Abstract
Jaundice caused by hyperbilirubinaemia is a common phenomenon during the neonatal period. Population-based studies evaluating assessment, management, and incidence of jaundice and need for phototherapy among otherwise healthy neonates are scarce. We prospectively explored these aspects in a primary care setting via assessing care as usual during the control phase of a stepped wedge cluster randomised controlled trial.
We conducted a prospective cohort study embedded in the Screening and TreAtment to Reduce Severe Hyperbilirubinaemia in Infants in Primary care (STARSHIP) Trial. Healthy neonates were included in seven primary care birth centres (PCBCs) in the Netherlands between July 2018 and March 2020. Neonates were eligible for inclusion if their gestational age was ≥ 35 weeks, they were admitted in a PCBC for at least 2 days during the first week of life, and if they did not previously receive phototherapy. Outcomes were the findings of visual assessment to detect jaundice, jaundice incidence and management, and the need for phototherapy treatment in the primary care setting.
860 neonates were included of whom 608 (71.9%) were visibly jaundiced at some point during admission in the PCBC, with 20 being ‘very yellow’. Of the latter, four (20%) did not receive total serum bilirubin (TSB) quantification. TSB levels were not associated with the degree of visible jaundice (p = 0.416). Thirty-one neonates (3.6%) received phototherapy and none received an exchange transfusion. Five neonates did not receive phototherapy despite having a TSB level above phototherapy threshold.
Jaundice is common in otherwise healthy neonates cared for in primary care. TSB quantification was not always performed in very jaundiced neonates, and not all neonates received phototherapy when indicated. Quality improvement initiatives are required, including alternative approaches to identifying potentially severe hyperbilirubinaemia.
Trial registration: NL6997 (Dutch Trial Register; Old NTR ID 7187), registered 3 May 2018.
Subject terms: Paediatric research, Neonatology
Introduction
Neonatal hyperbilirubinaemia is a common condition during the first days of life and typically presents as visible jaundice1. Hyperbilirubinaemia in the neonatal period is usually benign. In some neonates, unconjugated bilirubin may reach hazardous levels and cause acute bilirubin encephalopathy and later kernicterus spectrum disorder (KSD) when not timely recognised and treated2.
In several countries and settings, the first-line recognition of hyperbilirubinaemia is based on visual inspection of jaundice, followed by selective total serum bilirubin (TSB) quantification (i.e., if considered necessary). Transcutaneous bilirubin quantification is not widely used in the primary care setting. TSB levels are plotted on a nomogram to determine the need for treatment (Text Box 1)3–5. Phototherapy is a safe and effective treatment to decrease bilirubin levels and is usually applied in-hospital1. When bilirubin levels are extremely high or continue to increase despite intensive phototherapy, one or more exchange transfusions may be needed to decrease bilirubin levels.
Although neonatal jaundice is commonly observed, population-based data on the assessment, management, and incidence of visual jaundice and need for phototherapy among healthy neonates, especially if cared for in primary care, are scarce. Whereas the inaccuracy of visual inspection of jaundice to estimate TSB levels has previously been demonstrated6,7, the associations of visual jaundice assessment to the decision whether or not to quantify TSB, and of visual jaundice assessment to whether or not a neonate exceeded the individual phototherapy treatment threshold in primary care are unknown. Also, most population-based studies focus on hospitalised neonates having severe neonatal hyperbilirubinaemia or KSD8–11. Hence, these studies do not cover the complete scope of assessment, management, incidence, and burden of neonatal hyperbilirubinaemia. In addition, definitions of severe hyperbilirubinaemia vary, resulting in a wide variation in reported incidences of neonatal hyperbilirubinaemia between studies12–17.
The Screening and TreAtment to Reduce Severe Hyperbilirubinaemia in Primary care (STARSHIP) Trial is an ongoing factorial stepped-wedge cluster randomised controlled trial in seven Dutch primary care birth centres (PCBCs). It aims to assess the effectiveness of universal transcutaneous bilirubin (TcB) screening and of phototherapy applied in primary care18. See Text Box 2. In each participating PCBC, the initial phase of the STARSHIP trial evaluates usual care (i.e. no interventions are implemented). This provides a unique opportunity to explore the assessment, management, and incidence of neonatal jaundice and phototherapy in primary care among children included during this initial phase.
Methods
Study design
Prospective cohort study embedded in the factorial stepped-wedge cluster randomised controlled STARSHIP Trial18.
Setting
In the Netherlands, most healthy neonates are either born in primary care or discharged to primary care (i.e. the home or a PCBC) within the first few hours to days of life19. A maternity care assistant (MCA) provides postpartum care to mother and neonate during daytime for the first 8 days after delivery20. The MCA is supervised by a community midwife, who visits the family at least three times in the first week21. The MCA assesses each day whether the neonate is visually jaundiced and if so, to which degree. The MCA is expected to consult the community midwife if she considers the neonate ‘too jaundiced’ or if she feels that there are other reasons to quantify TSB. Medical doctors are only involved in the care of otherwise healthy neonates if consulted by the community midwife. The current national multidisciplinary guideline on neonatal hyperbilirubinaemia does not include universal screening, but alternatively states that each involved perinatal healthcare professional should be aware of a neonate’s a priori risk for developing hyperbilirubinaemia and that this risk should be documented and communicated among all involved perinatal healthcare professionals4. According to the guideline, the healthcare provider may decide to have blood taken to quantify TSB levels if hyperbilirubinaemia is suspected based on visual inspection (e.g., a neonate is assessed ‘too jaundiced’). The guideline does not provide objective criteria for having TSB quantified4. One of the PCBCs and a small number of primary care midwifery practices participating in the STARSHIP Trial used selective transcutaneous bilirubin (TcB) screening (i.e., TcB quantification if a neonate is assessed ‘too jaundiced’, followed by TSB quantification if the TcB level is above or < 50 µmol/L below the phototherapy threshold). TSB levels are plotted on the Dutch TSB nomogram (Text Box 1), which is based on the American Academy of Pediatrics guidelines3. A paediatrician of a nearby affiliated hospital can be consulted when hyperbilirubinaemia is confirmed, and this is then usually treated in the hospital.
The STARSHIP Trial is conducted in seven PCBCs throughout the Netherlands where MCAs provide postpartum care, supervised by community midwives. Women can choose to receive their care either at home or in a PCBC if the neonate is healthy. Neonates included in the control phase of the STARSHIP Trial, when usual care was evaluated, were included in this cohort. The control phase of the STARSHIP Trial ran between 2 July 2018 and 8 March 2020 (Supplementary Table 1)18.
