Abstract
Three exocellular enzymes of Thermoanaerobacterium thermosulfurigenes EM1 possess a C-terminal triplicated sequence related to a domain of bacterial cell surface proteins (S-layer proteins). At least one copy of this sequence, named the SLH (for S-layer homology) domain, is also present at the N terminus of the S-layer protein of this bacterium. The hypothesis that SLH domains serve to anchor proteins to the cell surface was investigated by using the SLH domain-containing xylanase. This enzyme was isolated from T. thermosulfurigenes EM1, and different forms with and without SLH domains were synthesized in Escherichia coli. The interaction of these proteins with isolated components of the cell envelope was determined to identify the attachment site in the cell wall. In addition, a polypeptide consisting of three SLH domains and the N terminus of the S-layer protein of T. thermosulfurigenes EM1 were included in these studies. The results indicate that SLH domains are necessary for the attachment of these proteins to peptidoglycan-containing sacculi. Extraction of the native sacculi with hydrofluoric acid led to the conclusion that not peptidoglycan but accessory cell wall polymers function as the adhesion component in the cell wall. Our results provide further evidence that attachment of proteins via their SLH domains represents an additional mode to display polypeptides on the cell surfaces of bacteria.
Thermoanaerobacterium thermosulfurigenes EM1 is an anaerobic thermophilic microorganism. An S-layer of hexagonal lattice symmetry is noncovalently attached to its gram-positive-type cell wall. It has been shown that at least two enzymes, such as a pullulanase and a xylanase, interact with the cell surface of T. thermosulfurigenes EM1 (7, 29). The C termini of those exocellular glycosidases consist of three SLH (S-layer homology) domains with almost identical amino acid sequences (21). Sequence comparison with the N terminus of the S-layer protein from T. thermosulfurigenes EM1 revealed a high level of similarity to the N-terminal SLH domain of the Thermoanaerobacter kivui S-layer protein, leading to the conclusion that the S-layer protein of strain EM1 also contains at least one SLH domain (7). SLH domains have been found in several other exocellular proteins (17). It has been shown for structural proteins that SLH domains serve as cell wall anchors. OlpB, an outer layer protein of Clostridium thermocellum, was shown to be linked to the cell envelope via its SLH domains (16). Olabarría et al. (23) reported that deletion of the SLH domains from the Thermus thermophilus S-layer protein results in the inability of the protein to attach to the underlying material in vivo. In both cases peptidoglycan was suggested to function as the adhesion component in the cell envelope. In agreement with these findings, Lemaire et al. (15) showed that an N-terminal region of the S-layer protein from Clostridium thermocellum, which contains SLH domains, was able to bind isolated cell walls. A different adhesion component was identified in Bacillus stearothermophilus PV72. The S-layer protein of this bacterium also possesses a typical SLH domain and was found to attach to a secondary cell wall polymer (24, 27).
In a recent study we found that the S-layer protein and the xylanase XynA of T. thermosulfurigenes EM1 share a common attachment site in the underlying peptidoglycan-containing layer (7). Here, we provide evidence that the SLH domains are necessary to anchor both the S-layer protein and the exocellular xylanase XynA from T. thermosulfurigenes EM1 to the cell wall and that accessory cell wall polymers and not peptidoglycan function as the adhesion component in the cell wall. To our knowledge, this is the first time an enzyme has been found to be anchored to the cell wall via SLH domains.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
T. thermosulfurigenes EM1 was grown anaerobically at 60°C on minimal medium with 0.5% glucose as described previously (2). Growth in continuous culture was performed as described by Specka et al. (29). Escherichia coli DH5α was used as the host for cloning and for expression of xynA and xynA derivatives. Overexpression of the gene fragment encoding the SLH polypeptide (7) was performed in E. coli BL21(DE3). E. coli was routinely grown in Luria-Bertani medium (25) at 37°C, with ampicillin (70 μg/ml), IPTG (isopropyl-β-d-thiogalactopyranoside) (48 μg/ml), and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (40 μg/ml) if required. T7 medium (31) was used for overproduction of the SLH polypeptide in BL21(DE3). Overexpression was induced at an optical density at 600 nm of 0.4 with 0.1 mM (final concentration) IPTG.
Plasmids.
Isolation of plasmids from E. coli was performed as described by Birnboim and Doly (4). pUC18 (Stratagene, San Diego, Calif.) served as the cloning and expression vector for xylanase-encoding DNA fragments. The SLH polypeptide was overproduced from plasmid pCT1611, which carried the part of xynA encoding three SLH domains (7). The plasmids constructed in this study and the corresponding proteins are listed below and summarized in Fig. 1.
FIG. 1.
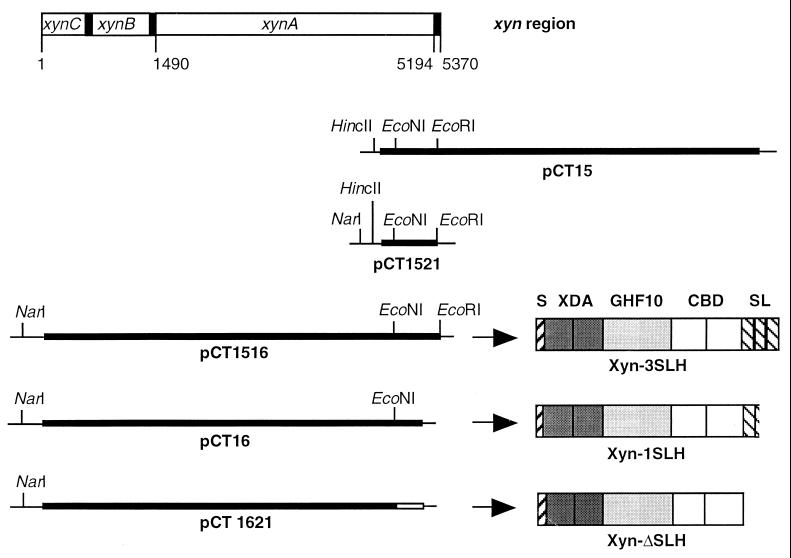
Schematic representation of the chromosomal xyn region from T. thermosulfurigenes EM1; DNA fragments cloned on plasmids pCT15, pCT1521, pCT1516, pCT16, and pCT1621; and the domain structures of XynA and XynA derivatives encoded on the plasmids. S, signal peptide; XDA, xylanase domains A; GHF10, catalytic domain of glycosyl hydrolase family 10; CBD, cellulose-binding domains; SL, SLH domains. Restriction sites in pUC18 (thin line) and the inserted gene fragments (thick line) are indicated.
