Abstract
Rationale & objective:
Primary hyperoxaluria type 1 (PH1) is an autosomal recessive disorder of glyoxylate metabolism that results in early onset kidney stone disease, nephrocalcinosis, and kidney failure. There is an unmet need for reliable markers of disease progression to test effectiveness of new treatments for patients with PH. In this study, we assessed the rate of eGFR decline across chronic kidney disease (CKD) stages in a cohort of patients with PH1.
Study Design:
Retrospective observational study.
Settings and Participants:
Patients with PH1 enrolled in the Rare Kidney Stone Consortium (RKSC) registry who did not have kidney failure at diagnosis and who had at least two eGFR values recorded from within 1 month of diagnosis until their last contact date or incident kidney failure event.
Predictors:
CKD stage, baseline patient and laboratory characteristics.
Outcomes:
Annualized rate of eGFR decline.
Analytical approach:
Generalized estimating equations (GEE) and linear regression were used to evaluate the associations between CKD stage, baseline patient and laboratory characteristics, and annual change in eGFR during follow-up.
Results:
Mean annual decline in eGFR was higher in CKD stage 3a (−5.3 ml/min/1.73 m2) than in stage 2 (−2.3 ml/min/1.73 m2). A greater rate of eGFR decline was observed in CKD stage 3b and 4 (−14.7 ml/min/1.73 m2 and−16.6 ml/min/1.73 m2, respectively). In CKD stage 2, older age was associated with a more rapid rate of eGFR decline. (p<0.001). The G170R variant of AGXT appears to have a favorable effect on eGFR annual decline.
Limitations:
Data at regular time points were not available for all patients due to reliance on voluntary reporting in a retrospective rare disease registry.
Conclusions:
eGFR decline was not uniform across CKD stages in this PH1 population, with a higher rate of eGFR decline in CKD stages 3b and 4. Thus, CKD stage needs to be accounted for when analyzing eGFR change in the setting of PH1.
Keywords: primary hyperoxaluria, eGFR slope, CKD, plasma oxalate, urinary oxalate
Plain Language Summary
Primary hyperoxaluria (PH) is a genetic disorder characterized by increased hepatic oxalate production. PH type 1 (PH1) accounts for the majority of PH cases. Approximately half of PH1 patients develop kidney failure by the 4th decade of life. The rate of change in eGFR over time has recently been proposed as a surrogate end point for clinical trials in patients with PH1. Using multivariable statistical analysis, eGFR slope estimates were compared across CKD stages in a PH1 cohort. Results suggest that eGFR decline occurs at a higher rate in more advanced CKD stages, an important consideration in design of clinical trials. Measures to prevent early loss of kidney function in PH1 could be particularly important, since eGFR decline accelerates in more advanced stages of CKD.
Introduction
The primary hyperoxalurias (PH) are inborn errors of metabolism that result in hepatic overproduction of oxalate1. To date, three forms of PH (PH types 1, 2 and 3) have been identified, each caused by a distinct enzyme deficiency in the glyoxylate metabolism pathway. Primary hyperoxaluria type 1 (PH1), the most severe of the three types, accounts for up to 80% of PH cases2 and results from pathogenic variants in the AGXT gene resulting in dysfunction of the hepatic peroxisomal enzyme alanine glyoxylate aminotransferase (AGT). PH1 is marked by high urinary oxalate (Uox) excretion which often is associated with recurrent urinary stone disease and/or nephrocalcinosis2,3. Over time, loss of kidney function is common, often leading to kidney failure4. Indeed, up to a half of PH1 patients have progressed to kidney failure by the time of diagnosis5,6.
Timely diagnosis and subsequent management play an important role in PH, since late diagnosis has been associated with adverse outcomes7–9. Up to 50% of PH1 patients progress to kidney failure by age 35, and 90% by age 60 years10. If kidney failure occurs, systemic oxalosis and/or recurrent oxalate nephropathy in a kidney allograft are frequent and potentially severe complications. Preservation of kidney function in those diagnosed earlier relies upon efforts to reduce calcium oxalate crystal-induced injury to the kidney through maintenance of high urine volume, use of crystallization inhibitors, and where possible reduction in hepatic oxalate production. In PH1, Uox excretion can be pharmacologically reduced in a subset of patients that are responsive to oral pyridoxine11, or by a small inhibitory RNA directed against hepatic glycolate oxidase (lumasiran)12. Currently, there are no approved therapies for PH2 or PH3. Clinical trials in PH have been challenging due to rarity of the disease and the long observation period necessary for clinically meaningful end points such as kidney failure or kidney stone events13. Thus, a Kidney Health Initiative (KHI) working group recently proposed Uox, plasma oxalate (Pox) and estimated glomerular function rate (eGFR) slope as potential surrogate end points that could be used to establish the efficacy of new treatments in PH1 clinical trials13.
In order for trials that employ eGFR as an endpoint to be sufficiently powered, knowledge regarding the expected rate of GFR decline in the disease under study is needed. Clinical experience suggests that PH1 patients can sometimes progress rapidly and unexpectedly to kidney failure, and that the loss of kidney function may not be linear over time. Thus, to better understand eGFR slope across the chronic kidney disease (CKD) stages, longitudinal data in the Rare Kidney Stone Consortium (RKSC) PH registry was analyzed. We also investigated the relationship between eGFR and another potential surrogate endpoint, Pox, since previous studies suggest these 2 measures are interrelated14.
Materials and Methods
Study Population
This study was approved by the institutional review board at the Mayo Clinic, Rochester. Our study population was derived from PH1 patients enrolled in the Oxalosis and Hyperoxaluria Foundation (OHF) Rare Kidney Stone Consortium (RKSC) PH Registry and who were free of kidney failure at time of PH diagnosis. Informed consent was obtained prior to enrollment in the RKSC registry. Patients were included in the analysis cohort if they had at least two eGFR values available for analysis after age 2, from within 1 month prior to PH1 diagnosis up to their last contact date or incident kidney failure. All available eGFR values in this timeframe were used in the analysis. For this analysis medical records abstracted and registry data entered at non-Mayo sites were excluded since follow up was limited and key laboratory values could not be verified.
Data Collection and Measurements
Serum creatinine, Pox, and Uox laboratory measurements were obtained from the OHF RKSC registry and augmented from Mayo laboratory databases. To eliminate inter-laboratory variability, all Pox values used for this study were measured in the Mayo Clinic Renal Testing laboratory by oxalate oxidase enzyme method (reference value < 2 μmol/L)15. eGFR was estimated using Pottel’s FAS equation, which allows for estimation of the glomerular filtration rate across the full age spectrum16. Kidney failure was defined as the first occurrence of transplant, initiation of dialysis, or eGFR<15 ml/min/1.73m2.
