Abstract
Autoimmunity linked to COVID-19 immunization has been recorded throughout the pandemic. Herein we present six new patients who experienced relapses of previous autoimmune disease (AD) or developed a new autoimmune or autoinflammatory condition following vaccination. In addition, we documented additional cases through a systematic review of the literature up to August 1st, 2022, in which 464 studies (928 cases) were included. The majority of patients (53.6%) were women, with a median age of 48 years (IQR: 34 to 66). The median period between immunization and the start of symptoms was eight days (IQR: 3 to 14). New-onset conditions were observed in 81.5% (n: 756) of the cases. The most common diseases associated with new-onset events following vaccination were immune thrombocytopenia, myocarditis, and Guillain-Barré syndrome. In contrast, immune thrombocytopenia, psoriasis, IgA nephropathy, and systemic lupus erythematosus were the most common illnesses associated with relapsing episodes (18.5%, n: 172). The first dosage was linked with new-onset events (69.8% vs. 59.3%, P = 0.0100), whereas the second dose was related to relapsing disease (29.5% vs. 59.3%, P = 0.0159). New-onset conditions and relapsing diseases were more common in women (51.5% and 62.9%, respectively; P = 0.0081). The groups were evenly balanced in age. No deaths were recorded after the disease relapsed, while 4.7% of patients with new-onset conditions died (P = 0.0013). In conclusion, there may be an association between COVID-19 vaccination and autoimmune and inflammatory diseases. Some ADs seem to be more common than others. Vaccines and SARS-CoV-2 may induce autoimmunity through similar mechanisms. Large, well-controlled studies are warranted to validate this relationship and assess additional variables such as genetic and other environmental factors.
Keywords: Autoimmununity, COVID-19, SARS-CoV-2, Vaccines
1. Introduction
The world witnessed a major infectious disease that first emerged in the Chinese city of Wuhan in 2019, an illness known as Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It quickly spread worldwide and was declared a pandemic within a few months. On August 6, 2022, the Johns Hopkins University Center for Systems Science and Engineering reported 583,840,223 cases, with 6,417,401 deaths [1].
The clinical spectrum of COVID-19 ranges from the absence of symptoms to the presence of severe pneumonia, associated with a hyperinflammatory state, which causes multiorgan failure [2,3]. The most severe disease cases are related to an increase in the production of inflammatory cytokines (i.e., cytokine storm) [4,5]. Because of the disease's fast spread and the lack of effective therapies, attempts were made worldwide to find vaccines to reduce the disease's severity and mortality. To date, 12,002,790,796 dosages have been administrated [1].
Over 200 vaccines against COVID-19 are currently being produced, with many already in clinical testing [6]. The main types include viral vector vaccines (Oxford/AstraZeneca, Sputnik V), genetic vaccines using messenger ribonucleic acid (mRNA) (Moderna and Pfizer/BioNTech), and inactivated vaccines (Sinovac, Sinopharm, Bharat Biotech Covaxin).
Vaccines save millions of lives each year, improving the quality of life. Vaccines have had great success over the last two centuries, but they are not free of side effects, including latent and overt autoimmunity via various pathways [7,8]. Although widespread vaccination against COVID-19 has reduced disease severity and mortality, vaccine-related adverse events such as autoimmune and autoinflammatory diseases have been documented. These include thrombotic thrombocytopenia, myocarditis, Guillain-Barré syndrome (GBS), demyelinating disorders, and systemic lupus erythematosus (SLE), among others [[9], [10], [11], [12], [13], [14], [15]]. We present six new patients who experienced relapses of autoimmune diseases (AD) or developed a new autoimmune or autoinflammatory disease following vaccination. In addition, we document the main cases of autoimmunity and autoinflammatory conditions associated with the COVID-19 vaccine in a systematic review. Finally, we discuss the possible hypotheses underlying this phenomenon based on the evidence gathered.
2. Materials and methods
2.1. Information sources and search strategy
A systematic literature review was conducted up to August 1st, 2022. The search was performed in PUBMED. We searched using the terms: (((((((((((((((((((((((((((((((((((((((((((((((“Thyroiditis, Autoimmune"[Mesh]) OR “Hashimoto Disease"[Mesh]) OR “Graves' Disease"[Mesh]) OR “Arthritis, Rheumatoid"[Mesh]) OR "Sjogren's Syndrome"[Mesh]) OR “Sarcoidosis"[Mesh]) OR “Diabetes Mellitus, Type 1"[Mesh]) OR “Multiple Sclerosis"[Mesh]) OR “Scleroderma, Systemic"[Mesh]) OR “Guillain-Barre Syndrome"[Mesh]) OR “Myasthenia Gravis"[Mesh]) OR “Hepatitis, Autoimmune"[Mesh]) OR “Liver Cirrhosis, Biliary"[Mesh]) OR “Cholangitis, Sclerosing"[Mesh]) OR “Crohn Disease"[Mesh]) OR “Colitis, Ulcerative"[Mesh]) OR “Anemia, Pernicious"[Mesh]) OR “Anemia, Hemolytic"[Mesh]) OR “Anemia, Hemolytic, Autoimmune"[Mesh]) OR “Purpura, Thrombocytopenic, Idiopathic"[Mesh]) OR “Celiac Disease"[Mesh]) OR “Vitiligo"[Mesh]) OR “Pemphigoid, Bullous"[Mesh]) OR “Dermatomyositis"[Mesh]) OR “Polymyositis"[Mesh]) OR “Kawasaki Disease” OR “Lupus Erythematosus, Systemic"[Mesh]) OR “Addison Disease"[Mesh]) OR “Primary biliary cholangitis” OR “Vasculitis"[Mesh]) OR “Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis"[Mesh]) OR “Systemic Vasculitis"[Mesh]) OR “Vasculitis, Leukocytoclastic, Cutaneous"[Mesh]) OR “Granulomatosis with Polyangiitis"[Mesh]) OR “Takayasu Arteritis"[Mesh]) OR “Giant Cell Arteritis"[Mesh]) OR “Thromboangiitis Obliterans"[Mesh]) OR “Mucocutaneous Lymph Node Syndrome"[Mesh]) OR “Polyarteritis Nodosa"[Mesh]) OR “Churg-Strauss Syndrome"[Mesh]) OR “IgA Vasculitis"[Mesh]) OR “Microscopic Polyangiitis"[Mesh]) OR “Antiphospholipid Syndrome"[Mesh]) AND “COVID-19 Vaccines"[Mesh]) OR “ChAdOx1 nCoV-19"[Mesh]) OR “2019-nCoV Vaccine mRNA-1273"[Mesh]) OR “BNT162 Vaccine"[Mesh]) OR “Ad26COVS1"[Mesh] OR SARS-CoV-2 vaccines). Additionally, a manual search was carried out through the articles referenced in the included studies to expand the number of articles.
2.2. Eligibility criteria
The articles included in this study described patients with autoimmunity, or inflammatory diseases, associated with a history of vaccination against COVID-19. Case reports and case series were included. Only articles in English or Spanish were included. Cases that did not report the population of interest and those reports that did not specify the type of vaccine were excluded.
2.3. Study selection and data extraction process
The eligibility assessment was made by two reviewers, who independently reviewed all the articles selected in the initial search. The two reviewers extracted information related to sociodemographic data, type of disease, clinical characteristics, laboratory data, histopathological data, type of vaccine received, treatment received, and response to treatment. Any differences were resolved by consensus. The PRISMA guidelines for reporting in systematic reviews were used during the selection and data analysis phases [16].
2.4. Statistical analysis
Studies reporting individual data of patients were included in the analysis. Univariate descriptive statistics were performed. Categorical variables were analyzed using frequencies, and continuous quantitative variables were expressed in the median and interquartile range (IQR). Fisher's exact or Mann–Whitney U tests were used to explore differences between new-onset and relapsing autoimmune/autoinflammatory conditions. The significance level of the study was set to 0.05. Statistical analyses were done using R software version 4.0.2.
3. Results
3.1. Case reports
Six patients attending the post-COVID unit at the Clínica del Occidente in Bogota, Colombia, who presented autoimmunity or autoinflammatory disease after receiving the SARS-CoV-2 vaccine are described in Table 1 . Two patients showed disease relapse after vaccination (none of them were on immunomodulatory management at the moment for vaccination since they were on disease remission). One patient debuted with the disease after vaccination, and three developed other inflammatory manifestations. The images of the clinical findings and histopathological findings of these three cases are shown in Fig. 1, Fig. 2, Fig. 3 .
Table 1.
Characteristics of six new cases of post-COVID vaccine autoimmune or inflammatory diseases.
| Disease | Age (years) | Sex | Type of vaccine | Clinical manifestations of autoimmune disease | Diagnostic tests | Symptoms onset after vaccination (days) | Comment and outcome | |
|---|---|---|---|---|---|---|---|---|
| Autoimmune Disease flare post-COVID vaccine | ||||||||
| Optic neuritis flare | 56 | F | Pfizer | Loss of visual acuity of the left eye | Ocular ultrasound: left eye retrobulbar optic neuritis | 6 days after 1st dose | In 2004, she was diagnosed with optic neuritis and received treatment with methylprednisolone. Since, she continued with progressive loss of vision, 6 cycles of cyclophosphamide were administered, and subsequently received mycophenolate for 2 years with adequate control of the disease. After the first dose of the vaccine, she once again developed loss of visual acuity. A month later she was diagnosed with an optic neuritis flare. At this instance, she received methylprednisolone (1 g/d) for 5 days, but since the symptoms persisted, plasmapheresis therapy was given for 5 days. Ambulatory management with prednisolone (10 mg/d) was prescribed. Since then, the patient's visual disturbances have improved slowly. |
|
| Rheumatoid arthritis flare | 47 | F | Jansen | Arthralgia and arthritis in 2, 3 and 4 bilateral metacarpophalangeal and proximal interphalangeal joints Right knee synovitis |
C-reactive protein 6.74 mg/L Erythrocyte sedimentation rate 40 mm/h |
8 days after vaccination | Since 2017, patient presents with bilateral symmetrical arthralgias in hands associated with morning stiffness, but never consulted neither received immunomodulatory therapy. After vaccination, the pain was sharply increased so she went to the emergency room where therapy with methylprednisolone (250 mg/d) for 3 days was initiated and ambulatory management with methotrexate (25 mg weekly), chloroquine (150 mg) and prednisolone was given. In a follow up consult, the patient presented modulation of her symptoms. |
|
| Autoimmune Disease post-COVID vaccine | ||||||||
| Autoimmune hepatitis | 69 | F | Pfizer | Jaundice Abdominal pain Choluria |
Hyperbilirubinemia (6.49 mg/dl) with direct bilirubin predominance (5.71 mg/dl) Elevated transaminases GOT: 559 U/L GPT:339 U/L Anti-smooth muscle antibodies 57.4 (Positive) Elevated IgG (3342 mg/dl) |
150 days after 2nd dose | After diagnosis, treatment with methylprednisolone (500 mg/d) for 5 days was initiated, and ambulatory treatment with azathioprine (50 mg twice a day) and tapering prednisolone of 10 mg per week was prescribed. In a follow up control in January 2022, patient clinical condition had resolved. | |
| Other disease post-COVID vaccine | ||||||||
| Sweet Syndrome | 53 | F | Pfizer | Erythematous painful plaques of different sizes scattered on 4 extremities (See Fig. 1) Fever Malaise |
Skin biopsy (See Fig. 1) C-reactive protein 89.77 mg/L White cells count 10.690/L Neutrophils 93.4% Peripheral blood smear without alterations Chest and abdominal CT scan within normal ranges. No visceral masses |
72 days after 2nd dose | After diagnosis, treatment with methylprednisolone (500 mg/d) for 3 days was initiated for 3 days which caused resolution of the skin lesions | |
| Urticarial Vasculitis | 56 | F | Sinovac | Erythematous lesions, with a pale center, pruritic, distributed on trunk and extremities (See Fig. 2) | Skin biopsy. (See Fig. 2) | 1 day after 2nd dose | After the appearance of the skin lesions treatment with loratadine and methylprednisolone (500 mg/d) was initiated for 3 days which caused improvement of the symptoms | |
| Leukocytoclastic vasculitis | 54 | F | Pfizer | Erythematous macular lesions with irregular borders in lower extremities (See Fig. 3) | Skin biopsy (See Fig. 3) | 8 days after 2nd dose | At first treatment with topic betamethasone and loratadine was initiated. Since there was no clinical improvement, methylprednisolone (500 mg/d) for 3 days, was established with which she presented resolution of her symptoms | |
GOT: Glutamic-oxaloacetic transaminase, GPT: Glutamic-pyruvate-transaminase, IgG: Immunoglobulin G, M: Male, F: Female.
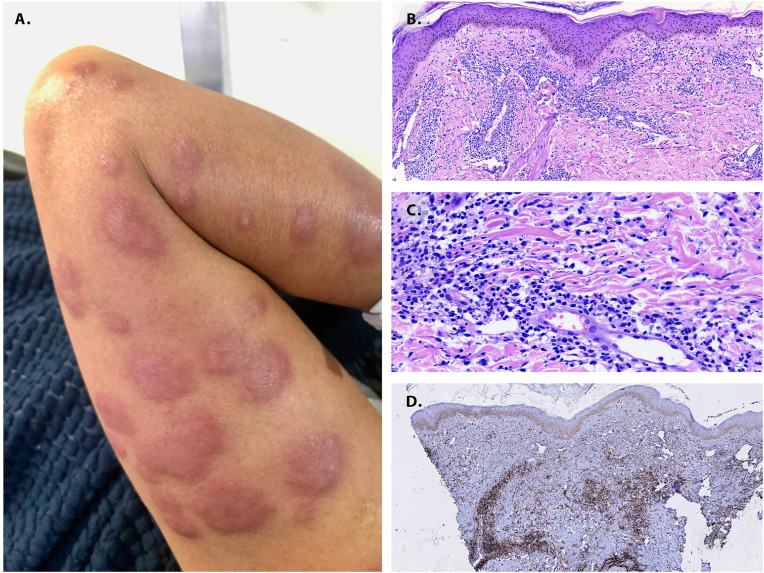
Fig. 1.
Sweet syndrome. A. Skin lesion nine days after vaccination (left arm). Painful erythematous plaques of various sizes were scattered on the four extremities. Skin biopsy histological findings. The infiltrates correspond to many histiocytes, lymphocytes, but largely hyposegmented “pelgeroid” and mature neutrophils at scanning magnification, B, at 10×, and C, at 40×. D. Immunohistochemistry with CD68 and Myeloperoxidase that discriminates histiocytes and “pelgeroid” polymorphonuclear cells, the MPO shows a diffuse coarse granular cytoplasmic reaction in the last.
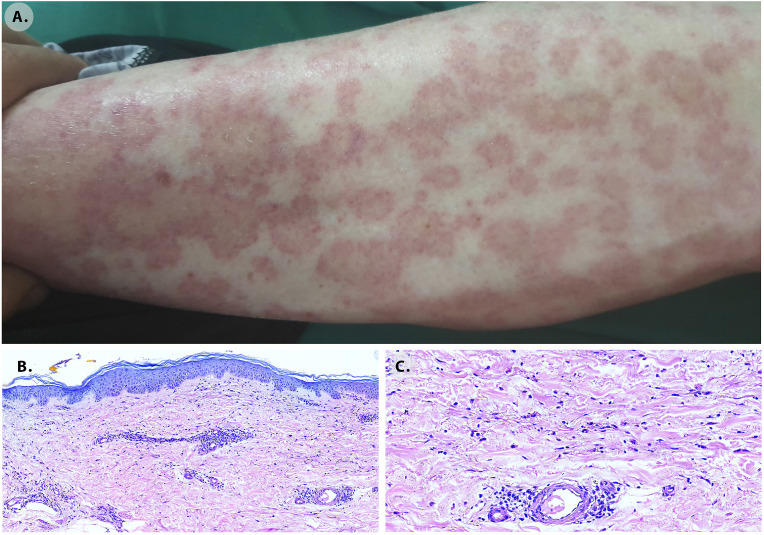
Fig. 2.
Urticarial vasculitis. A. Skin lesions seven days after the vaccination. Erythematous lesions, with a pale center, pruritic, distributed on the trunk and extremities. Skin biopsy histological findings. B. Dermis with oedema X10. C. Superficial and deep perivascular infiltrates of predominantly lymphocytes and histiocytes with prominent extravasated erythrocytes that suggest a late vasculitis consistent with chronic urticarial rash X40.
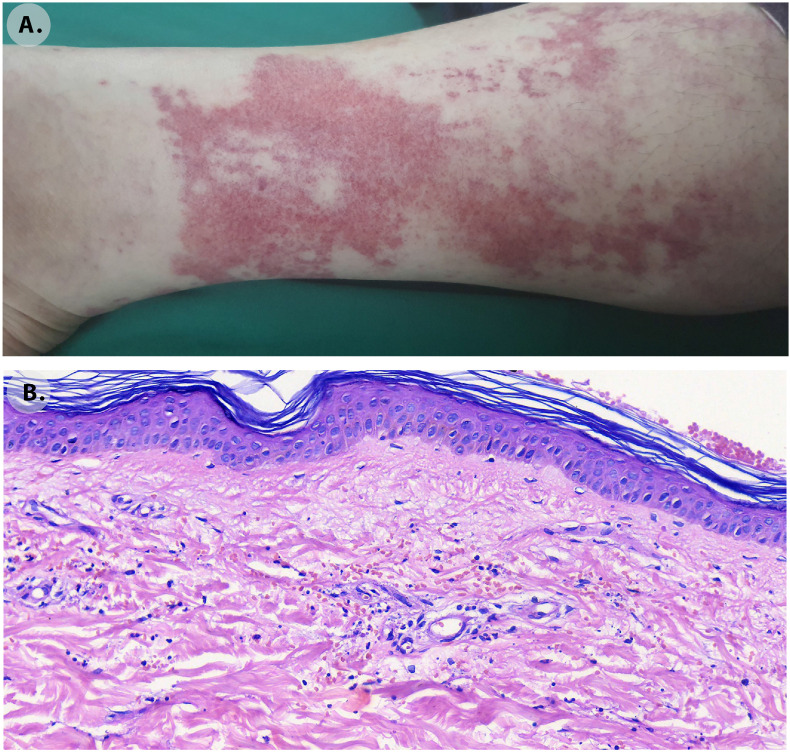
Fig. 3.
Leukocytoclastic vasculitis. A. Skin lesions appeared 19 days after the vaccination. Erythematous macular lesions with irregular borders in the lower extremities. B. Skin biopsy histological findings. In the superficial and deep dermis perivascular infiltration of neutrophils with leukocytoclasis, extravasated erythrocytes and capillary wall damage with some oedema X40.
3.2. Search results
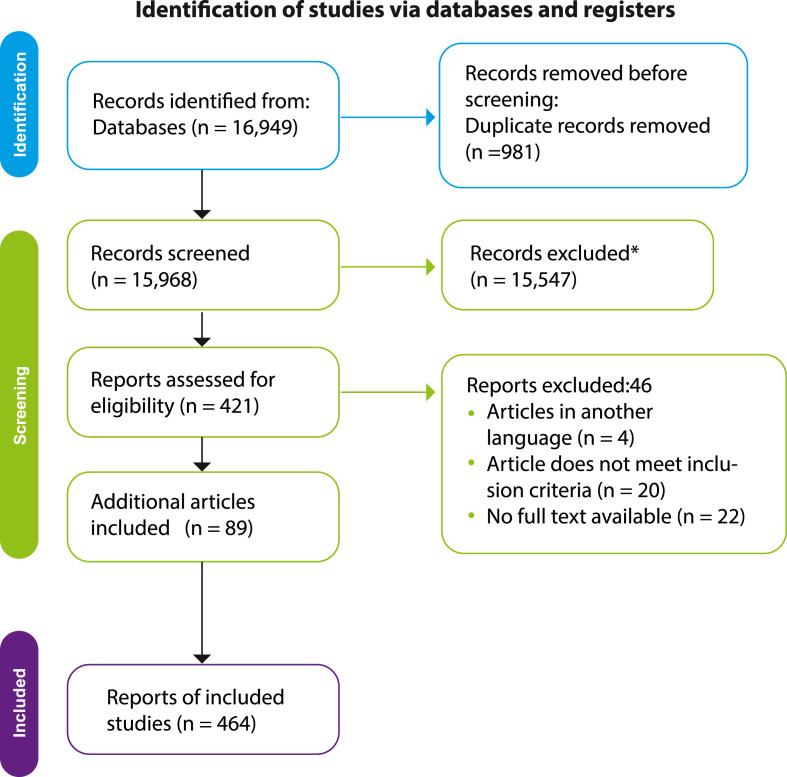
A total of 16,949 manuscripts were found through the main search. After duplication, 15,968 were obtained. Then, 421 articles remained after the title and abstract review. In the selection phase, 46 studies were excluded. After that, additional 85 articles were identified from other resources. Finally, 464 studies were included for qualitative and quantitative analysis [11], [[17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182], [183], [184], [185], [186], [187], [188], [189], [190], [191], [192], [193], [194], [195], [196], [197], [198], [199], [200], [201], [202], [203], [204], [205], [206], [207], [208], [209], [210], [211], [212], [213], [214], [215], [216], [217], [218], [219], [220], [221], [222], [223], [224], [225], [226], [227], [228], [229], [230], [231], [232], [233], [234], [235], [236], [237], [238], [239], [240], [241], [242], [243], [244], [245], [246], [247], [248], [249], [250], [251], [252], [253], [254], [255], [256], [257], [258], [259], [260], [261], [262], [263], [264], [265], [266], [267], [268], [269], [270], [271], [272], [273], [274], [275], [276], [277], [278], [279], [280], [281], [282], [283], [284], [285], [286], [287], [288], [289], [290], [291], [292], [293], [294], [295], [296], [297], [298], [299], [300], [301], [302], [303], [304], [305], [306], [307], [308], [309], [310], [311], [312], [313], [314], [315], [316], [317], [318], [319], [320], [321], [322], [323], [324], [325], [326], [327], [328], [329], [330], [331], [332], [333], [334], [335], [336], [337], [338], [339], [340], [341], [342], [343], [344], [345], [346], [347], [348], [349], [350], [351], [352], [353], [354], [355], [356], [357], [358], [359], [360], [361], [362], [363], [364], [365], [366], [367], [368], [369], [370], [371], [372], [373], [374], [375], [376], [377], [378], [379], [380], [381], [382], [383], [384], [385], [386], [387], [388], [389], [390], [391], [392], [393], [394], [395], [396], [397], [398], [399], [400], [401], [402], [403], [404], [405], [406], [407], [408], [409], [410], [411], [412], [413], [414], [415], [416], [417], [418], [419], [420], [421], [422], [423], [424], [425], [426], [427], [428], [429], [430], [431], [432], [433], [434], [435], [436], [437], [438], [439], [440], [441], [442], [443], [444], [445], [446], [447], [448], [449], [450], [451], [452], [453], [454], [455], [456], [457], [458], [459], [460], [461], [462], [463], [464], [465], [466], [467]] (Fig. 4 ).