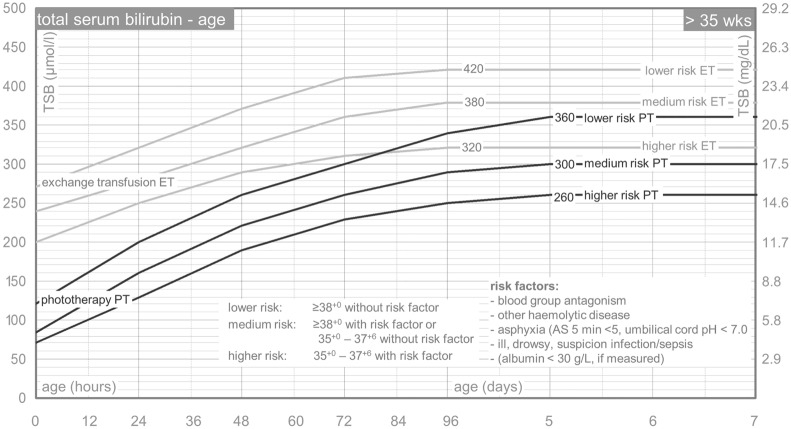
Text Box 1: the Dutch TSB nomogram.
The Dutch TSB nomogram is adapted from the American Academy of Pediatrics3. Treatment thresholds are based on postnatal age and risk assessment. Gestational age (< 38 weeks or ≥ 38 weeks) and risk factors (blood group antagonism, haemolytic disease; birth asphyxia; suspicion of infection; drowsy or ill neonate; and serum albumin level below 30 g/L) are combined to assess the risk category: lower, medium, or higher risk. See Fig. 1.
Figure 1.
Phototherapy and exchange transfusion thresholds for neonates born after more than 35 weeks of gestation. TSB total serum bilirubin, PT phototherapy, ET exchange transfusion, AS Apgar score. Translated from Dutch. The Dutch nomogram is available at: http://babyzietgeel.nl/kinderarts/hulpmiddelen/diagnostiek/bilicurve35wkn.php.
Participants
Neonates were eligible for inclusion in the STARSHIP Trial if:
Born ≥ 35 + 0 weeks of gestation;
Admitted to a participating PCBC during the first week of life;
Expected to remain admitted to the PCBC for at least 2 days;
Signed informed consent from parent(s) or primary caregiver(s) was obtained.
Neonates were not eligible if:
The neonate previously received phototherapy;
Parents did not have sufficient understanding of the Dutch language to be able to comprehend the patient information form.
For the analyses presented in this manuscript, all neonates included in the control phase of the STARSHIP Trial were eligible. Inclusion of the neonates was performed at admission to the PCBC and irrespective of the degree of jaundice of the neonate.
Variables
Outcomes of this study are: findings of assessment of jaundice by MCAs (ranging from ‘not yellow at all’ to ‘very yellow’; in the Netherlands no standardised colour scale is used for visual jaundice assessment), the number of neonates in whom TSB was quantified; TSB level; management of neonatal hyperbilirubinaemia (i.e., what treatment is needed and what treatment is performed); the incidence of neonatal hyperbilirubinaemia and of receiving phototherapy treatment; and risk factors associated with receiving phototherapy. An overview of all variables used for the current analyses and definitions of variables is shown in Supplementary Table 2.
Data sources
Baseline data regarding mother and neonate, and daily data regarding findings of screening and treatment of neonatal hyperbilirubinaemia were collected by MCAs of the participating PCBCs and by study personnel of the STARSHIP Trial and stored in a Limesurvey/Gemstracker database22. Additionally, parent(s) of all included neonates were asked to fill out a questionnaire, 2 weeks after discharge from the PCBC, that included questions regarding hospital admission for hyperbilirubinaemia. If a neonate was admitted to the hospital for neonatal hyperbilirubinaemia, additional information from the medical records regarding likely underlying causes, TSB levels, and treatment of hyperbilirubinaemia was requested from this hospital.
Statistical analysis
Analyses were performed using SPSS Statistics version 25.0. Data were summarised using descriptive statistics. Mean and standard deviation (SD) were calculated for continuous, normally distributed data. For non-normally distributed data, median and interquartile range (IQR) were calculated. As phototherapy treatment thresholds vary according to postnatal age and individual risk assessment for each neonate (Text Box 1), the difference between a neonate’s TSB level and the corresponding phototherapy threshold for each individual neonate was calculated4,5. In the absence of information on individual risk factors determining phototherapy thresholds, such as blood group incompatibility, the risk factor is generally considered to be absent. To compare whether or not TSB was quantified, and the difference between neonates’ TSB levels and corresponding phototherapy thresholds among neonates having different degrees of visual jaundice, χ2 and Kruskal–Wallis test were performed as appropriate. Logistic regression was performed to analyse which risk factors were independently associated with hyperbilirubinaemia necessitating treatment. A p-value < 0.05 was considered to indicate statistical significance.
Ethics
The STARSHIP Trial has been reviewed and approved by the Medical Research Ethics Committee of Erasmus MC Rotterdam, the Netherlands (MEC2017-473). The STARSHIP Trial was performed in accordance with the Declaration of Helsinki23.
Consent to participate
Parents provided written informed consent before participation of their neonate in the study.
Text Box 2: STARSHIP Trial.
The Screening and TreAtment to Reduce Severe Hyperbilirubinaemia in Infants in Primary care (STARSHIP) Trial is a factorial stepped-wedge cluster randomised controlled trial. In the STARSHIP Trial, universal transcutaneous bilirubin screening and phototherapy in primary care are evaluated. The STARSHIP Trial is conducted in seven primary care birth centres (PCBCs) in the Netherlands. MCAs provide postpartum care supervised by community midwives in PCBCs. Medical doctors are not involved in providing care, although in some PCBCs they can be consulted if a problem arises.
According to the factorial stepped-wedge cluster design of the STARSHIP Trial, each PCBC is allocated to a predefined timeline with three phases. Each PCBC starts with a control phase in which all included neonates receive standard care according to the national multidisciplinary hyperbilirubinaemia guideline (i.e., visual inspection of jaundice and selected TSB quantification to screen for hyperbilirubinaemia, and phototherapy in the hospital if treatment is indicated)4. The control phase is followed by a second phase in which one intervention is implemented (i.e., transcutaneous bilirubin screening or phototherapy in the PCBC rather than in-hospital) and eventually by a final phase in which both interventions are implemented (i.e., transcutaneous bilirubin screening and phototherapy in the PCBC)18.