Statistical Analysis
Data are presented as the mean (SD) or median [IQR] for continuous variables, and n (%) for categorical variables. Registry patients may contribute to more than one CKD stage group based on observed data using a landmark style analysis17,18. See Figure S1 for a visualization of the cohorts corresponding to each CKD stage. To be included in a particular stage for analysis, a patient had to have an eGFR value meeting or below each stage criteria (CKD2: <90; CKD 3a: <60 CKD3b: <45; CKD4: <30; CKD5: <15) plus at least one additional eGFR value measured at least one month later(see Figure S2). The first eGFR for a given stage represents the baseline. For example, a patient with eGFR values of 70, 55, 50, and 40 would contribute an observation as CKD2 with baseline eGFR 70 (follow up 55, 50, and 40) and contribute an observation as CKD 3a with baseline eGFR 55 (follow up 50 and 40), but would not contribute a CKD3b observation since there is not a second eGFR value after 40. Individual patient time points after kidney failure without a recorded eGFR were assigned a value of 15 ml/min/1.73m2. See Laboratory values closest to baseline and within the period of 1 year prior to and up to 1 week after baseline are reported.
For each observation unit (patient contributing to a CKD stage), annual absolute rate of change was calculated as the least squares slope of eGFR regressed on time from baseline using all eGFR values on or after the baseline. Annual percentage change was calculated as the percent change in geometric mean eGFR using least squares slope of eGFR on the log scale. Both absolute and percent change calculations utilized all follow-up eGFR values measured after entry into each CKD stage, until kidney failure or loss to follow-up. Mean of least squares slope within each CKD stage were compared to the null hypothesis of no annual change using one-sample t-tests. Generalized Estimating Equations (GEE) with compound symmetric working correlation were used to account for patients contributing multiple observations when assessing annual absolute and percent change in slope across CKD stages (multiple baselines with each entry into new stage). Overall p-values for trend were calculated considering CKD stage as an ordinal variable; estimates and p-values for each CKD stage were derived, with the earliest CKD stage 2 taken as the reference level. As a sensitivity analysis, the above analysis of absolute and annual percentage change in eGFR was also performed stratified by G170R mutation status, and within each CKD stage; 2-sample t-tests were used to assess differences in annual eGFR change across mutation types. It was not possible to analyze eGFR change across the CKD stages in the G170R homozygous genotype subgroup alone due to a small sample size. Linear regression was used to evaluate the association between baseline patient and laboratory characteristics and annual change in eGFR by stage.
The association between patient baseline characteristics and progression (to a worse CKD stage or kidney failure) was assessed using Cox proportional hazards models, fitted separately to each CKD stage. For this analysis, patients were considered to enter a CKD stage once their eGFR fell exactly within each CKD stage (CKD2: 61–90; CKD 3a: 46–60; CKD3b: 31–45; CKD4: 16–30; CKD5: <15). Each variable was analyzed univariately. Patients without progression were censored at date of last contact. Results are described as hazard ratios and 95% confidence intervals.
To evaluate the correlation between Pox laboratory measurements and eGFR values, all laboratory measurements which occurred +/−1 week of the eGFR value were retained for analysis. Linear mixed effects models with subject-specific intercept and fixed effects for all other covariates assessed the association between Pox and eGFR accounting for correlation within patients. The logarithmic transformations of both Pox and eGFR were used for this analysis to satisfy assumptions of normality of the error terms and a linear relationship between the two log-transformed variables to allow for calculation of the repeated measures correlation coefficient19. Along with coefficient estimates from the mixed models, predicted values and associated 95% confidence intervals for the mean, were reported for Pox measures across eGFR values. Slopes were also reported and were calculated by taking the derivative of the mixed model equation across eGFR values. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). All p-values were two-tailed and were considered statistically significant at the 0.05 alpha level.
Results
Baseline Characteristics
A total of 129 PH1 patients met inclusion criteria for this study (Figure 1). Median [IQR] age at PH diagnosis (dx) was 8.6 [3.3, 21.6] years, 70 (54.3%) were male, and 99 (76.7%) were Caucasian. Baseline characteristics by CKD stage are presented in Table 1. A total of 1,290 eGFR values were available throughout follow-up. Average (SD) follow-up time was 15.6 (1.2) years in this cohort (median [95% CI] 12.1 [9.7 to 15.2] years), and 48 (37.2%) subjects experienced kidney failure during follow-up.
Figure 1.
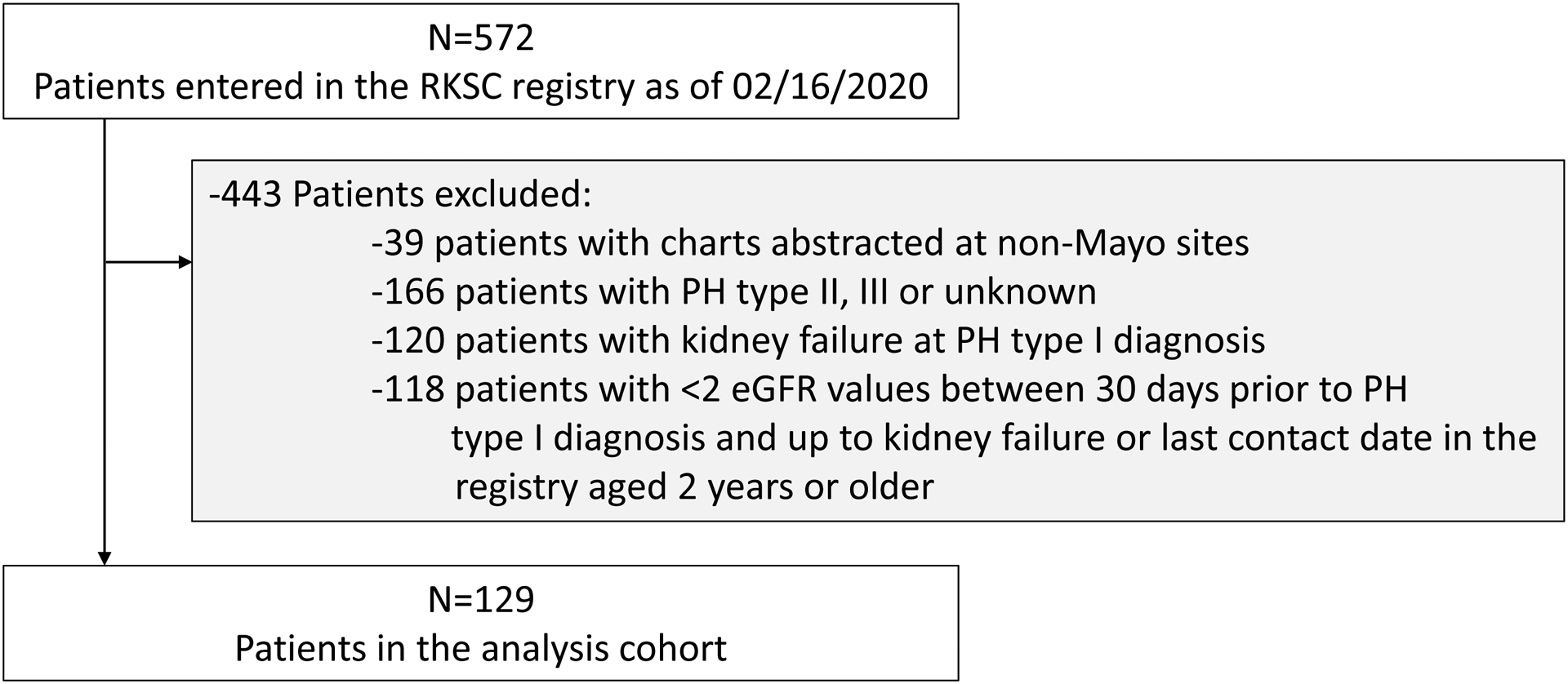
Flowchart of inclusion and exclusion criteria for analysis cohort
Table 1.
