Abstract
Transcription is the first step of gene expression and involves RNA polymerases. After transcription initiation, RNA polymerase enters elongation followed by transcription termination at the end of the gene. Only recently, structures of transcription elongation complexes bound to key transcription elongation factors have been determined in bacterial and eukaryotic systems. These structures have revealed numerous insights including the basis for transcriptional pausing, RNA polymerase interaction with large complexes such as the ribosome and the spliceosome, and the transition into productive elongation. Here, we review these structures and describe areas for future research.
Keywords: Transcription elongation, cryo-EM, RNA polymerase
Introduction
Transcription is a key step in gene expression and is carried out by RNA polymerases. Bacteria possess a single multi-subunit RNA polymerase (RNAP), whereas eukaryotes harbor three to five multi-subunit RNA polymerases. RNA polymerase II (Pol II) primarily transcribes protein-coding genes in eukaryotes [1].
Transcription can be divided into initiation, elongation, and termination phases. During initiation, gene promoters are recognized by general transcription factors and recruit RNA polymerase to form the pre-initiation complex (PIC) [2][3]. RNA polymerase, with the help of transcription initiation factors, unwinds the DNA to form a transcription bubble, and RNA synthesis can commence. After RNA polymerase has synthesized 9-10 RNA nucleotides, it escapes from the promoter region and enters the elongation phase of transcription. Elongation consists of the processive translocation of RNA polymerase along the gene body in single-base steps, concomitantly incorporating individual nucleotides into the nascent RNA (reviewed in: [4][5]). Termination occurs when nucleotide addition ends, and the RNA product is released.
Numerous structures of prokaryotic and eukaryotic RNA polymerase elongation complexes have been determined. Most of these structures, however, completely lack or include only a subset of elongation factors that regulate polymerase activity. With recent advances in cryo-electron microscopy (cryo-EM), cryo-electron tomography (cryo-ET) and crosslink-mass spectrometry (XL-MS), key regulatory steps in transcription elongation including pausing, mRNA capping, pause release, splicing, and termination have been visualized. This review will focus on the structural basis of elongation, emphasizing recent structures of paused, paused released, and activated elongation complexes. We also review structures of co-transcriptional processes and termination complexes.
Promoter escape and release into elongation
Distinct transcription factors are required for transcription initiation and transcription elongation. Initiation factors can be exchanged for elongation factors during the early stages of RNA synthesis. Structural studies of initiation and elongation complexes have allowed us to carefully map how these factors are exchanged during transcription. For example, σ70 is replaced by NusG on the RNAP clamp (albeit σ70 may not fully disassociate from RNAP) [6][7][8] (Fig 1A). Similarly, eukaryotic initiation factor TFIIB which is a structural and functional homolog of σ factors is exchanged for Spt4-5 (known as DRB-sensitivity inducing factor (DSIF) in mammals) near the RNA exit channel after Pol II has synthesized ~12-13 nts [9] (Fig 1B). The Pol II RPB2 protrusion domain serves as a key binding surface for heterodimeric, triple β-barrel domain containing complexes including initiation factor TFIIF, and elongation factors ELL2-EAF1 and Polymerase associated factor 1 (PAF) subunits PAF1-LEO1 (Fig 1C) [10–12]. TFIIF, unlike TFIIB, can associate with Pol II during elongation [9][13]. Finally, the Pol II stalk and foot region are bound by the Mediator complex during transcription initiation and these surfaces are exchanged for SPT6 and the PAF complex, respectively, during transcription elongation (Fig 1B). These transitions appear to be driven by cyclin dependent kinases (CDK) including CDK7 and CDK9, which phosphorylate the C-terminal domain (CTD) of the largest Pol II subunit and transcription factors [14]. In summary, overlapping binding surfaces utilized by initiation and elongation factors may be used to prevent or weaken binding of initiation factors after RNA polymerase has started to successfully elongate the RNA.
Figure 1. Initiation and elongation factors occupy overlapping binding surfaces on RNA polymerase.
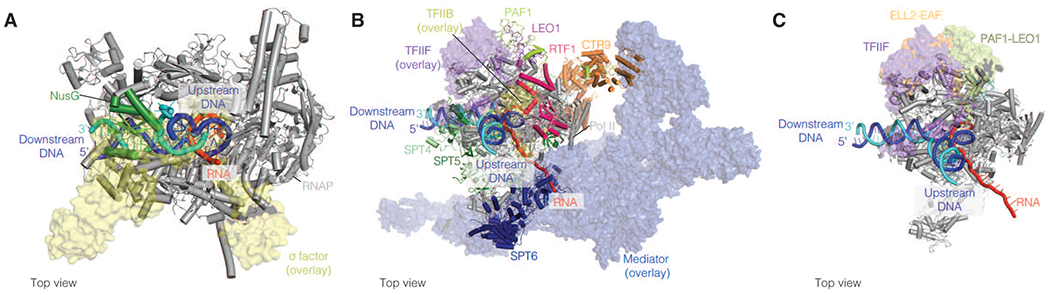
A. Comparison of transcription initiation and elongation complexes. Initiation factor σ (yellow) (Protein databank (PDB) ID: 6P1K) and elongation factor NusG (green) (PDB ID: 6C6U) occupy similar binding surfaces on the RNAP clamp (grey).
B. Initiation factors Mediator (blue), TFIIB (yellow), and TFIIF (purple) (PDB ID: 7ENC) and elongation factors SPT6 (dark blue), DSIF (green and light green), and PAF, respectively (PDB ID: 6TED), bind overlapping Pol II surfaces. Initiation factors are shown as transparent surfaces. Elongation factors are shown in cartoon representation.
C. Triple barrel domain containing proteins associate with an overlapping binding surface on the Pol II protrusion, TFIIF (purple) (PDB ID 7ENC), ELL2-EAF1 (orange) (PDB ID 7OKY), and LEO-PAF1 (chartreuse) (PDB ID 6TED). TFIIF and ELL2-EAF are overlaid on the EC* structure.
Conserved transcription elongation factor NusG/SPT4-5
NusG/SPT5 is the only elongation factor that is conserved among prokaryotes and eukaryotes and is the first elongation factor to bind RNA polymerase after initiation. NusG and SPT5 share significant sequence and structural homology [15]. NusG and its paralog RfaH are prokaryotic proteins consisting of a NusG N-terminal domain (NGN) and a Kyprides, Ouzounis, Woese (KOW) domain (Fig 2A). Similarly, SPT5 comprises an NGN domain followed by five to six KOW domains and a C-terminal repeat region. In metazoans, two additional KOW domains are found at the C-terminus of the protein (Fig 2B). SPT5 forms a heterodimer with SPT4 via its NGN domain.
