Abstract
Caries sensitivity varies between the two strains of inbred mice, BALB/cA has high sensitivity and C3H/HeN has low sensitivity. One potential reason seems to be a difference in pellicle-forming saliva protein composition. Here, we performed a proteomic analysis in order to identify differences of hydroxyapatite (HAP) adsorbed saliva proteins between these two mouse strains. HAP column chromatography revealed twice the quantity of high-affinity saliva proteins in C3H/HeN compared to BALB/cA. One- and two-dimensional electrophoresis showed 2 bands/spots with deviating migration. They were identified as murine carbonic anhydrase VI (CAVI) by peptide mass fingerprinting and confirmed with western blotting using a specific polyclonal antibody. Total RNA from the salivary glands of both mouse strains, PCR amplification of cDNA with a CAVI specific primer, and sequence analysis revealed one different base in codon 96, resulting in one different amino acid. Glyco-chains of CAVI deviate in one N-glycan, confirmed by mass analysis. CAVI activity was estimated from distinct circular dichroism spectra of the molecules and found higher in C3H/HeN mice. In summary, the CAVI composition of BALB/cA and C3H/HeN differs in one amino acid and a glyco-chain modification. Further, saliva from caries resistant C3H/HeN mice displayed higher CAVI activity and also overall hydroxyapatite adsorption, suggesting a relationship with caries susceptibility.
Keywords: Mouse saliva, Hydroxyapatite, Dental caries, Carbonic anhydrase VI
Highlights
-
•
CAVI was the salivary protein with high affinity for hydroxyapatite in two mice strains with different caries susceptibility.
-
•
CAVI of the two strains showed differences in molecular weight, amino acids and genes, glyco-chain modification and enzyme activity.
-
•
Differences in CAVI activity might contribute to caries susceptibility.
Mouse saliva; Hydroxyapatite; Dental caries; Carbonic anhydrase VI
1. Introduction
Maintaining healthy teeth plays an important role in maintaining a lifetime in good health, and it is said that losing teeth at older age implies the risk of dementia and being confined to bed. It has been reported that 40–50% of tooth loss in Japan is caused by dental caries [1]. The majority of 12-year-old children in developed countries has up to 1.2 teeth affected by caries (DMFT; decayed, missing and filled teeth) [2]. In Japan the ratio is 0.9, below the average and prevalence of dental caries is only 37.8%. However, with age, DMFT and dental caries incidence rise [3], and at the age of 40, the caries prevalence increases to 99% and DMFT to 16.5 [1]. Dental caries is the collapse of a tooth preceded by demineralization of the tooth's hard tissue by acid-producing cariogenic bacteria metabolizing sugar. Dental caries is caused by microbial factors (presence of caries causing bacteria), host factors (tooth quality and saliva resistance) and environmental factors (frequency of carbohydrate intake) [4]. However, the sugar intake in Japan is more than 30% lower than in Europe and the United States [5]. Ninety six percent of the people conduct daily tooth brushing to remove plaque, containing cariogenic bacteria, and about 40% brush their teeth twice a day or more [1]. As mentioned above, despite the progress of general countermeasures and oral care implementation to eliminate dental caries, the caries prevalence rate has not decreased for a long time in Japan. The reason might be missing oral hygiene measures, based on the understanding of each individual's accurate caries susceptibility. Dental caries damages the inorganic part of the tooth, and not only causes pain and eating disorders, but also serves as a reservoir for various kinds of microorganisms including cariogenic bacteria, potentially resulting in aspiration pneumonia in the elderly or inflammation of pulp and mucous membrane. Furthermore, bacteria entering the blood flow can also cause cardiovascular diseases such as infective endocarditis and arteriosclerosis [6]. Therefore, dental caries not only causes oral problems in children, but also harms the health of the whole body in adults, especially elderly people. Once dental caries evolves, it is known that the surrounding teeth of the repaired tooth also develop secondary caries with high probability [7]. Therefore caries should be prevented in the first place. At present, general caries susceptibility assessment is carried out by measuring saliva pH or saliva buffering capacity, and evaluating the number of cariogenic bacteria, but no evaluation standard for the degree of each contributing factor exists [8, 9]. Especially, individual differences regarding saliva function, except for the general buffering capacity are not understood. Saliva contains inorganic salts and various proteins, however the function of many proteins has not been clarified yet.
The enamel on the tooth surface is composed of 99% calcium phosphate called hydroxyapatite (HAP); saliva proteins adsorbed on its outmost layer bear an important role as a protective film called pellicle [10, 11], however the constituent proteins and details of their function are only partly understood. Regarding bacterial adhesion to the tooth surface [10], pellicle proteins might act as an intermediate between the HAP calcium ions and bacteria, but most details are still unknown [12]. To find the pellicle proteins, able to suppress dental caries by not-providing adhesion to bacteria and to clarify individual differences would make it possible to establish an oral hygiene program reflecting the individual caries risk and to lower caries prevalence. Animal experiments using two normal breeding inbred mice strains have revealed that caries susceptibility is higher in BALB/cA than in C3H/HeN [13, 14]. Since these studies showed that the tooth surface adhesion rate of caries-causing bacteria correlated with the caries incidence, it is expected that there are differences in the pellicle proteins that are receptors for oral bacterial adhesion. However, there are only few reports regarding comparative analysis of pellicle proteins in these mouse strains. Takahashi et al. showed in vitro that the salivary protein of BALB/cA was more likely to adhere and aggregate cariogenic bacteria than C3H/HeN, and the electrophoresis pattern of whole salivary protein from both strains had a slight different molecular weight around 40–45 kDa. They found that different major bands existed, but did not attain protein identification [13]. To analyze these differences in detail, in this study, proteomic analysis of hydroxyapatite (HAP) adsorbed salivary proteins was performed to identify the involved caries inhibiting proteins.
2. Materials and methods
The protocol was approved by the Animal Experiment Committee of Tsurumi University, School of Dental Medicine (approval number: 30A060). Animal welfare was fully conducted in accordance with the research guidelines of the Animal Experimental Committee.
2.1. Saliva sampling from mice
Whole saliva was collected from two strains of 8-week-old male conventional mice, BALB/cA and C3H/HeN. After purchase, the mice were only raised for acclimatization and were not used for other studies prior to the start of the study. These mice were bred in an environment with free access to tap water and regular feed CE2 (CLEA Japan, Inc. Tokyo, Japan), which is a GLP-compliant, standard rodent diet consisting mainly of vegetable protein (soybean meal) with a proper balance of animal protein. The environmental temperature was 23 ± 1 °C and the humidity was 55%.
Nine mice of each strain were stimulated with 0.5 ml of a mixture of 0.5 mg/ml pilocarpine sulphate and 0.5 ml of 2 mg/ml isoproterenol per mouse and saliva was collected by aspiration with a sterile micropipette tip for 5 min after drug stimulation. This sampling was performed by one researcher, and the order of animal sampling was random each time. The collected saliva was stored at -80 °C until analysis.
2.2. HAP-HPLC
Whole saliva samples were fractionated using a Hydroxyapatite-HPLC column (HAP-HPLC). 50 mL of a 10% hydroxyapatite particle suspension in a filling holder were poured into an empty column (Shodex® empty column, Showa Denko KK, Tokyo, Japan) at 1 mL/min for 50 min before use. The column had an inner diameter of 3 mm and a length of 10 cm and 1 mL of whole saliva was applied in 1 min. Elution of HAP-HPLC adsorbed proteins was carried out with a potassium phosphate buffer solution (pH 6.6) at a flow rate of 1 ml/min, stepwise with holding for 20 min at 50, 70, 100, 150, 200 and 400 mM for HAP affinity screening and high affinity fraction separation or with a linear gradient from 50 to 400 mM for sample preparation for ELISA analysis. Each eluted fraction was subjected to desalting treatment and kept as a sample for analysis.
2.3. Desalting treatment
Each eluted fraction was subject to centrifugal concentration (3,500 rpm for 2 hrs at 4 °C) using an ultrafiltration membrane (Amicon Ultra—15, Merck Millipore) with a molecular weight cutoff of 10,000 Da, followed by reconstituting the concentrated sample with 1 mL of pure water to desalt.