Results
In total, 860 neonates were included in the control phase of the STARSHIP Trial. Baseline characteristics are shown in Table 1. Median gestational age was 39.3 weeks (IQR 1.9) and mean birth weight was 3399 g (SD 487). Most neonates were born after a vaginal, non-instrumental delivery, had a Western ethnicity and a Rh D positive mother. Apgar score at 5 min was below 5 in 18 neonates (2.1%) and umbilical cord pH was below 7.0 in 11 (2.5%) out of 441 neonates in whom umbilical cord pH was quantified.
Table 1.
Baseline characteristics.
| n = 860 | ||
|---|---|---|
| Sex | ||
| Female | n (%) | 398 (46.7) |
| Male | n (%) | 454 (53.3) |
| Missing | n | 8 |
| Gestational age (weeks) | Median (IQR) | 39.3 (1.9) |
| Missing | n | 10 |
| Birth weight (grams) | Mean (SD) | 3399 (487) |
| Missing | n | 8 |
| Mode of delivery | ||
| Vaginal, non-instrumental | n (%) | 477 (56.1) |
| Vaginal, instrumental | n (%) | 68 (8.0) |
| C-section, non-instrumental | n (%) | 298 (35.0) |
| C-section, instrumentala | n (%) | 8 (0.9) |
| Missing | n | 9 |
| Apgar score < 5 at 5 min | n (%) | 18 (2.2) |
| Missing or unknown | n (%) | 24 |
| Umbilical cord pH quantified | n (%) | 441 (64.7) |
| Of which, umbilical cord pH < 7.0 | n (%) | 11 (2.5) |
| Umbilical cord pH not quantified | n (%) | 241 (35.3) |
| Missing or unknown | n | 178 |
| Maternal Rh D negative | n (%) | 119 (16.3) |
| Of which, fetal Rh D positive | n (%) | 42 (35.3) |
| Missing or unknown maternal Rh D | n | 131 |
| Non-western ethnicity neonateb | n (%) | 200 (28.1) |
| Missing | n | 149 |
| Type of feeding | ||
| Exclusive breastfeeding | n (%) | 533 (62.6) |
| Exclusive formula feeding | n (%) | 176 (20.7) |
| Combination | n (%) | 143 (16.8) |
| Missing | n | 8 |
| PCBCc | ||
| Fam, Tilburg | n (%) | 56 (6.5) |
| Haga, The Hague | n (%) | 98 (11.4) |
| Isala, Zwolle | n (%) | 207 (24.1) |
| Maasstad, Rotterdam | n (%) | 219 (25.5) |
| Noord, Rotterdam | n (%) | 83 (9.7) |
| Sophia, Rotterdam | n (%) | 187 (21.7) |
| Westeinde, The Hague | n (%) | 10 (1.2) |
SD standard deviation, IQR interquartile range, PCBC primary care birth centre.
aC-section, instrumental refers to (1) a vaginally, instrumental delivery that failed and subsequently a C-section was performed or (2) the use of vacuum extraction or forceps during C-section to assist the delivery of the neonate’s head.
bAccording to the definition of Statistics Netherlands24.
cDuration of inclusion period differed per PCBC. See Supplementary Table 1.
Assessment and incidence of neonatal hyperbilirubinaemia
The majority of neonates (n = 608, 71.9%) had some degree of jaundice at any point during admission in the PCBC; the maximum degree of jaundice was ‘slightly yellow’ in the vast majority of jaundiced neonates (n = 442, 72.7%). In most neonates, jaundice was first noted on postnatal day one or two (n = 390, 75.0% of neonates having some degree of jaundice); two neonates (0.3%) were jaundiced within 24 h after birth (i.e., on postnatal day 0). TSB was quantified at least once in 129 neonates (15.0%). Twenty-three neonates (2.7%) had a TSB level above the phototherapy threshold during PCBC admission, at a median age of 57 h (IQR 43)4. In an additional five neonates, TSB level was above phototherapy threshold after discharge home from the PCBC (postnatal age range: 40–142 h), see Table 2.
Table 2.
Assessment and incidence of neonatal hyperbilirubinaemia.
| n = 860 | ||
|---|---|---|
| Neonates having any degree of jaundice as assessed by MCA; maximum degree | n (%) | 608 (71.9) |
| Slightly yellow | n (%) | 442 (72.7) |
| Moderately yellow | n (%) | 91 (15.0) |
| Quite yellow | n (%) | 61 (10.0) |
| Very yellow | n (%) | 14 (2.3) |
| Missing visual jaundice assessment | n | 14 |
| First postnatal day of jaundice during admission in PCBC (n = 608)a | ||
| Day 0 (0–23 h) | n (%) | 2 (0.9) |
| Day 1–2 (24–71 h) | n (%) | 390 (75.0) |
| Day 3–5 (72–143 h) | n (%) | 202 (73.2) |
| Day 6–8 (144–215 h) | n (%) | 8 (23.5) |
| Missing first day of jaundice | n | 6 |
| Neonates who had TcB quantified in PCBC | n (%) | 116 (13.5) |
| 1 TcB quantification | n (%) | 80 (9.3) |
| 2 or more TcB quantifications | n (%) | 36 (4.2) |
| Neonates who had TSB quantified in PCBC (before start of phototherapy, if indicated) | n (%) | 129 (15.0) |
| 1 TSB quantification | n (%) | 96 (11.1) |
| 2 or more TSB quantifications | n (%) | 33 (3.8) |
| Bilirubin nomogram risk category | ||
| Lower risk | n (%) | 664 (77.2) |
| Medium risk | n (%) | 172 (20.0) |
| Higher risk | n (%) | 14 (1.6) |
| Missing | n | 10 |
| Highest TSB level during admission in PCBC (µmol/L; n = 124) | Mean (SD) | 223 (68) |
| TSB level missing | n | 5 |
| Neonates having a TSB level above phototherapy threshold during admission in PCBCb | n (%) | 26 (3.0) |
| TSB level missing | n | 2 |
| Postnatal age when exceeding phototherapy threshold in PCBC (hours; n = 23) | Median (IQR) | 57 (43) |
| Neonates having a TSB level above phototherapy threshold after discharge home | n (%) | 7 (0.8) |
| Missing | n | 148 |
MCA maternity care assistant, PCBC primary care birth centre, TcB transcutaneous bilirubin, TSB total serum bilirubin, SD standard deviation, IQR interquartile range.
aPercentage according to number of participating neonates that had some degree of jaundice during admission in the PCBC and were admitted in a participating PCBC at the time.
bPhototherapy threshold according to the Dutch TSB nomogram4,5.
