorized into four groups: 1, 2, 3, 4) was determined by questionnaire at GRAPHS enrollment. The main ethnic groups in the study area were Mo, Akan, Konkonba and other Northern ethnic tribes. Secondhand tobacco smoke exposure at the household level (categorical, yes or no) was determined by any report of secondhand tobacco smoke exposure via questionnaires at GRAPHS enrollment and again at the age four lung function visit. Questionnaires administered at GRAPHS enrollment identified household assets (e.g, number of livestock) which were employed to create an asset index, an index of relative socioeconomic status (continuous variable)28. Fieldworkers visited pregnant women weekly over pregnancy and queried the number of antenatal visits attended (dichotomous variable, categorized as ≥4 visits versus fewer). During pregnancy, four 72-hour personal carbon monoxide (CO) exposure assessments occurred to characterize integrated air pollution exposures21. As previously reported, linear interpolation was used to estimate an average prenatal CO exposure (continuous, in parts per million)10. Child sex was recorded at delivery (categorical, male versus female) and child age (continuous, in years) at the IOS session. Breastfeeding duration was determined by questionnaires administered every three months over the first year of life. Specifically, mothers were asked, “Are you still breastfeeding your baby?” We categorized breastfeeding duration (3, 6, 9, or 12 months) based on when the mother last reported breastfeeding her child. Body mass index (BMI), a measure of size and somatic height at the time of lung function, was calculated from height and weight measured at the IOS session. No imputation for missing data was performed.
Statistical Analyses
First, we examined associations between birth weight and length and small-for-gestational age (yes/no), considered separately, and age four lung function. We then examined the cross-sectional associations between age four WAZ, HAZ, WHZ, considered separately, and age four lung function. We used bivariate and multivariable generalized linear regression models for these analyses, adjusting for child sex, ethnicity, BMI and age at IOS, asset index, maternal age, number of antenatal visits and gestational age at delivery (Model 1). We then additionally adjusted for environmental exposures including secondhand smoke exposure and average prenatal CO exposure (Model 2).
Next, we constructed WAZ, HAZ, and WHZ trajectories using Latent Class Growth Analysis (LCGA), without consideration of lung function29–31. This data-driven approach allows us to identify characteristics of growth over four years of life and group study participants not by predefined groups but rather on data-defined classes (trajectories)32. Specifically, we used WAZ, HAZ, WHZ measured at ages three, six, nine, twelve months and four years to create z-score specific trajectories. Children with data at ≥1 timepoint and who were 48 months +/− 6 months (n=609) at the age four assessment were included. For each z-score, we constructed models with two to six trajectories where different models are defined by the number of trajectories. For each z-score, the best model (e.g., number of trajectories) was determined by: 1) Akaike information criteria (AIC) and Bayesian information criterion (BIC) where smaller numbers indicate better fit; 2) entropy where larger numbers (range 0 to 1) suggest better class separation; and 3) the number of children in each trajectory.
Following model selection and for each z-score trajectory, children were then assigned to the class for which they had the highest probability of correct assignment. Growth trajectory assignments were given a continuous, ordinal number zero to three, where zero was assigned the largest/highest trajectory and three the smallest/lowest trajectory. We then fit bivariate and multivariable generalized linear regression models (Models 1 and 2) to assess associations between WAZ, HAZ, WHZ trajectory assignments and age four lung function. Sensitivity models additionally adjusted for breastfeeding duration.
We used Stata version 14, zscore06 module to calculate z-scores. We constructed latent class growth trajectories using Mplus version 7.4. Regression analyses were performed in R software version 3.6.0. Collinearity was assessed in all regression models using the mctest package.
Results
GRAPHS enrolled N=1414 pregnant women, resulting in N=1306 (92%) live births. N=683 GRAPHS children attended the lung phenotyping visit. Of these, 567 (83%) performed acceptable and reproducible IOS. Mother-child dyads from a range of ethnic groups, including Akan, Gonja, Dagarti, Mo, Konkomba and others were enrolled. Approximately half of the children were female (n=289, 51%) and aged a median of 4 years (IQR 3.9, 4.2) at the time of IOS (Table 1). Baseline characteristics amongst children who attended the lung phenotyping visit and performed acceptable and reproducible IOS versus the rest of the GRAPHS cohort are shown Supplemental Table 1. On average, mothers of children included in these analyses were slightly older, attended more antenatal visits, and had lower average prenatal carbon monoxide exposures.
Table 1.
GRAPHS Cohort Characteristics (N=567)
| Maternal Age, Median (IQR) – Years | 27.6 | (23.3, 33.8) |
| Asset index – Median (IQR) | −0.43 | (−1.3, 0.7) |
| Ethnicity – no. (%) | ||
| 1 | 84 | 14.8% |
| 2 | 85 | 15% |
| 3 | 371 | 65.3% |
| 4 | 27 | 5% |
| Number of antenatal visits – no. (%) | ||
| Missing | 3 | |
| Four or more | 412 | 72.8% |
| Prenatal CO, Median (IQR) – ppm | 0.95 | 0.58, 1.57 |
| Secondhand Smoke Exposure – no. (%)* | 111 | 19.6% |
| Preterm birth (GA<37 weeks) – no. (%) | 20 | 3.5% |
| Child sex, female – no. (%) | 289 | 50.9% |
| Birth weight, Median (IQR) – kilograms | 2.95 | 2.64, 3.20 |
| Missing – no. (%) | 14 | 2.4% |
| Birth length, Median (IQR) – centimeters | 46.8 | 44.3, 49.0 |
| Missing – no. (%) | 14 | 2.4% |
| Small-for-gestational age – no. (%) | 122 | 22% |
| Missing – no. (%) | 15 | 2.6% |
| Impulse Oscillometry (IOS) | ||
| Age at IOS, Median (IQR) – years | 4.05 | 3.87, 4.23 |
| Height at IOS, Median (IQR) – centimeters | 98 | 95.4, 101.2 |
| Weight at IOS, Median (IQR) – kilograms | 14.6 | 13.6, 16 |
| Height-for-age z-score, Median (IQR) | −1.11 | −1.71, −0.41 |
| Weight-for-age z-score, Median (IQR) | −0.75 | −1.33, −0.16 |
| Weight-for-height z-score, Median (IQR) | −0.23 | −0.78, 0.43 |
| Body mass index, Median (IQR) – kilogram/meter2 | 15.08 | 14.4, 16 |
| R5, Median (IQR) – cmH2O/L/s | 14.3 | 12.3, 16.4 |
| X5, Median (IQR) – cmH2O/L/s | −3.4 | −4.5, −1.9 |
| R20, Median (IQR) – cmH2O/L/s | 8.4 | 6.9, 9.6 |
| R5–20, Median (IQR) – % | 71.7 | 50.4, 100.3 |
| Fres, Median (IQR) – Hertz | 28.2 | 24.3, 32.9 |
| AX, Median (IQR) – cmH2O/L | 5.7 | 4.5, 7.2 |
Secondhand smoke exposure determined by questionnaire
IQR, interquartile range; CO, carbon monoxide; ppm, parts per million; GA, gestational age
Associations between birth weight and length and small-for-gestational age and age four lung function
Of the children who performed acceptable and reproducible IOS, n=553 (99%) had valid birth weight and length measurements within 24 hours of birth. The Pearson’s correlation between birth weight and length was r=0.34, p<0.01. Multivariable models (Model 2, Table 2) found that birth weight was inversely associated with R5 (β=−0.90 cmH20/L/s, 95% CI −1.64, −0.16 per 1-kilogram increase in birth weight), R20 (β=−0.50 cmH20/L/s, 95% CI −0.92, −0.08 per 1-kilogram increase in birth weight), Fres (β=−2.14 Hertz, 95% CI −3.69, −0.60) and AX (β=−6.93 cmH2O, 95% CI −11.84, −2.03). Regression analyses did not identify an association between birth length or small-for-gestational age and lung function.
