Abstract
Competence for genetic transformation in Streptococcus pneumoniae is regulated by a quorum-sensing system encoded by two genetic loci, comCDE and comAB. Additional competence-specific operons, cilA, cilB, cilC, cilD, cilE, cinA-recA, coiA, and cfl, involved in the DNA uptake process and recombination, share an unusual consensus sequence at −10 and −25 in the promoter, which is absent from the promoters of comAB and comCDE. This pattern suggests that a factor regulating transcription of these transformation machinery genes but not involved with comCDE and comAB expression might be an alternative sigma factor. A search for such a global transcriptional regulator was begun by purifying pneumococcal RNA polymerase holoenzyme. In preparations from competent pneumococcal cultures a protein which seemed to be responsible for cilA transcription in vitro was identified. The corresponding gene was identified and found to be present in two copies, designated comX1 and comX2, located adjacent to two of the repeated rRNA operons. Expression of transformation machinery operons, such as cilA, cilD, cilE, and cfl, but not that of the quorum-sensing operons comAB and comCDE, was shown to depend on comX, while comX expression depended on ComE but not on ComX itself. We conclude that the factor is a competence-specific global transcription modulator which links quorum-sensing information transduced to ComE to competence and propose that it acts as an alternate sigma factor. We also report that comAB and comCDE are not sufficient for shutoff of competence-stimulating peptide-induced gene expression nor for the subsequent refractory period, suggesting that these phenomena depend on one or more ComX-dependent genes.
Streptococcus pneumoniae (pneumococcus) is naturally competent for genetic transformation. Competence, the state of cells able to take up DNA, develops suddenly in response to a cell-cell signal at some point during exponential growth phase, reaches a maximum about 20 min after its induction (induction phase), disappears abruptly (shutoff phase), and remains off for about 40 to 60 min during a period in which cells are refractory to the signal (7, 10, 20, 34, 48). During the period of competence, double-stranded DNA encountered by pneumococcus cells is bound to the cell surface and one strand of the DNA is degraded into short oligonucleotides in the medium while part of the other strand is imported inside the cell (29). The imported single strand of DNA is protected from nuclease activity by a single-stranded DNA binding protein (SSB) (32) and is finally incorporated into the chromosome by homologous recombination involving general recombination machinery, such as RecA (35). The quorum-sensing signal responsible for competence induction is a heptadecapeptide, named CSP (competence-stimulating peptide) (17), which derives from a precursor (ComC) by cleavage and transport into the medium by an ATP-binding cassette (ABC) transporter, ComAB (54). CSP acts through a receptor (ComD) and a response regulator (ComE) to activate both comAB and comCDE operons (39), establishing a positive feedback loop ensuring an abrupt rise in CSP levels, making all cells in a culture competent simultaneously. But how this quorum-sensing circuit (comCDE and comAB) evokes the expression of the genes for the machinery of genetic transformation is not understood, although it has been proposed that ComE (the quorum-sensing transducer) might act as a transcription factor to induce both the competence machinery genes and those of the CSP circuit (4). How competence is shut off after induction is also unknown, although it has been proposed that ComE has dual functions—activation at low doses of the CSP stimulus and repression at high doses—for the regulation of comCDE, which could account for the successive induction and suppression of competence in response to increasing levels of CSP (4).
Recently several competence-specific operons which are probably involved with the DNA uptake process and recombination were identified, such as cilA (ssb2, a gene for SSB), cilB (dal, like dprA in Haemophilus influenzae (22), cilC (ccl, like comC in Bacillus subtilis), cilD (cglABCDE), cilE (celAB), and coi (5, 40). These operons and additional competence-related operons, such as cinA-recA and cfl (like comF in B. subtilis) (24, 25), all contain an unusual perfectly conserved consensus sequence, TACGAATA (cin-box), at position −10 from the transcription start and a T-rich region at −25 (5). Campbell et al. showed that this consensus sequence is important for the transcription of the cinA-recA operon by measuring the activities of mutated promoters in vivo (5). The fact that this consensus sequence is not present in the promoters of comCDE and comAB suggests that a factor regulating transcription of these transformation machinery genes is different from the factor (ComE, apparently) responsible for comCDE regulation. Such a factor for expression of transformation machinery genes could be an alternative sigma factor, since the consensus sequences overlap the RNA polymerase holoenzyme binding site (15). Therefore we sought the putative global transcriptional modulator linking quorum-sensing information to the expression of the genes for transformation by purifying RNA polymerase holoenzyme from competent pneumococcal cultures. We report here identification of a competence-specific transcription modulator, ComX (probably an alternative sigma factor), which (i) is induced through ComE by the competence-stimulating peptide, (ii) is required for the expression of competence-specific operons which contain a cin-box but not of those of the quorum-sensing operons or of comX itself, and (iii) is present in two copies in the Rx derivative strain studied. We also report that the quorum-sensing system alone causes neither competence shutoff nor the CSP refractory period, and we propose that a ComX-induced gene may be responsible for these postcompetence phenomena.
MATERIALS AND METHODS
Bacterial strains, media, and DNA.
All pneumococcal strains, plasmids, and amplicons (DNA products amplified by PCR) used in this study are described in Table 1. CP1500 was used as the source of the template for in vitro transcription and the donor for transformation assays, and CP1250 (39) was a recipient strain and the source for targeting fragments in mutagenic plasmids and lacZ fusion plasmids. The insertion vector, pEVP3 (it carries a robust chloramphenicol resistance [Cmr] marker that is expressed as a single copy independent of target gene transcription, and it has a promoterless lacZ downstream of cloning sites allowing transcriptional fusion of a reporter to a targeted gene after integration [8]), was used for cloning pneumococcal targeting fragments in Escherichia coli DH10B (Gibco BRL). Plasmid pLS10, provided by S. A. Lacks, and the synthetic erythromycin resistance (Emr) marker cassette described previously (8) were used for developing new resistance markers. The sequences of all oligonucleotide primers used in this study are listed in Table 2. Luria-Bertani (LB) medium (45) was used for culture of E. coli, and the complete CAT medium (24) and its modified forms were used for that of pneumococcus (see below for details).
TABLE 1.
Pneumococcal strains and DNA constructs
| Strain, plasmid or amplicon | Relevant characteristics | Description | Sourcea or reference |
|---|---|---|---|
| Strains | |||
| CP1250 | hex malM511 str-1 bgl-1 | Rx derivative, low β-galactosidase background | 39 |
| CP1500 | hex nov-r1 bry-r str-1 ery-r1 ery-r2 | Donor of point markers | 6 |
| CPM1 | CP1250 rpoC::C-his-10::pEVP3 | 10× His tagged at C terminus of β′ of RNA polymerase with pEVP3; Cmr | CP1250 × pMSL1 |
| CPM2 | CP1250 ΔcomX1::PcEm | Insertion-deletion replacement of comX1 with synthetic amplicon, aMSL2, Emr | CP1250 × aMSL2 |
| CPM3 | CP1250 comX1′::(pEVP3)::comX1+ | lacZ fusion to comX1 by using pEVP3, Cmr ComX1+ | CP1250 × pMSL2 |
| CPM4 | CPM2 comX2′::(pEVP3)::′comX2 | lacZ fusion to comX2, ΔcomX1, ΔcomX2, Cmr Emr | CPM2 × pMSL3 |
| CPM5 | CP1250 ΔcomX2::PcTet | Insertion-deletion of comX2 with synthetic amplicon, aMSL5, Tetr | CP1250 × aMSL5 |
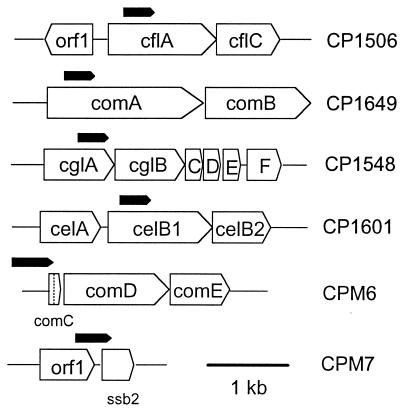
| CPM6 | CP1250 comC′::(pEVP3)::comC+ | lacZ fusion to comC by using pEVP3, Cmr ComC+ | CP1250 × pXF520 |
| CPM7 | CP1250 ssb2′::(pEVP3)::ssb2+ | lacZ fusion to ssb2 by using pEVP3, Cmr Ssb2+ | CP1250 × pMSL4 |
| CPM8 | CPM2 ΔcomX2::PcTet | ΔcomX1, ΔcomX2, Emr Tetr | CPM2 × CPM5 |
| CPM9 | CP1250 comX2′::(pEVP3)::′comX2 | lacZ fusion to comX2 by using pEVP3, Cmr ComX2+ | CP1250 × pMSL3 |
| CPM10 | CPM8 comC′::(pEVP3)::comC+ | lacZ fusion to comC, ΔcomX1, ΔcomX2, ComC+ Emr Tetr Cmr | CPM2 × CPM5 × CPM6 |
| CPM11 | CPM8 comA′::(pEVP3)::′comA | lacZ fusion to comA, comA, ΔcomX1, ΔcomX2; Emr Tetr Cmr | CPM2 × CPM5 × CP1649 |
| CPM12 | CPM8 ssb2′::(pEVP3)::ssb2+ | lacZ fusion to ssb2, ΔcomX1, ΔcomX2, Ssb2+ Emr Tetr Cmr | CPM2 × CPM5 × CPM7 |
| CPM13 | CPM8 cglA′::(pEVP3)::′cglA | lacZ fusion to cglA, cglA, ΔcomX1, ΔcomX2; Emr Tetr Cmr | CPM2 × CPM5 × CP1548 |
| CPM14 | CPM8 celB′::(pEVP3)::′celB | lacZ fusion to celB, celB, ΔcomX1, ΔcomX2; Emr Tetr Cmr | CPM2 × CPM5 × CP1601 |
| CPM15 | CPM8 cflA′::(pEVP3)::′cflA | lacZ fusion to cflA, cflA, ΔcomX1, ΔcomX2; Emr Tetr Cmr | CPM2 × CPM5 × CP1506 |
| CPM16 | CP1250 comX2′::(pEVP3)::comX2+ | lacZ fusion to comX2 by using pEVP3, Cmr ComX2+ | CP1250 × pMSL5 |
| CPM17 | CPM3 ΔcomE::PcEm | lacZ fusion to comX1, ΔcomE, Emr Cmr | CPM3 × aMSL6 |
| Plasmids | |||
| pEVP3 | Nonreplicative vector (CmrlacZ) | 8 | |
| pMSL1 | pEVP3::′rpoC::C-His-10 | Vector tagging rpoC with 10 × His | pEVP3::a(DAM211, DAM254) |
| pMSL2 | pEVP3::′ftsH comX1′ | comX1 targeting lacZ fusion insertion | pEVP3::a(MSL13, MSL14) |
| pMSL3 | pEVP3::′comX2′ | Mutation of comX and lacZ fusion | pEVP3::a(MSL27, MSL28) |
| pMSL4 | pEVP3::ssb2′ | ssb2 targeting and lacZ fusion | pEVP3::a(DAM214, DAM215) |
| pMSL5 | pEVP3::′nusG comX2′ | lacZ fusion to comX2, ComX2+ | pEVP3::a(MSL42, MSL28) |
| pXF520 | pEVP3::′orfL-comC′ | lacZ fusion to comC, ComC+ | 39 |
| Amplicons | |||
| aMSL1 | PcEm | Emr marker with synthetic promoter | a(DAM212, DAM213) |
| aMSL2 | ComX1up::PcEm::comX1dw | For insertion-deletion replacement for comX1 | See Fig. 3 |
| aMSL3 | Pc | Constitutive promoter | a(DAM212, MSL31) |
| aMSL4 | PcTet | New synthetic marker | a(DAM212, MSL33) |
| aMSL5 | ComX2up::PcTet::comX2dw | For insertion-deletion replacement for comX2 | See Materials and Methods |
| aMSL6 | ComEup::PcEm::comEdw | For insertion-deletion replacement for comE | See Materials and Methods |
Strain construction by a cross carried out by using transformation is indicated as recipient × DNA donor × additional DNA donor.
