Abstract
The rapid exploration of sp3-enriched chemical space is facilitated by fragment-coupling technologies that utilize simple and abundant alkyl precursors, among which alcohols are a highly desirable, commercially accessible, and synthetically versatile class of substrate. Herein, we describe an operationally convenient, N-heterocyclic carbene (NHC)-mediated deoxygenative Giese-type addition of alcohol-derived alkyl radicals to electron-deficient alkenes under mild photocatalytic conditions. The fragment coupling accommodates a broad range of primary, secondary, and tertiary alcohol partners, as well as structurally varied Michael acceptors containing traditionally reactive sites, such as electrophilic or oxidizable moieties. We demonstrate the late-stage diversification of densely functionalized molecular architectures, including drugs and biomolecules, and we further telescope our protocol with metallaphotoredox cross-coupling for step-economic access to sp3-rich complexity.
Keywords: photoredox, deoxygenation, alcohols, conjugate addition
Graphical Abstract

Alcohols are among the most commercially accessible alkyl fragments, yet their direct use in C(sp3)-based fragment couplings remains underdeveloped. In this report, a mild strategy for the in situ activation of alcohols by benzoxazolium salts is applied to deoxygenative conjugate alkylation for a broad range of alcohol and Michael acceptor substrates, including structurally diverse drugs, natural products, and biomolecules.
The ability to directly engage alkyl fragments in carbon–carbon bond-forming reactions is of great interest due to the ability of saturated architectural elements to modulate the physicochemical properties of chemical entities, including polymeric materials, biological probes, and pharmaceutical candidates.[1] As such, there is a strong impetus to invent carbon-carbon bond-forming technologies that employ C(sp3)-hybridized alkyl fragments as coupling partners. Despite notable advances in transition metal-catalyzed alkyl cross-coupling,[2] many opportunities remain to develop alternative fragment-coupling approaches.
Conjugate additions of alkyl nucleophiles represent an important class of C(sp3)-bond-forming reaction. These transformations, which historically required air- and moisture-sensitive organometallic reagents,[3] have undergone a renaissance in recent years with the resurgence of open-shell protocols.[4] Alkyl radicals generated through photoredox catalysis[5] can undergo Giese-type regioselective addition to polarized alkenes.[6] A variety of precursors, including halides,[7] amines,[8] carboxylates,[9] and sulfinates,[10] have been incorporated into this paradigm and applied in diverse contexts, such as total synthesis,[11] peptide bioconjugation,[12] and medicinal chemistry.[13] Given the importance of methods that facilitate the rapid exploration of sp3-enriched chemical space, we questioned whether simple and abundant aliphatic alcohols could be broadly engaged as radical precursors in 1,4-conjugate alkylation reactions.
Alcohols are among the most commercially abundant, synthetically versatile, and operationally convenient functional groups in organic synthesis (Figure 1).[14] Highly represented among biomolecules, alcohols serve as promising vectors for diversity-oriented preparation of biologically-active chemical libraries. Moreover, their extensive presence in pharmaceutical candidates underscores the potential for late-stage modification without the need for de novo synthesis.[15] Traditional two-electron chemistries that convert alcohols into more reactive electrophiles comprise the most common functional group interconversion in medicinal chemistry;[16] however, these methods typically require time- and resource-intensive chemical steps and purifications, while also forgoing the intrinsic advantages associated with the native alcohol functionality. The direct homolytic activation of strong C(sp3)–O bonds recently emerged as an orthogonal strategy, and various approaches have leveraged the redox chemistry of xanthate esters,[17] hemioxalates,[18] phosphoranyl radicals,[19] and low-valent oxophilic metals.[20] In 2021, our laboratory described the design and development of an N-heterocyclic carbene (NHC)-based reagent that facilitates direct deoxygenative radical generation from a wide range of primary, secondary, and tertiary alcohol substrates.[21] As a critical design feature, this scheme permits C(sp3)–O functionalization without isolation or purification of pre-activated intermediates.
Figure 1.
Deoxygenative conjugate alkylation with alcohols.
