Summary
Background
Prevalence of both multimorbidity and frailty increases with age, but more evidence is needed to elucidate their relationship and their association with other health-related outcomes. We analysed the dynamics of both conditions as people age and calculate the associated risk of death, nursing home admission, and need for home care.
Methods
Data were drawn from the primary care electronic health records of a longitudinal cohort of people aged 65 or older in Catalonia in 2010–2019. Frailty and multimorbidity were measured using validated instruments (eFRAGICAP, a cumulative deficit model; and SNAC-K, respectively), and their longitudinal evolution was described. Cox regression models accounted for the competing risk of death and adjusted by sex, socioeconomical status, and time-varying age, alcohol and smoking.
Findings
We included 1 456 052 patients. Prevalence of multimorbidity was consistently high regardless of age, while frailty almost quadrupled from 65 to 99 years. Frailty worsened and also changed with age: up to 84 years, it was more related to concurrent diseases, and afterwards, to frailty-related deficits. While concurrent diseases contributed more to mortality, frailty-related deficits increased the risk of institutionalisation and the need for home care.
Interpretation
The nature of people’s multimorbidity and frailty vary with age, as does their impact on health status. People become frailer as they age, and their frailty is more characterised by disability and other symptoms than by diseases. Mortality is most associated with the number of comorbidities, whereas frailty-related deficits are associated with needing specialised care.
Funding
Instituto de Salud Carlos III through PI19/00535, and the PFIS Grant FI20/00040 (Co-funded by European Regional Development Fund/European Social Fund).
Keywords: Multimorbidity, Frailty, Mortality, Aging, Cohort, Primary health care
Research in context.
Evidence before this study
The ageing population is characterised by both multimorbidity and frailty, but the relationship between the two concepts and their dynamics have not been fully elucidated. A search in PubMed for articles published from inception to 19 July 2022 using the terms (Dynamic OR Evolution OR Kinetics) AND Frailty AND (Multimorbidity OR Comorbidity) yielded 104 articles. Most articles analysed different aspects related to both conditions, such as the association with different outcomes. However, none analysed the simultaneous dynamics of multimorbidity and frailty using longitudinal, real-world data from routine clinical practice. A longitudinal analysis might be helpful to propose new hypotheses on the mechanisms of the joint evolution between multimorbidity and frailty.
Added value of this study
The study population (N = 1 456 052) had a mean follow-up of 70 years (standard deviation 32), allowing a longitudinal analysis of the relationship between multimorbidity and frailty as people age. A more detailed characterisation differentiating between frailty due to concurrent diseases versus that due to other frailty-related deficits, showed an evolving relationship as individuals age, with the characteristics of frailty varying as it worsens. In addition, the influence of frailty to health-related outcomes such as mortality, nursing home admission, and need for home care varies according to population multimorbidity.
Implications of all the available evidence
Health care plans should consider the interaction between individuals’ frailty and multimorbidity status along with other factors such as age. From age 85 years onwards, frailty is mainly characterised by deficits unrelated to multimorbidity. The specific characteristics of frailty appear to condition the risk for different health-related outcomes. Risk is therefore determined by the relationship between an individual’s frailty and multimorbidity, not only the severity of each. As indicators for both multimorbidity and frailty used in this research can be easily obtained from primary care electronic health records, the application of these findings to other international health care systems is feasible through a reference framework, allowing the standardisation of the criteria applied. These findings can guide decision-making of both policy-makers and primary care professionals when planning and designing care and treatment plans for the elderly population.
Alt-text: Unlabelled box
Introduction
As the ageing population continues to grow, related problems like multimorbidity and frailty are becoming major challenges for health systems. Defining how both concepts evolve over time is necessary to effectively address individual and population-level interventions, as both are strongly associated with other health outcomes such as death, falls, and institutionalisation.1, 2, 3, 4, 5 Multimorbidity, that is, the coexistence of more than one chronic disease,6 is more common in older people.7, 8 As there is no consensus on which chronic diseases should be considered when defining multimorbidity, its prevalence varies widely between studies.9 However, there are some validated proposals to standardise its definition in older people, such as the one described by the SNAC-K study, which defined 60 groups of chronic diseases.10
Frailty is grounded in a theoretical construct hypothesised to have an underlying biological basis. Diagnosis is not based on the state of specific organs, but rather by the age-related loss of homeostasis and resistance to stressors.2, 11 Different types of risk factors can lead to the development of frailty,12 including diseases, social or neurological problems, and disabilities. Frailty predisposes biologically older people to rapid, adverse changes in health status,13 so its prompt identification can enable interventions to manage it.11, 14 There are different instruments to assess frailty based on performance measures, routine data, questionnaires, or a combination of any of these.15 Nevertheless, there is no standard comprehensive assessment instrument and there is currently a move towards instruments that are specific to particular healthcare settings and populations.16 In primary healthcare setting, there is a need for instruments that are not time-consuming and that are helpful in making decisions about interventions and care allocation. In this regard, frailty indexes based on the deficit accumulation approach using electronic health records (EHR) may be assessment instruments that combine these features.17, 18, 19 These indexes include counts of signs, symptoms, diseases, laboratory measures, and social and functional impairments, generating a frailty score,20 with higher values indicating greater degrees of frailty. Clegg et al. proposed and validated an electronic frailty index (eFI) based on 36 deficits that can be identified in primary care EHR.18 Obtaining deficits from the EHR is a pragmatic approach that provides good-quality, accessible data. The eFI has been validated in other EHR systems, including in Catalonia, known as eFRAGICAP.21 For their part, frailty indexes have proven useful to measure the risk of mortality and other adverse health outcomes in older adults.18, 22
Multimorbidity and frailty are associated.13, 23, 24 Chronic diseases contribute to the development of frailty,11, 12, 25 while frailty-related health deterioration in health status may lead to the development of comorbidities, thus multimorbidity.12 Previous studies have reported the existence of this bidirectional relationship,13 suggesting some overlap between the two concepts. This evidence could inform health care planning, as both multimorbidity and frailty increases the risk of certain health-related outcomes. The more frail a person is, the higher their risk of mortality1, 2, 4 and other adverse outcomes, such as the need for home care or nursing home admission.1, 2 Likewise, multimorbidity increases the risk of mortality,3, 26 and institutionalisation.5 However, the relationship between these outcomes and both multimorbidity and frailty has yet to be studied. The main objective of this study is to describe the evolution between multimorbidity and frailty in the older population from Catalonia (Spain) over 10 years of follow-up, and to assess their relationship with all-cause mortality, nursing home admission, and the need for home care.