(i) pCT15 and pCT16.
The xylanase-encoding gene xynA was subcloned in two plasmids, pCT15 and pCT16 (21). pCT16 carried the 3′ end of xynC and xynB and the 5′ end of xynA. xynB and xynC were suspected to encode membrane components of an ABC transporter. The 5′ xynA fragment encoded a xylanase containing only one intact SLH domain (Xyn-1SLH [Fig. 1]). pCT15 contained the 3′ end of xynA (Fig. 1).
(ii) Construction of pCT1521.
Subcloning of a HincII-EcoRI fragment of pCT15 yielded pCT1521, which carried the 3′ end of xynA (Fig. 1).
(iii) Construction of pCT1516.
A NarI-EcoNI fragment of pCT16 containing the 5′ end of xynA was inserted between the NarI and EcoNI sites of pCT1521. The resulting plasmid contained the complete xynA gene, encoding a xylanase with three SLH domains (Xyn-3SLH [Fig. 1]).
(iv) Construction of pCT1621.
pCT16 was digested with EcoNI and then treated with DNA polymerase I and polynucleotide kinase in the presence of deoxynucleoside triphosphates. Subsequent ligation resulted in plasmid pCT1621, which exhibited a frameshift and a stop codon within the former EcoNI site of xynA. The corresponding xylanase did not contain any SLH domains (Xyn-ΔSLH [Fig. 1]).
DNA sequencing.
DNA was sequenced by the dideoxy chain termination method of Sanger et al. (26) with 35S-dATP and a T7 sequencing kit (Pharmacia, Freiburg, Germany).
Expression of xynA and xynA derivatives.
The genes or gene fragments carried by pCT1516, pCT16, and pCT1621 were expressed by growing E. coli DH5α containing the respective plasmid. When the cultures had reached an optical density at 600 nm of 0.8, cells were harvested by centrifugation (5,000 × g, 15 min, 4°C) and washed twice with 50 mM sodium-acetate buffer, pH 5.6. After resuspension in 3 volumes of the same buffer, cells were disrupted by sonication. Cell debris were removed by centrifugation at 20,000 × g for 30 min at 4°C, and the cell-free protein extract was used in the interaction studies.
Overproduction and purification of the SLH polypeptide.
Plasmid pCT1611 (7), encoding a His-tagged SLH polypeptide (composed of the three SLH domains of the xylanase from T. thermosulfurigenes EM1), was used to overexpress this protein in E. coli. Purification was achieved by affinity chromatography on Ni2+-nitriloacetic acid agarose as described previously (7).
Preparation and purification of antibodies against the SLH polypeptide.
Antibodies against the SLH polypeptide used in Western blotting experiments were produced as described by Brechtel et al. (7).
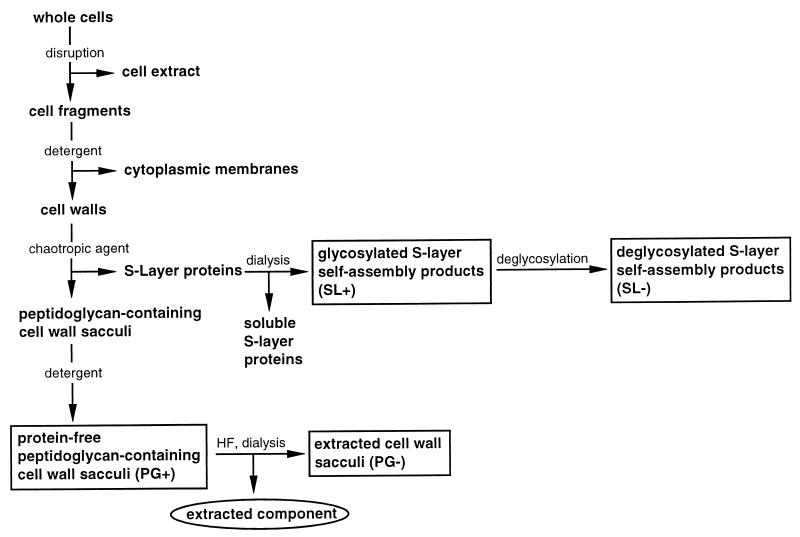
Isolation of S-layer- and peptidoglycan-containing cell wall polymers and extraction of peptidoglycan-containing sacculi with HF.
S-layer self-assembly products (SL+) and peptidoglycan-containing cell wall sacculi (PG+) (Fig. 2) were prepared by cell wall fractionation of T. thermosulfurigenes EM1 by the method of Messner and Sleytr (22) as described previously (7).
FIG. 2.
Schematic overview of the cell wall preparation procedure and the resulting fractions. Boxed fractions were used in interaction studies. Abbreviations used for the cell wall components are in parentheses. The circled fraction was used for analysis of the extracted component.
