Abstract
The antagonistic side effects of chemical medications led to the search for safe strategies such as biogenic agents. Correspondingly, this study aims to create biogenic, appropriate, auspicious and innovative therapeutic agents like Galaxaura elongata {GE}, Turbinaria ornata {TO} and Enteromorpha flexuosa {EF} macroalgae-based silver nanoparticles (Ag-NPs). The Ag+ reduction and the creation of Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs have been validated using UV–visible spectroscopy, Fourier transform infrared spectroscopy (FTIR), scanning electron microscope (SEM) and zeta potential analysis, and the chemical composition of macroalgae crude extracts was estimated through gas chromatography–mass spectrometry (GC–MS). Further, macroalgae-based Ag-NPs were tested for their free radical scavenging activity DPPH, ABTS, anticancer activity in human liver carcinoma (HepG2) cell line, distinctive inflammation forms and elevated α-amylase. Results showed that the biosynthesized Ag-NPs have unique mechanical and physicochemical characters attributed to their high relative surface area, nanosized dimensions and spherical shape. At dose of 200 µg/mL, the DPPH radical scavenging capacity was maximized with Ag[TO]-NPs (67.26%); however, Ag[EF]-NPs was the most potent as ABTs scavenger (97.74%). Additionally, Ag[GE]-NPs had the maximum proteinase inhibitory action with 59.78%. The 1000 µg/mL of Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs revealed significant inhibitions of cell growth of HepG2 resulting in cell viabilities 5.92%, 4.44% and 11.33%, respectively. These findings suggest that macroalgae bio-capped Ag-NPs have magnificent biological potentials for safe biomedical applications.
Keywords: Biological activities, Galaxaura elongate, Turbinaria ornata, Enteromorpha flexuosa
Introduction
The main drawback in most diseases is oxidative stress, which is imbalance between free radical production and reactive metabolites. This imbalance initiates the damage of imperative biomolecules and cells with a potential impression on the overall organisms. The removal of such free radicals by protective mechanisms is known collectively as an antioxidant defense mechanism (Perry et al. 2011; Ahmed et al. 2017a, b). The antioxidant defense mechanism plays an essential role in depleting free radicals by synchronizing their damaging action of free radicals. They mainly attack macromolecules causing cell impairment (Matough et al. 2012; Rosenzweig et al. 2019; Madkour 2020). Furthermore, free radicals are disproportionately created in diabetes by glucose oxidation in addition to nonenzymatic protein glycation (Povova et al. 2012; Mehta et al. 2012).
Pathophysiological conditions such as oxidative stress, cytotoxicity and inflammation play a main role in several chronic diseases. The damaged degrees of free radicals and the synchronized demolition of antioxidant defense mechanisms can cause precarious impairment of cellular organelles and enzymes, preeminent lipid peroxidation and insulin resistance development. These consequences of oxidative stress can evolve diabetes mellitus (DM) complications (Suganthy and Pandima Devi 2016). Similarly, oxidative stress and diminished acetylcholine (ACh) levels have a substantial role in the Alzheimer’s disease (AD) pathogenesis (Ames and Gold 1992; Guyton and Kensler 1993). Accordingly, the treatment of elevated oxidative stress blocks the route of different diseases (Rajendran et al. 2021).
In order to overcome the recent global problems, health problems and wastes which have increased substantially in many parts of the world, innovative ways must be developed. Hence, nanotechnology occupies the interest of most researcher and so as to create trustworthy nanomaterial to solve those growing problems (Badawi and Zaher 2021; Badawi, et al. 2021a; Badawi, et al. 2021b). Previous studies focused on the synthesis of nanomaterials by chemical and physical approaches. Nevertheless, these techniques are mostly expensive, need extensive complicated laboratory procedures, and consume toxic chemicals (Badawi, et al. 2021a; Dawadi et al. 2021).
Consequently, the plant- and alga-originated particles such as flavonoids, tannins, polyphenols, alkaloids, and polysaccharides are highly accepted for their wide-ranging biological roles and have an immense therapeutic possibility in nano-medicines for various types of diseases (Ahmed et al. 2014; Watkins et al. 2015; Saratale et al. 2018b; Barani et al. 2021; Javad Farhangi et al. 2021). So, the biosynthesis of NPs from plant and algal extracts is currently the greatest manipulated technique because it is eco-friendly, obtainable, cost-saving, biocompatible and safe (Pillai et al. 2020; Mahmoud et al. 2020, 2021; Barani et al. 2021; Hassanisaadi et al. 2021; Es-Haghi et al. 2021; Mohammadzadeh et al. 2022; Hassanisaadi et al. 2022).
The green synthesis processes of Ag-NPs utilize numerous crude extracts of different plant parts, such as fruit peels (Saratale et al. 2018a), leaves (Raman et al. 2012; Kumar et al. 2016), flowers and gums (Velmurugan et al. 2016) and roots (Rao et al. 2016) as well as of macroalga (Mahmoud et al. 2020, 2021), which are the most recently used methods in nanomaterial biosynthesis. The seaweeds used for green biosynthesis of inorganic nanoparticles (NPs) have a variety of bioactive constituents, which are broadly implicated in different biotechnological fields and biological applications (Fahim et al. 2008; Ahmed et al. 2008, 2014; Lomartire and Gonçalves 2022). Recently, researcher has validated that those distinctive compounds express valuable properties such as antioxidant, anti-diabetic, anticancer, anti-arthritic, antimicrobial, antiviral, water treatment and photo-bioreactor (Ahmed 2010a, b; Ahmed and Ahmed 2014; Abd-Ellatef et al. 2017; Ahmed et al. 2017a, b; Badawi et al. 2022).
Moreover, the created biosynthesized Ag-NPs are widely recommended for using in medical fields as anti-inflammatory (Prabakaran and Mani 2019), antioxidant and anticancer potentials (Gomaa 2017; Saratale et al. 2018a; Hashemzadeh et al. 2021), anti-diabetic (Rajaram et al. 2015; Popli et al. 2018), hepatoprotective (Mahmoud et al. 2021), renocardio protective (Mahmoud et al. 2020) and anti-Alzheimer potentials (Popli et al. 2018).
Regardless of the previous consideration, the biosynthesis of Ag-NPs still defies ongoing challenges as little yields, period spending and high cost. Hence, the procurement of biological, safe, available and inexpensive materials with high reducing/stabilizing potentials and production of Ag-NPs with good biological, chemical and physical properties is now a high urgency for medical field. Marine macroalgae such as Galaxaura elongata (G. elongata), Turbinaria ornata (T. ornata) and Enteromorpha flexuosa (E. flexuosa) crude extracts are innovative and promising green reducing and capping agents, and they contain various vitamins, minerals, organic and inorganic ingredients that could help human health (Pádua et al. 2004; Rao and Boominathan 2015; Ramkumar et al. 2017; Ahmed et al. 2020; Fathy et al. 2020; Abdelrheem et al. 2021; Abdel Azeem et al. 2021; Mohamed et al. 2022).
The applications of G. elongata, T. ornata and E. flexuosa macroalgae-based Ag-NPs for different in vitro effects were not investigated by previous publications. So, we need to assess whether these macroalgae-biosynthesized Ag-NPs have in vitro antioxidant, anticancer, anti-inflammatory, anti-diabetic and anti-Alzheimer or not. Accordingly, the present investigation was designed to create biogenic Ag-NPs from different types of crude extracts of G. elongate, T. ornata and E. flexuosa and also to originate biomedical agents with safe influences. As these bioactive Ag-NPs have distinctive biological, chemical and physical features due to their heightened relative surface area, size, distribution shape and quantum impacts as proved by ultraviolet–visible (UV–Vis) spectroscopy, Fourier transform infrared spectroscopy (FTIR) and gas chromatography (GC)-mass spectrum (MS), scanning electron microscope (SEM) and zeta potential analysis. These biosynthesized Ag-NPs are tested to assess their antioxidant activity by detecting the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and 2, 2’-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) scavenging activities and anticancer effects on human liver carcinoma cell line (HepG2) as well as anti-inflammatory (anti-proteinase and protein denaturation), anti-diabetic (alpha amylase enzyme inhibition) and anti-Alzheimer activities.
Materials and methods
Materials
Natural materials
The macroalgae samples (G. elongata, T. ornata and E. flexuosa) were collected from the Egyptian Red Seashores, identified in Phycology lab, Botany and Microbiology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt, according to the description of Chapman and Chapman 1980 & Robert 1989.
Chemicals
Silver nitrate was purchased from Sigma-Aldrich Chemicals, USA, DPPH, ABTS, α-amylase, acetyl cholinesterase enzyme and dimethyl sulfoxide (DMSO).
Methods
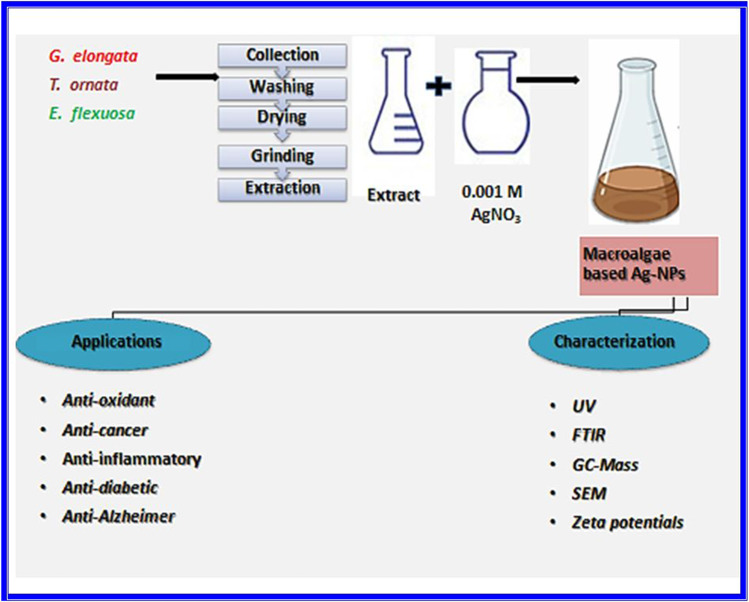
The methods of the preparation, characterization and applications of Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs are summarized in Fig. 1.