Methods
Design, data source, and population
This longitudinal cohort study drew data from the Sistema d’Informació pel Desenvolupament de la Investigació a l’Atenció Primària (SIDIAP, www.sidiap.org). Since 2005, the SIDIAP database collects the anonymised EHRs of approximately 6 million patients enrolled in primary care health services in Catalonia (Spain). This represents almost 80% of the Catalan population and is a reliable representation of the region as a whole.27 SIDIAP can be linked with hospital admissions data from all health care providers. Data collected included (i) sociodemographic information, (ii) visits to primary care, (iii) clinical measures, (iv) all diagnoses made in primary care (using International Classification of Diseases, 10th revision, ICD-10), (v) laboratory results, (vi) emergency admission episodes, (vii) medication dispensed in pharmacies (using Anatomical Therapeutic Classification, ATC), and (viii) inclusion in social assistance programmes.
Ethical considerations
This study was approved by the Scientific and Ethical Committees of SIDIAP (19/518-P) on the 18/12/2019. SIDIAP database is based on opt-out presumed consent. If a patient decides to opt out, their routine data would be excluded of the database.
Study setting
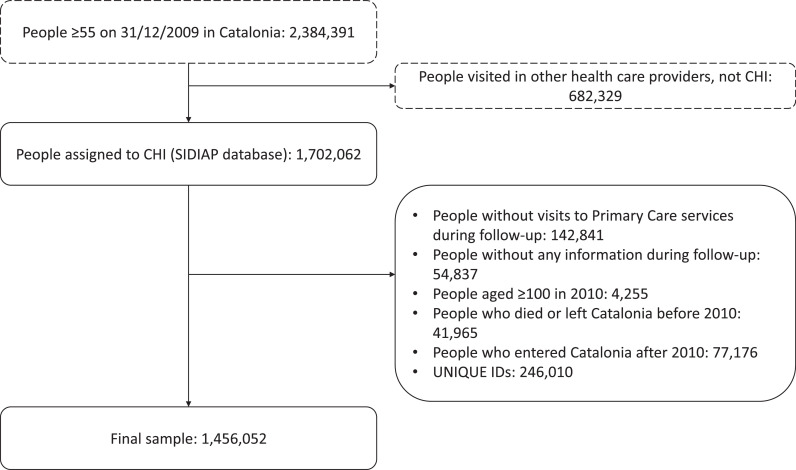
The study took place from 1 January 2010 to 31 December 2019. Participants were included at baseline if aged 65 years or older, or throughout the follow-up period as they turned 65, and followed until death or transfer out of the catchment area. Exclusion criteria were: no available data, did not visit a primary care centre during the follow-up years, and aged 100 years or older in 2010. From the initial sample of 1 702 062 individuals, 1 456 052 remained (see Figure 1).
Figure 1.
Flow chart of the study population. The figure reports the number of individuals who met each exclusion criterion, as well as the number of individuals that met all the criteria (unique IDs).
Exposures
Frailty was measured using eFRAGICAP, a validated adaptation of the eFI that uses EHR from Catalan primary care centres.21 The eFRAGICAP includes 36 possible deficits based on medical, pharmacy, and laboratory data from the EHR. Twenty deficits refer to diagnoses and 16 to signs/symptoms, laboratory results, and disabilities (SSLD) (see Table S1). The eFRAGICAP score, like the eFI, is calculated by dividing the deficit count by 36. Patients’ frailty was categorised using the cutoff points proposed by Clegg et al.18 (i.e. fit 0.12, mild 0.12 to 0.24, moderate 0.24 to 0.36, and severe 0.36.). Therefore, eFRAGICAP indicators were considered in three ways: (i) as a deficit count, (ii) as a deficit count per deficit type (disease-related versus SSLD), and (iii) as a category. We used an operational definition of multimorbidity based on 60 chronic disease categories determined in the SNAC-K study such as diabetes, hypertension, chronic kidney disease, autoimmune diseases, and ischemic heart diseases.10 SNAC-K was used to measure multimorbidity to increase the comparability of our results, as its use is more widespread. Multimorbidity was defined as the presence of concurrent diagnoses from at least two categories. An ordinal four category classification (non-multimorbid, 2–5 diseases, 6–10 diseases, >10 diseases), was also considered. Both exposures were calculated annually.
Study outcomes
The primary outcomes were: (i) all-cause mortality, (ii) nursing home admission, and (iii) need for home care. Outcomes (ii) and (iii) were measured using ICD-10 codes (see Supplementary Material). The start date for the calculation of risk was 1 January 2010, or date of inclusion for people under 65 in 2010. Follow-up stopped in case of death, transfer, or after the first event (for nursing home admission and need for home care).
Confounders
Age, sex, socioeconomic status, alcohol intake, and predominant smoking status were considered as confounders (Supplementary Material). Socioeconomic status was measured with a deprivation index tool from 2011.28 Mortality models also included nursing home admission and need for home care as confounders. Age, alcohol intake, smoking status, nursing home admission, and need for home care were measured annually.
Statistical analyses
Continuous variables were described using median, interquartile range, and range, after confirming the lack of Gaussianity, and categorical variables as absolute and relative frequencies. A Cox proportional hazard regression model was fitted to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) of mortality. Adjusted HRs were calculated using cause-specific Cox models for nursing home admission and need for home care, considering the competing risk of death. In all cases, stratified Cox models were assumed, considering multimorbidity in four categories as the strata variable. By defining multimorbidity as strata, different baseline risk functions for each category were calculated. Age, sex, socioeconomic status, alcohol intake, and smoking status were used as confounders. All covariates were time-varying, except for sex and socioeconomic status. The Akaike’s Information Criterion (AIC) from the models are reported. Missingness was 6.0% for smoking status, 31.5% for socioeconomic status, and 50.5% for alcohol intake, over the 10 years, and were imputed using multiple imputations (see Supplementary Material). Survival models were also fitted using complete cases as a sensitivity analysis to compare both Missing Completely At Random (MCAR) and Missing At Random (MAR) missingness schemes. Statistical significance was defined at 0.05, two-sided. The analyses were performed with R v4.1. and STATA v17.0.
Role of funding
The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of this work.
Results
Description of the population
In 2010, 943 671 patients were included; by the end of the study period, enrollment reached a total of 1 456 052 individuals (Figure S1). The mean length of follow-up was 7.04 years (standard deviation (SD) 3.15). Table 1 shows the characteristics of the baseline population. 57.6% were women, median age was 75 years, median eFRAGICAP was 0.11, and 61.5% of the were fit. Regarding morbidities, the sample had a median of five chronic diseases, and 89.3% were considered multimorbid. People with multimorbidity had higher eFRAGICAP (median 0.11, compared to 0.03 in non-multimorbid participants), and 43% of them were frail, versus just 0.7% in non-multimorbid patients. Frail people had more chronic diseases than fit subjects, being considered virtually all multimorbid (99.8%), while prevalence of multimorbidity dropped to 82.7% in fit individuals. At baseline, the most common status was multimorbid and fit (50.9%), whereas 38.4% were multimorbid and frail.
Table 1.
