Abstract
Diet influences onset, progression, and severity of several chronic diseases, including heart failure, diabetes, steatohepatitis, and a subset of cancers. The prevalence and clinical burden of these obesity-linked diseases has risen over the past two decades. These metabolic disorders are driven by ectopic lipid deposition in tissues not suited for fat storage, leading to lipotoxic disruption of cell function and survival. Sphingolipids such as ceramides are among the most deleterious and bioactive metabolites that accrue, as they participate in selective insulin resistance, dyslipidemia, oxidative stress and apoptosis. This review discusses our current understanding of biochemical pathways controlling ceramide synthesis, production and action; influences of diet on ceramide levels; application of circulating ceramides as clinical biomarkers of metabolic disease; and molecular mechanisms linking ceramides to altered metabolism and survival of cells. Development of nutritional or pharmacological strategies to lower ceramides could have therapeutic value in a wide range of prevalent diseases.
Keywords: nutrition, chronic disease, ceramide, sphingolipids, lipotoxicity
1. INTRODUCTION
Chronic diseases are multifactorial by nature, with nutrition playing an important but enigmatic role. The importance of a healthy diet is most commonly associated with obesity-related diseases, wherein energy intake exceeds demand and excess calories are stored as triglycerides. While triglyceride storage itself is likely inert, the capacity of adipose depots to safely store these extra calories is finite. As a result, lipids can eventually accumulate in tissues not suited for fat storage, such as the heart, liver, vasculature, and pancreas. This ectopic lipid accumulation creates a lipotoxic state that primes the body for cardiometabolic disease development, including heart failure, atherosclerosis, nonalcoholic fatty liver disease (NAFLD), obesity related cancers, and diabetes. Extensive studies carried out over the past 25 years indicate that ceramides, a bioactive and lipotoxic member of the sphingolipid class, elicit many of the cellular changes that underly the aforementioned diseases. The discoveries regarding ceramides in metabolic pathology present exciting opportunities for understanding the evolutionary forces that promote nutrition-linked chronic disease development and for identifying novel therapeutic and predictive targets for clinical implementation.
While ceramides comprise a minor proportion of the whole-body lipidome, they play potent roles in nutrition-linked chronic disease. For example, overexpression of ceramide synthesizing genes or ablation of genes required for ceramide degradation worsens features of metabolic disease (13, 46), while genetic or pharmacological inhibition of ceramide synthesis prevents cardiometabolic diseases (12, 58, 59, 141). In vitro studies in the 1990s demonstrated the signaling capacity of ceramides via direct participation in the insulin-responsive glucose uptake pathway, providing the initial link between ceramides and insulin resistance (135). This finding has now been widely confirmed in rodent studies and prospective human cohort studies (12, 31, 32, 66, 86). Additional studies have shown relationships between serum ceramides and terminal consequences of the metabolic syndrome, such as diabetes and major adverse cardiac events (31, 66, 78, 86). Nonetheless, more work remains if we are to achieve widespread use of ceramides as biomarkers or develop ceramide-lowering strategies to treat these pathologies.
Herein we summarize the modes of ceramide synthesis and transport, the clinical utility of ceramides as biomarkers for nutrition-linked chronic diseases, the role of dietary sphingolipid intake and dietary patterns on ceramide profiles, and the molecular mechanisms of ceramides that drive the pathogenesis underlying metabolic disease. Moreover, we discuss the potential modes of ceramide-lowering strategies, including lifestyle interventions and findings from preclinical pharmacological targeting of the sphingolipid synthesis pathway, to mitigate obesity-coupled diseases.
2. CERAMIDE SYNTHESIS AND METABOLISM
The sphingolipid class encompasses 4,000–5,000 unique lipid species with reported structures (LIPID MAPS Structure Database, Reference 130), which are metabolized in elegant anabolic and catabolic pathways characterized within multiple organisms.
2.1. De Novo Ceramide Synthesis
Ceramides are produced in a conserved four-step biosynthetic pathway within the endoplasmic reticulum (ER), which commences with the rate-limiting condensation of a saturated long-chain acyl-CoA and amino acid to produce 3-ketosphinganine via serine palmitoyltransferase (SPT) (Figure 1a). Three genes (i.e., SPTLC1, SPTLC2, and SPTLC3) encode the essential subunits of the SPT complex, serving as heterodimers with differential tissue expression and specificity for acyl-CoA and amino acid substrates (47, 61). Additional components termed small subunits of SPT and orosomucoid-like proteins interact directly with the SPT complex substrate binding sites to confer additional regulation of substrate specificity and activity (1, 5, 22, 47, 49, 150). The SPT complex predominantly produces an 18-carbon sphingoid backbone produced by the condensation of palmitoyl-CoA with serine but can incorporate alternative fatty acids (e.g., myristoyl-CoA, stearoyl-CoA) or initiate production of deoxysphingolipids by using alanine or glycine. The products of the SPT reaction are quickly acted upon by an NADPH-dependent reductase (i.e., 3-ketodihydrosphingosine reductase) to form the defining sphingoid scaffold dihydrosphingosine.
Figure 1.
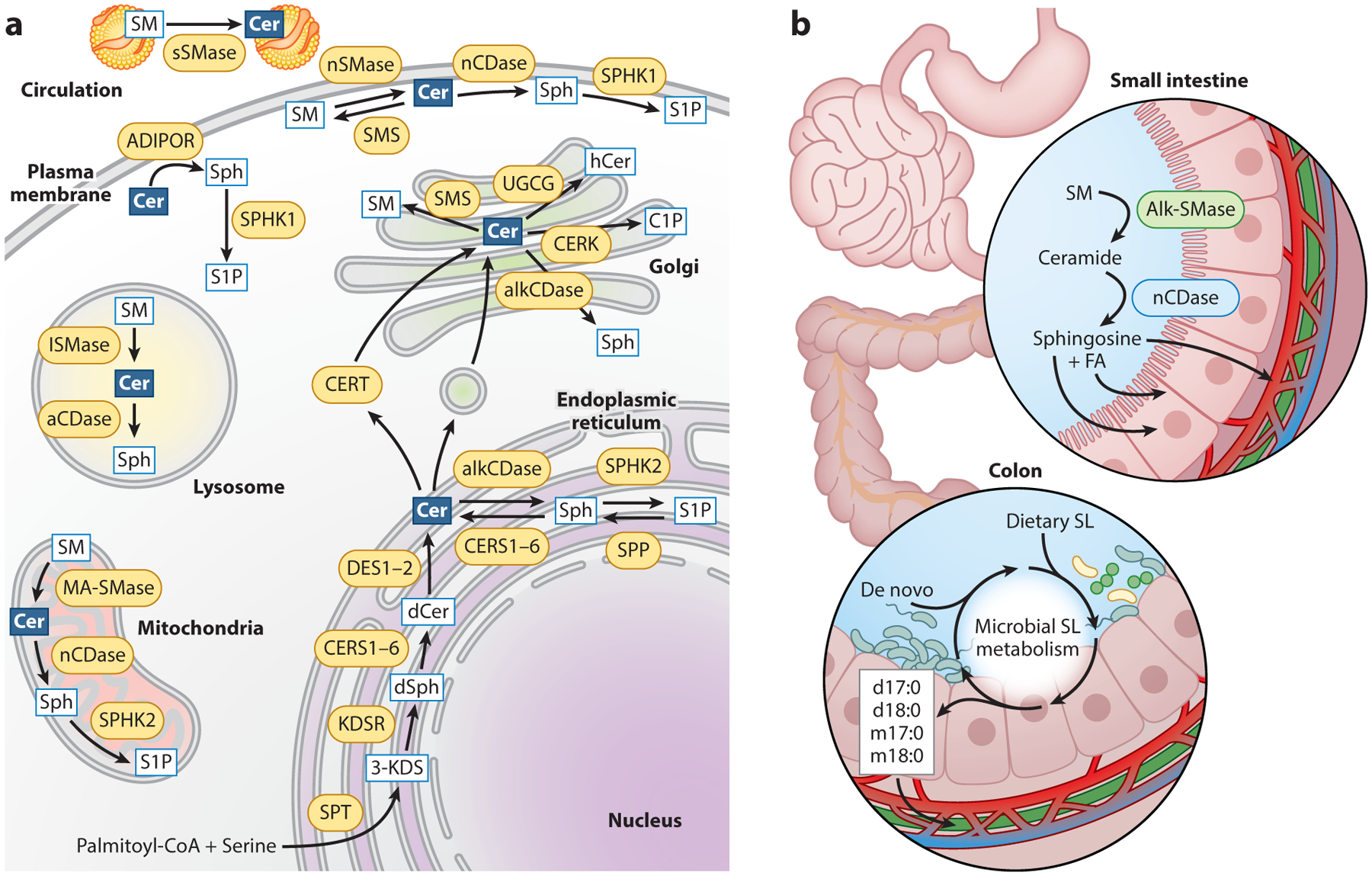
Intracellular and intestinal ceramide metabolism. (a) Sphingolipids are metabolized in various subcellular compartments. Ceramides are produced via a de novo biosynthetic pathway in the endoplasmic reticulum (ER) and are transported to the Golgi apparatus for synthesis of complex sphingolipids. Ceramide can be regenerated from SM hydrolysis in the mitochondria, lysosome, plasma membrane, and circulating lipoproteins. Deacylation of ceramides generates sphingosine, which can be phosphorylated to form S1P. (b) Dietary SM and ceramide are digested in the small intestine by alk-SMase and nCDase, respectively, to form sphingosine, which is absorbed by enterocytes. In the large intestine, dietary and de novo sphingolipids are metabolized by gut microbiota to generate unique odd-chain and deoxysphingolipid species. Abbreviations: 3-KDS, 3-ketosphinganine; aCDase, acid ceramidase; ADIPOR, adiponectin receptors; alkCDase, alkaline ceramidase; alk-SMase, alkaline sphingomyelinase; C1P, ceramide-1-phosphate; cer, ceramide; CERK, ceramide kinase; CERS, (dihydro)ceramide synthase; CERT, ceramide transport protein; dCer, dihydroceramide; DES, dihydroceramide desaturase; dSph, dihydrosphingosine; FA, fatty acid; hCer, hexosylceramide; KDSR, 3-ketodihydrosphingosine reductase; lSMase, lysosomal ceramidase; MA-SMase, mitochondria-associated sphingomyelinase; nCDase, neutral ceramidase; nSMase, neutral sphingomyelinase; S1P, sphingosine-1-phosphate; SL, sphingolipid; SM, sphingomyelin; SMS, sphingomyelin synthase; sph, sphingosine; SPHK1, sphingosine kinase 1; SPHK2, sphingosine kinase 2; SPP, sphingosine-1-phosphate phosphatase; SPT, serine palmitoyltransferase; sSMase, secretory sphingomyelinase; UGCG, glucosylceramide synthase.
The next branch point adding considerable diversity to the sphingolipid pool is the third reaction catalyzed by one of six n-acyltransferases. These (dihydro)ceramide synthases (CERS1–6) produce dihydroceramides by adding a second, variable acyl chain to the sphingoid backbone. The CERS enzymes differ immensely in both tissue expression pattern and substrate specificity, producing dihydroceramides with variable acyl chain lengths spanning 14–34 carbons (87). Despite their unique gene origins, the CERS enzymes’ primary sequences retain high homology, and substrate specificity is reportedly determined by a single ER luminal loop (137). Furthermore, all mammalian CERS, excepting CERS1, contain a conserved homeobox-like domain which has been shown in Drosophila melanogaster and cultured mammalian cells to mediate transcriptional regulation of lipase genes in response to intracellular fatty acid levels (9, 127). Transcriptional feedback of CERS enzymes has been observed, wherein knockdown or knockout of a specific CERS species elicits compensatory increases in alternative CERS isoforms and alterations in the composition of the cellular sphingolipidome (99, 113, 141). Additionally, the activity of CERS enzymes and the resulting diversity of the sphingolipid variable chain lengths is significantly affected by their formation of homo- or heterodimers (82).
In the ultimate step of the de novo ceramide synthesis cascade, dihydroceramide is converted to ceramide with the addition of a single 4,5-trans-double bond by the dihydroceramide desaturases (DES1–2), encoded by DEGS1 and DEGS2. DES1 is expressed ubiquitously in tissues, whereas DES2 is localized to the skin and intestinal epithelium and contains bifunctionality as a C4-hydroxylase that produces phytoceramides (96). The double bond introduced at this step elicits a marked change in lipid bioactivity and pathological fate. Dihydroceramides are considered benign or beneficial, while ceramides are deleterious mediators of various stress responses that drive the pathogenesis underlying nutrition-linked chronic disease (12, 123, 124).
2.2. Metabolism and Catabolism of Sphingolipids
Ceramides are shuttled from the ER to the Golgi apparatus to form complex sphingolipid species through the addition of various head groups to the first position oxygen molecule (37). For example, sphingomyelin (SM) is synthesized in the luminal Golgi with the addition of a choline head-group by SM synthase (37). Glucosylceramides are formed in the cis-Golgi by glucosylceramide synthase, with further glycosylation to form lactosylceramide or monosialodihexosylganglioside occurring in the trans-Golgi (44, 74). Ceramide can be phosphorylated by ceramide kinase in the Golgi to form ceramide-1-phosphate (C1P) (38). The transfer of ceramides to distinct Golgi regions is important for the regulation of complex sphingolipid synthesis, as delivery of ceramides for SM or C1P synthesis seems to be mediated by the ATP-dependent ceramide transport protein, whereas glycosphingolipids are generated from vesicularly delivered ceramides (21, 35, 48).
In addition to de novo biosynthesis, a large portion of intracellular ceramides are generated via hydrolysis of complex sphingolipids or salvaged with reacylation of sphingoid bases (74). Several sphingomyelinase (SMase) enzymes act to cleave the phosphocholine head group from SM to yield ceramide and phosphocholine. This process is mediated by alkaline, neutral, and acid SMase enzymes and is important for the modulation of cellular stress responses and digestion of dietary sphingolipids (74, 114). Alkaline SMase is expressed predominantly in the liver and intestinal mucosa and dissociates from the plasma membrane to the intestinal lumen by the actions of pancreatic trypsin or bile salts to digest dietary SM (Figure 1b) (153). Four mammalian genes encode the neutral SMases (nSMase1–3 and MA-SMase), which share the same optimal pH but differ in subcellular location (2). The most well characterized is nSMase2, which is relevant to nutrition-related chronic diseases due to its activation in the plasma membrane by the inflammatory cytokine tumor necrosis factor-α (17). Acid SMase is posttranslationally modified and trafficked to produce lysosomal SMase, which operates at low pHs, or secretory SMase, which hydrolyzes SMs present in circulating lipoproteins (65).