TABLE 2.
Oligonucleotides used in this study
| Name | Sequence (5′ → 3′)a |
|---|---|
| DAM138 | CTTCCACAGTAGTTCACCACCTT |
| DAM139 | TAGCTCTAGACACGCGTAGCAT |
| DAM140 | TAATGCTACGCGTGTCTAGAGCTA |
| DAM145 | ATCCCACTTTATCCAATTTTCGT |
| DAM206 | CTGACTTTCTCAAGATAAAAAGCC |
| DAM207 | TCAACCTCGTCGTGTTCAGA |
| DAM208 | GACTTCCCAATCCTCTGCAAC |
| DAM209 | TTTGTGCCAACAAGCCTAAAT |
| DAM211 | CCAATGCAT-AATCGGTGACAAACACATCG |
| DAM212 | CCGGGCCCAAAATTTGTTTGAT |
| DAM213 | AGTCGGCAGCGACTCATAGAAT |
| DAM214 | ATCAAACAAATTTTGGGCCCGG-AGACGTTAAACGTCCAATCAAGA |
| DAM215 | TCGCAACGGATATCAAGGTGAC |
| DAM250 | TTTTTGTTCATGTAATCACTCCTTC |
| DAM251 | CACGCCAAAGTAAACAATTTAAG |
| DAM254 | CTTAGTGATGGTGATGGTGATGGTGATGGTGATG-TTCTACTGTTTCTTCCACAGTTTCA |
| MSL13 | ATCAAACAAATTTTGGGCCCGG-CACAGTCCCTTGGACTTCTTCAT |
| MSL14 | TGCTATGCTTGGTGCACAGAGTC |
| MSL15 | AATTCTATGAGTCGCTGCCGACT-TTTCGAGGGCGTCAAAGAGTATT |
| MSL16 | ACAACTTACGTTGTAGCGCCCTG |
| MSL17 | CGTGCAATGGTTACAGAGTACGG |
| MSL18 | CTGAGCCAGGATCAAACTCTCAT |
| MSL23 | ATTGTCGGATTGGGAGCAAGAAG |
| MSL24 | TTCATTGCTTAAGACGCGTTCTA |
| MSL27 | GGACTGGTAGACGATATTCCACG |
| MSL28 | TGAAAGAGATAATAATCATCTAGCCA |
| MSL31 | CATGTAATCACTCCTTCTTAA |
| MSL32 | GTAATTAAGAAGGAGTGATTACATG-AATACATCCTATTCACAATCGAA |
| MSL33 | TCCCAAAGTTGATCCCTTAACGA |
| MSL34D | ACWCCWGGIGTIACIGGWTTYGTIGG |
| MSL35 | ACGCGTAGCATTACGTATTATTGATGG |
| MSL36 | TCGTTAAGGGATCAACTTTGGGA-TTTCGAGGGCGTCAAAGAGTATT |
| MSL37 | CCTGCACATTGGTTCGTCTTGTT |
| MSL38 | TTTGGTTGTGTCGTAAATTCGAT |
| MSL39 | AGTTGGCTGGTTACCTTGAATGT |
| MSL42 | TTGGAACAAGAAATTCGTGACAT |
| MSL51 | ATCAAACAAATTTTGGGCCCGG-CTCACTTGATGTTCAATAACATCTT |
| MSL52 | TGCTCAGTCAATTGTCTATGCTCG |
| MSL53 | AATTCTATGAGTCGCTGCCGACT-TTTGTATATTGAAACAACAGGGG |
| MSL54 | ACCAACGGACCTTCTATCTGTAGC |
| Q1 | CTTTACGAAACAGGAAAGATGCC |
| Q2 | GTGCTTTTGCAGACTACACTGGT |
| Q3 | CTTGACCAACGGACCTGAGTTGT |
-, border of 5′ extension.
Transformation of pneumococcal cells and selection of transformants.
Cells were exposed to DNA (0.2 μg/ml) for 45 min after treatment with CSP as described previously (24). Transformants were selected on CAT and 1.5% agar solid media with appropriate drugs at the following concentrations (see details in reference 33): novobiocin, 2.5 μg/ml; chloramphenicol, 2.5 μg/ml; erythromycin, 0.25 μg/ml; and tetracycline, 0.25 μg/ml.
Construction of new pneumococcal strains.
To construct the pneumococcal strain CPM1, containing a His-tagged RNA polymerase, we chose to tag the carboxyl terminus of RpoC (13, 41). A blunt-ended 530-bp fragment containing the 3′ end of rpoC (gene encoding the β′ subunit of RNA polymerase) and a 10-His-codon extension was amplified by PCR from CP1250 DNA with Pfu DNA polymerase (Stratagene), using primers DAM211 and DAM254. DAM211 includes the sequence of rpoC extending from −487 to −468 relative to its stop codon and a CCAATGCAT 5′ extension; DAM254 includes the sequence of rpoC from −1 to −25, with 10 His codons and a stop triplet as a 5′ extension. After insertion of the PCR product into the SmaI site of pEVP3, an E. coli DH10B clone carrying a chimeric plasmid having rpoC and lacZ on the same strand was identified. The plasmid (pMSL1) was integrated into CP1250 by insertion duplication at the rpoC locus by transformation, resulting in strain CPM1. The integrated structure was confirmed by sequencing PCR products at both junctions.
To construct the strain CPM2 (ΔcomX1), an Emr marker (PcEm) was amplified by PCR with DAM212 and DAM213 from the synthetic Emr cassette (8). comX1up (450 bp), which contained part of both comX1 and ftsH plus a sequence complementary to the end of PcEm, was amplified with MSL14 and MSL13 from CP1250 DNA. comX1dw (430 bp), which contained part of comX1 and a part of downstream sequence plus a terminus complementary to the other end of PcEm, was amplified with MSL15 and MSL16 from CP1250 DNA. The three PCR products were used as a mixed template for PCR with MSL14 and MSL16 to produce aMSL2. After transformation of CP1250 with aMSL2, the structures of the insertion-deletion mutations in Emr clones were confirmed by PCR, which demonstrated the expected junction fragments (620, 630, and 1,900 bp), using the primer pairs DAM250-MSL17, DAM251-MSL18, and MSL17-MSL18.
To construct the strain CPM3, with a comX1-lacZ fusion, the amplicon comX1up was inserted into the SmaI site in pEVP3, and the construct (pMSL2) was introduced into CP1250 by transformation. The correct orientation and integrity of the lacZ insertion in Cmr clones were confirmed by observing the expected product (570 bp) of PCR with MSL14 and DAM138.
To construct the strain CPM4, with a comX1 deletion and a comX2-lacZ fusion, the targeting fragment for comX2 was prepared by PCR from DNA of CPM2 (ΔcomX1) by using MSL27 and MSL28, which are complementary to an internal region of comX1. After insertion into the SmaI site of pEVP3, transforming CPM2 with the chimeric plasmid (pMSL3) yielded the double mutant CPM4, whose structure was confirmed by reading the sequences flanking comX1 and comX2 (see below).
To construct the CPM5, with a comX2 deletion, the coding region of the structural gene for tetracycline resistance (Tetr) was amplified by PCR with MSL32 and MSL33 from the template pLS10. The fragment Pc (constitutive promoter) was amplified with DAM212 and MSL31 from the synthetic Emr cassette (8). Both products were connected by PCR by using DAM212 and MSL33. ComX2up, which contains a comX2-specific upstream sequence (part of nusG) and a small 5′ segment of comX with an extension complementary to an end of PcTet, was amplified with MSL35 and MSL13 from a CP1250 template. comX2dw, which contains a small 3′ fragment of comX2 and adjacent downstream sequence with an extension complementary to the other end of PcTet, was amplified with MSL36 and MSL37 from the CP1250. The three DNA fragments, ComX2up, PcTet, and comX2dw, were used as a template for PCR with primers MSL35 and MSL37 to produce aMSL5. After transformation of CP1250 with aMSL5 and selection of Tetr colonies, the gene replacement structure was confirmed by PCR with the primer pairs MSL38-MSL42 and MSL39-MSL18.