Characteristics of the baseline population (year 2010).
Note: Categorical variables are described as n (%), excluding missing values, if any, from the calculation of the percentage. Quantitative variables are described as median (interquartile range) [min-max]. Multimorbidity was defined as having concurrent diseases in ≥2 SNAC-K categories.
| Baseline population (year 2010) ( 671) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Women | Men | Non-multimorbid | Multimorbid | Fit | Frail | Total | |
| ( 821; 57.6) | ( 850; 42.4) | ( 120; 10.7) | ( 551; 89.3) | ( 818 ; 61.5) | ( 853; 38.5) | |||
| Sex | ||||||||
| Female | 543 821 (100) | 0 (0) | 54 668 (54.1) | 489 153 (58.1) | 309 854 (53.3) | 233 967 (64.5) | 543 821 (57.6) | |
| Male | 0 (0) | 399 850 (100) | 46 452 (45.9) | 353 398 (41.9) | 270 964 (46.7) | 128 886 (35.5) | 399 850 (42.4) | |
| Age | 76 (12) [65-98] | 74 (10) [65-98] | 73 (12) [65-98] | 75 (12) [65-98] | 73 (10) [65-98] | 78 (11) [65-98] | 75 (12) [65-98] | |
| Deprivation index | ||||||||
| 1 (less deprived) | 80 428 (17.4) | 53 555 (15.8) | 19 136 (21.7) | 114 847 (16.1) | 89 880 (17.6) | 44 103 (15.2) | 133 983 (16.7) | |
| 2 | 71 503 (15.5) | 50 530 (14.9) | 13 905 (15.8) | 108 128 (15.2) | 79 744 (15.6) | 42 289 (14.6) | 122 033 (15.3) | |
| 3 | 71 938 (15.6) | 52 734 (15.6) | 12 949 (14.7) | 111 723 (15.7) | 80 019 (15.7) | 44 653 (15.4) | 124 672 (15.6) | |
| 4 | 67 925 (14.7) | 50 752 (15.0) | 11 049 (12.5) | 107 628 (15.1) | 74 583 (14.6) | 44 094 (15.2) | 118 677 (14.8) | |
| 5 (more deprived) | 59 908 (13.0) | 44 924 (13.3) | 8 950 (10.2) | 95 882 (13.5) | 61 947 (12.1) | 42 885 (14.8) | 104 832 (13.1) | |
| Missing | 82 006 | 61 581 | 12 987 | 60 407 | 70 894 | 72 693 | 143 587 | |
| eFRAGICAP | 0.11 (0.11) [0-0.64] | 0.08 (0.08) [0-0.56] | 0.03 (0.03) [0-0.28] | 0.11 (0.08) [0-0.64] | 0.08 (0.06) [0-0.11] | 0.17 (0.08) [0.14-0.64] | 0.11 (0.11) [0-0.64] | |
| Deficit count (total) | 4 (4) [0-13] | 3 (3) [0-20] | 1 (1) [0-10] | 4 (3) [0-23] | 3 (2) [0-4] | 6 (3) [5-23] | 4 (4) [0-23] | |
| Deficit count (diseases) | 2 (2) [0-15] | 2 (2) [0-14] | 0 (1) [0-4] | 2 (2) [0-15] | 1 (1) [0-4] | 4 (2) [0-15] | 2 (2) [0-15] | |
| Deficit count (SSLD) | 2 (2) [0-14] | 1 (1) [0-12] | 0 (1) [0-8] | 2 (2) [0-14] | 1 (2) [0-4] | 3 (2) [0-14] | 1 (2) [0-14] | |
| Frailty category | ||||||||
| Fit | 309 854 (57.0) | 270 964 (67.8) | 100 414 (99.3) | 480 404 (57.0) | 580 818 (100.0) | 0 (0) | 580 818 (61.5) | |
| Frail | 233 967 (43.0) | 128 886 (32.2) | 706 (0.7) | 362 147 (43.0) | 0 (0) | 362 853 (100) | 362 853 (38.5) | |
| Mild | 202 978 (37.3) | 116 523 (29.1) | 705 (0.7) | 318 796 (37.8) | 0 (0) | 319 501 (88.1) | 319 501 (33.9) | |
| Moderate | 27 489 (5.1) | 11 176 (2.8) | 1 (0) | 38 664 (4.6) | 0 (0) | 38 665 (10.7) | 38 665 (4.1) | |
| Severe | 3 500 (0.6) | 1 187 (0.3) | 0 (0) | 4 687 (0.6) | 0 (0) | 4 687 (1.3) | 4 687 (0.5) | |
| SNAC-K categories of chronic diseases | 5 (4) [0-26] | 5 (4) [0-23] | 1 (1) [0-1] | 5 (3) [2-26] | 4 (3) [0-17] | 7 (3) [0-26] | 5 (4) [0-26] | |
| Multimorbidity | ||||||||
| Non-multimorbid | 54 668 (10.1) | 46 452 (11.6) | 101 120 (0) | 0 (0) | 100 414 (17.3) | 706 (0.2) | 101 120 (10.7) | |
| Multimorbid | 489 153 (89.9) | 353 398 (88.4) | 0 (0) | 842 551 (100) | 480 404 (82.7) | 362 147 (99.8) | 842 551 (89.3) | |
| 2–5 diseases | 242 388 (44.6) | 197 908 (49.5) | 0 (0) | 440 296 (52.3) | 363 354 (62.6) | 76 942 (21.2) | 440 296 (46.7) | |
| 6–10 diseases | 213 328 (39.2) | 138 100 (34.5) | 0 (0) | 351 428 (41.7) | 115 360 (19.9) | 236 068 (65.1) | 351 428 (37.2) | |
| >10 diseases | 33 437 (6.1) | 17 390 (4.3) | 0 (0) | 50 827 (6.0) | 1 690 (0.3) | 49 137 (13.5) | 50 827 (5.4) | |
| Multimorbidity & frailty | ||||||||
| Non-multimorbid & Fit | 54 150 (10.0) | 46 264 (11.6) | 100 414 (99.3) | 0 (0) | 100 414 (17.3) | 0 (0) | 100 414 (10.6) | |
| Non-multimorbid & Frail | 518 (0.1) | 188 (0) | 706 (0.7) | 0 (0) | 0 (0) | 706 (0.2) | 706 (0.1) | |
| Multimorbid & Fit | 255 704 (47.0) | 224 700 (56.2) | 0 (0) | 480 404 (57.0) | 480 404 (82.7) | 0 (0) | 480 404 (50.9) | |
| Multimorbid & Frail | 233 449 (42.9) | 128 698 (32.2) | 0 (0) | 362 147 (43.0) | 0 (0) | 362 147 (99.8) | 362 147 (38.4) | |