Fig. 4.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow chart.
3.3. Systematic review of case reports
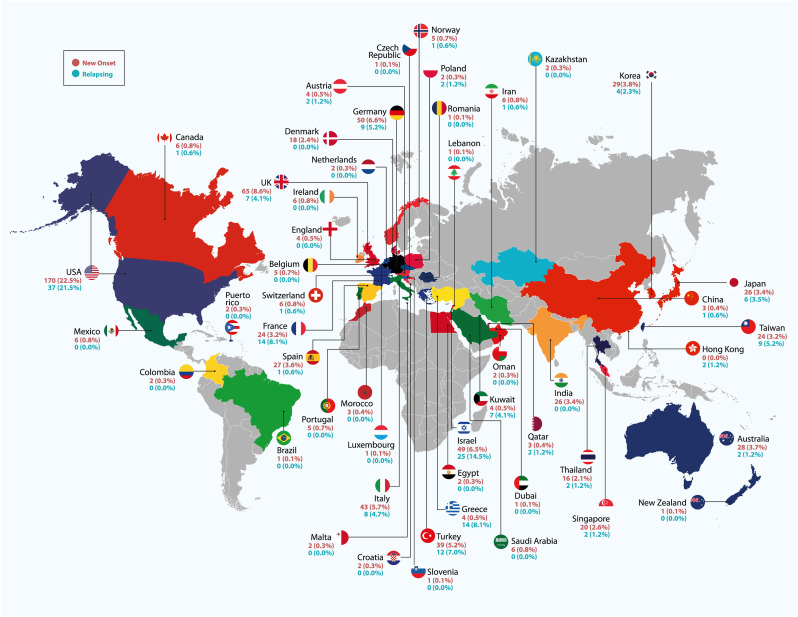
A total of 928 reports were obtained, each with its own data set. Most of them were women (488/910, 53.6%), with a median age of 48 (IQR: 34 to 66). The median period between immunization and the start of symptoms was eight days (IQR: 3 to 14). After immunization, the most common side effect was a new onset condition (756/928, or 81.5%). About 22.5% of new-onset and 21.5% of relapsing illness cases were reported in the United States (Fig. 5 ).
Fig. 5.
Distribution of reported cases by country, according to new onset (red) and relapsing (blue) of the autoimmune/inflammatory disease.
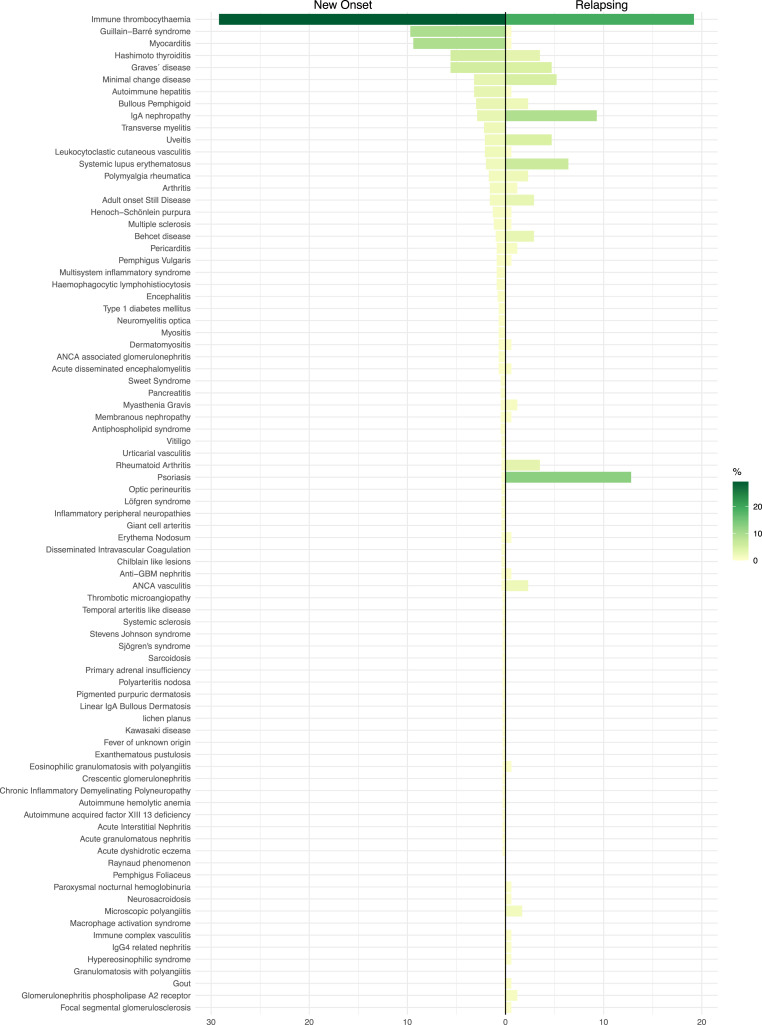
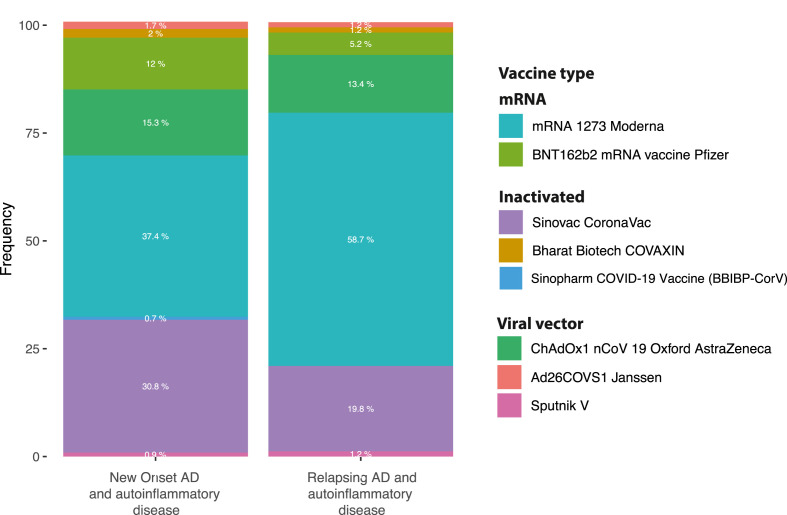
Following vaccination, the most commonly reported diseases associated with new-onset events were immune thrombocytopenia, GBS, and myocarditis (Fig. 6 ) (Table 2 ). Immune thrombocytopenia, psoriasis, IgA nephropathy, and SLE, on the other hand, were the most commonly reported illnesses associated with relapsing episodes (Fig. 6). Both occurrences were widely linked to the mRNA-1273 SARS-CoV-2 vaccine, which was followed by Sinovac-CoronaVac and ChAdOx1 nCoV-19 vaccine (AZD1222) (Fig. 7 ). The first dosage was linked with new-onset events (69.8% vs. 59.3%, P = 0.0100). In contrast, the second dose was associated with relapsing disease (29.5% vs. 39.5%, P = 0.0159). Few new-onset or relapsing events were reported after booster dose (0.7% vs. 1.2% respectively, P = 0.6216). New-onset conditions and relapsing disease were more common in women (51.5% and 62.9%, respectively; P = 0.0081). The groups were evenly balanced in age (P = 0.7851). No deaths were recorded after the disease relapsed, while 4.7% (35/920) of patients with new-onset conditions died (P = 0.0013).
Fig. 6.
Distribution of the main documented diseases after COVID-19 vaccination.
Table 2.
Demographic, laboratory, clinical characteristics, treatment, and clinical outcomes of the systematic review of patients.
| New onset (n: 756) | Relapsing (n: 172) | P value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (IQR) | 48 (33–66) | 46 (34–66) | 0.7851 |
| Days of onset of symptoms since vaccination (IQR) | 8 (3–14) | 7 (2–13.25) | 0.0094 |
| Gender | 0.0081 | ||
| Male | 359/740 (48.5%) | 63/170 (37.1%) | |
| Female | 381/740 (51.5%) | 107/170 (62.9%) | |
| Systemic lupus erythematosus | 15 (2.0%) | 11 (6.4%) | 0.0038 |
| Antiphospholipid syndrome | 4 (0.5%) | 0 (0.0%) | 1.0000 |
| Immune thrombocytopenia | 221 (29.2%) | 33 (19.2%) | 0.0079 |
| Disseminated intravascular coagulation | 3 (0.4%) | 0 (0.0%) | 1.0000 |
| Thrombotic microangiopathy | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Autoimmune acquired factor XIII/13 | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Autoimmune hemolytic anemia | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Acute disseminated encephalomyelitis | 5 (0.7%) | 1 (0.6%) | 1.0000 |
| Encephalitis | 6 (0.8%) | 0 (0.0%) | 0.5998 |
| Guillain-Barré syndrome | 73 (9.7%) | 1 (0.6%) | < 1e-04 |
| Chronic ínflammatory demyelinating polyneuropathy | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Multiple sclerosis | 9 (1.2%) | 1 (0.6%) | 0.6984 |
| Transverse myelitis | 17 (2.2%) | 0 (0.0%) | 0.0541 |
| Optic perineuritis | 3 (0.4%) | 0 (0.0%) | 1.0000 |
| Neuromyelitis_optica | 5 (0.7%) | 0 (0.0%) | 0.5907 |
| Inflammatory peripheral neuropathies | 3 (0.4%) | 0 (0.0%) | 1.0000 |
| Myasthenia Gravis | 4 (0.5%) | 2 (1.2%) | 0.3086 |
| Uveitis | 16 (2.1%) | 8 (4.7%) | 0.0658 |
| Graves' disease | 42 (5.6%) | 8 (4.7%) | 0.8513 |
| Hashimoto thyroiditis | 42 (5.6%) | 6 (3.5%) | 0.3415 |
| Type 1 diabetes mellitus | 5 (0.7%) | 0 (0.0%) | 0.5907 |
| Primary adrenal insufficiency | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Autoimmune hepatitis | 24 (3.2%) | 1 (0.6%) | 0.0662 |
| Pancreatitis | 4 (0.5%) | 0 (0.0%) | 1.0000 |
| Acute granulomatous nephritis | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Acute interstitial nephritis | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| ANCA associated glomerulonephritis | 5 (0.7%) | 0 (0.0%) | 0.5907 |
| Anti-GBM nephritis | 3 (0.4%) | 1 (0.6%) | 0.5602 |
| Minimal change disease | 24 (3.2%) | 9 (5.2%) | 0.1781 |
| IgG4 related nephritis | 1 (0.1%) | 1 (0.6%) | 0.3365 |
| Membranous nephropathy | 4 (0.5%) | 1 (0.6%) | 1.0000 |
| Crescentic glomerulonephritis | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| IgA nephropathy | 22 (2.9%) | 16 (9.3%) | 0.0008 |
| Focal segmental glomerulosclerosis | 1 (0.1%) | 1 (0.6%) | 0.3365 |
| Glomerulonephritis phospholipase A2 receptor | 1 (0.1%) | 2 (1.2%) | 0.0900 |
| Paroxysmal nocturnal hemoglobinuria | 1 (0.1%) | 1 (0.6%) | 0.3365 |
| Myocarditis | 71 (9.4%) | 1 (0.6%) | < 1e-04 |
| Pericarditis | 7 (0.9%) | 2 (1.2%) | 0.6758 |
| Sjogren′s syndrome | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Rheumatoid arthritis | 3 (0.4%) | 6 (3.5%) | 0.0019 |
| Arthritis | 12 (1.6%) | 2 (1.2%) | 1.0000 |
| Polymyalgia Rheumatica | 13 (1.7%) | 4 (2.3%) | 0.5361 |
| Myositis | 5 (0.7%) | 0 (0.0%) | 0.5907 |
| Gout | 1 (0.1%) | 1 (0.6%) | 0.3365 |
| Adult onset Still Disease | 12 (1.6%) | 5 (2.9%) | 0.2218 |
| Behcet disease | 1 (0.1%) | 5 (2.9%) | 0.0011 |
| ANCA vasculitis | 3 (0.4%) | 4 (2.3%) | 0.0251 |
| Granulomatosis with polyangiitis | 1 (0.1%) | 0 (0.0%) | 1.0000 |
| Raynaud phenomenon | 1 (0.1%) | 0 (0.0%) | 1.0000 |
| Giant cell arteritis | 3 (0.4%) | 0 (0.0%) | 1.0000 |
| Henoch-Schönlein purpura | 10 (1.3%) | 1 (0.6%) | 0.6996 |
| Leukocytoclastic vasculitis | 16 (2.1%) | 1 (0.6%) | 0.3389 |
| Urticarial vasculitis | 3 (0.4%) | 0 (0.0%) | 1.0000 |
| Microscopic polyangiitis | 1 (0.1%) | 3 (1.7%) | 0.0217 |
| Eosinophilic granulomatosis with polyangiitis | 2 (0.3%) | 1 (0.6%) | 0.4597 |
| Polyarteritis nodosa | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Immune complex vasculitis | 1 (0.1%) | 1 (0.6%) | 0.3365 |
| Kawasaki Disease | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Temporal arteritis like disease | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Löfgren syndrome | 3 (0.4%) | 0 (0.0%) | 1.0000 |
| Erythema nodosum | 3 (0.4%) | 1 (0.6%) | 0.5602 |
| Neurosacroidosis | 1 (0.1%) | 1 (0.6%) | 0.3365 |
| Macrophage activation syndrome | 1 (0.1%) | 0 (0.0%) | 1.0000 |
| Hypereosinophilic syndrome | 1 (0.1%) | 1 (0.6%) | 0.3365 |
| Hemophagocytic lymphohistiocytosis | 7 (0.9%) | 0 (0.0%) | 0.3599 |
| Fever of unknown origin | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Multisystem inflammatory syndrome | 7 (0.9%) | 0 (0.0%) | 0.3599 |
| Systemic sclerosis | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Vitiligo | 3 (0.4%) | 0 (0.0%) | 1.0000 |
| Dermatomyositis | 5 (0.7%) | 1 (0.6%) | 1.0000 |
| Psoriasis | 3 (0.4%) | 22 (12.8%) | < 1e-04 |
| Bullous pemphigoid | 23 (3.0%) | 4 (2.3%) | 0.8029 |
| Pemphigus vulgaris | 7 (0.9%) | 1 (0.6%) | 1.0000 |
| Pemphigus foliaceus | 1 (0.1%) | 0 (0.0%) | 1.0000 |
| Acute dyshidrotic eczema | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Stevens Johnson syndrome | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Linear IgA bullous dermatosis | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Chilblain like lesions | 3 (0.4%) | 0 (0.0%) | 1.0000 |
| Sweet syndrome | 4 (0.5%) | 0 (0.0%) | 1.0000 |
| Lichen planus | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Pigmented purpuric dermatosis | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Exanthematous pustulosis | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Sarcoidosis | 2 (0.3%) | 0 (0.0%) | 1.0000 |
| Laboratory characteristics | |||
| Elevated D Dimer | 95 (15.5%) | 1 (0.7%) | < 1e-04 |
| CSF albuminocytological dissociation | 48 (7.8%) | 1 (0.7%) | 0.0003 |
| Thrombocytopenia | 225 (35.5%) | 36 (23.2%) | 0.0032 |
| Proteinuria | 53 (8.7%) | 24 (16.1%) | 0.0098 |
| Haematuria | 40 (6.5%) | 20 (13.4%) | 0.0098 |
| Gadolinium enhancement of the myocardium pericardium | 35 (5.7%) | 0 (0.0%) | 0.0007 |
| Diffuse ST elevations | 28 (4.6%) | 1 (0.7%) | 0.0285 |
| Elevated troponin | 69 (11.3%) | 1 (0.7%) | < 1e-04 |
| Subepicardial enhancement | 21 (3.4%) | 0 (0.0%) | 0.0212 |
| Clinical characteristics | |||
| Arthralgia arthritis | 32 (5.2%) | 17 (11.3%) | 0.0092 |
| Headache | 39 (6.4%) | 3 (2.0%) | 0.0432 |
| Paraesthesia | 33 (5.3%) | 1 (0.7%) | 0.0075 |
| Weakness | 70 (11.1%) | 5 (3.3%) | 0.0020 |
| Pleurisy | 6 (1.0%) | 7 (4.6%) | 0.0062 |
| Treatment | |||
| Corticosteroids | 400 (52.9%) | 112 (65.1%) | 0.0039 |
| Anticoagulants/antiaggregants | 77 (10.2%) | 0 (0.0%) | < 1e-04 |
| IV immunoglobulins | 160 (21.2%) | 18 (10.5%) | 0.0009 |
| Transfusion of blood products: platelet | 56 (7.4%) | 5 (2.9%) | 0.0389 |
| NSAIDs | 61 (8.1%) | 6 (3.5%) | 0.0341 |
| Cyclosporine | 3 (0.4%) | 4 (2.3%) | 0.0251 |
| Diuretics/ACE inhibitor/calcium channel blocker/beta blocker/angiotensin II receptor antagonist | 49 (6.5%) | 3 (1.7%) | 0.0153 |
| Tacrolimus | 2 (0.3%) | 3 (1.7%) | 0.0468 |
| Ixekizumab | 0 (0.0%) | 2 (1.2%) | 0.0342 |
| Clinical outcomes | |||
| Good response | 342 (45.7%) | 81 (47.4%) | 0.7339 |
| Resistance | 236 (31.5%) | 57 (33.3%) | 0.6500 |
| Relapse | 20 (2.7%) | 1 (0.6%) | 0.1515 |
| Death | 35 (4.7%) | 0 (0.0%) | 0.0013 |
| IQR: Interquartile range, ANCA: Antineutrophil cytoplasmic antibody, CSF: cerebrospinal fluid, PF4: Platelet factor 4, NSAIDs: Non-steroidal anti-inflammatory drugs, ACE: Angiotensin‐converting enzyme. | |||
Fig. 7.
Autoimmune and autoinflammatory conditions after COVID-19 vaccine according to vaccine type.
4. Discussion
The pandemic's influence has boosted vaccine development, allowing them to be manufactured in record time. As a result, many vaccines with unique and promising modes of action have been developed. However, the quick deployment has raised several issues, including their safety, which could be linked to the dose given and the age of the patients (occurring before 55 years of age in most cases) [468].
We report six new patients who had autoimmune and autoinflammatory diseases, either for the first time or as a relapse. As revealed in the systematic review, these adverse effects have been previously reported in the literature. The most common diseases linked to immunization were thrombocytopenia, myocarditis, GBS, nephropathy, and thyroid disorders. It is remarkable since some of these diseases are usually triggered by infections and other vaccines [[469], [470], [471]]. It suggests similar immunopathogenic mechanisms between vaccines and infectious agents as triggering factors of ADs. This hypothesis could be supported through the anti-idiotype immune response, which shows that antibodies against a specific antigen can trigger the production of second particular antibodies against the first ones [472]. Surprisingly, the second antibodies may be capable of binding to receptors that the initial antigen may attach to. This is significant since many autoimmune or autoinflammatory reactions elicited by COVID-19 vaccinations have previously been reported with vaccines whose principal immunopathogenic mechanism is the anti-idiotype immune response [473,474].
SARS-CoV-2 might trigger ADs [475] through different mechanisms, including molecular mimicry [476,477]. Several studies have demonstrated that the history of past infections can alter the reactogenicity of mRNA vaccines through a cross-reactivity mechanism [468]. However, greater reactogenicity may confer higher protection but could generate more adverse events. Remarkably, patients with ADs are not at increased risk of adverse events associated with vaccination [478], possibly due to the effect of immunomodulatory drugs on vaccine immunogenicity.
Although RNA-based vaccines focus on synthesizing antigens that facilitate immunogenicity [479], the mRNA may bind to pattern recognition receptors (PRRs) in the cytosol or on the endosomes before translation. This binding is accomplished through Toll-like receptors (TLR), 8,7 or 3 in endosomes, or through melanoma differentiation-associated protein 5 (MDA5) or retinoic acid-inducible gene I (RIG-I) in the cytosol. As a consequence, the activation of inflammatory cascades associated with the activation of the type I interferon (IFN–I) and transcription of the nuclear factor (NF)-kB occurs [480].
These signaling pathways have been extensively studied in different ADs, which can be triggered by the antigenic effect of inadequately eliminated nucleic acids, generating an immune system activation [481,482]. A very high IFN-I response could negatively influence mRNA translation, affecting vaccine efficacy [483]. In addition, it has been described that an increase in the effect of IFN-I can trigger a loss of immune tolerance [483].
Some of the side effects of adenoviral vaccines have been linked to variations of the spike (S) protein, which attaches to endothelial cells in blood vessels via the angiotensin-converting enzyme 2 (ACE2), causing COVID- 19-like disorders [484].
The main autoimmune phenomena correspond to vaccine-induced immune thrombotic thrombocytopenia (VITT). Several studies have documented the presence of platelet-activating antibodies directed against platelet factor 4 (PF4), like Heparin-associated thrombocytopenia, which is characterized by the presence of antibodies against the heparin/(PF4) complex, generating thrombocytopenia and thrombosis due to platelet activation. It occurs due to the binding of PF4 with endothelial cells and platelets, facilitating platelet aggregation and thrombus formation [485]. PF4 platelet activation by these antibodies occurs through FcγRIIa [336]. The similarity between thrombocytopenia induced by heparins and SARS-CoV-2 vaccines is striking [486,487]. Patients with thrombocytopenia after ChAdOx1 nCov-19 IgG antibodies against PF4 have been described [488]. PF4 can interact with the double-stranded DNA of the vaccine vector. The PF4/DNA vector complex is taken up by antigen-presenting cells, later facilitating the production of antibodies against PF4 [489]. A recent study showed the structure of ChAdOx1/AZD-1222, evidencing a strong electronegative potential in the ChAdOx1 viral capsid, facilitating its binding with proteins such as PF4 [490].
Recently, the association between VITT and neutrophil activation was studied. It can occur through different signaling pathways and could be facilitated by NETosis and platelet activation [491]. Another mechanism that could explain the presence of VITT may be related to the activation of the NF–B pathway. Plasminogen activator inhibitor-1 (PAI-1) plays a relevant role in thrombotic events. It has been described that the presence of TNF alpha can promote an increase in serum concentrations of PAI 1 in sepsis. In addition, nuclear translocation of NF-kB in monocytes has been described, increasing the expression of tissue factor (TF) and increasing the expression of thrombin expression [492]. Besides being produced in monocytes, it is also expressed in the endothelium [493]. Due to the direct effect of NF-kB on monocytes, the production of cytokines, such as interleukin 1β, can generate procoagulant states [494,495].