To construct the strain CPM7, with a reporter of ssb2 gene expression, a fragment encompassing the putative promoter of ssb2 gene was amplified by PCR with DAM214 and DAM215. After the product was cloned in the SmaI site of pEVP3, the chimeric plasmid was introduced into CP1250 by transformation. The correct integration structure was confirmed by observing the expected product after PCR with primers specific for vector (DAM138) and for a site upstream of ssb2 (DAM214). The strains CPM8 through CPM15 were produced by crosses between the strains as described in Table 1.
To construct strain CPM16, which contains a lacZ fusion at comX2 at the same site as in CPM9 and CPM4 and also an intact comX2 allele, a comX2-specific targeting fragment extending from nusG into comX2 was amplified with MSL42 and MSL28. The amplified product was incorporated into the SmaI site of pEVP3. The correct chimeric plasmid (pMSL5) was identified by observing a product of the expected size after PCR with primers MSL42 and DAM138. After introduction into CP1250 by transformation, the correct integrated structure was confirmed by obtaining a product of the expected size by PCR with MSL42 and DAM138. To construct strain CPM17, which contains both a deletion of comE and a lacZ fusion at comX1, we assembled amplicon aMSL6, which contains PcEm flanked with terminal comE fragments, following a strategy similar to that outlined in Fig. 3. comEup and comEdw fragments were amplified with MSL51 and MSL52 and with MSL53 and MSL54, respectively. aMSL6 was synthesized by PCR with MSL52 and MSL54 by using comEup, comEdw, and PcEm as templates. After introduction of aMSL6 into CPM3 by transformation, the correct integrated structures in Emr Cmr colonies were confirmed by observing the expected products of PCR with MSL52 and DAM250 and with MSL54 and DAM251.
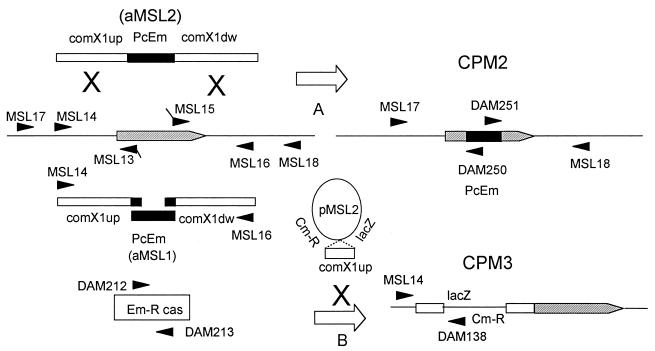
FIG. 3.
Strategies for construction and confirmation of an insertion-deletion mutation of comX1 and a comX1::lacZ fusion. Solid arrows and arrowheads mark primers for PCR; hollow arrows represent transformation processes. Hatched pentagon represents comX1 gene and its direction of transcription. Boxes and lines indicate double-stranded DNA. Black boxes in DNA represent PcEm markers. (A) Construction of CPM2 by transformation of CP1250 with the amplicon aMSL2, assembled by PCR from an Emr cassette and two DNA fragments flanking comX1. (B) Construction of CPM3 by transformation of CP1250 with pMSL2, an insertion plasmid targeting comX1. Details of construction of the strains and the verification of marker integration are described in the Materials and Methods section.
Purification of His-tagged RNA polymerase.
CPM1 was grown in 4 liters of CAT with 6 mM HCl to optical density (OD) of 0.1 at 37°C (24, 33). For some preparations, competence was induced by adding NaOH (11 mM) and CSP (100 ng/ml). After 10 min at 37°C, the cells were chilled to 4°C and harvested by centrifugation at 10,000 × g for 15 min. All further purification steps were done at 0 to 4°C unless otherwise specified. The cell pellet was washed with rinse buffer (10 mM Tris HCl [pH 8.0], 300 mM NaCl, 20% glycerol, 10 mM MgCl2, 5 mM β-mercaptoethanol) (28, 41) once and stored at −80°C for at least 1 h. After thawing, resuspension in 10 ml of rinse buffer, addition of 1 mM phenylmethylsulfonyl fluoride, 0.1% Triton X-100, and 5 μg of DNase I/ml, and incubation for 5 to 10 min at 37°C to lyse the cells and break the chromosomal DNA, the lysate was clarified (14,000 × g, 40 min) and applied to a 0.25-ml Ni-nitrilotriacetic acid agarose (Qiagen) affinity column at the rate of 0.4 ml/min. The Ni-nitrilotriacetic acid resin was previously equilibrated with lysis buffer (rinse buffer with 1 mM phenylmethylsulfonyl fluoride and 0.1% Triton X-100) in a 0.7 cm by 10 cm column. The adsorbed material was sequentially washed with lysis buffer containing 5 mM imidazole (three times with 7 ml each time) and 45 mM imidazole (one or three times with 5 ml each time). Bound proteins were eluted with 1 ml of 105 mM imidazole in lysis buffer and 1 ml of 205 mM imidazole in lysis buffer, sequentially. The combined eluates were dialyzed twice against 200 ml of storage buffer (50 mM Tris HCl [pH 8.0], 250 mM NaCl, 50% glycerol, 10 mM MgCl2, 0.1 mM EDTA, and 1 mM dithiothreitol) overnight and stored at −20°C. Protein concentrations were determined by comparison of bands stained with Colloidal Blue stain (Novex) to known amount of bovine serum albumin (BSA) on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels.
In vitro transcription assay.
Templates were amplified by PCR by using Pfu DNA polymerase and CP1500 DNA. A blunt-ended 369-bp fragment of the amiA locus (GenBank accession no. X17337) containing the promoter and a truncated amiA gene was amplified with primers DAM208 (positions 262 and 242) and DAM209 (positions −105 and −85), and a 605-bp fragment of ssb2 (The Institute for Genomic Research [TIGR]) containing the promoter and the complete gene was prepared with primers DAM206 (positions 447 and 425) and DAM207 (positions −159 and −140) (the parenthetical numbers refer to positions of primer ends relative to the first base of each gene). Expected run-off transcript sizes are 300 bp for amiA and 420 bp for ssb2. After purification with Millipore ultrafilter units, these PCR products were used for transcription assays in vitro. The transcription reaction (final volume, 20 μl) consisted of 0.4 pmol of the PCR product, 0.5 μg of pneumococcal RNA polymerase, 20 U of RNasin (RNase inhibitor; Promega), 10 nmol each of ATP, CTP, and GTP, 0.2 nmol of UTP, and 10 μCi of [α-32P]UTP (400 Ci/mM) (Amersham) in transcription buffer (40 mM Tris HCl [pH 8.0], 250 mM KCl, 10 mM MgCl2, 1 mM dithiothreitol, and 50 μg of BSA/ml (11, 43). Initiation complexes were allowed to form by incubation of the reaction mixture at 37°C for 10 min with neither nucleoside triphosphates nor [α-32P]UTP. After addition of the nucleoside triphosphates and labeled UTP, the reaction was continued for 50 min at 37°C and stopped by addition of 10 μl of loading dye (95% formamide, 20 mM EDTA, 0.05% bromophenol blue, and 0.05% xylene cyanol FF). Samples were denatured in boiling water for 5 min and electrophoresed in a 6% polyacrylamide gel containing 7 M urea. Dried gels were exposed to Kodak X-Omat film.
Protein sequencing.
After competence-specific RNA polymerase prepared from eight 4-liter cultures was dialyzed against distilled water, denatured by heat, and resolved by SDS-PAGE on a 10% NuPAGE Bis-Tris gel following the recommendation by the supplier (NOVEX), the resolved components were electrotransferred to polyvinylidene difluoride membrane and then chemically sequenced by The Protein Research Laboratory at the University of Illinois at Chicago.
PCRs.
All PCRs were carried out with the procedure described previously (24) except for variations in the annealing temperature, the extension time, and the enzyme composition. The annealing temperature chosen was 3°C below the annealing temperature predicted by the primer supplier (Operon Technology), and extension time was at least 1 min per kilobase of amplified product. For most analytical PCRs, cloned Taq DNA polymerase (Gibco BRL) was used. For preparative PCRs, cloned Pfu polymerase (Stratagene) was used, except for PCR to connect templates, where Taq (Gibco BRL) and Vent polymerases (New England Biolabs) were mixed 1:1 (50). Ligation-mediated PCR (LMPCR) (37) for obtaining upstream sequence of comX2 was carried out as described previously (24) by using Csp6I and a linker containing DAM139 and DAM140, followed by PCR amplification of the ligated fragments with DAM138 and DAM139. Annealing temperature for PCR with MSL34D and DAM138 to obtain the sequence of the comX2 upstream region was 40°C.
Southern analysis.
Chromosomal DNA from each strain was digested with EcoRI (New England Biolabs). Digested DNA (1 μg) was loaded on each lane and was electrophoresed for 3 h at 30 V in a 0.7% agarose gel in 0.5× TBE (Tris-borate-EDTA) buffer. All image-developing processes were based on the protocol provided by the supplier (Stratagene) of the kits for Southern analysis. DNA in the gel was depurinated by incubation in 0.25 N HCl for 10 min and then denatured in 1.5 M NaCl and 0.5 M NaOH for 30 min. After neutralization in 1.5 M NaCl and 0.5 M Tris HCl (pH 8.0), DNA was transferred to a Duralon-UV membrane in 10× SSC buffer (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) by capillary blotting for 16 h (45). After cross-linking by UV irradiation, prehybridization and hybridization reactions were done sequentially in 6× SSC buffer, 5× Denhardt’s reagent, 0.5% SDS, and 100 μg of sheared denatured salmon sperm DNA/ml without probe and with probe for 3 and 16 h, respectively, at 65°C. The probe was prepared by using Prime-It Fluor Fluorescence Labelling kit from the PCR-amplified 190-bp internal fragments of comX1 and boiled for 5 min before hybridization. After hybridization, the membrane was washed three times with 0.1× SSC buffer and 0.1% SDS at 65°C for 15 min. The bands were detected by the Illuminator Chemiluminescent Detection System.
Recovery of sequences flanking comX1 and comX2.