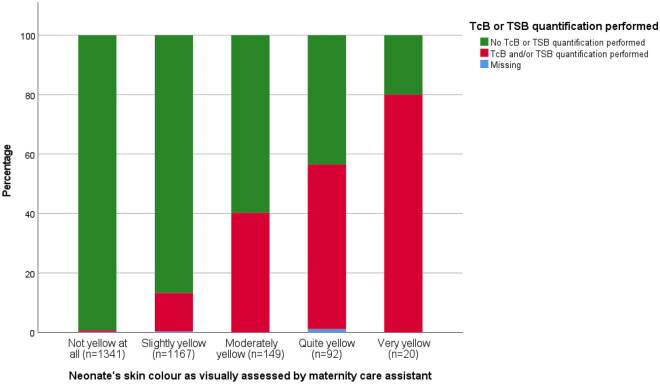
In total, 165 TcB and 171 TSB quantifications were performed during admission in the PCBC. Figure 2 shows the association between visual jaundice assessment by the MCA and whether or not TcB or TSB quantification was performed. Although there was a clear increase in the proportion of neonates having TcB or TSB quantified as jaundice was considered more severe (χ2 trend test p < 0.001), still no TcB or TSB was quantified in 44% of the neonates considered ‘quite yellow’ and in 20% in of the neonates considered ‘very yellow’.
Figure 2.
Proportion of assessment resulting in TcB or TSB being quantified according to degree of visible jaundice. TcB transcutaneous bilirubin, TSB total serum bilirubin.
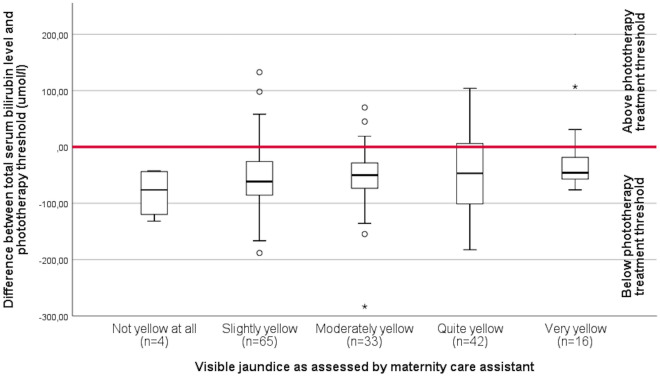
The difference between individual phototherapy treatment thresholds and TSB levels according to the visually assessed degree of jaundice is shown in Fig. 3. TSB was below the treatment threshold for all four assessments resulting in TSB being quantified in the absence of jaundice. There was no clear association between the degree of jaundice and the TSB level in those having TSB quantified (p = 0.416).
Figure 3.
Difference between individual phototherapy treatment threshold and total serum bilirubin level according to degree of jaundice as visually assessed. The area above the red bar indicates a total serum bilirubin level above phototherapy treatment threshold.
Management of hyperbilirubinaemia
Table 3 shows the management of hyperbilirubinaemia in neonates who received treatment. During the control period of the STARSHIP Trial, 33 neonates (3.8%) had a TSB level above the phototherapy treatment threshold4. Phototherapy was performed in 31 neonates (3.6%) with a median duration of 22 h (IQR 22.5). Three neonates (0.3%) received phototherapy despite having a TSB level below the phototherapy threshold, whereas five neonates (0.6%) did not receive phototherapy despite having a TSB level above the phototherapy threshold4. TSB levels of the latter five exceeded phototherapy threshold with a maximum of 31 µmol/L (1.81 mg/dL). One of these neonates was admitted to the hospital for another reason than hyperbilirubinaemia treatment. TSB levels exceeded the threshold for exchange transfusion (with a maximum of 71 µmol/L; 4.15 mg/dL) during admission in the PCBC in three neonates (0.3%) and during hospital admission in one additional neonate (0.1%)4,5, but no exchange transfusions were performed. The neonates with TSB levels that exceeded the exchange transfusion threshold during admission in the PCBC were slightly yellow (n = 2) and very yellow (n = 1). None of these neonates had a TSB quantified in the PCBC prior to exceeding the exchange transfusion threshold.
Table 3.
Hyperbilirubinaemia management.
| n = 858a | ||
|---|---|---|
| Neonates having hyperbilirubinaemia above the treatment threshold4,5 | n (%) | 33 (3.8) |
| Highest TSB level overall if necessitating treatment (µmol/L) | Mean (SD) | 318 (50) |
| Phototherapy performed | n (%) | 31 (3.6) |
| Total duration of phototherapy (hours) | Median (IQR) | 22 (22.5) |
| Missing duration of phototherapy | n | 1 |
| Exchange transfusion threshold exceededb | n (%) | 4 (0.5) |
| Exchange threshold exceeded in PCBC | n (%) | 3 (0.3) |
| Exchange threshold exceeded in hospital | n (%) | 1 (0.1) |
| Exchange transfusion performed | n (%) | 0 (0.0) |
Risk factors for receiving phototherapy treatment
Neonates who received phototherapy were more often born before 38 weeks of gestation when compared to neonates not receiving hyperbilirubinaemia treatment (56.7% vs. 12.8%; p < 0.001). The proportion of neonates born after an instrumental delivery was higher in the group receiving phototherapy than in the group not receiving phototherapy (26.7% vs. 8.3%; p = 0.004). Birth weight percentile25, perinatal asphyxia, Rh D incompatibility, type of feeding, sibling(s) who received phototherapy, and ethnicity were not significantly different between neonates who received phototherapy and those who did not (Table 4).
Table 4.
Association of risk factors with receiving treatment for hyperbilirubinaemia.
| Received phototherapy (n = 31) | Did not receive phototherapy (n = 827) | Total (n = 858)a | p value | |
|---|---|---|---|---|
| Gestational age | < 0.001* | |||
| < 38 weeks | 17 (56.7) | 105 (12.8) | 122 (14.4) | |
| ≥ 38 weeks | 13 (43.3) | 713 (87.2) | 726 (85.6) | |
| Missing | 1 | 9 | 10 | |
| Mode of delivery | 0.004* | |||
| Non-instrumental | 22 (73.3) | 751 (91.7) | 773 (91.0) | |
| Instrumental | 8 (26.7) | 68 (8.3) | 76 (9.0) | |
| Missing | 1 | 8 | 9 | |
| Birth weight percentile25 | 0.398 | |||
| < p10 | 5 (16.7) | 88 (10.8) | 93 (11.0) | |
| p10–p90 | 25 (83.3) | 647 (79.1) | 672 (79.2) | |
| > p90 | 0 (0.0) | 83 (10.1) | 83 (9.8) | |
| Missing | 1 | 9 | 10 | |
| Presence of perinatal asphyxia* | 0.191 | |||
| Yes | 2 (7.1) | 27 (3.3) | 29 (3.5) | |
| No | 26 (92.9) | 783 (96.7) | 809 (96.5) | |
| Missing | 3 | 17 | 20 | |
| Presence of Rh D incompatibility | 0.920 | |||
| Yes | 2 (7.1) | 40 (6.2) | 42 (6.2) | |
| No | 26 (92.9) | 608 (93.8) | 634 (93.8) | |
| Missing or unknown | 3 | 81 | 182 | |
| Type of feeding | 0.673 | |||
| Exclusive breastfeeding | 17 (56.7) | 516 (62.9) | 284 (34.5) | |
| Non-exclusive or no breastfeeding | 13 (43.3) | 304 (37.1) | 540 (65.5) | |
| Missing | 1 | 9 | 10 | |
| Sibling received hyperbilirubinaemia necessitating treatment | 0.964 | |||
| Yes | 4 (13.8) | 33 (4.8) | 37 (5.2) | |
| No | 25 (86.2) | 649 (95.2) | 674 (94.8) | |
| Missing | 2 | 145 | 147 | |
| Ethnicity neonate | 0.154 | |||
| Western | 22 (75.9) | 489 (71.7) | 511 (71.9) | |
| Non-Western | 7 (24.1) | 193 (28.3) | 200 (28.1) | |
| Missing | 2 | 147 | 149 |
aThe need for phototherapy was unknown for two neonates, as daily measurements and parental questionnaire were not filled out. These neonates were excluded from this analysis.