Fig. 1.
The outline of the experiments operated in this study
Biosynthesis of Ag-NPs by different forms of Egyptian macroalgae
Collection, extraction of G. elongata and biosynthesis of Ag [GE]-NPs
Initially, fresh G. elongata (J. Agardh) a red macroalga sample was collected and consecutively washed with tap and distilled water. Subsequently, the samples were dried at room temperature (25 °C) for one-week air drying with continuous stirring. Then, the dried macroalgae samples were milled through electric mixer until it turned into powder. Moreover, this powder was stored in dark place and finally became ready for different extraction methods.
Typically, 2 g of G. elongata powder was added into 100 mL of 95% ethanol. The combination was left on stirrer for 24 h to be extracted and filtered by Whatman no.1 filter paper, and a 50 mL volume of extract was finally added gradually into 450 mL 10–3 M of AgNO3 solution. The reduction progression of Ag+ to Ag0 NPs was associated with the change in solution color from pale yellow to reddish brown (Abdel Azeem et al. 2021).
Collection and extraction of T. ornata and biosynthesis of Ag[TO]-NPs
The brown macroalga T. ornata (Turn.) J. Agardh var ornate toylor was collected and washed with running tap water and distilled water; they were dried at room temperature ( 25 °C) and milled by electrical mixer until turned into powder, and then the powdered were stored in dark place. Last of all Ag[TO]-NPs were created according to the methods of (Deepak et al. 2017; Abdel Azeem et al. 2021).
Collection and extraction of E. flexuosa and biosynthesis of Ag [EF]-NPs
Primarily, E. flexuosa (L.) Nees. (green macroalga) was collected, washed several times with tap and distilled water and dried at room temperature (25 °C ). It was converted into powder by a mixer. Consequently, it was stored in a dark place and stand for various extraction methods.
The extraction of macroalgae samples were done according to the methods of (Yousefzadi et al. 2014; Abdel Azeem et al. 2021). Moreover, 50 mL of E. flexuosa extract was gently added into 450 mL of 10−3 M of AgNO3 solution for Ag [EF]-NPs biosynthesis. Lastly, the color of solution was converted to brown as a sign of Ag [EF]-NPs formation and the formed NPs were preserved in a dark place at room temperature (Yousefzadi et al. 2014).
Purification and drying Ag-NPs solution
The Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs solution was centrifuged at 12,000 rpm for 15 minutes, washed repeatedly with deionized water, dried at 50 0C and saved for various applications (Arunachalam et al. 2012).
Characterization of biosynthesized Ag-NPs
UV–Vis spectra analysis
The bioreduction of Ag+ to Ag0 was detected by visual judgment through appearance of brown color and by appraisal the UV–Vis spectrum of the reaction, at a wavelength of 250–800 nm in PerkinElmer UV WinLab UV–Vis spectrophotometer at the Nano-photonic Lab in Beni-Suef University, Beni-Suef, Egypt.
Fourier transform infrared spectroscopy (FTIR)
Fourier transform infrared spectroscopy spectral capacities were done to individualize functional groups in the biosynthesized Ag-NPs responsible for reducing and capping the bulk AgNO3. The formed Ag-NPs solution was analyzed using the FTIR instrument (VERTEX70 FT-IR spectrometer coupled to a RAM 11 FT-Raman module).
Gas chromatography–mass spectrometry (GC–MS) analysis
The chemical nature of crude extract of G. elongata, T. ornata and E. flexuosa was scrutinized by GC–MS chemical analysis assay. The investigation used a coupled Varian gas chromatography/mass spectrometry. The ionized voltage was 70 eV, with a mass range of m/z 39–400 amu. The ingredients were categorized by paralleling their mass spectra with the spectra of products in the library search report. The isolated peaks were categorized by matching with data from the mass spectra library (National Institute of Standard and Technology) and matched with authenticated compounds and available data (Adams 2007). The quantitative determination was carried out based on peak area integration.
Scanning electron microscope (SEM)
The morphological imaging SEM was performed using EDX-equipped JSM-6510.
Zeta potentials
Zeta potentials were scrutinized using Dynamic Light Scattering (DLS) at fixed angle of 173° at 25 °C.
In vitro biological analysis
DPPH free radical scavenging activity
The free radical scavenging capacity of macroalgae-based Ag-NPs on DPPH was evaluated at different concentrations {12.5, 25, 50, 100 and 200 µg/mL} of Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs and gallic acid as a standard. The Ag[GE]-NPs, Ag[TO]-NPs, Ag[EF]-NPs and gallic acid were mixed with 1.0 mL of methanol containing DPPH (4.0 mL of methanol per 4.0 mg of DPPH) radicals (0.2 mM). The mixture was forcefully shaken and left for 30 min in the dark, and the sample was diluted to be measured at the absorbance of 517 nm (Sousa et al. 2008). All assays were run in triplicate and averaged.
The scavenging ability was calculated using Eq. (1):
| 1 |
where As is the sample absorbance and A0 is the blank absorbance which contains all reagents except for the test samples.
The calculation of IC50 was done after charting of DPPH scavenging. Equation of straight lines was verified, and the IC50 was considered “x” in equation y = mx + b where y = 50 and values of m and b were given in the Trendline equation. The graphical slope of DPPH inhibition % was considered (Rahdar, et al. 2021).
ABTS free radical scavenging activity
In vitro antioxidant activity of macroalgae-based Ag-NPs was assessed by ABTS assay methods (Phull et al. 2016), the stock solution consisting of 7.4 mM ABTS and 2.6 mM potassium persulfate (Brand-Williams et al. 1995). The working mixtures were practiced by mixing the two stock solutions in equivalent volume and were permitted to react in the dark at room temperature for 12 h. The dilution process was done for the solution by mixing ABTS solution with methanol to obtain an absorbance of 0.736 ± 0.01 at 734 nm. The fresh solution was prepared for each ABTS assay. Then, 1.0 mL of different concentrations [12.5, 25, 50, 100 and 200 µg/mL] of Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs solutions and gallic acid as a standard reference was mixed with 1.0 mL of ABTS solution. After 10 min of incubation at room temperature, they were measured at the absorbance 734 nm. Finally, the inhibition percentage was calculated by Eq. (2):
| 2 |
Anticancer assessment of Ag-NPs against the HepG2 cell line
The cell viability potentials of macroalgae-based Ag-NPs were verified by 3-[4,5-Dimethylthiazol-2-yl] 2,5-diphenyltetrazolium bromide (MTT) which is a yellow water-soluble tetrazolium salt manipulating HepG2 cells similar to the approach of Sriranjani et al. 2016.
The cell viability percentage was estimated to get anticancer potential of the macroalgae-based Ag-NPs using calculated by Eq. (3):
| 3 |
where OD is the optical density ratio obtained from the spectrophotometer.
Evaluation of in vitro anti-inflammatory activity
Anti-proteinase action
The anti-proteinase activity of macroalgae-based Ag-NPs was measured in accordance with the methods of (Oyedapo and Famurewa 1995; Sakat et al. 2010), along with trivial modification. The reaction mixture (1.0 mL) included 0.06 mg trypsin, 0.5 mL 20 mM Tris–HCl buffer pH 7.4 and 0.5 mL of different Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs with various concentrations (12.5, 25, 50, 100 and 200 μg/mL). This combination was incubated at 370C for 5 min, and 0.5 mL of 0.8% (w/v) casein was supplemented. The mixture was then incubated for an additional 20 min, and 1.0 mL of 70% perchloric acid was added to block the reaction. The confused suspension was centrifuged, and the supernatant was read at an absorbance of 210 nm compared to blank buffer. These procedures were done in triplicate. The proteinase inhibitory activity ratio was calculated by Eq. (4):
| 4 |
Effect on protein denaturation
Protein denaturation analysis was completed as explained by Elias and Rao 1988, with slight modifications. Moreover, 1.0 mL of different concentrations of Ag [GE]-NPs, Ag[TO]-NPs, Ag[EF]-NPs and standard diclofenac sodium {12.5, 25, 50, 100 and 200 µg/mL} was admixed with 1.0 mL of fresh egg albumin solution and incubated at 27 ± 10C for 15 min. Denaturation was generated by keeping the reaction mixture for 10 min in water bath at 700C. The turbidity was assessed spectrophotometrically at 660 nm.
The inhibition ratio of denaturation was estimated from the blank where no samples were added. Every test was done in triplicate, and the mean was appropriated. The protein denaturation activity was calculated by Eq. (5):
| 5 |
In vitro anti-diabetic α-amylase inhibitory assay
The inhibition of α-amylase activity of biogenic Ag-NPs was finalized by assessing the maltose amount released during the test. This process is similar to Bhutkar and Bhise 2012, with some modifications. Different concentrations of Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs {12.5, 25, 50, 100 and 200 µg/mL} solution were pre-incubated with 100 μL of α-amylase solution (1.0 U/mL) at room temperature for 30 min; 100 μL of starch solution (1% w/v) was more supplemented to the mixture and incubated at room temperature for 10 min, and 100 μL of iodine solution was added to the mixture to stop the reaction; then, the solution was incubated for 5 min. Blank was provided where the identical enzyme quantity and the Ag-NPs were substituted by sodium phosphate buffer retained at a pH value of 6.9 and acarbose behaved as a positive control, and this procedure was done in triplicate. Finally, the absorbance of the tested samples was measured at 540 nm and the α-amylase inhibition percentage was estimated using Eq. (6):
| 6 |
Anti-acetyl cholinesterase activity
Acetyl-cholinesterase inhibition activity by innovative biogenic Ag-NPs was operated as stated by the procedures of Ellman et al. 1961, with little adjustments by adding 1.5 mL of 0.1 M phosphate buffer [Na2HPO4/NaH2PO4, pH 8.0], 50 µl of AChE source (600 U/mL) diluted 1:120, 50 µL of Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs and standard donepezil with different concentrations {12.5, 25, 50, 100 and 200 µg/mL}. Moreover, 10 µL of [0.075 M] acetylcholine iodide was then added and finally read at different periods at 412 nm. The experiment was done in triplicate. The AChE inhibition percentage was calculated using Eq. (7).
| 7 |
Statistical analysis
Data were given as mean ± standard error of 3 replicates using one-way analysis of variance (ANOVA) followed by Duncan’s test α = 0.05.