Table 2.
Association between birth outcomes and age four impulse oscillometry (IOS), linear regression (N=553)
| IOS Variable | Bivariate Analyses (N=553) | Model 1 (N=552) | Model 2 (N=542) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PE | 95% CI | p-value | PE | 95% CI | p-value | PE | 95% CI | p-value | |
| Birth Weight | |||||||||
| R5, cmH2O/L/s | −0.92 | −1.61, −0.23 | <0.01 | −1.03 | −1.78, −0.29 | <0.01 | −0.90 | −1.64, −0.16 | 0.02 |
| X5, cmH2O/L/s | 0.20 | −0.25, 0.65 | 0.39 | 0.03 | −0.46, 0.53 | 0.89 | 0.04 | −0.46, 0.54 | 0.88 |
| R20, cmH2O/L/s | −0.48 | −0.87, −0.09 | 0.02 | −0.53 | −0.95, −0.10 | 0.02 | −0.50 | −0.92, −0.08 | 0.02 |
| R5–20, % | −3.04 | −10.71, 4.62 | 0.44 | −3.00 | −11.44, 5.44 | 0.49 | −2.11 | −10.61, 6.38 | 0.63 |
| Fres, Hertz | −2.22 | −3.65, −0.79 | <0.01 | −1.96 | −3.53, −0.39 | 0.01 | −2.14 | −3.69, −0.60 | <0.01 |
| AX, cmH2O/L | −7.33 | −11.81, −2.85 | <0.01 | −6.99 | −11.90, −2.09 | <0.01 | −6.93 | −11.84, −2.03 | <0.01 |
| Birth Length | |||||||||
| R5, cmH2O/L/s | −0.03 | −0.12, 0.06 | 0.49 | −0.02 | −0.11, 0.06 | 0.59 | −0.03 | −0.11, 0.06 | 0.57 |
| X5, cmH2O/L/s | −0.01 | −0.07, 0.05 | 0.79 | −0.02 | −0.08, 0.04 | 0.51 | −0.01 | −0.07, 0.05 | 0.74 |
| R20, cmH2O/L/s | −0.02 | −0.07, 0.03 | 0.51 | −0.01 | −0.06, 0.04 | 0.58 | −0.02 | −0.08, 0.03 | 0.33 |
| R5–20, % | −0.18 | −1.16, 0.79 | 0.71 | −0.13 | −1.13, 0.88 | 0.81 | 0.11 | −0.91, 1.12 | 0.84 |
| Fres, Hertz | 0.05 | −0.13, 0.24 | 0.56 | 0.10 | −0.09, 0.29 | 0.30 | 0.08 | −0.11, 0.26 | 0.42 |
| AX, cmH2O/L | −0.09 | −0.66, 0.48 | 0.75 | 0.07 | −0.52, 0.65 | 0.83 | 0.03 | −0.55, 0.61 | 0.92 |
| Small-for-gestational age | |||||||||
| R5, cmH2O/L/s | 0.13 | −0.62, 0.88 | 0.73 | 0.19 | −0.57, 0.95 | 0.63 | 0.06 | −0.69, 0.81 | 0.87 |
| X5, cmH2O/L/s | −0.18 | −0.66, 0.31 | 0.47 | −0.09 | −0.58, 0.41 | 0.73 | −0.10 | −0.60, 0.41 | 0.71 |
| R20, cmH2O/L/s | 0.12 | −0.30, 0.54 | 0.57 | 0.14 | −0.29, 0.56 | 0.52 | 0.12 | −0.30, 0.55 | 0.58 |
| R5–20, % | 0.00 | −0.08, 0.09 | 0.91 | 0.00 | −0.08, 0.09 | 0.95 | −0.01 | −0.09, 0.08 | 0.91 |
| Fres, Hertz | 0.70 | −0.86, 2.25 | 0.38 | 0.65 | −0.93, 2.23 | 0.42 | 0.91 | −0.66, 2.48 | 0.26 |
| AX, cmH2O/L | 0.14 | −0.34, 0.62 | 0.57 | 0.16 | −0.32, 0.65 | 0.51 | 0.18 | −0.31, 0.67 | 0.47 |
Weight is per 1-kilogram increase in birth weight. Length is per 1-centimeter increase in birth length. Small-for-gestational age (yes vs. no) defined as a child born alive with a birth weight less than the 10th percentile for the specific gestational week at delivery after creating a Ghanaian specific curve using methodology described by the World Health Organization.
Model 1 adjusts for child sex, ethnicity, body mass index and age at time of IOS, asset index, maternal age, number of antenatal visits and preterm delivery.
Model 2 additionally adjusts for prenatal secondhand tobacco smoke exposure and prenatal household air pollution exposure as indexed by personal carbon monoxide exposure monitoring
Associations between age four anthropometrics and lung function
All children who performed acceptable and reproducible IOS had valid cross-sectional height and weight measures. Median age four WAZ, HAZ, and WHZ were −0.75 (IQR −1.33, −0.16), −1.11 (IQR −1.71, −0.41) and −0.23 (IQR −0.78, 0.43), respectively. In multivariable models, on average WAZ and HAZ were inversely associated with measures of airway resistance (Table 3). Specifically, in Model 2, children with higher WAZ and HAZ had lower R5 (WAZ β=−0.97 cmH20/L/s, 95% CI −1.41, −0.53; HAZ β=−0.84 cmH20/L/s, 95% CI −1.15, −0.53 per 1 unit z-score increase), R20 (WAZ β=−0.50 cmH20/L/s, 95% CI −0.75, −0.25, HAZ β=−0.40 cmH20/L/s, 95% CI −0.57, −0.22 per 1 unit z-score increase) and AX (WAZ β=−5.64 cmH20/L/s, 95% CI −8.57, −2.71; HAZ β=−4.70 cmH20/L/s, 95% CI −6.77, −2.63 per 1 unit z-score increase). No associations were seen with WHZ and IOS parameters.