Figure 2. Structures of early elongation complexes.

A. The NusG (green cartoon) and RfaH (green surface) NGN domains (PDB IDs 6C6U, 6C6S) flank the DNA cleft and associate with the upstream DNA as it exits RNAP. The RfaH KOW domain makes additional contacts with the upstream DNA (yellow surface).
B. Cryo-EM structure of human DSIF bound to Pol II (PDB ID 5OIK). DSIF forms a DNA clamp around the upstream DNA using SPT4 (light green) and the SPT5 NGN (dark green) and KOW1-L1 (yellow) domains. The RNA clamp is formed by SPT5 KOWx-4 (marine blue) and KOW5 (purple) domains. The KOW6-7 domain is superimposed and has not been visualized in DSIF bound Pol II structures (PDB ID: 5OHQ). Flexible regions associated with P-TEFb phosphorylation are indicated.
C. Cryo-EM structure of Integrator complex bound to mammalian paused elongation complex. Integrator endonuclease subunit INTS11 (purple) makes contacts with the exiting RNA. Phosphatase PP2A (yellow) binds near the Pol II CTD (PDB ID: 7PKS).
NusG, RfaH, and SPT4-5 bind RNAP or Pol II adjacent to the upstream DNA duplex and the end of transcription bubble using their NGN domains [16–19]. The NusG and RfaH NGN stabilize the RNAP active site by contacting the β’ and β RNAP domain pincers [17]. The SPT5 KOW1 and RfaH KOW domains also bind the upstream DNA next to the NGN (Fig 2A) [17]. SPT5 additionally forms a clamp around the exiting RNA via its KOWx-4 and KOW5 domains [18]. These conserved interactions serve as a baseline complex for all transcription elongation complexes.
Release into processive elongation
In eukaryotes, additional elongation factors are required for Pol II to achieve fast rates of RNA synthesis and to overcome and retain nucleosomes in the gene body [14]. These factors include PAF and SPT6. Pol II phosphorylation is required for SPT6 and PAF to bind Pol II [11][20]. These phosphorylations are installed by kinases Bur1/2 in S. cerevisiae and positive transcription elongation factor (P-TEF) b in mammals [21][11].
P-TEFb is a heterodimer consisting of cyclin dependent kinase 9 (CDK9) and cyclin-T1/cyclin-T2 (CYCT1/2). In addition to Pol II, P-TEFb phosphorylates negative elongation factor (NELF) and PAF, SPT6, and DSIF to form a complex called EC*, which contains Pol II, DSIF, PAF, and SPT6. NELF is specifically phosphorylated on subunit A, which makes contacts with Pol II and DSIF. This region is essential for NELF mediated pausing and phosphorylation may destabilize the NELF’s interaction with Pol II [22,23]. SPT5 is phosphorylated on a linker region between KOWx-4 and KOW5 (residue Ser666) as well as an unstructured C-terminal repeat region that precedes KOW6-7 [21][24][25]. Phosphorylation of SPT5 Ser666 is associated with escape from promoter proximal regions whereas phosphorylation of the C-terminal repeat region appears to counteract premature termination [26][27] (Fig 2B).
Recent structural and biochemical experiments have shown that EC* elongates RNA 5-10-fold faster than isolated Pol II, likely through an allosteric activation mechanism [28]. Specifically, the cryo-EM structure of EC* shows that PAF subunit RTF1 contacts the N-terminus of the Pol II bridge helix [28]. This interaction may enhance bending dynamics of the bridge helix that are thought to be associated with higher rates of nucleotide incorporation [29]. Together, elongation factors bind Pol II reversibly and are necessary for achieving fast rates of transcription.
Pausing and other offline states
RNA Polymerase pausing
Pausing is a key regulatory mechanism that controls the rate of RNA synthesis and is involved in mRNA processing, transcriptional proofreading, and RNA folding [4][30]. In prokaryotes, pausing is often coupled to co-transcriptional translation [31]. In metazoans, PoI II frequently pauses in the promoter proximal region (50-150 bp after the transcription start site) of genes (up to 70% of all genes) [14][32].
Structures of paused RNAP and Pol II were recently determined [33][16][23]. A common feature of these structures is the presence of an RNA•DNA hybrid in the polymerase active site that adopts a tilted conformation at the site of nucleotide addition. The nascent RNA adopts a post-translocated position whereas the template DNA adopts a pre-translocated position [16][23][33]. This offline state requires full forward translocation of the template DNA strand in the active site for RNA synthesis to continue. The exact mechanism for this is not yet known.
Pausing in metazoans is stabilized by factors DSIF and NELF. The cryo-EM structure of the mammalian paused elongation complex with Pol II-DSIF-NELF shows that NELF may use up to five different mechanisms to stabilize the paused state [23]. First, NELF restricts movement of the Pol II core and shelf modules, which may stabilize the tilted RNA•DNA hybrid state. Second, NELF obstructs TFIIS from associating with Pol II thereby preventing reactivation of Pol II, which involves shelf and core module movement. Third, NELF binds over the Pol II funnel which may interfere with diffusion of nucleotides into the Pol II active site. Fourth, NELF appears to block the binding of elongation factors such PAF. Fifth, a flexible region of NELF subunit A termed the NELF-A tentacle appears to contact DSIF and is essential for pausing [23][22]. Using this array of contacts, NELF can allosterically enhance Pol II pausing behavior.
Pausing is sometimes associated with the formation of RNA hairpins or other secondary structures in the nascent RNA [34][33][16]. These RNA hairpins can invade the RNAP RNA exit channel and may stabilize a swiveled RNAP clamp state by preventing it from unswiveling. The swivel RNAP clamp state may impair nucleotide incorporation by blocking trigger loop folding [17][16]. Elongation factor NusA binds both RNAP and the RNA hairpin to further stabilize the pause [16]. RNA hairpins found at eukaryotic pause sites likely serve a different function as they do not appear to be necessary for pausing [35][36]. The paused Pol II-DSIF-NELF structure was determined on a HIV-1 pause sequence containing the HIV-1 TAR hairpin. This hairpin that lies directly outside of the RNA exit channel was not visualized, suggesting a different behavior than the bacterial hairpin RNAs. The NELF-E RRM domain and P-TEFb also bind the HIV-1 TAR hairpin [37][35], implicating a potential role for the RNA in factor recruitment. Future studies are required to understand how eukaryotic RNA secondary structure may regulate pausing.