Results and discussion
An itemized study on biosynthesis, characterization and in vitro biological potentials of macroalgae-based Ag-NPs from the crude extract of G. elongata, T. ornata or E. flexuosa was elucidated in this paper.
Characterization
The UV–Vis, GC–MS and FTIR analysis were applied to analyze and characterize the marine macroalgae-based Ag-NPs properties.
Formation of macroalgae-based silver nanoparticles
After the addition of G. elongata, T. ornata and E. flexuosa crude extracts to AgNO3 initiate Ag-NPs formation, the change in color from colorless to brown or dark brown is the main sign of the reduction of Ag+ to Ag0 and formation of Ag-NPs as shown in Fig. 2.
Fig. 2.
Observation of macroalgae-based Ag-NPs synthesis: (A) formation of Ag[GE]-NPs; (B) formation of Ag[TO]-NPs; (C) formation of Ag[EF]-NPs
Optical properties (UV–visible spectroscopy)
The spectral optical absorption of Ag-NPs is ruled by SPR that shows a shift in the direction of the red or blue end hinges on the NPs size, structure, the surrounding dielectric medium, and aggregation (Mahmudin et al. 2015). The biosynthesis of Ag-NPs was confirmed by color alteration from light yellow to brown and UV–Vis spectroscopy using SPR in the range of 250–800 nm. The SPR peak represents different morphological features of the NPs, for example, the shape, size, and stability. The bands depicted in Fig. 3 are paralleled to the absorption by colloidal Ag-NPs in the [400–450 nm] region due to the SPR excitation which is good standard for choice of metal NPs ranging from 1 to 100 nm (Njagi et al. 2011; Arunachalam et al. 2012). The SPR spectrum of [GE]-NPs, Ag [TO]-NPs and Ag[E]-NPs is 440 nm, 436 nm and 434 nm, respectively, which indicate that the biosynthesized Ag-NPs have strong SPR, round and spherical in shape (Palithya et al. 2021).
Fig. 3.
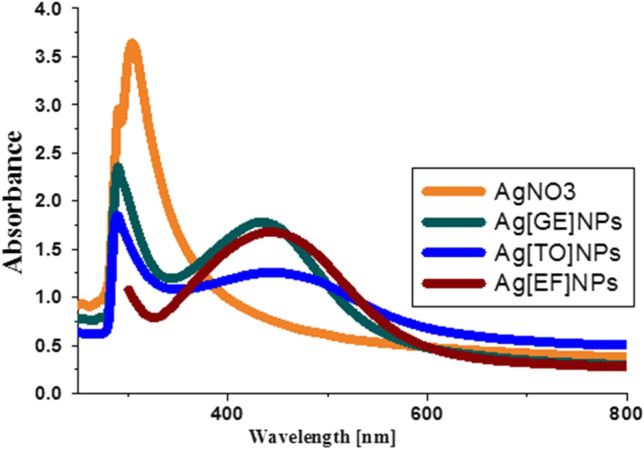
UV–Vis spectra of different forms of Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs
Fourier transform infrared spectroscopy (FTIR)
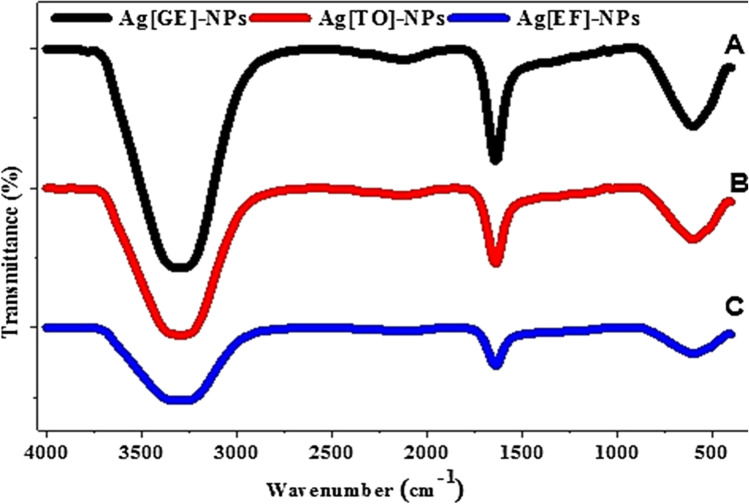
The spectra of FTIR of biogenic Ag-NPs by G. elongata, T. ornata and E. flexuosa demonstrate absorption peaks located in the 500–4000 cm−1 region. Table 1 presents the assignment of the observed bands in the three spectra. These spectra show the intense band at 3280, 3303 and 3285 cm−1 for Ag[GE]-NPs, Ag[TO]-NPs, and Ag[EF]-NPs, respectively, as depicted in Fig. 4, which is ascribed to the strong stretching hydroxyl O–H and H-bond of alcohols and phenols. The bands at 2119, 2132 and 2136 cm−1 for Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs, respectively, correspond to C≡C stretch of alkynes. The bands at 1637, 1636.75 and 1636.7 cm−1 for Ag[GE]-NPs, Ag[TO]-NPs, and Ag[EF]-NPs, respectively, to indicate to the N–H bend of primary amines. The bands at 1095.7 and 1043.7 cm−1 of Ag[GE]-NPs are due to C–N stretch of aliphatic amines. Finally, the bands at 604.2, 602.9 and 599.7 cm−1 for Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs, respectively, are related to the C–Cl stretch of alkyl halides. Also, the variation in the transmittance level suggests that biomolecules of the metal nanoparticles were functionalized with macroalgae extract. Thus, the carbonyl group can bind metal from amino acid residues and proteins, indicating that the proteins will possibly build a layer covering the metal nanoparticles (i.e., capping Ag-NPs) to prevent clusters and thereby stabilize the medium (Shankar et al. 2004; Ragupathi et al. 2012; Jeeva et al. 2014).
Table 1.
FTIR of different forms of macroalgae biosynthesized Ag-NPs
| Ag[GE]-NPs | Ag[TO]-NPs | Ag[EF]-NPs | Bond | Assignment | Reference |
|---|---|---|---|---|---|
| 604.275 | 602.907 | 599.766 | C–Cl stretch | Alkyl halides | Anandalakshmi et al. (2016) |
| 1043.476 | ––––––- | ––––––– | C–OH | Primary strong alcohol | Jeeva et al. (2014) |
| 1095.721 | ––––––- | ––––––– | C–O stretch | Aliphatic amines | Shankar et al. (2004) |
| 1637.730 | 1636.755 | 1636.764 | C = O | Aromatic group | Ragupathi et al. (2012) |
| 2119.551 | 2132.089 | 2136.005 | C≡C Stretch | Alkynes | Devaraj et al. (2013) |
| 3280.078 | 3303.658 | 3285.372 |
O–H stretch, H-bonded |
Alcohols, phenols | Abideen and Sankar (2015) |
| 3780.047 | 3779.339 | 3779.970 | O–H Stretch | Flavonoids and proteins | Anandalakshmi et al. (2016) |
Fig. 4.
FTIR of (A) Ag [GE]-NPs, (B) Ag[TO]-NPs and (C) Ag[EF]-NPs
Gas Chromatography–Mass Spectrometry (GC–MS) analysis of macroalgae crude extract
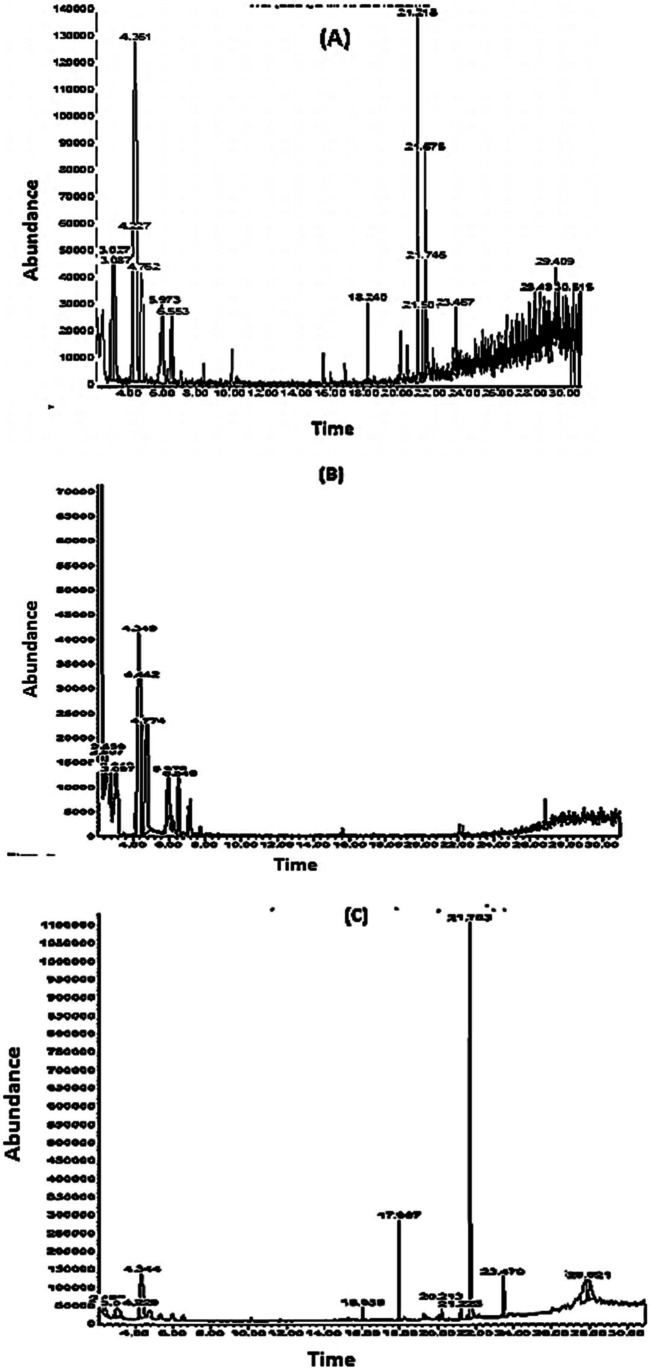
The chemical composition of G. elongata, T. ornata and E. flexuosa extracts was detected by GC–MS analysis. Data show the occurrence of multifarious phytochemicals components. The principal constituent concentrations of more than 1% of the total composition are displayed in Tables (2, 3, and 4) and Fig. 5(A, B, and C). The mass spectra of the compounds were compared with the standard library data bases and were characterized and identified.