Table 3.
Cross-sectional associations between age four z-scores and impulse oscillometry (IOS), linear regression
| IOS Variable | Bivariate Analyses (N=567) | Model 1 (N=565) | Model 2 (N=555) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PE | 95% CI | p-value | PE | 95% CI | p-value | PE | 95% CI | p-value | |
| Weight-for-Age Z-score | |||||||||
| R5, cmH2O/L/s | −0.58 | −0.93, −0.23 | <0.01 | −1.03 | −1.48, −0.59 | <0.01 | −0.97 | −1.41, −0.53 | <0.01 |
| X5, cmH2O/L/s | 0.26 | 0.03, 0.49 | 0.03 | 0.28 | −0.02, 0.58 | 0.07 | 0.22 | −0.09, 0.52 | 0.16 |
| R20, cmH2O/L/s | −0.28 | −0.47, −0.08 | <0.01 | −0.48 | −0.73, −0.23 | 0.08 | −0.50 | −0.75, −0.25 | <0.01 |
| R5–20, % | −1.62 | −5.57, 2.32 | 0.42 | −2.86 | −7.93, 2.20 | 0.27 | −1.80 | −6.94, 3.33 | 0.49 |
| Fres, Hertz | −0.57 | −1.31, 0.16 | 0.13 | −0.68 | −1.62, 0.27 | 0.16 | −0.69 | −1.64, 0.25 | 0.15 |
| AX, cmH2O/L | −0.37 | −0.60, −0.14 | <0.01 | −6.32 | −9.23, −3.42 | <0.01 | −5.64 | −8.57, −2.71 | <0.01 |
| Height-for-Age Z-score | |||||||||
| R5, cmH2O/L/s | −0.91 | −1.22, −0.61 | <0.01 | −0.84 | −1.17, −0.51 | <0.01 | −0.84 | −1.15, −0.53 | <0.01 |
| X5, cmH2O/L/s | 0.16 | −0.05, 0.37 | 0.13 | 0.20 | −0.02, 0.42 | 0.07 | 0.16 | −0.05, 0.38 | 0.14 |
| R20, cmH2O/L/s | −0.41 | −0.59, −0.24 | <0.01 | −0.34 | −0.53, −0.16 | <0.01 | −0.40 | −0.57, −0.22 | <0.01 |
| R5–20, % | −2.58 | −6.13, 0.97 | 0.15 | −2.96 | −6.71, 0.80 | 0.12 | −2.08 | −5.73, 1.57 | 0.26 |
| Fres, Hertz | −0.69 | −1.36, −0.03 | 0.04 | −0.43 | −1.12, 0.27 | 0.23 | −0.62 | −1.29, 0.05 | 0.07 |
| AX, cmH2O/L | −0.53 | −0.73, −0.33 | <0.01 | −4.89 | −7.03, −2.74 | <0.01 | −4.70 | −6.77, −2.63 | <0.01 |
| Weight-for-Height Z-score | |||||||||
| R5, cmH2O/L/s | 0.11 | −0.20, 0.43 | 0.47 | 0.08 | −0.76, 0.93 | 0.84 | 0.13 | −0.70, 0.96 | 0.76 |
| X5, cmH2O/L/s | 0.17 | −0.03, 0.38 | 0.09 | 0.19 | −0.37, 0.75 | 0.50 | 0.12 | −0.45, 0.68 | 0.69 |
| R20, cmH2O/L/s | 0.03 | −0.14, 0.21 | 0.72 | −0.13 | −0.60, 0.35 | 0.60 | −0.13 | −0.60, 0.34 | 0.60 |
| R5–20, % | 0.33 | −3.14, 3.81 | 0.85 | 2.66 | −6.76, 12.08 | 0.58 | 3.21 | −6.27, 12.68 | 0.51 |
| Fres, Hertz | −0.08 | −0.73, 0.58 | 0.82 | 0.27 | −1.51, 2.04 | 0.77 | 0.29 | −1.46, 2.05 | 0.74 |
| AX, cmH2O/L | 0.38 | −1.68, 2.43 | 0.72 | −0.68 | −6.21, 4.84 | 0.81 | −0.06 | −5.57, 5.45 | 0.98 |
Change in IOS variable per 1-unit z-score increase.
Model 1 adjusts for child sex, body mass index, ethnicity, asset index, ethnicity, maternal age, number of antenatal visits and gestational age at delivery. Model 2 additionally adjusts for prenatal secondhand tobacco smoke exposure, and prenatal household air pollution exposure as indexed by carbon monoxide
Latent Class Trajectory Modeling: Identification of the Number of Trajectories
Distribution of HAZ, WAZ and WHZ across all ages for the N=609 children aged 48 months +/− 6 months at the age four visit are shown in Figure S1. Data completion was high, with n=535 (88%), n=526 (86%) and n=525 (86%) of children having at least four out of five WAZ, HAZ and WHZ, respectively. Using LCGA fit data, we determined that four classes was the optimal fit for HAZ, WAZ and WHZ trajectories (Table S2). Posterior probabilities for LCGA class assignment was overall high (Table S3). Five hundred and eight children included in the growth trajectory construction had acceptable and reproducible lung function tests. Data completion amongst this subset was also high. Three hundred and sixty-three (71.5%), 353 (69.5%), and 349 (68.7%) children had five; 123 (24.2%), 128 (25.2%), and 133 (26.1%) children had four; 21 (4.1%), 26 (5.1%), and 23 (4.5%) had three; and 1 (0.2%), 1(0.2%) and 3 (0.6%) children had two WAZ, HAZ and WHZ measurements, respectively. Examination of growth trajectories suggested that the trajectories remain distinct from birth without crossing of lines, although changes in slope are noted (Figure 1). The mean z-scores for children assigned to the lowest and second lowest WAZ and HAZ were at or near −2 or −1, respectively, at each time point consistent with the definition of underweight and stunted or at-risk for being underweight or stunted. Assignment to these trajectories was common with 48% (n=243) of children in the two lowest WAZ trajectories and 54% (n=275) of children in the two lowest HAZ trajectories.
Figure 1. Latent class growth trajectories for (A) Weight-for-age, (B) Height-for-age and (C) Weight-for-Height z-score trajectories over the first four years of life in children with acceptable and reproducible lung function.