Ceramides are deacylated by a family of ceramidases to form sphingosine and a free fatty acid of variable length. Similar to SMase enzymes, ceramidase nomenclature (i.e., acid, ASAH1; neutral, ASAH2; or alkaline, ACER1–3) denotes the enzymes’ pH optima. ASAH2 is predominantly expressed in the gut epithelial brush border and plays a particular role in dietary ceramide digestion, as sphingosine is the only sphingolipid known to be readily absorbed by enterocytes (Figure 1b) (16). In addition to the ceramidase enzymes discussed above, adiponectin receptors (ADIPOR1 and 2) have ligand-activated ceramidase activity, which partially accounts for the insulin sensitizing effects of adiponectin (60, 129). Sphingosine formed from ceramide catabolism can be reacylated by CERS to form ceramides and complex sphingolipids, or phosphorylated by sphingosine kinases (SPHK1 and 2) to form the potent signaling molecule sphingosine-1-phosphate (S1P) (8, 122). In the gut, S1P is often fully degraded by S1P lyase to form phosphoethanolamine and a fatty aldehyde hexadecenal (144). Breakdown of glycosphingolipids by various glycosidases is important for dietary digestion and absorption of these lipids but is thought to occur less frequently extraintestinally and does not contribute to a significant fraction of ceramide regeneration (75, 131).
2.3. Dietary Sphingolipids
Sphingolipid content within foods is highly variable but is higher in dairy, eggs, fish, and soy products (146, 149). Consumption of dietary sphingolipids is not considered to contribute significantly to caloric intake (0.01–0.02% of intake by weight); however, the typical Western diet is estimated to provide approximately 0.3–0.4 grams of sphingolipid daily (146). While mammalian sources of dietary sphingolipid provide a broad spectrum of complex sphingolipids (e.g., SM, cerebro-sides, gangliosides, sulfatides) with SM as the predominant species, plant sources largely consist of a range of mono- and oligohexosylceramides with noncanonical sphingoid bases (i.e., d17:1, d18:1Δ8, and d18:2Δ4,8, reviewed in detail in 145, 146, 149). Thus, diets which differ in plant and animal product ingestion are likely reflected in relative abundance and diversity of dietary sphingolipid intake (159).
Dietary sphingolipids are differentially digested and absorbed according to class and composition of the sphingoid base and accessory acyl chain. As such, phosphosphingolipids, sphingosine, dihydrosphingosine, and SM are absorbed more readily than glycosphingolipids; sphingolipids with 16-carbon accessory chains are more readily taken up than very-long-chain species; and mammalian d18:1 sphingoid bases desaturated at the delta-4 position are selectively absorbed over alternative plant and fungal sources (33, 156). Dietary sphingolipids are not considered essential nutrients, and the extent to which digested sphingolipids are fully degraded and reassembled within enterocytes to contribute to the circulating and tissue lipidome is poorly understood (91); however, human and rodent studies have demonstrated that dietary sphingolipids effectively impair digestion and absorption of other dietary lipids (e.g., cholesterol, glycerolipids, free fatty acids) and may paradoxically confer some protection from systemic inflammation, insulin resistance, atherosclerosis, liver steatosis, and intestinal cancer (extensively reviewed in 103, 149, 156).
2.4. Gut Microbial Sphingolipids
Dietary sphingolipids are incompletely digested and absorbed in the small intestine and travel to the colon, where they can be absorbed and assimilated into the microbial lipidome (84). Additionally, the rate limiting enzyme of de novo ceramide synthesis, SPT, is conserved in some bacterial species (157). Interestingly, whereas mammalian systems predominantly produce sphingoid bases with even-numbered acyl chains, microbial sphingolipids often incorporate odd-chain backbones (128). Rodent tracing studies, as well as observations of odd-chain base sphingolipid species in human circulation, suggest that bacterially derived sphingolipids can enter host circulation to influence metabolism (34, 70). Brown and colleagues (6) have reported a feedback mechanism for host and microbe sphingolipid homeostasis, in which sphingolipids produced by the abundant Bacteroides species are essential to subdue the accumulation of host-derived ceramides and resulting intestinal inflammation and disease. Gonzalez and colleagues (39) have produced a dossier of work implicating a farnesoid X receptor (FXR) gut-liver-ceramide axis, in which the microbial degradation of bile acids stimulates FXR activity and upregulates biosynthesis of ceramide in the enterocyte. Consequently, decreases in portal vein and systemic ceramides in mice lacking intestinal FXR downregulated hepatic lipogenesis and glucogenesis and promoted liver lipid oxidation and adipocyte browning to confer protection from obesity-related metabolic disease (29, 67, 155). Conversely, Kayser and colleagues (72) have recently reported that circulating sphingolipid levels in overweight and obese humans are inversely correlated with gut microbial diversity and bacterial expression of genes involved in bile acid degradation. Instead, resolution of gut dysbiosis and circulating ceramides with diet-induced weight loss was attributed to increased colonization of anti-inflammatory Bifidobacterium species and suppression of lipopolysaccharide biosynthesis. Thus, a knowledge gap exists regarding ways in which dietary patterns and nutrition-linked diseases alter gut microbiota and the intestinal sphingolipidome to influence whole-body metabolic health and disease.
3. CERAMIDES AS MARKERS OF DISEASE
Clinical care precision and effective resource partitioning through personalized medicine are aided via diagnostic, risk, prognostic, and predictive biomarkers (101). Ceramides are an ideal biomarker due to their sensitivity and specificity in association with discrete clinical outcomes and reliably detected presence in noninvasive body fluids, including serum, plasma, and urine. Moreover, ceramides are conditionally independent from already established lipid markers, triglycerides and cholesterol (109). While ceramides are proposed biomarkers for myriad metabolic conditions, they are most extensively studied in relation to cardiovascular disease (CVD) (Table 1). In fact, a ceramide score, CERT1, has been implemented clinically in both private and public practice in Finland as well as at the Cleveland and Mayo Clinics in the United States.
Table 1.
Ceramides as biomarkers of nutrition-linked chronic disease
| Participants/region | Study design | Disease outcome | Measurement | Sphingolipid associations |
|---|---|---|---|---|
| FINRISK 2002 (n = 8,101 adults), Finland (51) | Prospective cohort (13 years follow-up) | Incident MACE, recurrent MACE, fatal incident MACE | 4 ceramides (in serum), targeted LC-MS/MS | Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/24:1) were positively associated with incident MACE, fatal incident MACE, and recurrent MACE. |
| Corogene (n = 160), Finland; Special Program University Medicine-Inflammation in Acute Coronary Syndrome (SPUM-ACS) (n = 1,637), Switzerland; Bergen Coronary Angiography Cohort (BECAC) (n = 1,587), Norway (78) | Prospective cohort studies | Cardiovascular death | 4 ceramides (in serum), targeted LC-MS/MS | Cer(d18:1/16:0), Cer(d18:1/24:1) were positively associated with cardiovascular death in Corogene and BECAC; Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/24:1) were positively associated with cardiovascular death in SPUM-ACS; Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/24:1) relative to Cer(d18:1/24:0) were significantly associated with cardiovascular death in all 3 cohorts. |
| Utah CAD Study (n = 674), United States (109) | Case control study | CAD prevalence | 6 ceramides, 6 dihydroceramides, 6 glucosylceramides, 6 dihydro-sphingomyelins, 6 sphingomyelins, sphinganine, sphingosine (in serum), targeted LC-MS/MS | All ceramides were significantly positively associated with CAD; study generated the sphingolipid-inclusive CAD risk score. |
| Ludwigshafen Risk and Cardiovascular Health (LURIC) study, patients with CAD (n = 445); separate randomized parallel arm treated with simvastatin (n = 24) or ezetimibe (n = 24) or both (n = 24); participants with LOF (R46L) mutation in PCSK9 gene (n = 19), Germany (136) | Case control derived from prospective cohort study with parallel randomized control study arm | CAD death; ceramide concentration in response to lipid lowering medication | 8 ceramides (in plasma), UPLC-MS | Simvastatin decreased ceramides by 25% but ezetimibe had no effect on ceramide concentrations; patients harboring the R46L PCSK9 mutation had decreased ceramide concentrations. |
| Study including all patients admitted to the Department of Emergency of the First Affiliated Hospital of Xinxiang Medical University with first-ever ischemic stroke (n = 404), China (43) | Case control study | Acute ischemic stroke | 3 ceramides (in plasma), targeted LC-MS/MS | Plasma ceramides are significantly increased in patients with ischemic stroke compared with age- and sex-matched controls; increased ceramides are associated with moderate to high risk of stroke. |
| Western Norway Coronary Angiography Cohort (WECAC) (n = 3,789), Norway; Long-Term Intervention with Pravastatin in Ischemic Disease trial (LIPID) (n = 5,991); Langzeiterfolge der KARdiOLogischen Anschlussheilbehandlung (KAROLA) (n = 1,023), Germany (54) |
Prospective cohort studies | Cardiovascular death | 4 ceramides, 3 phospholipids, targeted LC-MS/MS | Study used ceramide and phospholipid data to develop the CERT2 score, which is highly significant in predicting cardiovascular death in all 3 cohorts. |
| Cardiovascular Health Study (n = 4,249), United States (85) |
Prospective cohort study | Incident heart failure | 4 ceramides, 4 sphingomyelins (in plasma), targeted LC-MS/MS |
Cer(d18:1/16:0) was significantly associated with incident heart failure. |
| Stabilization of Atherosclerotic Plaque by Inhibition of Darapladib Therapy (STABILTY) (n = 11,222), multiple geographical locations (57) | Case control study | MACE, MI, stroke, cardiovascular death, hospitalization due to heart failure | 3 ceramides, 3 phospholipids, targeted LC-MS/MS | CERT2 score and its components were significantly associated with MACE, MI, stroke, cardiovascular death, and hospitalization due to heart failure. |
| Framingham Heart Study (n = 2,642), United States; Study of Health in Pomerania (SHIP) (n = 3,134), Germany (108) |
Prospective cohort studies | Coronary heart disease and mortality | 3 ceramides, LC-MS | Cer(d19:1/24:0)/Cer(d18:1/16:0) ratio was inversely associated with cardiovascular disease risk; Cer(d19:1/24:0)/Cer(d18:1/16:0) and Cer(d181/22:0)/Cer(d18:1/16:0) ratios were inversely associated with all-cause mortality. |
| Cohorte LausannoiseCohort (CoLaus) (n = 150), Switzerland; Devenir des Spondyloarthrites Indifferenciees Recentes (DESIR) cohort (n = 160), France (152) | Longitudinal cohort studies | Prevalent and incidence diabetes | 5 dihydroceramides, 9 ceramides, 7 globotriaosyl ceramides, 9 glucosyl/galactosyl ceramides, 7 lactosyl ceramides, targeted LC-MS/MS | Cer(d18:0/16:0), Cer(d18:1/22:0), Cer(d18:0/23:0), Cer(d18:0/24:0), Cer(d18:0/24:1), Cer(d18:0/23:0), Cer(d18:1/18:0), and Cer(d18:1/22:0) were elevated 5 years before diabetes diagnosis; Cer(d18:0/24:0), Cer(d18:0/24:1), Cer(d18:0/22:0), and Cer(D18:0/23:0) were elevated 9 years before diabetes diagnosis. |
| European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis (ATHEROREMO) (n = 581), Europe (3) | Prospective cohort study | Incident MACE in patients with CAD | 5 cholesterol esters, 4 ceramides (in serum), targeted LC-MS/MS | Cer(d18:1/16:0) was associated with MACE; Cer(d18:1/16:0), Cer(d18:1/20:0), and Cer(d18:1/24:1) relative to Cer(d18:1/24:0) were associated with death and nonfatal acute coronary syndrome. |
| Cardiovascular Health Study (n = 3,645), United States (32) | Prospective cohort study | Incident diabetes | 5 ceramides, 6 sphingomyelins, 3 hexosyl-ceramides (in plasma), targeted LC-MS/MS | Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/20), and Cer(d18:1/22:0) were associated with higher risk of diabetes. |
| Strong Heart Study (n = 435), Strong Heart Family Study (n = 1,902), United States (American Indian population) (31) | Nested case control stud, parallel prospective cohort | Incident diabetes | 6 ceramides, 6 sphingomyelin species, 3 glucosyl ceramides, 1 lactosyl ceramide (in plasma), sequential LC-MS (in plasma) | Cer(d18:1/18:0), Cer(d18:1/20:0), and Cer(d18:1/22:0) were associated with a higher risk of diabetes. |
| Strong Heart Family Study (n = 2,086), United States (American Indian population) (86) | Prospective cohort study | Baseline and follow-up HOMA-IR and insulin; associations with BMI | 5 ceramides, 6 sphingomyelins, 3 glucosyl ceramides, 1 lactosyl ceramide, targeted reverse phase chromatography | Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/20:0), Cer(d18:1/22:0) were associated with higher plasma insulin and HOMA-IR at baseline; SM(d18:1/18:0), SM(d18:1.20:0), SM(d18:1//22:0), and SM (d18:1/24:0) were inversely associated with insulin and HOMA-IR in individuals with a normal BMI. |
| FINRISK 2002 (n = 8,045 adults), Finland; Western Norway Coronary Angiography Cohort (WECAC) (n = 3,344), Norway; Prevent Metabolic Syndrome Cohort (PrevMetSyn) (n = 371) (55) | Prospective cohort studies, interventional trial | Incident diabetes | 4 ceramides, targeted LC-MS/MS | Cer(d18:1/18:0)/Cer(d18:1/16:0) ceramide ratio was predictive of incident diabetes; ceramide ratio decreased in individuals with 5% or more weight loss. |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; Cer, ceramide; CERT2, Cardiac Event Risk Test 2; HOMA-IR, homeostatic model assessment of insulin resistance; LC-MS, liquid chromatography tandem mass spectrometry; LOF, loss of function; MACE, major adverse cardiovascular event; MI, myocardial infarctions; MS, mass spectrometry; SM, sphingomyelin; UPLC-MS, ultra performance liquid chromatography tandem mass spectrometry.