Fig. 5.
GC–MS of the crude ethanolic extract of (A) G. elongata, (B) T. ornata and (C) E. flexuosa
Table 2 represents the fourteen bioactive compounds with ratio more than 1% from methanolic extract of G. elongata with their chemical constituents, while Fig. 5A. shows the abundance them with the retention time.
Table 2.
GC–MS analysis of the crude methanolic extract of G. elongata
| No | Retention time | Chemical constituents |
Area % (higher than 1%) |
|---|---|---|---|
| 1 | 3.025 | • Toluene | 8.91 |
| 2 | 3.088 | • Toluene | 7.39 |
| 3 | 4.227 | • Ethylbenzene | 4.87 |
| 4 | 4.359 |
• o-Xylene, • Ethylbenzene • P-xylene |
4.48 |
| 5 | 4.765 |
• Benzene propanoic acid, octyl ester, • Benzyl glycolate, • Carbamic acid, (4-nitrophenyl)-, phenylmethyl ester |
4.765 |
| 6 | 5.972 |
• Diethylmalonic acid, monochloride, phenethyl ester, • Succinic acid, di(2-phenylethyl) ester, • Dimethylmalonic acid, ethyl 2-phenethyl ester |
7.33 |
| 7 | 6.55 |
• p-Toluic acid, 2-phenylethyl ester • Styrene • Thiourea, N,N'-dimethyl |
3.52 |
| 8 | 18.24 |
• Decane, 2,3,7-trimethyl, Heptacosane • Tridecane, 1-iodo |
1.04 |
| 9 | 21.216 |
• Nonanoic acid, methyl ester • Decanoic acid, methyl ester • Hexadecanoic acid, methyl ester |
7.44 |
| 10 | 21.502 |
• 1H-Pyrrolo[3,2,1-i,j]quinolin-2-one,2,4,5,6-tetrahydro-4,4,6-trimethyl-6-phenyl- • 4,5-Diamino-6-[3,4,5-trimethoxyanilino]pyrimidine • 2-(4-Acetylphenylamino)-1,4-naphthoquinone |
1.5 |
| 11 | 21.673 |
• 5–Acetamido-5-propyldecane • 4-Acetamido-4-ethyloctane • Undecanoic acid |
6.73 |
| 12 | 21.748 |
• 5,8-Methano-3H-2-benzopyran-3-one, • 4,4a,5,6,7,8-hexahydro-exo-4-(4-methoxyphenyl)-1-phenyl- • Dipropetryn 2-(1-propenyl)-6-methylphenol |
3.43 |
| 13 | 23.464 |
• Cyclododecanol • 1H-Indene, octahydro-, cis- cis-11-Tetradecen-1-ol |
1.16 |
| 14 | 30.514 |
• 2,5-Dibromobenzoic acid • 7,10-Dimethylbenzo(a)pyrene • Perhydro-htx, 1-acetyl-, acetate(ester) |
1.26 |
Data shown in Table 3 represent the details of the bioactive compounds of T. ornata which has fifteen compounds more than 1%; Fig. 5B reveals the chromatogram of the identified compounds by GC–MS. Additionally, Table 4 shows the only thirteen bioactive compounds more than 1% in GC–MS analysis of E. flexuosa which is depicted in Fig. 5C.
Table 3.
GC–MS analysis of the crude methanolic extract of T. ornata
| No | Retention time | Chemical constituents |
Area % (higher than 1%) |
|---|---|---|---|
| 1 | 2.487 |
• Furan, tetrahydro-2,4-dimethyl-, cis N- Aminopyrrolidine • Silane, ethenyltrimethyl- |
1.48 |
| 2 | 2.544 |
• 2(3H)-Furanone, dihydro-5-methyl- Piperazine • Piperazine • Furan, tetrahydro-2,5-dimethyl-, cis |
1.69 |
| 3 | 2.562 |
• Tetrahydropyran • Tetrahydropyran • Piperazine |
3.1 |
| 4 | 2.796 | • 1,2-Propanedione, 1-phenyl-Pyridine, 2-ethenyl-2-Methyl-3-thiosemicarbazide | 3.45 |
| 5 | 3.019 |
• 1,5-Hexadien-3-yne, 2-methyl- • 1,5-Hexadien-3-yne, 2-methyl- • Toluene |
1.14 |
| 6 | 4.341 |
• Ethylbenzene • o-Xylene • p-Xylene |
34.92 |
| 7 | 4.438 |
• Ethylbenzene • Benzene, 1,3-dimethyl- • p-Xylene |
10.65 |
| 8 | 4.719 |
• Ethylbenzene • Ethylbenzene • Ethylbenzene |
6.02 |
| 9 | 4.747 |
• Ethylbenzene • p-Xylene • 1,6-Heptadiyne |
3.11 |
| 10 | 4.77 |
• 1-Propanol, 2-chloro-Ethylbenzene • Ethylbenzene |
10.17 |
| 11 | 5.972 |
• 2,4-Nonadiyne • Benzene, 1-ethyl-4-methyl- • Benzene, 1-ethyl-4-methyl- |
9.11 |
| 12 | 6.55 |
• 3-Heptadien-5-yne, 2,4-dimethyl- • Benzene, 1,2,3-trimethyl- • Benzene, 1,2,3-trimethyl- |
6.12 |
| 13 | 7.105 |
• 1,5-Dimethyl-2-pyrrolecarbonitrile • 3-Pyridinecarbonitrile, 1,4-dihydro-1-methyl • 1,5-Dimethyl-2-pyrrolecarbonitrile |
2.37 |
| 14 | 7.196 |
• trans-.beta.-Ocimene • (1S)-2,6,6-Trimethylbicyclo[3.1.1] • hept-2-ene • trans-.beta.-Ocimene |
2.36 |
| 15 | 26.84 |
• yrido[3,2-d]pyrimidin-4(3H)-one, 3-hydroxy-2-methyl- 1H-Indole-2-carboxylic acid, 5-chloro-, ethyl ester • (2-Oxo-3,4-dihydro-2H-benzo[1,4]thiazin-3-yl)acetic acid, methyl ester |
1.72 |
Table 4.
GC–MS analysis of the crude methanolic extract of E. flexuosa
| No | Retention time | Chemical constituents |
Area % (higher than 1%) |
|---|---|---|---|
| 1 | 2.453 |
• 1-Benzyl-3-phenyl-1H-1,2,4-triazol-4-oxide • 1-(2-Hydroxyimino-2-phenyl–ethyl)-pyrrolidine-2,5-dione • 1H-Pyrimidine-2,4-dione, 1-benzyloxymethyl-5-bromo-6-methyl- |
3.35 |
| 2 | 3.054 |
• 1,5-Heptadien-3-yne • 10-Phenyldecanoic acid • Benzene, undecyl |
3.39 |
| 3 | 4.227 |
• 3-Benzylthio-5-hydroxy-6,6-dimethy • l-1,6-dihydro-1,2,4-triazine • 1H-1,2,3-Triazole-4,5-dimethanol, 1-(phenylmethyl) • Benzyl isopentyl ether |
1.02 |
| 4 | 4.341 |
• 1,2-Benzisoxazole • 1,5-Heptadien-3-yne • Pyridine, 4-[2-(phenylmethyl)-2H-1,2,3,4-tetrazol-5-yl] |
14.68 |
| 5 | 16.037 |
• 1-Methoxybicyclo[2,2,2]oct-5-en-2-yl methyl ketone • 2,4-Dichloro-N-(4-ethoxy-phenyl)-3-methyl-benzenesulfonamide • 3,6-Dimethyl-6-hepten-4-yn-3-ol |
1.09 |
| 6 | 17.965 |
• 1-Octadecanol • n-Nonadecanol-1 • 1-Hexadecanol |
7.56 |
| 7 | 20.214 | • 2-Pentadecanone, 6,10,14-trimethyl | 1.40 |
| 8 | 21.221 |
• Nonanoic acid, methyl ester • Hexadecanoic acid, 15-methyl-, methyl ester • Decanoic acid, methyl ester |
1.48 |
| 9 | 21.702 |
• Pentadecanoic acid • alpha.-D-Glucopyranose, 4-O-.beta-D-galactopyranosyl- • Tetradecanoic acid |
43.55 |
| 10 | 23.470 | • 6-Nonen-1-ol, acetate, (Z)- 1,1'-Bicyclopropyl, 2,2,2',2'-tetramethyl-1H-Imidazole-4-propanamine | 3.30 |
| 11 | 27.819 |
• Nicotinamide, 5-chloro-2-(4-fluorophenoxy)-4,6-dimethyl-N-phenethyl- • Imidazo[1,2-a]pyridine, 6-bromo-2-thiophen-2-yl- 4-Bromophenoxathiin] |
8.79 |
| 12 | 27.882 |
• 4-Bromophenoxathiin • 3,5-Dibromobenzoic acid • 2-(p-Methoxyphenyl)-4-quinolinecarboxamide |
4.70 |
| 13 | 28.019 | • Imidazo[1,2-a]pyridine, 6-bromo-2- thiophen-2-yl- 1,3-Dithiolo[4,5-c]pyridine-6-carbonitrile, 4,7-dichloro-2-thioxo- 2,4,6,8-Tetrathiatricyclo[3.3.1.1(3,7)]decane-1-carboxylic acid, 3,5 –dimethyl | 5.08 |
Zeta potential analysis for macroalgae biosynthesized Ag-NPs
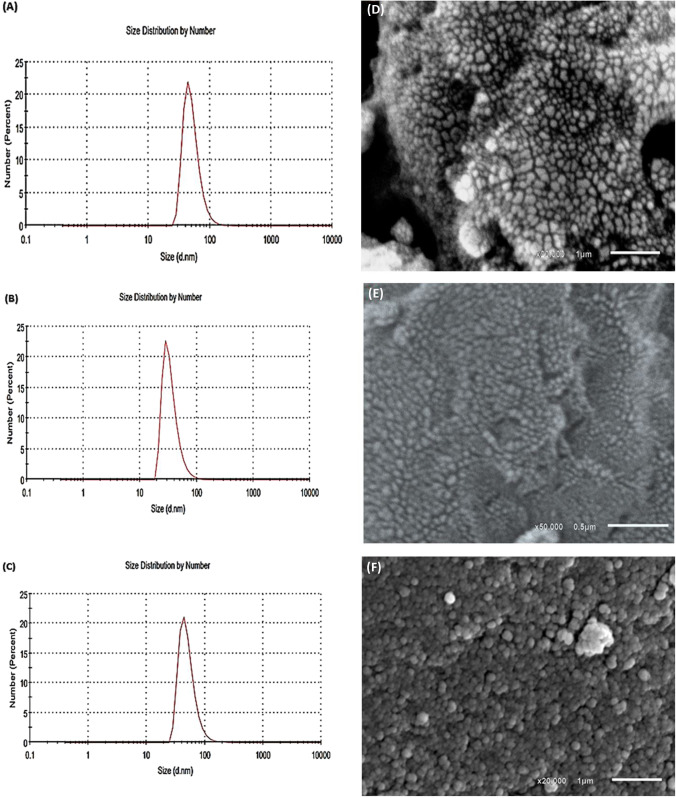
Zeta potentials of macroalgae-based Ag-NPs were assessed by DLS as illustrated in Fig. 6(A, B and C) . The obtained data recommend that zeta potential of macroalgae-based Ag-Nps is stable colloid. Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs have a zeta potential of [-24.4, -20.8 and -18.3] mV, respectively. These high negative potential values support long-term stability and appropriate colloidal nature of Ag-NPs as a result of negative repulsion (Mukherjee et al. 2014).