5. Conclusions
There is likely an association between COVID-19 vaccination and autoimmune and inflammatory diseases. Some ADs seem to be more common than others. The mechanisms of autoimmunity induction by COVID-19 vaccines and SARS-CoV-2 infection may be similar. Large, well-controlled studies are warranted to validate this relationship and assess additional variables such as genetic and environmental influences. Further detailed studies focusing on mechanisms, including molecular mimicry and bystander activation, will be essential to explain these rare events. Noteworthy, these rare events should not deter the use of this and other necessary vaccinations.
Funding
This study was supported by Universidad del Rosario (grant ABN011) and LifeFactors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
Data availability
Data will be made available on request.
References
- 1.COVID-19 Dashboard 2022, Cent. Syst. Sci. Eng. Johns Hopkins Univ. (2022). https://coronavirus.jhu.edu/map.html (accessed June 1, 2022).
- 2.Zhang Q., Wang Z., Lv Y., Zhao J., Dang Q., Xu D., Zhao D., Liu H., Wang Z., Zhao X., Xu Z., Zhang X. Clinical features and prognostic factors of patients with COVID-19 in Henan Province, China. Hum. Cell. 2021;34:419–435. doi: 10.1007/s13577-021-00499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., Du B., Li L.-J., Zeng G., Yuen K.-Y., Chen R.-C., Tang C.-L., Wang T., Chen P.-Y., Xiang J., Li S.-Y., Wang J.-L., Liang Z.-J., Peng Y.-X., Wei L., Liu Y., Hu Y.-H., Peng P., Wang J.-M., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S. China medical treatment expert group for covid-19, clinical characteristics of coronavirus disease 2019 in China., N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hojyo S., Uchida M., Tanaka K., Hasebe R., Tanaka Y., Murakami M., Hirano T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Q., Wu X., Zheng X., Luo S., Xu S., Weng J. Targeting inflammation and cytokine storm in COVID-19. Pharmacol. Res. 2020;159:105051. doi: 10.1016/j.phrs.2020.105051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellet J., Pepper M.S. A COVID-19 vaccine: big strides come with big challenges., Vaccines. 2021;9 doi: 10.3390/vaccines9010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wraith D.C., Goldman M., Lambert P.-H. Vaccination and autoimmune disease: what is the evidence? Lancet (London, England) 2003;362:1659–1666. doi: 10.1016/S0140-6736(03)14802-7. [DOI] [PubMed] [Google Scholar]
- 8.Segal Y., Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell. Mol. Immunol. 2018;15:586–594. doi: 10.1038/cmi.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y., Xu Z., Wang P., Li X.-M., Shuai Z.-W., Ye D.-Q., Pan H.-F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165:386–401. doi: 10.1111/imm.13443. [DOI] [PubMed] [Google Scholar]
- 10.Abu S., Roguin A., Hellou E., Ishai A., Shoshan U., Mahamid L. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect, the company ’ s public news and information. 2020 [Google Scholar]
- 11.Baimukhamedov C., Makhmudov S., Botabekova A. Seropositive rheumatoid arthritis after vaccination against SARS-CoV-2 infection. Int. J. Rheum. Dis. 2021;24:1440–1441. doi: 10.1111/1756-185X.14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gi Y., Ju W., Ha Y., Ban J., Ah S., Sung J. Sensory Guillain-Barre syndrome following the ChAdOx1 nCov-19 vaccine : Report of two cases and review of literature. 2020 doi: 10.1016/j.jneuroim.2021.577691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jawed M., Khalid A., Rubin M., Shafiq R., Cemalovic N. Acute immune thrombocytopenia (ITP) following COVID-19 vaccination in a patient with previously stable ITP, open forum infect. Dis. 2021;8:2018–2020. doi: 10.1093/ofid/ofab343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaur I., Zafar S., Capitle E., Khianey R. COVID-19 vaccination as a potential trigger for new-onset systemic lupus erythematosus. Cureus. 2022;14:1–7. doi: 10.7759/cureus.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toljan K., Amin M., Kunchok A., Ontaneda D. New diagnosis of multiple sclerosis in the setting of mRNA COVID-19 vaccine exposure. J. Neuroimmunol. 2022;362 doi: 10.1016/j.jneuroim.2021.577785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting Items for systematic reviews and meta-analyses: the PRISMA statement, PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S., Fan X.-R., He S., Zhang J.-W., Li S.-J. Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2021;42:3537–3539. doi: 10.1007/s10072-021-05427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez C., Pérez-Nieva A., Máiz L., Meijón M.D.M., Llamas P., Monreal M., Bikdeli B., Jiménez D. Vaccine-induced immune thrombotic thrombocytopenia after the BNT162b2 mRNA Covid-19 vaccine: a case study., Thromb. Res. 2021;208:1–3. doi: 10.1016/j.thromres.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulsirichawaroj P., Sanmaneechai O., Wittawatmongkol O., Chokephaibulkit K. Polyneuritis cranialis associated with BNT162b2 mRNA COVID-19 vaccine in a healthy adolescent. Vaccines. 2022;10 doi: 10.3390/vaccines10010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manzo C., Natale M., Castagna A. Polymyalgia rheumatica as uncommon adverse event following immunization with COVID-19 vaccine: a case report and review of literature. Aging Med. (Milt. 2021;4:234–238. doi: 10.1002/agm2.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyun H., Song J.Y., Seong H., Yoon J.G., Noh J.Y., Cheong H.J., Kim W.J., Polyarthralgia, Syndrome Myalgia. After ChAdOx1 nCOV-19 vaccination. J. Kor. Med. Sci. 2021;36 doi: 10.3346/jkms.2021.36.e245. e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atak M.F., Farabi B., Kalelioglu M.B., Rao B.K. Pigmented purpuric dermatosis after BNT162B2 mRNA COVID-19 vaccine administration. J. Cosmet. Dermatol. 2022;21:435–437. doi: 10.1111/jocd.14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tano E., San Martin S., Girgis S., Martinez-Fernandez Y., Sanchez Vegas C. Perimyocarditis in adolescents after pfizer-BioNTech COVID-19 vaccine., J. Pediatric infect. Disabil. Soc. 2021;10:962–966. doi: 10.1093/jpids/piab060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasnie A.A., Hasnie U.A., Patel N., Aziz M.U., Xie M., Lloyd S.G., Prabhu S.D. Perimyocarditis following first dose of the mRNA-1273 SARS-CoV-2 (Moderna) vaccine in a healthy young male: a case report., BMC Cardiovasc. Disord. 2021;21:375. doi: 10.1186/s12872-021-02183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H.W., Jenista E.R., Wendell D.C., Azevedo C.F., Campbell M.J., Darty S.N., Parker M.A., Kim R.J. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6:1196–1201. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scully M., Singh D., Lown R., Poles A., Solomon T., Levi M., Goldblatt D., Kotoucek P., Thomas W., Lester W. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination., N. Engl. J. Med. 2021;384:2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mudie L.I., Zick J.D., Dacey M.S., Palestine A.G. Panuveitis following vaccination for COVID-19. Ocul. Immunol. Inflamm. 2021;29:741–742. doi: 10.1080/09273948.2021.1949478. [DOI] [PubMed] [Google Scholar]
- 28.García-Estrada C., Gómez-Figueroa E., Alban L., Arias-Cárdenas A. Optic neuritis after COVID-19 vaccine application., Clin. Exp. Neuroimmunol. 2021 doi: 10.1111/cen3.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavin M., Elder P.T., O'Keeffe D., Enright H., Ryan E., Kelly A., El Hassadi E., McNicholl F.P., Benson G., Le G.N., Byrne M., Ryan K., O'Connell N.M., O'Donnell J.S. Vaccine-induced immune thrombotic thrombocytopenia (VITT) - a novel clinico-pathological entity with heterogeneous clinical presentations. Br. J. Haematol. 2021;195:76–84. doi: 10.1111/bjh.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaminetsky J., Rudikoff D. New-onset vitiligo following mRNA-1273 (Moderna) COVID-19 vaccination. Clin. Case Reports. 2021;9 doi: 10.1002/ccr3.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zavala-Miranda M.F., González-Ibarra S.G., Pérez-Arias A.A., Uribe-Uribe N.O., Mejia-Vilet J.M. New-onset systemic lupus erythematosus beginning as class V lupus nephritis after COVID-19 vaccination. Kidney Int. 2021;100:1340–1341. doi: 10.1016/j.kint.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Báez-Negrón L., Vilá L.M. New-onset systemic lupus erythematosus after mRNA SARS-CoV-2 vaccination., case rep. Rheumatol. 2022;2022:6436839. doi: 10.1155/2022/6436839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim J.H., Han M.H., Kim Y.J., Kim M.S., Jung H.Y., Choi J.Y., Cho J.H., Kim C.D., Kim Y.L., Park S.H. New-onset nephrotic syndrome after janssen COVID-19 vaccination: a case report and literature review. J. Kor. Med. Sci. 2021;36 doi: 10.3346/jkms.2021.36.e218. e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bostan E., Gulseren D., Gokoz O. New-onset leukocytoclastic vasculitis after COVID-19 vaccine. Int. J. Dermatol. 2021;60:1305–1306. doi: 10.1111/ijd.15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakatani S., Mori K., Morioka F., Hirata C., Tsuda A., Uedono H., Ishimura E., Tsuruta D., Emoto M. New-onset kidney biopsy-proven IgA vasculitis after receiving mRNA-1273 COVID-19 vaccine: case report., CEN Case Reports. 2022:1–5. doi: 10.1007/s13730-021-00677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hidaka D., Ogasawara R., Sugimura S., Fujii F., Kojima K., Nagai J., Ebata K., Okada K., Kobayashi N., Ogasawara M., Imamura M., Ota S. New-onset Evans syndrome associated with systemic lupus erythematosus after BNT162b2 mRNA COVID-19 vaccination. Int. J. Hematol. 2022;115:424–427. doi: 10.1007/s12185-021-03243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merhy R., Sarkis A.-S., Kaikati J., El Khoury L., Ghosn S., Stephan F. New-onset cutaneous lichen planus triggered by COVID-19 vaccination. J. Eur. Acad. Dermatol. Venereol. 2021;35:e729–e730. doi: 10.1111/jdv.17504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou T., Fronhoffs F., Dold L., Strassburg C.P., Weismüller T.J. New-onset autoimmune hepatitis following mRNA COVID-19 vaccination in a 36-year-old woman with primary sclerosing cholangitis - should we be more vigilant? J. Hepatol. 2022;76:218–220. doi: 10.1016/j.jhep.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H.J., Jung M., Lim B.J., Han S.H. New-onset class III lupus nephritis with multi-organ involvement after COVID-19 vaccination. Kidney Int. 2022;101:826–828. doi: 10.1016/j.kint.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabinovitch T., Ben-Arie-Weintrob Y., Hareuveni-Blum T., Shaer B., Vishnevskia-Dai V., Shulman S., Newman H., Biadsy M., Masarwa D., Fischer N., Yovel O., Goldfeather-Ben Zaken S., Habot-Wilner Z. UVEITIS after the BNT162b2 mRNA vaccination against SARS-CoV-2 infection: a possible association., Retina. 2021;41:2462–2471. doi: 10.1097/IAE.0000000000003277. [DOI] [PubMed] [Google Scholar]
- 41.Padiyar S., Kamath N., Mathew J., Chandu A.S., Deodhar D., Shastry B.A., Shashikala T., Ganapati A. New-onset Adult-onset Still's disease-like syndrome after ChAdOx1 nCoV-19 vaccination-a case series with review of literature., Clin. Rheumatol. 2022:1–7. doi: 10.1007/s10067-022-06065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett C., Chambers L.M., Son J., Goje O. Newly diagnosed immune thrombocytopenia in a pregnant patient after coronavirus disease 2019 vaccination. J. Obstet. Gynaecol. Res. 2021;47:4077–4080. doi: 10.1111/jog.14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horino T., Sawamura D., Inotani S., Ishihara M., Komori M., Ichii O. Newly diagnosed IgA nephropathy with gross haematuria following COVID-19 vaccination. QJM. 2022;115:28–29. doi: 10.1093/qjmed/hcab305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toljan K., Amin M., Kunchok A., Ontaneda D. New diagnosis of multiple sclerosis in the setting of mRNA COVID-19 vaccine exposure. J. Neuroimmunol. 2022;362:577785. doi: 10.1016/j.jneuroim.2021.577785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujikawa P., Shah F.A., Braford M., Patel K., Madey J. Neuromyelitis optica in a healthy female after severe acute respiratory syndrome coronavirus 2 mRNA-1273 vaccine. Cureus. 2021;13 doi: 10.7759/cureus.17961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waheed S., Bayas A., Hindi F., Rizvi Z., Espinosa P.S. Neurological complications of COVID-19: guillain-barre syndrome following pfizer COVID-19 vaccine. Cureus. 2021;13 doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anupama Y.J., Patel R.G.N., Vankalakunti M. Nephrotic syndrome following ChAdOx1 nCoV-19 vaccine against SARScoV-2., kidney int. Report. 2021;6:2248. doi: 10.1016/j.ekir.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Unver S., Haholu A., Yildirim S. Nephrotic syndrome and acute kidney injury following CoronaVac anti-SARS-CoV-2 vaccine. Clin. Kidney J. 2021;14:2608–2611. doi: 10.1093/ckj/sfab155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Godoy I.R.B., Rodrigues T.C., Skaf A. Myositis ossificans following COVID-19 vaccination. QJM. 2021;114:659–660. doi: 10.1093/qjmed/hcab161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Rasbi S., Al-Maqbali J.S., Al-Farsi R., Al Shukaili M.A., Al-Riyami M.H., Al Falahi Z., Al Farhan H., Al Alawi A.M., Myocarditis, Hemorrhage Pulmonary, Myositis Extensive. With rhabdomyolysis 12 Days after first dose of pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine: a case report., Am.. J. Case Rep. 2022;23 doi: 10.12659/AJCR.934399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zettinig G., Krebs M. Two further cases of Graves' disease following SARS-Cov-2 vaccination. J. Endocrinol. Invest. 2022;45:227–228. doi: 10.1007/s40618-021-01650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosner C.M., Genovese L., Tehrani B.N., Atkins M., Bakhshi H., Chaudhri S., Damluji A.A., de Lemos J.A., Desai S.S., Emaminia A., Flanagan M.C., Khera A., Maghsoudi A., Mekonnen G., Muthukumar A., Saeed I.M., Sherwood M.W., Sinha S.S., O'Connor C.M. C.R. deFilippi, myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021;144:502–505. doi: 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levin D., Shimon G., Fadlon-Derai M., Gershovitz L., Shovali A., Sebbag A., Bader S., Fink N., Gordon B. Myocarditis following COVID-19 vaccination - a case series. Vaccine. 2021;39:6195–6200. doi: 10.1016/j.vaccine.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albert E., Aurigemma G., Saucedo J., Gerson D.S. Myocarditis following COVID-19 vaccination. Radiol. Case Reports. 2021;16:2142–2145. doi: 10.1016/j.radcr.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abu Mouch S., Roguin A., Hellou E., Ishai A., Shoshan U., Mahamid L., Zoabi M., Aisman M., Goldschmid N., erar Yanay N. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 39. 2021:3790–3793. doi: 10.1016/j.vaccine.2021.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Starekova J., Bluemke D.A., Bradham W.S., Grist T.M., Schiebler M.L., Reeder S.B. Myocarditis associated with mRNA COVID-19 vaccination. Radiology. 2021;301:E409–E411. doi: 10.1148/radiol.2021211430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D'Angelo T., Cattafi A., Carerj M.L., Booz C., Ascenti G., Cicero G., Blandino A., Mazziotti S. Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced reaction?, Can. J. Cardiol. 2021;37:1665–1667. doi: 10.1016/j.cjca.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takenaka T., Matsuzaki M., Fujiwara S., Hayashida M., Suyama H., Kawamoto M. Myeloperoxidase anti-neutrophil cytoplasmic antibody positive optic perineuritis after mRNA coronavirus disease-19 vaccine. QJM. 2021;114:737–738. doi: 10.1093/qjmed/hcab227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nune A., Iyengar K.P., Goddard C., Ahmed A.E. Multisystem inflammatory syndrome in an adult following the SARS-CoV-2 vaccine (MIS-V) BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-243888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Belay E.D., Godfred Cato S., Rao A.K., Abrams J., Wilson W.W., Lim S., Newton-Cheh C., Melgar M., DeCuir J., Webb B., Marquez P., Su J.R., Meng L., Grome H.N., Schlaudecker E., Talaat K., Edwards K., Barnett E., Campbell A.P., Broder K.R., Bamrah Morris S. Multisystem Inflammatory Syndrome in Adults after SARS-CoV-2 infection and COVID-19 vaccination., Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. Am. 2021 doi: 10.1093/cid/ciab936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leclerc S., Royal V., Lamarche C., Laurin L.-P. Minimal change disease with severe acute kidney injury following the oxford-AstraZeneca COVID-19 vaccine: a case report., Am.. J. Kidney dis. Off. J. Natl. Kidney Found. 2021;78:607–610. doi: 10.1053/j.ajkd.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Psyllaki A., Stavrakaki I., Androvitsanea A., Gakiopoulou H., Petrakis I., Stylianou K. Two cases of glomerular involvement after vaccination against COVID-19: epiphenomenon or causality? Clin. Kidney J. 2022;15:574–575. doi: 10.1093/ckj/sfab252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kobayashi S., Fugo K., Yamazaki K., Terawaki H. Minimal change disease soon after Pfizer-BioNTech COVID-19 vaccination. Clin. Kidney J. 2021;14:2606–2607. doi: 10.1093/ckj/sfab156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kervella D., Jacquemont L., Chapelet-Debout A., Deltombe C., Ville S. Minimal change disease relapse following SARS-CoV-2 mRNA vaccine. Kidney Int. 2021;100:457–458. doi: 10.1016/j.kint.2021.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dirim A.B., Safak S., Andac B., Garayeva N., Demir E., Artan A.S., Ozluk Y., Kilicaslan I., Oto O.A., Ozturk S., Yazici H. Minimal change disease following vaccination with CoronaVac. Clin. Kidney J. 2021;14:2268–2269. doi: 10.1093/ckj/sfab123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mancianti N., Guarnieri A., Tripodi S., Salvo D.P., Garosi G. Minimal change disease following vaccination for SARS-CoV-2. J. Nephrol. 2021;34:1039–1040. doi: 10.1007/s40620-021-01091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lebedev L., Sapojnikov M., Wechsler A., Varadi-Levi R., Zamir D., Tobar A., Levin-Iaina N., Fytlovich S., Yagil Y. Minimal change disease following the pfizer-BioNTech COVID-19 vaccine., Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2021;78:142–145. doi: 10.1053/j.ajkd.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holzworth A., Couchot P., Cruz-Knight W., Brucculeri M. Minimal change disease following the Moderna mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;100:463–464. doi: 10.1016/j.kint.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thappy S., Thalappil S.R., Abbarh S., Al-Mashdali A., Akhtar M., Alkadi M.M. Minimal change disease following the Moderna COVID-19 vaccine: first case report. BMC Nephrol. 2021;22:376. doi: 10.1186/s12882-021-02583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.D'Agati V.D., Kudose S., Bomback A.S., Adamidis A., Tartini A. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int. 2021;100:461–463. doi: 10.1016/j.kint.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jongvilaikasem P., Rianthavorn P. Minimal change disease and acute interstitial nephritis following SARS-CoV-2 BNT162b2 vaccination. Pediatr. Nephrol. 2022;37:1419–1421. doi: 10.1007/s00467-022-05470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanna J., Ingram A., Shao T. Minimal change disease after first dose of pfizer-BioNTech COVID-19 vaccine: a case report and review of minimal change disease related to COVID-19 vaccine. Can. J. Kidney Heal. Dis. 2021;8 doi: 10.1177/20543581211058271. 20543581211058270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kenda J., Lovrič D., Škerget M., Milivojević N. Treatment of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia related acute ischemic stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2021;30:106072. doi: 10.1016/j.jstrokecerebrovasdis.2021.106072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dang Y.L., Bryson A., Syndrome Miller-Fisher, Guillain-Barre Syndrome overlap syndrome in a patient post Oxford-AstraZeneca SARS-CoV-2 vaccination. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-246701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishiguchi Y., Matsuyama H., Maeda K., Shindo A., Tomimoto H. Miller Fisher syndrome following BNT162b2 mRNA coronavirus 2019 vaccination. BMC Neurol. 2021;21:452. doi: 10.1186/s12883-021-02489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gueguen L., Loheac C., Saidani N., Khatchatourian L. Membranous nephropathy following anti-COVID-19 mRNA vaccination. Kidney Int. 2021;100:1140–1141. doi: 10.1016/j.kint.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Michele M., Iacobucci M., Chistolini A., Nicolini E., Pulcinelli F., Cerbelli B., Merenda E., Schiavo O.G., Sbardella E., Berto I., Petraglia L., Caracciolo N., Chiara M., Truglia S., Toni D. Malignant cerebral infarction after ChAdOx1 nCov-19 vaccination: a catastrophic variant of vaccine-induced immune thrombotic thrombocytopenia. Nat. Commun. 2021;12:4663. doi: 10.1038/s41467-021-25010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muench F., Krusche M., Sander L.E., Rose T., Burmester G.-R., Schneider U. Macrophage activation syndrome in a patient with adult-onset Still's disease following first COVID-19 vaccination with BNT162b2. BMC Rheumatol. 2021;5:60. doi: 10.1186/s41927-021-00237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sekar A. Lupus nephritis flare post Moderna mRNA-1273 coronavirus vaccine. QJM. 2022;114:882–883. doi: 10.1093/qjmed/hcab284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cohen S.R., Prussick L., Kahn J.S., Gao D.X., Radfar A., Rosmarin D. Leukocytoclastic vasculitis flare following the COVID-19 vaccine. Int. J. Dermatol. 2021;60:1032–1033. doi: 10.1111/ijd.