*Defined as Apgar score < 5 at 5 min and/or umbilical cord pH < 7.0. According to the definition of the Dutch TSB nomogram.
Discussion
In our prospective cohort study evaluating the assessment, management, and incidence of neonatal hyperbilirubinaemia and the need for phototherapy among neonates cared for in primary care, we found that approximately 70% of neonates became jaundiced at any point during the first days of life and that 3.6% received treatment for hyperbilirubinaemia. However, not all neonates who had a TSB level that exceeded the phototherapy threshold received phototherapy. Also, TcB or TSB levels were not quantified in a substantial proportion of neonates assessed as moderately to severely jaundiced. Visual jaundice assessment was not reliable in estimating TSB levels.
To the best of our knowledge, this study is the first to prospectively describe the full scope of assessment, management, and corresponding incidence of hyperbilirubinaemia in otherwise healthy neonates cared for in primary care. This provides insight in the overall burden of neonatal hyperbilirubinaemia in primary care. We were able to identify neonates requiring phototherapy following discharge home by using parental questionnaires. Using parents as a source for data also has some pitfalls. First, if parents indicated that their neonate received phototherapy after discharge from the PCBC, this was not always in agreement with the actual data from the medical records in the hospital. Second, despite the prospective nature of the study, a proportion of included neonates had missing data, primarily due to missing parental questionnaires (17.3%). This may have led to an underestimation of the proportion of neonates who needed treatment. However, among the 711 (out of 860) neonates whose parents did respond, only five extra neonates who received treatment were identified using the questionnaires. Thus, we expect minimal influence of the missing data on this outcome. Neonates born after a C-section were overrepresented in our study (36% vs. 15% nationally)26, probably because their mothers were more likely to stay (longer) in the PCBC. As C-section is not known as a protective or risk factor for neonatal hyperbilirubinaemia, we expect negligible impact on our results. Additionally, the informed consent procedure may have induced selection (e.g., neonates whose parents refused participation in the STARSHIP Trial may have had other demographic characteristics). In contrast, overestimation of the proportion of neonates receiving hyperbilirubinaemia treatment in the whole population may have occurred as well. This is because we were dependent on parental consent for participation of their neonate in the STARSHIP trial and parents having a previous child with hyperbilirubinaemia may have been more likely to provide informed consent. Unfortunately, we were unable to assess the incidence of receiving phototherapy and associated risk factors (e.g., siblings having received phototherapy) among neonates without consent. Other findings may also have been influenced by the trial itself. Before the start of the STARSHIP Trial, all maternity care professionals were trained regarding neonatal hyperbilirubinaemia and study procedures. The training and the trial may have raised awareness on neonatal hyperbilirubinaemia, potentially resulting in a lower threshold to assess the neonate as jaundiced and to quantify TSB. From a clinical perspective, this can be considered a positive development in the context of preventing severe hyperbilirubinaemia.
The incidence of visible jaundice in our study is comparable to other studies in (near) term neonates in which 60–90% became jaundiced27–29. The finding that visual jaundice assessment is not reliable to estimate TSB levels is in line with other studies describing the inaccuracy of visual jaundice assessment6,7. Strikingly, in a substantial proportion of neonates being assessed as ‘quite yellow’ or ‘very yellow’, no TcB or TSB was quantified. This observation corresponds with a previous study among MCAs regarding neonatal hyperbilirubinaemia, which showed structural underestimation of TSB levels and common application of a so-called ‘wait-and-see approach’ in visibly jaundiced neonates30. Moreover, despite being strongly recommended by the national guideline4, TSB was not quantified in two neonates who developed visible jaundice within 24 h after birth. Also, five neonates did not receive phototherapy despite having a TSB level that exceeded the phototherapy threshold as defined by the national guideline4. Our evaluation of standard practice in this cohort highlights significant gaps in guideline application. In the current study, we did not prospectively explore the considerations underlying these decisions. Previous studies indicate that lack of knowledge on guideline recommendations31,32, and systematic underestimation of the severity of jaundice based on visual assessment likely contributed30. Other potential reasons for non-compliance may include a belief that the recommendations in the guideline do not reflect the best care for the neonate (e.g., a healthcare provider may consider the phototherapy thresholds too conservative as evidence on exact phototherapy thresholds is lacking33, and TSB quantification is avoided to keep the neonate in primary care), or practical challenges regarding feasibility of guideline compliance in daily practice. Research focused on these considerations may be useful to improve guideline adherence. Non-compliance to neonatal jaundice guidelines can have potentially severe consequences, as demonstrated by Rennie et al. in a Swedish study where KSD was (potentially) avoidable in 11 out of 13 neonates having KSD9. Additionally, a national audit indicated that non-compliance to the guideline was an important contributing factor to severe neonatal hyperbilirubinaemia in the Netherlands34.