2.4. SDS-PAGE
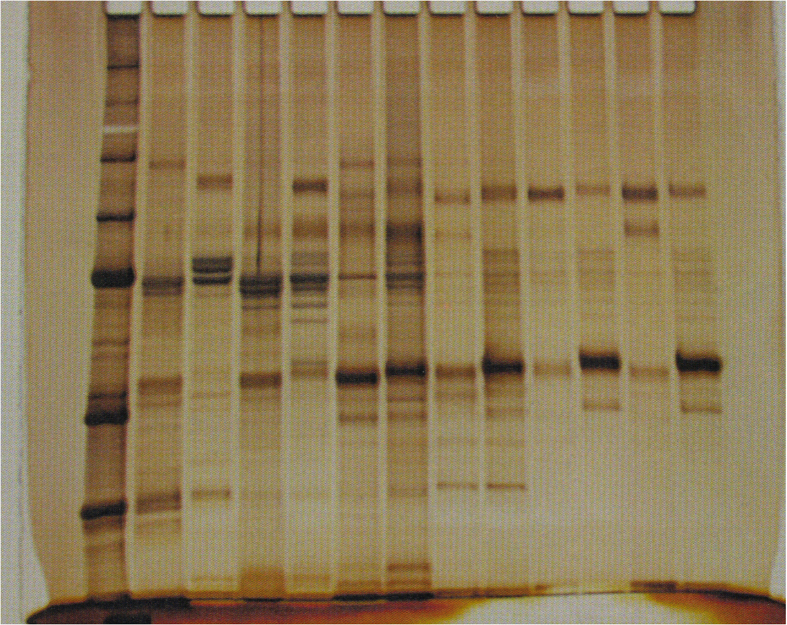
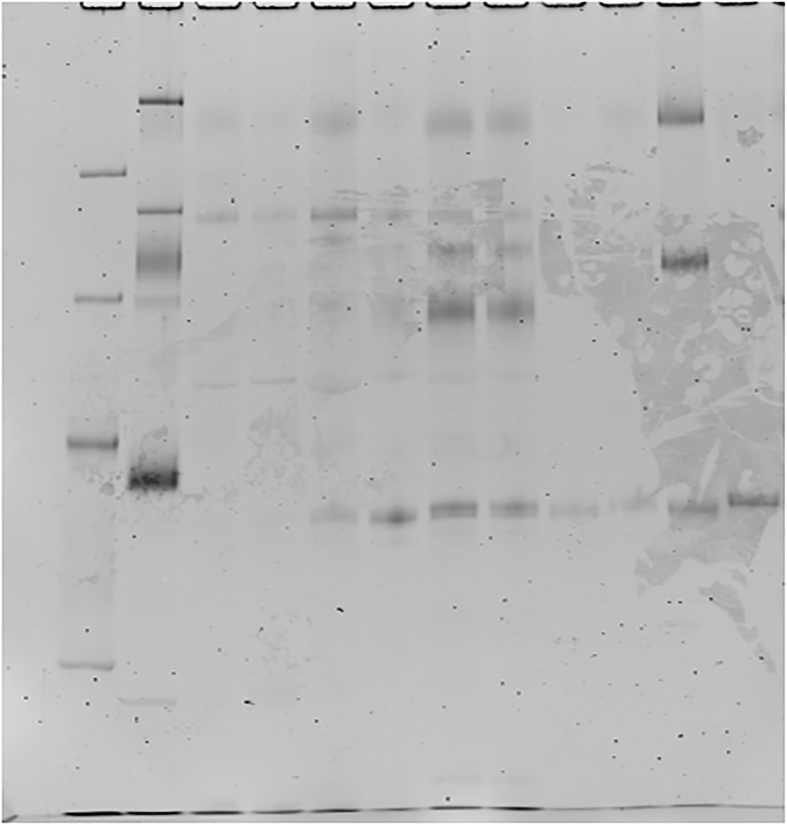
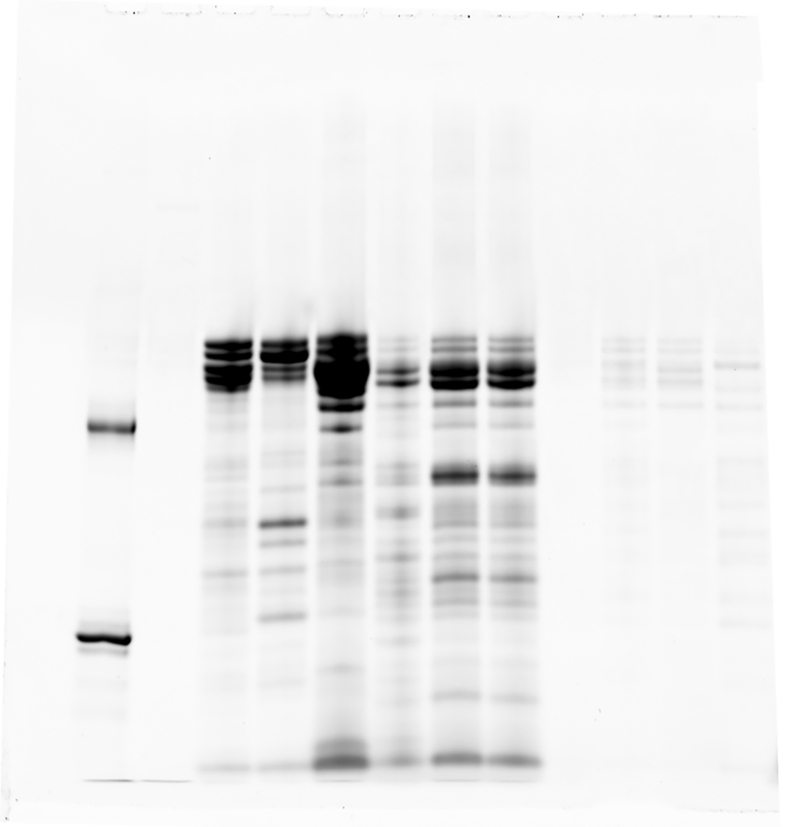
Proteins from nine mice of each strain were separated by 12% SDS polyacrylamide gel electrophoresis using the Laemmli system with a gel size of 7.5 cm × 7.5 cm and a constant current of 20 mA/sheet, followed by CBB (Coomassie Brilliant Blue) stain which was carried out with EzStainAQua® (ATTO Corporation, Tokyo Japan), and silver stain which was performed with Silver strain plus ® kit (BioRad, Hercules, CA, USA) according to the manufacturers' instruction. Glycoproteins were stained using Pro-Q Emerald 488 Glycoprotein Gel and Bio Stain Kit P21875 (Invitrogen; Life Technologies Japan Ltd., Tokyo, Japan), according to the manufacturers' instruction. Shortly after performing SDS-PAGE and fixation with 50% methanol and 10% acetic acid, the washed gel was oxidized with 3% acetic acid and periodic acid and then stained with Pro-Q® Emerald 488 Staining Solution for 2 h s. Detection was performed with Amersham Typhoon Biomolecular Imager (GE Healthcare Bio-Sciences Corp., Marlborough, USA) at Ex/Em; 510 nm/520 nm. Phosphorylated proteins were stained using Pro-Q Diamond Phosphoprotein Gel Stain (Invitrogen; Life Technologies Japan Ltd., Tokyo, Japan) also according to the manufacturers’ instruction as follows. The fixed and washed gel was incubated with Pro-Q® Diamond staining solution in a dark place for 15min and de-stained in a solution of 20% acetonitrile and 50 mM sodium acetate at pH 4.0 for 1hr. Detection was carried out with the same Typhoon Imager (GE Healthcare Bio-Sciences Corp., Marlborough, USA) at Ex/Em: 555 nm/580 nm. The total amount of protein of the eluted HAP-HPLC fractions of each strain were adjusted to a constant level. The fractions of BALB/cA were labeled with Amersham CyDye DIGE Fluor Cy3 minimal dye (GE Healthcare, Tokyo, Japan), and that of C3H/HeN were labeled with Amersham CyDye DIGE Fluor Cy5 minimal dye (GE Healthcare, Tokyo, Japan). An equal amount of each was mixed and two-dimensional gel electrophoresis performed. The first dimension was isoelectric focusing with a gradient from PI 3 to PI 10 using 7 cm Immobiline ™ Dry Strip gel strips, containing an immobilized pH gradient (IPG), pH 3–11 were immersed in the sample mixed with a hydrolysis buffer [8 M urea, 4% CHAPS, 2% IPG buffer (carrier ampholytes), 40 mM DTT] at 20 °C for 10 h. The following 6-step condition was applied at 20 °C. Step 1: stepwise at 300V for 40min, Step 2: gradient at 1000V, 20min, Step 3: gradient at 5000V, 48min, Step 4: stepwise at 5000V for 72min, Step 5: stepwise at 300V for 40min, Step 6: stepwise at 100V for 240min. The IPG strip was removed and the second-dimension electrophoresis was performed with the Laemmli system and a constant current of 20mA/sheet using a 12.5% PA gel sized 7.5 cm × 7.5 cm. The proteins were visualized by fluorescence detection with Cy 3 at 580 nm and Cy 5 at 670 nm, using Amersham Typhoon Biomolecular Imager (GE Healthcare Bio-Sciences Corp., Marlborough, USA).