We sought to use this facile, NHC-mediated activation strategy for deoxygenative conjugate alkylation via direct, in situ activation of alcohol precursors. From the outset, we recognized that the absence of a transition metal coupling catalyst could mitigate potential concerns about the sensitivity and compatibility of organometallic species. To fully harness the structural variety and commercial abundance of alcohols, we identified the need for a robust deoxyalkylation platform that would (i) operate rapidly under visible light conditions at room temperature, (ii) utilize alkyl radicals from a diverse spectrum of alcohol substrates, and (iii) support late-stage applications with extensive functional group compatibility. In alignment with these ideals, we report herein the development of a mild and simple deoxygenative conjugate alkylation platform that enables modular access to a broad array of C(sp3)-rich products in a single step from commercial alcohols.
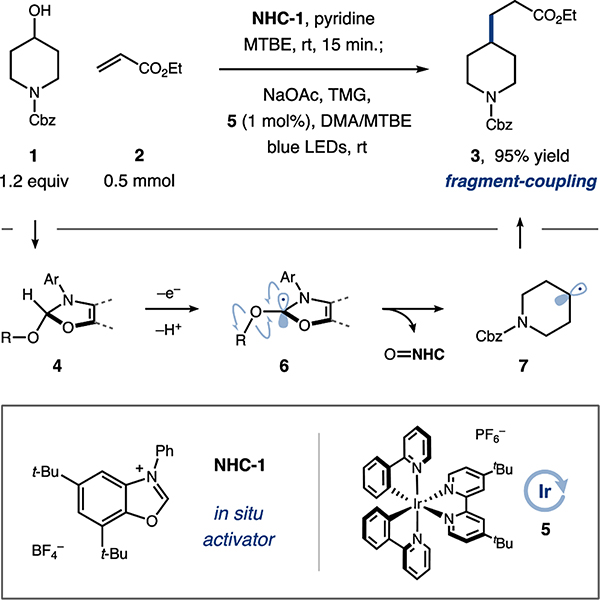
We envisioned that alcohols (1) and electrophilic alkenes (2) would undergo deoxygenative conjugate alkylation to form fragment-coupled products (3) as shown in Figure 2. Condensation of 1 with benzoxazolium salt NHC-1 forms adduct 4 in situ. Visible-light excitation of photocatalyst 5 accesses a long-lived, triplet excited state (E1/2red [*Ir(III)/Ir(II)] = +0.66 V vs. saturated calomel electrode (SCE) in MeCN, with lifetime τ = 1.3 μs).[22] Reductive quenching by 4 and subsequent deprotonation delivers an α-amino radical (6).[23] Rapid β-scission[24,25] liberates an aromatized byproduct and an alkyl radical (7), which undergoes Giese-type addition to 2 and subsequent reduction to deliver the desired adduct (3).[26] Central to our reaction design, the NHC–alcohol adduct 4 would be susceptible to mild redox activation, preferentially undergoing oxidation over traditionally photoredox-labile tertiary alkylamines[27] and carboxylic acids.[28] In contrast to two-step methods for alcohol activation, we anticipated that these mild conditions would permit broad functional group compatibility and allow us to productively engage a wide range of substrates.
Figure 2.
Reaction design and optimized conditions.
Upon reaction optimization (see Supporting Information, Tables S1–S4), we found that deoxyalkylation proceeded efficiently upon pre-stirring alcohol substrate (1, 1.2 equiv) with NHC-1 (1.1 equiv) and pyridine (2.2 equiv) in MTBE (0.1 M) for 15 minutes, followed by addition of ethyl acrylate (2, 1.0 equiv), sodium acetate (2.2 equiv), 1,1,3,3-tetramethylguanidine (TMG, 1.0 equiv), and photocatalyst 5 (1 mol%) in MTBE/DMA (1:2, 0.033 M). Subsequent irradiation (450 nm light, 2 h) afforded the desired product in excellent yield (Figure 2). This straightforward protocol is executed in a single reaction vessel with no intermediate workup or chromatography, and without the need for solvent removal or transfer. In addition, the low stoichiometric ratio of the alcohol to the Michael acceptor leads to minimal substrate waste, an important consideration for high-value coupling partners and late-stage applications.