Most studies assessing the burden of neonatal hyperbilirubinaemia focused on severe neonatal hyperbilirubinaemia or on KSD12–17. Studies assessing the hospitalisation rate for neonatal hyperbilirubinaemia showed incidences for hyperbilirubinaemia treatment ranging from 0.55 to 2.62%35–39. The retrospective nature of these studies in which the researchers depended on correct registration of the diagnosis of hyperbilirubinaemia may have contributed to the lower published incidence. Studies having phototherapy use as secondary outcome when assessing the institution of a bilirubin screening programme found (slightly) higher percentages in their control group (4.2–6.1%)38–40. The difference in the percentage of neonates necessitating hyperbilirubinaemia treatment between these studies and ours may also be attributed to other hyperbilirubinaemia assessment and management strategies. In the Netherlands, neonates are typically screened visually for neonatal hyperbilirubinaemia, followed by selective TSB quantification; universal TcB or TSB screening is not performed. Additionally, in the Netherlands a relatively high proportion of neonates are cared for in primary care shortly after birth, where transcutaneous bilirubinometers are not widely used yet. Our current evaluation of care-as-usual indicates that TcB or TSB is often not quantified even in neonates who were considered quite yellow or very yellow. As such, it is possible that some neonates requiring phototherapy were not identified. In other countries, most neonates remain admitted in the hospital for several days after birth and TcB or TSB is quantified before discharge3,15.
Potential risk factors for developing severe hyperbilirubinaemia have been widely investigated3,33. Whereas gestational age < 38 weeks is a well-known risk factor, instrumental delivery itself is not widely investigated as risk factor16,41–43. Most studies focus on bruising and cephalic haematomas, that may arise from an instrumental delivery, which increases the risk for severe neonatal hyperbilirubinaemia16,41. Instrumental delivery may be a marker for another risk factor (e.g., large for gestational age; LGA)44. However, we did not find a higher LGA incidence in neonates receiving phototherapy. Other well-known risk factors for hyperbilirubinaemia, such as Rh D incompatibility, previous siblings who received phototherapy, and exclusive breastfeeding, did not differ significantly between neonates who received phototherapy and those who did not. This may in part be due to limited power.
Findings from this study are useful for perinatal healthcare providers in primary care as well as in secondary and tertiary care (e.g., if a neonate is admitted together with mother). Data on the incidence of jaundice and the need for hyperbilirubinaemia treatment can help raise awareness regarding the extent of the problem. This awareness should also include the inaccuracy of visual jaundice assessment. Although this inaccuracy has been demonstrated previously29,45,46, our current study indicates that many healthcare professionals still strongly rely on visual assessment, and this is in fact in line with the current Dutch guideline, which is now undergoing revision. Although not every case of severe hyperbilirubinaemia results in KSD, KSD is entirely preventable and should clearly be a never-event. As such, regarding severe hyperbilirubinaemia as a healthcare system failure may strengthen implementation of new strategies to prevent KSD47. More objective approaches to universal hyperbilirubinaemia screening, for example using a transcutaneous bilirubinometer, should be considered to improve early recognition of potentially severe hyperbilirubinaemia3,48,49. Even though the PCBCs and their healthcare professionals took part in a trial focused on hyperbilirubinaemia assessment and management, the recommendations of the national guideline regarding TSB quantification and start of phototherapy treatment were not adhered to in some cases. Hence, more knowledge regarding risk factors for hyperbilirubinaemia, when to quantify TSB, treatment thresholds, and adherence to the national guideline are important as well. Future research should focus on objective approaches of universal screening for potentially severe neonatal hyperbilirubinaemia in a primary care setting. The STARSHIP Trial will present results from implementing a universal screening programme in primary care using TcB in the next year or two.
In this prospective cohort study embedded in the STARSHIP Trial, assessment, management and incidence of neonatal jaundice and the need for phototherapy were evaluated. We demonstrated that the vast majority of neonates had some degree of jaundice during admission and that phototherapy was provided in 3.6% of neonates. Also, we showed that visual jaundice assessment was inaccurate in determining hyperbilirubinaemia and that compliance to the guideline requires improvement. We suggest that awareness regarding neonatal hyperbilirubinaemia and its potentially devastating consequences should be raised. Additionally, the benefits of objective universal screening to improve recognition of hyperbilirubinaemia need to be assessed in an attempt to reduce the burden of neonatal hyperbilirubinaemia.
Supplementary Information
Acknowledgements
We acknowledge the participants, their parent(s), and the personnel of the participating primary care birth centres for their efforts. We thank Martin Baartmans, Jolita Bekhof, Esther Bijl, Harry Buijs, Jan Erik Bunt, Peter Dijk, Marja Huizer, Christian Hulzebos, Ralph Leunissen, Beata Pazur, Ben Snoeren, Bente de Vries, and Leo Wewerinke for their support in initiating and conducting the STARSHIP Trial; Paul den Butter, Maurits Schreuder, and Gerdien Tramper-Stranders for providing data regarding hospital admissions; and Melissa Gouvernante, Janine Hageman, Joen IJsselmuiden, Kristel Kolloen, Deborah van Urk, Lisanne Vermeulen-Nieuwelink, Sanne van de Walle, Lina Wang, Senna Worlanyoh, and Miranda van Zwet for their help in data collection.
Author contributions
The STARSHIP Trial was designed by J.V.B., J.P.D.G., R.F.K., L.C.M.B., M.J.P., E.I., I.K.M.R., and E.A.P.S. Funding for the STARSHIP Trial was secured by J.V.B., J.P.D.G., R.F.K., I.K.M.R., and E.A.P.S. The members of the STARSHIP study group were involved in initiating and conducting the research in the participating PCBCs. B.A.M.v.d.G. and J.V.B. designed the current analysis of the control period of the STARSHIP Trial. Data was collected by B.A.M.v.d.G., M.J.S.d.M., and I.S.A.B. B.A.M.v.d.G., M.J.S.d.M., and I.S.A.B. analysed the data. B.A.M.v.d.G. and J.V.B. wrote the first version of the manuscript. All authors were involved in the interpretation of the data. The manuscript was critically revised and approved for final submission by all authors.
Funding
This work was supported by The Netherlands Organisation for Health Research and Development (ZonMw), grant number 843002805, and an Erasmus MC Efficiency Research grant, grant number 2016-16107. The funders had no role in the design of the study, data collection, analysis and interpretation of the data, writing the manuscript, nor in the decision to submit the manuscript for publication.
Data availability
The anonymised datasets from the current study are available from the corresponding author on reasonable request.
Code availability
The syntaxes from the current analyses are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Berthe A. M. van der Geest, Email: b.vandergeest@erasmusmc.nl
Jasper V. Been, Email: j.been@erasmusmc.nl
STARSHIP Study Group:
Martin G. A. Baartmans, Jolita Bekhof, Harry Buijs, Jan Erik Bunt, Peter H. Dijk, Christian V. Hulzebos, Ralph W. J. Leunissen, Ben J. P. W. Snoeren, Bente de Vries, and Leo Wewerinke
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-17933-2.