In order to remove covalently bound accessory cell wall polymers from the peptidoglycan layer, the extraction method of Ries et al. (24) was used. Native sacculi were treated with 48% HF for 96 h at 4°C. After centrifugation at 40,000 × g for 20 min at 4°C, the pellet representing the extracted peptidoglycan fraction (PG−) (Fig. 2) was washed once with 48% HF and three times with distilled water. The extract was dialyzed against distilled water overnight.
Deglycosylation of the S-layer protein.
Deglycosylation of the S-layer protein (yielding fraction SL−) (Fig. 2) was performed as described by Edge et al. (8).
Digestion of the S-layer protein with Lys-C.
S-layer self-assembly products (50 to 100 μg) were incubated with 2 μg of endoproteinase Lys-C (Boehringer, Mannheim, Germany) in 1 M GHCl in 10 mM Tris buffer (pH 7.5) under conditions suggested by the producer. The mixture was subsequently dialyzed for 16 h at 4°C against distilled water and centrifugated at 40,000 × g for 20 min at 4°C. The supernatant, which contained an N-terminal fragment of the S-layer protein, was used in interaction studies.
Analysis of the component extracted from peptidoglycan-containing cell wall sacculi.
Determination of the protein contents in the extract was done by measuring the absorbance at 280 nm by the method of Bradford (6). Detection of reducing sugars was performed with DNSA reagent (3). Detection of periodate-oxidizable polysaccharides was performed by a method described by Mantle and Allen (18). The method is based on the periodic acid-Schiff stain; periodate treatment leads to the formation of aldehyde groups, which form colored complexes with Schiff reagent.
Determination of xylanase activity.
Xylanase activity was measured by the DNSA method as described before (7).
SDS-PAGE and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out as described by Laemmli (14) with a minigel system (Biometra, Göttingen, Germany). Proteins were detected by Coomassie blue or silver staining (5). Molecular weights were estimated with the high-molecular-weight standard (Fluka, Neu-Ulm, Germany) or with the SDS–7-Da mark VII-L standard (Sigma, Deisenhofen, Germany). Proteins displaying xylanolytic activity were visualized with Congo red as described by Schwarz et al. (28).
Western blotting was performed with cellulose nitrate membranes in a semidry-fast blot apparatus (Biometra). SLH domains were detected with antibodies against the SLH polypeptide (7).
Interaction studies.
Exocellular proteins or protein constructs were tested for their ability to interact with isolated cell wall components. The respective protein was incubated with the isolated lyophylized cell wall component in buffer (xylanase and SLH polypeptide in 20 mM sodium-acetate buffer, pH 5.6; N terminus of S-layer protein in 20 mM Tris-HCl, pH 7.5) overnight at room temperature. The mixture was centrifuged (40,000 × g, 20 min, 4°C). The supernatant contained unbound proteins (fraction s). The pellet, consisting of the insoluble cell wall component and attached proteins, was washed twice and then resuspended in incubation buffer (volume equivalent to the volume of the supernatant) (fraction p). Both fraction s and p were investigated by SDS-PAGE and, if possible, by Western blotting and by measuring xylanase activity.
RESULTS
Extraction of peptidoglycan-containing cell wall sacculi with HF.
Previously, we reported on the isolation of S-layer- and peptidoglycan-containing cell wall sacculi (7). To obtain more detailed information about the type and location of the component responsible for attachment of exocellular proteins, the cell wall preparation was specified. Treatment of protein-free peptidoglycan-containing sacculi with HF, which had been shown to cleave phosphodiester linkages in cell wall polymers (24), led to remarkable changes in binding properties of the sacculi. In the interaction studies described below, the HF-extracted peptidoglycan fraction (PG−) was compared to native peptidoglycan-containing cell wall sacculi (PG+) in regard to its ability to bind different protein species from T. thermosulfurigenes EM1 containing SLH domains.
Analysis of the fraction extracted from the native sacculi revealed that it was protein free. Furthermore, no reducing sugars could be detected with DNSA reagent. However, a positive reaction was obtained after treatment with periodate and Schiff reagent. Therefore, we conclude that the extracted component is an accessory sugar-rich cell wall polymer. Evidence for such a polymer in the cell wall of T. thermosulfurigenes EM1 has been previously described (1).
Interaction of the SLH polypeptide with isolated cell wall components.