Fig. 6.
Zeta potentials and SEM images of biosynthesized (A,D) Ag[GE]-NPs, (B,E) Ag[TO]-NPs, and (C,F) Ag[EF]-NPs
Scanning electron microscope.
The image analysis was performed using analytical low vacuum SEM (JEOL, JSM-6490LA, Japan) at 20 kV. Figure 6(D-F) reveals the SEM images of Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs, which show almost spherical NPs with non-uniform size.
The particle size of Ag[GE]-NPs ranges from 30 to 90 nm (Fig. 6D), while Ag[TO]-NPs particle size range from 20 to 60 nm. Comparably, the Ag[EF]-NPs particle size is diverse from 30 to 90 nm with a mean value of 55 nm.
In vitro potentials of marine macroalgae-based Ag-NPs
Antioxidant Activity of macroalgae-based Ag-NPs
Antioxidants are essential in alleviating many syndromes like inflammation, diabetes mellitus, and cancer due to oxidative stress suppression. FTIR and GC–MS analysis confirmed that G. elongata, T. ornata and E. flexuosa are reducing and stabilizing agents for Ag-NPs biosynthesis consisting of phenol, flavonoids, terpenoids which significantly scavenged DPPH and ABTS.
DPPH scavenging activity
The DPPH radicals react with appropriate reducing agents, wherein the electrons are paired off and the solution color disappears stoichiometrically hanging on the number of electrons taken up as explained by Subhasree et al. 2009. Herein, the color solution is increasingly converted from purple to yellow, diphenyl picryl hydrazine, the free radical scavenging was found to be ameliorated with increased macroalgae-based Ag-NPs concentrations.
The DPPH scavenging activity for the tested biogenic NPs exhibited an enhancement in a concentration-dependent manner. The antioxidant potential of macroalgae-based Ag-NPs could be assigned to the presence of flavonoids and phenols originating from macroalgae crude extract as explained in the FTIR analysis. Hence, the decrease in DPPH radical absorbance was because of the radical scavenging by electron supply. As observed in the results of the current study, all treatments including Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs have a significant inhibitory action against the DPPH radical recording of 63.69%, 67.26% and 58.92%, respectively, compared with standard gallic acid 72.96% at 200 µg/mL (Fig. 7A).
Fig. 7.
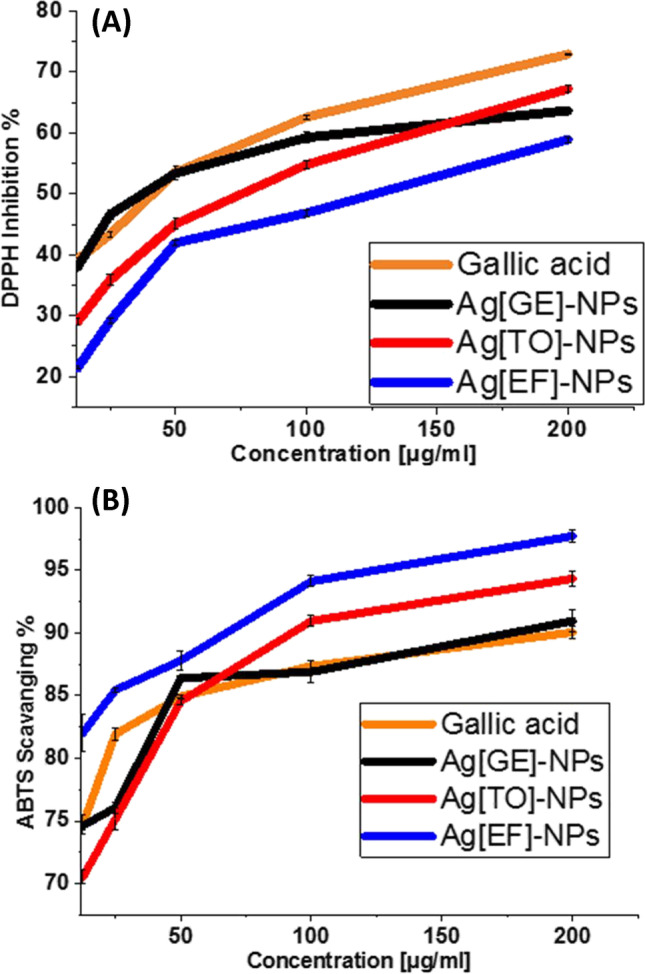
Impacts of Ag[GE]-NPs, Ag[TO]-NPs, Ag[EF]-NPs and gallic acid on [A] DPPH and [B] ABTS radical scavenging. Values are mean ± SE, n = 3, using Duncan’s test p < 0.05
The greater antioxidant activity is signified by the lower IC50 value, shown by the IC50 calculations for gallic acid 82.58 µg/mL which is followed by Ag[GE]-NPs 98.37 µg/mL, Ag[TO]-NPs 98.68 µg/mL, and finally, Ag[EF]-NPs 140.38 µg/mL, These findings are in concurrence with the previous investigations of Popli et al. (2018) and Saratale et al. (2018a).
ABTS scavenging activity
Free radicals are extremely reactive species that are created by cells in immune function and respiration. This reaction is fetal because generates cardiovascular disorders, cancer, atherosclerosis and inflammatory complaints (Chang et al. 2012). The ABTS scavenging activity of macroalgae biosynthesized NPs exhibited an increase in a dose dependent manner. Except for the highest concentration the effect of Ag[GE]-NPs was the most potent and was analogous to the effect of gallic acid. The antioxidant proficiency of 200 μg/mL of Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs shows the highest ability to scavenge ABTS (Fig. 7B).
The IC50 values for Ag[GE]-NPs 48.12 µg/mL, Ag[TO]-NPs 50.63 µg/mL and Ag[EF]-NPs 50.9 µg/mL are near the gallic acid 55.37 µg/mL. This finding is more significant than those conveyed in preceding investigations (Gomaa 2017; Sudha et al. 2017; Saratale et al. 2018a; Salem et al. 2022) which confirm the scavenging potentials of biogenic Ag-NPs.
Anticancer activity against the HepG2 cell line
The anticancer activity of Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs against HepG2 cell line was scrutinized by MTT assay utilizing different doses of macroalgae-based Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs (0.97, 1.9, 3.9, 7.8, 15.6, 31.25, 62.5, 125, 250, 500 and 1000 μg/mL). The results indicate that macroalgae biosynthesized Ag-NPs possess significant anticancer activity affected by time and concentrations as illustrated in Fig. 8. Interestingly, these data displayed the highest concentrations of Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs (1000 µg/mL) exhibited a significant inhibitions of cell growth of HepG2 resulting in cell viabilities of 5.92%, 4.44% and 11.33%, respectively. The results propose that macroalgae biosynthesized NPs are more efficient in a dose reliant manner as it contain functional biological groups which hinder the growth of HepG2. Accordingly, the anticancer effects of Ag-NPs are associated with interactions of NPs and cellular functional proteins which ultimately cause cellular changes (A´valos et al. 2018). Moreover, the IC50 values for all treatment agents seem to be equal as indicated Ag[GE]-NPs 104.15 µg/mL, Ag[TO]-NPs 104.81 µg/mL and Ag[GE]-NPs 104.91 µg/mL.
Fig. 8.