| Living in a nursery home | 21 236 (3.9) | 7 850 (2.0) | 1 539 (1.5) | 27 547 (3.3) | 6 749 (1.2) | 22 337 (6.2) | 29 086 (3.1) | |
| Receiving home care | 29 356 (5.4) | 11 962 (3.0) | 721 (0.7) | 40 597 (4.8) | 2 463 (0.4) | 38 855 (10.7) | 41 318 (4.4) | |
| Alcohol intake | ||||||||
| Non-drinker | 193 229 (86.6) | 91 264 (54.2) | 145 619 (67.3) | 138 874 (79.2) | 274 962 (72.9) | 9 531 (67.2) | 284,493 (72.6) | |
| Low-risk drinker | 28 987 (13.0) | 72 262 (42.9) | 66 582 (30.8) | 34 667 (19.8) | 96 884 (25.7) | 4 365 (30.8) | 101,249 (25.9) | |
| High-risk drinker | 946 (0.4) | 4 916 (2.9) | 4 076 (1.9) | 1 786 (1.0) | 5 581 (1.5) | 281 (2.0) | 5862 (1.5) | |
| Missing | 320 659 | 231 408 | 364 541 | 187 526 | 465 124 | 86 943 | 552 067 | |
| Smoking status | ||||||||
| Non-smoker | 50 581 (10.8) | 120 681 (34.7) | 91 126 (19.2) | 80 136 (23.5) | 162 939 (21.3) | 8 323 (16.4) | 171 262 (21.0) | |
| Exsmoker | 400 954 (85.7) | 165 264 (47.5) | 328 541 (69.3) | 237 677 (69.6) | 529 643 (69.2) | 365.75 (72.0) | 566 218 (69.4) | |
| Smoker | 16 129 (3.4) | 62 313 (17.9) | 54 701 (11.5) | 23 741 (7.0) | 72 532 (9.5) | 5 910 (11.6) | 78 442 (9.6) | |
| Missing | 76 157 | 51 592 | 106 450 | 21 299 | 77 437 | 50 312 | 127 749 | |
When considering the complete study period, mean prevalence of multimorbidity and frailty was 94.1% and 51.3%, respectively. Prevalence of multimorbidity in frail patients was 99.9%, while the prevalence of frailty in people with multimorbidity was 54.5%. Over the entire study period, 42.8% of patients had multimorbidity and were fit, and the percentage who had multimorbidity and frailty rose to 51.2%. Tables S1, and S2 present the population characteristics and prevalence of individual frailty deficits both at baseline and over follow-up.
Dynamics of frailty and multimorbidity
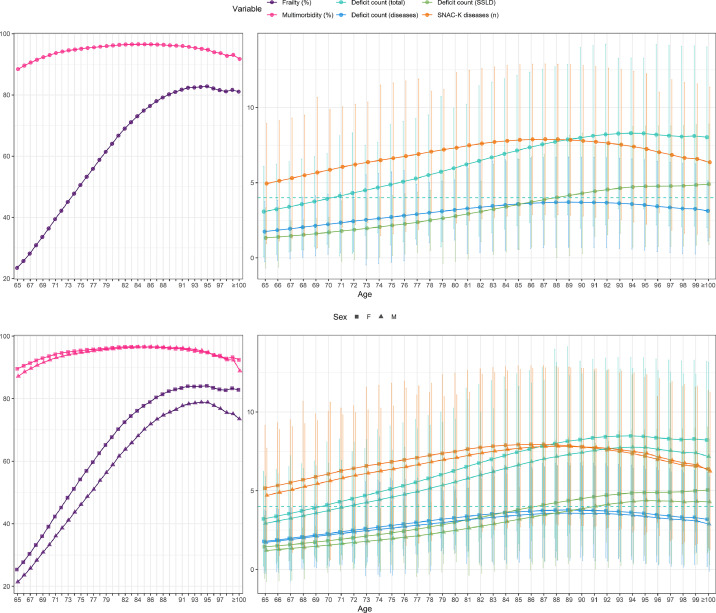
Figure 2 shows the dynamics of multimorbidity and frailty with age. While the prevalence of frailty increased with age, from 23.5% at age 65 up to 82.8% at age 95, the prevalence of multimorbidity remained roughly constant. From age 85 onwards the contribution to frailty from disease-related deficits decreased, while deficits related to other causes (SSLD) continued increasing. This trend was similar for men and women. Thus, from the age of 89 years, the number of frailty deficits exceeded the number of chronic diseases.
Figure 2.
Dynamics of frailty and multimorbidity with age. Complete population, and stratified by sex. Note: Multimorbidity is described as the percentage of the population that was multimorbid at each timepoint. A person was considered multimorbid if they had active diagnoses belonging to two or more distinct SNAC-K disease categories. The rest of variables are described as median interquartile range. Deficit were divided into two categories: disease-related deficits and deficits related to symptoms/signs, laboratory, and disability (SSLD). The dashed blue line represents the number of deficits from which the individual would be considered frail. Data from people aged 100 or more are aggregated. F: women; M: men. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
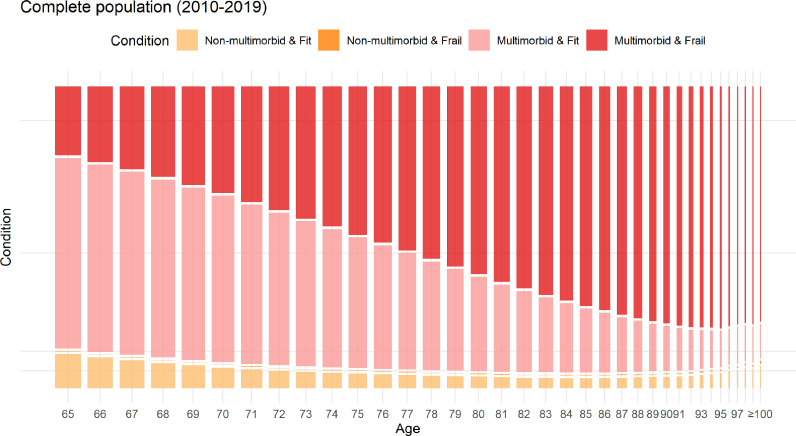
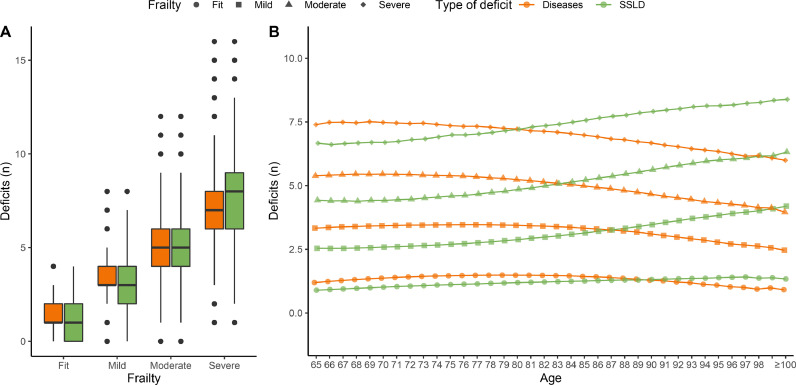
Figure 3 shows the evolution of the combination of frailty plus multimorbidity status over the complete follow-up. As the population aged, the prevalence of frailty increased, while that of multimorbidity remained high. The combination of frailty and non-multimorbidity existed, but was infrequent (Table 1). Figure S2 shows the individual dynamics of frailty over 10 years in random subsamples of 20,000 individuals whose follow-up started at age 65, 75 or 85. This figure shows how the dynamics of frailty varies according to baseline age. For example, the proportion of people who remained fit throughout the 10-year study period was higher in patients enrolled at age 65 versus 75 or 85. The composition of frailty was different depending on its severity, with the number of SSLD deficits (Figure 4A) increasing as frailty worsened. The more severe the frailty was, the earlier (in age) SSLD deficits began to outnumber disease-related deficits (Figure 4B).