Patient and laboratory characteristics at time of entry into each CKD stage
| Patient characteristic | CKD Stage 2 (N=121) | CKD Stage 3a (N=72) | CKD Stage 3b (N=41) | CKD Stage 4 (N=14) |
|---|---|---|---|---|
| Male, n(%) | 66 (54.5%) | 36 (50.0%) | 22 (53.7%) | 9 (64.3%) |
| Age at PH dx (years) | ||||
| Median [IQR] | 7.8 [3.3, 21.9] | 7.7 [4.2, 21.7] | 9.4 [4.4, 28.1] | 10.8 [6.9, 28.9] |
| Range | (0.0–53.4) | (0.0–53.4) | (0.0–53.4) | (0.5–53.4) |
| Time from Symptom Onset to PH diagnosis* (years) | ||||
| N | 106 | 66 | 36 | 13 |
| Median [IQR] | 2.0 [0.1, 8.0] | 1.3 [0.0, 6.0] | 2.0 [0.1, 9.0] | 3.9 [2.0, 11.1] |
| Range | (−12.3–51.0) | (−0.7–51.0) | (−0.5–51.0) | (−0.5–35.3) |
| Age at 1st entry into CKD stage (years) | ||||
| Median [IQR] | 10.7 [4.7, 28.1] | 14.0 [6.0, 31.4] | 19.0 [8.4, 40.8] | 22.6 [10.2, 46.2] |
| Range | (2.0–55.0) | (2.1–55.0) | (2.7–65.0) | (2.7–58.4) |
| eGFR at 1st entry into CKD stage, mL/min/1.73 m2 | ||||
| Median [IQR] | 66.6 [54.6, 77.5] | 52.2 [45.1, 56.9] | 38.2 [33.2, 43.0] | 24.6 [22.5, 27.6] |
| Range | (17.0–89.7) | (17.0–59.9) | (17.0–44.7) | (16.0–29.1) |
| Nephrocalcinosis, n(%) | 37 (30.6%) | 29 (40.3%) | 15 (36.6%) | 7 (50.0%) |
| Plasma oxalate, μmol/L | ||||
| N | 28 | 20 | 18 | 8 |
| Median [IQR] | 4.1 [3.0, 9.2] | 5.4 [2.7, 8.4] | 13.4 [6.5, 16.0] | 27.1 [11.7, 34.3] |
| Range | (0.9–19.1) | (0.9–14.9) | (1.4–32.3) | (2.9–49.7) |
| Urine oxalate, mmol/1.73 m2/24 h | ||||
| N | 80 | 47 | 29 | 11 |
| Median [IQR] | 1.7 [1.1, 2.7] | 1.9 [1.0, 3.0] | 2.1 [1.1, 2.7] | 2.3 [1.4, 3.4] |
| Range | (0.4–5.7) | (0.3–5.6) | (0.5–4.8) | (0.7–3.8) |
Definition of “1st entry into each CKD stage” is the 1st time the patient had a reported eGFR that was at or lower than the CKD stage cut-off
Lab values at baseline were defined as occurring between 1 year prior to and up to 1 week after entry into CKD stage
PH diagnosis preceded onset of symptoms in some patients identified by family screening.
Change in eGFR across CKD stages
Among the 129 PH1 patients, 7 patients did not have any eGFR value below 90 during follow-up, and 1 patient did not have a second eGFR measure occurring at least 1 month after the first eGFR measure <90 during follow-up. For the remaining patients, there were 121 patients available for follow-up in CKD stage 2; 72 in CKD stage 3a; 41 in CKD stage 3b; and 14 in CKD stage 4. The median [IQR] number of eGFR measurements per patient available for the study participants was 8 [4, 13] in CKD stage 2; 8 [4, 14] in stage 3a; 6 [3, 12] in stage 3b; and 3 [3, 6] in stage 4. Patients in CKD stages 3a, 3b and 4 all experienced a significant decline in eGFR during follow-up when expressed as annualized change. When compared across stages, annualized eGFR declined more rapidly in stages 3a, 3b and 4 as compared to stage 2 (Table 2 and Figure 2). Results were similar when expressed as percent change in eGFR (see Table S1). The eGFR trajectories during follow-up stratified by entry into each CKD stage are presented in Figure S1. As expected, progressive eGFR decline was the rule. However, a single patient experienced a substantial improvement in eGFR after reaching CKD stage 4 due to an episode of acute post-infectious nephritis in childhood unrelated to PH that caused a transient kidney injury (see Figure S1).
Table 2.
eGFR annual rates of absolute change during follow-up
| N | N eGFR | N follow-up eGFR per patient, Median [IQR] | Annual rate of change (ml/min/1.73m2/year) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median [IQR] | Beta Coefficient* (SE) | P | P-trend | ||||
| CKD Stage | 0.007 | |||||||
| 2 (<90) | 121 | 1239 | 8 [4, 13] | −2.3 (14.0) | −0.85 [−3.2, 1.2] | Ref | Ref | |
| 3a (<60) | 72 | 767 | 8 [4, 14] | −5.3 (21.3)** | −1.2 [−6.3, 0.64] | −2.5 (1.6) | 0.1 | |
| 3b (<45) | 41 | 361 | 6 [3, 12] | −14.7 (24.5)** | −4.2 [−13, −1.7] | −6.5 (2.7) | 0.02 | |
| 4 (<30) | 14 | 93 | 3 [3, 6] | −16.6 (20.0)** | −9.6 [−31, −1.7] | −10 (4.1) | 0.01 | |
Beta coefficient represents the estimated change in eGFR slope (ml/min/1.73m2/year) for each subsequent CKD stage, relative to CKD stage 2. Results were calculated using Generalized Estimating Equations (GEE) with compound symmetric working correlation to account for patients contributing multiple observations to the data.
Significantly different from 0 at the 0.05 alpha level.
P-values in bold denote statistical significance at the 0.05 alpha level.
Figure 2.