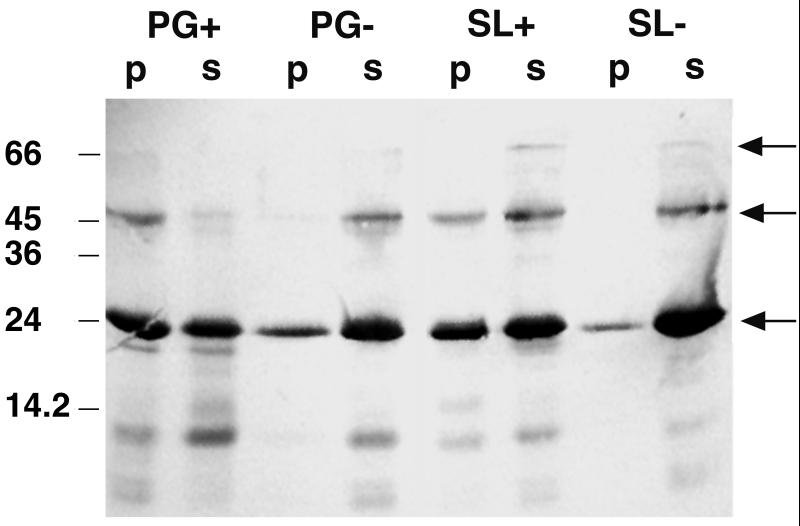
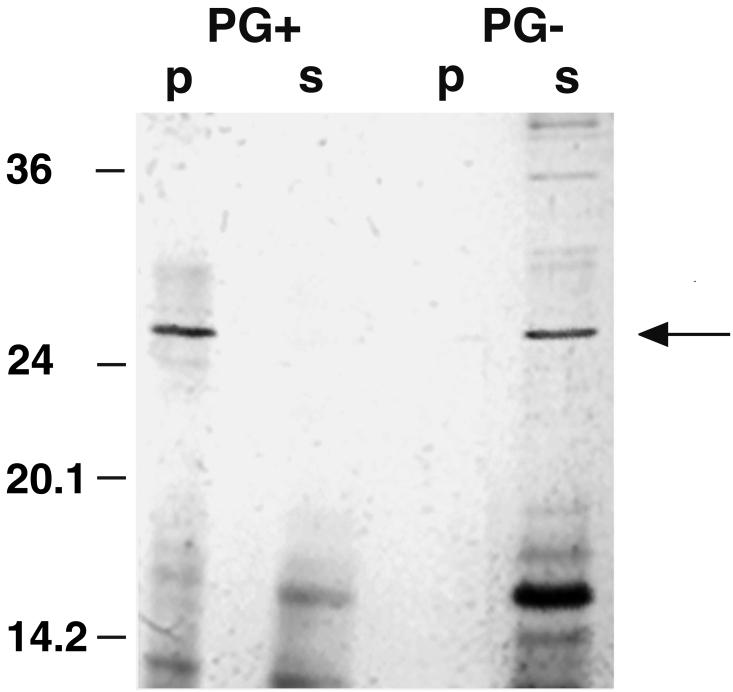
Recently, an SLH polypeptide consisting of the three SLH domains of the xylanase from T. thermosulfurigenes EM1 was overexpressed in E. coli (7). Here, we used this purified protein to investigate its interaction with isolated cell wall components. The SLH polypeptide was incubated with the respective cell wall components as described above. Two fractions resulted after centrifugation and washing of the pellet: fraction p contained the bound part of the SLH polypeptide, and fraction s (supernatant) consisted of the unbound polypeptide. SDS-PAGE of both fractions s and p and subsequent immunoblotting with antibodies against the SLH polypeptide gave the following results (Fig. 3). First, even under denaturing conditions, the SLH polypeptide exhibited high affinity to itself. In addition to the monomers (23 kDa), dimers and trimers of the SLH polypeptide were detected. Second, the highest affinity of the SLH polypeptide was to native sacculi (PG+) whereas only a small amount of the protein could bind to HF-extracted sacculi (PG−). Furthermore, it appeared that almost half of the polypeptide interacted with the glycosylated self-assembly products of the S-layer protein (SL+). In contrast, very little interaction was observed with deglycosylated S-layer (SL−).
FIG. 3.
Western blot showing the affinity of the SLH polypeptide to itself and to isolated cell wall components. Ten micrograms of the SLH polypeptide and 0.5 mg of the cell wall component in a total volume of 100 μl were used in the interaction study. Twenty microliters of each fraction (p and s) was applied to an SDS gel. The blot was prepared with antibodies against the SLH polypeptide. Fractions p and s represent bound and unbound proteins, respectively. Arrows indicate bands representing the SLH polypeptide monomer (23 kDa), dimer, and trimer.
Purification of the xylanase XynA from T. thermosulfurigenes EM1.
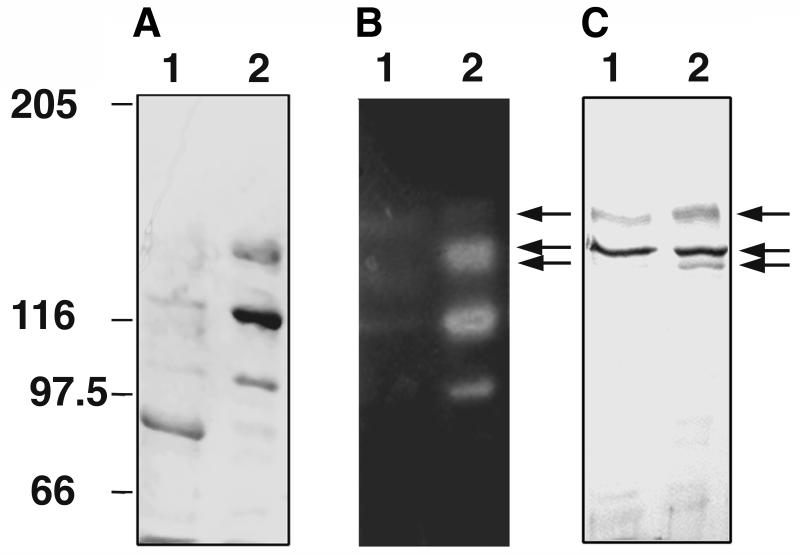
In addition to the SLH polypeptide, the affinity of an SLH domain-containing enzyme to isolated cell wall components was determined. For that purpose, the xylanase of T. thermosulfurigenes EM1 was purified and used in interaction studies (see below). The enzyme contains two cellulose-binding domains and therefore exhibits high affinity to microcrystalline cellulose. Thus, the enzyme could be purified in a simple one-step procedure by affinity chromatography on microcrystalline cellulose. A culture grown on minimal medium with 0.5% xylose was used for isolation of the xylanase. Under these conditions, about 80% of the enzyme was secreted into the medium at the stationary phase of the culture. Affinity chromatography on microcrystalline cellulose led to a 3.4-fold enrichment of the xylanase with a yield of 16.5%. In activity-stained SDS gels, five different xylanolytic forms could be detected after purification (Fig. 4). Their molecular masses, estimated from SDS gels, were 150, 136, 132, 115, and 100 kDa. The three largest forms gave signals on a Western blot prepared with antibodies against the SLH polypeptide and thus contained SLH domains (Fig. 4C). Since only one xylanase with SLH domains exists in T. thermosulfurigenes EM1 (21), we assume that at least the larger forms are derivatives of one gene product. According to the DNA sequence, the expected molecular masses of XynA are 136.5 and 133 kDa with and without a signal peptide, respectively. Therefore, the 150-kDa protein might be a glycosylated form of the xylanase. Glycosylation as well as the presence of multiple C-terminally truncated enzyme forms are well known in the case of the pullulanase from T. thermosulfurigenes EM1 (30). Since we did not find any evidence for the existence of several xylanase-encoding genes in T. thermosulfurigenes EM1, we expect that the two smaller xylanase forms (115 and 100 kDa) also are C-terminally truncated forms of the xynA gene product containing no SLH domain.