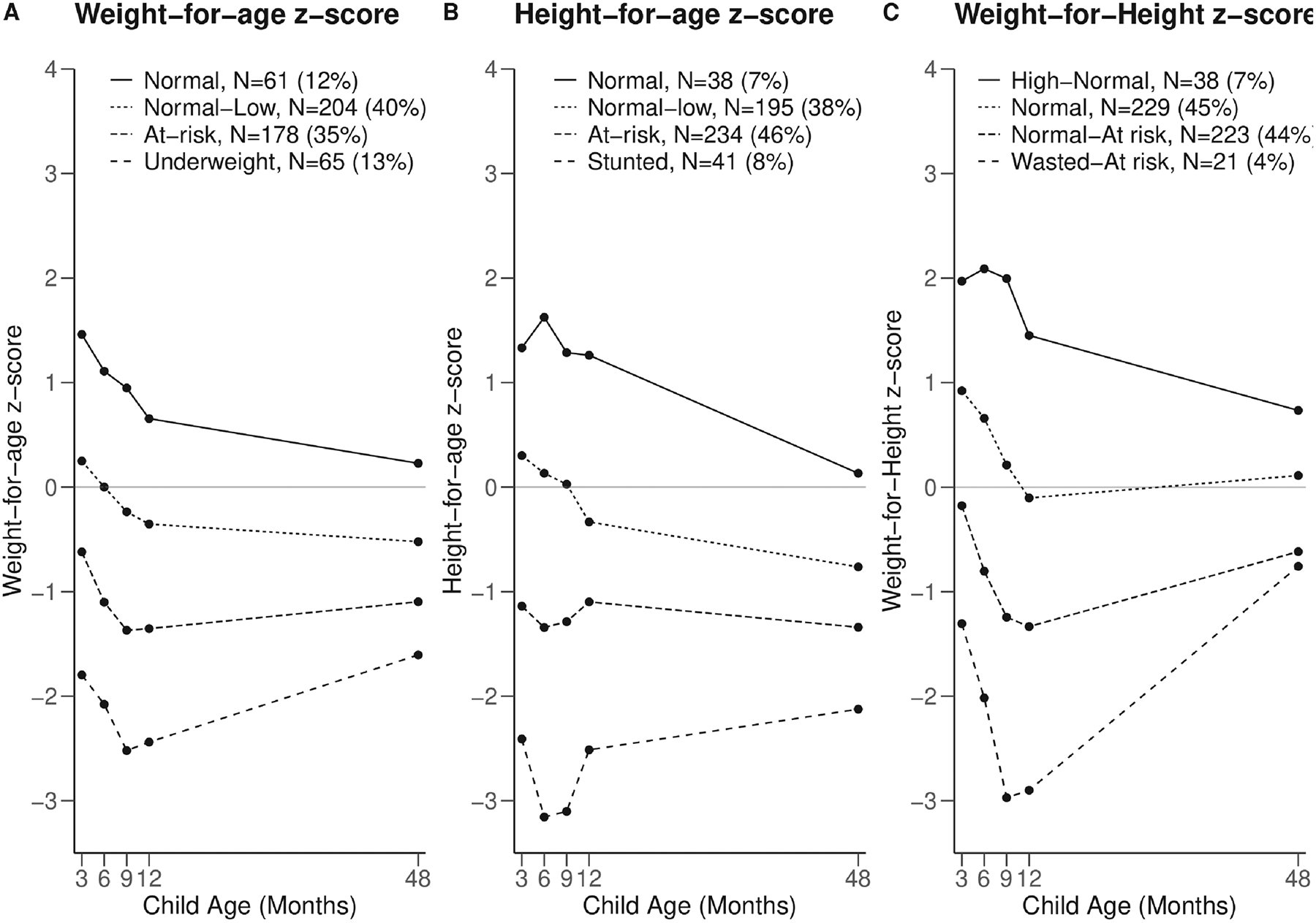
Height and weight were measured at 3, 6, 9, 12 and 48 months and WHO z-scores calculated using the 2006 WHO child growth standards. Latent class growth analyses were employed to construct trajectories for each z-score over the first four years of life.
Associations between growth trajectories and age four lung function
In multivariable models, on average, children in poorer WAZ trajectories had higher R5, R20 and AX, as compared to the normal trajectory (Figure 2A, Table S4). Specifically, in Model 2, children in the WAZ “Underweight” had R5 values that were 2.10 cmH20/L/s (95% CI 0.75, 3.45) higher, respectively, than children in the “Normal” trajectory (ptrend<0.01). Children in the WAZ trajectories “At risk” and “Underweight” had R20 values that were 0.74 cmH20/L/s (95% CI 0.11, 1.37) and 1.57 cmH20/L/s (95% CI 0.80, 2.34) higher, respectively, than children in the “Normal” trajectory (ptrend<0.01). Finally, children in the WAZ “Underweight” had higher AX (β=13.09 cmH20/L, 95% CI 3.99, 22.19) than children in the “Normal trajectory (ptrend<0.01).
Figure 2. Associations between (A) Weight-for-Age, (B) Height-for-Age, and (C) Weight-for-Height z-score trajectories through age 4 years and impulse oscillometry.
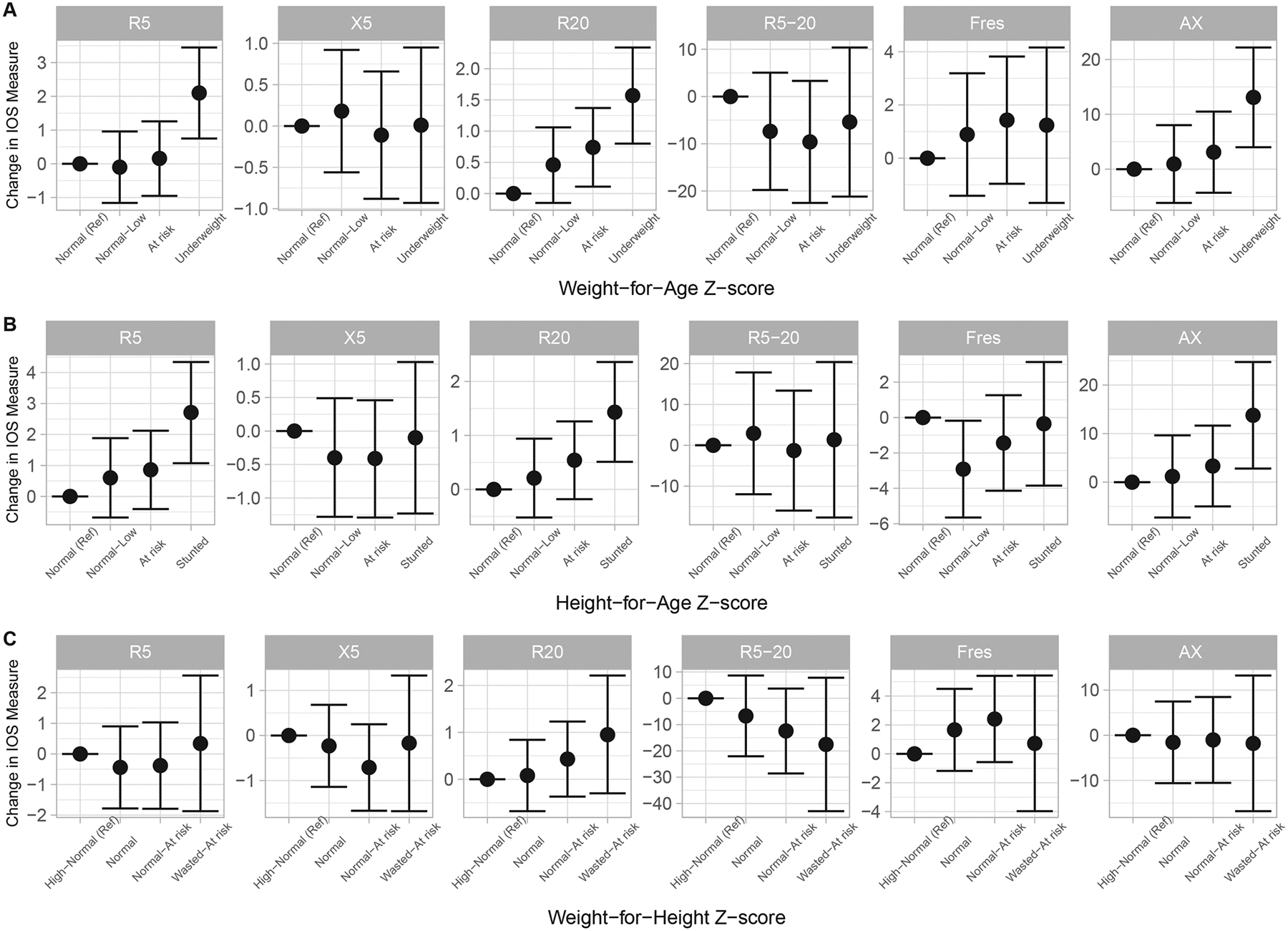
Height and weight were measured at 3, 6, 9, 12 and 48 months and WHO z-scores calculated using the 2006 WHO child growth standards. Latent class growth analyses were employed to construct trajectories for each z-score over the first four years of life. Linear regression models adjust for child sex, ethnicity, body mass index and age at the time of IOS, wealth index, maternal age, number of antenatal visits, preterm delivery, secondhand tobacco smoke exposure and prenatal household air pollution exposure as indexed by carbon monoxide. Abbreviations: R5, resistance at 5 Hertz (Hz) in cmH20/L/s; X5, reactance at 5Hz in cmH20/L/s; R20, resistance at 20Hz in cmH20/L/s; R5–20, difference between R5 and R20 in %; Fres, resonant frequency in Hertz; AX, reactance area in cmH20/L.
Similarly, on average children in poorer HAZ trajectories had higher R5, R20 and AX (Figure 2B, Table S5). Specifically, in Model 2, children in the HAZ “Stunted” had R5, R20 and AX values that were 2.71 cmH20/L/s (95% CI 1.07, 4.34), 1.43 cmH20/L/s (95% CI 0.51, 2.36) and 13.77 cmH20/L (95% CI 2.80, 24.74) higher, respectively, than children in the “Normal” trajectory (R5 and R20 ptrend<0.01, AX ptrend=0.02).
No associations were seen between WHZ trajectories and IOS parameters (Figure 2C, Table S6). Sensitivity models additionally adjusting for breastfeeding duration did not substantively change results (data not shown).
Discussion
Despite the fact that approximately 39% of the 141 million African children under the age of five are stunted and 21% (31 million) are underweight, the effects of impaired early childhood growth on lung health in this population is poorly defined6. In this prospective pregnancy cohort study from rural Ghana, we investigated associations between anthropometry at birth and age four years and growth trajectories from birth through age four years and early childhood lung function. Overall, we found a consistent association between poorer anthropometry and higher airway resistance independent of current body size as indexed by age four body mass index, suggesting smaller airway caliber per lung parenchyma, or dysanapsis33. Specifically, children with lower weight at birth, lower WAZ and HAZ at four years, and lower WAZ and HAZ trajectories over the first four years of life had higher R5, or total airway resistance, and R20, or large airway resistance. Given the importance of early life lung health on respiratory morbidity over the life course, these data add to the urgency of understanding etiologies of poor growth, particularly in sub-Saharan Africa.