With optimized conditions in hand, we first explored the scope of the alcohol partner (Table 1). For primary substrates that undergo slower β-scission and generate higher-energy, less nucleophilic radicals,[29] we discovered that NHC-2 was most effective for ensuring efficient deoxygenation and for suppressing undesired side reactivity between 2 and 6 prior to β-scission. Primary alcohols with appended saturated scaffolds, such as piperidine, piperazine, and cyclobutane were viable substrates in this reaction (8–10). Notably, preservation of the tertiary alkylamine[27] of adduct 9 highlights the advantage of our NHC-mediated method over more oxidizing alternatives. Despite the decreased SOMO-nucleophilicity of radicals adjacent to extended π-systems,[30] we found that α-pyrazolyl (11) and resorcinol-containing (12) alcohols could be efficiently alkylated. Amino alcohols (13) and free diols (14) underwent chemoselective activation of the more sterically accessible primary alcohol. For the fragment-coupling of secondary alcohols with high-energy s-rich C(sp3)–O bonds, we observed that in situ condensation with activator NHC-2 enabled Giese addition (15 and 16); the corresponding alkyl radicals are recognized as less nucleophilic, and lower yields were observed with other activators. By contrast, unstrained secondary alcohols that produce unbiased alkyl radicals were productively activated with NHC-1. The d8-isotopolog of leucine was prepared in excellent yield (17), indicating potential application to the preparation of peptide mass spectrometry standards.[31] Medicinally relevant, heterocyclic scaffolds (18 and 19) were readily alkylated, and acyclic secondary substrates, including fluconazole- and threonine-derived alcohols, were also successfully coupled (20–22). The assembly of alkyl-substituted quaternary carbon centers—a longstanding challenge in organic synthesis—was achieved by employing the deoxygenation mediator NHC-3, a more electrophilic reagent that effectively condenses with sterically congested alcohols. Using this activator, an array of quaternized products were prepared from the corresponding cyclic and acyclic tertiary alcohols in near-quantitative yields (23–27).
Table 1.
Photoredox Deoxygenative Conjugate Addition: Substrate Scope[a]

|
Alcohol (1.2 equiv), NHC-1 (1.1 equiv), and pyridine (2.2 equiv) in MTBE (0.1 M), then Michael acceptor (0.50 mmol, 1.0 equiv), NaOAc (2.2 equiv), TMG (1.0 equiv), and 5 (1 mol%) in MTBE/DMA (1:2, 0.033 M). Yields are isolated. See Supporting Information (SI) for full experimental details.
With NHC-2.
Condensation in DCM, reverse addition.
Between 1:1 and 2:1 d.r.
With 20 equiv D2O.
With NHC-3 in PhCF3, −20 °C to 0 °C for 2 h; [Ir(F(Me)ppy)2(dtbbpy)](PF6) instead of 5.
With DMSO instead of DMA.
With quinuclidine instead of NaOAc.
Irradiation for 4 h.
We then turned our attention to the scope of the Michael acceptor. The reaction tolerated a wide range of functionality on the electrophilic alkene fragment. Acrylates containing reactive functional groups, such as primary halides, epoxides, and terminal alkynes, were well-tolerated under these reaction conditions (29–32). Furthermore, amino acid derivatives and α-alkyl acrylates were viable substrates (33 and 34). For less-electrophilic acrylamides (35–37), we observed that tertiary, alcohol-containing secondary, and primary amides were competent. Unprotected carboxylate, nitrile, sulfone, boronate, styrenyl, and phosphonate acceptors were also found to be excellent reaction partners (38–43). Moreover, sterically hindered bicyclic systems (44), β-substituted malonates (45), and cyclic ketones (46) underwent efficient fragment coupling under these conditions.
To demonstrate the applicability of this deoxygenative fragment-coupling protocol in late-stage settings, we sought to derivatize a wide variety of structurally complex architectures, such as biomolecules and pharmaceutical agents (Table 2). We found that mannofuranose and glucopyranose underwent deoxyalkylation at the anomeric position to provide the corresponding adducts in excellent yields (47 and 48). The unactivated secondary alcohol in glucofuranose (49), as well the primary alcohols in fructopyranose (50) and ribonic acid (51), served as competent vectors for fragment coupling. In addition, the tertiary amine-containing pharmaceutical agents dasatinib and ranolazine could be successfully subjected to our optimized conditions (52 and 53), illustrating the compatibility of NHC-mediated deoxygenation with oxidizable functionality in photoredox catalysis. Ethacrynic acid (54), ibrutinib (55), and axitinib (56) were efficiently employed as alkene acceptors, demonstrating tolerance of free carboxylic acid, aryl chloride, aniline, and thioether moieties. Finally, we sought to showcase the compatibility of our protocol with subsequent metallaphotoredox reactions in an operationally facile, three-component iterative coupling. Following 1,4-conjugate alkylation, we were able to rapidly access telescoped products in a one-pot fashion by directly introducing the additional reaction components needed for either cross-electrophile coupling[32] (57) or deoxygenative arylation[21a] (58).