FIG. 4.
Analysis of xylanase purified from the culture supernatant of T. thermosulfurigenes EM1. (A) Coomassie blue-stained SDS gel; (B) activity staining for xylanases; (C) Western blot prepared with antibodies against the SLH domains of the xylanase. Lanes 1, concentrated culture supernatant; lanes 2, concentrated fraction obtained after affinity chromatography. One to 2 μg of protein was applied in each lane. Five different xylanase forms were observed. Arrows indicate xylanase derivatives containing SLH domains. Numbers on the left indicate migrations of marker proteins (in kilodaltons).
Production of intact and C-terminally truncated forms of the xylanase XynA from T. thermosulfurigenes EM1 in E. coli.
The role of SLH domains in cell wall attachment of the xylanase was also studied with heterologously expressed forms of the enzyme. An intact form and two truncated forms of the xylanase-encoding gene were constructed and expressed in E. coli. The resulting enzymes (Fig. 1) also exhibited xylanolytic activity but existed in multiple, relatively unstable forms in the crude extracts of E. coli. Besides proteins of the expected sizes (Xyn-3SLH, 136.5 kDa; Xyn-1SLH, 130.5 kDa; and Xyn-ΔSLH, 116 kDa [including the signal peptide]), many truncated, active and inactive forms of the enzymes appeared, which did not allow purification of the original full-length proteins. Therefore, the crude extracts were used in the following interaction studies.
Interaction of the xylanase from T. thermosulfurigenes EM1 and heterologously expressed forms of the enzyme with isolated cell wall components.
Interaction of SLH domains with cell wall components was quantitated in interaction studies by determining the amount of xylanase activity able to attach to the respective cell wall polymer. The purified xylanase XynA and the crude E. coli extracts containing Xyn-3SLH, Xyn-1SLH, or Xyn-ΔSLH were incubated with each of the following cell wall components: native peptidoglycan-containing cell wall sacculi (PG+), extracted cell wall sacculi (PG−), peptidoglycan from Staphylococcus aureus (PGS), S-layer self-assembly products (SL+), and deglycosylated self-assembly products (SL−) (Fig. 2). The distribution of xylanase activity in fractions p and s, representing bound and unbound xylanase, respectively, was determined. The results are listed in Table 1. A total of 48% of the xylanase XynA from T. thermosulfurigenes EM1 and 46% of Xyn-3SLH were found attached to the native cell wall sacculi (PG+), whereas much less of the xylanase could bind to the other cell wall components. The ability to bind to peptidoglycan-containing sacculi was drastically reduced in the case of Xyn-ΔSLH as well as Xyn-1SLH, indicating that the xylanase might need all three SLH domains for interaction. Since the purified xylanase XynA consisted of different forms with and without SLH domains, it is not surprising that the percentage of XynA bound to the sacculi was only 48%. It could be confirmed by use of activity-stained SDS gels and Western blot analysis that only the three largest forms of XynA with SLH domains were bound to cell wall sacculi (data not shown). Similarly, the fact that only 46% of the activity of Xyn-3SLH could bind to cell wall sacculi is most likely the result of xylanase degradation. The extent of the breakdown becomes obvious from the SDS gel depicted in Fig. 5, which shows that a high-molecular-weight fraction of active xylanases was bound to the cell wall sacculi. In contrast, a low-molecular-weight fraction of xylanases exhibited enzymatic activity but could not interact with the cell wall sacculi (Fig. 5A). As demonstrated by Western blot analysis, almost all xylanases and xylanase fragments, which retained their SLH domains, appeared in the insoluble fraction p, demonstrating that they were able to bind to cell wall sacculi (Fig. 5B).
TABLE 1.
Interaction of the xylanases XynA, Xyn-3SLH, and the C-terminally truncated forms Xyn-1SLH and Xyn-ΔSLH with isolated cell wall components of T. thermosulfurigenes EM1
| Xylanase | % of xylanase activity attached to the following cell wall componenta:
|
||||
|---|---|---|---|---|---|
| PG+ | PG− | PGS | SL+ | SL− | |
| XynA | 48 | 18 | 17 | 23 | 9 |
| Xyn-3SLH | 46 | 17 | 11 | 10 | 5 |
| Xyn-1SLH | 7 | 7 | 7 | 8 | 7 |
| Xyn-ΔSLH | 8 | 9 | 8 | 7 | 6 |
The xylanase (150 mU) was incubated with 1 mg of the indicated cell wall component in a total volume of 300 μl. The sum of the xylanase activities from the fractions containing bound (p) and unbound protein (s) was defined as 100%.
FIG. 5.
Interaction of the Xyn-3SLH produced from E. coli(pCT1516) with native peptidoglycan-containing cell wall sacculi (PG+). The xylanase (150 mU) was incubated with 1 mg of the PG+ fraction (total volume, 300 μl), and 20 μl of each sample was used for SDS-PAGE. (A) activity-stained SDS gel; (B) Western blot prepared with antibodies against the SLH polypeptide. Fractions p and s represent bound and unbound proteins, respectively. The migrations of marker proteins (in kilodaltons) are indicated on the left.