Impaired growth prenatally and in early childhood are pervasive in LMICs and inextricably linked to poverty. Between 2000 and 2018, Africa, including West Africa, saw an increase in the number of stunted children under age five, whereas Asia and Latin America saw a decrease34. Indeed, our study WHO z-scores suggest an overall frail cohort with study mean below zero (see Figure S1). Our data from Ghana suggest that poorer anthropometry beginning at birth and through age four years are independently associated with impaired respiratory mechanics in early childhood. The observed difference in R5 and AX in children assigned to trajectories consistent with stunting or being underweight as compared to normal is similar to or greater than previously reported for children with uncontrolled asthma and bronchopulmonary dysplasia, as compared to normal children and similar in magnitude to baseline differences predictive of future asthma exacerbations35–37. Given the high prevalence of stunting and being underweight in the region, the population at-risk for poorer lung function is significant. Establishing good early life lung function is critical for lung health in childhood and attaining maximal lung health over the life course38. These data point to poor early childhood growth as an important and potentially modifiable driver of poor lung health in LMICs.
We found that infants with lower birth weight have increased R5, or total airway resistance, and R20, or large airway resistance, at age four years, independent of size at the time of lung function testing. Lung development occurs rapidly in utero, beginning at approximately four weeks and extending over gestation39. Low birth weight reflects impaired global fetal development and these findings suggest altered organ structure and tissue remodeling with wide ranging effects. Infants born with fetal growth restriction and bronchopulmonary dysplasia have altered alveolar and pulmonary vascular development resulting in an increased airway resistance phenotype35. Supporting evidence from animal studies suggest that fetal growth restriction results in airway remodeling, thickened airspace walls including increased smooth muscle mass and altered extracellular matrix composition and smaller cross-sectional size of conductive airways40–43.
Lung development continues postnatally through adolescence with rapid alveolar expansion and enlargement of airways. These data suggest that children with impaired growth through and at age four had altered lung mechanics. Indeed, nutrition and growth play a key role in lung development, directly through lung growth and indirectly through, for example, epigenetic changes that program future lung growth44; 45. Underlying mechanisms of postnatal growth on lung function impairments are not known, however it has been postulated that growth restricted children may have reductions in airway caliber, a major component of airway resistance, which may result in airway obstruction and predispose to obstructive airways disease46.
We observed that lower birth weight, WAZ and HAZ at age four years and being underweight and stunted were associated with increased AX, suggesting increased small airways resistance. All analyses identify consistent associations with R5; changes in resistance at low frequencies such as R5 may also be indicative of small airways disease47. Spirometry is considered the gold standard for lung function evaluation however the forced maneuver may be difficult for young children to perform and does not sensitively evaluate the small airways. Further, signals of small airways impairment may precede spirometry airflow limitation48. For example, Cosio et al suggest that ~75% of the small airways must be obstructed before changes in forced expiratory volumes are seen49.