RNA Polymerase Backtracking
RNA polymerase can backtrack when it encounters DNA lesions or transcriptional barriers such as nucleosomes, or when it misincorporates nucleotides. Long pausing events are associated with backtracked RNA polymerases. During this process, the 3’ end of the RNA is extruded through the secondary channel as the polymerase travels backwards on the DNA template [38][39]. Elongation factors GreA and GreB in prokaryotes and TFIIS in eukaryotes bind near the secondary channel and insert a domain containing acidic residues into the active site to stimulate cleavage of the 3’ end of the RNA [38][39]. TFIIS can additionally realign the RNA•DNA hybrid in the active site [39]. Understanding the role of TFIIS in processes such as nucleosomal transcription where pausing frequently occurs are still open areas in structural biology [40].
Transcriptional attenuation
Paused RNAP and Pol II can prematurely terminate in gene bodies in a process termed transcriptional attenuation. In mammals and other multi-cellular eukaryotes, transcriptional attenuation is intimately linked to promoter-proximal pausing and involves the 14-subunit Integrator complex [41][42]. Recent cryo-EM structures of the mammalian paused elongation complex in complex with the 1.5 MDa Integrator complex resolved a pre-termination state [43][44]. Specifically, the exiting RNA makes contacts with Integrator subunit INTS11, the known endonuclease subunit of Integrator. It is currently thought that RNA cleavage by INTS11 stimulates premature termination, although the mechanism for this remains unclear (Fig 2C) [45][46][47]. Additionally, the Integrator complex localizes Protein Phosphatase (PP)2A near the Pol II CTD. This positioning appears to antagonize CDK9 phosphorylation of the Pol II CTD and SPT5, thereby hampering elongation and supporting termination (Fig 2C) [27][48]. The Integrator complex does not bind to the upstream DNA clamp formed by DSIF, which may enable the release of DNA from Pol II once the RNA is cleaved and premature termination has occurred. Finally, Integrator appears to block binding of elongation factors SPT6 and PAF to Pol II [43].
In bacteria, transcriptional attenuation is frequently associated with transcription coupled translation. This coupling is used to finely regulate gene expression in response to stress and nutrient levels [49]. The structures of RNAP-ribosome complexes, containing elongation factors NusG and NusA, were recently determined by three groups using cryo-EM, cryo-ET and crosslinking followed by mass spectrometry (XL-MS) [50][51][52]. Together, they show at least two arrangements of RNAP and the ribosome. The Weixlbaumer and Ebright groups show that E. coli NusG bridges RNAP and the head of the 30S ribosomal subunit (Fig 3A) when the RNAP active site and ribosomal P-site are separated by 38-47 nucleotides [50][51]. Shorter RNA spacers are incompatible with NusG bridging and resemble a collided or uncoupled state previously termed the “expressome” [53].
Figure 3. Events coupled to transcription elongation.

A. RNAP-30S ribosome structures from Escherichia coli and Mycoplasma pneumoniae (PDB IDs: 6ZTJ, PDVDEV: 00000049). The 50S has been omitted for simplicity. NusG orientation is indicated by the position of the NGN and KOW domains (green). NusA by the CTD, KH1, KH2 and NTD domains (yellow).
B. Cryo-EM structure of EC* bound to the U1 spliceosome (PDB ID: 7B0Y). RTF1 was excluded in this structure, and its binding position in EC* is shown as a silhouette (hot pink) (PDB ID: 6TED). RTF1 and the U1-70K and U1-SnRNA share an overlapping binding surface on Pol II subunit RPB12.
C. Structures of chromatin remodeller Chd1 and histone chaperone FACT during nucleosomal transcription. As S. cerevisiae Pol II begins to transcribe through a nucleosome, Chd1 is bound (left panel) (PDB ID: 7NKX). The interaction between Chd1 and FACT is indicated by a dotted line. In the right panel, Pol II has transcribed further into the nucleosome, exposing the H2A-H2B dimer. FACT binds the exposed histone dimer. Chd1 is displaced but retains its interaction with FACT (dotted line) (PDB ID: 7NKY).
A different arrangement was observed in Mycoplasma pneumoniae using cryo-ET and XL-MS. In these structures, NusA mediates the RNAP-ribosome interaction and NusG bridging is absent (Fig 3A). Although these structures are not mutually exclusive, these two states could recapitulate different RNAP-ribosome complexes or indicate that NusG is not required for coupling in some bacterial species [54]. The latter possibility is supported by a proline rich motif, which is crucial for the NusA interaction and the 30S ribosome subunit in M. pneumoniae yet is poorly conserved in E. coli [55]. Similarly, the residues of E. coli NusA that mediate the 30S interaction are not conserved in M. pneumoniae NusA [55]. This suggests that the RNAP-ribosome complex may assemble differently across the prokaryotic domain and in some species there may not be any direct coupling of transcription and translation [56][55].
Transcription-coupled DNA repair
Bulky DNA adducts in the template DNA strand induce RNA polymerase stalling. This stalled polymerase complex can continue elongation either by error-prone or error-free translesion synthesis (TLS) [57][58]. Alternatively, stalled RNA polymerase is used to recruit DNA repair factors, resulting in transcription coupled DNA repair (TCR) [59]. In mammals, a complex comprising the helicase CSB, the E3 ubiquitin ligase CRL4CSA, and the scaffold protein UVSSA, binds Pol II [60]. Five new cryo-EM structures recapitulate different stages of mammalian TCR [59]. Based on these structures, a model for TCR is proposed. Upon encountering a lesion in the DNA, Pol II stalls and CSB replaces DSIF on the upstream side of Pol II. CSB then uses its ATPase activity to push Pol II forward. If the lesion containing DNA is bypassed by Pol II, transcription elongation can resume. When such obstacles are not overcome, CRL4CSA ubiquitylates Pol II and TFIIH may push Pol II backwards on the DNA template. This can result in complete disassembly and degradation of the EC by the proteosome and repair of the lesion by global-genome nucleotide excision repair (GG-NER) [59][61]. Future structural work is required to understand how repair factors coordinate with Pol II and how the process of TCR is mediated in conjunction with RNAP.
Co-transcriptional RNA processing
RNA is co-transcriptionally processed. This includes but is not limited to capping, splicing, post-transcriptional modification of the nascent RNA, 3’ end processing, and polyadenylation. After the synthesis of ~15 RNA bases, a 7-methyl guanosine triphosphate cap is added to eukaryotic mRNAs to protect the 5’ end from exonucleases and to recruit the cap binding complex (reviewed in: [62]). RNA capping is performed by capping enzyme (CE). CE is recruited to the 5’ end of the nascent RNA by Spt5 and the phosphorylated Rpb1 CTD [63][64][65]. The structure of S. cerevisiae CE bound to Pol II revealed that the CE binds near the RNA exit tunnel [66]. Future structural work is required to understand how Spt4-5 coordinates with CE to promote mRNA capping.