References
- 1.Olusanya BO, Kaplan M, Hansen TWR. Neonatal hyperbilirubinaemia: A global perspective. Lancet Child Adolesc. Health. 2018;2:610–620. doi: 10.1016/S2352-4642(18)30139-1. [DOI] [PubMed] [Google Scholar]
- 2.Le Pichon JB, Riordan SM, Watchko J, Shapiro SM. The neurological sequelae of neonatal hyperbilirubinemia: Definitions, diagnosis and treatment of the kernicterus spectrum disorders (KSDs) Curr. Pediatr. Rev. 2017;13:199–209. doi: 10.2174/1573396313666170815100214. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- 4.Nederlandse Vereniging voor Kindergeneeskunde, Kwaliteitsinstituut voor de Gezondheidszorg CBO. Guideline prevention, diagnosis and treatment of hyperbilirubinaemia among newborns born at a gestational age of more than 35 weeks. Richtlijn preventie, diagnostiek en behandeling van hyperbilirubinemie bij de pasgeborene, geboren na een zwangerschapsduur van meer dan 35 weken. https://www.nvk.nl/Portals/0/richtlijnen/hyperbili/richtlijnhyperbili.pdf (2008). [PubMed]
- 5.Paediatric Association of the Netherlands. Nederlandse Vereniging voor Kindergeneeskunde. Bilirubin nomograms >35 weeks. Bilicurve >35 wkn. [Paediatric Association of the Netherlands] Nederlandse Vereniging voor Kindergeneeskunde. http://babyzietgeel.nl/kinderarts/hulpmiddelen/diagnostiek/bilicurve35wkn.php (2008).
- 6.Lease M, Whalen B. Assessing jaundice in infants of 35-week gestation and greater. Curr. Opin. Pediatr. 2010;22:352–365. doi: 10.1097/MOP.0b013e328339603f. [DOI] [PubMed] [Google Scholar]
- 7.Maisels MJ, Clune S, Coleman K, Gendelman B, Kendall A, McManus S, Smyth M. The natural history of jaundice in predominantly breastfed infants. Pediatrics. 2014;134:e340–345. doi: 10.1542/peds.2013-4299. [DOI] [PubMed] [Google Scholar]
- 8.Donneborg ML, Hansen BM, Vandborg PK, Rodrigo-Domingo M, Ebbesen F. Extreme neonatal hyperbilirubinemia and kernicterus spectrum disorder in Denmark during the years 2000–2015. J. Perinatol. 2020;40:194–202. doi: 10.1038/s41372-019-0566-8. [DOI] [PubMed] [Google Scholar]
- 9.Alkén J, Håkansson S, Ekéus C, Gustafson P, Norman M. Rates of extreme neonatal hyperbilirubinemia and kernicterus in children and adherence to national guidelines for screening, diagnosis, and treatment in Sweden. JAMA Netw. Open. 2019;2:e190858. doi: 10.1001/jamanetworkopen.2019.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjerre JV, Petersen JR, Ebbesen F. Surveillance of extreme hyperbilirubinaemia in Denmark. A method to identify the newborn infants. Acta Paediatr. 2008;97:1030–1034. doi: 10.1111/j.1651-2227.2008.00879.x. [DOI] [PubMed] [Google Scholar]
- 11.Bhutani VK, Meng NF, Knauer Y, Danielsen BH, Wong RJ, Stevenson DK, Gould JB. Extreme hyperbilirubinemia and rescue exchange transfusion in California from 2007 to 2012. J. Perinatol. 2016;36:853–857. doi: 10.1038/jp.2016.106. [DOI] [PubMed] [Google Scholar]
- 12.Gotink MJ, Benders MJ, Lavrijsen SW, Rodrigues Pereira R, Hulzebos CV, Dijk PH. Severe neonatal hyperbilirubinemia in the Netherlands. Neonatology. 2013;104:137–142. doi: 10.1159/000351274. [DOI] [PubMed] [Google Scholar]
- 13.Sgro M, Campbell D, Shah V. Incidence and causes of severe neonatal hyperbilirubinemia in Canada. CMAJ. 2006;175:587–590. doi: 10.1503/cmaj.060328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sgro M, Campbell DM, Kandasamy S, Shah V. Incidence of chronic bilirubin encephalopathy in Canada, 2007–2008. Pediatrics. 2012;130:e886–890. doi: 10.1542/peds.2012-0253. [DOI] [PubMed] [Google Scholar]
- 15.Barrington KJ, Sankaran K, Canadian Paediatric Society, Fetus and Newborn Committee Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants. Paediatr. Child Health. 2007;12:1B–12B. doi: 10.1093/pch/12.5.401. [DOI] [Google Scholar]
- 16.Kuzniewicz MW, Escobar GJ, Wi S, Liljestrand P, McCulloch C, Newman TB. Risk factors for severe hyperbilirubinemia among infants with borderline bilirubin levels: A nested case-control study. J. Pediatr. 2008;153:234–240. doi: 10.1016/j.jpeds.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slusher TM, Zamora TG, Appiah D, et al. Burden of severe neonatal jaundice: A systematic review and meta-analysis. BMJ Paediatr. Open. 2017;1:e000105. doi: 10.1136/bmjpo-2017-000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Geest BAM, de Graaf JP, Bertens LCM, et al. Screening and treatment to reduce severe hyperbilirubinaemia in infants in primary care (STARSHIP): A factorial stepped-wedge cluster randomised controlled trial protocol. BMJ Open. 2019;9:e028270. doi: 10.1136/bmjopen-2018-028270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perined . Perinatal care in the Netherlands anno 2018: national perinatal figures and interpretation. Perinatale zorg in Nederland anno 2018: landelijke perinatale cijfers en duiding. Perined; 2019. [Google Scholar]
- 20.Lagendijk J, Steegers EAP, Been JV. Inequity in postpartum healthcare provision at home and its association with subsequent healthcare expenditure. Eur. J. Public Health. 2019;29:849–855. doi: 10.1093/eurpub/ckz076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Boer, J. & Zondag, L. Multidisciplinary guideline postnatal care. Multidisciplinaire richtlijn Postnatale Zorg - Verloskundige basiszorg voor moeder en kind. Koninklijke Nederlandse Organisatie van Verloskundigen. https://www.knov.nl/serve/file/knov.nl/knov_downloads/2882/file/Postnatale_zorg_opgemaakte_versie_door_IB_md_10_aug_2018.pdf (2018).
- 22.Schmitz, C. LimeSurvey: An open source survey tool. LimeSurvey Project, Hamburg, Germany (2012).
- 23.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Guideline for Good Clinical Practice ICH (2016).
- 24.Statistics Netherlands. Centraal Bureau voor de Statistiek. Person with a western migration background. Statistics Netherlands Centraal Bureau voor de Statistiek. https://www.cbs.nl/en-gb/onze-diensten/methods/definitions/person-with-a-western-migration-background.2021 (2016).