Figure 3.
Mosaic plot showing the flow of the combination of frailty plus multimorbidity in included patients at each age. Note: A person was considered as multimorbid if they had active diagnoses in two or more distinct SNAC-K disease categories, and frail if they had a frailty index 0.12. In mosaic plots, the area of each box corresponds to the proportion of persons included in each category. Data from registers aged 100 or more are aggregated.
Figure 4.
Number of deficits of each type by frailty category. Note: The distributions of the number of deficits were compared within frailty class using a Mann-Whitney-Wilcoxon test. All differences were significant (-value 0.001).
Risk of all-cause mortality, nursing home admission, and need for home care
Patients were followed for 7.0 years (SD 3.15) for the outcome of mortality, 8.5 (SD 2.3) years for nursing home admission, and 8.5 (SD 2.3) for the need for home care. 30 891 (2.12%) and 43 750 (3.00%) individiduals were removed from the nursing home admission and need for home care models, respectively, as they already started the follow-up with that status. Figures S3, S4, and S5 present the Kaplan-Meier survival curve and the cumulative incidence functions (CIFs) of the models fitted with baseline covariates, showing a significant increase in the risk of all outcomes as frailty status worsened.
Table 2 reports estimated hazard ratios (HR) from the Cox regression models. The risk of all-cause mortality was similar in all frailty categories (mild: HR 1.25, moderate: HR 1.44, and severe: HR 1.38). Each additional deficit increased the risk of death by 3%, or 7% if the deficit was disease-related. Men were more likely to die than women. The risk of death also increased with deprivation index and exposure to tobacco. Living in a nursing home and receiving home care were likewise important factors (HR (2.71, 2.86), and (2.44, 2.90), respectively, depending on how frailty was considered). The risk of nursing home admission increased sharply as frailty worsened (mild: HR 9.70, moderate: HR 40, and severe: HR 87). Each additional deficit increased the risk by 40%, or 90% if it was SSLD-related. The risk of nursing home admission was higher in women, people living in less deprived neighbourhoods, smokers and people who drank. As for the need for home care, the risk also increased sharply as frailty worsened (mild: HR 21, moderate: HR 160, and severe: HR 620). Each additional deficit increased the risk by 50%, rising to HR = 3.4 if the deficit was SSLD-related. Risk was higher for men and people from more deprived neighbourhoods. The HRs obtained in models fitted using the complete cases showed the same trends described above (Table S3).
Table 2.
Association between frailty and the risk of all-cause mortality, nursing home admission, and needing home care. Note: Three models are reported for each outcome, one for each frailty measure: sum of all deficits; sum of deficits, per deficit type; and frailty category. Models for nursing home admission and needing home care accounted the competing risk of death. Reference groups were the following: woman, for sex; non-drinker, for alcohol consumption; non-smoker for smoking status; least deprived, for deprivation index; and fit, for frailty. In (*) both values were < 1, but due to rounding they appear as 0.99, 1. p-values are not reported as all of them were <0.01, except for (**), where -value = 0.66, and (***), where -value = 0.86. HR: Hazard Ratio, CI: Confidence Interval, AIC: Akaike’s Information Criterion.
| Variable | Level | Mortality |
Nursing home admission |
Need for home care |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deficit count |
Deficit count, per deficit type |
Frailty category |
Deficit count |
Deficit count, per deficit type |
Frailty category |
Deficit count |
Deficit count, per deficit type |
Frailty category |
|||||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Deficit count (total) | - | 1.03 | 1.03, 1.03 | - | - | - | - | 1.4 | 1.40, 1.40 | - | - | - | - | 1.5 | 1.50, 1.50 | - | - | - | - |
| Deficit count (diseases) | - | - | - | 1.07 | 1.06, 1.07 | - | - | - | - | 1.1 | 1.1, 1.1 | - | - | - | - | 0.99 | 0.99, 1 (*) | - | - |
| Deficit count (SSLD) | - | - | - | 0.93 | 0.93, 0.94 | - | - | - | - | 1.9 | 1.90, 1.90 | - | - | - | - | 3.4 | 3.4, 3.4 | - | - |
| Frailty | Mild | - | - | - | - | 1.25 | 1.24, 1.27 | - | - | - | - | 9.7 | 9.68, 9.72 | - | - | - | - | 21 | 20.98, 21.02 |
| Moderate | - | - | - | - | 1.44 | 1.42, 1.46 | - | - | - | - | 40 | 39.98, 40.02 | - | - | - | - | 160 | 159.98, 160.02 | |
| Severe | - | - | - | - | 1.38 | 1.36, 1.41 | - | - | - | - | 87 | 86.98, 87.02 | - | - | - | - | 620 | 619.98, 620.02 | |
| Sex | Man | 1.69 | 1.68, 1.70 | 1.7 | 1.69, 1.71 | 1.69 | 1.68, 1.70 | 0.92 | 0.91, 0.93 | 0.89 | 0.88, 0.90 | 0.91 | 0.90, 0.92 | 1.2 | 1.19, 1.21 | 0.97 | 0.96, 0.98 | 1.2 | 1.19, 1.21 |
| Age | - | 1.1 | 1.10, 1.10 | 1.1 | 1.10, 1.10 | 1.1 | 1.10, 1.10 | 1.1 | 1.10, 1.10 | 1.1 | 1.10, 1.10 | 1.1 | 1.10, 1.10 | 1.1 | 1.10, 1.10 | 1 | 1.10, 1.10 | 1.1 | 1.10, 1.10 |
| Deprivation index | 2 | 1.11 | 1.10, 1.12 | 1.11 | 1.10, 1.12 | 1.11 | 1.10, 1.12 | 0.75 | 0.74, 0.76 | 0.74 | 0.73, 0.75 | 0.74 | 0.73, 0.75 | 1.1 | 1.09, 1.11 | 1.1 | 1.09, 1.11 | 1.1 | 1.09, 1.11 |