Exon recognition and intron splicing are coordinated with transcription elongation. The structure of mammalian EC* bound to the U1 snRNP spliceosomal complex was recently obtained using cryo-EM [67]. The structure shows a direct contact between the U1-70K RNA recognition motif via charged electrostatic surfaces along the RPB2 protrusion. The nature of the interaction is consistent with U1 recruitment to the transcription machinery in the absence of splicing [68]. In contrast with the RNAP-ribosome structures, different RNA linker lengths do not alter the U1 interaction with Pol II. Importantly, the dissociable PAF subunit RTF1 was not included in this structure. RTF1 and U1 share a binding surface on RPB12 (Fig 3B), hence the composition of EC* during spliceosome assembling remains elusive. Based on these findings and previously reported spliceosome assembly structures [69][70][71], a topological intron loop model has been proposed. In this intron-defined model, U1 remains associated with Pol II until formation of the B complex. At this stage, the U1 is displaced from Pol II to allow for Poll II-independent splicing. How these structural findings are consistent with previous observations that support exon-defined models is unclear [72].
Finally, RNA can be co-transcriptionally modified with marks such as N6-methyladenosine. The function of these marks is poorly understood; however, recent data suggest that N6-methyladenosine is used to prevent Integrator from associating with Pol II [73]. Future structural studies are needed to understand how RNA modification enzymes interact with both nascent transcripts and Pol II.
Roadblocks to elongation
RNAP and Pol II must overcome protein complexes bound to the DNA substrate including nucleosomes. A nucleosome consists of 145-147 base pairs (bp) of DNA wrapped around a histone octamer core [74]. Nucleosomes impair transcription elongation [75] [76][77][78] [79][80]. Conversely, transcription promotes the loss of nucleosomes [81]. To efficiently transcribe and retain nucleosomes on the DNA template, Pol II requires elongation factors and transcription-associated histone chaperones.
Pol II pauses at specific sites along the proximal side of the nucleosome. These paused states form stable complexes, enabling structure determination by cryo-EM [82][80]. Structures of Pol II on nucleosomal substrates have shown that Pol II makes specific contacts with the nucleosomal DNA using its RPB1 and RPB2 subunits. Elongation factors Spt4-5, TFIIS, and Elf1 act as spacers between Pol II and the nucleosome, and block an interaction between Rpb2 and the nucleosome that can potentially arrest Pol II progression [83][84][80]. Elf1 directly contacts the H3/H4 tetramer whereas the Spt5 N-terminus binds the H2A/H2B dimer to prevent reassociation of DNA with histones [83][85][86][87][82][88][80].
Histone chaperones and chromatin remodelers help Pol II progress through chromatin. Cryo-EM structures of S. cerevisiae Pol II-Spt4-5-nucleosome in complex with the chromatin remodeler Chd1 and the histone chaperone FACT reveal different functions for these factors [82] (Fig 3C). As Pol II progresses through the nucleosome, it breaks contacts between the DNA and histones. This process releases the autoinhibitory Chd1 DNA binding region from the DNA and activates Chd1 ATPase activity. Chd1 pushes DNA towards the Pol II active site to facilitate progression through the nucleosome. Subsequently, FACT associates with the proximal H2A-H2B dimer to prevent dimer loss during transcription [82][89][90] (Fig 3C). Biochemical and biophysical data support the idea that nucleosomes are retained during transcription by reloading them on the DNA directly upstream of elongating Pol II [81][85][91][87]. Consistent with this model, recent observations in S. cerevisiae suggest that nucleosomes remain in neighboring positions after Pol II transcription [92]. How exactly nucleosomes are maintained on template DNA remains elusive and will be an exciting avenue for future structural studies.
Transcription termination
Transcription terminates when RNA is released from RNA polymerase for further processing. Transcription termination at the end of gene bodies occurs either intrinsically, through specific DNA/RNA sequences, or extrinsically, through specific protein factors [93][94][95]. One of the most studied termination factors is the bacterial RNA helicase ρ. The paradigm of ρ-dependent termination proposes that ρ associates with C-rich sequences within the RNA, and translocates in the 5’-3’ direction until it reaches RNAP and expels it [96]. Remarkably, recent cryo-EM structures support an alternative, but complementary, mechanism. This work shows that ρ can engage with NusG, NusA and RNAP during transcription elongation. When RNAP slows, ρ causes NusG to dissociate and RNAP to transition into a “moribund” state that results in RNAP dissociation from DNA (Fig 4A) [97][98]. Structural studies of eukaryotic transcription termination are currently limited, and it is thus unclear how DNA and RNA sequence and factors collaborate at the 3’ end of genes to stimulate termination.
Figure 4-. Structures of transcription termination and antitermination complexes.

A. The cryo-EM structure of the moribund E. coli ρ-RNAP complex. ρ is turquoise and NusA is yellow. NusG is lost in the moribund state and NusA reorients its binding position on the RNAP surface. The location of NusA in the elongation competent state is indicated as a yellow surface (PDB IDs: 6Z9P and 6Z9T).
B. λ-N antitermination complex. Interactions between λ-N and elongation factors NusE, NusG, and NusAare shown (PDB ID: 6GOV).
C. The C-terminal extension of PAF subunit LEO1 (purple) is superimposed on the λ-N structure shown in panel B (PDB IDs: 6GOV and 6TED). LEO1 and λ-N may use their C-terminal extensions to stabilize upstream DNA rewinding.
Suppression of pausing and termination plays a critical role in viral gene expression, particularly during the lytic stage of viral development. The Ebright and Wahl groups obtained structures of the Q anti-pausing factor from bacteriophage 21 (Q21) and N of phage λ (λN), respectively, in complex with elongating RNAP [99][100]. The binding of Q21 to elongating RNAP counteracts the formation of pausing or termination RNA hairpins [99]. λN makes an intricate series of contacts with NusA, G, and E, the RNA•DNA hybrid, and nascent RNA (Fig 4B). Specific contacts made by λN with the RNAP β flap tip (FT) and β’ zinc binding domain (ZBD) appear to block RNA hairpins from forming between the FT and ZBD. λN binding also repositions NusA, blocking its pause supporting role [33]. Additionally, a C-terminal fragment of λN makes contacts with the upstream DNA, possibly to support DNA rewinding. Finally, λN appears to block ρ mediated termination [100]. In comparison with EC*, the position of λN partially resembles a C-terminal loop of the LEO1 subunit of PAF complex (Fig 4C), suggesting that elongation competent complexes may share some conserved features to support DNA rewinding and stabilize an active enzyme conformation [100]. Together, Q21 and λN protein block pausing and intrinsic termination pathways by interfering with hairpin formation and supporting transcription elongation.