3.1. Individual Ceramides and Cardiovascular Disease
Associations between CVD and individual ceramides have been shown in numerous case-control and cohort studies, as well as clinical trials (3, 51, 78, 108, 136). Ceramide species Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/20:0), and Cer(d18:1/24:1) and their ratios to Cer(d18:1/24:0) have demonstrated predictive power for myocardial infarctions (MI) and cardiovascular death (51). Additionally, these same ceramide species and ratios predicted MI and cardiovascular death in patients with stable coronary heart disease or in secondary prevention following an MI (78). Interestingly, the associations between ceramides and CVD are stronger for recurrent events and fatal outcomes (3, 51, 78).
Far less is known regarding distinct ceramide species in association with heart failure and stroke. Lemaitre et al. (85) report associations between plasma ceramides in 1,179 cases of incident heart failure in the Cardiovascular Health Study. Specifically, Cer(d18:1/16:0) demonstrated a positive association, while Cer(d18:1/22:0) was inversely associated. Gui et al. (43) report increased levels of plasma Cer(d18:1/16:0), Cer(d18:1/22:0), and Cer(d18:1/24:0) in a matched case control study including 202 patients with acute ischemic stroke and 202 age- and sex-matched controls. Of note, ceramides were significantly higher in patients with moderate-to-high clinical severity (n = 99) than patients with minor stroke (n = 103), indicating the capacity for ceramide to predict both risk and severity of stroke. Further research in the areas of heart failure and stroke in association with ceramides may yield powerful diagnostic and risk predictive tools.
3.2. Ceramide-Based Scores and Cardiovascular Disease
Three predictive algorithms comprising ceramides have been published to date: Cardiac Event Risk Test 1 (CERT1) (51, 78), Cardiac Event Risk Test 2 (CERT2) (54), and sphingolipid-inclusive coronary artery disease score (SIC) (109). CERT1 is the most long-standing and established score, and it is used clinically in Finland and parts of the Unites States. This score, which was developed by Zora Biosciences and recently licensed by Quest Diagnostics, comprises Cer(d18:1/16:0), Cer(d18:1/18:0), and Cer(d18:1/24:1) concentrations and each respective lipid’s ratio to Cer(d18:1/24:0). These six score components are broken into quartiles, with the highest quartile assigned 2 points, the second-highest quartile 1 point, and the lower two quartiles 0 points (51, 78). Hence, the score ranges from 0–12 points with discrete risk categories (0–2, low; 3–6, moderate; 7–9, increased; 10–12, high), demonstrating a positive linear relationship with CVD risk. Of note, this linear increase is not seen with conventional CVD marker low-density lipoprotein (LDL) cholesterol (56). The CERT1 score is an impressive clinical score, demonstrating a disease-severity dependent scale with promise for use in risk stratification. A revised iteration of the CERT1 score, CERT2, is composed of ceramide and phospholipid species selected in a stepwise fashion and validated in four large independent cohorts, including the Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy (STABILITY) trial, which includes participants from multiple geographical locations worldwide (54, 57). The third published ceramide-based score is SIC, which includes nonabundant sphingolipids in addition to the most prevalent ceramide species (109). SIC performs conditionally independently from and more effectively than conventional CVD markers, including LDL cholesterol and triglycerides, indicating that ceramides are robust, nonredundant, and novel biomarkers. However, SIC was generated in a case-control study and has not been validated in multiple cohorts or in a prospective study design. Ceramide-based scores are an important and rapidly translatable area of research, and they overcome the cumbersome nature of large sphingolipid panels by presenting an appealing and easily interpretable diagnostic reporting option. Of note, the improved disease prediction and risk stratification power yielded by ceramide-based scores has considerable potential to improve patient care and effective utilization of healthcare resources.
3.3. Ceramides in Association with Diabetes, Insulin Resistance, and Metabolic Syndrome
Ceramides are implicated as causal factors in insulin resistance and type 2 diabetes mellitus (T2DM) metabolism and are obligate intermediates in beta cell death (10). They are also potent prognostic markers of incident T2DM in mice and humans. Fretts et al. (32) recently identified significant associations of Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/20:0), and Cer(d18:1/22:0) with higher risk of incident diabetes in the Cardiovascular Health Study (n = 3,645), which comprises older adults with 26 years of follow-up. Accordingly, ceramides also associate with markers of glycemic control: insulin, homeostatic model assessment of insulin resistance (HOMA-IR), and homeostatic model assessment of β-cell function (HOMA-β) in the Strong Heart Study (31, 86). Interestingly, SMs are associated with body mass index in the Strong Heart Study. Moreover, the ratio of Cer(d18:1/18:0)/Cer(d18:1/16:0) was predictive of incident diabetes in the FINRISK cohort, Western Norway Coronary Angiography Study, and interventional Prevent Metabolic Syndrome Trial (combined n = 11,760) (54). Notably, while not implicated as causative agents in diabetes, dihydroceramides may be more sensitive disease biomarkers than ceramides, as they are immediately adjacent to ceramides in the de novo synthesis pathway and considerably less abundant, rendering them a more sensitive readout of alterations in sphingolipid biosynthesis. In two independent cohorts, dihydroceramide species Cer(d18:0/18:0) and Cer(d18:0/22:0) predict diabetes up to 9 years before onset more potently than their corresponding ceramide species with the same acyl chain lengths (152). However, a majority of human cohort studies evaluating ceramides in diabetes do not measure dihydroceramides, and this finding has not been widely replicated. Mechanistic studies have definitively established the causal link between ceramides and diabetes development. Further epidemiological studies, particularly prospective cohorts with diverse populations, are necessary to fully interrogate the predictive and prognostic utility of ceramide, and potentially dihydroceramide, as biomarkers in diabetes.
3.4. Ceramides and Obesity-Related Cancers
Elevated body weight and corresponding metabolic changes are associated with certain cancers, including colorectal, breast, endometrial, and liver cancers (4). The role of ceramides in cancer is paradoxical, as proapoptotic ceramides prevent tumor growth but also participate in the metabolic milieu (e.g., insulin resistance and dyslipidemia) driving onset of obesity-related cancers. An additional layer of complexity lies within differences in interpretation of ceramide concentrations in circulation and in tumor tissue. In colorectal cancer, concentrations of circulating Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/18:1), and Cer(d18:1/24:1), but not hexosyl-ceramides or SMs, were significantly associated with stage IV colorectal cancer (121). In breast cancer, higher ceramide concentrations in tumors have been associated with less aggressive breast cancer by the Ki67 index and nuclear grade; however, higher expression of de novo ceramide synthesizing genes is associated with poorer outcomes (98). In the setting of ovarian cancer, an insulin resistance–related disease, Cer(d18:1/16:0), Cer(d18:1/18:1), and Cer(d18:1/18:0) are elevated in plasma of patients with advanced ovarian cancer (76). In hepatocellular carcinoma, ceramides are decreased in tumor tissue compared with adjacent nontumor tissue, yet they are increased in circulation. Furthermore, plasma Cer(d18:1/16:0) correlates with markers of hepatocellular injury (41, 77). Moreover, ceramides are proposed as an early indicator of response to radiotherapy for hepatocellular carcinoma (28). Circulating ceramides, specifically Cer(d18:1/16:0) and Cer(d18:1/24:1), are also proposed as early (before weight loss) markers of cancer cachexia (97). Additional studies with larger sample sizes and prospective designs are merited to elucidate the relationships between tumor and circulating ceramide and cancer incidence, severity, and outcome.
3.5. Origins of Circulating Ceramides that Serve as Biomarkers of Cardiometabolic Disease
As ceramide scores become more widely reported in the literature and utilized in the clinic, it is important to determine why distinct ceramide species associate with discrete disease outcomes (91, 160). For example, why is Cer(d18:1/16:0) positively associated with cardiovascular mortality, while Cer(d18:1/24:0) is inversely associated? Multiple explanations exist, including the tissue sources of particular ceramide species and unique biological roles of specific ceramide species (113, 141). The CERS enzymes that add the variable acyl chain to the sphingoid backbone have unique tissue distributions and substrate specificities that cause particular tissues to be rich in specific acyl chains (Figure 2). For example, muscle has abundant CERS1 and therefore contains predominately Cer(d18:1/18:0) ceramides. Likewise, circulating and intramuscular Cer(d18:1/18:0) is tightly linked with insulin resistance and diabetes, which aligns with muscle ceramides and their role in blunting insulin-stimulated glucose uptake in the muscle in the insulin resistant state (54, 142, 152). The liver predominately expresses CERS2, with resulting biosynthesis of very long chain ceramides, including Cer(d18:1/24:0), Cer(d18:1/24:1), and Cer(d18:1/26:0). Likewise, Cer(d18:1/16:0) is enriched in adipose tissue, which primarily expresses CERS6. Cross-talk between organs is apparent in preclinical models, as genetic lowering of ceramide in liver or adipose attenuates sphingolipid levels in the alternate organ and improves glycemic control, liver steatosis, and adipose morphology (12, 154).
Figure 2.
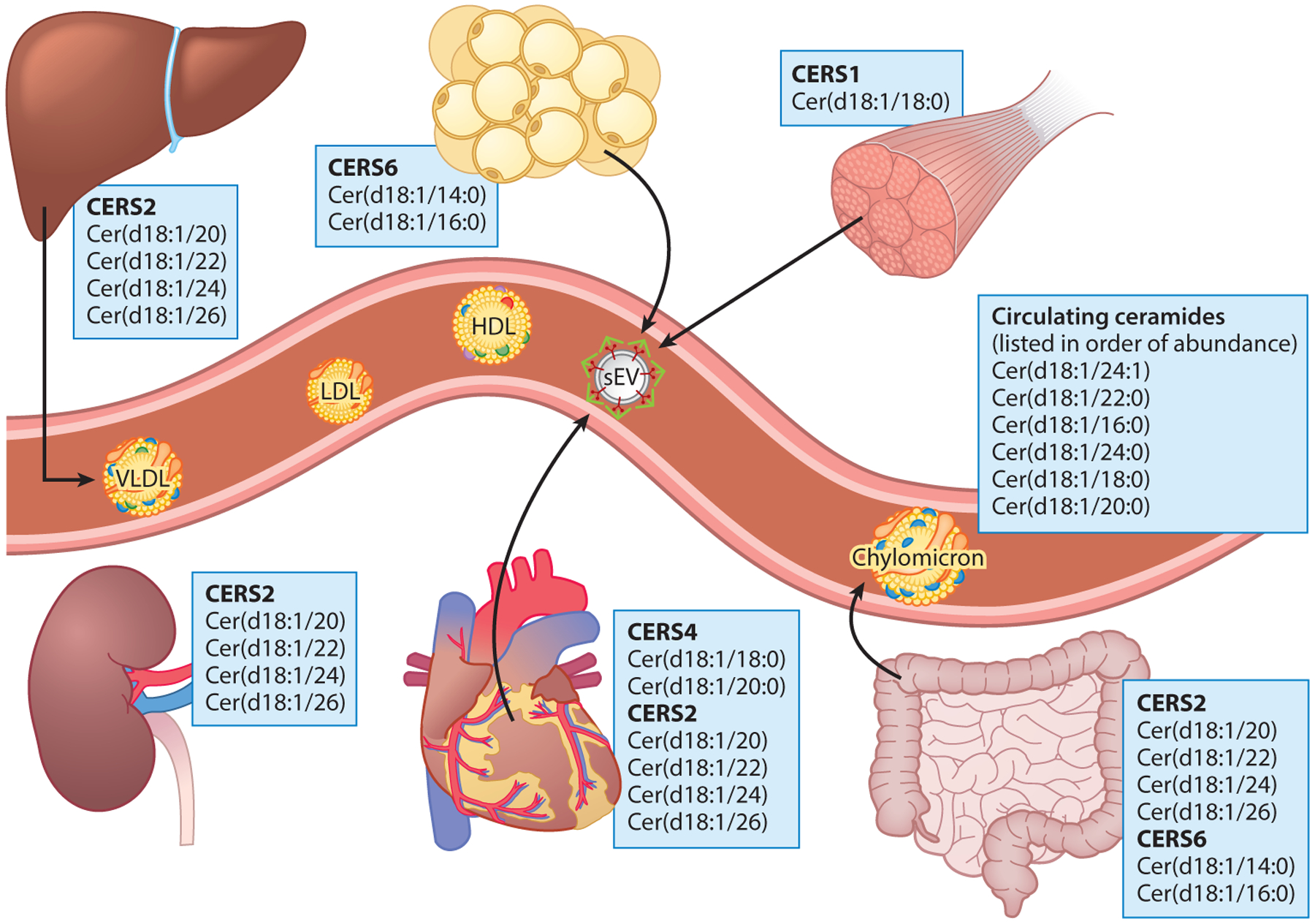
Species-level ceramide sources based on CERS enzyme expression profile and substrate specificity. Ceramide synthases, the step in the de novo synthesis pathway that adds a variable acyl chain to the sphingoid backbone, result in much of the diversity of the sphingolipid pool. Varied tissue expression and substrate selectivity result in unique tissue distributions of ceramide species. The circulating ceramide pool reflects the tissue distribution of the CERS enzymes and their substrate preferences. Abbreviations: CERS1–6, ceramide synthases 1–6; VLDL, very-low-density lipoprotein; LDL, low-density lipoprotein; HDL, high-density lipoprotein; sEV, small extracellular vesicle).