The fragments flanking comX1 were obtained by PCR by using PcEm-specific primers and comX1-specific primers (MSL17-DAM250 and MSL18-DAM251) from CPM4 chromosomal DNA. PCR with DAM145-MSL18 yielded a product of the size expected if the duplicated region of comX2 extended downstream beyond MSL18 from CPM4. As PCR with MSL17-DAM138 failed, a sequence upstream of comX2 was recovered by LMPCR as described above. As the product exhibited significant homology to nusG of B. subtilis, PCR using a degenerate primer (MSL34D) complementary to a conserved region of nusG and DAM138 was used to obtain an 800-bp product, which extended the homology to nusG further. To get more reliable sequences, both comX1 and comX2 regions were sequenced forward and backward twice by using sequence primers (Q1, MSL27, Q3, and MSL28 for comX1 and Q2, MSL27, MSL28, and Q3 for comX2) after obtaining fragments from both loci from CP1250 by PCR with primers MSL17-MSL18 (comX1) and MSL42-MSL18 (comX2).
β-Galactosidase assay.
Cultures grown in CAT plus 6 mM HCl at 37°C were split for treatment of one half with 200 ng of CSP/ml and 11 mM NaOH. After incubation for 40 min at 37°C for competence induction, cells were chilled, collected by centrifugation at 12,000 × g for 10 min at 4°C, resuspended in 40 μl of 0.1 M sodium phosphate buffer–0.1% Triton X-100, and lysed for 10 min at 37°C. A β-galactosidase assay with ONPG (o-nitrophenyl-β-d-galactopyranoside) as a substrate followed the protocol described previously (45); the activity is expressed in Miller units with respect to the OD of the culture when CSP was added (30).
RESULTS
Identification of a factor associated with RNA polymerase from competent cells and structure of its gene.
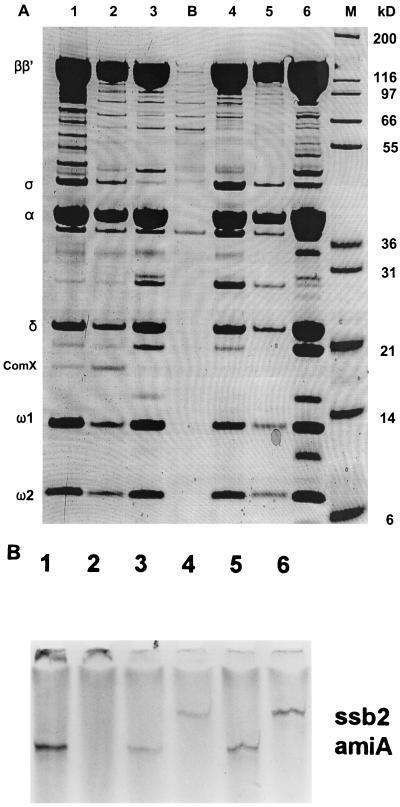
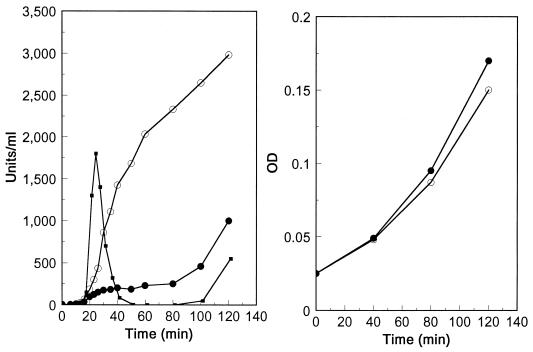
Because the putative global competence transcriptional modulator might act as an alternative sigma factor, we chose to search for a factor present specifically in RNA polymerase preparations from competent cultures. Since a procedure for purifying pneumococcal RNA polymerase had not been reported, we employed a His tagging approach (13) to simplify the purification. rpoC, the gene for the largest subunit (β′) among the RNA polymerase components, positioned at the end of the putative rpoBC operon, was tagged with a sequence encoding 10 histidine residues at the C terminus in CP1250, as described in the Materials and Methods section. To prepare the His-tagged RNA polymerases from both competent and noncompetent cultures, several cultures of strain CPM1 were treated with CSP, but other parallel cultures were not. RNA polymerases were prepared from those cultures as described in the Materials and Methods section. Figure 1A shows six such RNA polymerase preparations, three from independent competent cultures and three from independent untreated, noncompetent cultures. Figure 1B shows that they were active in RNA synthesis. While some polypeptide components of the RNA polymerase preparations obtained varied among the preparations, the seven principal bands in all preparations were similar in molecular weight (MW) to seven which were also reported to be the primary components of similar partially purified RNA polymerase preparations from B. subtilis (14). As those bands appear to be the major components of the RNA polymerase holoenzyme, we designate them β, β′, ς, α, δ, ω1, and ω2, following the terminology used for bacillus. Three of these identifications were verified by N-terminal sequence determination (ς, α, and δ) and comparison of the corresponding genes with those of B. subtilis (data not shown). An additional band (MW, 19,000) was observed in most RNA polymerase preparations from competent cultures (8 of 10 independent preparations, 3 of which are shown in Fig. 1A) but was absent from all of 10 independent preparations from uninduced cultures. To determine whether RNA polymerase preparations with this particular additional band (e.g., lanes 1 and 2 in Fig. 1A) could direct transcription of a known competence-specific gene, we performed in vitro transcription assays with two linear templates (see Materials and Methods), i.e., an amiA fragment which contains the constitutive promoter of the amiA gene (2) and a cilA (ssb2) fragment which contains the cin-box promoter of the ssb2 gene, which is expressed specifically at competence (5). Polymerase prepared from noncompetent cultures directed transcription from the amiA fragment (300 bp) but not at all from the ssb2 fragment (420 bp) (one pair of results is shown in Fig. 1B). In contrast, polymerase prepared from competent cultures and displaying the additional protein transcribed both the ssb2 fragment and the amiA fragment (two pairs of results are shown in Fig. 1B). These results suggested that the 19-kDa additional RNA polymerase component might be responsible for the transcription of the competence-specific gene, ssb2, since the presence of no other protein observed in the RNA polymerase preparations correlated with successful in vitro transcription of ssb2.
FIG. 1.
Specific transcription of a competence gene, ssb2, by preparations of His-tagged RNA polymerase with ComX. (A) SDS-PAGE analysis of RNA polymerase preparations from competent and noncompetent cultures is shown. RNA polymerase was prepared from three competent cultures (lanes 1, 2, and 3) and from three independent noncompetent cultures (lanes 4, 5, and 6). Lane B was blank (a control preparation with no His tag from CP1250), and lane M contained standards. The amounts of protein loaded were approximately 25, 10, 25, 25, 10, and 50 μg, from left to right, respectively. The gel was stained with Colloidal Blue stain. (B) Transcriptional specificity of RNA polymerase preparations. In vitro transcription of two genes, amiA and ssb2, was performed with three RNA polymerase preparations as described in the Materials and Methods section. The reaction products were analyzed on a denaturing 6% polyacrylamide gel. Products are shown for two preparations from competent cultures (lanes 3 and 4, enzyme displayed in panel A, lane 1; lanes 5 and 6, enzyme displayed in panel A, lane 2) and one from a noncompetent culture (lanes 1 and 2, enzyme displayed in panel A, lane 6). Templates were ssb2 for lanes 2, 4, and 6 and amiA for lanes 1, 3, and 5. The predicted transcript sizes are 420 bp for ssb2 and 300 bp for amiA.
To analyze the function of this 19-kDa protein further, we obtained its N-terminal amino acid sequence. From 2 μg of gel-purified material, a partial N-terminal sequence, N-XXKELYXXVQXXVY-C, was obtained by Edman degradation and microsequencing (see Materials and Methods). In the available partial genome sequence of pneumococcus (the type 4 strain, [1, 47]), a single match to this sequence was found, in contig 4125. Translation beginning at the matched region could yield a 159-amino-acid protein with a translational start site 10 bp downstream of an apparent ribosome binding (Shine-Dalgarno) sequence. The size of the predicted protein, 19.9 kDa, was consistent with the gel position of the competence-specific band shown in Fig. 1A. Since the additional polymerase component appears to be encoded by this open reading frame (ORF), and since the gene proved to be involved in competence regulation (see below), we name the corresponding gene comX1 (to distinguish it from an additional copy present at a different locus in strain CP1250, as described below).
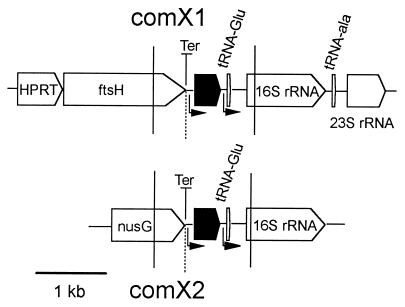
Since comX1 is positioned in the middle of contig 4125, we could map the gene (shown in Fig. 2). ftsH, a cell division gene, is positioned upstream of comX1 and is separated from it by a strong rho-independent terminator. There was no obvious consensus −35 promoter signal, but a putative pneumococcal extended −10 sequence, TcTGtTAgAcT (consensus sequence appears in capital letters), sufficient for promoter activity in several documented cases (44), is found upstream of comX1. Although no obvious terminator structure is recognized downstream of comX1, a perfect −35 and −10 consensus sequence is followed by tRNA genes and rRNA genes. Therefore, comX1 seems to be in a monocistronic operon.
FIG. 2.
Map of regions neighboring comX1 and comX2. Solid vertical lines represent the ends of sequence read for each locus, and the dotted vertical line marks the border of structural identity between the loci. Open pentagons represent putative gene assignments; filled pentagons indicate comX copies. Ter represents rho-independent terminator; bent arrows represent promoters. The complete map at the top is derived from the partial type 4 genome sequence. The organization of the sequence flanking comX1 in CP1250 was the same as that of contig 4125 from type 4. The comX2 locus was absent from the type 4 genome sequence database and was determined in CP1250 as described in the text.
comX1 is induced during competence but is not required for competence.