- 25.Hoftiezer L, Hof MHP, Dijs-Elsinga J, Hogeveen M, Hukkelhoven CWPM, van Lingen RA. From population reference to national standard: New and improved birthweight charts. Am. J. Obstet. Gynecol. 2019;220:383.e381–383.e317. doi: 10.1016/j.ajog.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Perined. Peristat. http://www.peristat.nl (2020).
- 27.Hansen TWR. The epidemiology of neonatal jaundice. Pediatr. Med. 2021 doi: 10.21037/pm-21-4. [DOI] [Google Scholar]
- 28.Osborn LM, Reiff MI, Bolus R. Jaundice in the full-term neonate. Pediatrics. 1984;73:520–525. doi: 10.1542/peds.73.4.520. [DOI] [PubMed] [Google Scholar]
- 29.Keren R, Tremont K, Luan X, Cnaan A. Visual assessment of jaundice in term and late preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 2009;94:F317–F322. doi: 10.1136/adc.2008.150714. [DOI] [PubMed] [Google Scholar]
- 30.van der Geest BAM, Theeuwen IM, Reiss IKM, Steegers EAP, Been JV. Assessing knowledge and skills of maternity care professionals regarding neonatal hyperbilirubinaemia: A nationwide survey. BMC Pregnancy Childbirth. 2021;21:63. doi: 10.1186/s12884-020-03463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakkee M, Lugtenberg M, Spuls PI, de Jong EM, Thio HB, Westert GP, Nijsten T. Knowledge, attitudes and use of the guidelines for the treatment of moderate to severe plaque psoriasis among Dutch dermatologists. Br. J. Dermatol. 2008;159:426–432. doi: 10.1111/j.1365-2133.2008.08692.x. [DOI] [PubMed] [Google Scholar]
- 32.Lugtenberg M, Zegers-van Schaick JM, Westert GP, Burgers JS. Why don't physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implement Sci. 2009;4:54. doi: 10.1186/1748-5908-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Collaborating Centre for Women's and Children's Health. Neonatal jaundice. National Institute for Health and Clinical Excellence. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0016565/pdf/PubMedHealth_PMH0016565.pdf (2010).
- 34.van der Geest BAM, Rosman AN, Bergman KA, Smit BJ, Dijk PH, Been JV, Hulzebos CV. Severe neonatal hyperbilirubinaemia: Lessons learnt from a national perinatal audit. Arch. Dis. Child Fetal Neonatal Ed. 2022 doi: 10.1136/archdischild-2021-322891. [DOI] [PubMed] [Google Scholar]
- 35.Eggert LD, Wiedmeier SE, Wilson J, Christensen RD. The effect of instituting a prehospital-discharge newborn bilirubin screening program in an 18-hospital health system. Pediatrics. 2006;117:e855–e862. doi: 10.1542/peds.2005-1338. [DOI] [PubMed] [Google Scholar]
- 36.Burgos AE, Schmitt SK, Stevenson DK, Phibbs CS. Readmission for neonatal jaundice in California, 1991–2000: Trends and implications. Pediatrics. 2008;121:e864–869. doi: 10.1542/peds.2007-1214. [DOI] [PubMed] [Google Scholar]
- 37.Burke BL, Robbins JM, Bird TM, Hobbs CA, Nesmith C, Tilford JM. Trends in hospitalizations for neonatal jaundice and kernicterus in the United States, 1988–2005. Pediatrics. 2009;123:524–532. doi: 10.1542/peds.2007-2915. [DOI] [PubMed] [Google Scholar]
- 38.Wainer S, Parmar SM, Allegro D, Rabi Y, Lyon ME. Impact of a transcutaneous bilirubinometry program on resource utilization and severe hyperbilirubinemia. Pediatrics. 2012;129:77–86. doi: 10.1542/peds.2011-0599. [DOI] [PubMed] [Google Scholar]
- 39.Kuzniewicz MW, Escobar GJ, Newman TB. Impact of universal bilirubin screening on severe hyperbilirubinemia and phototherapy use. Pediatrics. 2009;124:1031–1039. doi: 10.1542/peds.2008-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mah MP, Clark SL, Akhigbe E, Englebright J, Frye DK, Meyers JA, Perlin JB, Rodriguez M, Shepard A. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics. 2010;125:e1143–e1148. doi: 10.1542/peds.2009-1412. [DOI] [PubMed] [Google Scholar]
- 41.Newman TB, Xiong B, Gonzales VM, Escobar GJ. Prediction and prevention of extreme neonatal hyperbilirubinemia in a mature health maintenance organization. Arch. Pediatr. Adolesc. Med. 2000;154:1140–1147. doi: 10.1001/archpedi.154.11.1140. [DOI] [PubMed] [Google Scholar]
- 42.Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101:995–998. doi: 10.1542/peds.101.6.995. [DOI] [PubMed] [Google Scholar]
- 43.Keren R, Luan X, Friedman S, Saddlemire S, Cnaan A, Bhutani VK. A comparison of alternative risk-assessment strategies for predicting significant neonatal hyperbilirubinemia in term and near-term infants. Pediatrics. 2008;121:e170–e179. doi: 10.1542/peds.2006-3499. [DOI] [PubMed] [Google Scholar]
- 44.Keren R, Bhutani VK, Luan X, Nihtianova S, Cnaan A, Schwartz JS. Identifying newborns at risk of significant hyperbilirubinaemia: A comparison of two recommended approaches. Arch. Dis. Child. 2005;90:415–421. doi: 10.1136/adc.2004.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moyer VA, Ahn C, Sneed S. Accuracy of clinical judgment in neonatal jaundice. Arch. Pediatr. Adolesc. Med. 2000;154:391–394. doi: 10.1001/archpedi.154.4.391. [DOI] [PubMed] [Google Scholar]
- 46.Riskin A, Tamir A, Kugelman A, Hemo M, Bader D. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia? J. Pediatr. 2008;152(782–787):787.e1–2. doi: 10.1016/j.jpeds.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Bhutani VK, Johnson L. A proposal to prevent severe neonatal hyperbilirubinemia and kernicterus. J. Perinatol. 2009;29:S61–S67. doi: 10.1038/jp.2008.213. [DOI] [PubMed] [Google Scholar]
- 48.Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J. Perinatol. 2010;30(Suppl):S6–15. doi: 10.1038/jp.2010.98. [DOI] [PubMed] [Google Scholar]
- 49.Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant > or =35 weeks' gestation: an update with clarifications. Pediatrics. 2009;124:1193–1198. doi: 10.1542/peds.2009-0329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The anonymised datasets from the current study are available from the corresponding author on reasonable request.
The syntaxes from the current analyses are available from the corresponding author on reasonable request.