2.5. Peptide mass fingerprinting by MALDI-TOF MS analysis
The spots excised from the 2D-PAGE (gel) were subjected to S-pyridine-ethylation treatment, digested with trypsin (overnight at 37 °C), and desalted with ZipTip C18 (Millipore). The eluted sample was applied directly onto the matrix of the MALDI plate, and after being air-dried, mass spectrometry was performed under the following conditions.
Equipment used: AXIMA-CFR MALDI-TOFMS device, extraction voltage: 20kv, flight mode: Reflectron, detection ion: Positive, matrix: α-cyano-4-hydroxycinnamic acid (CHCA).
Identification was performed by Mascot database search using Mascot Server (Matrix Science K.K., Tokyo Japan, https://www.matrixscience.com/search_form_select.html).
2.6. Production of polyclonal antibody
Invitrogen (Life Technologies Japan Ltd., Tokyo, Japan) was commissioned with peptide design and synthesis, KLH conjugation, and immunization for antibody production. Two 15 amino-acid-residue sequences (CKEYGTYENAKDQKN and CIQNGYRSTQPNNHR) from the amino acid sequence database (advanced-BLAST search; https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome) of the identified protein are considered highly antigenic. 30 mg (of these peptides) were synthesized by the Fmoc immobilization method. Residues KLH (7 mg for immunization) and GGG (3 mg for ELISA) were conjugated to above peptide to make it a carrier. Two rabbits (JW, SPF) were immunized with the carrier-attached synthetic peptide, and ELISA was performed with rabbit serum collected after 7 days using BALB/cA, C3H/HeN mouse saliva protein as antigen. The antibody titer of the polyclonal antibodies was purified with affinity column chromatography (heparin sephacryl) and the antibody titer increase confirmed.
2.7. Glyco-chain analysis and western blotting
The HAP-HPLC elution fractions were treated with deglycosylation enzymes (PNGase F, Neuraminidase and O-Glycosidase) of the Enzymatic Protein Deglycosylation Kit (Sigma-Aldrich, Tokyo, Japan) followed by Western blotting. After separating the sample by 12.5% SDS-PAGE, a semi-dry blotting device (WSE-4045HorizeBLOT 4M, ATTO CORPORATION, Tokyo, Japan) was used with a polyvinylidene difluoride (PVDF) membrane (Immobilon®-P PVDF Membrane, Millipore® Merck Japan, Tokyo, Japan) for Western blotting. On the anode side, a filter soaked with 0.3M Tris, 5% Methanol and 25mM Tris, 5% Methanol was placed and on top of it, membrane and gel. On the cathode side, a filter paper soaked with 25mM Tris, 40mM 6-Aminohexanoic acid and 5% Methanol was placed, sandwiched between electrode plates and charged with a constant current of 100 mA for 1 h for transfer. For detection, ECL Plus, anti-rabbit HRP labeled secondary antibody kit (GE Healthcare, Tokyo, Japan) was used.
2.8. Analysis of glyco-chains by MALDI-TOF MS
N-glycans were cleaved from the proteins of the HAP-HPLC elution fractions by PNGase F and sialic acid was removed by sialidase treatment. Glyco-chains for analysis were prepared and used as samples for MS analysis. The samples were mixed with a matrix (2,5-DHB) and analyzed by MALDI-TOF/MS (Reflex VI; Bruker Japan KK, Yokohama, Japan). The m/z value obtained by MS was checked with a software tool for determining glycosylation compositions from mass spectrometric data (GlycoMod, http://www.expasy.ch/tools/glycomod/). The carbohydrate chain was predicted by searching within 0.5 Da.
2.9. Determination of the protein quantity by ELISA
The protein from the linear gradient elution fraction of HAP-HPLC was quantified and immobilized on the ELISA plate Invitrogen Nunc MaxiSorp ™ flat-bottom (Thermo Fisher Scientific KK., Tokyo, Japan) to react with the polyclonal antibody. Thereafter, an anti-rabbit HRP-labeled secondary antibody was used, and the level of coloration of the chromogenic substrate ABTS was measured (OD: 405 nm) for quantification.
2.10. Genetic analysis
The parotid and submaxillary glands of each mouse were excised, homogenized on ice, and total RNA was extracted using High pure RNA Isolation Kit (Roche Diagnostics Corporation, Indianapolis, USA). The extracted RNA was subjected to reverse transcription reaction using SuperScript II Rnase H-Reverse Transcriptase (Thermo Fisher Scientific KK, Tokyo, Japan) and random hexamer primer (Thermo Fisher Scientific KK, Tokyo, Japan) to synthesize cDNA. Mouse CAVI-specific primer sets 33F (5′-GCCCTGGTGAGCGTGGTGTC-3′), 974R (5′-CCGGCTCCAA AAGTGCCGGT-3′), 667F (5′-ACCCAGGCTCGCTCACCACA-3′), 1280R (5′-TTCGGCGCTGGGGGTCAAAC-3′) were used to perform PCR with HotStarTaq Master Mix Kit (Qiagen KK, Tokyo, Japan) according to the manufacturer's instructions to amplify 1247 bp including the coding region from codon 3 (nt 33) to the final codon of the gene. Reaction conditions were as following: denaturation at 95 °C for 15 min, then 40 cycles at 94 °C for 30 s, 60 °C for 60 s and 72 °C for 90 s, and the final extension reaction at 72 °C for 10 min. For the sequence analysis, 10 μL of the PCR product was used for agarose electrophoresis to confirm the amplification of the DNA fragment, followed by purification using High Pure PCR Product Purification Kit (Roche Diagnostics Corporation, Indianapolis, USA). The sequencing was performed using ABI Prism BigDye Terminator V 3.1 cycle sequencing kit (Applied Biosystems, Foster, CA, USA) according to the manufacturer's instruction and the sequence was read with an automatic sequencer (3730X DNA Analyzer; Applied Biosystems, Foster, CA, USA).
2.11. Far-UV circular dichroism spectroscopy
Far-UV circular dichroism (CD) measurements were performed on the spectropolarimeter J-720 W (Japan Spectroscopic Co., Ltd.; Tokyo, Japan) using a 1-mm path-length quartz cuvette. The CD spectra of the C6 and B6 fractions in deionized solutions at 0.1 mg/ml concentration were measured at 25 °C. The CD spectra of the samples were corrected by subtracting the spectra of the deionized water.
2.12. Measurement of buffering capacity
The measurement was carried out according to the method of Erik R. Swenson et al [4]. 10 μL (1 mg/mL) of each desalted HAP-HPLC elution fraction and the de-glycosylated fraction were added to 500 μL of 10 mM NaHCO3, saturated with CO2, and the buffering capacity was measured as pH change with an electrode.
2.13. Statistical analysis
The data obtained were statistically analyzed using IBN SPSS Statistics version 19 (IBM, Armonk, NY, USA). Using a Wilcoxon signed-rank test, we analyzed data of molecular weight bands obtained in a polyacrylamide gel of electrophoresis. In other tests, the data of each sample compared with the amount of HAP-high affinity proteins or buffering capacity level within Balb/cA and C3H/HeN was analyzed by Mann-Whitney U test. The significance was set as p < 0.05.