Table 2.
Late-stage functionalization and synthetic applications[a]

|
Isolated yields. See SI for full experimental details.
In summary, we describe a general, direct deoxygenative hydroalkylation of many classes of electrophilic olefins with primary, secondary, and tertiary alcohols through the application of a recently developed NHC-based activator. The protocol tolerates traditionally reactive sites, such as primary halides, epoxides, free carboxylic acids, and easily oxidizable tertiary amines, highlighting the mild nature of this approach. The late-stage alkylation of complex saccharides and pharmaceuticals further showcases the applicability of our deoxygenative conjugate addition in densely functionalized molecular contexts.
Supplementary Material
Acknowledgements
The authors are grateful for financial support provided by the National Institute of General Medical Sciences (NIGMS), the NIH (under award no. R35GM134897–03), the Princeton Catalysis Initiative, and kind gifts from Merck, Janssen, BMS, Genentech, Celgene and Pfizer. J.Z.W. acknowledges Princeton University for a first-year fellowship. We thank Prof. Dr. Zhe Dong, Nathan Dow, and John F. Hoskin for helpful discussions.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].a) Lovering F, Bikker J, Humblet J, J. Med. Chem. 2009, 52, 6752; [DOI] [PubMed] [Google Scholar]; b) Lovering F, Med. Chem. Commun. 2013, 4, 515. [Google Scholar]
- [2].a) Devasagayaraj A, Stüdemann T, Knochel P, Angew. Chem., Int. Ed. Engl. 1996, 34, 2723–2725; [Google Scholar]; b) Giovannini R, Stüdemann T, Dussin G, Knochel P, Angew. Chem., Int. Ed. 1998, 37, 2387–2390; [DOI] [PubMed] [Google Scholar]; c) Terao J, Ikumi H, Watanabe A, Kuniyasu H, Kambe N, J. Am. Chem. Soc. 2002, 124, 4222–4223; [DOI] [PubMed] [Google Scholar]; d) Zhou J, Fu GC, J. Am. Chem. Soc. 2003, 125, 14726–14727. [DOI] [PubMed] [Google Scholar]
- [3].a) Komnenos T, Lieb. Ann. Chem. 1883, 218, 145–167; [Google Scholar]; b) Michael A, J. Prakt. Chem. 1887, 35, 349; [Google Scholar]; c) Perlmutter P; Tetrahedron Organic Chemistry Series, No. 9; Pergamon: Oxford, 1992. [Google Scholar]
- [4].a) Shaw MH, Twilton J, MacMillan DWC, J. Org. Chem. 2016, 81, 6898–6926; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gant Kanegusuku AL, Roizen JL, Angew. Chem., Int. Ed. 2021, 60, 21116– 21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Chan AY, Perry IB, Bissonnette NB, Buksh BF, Edwards GA, Frye LI, Garry OL, Lavagnino MN, Li BX, Liang Y, Mao E, Millet A, Oakley JV, Reed NL, Sakai HA, Seath CP, MacMillan DWC, Chem. Rev. 2022, 122, 1485–1542; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Skubi KL, Blum TR, Yoon TP, Chem. Rev. 2016, 116, 10035–10074; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Levin MD, Kim S, Toste FD, ACS Cent. Sci. 2016, 2, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) Giese B, Lachhein S, Angew. Chem., Int. Ed. Engl, 1981, 20, 967; [Google Scholar]; b) Giese B, Angew. Chem., Int. Ed. Engl, 1983, 22, 753–764; [Google Scholar]; c) Giese B, Dupuis J, Angew. Chem., Int. Ed. Engl. 1983, 22, 622–623; [Google Scholar]; d) Giese B, González-Gómez JA, Witzel T, Angew. Chem., Int. Ed. Engl, 1984, 23, 69–70. [Google Scholar]