| 3 | 1.19 | 1.17, 1.20 | 1.18 | 1.17, 1.19 | 1.18 | 1.17, 1.20 | 0.75 | 0.74, 0.76 | 0.75 | 0.74. 0.76 | 0.74 | 0.73, 0.75 | 1.2 | 1.19, 1.21 | 1.2 | 1.19, 1.21 | 1.1 | 1.09, 1.11 | |
| 4 | 1.24 | 1.22, 1.25 | 1.23 | 1.22, 1.25 | 1.24 | 1.22, 1.25 | 0.59 | 0.58, 0.60 | 0.6 | 0.59, 0.61 | 0.59 | 0.58, 0.60 | 1.1 | 1.09, 1.11 | 1.2 | 1.19, 1.21 | 1.1 | 1.09, 1.11 | |
| 5 (More deprived) | 1.32 | 1.30, 1.34 | 1.31 | 1.29, 1.34 | 1.32 | 1.29, 1.34 | 0.6 | 0.58, 0.62 | 0.6 | 0.58, 0.62 | 0.6 | 0.58, 0.62 | 1.2 | 1.19, 1.21 | 1.2 | 1.19, 1.21 | 1.2 | 1.19, 1.21 | |
| Alcohol consumption | Low-risk drinker | 0.99 | 0.98, 1 | 0.98 | 0.98, 0.99 | 0.99 | 0.98, 1 | 1.1 | 1.09, 1.11 | 1.1 | 1.09, 1.11 | 1 | 0.99, 1 | 0.69 | 0.68, 0.70 | 0.78 | 0.77, 0.79 | 0.68 | 0.67, 0.69 |
| High-risk drinker | 1.05 | 1.02, 1.09 | 1.05 | 1.01, 1.08 | 1.05 | 1.02, 1.09 | 1.3 | 1.26, 1.34 | 1.3 | 1.26, 1.34 | 1.3 | 1.26, 1.33 | 0.82 | 0.79, 0.85 | 0.86 | 0.83, 0.89 | 0.81 | 0.78, 0.84 | |
| Smoking status | Ex-smoker | 1.27 | 1.26, 1.28 | 1.27 | 1.26, 1.28 | 1.27 | 1.26, 1.28 | 0.91 | 0.90, 0.92 | 0.91 | 0.90, 0.92 | 0.94 | 0.93, 0.95 | 0.97 | 0.96, 0.98 | 1 | 0.99, 1 | 1 | 0.99, 1.01 |
| Smoker | 1.51 | 1.50, 1.53 | 1.52 | 1.50, 1.54 | 1.52 | 1.50, 1.53 | 1.3 | 1.28, 1.32 | 1.2 | 1.18, 1.22 | 1.3 | 1.28, 1.32 | 1 | 0.99, 1 | 0.94 | 0.93, 0.95 | 1.1 | 1.09, 1.11 | |
| Nursing home admission | Yes | 2.71 | 2.68, 2.73 | 2.86 | 2.84, 2.89 | 2.71 | 2.69, 2.73 | - | - | - | - | - | - | - | - | - | - | - | - |
| Need for home care | Yes | 2.44 | 2.42, 2.46 | 2.9 | 2.87, 2.93 | 2.49 | 2.47, 2.51 | - | - | - | - | - | - | - | - | - | - | - | - |
| AIC | 8 503 746 | 8 499 722 | 8 502 620 | 8 884 381 | 8 885 905 | 8 885 551 | 9 569 799 | 9 309 981 | 9 584 591 | ||||||||||
Discussion
In a cohort of 1 456 052 people aged 65 or over, this study presented the evolution of multimorbidity and frailty. The key findings are as follows: (i) the types of frailty deficits present (disease-related or SSLD) differ depending on the individual’s age; (ii) frailty increases up to 84 years of age due to multimorbidity; (iii) from age 85, it increases due to SSLD deficits; (iv) the risk of other health-related outcomes varies depending on the type of deficits present.
Prevalence of multimorbidity was consistently high, regardless of age, which could be due to the consideration of very prevalent chronic diseases such as hypertension, arthritis and diabetes in its definition. On the other hand, prevalence of frailty almost quadrupled from the youngest versus the oldest patients, especially women. The mean prevalence of multimorbidity in frail patients and of frailty in people with multimorbidity was higher than published estimates13 possibly reflecting the influence of multimorbidity on frailty.
Although eFRAGICAP and eFI were constructed by assessing frailty as a single construct, a differential analysis of disease- and SSLD-related deficits allowed a deeper study of the dynamics of frailty as people age, its characterisation, and its influence on health-related outcomes. As frailty worsened, both the number of chronic diseases and SSLD deficits increased, with the latter eventually surpassing the former as age advanced. From 65 to 84 years of age, a high eFRAGICAP was more likely to be due to the number of concurrent diseases than frailty-related deficits, whereas from age 85 onwards the opposite was true. Therefore, as people aged and became frailer, their frailty evolved into one characterised by disability and other signs and symptoms, and not diseases. This may be because of the natural limits on the number of concurrent chronic diseases that can coexist without causing death. Stoltz et al. identified that in the last 2–3 years before death the frailty index rose sharply, which could be due to the rapid appearance and accumulation of either diseases or SSLD deficits that lead to death.29
Mortality was more closely associated with disease-related compared to SSLD deficits. Male smokers and high-risk drinkers from the most deprived neighbourhoods were at the highest risk of death. Admission to a nursing home and need for home care was more related to SSLD than disease-related deficits. Female smokers and high-risk drinkers from less deprived areas were the most likely to be admitted to a nursing home. Men from the most deprived neighbourhoods were more likely to need home care. Adjustment for these variables suggests that specialised strategies are needed for populations with these pre-existing characteristics. The HR of SSLD deficits was higher for home care need (HR = 3) than nursing home admission (HR = 1.9), suggesting that frailty may have more impact on this community care service. This finding can help healthcare planning in primary care, which budget for home care or other community care services may vary depending on the frailty of each primary care centre’s population. This could demonstrate the usefulness of eFRAGICAP in supporting clinical decision-making.16 To our knowledge, this is the first study identifying which frailty and multimorbidity factors are more important for each health-related outcome using longitudinal data from the same population. Finally, as mortality risk is higher in people with more diseases than in those who mainly have SSLD deficits, individuals reaching the age of 85 will have fewer diseases but need more support to address their SSLD deficits.