To begin characterization of this gene, it was mutated by replacement with an Emr marker by using natural transformation and it was fused to a promoterless lacZ reporter gene by using pEVP3 (Fig. 3) to produce CPM2 and CPM3, respectively. Enzyme assays of the reporter gene product showed that the expression of comX1 was turned on in response to treatment with CSP (Table 3). However, in contrast to the other genes known to be expressed specifically during competence, the ΔcomX1 strain CPM2 was transformed at normal levels under the same conditions (Table 4). These observations suggested the possibility of an additional, functional, copy of comX in strain Rx.
TABLE 3.
β-Galactosidase production in various strains showing effects of comX mutations on CSP-dependent regulation of transformation genes
| Strain | lacZ reporter fusion sites | Phenotypeb | β-Galactosidase activitya
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clone | Expt 1
|
Expt 2
|
Expt 3
|
Expt 4
|
|||||||
| −CSP | +CSP | −CSP | +CSP | −CSP | +CSP | −CSP | +CSP | ||||
| CPM6 | comC | WT | 330 | 360 | 150 | 150 | |||||
| CPM10 | comC | ComX1− ComX2− | 1 | 150 | 300 | 390 | |||||
| 2 | 240 | 430 | |||||||||
| CP1649 | comA | ComA− | 2.2 | 130 | 2.3 | 130 | |||||
| CPM11 | comA | ComX1− ComX2− ComA− | 1 | 2.0 | 150 | 2.7 | 210 | ||||
| 2 | 0.9 | 270 | |||||||||
| CPM3 | comX1 | WT | 0.9 | 100 | 3.2 | 50 | |||||
| CPM16 | comX2 | WT | 1 | 3.5 | 130 | 4.1 | 190 | ||||
| 2 | 3.2 | 140 | 4.1 | 180 | |||||||
| CPM17 | comX1 | ComE− | 1 | 2.7 | 2.6 | 2.5 | 3.4 | ||||
| 2 | 3.6 | 3.5 | |||||||||
| CPM9 | comX2 | ComX2− | 1 | 8.7 | 260 | 3.2 | 150 | 4.6 | 170 | ||
| 2 | 2.4 | 190 | |||||||||
| CPM4 | comX2 | ComX1− ComX2− | 1 | 3.1 | 570 | 3.3 | 330 | 3.1 | 390 | ||
| 2 | 2.7 | 390 | |||||||||
| CPM7 | ssb2 | WT | 0.9 | 130 | 2.2 | 110 | 1.7 | 60 | |||
| CPM12 | ssb2 | ComX1− ComX2− | 1 | 1.8 | 2.0 | 3.3 | 3.2 | ||||
| 2 | 3.9 | 4.5 | |||||||||
| CP1548 | cglA | CglA− | 3.4 | 370 | 160 | 3.5 | 190 | ||||
| CPM13 | cglA | ComX1− ComX2− CglA− | 1 | 1.8 | 1.8 | 2.9 | 2.9 | ||||
| 2 | 3.9 | 3.1 | |||||||||
| CP1601 | celB | CelB− | 1.5 | 90 | 1.8 | 2.5 | 50 | ||||
| CPM14 | celB | ComX1− ComX2− CelB− | 1 | 2.2 | 2.1 | 2.9 | 3.2 | ||||
| 2 | 3.5 | 4.4 | |||||||||
| CP1506 | cflA | CflA− | 0.5 | 90 | 70 | ||||||
| CPM15 | cflA | ComX1− ComX2− CflA− | 1 | 1.6 | 3.0 | 3.2 | |||||
| 2 | 3.7 | 4.1 | |||||||||
| CP1250 | None | WT | 0.4 | 1.5 | 2.9 | 2.8 | 3.1 | 2.5 | |||
Two independent clones of each strain were grown in CAT containing 6 mM HCl to an OD550 of 0.05 in several experiments. After 1 ml of culture was either not treated (−CSP) or treated (+CSP) with CSP and NaOH, incubation was continued for 40 min. Enzyme activity in crude cell extract at 40 min was measured by the ONPG assay and is expressed in Miller units (30).
WT, wild type.
TABLE 4.
One copy of comX is sufficient and required for transformation
| Strain | Relevant genotype | Clone | Competencea (Novr transformants/μl)
|
||
|---|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | |||
| CP1250 | WT | 830 | 770 | 1,560 | |
| CPM2 | ΔcomX1 comX2+ | 1 | 400 | 540 | 1,480 |
| 2 | 2,000 | ||||
| 3 | 570 | ||||
| CPM5 | comX1+ ΔcomX2 | 810 | 1,350 | ||
| CPM8 | ΔcomX1 ΔcomX2 | <0.01b | <0.01 | ||
| CPM4 | ΔcomX1 comX2 | 1 | <1 | ||
| 2 | <1 | ||||
Several independent clones of each strain were grown in CAT containing 6 mM HCl to an OD550 of 0.025 in several experiments. 5MC (Novr) DNA was added to 10 μg/ml with CSP and NaOH. Incubation was continued for 40 min. Competence was measured as the number of Novr transformants in 1 μl (105 cells) of transformed culture except for CPM8, where it was measured in 100 μl of the culture. Where transformants were observed, at least 100 colonies were counted.
No transformants were observed when the designated portion of culture was challenged.
comX is present in two copies which are flanked by different upstream genes but by identical downstream genes.
To test for the presence of an additional, possibly functional, copy of comX in strain CP1250, CPM2 (ΔcomX1) was subjected to PCR with primers internal to comX1 (MSL23 and MSL24 or MSL27 and MSL28) and to Southern blot analysis by using an amplified internal comX1 fragment as a probe. PCR amplification products were observed (320 and 190 bp) corresponding to internal regions of comX1 (data not shown). Furthermore, Southern blot analysis revealed two EcoRI fragments hybridizing to comX for CP1250 and one for CPM2 (data not shown). These data together implied that there was an additional copy of comX present in the wild-type genome of Rx. Indeed, as subsequent deletion of a second comX gene (see below) produced a strain, CPM8, that was negative on PCR with the same primers used for detecting the presence of comX1 and comX2 (data not shown), we conclude that comX is present in two copies in strain CP1250, an Rx derivative.
To map both comX copies more exactly, their flanking sequences were obtained by LMPCR and direct PCR from strain CPM4 (see below), using the inserted marker sequences to provide copy-specific tags, as described in the Materials and Methods section. The results showed that the region containing comX1 in strain Rx had exactly the same structure, except for several single-base substitutions, as that of the comX allele contained in the type 4 genome database, while comX2 (absent from the partial genome database) was flanked upstream by nusG, an antiterminator homologue, and downstream by tRNA and rRNA genes were identical to those adjacent to comX1. The detailed sequences read at least twice in each strand for each comX region (GenBank accession no. AF161700 and AF161701). The results showed that the sequences of comX1 and comX2 in CP1250 were perfectly identical to one another. Furthermore, their putative promoter regions were identical up to the terminal triplets of the upstream genes. Several base differences were observed between the regions downstream of comX1 and comX2. Finally, although the sequence of the comX1 gene in the TIGR database had several base changes compared to comX1 in CP1250, the predicted protein products differed at only one position (Glu31Asp).
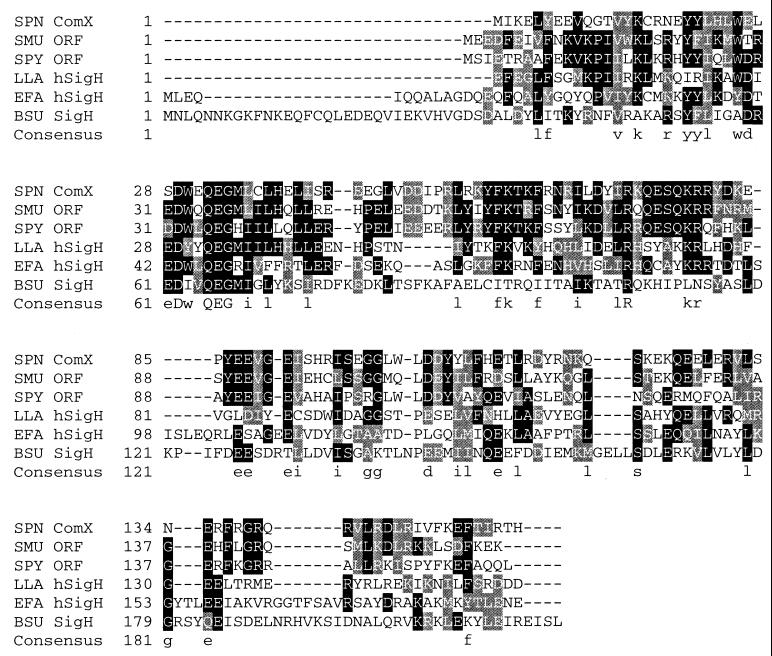
A comparison of the deduced amino acid sequence of comX with other known protein sequences was done by using BLAST to search both the GenBank database and the unfinished microbial genome databases at the National Center for Biotechnology Information (NCBI [36]) and at WIT (52). It identified two genes of unknown function encoding very similar proteins: an ORF in Streptococcus pyogenes (expectation value [E] = 10−31, 40% identical and 65% similar residues; Fig. 4) and another ORF in Streptococcus mutans (E = 10−36, 46% identical and 66% similar residues). In addition, the search revealed two rather more-distant homologues; one from Enterococcus faecalis (E = 10−9, 32% identical and 46% similar residues) and the other from Lactococcus lactis (E = 10−8, 26% identical and 45% similar residues). The two distant homologues of ComX are classified as sigma H homologues within the sigma 70 family of sigma factors on the basis of weak similarity to sigma-H of B. subtilis, an accessory sigma factor which is involved in sporulation and stationary-phase transcription (12, 21). Furthermore, this set of six putative sigmas exhibited their greatest similarities in a highly conserved region, subregion 2.2 (26, 27), which is the most highly conserved region among sigma 70 family members; 11 of 20 residues in the region were more than 80% similar among the six sigmas, and four residues were identical in all six (Fig. 4).
FIG. 4.