Conclusions and Future Directions
Advances in cryo-EM and protein expression technology have allowed us to capture the high-resolution structures of large, dynamic transcription elongation complexes for the first time. However, multiple outstanding questions remain.
For example, promoter-proximal pausing is associated with the presence of the first gene body nucleosome and mRNA capping [14]. Understanding structurally how the paused elongation complex may be coupled with chromatin context and RNA processing will answer important questions in pause regulation. Additionally, it is unclear how P-TEFb is recruited to the paused elongation complex. Structural characterization of P-TEFb in various factor contexts will enable us to understand mechanisms of P-TEFb recruitment and Pol II release from the paused to the elongation state.
How nucleosomes are maintained on the DNA template during transcription and how chromatin remodelers, histone chaperones, and chromatin modification enzymes interact with transcription elongation complexes are areas that are ripe for future structural studies. Structures of these complexes will help define when nucleosomes are modified and how factors collaborate to redeposit nucleosomes on the DNA template after Pol II has passed through them. Various DNA binding proteins tightly associate with DNA, and their presence may affect transcription elongation. Studying how these proteins interact with RNA polymerase biochemically and structurally will explain how these factors affect transcription dynamics, including pausing and transcriptional attenuation.
Finally, the recent cryo-ET structures of RNAP-ribosome structures have elucidated how transcription and translation are directly coupled in a cellular context [52]. As transcription elongation complexes get larger and more complicated, cryo-ET efforts could be applied to understand how complex processes like splicing and transcription are coupled. These efforts will be challenging but will likely reveal many unexpected interactions.
Our structural understanding of transcriptional elongation complexes has rapidly expanded in the last five years, and it will be exciting to see how these structures are used as a foundation to expand our view on regulatory processes that are associated with transcription elongation.
Acknowledgements
We apologize to colleagues we could not cite due to space constraints. We thank members of the Vos Lab for their support and comments. AAM acknowledges funding from the National Science Foundation Graduate Research Fellowship (1745302). RVN acknowledges funding from Swiss National Science Foundation Early Postdoc Fellowship (P2LAP3_195061). Work in the Vos Lab is supported by Alex’s Lemonade Foundation, the Smith Family Foundation, and the NIH New Innovator Award (DP2-GM146254).
Footnotes
Conflict of interest statement
The authors declare no conflict of interest.
References
Papers of particular interest:
• of special interest
•• of outstanding interest
- 1.Cramer P: Multisubunit RNA polymerases. Curr Opin Struct Biol 2002, 12:89–97. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Chiu C, Gopalkrishnan S, Chen AY, Olinares PDB, Saecker RM, Winkelman JT, Maloney MF, Chait BT, Ross W: Stepwise promoter melting by bacterial RNA polymerase. Mol Cell 2020, 78:275–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farnung L, Vos SM: Assembly of RNA polymerase II transcription initiation complexes. Curr Opin Struct Biol 2022, 73:102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonkers I, Lis JT: Getting up to speed with transcription elongation by RNA polymerase II. Nat Rev Mol Cell Biol 2015, 16:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mustaev A, Roberts J, Gottesman M: Transcription elongation. Transcription 2017, 8:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bar-Nahum G, Nudler E: Isolation and characterization of σ70-retaining transcription elongation complexes from Escherichia coli. Cell 2001, 106:443–451. [DOI] [PubMed] [Google Scholar]
- 7.Deighan P, Pukhrambam C, Nickels BE, Hochschild A: Initial transcribed region sequences influence the composition and functional properties of the bacterial elongation complex. Genes Dev 2011, 25:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harden TT, Wells CD, Friedman LJ, Landick R, Hochschild A, Kondev J, Gelles J: Bacterial RNA polymerase can retain σ70 throughout transcription. Proc Natl Acad Sci 2016, 113:602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sainsbury S, Niesser J, Cramer P: Structure and function of the initially transcribing RNA polymerase II–TFIIB complex. Nature 2013, 493:437–440. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Vos SM, Dienemann C, Ninov M, Urlaub H, Cramer P: Allosteric transcription stimulation by RNA polymerase II super elongation complex. Mol Cell 2021, 81. [DOI] [PubMed] [Google Scholar]
- 11.Vos SM, Farnung L, Boehning M, Wigge C, Linden A, Urlaub H, Cramer P: Structure of activated transcription complex Pol II-DSIF-PAF-SPT6. Nature 2018, 560:607–612. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Yin X, Li J, Wu Z, Qi Y, Wang X, Liu W, Xu Y: Structures of the human Mediator and Mediator-bound preinitiation complex. Science 2021, 372. [DOI] [PubMed] [Google Scholar]
- 13.Schweikhard V, Meng C, Murakami K, Kaplan CD, Kornberg RD, Block SM: Transcription factors TFIIF and TFIIS promote transcript elongation by RNA polymerase II by synergistic and independent mechanisms. Proc Natl Acad Sci U S A 2014, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Core L, Adelman K: Promoter-proximal pausing of RNA polymerase II: a nexus of gene regulation. Genes Dev 2019, 33:960–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang B, Artsimovitch I: NusG, an Ancient Yet Rapidly Evolving Transcription Factor. Front Microbiol 2021, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo X, Myasnikov AG, Chen J, Crucifix C, Papai G, Takacs M, Schultz P, Weixlbaumer A: Structural basis for nusa stabilized transcriptional pausing. Mol Cell 2018, 69:816–827.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang JY, Mooney RA, Nedialkov Y, Saba J, Mishanina TV, Artsimovitch I, Landick R, Darst SA: Structural basis for transcript elongation control by nusg family universal regulators. Cell 2018, 173:1650–1662.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernecky C, Plitzko JM, Cramer P: Structure of a transcribing RNA polymerase II–DSIF complex reveals a multidentate DNA–RNA clamp. Nat Struct Mol Biol 2017, 24:809–815. [DOI] [PubMed] [Google Scholar]