3. Results
3.1. HAP-HPLC
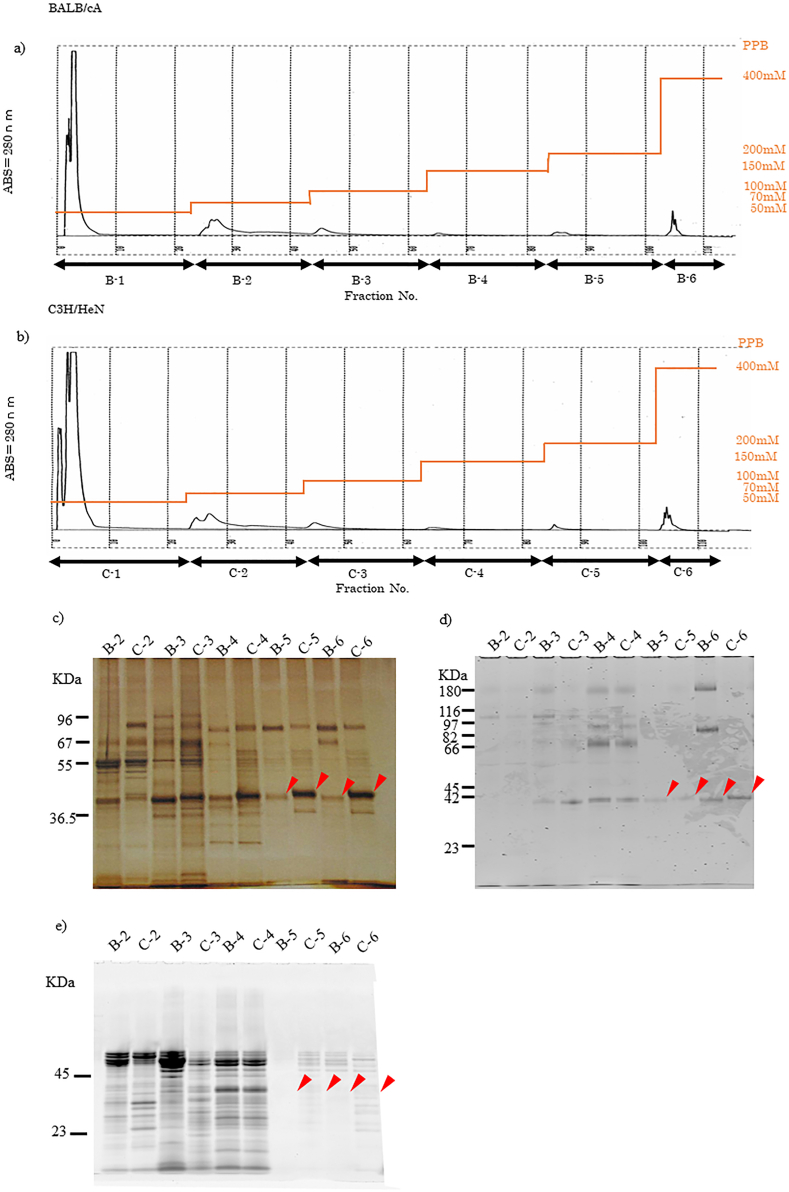
Whole saliva was eluted stepwise by HAP-HPLC chromatography (Fig. 1a, b). The proportion of strongly adsorbed proteins on HAP (as shown in Table 1) was 1.44% (B5; 0.61%, B6; 0.82%) of total adsorbed proteins for BALB/cA, whereas for C3H/HeN it was more than twice with 3.25% (C 5; 1.02%, C 6; 2.23%) (as in Table1). We repeated HAP-HPLC separation five times independently, and it was revealed that the proportion of HAP adsorbed proteins with the highest affinity was significantly (Mann-Whitney U-test, p < 0.01) higher in caries resistant C3H/HeN compared to the caries susceptible BALB/cA mice.
Figure 1.
HAP-HPLC and SDS-PAGE staining. Stepwise elution of saliva proteins for BALB/cA (a) and C3H/HeN (b). Each fraction of HAP-HPLC was adjusted in protein quantity and separated by 12% SDS-PAGE. All proteins and glycoproteins were visualized by c) silver, d) glycoprotein and e) phosphorylation staining, respectively, and each original photograph was shown in the supplementary file.
Table 1.
The protein ratio of each affinity fraction.
| Fraction No. | Protein w/v % | HAP Affinity | Protein Subtotal% |
|---|---|---|---|
| BALB/cA | |||
| Non-adsorbed | 71.55 | no | 71.55 |
| B-1 | 15.21 | low | 19.54 |
| B-2 | 4.32 | ||
| B-3 | 4.82 | medium | 7.47 |
| B-4 | 2.64 | ||
| B-5 | 0.61 | high | 1.44 |
| B-6 | 0.82 | ||
| total protein | 10.3 mg/ml | ||
| C3H/HeN | |||
| Non-adsorbed | 70.55 | no | 70.55 |
| C-1 | 8.98 | low | 18.73 |
| C-2 | 9.75 | ||
| C-3 | 4.90 | medium | 7.47 |
| C-4 | 2.56 | ||
| C-5 | 1.02 | high | 3.25 |
| C-6 | 2.23 | ||
| total protein | 11.0 mg/ml | ||
3.2. Analysis by SDS-PAGE
As shown in Figure 1c, there was a difference in the migration of bands around the molecular weight of 40 kDa for the results of silver-stained strongly adsorbed fractions B5, C5, B6, C6 on HAP. C3H/HeN showed less migration than BALB/cA. In the electrophoresis pattern of six gels, the molecular weight corresponding to the migration of the bands of both strains was calculated from the migration of the standard, and revealed that the band of Balb/cA was 40.8 ± 1.06 kDa and that of C3H/HeN was 41.9 ± 0.97 kDa, resulting in a significant difference (Wilcoxon signed rank test, p < 0.05). As a result of glyco-chain staining with Pro-Q Emerald, glycosylated proteins were observed in moderate and strong HAP-adsorption fractions for both BALB/cA and C3H/HeN. In Figure 1d the lanes B5, C5, B6, and C6 showed a difference in the migration of proteins near 40 kDa similar to silver staining (Figure 1c). Phosphorylated proteins detected by Pro-Q Diamond in both BALB/cA and C3H/HeN were observed in weakly and moderately adsorbed fractions to HAP, but not in the strongly adsorbed proteins B5, C5, B6 and C6 (Figure 1e). These results indicate that the salivary proteins of major interest in BALB/cA and C3H/HeN strongly adsorbed to HAP and are present in a band near the molecular weight of 40 kDa. Higher molecular mass of the protein band in C3H/HeN compared to BALB/cA, is due to glyco-chains.
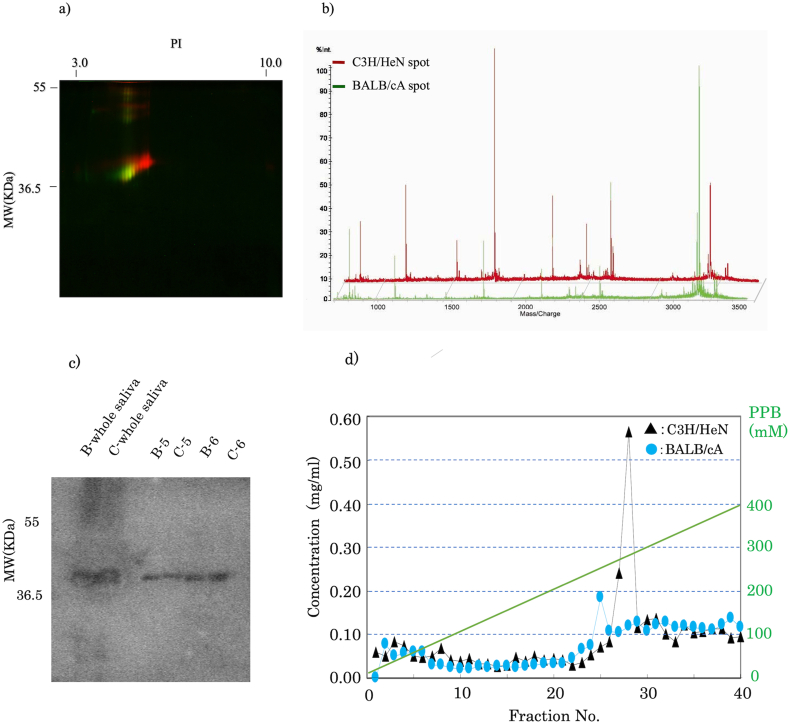
In order to see the difference between B6 and C6, different labels were applied and equal amounts were run on the same gel of 2D-PAGE (Figure 2a). B6 was observed as a green and C6 as a red spot, and the result revealed a lower pI and a lower molecular weight for B6 than C6.
Figure 2.
Qualitative and quantitative analysis of HAP-high affinity fractions. a) Differential 2D-PAGE. HAP-HPLC fractions eluted at 400 mM in both mouse strains were chemically labeled with Cy5 (red; C3H/HeN) and Cy3 (green; BALB/cA) fluorescent dye and separated by 2D-PAGE in equivalent gels. b) PMF analysis of each spot (MALDI-TOF/MS). BALB/cA is shown as green line and C3H/HeN as red line. c) Western blot analysis using HAP-HPLC fractions and polyclonal antibody. The original photograph was shown in the supplementary file. d) CAVI concentration of HAP-HPLC linear gradient fractions determined by ELISA.
3.3. Peptide mass fingerprinting by MALDI-TOF MS analysis
After separating fractions B5, B6, C5 and C6 by 2D-PAGE followed by CBB staining, a spot was observed near pI 4.0–5.0, at approximately the same position as the spot observed by glyco-chain staining with Pro-Q Emerald (data not shown). Therefore, these spots of fractions B6 and C6 were cut out and MALDI-TOF MS analysis was performed.