- [7].a) Fukuzumi S, Mochizuki S, Tanaka T, J. Phys. Chem. 1990, 94, 722; [Google Scholar]; b) Narayanam JM, Tucker JW, Stephenson CR, J. Am. Chem. Soc. 2009, 131, 8756–8757; [DOI] [PubMed] [Google Scholar]; c) Maji T, Karmakar A, Reiser O, J. Org. Chem. 2011, 77, 1216; [DOI] [PubMed] [Google Scholar]; d) Nguyen JD, D’Amato EM, Narayanam JMR, Stephenson CRJ, Nature Chem. 2012, 4, 854–859; [DOI] [PubMed] [Google Scholar]; e) Constantin T, Zanini M, Regni A, Sheikh NS, Juliá F, Leonori D, Science 2020, 367, 1021–1026 [DOI] [PubMed] [Google Scholar]
- [8].Wu J, Grant PS, Li X, Noble A, Aggarwal VK, Angew. Chem., Int. Ed. 2019, 58, 5697–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kitcatt D, Nicolle S, Lee AL, Chem. Soc. Rev. 2022, 51, 1415–1453. [DOI] [PubMed] [Google Scholar]
- [10].Gualandi A, Mazzarella D, Ortega-Martinez A, Mengozzi L, Calcinelli F, Matteucci E, Monti F, Armaroli N, Sambri L, Cozzi PG, ACS Catal. 2017, 7, 5357–5362. [Google Scholar]
- [11].a) Jamison CR, Overman LE, Acc. Chem. Res. 2016, 49, 1578–1586; [DOI] [PubMed] [Google Scholar]; b) Smith JM, Harwood SJ, Baran PS, Acc. Chem. Res. 2018, 51, 1807–1817; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Pitre SP, Overman LE, Chem. Rev. 2021, 122, 1717–1751. [DOI] [PubMed] [Google Scholar]
- [12].a) Kim J, Li BX, Huang RY-C, Qiao JX, Ewing WR, MacMillan DWC, J. Am. Chem. Soc. 2020, 142, 21260– 21266; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bloom S, Liu C, Kölmel DK, Qiao JX, Zhang Y, Poss MA, Ewing WR, MacMillan DWC, Nature Chem. 2018, 10, 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ritts A, ElMarrouni CB, Balsells J, Chem. Sci. 2018, 9, 6639–6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].a) Ertl P, 2017, 36, 1–7; [Google Scholar]; b) Ertl P, Schuhmann T, J. Nat. Prod. 2019, 82, 1258–1263; [DOI] [PubMed] [Google Scholar]; c) Henkel T, Brunne RM, Müller H, Reichel F, Angew. Chem., Int. Ed. 1999, 38, 643–647; [DOI] [PubMed] [Google Scholar]; d) Reaxys search (January 2022) of commercially available alkyl fragments: alcohols (177,700), carboxylic acids (98,451), bromides (26,966), iodides (3,232), boron/zinc/Grignard/lithium (1,297).
- [15]. Reaxys search (March 2022) of alkyl alcohol fragments with pharmacological data (1,085,251).
- [16].a) Dombrowski AW, Gesmundo NJ, Aguirre AL, Sarris KA, Young JM, Bogdan AR, Martin MC, Gedeon S, Wang Y, ACS Med. Chem. Lett. 2020, 11, 597–604; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Carey JS, Laffan D, Thomson C, Williams MT, Org. Biomol. Chem. 2006, 4, 2337– 2347; [DOI] [PubMed] [Google Scholar]; c) Roughley SD, Jordan AM, J. Med. Chem. 2011, 54, 3451– 3479; [DOI] [PubMed] [Google Scholar]; d) Schneider N, Lowe DM, Sayle RA, Tarselli MA, Landrum GA, J. Med. Chem. 2016, 59, 4385– 4402. [DOI] [PubMed] [Google Scholar]
- [17].a) Lopez RM, Hays DS, Fu GC, J. Am. Chem. Soc. 1997, 119, 6949–6950; [Google Scholar]; b) Zard SZ, Angew. Chem., Int. Ed. Engl. 1997, 36, 672–685; [Google Scholar]; c) Togo H, Matsubayashi S, Yamazaki O, Yokoyama MJ, Org. Chem. 2000, 65, 2816–2819; [DOI] [PubMed] [Google Scholar]; d) Spiegel DA, Wiberg KB, Schacherer LN, Medeiros MR, Wood JL, J. Am. Chem. Soc. 2005, 127, 12513–12515; [DOI] [PubMed] [Google Scholar]; e) Chenneberg L, Baralle A, Daniel M, Fensterbank L, Goddard J-P, Ollivier C, Adv. Synth. Catal. 2014, 356, 2756–2762; [Google Scholar]; f) Friese FW, Studer A, Angew. Chem., Int. Ed. 2019, 58, 9561–9564; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Wu J, Bär RM, Guo L, Noble A, Aggarwal VK, Angew. Chem., Int. Ed. 2019, 58, 18830–18834; [DOI] [PubMed] [Google Scholar]; h) Guo HM, He BQ, X. W, Org. Lett. 2022, 24, 3199–3204. [DOI] [PubMed] [Google Scholar]