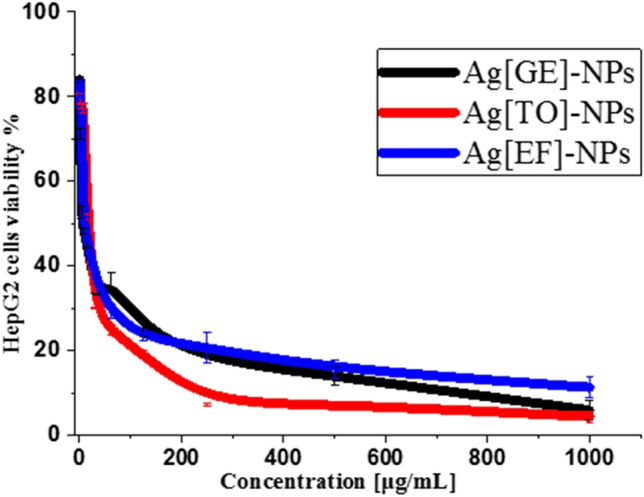
Anticancer activity of different concentrations of Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs against HepG2 cell line. Values are mean ± SE, n = 3, p < 0.001, using Duncan’s test
In vitro α-amylase inhibitory activity
Alpha-amylase is the key enzyme associated with starch breakdown and carbohydrate also liberates sugar into the bloodstream, which is an auxiliary cause of the increase in blood glucose level and eventually diabetes. The current finding reveals that macroalgae-based Ag [GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs powerfully inhibit the α-amylase enzyme. The standard acarbose is complex oligosaccharide that obstructs the carbohydrates digestion. It inhibits the action of pancreatic amylase in the starch breakdown (Narkhede et al. 2011). The reaction mechanisms involved in inhibiting α-amylase by protein inhibitors are not understood. However, some recommendations exist that protein (flavanols) may cause conformational structural changes (Kim et al. 2000). The FTIR and GC–MS analyses of crude extracts of G. elongata, T. ornata and E. flexuosa have shown the presence of flavonoids, phenols, proteins, aliphatic amines and tannins.
Figure 9 illustrates the impacts of macroalgae-based Ag-NPs on enzyme inhibition. These results verify that macroalgae-based Ag-NPs exhibit an inhibitory impact on α-amylase in a dose-dependent way and when Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs dose was 200 μg/mL, the inhibitory action was 64.20%, 67.46% and 67.46%, respectively, while inhibition caused by standard acarbose was 73.25%.
Fig. 9.
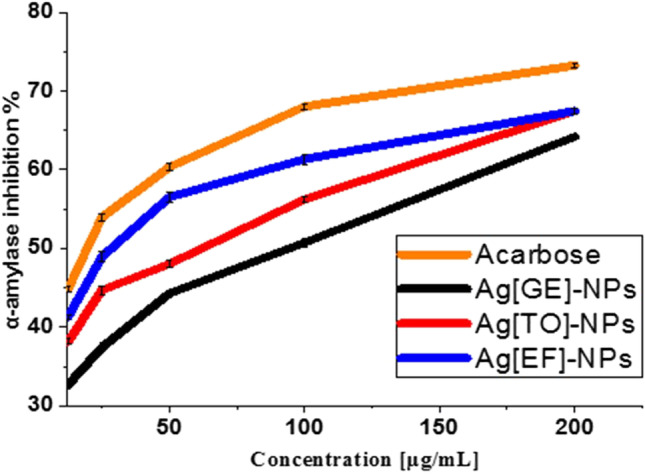
The α-amylase inhibitory activity of Ag[GE]-NPs, Ag[TO]-NPs, Ag[EF]-NPs and acarbose. Values are mean ± SE, n = 3, using Duncan’s test p < 0.05
The α-amylase IC50 value for acarbose was 74.66% µg/mL which is near to Ag[EF]-NPs 87.33 µg/mL, Ag[TO]-NPs 90.0 µg/mL and lastly Ag[GE]-NPs with 102.55 µg/mL; this finding is reliable with those reported in previous studies (Rajaram et al. 2015). Subsequently, it could be elucidated from these results that macroalgae-based Ag-NPs possibly will be utilized as a substitution to acarbose because they have a prospective inhibitory influence on α-amylase and do not have toxic side effects when they are used in an appropriate doses.
Evaluation of in vitro anti-inflammatory activity
Proteinase inhibitory action
Neutrophils are a common brilliant supply of serine proteinase and are concentrated in lysosomes. Leukocytes proteinase formerly shared a vital part in the tissue damage progression for the duration of inflammatory reactions and proteinase inhibitors offered a noteworthy defense. Proteinase inhibitory process is evaluated via the decreased IC50 as given by standard diclofenac sodium 86.82 µg/mL which is close to Ag[GE]-NPs 90.8 µg/mL, Ag[EF]-NPs 125.24 µg/mL and finally 127.01 µg/mL, biosynthesized Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs generate significant anti-inflammatory actions using membrane stabilization and inhibiting protein denaturation in a dose dependent behavior as revealed in Fig. 10. Maximum inhibitions are shown at 59.78%, 44.40%, and 47.38% at 200 μg/mL. Diclofenac sodium displayed the maximum inhibition 64.28% at 200 μg/mL. However, the effects of Ag [GE]-NPs were the most potent and were comparable to the effect of diclofenac sodium. These findings are in harmony with Prabakaran and Mani 2019.
Fig. 10.
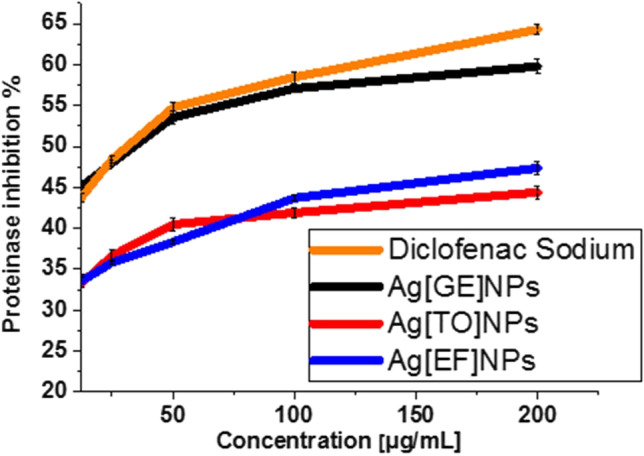
Anti-proteinase action of Ag[GE]-NPs, Ag[TO]-NPs, Ag[EF]-NPs and diclofenac sodium. Values are mean ± SE, n = 3, using Duncan’s test p < 0.05
The inhibitory efficiency of biogenic Ag-NPs on protein denaturation, cyclooxygenase and 5-lipoxygenase indicates that G. elongate, T. ornata or E. flexuosa macroalgae-based Ag-NPs are capable of hindering the creation of prostaglandins and leukotrienes, which assigns the anti-inflammatory properties of macroalgae-based Ag-NPs.
Inhibition of albumin denaturation
Protein denaturation is a mislaying progression of tertiary and secondary protein structures in response to external pressure or composites, (e.g., heating, strong base or acid, a concerted inorganic salt or organic solvents). Furthermore, biotic proteins lose their biological function once denatured.
The albumin denaturation inhibition process is evaluated via the decreased IC50 as given by standard diclofenac sodium 105.41 µg/mL which is higher than Ag[EF]-NPs 126.87 µg/mL, Ag[TO]-NPs 135.27 µg/mL and Ag[GE]-NPs 163.53 µg/mL; the inhibition activity of 200 μg/mL of Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs was 57.46%, 54.8% and 59.46%, respectively, which approaches 200 μg/mL of standard diclofenac sodium which offered 64.28% as depicted in Fig. 11. The current study’s findings illustrate that macroalgae-based Ag-NPs have marked in vitro anti-inflammatory effects on protein denaturation. These findings are more ameliorated than the findings of Mani et al. 2015; Prabakaran and Mani 2019.
Fig. 11.
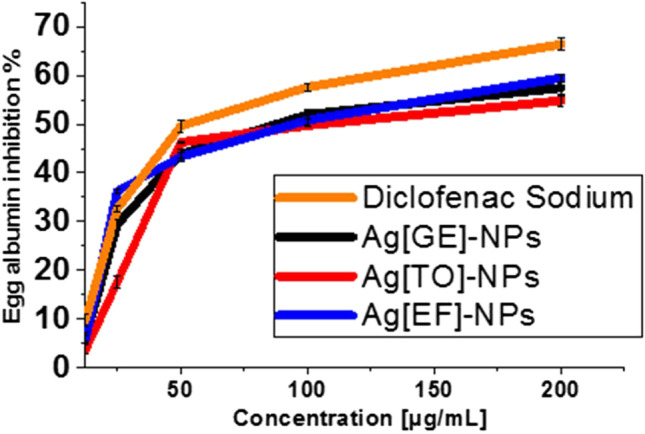
Protein denaturation (fresh egg albumin) activity of Ag[GE]-NPs, Ag[TO]-NPs, Ag[EF]-NPs and diclofenac sodium. Values are mean ± SE, n = 3, using Duncan’s test p < 0.05
Acetyl cholinesterase (AChE) inhibition percentage
Alzheimer’s disease is a developing syndrome that accompanies with AChE deficiency which damages nerve cells in different parts of the brain including memory, learning, language, and reasoning. Thus, the treatment of this disease must have the potency to inhibit AChE. When biogenic Ag-NPs cooperate with AChE proteins, they inhibit AChE proteins and show that NPs have binding affinity to ChE. This interaction may occur because of the lithiophilicity of the NPs and hydrophobicity nature of the enzyme ChE molecule (Wang et al. 2009; Rajakumar et al. 2017; Moawad et al. 2020).
The IC50 values for AChE inhibition indicated that Ag[EF]-NPs is the best with 19.78 µg/mL, while the standard donepezil value is 71.4 µg/mL which is near the values of Ag[TO]-NPs 76.14 µg/mL and Ag[GE]-NPs 71.92 µg/mL. Figure 12 illustrates the inhibitory effects of Ag[GE]-NPs, Ag[TO]-NPs and Ag[EF]-NPs on AChE with the results of 73.75%, 70.53% and 63.46%, respectively, and the reference standard donepezil was of 83.6%. The increase in AChE inhibition was concentration dependent. The effect of Ag [GE]-NPs was the most potent and was comparable to that of donepezil.
Fig. 12.