Sequence alignment of ComX homologues. The sequences of the homologues were aligned with the program Clustal W (9). Amino acid similarity is indicated by highlighting (black background, ≥50% identity; shaded background, ≥50% similarity). In the consensus sequence, residues with ≥80% similarity are shown in lowercase and 100% matches are shown in uppercase. The most highly conserved region of the sigma 70 family (subregion 2.2) is located between residues 56 and 75 of B. subtilis sigH. Protein abbreviations and sources are as follows: SPN ComX, ComX in S. pneumoniae, accession no. RPN00272 in the WIT database (52); SMU ORF, S. mutans, contig 746 (bp 2275 through 1796 of the sequence in the NCBI database [36]); SPY ORF, S. pyogenes accession no. RST00265 in the WIT database; LLA hSigH, L. lactis tr/Q48591; EFA hSigH, E. faecalis REF02274 in the WIT database; BSU SigH, B. subtilis RBS00098 in the WIT database.
Either copy of comX is sufficient for competence.
To test whether ComX is required for transformation, we mutated the second copy of comX carried by the ΔcomX1 strain CPM2 by insertion-duplication mutagenesis using 190 bp internal to comX as a targeting fragment. In the resulting double mutant, CPM4, transformation was reduced to less than 0.2% of wild-type levels (Table 4). In the comX double deletion strain, CPM8, constructed as described below, transformation was reduced still further, to less than 0.002% of wild-type level (Table 4). Therefore, the comX2 gene is required and sufficient for genetic transformation in the ΔcomX1 background.
The results presented above establish that comX1 is induced by CSP and that comX2 is sufficient for competence. To confirm that comX1 is also active, as suggested by the identity of sequences in the promoters of the two comX copies and by its induction by CSP, we constructed a tagged comX2 deletion, using a new synthetic marker, PcTet (aMSL4), composed of a Tetr gene under the control of a synthetic pneumococcal constitutive promoter (8). The new marker was substituted for comX2 in CP1250 by the strategy described in the Materials and Methods section. As the resulting ΔcomX2 strain, CPM5, transformed at normal levels (Table 4), we conclude that comX1 is also sufficient for transformation and thus that both copies of comX are active, with either one being sufficient for wild-type levels of competence.
comX is a regulatory gene, upstream of cin-box genes but downstream of comE.
comX is induced by CSP and required for genetic transformation, properties shared with many competence-related genes. Since ComX was associated with RNA polymerase, seemed to be responsible for specific in vitro transcription of ssb2, and was similar only to hypothetical sigma H homologues among genes whose function is known, it was a reasonable candidate for the hypothetical global transcription modulator of the transformation machinery genes mentioned in the introduction. To determine independently the functional position of ComX in the hierarchy of competence regulation, we carried out a series of epistasis experiments with lacZ reporter fusions, examining both the dependence of comX expression on other competence genes and the dependence of other competence genes’ expression on comX. First, six strains, which contained each of the competence genes comC, comA, ssb2, cglA, celB, and cflA fused to lacZ (a fusion map is shown in Fig. 5), were crossed into the comX double-mutation background, by transforming CPM2 simultaneously with the comX2 deletion construct from CPM5 and individual lacZ fusion constructs, to make CPM10 (comC::lacZ), CPM11 (comA::lacZ), CPM12 (ssb2::lacZ), CPM13 (cglA::lacZ), CPM14 (celB::lacZ), and CPM15 (cflA::lacZ). All these reporters exhibited the expected low or negligible expression in noncompetent cultures (except for those of CPM6 and CPM10). As shown in Table 3, comAB, an operon of the quorum-sensing system, was induced by CSP equally well in the comX double-mutation background and in the comX-positive background (compare data for CPM11 and CP1649). The expression of comCDE, the other quorum-sensing operon, was not affected by comX mutation (compare data for CPM10 and CPM6; CPM6 was previously noticed to exhibit higher endogenous comCDE expression in competence-inhibiting acidic media for unknown reasons [39]). In contrast, induction of four competence operons containing the cin-box (ssb2, cgl, cel, and cfl) was abolished in the comX double-mutation background, but it was clearly observable in the comX-positive background (compare data for CPM12, CPM13, CPM14, and CPM15 with those for CPM7, CP1548, CP1601, and CP1506). Thus, comX is required for the expression of competence-specific genes containing the consensus (cin-box) but not for the expression of the quorum-sensing operons comCDE and comAB. To test whether comX gene products affect the expression of comX itself, we made isogenic comX2::lacZ fusion strains that were ComX positive (CPM16), ComX2 negative (CPM9), and negative for both ComX1 and ComX2 (CPM4). In all these strains, lacZ expression was found to be induced by CSP (Table 3), showing that the induction of comX2 is not dependent on the comX product. Thus, comX is a regulatory gene that divides the class of genes induced by CSP into two groups: those dependent on comX function and those that are inducible without comX function. The former appear to be principally those thought of as structural genes of DNA processing; the latter appear to be quorum-sensing genes and comX. These data are perfectly consistent with the idea that ComX could act on the cin-box directly for the expression of the structural genes since it affects only cin-box genes among genes known to be induced by CSP.
FIG. 5.
Map of lacZ fusion sites at six competence loci. Each targeting fragment (filled pentagons) directed the nonreplicative vector, pEVP3, to introduce a lacZ fusion at the site marked by the point of the pentagon. Fusion constructs except that of CPM7 (see the Methods and Materials section) were described previously (24). The exact positions of the targeting fragments in the competence loci in the mutants are as follows: strain CP1506, contig 4155 (bp 6235 through 5935); CP1649, contig 4105 (bp 4920 through 5220); CP1548, contig 4194 (bp 16895 through 16595); CP1601, contig 4139 (bp 4806 through 4506); CPM6, U33315 (bp 332 through 721); CPM7, contig 4219 (bp 836 through 476). Gene positions are as follows: cflA, contig 4155 (bp 6454 through 5156); celB1, contig 4139 (bp 5029 through 3506); comA, contig 4105 (bp 4693 through 6852); cglA, contig 4194 (bp 17495 through 16554); ssb2, contig 4219 (bp 514 through 118).
Since comX was induced by CSP and expression of the comCDE operon was not affected by comX loss, the quorum-sensing system seemed likely to be upstream of ComX in the process of competence induction. To locate ComX more precisely, we examined the expression of comX in a comE (quorum-sensing transducer) deletion background. The results (Table 3) show that ComE is upstream of ComX for competence induction, since CSP elicited no detectable β-galactosidase activity in CPM17 (ΔcomE, comX1::lacZ). Since ComE is upstream of ComX and there is a report that ComE binds a direct repeat region common to the putative promoters of the comCDE and comAB operons, we searched for any similar conserved sequences in the putative promoters of comX and comAB. Indeed, similar direct repeats are observable upstream of all three operons, with constant separation and a constant distance from an apparent −10 consensus. They are -68TTGgGAGAA-60 and -48TTGaGAGAA-40 at comCDE, -68AtGgagaAA-60 and -48AgGagctAA-40 at comX, and -68TGGaggGag-60 and -48TGGgaaGga-40 at comAB. The consensus among the six repeat units was (T70A30)(G50T50)G(G50A50) (G70A30)(A50G30)(G70) (A80G20)(A80G20). Thus, the mechanism of regulation of comX may share some components with that for regulation of comAB and comCDE, and ComE may act at the direct repeats in the promoters of all three operons.
When the relative activities of comX2 promoters in the wild type (CPM16) and in the comX double-deletion background (CPM4) were compared by using identical reporter fusion sites, cumulative β-galactosidase activities after 40-min incubation with CSP were always found to be more than twice as high for CPM4 as for CPM16 (Table 3). This pattern suggests a negative feedback effect on expression of comX by comX gene products or by comX-regulated genes. To address the question of whether the increase in cumulative β-galactosidase in the comX mutant background arose via increased expression during the induction phase or from a failure of competence shutoff (34) at later stages in the response to CSP, we compared in detail the comX expression kinetics of these two strains from the moment of treatment with CSP through the shutoff phase and refractory period (Fig. 6). The results revealed a strong influence of comX on comX expression. In CPM4, lacZ expression was higher than in CPM16, both during the competence induction phase (15 to 25 min) and at the time of competence shutoff (25 to 40 min); lacZ synthesis continued even during the refractory period (40 to 90 min). In CPM16, in contrast, lacZ expression ceased during the shutoff phase and refractory period, reappearing only as competence reappeared, after 100 min. These observations suggest that during competence induction, the comX product inhibits its own synthesis to some degree and show that during the later competence shutoff phase and refractory period, either the comX gene product or the product of a comX-induced gene inhibits comX gene expression almost completely. Thus, comX affects its own expression levels during all phases of the response to CSP.
FIG. 6.
Comparison of comX promoter activities in CPM16 and CPM4 after competence induction. Competence was induced by CSP and NaOH (at time 0) for each strain at OD550 of 0.025. Left panel: β-galactosidase activities (Miller units) were monitored for both strains CPM16 (●) and strain CPM4 (○). Transformation (1 unit = 100 Novr transformants) for CPM16 (■) was also monitored at the same time by determining the number of Novr transformants after exposing a portion of the culture to donor DNA for 90 s. Right panel: Growth of CPM16 (●) and CPM4 (○) was monitored by measuring OD550.
DISCUSSION
We have identified a new competence-induced gene (comX) in pneumococcus, present in two identical copies, that is required for competence. Epistasis experiments showed that cin-box operons depended on this gene for expression but that two known competence operons involved in quorum-sensing (comCDE and comAB), with different promoter characteristics, did not. Additionally, the comX gene product was not required for its own expression. Since the expression of comX did depend on comE, we conclude that ComX establishes a link between the quorum-sensing information transduced to comE and the later steps of competence induction.