As a result of MASCOT analysis from the obtained mass distribution chart (Figure 2b), the eluted fractions B6, C6 of BALB/cA and C3H/HeN respectively showed an almost identical peptide peak pattern, and all 4 fractions showed the mouse carbonic anhydrase (CAVI) with a score of 85 or more, expected value 2.5-e5 or less, peptide matching score 10 or more and sequence coverage rate over 45% against CAVI.
3.4. Confirmation of CAVI in saliva (western blotting)
Part of the amino acid sequence of the mouse CAVI protein published in a database was synthesized for use as an antigen and a rabbit polyclonal antibody was prepared. The results of western blotting using the prepared polyclonal antibody are shown in Figure 2c. The CAVI band was confirmed in all saliva HAP-HPLC elution fractions B5, B6 of BALB/cA, and C5, C6 of C3H/HeN (Figure 2c). It shows that the CAVI of C3H/HeN has a somewhat higher molecular weight than BALB/cA.
3.5. Determination of CAⅥ quantity in HAP-HPLC fractions using ELISA
Figure 2d shows the quantitative results of HAP-HPLC linear gradient fractionation by ELISA. A difference in retention time and concentration of CAVI between BALB/cA and C3H/HeN was confirmed. The retention time of C3H/HeN was longer indicating a strong adsorption, and the HAP adsorbed quantity was also high.
3.6. Analysis of glyco-chain modification (western blotting and MALDI TOF/MS analysis)
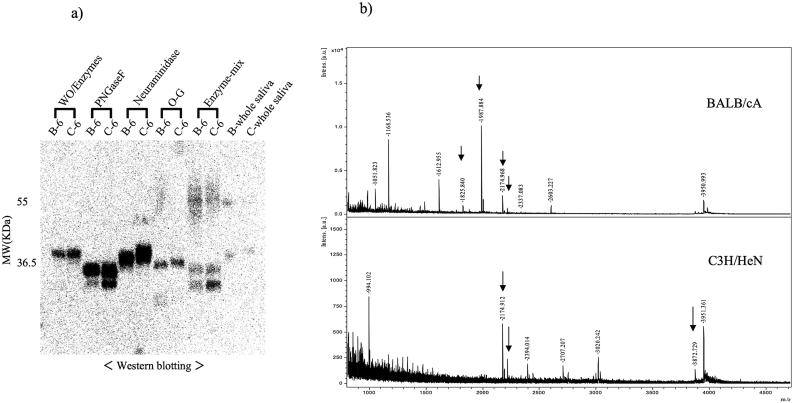
The HAP-HPLC elution fractions B6 and C6 of BALB/cA and C3H/HeN respectively were treated with the deglycosylation enzymes PNGase-F, Neuraminidase and O-Glycosidase, followed by SDS-PAGE and Western blotting (Figure 3a). When the three enzymes were mixed and the proteins digested, the migration speed of B6 and C6 proteins increased, bands around 40 kDa shifted to 35 kDa and 30 kDa respectively, and the 30 kDa band became stronger. Digestion with PNGaseF only gave two bands at 35 kDa and 30 kDa, with a stronger band at 35 kDa. When digestion was performed only with Neuraminidase, a shift in molecular weight could not be confirmed. Digestion with O-Glycosidase only showed a molecular weight decrease to 36.5 kDa and ca. 36 KDa while maintaining the molecular weight difference between B6 and C6 of the HAP-HPLC eluted fractions. Therefore, the results of analyzing the glyco-chains by MALDI TOF/MS (Figure 3b) show N-type glyco-chains of different structure. For BALB/cA at least five kinds with lower molecular weight, and for C3H/HeN three kinds with higher molecular weight were identified.
Figure 3.
Glycosylation modification analysis of high affinity fractions. a) Western blot analysis using de-glycosylation enzymes. The original photograph was shown in the supplementary file. The eluted fractions of B6 and C6 were treated with the de-glycosylation enzymes, and analyzed by western-blot with a polyclonal antibody. b) Glycan analysis with MALDI-TOF MS.
3.7. Sequence analysis of cDNA
The synthesized cDNA from the RNA of each mouse salivary glands was amplified with a CAVI specific primer followed by sequencing (Figure 4). Comparing the results with the database, C3H/HeN had an adenine at position 836 replaced by cytosine, and the amino acid changed from Gln to Pro. Regarding the BALB/cA sequence thymine at position 109 was replaced by cytosine, cytosine at position 281 was replaced by adenine and adenine at 836 was replaced by cytosine. The amino acid sequence remained unchanged at 109, however at 281 Leu was replaced by Ile and at 836 Gln was replaced by Pro. As a result of comparison with the CAVI gene database, it was confirmed that CAVIs of C3H/HeN and BALB/cA are polymorphic due to SNPs at positions 109, 281 and 836 (https://www.ncbi.nlm.nih.gov/gene?cmd=Retrieve&dopt=Graphics&list_uids=12353).
Figure 4.
Comparison of DNA sequences and coded amino acids of CA6 for two mouse strains. Nucleotide sequence data reported are available in the DBJ/EMBL/GenBank databases under the accession numbers AB911240-AB911241.
3.8. CD spectrum analysis
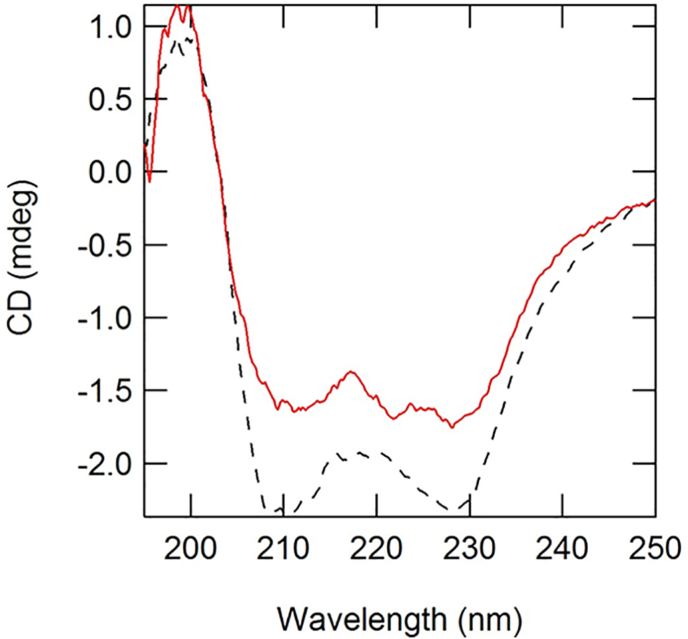
For conformational analysis, we measured far-UV CD spectra of B6 and C6 (Figure 5). The CD spectra of C6 and B6 were very similar within experimental errors. However the CD spectrum intensity of C6 was lower at 200 nm and higher at 210–240 nm than that of B6. Further, the CD spectrum of B6 showed a small negative peak at 222 nm. These results show that the conformations of B6 and C6 are very similar, but not identical.
Figure 5.
Far-UV CD spectra of B6 and C6. Spectra of B6 (red solid line) and C6 (black broken line) measured at 25 °C.
3.9. Measurement of buffering capacity
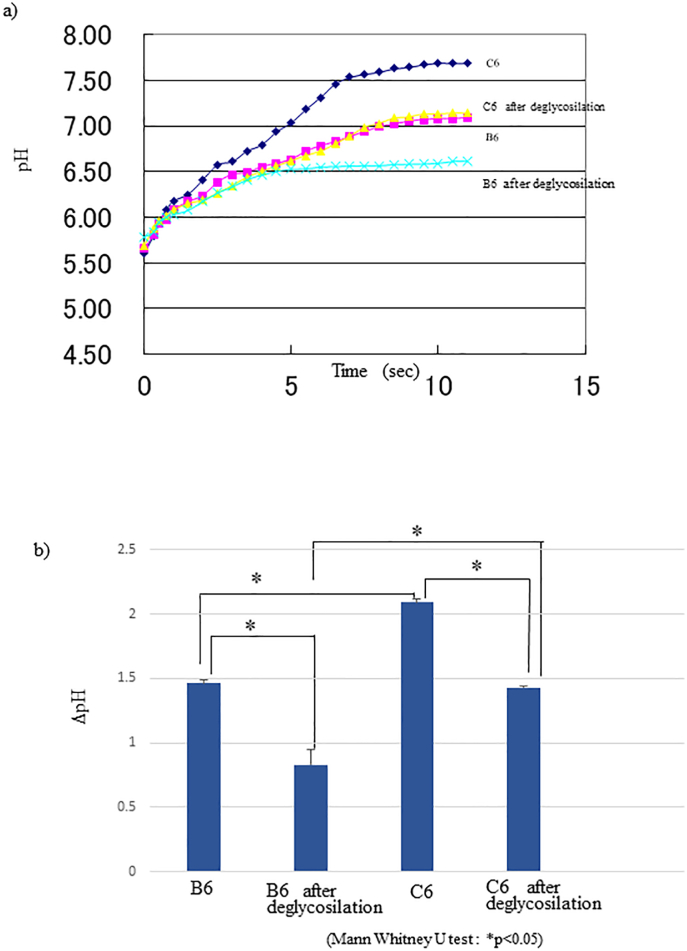
Figure 6 shows the buffering capacity of the HAP-HPLC elution fractions. Regarding C6 of C3H/HeN, the initial pH increased to pH 7.69 in 10 min, whereas for B6 of BALB/cA, the initial pH increased to pH 7.13 in about 10 min, but no further pH increase was observed afterwards (Figure 6a).