We also found a lack of association between WHZ at age four and age four IOS parameters as well as WHZ trajectory and age four IOS parameters. Low WHZ is an indicator of acute malnutrition, and this lack of association suggests that lung function is preserved in the acute phase of malnutrition during which tissue remodelling and physiological adaptations may not yet have resulted in dysanapsis. Supporting these findings, a study from Malawi of children surviving acute malnutrition episodes found no association with lung function18.
Poorer lung function in early childhood may increase risk for respiratory infection and wheezing50, further impairing lung function growth51. Diminished lung function may result in reduced maximally attainable lung function52 with greater predisposition to respiratory disease later in life53. The associations observed between weight at birth, WAZ and HAZ at age four and WAZ and HAZ trajectories over early childhood and higher airway resistance suggest that smaller children have reduced airway caliber independent of somatic growth and thus conceptually low lung volume, consistent with the definition of dysanapsis33; 54, a likely critical component of the low early-life lung function phenotype55. If replicated, our findings would support impaired early childhood growth as a risk factor for dysanapsis, with implications for lung health over the life course and highlight the need to understand the wide-ranging etiologies6; 9 of poor growth.
We note several study strengths. We leverage GRAPHS, a well-characterized prospective pregnancy cohort with anthropometrics measured every three months over the first year of life and again at age four years. We employed a validated measure of lung function to allow rigorous examination of associations between anthropometry in early life and lung function. Strengths of IOS include its effort-independent nature making the test ideal for use in small children, ability to characterize small airways, and greater precision in detecting lung mechanics, as compared to spirometry56; 57. Questionnaires captured a number of important covariates and ultrasound in early pregnancy provided accurate gestational dating. We also note limitations. Anthropometrics in the first year of life were measured only once at each age; repeated measurements may reduce measurement error. Given the young age of study children, we did not perform post-bronchodilator IOS nor spirometry, considered the gold standard of lung function assessment, nor multiple breath washout testing, however these assessments will be important for future evaluations. Comparing lung function in our cohort with a healthy standard would be an important future analysis; to our knowledge no impulse oscillometry reference lung function equations are available for children from sub-Saharan Africa. These analyses within a rural Ghanaian cohort may not be generalizable to other populations.
Supplementary Material
E-Figure 1. Distribution of z-scores from 3 months to 4 years of life (N=609). Children who were aged 54 months or younger at the time of lung phenotyping visit were included in construction of growth trajectories. Shown here are the distributions of: (A) Weight-for-Age, (B) Height-for-Age, and (C) Weight-for-Height z-scores across all ages (3, 6, 9, 12 months and 4 years). Across all ages, 369 (61%), 166 (27%), 61 (10%), 10 (2%), 3 (0.4%) children had five, four, three, two and one Weight-for-Age z-score measure, respectively. Across all ages, 358 (59%), 168 (28%), 69 (11%), 11 (2%), and 3 (0.4%) children had five, four, three, two and one Height-for-Age z-score measure, respectively. Across all ages, 354 (58%), 171 (28%), 69 (11%), 11 (2%) and 4 (0.7%) children had five, four, three, two and one Weight-for-Height z-score measure respectively.
Implications:
In summary, these data suggest that poor anthropometry and growth in early childhood are associated with impaired lung function, with implications for lung health in both childhood and adulthood.
Acknowledgements:
Funding:
GRAPHS was supported by the National Institute of Environmental Health Sciences (NIEHS) Grant R01 ES019547, R01 ES026991, P30 ES 009089 and 1S10OD016219, Fogarty International Center R21 TW010957, Thrasher Research Fund, and the Clean Cooking Alliance. BJW was additionally supported by the NIEHS K23 ES021471. AGL was additionally supported by the National Heart, Lung and Blood Institute K23 HL135349. CFG was additionally supported by NIEHS F31 ES031833. The funders played no role in any aspect of the present study.
Abbreviations and Acronyms:
- AX
area of reactance
- BMI
body mass index
- CO
carbon monoxide
- COPD
chronic respiratory diseases including chronic obstructive pulmonary disease
- Fres
resonant frequency
- GRAPHS
The Ghana Randomized Air Pollution and Health Study
- HAZ
height-for-age z-score
- Hz
Hertz
- IOS
impulse oscillometry
- LCGA
Latent Class Growth Analyses
- LMICs
low- and middle-income countries
- R5
resistance at 5 Hertz
- R20
resistance at 20 Hertz
- R5–20
the difference in resistance between 5Hz and 20Hz
- WAZ
weight-for-age z-score
- WHZ
weight-for-height z-score
- X5
reactance at 5 Hz
Footnotes
Declaration of interests: All authors declare no relevant conflicts of interest.
Data availability statement:
Anonymized data that underlie the results reported herein are available upon request. Proposals should be directed to kwakupoku.asante@kintampo-hrc.org and to Alison.Lee@mssm.edu; to gain access, data requestors will need to sign a data access agreement.