ComX was initially identified as a protein present in preparations of RNA polymerase from competent cultures but absent from parallel RNA polymerase preparations from noncompetent cultures. His-tagged RNA polymerase containing the ComX protein directed transcription of a competence-specific late gene, ssb2, but the polymerase from noncompetent cultures did not, even though it was active in transcription from a constitutive gene, amiA. On the basis of the epistasis results, the association of ComX with competent polymerase, the cin-specific activity of ComX-containing polymerase, and the similarity of ComX to sigma H, we hypothesize that the competence-specific factor (ComX) induced by the quorum-sensing system is an alternative sigma factor which recognizes the cin-box and T-rich region in the promoter of transformation machinery genes and directs their transcription. It is interesting to note that the central four bases of the cin-box (GAAT) are identical to four of five bases of the −10 consensus sequence recognized by the B. subtilis sigma H (53).
It has long been recognized (31, 42) that streptococcal competence for genetic transformation can entail a transient but global shift of protein synthesis, occurring simultaneously throughout a growing culture, such that expression of many genes is shut down just when a specific set of genes is activated. This global protein shift accompanying competence induction would be explained by the proposed role of comX as a competitive sigma in which it could block transiently not only the transcription of quorum-sensing operons and the comX gene itself but also the large number of genes transcribed by the primary RNA polymerase.
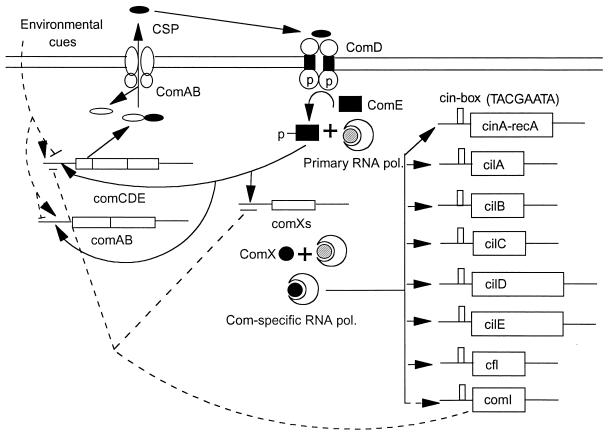
We propose the following model for regulation of genetic transformation incorporating a role of comX in mediating the connection of quorum sensing to competence induction, based on results from previous studies and the present study, illustrated in Fig. 7. Environmental cues that affect competence, such as pH (7), phosphate concentration, and peptides (3) might modulate the basal level of comCDE and comAB expression or modulate the effectiveness of CSP interaction with its cognate receptor, ComD (18). As cell density in a growing culture increases, basal expression of these operons causes the absolute concentration of CSP outside the cells to increase, reaching a threshold concentration at which CSP can effectively activate the cognate receptor. Activated ComD (sensor of the two-component system), in turn, may activate ComE (response regulator) by phosphorylating it, as in many other bacterial two-component systems (46). Phosphorylated ComE may activate three competence-specific operons, comCDE, comAB, and comX, directly by interacting with the major RNA polymerase (primary sigma and core RNA polymerase) and the direct repeats in these promoters, since it has been reported that ComE binds to a direct repeat at the comCDE promoter strongly and to a similar site at the comAB promoter weakly (51) and since similar putative binding sites are identifiable in the promoter regions of these three operons. With ensuing accumulation of ComX, it replaces the primary sigma (sigma A homologue) from RNA polymerase holoenzyme, to create a competence-specific RNA polymerase holoenzyme, directing expression of cin-box-containing genes and thus allowing the synthesis of the genes for DNA transport and recombination machinery.
FIG. 7.
Hypothetical model for the regulation of genetic transformation. Solid arrows indicate processing steps or transcriptional activation steps that have been shown to take place or for which supporting observations are described in the text. T bars indicate negative regulation. Dashed lines indicate hypothetical links. comI, a putative gene responsible for competence shutoff and refractory period.
With the addition of ComX to the signal transduction chain linking CSP to competence, it becomes possible to begin to assign responsibility for other aspects of competence to either of two experimentally distinguishable sets of induced genes—those of the quorum-sensing circuit versus comX and those dependent on comX action. One such aspect is the transient nature of competence, which disappears rather rapidly despite the continued presence of high levels of CSP. The activity of the comX promoter measured during competence induction was increased by mutation of comX, and in the comX mutant background, the expression continued without interruption during the competence shutoff phase and refractory period, whereas the same gene was silenced in the comX-positive background. The first phenomenon suggests that the comX product inhibits its own synthesis to some degree, which could be explained simply on the basis of sigma factor displacement. Such a replacement of the sigma A homologue by comX is also likely to reduce expression of the two quorum-sensing operons, for which the major RNA polymerase also seems to be necessary, and could be the reason for the precocious reduction of comCDE expression during competence induction previously observed by Alloing et al. (4). But such a reduction of the expression of quorum-sensing operons and of comX through the role of ComX as a competitive sigma factor seems insufficient to shut off competence completely soon after induction or to keep it off for another generation, since the overall culture growth rate is not perturbed by exposure to CSP (Fig. 6). Therefore, the complete shutoff of competence and the long refractory period both seem to require an additional negative control mechanism. Alloing et al. suggested that the expression of comCDE induced by a phosphorylated ComE might be inhibited by a different, more highly phosphorylated form of ComE (4). However, the observation that comX expression persisted for a long time in comX double mutants suggests that the quorum-sensing system upstream of comX cannot accomplish the inhibition alone. Therefore, we conclude that one of the genes regulated by comX effects the complete shutoff of competence after induction and its continued suppression. Possible targets for the product of such a gene include the promoters of the comCDE operon or comX or the proteins ComD or ComE.
Many species (including Streptococcus milleri, Streptococcus anginosus, Streptococcus constellatus, Streptococcus oralis, Streptococcus mitis, Streptococcus crista, Streptococcus intermedius, Streptococcus gordonii, Streptococcus sanguis, and S. pneumoniae) in the genus Streptococcus are known to be naturally competent (19). Streptococcus pyogenes, although not known to be competent naturally, appears to carry homologues for many transformation machinery genes, such as celAB, cglAB, recA, and ssb, and has homologues to the quorum sensor, ComD, and to the transducer, ComE (19). In addition, there are homologues to dal and cfl (data not shown). Thus, S. pyogenes has essentially all major components of the transformation machinery genes. Furthermore, there is also a copy of the cin-box in the promoter region of each of these apparent transformation machinery genes (19). As S. pyogenes also carries a homologue to ComX, the apparatus for transformation in S. pneumoniae and S. pyogenes seems to be broadly conserved, including elements for quorum sensing, for activation of transformation genes by ComX, and for transformation itself. This conservation suggests that even though a ComC homologue has not been found, some kind of quorum-sensing effector might be present in S. pyogenes. It would be interesting to learn whether expression of the ComX homologue in S. pyogenes makes the species competent, which could greatly facilitate the genetic manipulation of the species. S. mutans is also naturally transformable (38); although the sequencing of S. mutans is not far advanced, it has already revealed possible homologues to celAB, comDE, and comX. Therefore, it seems possible that S. mutans will also turn out to carry a transformation system much like that in pneumococcus.
Natural competence in another gram-positive bacterium, B. subtilis, is known to depend on a quorum-sensing system (23). Accumulated small peptide pheromones (ComX and CSF) in the bacillus growing culture stimulate comS expression through a bacterial two-component system, comPA, at high cell density, and in turn, ComS activates ComK by releasing the inhibitor MecA. ComK is the key transcription factor for expression of all of the late competence genes that encode the DNA processing and uptake machinery (16, 49). Thus, cell density signals in B. subtilis lead to activation of a transcription factor which directs the major RNA polymerase to promoters of transformation machinery genes, while in S. pneumoniae, a cell density signal (CSP) induces expression of ComX, which appears to act as a competence-specific sigma factor to focus transcription on a regulon composed of transformation machinery genes.
ACKNOWLEDGMENTS
This work was supported in part by the U.S. National Science Foundation (MCB-9722821).
Assistance with protein sequencing by Bao-Shiang Lee from Protein Research Laboratory at the University of Illinois at Chicago is gratefully acknowledged. The generosity of TIGR in making available genome sequences prior to publication (47) is also acknowledged.
REFERENCES
- 1.Aaberge I S, Eng J, Lermark G, Løvik M. Virulence of Streptococcus pneumoniae in mice: a standardized method for preparation and frozen storage of the experimental bacterial inoculum. Microb Pathog. 1995;18:141–152. doi: 10.1016/s0882-4010(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 2.Alloing G, Trombe M C, Claverys J P. The ami locus of the gram-positive bacterium Streptococcus pneumoniae is similar to binding protein-dependent transport operons of gram-negative bacteria. Mol Microbiol. 1990;4:633–644. doi: 10.1111/j.1365-2958.1990.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 3.Alloing G, Granadel C, Morrison D A, Claverys J P. Competence pheromone, oligopeptide permease, and induction of competence in Streptococcus pneumoniae. Mol Microbiol. 1996;21:471–478. doi: 10.1111/j.1365-2958.1996.tb02556.x. [DOI] [PubMed] [Google Scholar]
- 4.Alloing G, Martin B, Granadel C, Claverys J P. Development of competence in Streptococcus pneumoniae: pheromone autoinduction and control of quorum-sensing by the oligopeptide permease. Mol Microbiol. 1998;29:75–83. doi: 10.1046/j.1365-2958.1998.00904.x. [DOI] [PubMed] [Google Scholar]
- 5.Campbell E A, Choi S Y, Masure H R. A competence regulon in Streptococcus pneumoniae revealed by genomic analysis. Mol Microbiol. 1998;27:929–939. doi: 10.1046/j.1365-2958.1998.00737.x. [DOI] [PubMed] [Google Scholar]
- 6.Cato A, Guild W R. Transformation and DNA size. J Mol Biol. 1968;37:157–178. doi: 10.1016/0022-2836(68)90080-6. [DOI] [PubMed] [Google Scholar]
- 7.Chen J D, Morrison D A. Modulation of competence for genetic transformation in Streptococcus pneumoniae. J Gen Microbiol. 1987;133:1959–1967. doi: 10.1099/00221287-133-7-1959. [DOI] [PubMed] [Google Scholar]
- 8.Claverys J P, Dintilhac A, Pestova E V, Martin B, Morrison D A. Construction and evaluation of new drug-resistance cassettes for gene disruption mutagenesis in Streptococcus pneumoniae, using an ami test platform. Gene. 1995;164:123–128. doi: 10.1016/0378-1119(95)00485-o. [DOI] [PubMed] [Google Scholar]
- 9.Clustal W Website. Program server. [Online.] http://www.clustalw.genome.ad.jp. [12 April 1999, last date accessed.]