Figure 6.
pH buffering capacity. a) Measurement of pH buffering capacity by the modified kinetic method of Maren et al. using 10 mM NaHCO3 saturated with CO2. ▲; B6, x; B6 after de-glycosylation, ♦; C6, ■; C6 after de-glycosylation. b) Comparison of changes in pH value. The pH value at 10 min was compared between both proteins as well as before and after de-glycosylation.
The glyco-chains of fractions C6 and B6 were enzymatically cleaved and the buffering capacity was measured. As a result, the activity value of C6 decreased to 39.7% (Figure 6b). Even with cut off glyco-chains, C6 showed a higher activity value than B6 (Figure 6a).
4. Discussion
BALB/cA is known as a caries-sensitive mouse and C3H/HeN is known as a caries-resistant mouse, but the reason has not been clarified. It was hypothesized that clarifying this difference could lead to factors determining the susceptibility of human caries. We predicted that pellicle proteins attached to enamel were one of the factors behind the difference, and we succeeded in finding a difference in this study. If this difference can be applied to monitor human caries susceptibility, it will lead to an evaluation of caries resistance of the host, which is lacking in current caries risk diagnosis. This will lead to the realization of new and more accurate diagnosis and preventive measures can be expected.
Dental pellicles form by adsorption of more than 100 kinds of saliva proteins and peptides to the enamel surface layer and play an important role in protection and remineralization of enamel [15]. According to previous studies, histatins, statherin and acidic proline-rich proteins (PRPs) firmly attach to the hydroxyapatite of enamel and seem to play a central role as pellicle proteins among salivary proteins [16, 17]. Histatins are basic peptides with a broad antimicrobial [18] spectrum and are thought to suppress dental caries-related bacteria [19], but epidemiologic investigations are mixed with some indicating a relationship with caries and some not [20, 21]. Statherin is a small protein (5.4kDa) acting on remineralization of enamel and is known to reduce adhesion of Streptococcus mutans to enamel [21]. Epidemiological investigations of statherin also show mixed results regarding the involvement of caries [20, 21, 22]. Acidic PRPs serve as a source of calcium in saliva [23], so they seem important for remineralization. However, Protein Antigen C (Pac), an adhesin of Streptococcus mutans [36], uses PRPs as initial receptor [12, 24, 25] and there are reports that acidic PRPs correlate with the incidence of early childhood caries [26]. Therefore, when considering caries risk and prevention methods based on host specific pellicle protein composition, the significance of the candidates has not been clarified and has not been put into practical use. In order to find new candidates, this study used an in vitro model of saliva proteins adsorbed by HAP.
We considered that the pellicle-forming protein, which has the strongest adsorption property to HAP, could be one of the candidates for affecting the onset of caries. The purpose of this study was to compare pellicle-forming salivary proteins of two mouse groups with different caries susceptibility in vitro and identify their candidates.
The ultimate goal is to identify the factors that affect human caries, but analysis by comparing humans with environmental and genetic factors aligned is not possible. Therefore, as a first step, we used an experimental animal that can do just that as a model. In many studies related to human life phenomena, first of all a method is adopted in which quantitative and qualitative significance is evaluated in vitro, then verified with experimental animals, which are model organisms, and finally confirmed in humans, which is considered appropriate from an ethical point of view.
On the other hand, in some cases, epidemiological studies in humans have been conducted from the beginning without in vitro or animal experiments. The reason is the possibility of discovering something that has not been found as a candidate in an in vitro experiment. This approach might be suitable for studying diseases that are affected by multiple factors, such as dental caries. However, because the background of the subjects cannot be aligned, the required sample size becomes large, the number of survey items is exhaustive and the research scale enormous. And it frequently happens that a sufficient scale cannot be secured, and a significant difference cannot be obtained [37, 38]. Tautman et al. reported that there were significant differences between groups with different caries susceptibility regarding a large number of candidate proteins, but due to the small sample size, large-scale studies will be necessary in future to obtain clear differences [39].
Here, we attempted to identify caries inhibitory proteins by a proteome analysis of tightly adhering pellicle proteins with high molecular weight. As a result of our examination in two mouse strains with different caries susceptibility (higher for BALB/cA and lower for C3H/HeN), we found a protein with high HAP-affinity and properties differing between the two strains and identified it as CAVI. CAVI, existing in secretion fluid, is a member of the carbonic anhydrase family, which matches the CAVI annotation result as glycosylated but non-phosphorylated protein. CAVI catalyzes the reaction of hydrogen ions produced by bacteria in the oral cavity with bicarbonate ions to produce water and carbon dioxide in order to neutralize acids [27]. It is also present in human saliva, adhering to the tooth surface [28]. In a study of pellicle constituent proteins over time, it has been reported that CAVI exists as a pellicle constituent protein from the initial stage over a long period of time [29, 30, 31]. Meanwhile, epidemiologic investigations indicate that salivary CAVI levels and DMFT are inversely correlated in humans [31]. It is considered that CAVI is present on enamel in a stable manner from the early stage of pellicle formation and participates in the prevention of dental caries by buffering the pH and protecting the enamel. Thus, it is also known that some pellicle proteins like acidic PRPs while protecting the tooth surface, at the same time become targets as anchors for early attachment bacteria [10]. When an agglutination test of S. mutans was carried out using CAVI fractions of two mouse strains, an aggregation effect could not be observed (data not shown). However careful consideration is necessary due to the possibility that the ligand is hidden inside the protein and not exposed to the receptor, as it is the case for acidic PRPs and statherin. However, since there have been no reports of CAVI as a ligand, it can be said that there is no evidence of being actively involved in the colonization of S. mutans. Therefore, since CAVI does not act as a ligand involved in bacterial adhesion on the tooth surface, it also does not promote fixation of S. mutans, and is considered not to inhibit a caries suppression effect. It has been clarified that CAVI has an effect on bicarbonate buffering capacity [27], but comparison of CAVI enzyme activity between mouse strains has not been reported yet. Therefore here, comparison of enzyme activity per constant saliva volume between the two mouse strains showed that C3H/HeN had a higher activity than BALB/cA (Figure 6). From these results, it can be predicted, that in a state adsorbed to enamel as a pellicle protein CAVI of C3H/HeN is both more abundant and has higher enzyme activity than BALB/cA. Therefore, even when the pH in plaque declines, it is possible that due to the enzymatic activity of CAVI on the surface of HAP, the pH is buffered and does not drop to the critical enamel demineralizing pH. Hence the development of caries is suppressed; this effect might be higher in C3H/HeN than in BALB/cA. Considering the data on the caries suppression effect of CAVI in humans [31], it is possible that the enzyme activity plays a more important role rather than the abundance of CAVI. These findings suggest that the difference in caries sensitivity of BALB/cA and C3H/HeN may be based on the activity difference of the strongly absorbed CAVI on HAP. The activity gap might stem from differences in CAVI protein structure. We analyzed the primary structure and higher order structure of CAVI proteins of both mice strains, predicting that the qualitative differences also produce differences in activity. To analyze the primary structure the nucleotide sequence for the amino acid coding region was compared to the database and revealed three SNPs. Among them, the polymorphism at nucleotide position 283 resulted in the primary structure change Leu-Ile of the CAVI protein. Therefore, different higher order structures might be possible.
In fact, the results of CD spectral analysis showed similarity, but not complete identity (Figure 5). Since the protein conformation is also affected by posttranslational modification, phosphorylation and modification patterns of the glyco-chains were compared. The CAVI protein was rarely phosphorylated, but the analysis pattern of glyco-chains was different.