The majority of circulating sphingolipids are trafficked within lipoproteins and are present in very-low-density lipoprotein, LDL, and high-density lipoprotein (HDL) (45, 119) (Figure 2). Per particle, very-low-density lipoproteins have the highest concentration of SM, ceramide, and S1P, emphasizing the major contribution of hepatic-derived ceramides in circulation (45). Accordingly, apolipoprotein B- (ApoB)-containing lipoprotein deficiencies observed in abetalipoproteinemia or microsomal transfer protein knockout mice confer an 80–90% reduction in plasma ceramides, but not glycosphingolipids (64). Microsomal transfer protein is also essential for packaging of lipids into intestinally derived chylomicrons. Early rat feeding studies with radiolabeled SM or dihydrosphingosine indicate absorption of sphingolipid-derived fatty acids into lymph triglycerides and lecithin (102), and human trials have detected sphingolipids in intestinally derived chylomicrons (83). As such, a portion of sphingolipids shuttled within ApoB-containing lipoproteins could be supplied by enterocytes.
Due to the relative abundance of circulating lipoproteins, most SM is carried within LDL and the larger HDL subfraction HDL2 (45). Ceramide, glycosphingolipids, and dihydrosphingosine are predominantly carried within LDL, sphingosine and dihydroS1P within the smaller HDL3 subfraction, and S1P within HDL3 and bound to albumin (45, 119). While lipoprotein sphingolipid proportions may mirror their tissue of origin, sphingolipids are likely modified within circulation, although these mechanisms are incompletely characterized. SM within LDL can be metabolized to ceramide by secretory SMase (23) (Figure 1). S1P is exported via the transporter Spinster2 (100), and its delivery to and uptake from HDL may be facilitated by phospholipid transfer protein and apolipoprotein M, respectively (15, 158). Yet, little else is known regarding the delivery of sphingolipids to tissues via lipoprotein lipase, lipoprotein endocytosis, or alternative mechanisms.
An emerging field of research regarding lipid transport via small extracellular vesicles (sEVs) has provided an additional method of sphingolipid shuttling aside from lipoprotein packaging and transport. Thus far, sphingolipids have been quantified in sEVs derived from adipose tissue, skeletal muscle, heart, and the endothelium (7, 20, 143). Indeed, ceramides may themselves play a role in sEV sphingolipid shuttling via stimulation of nSMase-dependent formation and release of exosomes (126). The consequences of sphingolipid delivery to distal tissue compartments via sEVs has not been determined but may play a role in whole-body sphingolipid metabolism and cardiometabolic pathogenesis.
4. NUTRITION-RELATED INTERVENTIONS AND CIRCULATING CERAMIDES
Though ceramide-based risk scores are utilized in the clinic, evidence-based recommendations are nonexistent for patients deemed high risk to manage their hyperceramidemia (132). Exploration of lifestyle interventions, including diet, weight loss, and metabolic surgery, and their effects on circulating ceramide profiles, will contribute to the effective implementation of ceramide-based clinical algorithms and successful ceramide-associated cardiometabolic disease risk mitigation.
4.1. Dietary Patterns and Ceramides
In the past decade, a series of human studies have been conducted to probe for impacts of dietary intake on circulating sphingolipids. An emerging pattern suggests that the degree of unsaturation of dietary lipids modulates the circulating sphingolipidome, although there is little consensus regarding which specific sphingolipids are significantly altered and in which direction (26, 73, 88, 90, 115, 120, 139, 147). Perhaps the most consistent pairing of dietary intervention and lipid outcome in human studies is with palmitate supplementation, which increases long-chain (139) or total circulating ceramides (73, 115). Additionally, overfeeding studies in which fat consumption is raised to 40–60% of energy intake have reported increases in total intramuscular (19) or circulating ceramides (52, 90). Conversely, interventions that increase polyunsaturated fat intake decrease circulating ceramides (79, 115, 140) or shift the balance of accessory chains toward a higher proportion of very-long-chain ceramides (92, 120, 147). These observations suggest that ceramide levels may be induced by increasing the availability of de novo sphingolipid synthesis substrates (e.g., palmitate) or increasing the overall lipid load. Moreover, sphingolipid metabolism may be altered by the anti-inflammatory properties of certain dietary polyunsaturated fats (e.g., docosahexaenoic acid) or their direct actions on expression of ceramide biosynthesis machinery (25, 69).
Interestingly, preliminary studies have linked consumption of dairy, typically high in saturated fat and sphingolipid content, to improvements in circulating sphingolipids (14, 36, 83). Several metabolic improvements with milk SM or polar lipid mixture feeding have been observed in rodent studies, including resolution of high-fat-diet-induced dyslipidemia, gut dysbiosis, liver steatosis, and adipose inflammation (104). Le Barz et al. (83) examined the effects of milk polar lipid feeding in postmenopausal women and ileostomy patients. Despite the 4-week dietary supplementation with milk-derived SM and ceramide, serum Cer(d18:1/16:0), Cer(d18:1/18:0), and Cer(d18:1/24:1) paradoxically decreased. Furthermore, ceramide and SM content of intestinally derived chylomicrons decreased, whereas their concentrations in ileal efflux and fecal material increased. Together, these data suggest that dietary composition may significantly impact sphingolipid absorption and/or enterocyte and microbial sphingolipid metabolism to modulate circulating ceramides. Furthermore, reports of neutral or inverse associations of dairy intake with CVD and mortality risk may be partially explained by favorable alterations in sphingolipid homeostasis (138).
At present, we lack experimental evidence and scientific consensus on dietary approaches to modulate ceramide levels and associated disease risk. Future studies must address important questions regarding the mechanisms by which diet affects ceramide synthesis or gut metabolism, which nutrients most significantly impact sphingolipid metabolism, and how these interactions perform within larger dietary patterns. Current studies are limited by differences in lipid measurement and target resolution. Although the majority of human diet studies cited herein utilized targeted platforms for sphingolipidomics, many quantified only a select few ceramide species to compare between diet groups (Table 2). Future investigations may benefit from inclusion of a wider range of sphingolipid species. Additionally, large studies have typically been observational, and most reports, including interventional trials, rely heavily on self-reported intake data. Lastly, studies conducted thus far primarily represent predominantly white populations from the US and northern Europe, which limits translatability to the black and indigenous populations that experience the highest rates of nutrition-linked chronic disease. Further research is required to distinguish signatures of diverse cultural, racial, and socioeconomic dietary patterns in the context of circulating ceramides and disease risk.
Table 2.
Circulating ceramides with dietary pattern interventions
| Participants/region | Study design | Experimental design | Measurement/class | Sphingolipid outcomes |
|---|---|---|---|---|
| 980 participants from the PREDIMED Trial, 25% with incident cases of CVD, Spain (148) | Case-cohort | 1 year of Mediterranean diet supplemented with EVOO or nuts, with low-fat diet control | Untargeted LC-MS/MS metabolomics; plasma Cer(d18:1/16:0), Cer(d18:1/22:0), Cer(d18:1/24:0), and Cer(d18:1/24:1) | No change was detected in plasma ceramides. |
| 5,124 adults from the Framingham Offspring Study, United States (147) | Cross-sectional | Dietary pattern scores (DGAI, MDS) assessed from Harvard FFQ | Targeted LC-MS/MS; plasma Cer(d18:1/16:0), Cer(d18:1/22:0), and Cer(d18:1/24:0) | DGAI score was associated with lower Cer(d18:1/16:0), Cer(d18:1/22:0), Cer(d18:1/24:0), and Cer(d18:1/22:0)/Cer(d18:1/16:0) ratio; SFA intake was associated with Cer(d18:1/16:0); fat and sugar intake were inversely associated with Cer(d18:1/22:0)/Cer(d18:1/16:0) ratio; MDS was associated with lower Cer(d18:1/16:0) and Cer(d18:1/22:0) and higher Cer(d18:1/24:0) and Cer(d18:1/24:0)/Cer(d18:1/16:0) ratio; Cer(d18:1/24:0) was associated with nut intake and MUFA/SFA intake ratio and negatively associated with fruit intake; and Cer(d18:1/24:0)/Cer(d18:1/16:0) ratio was associated with nut intake, MUFA/SFA intake ratio, and vegetable intake. |
| 106 adults with metabolic syndrome, Finland (81) | Randomized controlled trial | 12-week parallel study of healthy diet with whole grain products, fatty fish (3 servings/week) and bilberries (300 g/day); whole grain enriched diet with whole grain products; or control diet with refined grain products | Targeted UPLC-MS; plasma ceramides | No change was present in plasma ceramides. |
| 200 adults with metabolic syndrome from the Systems Biology in Controlled Dietary Interventions and Cohort studies (SYSDIET) study, Finland, Denmark, Sweden, Iceland (80) | Randomized controlled trial | 18–24 week parallel study of healthy Nordic diet (whole grains, fruits, vegetables, berries, vegetable oils and margarines, fish, low-fat dairy, low-fat meat) and average Nordic diet control | Targeted UPLC-MS; plasma ceramides | Cer(d18:1/22:0), Cer(d18:1/23:0), and Cer(d18:1/24:0) decreased after 8 weeks in the Healthy Nordic diet group; differences normalized by 18 and 24 weeks. |
| 96 middle-aged adults, United States (26) | Cross-sectional | HEI-2015 assessed from 24-hour dietary recall | Targeted LC-MS; plasma Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/22:0), and Cer(d18:1/24:0) | Higher HEI-2015 score and total vegetable and whole grain intake were inversely associated with Cer(d18:1/22:0); saturated fat and sugar intake were positively associated with Cer(d18:1/22:0). |
| 36 overweight young adults, United States (93) | Non-randomized, repeated measures | 8-week high-fruit, high-vegetable diet (FRUVED) according to USDA MyPlate, alone or with low-refined-carbohydrate (FRUVED+LRC) or low-fat (FRUVED+LF) guidelines | Targeted LC-MS/MS; serum ceramides, glucosylceramides, and lactosylceramides | Total ceramide, Cer(d18:1/22:0), Cer(d18:1/24:1), and Cer(d18:1/26:0) were significantly lower at 5 weeks in FRUVED+LRC and FRUVED+LF groups compared with baseline. GluCer(d18:1/22:0), GluCer(d18:1/24:0), GluCer(d18:1/24:1), LacCer(d18:1/22:0), and LacCer(d18:1/24:0) levels decreased with time in FRUVED+LRC and FRUVED+LF groups. Cer(d18:1/16:0) was higher in all study groups at 8 weeks compared with baseline. |
| 31 CAD patients previously treated with PCI, Sweden (24) | Cross-over randomized controlled trial | 4-week lacto-ovo-vegetarian diet (VD) or isocaloric diet with daily meat consumption (MD) separated by 4-week washout period | Targeted UPLC-MS/MS; plasma ceramides, hexosylceramides, lactosylceramides, and sphingomyelins | Cer(d18:1/16:0) and a WGCNA lipid cluster dominated by SM and ceramide species were lower in VD than MD. Cer(d18:1/16:0) and Cer(d18:1/22:0), HexCer(d18:1/22:0), and LacCer(d18:1/16:0) decreased in VD from baseline. |
| 5,124 adults from the Framingham Offspring Study, United States (147) | Cross-sectional | SSB intake assessed from Harvard FFQ | Targeted LC-MS/MS; plasma Cer(d18:1/16:0), Cer(d18:1/22:0), and Cer(d18:1/24:0) |
SSB intake was positively associated with Cer(d18:1/16:0) and Cer(d18:1/22:0). SSB intake was positively associated with Cer(d18:1/24:0) only in prediabetic or diabetic individuals. |
| 30 adolescent and overweight male habitual SSB consumers, United States (14) | Cross-over randomized controlled trial | 3 weeks of daily consumption of 24 oz soda or 22 oz 2% milk separated by a 2-week washout period | Targeted UPLC-MS/MS; plasma sphingoid bases, ceramides, hexosylceramides, and spingomyelins | GluCer(d18:1/16:0), LacCer(d18:1/16:0), and LacCer(d18:1/18:0) significantly decreased during the milk consumption period. |
| 1,099 participants in the Nurses’ Health Study (NHS), NHS II, and Health Professionals Follow-up Study, United States (92) | Cross-sectional | Frequency of nut intake assessed from FFQs | Untargeted LC-MS; plasma sphingolipids | Nut consumption was positively correlated with Cer(d18:1/24:0), SM(d18:1/22:0), and SM(d18:1/24:0) and negatively associated with SM(d18:1/18:0). |
| 10 obese adults, United States (140) | Cross-over, double-blinded, randomized controlled trial | 5-day inpatient study with daily ingestion of 48 g walnuts or placebo with a 1-month washout period | Untargeted LC-MS; plasma sphingolipids | Walnut feeding was inversely associated with total ceramide, SM, and mono- and di-hexosylceramide. |
| 20 healthy adults, United States (139) | Cross-over, double-blinded, randomized controlled trial | 2 weeks of daily consumption of chocolate spread enriched with EVOO or palm oil | Targeted LC-MS/MS; 25 plasma sphingolipids | Cer(d18:1/16:0), Cer(d18:1/16:0)/Cer(d18:1/22:0)+Cer(d18:1/24:0) ratio, and SM(d18:1/18:0) were higher with palm oil feeding than EVOO. |