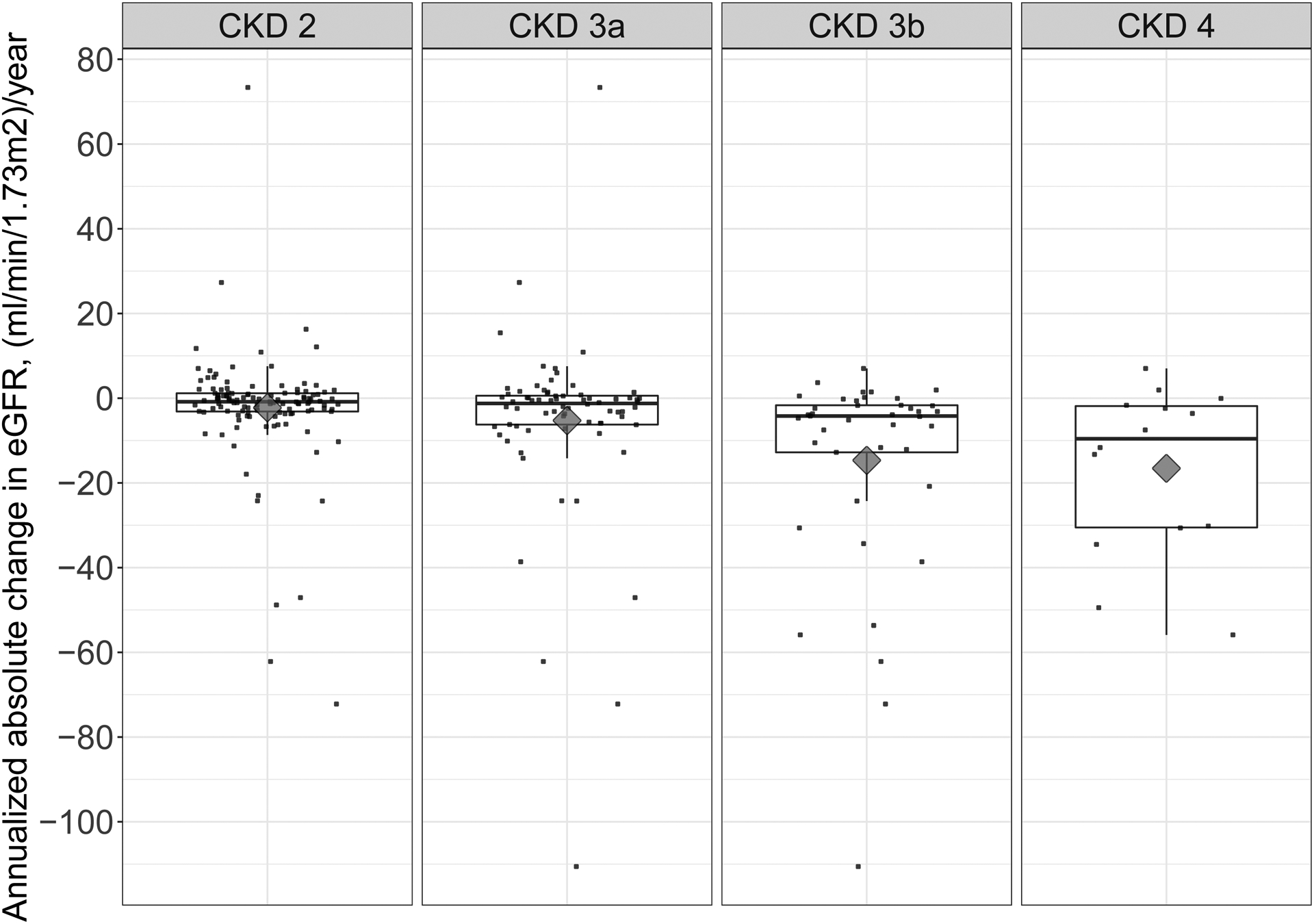
Boxplots overlaid with scatterplots of eGFR annual rates of change during follow-up, by CKD stage. Black diamonds denote the mean rate of change in each CKD stage.
When stratifying by G170R mutation status (Table S4), 11 of the 13 patients homozygous for G170R met inclusion for entry into CKD2 and 1 met inclusion for entry into CKD3a. Accordingly, this small subgroup appeared less likely to lose kidney function over time. Patients heterozygous for a G170R mutation did not show significant differences in annualized eGFR decline across CKD stages. Patients without a G170R mutation had a similar, albeit slightly greater annualized loss in eGFR compared to the overall cohort in the main analysis, and when compared across stages their annualized eGFR also declined more rapidly in the more advanced CKD stages. However, there was no overall statistically significant difference in annual rates of eGFR change across mutation type within any CKD stage (Table S4), perhaps due to the reduced sample size rather that lack of a true biologic difference.
Association between baseline characteristics and change in eGFR across CKD stages
Older age was associated with a more rapid rate of decline in eGFR for patients in CKD stage 2 (p<0.01). For patients in CKD stage 3b, higher Uox was also associated with a more rapid rate of decline in eGFR (average per 0.1 mmol/1.73m2/24hr of Uox: −0.50; p<0.05 for both). However results were no longer significant for Uox for patients in CKD stage 4 (Table 3). Findings were similar when assessing annual percent in eGFR as the outcome (see Table S2).
Table 3.
Univariate associations between eGFR change and factors at entry into that CKD stage
| Patient characteristic | N | Estimate* | Lower 95% CI | Upper 95% CI | P | |
|---|---|---|---|---|---|---|
| Stage 2 (eGFR<90) | Male, n(%) | 121 | 2.49 | −2.50 | 7.49 | 0.3 |
| Age at PH dx (per 10 years) | 121 | −1.19 | −2.90 | 0.52 | 0.2 | |
| Difference b/t Symptom Onset and PH dx (per year) | 106 | −0.11 | −0.34 | 0.12 | 0.3 | |
| Age at 1st entry into CKD stage (per decade) | 121 | −2.29 | −3.93 | −0.65 | 0.01 | |
| eGFR at 1st entry into CKD stage (per 10 mL/min/1.73 m2) | 121 | 1.22 | −0.30 | 2.75 | 0.1 | |
| Nephrocalcinosis, n(%) | 121 | 3.44 | −1.94 | 8.83 | 0.2 | |
| Plasma oxalate† (per 1 μmol/L) | 28 | 0.39 | −0.55 | 1.34 | 0.4 | |
| BSA adjusted urine oxalate† (per 0.1 mmol/1.73 m2/24 h) | 80 | −0.14 | −0.35 | 0.07 | 0.2 | |
| Stage 3a (eGFR<60) | Male, n(%) | 72 | 8.83 | −0.85 | 18.51 | 0.08 |
| Age at PH dx (per 10 years) | 72 | −1.20 | −4.51 | 2.10 | 0.5 | |
| Difference b/t Symptom Onset and PH dx (years) | 66 | −0.08 | −0.55 | 0.40 | 0.8 | |
| Age at 1st entry into CKD stage (per decade) | 72 | −2.60 | −5.40 | 0.19 | 0.07 | |
| eGFR at 1st entry into CKD stage (per 10 mL/min/1.73 m2) | 72 | 4.12 | −1.13 | 9.38 | 0.1 | |
| Nephrocalcinosis, n(%) | 72 | 5.17 | −4.85 | 15.20 | 0.3 | |
| Plasma oxalate† (per 1 μmol/L) | 20 | 0.98 | −0.31 | 2.27 | 0.2 | |
| BSA adjusted urine oxalate†(per 0.1 mmol/1.73 m2/24 h) | 47 | −0.16 | −0.48 | 0.16 | 0.3 | |
| Stage 3b (eGFR<45) | Male, n(%) | 41 | 5.55 | −9.60 | 20.70 | 0.6 |
| Age at PH dx (per 10 years) | 41 | −0.40 | −5.26 | 4.46 | 0.9 | |
| Difference b/t Symptom Onset and PH dx (years) | 36 | 0.15 | −0.50 | 0.79 | 0.7 | |
| Age at 1st entry into CKD stage (per decade) | 41 | −1.44 | −5.43 | 2.56 | 0.5 | |
| eGFR at 1st entry into CKD stage (per 10 mL/min/1.73 m2) | 41 | −1.12 | −11.64 | 9.40 | 0.8 | |
| Nephrocalcinosis, n(%) | 41 | 7.13 | −8.49 | 22.75 | 0.4 | |
| Plasma oxalate† (per 1 μmol/L) | 18 | −0.37 | −1.20 | 0.45 | 0.4 | |
| BSA adjusted urine oxalate† (per 0.1 mmol/1.73 m2/24 h) | 29 | −0.50 | −0.96 | −0.04 | 0.04 | |
| Stage 4 (eGFR<30) | Male, n(%) | 14 | −6.75 | −29.20 | 15.70 | 0.6 |
| Age at PH dx (per 10 years) | 14 | 3.19 | −4.03 | 10.43 | 0.4 | |
| Difference b/t Symptom Onset and PH dx (years) | 13 | 0.64 | −0.34 | 1.63 | 0.2 | |
| Age at 1st entry into CKD stage (per decade) | 14 | 1.40 | −4.49 | 7.30 | 0.7 | |
| eGFR at 1st entry into CKD stage (per 10 mL/min/1.73 m2) | 14 | −6.11 | −32.72 | 20.50 | 0.7 | |
| Nephrocalcinosis, n(%) | 14 | −17.50 | −36.96 | 1.95 | 0.1 | |
| Plasma oxalate† (per 1 μmol/L) | 8 | −0.62 | −1.54 | 0.30 | 0.2 | |
| BSA adjusted urine oxalate† (per 0.1 mmol/1.73 m2/24 h) | 11 | −1.15 | −2.24 | −0.05 | 0.07 |
Models are fit separately for different CKD stages.