- 19.Ehara H, Yokoyama T, Shigematsu H, Yokoyama S, Shirouzu M, Sekine S: Structure of the complete elongation complex of RNA polymerase II with basal factors. Science 2017, 357:921–924. [DOI] [PubMed] [Google Scholar]
- 20.Sdano MA, Fulcher JM, Palani S, Chandrasekharan MB, Parnell TJ, Whitby FG, Formosa T, Hill CP: A novel SH2 recognition mechanism recruits Spt6 to the doubly phosphorylated RNA polymerase II linker at sites of transcription. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chun Y, Joo YJ, Suh H, Batot G, Hill CP, Formosa T, Buratowski S: Selective kinase inhibition shows that Bur1 (Cdk9) phosphorylates the Rpb1 linker in vivo. Mol Cell Biol 2019, 39:e00602–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narita T, Yamaguchi Y, Yano K, Sugimoto S, Chanarat S, Wada T, Kim D, Hasegawa J, Omori M, Inukai N, et al. : Human transcription elongation factor NELF: identification of novel subunits and reconstitution of the functionally active complex. Mol Cell Biol 2003, 23:1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vos SM, Farnung L, Urlaub H, Cramer P: Structure of paused transcription complex Pol II-DSIF-NELF. Nature 2018, 560:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sansó M, Levin RS, Lipp JJ, Wang VY-F, Greifenberg AK, Quezada EM, Ali A, Ghosh A, Larochelle S, Rana TM: P-TEFb regulation of transcription termination factor Xrn2 revealed by a chemical genetic screen for Cdk9 substrates. Genes Dev 2016, 30:117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada T, Yamaguchi Y, Inukai N, Okamoto S, Mura T, Handa H: P-TEFb-mediated phosphorylation of hSpt5 C-terminal repeats is critical for processive transcription elongation. Mol Cell 2006, 21:227–237. [DOI] [PubMed] [Google Scholar]
- 26.Hu S, Peng L, Xu C, Wang Z, Song A, Chen FX: SPT5 stabilizes RNA polymerase II, orchestrates transcription cycles, and maintains the enhancer landscape. Mol Cell 2021, 81:4425–4439. [DOI] [PubMed] [Google Scholar]
- 27.Parua PK, Kalan S, Benjamin B, Sansó M, Fisher RP: Distinct Cdk9-phosphatase switches act at the beginning and end of elongation by RNA polymerase II. Nat Commun 2020, 11:4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.•.Vos SM, Farnung L, Linden A, Urlaub H, Cramer P: Structure of complete Pol II–DSIF–PAF–SPT6 transcription complex reveals RTF1 allosteric activation. Nat Struct Mol Biol 2020, 27:668–677 [DOI] [PubMed] [Google Scholar]; This paper describes the EC* complex bound to RTF1 and an allosteric activation mechanism for RTF1.
- 29.Weinzierl ROJ: The nucleotide addition cycle of RNA polymerase is controlled by two molecular hinges in the Bridge Helix domain. BMC Biol 2010, 8:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang JY, Mishanina TV, Landick R, Darst SA: Mechanisms of transcriptional pausing in bacteria. J Mol Biol 2019, 431:4007–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irastortza-Olaziregi M, Amster-Choder O: Coupled transcription-translation in prokaryotes: an old couple with new surprises. Front Microbiol 2021, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day DS, Zhang B, Stevens SM, Ferrari F, Larschan EN, Park PJ, Pu WT: Comprehensive analysis of promoter-proximal RNA polymerase II pausing across mammalian cell types. Genome Biol 2016, 17:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang JY, Mishanina TV, Bellecourt MJ, Mooney RA, Darst SA, Landick R: RNA Polymerase Accommodates a Pause RNA Hairpin by Global Conformational Rearrangements that Prolong Pausing. Mol Cell 2018, 69:802–815.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gressel S, Schwalb B, Decker TM, Qin W, Leonhardt H, Eick D, Cramer P: CDK9-dependent RNA polymerase II pausing controls transcription initiation. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagano JM, Kwak H, Waters CT, Sprouse RO, White BS, Ozer A, Szeto K, Shalloway D, Craighead HG, Lis JT: Defining NELF-E RNA binding in HIV-1 and promoter-proximal pause regions. PLoS Genet 2014, 10:e1004090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kireeva ML, Kashlev M: Mechanism of sequence-specific pausing of bacterial RNA polymerase. Proc Natl Acad Sci U S A 2009, 106:8900–8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulze-Gahmen U, Echeverria I, Stjepanovic G, Bai Y, Lu H, Schneidman-Duhovny D, Doudna JA, Zhou Q, Sali A, Hurley JH: Insights into HIV-1 proviral transcription from integrative structure and dynamics of the Tat: AFF4: P-TEFb: TAR complex. Elife 2016, 5:e15910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdelkareem M, Saint-André C, Takacs M, Papai G, Crucifix C, Guo X, Ortiz J, Weixlbaumer A: Structural basis of transcription: RNA polymerase backtracking and its reactivation. Mol Cell 2019, 75:298–309.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheung ACM, Cramer P: Structural basis of RNA polymerase II backtracking, arrest and reactivation. Nature 2011, 471:249–253. [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Guermah M, Roeder RG: The human PAF1 complex acts in chromatin transcription elongation both independently and cooperatively with SII/TFIIS. Cell 2010, 140:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto J, Hagiwara Y, Chiba K, Isobe T, Narita T, Handa H, Yamaguchi Y: DSIF and NELF interact with Integrator to specify the correct post-transcriptional fate of snRNA genes. Nat Commun 2014, 5:1–10. [DOI] [PubMed] [Google Scholar]
- 42.Stadelmayer B, Micas G, Gamot A, Martin P, Malirat N, Koval S, Raffel R, Sobhian B, Severac D, Rialle S, et al. : Integrator complex regulates NELF-mediated RNA polymerase II pause/release and processivity at coding genes. Nat Commun 2014, 5:5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.••.Fianu I, Chen Y, Dienemann C, Dybkov O, Linden A, Urlaub H, Cramer P: Structural basis of Integrator-mediated transcription regulation. Science 2021, 374:883–887. [DOI] [PubMed] [Google Scholar]; This paper describes a paused mammalian Pol II complex bound to the Integrator complex. The structure shows how Integrator is postioned to cleave the nascent RNA in order to accomplish transcriptional attenuation.