- 10.Coomaraswamy G. Induction of genetic transformation in Streptococcus pneumoniae by a pheromone peptide and its synthetic analogues. Ph.D. thesis. Chicago: University of Illinois; 1996. [Google Scholar]
- 11.Deora R, Misra T K. Characterization of the primary ς factor of Staphylococcus aureus. J Biol Chem. 1996;271:21828–21834. doi: 10.1074/jbc.271.36.21828. [DOI] [PubMed] [Google Scholar]
- 12.Dubnau E, Weir J, Nair G, Carter III L, Moran C, Jr, Smith I. Bacillus sporulation gene spo0H codes for ς30 (ςH) J Bacteriol. 1988;170:1054–1062. doi: 10.1128/jb.170.3.1054-1062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita M, Sadaie Y. Rapid isolation of RNA polymerase from sporulating cells of Bacillus subtilis. Gene. 1998;221:185–190. doi: 10.1016/s0378-1119(98)00452-1. [DOI] [PubMed] [Google Scholar]
- 14.Haldenwang W G, Lang N, Losick R. A sporulation-induced sigma-like regulatory protein from B. subtilis. Cell. 1981;23:615–624. doi: 10.1016/0092-8674(81)90157-4. [DOI] [PubMed] [Google Scholar]
- 15.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamoen L W, Van Werkhoven A F, Bijlsma J J, Dubnau D, Venema G. The competence transcription factor of Bacillus subtilis recognizes short A/T-rich sequences arranged in a unique, flexible pattern along the DNA helix. Genes Dev. 1998;12:1539–1550. doi: 10.1101/gad.12.10.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Håvarstein L S, Coomaraswamy G, Morrison D A. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci USA. 1995;92:11140–11144. doi: 10.1073/pnas.92.24.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Håvarstein L S, Gaustad P, Nes I F, Morrison D A. Identification of the streptococcal competence pheromone receptor. Mol Microbiol. 1996;21:863–869. doi: 10.1046/j.1365-2958.1996.521416.x. [DOI] [PubMed] [Google Scholar]
- 19.Håvarstein L S, Morrison D A. Quorum sensing and peptide pheromones in streptococcal competence for genetic transformation. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C: ASM Press; 1999. pp. 9–26. [Google Scholar]
- 20.Hotchkiss R D. Cyclical behavior in pneumococcal growth and transformability occasioned by environmental changes. Proc Natl Acad Sci USA. 1954;40:49–55. doi: 10.1073/pnas.40.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong S, Yoshikawa H, Takahashi H. Isolation and characterization of the secE homologue gene of Bacillus subtilis. Mol Microbiol. 1993;10:133–142. doi: 10.1111/j.1365-2958.1993.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 22.Karudapuram S, Zhao X, Barcak G J. DNA sequence and characterization of Haemophilus influenzae dprA+, a gene required for chromosomal but not plasmid DNA transformation. J Bacteriol. 1995;177:3235–3240. doi: 10.1128/jb.177.11.3235-3240.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazazzera B A, Palmer T, Quisel J, Grossman A D. Cell density control of gene expression and development in Bacillus subtilis. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C: ASM Press; 1999. pp. 27–46. [Google Scholar]
- 24.Lee M S, Dougherty B A, Madeo A C, Morrison D A. Construction and analysis of a library for random insertional mutagenesis in Streptococcus pneumoniae: use for recovery of mutants defective in genetic transformation and for identification of essential genes. Appl Environ Microbiol. 1999;65:1883–1890. doi: 10.1128/aem.65.5.1883-1890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Londono-Vallejo J A, Dubnau D. comF, a Bacillus subtilis late competence locus, encodes a protein similar to ATP-dependent RNA/DNA helicases. Mol Microbiol. 1993;9:119–131. doi: 10.1111/j.1365-2958.1993.tb01674.x. [DOI] [PubMed] [Google Scholar]
- 26.Lonetto M, Gribskov M, Gross C A. The ς70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lonetto M A, Brown K L, Rudd K E, Buttner M J. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase ς factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci USA. 1994;91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Losick R, Chamberlin M. RNA polymerase. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1976. [Google Scholar]
- 29.Mejean V, Claverys J P. Polarity of DNA entry in transformation of Streptococcus pneumoniae. Mol Gen Genet. 1988;213:444–448. doi: 10.1007/BF00339614. [DOI] [PubMed] [Google Scholar]
- 30.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1972. [Google Scholar]
- 31.Morrison D A, Baker M F. Competence for genetic transformation in pneumococcus depends on synthesis of a small set of proteins. Nature. 1979;282:215–217. doi: 10.1038/282215a0. [DOI] [PubMed] [Google Scholar]
- 32.Morrison D A, Mannarelli B. Transformation in pneumococcus: nuclease resistance of deoxyribonucleic acid in the eclipse complex. J Bacteriol. 1979;140:655–665. doi: 10.1128/jb.140.2.655-665.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison D A, Lacks S A, Guild W R, Hageman J M. Isolation and characterization of three new classes of transformation-deficient mutants of Streptococcus pneumoniae that are defective in DNA transport and genetic recombination. J Bacteriol. 1983;156:281–290. doi: 10.1128/jb.156.1.281-290.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison D A. Streptococcal competence for genetic transformation: regulation by peptide pheromones. Microbial Drug Res. 1997;3:27–38. doi: 10.1089/mdr.1997.3.27. [DOI] [PubMed] [Google Scholar]
- 35.Mortier-Barriere I, de Saizieu A, Claverys J P, Martin B. Competence-specific induction of recA is required for full recombination proficiency during transformation in Streptococcus pneumoniae. Mol Microbiol. 1998;27:159–170. doi: 10.1046/j.1365-2958.1998.00668.x. [DOI] [PubMed] [Google Scholar]
- 36.National Center for Biotechnology Information Website. Unfinished microbial genome database under BLAST server. [Online.] http://www.ncbi.nlm.nih.gov. [19 March 1999, last date revised.]
- 37.Palittapongarnpim P, Chomyc S, Fanning A, Kunimoto D. DNA fingerprinting of Mycobacterium tuberculosis isolates by ligation-mediated polymerase chain reaction. Nucleic Acids Res. 1993;21:761–762. doi: 10.1093/nar/21.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry D, Kuramitsu H K. Genetic transformation of Streptococcus mutans. Infect Immun. 1981;32:1295–1297. doi: 10.1128/iai.32.3.1295-1297.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pestova E V, Håvarstein L S, Morrison D A. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol Microbiol. 1996;21:853–862. doi: 10.1046/j.1365-2958.1996.501417.x. [DOI] [PubMed] [Google Scholar]
- 40.Pestova E V, Morrison D A. Isolation and characterization of three Streptococcus pneumoniae transformation-specific loci by use of a lacZ reporter insertion vector. J Bacteriol. 1998;180:2701–2710. doi: 10.1128/jb.180.10.2701-2710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi Y, Hulett M F. PhoP-P and RNA polymerase sigmaA holoenzyme are sufficient for transcription of pho regulon promoters in Bacillus subtilis: phoP-P activator sites within the coding region stimulate transcription in vitro. Mol Microbiol. 1998;28:1187–1197. doi: 10.1046/j.1365-2958.1998.00882.x. [DOI] [PubMed] [Google Scholar]
- 42.Raina J L, Ravin A W. Switches in macromolecular synthesis during induction of competence for transformation of Streptococcus sanguis. Proc Natl Acad Sci USA. 1980;77:6062–6066. doi: 10.1073/pnas.77.10.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao L, Karls R K, Betley M J. In vitro transcription of pathogenesis-related genes by purified RNA polymerase from Staphylococcus aureus. J Bacteriol. 1995;177:2609–2614. doi: 10.1128/jb.177.10.2609-2614.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabelnikov A G, Greenberg B, Lacks S A. An extended −10 promoter alone directs transcription of the DpnII operon of Streptococcus pneumoniae. J Mol Biol. 1995;250:144–155. doi: 10.1006/jmbi.1995.0366. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 46.Stock J B, Surette M G, Levit M, Park P. Two-component signal transduction systems: structure-function relationships and mechanism of catalysis. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: American Society for Microbiology; 1995. pp. 25–51. [Google Scholar]
- 47.TIGR Website. Sequences on the Institute for Genomic Research web site. Nov. 21, 1997, release date. [Online.] http://www.tigr.org. [18 March 1999, last date accessed.]
- 48.Tomasz A, Hotchkiss R D. Regulation of the transformability of pneumococcal cultures by macromolecular cell products. Proc Natl Acad Sci USA. 1964;51:480–487. doi: 10.1073/pnas.51.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Sinderen D, Luttinger A, Kong L, Dubnau D, Venema G, Hamoen L. ComK encodes the competence transcription factor, the key regulatory protein for competence development in Bacillus subtilis. Mol Microbiol. 1995;15:455–462. doi: 10.1111/j.1365-2958.1995.tb02259.x. [DOI] [PubMed] [Google Scholar]
- 50.Wach A. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast. 1996;12:259–265. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C259::AID-YEA901%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 51.Ween, O., P. Gaustad, and L. S. Håvarstein. Identification of DNA binding sites for ComE, a key regulator of natural competence in Streptococcus pneumoniae. Mol. Microbiol., in press. [DOI] [PubMed]
- 52.WIT Website. Genome annotations. [Online.] http://wit.mcs.anl.gov.
- 53.Wösten M M S M. Eubacterial sigma-factors. FEMS Microbiol Rev. 1998;22:127–150. doi: 10.1111/j.1574-6976.1998.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhou L, Hui F M, Morrison D A. Competence for genetic transformation in Streptococcus pneumoniae: organization of a regulatory locus with homology to two lactococcin A secretion genes. Gene. 1995;153:25–31. doi: 10.1016/0378-1119(94)00841-f. [DOI] [PubMed] [Google Scholar]