| 61 overweight adults, Sweden (115) | Double-blinded, randomized controlled trial | 8-week parallel feeding study of overconsumption with muffins enriched in sunflower oil (PUFA) or palm oil (SFA) followed by a 4-week caloric restriction period | Targeted UPLC-MS; serum and adipose dihydroceramides, ceramides, hexosylceramides, sphingoid bases, and sphingomyelin | SFA overfeeding led to an increase in the majority of serum sphingolipids, whereas PUFA overfeeding was associated with a decrease in all serum sphingolipids excepting Cer(d18:1/24:0) and Cer(d18:0/24:0). Serum sphingolipids normalized following caloric restriction period. Differences in adipose sphingolipids were not specified. |
| 18 healthy adults, United States (73) | Cross-over, randomized controlled trial | 3-week high-palmitate (HPA) or low-PA, high-oleic acid (HOA) diet separated by a 1-week washout period | Targeted LC-MS/MS; serum ceramides | Serum ceramides were significantly higher with HPA versus HOA feeding. |
| 2,860 Chinese Singaporeans from the Singapore Prospective Study Program, Singapore (120) | Cross-sectional | Dietary fat and protein intake estimated from FFQs | Targeted UPLC-MS; plasma ceramides, hexosylceramides, sphingoid bases, and sphingomyelins | SFA intake was associated with total SM; long chain SM; and ceramides, hexosylceramides, SM, and phosphorylated sphingoid bases with a 16:1 backbone. PUFA intake was negatively associated with Cer(d18:1/16:0), HexCer(d18:1/16:0), Cer(d18:1/18:0), and HexCer(d18:1/18:0). No associations were present between MUFA intake and plasma sphingolipids. Protein intake was negatively associated with all sphingolipid classes and individual species with 18:1 and 18:2 backbones, excepting Cer(d18:1/16:0) and Cer(d18:1/18:0) ceramides; protein intake was positively associated with 16:1 backbone SMs. |
| 33 subjects with recent acute myocardial infarction or unstable ischemic attack, Finland (79) | Randomized controlled trial | 8-week parallel study of fatty fish, lean fish, or control diet | Targeted UPLC-MS; plasma sphingolipids | Total ceramides decreased from the baseline in the fatty fish group. |
| 33 healthy adults, Norway (105) | Double-blinded, randomized controlled trial | 7-week parallel study of supplementation with 8 g/day fish oil or sunflower oil | Targeted UPLC-QTOFMS; plasma ceramides and sphingomyelins | Very-long-chain SM species significantly increased in the fish oil group. No change was reported in ceramides. |
| 58 postmenopausal women in the Milk Polar Lipids Consumption, Lipid Metabolism, and Inflammation in Menopausal Women cohort (VALOBAB-C) and 4 ileostomy patients (VALOBAB-D), France (83) | Double-blinded, randomized controlled trial | VALOBAB-C: 4-week parallel study of daily supplementation with milk polar lipid (PL)-enriched cream cheese (0, 3, or 5 g PL); VALOBAB-D: Cross-over study with acute milk PL-enriched cream cheese intake (0, 3, or 5 g PL) separated by 4–6 week washout periods |
Targeted HPLC-MS/MS; serum, chylomicron, ileal efflux, and fecal ceramides and SM | Milk PL intake decreased serum SM(d18:1/16:1), SM(d18:1/18:1), SM(d18:1/20:1), and Cer(d18:1/24:1); serum Cer(d18:1/20:0), SM(d18:1/20:0), and SM(d18:1/22:1) increased with PL feeding. Milk PL intake decreased intestinally derived chylomicron ceramide and SM content. Milk PL intake increased ceramide and SM levels in ileal efflux and feces. |
| 105 healthy adults from the DESIR cohort, France (36) | Observational | Cheese and noncheese dairy consumption was assessed with FFQs | Shotgun lipidomics; plasma dihydroceramides and ceramides | Noncheese dairy intake was inversely correlated with total dihydroceramide, Cer(d18:0/16:0), Cer(d18:0/22:0), Cer(d18:0/23:0), Cer(d18:0/24:0), Cer(d18:0/24:1), Cer(d18:1/26:0), and Cer(d18:1/26:1) levels in women. |
| 16 healthy adult men, Australia (94) | Cross-over, randomized controlled trial | Breakfast meals consisting of dairy fat or soy oil were consumed 4–6 weeks apart | Targeted ESI-MS/MS; plasma dihydroceramide, ceramide, hexosylceramide, and sphingomyelin | Total dihydroceramide and sphingomyelin levels increased postprandially 4 h following the dairy fat meal. Total dihydroceramide, ceramide, GM3, and sphingomyelin decreased postprandially 1 h following the soy oil meal. |
| 29 adult men, United States (19) | Prospective cohort | 8-week period of overfeeding: 140% of kcal to maintain body weight, 44% of kcal from fat | Targeted LC-MS/MS; muscle ceramides | Muscle Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/18:1), Cer(d18:1/20:0), Cer(d18:1/22:0), Cer(d18:1/24:0), Cer(d18:1/24:1), and total ceramides increased post-overfeeding compared with baseline. |
| 40 healthy adults, Australia (52) | Prospective cohort | 4-week period of overfeeding: 1,250 kcal above baseline, 45% kcal from fat | Targeted HPLC-MS; serum sphingosine, dihydroceramides, ceramides, hexosylceramides, GM3, and sphingomyelins | Total ceramides increased with overfeeding. Cer(d18:1/22:0), Cer(d18:0/22:0), HexCer(d18:1/22:0), GM3(d18:1/22:0), Cer(d18:1/24:0), Cer(d18:0/24:0), HexCer(d18:1/24:0), and GM3(d18:1/24:0) increased with overfeeding. Cer(d18:1/18:0), Cer(d18:0/18:0), GM3(18:1/18:0), Cer(d18:1/24:1), HexCer(d18:1/24:1), and GM3(d18:1/24:1) decreased with overfeeding. |
| 38 overweight adults, Finland (90) | Randomized controlled trial | 3-week parallel study of 1,000 kcal/day overfeeding with saturated fat (SAT), unsaturated fat (UNSAT), or simple sugars (CARB) | UPLC-QTOFMS; plasma ceramides, and dihydroceramides | Total ceramide, total dihydroceramide, Cer(d18:1/24:0), Cer(d18:1/24:1), Cer(d18:0/24:0), and Cer(d18:2/23:0) increased in the SAT overfeeding group. No changes were reported in sphingolipids in the UNSAT or CARB groups. |
| 50 adults with active rheumatoid arthritis, Sweden (88) | Cross-over, randomized controlled trial | 10-week Mediterranean-style diet intervention or Western-style diet control separated by a 2–5 month washout | Targeted LC-MS/MS; serum ceramides, lactosylceramide, glucosyl/galactosylceramide, globoceramide, and sphingomyelin | CERT2 risk score and total serum ceramides increased during control diet feeding period but did not decrease after Mediterranean diet intervention. |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; Cer, ceramide; CERT2, Cardiac Event Risk Test 2; CVD, cardiovascular disease; DGAI, Dietary Guidelines for American Adherence Index; EVOO, extra-virgin olive oil; ESI-MS, electrospray ionization mass spectrometry; FFQ, food frequency questionnaire; GluCer, glucosylceramide; GM3, monosialodihexosylganglioside; HEI-2015, healthy eating index-2015; HexCer, hexosylceramide; LacCer, lactosylceramide; LC-MS, liquid chromatography tandem mass spectrometry; MDS, Mediterranean Diet Score; MUFA, mono-unsaturated fatty acid; PA, palmitic acid; PCI, percutaneous coronary intervention; PUFA, poly-unsaturated fatty acid; SFA, saturated fatty acid; SM, sphingomyelin; SSB, sugar sweetened beverages; UPLC-MS, ultra-performance liquid chromatography tandem mass spectrometry; UPLC-QTOFMS, ultra-performance liquid chromatography quadrupole time of flight mass spectrometry; USDA, United States Department of Agriculture; WGCNA, weighted correlation network analysis.
4.2. Ceramides with Weight Loss or Metabolic Surgery
Circulating ceramides are elevated in obese individuals with T2DM or NAFLD (50, 66, 89, 151), and moderate weight loss of 3–5% is a currently recommended therapy to reduce cardiometabolic comorbidities of obesity (116). Yet, it remains unknown whether metabolic improvements observed with clinically significant weight loss are associated with reductions in ceramide levels. Several studies have investigated the impact of weight loss on tissue and circulating ceramides. Kayser et al. (72) reported significant decreases in serum dihydroceramides, d18:1 ceramides, d18:2 ceramides, and SM by 47%, 35%, 39%, and 26%, respectively, in nondiabetic obese adults after 6 weeks of caloric restriction. Promrat and colleagues (112) also reported significant decreases in serum ceramides of obese patients with steatohepatitis after a 1-year diet, exercise, and behavioral weight loss intervention. Alternatively, Dube et al. (27) reported that exercise more effectively decreased intramuscular ceramides than diet-induced weight loss. Translation of these findings, although promising, is limited by differences in methods for sphingolipid quantification, weight loss intervention and duration, and patient population, as well as limited sample size (combined n = 96). Considerably more evidence is required to delineate the impacts of diet and lifestyle-related weight loss interventions on levels of circulating ceramides and attenuation of cardiometabolic disease risk.
Bariatric surgery is a durable, long-term weight loss intervention that elicits metabolic improvements, including remission of diabetes, that exceed those achieved by nonsurgical weight loss. Thus far, seven small studies have investigated the effects of bariatric surgery, primarily Roux-en-Y gastric bypass, on circulating (40, 53, 62, 71, 95, 106) or intramuscular (18) sphingolipids. In these reports, bariatric surgery consistently decreased circulating sphingolipids, particularly total and very-long-chain ceramides, up to 6 months after surgery. Decreases in very-long-chain ceramides correlated with improvements in measures of glycemic control and insulin sensitivity (62, 71). Accordingly, very-long-chain ceramides positively correlated with HOMA-IR (106) and glycated hemoglobin (40). Thus, lowering circulating ceramides may contribute to the striking metabolic improvements observed with bariatric surgery and extreme weight loss.
5. MOLECULAR MECHANISMS OF CERAMIDE ACTION
Ceramide concentrations are tightly regulated, and disruptions in this organized system lead to disease pathogenesis. We hypothesize that these outcomes stem from an evolutionarily conserved, two-phase mechanism of ceramide action, with the molecule serving as a nutrient sensor that protects cells from acute intracellular elevations in detergent-like free fatty acids. The two mechanisms are delineated below as the (a) metabolic program and (b) apoptotic/fibrotic program.
5.1. Metabolic Program
Once inside a cell, free fatty acids are rapidly neutralized via esterification to form acyl-CoAs, which are metabolized according to the cellular energy status. Acyl-CoAs may be joined with glycerol to produce triglycerides as an inert energy store and other glycerolipids integral to lipid bilayer formation (134). Alternatively, acyl-CoAs can also be coupled to carnitine and shuttled into mitochondria for beta oxidation (134). A less common fate for acyl-CoAs is their entry into the sphingolipid synthesis pathway. We hypothesize that in states of free fatty acid overload, acyl-CoA flux into the sphingolipid pathway is enriched, increasing cellular sphingolipid concentrations and initiating the metabolic program. This adaptive metabolic program mitigates fatty-acid driven damage by (a) altering fuel choice (e.g., decreasing glucose and amino acid uptake), (b) promoting fatty acid esterification and storage, and (c) decreasing mitochondrial efficiency (Figure 3). When these adaptations are insufficient and ceramides increase beyond a critical threshold, they elicit an apoptotic/fibrotic program to minimize tissue and organismal damage resulting from cell lysis and the release of harmful cellular debris (Figure 3). Ceramide-mediated cellular reprogramming is protective in the short-term but maladaptive with chronic overnutrition. We hypothesize that extended activation of ceramide signaling drives many features of the metabolic syndrome and ultimately drives the pathogenesis of nutrition-related chronic disease.
Figure 3.
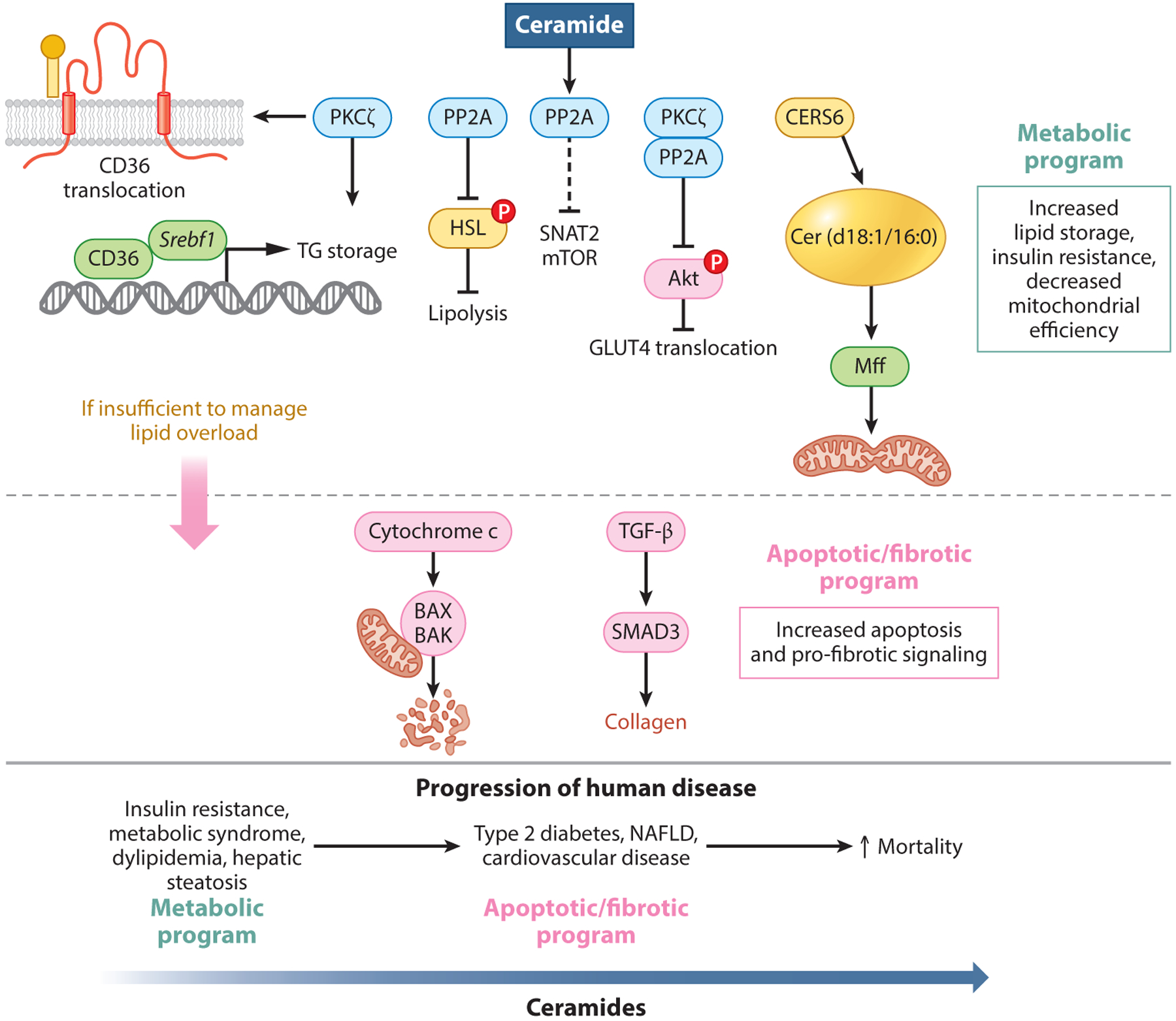
Ceramide-mediated alterations of cellular metabolism in states of fatty acid overload. In the protective metabolic program, ceramides activate PP2A, which enacts downstream mechanisms promoting fatty acid esterification and storage, inhibiting lipolysis via inhibitory phosphorylation of HSL and reducing glucose and amino acid metabolism. Ceramides also activate PKCζ, which inhibits glucose uptake by preventing phosphorylation of Akt. Additionally, CERS6 derived Cer(d18:1/16:0) species interact directly with mff, promoting mitochondrial fragmentation and reduced efficiency. If this metabolic program is unable to quench incoming fatty acids, ceramides trigger an apoptotic/fibrotic program to minimize lipid-mediated cellular damage. This program includes programmed cell death and collagen deposition. While mechanisms of ceramide are protective in the acute setting, chronic overnutrition leads to maladaptive ceramide signaling driving disease pathogenesis. Abbreviations: Akt, protein kinase B; BAK, BCL2-antagnist/killer 1; BAX, BCL2 associated X protein; CERS6, (dihydro) ceramide synthase 6; CD36, cluster of differentiation 36; cytochrome c, cytochrome complex; GLUT4, glucose transporter 4; HSL, hormone sensitive lipase; mff, mitochondrial fission factor; mTOR, mechanistic target of rapamycin; NAFLD, nonalcoholic fatty liver disease; P, phosphorylation; PKc, protein kinase C; PP2A, protein phosphatase 2A; SMAD3, mothers against decapentaplegic homolog 3; SNAT2, sodium-coupled neutral amino acid transporter 2; Srebf1, sterol regulatory element binding transcription factor 1; TG storage, triglyceride storage; TGF-β, transforming growth factor beta.