In summary, interaction studies with the wild-type and recombinant forms of the xylanase revealed that only those enzymes containing three SLH domains could bind to native cell wall sacculi. No significant affinity was found for HF-extracted sacculi (Table 1).
Interaction of the S-layer N terminus with isolated cell wall components.
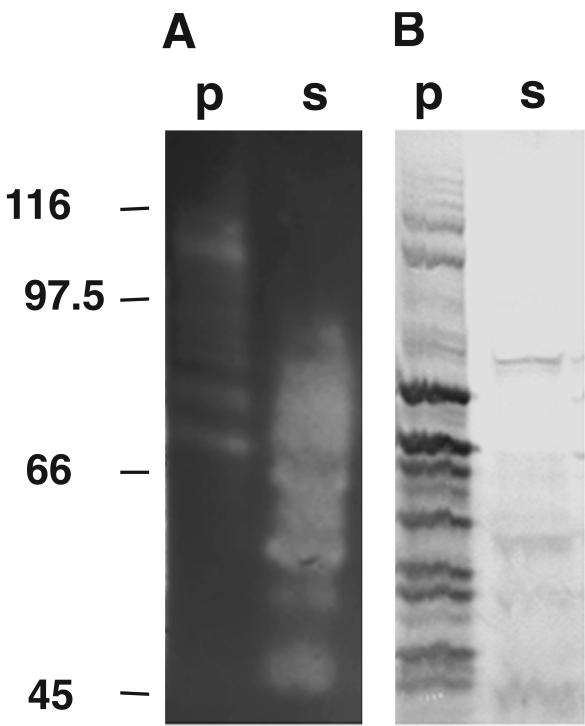
Digestion of the S-layer protein with endoproteinase Lys-C yielded a 27-kDa fragment which could be identified as the N terminus of the protein by amino acid sequence analysis. Comparison of the N-terminal amino acid sequence to the N terminus of the S-layer protein from T. kivui had revealed that the S-layer protein of T. thermosulfurigenes EM1 possesses at least one SLH domain at its N terminus (7). The results of interaction studies with Lys-C-digested S-layer protein and native (PG+) and extracted (PG−) cell wall sacculi are displayed in Fig. 6. High affinity of the N-terminal fragment of the S-layer protein, which contained an SLH domain, to peptidoglycan-containing sacculi was observed. In contrast, no interaction with HF-treated sacculi was found.
FIG. 6.
Interaction of an N-terminal cleavage fragment of the S-layer protein with native peptidoglycan-containing cell wall sacculi (PG+). A silver-stained SDS gel is shown. Fractions containing 20 μg of the cleavage fragments and 0.5 mg of the cell wall components were used (total volume, 100 μl), and 20 μl of each sample was applied to the gel. Bands representing the N-terminal cleavage fragment of the S-layer protein (27 kDa) are indicated by an arrow. p, fraction containing protein bound to the corresponding cell wall component; s, unbound protein. The migrations of marker proteins (in kilodaltons) are indicated on the left.
The interaction studies revealed that the SLH polypeptide, the xylanase XynA from T. thermosulfurigenes EM1, the xylanase Xyn-3SLH synthesized in E. coli, and the N terminus of the S-layer protein were able to bind to native peptidoglycan-containing sacculi. Their affinity for HF-treated peptidoglycan sacculi was very low. Almost no affinity for native peptidoglycan-containing sacculi was observed in the case of the xylanase forms with only one SLH domain (Xyn-1SLH) and without SLH domains (Xyn-ΔSLH).
DISCUSSION
It has been proposed before that exocellular enzymes of T. thermosulfurigenes EM1 are attached to the cell envelope via their SLH domains (7, 20). Previous studies led us to the assumption that there is a common anchoring mechanism for the attachment of S-layer protein and exoenzymes to the cell surface of T. thermosulfurigenes EM1 (7). In this study, we report on studies of interaction of proteins with and without SLH domains and cell wall components. The results of the studies are consistent. Proteins with SLH domains (the SLH polypeptide, XynA, Xyn-3SLH, and the N-terminal fragment of the S-layer protein) were able to interact with native cell wall sacculi. In contrast, a truncated form of the xylanase without SLH domains (Xyn-ΔSLH) could not bind to cell wall material. Xyn-1SLH, which contained only one intact SLH domain, was also not able to bind to cell wall sacculi, indicating that all three SLH domains might be necessary for cell wall attachment of the xylanase. However, it cannot be excluded that a defect in protein folding of Xyn-1SLH might be responsible for the loss of affinity to peptidoglycan-containing sacculi. It becomes evident that SLH domains serve to anchor exocellular proteins to the cell envelope. The molecular mechanism of this interaction has not yet been elucidated. In this context it is interesting that isolated SLH domains also exhibit high affinity to each other. Even incubation in SDS-containing buffer did not completely prevent interaction of on SLH polypeptide with another. This led to the formation of oligomers, which could be detected in Western blots (Fig. 3). This finding confirms the prediction of Lupas et al. (17) and the data of Lemaire et al. (16) that SLH domains also mediate association of SLH domain-bearing polypeptides. The molecular basis of this affinity to each other is probably related to the mechanism of interaction with the cell envelope in vivo, yet it is unknown which amino acids or structures are responsible for the affinity. Further investigations will be performed to specify the interaction.
Different cell wall components have been proposed to function as binding sites for exocellular proteins with SLH domains. Lemaire et al. (16), Engel et al. (11), Lupas et al. (17), and Olabarría et al. (23) suggest that the peptidoglycan is responsible for cell wall attachment of structural proteins. Egelseer et al. (10) found that a high-molecular-weight amylase of B. stearothermophilus DSM 2358 adheres to its S-layer. A common attachment site for S-layer proteins seems to be accessory, so-called secondary cell wall polymers, which are covalently linked to the peptidoglycan layer (9, 12, 19, 24, 27).