Other studies had previously investigated the relationship between both concepts with other outcomes. Hanlon et al. concluded that in middle-aged people with multimorbidity, frailty was associated with mortality.30 Woo et al. showed that individuals with multimorbidity had higher risk of death than those with frailty only, as our results suggests.15 Middleton et al. fitted a survival model using frailty categories, reporting a two- and four-fold increased risk in people with moderate and severe frailty compared to the fit group.4 This effect is not apparent in our cohort, who showed a similar risk of death regardless of frailty. This might be due to the inclusion of multimorbidity as a strata covariate in the model, modulating the risk of death according to the number of diseases rather than the frailty. Prevalence of frailty deficits was similar to those reported by previous papers using SIDIAP databases.4, 21
This study has strengths and limitations. We used a large and representative database27, 31 based on electronic health records. EHRs, although previously cleaned, represent real-world data and may contain mistakes inherent to data from daily clinical practice. Multiple imputations based on the MAR assumption were performed to minimise the effect of missing data. The smoking and alcohol consume variables are measured most often in population at risk, which can depend on observed variables, therefore MAR assumption is assumed. On the other hand, socioeconomic status is calculated based on the address information and might be missing for different causes, such no information, recently moved, etc. and can be related with sociodemographic variables. Then, we also assumed a MAR mechanism. Finally, according to sensitivity analysis performed, both models fitted with imputed data and complete cases obtained results in the same direction. Therefore, the MAR mechanism seems reasonable. Some results such as the small proportion of people who were frail and not multimorbid, could be due to poor follow-up in older patients. We also used standardised validated tools for measuring both multimorbidity10 and frailty.18, 21 Similarly to other frailty indices based on EHR, eFRAGICAP may show a greater multimorbidity burden because diagnoses are more routinely collected than other frailty-related information. For this reason, eFRAGICAP was also considered according to the nature of the deficits considered, i.e., disease- or SSLD-related. This allowed a more detailed description of the ageing of the study population, and the study on the influence of each type of deficit in each outcome. Other methods to measure frailty, such as gait speed or self-reported exhaustion,1, 11 might be more precise but also less convenient in a primary care setting. In addition, the validation of eFRAGICAP already demonstrated its correlation with other scales that consider only frailty (i.e. CFS, RISC).21 The frailty index could be improved by including inflammatory biomarkers that are precursors driving frailty and late-life decline,32 and other geriatric health indicators (Health Assessment Tool and Walking Speed33). However, any additional information can be easily included in the frailty index if the data are available. Finally, some limitations regarding the lack of some well-known confounding factors need to be considered. Body mass index and ethnicity could not be included in the analysis, as the former was not registered yearly in most of the included individuals and the latter is not recorded in SIDIAP.
There is still no international consensus on the assessment of multimorbidity and frailty. However, as shown here, there are guidelines for their measurement using EHRs. Our proposal includes mostly ICD-10 codes and other parameters that can be analysed and standardised in different EHR systems. Furthermore, this would increase its validity internationally, helping to establish its use and predict the onset of patients’ worsening health, which might lead to needing home care, nursing home admission, or death. Based on these results, the assessment of multimorbidity and eFRAGICAP in daily clinical practice would allow estimating which type of outcome is more likely for each patient given their situation. This would allow better decision-making on treatment and care planning, especially for older patients, by identifying which are in higher risk of death or needing special care. Different prevention strategies can be implemented depending on the level of impairment and the predominance of each type of frailty deficit of the individual.16 Findings of this study can be useful in informing the Catalan guidelines for the care of people with frailty and complex care needs, as well as other national public health services guidelines.34 In addition, the assessment of multimorbidity and eFRAGICAP can be done automatically with the data routinely collected in the EHR.
Future studies analysing which patterns of multimorbidity are most closely associated with frailty could help disentangle the biological, inflammatory, and genetic causes of frailty from those of multimorbidity. This would open the door to more personalised patient care and informing estimates of the type of patients that health systems will serve in the coming years. A deeper study of the subpopulation of individuals with negligible health-related problems is necessary to understand their characteristics and ageing process. A similar work to that done by Stoltz et al. but dividing into SSLD and disease-related deficits could help to identify which type of deficits and at what time increase the most for each health outcome, to allow a deeper monitoring of the patients.
Multimorbidity and frailty are both characteristics of the ageing population. However, their presence and influence on well-being vary with age. As a person ages, disease-related frailty decreases, while frailty due to other deficits, such as activity limitation or memory loss, increases, and frailty worsens. This trend reversal occurs at the age of 85. Multimorbidity and frailty are positively associated in people over 65. Regarding age-related outcomes, the number of diseases increases the risk of all-cause mortality, while the number of frailty-related deficits sharply increases the risk of nursing home admission and the need for home care. Finally, the frailty index used can easily be applied to other EHR systems, making international implementation feasible.
This study shows the change in frailty over the years. Early on, it is characterised by the person’s diseases and evolves towards a loss of homeostasis. Future studies might focus on a definition and further description of frailty in people over 85, when its presence may be more important to a person’s health than their diseases.
Contributors
CVF, MCB, ARL, and LACR participated in the conceptualisation of the study, investigation, funding acquisition, and methodology definition. LACR and ARL performed the data curation, formal analysis and visualisation. LACR and CVF drafted the manuscript,and all authors participated in the review and editing of the final manuscript.
Data sharing statement
The data used in this study are only available for the participating researchers, in accordance with current European and national laws. Thus, the distribution of the data is not allowed. However, researchers from public institutions can request data from SIDIAP. Further information is available online (https://www.sidiap.org/index.php/menu-solicitudes-en/application-proccedure).
Declaration of interests
The authors declare no conflict of interest.
Acknowledgment
Authors thank Meggan Harris for her role in editing and proofreading the manuscript.
Funding
The project received a research grant from the Carlos III Institute of Health, Ministry of Economy and Competitiveness (Spain), awarded on the 2019 call under the Health Strategy Action 2013–2016, within the National Research Programme oriented to Societal Challenges, within the Technical, Scientific and Innovation Research National Plan 2013–2016, (reference PI19/00535), and the PFIS Grant FI20/00040, co-funded with European Union ERDF (European Regional Development Fund) funds. The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of this work.
Footnotes
Supplementary material associated with this article can be found in the online version at doi: 10.1016/j.eclinm.2022.101610.