5.1.1. Decreased uptake and utilization of glucose and amino acids.
Insulin stimulates glucose uptake into muscle and adipose tissues by initiating the translocation of glucose transporter 4 (GLUT4) from the cytoplasm to the plasma membrane (135). Insulin initiates a cascade of signaling events that directly phosphorylates and activates Akt (also known as protein kinase B), which stimulates GLUT4 translocation, allowing glucose to enter the cell (133). Ceramides inhibit Akt phosphorylation downstream of insulin signaling via protein phosphatase 2A (PP2A) and protein kinase C zeta (PKCζ) (135, 161). PP2A dephosphorylates two activating residues, while PKCζ phosphorylates Akt at an inhibitory site (11, 111, 117, 163). Physiologically, this culminates in impaired insulin-stimulated glucose uptake (135). In addition to decreasing uptake and utilization of glucose, ceramides decrease the intracellular amino acid pool and diminish signaling through the mTOR (mammalian target of rapamycin) pathway by sequestering the amino acid transporter, SNAT2, away from the plasma membrane (30, 42, 63). The molecular mechanisms by which ceramides inhibit amino acid uptake are not clearly understood but are also potentially mediated by PP2A (30, 42). By downregulating amino acid transporters, ceramides essentially starve the cell of nonfatty acid energy sources and induce homeostatic autophagy to increase consumption of the overflowing fatty acids (42).
In alignment, genetic and pharmacological inhibition of de novo ceramide synthesis alleviates insulin resistance in multiple rodent models of diabetes (12, 107, 134). Treatment with myriocin, an irreversible, high-affinity inhibitor of SPT, or fenretinide, a synthetic retinoid inhibitor of DES1, prevents or reverses insulin resistance (11, 59, 134). Sptlc2 deletion in the liver, or whole-body haploinsufficiency, elicits similar glycemic improvements (107, 134). Furthermore, excising the Degs1 gene (encoding DES1) from whole body, liver, or adipose tissue of adult mice improved glucose tolerance and insulin sensitivity in animals with diet-induced obesity or leptin deficiency (12).
5.1.2. Increased fatty acid uptake, esterification, and storage.
In addition to altering glucose metabolism, ceramides also modify lipid handling. Via PKCζ activation, ceramides increase expression and promote translocation of fatty acid transporter cluster of differentiation 36 (CD36) to the cell membrane, which facilitates passage of fatty acids through lipid bilayers and promotes their esterification (11, 12, 60). Additionally, PKCζ induces sterol regulatory element binding transcription factor 1 (Srebf1) and its downstream transcriptional targets, thereby stimulating the transformation of deleterious free fatty acids into inert triglycerides for storage (11, 12). Analogously, ceramides also inhibit lipolysis through PP2A activation, which negatively regulates hormone-sensitive lipase (HSL) (12). En masse, these mechanisms lower cellular free fatty acid levels by enhancing uptake and storage while simultaneously blunting lipolysis, which ultimately protects the cell from free fatty acid overload.
Again, genetic and pharmacological inhibition of de novo ceramide synthesis in mouse models ameliorates symptoms of lipid accumulation. Inducible Degs1 ablation in mice dramatically lowers hepatic expression of Srebf1 and its transcriptional targets encoding proteins facilitating triglyceride storage (12). Additionally, Degs1 ablation inhibits fatty acid uptake into hepatocytes by decreasing activation of PKCζ (12).
5.1.3. Decreased mitochondrial efficiency.
Cardiometabolic diseases are frequently associated with impaired mitochondrial function and increased oxidative stress, which may be partially explained by ceramide actions. Ceramides decrease electron-transport chain activity, increase membrane permeability, and promote mitochondrial fission (11). Elevated Cer(d18:1/16:0), in particular, is demonstrated to inhibit electron-transport chain complex II and IV activity and increase reactive oxygen species production (162). Specifically, Cer(d18:1/16:0) derived from CERS6 promotes mitochondrial fragmentation by interacting with mitochondrial fission factor, leading to a change in mitochondrial morphology and a decrease in respiratory capacity (46). Ultimately, these actions make mitochondria less efficient, which we presume allows the cell to consume more fatty acid substrate at the expense of increasing reactive oxygen species generation.
Several studies show that inhibiting ceramide synthesis leads to improved oxidative phosphorylation. Firstly, Cers6 knockout mice were protected from high fat diet–induced mitochondrial dysfunction and displayed significantly increased oxygen consumption rates and extracellular acidification rates compared with controls (46). Additionally, Degs1 depleted mice showed enhanced mitochondrial complex activity in white adipose tissue compared with controls (12).
5.2. Apoptotic and Fibrotic Program
Ceramide induction of apoptosis allows for controlled cell death, preventing the release of cytosolic content into the extracellular space, which would otherwise occur following uncontrolled cell lysis. Similarly, the induction of fibrosis allows the organism to minimize widespread damage related to tissue inflammation and necrosis. Thus, ceramide-mediated apoptosis and fibrosis protect the organism from damage resulting from uncontrolled injury.
As ceramides accumulate, they increase mitochondrial outer membrane permeability, which stimulates cytochrome c release and apoptosis initiation (125). Blocking ceramide production reverses the proapoptotic cascade (110). In addition to apoptosis, ceramides are suspected to activate TGF-β signaling, which is a key regulator of collagen expression (110). More specifically, ceramides have a synergistic effect on the intensity of TGF-β signaling cascade by inducing mothers against decapentaplegic homolog 3 (SMAD3) phosphorylation and increasing collagen promoter activity (118). Correspondingly, myriocin treatment of rats fed a high-fat diet attenuated hepatic ceramide accumulation, fibrosis, and cleaved caspase 3 levels (68).
Collectively, the aforementioned studies suggest that ceramides are influential regulators of glucose homeostasis, lipid metabolism, and programmed cell death. Ceramides could therefore be a therapeutic target to ameliorate the pathological mechanisms and clinical endpoints of nutrition-linked chronic disease.
6. SUMMARY AND CONCLUSION
A rapidly growing and clinically translatable body of evidence implicates ceramides as lipotoxic drivers and potent biomarkers of nutrition-linked chronic diseases. Further technical refinement and study in globally representative populations are required to facilitate widespread clinical application. Harmonization of sphingolipid measurement methods and development of reference populations is a critical technical bottleneck that demands attention. Furthermore, effective ceramide-lowering recommendations merit development in order for patients to reduce their hyperceramidemia-related cardiometabolic disease risk. A detailed understanding of dietary ingestion of sphingolipids, gut microbiota sphingolipid synthesis, and metabolic disease state influence on ceramides—as well as a map of the downstream mechanisms of action—is necessary to effectively reap the therapeutic and prognostic potential of ceramides in nutrition-linked chronic disease. Understanding the genetic basis of hyperceramidemia, as well as the response to lifestyle changes including exercise, metabolic surgery, and dietary interventions on ceramide concentrations, will all contribute to the effective implementation of ceramide-based clinical algorithms and successful ceramide-associated cardiometabolic disease risk mitigation.
ACKNOWLEDGMENTS
The authors received research support from the National Institutes of Health (DK115824, DK131609, and DK116450 to S.A.S., 5T32DK091317 to R.J.N., and DK127603-02 to A.M.P.), the Juvenile Diabetes Research Foundation (JDRF 3-SRA-2019-768-A-B to S.A.S.), the American Diabetes Association (to S.A.S.), the American Heart Association (to S.A.S.), and the Margolis Foundation (to S.A.S.).
DISCLOSURE STATEMENT
A.M.P., R.J.N., and M.K.N. are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review. S.A.S is a shareholder and consultant for Centaurus Therapeutics.
LITERATURE CITED
- 1.Adams JM 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, et al. 2004. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53:25–31 [DOI] [PubMed] [Google Scholar]
- 2.Airola MV, Hannun YA. 2013. Sphingolipid metabolism and neutral sphingomyelinases. Handb. Exp. Pharmacol 215:57–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anroedh S, Hilvo M, Akkerhuis KM, Kauhanen D, Koistinen K, et al. 2018. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J. Lipid Res 59:1729–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. 2019. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism 92:121–35 [DOI] [PubMed] [Google Scholar]
- 5.Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, et al. 2010. Orm family proteins mediate sphingolipid homeostasis. Nature 463:1048–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown EM, Ke X, Hitchcock D, Jeanfavre S, Avila-Pacheco J, et al. 2019. Bacteroides-derived sphingolipids are critical for maintaining intestinal homeostasis and symbiosis. Cell Host Microbe 25:668–80.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burrello J, Biemmi V, Dei Cas M, Amongero M, Bolis S, et al. 2020. Sphingolipid composition of circulating extracellular vesicles after myocardial ischemia. Sci. Rep 10:16182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cartier A, Hla T. 2019. Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science 366:6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaurasia B, Holland WL, Summers SA. 2018. Does this schlank make me look fat? Trends Endocrinol. Metab 29:597–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaurasia B, Summers SA. 2015. Ceramides—lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab 26:538–50 [DOI] [PubMed] [Google Scholar]
- 11.Chaurasia B, Summers SA. 2021. Ceramides in metabolism: key lipotoxic players. Annu. Rev. Physiol 83:303–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaurasia B, Tippetts TS, Mayoral Monibas R, Liu J, Li Y, et al. 2019. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 365:386–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaurasia B, Ying L, Talbot CL, Maschek JA, Cox J, et al. 2021. Ceramides are necessary and sufficient for diet-induced impairment of thermogenic adipocytes. Mol. Metab 45:101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu S, Siri-Tarino P, Bergeron N, Suh JH, Krauss RM. 2020. A randomized study of the effect of replacing sugar-sweetened soda by reduced fat milk on cardiometabolic health in male adolescent soda drinkers. Nutrients 12(2):405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnström J, et al. 2011. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. PNAS 108:9613–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke BA, Majumder S, Zhu H, Lee YT, Kono M, et al. 2019. The Ormdl genes regulate the sphingolipid synthesis pathway to ensure proper myelination and neurologic function in mice. eLife 8:e51067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke CJ, Guthrie JM, Hannun YA. 2008. Regulation of neutral sphingomyelinase-2 (nSMase2) by tumor necrosis factor–alpha involves protein kinase C-delta in lung epithelial cells. Mol. Pharmacol 74:1022–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coen PM, Menshikova EV, Distefano G, Zheng D, Tanner CJ, et al. 2015. Exercise and weight loss improve muscle mitochondrial respiration, lipid partitioning, and insulin sensitivity after gastric bypass surgery. Diabetes 64:3737–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Covington JD, Johannsen DL, Coen PM, Burk DH, Obanda DN, et al. 2017. Intramyocellular lipid droplet size rather than total lipid content is related to insulin sensitivity after 8 weeks of overfeeding. Obesity 25:2079–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crewe C, Joffin N, Rutkowski JM, Kim M, Zhang F, et al. 2018. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell 175:695–708.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Angelo G, Uemura T, Chuang CC, Polishchuk E, Santoro M, et al. 2013. Vesicular and non-vesicular transport feed distinct glycosylation pathways in the Golgi. Nature 501:116–20 [DOI] [PubMed] [Google Scholar]
- 22.Davis DL, Gable K, Suemitsu J, Dunn TM, Wattenberg BW. 2019. The ORMDL/Orm-serine palmitoyltransferase (SPT) complex is directly regulated by ceramide: reconstitution of SPT regulation in isolated membranes. J. Biol. Chem 294:5146–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deevska GM, Sunkara M, Morris AJ, Nikolova-Karakashian MN. 2012. Characterization of secretory sphingomyelinase activity, lipoprotein sphingolipid content and LDL aggregation in ldlr−/− mice fed on a high-fat diet. Biosci. Rep 32:479–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djekic D, Shi L, Calais F, Carlsson F, Landberg R, et al. 2020. Effects of a lacto-ovo-vegetarian diet on the plasma lipidome and its association with atherosclerotic burden in patients with coronary artery disease-a randomized, open-label, cross-over study. Nutrients 12(11):3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong Y-q, Zhang X-z, Sun L-l, Zhang S-y, Liu B, et al. 2017. Omega-3 PUFA ameliorates hyperhomocysteinemia-induced hepatic steatosis in mice by inhibiting hepatic ceramide synthesis. Acta Pharmacol. Sin 38:1601–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drazba MA, Holaskova I, Sahyoun NR, Ventura Marra M. 2019. Associations of adiposity and diet quality with serum ceramides in middle-aged adults with cardiovascular risk factors. J. Clin. Med 8(4):527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dube JJ, Amati F, Toledo FG, Stefanovic-Racic M, Rossi A, et al. 2011. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 54:1147–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubois N, Rio E, Ripoche N, Ferchaud-Roucher V, Gaugler MH, et al. 2016. Plasma ceramide, a real-time predictive marker of pulmonary and hepatic metastases response to stereotactic body radiation therapy combined with irinotecan. Radiother. Oncol 119:229–35 [DOI] [PubMed] [Google Scholar]