Estimate: Change in annual absolute rate of change of eGFR (ml/min/1.73m2/year) per 1 unit increase in patient characteristic
Lab values at baseline were defined as occurring between 1 year prior to and up to 1 week after entry into CKD stage
P-values in bold denote statistical significance at the 0.05 alpha level.
N=number of patients.
Results were calculated using linear regression.
Association between baseline characteristics and time to entry into subsequent CKD stage, across CKD stages
In time to event analyses, higher eGFR at first entry into CKD stage 2 was associated with a lower risk of entry into subsequent CKD stages or kidney failure; older age at first entry into CKD stages 3a and 3b was associated with a higher risk of entry into subsequent stages; older age at PH diagnosis and longer time between first symptom onset and PH diagnosis were associated with higher risk of entry into subsequent stages in CKD stage 3a; and a history of nephrocalcinosis was associated with a higher risk of entry into kidney failure in CKD stage 4 (Table 4).
Table 4.
Univariate associations between baseline factors prior to or at CKD stage and time to entry into subsequent CKD stage
| Patient characteristic | Patients (N) | Events (N) | HR | Lower 95% CI | Upper 95% CI | P | |
|---|---|---|---|---|---|---|---|
| Stage 2 (eGFR<90) | Male, n(%) | 81 | 43 | 0.80 | 0.44 | 1.47 | 0.5 |
| Age at PH dx (per 10 years) | 81 | 43 | 1.11 | 0.88 | 1.39 | 0.4 | |
| Difference b/t Symptom Onset and PH dx (per year) | 70 | 40 | 1.02 | 0.98 | 1.05 | 0.3 | |
| Age at 1st entry into CKD stage (per decade) | 81 | 43 | 1.10 | 0.87 | 1.40 | 0.4 | |
| eGFR at 1st entry into CKD stage (per 10 mL/min/1.73 m2) | 81 | 43 | 0.42 | 0.27 | 0.64 | <.001 | |
| Nephrocalcinosis, n(%) | 81 | 43 | 1.21 | 0.59 | 2.50 | 0.6 | |
| Plasma oxalate† (per 1 μmol/L) | 20 | 11 | 0.96 | 0.79 | 1.16 | 0.7 | |
| BSA adjusted urine oxalate†(per 0.1 mmol/1.73 m2/24 h) | 54 | 31 | 1.02 | 0.98 | 1.05 | 0.4 | |
| Stage 3a (eGFR<60) | Male, n(%) | 54 | 32 | 0.70 | 0.34 | 1.45 | 0.3 |
| Age at PH dx (per 10 years) | 54 | 32 | 1.37 | 1.07 | 1.75 | 0.01 | |
| Difference b/t Symptom Onset and PH dx (years) | 52 | 31 | 1.05 | 1.01 | 1.09 | 0.01 | |
| Age at 1st entry into CKD stage (per decade) | 54 | 32 | 1.32 | 1.08 | 1.61 | 0.01 | |
| eGFR at 1st entry into CKD stage (per 10 mL/min/1.73 m2) | 54 | 32 | 0.75 | 0.34 | 1.64 | 0.5 | |
| Nephrocalcinosis, n(%) | 54 | 32 | 1.23 | 0.61 | 2.47 | 0.6 | |
| Plasma oxalate† (per 1 μmol/L) | 17 | 7 | 1.00 | 0.79 | 1.27 | 0.9 | |
| BSA adjusted urine oxalate†(per 0.1 mmol/1.73 m2/24 h) | 37 | 21 | 0.99 | 0.97 | 1.03 | 0.9 | |
| Stage 3b (eGFR<45) | Male, n(%) | 32 | 26 | 1.20 | 0.54 | 2.67 | 0.7 |
| Age at PH dx (per 10 years) | 32 | 26 | 1.23 | 0.95 | 1.58 | 0.1 | |
| Difference b/t Symptom Onset and PH dx (years) | 28 | 23 | 1.01 | 0.98 | 1.05 | 0.4 | |
| Age at 1st entry into CKD stage (per decade) | 32 | 26 | 1.29 | 1.03 | 1.61 | 0.03 | |
| eGFR at 1st entry into CKD stage (per 10 mL/min/1.73 m2) | 32 | 26 | 0.56 | 0.17 | 1.85 | 0.3 | |
| Nephrocalcinosis, n(%) | 32 | 26 | 0.45 | 0.19 | 1.05 | 0.07 | |
| Plasma oxalate† (per 1 μmol/L) | 13 | 11 | 1.12 | 0.99 | 1.25 | 0.07 | |
| BSA adjusted urine oxalate†(per 0.1 mmol/1.73 m2/24 h) | 21 | 17 | 1.03 | 0.99 | 1.08 | 0.1 | |
| Stage 4 (eGFR<30) | Male, n(%) | 14 | 11 | 1.34 | 0.39 | 4.64 | 0.6 |
| Age at PH dx (per 10 years) | 14 | 11 | 1.06 | 0.75 | 1.49 | 0.7 | |
| Difference b/t Symptom Onset and PH dx (years) | 13 | 11 | 0.99 | 0.94 | 1.04 | 0.8 | |
| Age at 1st entry into CKD stage (per decade) | 14 | 11 | 1.16 | 0.86 | 1.57 | 0.3 | |
| eGFR at 1st entry into CKD stage (per 10 mL/min/1.73 m2) | 14 | 11 | 0.75 | 0.21 | 2.73 | 0.7 | |
| Nephrocalcinosis, n(%) | 14 | 11 | 5.30 | 1.07 | 26.28 | 0.04 | |
| Plasma oxalate† (per 1 μmol/L) | 8 | 6 | 1.062 | 0.95 | 1.09 | 0.6 | |
| BSA adjusted urine oxalate†(per 0.1 mmol/1.73 m2/24 h) | 11 | 9 | 1.03 | 0.96 | 1.12 | 0.4 |
Models are fit separately for different CKD stages.