To determine the component facilitating the attachment of exoproteins from T. thermosulfurigenes EM1, we extracted native peptidoglycan-containing sacculi with HF as described by Ries et al. (24) and detected a protein-free accessory cell wall polymer in the extract. In all assays, interaction of the SLH domain-containing protein was drastically reduced when HF-extracted peptidoglycan lacking the polymer was used as the cell wall component. These results strongly suggest that not the peptidoglycan itself but accessory cell wall polymers which are covalently bound to the peptidoglycan layer function as the adhesion site for exocellular proteins. In B. stearothermophilus PV72/p2, the accessory cell wall polymer which interacts with the SLH domain-containing N terminus of the S-layer protein is composed mainly of N-acetylglucosamine (GlcNAc) and N-acetylmannosamine (ManNAc) (24). Previous analysis of the cell wall of T. thermosulfurigenes EM1 revealed a ratio of GlcNAc to N-aetylmuramic acid of 2.1 to 1, indicating the presence of another polymer in addition to peptidoglycan (1). Furthermore, fucosamine and galactosamine have been detected in the cell wall (13).
Since the affinity of the xylanase XynA and the SLH polypeptide for glycosylated S-layer was higher than that for deglycosylated self-assembly products, we assume that part of the secondary cell wall polymer remained attached to the S-layer during the preparation process. This assumption is in agreement with the studies of Ries et al. (24), who extracted a secondary cell wall polymer from S-layer self-assembly products of B. stearothermophilus PV72/p2.
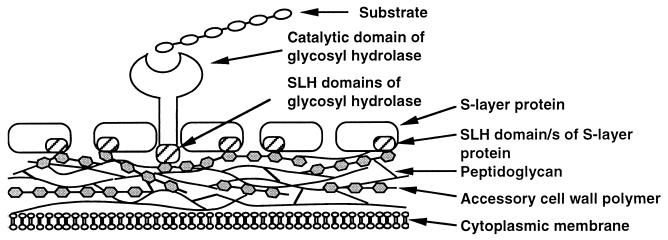
A common model for the cell wall attachment of extracellular enzymes from T. thermosulfurigenes EM1 was proposed by Matuschek et al. (20). Here we present an updated model for the cell surface attachment of exocellular proteins of T. thermosulfurigenes EM1 (Fig. 7), in which the interaction of the SLH domains with the accessory cell wall polymers is depicted.
FIG. 7.
Hypothetic model for the attachment of exocellular proteins from T. thermosulfurigenes EM1 to the cell envelope via their SLH domains. The SLH domains of the S-layer as well as the SLH domains of exocellular glycosyl hydrolases interact with secondary cell wall polymers, which are covalently linked to the underlying peptidoglycan layer.
The multiple xylanase forms present in cultures of T. thermosulfurigenes EM1 were mentioned above. Multiplicity was observed also for the pullulanase of T. thermosulfurigenes EM1. It had been shown that the enzyme appears as multiple intact and truncated glycosylated and nonglycosylated modification products of one precursor (30). Multiplicity of exocellular enzymes in microorganisms is well known. Wong et al. (32) discussed the multiplicity of xylanases as a cooperation mechanism, which enhances substrate accessibility and hydrolysis.
A C-terminally truncated form of the xylanase with a molecular mass of 115 kDa was found in the culture supernatant of T. thermosulfurigenes EM1 (Fig. 4) and also existed in crude extracts of E. coli containing plasmid pCT1516, pCT16, or pCT1621 (data not shown). This 115-kDa xylanase, containing no SLH domains, was not found in cell walls of T. thermosulfurigenes EM1 (7). We assume that under certain conditions, a specific protease, which either is cell bound or is secreted in the culture medium, cuts off the SLH domains and thereby releases the enzyme into the medium. The phenomenon is currently being investigated.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
We thank Uta Meyer and Roland Schmid for their participation in the analysis of fragments of the S-layer protein.