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sandhu S., Bhatnagar A., Kumar H., Dixit P.K., Paliwal G., Suhag D.K., Patil C., Mitra D. Leukocytoclastic vasculitis as a cutaneous manifestation of ChAdOx1 nCoV-19 corona virus vaccine (recombinant) Dermatol. Ther. 2021;34 doi: 10.1111/dth.15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erler A., Fiedler J., Koch A., Heldmann F., Schütz A. Leukocytoclastic vasculitis after vaccination with a SARS-CoV-2 vaccine. Arthritis Rheumatol. 2021;73:2188. doi: 10.1002/art.41910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jin W.J., Ahn S.W., Jang S.H., Hong S.M., Seol J.E., Kim H. Leukocytoclastic vasculitis after coronavirus disease 2019 vaccination. J. Dermatol. 2022;49:e34–e35. doi: 10.1111/1346-8138.16212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kreuter A., Burmann S.-N., Burkert B., Oellig F., Michalowitz A.-L. Transition of cutaneous into systemic lupus erythematosus following adenoviral vector-based SARS-CoV-2 vaccination. J. Eur. Acad. Dermatol. Venereol. 2021;35:e733–e735. doi: 10.1111/jdv.17514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fiorillo G., Pancetti S., Cortese A., Toso F., Manara S., Costanzo A., Borroni R.G. Leukocytoclastic vasculitis (cutaneous small-vessel vasculitis) after COVID-19 vaccination. J. Autoimmun. 2022;127:102783. doi: 10.1016/j.jaut.2021.102783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palla P., Vergadis C., Sakellariou S., Androutsakos T. Letter to the editor: autoimmune hepatitis after COVID-19 vaccination. Hepatology. 2022;vol. 75:489–490. doi: 10.1002/hep.32156. A Rare Adverse Effect?, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park K., Miyake S., Tai C., Tseng M., Andeen N.K., Kung V.L. Letter regarding: “A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination”., kidney int. Report. 2021;6:2246–2247. doi: 10.1016/j.ekir.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schwotzer N., Kissling S., Fakhouri F. Letter regarding “Minimal change disease relapse following SARS-CoV-2 mRNA vaccine”. Kidney Int. 2021;100:458–459. doi: 10.1016/j.kint.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cory P., Lawrence H., Abdulrahim H., Mahmood-Rao H., Hussein A., Gane J. Lessons of the month 3: haemophagocytic lymphohistiocytosis following COVID-19 vaccination (ChAdOx1 nCoV-19) Clin. Med. 2021;21:e677–e679. doi: 10.7861/clinmed.2021-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Notghi A.A., Atley J., Silva M. Lessons of the month 1: longitudinal extensive transverse myelitis following AstraZeneca COVID-19 vaccination. Clin. Med. 2021;21:e535–e538. doi: 10.7861/clinmed.2021-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tan H.Z., Tan R.Y., Choo J.C.J., Lim C.C., Tan C.S., Loh A.H.L., Tien C.S.-Y., Tan P.H., Woo K.T. Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int. 2021;100:469–471. doi: 10.1016/j.kint.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kreuter A., Licciardi-Fernandez M.J., Burmann S.-N., Burkert B., Oellig F., Michalowitz A.-L. Induction and exacerbation of subacute cutaneous lupus erythematosus following mRNA-based or adenoviral vector-based SARS-CoV-2 vaccination. Clin. Exp. Dermatol. 2022;47:161–163. doi: 10.1111/ced.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alabkal J., Rebchuk A.D., Lyndon D., Randhawa N. Incomplete subacute transverse myelitis following vaccination with pfizer-BioNTech COVID-19 mRNA vaccine: a case report., Cureus. 2021;13 doi: 10.7759/cureus.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Güney T., Can F., Akıncı S., Soyer Kösemehmetoğlu Ö., Dilek İ. Immune-Mediated thrombotic thrombocytopenic purpura after BNT162b2 vaccine. Turkish J. Haematol. Off. J. Turkish Soc. Haematol. 2022;39:74–75. doi: 10.4274/tjh.galenos.2021.2021.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee K.A., Kim Y.J., Jin H.Y. Thyrotoxicosis after COVID-19 vaccination: seven case reports and a literature review. Endocrine. 2021;74:470–472. doi: 10.1007/s12020-021-02898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Banerjee S., Sandhu M., Tonzi E., Tambe A., Gambhir H.S. Immune-Mediated thrombocytopenia associated with Ad26.COV2.S (janssen; johnson & johnson) vaccine. Am. J. Therapeut. 2021;28:e604–e606. doi: 10.1097/MJT.0000000000001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Watad A., De Marco G., Mahajna H., Druyan A., Eltity M., Hijazi N., Haddad A., Elias M., Zisman D., Naffaa M.E., Brodavka M., Cohen Y., Abu-Much A., Elhija M.A., Bridgewood C., Langevitz P., McLorinan J., Bragazzi N.L., Marzo-Ortega H., Lidar M., Calabrese C., Calabrese L., Vital E., Shoenfeld Y., Amital H., McGonagle D. Immune-mediated disease flares or new-onset disease in 27 subjects following mrna/dna sars-cov-2 vaccination. Vaccines. 2021;9 doi: 10.3390/VACCINES9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jasaraj R.B., Shrestha D.B., Gaire S., Kassem M. Immune thrombocytopenic purpura following pfizer-BioNTech COVID-19 vaccine in an elderly female. Cureus. 2021;13 doi: 10.7759/cureus.16871. e16871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krajewski P.K., Szepietowski J.C. Immune thrombocytopenic purpura associated with COVID-19 Pfizer-BioNTech BNT16B2b2 mRNA vaccine. J. Eur. Acad. Dermatol. Venereol. 2021;35:e626–e627. doi: 10.1111/jdv.17444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hines A., Shen J.G., Olazagasti C., Shams S. Immune thrombocytopenic purpura and acute liver injury after COVID-19 vaccine. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-242678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paulsen F.-O., Schaefers C., Langer F., Frenzel C., Wenzel U., Hengel F.E., Bokemeyer C., Seidel C. Immune thrombocytopenic purpura after vaccination with COVID-19 vaccine (ChAdOx1 nCov-19) Blood. 2021;138:996–999. doi: 10.1182/blood.2021012790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Candelli M., Rossi E., Valletta F., De Stefano V., Franceschi F. Immune thrombocytopenic purpura after SARS-CoV-2 vaccine. Br. J. Haematol. 2021;194:547–549. doi: 10.1111/bjh.17508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qasim H., Ali E., Yassin M.A. Immune thrombocytopenia relapse post covid-19 vaccine in young male patient. IDCases. 2021;26 doi: 10.1016/j.idcr.2021.e01344. e01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.A Ali E., Al-Maharmeh Q., Rozi W.M., Habib M.B., Yassin M. Immune thrombocytopenia purpura flare post COVID-19 vaccine. Ann. Med. Surg. 2022;75:103164. doi: 10.1016/j.amsu.2021.103164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tarawneh O., Tarawneh H. Immune thrombocytopenia in a 22-year-old post Covid-19 vaccine. Am. J. Hematol. 2021;96:E133–E134. doi: 10.1002/ajh.26106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pandya M., Thota G., Wang X., Luo H. Thyroiditis after COVID-19 mRNA vaccine: a case series., aace Clin. Case Reports. 2022;8:116–118. doi: 10.1016/j.aace.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ganzel C., Ben-Chetrit E. Immune thrombocytopenia following the pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine. Isr. Med. Assoc. J. 2021;23:341. [PubMed] [Google Scholar]
- 108.Fujita M., Ureshino H., Sugihara A., Nishioka A., Kimura S. Immune thrombocytopenia exacerbation after COVID-19 vaccination in a young woman. Cureus. 2021;13 doi: 10.7759/cureus.17942. e17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Akiyama H., Kakiuchi S., Rikitake J., Matsuba H., Sekinada D., Kozuki Y., Iwata N. Immune thrombocytopenia associated with Pfizer-BioNTech’s BNT162b2 mRNA COVID-19 vaccine. IDCases. 2021;25 doi: 10.1016/j.idcr.2021.e01245. e01245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Battegay R., Istampoulouoglou I., Holbro A., Buser A., Hirsiger J.R., Eckstein J., Berger C.T., Koechlin S., Leuppi-Taegtmeyer A.B. Immune thrombocytopenia associated with COVID-19 mRNA vaccine tozinameran - a clinical case and global pharmacovigilance data., Swiss Med. Wkly. Times. 2021;151 doi: 10.4414/smw.2021.w30084. w30084. [DOI] [PubMed] [Google Scholar]
- 111.Al-Ahmad M., Al Rasheed M., Shalaby N., Rodriguez-Bouza T., Altourah L. Immune Thrombocytopenia (ITP): relapse Versus de novo After COVID-19 Vaccination., Clin. Appl. Thromb. Off. J. Int. Acad. Clin. Appl. Thromb. 2022;28 doi: 10.1177/10760296211073920. 10760296211073920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Badier L., Toledano A., Porel T., Dumond S., Jouglen J., Sailler L., Bagheri H., Moulis G., Lafaurie M. IgA vasculitis in adult patient following vaccination by ChadOx1 nCoV-19. Autoimmun. Rev. 2021;20:102951. doi: 10.1016/j.autrev.2021.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Niel O., Florescu C. IgA nephropathy presenting as rapidly progressive glomerulonephritis following first dose of COVID-19 vaccine. Pediatr. Nephrol. 2022;37:461–462. doi: 10.1007/s00467-021-05351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hanna C., Herrera Hernandez L.P., Bu L., Kizilbash S., Najera L., Rheault M.N., Czyzyk J., Kouri A.M. IgA nephropathy presenting as macroscopic hematuria in 2 pediatric patients after receiving the Pfizer COVID-19 vaccine. Kidney Int. 2021;100:705–706. doi: 10.1016/j.kint.2021.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Horino T. IgA nephropathy flare-up following SARS-CoV-2 vaccination. QJM. 2021;114:735–736. doi: 10.1093/qjmed/hcab223. [DOI] [PubMed] [Google Scholar]
- 116.Abramson M., Mon-Wei Yu S., Campbell K.N., Chung M., Salem F. IgA nephropathy after SARS-CoV-2 vaccination. Kidney Med. 2021;3:860–863. doi: 10.1016/j.xkme.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rubinstein T.J. Thyroid eye disease following COVID-19 vaccine in a patient with a history graves' disease: a case report., ophthal. Plast. Reconstr. Surgery. 2021;37:e221–e223. doi: 10.1097/IOP.0000000000002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Julian J.A., Mathern D.R., Fernando D. Idiopathic thrombocytopenic purpura and the moderna covid-19 vaccine. Ann. Emerg. Med. 2021;77:654–656. doi: 10.1016/j.annemergmed.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kudose S., Friedmann P., Albajrami O., D'Agati V.D. Histologic correlates of gross hematuria following Moderna COVID-19 vaccine in patients with IgA nephropathy. Kidney Int. 2021;100:468–469. doi: 10.1016/j.kint.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lindhoff-Last E., Schoenborn L., Piorkowski M., Herold J., Greinacher A., Sheppard J.-A., Warkentin T.E. Heterogeneity of vaccine-induced immune thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination and safety of second vaccination with BNT162b2. Thromb. Haemostasis. 2022;122:304–307. doi: 10.1055/a-1701-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hines A.M., Murphy N., Mullin C., Barillas J., Barrientos J.C. Henoch-Schönlein purpura presenting post COVID-19 vaccination. Vaccine. 2021;39:4571–4572. doi: 10.1016/j.vaccine.2021.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sirufo M.M., Raggiunti M., Magnanimi L.M., Ginaldi L., De Martinis M. Henoch-schönlein purpura following the first dose of covid-19 viral vector vaccine: a case report, Vaccines. 2021;9 doi: 10.3390/VACCINES9101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ai S., Awford A., Roncolato F. Hemophagocytic lymphohistiocytosis following ChAdOx1 nCov-19 vaccination. J. Med. Virol. 2022;94:14–16. doi: 10.1002/jmv.27279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tang L.V., Hu Y. Hemophagocytic lymphohistiocytosis after COVID-19 vaccination. J. Hematol. Oncol. 2021;14:87. doi: 10.1186/s13045-021-01100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Attwell L., Zaw T., McCormick J., Marks J., McCarthy H. Haemophagocytic lymphohistiocytosis after ChAdOx1 nCoV-19 vaccination. J. Clin. Pathol. 2022;75:282–284. doi: 10.1136/jclinpath-2021-207760. [DOI] [PubMed] [Google Scholar]
- 126.Čenščák D., Ungermann L., Štětkářová I., Ehler E. Guillan-Barré Syndrome after First Vaccination Dose against COVID-19: Case Report., Acta Medica (Hradec Kral. 2021;64:183–186. doi: 10.14712/18059694.2021.31. [DOI] [PubMed] [Google Scholar]
- 127.Allen C.M., Ramsamy S., Tarr A.W., Tighe P.J., Irving W.L., Tanasescu R., Evans J.R. Guillain-barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann. Neurol. 2021;90:315–318. doi: 10.1002/ana.26144. [DOI] [PubMed] [Google Scholar]
- 128.Aktas H., Ertuğrul G. Vitiligo in a COVID-19-vaccinated patient with ulcerative colitis: coincidence? Clin. Exp. Dermatol. 2022;47:143–144. doi: 10.1111/ced.14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pujol A., Gómez L.-A., Gallegos C., Nicolau J., Sanchís P., González-Freire M. Á.A. López-González, K. Dotres, L. Masmiquel, Thyroid as a target of adjuvant autoimmunity/inflammatory syndrome due to mRNA-based SARS-CoV2 vaccination: from Graves' disease to silent thyroiditis. J. Endocrinol. Invest. 2022;45:875–882. doi: 10.1007/s40618-021-01707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kanabar G., Wilkinson P. Guillain-Barré syndrome presenting with facial diplegia following COVID-19 vaccination in two patients. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-244527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rossetti A., Gheihman G., O'Hare M., Kosowsky J.M. Guillain-barré syndrome presenting as facial diplegia after COVID-19 vaccination: a case report., J. Emerg. Med. 2021;61:e141–e145. doi: 10.1016/j.jemermed.2021.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Osowicki J., Morgan H., Harris A., Crawford N.W., Buttery J.P., Kiers L. Guillain-barré syndrome in an Australian state using both mRNA and adenovirus-vector SARS-CoV-2 vaccines. Ann. Neurol. 2021;90:856–858. doi: 10.1002/ana.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Azam S., Khalil A., Taha A. Guillain-barré-syndrome in a 67-year-old male post COVID-19 vaccination (astra zeneca), Am. J. Med. Case Rep. 2021;9:424–427. http://pubs.sciepub.com/ [Google Scholar]
- 134.Patel S.U., Khurram R., Lakhani A., Quirk B. Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-242956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Theuriet J., Richard C., Becker J., Pegat A., Bernard E., Vukusic S. Guillain-Barré syndrome following first injection of ChAdOx1 nCoV-19 vaccine: first report. Rev. Neurol. (Paris) 2021;177:1305–1307. doi: 10.1016/j.neurol.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 136.V Maramattom B., Krishnan P., Paul R., Padmanabhan S., Cherukudal Vishnu Nampoothiri S., Syed A.A., Mangat H.S. Guillain-barré syndrome following ChAdOx1-S/nCoV-19 vaccine. Ann. Neurol. 2021;90:312–314. doi: 10.1002/ana.26143. [DOI] [PubMed] [Google Scholar]
- 137.Trimboli M., Zoleo P., Arabia G., Gambardella A. Guillain-Barré syndrome following BNT162b2 COVID-19 vaccine. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2021;42:4401–4402. doi: 10.1007/s10072-021-05523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ling L., Bagshaw S.M., Villeneuve P.-M. Guillain-Barré syndrome after SARS-CoV-2 vaccination in a patient with previous vaccine-associated Guillain-Barré syndrome. C. Can. Med. Assoc. J. = J. l’Association Medicale Can. 2021;193:E1766–E1769. doi: 10.1503/cmaj.210947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Malamud E., Caress J.B., Lapid D.J., Otallah S.I. “Guillain-Barré Syndrome After COVID-19 Vaccination in an Adolescent” [Pediatric Neurology. 2022;126:77. doi: 10.1016/j.pediatrneurol.2021.11.001. January 2022, Pages 9-10]., Pediatr. Neurol. 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bin Waqar S.H., Khan A.A., Memon S. Thrombotic thrombocytopenic purpura: a new menace after COVID bnt162b2 vaccine. Int. J. Hematol. 2021;114:626–629. doi: 10.1007/s12185-021-03190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McKean N., Chircop C. Guillain-Barré syndrome after COVID-19 vaccination. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-244125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.da Silva G.F., da Silva C.F. R.E.N. da N. Oliveira, F. Romancini, R.M. Mendes, A. Locks, M.F.M. Longo, C.H.C. Moro, A.L. Longo, V.L. Braatz, Guillain-Barré syndrome after coronavirus disease 2019 vaccine: a temporal association., Clin. Exp. Neuroimmunol. 2021 doi: 10.1111/cen3.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Introna A., Caputo F., Santoro C., Guerra T., Ucci M., Mezzapesa D.M., Trojano M. Guillain-Barré syndrome after AstraZeneca COVID-19-vaccination: a causal or casual association?, Clin. Neurol. Neurosurgery. 2021;208:106887. doi: 10.1016/j.clineuro.2021.106887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Negrea L., Rovin B.H. Gross hematuria following vaccination for severe acute respiratory syndrome coronavirus 2 in 2 patients with IgA nephropathy. Kidney Int. 2021;99:1487. doi: 10.1016/j.kint.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Perrin P., Bassand X., Benotmane I., Bouvier N. Gross hematuria following SARS-CoV-2 vaccination in patients with IgA nephropathy. Kidney Int. 2021;100:466–468. doi: 10.1016/j.kint.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lo W.K., Chan K.W. Gross haematuria after mRNA COVID-19 vaccination in two patients with histological and clinical diagnosis of IgA nephropathy. Nephrology. 2022;27:110–111. doi: 10.1111/nep.13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Weintraub M.A., Ameer B., Sinha Gregory N. Graves disease following the SARS-CoV-2 vaccine: case series. J. Investig. Med. High Impact Case Reports. 2021;9 doi: 10.1177/23247096211063356. 23247096211063356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sriphrapradang C., Shantavasinkul P.C. Graves' disease following SARS-CoV-2 vaccination. Endocrine. 2021;74:473–474. doi: 10.1007/s12020-021-02902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Goblirsch T.J., Paulson A.E., Tashko G., Mekonnen A.J. Graves' disease following administration of second dose of SARS-CoV-2 vaccine. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-246432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gillion V., Jadoul M., Demoulin N., Aydin S., Devresse A. Granulomatous vasculitis after the AstraZeneca anti-SARS-CoV-2 vaccine. Kidney Int. 2021;100:706–707. doi: 10.1016/j.kint.2021.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee H.P., Selvaratnam V., Rajasuriar J.S. Thrombotic thrombocytopenic purpura after ChAdOx1 nCoV-19 vaccine. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-246049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yamamoto S., Nishimura K., Yo K., Waki D., Murabe H., Yokota T. Flare-up of adult-onset Still's disease after receiving a second dose of BNT162b2 COVID-19 mRNA vaccine. Clin. Exp. Rheumatol. 2021;39(Suppl 1):139–140. doi: 10.55563/clinexprheumatol/tvlpnc. [DOI] [PubMed] [Google Scholar]
- 153.Terracina K.A., Tan F.K. Flare of rheumatoid arthritis after COVID-19 vaccination. Lancet. Rheumatol. 2021;3:e469–e470. doi: 10.1016/S2665-9913(21)00108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim J.-W., Jung J.-Y., Suh C.-H., Kim H.-A. Flare of adult-onset Still's disease following mRNA COVID-19 vaccination: a case report and review of literature. Clin. Rheumatol. 2022;41:1583–1589. doi: 10.1007/s10067-022-06106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rademacher J.-G., Tampe B., Korsten P. First report of two cases of löfgren’s syndrome after SARS-CoV-2 vaccination-coincidence or causality? Vaccines. 2021;9 doi: 10.3390/vaccines9111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.de Bruijn S., Maes M.-B., De Waele L., Vanhoorelbeke K., Gadisseur A. First report of a de novo iTTP episode associated with an mRNA-based anti-COVID-19 vaccination. J. Thromb. Haemostasis. 2021;19:2014–2018. doi: 10.1111/jth.15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Havla J., Schultz Y., Zimmermann H., Hohlfeld R., Danek A., Kümpfel T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J. Neurol. 2022;269:55–58. doi: 10.1007/s00415-021-10648-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Osmanodja B., Schreiber A., Schrezenmeier E., Seelow E. First diagnosis of thrombotic thrombocytopenic purpura after SARS-CoV-2 vaccine - case report. BMC Nephrol. 2021;22:411. doi: 10.1186/s12882-021-02616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Aly M.H., Alshehri A.A., Mohammed A., Almalki A.M., Ahmed W.A., Almuflihi A.M., Alwafi A.A. First case of erythema nodosum associated with pfizer vaccine. Cureus. 2021;13 doi: 10.7759/cureus.19529. e19529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shimoyama S., Kanisawa Y., Ono K., Souri M., Ichinose A. First and fatal case of autoimmune acquired factor XIII/13 deficiency after COVID-19/SARS-CoV-2 vaccination. Am. J. Hematol. 2022;97:243–245. doi: 10.1002/ajh.26426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rodriguez E.V.C., Bouazza F.-Z., Dauby N., Mullier F., d'Otreppe S., Jissendi Tchofo P., Bartiaux M., Sirjacques C., Roman A., Hermans C., Cliquennois M. Fatal vaccine-induced immune thrombotic thrombocytopenia (VITT) post Ad26.COV2.S: first documented case outside US. Infection. 2022;50:531–536. doi: 10.1007/s15010-021-01712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yocum A., Simon E.L. Thrombotic thrombocytopenic purpura after Ad26.COV2-S vaccination., Am. J. Emerg. Med. 2021;49:441. doi: 10.1016/j.ajem.2021.05.001. e3-441.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jamme M., Mosnino E., Hayon J., Franchineau G. Fatal cerebral venous sinus thrombosis after COVID-19 vaccination. Intensive Care Med. 2021;47:790–791. doi: 10.1007/s00134-021-06425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Toom S., Wolf B., Avula A., Peeke S., Becker K. Familial thrombocytopenia flare-up following the first dose of mRNA-1273 Covid-19 vaccine. Am. J. Hematol. 2021;96:E134–E135. doi: 10.1002/ajh.26128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Niebel D., Ralser-Isselstein V., Jaschke K., Braegelmann C., Bieber T., Wenzel J. Exacerbation of subacute cutaneous lupus erythematosus following vaccination with BNT162b2 mRNA vaccine. Dermatol. Ther. 2021;34 doi: 10.1111/dth.15017. e15017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mieczkowska K., Kaubisch A., McLellan B.N. Exacerbation of psoriasis following COVID-19 vaccination in a patient previously treated with PD-1 inhibitor. Dermatol. Ther. 2021;34 doi: 10.1111/dth.15055. e15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Finsterer J. Exacerbating guillain-barré syndrome eight days after vector-based COVID-19 vaccination. Case Rep. Infect. Dis. 2021;2021:3619131. doi: 10.1155/2021/3619131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Costanzo G., Ledda A.G., Ghisu A., Vacca M., Firinu D., Del Giacco S. Eosinophilic granulomatosis with polyangiitis relapse after COVID-19 vaccination: a case report., Vaccines. 2021;10 doi: 10.3390/vaccines10010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chan-Chung C., Ong C.S., Chan L.L., Tan E.K. Eosinophilic granulomatosis with polyangiitis after COVID-19 vaccination. QJM. 2022;114:807–809. doi: 10.1093/qjmed/hcab273. [DOI] [PubMed] [Google Scholar]