Lab values at baseline were defined as occurring between 1 year prior to and up to 1 week after entry into CKD
P-values in bold denote statistical significance at the 0.05 alpha level.
N=number of patients or number of events.
Results were calculated using cox proportional hazards regression.
Relationship between Pox and eGFR
For analyses comparing laboratory values over the course of disease, 75 individuals had at least 1 Pox obtained within 1 week of a serum creatinine (SCr) result, resulting in a total of 399 paired measurements available for analysis.
A repeated measures correlation coefficient demonstrated a strong negative association between Pox and eGFR (r=−0.41 (95% CI: −0.50, −0.32), p<0.01). Model coefficients from fitting the linear mixed model are presented in Table S3. Fitted average Pox trajectories by eGFR from the model along with the actual patient trajectories are plotted in Figure 3. Each 10% decrease in eGFR was associated with a 13.9% increase in Pox. Predicted Pox values and slopes across eGFR levels are presented in Table S5. Pox concentrations appear to show modest change for eGFR levels greater than 60, and then sharply increase with decreasing eGFR values <30 ml/min/1.73m2.
Figure 3.
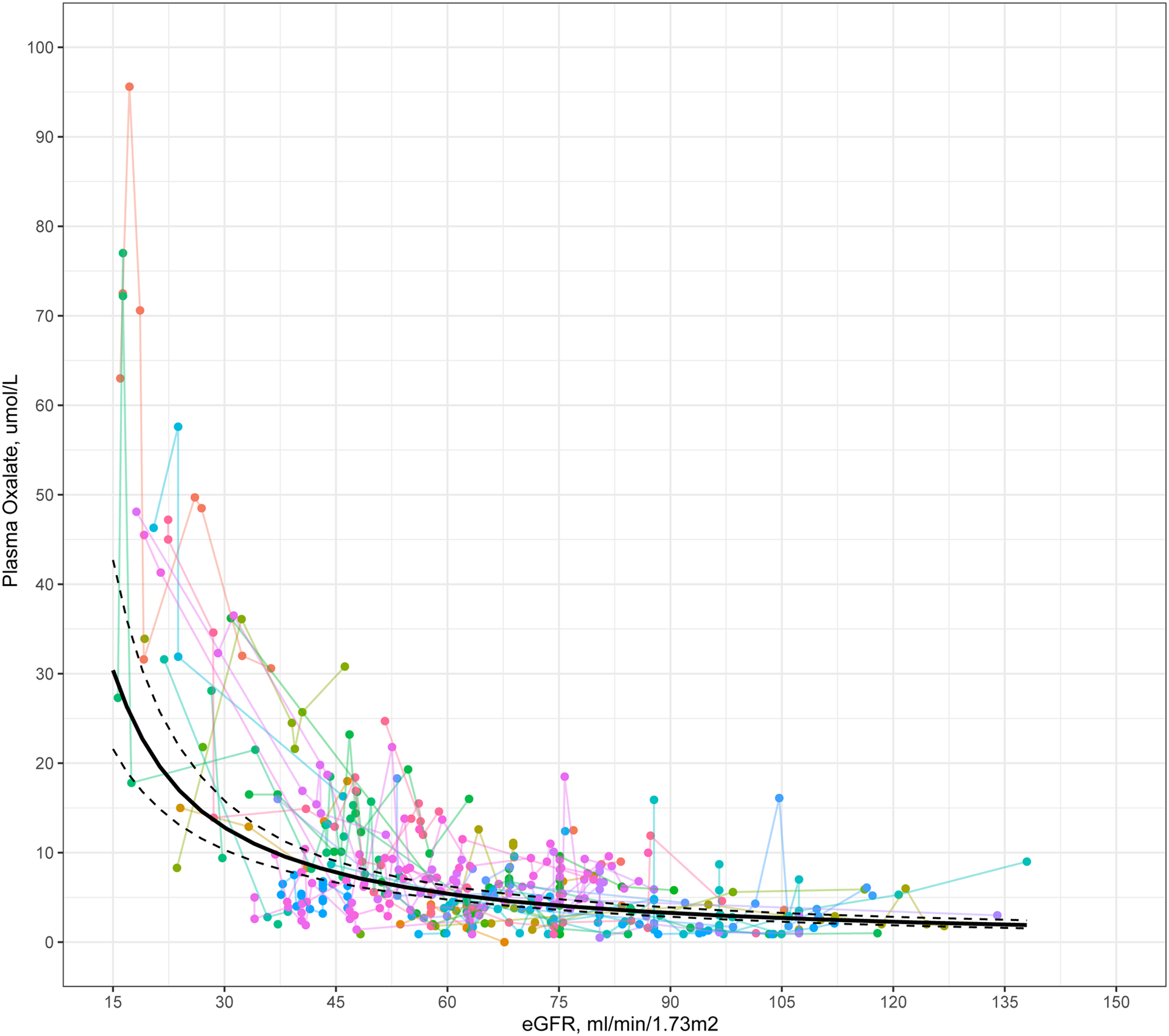
Plot of plasma oxalate versus eGFR. Individual patient trajectories of plasma oxalate by eGFR are plotted in color. Black line indicates fitted average plasma oxalate versus eGFR using a linear mixed model, using logarithmic transformations of both Pox and eGFR. Dotted lines indicate the 95% confidence intervals of the fixed effects.
Discussion
Given the marked morbidity PH1 patients can experience, particularly kidney failure in up to half of subjects by the 4th decade, there is a pressing need for improved treatments13. End points for clinical trials to test effectiveness of new agents for this rare disorder have been challenging due to rarity of the disease and the long observation period necessary for clinically meaningful end points. The slope of eGFR over time has been proposed as a surrogate endpoint13,20,21. Recent studies have led to the general acceptance of eGFR slope as a surrogate for kidney failure in several specific kidney diseases by the US Food and Drug Administration (FDA), the National Kidney Foundation (NKF) and the European Medicines Agency21,22. In a recent metanalysis of over 3 million participants, slower eGFR decline by 0.75 ml/min/1.73m2 per year over 2 years was associated with a lower risk of kidney failure among those participants with baseline eGFR both above and below 60ml/min/1.73 m2 20. Thus, the NKF deemed that a difference of 0.5–1.0 ml/min/1.73 m2/year in eGFR portends a meaningful clinical benefit23,24. However, the validity of eGFR slope as a surrogate for clinical endpoints in patients with rarer causes of CKD requires that the decline in eGFR be consistent over time and by CKD stage.
The current study examined eGFR slope over time across progressive CKD stages in a large cohort of PH1 patients and annual decline of eGFR was shown to vary by CKD stage. Our method included all eGFR measurements for each patient from the time of assignment to a CKD cohort until kidney failure, death, or loss to follow-up in order to account for the full clinical course. Mean annual change was −2.3 and −5.3 ml/min/1.73m2 in CKD stages 2 and 3a (Figure 2 and Table 2). Thus, improvements in eGFR slope of the magnitude suggested by the NKF guidelines would likely be meaningful. A greater rate of eGFR decline was observed in CKD stages 3b and 4, with annual rates of decline of −14.7 and −16.6 ml/min/1.73m2, respectively. This study supports the clinical impression that eGFR decline tends to accelerate as patients progress through CKD stages 3b and 4. Thus, if eGFR is used as a surrogate endpoint in future studies, it would be important to take baseline CKD stage into account.