- [18].a) Lackner GL, Quasdorf KW, Overman LE, J. Am. Chem. Soc. 2013, 135, 15342–15345; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nawrat CC, Jamison CR, Slutskyy Y, MacMillan DWC, Overman LE, J. Am. Chem. Soc. 2015, 137, 11270–11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].a) Zhang L, Koreeda M, J. Am. Chem. Soc. 2004, 126, 13190–13191; [DOI] [PubMed] [Google Scholar]; b) Stache EE, Ertel AB, Rovis T, Doyle AG, ACS Catal. 2018, 8, 11134–11139; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Guo H-M, Wu X, Nat. Commun. 2021, 12, 5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].a) Xie H, Guo J, Wang Y-Q, Wang K, Guo P, Su P-F, Wang X, Shu X-Z, J. Am. Chem. Soc. 2020, 142, 16787–16794; [DOI] [PubMed] [Google Scholar]; b) Xie H, Wang S, Wang Y, Guo P, Shu X-Z, ACS Catal. 2022, 12, 1018–1023; [Google Scholar]; c) Suga T, Takahashi Y, Miki C, Ukaji Y, Angew. Chem., Int. Ed. 2022, e202112533. [DOI] [PubMed] [Google Scholar]
- [21].a) Dong Z, MacMillan DWC, Nature 2021, 598, 451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]; B) Sakai HA, MacMillan DWC, J. Am. Chem. Soc. 2022, 144, 6185–6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Slinker JD, Gorodetsky AA, Lowry MS, Wang J, Parker S, Rohl R, Bernhard S, Malliaras GG, J. Am. Chem. Soc. 2004, 126, 2763–2767 [DOI] [PubMed] [Google Scholar]
- [23].a) McNally A, Prier CK, MacMillan DWC, Science 2011, 334, 1114–1117; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dinnocenzo JP, Banach TE, J. Am. Chem. Soc. 1989, 111, 8646–8653. [Google Scholar]
- [24].Kariofillis SK, Shields BJ, Tekle-Smith MA, Zacuto MJ, Doyle AG, J. Am. Chem. Soc. 2020, 142, 7683–7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].a) Huang L, Ji T, Rueping M, J. Am. Chem. Soc. 2020, 142, 3532–3539; [DOI] [PubMed] [Google Scholar]; b) Cong F, Lv X-Y, Day CS, Martin R, J. Am. Chem. Soc. 2020, 142, 20594–20599; [DOI] [PubMed] [Google Scholar]; c) Chen Y, Wang X, He X, An Q, Zuo Z, J. Am. Chem. Soc. 2021, 143, 4896–4902. [DOI] [PubMed] [Google Scholar]
- [26].Bortolamei N, Isse AA, Gennaro A, Electrochim. Acta. 2010, 55, 8312. [Google Scholar]
- [27].Beatty JW, Stephenson CRJ, Acc. Chem. Res. 2015, 48, 1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].a) Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, MacMillan DWC, Science 2014, 345, 437; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Noble A, McCarver SJ, MacMillan DWC, J. Am. Chem. Soc. 2015, 137, 624; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chu L, Ohta C, Zuo Z, MacMillan DWC, J. Am. Chem. Soc. 2014, 136, 10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Parsaee F, Senarathna MC, Kannangara PB, Alexander SN, Arche PDE, Welin ER, Nat. Rev. Chem. 2021, 5, 486–499. [DOI] [PubMed] [Google Scholar]
- [30].Hoomissen DJV, Vyas S, J. Org. Chem. 2017, 82, 5731–5742. [DOI] [PubMed] [Google Scholar]
- [31].a) Gant TG, J. Med. Chem. 2014, 57, 3595–3611; [DOI] [PubMed] [Google Scholar]; b) Hell SM, Meyer CF, Ortalli S, Sap JBI, Chen X, Gouverneur V, Chem. Sci. 2021, 12, 12149–12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang P, Le CC, MacMillan DWC, J. Am. Chem. Soc. 2016, 138, 8084–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.