- 170.Maraziti G., Becattini C. Eltrombopag for refractory vaccine-induced immune thrombotic thrombocytopenia. J. Thromb. Thrombolysis. 2022;53:954–958. doi: 10.1007/s11239-021-02604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cole A., Thomas R., Goldman N., Howell K., Chakravarty K., Denton C.P., Ong V.H. Diffuse cutaneous systemic sclerosis following SARS-Co V-2 vaccination. J. Autoimmun. 2022;128 doi: 10.1016/j.jaut.2022.102812. 102812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Solimani F., Mansour Y., Didona D., Dilling A., Ghoreschi K., Meier K. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J. Eur. Acad. Dermatol. Venereol. 2021;35:e649–e651. doi: 10.1111/jdv.17480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jafri A., Prieto A., Gashau H., Bartlett J.D. Thrombotic thrombocytopenia following ChAdOx1 nCov-19 vaccination. Acute Med. 2021;20:223–226. [PubMed] [Google Scholar]
- 174.Sugita K., Kaneko S., Hisada R., Harano M., Anno E., Hagiwara S., Imai E., Nagata M., Tsukamoto Y. Development of IgA vasculitis with severe glomerulonephritis after COVID-19 vaccination: a case report and literature review. CEN Case Reports. 2022:1–6. doi: 10.1007/s13730-022-00695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lui D.T.W., Lee K.K., Lee C.H., Lee A.C.H., Hung I.F.N., Tan K.C.B. Development of graves' disease after SARS-CoV-2 mRNA vaccination: a case report and literature review., front. Publ. Health. 2021;9 doi: 10.3389/fpubh.2021.778964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee A.Y.S., Lee C., Brown D.A., Suan D. Development of anti-NXP2 dermatomyositis following Comirnaty COVID-19 vaccination, Postgrad. Med. J. 2022 doi: 10.1136/postgradmedj-2022-141510. postgradmedj- [DOI] [PubMed] [Google Scholar]
- 177.Camargo Coronel A., Jiménez Balderas F.J., Quiñones Moya H., Hernández Zavala M.R., Mandinabeitia Rodríguez P., Hernández Vázquez J.R., Zamora Zarco S., Aguilar Castillo S.D.J. Dermatomyositis post vaccine against SARS-COV2. BMC Rheumatol. 2022;6:20. doi: 10.1186/s41927-022-00250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Venkateswaran K., Aw D.C.-W., Huang J., Angkodjojo S. Dermatomyositis following COVID-19 vaccination. Dermatol. Ther. 2022 doi: 10.1111/dth.15479. e15479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gouda W., Albasri A., Alsaqabi F., Al Sabah H.Y., Alkandari M., Abdelnaby H. Dermatomyositis following BNT162b2 mRNA COVID-19 vaccination. J. Kor. Med. Sci. 2022;37 doi: 10.3346/jkms.2022.37.e32. e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Carli G., Nichele I., Ruggeri M., Barra S., Tosetto A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern. Emerg. Med. 2021;16:803–804. doi: 10.1007/s11739-021-02685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fillon A., Sautenet B., Barbet C., Moret L., Thillard E.M., Jonville-Béra A.P., Halimi J.M. De novo and relapsing necrotizing vasculitis after COVID-19 vaccination. Clin. Kidney J. 2022;15:560–563. doi: 10.1093/ckj/sfab285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Anderegg M.A., Liu M., Saganas C., Montani M., Vogt B., Huynh-Do U., Fuster D.G. De novo vasculitis after mRNA-1273 (Moderna) vaccination. Kidney Int. 2021;100:474–476. doi: 10.1016/j.kint.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Marinaki S., Kolovou K., Liapis G., Skalioti C., Tsiakas S., Boletis I. De novo minimal change disease following vaccination with the pfizer/BioNTech SARS-CoV-2 vaccine in a living kidney donor. Medicina. 2021;58 doi: 10.3390/medicina58010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Muir K.-L., Kallam A., Koepsell S.A., Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination., N. Engl. J. Med. 2021;384:1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mohamed M.M.B., Wickman T.J., Fogo A.B., Velez J.C.Q. De novo immunoglobulin A vasculitis following exposure to SARS-CoV-2 immunization. Ochsner J. 2021;21:395–401. doi: 10.31486/toj.21.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ramessur R., Saffar N., Czako B., Agarwal A., Batta K. Cutaneous thrombosis associated with skin necrosis following Oxford-AstraZeneca COVID-19 vaccination. Clin. Exp. Dermatol. 2021;46:1610–1612. doi: 10.1111/ced.14819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kar B.R., Singh B.S., Mohapatra L., Agrawal I. Cutaneous small-vessel vasculitis following COVID-19 vaccine. J. Cosmet. Dermatol. 2021;20:3382–3383. doi: 10.1111/jocd.14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ungari M., Pezzarossa E. Cutaneous lymphocytic vasculitis after administration of the second dose of AZD1222 (Oxford-AstraZeneca) severe acute respiratory syndrome coronavirus 2 vaccination: casuality or causality? Am. J. Dermatopathol. 2022;44:80–82. doi: 10.1097/DAD.0000000000002104. [DOI] [PubMed] [Google Scholar]
- 189.Vassallo C., Boveri E., Brazzelli V., Rampino T., Bruno R., Bonometti A., Gregorini M. Cutaneous lymphocytic vasculitis after administration of COVID-19 mRNA vaccine. Dermatol. Ther. 2021;34 doi: 10.1111/dth.15076. e15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Theodorou D.J., Theodorou S.J., Axiotis A., Gianniki M., Tsifetaki N. COVID-19 vaccine-related myositis. QJM. 2021;114:424–425. doi: 10.1093/qjmed/hcab043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dash S., Behera B., Sethy M., Mishra J., Garg S. COVID-19 vaccine-induced urticarial vasculitis. Dermatol. Ther. 2021;34 doi: 10.1111/dth.15093. e15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Farooq M., Mohammed Y., Zafar M., Dharmasena D., Rana U.I., Kankam O. COVID-19 vaccine-induced pneumonitis, myositis and myopericarditis. Cureus. 2022;14 doi: 10.7759/cureus.20979. e20979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ramalingam S., Arora H., Lewis S., Gunasekaran K., Muruganandam M., Nagaraju S., Padmanabhan P. COVID-19 vaccine-induced cellulitis and myositis. Cleve. Clin. J. Med. 2021;88:648–650. doi: 10.3949/ccjm.88a.21038. [DOI] [PubMed] [Google Scholar]
- 194.Wu M., Karim M., Ashinoff R. COVID-19 vaccine-associated dermatomyositis. JAAD Case Reports. 2022;23:58–60. doi: 10.1016/j.jdcr.2022.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sangli S., Virani A., Cheronis N., Vannatter B., Minich C., Noronha S., Bhagavatula R., Speredelozzi D., Sareen M., Kaplan R.B. Thrombosis with thrombocytopenia after the messenger RNA-1273 vaccine. Ann. Intern. Med. 2021;174:1480–1482. doi: 10.7326/L21-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Camacho-Domínguez L., Rodríguez Y., Polo F., Restrepo Gutierrez J.C., Zapata E., Rojas M., Anaya J.-M. COVID-19 vaccine and autoimmunity. A new case of autoimmune hepatitis and review of the literature, J. Transl. Autoimmun. 2022;5:100140. doi: 10.1016/J.JTAUTO.2022.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Malhotra H.S., Gupta P., Prabhu V., Kumar Garg R., Dandu H., Agarwal V. COVID-19 vaccination-associated myelitis. QJM. 2021;114:591–593. doi: 10.1093/qjmed/hcab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gupta K., Sharma G.S., Kumar A. COVID-19 vaccination-associated anti-Jo-1 syndrome. Reumatologia. 2021;59:420–422. doi: 10.5114/reum.2021.111836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ramos-Casals M., Sainz-de-la-Maza M., Muxí A. COVID-19 vaccination unveiling subclinical Sjögren’s syndrome. Clin. Exp. Rheumatol. 2021;39(Suppl 1):228–229. doi: 10.55563/clinexprheumatol/u1v6z1. [DOI] [PubMed] [Google Scholar]
- 200.Shah S.R.A., Dolkar S., Mathew J., Vishnu P. COVID-19 vaccination associated severe immune thrombocytopenia. Exp. Hematol. Oncol. 2021;10:42. doi: 10.1186/s40164-021-00235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kaur I., Zafar S., Capitle E., Khianey R. COVID-19 vaccination as a potential trigger for new-onset systemic lupus erythematosus. Cureus. 2022;14 doi: 10.7759/cureus.21917. e21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Klomjit N., Alexander M.P., Fervenza F.C., Zoghby Z., Garg A., Hogan M.C., Nasr S.H., Minshar M.A., Zand L. COVID-19 vaccination and glomerulonephritis., kidney int. Report. 2021;6:2969–2978. doi: 10.1016/j.ekir.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Singh B., Kaur P., Cedeno L., Brahimi T., Patel P., Virk H., Shamoon F., Bikkina M. COVID-19 mRNA vaccine and myocarditis., eur.. J. Case reports intern. Med. 2021;8:2681. doi: 10.12890/2021_002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Badoiu A., Moranne O., Coudray S., Ion I.M. Clinical variant of guillain-barre syndrome with prominent facial diplegia after AstraZeneca coronavirus disease 2019 vaccine. J. Clin. Neuromuscul. Dis. 2021;23:115–116. doi: 10.1097/CND.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 205.Deucher W., Sukumar S., Cataland S.R. Clinical relapse of immune-mediated thrombotic thrombocytopenic purpura following COVID-19 vaccination., Res. Pract. Thromb. Haemostasis. 2022;6 doi: 10.1002/rth2.12658. e12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., Wiedmann M., Aamodt A.-H., Skattør T.H., Tjønnfjord G.E., Holme P.A. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination., N. Engl. J. Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Larson V., Seidenberg R., Caplan A., Brinster N.K., Meehan S.A., Kim R.H. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination. J. Cutan. Pathol. 2022;49:34–41. doi: 10.1111/cup.14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bagella C.F., Corda D.G., Zara P., Elia A.E., Ruiu E., Sechi E., Solla P. Chronic inflammatory demyelinating polyneuropathy after ChAdOx1 nCoV-19 vaccination. Vaccines. 2021;9 doi: 10.3390/vaccines9121502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lesort C., Kanitakis J., Donzier L., Jullien D. Chilblain-like lesions after BNT162b2 mRNA COVID-19 vaccine: a case report suggesting that “COVID toes” are due to the immune reaction to SARS-CoV-2. J. Eur. Acad. Dermatol. Venereol. 2021;35:e630–e632. doi: 10.1111/jdv.17451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Suresh P., Petchey W. ChAdOx1 nCOV-19 vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis (CVST) BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-243931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Uaprasert N., Panrong K., Tungjitviboonkun S., Dussadee K., Decharatanachart P., Kaveevorayan P., Shoosanglertwijit R., Watanaboonyongcharoen P., Bunworasate U., Rojnuckarin P. ChAdOx1 nCoV-19 vaccine-associated thrombocytopenia: three cases of immune thrombocytopenia after 107 720 doses of ChAdOx1 vaccination in Thailand., Blood Coagul. Fibrinolysis an Int. J. Haemost. Thromb. 2022;33:67–70. doi: 10.1097/MBC.0000000000001082. [DOI] [PubMed] [Google Scholar]
- 212.Wang Y.-H., Huang L.-Y., Chen Y.-L., Chan J.-S., Chiang W.-F., Lin C.-Y., Chen M.-H., Shyu H.-Y., Hsiao P.-J. ChAdOx1 COVID-19 vaccine-induced thrombocytopenia syndrome. QJM. 2021;114:733–734. doi: 10.1093/qjmed/hcab221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dias L., Soares-Dos-Reis R., Meira J., Ferrão D., Soares P.R., Pastor A., Gama G., Fonseca L., Fagundes V., Carvalho M. Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2021;30:105906. doi: 10.1016/j.jstrokecerebrovasdis.2021.105906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Atta S.N., Othman N., Babar M. Cerebral venous sinus thrombosis secondary to ChAdOx-1 nCov-19 vaccine. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mehta P.R., Apap Mangion S., Benger M., Stanton B.R., Czuprynska J., Arya R., Sztriha L.K. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination - a report of two UK cases. Brain Behav. Immun. 2021;95:514–517. doi: 10.1016/j.bbi.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jinno S., Naka I., Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol. Adv. Pract. 2021;5 doi: 10.1093/rap/rkab096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bano F., Badugama B., Chandra D. Thrombosis and thrombocytopaenia after ChAdOx1 nCoV-19 vaccination: a single UK centre experience. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-243894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Saleh A., Collins J. Case study of thrombosis and thrombocytopenia syndrome following administration of the AstraZeneca COVID-19 vaccine. Aust. J. Gen. Pract. 2021;50 [PubMed] [Google Scholar]
- 219.Eom H., Kim S.W., Kim M., Kim Y.E., Kim J.H., Shin H.Y., Lee H.L. Case reports of acute transverse myelitis associated with mRNA vaccine for COVID-19. J. Kor. Med. Sci. 2022;37 doi: 10.3346/jkms.2022.37.e52. e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Costello A., Pandita A., Devitt J. Case report: thrombotic thrombocytopenia after COVID-19 janssen vaccination. Am. Fam. Physician. 2021;103:646–647. [PubMed] [Google Scholar]
- 221.Hakroush S., Tampe B. Case report: ANCA-associated vasculitis presenting with rhabdomyolysis and pauci-immune crescentic glomerulonephritis after pfizer-BioNTech COVID-19 mRNA vaccination. Front. Immunol. 2021;12:762006. doi: 10.3389/fimmu.2021.762006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tailor P.D., Feighery A.M., El-Sabawi B., Prasad A. Case report: acute myocarditis following the second dose of mRNA-1273 SARS-CoV-2 vaccine., Eur. Hear. Journal. Case Reports. 2021;5 doi: 10.1093/ehjcr/ytab319. ytab319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Razok A., Shams A., Almeer A., Zahid M. Post-COVID-19 vaccine Guillain-Barré syndrome; first reported case from Qatar. Ann. Med. Surg. 2021;67:102540. doi: 10.1016/j.amsu.2021.102540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hasan T., Khan M., Khan F., Hamza G. Case of Guillain-Barré syndrome following COVID-19 vaccine. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-243629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sauret A., Stievenart J., Smets P., Olagne L., Guelon B., Aumaître O., André M., Trefond L. Case of giant cell arteritis after SARS-CoV-2 vaccination: a particular phenotype?, J. Rheumatol. 2022;49:120. doi: 10.3899/jrheum.210724. [DOI] [PubMed] [Google Scholar]
- 226.Nakamura K., Kosano M., Sakai Y., Saito N., Takazawa Y., Omodaka T., Kiniwa Y., Okuyama R. Case of bullous pemphigoid following coronavirus disease 2019 vaccination. J. Dermatol. 2021;48:e606–e607. doi: 10.1111/1346-8138.16170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dell'Antonia M., Anedda S., Usai F., Atzori L., Ferreli C. Bullous pemphigoid triggered by COVID-19 vaccine: rapid resolution with corticosteroid therapy. Dermatol. Ther. 2022;35 doi: 10.1111/dth.15208. e15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mauriello A., Scimeca M., Amelio I., Massoud R., Novelli A., Di Lorenzo F., Finocchiaro S., Cimino C., Telesca R., Chiocchi M., Sun Q., Wang Y., Shi Y., Novelli G., Melino G. Thromboembolism after COVID-19 vaccine in patients with preexisting thrombocytopenia. Cell Death Dis. 2021;12:762. doi: 10.1038/s41419-021-04058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pérez-López I., Moyano-Bueno D., Ruiz-Villaverde R. Bullous pemphigoid and COVID-19 vaccine. Med. Clin. 2021;157:e333–e334. doi: 10.1016/j.medcli.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Schmidt V., Blum R., Möhrenschlager M. Biphasic bullous pemphigoid starting after first dose and boosted by second dose of mRNA-1273 vaccine in an 84-year-old female with polymorbidity and polypharmacy. J. Eur. Acad. Dermatol. Venereol. 2022;36:e88–e90. doi: 10.1111/jdv.17722. [DOI] [PubMed] [Google Scholar]
- 231.Bayas A., Menacher M., Christ M., Behrens L., Rank A., Naumann M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination., Lancet (London. England) 2021;397 doi: 10.1016/S0140-6736(21)00872-2. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bonifacio G.B., Patel D., Cook S., Purcaru E., Couzins M., Domjan J., Ryan S., Alareed A., Tuohy O., Slaght S., Furby J., Allen D., Katifi H.A., Kinton L. Bilateral facial weakness with paraesthesia variant of Guillain-Barré syndrome following Vaxzevria COVID-19 vaccine. J. Neurol. Neurosurg. Psychiatry. 2022;93:341–342. doi: 10.1136/jnnp-2021-327027. [DOI] [PubMed] [Google Scholar]
- 233.Ryan E., Benjamin D., McDonald I., Barrett A., McHugh J., Ryan K., Enright H. AZD1222 vaccine-related coagulopathy and thrombocytopenia without thrombosis in a young female. Br. J. Haematol. 2021;194:553–556. doi: 10.1111/bjh.17530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dalwadi V., Hancock D., Ballout A.A., Geraci A. Axonal-Variant guillian-barre syndrome temporally associated with mRNA-based moderna SARS-CoV-2 vaccine. Cureus. 2021;13 doi: 10.7759/cureus.18291. e18291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ishay Y., Kenig A., Tsemach-Toren T., Amer R., Rubin L., Hershkovitz Y., Kharouf F. Autoimmune phenomena following SARS-CoV-2 vaccination. Int. Immunopharm. 2021;99:107970. doi: 10.1016/j.intimp.2021.107970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Calabria E., Canfora F., Mascolo M., Varricchio S., Mignogna M.D., Adamo D. Autoimmune mucocutaneous blistering diseases after SARS-Cov-2 vaccination: a Case report of Pemphigus Vulgaris and a literature review., Pathol. Res. Pract. 2022;232:153834. doi: 10.1016/j.prp.2022.153834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Vuille-Lessard É., Montani M., Bosch J., Semmo N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J. Autoimmun. 2021;123:102710. doi: 10.1016/j.jaut.2021.102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rocco A., Sgamato C., Compare D., Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: may not be a casuality. J. Hepatol. 2021;75:728–729. doi: 10.1016/j.jhep.2021.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Coto-Segura P., Fernández-Prada M., Mir-Bonafé M., García-García B., González-Iglesias I., Alonso-Penanes P., González-Guerrero M., Gutiérrez-Palacios A., Miranda-Martínez E., Martinón-Torres F. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature. Clin. Exp. Dermatol. 2022;47:141–143. doi: 10.1111/ced.14835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Blauenfeldt R.A., Kristensen S.R., Ernstsen S.L., Kristensen C.C.H., Simonsen C.Z., Hvas A.-M. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J. Thromb. Haemostasis. 2021;19:1771–1775. doi: 10.1111/jth.15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tan C.K., Wong Y.J., Wang L.M., Ang T.L., Kumar R. Autoimmune hepatitis following COVID-19 vaccination: true causality or mere association? J. Hepatol. 2021;75:1250–1252. doi: 10.1016/j.jhep.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rela M., Jothimani D., Vij M., Rajakumar A., Rammohan A. Auto-immune hepatitis following COVID vaccination. J. Autoimmun. 2021;123:102688. doi: 10.1016/j.jaut.2021.102688. [DOI] [PubMed] [Google Scholar]
- 243.Clayton-Chubb D., Schneider D., Freeman E., Kemp W., Roberts S.K. Autoimmune hepatitis developing after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine. J. Hepatol. 2021;75:1249–1250. doi: 10.1016/j.jhep.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bril F., Al Diffalha S., Dean M., Fettig D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J. Hepatol. 2021;75:222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Avci E., Abasiyanik F. Autoimmune hepatitis after SARS-CoV-2 vaccine: new-onset or flare-up? J. Autoimmun. 2021;125:102745. doi: 10.1016/j.jaut.2021.102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Garrido I., Lopes S., Simões M.S., Liberal R., Lopes J., Carneiro F., Macedo G. Autoimmune hepatitis after COVID-19 vaccine - more than a coincidence. J. Autoimmun. 2021;125:102741. doi: 10.1016/j.jaut.2021.102741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Portuguese A.J., Sunga C., Kruse-Jarres R., Gernsheimer T., Abkowitz J. Autoimmune- and complement-mediated hematologic condition recrudescence following SARS-CoV-2 vaccination. Blood Adv. 2021;5:2794–2798. doi: 10.1182/bloodadvances.2021004957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Peralta-Amaro A.L., Tejada-Ruiz M.I., Rivera-Alvarado K.L., de J. Cobos-Quevedo O., Romero-Hernández P., Macías-Arroyo W., Avendaño-Ponce A., Hurtado-Díaz J., Vera-Lastra O., Lucas-Hernández A. Atypical kawasaki disease after COVID-19 vaccination: a new form of adverse event following immunization., Vaccines. 2022;10 doi: 10.3390/vaccines10010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kharkar V., Vishwanath T., Mahajan S., Joshi R., Gole P. Asymmetrical cutaneous vasculitis following COVID-19 vaccination with unusual eosinophil preponderance. Clin. Exp. Dermatol. 2021;46:1596–1597. doi: 10.1111/ced.14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Oo W.M., Giri P., de Souza A. AstraZeneca COVID-19 vaccine and Guillain- barré syndrome in tasmania: a causal link?, J. Neuroimmunol. 2021;360:577719. doi: 10.1016/j.jneuroim.2021.577719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Welsh K.J., Baumblatt J., Chege W., Goud R., Nair N. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS) Vaccine. 2021;39:3329–3332. doi: 10.1016/j.vaccine.2021.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Baimukhamedov C. Arthritis of the left elbow joint after vaccination against SARS-CoV-2 infection. Int. J. Rheum. Dis. 2021;24:1218–1220. doi: 10.1111/1756-185X.14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Unal Enginar A. Arthritis following COVID-19 vaccination: report of two cases. Int. Immunopharm. 2021;101:108256. doi: 10.1016/j.intimp.2021.108256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Dutta A., Ghosh R., Bhattacharya D., Bhat S., Ray A., Pandit A., Das S., Dubey S. Anti-PF4 antibody negative cerebral venous sinus thrombosis without thrombocytopenia following immunization with COVID-19 vaccine in an elderly non-comorbid Indian male, managed with conventional heparin-warfarin based anticoagulation., Diabetes Metab. Syndr. 2021;15:102184. doi: 10.1016/j.dsx.2021.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Dube G.K., Benvenuto L.J., Batal I. Antineutrophil cytoplasmic autoantibody-associated glomerulonephritis following the pfizer-BioNTech COVID-19 vaccine., kidney int. Report. 2021;6:3087–3089. doi: 10.1016/j.ekir.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sacker A., Kung V., Andeen N. Anti-GBM nephritis with mesangial IgA deposits after SARS-CoV-2 mRNA vaccination. Kidney Int. 2021;100:471–472. doi: 10.1016/j.kint.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Renisi G., Lombardi A., Stanzione M., Invernizzi A., Bandera A., Gori A. Anterior uveitis onset after bnt162b2 vaccination: is this just a coincidence? Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021;110:95–97. doi: 10.1016/j.ijid.2021.07.035. [DOI] [PubMed] [Google Scholar]