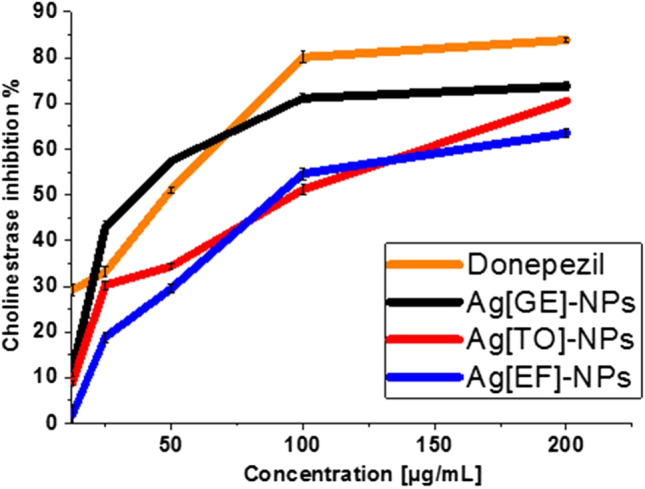
AChE inhibition ratio Ag[GE]-NPs, Ag[TO]-NPs, Ag[EF]-NPs and diclofenac sodium. Values are mean ± SE, n = 3, using Duncan’s test p < 0.05
Conclusion
Based on the obtained results, the marine macroalgae crude extracts of G. elongate, T. ornata or E. flexuosa have been efficiently used as a natural, non-toxic, low-cost, bio-reducing and capping agents for Ag-NPs biosynthesis. The reduction of Ag+ and formation of Ag0 have been verified by UV–Vis spectroscopy and FTIR analyses. The formed Ag-NPs were shaped in sphere having a crystalline nature with size ranging from 20 to 25 nm. Moreover, our findings powerfully advocate that the G. elongate, T. ornata or E. flexuosa biogenic Ag-NPs have many potent in vitro biological activities including antioxidant, anticancer, anti-inflammatory, anti-diabetic and anti-Alzheimer. These biogenic Ag-NPs may be used as medication in near future. However, further in vivo studies in animals and clinical studies are required to ensure their safeties and efficacies before their approval for treatment of diseases in human beings. One limitation for the clinical use of these innovative macroalgae bio-capped Ag-NPs as antioxidant, anticancer, anti-inflammatory, anti-diabetic and anti-Alzheimer is that most studies supporting their efficiency are performed in vitro or in vivo on animal models. Analogously, there are concerns regarding the possible side effects associated with their use in medical fields on human beings. Addressing these productions may help apply these medications in the future in clinical.
Author Contributions
M.N.A., O.M.A., K.N.M.E. and M.S. contributed to the conceptualization; M.N.A. and O.M.A. contributed to the approaches; M.N.A. and O.M.A helped in the assessment; M.N.A., O.M.A., K.N.M.E. and M.S. contributed to the resources; M.N.A. was involved in writing—original draft preparation; O.M.A., K.N.M.E. and M.S. contributed to writing—review and editing.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data Availability Statement
Supporting data presented in this paper are available on request from the corresponding author.
Declarations
Ethical Approval
All animal approaches are in harmony with the standard guidelines of the Experimental Animal Ethics Committee of Faculty of Science, Beni-Suef University, Egypt (Ethical Approval number: BSU/FS/020–99).
Consent to Participate
We understand that all information we provide for this study will be treated confidentially.
Consent to Publish
All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s11356-024-34308-4
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/9/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s11356-024-34308-4
References
- A´valos A, Morales P, Haza AI (2018) Manufactured silver and gold nanoparticles-induced apoptosis by caspase-pathway in human cell lines. Toxicol Environ Chem 100:629–643
- Abdel Azeem MN, Hassaballa S, Ahmed OM, Elsayed KN, Shaban M. Photocatalytic Activity of Revolutionary Galaxaura elongata, Turbinaria ornata, and Enteromorpha flexuosa’s Bio-Capped Silver Nanoparticles for Industrial Wastewater Treatment. Nanomaterials. 2021;11(12):3241. doi: 10.3390/nano11123241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-Ellatef GF, Ahmed OM, Abdel-Reheim ES, Abdel-Hamid AZ (2017) Ulva lactuca polysaccharides prevent Wistar rat breast carcinogenesis through the augmentation of apoptosis, enhancement of antioxidant defense system, and suppression of inflammation. Breast Cancer - Targets and Therapy 9:67–83 [DOI] [PMC free article] [PubMed]
- Abdelrheem DA, Rahman AA, Elsayed KN, Abd El-Mageed HR, Mohamed HS, Ahmed SA (2021) Isolation, characterization, in vitro anticancer activity, dft calculations, molecular docking, bioactivity score, drug-likeness and admet studies of eight phytoconstituents from brown alga Sargassum platycarpum. J Mol Struct 1225:129245
- Abideen S, Sankar M (2015) In-vitro screening of antidiabetic and antimicrobial activity against green synthesized AgNO3 using seaweeds. J Nanomed Nanotechnol S6–001:10.2157–7439
- Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry, vol 456. Allured Publishing Corporation, Carol Stream, IL
- Ahmed OM (2010a) Antihyperglycemic effects of water extract of Ulva lactuca and its polysaccharides in nicotinamide-streptozotocin-induced diabetic rats. Egypt J Zoo 54:273–297
- Ahmed OM (2010b) Anti-hyperlipidemic, antioxidant and cardiac improving effects of water extract of Ulva lactuca and its polysaccharides in nicotinamide-streptozotocin-induced diabetic rats. Egypt J Zoo 54:253–272
- Ahmed OM, Ahmed RR (2014) Anti-proliferative and apoptotic efficacies of ulvan polysaccharides against different types of carcinoma cells in vitro and in vivo. J Cancer Sci Ther 6(6):202–208
- Ahmed OM, Ashour MB, Fahim EF, Mahmoud AM, Ahmed NA (2014) Preventive effect of Spirulina versicolor and Enteromorpha flexuosa ethanolic extracts against diethylnitrosamine/Benzo(A)Pyrene-Induced hapatocarcinogencity in Rats. Int J Multidiscip Acad Res 2(6):633–650
- Ahmed OM, Fahim HE, Ahmed RR, Khedr ME, Mekhaeed TH, Abou Seif SH (2008) Protective effects of Ulva lactuca against acetaminophen-induced kidney injury. Journal of Egyptian German Society of Zoology 56A:281–306
- Ahmed OM, Hassan MA, Abdel-Twab SM, Azeem MNA (2017a) Navel orange peel hydroethanolic extract, naringin and naringenin have anti-diabetic potentials in type 2 diabetic rats. Biomed Pharmacother 94:197–205 [DOI] [PubMed]
- Ahmed OM, Soliman HA, Mahmoud B, Gheryany RR (2017b) Ulva lactuca hydroethanolic extract suppresses experimental arthritis via its anti-inflammatory and antioxidant activities. Beni-Suef Univ J Basic Appl Sci 6(4):394–408
- Ahmed SA, Rahman AA, Elsayed KN, Abd El-Mageed HR, Mohames HS, & Ahmed SA (2020). Cytotoxic activity, molecular docking, pharmacokinetic properties and quantum mechanics calculations of the brown macroalga Cystoseira trinodis compounds. J Biomol Struct Dy 1–31 [DOI] [PubMed]
- Ames BN, Gold LS (1992) Animal cancer tests and cancer prevention. J Natl Cancer Inst Monogr 12:125–132 [PubMed]
- Anandalakshmi K, Venugobal J, Ramasamy V (2016) Characterization of Silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl Nanosci 6(3):399–440
- Arunachalam R, Dhanasingh S, Kalimuthu B, Uthirappan M, Rose C, Mandal AB (2012) Phytosynthesis of silver nanoparticles using Coccinia grandis leaf extract and its application in the photocatalytic degradation. Colloids Surf B 94:226–230 [DOI] [PubMed]
- Badawi AK, Zaher K (2021) Hybrid treatment system for real textile wastewater remediation based on coagulation/flocculation, adsorption and filtration processes: performance and economic evaluation. J Water Process Eng 40:101963
- Badawi AK, Abd Elkodous M, Ali GA (2021a) Recent advances in dye and metal ion removal using efficient adsorbents and novel nano-based materials: an overview. RSC Adv 11(58):36528–36553 [DOI] [PMC free article] [PubMed]
- Badawi AK, Bakhoum ES, Zaher K. Sustainable evaluation of using nanozero-valent iron and activated carbon for real textile effluent remediation. Arab J Sci Eng. 2021;46(11):10365–10380. doi: 10.1007/s13369-021-05349-5. [DOI] [Google Scholar]
- Badawi AK, Ismail B, Baaloudj O, Abdalla KZ. Advanced wastewater treatment process using algal photo-bioreactor associated with dissolved-air flotation system: A pilot-scale demonstration. J Water Process Eng. 2022;46:102565. doi: 10.1016/j.jwpe.2022.102565. [DOI] [Google Scholar]
- Barani M, Rahdar A, Sargazi S, Amiri MS, Sharma PK, Bhalla N. Nanotechnology for inflammatory bowel disease management: Detection, imaging and treatment. Sens Bio-Sensing Res. 2021;32:100417. doi: 10.1016/j.sbsr.2021.100417. [DOI] [Google Scholar]
- Bhutkar MA, Bhise SB. In-vitro assay of alpha amylase inhibitory activity of some indigenous plants. Int J Chem Sci. 2012;10(1):457–462. [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset CLWT. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chang CL, Lin CS, La GH. Phytochemical characteristics, free radical scavenging activities, and neuroprotection of five medicinal plant extracts. Evid based Complement Alternat Med. 2012;8:984295. doi: 10.1155/2012/984295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman VJ, Chapman DJ (1980) Seaweeds and their uses. Third edition. London, New York, pp.1, 30, 64, 96, 234–237
- Dawadi S, Katuwal S, Gupta A, Lamichhane U, Thapa R, Jaisi S, ..., Parajuli N (2021) Current research on silver nanoparticles: synthesis, characterization, and applications. J Nanomater 2021 [DOI] [PMC free article] [PubMed]
- Deepak P, Sowmiya R, Ramkumar R, Balasubramani G, Aiswary D, Perumal P (2017) Structural characterization and evaluation of mosquito-larvicidal property of silver nanoparticles synthesized from the seaweed, Turbinaria ornata (Turner). Journal of Agardh 1848 Artificial Cells, Nanomedicine, and Biotechnology 45(5):990–998 [DOI] [PubMed]
- Devaraj P, Kumari P, Aarti C, Renganathan A (2013) Synthesis and characterization of silver nanoparticles using cannonball leaves and their cytotoxic activity against MCF-7 cell line. J Nanotechnol 2013
- Elias G, Rao MN. Inhibition of albumin denaturation and anti-inflammatory activity of dehydrozingerone and its analogs. Indian J Exp Biol. 1988;26(10):540–542. [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres JV, Featherstone RM. A new and rapid colorimetric determination of acetyl cholinesterase activity. Biochem Pharmacol. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Es-Haghi A, Taghavizadeh Yazdi ME, Sharifalhoseini M, Baghani M, Yousefi E, Rahdar A, Baino F. Application of response surface methodology for optimizing the therapeutic activity of ZnO nanoparticles biosynthesized from Aspergillus niger. Biomimetics. 2021;6(2):34. doi: 10.3390/biomimetics6020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim HE, Ahmed OM, Ahmed RR, Khedr ME, Mekhaeel TH, Abou Seif SH. Protective effects of Ulva lactuca against acetaminophen-induced liver injury. J Egypt German Soc Zool. 2008;56A:377–415. [Google Scholar]
- Fathy W, Elsayed K, Essawy E, Tawfik E, Zaki A, Abdelhameed MS, Hammouda O. Biosynthesis of Silver Nanoparticles from Synechocystis sp to be Used as a Flocculant Agent with Different Microalgae Strains. Curr Nanomater. 2020;5:00–00. doi: 10.2174/2468187310999200605161200. [DOI] [Google Scholar]
- Gomaa EZ. Antimicrobial, antioxidant and antitumor activities of silver nanoparticles synthesized by Allium cepa extract: a green approach. J Genet Eng Biotechnol. 2017;15(1):49–57. doi: 10.1016/j.jgeb.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton KZ, Kensler TW. Oxidative mechanisms in carcinogenesis. Br Med Bull. 1993;49(3):523–544. doi: 10.1093/oxfordjournals.bmb.a072628. [DOI] [PubMed] [Google Scholar]
- Hashemzadeh MR, Yazdi MET, Amiri MS, Mousavi SH. Stem cell therapy in the heart: biomaterials as a key route. Tissue and Cell. 2021;71:101504. doi: 10.1016/j.tice.2021.101504. [DOI] [PubMed] [Google Scholar]
- Hassanisaadi M, Barani M, Rahdar A, Heidary M, Thysiadou A, Kyzas GZ (2022) Role of agrochemical-based nanomaterials in plants: biotic and abiotic stress with germination improvement of seeds. Plant Growth Regulation 1–44.