Appendix A. Supplementary materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/
References
- 1.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Rockwood K., Mitnitski A., Song X., Steen B., Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Assoc. 2006;54(6):975–979. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 3.Loprinzi P.D., Addoh O., Joyner C. Multimorbidity, mortality, and physical activity. Chronic Illn. 2016;12(4):272–280. doi: 10.1177/1742395316644306. [DOI] [PubMed] [Google Scholar]
- 4.Middleton R., Poveda J.L., Pernas F.O., et al. Mortality, falls, and fracture risk are positively associated with frailty: a SIDIAP cohort study of 890 000 patients. J Gerontol A Biol Sci Med Sci. 2022;77(1):148–154. doi: 10.1093/gerona/glab102. PMID: 33885746; PMCID: PMC8751782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viljanen A., Salminen M., Irjala K., et al. Chronic conditions and multimorbidity associated with institutionalization among finnish community-dwelling older people: an 18-year population-based follow-up study. Eur Geriatr Med. 2021;12:1275–1284. doi: 10.1007/s41999-021-00535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sciences T.A.o.M. The Academy of Medical Sciences; 2018. Multimorbidity: a Priority for Global Health Research Vol. 1. [Google Scholar]; https://acmedsci.ac.uk/file-download/82222577
- 7.Schiøtz M.L., Stockmarr A., Høst D., Glümer C., Frølich A. Social disparities in the prevalence of multimorbidity – a register-based population study. BMC Public Health. 2017;17(1):422. doi: 10.1186/s12889-017-4314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thygesen L.C., Christensen K., Rørth M., Sørensen H.T., Vandenbroucke J.P., Westendorp R.G.J. Tipping points – do the prognostic values of multimorbidity and functional status vary with age? Clin Epidemiol. 2021;13:853–857. doi: 10.2147/clep.s325348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho I.S.-S., Azcoaga-Lorenzo A., Akbari A., et al. Examining variation in the measurement of multimorbidity in research: a systematic review of 566 studies. Lancet Public Health. 2021;6(8):e587–e597. doi: 10.1016/s2468-2667(21)00107-9. [DOI] [PubMed] [Google Scholar]
- 10.Calderón-Larrañaga A., Vetrano D.L., Onder G., et al. Assessing and measuring chronic multimorbidity in the older population: a proposal for its operationalization. J Gerontol A Biol Sci Med Sci. 2017;72(10):1417–1423. doi: 10.1093/gerona/glw233. PMID: 28003375; PMCID: PMC5861938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clegg A., Young J., Iliffe S., Rikkert M.O., Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/s0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villacampa-Fernández P., Navarro-Pardo E., Tarín J.J., Cano A. Frailty and multimorbidity: two related yet different concepts. Maturitas. 2017;95:31–35. doi: 10.1016/j.maturitas.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Vetrano D.L., Palmer K., Marengoni A. Frailty and multimorbidity: a systematic review and meta-analysis. J Gerontol Ser A. 2018;74(5):659–666. doi: 10.1093/gerona/gly110. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Mañas L., Fried L.P. Frailty in the clinical scenario. Lancet. 2015;385(9968):e7–e9. doi: 10.1016/s0140-6736(14)61595-6. [DOI] [PubMed] [Google Scholar]
- 15.Woo J., Leung J. Multi-morbidity, dependency, and frailty singly or in combination have different impact on health outcomes. Age. 2013;36(2):923–931. doi: 10.1007/s11357-013-9590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoogendijk E.O., Afilalo J., Ensrud K.E., Kowal P., Onder G., Fried L.P. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–1375. doi: 10.1016/s0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 17.Mitnitski A.B., Mogilner A.J., Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clegg A., Bates C., Young J., et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–360. doi: 10.1093/ageing/afw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitnitski A., Collerton J., Martin-Ruiz C., et al. Age-related frailty and its association with biological markers of ageing. BMC Med. 2015;13(1):161. doi: 10.1186/s12916-015-0400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walston J., Hadley E.C., Ferrucci L., et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the american geriatrics society/national institute on aging research conference on frailty in older adults. J Am Geriatr Soc. 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 21.Orfila F., Carrasco-Ribelles L.A., Abellana R., et al. Validation of an electronic frailty index with electronic health records: eFRAGICAP index. BMC Geriatr. 2022;22(1):404. doi: 10.1186/s12877-022-03090-8. PMID: 35525922; PMCID: PMC9080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walston J.D., Bandeen-Roche K. Frailty: a tale of two concepts. BMC Med. 2015;13(1):185. doi: 10.1186/s12916-015-0420-6. PMID: 26265077; PMCID: PMC4531437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C., Park J., Wu C., et al. Cognitive frailty in relation to adverse health outcomes independent of multimorbidity: results from the China health and retirement longitudinal study. Aging (Albany NY) 2020;12(22):23129–23145. doi: 10.18632/aging.104078. Epub 2020 Nov 18. PMID: 33221750; PMCID: PMC7746379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholson K., Griffith L.E., Sohel N., Raina P. Examining early and late onset of multimorbidity in the canadian longitudinal study on aging. J Am Geriatr Soc. 2021;69(6):1579–1591. doi: 10.1111/jgs.17096. [DOI] [PubMed] [Google Scholar]
- 25.Guaraldi G., Brothers T.D., Zona S., et al. A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. AIDS. 2015;29(13):1633–1641. doi: 10.1097/qad.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 26.Nunes B.P., Flores T.a.R., Mielke G.I., Thumé E., Facchini L.A. Multimorbidity and mortality in older adults: asystematic review and meta-analysis. Arch Geriatr Gerontol. 2016;67:130–138. doi: 10.1016/j.archger.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 27.del Mar García-Gil M., Hermosilla E., Prieto-Alhambra D., et al. Construction and validation of a scoring system for the selection of high-quality data in a spanish population primary care database (SIDIAP) J Innov Health Inform. 2011;19(3):135–145. doi: 10.14236/jhi.v19i3.806. [DOI] [PubMed] [Google Scholar]
- 28.Duque I., Domínguez-Berjón M.F., Cebrecos A., et al. Índice de privación en españa por sección censal en 2011. Gaceta Sanitaria. 2021;35(2):113–122. doi: 10.1016/j.gaceta.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Stolz E., Mayerl H., Hoogendijk E.O., Armstrong J.J., Roller-Wirnsberger R., Freidl W. Acceleration of health deficit accumulation in late-life: evidence of terminal decline in frailty index three years before death in the US health and retirement study. Ann Epidemiol. 2021;58:156–161. doi: 10.1016/j.annepidem.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Hanlon P., Nicholl B.I., Jani B.D., Lee D., McQueenie R., Mair F.S. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493737 UK biobank participants. Lancet Public Health. 2018;3(7):e323–e332. doi: 10.1016/s2468-2667(18)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Recalde M., Rodríguez C., Burn E., et al. Data resource profile: the information system for research in primary care (SIDIAP) Int J Epidemiol. 2022 doi: 10.1093/ije/dyac068. Epub ahead of print. PMID: 35415748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bektas A., Schurman S.H., Ranjan S., Ferrucci L. Aging, inflammation and the environment. Exp Gerontol. 2018;105:10–18. doi: 10.1016/j.exger.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zucchelli A., Vetrano D.L., Grande G., et al. Comparing the prognostic value of geriatric health indicators: a population-based study. BMC Med. 2019;17(1) doi: 10.1186/s12916-019-1418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Attention to chronicity. https://salutweb.gencat.cat/ca/el_departament/ambits-estrategics/atencio-sociosanitaria/cronicitat/index.html/googtrans(calen). Accessed 1 July 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/