Some examples that modifications of glyco-chains affect the activity of enzymatic proteins have been reported so far [32, 33, 34, 35]. When the glyco-chains of CAVI protein from both mice strains were cleaved and the enzyme activities compared, the activity decreased for both strains, however with a difference in the attenuation rate. Therefore, in addition to the change of the primary structure, it is possible that the difference in glycosylation modification also influences a change of the higher order structure, and it is likely that the CAVI enzyme activity also differs. Since this study is the result of a mouse model, it is of course necessary to verify it in humans in future. Additionally, the enzyme activity as carbonic anhydrase was detected in the identified protein CAVI, but since this is only a detection system in liquid, it is necessary to establish a system to detect the activity in the solid phase of the pellicle [40].
Further research is needed as to whether CAVI is related to a suppression effect of human caries as a pellicle protein and whether it is useful for caries risk diagnosis and prevention strategy.
5. Conclusions
CAVI is a HAP-high-affinity mouse saliva protein, which shows differences in glyco-chain modification between two mice strains. We found a CAVI coding gene polymorphism in two bases causing one amino acid difference. In addition, differences in CAVI three-dimensional structure and function of pH buffering were also shown. In other words, the pellicle protein, expressing CAVI, which adheres more firmly to enamel and exerts a high buffering action in C3H/HeN compared to BALB/cA, might exhibit caries resistance. Therefore, a possible involvement of CAVI in caries prevention was suggested.
In our study, the difference in the structure and modification of CAVI, reflected in the difference in buffer function could not be clarified. Another limitation is, that saliva samples separated by HAP-HPLC were concentrated with a molecular weight cut of 10,000 Da; therefore smaller molecules should be analyzed with other methods.
It is a future task to verify whether the difference in the structure and modification of CAVI really affects caries susceptibility. Furthermore, clinical research in humans will be necessary in future to verify whether CAVI is an influential factor even in humans, which is the original purpose.
Declarations
Author contribution statement
Keijiro Ohshima, Tomoko Ohshima: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Karen Meyer, Nobuko Maeda: Analyzed and interpreted the data; Wrote the paper.
Eisuke Takai, Shunsuke Yoshizawa, Kentaro Shiraki: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Nobuko Maeda was supported by Japan Society for the Promotion of Science KAKENHI Grant Number 10877340.
Data availability statement
Nucleotide sequence data reported are available in the DBJ/EMBL/GenBank databases under the accession numbers AB911240-AB911241.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
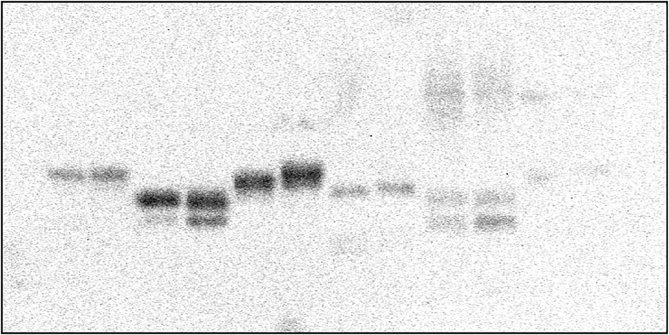
Fig1c-P2.tif.
Fig1d-P.tif.
Fig1e-P.tif.
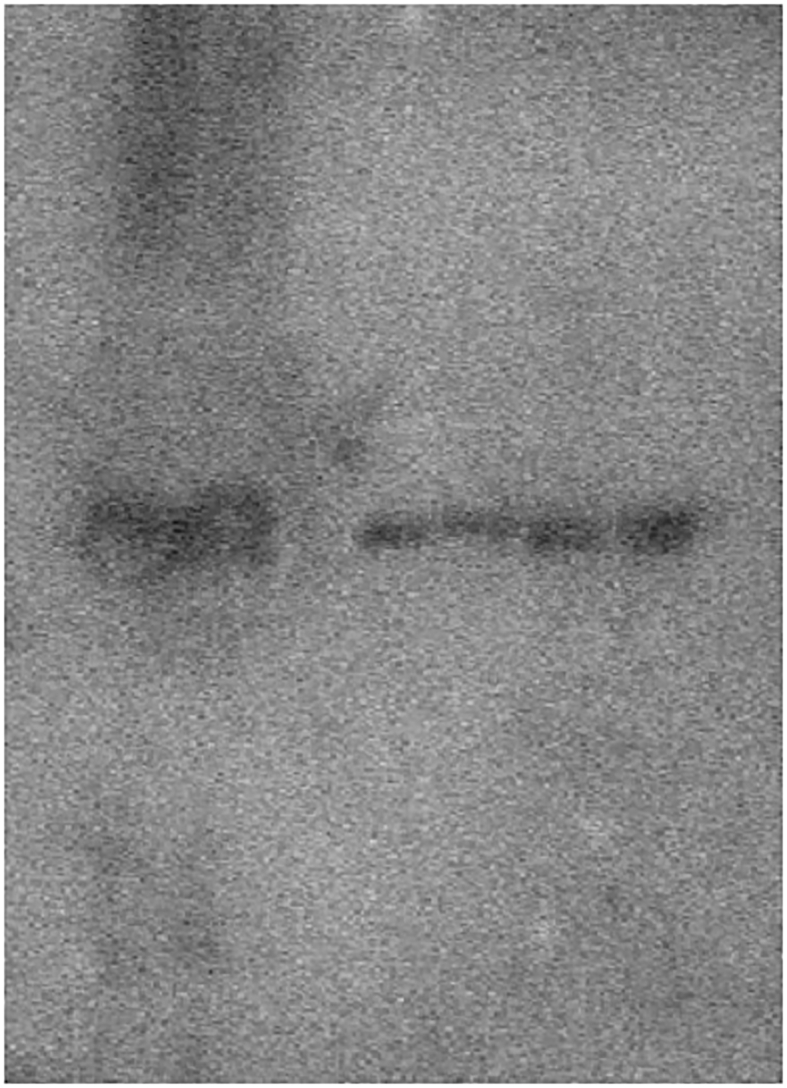
Fig2c-P.tif
Fig3a-P.bmp.
References
- 1.Ministry of Health, Labour and Welfare-Japan . 2017. Survey Results of Dental Diseases in 2017. [Google Scholar]
- 2.Petersen P.E. Challenges to improvement of oral health in the 21st century – the approach of the WHO Global Oral Health Programme. Int. Dent. J. 2004;54:329–343. doi: 10.1111/j.1875-595x.2004.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 3.Ministry of Education Japan . 2016. Statistical Abstract 2016 Edition Physical Education and Sport Disease Rate Among Students. [Google Scholar]
- 4.Maren T.H. A simplified micromethod for the determination of carbonic anhydrase and its inhibitors. J. Pharmacol. Exp. Therapeut. 1960;130:26–29. [PubMed] [Google Scholar]
- 5.Ministry of Agriculture . 2014. Forestry and Fisheries. -Japan, Production of Sugar and Starch. [Google Scholar]
- 6.Tonomura S., Ihara M., Kawano T., Tanaka T., Okuno Y., Saito S., Robert P.F., Kuriyama N., Nomura R., Watanabe Y., Nakano K., Toyoda K. Intracerebral hemorrhage and deep microbleeds associated with cnm-positive Streptococcus mutans; a hospital cohort study. K. Sci Rep. 2016;6 doi: 10.1038/srep20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fure S. Ten-year cross-sectional and incidence study of coronal and root caries and some related factors in elderly Swedish individuals. Gerodontology. 2004;21:130–140. doi: 10.1111/j.1741-2358.2004.00025.x. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi M., Masao O., Mariko I., Harumi H., Junko K., Ichiro H., Wataru M. Study of the assessment of caries risk for school children period. J Fukuoka Dental College. 2002;29:213–219. [Google Scholar]
- 9.Nakashima T., Anzai T., Kusaba A., Kosimune S., Takehara T. Trial and outcome of school dental health activities based on caries risk tests. J Dent Health-Tokyo. 2002;52:196–202. [Google Scholar]
- 10.Kolenbrander P.E., London J. Adhere today, here tomorrow: oeal bacterial adherence. J. Bacteriol. 1993;175:3247–3252. doi: 10.1128/jb.175.11.3247-3252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee Y.H., Zimmerman J.N., Custodio W., Xiao Y., Basiri T., Hatibovic-Kofman S., Siqueira W.L. Proteomic evaluation of acquired enamel pellicle during in vivo formation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell M.W.C., Mansson-Rahemtulla B. Interaction between surface protein antigens of Streptococcus mutans and human salivary components. Oral Microbiol. Immunol. 1989;4:106–111. doi: 10.1111/j.1399-302x.1989.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi A., Ohshima T., Maeda N. Differences in salivatory factors contributing to colonization of the oral bacteria among inbred mouse strains. J. Oral Biol. 1999;41:121–132. [Google Scholar]
- 14.Kurihara Y., Naito T., Obayashi K., Hirasawa M., Kurihara Y., Moriwaki K. Caries susceptibility in inbred mouse strains and inheritance patterns in F1 and backcross (N2) progeny from strains with high and low caries susceptibility. Caries Res. 1991;25:341–346. doi: 10.1159/000261389. [DOI] [PubMed] [Google Scholar]
- 15.Siqueira W.L., Custodio W., McDonald E.E. New insights into the composition and functions of the acquired enamel pellicle. J. Dent. Res. 2012;91:1110–1118. doi: 10.1177/0022034512462578. [DOI] [PubMed] [Google Scholar]