- 44.Zheng H, Jin Q, Qi Y, Liu W, Ren Y, Wang X, Chen F, Cheng J, Chen X, Xu Y: Structural basis of INTAC-regulated transcription. bioRxiv 2021, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dasilva LF, Blumenthal E, Beckedorff F, Cingaram PR, Gomes Dos Santos H, Edupuganti RR, Zhang A, Dokaneheifard S, Aoi Y, Yue J: Integrator enforces the fidelity of transcriptional termination at protein-coding genes. Sci Adv 2021, 7:eabe3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfleiderer MM, Galej WP: Structure of the catalytic core of the Integrator complex. Mol Cell 2021, 81:1246–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albrecht TR, Shevtsov SP, Wu Y, Mascibroda LG, Peart NJ, Huang K-L, Sawyer IA, Tong L, Dundr M, Wagner EJ: Integrator subunit 4 is a ‘Symplekin-like’scaffold that associates with INTS9/11 to form the Integrator cleavage module. Nucleic Acids Res 2018, 46:4241–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vervoort SJ, Welsh SA, Devlin JR, Barbieri E, Knight DA, Offley S, Bjelosevic S, Costacurta M, Todorovski I, Kearney CJ, et al. : The PP2A-Integrator-CDK9 axis fine-tunes transcription and can be targeted therapeutically in cancer. Cell 2021, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turnbough CL Jr: Regulation of bacterial gene expression by transcription attenuation. Microbiol Mol Biol Rev 2019, 83:e00019–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.•.Webster MW, Takacs M, Zhu C, Vidmar V, Eduljee A, Abdelkareem M, Weixlbaumer A: Structural basis of transcription-translation coupling and collision in bacteria. Science 2020, 369:1355–1359. [DOI] [PubMed] [Google Scholar]; This paper shows the complete architecture of NusG bridging RNAP and the 30S ribosome by varying the RNA linker length between RNAP and the 30S.
- 51.•.Wang C, Molodtsov V, Firlar E, Kaelber JT, Blaha G, Su M, Ebright RH: Structural basis of transcription-translation coupling. Science 2020, 369:1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows the complete architecture of NusG bridging RNAP and the 30S ribosome by varying the RNA linker length between RNAP and the 30S.
- 52.••.O’Reilly FJ, Xue L, Graziadei A, Sinn L, Lenz S, Tegunov D, Blötz C, Singh N, Hagen WJH, Cramer P: In-cell architecture of an actively transcribing-translating expressome. Science 2020, 369:554–557. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows the complete architecture of NusA bridging RNAP and the 30S ribosome by visualizing the complex in cells using cryo-ET.
- 53.Kohler R, Mooney RA, Mills DJ, Landick R, Cramer P: Architecture of a transcribing-translating expressome. Science 2017, 356:194–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan H, Conn AB, Williams PB, Diggs S, Hahm J, Gamper HB, Hou Y-M, O’Leary SE, Wang Y, Blaha GM: Transcription–translation coupling: direct interactions of RNA polymerase with ribosomes and ribosomal subunits. Nucleic Acids Res 2017, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Webster MW, Weixlbaumer A: Macromolecular assemblies supporting transcription-translation coupling. Transcription 2021, 12:103–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson GE, Lalanne J-B, Peters ML, Li G-W: Functionally uncoupled transcription–translation in Bacillus subtilis. Nature 2020, 585:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walmacq C, Cheung ACM, Kireeva ML, Lubkowska L, Ye C, Gotte D, Strathern JN, Carell T, Cramer P, Kashlev M: Mechanism of translesion transcription by RNA polymerase II and its role in cellular resistance to DNA damage. Mol Cell 2012, 46:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walmacq C, Wang L, Chong J, Scibelli K, Lubkowska L, Gnatt A, Brooks PJ, Wang D, Kashlev M: Mechanism of RNA polymerase II bypass of oxidative cyclopurine DNA lesions. Proc Natl Acad Sci 2015, 112:E410–E419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kokic G, Wagner FR, Chernev A, Urlaub H, Cramer P: Structural basis of human transcription–DNA repair coupling. Nature 2021, 598:368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J, Lahiri I, Wang W, Wier A, Cianfrocco MA, Chong J, Hare AA, Dervan PB, DiMaio F, Leschziner AE: Structural basis for the initiation of eukaryotic transcription-coupled DNA repair. Nature 2017, 551:653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Weegen Y, Golan-Berman H, Mevissen TET, Apelt K, González-Prieto R, Goedhart J, Heilbrun EE, Vertegaal ACO, van den Heuvel D, Walter JC: The cooperative action of CSB, CSA, and UVSSA target TFIIH to DNA damage-stalled RNA polymerase II. Nat Commun 2020, 11:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Galloway A, Cowling VH: mRNA cap regulation in mammalian cell function and fate. Biochim Biophys Acta (BBA)-Gene Regul Mech 2019, 1862:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doamekpor SK, Schwer B, Sanchez AM, Shuman S, Lima CD: Fission yeast RNA triphosphatase reads an Spt5 CTD code. Rna 2015, 21:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mandal SS, Chu C, Wada T, Handa H, Shatkin AJ, Reinberg D: Functional interactions of RNA-capping enzyme with factors that positively and negatively regulate promoter escape by RNA polymerase II. Proc Natl Acad Sci U S A 2004, 101:7572–7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noe Gonzalez M, Sato S, Tomomori-Sato C, Conaway JW, Conaway RC: CTD-dependent and -independent mechanisms govern co-transcriptional capping of Pol II transcripts. Nat Commun 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinez-Rucobo FW, Kohler R, van de Waterbeemd M, Heck AJR, Hemann M, Herzog F, Stark H, Cramer P: Molecular basis of transcription-coupled pre-mRNA capping. Mol Cell 2015, 58:1079–1089. [DOI] [PubMed] [Google Scholar]
- 67.••.Zhang S, Aibara S, Vos SM, Agafonov DE, Lührmann R, Cramer P: Structure of a transcribing RNA polymerase II–U1 snRNP complex. Science 2021, 371:305–309. [DOI] [PubMed] [Google Scholar]; This paper describes the cryo-EM structure of metazoan Pol II in complex with the U1 spliceosome and suggests a mechanism for transcription-coupled splicing.