- 258.Londoño M.-C., Gratacós-Ginès J., Sáez-Peñataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination - still casualty? J. Hepatol. 2021;75:1248–1249. doi: 10.1016/j.jhep.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Shakoor M.T., Birkenbach M.P., Lynch M. ANCA-associated vasculitis following pfizer-BioNTech COVID-19 vaccine., Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2021;78:611–613. doi: 10.1053/j.ajkd.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sekar A., Campbell R., Tabbara J., Rastogi P. ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney Int. 2021;100:473–474. doi: 10.1016/j.kint.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bhan C., Bheesham N., Shakuntulla F., Sharma M., Sun C., Weinstein M. An unusual presentation of acute deep vein thrombosis after the Moderna COVID-19 vaccine-a case report. Ann. Transl. Med. 2021;9:1605. doi: 10.21037/atm-21-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lee E.-J., Cines D.B., Gernsheimer T., Kessler C., Michel M., Tarantino M.D., Semple J.W., Arnold D.M., Godeau B., Lambert M.P., Bussel J.B. Thrombocytopenia following pfizer and moderna SARS-CoV-2 vaccination. Am. J. Hematol. 2021;96:534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jin W., Tang Y., Wen C. An ocular adverse event in temporal association with COVID-19 vaccination in a patient with systemic lupus erythematosus: a case report. Hum. Vaccines Immunother. 2021;17:4102–4104. doi: 10.1080/21645515.2021.1976036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Okada M., Kikuchi E., Nagasawa M., Oshiba A., Shimoda M. An adolescent girl diagnosed with IgA nephropathy following the first dose of the COVID-19 vaccine. CEN Case Reports. 2022:1–4. doi: 10.1007/s13730-021-00679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Maas R.J., Gianotten S., van der Meijden W.A.G. An additional case of minimal change disease following the pfizer-BioNTech COVID-19 vaccine., Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2021;78:312. doi: 10.1053/j.ajkd.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sriphrapradang C. Aggravation of hyperthyroidism after heterologous prime-boost immunization with inactivated and adenovirus-vectored SARS-CoV-2 vaccine in a patient with Graves' disease. Endocrine. 2021;74:226–227. doi: 10.1007/s12020-021-02879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Sharabi A., Shiber S., Molad Y. Adult-onset Still's disease following mRNA COVID-19 vaccination. Clin. Immunol. 2021;233:108878. doi: 10.1016/j.clim.2021.108878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Park S.Y., Lee K.-H. Adult-onset Still's disease after BNT162b2 mRNA COVID-19 vaccine. J. Kor. Med. Sci. 2021;36 doi: 10.3346/jkms.2021.36.e344. e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Leone F., Cerasuolo P.G., Bosello S.L., Verardi L., Fiori E., Cocciolillo F., Merlino B., Zoli A., D'Agostino M.A. Adult-onset Still's disease following COVID-19 vaccination. Lancet. Rheumatol. 2021;3:e678–e680. doi: 10.1016/S2665-9913(21)00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sweeney A., Tracey G., Garnham K. Adult-onset Still disease post-adenovirus vector COVID-19 vaccine. Intern. Med. J. 2021;51:2144–2145. doi: 10.1111/imj.15563. [DOI] [PubMed] [Google Scholar]
- 271.Biradar V., Konnur A., Gang S., Hegde U., Rajapurkar M., Patel H., Pandey S.N., Soni S. Adult-onset nephrotic syndrome following coronavirus disease vaccination. Clin. Kidney J. 2022;15:168–170. doi: 10.1093/ckj/sfab153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Suri V., Pandey S., Singh J., Jena A. Acute-onset chronic inflammatory demyelinating polyneuropathy after COVID-19 infection and subsequent ChAdOx1 nCoV-19 vaccination. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-245816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wolf M.E., Luz B., Niehaus L., Bhogal P., Bäzner H., Henkes H., Thrombocytopenia, Venous Intracranial. Sinus thrombosis after “COVID-19 vaccine AstraZeneca” exposure. J. Clin. Med. 2021;10 doi: 10.3390/jcm10081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gao J.-J., Tseng H.-P., Lin C.-L., Shiu J.-S., Lee M.-H., Liu C.-H. Acute transverse myelitis following COVID-19 vaccination. Vaccines. 2021;9 doi: 10.3390/vaccines9091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hsiao Y.-T., Tsai M.-J., Chen Y.-H., Hsu C.-F. Acute transverse myelitis after COVID-19 vaccination. Medicina. 2021;57 doi: 10.3390/medicina57101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Flower L., Bares Z., Santiapillai G., Harris S. Acute ST-segment elevation myocardial infarction secondary to vaccine-induced immune thrombosis with thrombocytopaenia (VITT) BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-245218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Parkash O., Sharko A., Farooqi A., Ying G.W., Sura P. Acute pancreatitis: a possible side effect of COVID-19 vaccine., Cureus. 2021;13 doi: 10.7759/cureus.14741. e14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Simone A., Herald J., Chen A., Gulati N., Shen A.Y.-J., Lewin B., Lee M.-S. Acute myocarditis following COVID-19 mRNA vaccination in adults aged 18 Years or older., JAMA intern. Med. 2021;181:1668–1670. doi: 10.1001/jamainternmed.2021.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Habib M.B., Hamamyh T., Elyas A., Altermanini M., Elhassan M. Acute myocarditis following administration of BNT162b2 vaccine. IDCases. 2021;25 doi: 10.1016/j.idcr.2021.e01197. e01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Nevet A. Acute myocarditis associated with anti-COVID-19 vaccination. Clin. Exp. Vaccine Res. 2021;10:196–197. doi: 10.7774/cevr.2021.10.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Cereda A., Conca C., Barbieri L., Ferrante G., Tumminello G., Lucreziotti S., Guazzi M., Mafrici A. Acute myocarditis after the second dose of SARS-CoV-2 vaccine: serendipity or atypical causal relationship? Anatol. J. Cardiol. 2021;25:522–523. doi: 10.5152/AnatolJCardiol. 2021.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Schmitt P., Demoulin R., Poyet R., Capilla E., Rohel G., Pons F., Jégo C., Sidibe S., Druelle A., Brocq F.-X., Dutasta F., Cellarier G.R. Acute Myocarditis after COVID-19 vaccination: a case report., Rev. Med. Interne. 2021;42:797–800. doi: 10.1016/j.revmed.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Nguyen T.D., Mall G., Westphal J.G., Weingärtner O., Möbius-Winkler S., Schulze P.C. Acute myocarditis after COVID-19 vaccination with mRNA-1273 in a patient with former SARS-CoV-2 infection. ESC Hear. Fail. 2021;8:4710–4714. doi: 10.1002/ehf2.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Juay L., Chandran N.S. Three cases of vesiculobullous non-IgE-mediated cutaneous reactions to tozinameran (Pfizer-BioNTech COVID-19 vaccine) J. Eur. Acad. Dermatol. Venereol. 2021;35:e855–e857. doi: 10.1111/jdv.17581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bautista García J., Peña Ortega P., Bonilla Fernández J.A., Cárdenes León A., Ramírez Burgos L., Caballero Dorta E. Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19. Rev. Esp. Cardiol. 2021;74:812–814. doi: 10.1016/j.rec.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Mansour J., Short R.G., Bhalla S., Woodard P.K., Verma A., Robinson X., Raptis D.A. Acute myocarditis after a second dose of the mRNA COVID-19 vaccine: a report of two cases. Clin. Imag. 2021;78:247–249. doi: 10.1016/j.clinimag.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Vegezzi E., Ravaglia S., Buongarzone G., Bini P., Diamanti L., Gastaldi M., Prunetti P., Rognone E., Marchioni E. Acute myelitis and ChAdOx1 nCoV-19 vaccine: casual or causal association? J. Neuroimmunol. 2021;359:577686. doi: 10.1016/j.jneuroim.2021.577686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Plasse R., Nee R., Gao S., Olson S. Acute kidney injury with gross hematuria and IgA nephropathy after COVID-19 vaccination. Kidney Int. 2021;100:944–945. doi: 10.1016/j.kint.2021.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Costentin G., Ozkul-Wermester O., Triquenot A., Le Cam-Duchez V., Massy N., Benhamou Y., Massardier E. Acute ischemic stroke revealing ChAdOx1 nCov-19 vaccine-induced immune thrombotic thrombocytopenia: impact on recanalization strategy. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2021;30:105942. doi: 10.1016/j.jstrokecerebrovasdis.2021.105942. [DOI] [PubMed] [Google Scholar]
- 290.Jawed M., Khalid A., Rubin M., Shafiq R., Cemalovic N. Acute immune thrombocytopenia (ITP) following COVID-19 vaccination in a patient with previously stable ITP., open forum infect. Dis. 2021;8 doi: 10.1093/ofid/ofab343. ofab343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ozgen Kenangil G., Ari B.C., Guler C., Demir M.K. Acute disseminated encephalomyelitis-like presentation after an inactivated coronavirus vaccine., Acta Neurol. Belgeler. 2021;121:1089–1091. doi: 10.1007/s13760-021-01699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Vogrig A., Janes F., Gigli G.L., Curcio F., Del Negro I., D'Agostini S., Fabris M., Valente M. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin. Neurol. Neurosurg. 2021;208:106839. doi: 10.1016/j.clineuro.2021.106839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ghielmetti M., Schaufelberger H.D., Mieli-Vergani G., Cerny A., Dayer E., Vergani D., Terziroli Beretta-Piccoli B. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: a novel clinical entity?, J. Autoimmun. 2021;123:102706. doi: 10.1016/j.jaut.2021.102706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Maayan H., Kirgner I., Gutwein O., Herzog-Tzarfati K., Rahimi-Levene N., Koren-Michowitz M., Blickstein D. Acquired thrombotic thrombocytopenic purpura: a rare disease associated with BNT162b2 vaccine., J. Thromb. Haemostasis. 2021;19:2314–2317. doi: 10.1111/jth.15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.İremli B.G., Şendur S.N., Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: postvaccination ASIA syndrome. J. Clin. Endocrinol. Metab. 2021;106:2600–2605. doi: 10.1210/clinem/dgab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Al-Ahmad M., Al-Rasheed M., Shalaby N.A.B. Acquired thrombotic thrombocytopenic purpura with possible association with AstraZeneca-Oxford COVID-19 vaccine. EJH. 2021 doi: 10.1002/jha2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Fujita Y., Yoshida K., Ichikawa D., Shibagaki Y., Yazawa M. Abrupt worsening of occult IgA nephropathy after the first dose of SARS-CoV-2 vaccination. CEN Case Reports. 2022:1–7. doi: 10.1007/s13730-021-00670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tagliaferri A.R., Horani G., Stephens K., Michael P. A rare presentation of undiagnosed multiple sclerosis after the COVID-19 vaccine., J. Community Hosp. Intern. Med. Perspecta. 2021;11:772–775. doi: 10.1080/20009666.2021.1979745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kotal R., Jacob I., Rangappa P., Rao K., Hosurkar G., Anumula S.K., Kuberappa A.M. A rare case of vaccine-induced immune thrombosis and thrombocytopenia and approach to management., Surg. Neurol. Bar Int. 2021;12:408. doi: 10.25259/SNI_689_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Naitlho A., Lahlou W., Bourial A., Rais H., Ismaili N., Abousahfa I., Belyamani L. A rare case of henoch-schönlein purpura following a COVID-19 vaccine-case report. SN Compr. Clin. Med. 2021:1–4. doi: 10.1007/s42399-021-01025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kripalani Y., Lakkappan V., Parulekar L., Shaikh A., Singh R., Vyas P. A rare case of guillain-barré syndrome following COVID-19 vaccination., eur.. J. Case reports intern. Med. 2021;8:2707. doi: 10.12890/2021_002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.D'Agostino V., Caranci F., Negro A., Piscitelli V., Tuccillo B., Fasano F., Sirabella G., Marano I., Granata V., Grassi R., Pupo D., Grassi R. A rare case of cerebral venous thrombosis and disseminated intravascular coagulation temporally associated to the COVID-19 vaccine administration., J. Personalized Med. 2021;11 doi: 10.3390/jpm11040285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Abobaker A., Idris M.A., Ogunjimi O. A localised vasculitic-like skin rash following the second dose of COVID-19 vaccine., Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2022;114:29–30. doi: 10.1016/j.ijid.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Jeon Y.H., Lim D.-H., Choi S.W., Choi S.J. A flare of Still's disease following COVID-19 vaccination in a 34-year-old patient., Rheumatol. Bar Int. 2022;42:743–748. doi: 10.1007/s00296-021-05052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Shazley O., Alshazley M. A COVID-positive 52-year-old man presented with venous thromboembolism and disseminated intravascular coagulation following johnson & johnson vaccination: a case-study., Cureus. 2021;13 doi: 10.7759/cureus.16383. e16383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Hocking J., Chunilal S.D., Chen V.M., Brighton T., Nguyen J., Tan J., Ting S.B., Tran H. The first known case of vaccine-induced thrombotic thrombocytopenia in Australia., Med. J. Aust. 2021;215:19–20. doi: 10.5694/mja2.51135. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Son Y.-B., Kim T.-B., Min H.-J., Lee J., Yang J., Kim M.-G., Jo S.K., Cho W.Y., Oh S.W. A case report of thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination and heparin use during hemodialysis., J. Korean Med. Sci. 2022;37 doi: 10.3346/jkms.2022.37.e75. e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Hamouche W., El Soufi Y., Alzaraq S., Okafor B.V., Zhang F., Paras C. A case report of new onset graves' disease induced by SARS-CoV-2 infection or vaccine?, J. Clin. Transl. Endocrinol. Case Reports. 2022;23:100104. doi: 10.1016/j.jecr.2021.100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kim G., Choi E.-J., Park H.-S., Lee J.-H., Lee J.-H., Lee K.-H. A case report of immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. J. Kor. Med. Sci. 2021;36 doi: 10.3346/jkms.2021.36.e306. e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Bailly-Caillé B., Jouen F., Dompmartin A., Morice C. A case report of anti-P200 pemphigoid following COVID-19 vaccination., JAAD Case Reports. 2022;23:83–86. doi: 10.1016/j.jdcr.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Kim D., Choi J.H., Jang J.Y., So O., Cho E., Choi H., Hong K.S., Park K.T. A case report for myopericarditis after BNT162b2 COVID-19 mRNA vaccination in a Korean young male. J. Kor. Med. Sci. 2021;36 doi: 10.3346/jkms.2021.36.e277. e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Tølbøll Sørensen A.L., Rolland M., Hartmann J., Harboe Z.B., Roed C., Jensen T.Ø., Kolte L., El Fassi D., Hillingsø J., Radziwon-Balicka A., Soyka R.S., Hansen K., Kirkby N., Goetze J.P., Gybel-Brask M., Leinøe E.B., Hvas A.-M., Kampmann P., Stensballe J. A case of thrombocytopenia and multiple thromboses after vaccination with ChAdOx1 nCoV-19 against SARS-CoV-2., Blood Adv. 2021;5:2569–2574. doi: 10.1182/bloodadvances.2021004904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Miyaue N., Yoshida A., Yamanishi Y., Tada S., Ando R., Hosokawa Y., Yabe H., Nagai M. Refractory longitudinally extensive transverse myelitis after severe acute respiratory syndrome coronavirus 2 vaccination in a Japanese man. Intern. Med. 2022;61:739–742. doi: 10.2169/internalmedicine.8747-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Hung Y.-P., Sun K.-S. A case of myopericarditis with pleuritis following AstraZeneca Covid-19 vaccination., QJM. 2022;114:879–881. doi: 10.1093/qjmed/hcab278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Da Y., Goh G.H., Khatri P. A case of membranous nephropathy following Pfizer-BioNTech mRNA vaccination against COVID-19., Kidney Int. 2021;100:938–939. doi: 10.1016/j.kint.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Pagenkopf C., Südmeyer M. A case of longitudinally extensive transverse myelitis following vaccination against Covid-19., J. Neuroimmunol. 2021;358:577606. doi: 10.1016/j.jneuroim.2021.577606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Su S.-C., Lyu R.-K., Chang C.-W., Tseng W.-E.J. The first guillain-barr? Syndrome after SARS-CoV-2 vaccination in taiwan., acta neurol. Taiwania. 2022;31(1):46–51. [PubMed] [Google Scholar]
- 318.King E.R., Towner E. A case of immune thrombocytopenia after BNT162b2 mRNA COVID-19 vaccination. Am.. J. Case Rep. 2021;22 doi: 10.12659/AJCR.931478. e931478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.V Malayala S., Papudesi B.N., Sharma R., Vusqa U.T., Raza A. A case of idiopathic thrombocytopenic purpura after booster dose of BNT162b2 (Pfizer-Biontech) COVID-19 vaccine., Cureus. 2021;13 doi: 10.7759/cureus.18985. e18985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Rao S.J., Khurana S., Murthy G., Dawson E.T., Jazebi N., Haas C.J. A case of Guillain-Barre syndrome following Pfizer COVID-19 vaccine., J. Community Hosp. Intern. Med. Perspecta. 2021;11:597–600. doi: 10.1080/20009666.2021.1954284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Rahim S.E.G., Lin J.T., Wang J.C. A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination., Kidney Int. 2021;100:238. doi: 10.1016/j.kint.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Patrizio A., Ferrari S.M., Antonelli A., Fallahi P. A case of Graves' disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination., J. Autoimmun. 2021;125:102738. doi: 10.1016/j.jaut.2021.102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Yamamoto K., Mashiba T., Takano K., Suzuki T., Kami M., Takita M., Kusumi E., Mizuno Y., Hamaki T. A case of exacerbation of subclinical hyperthyroidism after first administration of BNT162b2 mRNA COVID-19 vaccine., Vaccines. 2021;9 doi: 10.3390/vaccines9101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Elamin S., Hinds F., Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin. Exp. Dermatol. 2022;47:153–155. doi: 10.1111/ced.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Chittal A., Rao S., Lakra P., Nacu N., Haas C. A case of COVID-19 vaccine-induced thrombotic thrombocytopenia., J. Community Hosp. intern. Med. Perspecta. 2021;11:776–778. doi: 10.1080/20009666.2021.1980966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Tagliaferri A.R., Narvaneni S., Azzam M.H., Grist W. A case of COVID-19 vaccine causing a myasthenia Gravis crisis. Cureus. 2021 doi: 10.7759/cureus.15581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Chavez A., Pougnier C. A case of COVID-19 vaccine associated new diagnosis myasthenia Gravis., J. Prim. Care Community Health. 2021;12 doi: 10.1177/21501327211051933. 21501327211051932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ammirati E., Cavalotti C., Milazzo A., Pedrotti P., Soriano F., Schroeder J.W., Morici N., Giannattasio C., Frigerio M., Metra M., Camici P.G., Oliva F. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection. Int. J. Cardiol. Hear. Vasc. 2021;34:100774. doi: 10.1016/j.ijcha.2021.100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Young J., Mercieca L., Ceci M., Pisani D., Betts A., Boffa M.J. A case of bullous pemphigoid after the SARS-CoV-2 mRNA vaccine., J. Eur. Acad. Dermatol. Venereol. 2022;36:e13–e16. doi: 10.1111/jdv.17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Villa M., Díaz-Crespo F., Pérez de José A. Ú. Verdalles, E. Verde, F. Almeida Ruiz, A. Acosta, A. Mijaylova, M. Goicoechea, A case of ANCA-associated vasculitis after AZD1222 (Oxford-AstraZeneca) SARS-CoV-2 vaccination: casualty or causality?, Kidney Int. 2021;100:937–938. doi: 10.1016/j.kint.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Wiest N.E., Johns G.S., Edwards E. A case of acute pulmonary embolus after mRNA SARS-CoV-2 immunization., Vaccines. 2021;9 doi: 10.3390/vaccines9080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Walter T., Connor S., Stedman C., Doogue M. A case of acute necrotising pancreatitis following the second dose of Pfizer-BioNTech COVID-19 mRNA vaccine., Br. J. Clin. Pharmacol. 2022;88:1385–1386. doi: 10.1111/bcp.15039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Mira F.S., Costa Carvalho J., de Almeida P.A., Pimenta A.C., Alen Coutinho I., Figueiredo C., Rodrigues L., Sousa V., Ferreira E., Pinto H., Escada L., Galvão A., Alves R. A case of acute interstitial nephritis after two doses of the BNT162b2 SARS-CoV-2 vaccine., int. J. Nephrol. Renovasc. Dis. 2021;14:421–426. doi: 10.2147/IJNRD.S345898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Nasuelli N.A., De Marchi F., Cecchin M., De Paoli I., Onorato S., Pettinaroli R., Savoini G., Godi L. A case of acute demyelinating polyradiculoneuropathy with bilateral facial palsy after ChAdOx1 nCoV-19 vaccine., Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2021;42:4747–4749. doi: 10.1007/s10072-021-05467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Al-Maqbali J.S., Al Rasbi S., Kashoub M.S., Al Hinaai A.M., Farhan H., Al Rawahi B., Al Alawi A.M. A 59-year-old woman with extensive deep vein thrombosis and pulmonary thromboembolism 7 Days following a first dose of the pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine., Am.. J. Case Rep. 2021;22 doi: 10.12659/AJCR.932946. e932946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination., N. Engl. J. Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.McShane C., Kiat C., Rigby J. Ó. Crosbie, the mRNA COVID-19 vaccine - a rare trigger of autoimmune hepatitis?, J. Hepatol. 2021;75:1252–1254. doi: 10.1016/j.jhep.2021.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Idogun P.O., Ward M.C., Teklie Y., Wiese-Rometsch W., Baker J. Newly diagnosed idiopathic thrombocytopenia post COVID-19 vaccine administration. Cureus. 2021;13 doi: 10.7759/cureus.14853. e14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.A.M. N, Saleh A.M., Khalid A., Alshaya A.K., Alanazi S.M.M. Systemic lupus erythematosus with acute pancreatitis and vasculitic rash following COVID-19 vaccine: a case report and literature review. Clin. Rheumatol. 2022;41:1577–1582. doi: 10.1007/s10067-022-06097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Magliulo D., Narayan S., Ue F., Boulougoura A., Badlissi F. Adult-onset Still's disease after mRNA COVID-19 vaccine. Lancet. Rheumatol. 2021;3:e680–e682. doi: 10.1016/S2665-9913(21)00219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Brubaker J.E.D., Casaccio C.L., Brazeau M.J. Recurrence of autoimmune hepatitis after COVID-19 vaccination. Cureus. 2022;14 doi: 10.7759/cureus.25339. e25339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Mekritthikrai K., Jaru-Ampornpan P., Komolmit P., Thanapirom K. Autoimmune hepatitis triggered by COVID-19 vaccine: the first case from inactivated vaccine., ACG Case Reports J. 2022;9 doi: 10.14309/crj.0000000000000811. e00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Kim B.C., Kim H.S., Han K.H., Han S.Y., Jo H.A. A case report of MPO-ANCA-associated vasculitis following heterologous mRNA1273 COVID-19 booster vaccination. J. Kor. Med. Sci. 2022;37 doi: 10.3346/jkms.2022.37.e204. e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Finsterer J. Letter to the editor: pre-existing neuropathy favours SARS-CoV-2 vaccination associated guillain-barre syndrome. J. Kor. Med. Sci. 2022;37 doi: 10.3346/jkms.2022.37.e217. e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Kanamori H. Gross hematuria can be an impact of severe acute respiratory syndrome coronavirus 2 vaccination on immunoglobulin A nephropathy: a case report. J. Med. Case Rep. 2022;16:273. doi: 10.1186/s13256-022-03514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Ruggeri R.M., Giovanellla L., Campennì A. SARS-CoV-2 vaccine may trigger thyroid autoimmunity: real-life experience and review of the literature. J. Endocrinol. Invest. 2022:1–7. doi: 10.1007/s40618-022-01863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Khan E., Shrestha A.K., Colantonio M.A., Liberio R.N., Sriwastava S. Acute transverse myelitis following SARS-CoV-2 vaccination: a case report and review of literature. J. Neurol. 2022;269:1121–1132. doi: 10.1007/s00415-021-10785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Baicus C., Delcea C., Pinte L., Dan G.A. Hyper-inflammation after COVID-19 mARN vaccination: at the crossroads of multisystem inflammatory disease and adult-onset Still's disease. Does terminology matter? Rom. J. Intern. Med. 2022;60:3–5. doi: 10.2478/rjim-2021-0035. [DOI] [PubMed] [Google Scholar]
- 349.Nagai K., Iwase M., Ueda A. A case of anti-GBM nephritis following centipede bites and COVID-19 vaccination., CEN Case Reports. 2022;11:166–170. doi: 10.1007/s13730-021-00646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Abou-Ismail M.Y., Moser K.A., Smock K.J., Lim M.Y. Vaccine-induced thrombotic thrombocytopenia following Ad26.COV2.S vaccine in a man presenting as acute venous thromboembolism. Am. J. Hematol. 2021;96:E346–E349. doi: 10.1002/ajh.26265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Molina Rios S., Rojas Martinez R., Estévez Ramirez G.M., Medina Y.F., Lupus Erythematosus Systemic, Syndrome Antiphospholipid. After COVID-19 vaccination. A Case Report., Mod. Rheumatol. Case Reports. 2022 doi: 10.1093/mrcr/rxac018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Mejren A., Sørensen C.M., Gormsen L.C., Tougaard R.S., Nielsen B.D. Large-vessel giant cell arteritis after COVID-19 vaccine. Scand. J. Rheumatol. 2022;51:154–155. doi: 10.1080/03009742.2021.1961401. [DOI] [PubMed] [Google Scholar]
- 353.Rinaldi V., Bellucci G., Romano A., Bozzao A., Salvetti M. ADEM after ChAdOx1 nCoV-19 vaccine: a case report., Mult. Scler. 2022;28:1151–1154. doi: 10.1177/13524585211040222. [DOI] [PubMed] [Google Scholar]
- 354.Saito K., Ichikawa S., Hatta S., Katsuoka Y., Harigae H., Izumi T. Vincristine therapy for severe and refractory immune thrombocytopenia following COVID-19 vaccination. Ann. Hematol. 2022;101:885–887. doi: 10.1007/s00277-021-04666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Zin Tun G.S., Gleeson D., Al-Joudeh A., Dube A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J. Hepatol. 2022;76:747–749. doi: 10.1016/j.jhep.2021.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Agharbi F.-Z., Eljazouly M., Basri G., Faik M., Benkirane A., Albouzidi A., Chiheb S. Bullous pemphigoid induced by the AstraZeneca COVID-19 vaccine. Ann. Dermatol. Venereol. 2022;149:56–57. doi: 10.1016/j.annder.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Permezel F., Borojevic B., Lau S., de Boer H.H. Acute disseminated encephalomyelitis (ADEM) following recent Oxford/AstraZeneca COVID-19 vaccination. Forensic Sci. Med. Pathol. 2022;18:74–79. doi: 10.1007/s12024-021-00440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Torrente S., Castiella A., Garmendia M., Zapata E. Probable autoimmune hepatitis reactivated after COVID-19 vaccination. Gastroenterol. Hepatol. 2022;45(Suppl 1):115–116. doi: 10.1016/j.gastrohep.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Hali F., Kerouach A., Alatawna H., Chiheb S., Lakhdar H. Linear IgA bullous dermatosis following Oxford AstraZeneca COVID-19 vaccine. Clin. Exp. Dermatol. 2022;47:611–613. doi: 10.1111/ced.15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Gambichler T., Hamdani N., Budde H., Sieme M., Skrygan M., Scholl L., Dickel H., Behle B., Ganjuur N., Scheel C., Abu Rached N., Ocker L., Stranzenbach R., Doerler M., Pfeiffer L., Becker J.C. Bullous pemphigoid after SARS-CoV-2 vaccination: spike-protein-directed immunofluorescence confocal microscopy and T-cell-receptor studies. Br. J. Dermatol. 2022;186:728–731. doi: 10.1111/bjd.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Zengarini C., Pileri A., Salamone F.P., Piraccini B.M., Vitale G., La Placa M. Subacute cutaneous lupus erythematosus induction after SARS-CoV-2 vaccine in a patient with primary biliary cholangitis. J. Eur. Acad. Dermatol. Venereol. 2022;36:e179–e180. doi: 10.1111/jdv.17827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Patil S., Patil A. Systemic lupus erythematosus after COVID-19 vaccination: a case report., J. Cosmet. Dermatol. 2021;20:3103–3104. doi: 10.1111/jocd.14386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Mokos M., Bašić-Jukić N. IgA nephropathy following SARS-CoV-2 vaccination in a renal transplant recipient with a history of aristolochic acid nephropathy., Ther. Apher. Dial. Off. Peer-Reviewed J. Int. Soc. Apher. Japanese Soc. Apher. Japanese Soc. Dial. Ther. 2022;26:667–668. doi: 10.1111/1744-9987.13765. [DOI] [PubMed] [Google Scholar]
- 364.Abičić A., Adamec I., Habek M. Miller Fisher syndrome following Pfizer COVID-19 vaccine. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2022;43:1495–1497. doi: 10.1007/s10072-021-05776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Moreno-Torres V. Á. Gutiérrez, M. Valdenebro, A. Ortega, M.-J. Cítores, E. Montero, Catastrophic antiphospholipid syndrome triggered by mRNA COVID-19 vaccine. Clin. Exp. Rheumatol. 2022;40:1054–1055. doi: 10.55563/clinexprheumatol/s3sbgu. [DOI] [PubMed] [Google Scholar]
- 366.V Knechtl G., Seyed Jafari S.M., Berger T., Rammlmair A., Feldmeyer L., Borradori L. Development of pemphigus vulgaris following mRNA SARS-CoV-19 BNT162b2 vaccination in an 89-year-old patient. J. Eur. Acad. Dermatol. Venereol. 2022;36:e251–e253. doi: 10.1111/jdv.17868. [DOI] [PubMed] [Google Scholar]
- 367.Erard D., Villeret F., Lavrut P.-M., Dumortier J. Autoimmune hepatitis developing after COVID 19 vaccine: presumed guilty? Clin. Res. Hepatol. Gastroenterol. 2022;46:101841. doi: 10.1016/j.clinre.2021.101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Pauluzzi M., Stinco G., Errichetti E. Bullous pemphigoid in a young male after COVID-19 mRNA vaccine: a report and brief literature review. J. Eur. Acad. Dermatol. Venereol. 2022;36:e257–e259. doi: 10.1111/jdv.17891. [DOI] [PubMed] [Google Scholar]
- 369.Dams L., Kraemer M., Becker J. MOG-antibody-associated longitudinal extensive myelitis after ChAdOx1 nCoV-19 vaccination. Mult. Scler. 2022;28:1159–1162. doi: 10.1177/13524585211057512. [DOI] [PubMed] [Google Scholar]
- 370.Nakano H., Yamaguchi K., Kawabata K., Asakawa M., Matsumoto Y. Acute transverse myelitis after BNT162b2 vaccination against COVID-19: report of a fatal case and review of the literature. J. Neurol. Sci. 2022;434:120102. doi: 10.1016/j.jns.2021.120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Ottaviani S., Juge P.-A., Forien M., Ebstein E., Palazzo E., Dieudé P. Polymyalgia rheumatica following COVID-19 vaccination: a case-series of ten patients., Jt. Bone Spine. 2022;89:105334. doi: 10.1016/j.jbspin.2021.105334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Abu Rached N., Mansour R., Susok L., Fried S., Abolmaali N., Lee Y.-P., Gambichler T. Sarcoidal immune reaction following SARS-CoV-2 vaccination. Clin. Exp. Dermatol. 2022;47:970–972. doi: 10.1111/ced.15082. [DOI] [PubMed] [Google Scholar]
- 373.Finsterer J., Redzic Z. Symptomatic peduncular, cavernous bleeding following SARS-CoV-2 vaccination induced immune thrombocytopenia. Brain Hemorrhages. 2021;2:169–171. doi: 10.1016/j.hest.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Kim N., Kim J.-H., Park J.-S. Guillain-Barré syndrome associated with BNT162b2 COVID vaccination: a first case report from South Korea. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2022;43:1491–1493. doi: 10.1007/s10072-021-05849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Pla Peris B., Merchante Alfaro A.Á., Maravall Royo F.J., Abellán Galiana P., Pérez Naranjo S., González Boillos M. Thyrotoxicosis following SARS-COV-2 vaccination: a case series and discussion. J. Endocrinol. Invest. 2022;45:1071–1077. doi: 10.1007/s40618-022-01739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Capezzone M., Tosti-Balducci M., Morabito E.M., Caldarelli G.P., Sagnella A., Cantara S., Alessandri M., Castagna M.G. Silent thyroiditis following vaccination against COVID-19: report of two cases. J. Endocrinol. Invest. 2022;45:1079–1083. doi: 10.1007/s40618-021-01725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Hung W.-K., Chi C.-C. Incident bullous pemphigoid in a psoriatic patient following mRNA-1273 SARS-CoV-2 vaccination. J. Eur. Acad. Dermatol. Venereol. 2022;36:e407–e409. doi: 10.1111/jdv.17955. [DOI] [PubMed] [Google Scholar]
- 378.Afacan E., Edek Y.C., İlter N., Gülekon A. Can Covid-19 vaccines cause or exacerbate bullous pemphigoid? A report of seven cases from one center., Int. J. Dermatol. 2022;61:626–627. doi: 10.1111/ijd.16086. [DOI] [PubMed] [Google Scholar]
- 379.Sasaki H., Itoh A., Watanabe Y., Nakajima Y., Saisho Y., Irie J., Meguro S., Itoh H. Newly developed type 1 diabetes after coronavirus disease 2019 vaccination: a case report. J. Diabetes Investig. 2022;13:1105–1108. doi: 10.1111/jdi.13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Tang X., He B., Liu Z., Zhou Z., Li X. Fulminant type 1 diabetes after COVID-19 vaccination. Diabetes Metab. 2022;48:101324. doi: 10.1016/j.diabet.2022.101324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Oğuz S.H., Şendur S.N., İremli B.G., Gürlek A., Erbas T., Ünlütürk U. SARS-CoV-2 vaccine-induced thyroiditis: safety of revaccinations and clinical follow-up. J. Clin. Endocrinol. Metab. 2022;107:e1823–e1834. doi: 10.1210/clinem/dgac049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.AlQudari E.A., Alabdan L.I., Alkhathami A.A., Alotaibi M.D., Alhamzi H.A. Adult-onset Still's disease after the ChAdOx1 nCoV-19 vaccine. Cureus. 2022;14 doi: 10.7759/cureus.21279. e21279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Akoglu G. Pemphigus vulgaris after SARS-CoV-2 vaccination: a case with new-onset and two cases with severe aggravation., Dermatol. Ther. 2022;35 doi: 10.1111/dth.15396. e15396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Darrigade A.-S., Théophile H., Sanchez-Pena P., Milpied B., Colbert M., Pedeboscq S., Pistone T., Jullié M.-L., Seneschal J. Sweet syndrome induced by SARS-CoV-2 Pfizer-BioNTech mRNA vaccine. Allergy. 2021;76:3194–3196. doi: 10.1111/all.14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Kim J.W., Kim Y.G., Park Y.C., Choi S., Lee S., Min H.J., Kim M.J. Guillain-barre syndrome after two COVID-19 vaccinations: two case reports with follow-up electrodiagnostic study. J. Kor. Med. Sci. 2022;37 doi: 10.3346/jkms.2022.37.e58. e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Sugimoto T., Yorishima A., Oka N., Masuda S., Yoshida Y., Hirata S. Exacerbation of systemic lupus erythematosus after receiving mRNA-1273-based coronavirus disease 2019 vaccine. J. Dermatol. 2022;49:e199–e200. doi: 10.1111/1346-8138.16327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Chou S.-C., Chang Y.-C., Liao C.-K., Chen T.-C., Sun K.-J., Huang W.-H., Wu Y.-F. New presentations and exacerbations of immune thrombocytopenia after coronavirus disease 2019 vaccinations: the Taiwan experience. Platelets. 2022;33:531–535. doi: 10.1080/09537104.2022.2042237. [DOI] [PubMed] [Google Scholar]
- 388.Stasiak M., Zawadzka-Starczewska K., Lewiński A. Significance of HLA haplotypes in two patients with subacute thyroiditis triggered by mRNA-based COVID-19 vaccine. Vaccines. 2022;10 doi: 10.3390/vaccines10020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Anamnart C., Tisavipat N., Owattanapanich W., Apiwattanakul M., Savangned P., Prayoonwiwat N., Siritho S., Rattanathamsakul N., Jitprapaikulsan J. Newly diagnosed neuromyelitis optica spectrum disorders following vaccination: case report and systematic review. Mult. Scler. Relat. Disord. 2022;58:103414. doi: 10.1016/j.msard.2021.103414. [DOI] [PubMed] [Google Scholar]
- 390.Bostan H., Ucan B., Kizilgul M., Calapkulu M., Hepsen S., Gul U., Ozturk Unsal I., Cakal E. Relapsed and newly diagnosed Graves' disease due to immunization against COVID-19: a case series and review of the literature., J. Autoimmun. 2022;128:102809. doi: 10.1016/j.jaut.2022.102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Sakurai K., Narita D., Saito N., Ueno T., Sato R., Niitsuma S., Takahashi K., Arihara Z. Type 1 diabetes mellitus following COVID-19 RNA-based vaccine. J. Diabetes Investig. 2022;13:1290–1292. doi: 10.1111/jdi.13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Al-Quliti K., Qureshi A., Quadri M., Abdulhameed B., Alanazi A., Alhujeily R. Acute demyelinating encephalomyelitis post-COVID-19 vaccination: a case report and literature review., dis. (Basel, Switzerland) 2022;10 doi: 10.3390/diseases10010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Dharmasaroja P. Early flare-ups of myasthenia Gravis after thoracoscopic thymectomy in a patient recently receiving BNT162b2 mRNA COVID-19 vaccination. Cureus. 2022;14 doi: 10.7759/cureus.21571. e21571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Hashizume H., Ajima S., Ishikawa Y. Immunoglobulin A vasculitis post-severe acute respiratory syndrome coronavirus 2 vaccination and review of reported cases. J. Dermatol. 2022;49:560–563. doi: 10.1111/1346-8138.16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Baffa M.E., Maglie R., Giovannozzi N., Montefusco F., Senatore S., Massi D., Antiga E. Sweet syndrome following SARS-CoV2 vaccination. Vaccines. 2021;9 doi: 10.3390/vaccines9111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Fimiano F., D'Amato D., Gambella A., Marzano A., Saracco G.M., Morgando A. Autoimmune hepatitis or drug-induced autoimmune hepatitis following Covid-19 vaccination? Liver Int. Off. J. Int. Assoc. Study Liver. 2022;42:1204–1205. doi: 10.1111/liv.15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Wong J.S.Y., Kang J.H.-E., Maw K.Z. Acute immune thrombocytopenic purpura post first dose of COVID-19 vaccination. Postgrad. Med. 2022;98:e129–e130. doi: 10.1136/postgradmedj-2021-140947. [DOI] [PubMed] [Google Scholar]
- 398.Chee Y.J., Liew H., Hoi W.H., Lee Y., Lim B., Chin H.X., Lai R.T.R., Koh Y., Tham M., Seow C.J., Quek Z.H., Chen A.W., Quek T.P.L., Tan A.W.K., Dalan R. SARS-CoV-2 mRNA vaccination and graves' disease: a report of 12 cases and review of the literature., J. Clin. Endocrinol. Metab. 2022;107:e2324–e2330. doi: 10.1210/clinem/dgac119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Etemadifar M., Nouri H., Salari M., Sedaghat N. Detection of anti-NMDA receptor antibodies following BBIBP-CorV COVID-19 vaccination in a rituximab-treated person with multiple sclerosis presenting with manifestations of an acute relapse. Hum. Vaccines Immunother. 2022;18:2033540. doi: 10.1080/21645515.2022.2033540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Tabatabaee S., Rezania F., Alwedaie S.M.J., Malekdar E., Badi Z., Tabatabaei S.M., Mirzaasgari Z. Post COVID-19 vaccination Guillain-Barre syndrome: three cases. Hum. Vaccines Immunother. 2022;18:2045153. doi: 10.1080/21645515.2022.2045153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Roongta R., Mondal S., Haldar S., Kumar M.S., Ghosh A. Two flares of Still's disease after two doses of the ChAdOx1 vaccine. Clin. Rheumatol. 2022;41:1591–1596. doi: 10.1007/s10067-022-06124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Alshammari F., Abuzied Y., Korairi A., Alajlan M., Alzomia M., AlSheef M. Bullous pemphigoid after second dose of mRNA- (Pfizer-BioNTech) Covid-19 vaccine: a case report., Ann. Med. Surg. 2022;75:103420. doi: 10.1016/j.amsu.2022.103420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Kishimoto M., Ishikawa T., Odawara M. Subacute thyroiditis with liver dysfunction following coronavirus disease 2019 (COVID-19) vaccination: report of two cases and a literature review. Endocr. J. 2022 doi: 10.1507/endocrj.EJ21-0629. [DOI] [PubMed] [Google Scholar]
- 404.De Bruyne S., Van Landeghem S., Schauwvlieghe A., Noens L. Life-threatening autoimmune hemolytic anemia following mRNA COVID-19 vaccination: don't be too prudent with the red gold. Clin. Chem. Lab. Med. 2022;60:e125–e128. doi: 10.1515/cclm-2022-0118. [DOI] [PubMed] [Google Scholar]
- 405.Senda J., Ashida R., Sugawara K., Kawaguchi K. Acute meningoencephalitis after COVID-19 vaccination in an adult patient with rheumatoid vasculitis. Intern. Med. 2022;61:1609–1612. doi: 10.2169/internalmedicine.8815-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Majid I., Mearaj S. Sweet syndrome after Oxford-AstraZeneca COVID-19 vaccine (AZD1222) in an elderly female. Dermatol. Ther. 2021;34 doi: 10.1111/dth.15146. e15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Ghorbani H., Rouhi T., Vosough Z., Shokri-Shirvani J. Drug-induced hepatitis after Sinopharm COVID-19 vaccination: a case study of a 62-year-old patient., Int. J. Surg. Case Rep. 2022;93:106926. doi: 10.1016/j.ijscr.2022.106926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Saffarian Z., Samii R., Ghanadan A., Vahidnezhad H. De novo severe pemphigus vulgaris following SARS-CoV-2 vaccination with BBIBP-CorV. Dermatol. Ther. 2022;35 doi: 10.1111/dth.15448. e15448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Thaler J., Ay C., V Gleixner K., Hauswirth A.W., Cacioppo F., Grafeneder J., Quehenberger P., Pabinger I., Knöbl P. Successful treatment of vaccine-induced prothrombotic immune thrombocytopenia (VIPIT) J. Thromb. Haemostasis. 2021;19:1819–1822. doi: 10.1111/jth.15346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Chanut M., Jaidi R., Kohn M., Grange T., Brones C., Lombion N., Rousselot P., Longval T. Successful mRNA SARS-Cov-2 vaccine rechallenge after a first episode of immune thrombocytopenic purpura. Platelets. 2022;33:652–653. doi: 10.1080/09537104.2022.2044463. [DOI] [PubMed] [Google Scholar]
- 411.Soltanpoor P., Norouzi G. Subacute thyroiditis following COVID-19 vaccination. Clin. Case Reports. 2021;9 doi: 10.1002/ccr3.4812. e04812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Şahin Tekin M., Şaylısoy S., Yorulmaz G. Subacute thyroiditis following COVID-19 vaccination in a 67-year-old male patient: a case report. Hum. Vaccines Immunother. 2021;17:4090–4092. doi: 10.1080/21645515.2021.1947102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Fanni D., Saba L., Demontis R., Gerosa C., Chighine A., Nioi M., Suri J.S., Ravarino A., Cau F., Barcellona D., Botta M.C., Porcu M., Scano A., Coghe F., Orrù G., Van Eyken P., Gibo Y., La Nasa G., D’aloja E., Marongiu F., Faa G. Vaccine-induced severe thrombotic thrombocytopenia following COVID-19 vaccination: a report of an autoptic case and review of the literature., Eur. Rev. Med. Pharmacol. Sci. 2021;25:5063–5069. doi: 10.26355/eurrev_202108_26464. [DOI] [PubMed] [Google Scholar]
- 414.Sigstad E., Grøholt K.K., Westerheim O. Subacute thyroiditis after vaccination against SARS-CoV-2. Tidsskr. Nor. Laegeforen. 2021;141 doi: 10.4045/tidsskr.21.0554. [DOI] [PubMed] [Google Scholar]