REFERENCES
- 1.Antranikian G. Physiology and enzymology of thermophilic anaerobic bacteria degrading starch. FEMS Microbiol Rev. 1990;75:201–218. [Google Scholar]
- 2.Antranikian G, Herzberg C, Gottschalk G. Production of thermostable α-amylase, pullulanase, and α-glucosidase in continuous culture by a new Clostridium isolate. Appl Environ Microbiol. 1987;53:1668–1673. doi: 10.1128/aem.53.7.1668-1673.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmeyer H U, Gawehn K, Grabe M. Enzyme als biochemische Reagenzien. In: Bergmeyer H U, editor. Methoden der enzymatischen Analyse. Weinheim, Germany: Verlag Chemie; 1974. pp. 454–558. [Google Scholar]
- 4.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blum H, Beier H, Gross H J. Improved silver staining method of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Brechtel E, Matuschek M, Hellberg A, Egelseer E M, Schmid R, Bahl H. Cell wall of Thermoanaerobacterium thermosulfurigenes EM1: isolation of its components and attachment of the xylanase XynA. Arch Microbiol. 1999;171:159–165. doi: 10.1007/s002030050694. [DOI] [PubMed] [Google Scholar]
- 8.Edge A S B, Faltynek C R, Hof L, Reichert L E, Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981;118:131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- 9.Egelseer E M, Leitner K, Jarosch M, Hotzy C, Zayni S, Sleytr U B, Sára M. The S-layer proteins of two Bacillus stearothermophilus wild-type strains are bound via their N-terminal region to a secondary cell wall polymer of identical chemical composition. J Bacteriol. 1998;180:1488–1495. doi: 10.1128/jb.180.6.1488-1495.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egelseer E M, Schocher I, Sára M, Sleytr U B. The S-layer from Bacillus stearothermophilus DSM 2358 functions as an adhesion site for a high-molecular-weight amylase. J Bacteriol. 1995;177:1444–1451. doi: 10.1128/jb.177.6.1444-1451.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel A, Cejka Z, Lupas A, Lottspeich F, Baumeister W. Isolation and cloning of Ompα, a coiled-coil protein spanning the periplasmic space of the ancestral eubacterium Thermotoga maritima. EMBO J. 1992;11:4369–4378. doi: 10.1002/j.1460-2075.1992.tb05537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hastie A T, Brinton C C., Jr Specific interaction of the tetragonally arrayed protein layer of Bacillus sphaericus with its peptidoglycan sacculus. J Bacteriol. 1979;138:1010–1021. doi: 10.1128/jb.138.3.1010-1021.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozianowski G. Charakterisierung der Zellwand von Clostridium thermosulfurogenes EM1 und Einfluβ der Wachstumsbedingungen auf die Zellwandzusammensetzung. Diploma thesis. Göttingen, Germany: University of Göttingen; 1988. [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Lemaire M, Miras I, Gounon P, Béguin P. Identification of a region responsible for binding to the cell wall within the S-layer protein of Clostridium thermocellum. Microbiology. 1998;144:211–217. doi: 10.1099/00221287-144-1-211. [DOI] [PubMed] [Google Scholar]
- 16.Lemaire M, Ohayon H, Gounon P, Fujino T, Béguin P. OlpB, a new outer layer protein of Clostridium thermocellum, and binding of its S-layer-like domains to components of the cell envelope. J Bacteriol. 1995;177:2451–2459. doi: 10.1128/jb.177.9.2451-2459.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lupas A, Engelhardt H, Peters J, Santarius U, Volker S, Baumeister W. Domain structure of the Acetogenium kivui surface layer revealed by electron crystallography and sequence analysis. J Bacteriol. 1994;176:1224–1233. doi: 10.1128/jb.176.5.1224-1233.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantle M, Allen A. A colorimetric assay for glycoproteins based on the periodic acid/Schiff stain. Biochem Soc Trans. 1978;6:607–609. doi: 10.1042/bst0060607. [DOI] [PubMed] [Google Scholar]
- 19.Masuda K, Kawata T. Characterization of a regular array in the wall of Lactobacillus buchneri and its reattachment to the other wall components. J Gen Microbiol. 1981;124:81–90. [Google Scholar]
- 20.Matuschek M, Burchhardt G, Sahm K, Bahl H. Pullulanase of Thermoanaerobacterium thermosulfurigenes EM1 (Clostridium thermosulfurigenes): molecular analysis of the gene, composite structure of the enzyme, and a common model for its attachment to the cell surface. J Bacteriol. 1994;176:3295–3302. doi: 10.1128/jb.176.11.3295-3302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matuschek M, Sahm K, Zibat A, Bahl H. Characterization of genes from Thermoanaerobacterium thermosulfurigenes EM1 that encode two glycosyl hydrolases with conserved S-layer-like domains. Mol Gen Genet. 1996;252:493–496. doi: 10.1007/BF02173016. [DOI] [PubMed] [Google Scholar]
- 22.Messner P, Sleytr U B. Separation and purification of S-layers from Gram-positive and Gram-negative bacteria. In: Hancock I C, Poxton I R, editors. Bacterial cell surface techniques. Chichester, Great Britain: Wiley & Sons; 1988. pp. 97–104. [Google Scholar]
- 23.Olabarría G, Carrascosa J L, de Pedro M A, Berenguer J. A conserved motif in S-layer proteins is involved in peptidoglycan binding in Thermus thermophilus. J Bacteriol. 1996;178:4765–4772. doi: 10.1128/jb.178.16.4765-4772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ries W, Hotzy C, Schocher I, Sleytr U B, Sára M. Evidence that the N-terminal part of the S-layer protein from Bacillus stearothermophilus PV72/p2 recognizes a secondary cell wall polymer. J Bacteriol. 1997;179:3892–3898. doi: 10.1128/jb.179.12.3892-3898.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sára M, Kuen B, Mayer H F, Mandl F, Schuster K C, Sleytr U B. Dynamics in oxygen-induced changes in S-layer protein synthesis from Bacillus stearothermophilus PV72 and the S-layer-deficient variant T5 in continuous culture and studies of the cell wall composition. J Bacteriol. 1996;178:2108–2117. doi: 10.1128/jb.178.7.2108-2117.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz W H, Bronnenmeier K, Grabnitz F, Staudenbauer W L. Activity staining of cellulases in polyacrylamide gels containing mixed linkage beta-glucans. Anal Biochem. 1987;164:72–77. doi: 10.1016/0003-2697(87)90369-1. [DOI] [PubMed] [Google Scholar]
- 29.Specka U, Spreinat A, Antranikian G, Mayer F. Immunocytochemical identification and localization of active and inactive α-amylase and pullulanase in cells of Clostridium thermosulfurogenes EM1. Appl Environ Microbiol. 1991;57:1062–1069. doi: 10.1128/aem.57.4.1062-1069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spreinat A, Antranikian G. Analysis of the amylolytic enzyme system of Clostridium thermosulfurogenes EM1: purification and synergistic action of pullulanases and maltohexaose forming α-amylase. Starch/stärke. 1992;44:305–312. [Google Scholar]
- 31.Studier F W, Moffat B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 32.Wong K K Y, Tan L U L, Saddler J N. Multiplicity of β-1,4-xylanases in microorganisms: functions and applications. Microbiol Rev. 1988;52:305–317. doi: 10.1128/mr.52.3.305-317.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]