- 16.Vitorino R., Lobo M.J.C., Williams J., Ferrer-Correia A.J., Tomer K.B., Duarte J.A., Domingues P.M., Amado F.M.L. Peptidomic analysis of human acquired enamel pellicle. Biomed. Chromatogr. 2007;21:1107–1117. doi: 10.1002/bmc.830. [DOI] [PubMed] [Google Scholar]
- 17.Siqueira W.L., Oppenheim F.G. Small molecular weight proteins/peptides present in the in vivo formed human acquired enamel pellicle. Arch. Oral Biol. 2009;54:437–444. doi: 10.1016/j.archoralbio.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helmerhorst E.J., Hodgson R., van't Hof W., Veerman E.C.I., Allison C., Nieuw A.V. The effects of histatin-derived basic antimicrobial peptides on oral biofilms. J. Dent. Res. 1999;78:1245–1250. doi: 10.1177/00220345990780060801. [DOI] [PubMed] [Google Scholar]
- 19.De Smet K., Contreras R. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol. Lett. 2005;27:1337–1347. doi: 10.1007/s10529-005-0936-5. [DOI] [PubMed] [Google Scholar]
- 20.Dodds M.W., Johnson D.A., Mobley C.C., Hattaway K.M. Parotid saliva protein profiles in caries-free and caries-active adults. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997;83:244–251. doi: 10.1016/s1079-2104(97)90012-3. [DOI] [PubMed] [Google Scholar]
- 21.Shimotoyodome A., Kobayashi H., Tokimitsu I., Matsukubo T., Takaesu Y. Statherin and histatin 1 reduce parotid saliva-promoted Streptococcus mutans strain MT8148 adhesion to hydroxyapatite surfaces. Caries Res. 2006;40:403–411. doi: 10.1159/000094286. [DOI] [PubMed] [Google Scholar]
- 22.Vitorino R., Guedes S.D.M., Ferreira R., Lobo M.J.C., Duarte J., Ferrer-Correia A.J., Tomer K.B., Domingues P.M., Amado F.M.L. Two-dimensional electrophoresis study of in vitro pellicle formation and dental caries susceptibility. Eur. J. Oral Sci. 2006;114:147–153. doi: 10.1111/j.1600-0722.2006.00328.x. [DOI] [PubMed] [Google Scholar]
- 23.Buzalaf M.A., Hannas A.R., Kato M.T. Saliva and dental erosion. J. Appl. Oral Sci. 2012;20:493–502. doi: 10.1590/S1678-77572012000500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimotoyodome A., Kobayashi H., Nakamura J., Tokimitsu I., Hase T., Inoue T. Reduction of saliva-promoted adhesion of Streptococcus mutans MT8148 and dental biofilm development by tragacanth gum and yeast-derived phosphomannan. Biofouling. 2006;22:261–268. doi: 10.1080/08927010600902821. [DOI] [PubMed] [Google Scholar]
- 25.Vitorino R., Lobo M.J.C., Duarte J.A., Domingues P.M., Amado F.M.L. Peptide profile of human acquired enamel pellicle using MALDI tandem MS. J. Separ. Sci. 2008;31:523–537. doi: 10.1002/jssc.200700486. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro T.R., Dria K.J., de Carvalho C.B.M., Monteiro A.J., Fonteles M.C., Carvalho K.M., Fonteles C.S.R. Salivary peptide profile and its association with early childhood caries. Int. J. Paediatr. Dent. 2013;23:225–234. doi: 10.1111/j.1365-263X.2012.01258.x. [DOI] [PubMed] [Google Scholar]
- 27.Leinonen J., Kivela J., Parkkila S., Parkkila A.K., Rajaniemi H. Salivary carbonic anhydrase isoenzyme VI is located in the human enamel pellicle. Caries Res. 1999;33:185–190. doi: 10.1159/000016515. [DOI] [PubMed] [Google Scholar]
- 28.Vitorino R., Lobo M.J.C., Duarte J., Ferrer-Correia A.J., Tomer K.B., Dubin J.R., Domingues P.M., Amado F.M.L. In vitro hydroxyapatite adsorbed salivary proteins. Biochem. Biophys. Res. Commun. 2004;320:342–346. doi: 10.1016/j.bbrc.2004.05.169. [DOI] [PubMed] [Google Scholar]
- 29.Parkkila S., Parkkila A.K., Vierjoki T., Ståhlberg T., Rajaniemi H. Competitive time-resolved immunofluorometric assay for quantifying carbonic anhydrase VI in saliva. Clin. Chem. 1993;39:2154–2157. [PubMed] [Google Scholar]
- 30.Hurt J.D., Tu C., Laipis P.J., Silverman D.N. Catalytic properties of murine carbonic anhydrase IV. J. Biol. Chem. 1997;272:13512–13518. doi: 10.1074/jbc.272.21.13512. [DOI] [PubMed] [Google Scholar]
- 31.Kivelä J., Parkkila S., Parkkila A.K., Rajaniemi H.A. A low concentration of carbonic anhydrase isoenzyme VI in whole saliva is associated with caries prevalence. Caries Res. 1999;33:178–184. doi: 10.1159/000016514. [DOI] [PubMed] [Google Scholar]
- 32.Cooper C.A., Gasteiger E., Packer N.H. GlycoMod – a software tool for determining glycolysation compositions from mass spectrometric data. Proteomics. 2001;1:340–349. doi: 10.1002/1615-9861(200102)1:2<340::AID-PROT340>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 33.Okajima T., Matsuura A., Matsuda T. Biological functions of glycosyltransferase genes involved in O-fucose glycan synthesis. J. Biochem. 2008;144:1–6. doi: 10.1093/jb/mvn016. [DOI] [PubMed] [Google Scholar]
- 34.Matsuura A., Ito M., Sakaidani Y., Kondo T., Murakami K., Furukawa K., Nadano D., Matsuda T., Okajima T. O-linked N-acetylglucosamine is present on the extracellular domain of notch receptors. J. Biol. Chem. 2008;283:35486–35495. doi: 10.1074/jbc.M806202200. [DOI] [PubMed] [Google Scholar]
- 35.Yamakoshi Y., Yamakoshi F., Hu J.C.C., Simmer J.P. Characterization of kallikrein-related peptidase 4 glycosylations. Eur. J. Oral Sci. 2011;119:234–240. doi: 10.1111/j.1600-0722.2011.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Cloning of a surface protein antigen gene from serotype c Streptococcus mutans. Molecular microbiol. 1989;3(2):221–228. doi: 10.1111/j.1365-2958.1989.tb01811.x. [DOI] [PubMed] [Google Scholar]
- 37.Kirsch J., Hannig C., Pötschke S., Basche S., Bowen W.H., Rupf S., Trautmann S., Umanskaya N., Hannig M. Enzymology and ultrastructure of the in situ pellicle in caries-active and caries-inactive patients. Caries Res. 2017;51:109–118. doi: 10.1159/000452226. [DOI] [PubMed] [Google Scholar]
- 38.Schulz A., Lang R., Behr J., Hertel S., Reich M., Kümmerer K., Hannig M., Hanni C., Hofmann T. Targeted metabolomics of pellicle and saliva in children with different caries activity. Sci. Rep. 2020;10:697–708. doi: 10.1038/s41598-020-57531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trautmann S., Barghash A., Fecher-Trost C., Schalkowsky P., Hannig C., Kirsch J., Rupf S., Keller A., Helms V., Hannig M. Proteomic analysis of the initial oral pellicle in caries-active and caries-free individuals. Proteonomics Clin. Appl. 2019;13(1-11) doi: 10.1002/prca.201800143. [DOI] [PubMed] [Google Scholar]
- 40.Hannig C., Attin T., Hannig M., Henze E., Brinkmann K., Zech R. Immobilisation and activity of human alpha-amylase in the acquired enamel pellicle. Arch. Oral Biol. 2004;49:469–475. doi: 10.1016/j.archoralbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Nucleotide sequence data reported are available in the DBJ/EMBL/GenBank databases under the accession numbers AB911240-AB911241.