References
- 1.Berry CE, Billheimer D, Jenkins IC, Lu ZJ, Stern DA, Gerald LB, Carr TF, Guerra S, Morgan WJ, Wright AL et al. 2016. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med. 194(5):607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tennant PW, Gibson GJ, Pearce MS. 2008. Lifecourse predictors of adult respiratory function: Results from the newcastle thousand families study. Thorax. 63(9):823–830. [DOI] [PubMed] [Google Scholar]
- 3.McAllister DA, Liu L, Shi T, Chu Y, Reed C, Burrows J, Adeloye D, Rudan I, Black RE, Campbell H et al. 2019. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: A systematic analysis. Lancet Glob Health. 7(1):e47–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, Bowatte G, Gurrin L, Johns DP, Thompson BR et al. 2018. Childhood predictors of lung function trajectories and future copd risk: A prospective cohort study from the first to the sixth decade of life. The Lancet Respiratory medicine. 6(7):535–544. [DOI] [PubMed] [Google Scholar]
- 5.Martinez FD. 2016. Early-life origins of chronic obstructive pulmonary disease. The New England journal of medicine. 375(9):871–878. [DOI] [PubMed] [Google Scholar]
- 6.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. 2008. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet (London, England). 371(9608):243–260. [DOI] [PubMed] [Google Scholar]
- 7.Prentice AM, Moore SE. 2005. Early programming of adult diseases in resource poor countries. Archives of disease in childhood. 90(4):429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arigliani M, Spinelli AM, Liguoro I, Cogo P. 2018. Nutrition and lung growth. Nutrients. 10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boamah-Kaali E, Jack DW, Ae-Ngibise KA, Quinn A, Kaali S, Dubowski K, Oppong FB, Wylie BJ, Mujtaba MN, Gould CF et al. 2021. Prenatal and postnatal household air pollution exposure and infant growth trajectories: Evidence from a rural ghanaian pregnancy cohort. Environ Health Perspect. 129(11):117009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee AG, Kaali S, Quinn A, Delimini R, Burkart K, Opoku-Mensah J, Wylie BJ, Yawson AK, Kinney PL, Ae-Ngibise KA et al. 2019. Prenatal household air pollution is associated with impaired infant lung function with sex-specific effects. Evidence from graphs, a cluster randomized cookstove intervention trial. American journal of respiratory and critical care medicine. 199(6):738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saad NJ, Patel J, Burney P, Minelli C. 2017. Birth weight and lung function in adulthood: A systematic review and meta-analysis. Annals of the American Thoracic Society. 14(6):994–1004. [DOI] [PubMed] [Google Scholar]
- 12.Gray D, Willemse L, Visagie A, Czövek D, Nduru P, Vanker A, Stein DJ, Koen N, Sly PD, Hantos Z et al. 2017. Determinants of early-life lung function in african infants. Thorax. 72(5):445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lima Rda C, Victora CG, Menezes AM, Barros FC. 2005. Respiratory function in adolescence in relation to low birth weight, preterm delivery, and intrauterine growth restriction. Chest. 128(4):2400–2407. [DOI] [PubMed] [Google Scholar]
- 14.Stein CE, Kumaran K, Fall CH, Shaheen SO, Osmond C, Barker DJ. 1997. Relation of fetal growth to adult lung function in south india. Thorax. 52(10):895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peralta GP, Fuertes E, Granell R, Mahmoud O, Roda C, Serra I, Jarvis D, Henderson J, Garcia-Aymerich J. 2019. Childhood body composition trajectories and adolescent lung function. Findings from the alspac study. American journal of respiratory and critical care medicine. 200(1):75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassaye T, Becklake MR, Receveur O, Hanley JA, Johns T. 2001. Association between vitamin a status and lung function level in children aged 6−−9 years in wukro wereda, northern ethiopia. International journal of epidemiology. 30(3):457–464. [DOI] [PubMed] [Google Scholar]
- 17.Arigliani M, Canciani MC, Mottini G, Altomare M, Magnolato A, Loa Clemente SV, Tshilolo L, Cogo P, Quanjer PH. 2017. Evaluation of the global lung initiative 2012 reference values for spirometry in african children. American journal of respiratory and critical care medicine. 195(2):229–236. [DOI] [PubMed] [Google Scholar]
- 18.Lelijveld N, Kerac M, Seal A, Chimwezi E, Wells JC, Heyderman RS, Nyirenda MJ, Stocks J, Kirkby J. 2017. Long-term effects of severe acute malnutrition on lung function in malawian children: A cohort study. The European respiratory journal. 49(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jack DW, Asante KP, Wylie BJ, Chillrud SN, Whyatt RM, Ae-Ngibise KA, Quinn AK, Yawson AK, Boamah EA, Agyei O et al. 2015. Ghana randomized air pollution and health study (graphs): Study protocol for a randomized controlled trial. Trials. 16:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jack DW, Ae-Ngibise KA, Gould CF, Boamah-Kaali E, Lee AG, Mujtaba MN, Chillrud S, Kaali S, Quinn AK, Gyaase S et al. 2021. A cluster randomised trial of cookstove interventions to improve infant health in ghana. BMJ Global Health. 6(8):e005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chillrud SN, Ae-Ngibise KA, Gould CF, Owusu-Agyei S, Mujtaba M, Manu G, Burkart K, Kinney PL, Quinn A, Jack DW et al. 2021. The effect of clean cooking interventions on mother and child personal exposure to air pollution: Results from the ghana randomized air pollution and health study (graphs). Journal of exposure science & environmental epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boamah EA, Asante K, Ae-Ngibise K, Kinney PL, Jack DW, Manu G, Azindow IT, Owusu-Agyei S, Wylie BJ. 2014. Gestational age assessment in the ghana randomized air pollution and health study (graphs): Ultrasound capacity building, fetal biometry protocol development, and ongoing quality control. JMIR research protocols. 3(4):e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinn AK AI, Ae-Ngibise KA, Agyei O, Boamah-Kaali EA, Burkart K, Carrión D, Chillrud SN, Gould CF, Gyaase S, Jack DW, Kaali S, Kinney PL, Lee AG, Mujtaba MN, Oppong FB, Owusu-Agyei S, Yawson A, Wylie BJ, Asante KP. 2021. Jun 13. Prenatal household air pollutant exposure is associated with reduced size and gestational age at birth among a cohort of ghanaian infants. . Environ Int. 155:106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinn AK, Adjei IA, Ae-Ngibise KA, Agyei O, Boamah-Kaali EA, Burkart K, Carrión D, Chillrud SN, Gould CF, Gyaase S et al. 2021. Prenatal household air pollutant exposure is associated with reduced size and gestational age at birth among a cohort of ghanaian infants. Environment international. 155:106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Organization WH. 2006. Who child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. World Health Organization. [Google Scholar]
- 26.Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, Bisgaard H, Davis GM, Ducharme FM, Eigen H et al. 2007. An official american thoracic society/european respiratory society statement: Pulmonary function testing in preschool children. American journal of respiratory and critical care medicine. 175(12):1304–1345. [DOI] [PubMed] [Google Scholar]
- 27.King GG, Bates J, Berger KI, Calverley P, de Melo PL, Dellacà RL, Farré R, Hall GL, Ioan I, Irvin CG et al. 2020. Technical standards for respiratory oscillometry. The European respiratory journal. 55(2). [DOI] [PubMed] [Google Scholar]
- 28.Gunnsteinsson S, Labrique AB, West KP Jr., Christian P, Mehra S, Shamim AA, Rashid M, Katz J, Klemm RD. 2010. Constructing indices of rural living standards in northwestern bangladesh. Journal of health, population, and nutrition. 28(5):509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berlin KS, Parra GR, Williams NA. 2014. An introduction to latent variable mixture modeling (part 2): Longitudinal latent class growth analysis and growth mixture models. Journal of pediatric psychology. 39(2):188–203. [DOI] [PubMed] [Google Scholar]
- 30.Nagin DS, Odgers CL. 2010. Group-based trajectory modeling in clinical research. Annual review of clinical psychology. 6:109–138. [DOI] [PubMed] [Google Scholar]
- 31.Muthén B 2006. The potential of growth mixture modelling. Infant and Child Development. 15(6):623–625. [Google Scholar]
- 32.Hoekstra T, Twisk JWR. 2015. The analysis of individual health trajectories across the life course: Latent class growth models versus mixed models. In: Burton-Jeangros C, Cullati S, Sacker A, Blane D, editors. A life course perspective on health trajectories and transitions. Cham (CH): Springer; Copyright 2015, The Author(s). p. 179–195. [PubMed] [Google Scholar]
- 33.Green M, Mead J, Turner JM. 1974. Variability of maximum expiratory flow-volume curves. J Appl Physiol. 37(1):67–74. [DOI] [PubMed] [Google Scholar]
- 34.Fund UNCs, Organization WH, Bank IBfRaDTW. 2019. Levels and trends in child malnutrition: Key findings of the 2019 edition of the joint child malnutrition estimates.