Because Pox is the net result of both oxalate generation and renal elimination of oxalate, elevations in Pox are relatively modest in patients with PH1 when GFR is well preserved. However, as GFR declines, Pox increases rapidly. These interrelationships might explain why associations between Pox and GFR decline were more notable at advanced CKD stages in the current study (Figure 3 and Table S5). Figure 3 also demonstrates an inflection point for Pox at an eGFR of approximately 30 ml/min/1.73 m2. The risk for systemic oxalosis increases exponentially as Pox exceeds 30–50 μmol/L25–27, which corresponds to the oxalate concentration at which the calcium oxalate ion product in blood exceeds the solubility product. Thus, these data suggest that patients need to be monitored closely as GFR approaches 30 ml/min/1.73m2, i.e. CKD stage 3b and 4, with frequent checks of Pox and early initiation of dialysis as indicated to prevent systemic oxalosis.
Progression to renal failure has been shown to correlate with urine oxalate excretion rate in PH patients with preserved kidney function5,28. High Uox favors CaOx crystal formation. CaOx crystals in kidney tubules and interstitium cause injury and trigger an inflammatory pathway (NLRP3) resulting in progressive renal fibrosis and loss of kidney function28,29. However, since urinary oxalate excretion is expected to decrease as GFR declines, the causative role of Uox in advanced CKD has been unclear. Markedly elevated Pox in CKD stages 4–5 has been implicated in systemic deposition of oxalate (oxalosis) causing damage to diverse organs including bone, skin, eyes, and heart30. However, Pox levels are typically only mildly elevated in CKD stages 1–3a and a role for Pox as a predictor of CKD progression at early stages has not yet been defined. Based on the overall evidence, we suggest that Pox and Uox may play complementary roles for predicting kidney function decline, with Uox being more informative during early CKD stages (1–3a) and Pox in more advanced CKD stages13,31.
The current study has notable strengths. We have data available for a relatively large number of individuals with this rare disease, including multiple laboratory measurements such as SCr and Pox over decades of follow-up. Such data allows for longitudinal analysis of eGFR and Pox trends over time, as well as the ability to investigate potential associations with other laboratory and clinical features.
This study has certain limitations. As data were obtained from retrospective review of registry data, causality cannot be inferred from the observed associations of Pox and eGFR decline. The Oxalosis and Hyperoxaluria Foundation (OHF) RKSC PH registry is a voluntary retrospective registry and therefore this cohort may not represent all patients with PH1. Moreover, comprehensive data at regular time points were not available for all patients due to voluntary reporting at the time of medical care sought at variable time intervals.
In conclusion, the current study demonstrates that CKD stage needs to be accounted for when analyzing eGFR change over time in a PH1 population. Importantly, these data suggest that measures to prevent eGFR decline below 45 ml/min/1.73m2 are particularly important in PH1 since the disease course appears to accelerate rapidly thereafter.
Supplementary Material
Supplementary Material
Figure S1: Individual patient trajectories of eGFR vs years after entry into CKD stage (A) 2, (B) 3a, (C) 3b, and (D) 4. Black dashed lined indicates time of entry into respective CKD stage.
Figure S2: Flowchart of patient entry into each CKD stage
Table S1. eGFR annual rates of percent change during follow-up
Table S2: Associations between eGFR percentage change and factors at entry into CKD stage
Table S3. Coefficients from linear mixed models for plasma oxalate vs eGFR
Table S4. eGFR annual rates of change during follow-up, by G170R mutation status
Table S5. Predicted Pox and rate of change across different CKD stages using a linear mixed model framework
Acknowledgments:
We thank all patients and families who have participated in the RKSC PH registry as well as the many physicians who collected detailed clinical records. Further, we thank the study coordinators who collected the clinical data and biological samples and particularly thank referring nephrologists Dr. Baum, Dr. Greenbaum, Dr. Saland, Dr. Bartosh and Dr. Chandra.
Support:
This work was funded by the Rare Kidney Stone Consortium (U54DK83908), which is part of Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Sciences (NCATS). This consortium was funded through collaboration between NCATS, and the National Institute of Diabetes and Digestive and Kidney Diseases. This work was also supported by funds form the Oxalosis and Hyperoxaluria Foundation, Mayo Foundation, and an industry grant from OxThera, Inc. Those funding the study had no role in study design, analysis, reporting or decision to submit the manuscript for publication.
Financial Disclosure:
Dr. Milliner has received consulting fees from OxThera, Dicerna, Allena, Alynam, and Synlogic and grant support from the NIDDK, OxThera, Dicerna, and Alnylam. Dr. Lieske receives consulting fees from the American Board of Internal Medicine, Alnylam, OxThera, Dicerna, Synlogoic, Orfan, and Novobiome, and grant support from Alnylam, Allena, Retrophin, OxThera, NIDDK, and Dicerna. Dr. Sas has received consulting fees from Advicenne, and lecture fees from Retrophin. Dr. Singh has received honorarium from Deerfield Institute. Dr. Schulte has received consulting fees from OxThera. Lisa Vaughan has nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Cochat P, Rumsby G. Primary hyperoxaluria. N Engl J Med. Aug 15 2013;369(7):649–58. doi: 10.1056/NEJMra1301564 [DOI] [PubMed] [Google Scholar]
- 2.Singh P, Viehman JK, Mehta RA, et al. Clinical characterization of primary hyperoxaluria type 3 in comparison to types 1 and 2: a retrospective cohort study. Nephrol Dial Transplant. Feb 5 2021;doi: 10.1093/ndt/gfab027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geraghty R, Wood K, Sayer JA. Calcium oxalate crystal deposition in the kidney: identification, causes and consequences. Urolithiasis. Oct 2020;48(5):377–384. doi: 10.1007/s00240-020-01202-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoppe B, Beck BB, Milliner DS. The primary hyperoxalurias. Kidney Int. Jun 2009;75(12):1264–1271. doi: 10.1038/ki.2009.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang X, Bergstralh EJ, Mehta RA, Vrtiska TJ, Milliner DS, Lieske JC. Nephrocalcinosis is a risk factor for kidney failure in primary hyperoxaluria. Kidney Int. Mar 2015;87(3):623–31. doi: 10.1038/ki.2014.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Woerden CS, Groothoff JW, Wanders RJ, Davin JC, Wijburg FA. Primary hyperoxaluria type 1 in The Netherlands: prevalence and outcome. Nephrol Dial Transplant. Feb 2003;18(2):273–9. doi: 10.1093/ndt/18.2.273 [DOI] [PubMed] [Google Scholar]