- 68.Spiluttini B, Gu B, Belagal P, Smirnova AS, Nguyen VT, Hébert C, Schmidt U, Bertrand E, Darzacq X, Bensaude O: Splicing-independent recruitment of U1 snRNP to a transcription unit in living cells. J Cell Sci 2010, 123:2085–2093. [DOI] [PubMed] [Google Scholar]
- 69.Plaschka C, Lin P-C, Charenton C, Nagai K: Prespliceosome structure provides insights into spliceosome assembly and regulation. Nature 2018, 559:419–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bertram K, Agafonov DE, Liu W-T, Dybkov O, Will CL, Hartmuth K, Urlaub H, Kastner B, Stark H, Lührmann R: Cryo-EM structure of a human spliceosome activated for step 2 of splicing. Nature 2017, 542:318–323. [DOI] [PubMed] [Google Scholar]
- 71.Zhan X, Yan C, Zhang X, Lei J, Shi Y: Structure of a human catalytic step I spliceosome. Science 2018, 359:537–545. [DOI] [PubMed] [Google Scholar]
- 72.Berget SM: Exon Recognition in Vertebrate Splicing (∗). J Biol Chem 1995, 270:2411–2414. [DOI] [PubMed] [Google Scholar]
- 73.Xu W, He C, Kaye EG, Li J, Mu M, Nelson GM, Dong L, Wang J, Wu F, Shi YG: Dynamic control of chromatin-associated m6A methylation regulates nascent RNA synthesis. Mol Cell 2022, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ: Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 1997, 389:251–260. [DOI] [PubMed] [Google Scholar]
- 75.Bondarenko VA, Steele LM, Ujvári A, Gaykalova DA, Kulaeva OI, Polikanov YS, Luse DS, Studitsky VM: Nucleosomes can form a polar barrier to transcript elongation by RNA polymerase II. Mol Cell 2006, 24:469–479. [DOI] [PubMed] [Google Scholar]
- 76.Mg Izban, Luse DS: Factor-stimulated RNA polymerase II transcribes at physiological elongation rates on naked DNA but very poorly on chromatin templates. J Biol Chem 1992, 267:13647–13655. [PubMed] [Google Scholar]
- 77.Lorch Y, LaPointe JW, Kornberg RD: Nucleosomes inhibit the initiation of transcription but allow chain elongation with the displacement of histones. Cell 1987, 49:203–210. [DOI] [PubMed] [Google Scholar]
- 78.Kireeva ML, Hancock B, Cremona GH, Walter W, Studitsky VM, Kashlev M: Nature of the nucleosomal barrier to RNA polymerase II. Mol Cell 2005, 18:97–108. [DOI] [PubMed] [Google Scholar]
- 79.Bednar J, Studitsky VM, Grigoryev SA, Felsenfeld G, Woodcock CL: The nature of the nucleosomal barrier to transcription: direct observation of paused intermediates by electron cryomicroscopy. Mol Cell 1999, 4:377–386. [DOI] [PubMed] [Google Scholar]
- 80.Kujirai T, Ehara H, Fujino Y, Shirouzu M, Sekine S-I, Kurumizaka H: Structural basis of the nucleosome transition during RNA polymerase II passage. Science 2018, 362:595–598. [DOI] [PubMed] [Google Scholar]
- 81.Studitsky VM, Clark DJ, Felsenfeld G: A histone octamer can step around a transcribing polymerase without leaving the template. Cell 1994, 76:371–382. [DOI] [PubMed] [Google Scholar]
- 82.••.Farnung L, Ochmann M, Engeholm M, Cramer P: Structural basis of nucleosome transcription mediated by Chd1 and FACT. Nat Struct Mol Biol 2021, 28:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes the structures of yeast Pol II transcribing through a nucleosome in complex with Chd1 and FACT. The structures provide models for understanding factor assisted nucleosomal transcription.
- 83.Ehara H, Kujirai T, Fujino Y, Shirouzu M, Kurumizaka H, Sekine S: Structural insight into nucleosome transcription by RNA polymerase II with elongation factors. Science 2019, 363:744–747. [DOI] [PubMed] [Google Scholar]
- 84.Farnung L, Vos SM, Cramer P: Structure of transcribing RNA polymerase II-nucleosome complex. Nat Commun 2018, 9:5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Studitsky VM, Kassavetis GA, Geiduschek EP, Felsenfeld G: Mechanism of transcription through the nucleosome by eukaryotic RNA polymerase. Science 1997, 278:1960–1963. [DOI] [PubMed] [Google Scholar]
- 86.Hodges C, Bintu L, Lubkowska L, Kashlev M, Bustamante C: Nucleosomal fluctuations govern the transcription dynamics of RNA polymerase II. Science 2009, 325:626–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bintu L, Kopaczynska M, Hodges C, Lubkowska L, Kashlev M, Bustamante C: The elongation rate of RNA polymerase determines the fate of transcribed nucleosomes. Nat Struct Mol Biol 2011, 18:1394–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Evrin C, Serra‐Cardona A, Duan S, Mukherjee PP, Zhang Z, Labib KPM: Spt5 histone binding activity preserves chromatin during transcription by RNA polymerase II. EMBO J 2022, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mayanagi K, Saikusa K, Miyazaki N, Akashi S, Iwasaki K, Nishimura Y, Morikawa K, Tsunaka Y: Structural visualization of key steps in nucleosome reorganization by human FACT. Sci Rep 2019, 9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y, Zhou K, Zhang N, Wei H, Tan YZ, Zhang Z, Carragher B, Potter CS, D’Arcy S, Luger K: FACT caught in the act of manipulating the nucleosome. Nature 2020, 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kulaeva OI, Gaykalova DA, Pestov NA, Golovastov VV., Vassylyev DG, Artsimovitch I, Studitsky VM: Mechanism of chromatin remodeling and recovery during passage of RNA polymerase II. Nat Struct Mol Biol 2009, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schlissel G, Rine J: The nucleosome core particle remembers its position through DNA replication and RNA transcription. Proc Natl Acad Sci 2019, 116:20605–20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peters JM, Vangeloff AD, Landick R: Bacterial transcription terminators: the RNA 3′-end chronicles. J Mol Biol 2011, 412:793–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schwalb B, Michel M, Zacher B, Frühauf K, Demel C, Tresch A, Gagneur J, Cramer P: TT-seq maps the human transient transcriptome. Science 2016, 352:1225–1228. [DOI] [PubMed] [Google Scholar]
- 95.Proudfoot NJ: Transcriptional termination in mammals: Stopping the RNA polymerase II juggernaut. Science 2016, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin DJ, Burgess RR, Richardson JP, Gross CA: Termination efficiency at rho-dependent terminators depends on kinetic coupling between RNA polymerase and rho. Proc Natl Acad Sci 1992, 89:1453–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Said N, Hilal T, Sunday ND, Khatri A, Bürger J, Mielke T, Belogurov GA, Loll B, Sen R, Artsimovitch I: Steps toward translocation-independent RNA polymerase inactivation by terminator ATPase ρ. Science 2021, 371:eabd1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hao Z, Epshtein V, Kim KH, Proshkin S, Svetlov V, Kamarthapu V, Bharati B, Mironov A, Walz T, Nudler E: Pre-termination Transcription Complex: Structure and Function. Mol Cell 2021, 81:281–292.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yin Z, Kaelber JT, Ebright RH: Structural basis of Q-dependent antitermination. Proc Natl Acad Sci 2019, 116:18384–18390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krupp F, Said N, Huang Y-H, Loll B, Bürger J, Mielke T, Spahn CMT, Wahl MC: Structural basis for the action of an all-purpose transcription anti-termination factor. Mol Cell 2019, 74:143–157. [DOI] [PubMed] [Google Scholar]