- 35.Durlak W, Klimek M, Wroński M, Trybulska A, Kwinta P. 2021. Multimodal longitudinal respiratory function assessment in very low birth weight 7-year-old children. Advances in medical sciences. 66(1):81–88. [DOI] [PubMed] [Google Scholar]
- 36.Shi Y, Aledia AS, Galant SP, George SC. 2013. Peripheral airway impairment measured by oscillometry predicts loss of asthma control in children. The Journal of allergy and clinical immunology. 131(3):718–723. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y, Aledia AS, Tatavoosian AV, Vijayalakshmi S, Galant SP, George SC. 2012. Relating small airways to asthma control by using impulse oscillometry in children. The Journal of allergy and clinical immunology. 129(3):671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lange P, Ahmed E, Lahmar ZM, Martinez FJ, Bourdin A. 2021. Natural history and mechanisms of copd. Respirology (Carlton, Vic). 26(4):298–321. [DOI] [PubMed] [Google Scholar]
- 39.Schittny JC. 2017. Development of the lung. Cell and tissue research. 367(3):427–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ganguly A, Martin RJ. 2019. Vulnerability of the developing airway. Respiratory physiology & neurobiology. 270:103263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogel ER, Britt RD Jr., Faksh A, Kuipers I, Pandya H, Prakash YS, Martin RJ, Pabelick CM. 2017. Moderate hyperoxia induces extracellular matrix remodeling by human fetal airway smooth muscle cells. Pediatric research. 81(2):376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Brien EA, Barnes V, Zhao L, McKnight RA, Yu X, Callaway CW, Wang L, Sun JC, Dahl MJ, Wint A et al. 2007. Uteroplacental insufficiency decreases p53 serine-15 phosphorylation in term iugr rat lungs. American journal of physiology Regulatory, integrative and comparative physiology. 293(1):R314–322. [DOI] [PubMed] [Google Scholar]
- 43.Wignarajah D, Cock ML, Pinkerton KE, Harding R. 2002. Influence of intrauterine growth restriction on airway development in fetal and postnatal sheep. Pediatric research. 51(6):681–688. [DOI] [PubMed] [Google Scholar]
- 44.Joss-Moore LA, Albertine KH, Lane RH. 2011. Epigenetics and the developmental origins of lung disease. Molecular genetics and metabolism. 104(1–2):61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harding R, Maritz G. 2012. Maternal and fetal origins of lung disease in adulthood. Seminars in fetal & neonatal medicine. 17(2):67–72. [DOI] [PubMed] [Google Scholar]
- 46.den Dekker HT, Sonnenschein-van der Voort AMM, de Jongste JC, Anessi-Maesano I, Arshad SH, Barros H, Beardsmore CS, Bisgaard H, Phar SC, Craig L et al. 2016. Early growth characteristics and the risk of reduced lung function and asthma: A meta-analysis of 25,000 children. The Journal of allergy and clinical immunology. 137(4):1026–1035. [DOI] [PubMed] [Google Scholar]
- 47.Bickel S, Popler J, Lesnick B, Eid N. 2014. Impulse oscillometry: Interpretation and practical applications. Chest. 146(3):841–847. [DOI] [PubMed] [Google Scholar]
- 48.Arshad SH, Hodgekiss C, Holloway JW, Kurukulaaratchy R, Karmaus W, Zhang H, Roberts G. 2020. Association of asthma and smoking with lung function impairment in adolescence and early adulthood: The isle of wight birth cohort study. The European respiratory journal. 55(3). [DOI] [PubMed] [Google Scholar]
- 49.Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, Macklem PT. 1978. The relations between structural changes in small airways and pulmonary-function tests. The New England journal of medicine. 298(23):1277–1281. [DOI] [PubMed] [Google Scholar]
- 50.Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. 1988. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. The New England journal of medicine. 319(17):1112–1117. [DOI] [PubMed] [Google Scholar]
- 51.Chan JY, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD. 2015. Pneumonia in childhood and impaired lung function in adults: A longitudinal study. Pediatrics. 135(4):607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agusti A, Faner R. 2019. Lung function trajectories in health and disease. The Lancet Respiratory Medicine. 7(4):358–364. [DOI] [PubMed] [Google Scholar]
- 53.Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, Bowatte G, Gurrin L, Johns DP, Thompson BR. 2018. Childhood predictors of lung function trajectories and future copd risk: A prospective cohort study from the first to the sixth decade of life. The lancet Respiratory medicine. 6(7):535–544. [DOI] [PubMed] [Google Scholar]
- 54.Mead J 1980. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis. 121(2):339–342. [DOI] [PubMed] [Google Scholar]
- 55.Smith BM, Kirby M, Hoffman EA, Kronmal RA, Aaron SD, Allen NB, Bertoni A, Coxson HO, Cooper C, Couper DJ et al. 2020. Association of dysanapsis with chronic obstructive pulmonary disease among older adults. Jama. 323(22):2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsen GL, Morgan W, Heldt GP, Mauger DT, Boehmer SJ, Chinchilli VM, Lemanske RF Jr., Martinez F, Strunk RC, Szefler SJ et al. 2009. Impulse oscillometry versus spirometry in a long-term study of controller therapy for pediatric asthma. The Journal of allergy and clinical immunology. 123(4):861–867.e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saadeh C, Saadeh C, Cross B, Gaylor M, Griffith M. 2015. Advantage of impulse oscillometry over spirometry to diagnose chronic obstructive pulmonary disease and monitor pulmonary responses to bronchodilators: An observational study. SAGE Open Med. 3:2050312115578957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
E-Figure 1. Distribution of z-scores from 3 months to 4 years of life (N=609). Children who were aged 54 months or younger at the time of lung phenotyping visit were included in construction of growth trajectories. Shown here are the distributions of: (A) Weight-for-Age, (B) Height-for-Age, and (C) Weight-for-Height z-scores across all ages (3, 6, 9, 12 months and 4 years). Across all ages, 369 (61%), 166 (27%), 61 (10%), 10 (2%), 3 (0.4%) children had five, four, three, two and one Weight-for-Age z-score measure, respectively. Across all ages, 358 (59%), 168 (28%), 69 (11%), 11 (2%), and 3 (0.4%) children had five, four, three, two and one Height-for-Age z-score measure, respectively. Across all ages, 354 (58%), 171 (28%), 69 (11%), 11 (2%) and 4 (0.7%) children had five, four, three, two and one Weight-for-Height z-score measure respectively.
Data Availability Statement
Anonymized data that underlie the results reported herein are available upon request. Proposals should be directed to kwakupoku.asante@kintampo-hrc.org and to Alison.Lee@mssm.edu; to gain access, data requestors will need to sign a data access agreement.