- 7.Fargue S, Harambat J, Gagnadoux MF, et al. Effect of conservative treatment on the renal outcome of children with primary hyperoxaluria type 1. Kidney Int. Oct 2009;76(7):767–73. doi: 10.1038/ki.2009.237 [DOI] [PubMed] [Google Scholar]
- 8.Lieske JC, Monico CG, Holmes WS, et al. International registry for primary hyperoxaluria. Am J Nephrol. May-Jun 2005;25(3):290–6. doi: 10.1159/000086360 [DOI] [PubMed] [Google Scholar]
- 9.Kopp N, Leumann E. Changing pattern of primary hyperoxaluria in Switzerland. Nephrol Dial Transplant. Dec 1995;10(12):2224–7. doi: 10.1093/ndt/10.12.2224 [DOI] [PubMed] [Google Scholar]
- 10.Hopp K, Cogal AG, Bergstralh EJ, et al. Phenotype-Genotype Correlations and Estimated Carrier Frequencies of Primary Hyperoxaluria. Journal of the American Society of Nephrology. 2015;26(10):2559–2570. doi: 10.1681/asn.2014070698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh P, Chebib FT, Cogal AG, Gavrilov DK, Harris PC, Lieske JC. Pyridoxine Responsiveness in a Type 1 Primary Hyperoxaluria Patient With a Rare (Atypical) AGXT Gene Mutation. Kidney Int Rep. Jun 2020;5(6):955–958. doi: 10.1016/j.ekir.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrelfs SF, Frishberg Y, Hulton SA, et al. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. N Engl J Med. Apr 1 2021;384(13):1216–1226. doi: 10.1056/NEJMoa2021712 [DOI] [PubMed] [Google Scholar]
- 13.Milliner DS, McGregor TL, Thompson A, et al. End Points for Clinical Trials in Primary Hyperoxaluria. Clin J Am Soc Nephrol. Jul 1 2020;15(7):1056–1065. doi: 10.2215/cjn.13821119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perinpam M, Enders FT, Mara KC, et al. Plasma oxalate in relation to eGFR in patients with primary hyperoxaluria, enteric hyperoxaluria and urinary stone disease. Clin Biochem. Dec 2017;50(18):1014–1019. doi: 10.1016/j.clinbiochem.2017.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ladwig PM, Liedtke RR, Larson TS, Lieske JC. Sensitive Spectrophotometric Assay for Plasma Oxalate. Clinical Chemistry. 2005;51(12):2377–2380. doi: 10.1373/clinchem.2005.054353 [DOI] [PubMed] [Google Scholar]
- 16.Pottel H, Hoste L, Dubourg L, et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant. May 2016;31(5):798–806. doi: 10.1093/ndt/gfv454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan CJ. Landmark analysis: A primer. Journal of Nuclear Cardiology. 2019/April/01 2019;26(2):391–393. doi: 10.1007/s12350-019-01624-z [DOI] [PubMed] [Google Scholar]
- 18.Dafni U Landmark Analysis at the 25-Year Landmark Point. Circulation: Cardiovascular Quality and Outcomes. 2011;4(3):363–371. doi:doi: 10.1161/CIRCOUTCOMES.110.957951 [DOI] [PubMed] [Google Scholar]
- 19.Bakdash JZ, Marusich LR. Repeated Measures Correlation. Methods. Frontiers in Psychology. 2017-April-07 2017;8(456)doi: 10.3389/fpsyg.2017.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grams ME, Sang Y, Ballew SH, et al. Evaluating Glomerular Filtration Rate Slope as a Surrogate End Point for ESKD in Clinical Trials: An Individual Participant Meta-Analysis of Observational Data. J Am Soc Nephrol. Sep 2019;30(9):1746–1755. doi: 10.1681/asn.2019010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foundation NK. Accelerating New Clinical Trials and Treatments for Kidney Disease. https://www.kidney.org/news/accelerating-new-clinical-trials-and-treatments-kidney-disease. Published 2018. Accessed Jan 2022.
- 22.Levey AS, Gansevoort RT, Coresh J, et al. Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD: A Scientific Workshop Sponsored by the National Kidney Foundation in Collaboration With the US Food and Drug Administration and European Medicines Agency. American Journal of Kidney Diseases. 2020;75(1):84–104. doi: 10.1053/j.ajkd.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 23.statement Np. https://www.kidney.org/CKDEndpoints. Published 2018. Accessed Jan 2022.
- 24.Inker LA, Heerspink HJL, Tighiouart H, et al. GFR Slope as a Surrogate End Point for Kidney Disease Progression in Clinical Trials: A Meta-Analysis of Treatment Effects of Randomized Controlled Trials. Journal of the American Society of Nephrology. 2019;30(9):1735–1745. doi: 10.1681/asn.2019010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barratt TM, van’t Hoff WG. Are there guidelines for a strategy according to glomerular filtration rate, plasma oxalate determination and the risk of oxalate accumulation? Nephrol Dial Transplant. 1995;10 Suppl 8:22–3. doi: 10.1093/ndt/10.supp8.22 [DOI] [PubMed] [Google Scholar]
- 26.Hoppe B, Kemper MJ, Bökenkamp A, Portale AA, Cohn RA, Langman CB. Plasma calcium oxalate supersaturation in children with primary hyperoxaluria and end-stage renal failure. Kidney Int. Jul 1999;56(1):268–74. doi: 10.1046/j.1523-1755.1999.00546.x [DOI] [PubMed] [Google Scholar]
- 27.Marangella M, Cosseddu D, Petrarulo M, Vitale C, Linari F. Thresholds of serum calcium oxalate supersaturation in relation to renal function in patients with or without primary hyperoxaluria. Nephrol Dial Transplant. 1993;8(12):1333–7. [PubMed] [Google Scholar]
- 28.Zhao F, Bergstralh EJ, Mehta RA, et al. Predictors of Incident ESRD among Patients with Primary Hyperoxaluria Presenting Prior to Kidney Failure. Clin J Am Soc Nephrol. Jan 7 2016;11(1):119–26. doi: 10.2215/cjn.02810315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pak CY, Adams-Huet B, Poindexter JR, Pearle MS, Peterson RD, Moe OW. Rapid Communication: relative effect of urinary calcium and oxalate on saturation of calcium oxalate. Kidney Int. Nov 2004;66(5):2032–7. doi: 10.1111/j.1523-1755.2004.00975.x [DOI] [PubMed] [Google Scholar]
- 30.Sas DJ, Enders FT, Gunderson TM, et al. Natural History of Clinical, Laboratory, and Echocardiographic Parameters of a Primary Hyperoxaluria Cohort on Long Term Hemodialysis. Front Med (Lausanne). 2021;8:592357. doi: 10.3389/fmed.2021.592357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah RJ, Vaughan LE, Enders FT, Milliner DS, Lieske JC. Plasma Oxalate as a Predictor of Kidney Function Decline in a Primary Hyperoxaluria Cohort. Int J Mol Sci. May 20 2020;21(10)doi: 10.3390/ijms21103608 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Figure S1: Individual patient trajectories of eGFR vs years after entry into CKD stage (A) 2, (B) 3a, (C) 3b, and (D) 4. Black dashed lined indicates time of entry into respective CKD stage.
Figure S2: Flowchart of patient entry into each CKD stage
Table S1. eGFR annual rates of percent change during follow-up
Table S2: Associations between eGFR percentage change and factors at entry into CKD stage
Table S3. Coefficients from linear mixed models for plasma oxalate vs eGFR
Table S4. eGFR annual rates of change during follow-up, by G170R mutation status
Table S5. Predicted Pox and rate of change across different CKD stages using a linear mixed model framework