- 29.Fang S, Suh JM, Reilly SM, Yu E, Osborn O, et al. 2015. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat. Med 21:159–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finicle BT, Ramirez MU, Liu G, Selwan EM, McCracken AN, et al. 2018. Sphingolipids inhibit endosomal recycling of nutrient transporters by inactivating ARF6. J. Cell Sci 131(12):jcs213314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fretts AM, Jensen PN, Hoofnagle A, McKnight B, Howard BV, et al. 2020. Plasma ceramide species are associated with diabetes risk in participants of the Strong Heart Study. J. Nutr 150:1214–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fretts AM, Jensen PN, Hoofnagle AN, McKnight B, Howard BV, et al. 2021. Plasma ceramides containing saturated fatty acids are associated with risk of type 2 diabetes. J. Lipid Res 62:100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujii A, Manabe Y, Aida K, Tsuduki T, Hirata T, Sugawara T. 2017. Selective absorption of dietary sphingoid bases from the intestine via efflux by P-glycoprotein in rats. J. Nutr. Sci. Vitaminol 63:44–50 [DOI] [PubMed] [Google Scholar]
- 34.Fukami H, Tachimoto H, Kishi M, Kaga T, Waki H, et al. 2010. Preparation of (13)C-labeled ceramide by acetic acid bacteria and its incorporation in mice. J. Lipid Res 51:3389–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukasawa M, Nishijima M, Hanada K. 1999. Genetic evidence for ATP-dependent endoplasmic reticulum-to-Golgi apparatus trafficking of ceramide for sphingomyelin synthesis in Chinese hamster ovary cells. J. Cell Biol 144:673–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fumeron F, Nicolas A, Bastard JP, Fellahi S, Wigger L, et al. 2020. Dairy consumption is associated with lower plasma dihydroceramides in women from the D.E.S.I.R. cohort. Diabetes Metab 46:144–49 [DOI] [PubMed] [Google Scholar]
- 37.Futerman AH, Riezman H. 2005. The ins and outs of sphingolipid synthesis. Trends Cell Biol 15:312–8 [DOI] [PubMed] [Google Scholar]
- 38.Gomez-Munoz A, Gangoiti P, Granado MH, Arana L, Ouro A. 2010. Ceramide-1-phosphate in cell survival and inflammatory signaling. Adv. Exp. Med. Biol 688:118–30 [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez FJ, Jiang C, Patterson AD. 2016. An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology 151:845–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graessler J, Bornstein TD, Goel D, Bhalla VP, Lohmann T, et al. 2014. Lipidomic profiling before and after Roux-en-Y gastric bypass in obese patients with diabetes. Pharmacogenom. J 14:201–7 [DOI] [PubMed] [Google Scholar]
- 41.Grammatikos G, Schoell N, Ferreirós N, Bon D, Herrmann E, et al. 2016. Serum sphingolipidomic analyses reveal an upregulation of C16-ceramide and sphingosine-1-phosphate in hepatocellular carcinoma. Oncotarget 7:18095–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guenther GG, Peralta ER, Rosales KR, Wong SY, Siskind LJ, Edinger AL. 2008. Ceramide starves cells to death by downregulating nutrient transporter proteins. PNAS 105:17402–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gui YK, Li Q, Liu L, Zeng P, Ren RF, et al. 2020. Plasma levels of ceramides relate to ischemic stroke risk and clinical severity. Brain Res. Bull 158:122–27 [DOI] [PubMed] [Google Scholar]
- 44.Halter D, Neumann S, van Dijk SM, Wolthoorn J, de Mazière AM, et al. 2007. Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J. Cell Biol 179:101–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, et al. 2010. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J. Lipid Res 51:3074–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammerschmidt P, Ostkotte D, Nolte H, Gerl MJ, Jais A, et al. 2019. CerS6-derived sphingolipids interact with Mff and promote mitochondrial fragmentation in obesity. Cell 177:1536–52 [DOI] [PubMed] [Google Scholar]
- 47.Han G, Gupta SD, Gable K, Niranjanakumari S, Moitra P, et al. 2009. Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. PNAS 106:8186–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, et al. 2003. Molecular machinery for non-vesicular trafficking of ceramide. Nature 426:803–9 [DOI] [PubMed] [Google Scholar]
- 49.Harmon JM, Bacikova D, Gable K, Gupta SD, Han G, et al. 2013. Topological and functional characterization of the ssSPTs, small activating subunits of serine palmitoyltransferase. J. Biol. Chem 288:10144–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, et al. 2009. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58:337–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Havulinna AS, Sysi-Aho M, Hilvo M, Kauhanen D, Hurme R, et al. 2016. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler. Thromb. Vasc. Biol 36:2424–30 [DOI] [PubMed] [Google Scholar]
- 52.Heilbronn LK, Coster AC, Campbell LV, Greenfield JR, Lange K, et al. 2013. The effect of short-term overfeeding on serum lipids in healthy humans. Obesity 21:e649–59 [DOI] [PubMed] [Google Scholar]
- 53.Heneghan HM, Huang H, Kashyap SR, Gornik HL, McCullough AJ, et al. 2013. Reduced cardiovascular risk after bariatric surgery is linked to plasma ceramides, apolipoprotein-B100, and ApoB100/A1 ratio. Surg. Obes. Relat. Dis 9:100–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, et al. 2020. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J 41:371–80 [DOI] [PubMed] [Google Scholar]
- 55.Hilvo M, Salonurmi T, Havulinna AS, Kauhanen D, Pedersen ER, et al. 2018. Ceramide stearic to palmitic acid ratio predicts incident diabetes. Diabetologia 61:1424–34 [DOI] [PubMed] [Google Scholar]
- 56.Hilvo M, Vasile VC, Donato LJ, Hurme R, Laaksonen R. 2020. Ceramides and ceramide scores: clinical applications for cardiometabolic risk stratification. Front. Endocrinol 11:570628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hilvo M, Wallentin L, Ghukasyan Lakic T, Held C, Kauhanen D, et al. 2020. Prediction of residual risk by ceramide-phospholipid score in patients with stable coronary heart disease on optimal medical therapy. J. Am. Heart Assoc 9:e015258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hojjati MR, Li Z, Zhou H, Tang S, Huan C, et al. 2005. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J. Biol. Chem 280:10284–9 [DOI] [PubMed] [Google Scholar]
- 59.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, et al. 2007. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5:167–79 [DOI] [PubMed] [Google Scholar]
- 60.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, et al. 2011. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med 17:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hornemann T, Richard S, Rütti MF, Wei Y, von Eckardstein A. 2006. Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. J. Biol. Chem 281:37275–81 [DOI] [PubMed] [Google Scholar]
- 62.Huang H, Kasumov T, Gatmaitan P, Heneghan HM, Kashyap SR, et al. 2011. Gastric bypass surgery reduces plasma ceramide subspecies and improves insulin sensitivity in severely obese patients. Obesity 19:2235–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. 2005. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J 19:461–63 [DOI] [PubMed] [Google Scholar]
- 64.Iqbal J, Walsh MT, Hammad SM, Cuchel M, Tarugi P, et al. 2015. Microsomal triglyceride transfer protein transfers and determines plasma concentrations of ceramide and sphingomyelin but not glycolsylceramide. J. Biol. Chem 290:25863–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jenkins RW, Canals D, Hannun YA. 2009. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell Signal 21:836–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jensen PN, Fretts AM, Yu C, Hoofnagle AN, Umans JG, et al. 2019. Circulating sphingolipids, fasting glucose, and impaired fasting glucose: the Strong Heart Family Study. EBioMedicine 41:44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang C, Xie C, Li F, Zhang L, Nichols RG, et al. 2015. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Investig 125:386–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang M, Li C, Liu Q, Wang A, Lei M. 2019. Inhibiting ceramide synthesis attenuates hepatic steatosis and fibrosis in rats with non-alcoholic fatty liver disease. Front. Endocrinol 10:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin J, Lu Z, Li Y, Cowart LA, Lopes-Virella MF, Huang Y. 2018. Docosahexaenoic acid antagonizes the boosting effect of palmitic acid on LPS inflammatory signaling by inhibiting gene transcription and ceramide synthesis. PLOS ONE 13:e0193343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson EL, Heaver SL, Waters JL, Kim BI, Bretin A, et al. 2020. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat. Commun 11:2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kayser BD, Lhomme M, Dao MC, Ichou F, Bouillot JL, et al. 2017. Serum lipidomics reveals early differential effects of gastric bypass compared with banding on phospholipids and sphingolipids independent of differences in weight loss. Int. J. Obes 41:917–25 [DOI] [PubMed] [Google Scholar]
- 72.Kayser BD, Prifti E, Lhomme M, Belda E, Dao MC, et al. 2019. Elevated serum ceramides are linked with obesity-associated gut dysbiosis and impaired glucose metabolism. Metabolomics 15:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kien CL, Bunn JY, Poynter ME, Stevens R, Bain J, et al. 2013. A lipidomics analysis of the relationship between dietary fatty acid composition and insulin sensitivity in young adults. Diabetes 62:1054–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kitatani K, Idkowiak-Baldys J, Hannun YA. 2008. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal 20:1010–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kitatani K, Sheldon K, Rajagopalan V, Anelli V, Jenkins RW, et al. 2009. Involvement of acid beta-glucosidase 1 in the salvage pathway of ceramide formation. J. Biol. Chem 284:12972–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knapp P, Bodnar L, Błachnio-Zabielska A, Reszeć J, Świderska M, Chabowski A. 2021. Blood bioactive sphingolipids in patients with advanced serous epithelial ovarian cancer—mass spectrometry analysis. Arch. Med. Sci 17:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krautbauer S, Meier EM, Rein-Fischboeck L, Pohl R, Weiss TS, et al. 2016. Ceramide and polyunsaturated phospholipids are strongly reduced in human hepatocellular carcinoma. Biochim. Biophys. Acta 1861:1767–74 [DOI] [PubMed] [Google Scholar]
- 78.Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, et al. 2016. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J 37:1967–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lankinen M, Schwab U, Erkkila A, Seppanen-Laakso T, Hannila ML, et al. 2009. Fatty fish intake decreases lipids related to inflammation and insulin signaling—a lipidomics approach. PLOS ONE 4:e5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lankinen M, Schwab U, Kolehmainen M, Paananen J, Nygren H, et al. 2016. A healthy Nordic diet alters the plasma lipidomic profile in adults with features of metabolic syndrome in a multicenter randomized dietary intervention. J. Nutr 146:662–72 [DOI] [PubMed] [Google Scholar]
- 81.Lankinen M, Schwab U, Kolehmainen M, Paananen J, Poutanen K, et al. 2011. Whole grain products, fish and bilberries alter glucose and lipid metabolism in a randomized, controlled trial: the Sysdimet study. PLOS ONE 6:e22646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laviad EL, Kelly S, Merrill AH Jr., Futerman AH. 2012. Modulation of ceramide synthase activity via dimerization. J. Biol. Chem 287:21025–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Le Barz M, Vors C, Combe E, Joumard-Cubizolles L, Lecomte M, et al. 2021. Milk polar lipids favorably alter circulating and intestinal ceramide and sphingomyelin species in postmenopausal women. JCI Insight 6(10):e146161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee MT, Le HH, Johnson EL. 2021. Dietary sphinganine is selectively assimilated by members of the mammalian gut microbiome. J. Lipid Res 62:100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lemaitre RN, Jensen PN, Hoofnagle A, McKnight B, Fretts AM, et al. 2019. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circ. Heart Fail 12:e005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lemaitre RN, Yu C, Hoofnagle A, Hari N, Jensen P, et al. 2018. Circulating sphingolipids, insulin, HOMA-IR and HOMA-B: the Strong Heart Family Study. Diabetes 67(8):1663–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Levy M, Futerman AH. 2010. Mammalian ceramide synthases. IUBMB Life 62:347–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lindqvist HM, Bärebring L, Gjertsson I, Jylhä A, Laaksonen R, et al. 2021. A randomized controlled dietary intervention improved the serum lipid signature towards a less atherogenic profile in patients with rheumatoid arthritis. Metabolites 11(9):632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lopez X, Goldfine AB, Holland WL, Gordillo R, Scherer PE. 2013. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J. Pediatr. Endocrinol. Metab 26:995–8 [DOI] [PubMed] [Google Scholar]
- 90.Luukkonen PK, Sadevirta S, Zhou Y, Kayser B, Ali A, et al. 2018. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care 41:1732–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mah M, Febbraio M, Turpin-Nolan S. 2021. Circulating ceramides—are origins important for sphingolipid biomarkers and treatments? Front. Endocrinol 12:684448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Malik VS, Guasch-Ferre M, Hu FB, Townsend MK, Zeleznik OA, et al. 2019. Identification of plasma lipid metabolites associated with nut consumption in US men and women. J. Nutr 149:1215–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mathews AT, Famodu OA, Olfert MD, Murray PJ, Cuff CF, et al. 2017. Efficacy of nutritional interventions to lower circulating ceramides in young adults: FRUVEDomic pilot study. Physiol. Rep 5(13):e13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meikle PJ, Barlow CK, Mellett NA, Mundra PA, Bonham MP, et al. 2015. Postprandial plasma phospholipids in men are influenced by the source of dietary fat. J. Nutr 145:2012–18 [DOI] [PubMed] [Google Scholar]