- 415.Borges Canha M., Neves J.S., Oliveira A.I., Sarmento A., Carvalho D. Subacute thyroiditis after severe acute respiratory syndrome coronavirus 2 vaxzevria vaccination in a patient with thyroid autoimmunity. Cureus. 2022;14 doi: 10.7759/cureus.22353. e22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Jhon M., Lee S.-H., Oh T.-H., Kang H.-C. Subacute thyroiditis after receiving the mRNA COVID-19 vaccine (moderna): the first case report and literature review in korea., J. Korean Med. Sci. 2022;37 doi: 10.3346/jkms.2022.37.e39. e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Oyibo S.O. Subacute thyroiditis after receiving the adenovirus-vectored vaccine for coronavirus disease (COVID-19) Cureus. 2021;13 doi: 10.7759/cureus.16045. e16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Saygılı E.S., Karakilic E. Subacute thyroiditis after inactive SARS-CoV-2 vaccine. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-244711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Nagalli S., Shankar Kikkeri N. Sub-acute onset of guillain-barré syndrome post-mRNA-1273 vaccination: a case report., SN compr. Clin. Med. 2022;4:41. doi: 10.1007/s42399-022-01124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Joseph A.K., Chong B.F. Subacute cutaneous lupus erythematosus flare triggered by COVID-19 vaccine. Dermatol. Ther. 2021;34 doi: 10.1111/dth.15114. e15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Elboraey M.O., Essa E.E.S.F. Stevens-Johnson syndrome post second dose of Pfizer COVID-19 vaccine: a case report., Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021;132:e139–e142. doi: 10.1016/j.oooo.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Bencharattanaphakhi R., Rerknimitr P. Sinovac COVID-19 vaccine-induced cutaneous leukocytoclastic vasculitis. JAAD Case Reports. 2021;18:1–3. doi: 10.1016/j.jdcr.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Niebel D., Wenzel J., Wilsmann-Theis D., Ziob J., Wilhelmi J., Braegelmann C. Single-Center clinico-pathological case study of 19 patients with cutaneous adverse reactions following COVID-19 vaccines. Dermatopathol. (Basel, Switzerland) 2021;8:463–476. doi: 10.3390/dermatopathology8040049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Aladdin Y., Algahtani H., Shirah B. Vaccine-induced immune thrombotic thrombocytopenia with disseminated intravascular coagulation and death following the ChAdOx1 nCoV-19 vaccine. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2021;30:105938. doi: 10.1016/j.jstrokecerebrovasdis.2021.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Helms J.M., Ansteatt K.T., Roberts J.C., Kamatam S., Foong K.S., Labayog J.-M.S., Tarantino M.D. Severe, refractory immune thrombocytopenia occurring after SARS-CoV-2 vaccine. Hematol. Res. Rev. 2021;12:221–224. doi: 10.2147/JBM.S307047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Maniscalco G.T., Manzo V., Di Battista M.E., Salvatore S., Moreggia O., Scavone C., Capuano A. Severe multiple sclerosis relapse after COVID-19 vaccination: a case report., front. Neurol. 2021;12:721502. doi: 10.3389/fneur.2021.721502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Cooper K.M., Switzer B. Severe immune thrombocytopenic purpura after SARS-CoV-2 vaccine. Arch. Clin. Cases. 2021;8:31–36. doi: 10.22551/2021.31.0802.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Gardellini A., Guidotti F., Maino E., Steffanoni S., Zancanella M., Turrini M. Severe immune thrombocytopenia after COVID-19 vaccination: report of four cases and review of the literature. Blood Cells Mol. Dis. 2021;92:102615. doi: 10.1016/j.bcmd.2021.102615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Bianchi S., Angi A., Passucci M., Palumbo G., Baldacci E., Testi A.M. Severe immune thrombocytopenia (ITP) following SARS-CoV-2 mRNA vaccine in a girl on immunosuppressive treatment and in prolonged stable phase of ITP. Mediterr. J. Hematol. Infect. Dis. 2022;14 doi: 10.4084/MJHID. e2022011. 2022.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.V Gadi S.R., Brunker P.A.R., Al-Samkari H., Sykes D.B., Saff R.R., Lo J., Bendapudi P., Leaf D.E., Leaf R.K. Severe autoimmune hemolytic anemia following receipt of SARS-CoV-2 mRNA vaccine. Transfusion. 2021;61:3267–3271. doi: 10.1111/trf.16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Min Y.G., Ju W., Ha Y.-E., Ban J.-J., Lee S.A., Sung J.-J., Shin J.-Y. Sensory Guillain-Barre syndrome following the ChAdOx1 nCov-19 vaccine: report of two cases and review of literature. J. Neuroimmunol. 2021;359:577691. doi: 10.1016/j.jneuroim.2021.577691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Fueyo-Rodriguez O., Valente-Acosta B., Jimenez-Soto R., Neme-Yunes Y., Inclán-Alarcón S.I., Trejo-Gonzalez R. M.Á. García-Salcido, Secondary immune thrombocytopenia supposedly attributable to COVID-19 vaccination. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-242220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Cadiou S., Perdriger A., Ardois S., Albert J.-D., Berthoud O., Lescoat A., Guggenbuhl P., Robin F. SARS-CoV-2, polymyalgia rheumatica and giant cell arteritis: COVID-19 vaccine shot as a trigger? Comment on: “Can SARS-CoV-2 trigger relapse of polymyalgia rheumatica?” by Manzo et al. Joint Bone Spine. 2022;2021(88):105282. doi: 10.1016/j.jbspin.2021.105282. 105150., Jt. Bone Spine. 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Patel K.R., Cunnane M.E., Deschler D.G. SARS-CoV-2 vaccine-induced subacute thyroiditis. Am. J. Otolaryngol. 2022;43:103211. doi: 10.1016/j.amjoto.2021.103211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Charidimou A., Samudrala S., Cervantes-Arslanian A.M., Sloan J.M., Dasenbrock H.H., Daneshmand A. Vaccine-induced immune thrombotic thrombocytopenia with concurrent arterial and venous thrombi following Ad26.COV2.S vaccination. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2021;30:106113. doi: 10.1016/j.jstrokecerebrovasdis.2021.106113. [DOI] [PubMed] [Google Scholar]
- 436.Baskaran K., Cohen A.W.S., Weerasinghe N., Vilayur E. Report of two cases of minimal change disease following vaccination for COVID -19. Nephrology. 2022;27:111–112. doi: 10.1111/nep.13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Salem F., Rein J.L., Yu S.M.-W., Abramson M., Cravedi P., Chung M. Report of three cases of minimal change disease following the second dose of mRNA SARS-CoV-2 COVID-19 vaccine., kidney int. Report. 2021;6:2523–2524. doi: 10.1016/j.ekir.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.De Fabritiis M., Angelini M.L., Fabbrizio B., Cenacchi G., Americo C., Cristino S., Lifrieri M.F., Cappuccilli M., Spazzoli A., Zambianchi L., Mosconi G. Renal thrombotic microangiopathy in concurrent COVID-19 vaccination and infection. Pathogens. 2021;10 doi: 10.3390/pathogens10081045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Parperis K., Constantinou M. Remitting seronegative symmetrical synovitis with pitting oedema following BNT162b2 mRNA COVID-19 vaccination. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-244479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.David R., Hanna P., Lee K., Ritchie A. Relapsed ANCA associated vasculitis following Oxford AstraZeneca ChAdOx1-S COVID-19 vaccination: a case series of two patients., Nephrology. 2022;27:109–110. doi: 10.1111/nep.13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Sissa C., Al-Khaffaf A., Frattini F., Gaiardoni R., Mimiola E., Montorsi P., Melara B., Amato M., Peyvandi F., Franchini M. Relapse of thrombotic thrombocytopenic purpura after COVID-19 vaccine., Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapheresis. 2021;60:103145. doi: 10.1016/j.transci.2021.103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Aydın M.F., Yıldız A., Oruç A., Sezen M., Dilek K., Güllülü M., Yavuz M., Ersoy A. Relapse of primary membranous nephropathy after inactivated SARS-CoV-2 virus vaccination. Kidney Int. 2021;100:464–465. doi: 10.1016/j.kint.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Komaba H., Wada T., Fukagawa M. Relapse of minimal change disease following the pfizer-BioNTech COVID-19 vaccine., Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2021;78:469–470. doi: 10.1053/j.ajkd.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Morlidge C., El-Kateb S., Jeevaratnam P., Thompson B. Relapse of minimal change disease following the AstraZeneca COVID-19 vaccine. Kidney Int. 2021;100:459. doi: 10.1016/j.kint.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Conticini E., d'Alessandro M., Bergantini L., Bargagli E., Gentili F., Mazzei M.A., Cantarini L., Frediani B. Relapse of microscopic polyangiitis after vaccination against COVID-19: a case report., J. Med. Virol. 2021;93:6439–6441. doi: 10.1002/jmv.27192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Boonyawat K., Angchaisuksiri P. Vaccine-induced immune thrombotic thrombocytopenia with ChAdOx1 nCoV-19 is rare in Asia., Res. Pract. Thromb. Haemostasis. 2022;6 doi: 10.1002/rth2.12644. e12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Masset C., Kervella D., Kandel-Aznar C., Fantou A., Blancho G., Hamidou M. Relapse of IgG4-related nephritis following mRNA COVID-19 vaccine. Kidney Int. 2021;100:465–466. doi: 10.1016/j.kint.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Tuschen K., Bräsen J.H., Schmitz J., Vischedyk M., Weidemann A. Relapse of class V lupus nephritis after vaccination with COVID-19 mRNA vaccine. Kidney Int. 2021;100:941–944. doi: 10.1016/j.kint.2021.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Major A., Carll T., Chan C.W., Christenson C., Aldarweesh F., Wool G.D., Cohen K.S. Refractory vaccine-induced immune thrombotic thrombocytopenia (VITT) managed with delayed therapeutic plasma exchange (TPE) J. Clin. Apher. 2022;37:117–121. doi: 10.1002/jca.21945. [DOI] [PubMed] [Google Scholar]
- 450.Wu X., Lim J.H.L., Lee J.S.S., Chio M.T.-W. Recurrent erythema nodosum after the second dose of the Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Int. 2022;6:107–108. doi: 10.1016/j.jdin.2021.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.An Q.-J., Qin D.-A., Pei J.-X. Reactive arthritis after COVID-19 vaccination. Hum. Vaccines Immunother. 2021;17:2954–2956. doi: 10.1080/21645515.2021.1920274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Maye J.A., Chong H.P., Rajagopal V., Petchey W. Reactivation of IgA vasculitis following COVID-19 vaccination. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-247188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Krajewski P.K. Ł. Matusiak, J.C. Szepietowski, Psoriasis flare-up associated with second dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine. J. Eur. Acad. Dermatol. Venereol. 2021;35:e632–e634. doi: 10.1111/jdv.17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Chao J.-P., Tsai T.-F. Psoriasis flare following ChAdOx1-S/nCoV-19 vaccination in patients with psoriasis under biologic treatment. Dermatol. Sin. 2021;39:208–209. doi: 10.4103/ds.ds_45_21. [DOI] [Google Scholar]
- 455.Fang W.-C., Chiu L.-W., Hu S.C.-S. Psoriasis exacerbation after first dose of AstraZeneca coronavirus disease 2019 vaccine. J. Dermatol. 2021;48:e566–e567. doi: 10.1111/1346-8138.16137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Sotiriou E., Tsentemeidou A., Bakirtzi K., Lallas A., Ioannides D., Vakirlis E. Psoriasis exacerbation after COVID-19 vaccination: a report of 14 cases from a single centre. J. Eur. Acad. Dermatol. Venereol. 2021;35:e857–e859. doi: 10.1111/jdv.17582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Page D., Zhu N., Sawler D., Sun H.W., Turley E., Pai M., Wu C. Vaccine-induced immune thrombotic thrombocytopenia presenting with normal platelet count., Res. Pract. Thromb. Haemostasis. 2021;5 doi: 10.1002/rth2.12596. e12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Tiede A., Sachs U.J., Czwalinna A., Werwitzke S., Bikker R., Krauss J.K., Donnerstag F., Weißenborn K., Höglinger G., Maasoumy B., Wedemeyer H., Ganser A. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood. 2021;138:350–353. doi: 10.1182/blood.2021011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Gambichler T., Scholl L., Dickel H., Ocker L., Stranzenbach R. Prompt onset of Rowell's syndrome following the first BNT162b2 SARS-CoV-2 vaccination. J. Eur. Acad. Dermatol. Venereol. 2021;35:e415–e416. doi: 10.1111/jdv.17225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Varona J.F., García-Isidro M., Moeinvaziri M., Ramos-López M., Fernández-Domínguez M. Primary adrenal insufficiency associated with Oxford-AstraZeneca ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia (VITT)., Eur. J. Intern. Med. 2021;91:90–92. doi: 10.1016/j.ejim.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Weijers J., Alvarez C., Hermans M.M.H. Post-vaccinal minimal change disease. Kidney Int. 2021;100:459–461. doi: 10.1016/j.kint.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Zuhorn F., Graf T., Klingebiel R., Schäbitz W.-R., Rogalewski A. Postvaccinal encephalitis after ChAdOx1 nCov-19. Ann. Neurol. 2021;90:506–511. doi: 10.1002/ana.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Matta A., Kallamadi R., Matta D., Bande D. Post-mRNA COVID-19 vaccination myocarditis., eur. J. Case Reports Intern. Med. 2021;8:2769. doi: 10.12890/2021_002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Grossman M.E., Appel G., Little A.J., Ko C.J. Post-COVID-19 vaccination IgA vasculitis in an adult. J. Cutan. Pathol. 2022;49:385–387. doi: 10.1111/cup.14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Waheed W., Carey M.E., Tandan S.R., Tandan R. Post COVID-19 vaccine small fiber neuropathy. Muscle Nerve. 2021;64:E1–E2. doi: 10.1002/mus.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Badrawi N., Kumar N., Albastaki U. Post COVID-19 vaccination neuromyelitis optica spectrum disorder: case report & MRI findings. Radiol. Case Reports. 2021;16:3864–3867. doi: 10.1016/j.radcr.2021.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Nygaard U., Holm M., Bohnstedt C., Chai Q., Schmidt L.S., Hartling U.B., Petersen J.J.H., Thaarup J., Bjerre J., Vejlstrup N.G., Juul K., Stensballe L.G. Population-based incidence of myopericarditis after COVID-19 vaccination in Danish adolescents. Pediatr. Infect. Dis. J. 2022;41:e25–e28. doi: 10.1097/INF.0000000000003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Vizcarra P., Haemmerle J., Velasco H., Velasco T., Fernández-Escribano M., Vallejo A., Casado J.L. BNT162b2 mRNA COVID-19 vaccine Reactogenicity: the key role of immunity., Vaccine. 2021;39:7367–7374. doi: 10.1016/j.vaccine.2021.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Matthews A.W., Griffiths I.D. Post-vaccinial pericarditis and myocarditis. Br. Heart J. 1974;36:1043–1045. doi: 10.1136/hrt.36.10.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Klok F.A., Pai M., V Huisman M., Makris M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet. Haematol. 2022;9:e73–e80. doi: 10.1016/S2352-3026(21)00306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Haber P., Sejvar J., Mikaeloff Y., DeStefano F. Vaccines and guillain-barré syndrome. Drug Saf. 2009;32:309–323. doi: 10.2165/00002018-200932040-00005. [DOI] [PubMed] [Google Scholar]
- 472.Jerne N.K. Towards a network theory of the immune system. Ann. Immunol. (Paris) 1974;125C:373–389. [PubMed] [Google Scholar]
- 473.Paque R.E., Miller R. Autoanti-idiotypes exhibit mimicry of myocyte antigens in virus-induced myocarditis. J. Virol. 1991;65:16–22. doi: 10.1128/JVI.65.1.16-22.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Murphy W.J., Longo D.L. A possible role for anti-idiotype Antibodies in SARS-CoV-2 infection and vaccination, N. Engl. J. Med. 2022;386:394–396. doi: 10.1056/nejmcibr2113694. [DOI] [PubMed] [Google Scholar]
- 475.Rodríguez Y., Novelli L., Rojas M., De Santis M., Acosta-Ampudia Y., Monsalve D.M., Ramírez-Santana C., Costanzo A., Ridgway W.M., Ansari A.A., Gershwin M.E., Selmi C., Anaya J.-M. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J. Autoimmun. 2020;114:102506. doi: 10.1016/j.jaut.2020.102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Liu Y., Sawalha A.H., Lu Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2021;33:155–162. doi: 10.1097/BOR.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Anaya J.-M., Herrán M., Beltrán S., Rojas M. Is post-COVID syndrome an autoimmune disease? Expet Rev. Clin. Immunol. 2022 doi: 10.1080/1744666X.2022.2085561. [DOI] [PubMed] [Google Scholar]
- 478.Wieske L., Kummer L.Y.L., van Dam K.P.J., Stalman E.W., van der Kooi A.J., Raaphorst J., Löwenberg M., Takkenberg R.B., Volkers A.G., D'Haens G.R.A.M., Tas S.W., Spuls P.I., Bekkenk M.W., Musters A.H., Post N.F., Bosma A.L., Hilhorst M.L., Vegting Y., Bemelman F.J., Killestein J., van Kempen Z.L.E., Voskuyl A.E., Broens B., Sanchez A.P., Wolbink G., Boekel L., Rutgers A., de Leeuw K., Horváth B., Verschuuren J.J.G.M., Ruiter A.M., van Ouwerkerk L., van der Woude D., Allaart C.F., Teng Y.K.O., van Paassen P., Busch M.H., Jallah B.P., Brusse E., van Doorn P.A., Baars A.E., Hijnen D., Schreurs C.R.G., van der Pol W.L., Goedee H.S., Steenhuis M., Rispens T., Ten Brinke A., Verstegen N.J.M., Zwinderman K.A.H., van Ham S.M., Kuijpers T.W., Eftimov F. Risk factors associated with short-term adverse events after SARS-CoV-2 vaccination in patients with immune-mediated inflammatory diseases. BMC Med. 2022;20:100. doi: 10.1186/s12916-022-02310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Marć M.A., Domínguez-Álvarez E., Gamazo C. Nucleic acid vaccination strategies against infectious diseases., Expert Opin. Drug Deliv. 2015;12:1851–1865. doi: 10.1517/17425247.2015.1077559. [DOI] [PubMed] [Google Scholar]
- 480.Reikine S., Nguyen J.B., Modis Y., Recognition Pattern, Mechanisms Signaling. Of RIG-I and MDA5. Front. Immunol. 2014;5:342. doi: 10.3389/fimmu.2014.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Schurz H., Salie M., Tromp G., Hoal E.G., Kinnear C.J., Möller M. The X chromosome and sex-specific effects in infectious disease susceptibility., Hum. Genomics. 2019;13:2. doi: 10.1186/s40246-018-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Pelka K., Shibata T., Miyake K., Latz E. Nucleic acid-sensing TLRs and autoimmunity: novel insights from structural and cell biology. Immunol. Rev. 2016;269:60–75. doi: 10.1111/imr.12375. [DOI] [PubMed] [Google Scholar]
- 483.D’haese S., Lacroix C., Garcia F., Plana M., Ruta S., Vanham G., Verrier B., Aerts J.L. Off the beaten path: novel mRNA-nanoformulations for therapeutic vaccination against HIV. J. Control. Release Off. J. Control. Release Soc. 2021;330:1016–1033. doi: 10.1016/j.jconrel.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 484.Kowarz E., Krutzke L., Reis J., Bracharz S., Kochanek S., Marschalek R. v1“Vaccine-Induced Covid-19 Mimicry” Syndrome:Splice reactions within the SARS-CoV-2 Spike open reading frame result in Spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines. 2021 doi: 10.21203/rs.3.rs-558954/. [DOI] [Google Scholar]
- 485.Cai Z., Greene M.I., Zhu Z., Zhang H., Features Structural, Functions P.F.4. That occur in heparin-induced thrombocytopenia (HIT) complicated by COVID-19. Antibodies. 2020;9 doi: 10.3390/antib9040052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Greinacher A., Selleng K., Warkentin T.E. Autoimmune heparin-induced thrombocytopenia. J. Thromb. Haemostasis. 2017;15:2099–2114. doi: 10.1111/jth.13813. [DOI] [PubMed] [Google Scholar]
- 487.Greinacher A. CLINICAL PRACTICE. Heparin-induced thrombocytopenia. N. Engl. J. Med. 2015;373:252–261. doi: 10.1056/NEJMcp1411910. [DOI] [PubMed] [Google Scholar]
- 488.Handtke S., Wolff M., Zaninetti C., Wesche J., Schönborn L., Aurich K., Ulm L., Hübner N.-O., Becker K., Thiele T., Greinacher A. A flow cytometric assay to detect platelet-activating antibodies in VITT after ChAdOx1 nCov-19 vaccination. Blood. 2021;137:3656–3659. doi: 10.1182/blood.2021012064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.McGonagle D., De Marco G., Bridgewood C. Mechanisms of immunothrombosis in vaccine-induced thrombotic thrombocytopenia (VITT) compared to natural SARS-CoV-2 infection. J. Autoimmun. 2021;121:102662. doi: 10.1016/j.jaut.2021.102662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Baker A.T., Boyd R.J., Sarkar D., Teijeira-Crespo A., Chan C.K., Bates E., Waraich K., Vant J., Wilson E., Truong C.D., Lipka-Lloyd M., Fromme P., Vermaas J., Williams D., Machiesky L., Heurich M., Nagalo B.M., Coughlan L., Umlauf S., Chiu P.-L., Rizkallah P.J., Cohen T.S., Parker A.L., Singharoy A., Borad M.J. ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abl8213. eabl8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Holm S., Kared H., Michelsen A.E., Kong X.Y., Dahl T.B., Schultz N.H., Nyman T.A., Fladeby C., Seljeflot I., Ueland T., Stensland M., Mjaaland S., Goll G.L., Nissen-Meyer L.S., Aukrust P., Skagen K., Gregersen I., Skjelland M., Holme P.A., Munthe L.A., Halvorsen B. Immune complexes, innate immunity, and NETosis in ChAdOx1 vaccine-induced thrombocytopenia. Eur. Heart J. 2021;42:4064–4072. doi: 10.1093/eurheartj/ehab506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Morgan E.N., Pohlman T.H., Vocelka C., Farr A., Lindley G., Chandler W., Griscavage-Ennis J.M., Verrier E.D. Nuclear factor kappaB mediates a procoagulant response in monocytes during extracorporeal circulation. J. Thorac. Cardiovasc. Surg. 2003;125:165–171. doi: 10.1067/mtc.2003.99. [DOI] [PubMed] [Google Scholar]
- 493.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pão C.R.R., Righy C., Franco S., Souza T.M.L., Kurtz P., Bozza F.A., Bozza P.T. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136:1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Mattsson E., Herwald H., Egesten A. Superantigens from Staphylococcus aureus induce procoagulant activity and monocyte tissue factor expression in whole blood and mononuclear cells via IL-1 beta. J. Thromb. Haemostasis. 2003;1:2569–2576. doi: 10.1111/j.1538-7836.2003.00498.x. [DOI] [PubMed] [Google Scholar]
- 495.Iba T., Umemura Y., Wada H., Levy J.H. Roles of coagulation abnormalities and microthrombosis in sepsis: pathophysiology, diagnosis, and treatment. Arch. Med. Res. 2021;52:788–797. doi: 10.1016/j.arcmed.2021.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.