- 95.Mikhalkova D, Holman SR, Jiang H, Saghir M, Novak E, et al. 2018. Bariatric surgery-induced cardiac and lipidomic changes in obesity-related heart failure with preserved ejection fraction. Obesity 26:284–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mizutani Y, Kihara A, Igarashi Y. 2004. Identification of the human sphingolipid C4-hydroxylase, hDES2, and its up-regulation during keratinocyte differentiation. FEBS Lett 563:93–97 [DOI] [PubMed] [Google Scholar]
- 97.Morigny P, Zuber J, Haid M, Kaltenecker D, Riols F, et al. 2020. High levels of modified ceramides are a defining feature of murine and human cancer cachexia. J. Cachexia Sarcopenia Muscle 11:1459–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moro K, Kawaguchi T, Tsuchida J, Gabriel E, Qi Q, et al. 2018. Ceramide species are elevated in human breast cancer and are associated with less aggressiveness. Oncotarget 9:19874–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mullen TD, Spassieva S, Jenkins RW, Kitatani K, Bielawski J, et al. 2011. Selective knockdown of ceramide synthases reveals complex interregulation of sphingolipid metabolism [S]. J. Lipid Res 52:68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nagahashi M, Kim EY, Yamada A, Ramachandran S, Allegood JC, et al. 2013. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J 27:1001–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nature Biotechnology Editorial Board. 2010. Biomarkers on a roll. Nat. Biotechnol 28:431–31 [DOI] [PubMed] [Google Scholar]
- 102.Nilsson A 1968. Metabolism of sphingomyelin in the intestinal tract of the rat. Biochim. Biophys. Acta 164:575–84 [DOI] [PubMed] [Google Scholar]
- 103.Norris GH, Blesso CN. 2017. Dietary sphingolipids: potential for management of dyslipidemia and nonalcoholic fatty liver disease. Nutr. Rev 75:274–85 [DOI] [PubMed] [Google Scholar]
- 104.Norris GH, Milard M, Michalski MC, Blesso CN. 2019. Protective properties of milk sphingomyelin against dysfunctional lipid metabolism, gut dysbiosis, and inflammation. J. Nutr. Biochem 73:108224. [DOI] [PubMed] [Google Scholar]
- 105.Ottestad I, Hassani S, Borge GI, Kohler A, Vogt G, et al. 2012. Fish oil supplementation alters the plasma lipidomic profile and increases long-chain PUFAs of phospholipids and triglycerides in healthy subjects. PLOS ONE 7:e42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Özer H, Aslan İ, Oruç MT, Çöpelci Y, Afşar E, et al. 2018. Early postoperative changes of sphingomyelins and ceramides after laparoscopic sleeve gastrectomy. Lipids Health Disease 17:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, et al. 2008. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res 49:2101–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peterson LR, Xanthakis V, Duncan MS, Gross S, Friedrich N, et al. 2018. Ceramide remodeling and risk of cardiovascular events and mortality. J. Am. Heart Assoc 7(10):e007931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poss AM, Maschek JA, Cox JE, Hauner BJ, Hopkins PN, et al. 2019. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J. Clin. Investig 130(3):1363–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Poss AM, Summers SA. 2020. Too much of a good thing? An evolutionary theory to explain the role of ceramides in NAFLD. Front. Endocrinol 11:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Powell DJ, Hajduch E, Kular G, Hundal HS. 2003. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol. Cell. Biol 23:7794–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Promrat K, Longato L, Wands JR, de la Monte SM. 2011. Weight loss amelioration of non-alcoholic steatohepatitis linked to shifts in hepatic ceramide expression and serum ceramide levels. Hepatol. Res 41:754–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raichur S, Wang ST, Chan PW, Li Y, Ching J, et al. 2014. CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab 20:687–95 [DOI] [PubMed] [Google Scholar]
- 114.Rohrhofer J, Zwirzitz B, Selberherr E, Untersmayr E. 2021. The impact of dietary sphingolipids on intestinal microbiota and gastrointestinal immune homeostasis. Front. Immunol 12:635704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rosqvist F, Kullberg J, Stahlman M, Cedernaes J, Heurling K, et al. 2019. Overeating saturated fat promotes fatty liver and ceramides compared with polyunsaturated fat: a randomized trial. J. Clin. Endocrinol. Metab 104:6207–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ryan D, Heaner M. 2014. Guidelines 2013 for managing overweight and obesity in adults. Preface to the full report. Obesity 22(suppl. 2):S1–3 [DOI] [PubMed] [Google Scholar]
- 117.Salinas M, Lopez-Valdaliso R, Martin D, Alvarez A, Cuadrado A. 2000. Inhibition of PKB/Akt1 by C2-ceramide involves activation of ceramide-activated protein phosphatase in PC12 cells. Mol. Cell. Neurosci 15:156–69 [DOI] [PubMed] [Google Scholar]
- 118.Sato M, Markiewicz M, Yamanaka M, Bielawska A, Mao C, et al. 2003. Modulation of transforming growth factor-beta (TGF-beta) signaling by endogenous sphingolipid mediators. J. Biol. Chem 278:9276–82 [DOI] [PubMed] [Google Scholar]
- 119.Scherer M, Bottcher A, Schmitz G, Liebisch G. 2011. Sphingolipid profiling of human plasma and FPLC-separated lipoprotein fractions by hydrophilic interaction chromatography tandem mass spectrometry. Biochim. Biophys. Acta 1811:68–75 [DOI] [PubMed] [Google Scholar]
- 120.Seah JYH, Chew WS, Torta F, Khoo CM, Wenk MR, et al. 2021. Dietary fat and protein intake in relation to plasma sphingolipids as determined by a large-scale lipidomic analysis. Metabolites 11(2):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Separovic D, Shields AF, Philip PA, Bielawski J, Bielawska A, et al. 2017. Altered levels of serum ceramide, sphingosine and sphingomyelin are associated with colorectal cancer: a retrospective pilot study. Anticancer Res 37:1213–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Serra M, Saba JD. 2010. Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Adv. Enzyme Regul 50:349–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Siddique MM, Li Y, Chaurasia B, Kaddai VA, Summers SA. 2015. Dihydroceramides: from bit players to lead actors. J. Biol. Chem 290:15371–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Siddique MM, Li Y, Wang L, Ching J, Mal M, et al. 2013. Ablation of dihydroceramide desaturase 1, a therapeutic target for the treatment of metabolic diseases, simultaneously stimulates anabolic and catabolic signaling. Mol. Cell. Biol 33:2353–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Siskind LJ, Kolesnick RN, Colombini M. 2002. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J. Biol. Chem 277:26796–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Skotland T, Sagini K, Sandvig K, Llorente A. 2020. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev 159:308–21 [DOI] [PubMed] [Google Scholar]
- 127.Sociale M, Wulf AL, Breiden B, Klee K, Thielisch M, et al. 2018. Ceramide synthase schlank is a transcriptional regulator adapting gene expression to energy requirements. Cell Rep 22:967–78 [DOI] [PubMed] [Google Scholar]
- 128.Stoffel W, Dittmar K, Wilmes R. 1975. Sphingolipid metabolism in Bacteroideaceae. Hoppe Seylers Z. Physiol. Chem 356:715–25 [DOI] [PubMed] [Google Scholar]
- 129.Straub LG, Scherer PE. 2019. Metabolic messengers: adiponectin. Nat. Metab 1:334–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sud M, Fahy E, Cotter D, Brown A, Dennis EA, et al. 2007. LMSD: LIPID MAPS structure database. Nucleic Acids Res 35:D527–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sugawara T, Kinoshita M, Ohnishi M, Nagata J, Saito M. 2003. Digestion of maize sphingolipids in rats and uptake of sphingadienine by Caco-2 cells. J. Nutr 133:2777–82 [DOI] [PubMed] [Google Scholar]
- 132.Summers SA. 2018. Could ceramides become the new cholesterol? Cell Metab 27:276–80 [DOI] [PubMed] [Google Scholar]
- 133.Summers SA, Birnbaum MJ. 1997. A role for the serine/threonine kinase, Akt, in insulin-stimulated glucose uptake. Biochem. Soc. Trans 25:981–88 [DOI] [PubMed] [Google Scholar]
- 134.Summers SA, Chaurasia B, Holland WL. 2019. Metabolic messengers: ceramides. Nat. Metab 1:1051–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Summers SA, Garza LA, Zhou H, Birnbaum MJ. 1998. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol 18:5457–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tarasov K, Ekroos K, Suoniemi M, Kauhanen D, Sylvanne T, et al. 2014. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J. Clin. Endocrinol. Metab 99:e45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tidhar R, Zelnik ID, Volpert G, Ben-Dor S, Kelly S, et al. 2018. Eleven residues determine the acyl chain specificity of ceramide synthases. J. Biol. Chem 293:9912–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Trieu K, Bhat S, Dai Z, Leander K, Gigante B, et al. 2021. Biomarkers of dairy fat intake, incident cardiovascular disease, and all-cause mortality: a cohort study, systematic review, and meta-analysis. PLOS Med 18:e1003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tuccinardi D, Di Mauro A, Lattanzi G, Rossini G, Monte L, et al. 2021. An extra virgin olive oil–enriched chocolate spread positively modulates insulin-resistance markers compared with a palm oil–enriched one in healthy young adults: a double-blind, cross-over, randomized controlled trial. Diabetes Metab. Res. Rev 38(2):e3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tuccinardi D, Farr OM, Upadhyay J, Oussaada SM, Klapa MI, et al. 2019. Mechanisms underlying the cardiometabolic protective effect of walnut consumption in obese people: a cross-over, randomized, double-blind, controlled inpatient physiology study. Diabetes Obes. Metab 21:2086–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, et al. 2014. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab 20:678–86 [DOI] [PubMed] [Google Scholar]
- 142.Turpin-Nolan SM, Hammerschmidt P, Chen W, Jais A, Timper K, et al. 2019. CerS1-derived C18:0 ceramide in skeletal muscle promotes obesity-induced insulin resistance. Cell Rep 26:1–10.e7 [DOI] [PubMed] [Google Scholar]
- 143.Valentino TR, Rule BD, Mobley CB, Nikolova-Karakashian M, Vechetti IJ. 2021. Skeletal muscle cell growth alters the lipid composition of extracellular vesicles. Membranes 11(8):619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Van Veldhoven PP. 2000. [28]Sphingosine-1-phosphate lyase. Methods Enzymol 311:244–54 [DOI] [PubMed] [Google Scholar]
- 145.Vance DE, Vance JE. 1996. Biochemistry of Lipids, Lipoproteins and Membranes Amsterdam: Elsevier [Google Scholar]
- 146.Vesper H, Schmelz EM, Nikolova-Karakashian MN, Dillehay DL, Lynch DV, Merrill AH Jr. 1999. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J. Nutr 129:1239–50 [DOI] [PubMed] [Google Scholar]
- 147.Walker ME, Xanthakis V, Peterson LR, Duncan MS, Lee J, et al. 2020. Dietary patterns, ceramide ratios, and risk of all-cause and cause-specific mortality: the Framingham Offspring Study. J. Nutr 150(11):2994–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang DD, Toledo E, Hruby A, Rosner BA, Willett WC, et al. 2017. Plasma ceramides, Mediterranean diet, and incident cardiovascular disease in the PREDIMED trial (Prevencion con Dieta Mediterranea). Circulation 135:2028–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang X, Wang Y, Xu J, Xue C. 2021. Sphingolipids in food and their critical roles in human health. Crit. Rev. Food Sci. Nutr 61:462–91 [DOI] [PubMed] [Google Scholar]
- 150.Wang Y, Niu Y, Zhang Z, Gable K, Gupta SD, et al. 2021. Structural insights into the regulation of human serine palmitoyltransferase complexes. Nat. Struct. Mol. Biol 28:240–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wasilewska N, Bobrus-Chociej A, Harasim-Symbor E, Tarasow E, Wojtkowska M, et al. 2018. Increased serum concentration of ceramides in obese children with nonalcoholic fatty liver disease. Lipids Health Dis 17:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wigger L, Cruciani-Guglielmacci C, Nicolas A, Denom J, Fernandez N, et al. 2017. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Rep 18:2269–79 [DOI] [PubMed] [Google Scholar]
- 153.Wu J, Liu F, Nilsson A, Duan RD. 2004. Pancreatic trypsin cleaves intestinal alkaline sphingomyelinase from mucosa and enhances the sphingomyelinase activity. Am. J. Physiol. Gastrointest. Liver Physiol 287:G967–73 [DOI] [PubMed] [Google Scholar]
- 154.Xia JY, Holland WL, Kusminski CM, Sun K, Sharma AX, et al. 2015. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab 22:266–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xie C, Jiang C, Shi J, Gao X, Sun D, et al. 2017. An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 66:613–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yamashita S, Kinoshita M, Miyazawa T. 2021. Dietary sphingolipids contribute to health via intestinal maintenance. Int. J. Mol. Sci 22(13):7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yard BA, Carter LG, Johnson KA, Overton IM, Dorward M, et al. 2007. The structure of serine palmitoyltransferase; gateway to sphingolipid biosynthesis. J. Mol. Biol 370:870–86 [DOI] [PubMed] [Google Scholar]
- 158.Yu Y, Guo S, Feng Y, Feng L, Cui Y, et al. 2014. Phospholipid transfer protein deficiency decreases the content of S1P in HDL via the loss of its transfer capability. Lipids 49:183–90 [DOI] [PubMed] [Google Scholar]
- 159.Yunoki K, Ogawa T, Ono J, Miyashita R, Aida K, et al. 2008. Analysis of sphingolipid classes and their contents in meals. Biosci. Biotechnol. Biochem 72:222–25 [DOI] [PubMed] [Google Scholar]
- 160.Zelnik ID, Kim JL, Futerman AH. 2021. The complex tail of circulating sphingolipids in atherosclerosis and cardiovascular disease. J. Lipid Atheroscler 10:268–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou H, Summers SA, Birnbaum MJ, Pittman RN. 1998. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J. Biol. Chem 273:16568–75 [DOI] [PubMed] [Google Scholar]
- 162.Zigdon H, Kogot-Levin A, Park JW, Goldschmidt R, Kelly S, et al. 2013. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J. Biol. Chem 288:4947–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zinda MJ, Vlahos CJ, Lai MT. 2001. Ceramide induces the dephosphorylation and inhibition of constitutively activated Akt in PTEN negative U87mg cells. Biochem. Biophys. Res. Commun 280:1107–15 [DOI] [PubMed] [Google Scholar]