- Hassanisaadi M, Bonjar GHS, Rahdar A, Pandey S, Hosseinipour A, Abdolshahi R. Environmentally safe biosynthesis of gold nanoparticles using plant water extracts. Nanomaterials. 2021;11(8):2033. doi: 10.3390/nano11082033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javad Farhangi M, Es-Haghi A, Taghavizadeh Yazdi ME, Rahda A, Baino F. MOF-Mediated Synthesis of CuO/CeO2 Composite Nanoparticles: Characterization and Estimation of the Cellular Toxicity against Breast Cancer Cell Line (MCF-7) J Funct Biomater. 2021;12(4):53. doi: 10.3390/jfb12040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeeva K, Thiyagarajan M, Langovan V, Geetha N, Venkatachalam P. Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind Crops Prod. 2014;52:714–720. doi: 10.1016/j.indcrop.2013.11.037. [DOI] [Google Scholar]
- Kim JS, Kwon CS, Son KH. Inhibition of α-gluosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem. 2000;64(11):2458–2461. doi: 10.1271/bbb.64.2458. [DOI] [PubMed] [Google Scholar]
- Kumar B, Kumari S, Rachid S, Karen B, Marcelo G, Luis C. In vitro evaluation of silver nanoparticles cytotoxicity on hepatic cancer (Hep-G2) cell line and their antioxidant activity: green approach for fabrication and application. J Photochem Photobiol B. 2016;159:8–13. doi: 10.1016/j.jphotobiol.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Lomartire S, Gonçalves AM. An Overview of potential seaweed-derived bioactive compounds for pharmaceutical applications. Mar Drugs. 2022;20(2):141. doi: 10.3390/md20020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madkour LH (2020) Antioxidant therapeutic defenses toward redox biology and oxidative stress. in nanoparticles induce oxidative and endoplasmic reticulum stresses (pp. 557–629). Springer, Cham.
- Mahmoud AM, Ahmed OM, Mohamed IB, Soliman HA, Mohamed BM (2021) The preventive effects and mode of actions of Ulva fasciata synthesized silver nanoparticles in doxorubicin-induced hepatotoxicity in Wistar rats. J Pharm Res Int 33(24A), 24–48; Article no.JPRI.67485
- Mahmoud AM, Mohamed BM, Ibraheem IBM, Soliman HA, Ahmed OM. Characterization of Ulva fasciata ethanolic extract-mediated biosynthesized silver nanoparticles and evaluation of their nephrocadiopreventive effects in doxorubicin-injected Wistar rats. Adv Anim Vet Sci. 2020;8(s2):98–111. doi: 10.17582/journal.aavs/2020/8.s2.98.111. [DOI] [Google Scholar]
- Mahmudin L, Suharyadi E, Utomo ABS, Abraha K (2015) Optical properties of silver nanoparticles for surface plasmon resonance (SPR)-based biosensor applications. J Modern Phys 6
- Mani AK, Seethalakshmi S, Gopal V. Evaluation of in-vitro anti-inflammatory activity of silver nanoparticles synthesized using piper nigrum extract. J Nanomed Nanotechnol. 2015;6(2):1. [Google Scholar]
- Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12(1):5–18. doi: 10.12816/0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta M, Adem A, Sabbagh M. New acetyl cholinesterase inhibitors for Alzheimer’s disease. Int J Alzheimer’s Dis. 2012;2012:728983. doi: 10.1155/2012/728983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad A, Abuzaid H, Arafa WM, Ahmed O, Hetta M, Mohammed R (2020) Anticholinesterase and acaricidal activities of steroids isolated from Trianthema portulacastrum L. against Rhipicephalus annulatus tick. J Appl Pharm Sci 10(04):047–055
- Mohammadzadeh V, Barani M, Amiri MS, Yazdi MET, Hassanisaadi M, Rahdar A, Varma RS (2022) Applications of plant-based nanoparticles in nanomedicine: A review. Sustain Chem Pharm 25:100606
- Mohamed MH, Zaki AH, Abdel-Raouf N, Alsamhary KI, Fathy WA, Abdelhameed MS, & Elsayed KNM (2022). Flocculation of microalgae using calcium oxide nanoparticles; process optimization and characterization. Int Aquat Res. 10.22034/IAR.2022.1943339.1206.
- Mukherjee S, Chowdhury D, Kotcherlakota R, Patra S, Vinothkumar B, Bhadra MB, et al. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system) Theranostics. 2014;4:316–335. doi: 10.7150/thno.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkhede MB, Ajimire PV, Wagh AE, Mohan M, Shivashanmugam AT. In-vitro antidiabetic activity of Caesalpina digyna (R.) methanol root extract. J Plant Sci Res. 2011;1(2):101–106. [Google Scholar]
- Njagi EC, Huang H, Stafford L, Genuino H, Galindo HM, Collins JB, Hoag GE, Suib SL. Biosynthesis of iron and silver nanoparticles at room temperature using aqueous Sorghum bran extracts. Langmuir. 2011;27:264–271. doi: 10.1021/la103190n. [DOI] [PubMed] [Google Scholar]
- Oyedapo OO, Famurewa AJ. Antiprotease and membrane stabilizing activities of extracts of Fagara zanthoxyloides, Olax subscorpioides and Tetrapleura tetraptera. Int J Pharmacogn. 1995;33(1):65–69. doi: 10.3109/13880209509088150. [DOI] [Google Scholar]
- Pádua MD, Fontoura PSG, Mathias AL. Chemical composition of Ulvaria oxysperma (Kützing) bliding, Ulva lactuca (Linnaeus) and Ulva fascita (Delile) Braz Arch Biol Technol. 2004;47(1):49–55. doi: 10.1590/S1516-89132004000100007. [DOI] [Google Scholar]
- Palithya S, Gaddam SA, Kotakadi VS, Penchalaneni J, Challagundla VN. Biosynthesis of silver nanoparticles using leaf extract of Decaschistia crotonifolia and its antibacterial, antioxidant, and catalytic applications. Green Chem Lett Rev. 2021;14(1):137–152. doi: 10.1080/17518253.2021.1876172. [DOI] [Google Scholar]
- Perry SW, Norman HP, Barbieri J, Browwn EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Bio-Techniques. 2011;50(2):98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phull AR, Abbas Q, Ali A, Raza H, Zia M, Haq IU. Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata. Future J Pharm Sci. 2016;2(1):31–36. doi: 10.1016/j.fjps.2016.03.001. [DOI] [Google Scholar]
- Pillai AM, Sivasankarapillai VS, Rahdar A, Joseph J, Sadeghfar F, Rajesh K, Kyzas GZ. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J Mol Struct. 2020;1211:128107. doi: 10.1016/j.molstruc.2020.128107. [DOI] [Google Scholar]
- Popli D, Anil V, Subramanyam ABMNNVRR, Rao SN, ..., Govindappa M (2018) Endophyte fungi, Cladosporium species-mediated synthesis of silver nanoparticles possessing in-vitro antioxidant, anti-diabetic and anti-Alzheimer activity. Artificial Cells, Nanomedicine, and Biotechnology, 46(sup1), 676-683 [DOI] [PubMed]
- Povova J, Ambroz P, Bar M, Pavukova V, Sery O, Tomaskova H, Janout V. Epidemiological of and risk factors for Alzheimer's disease: a review. Biomedical Paper Medicine Faculty, University of Palacky Olomouc Czech Republic. 2012;156(2):108–114. doi: 10.5507/bp.2012.055. [DOI] [PubMed] [Google Scholar]
- Prabakaran AS, Mani N. Anti-inflammatory activity of silver nanoparticles synthesized from Eichhornia crassipes: An in-vitro study. J Pharmacogn Phytochem. 2019;8(4):2556–2558. [Google Scholar]
- Ragupathi Raja Kannan R, Arumugam R, Ramya D, Manivannan K, Anantharaman P. Green synthesis of silver nanoparticles using marine macroalga Chaetomorphalinum. Appl Nanosci. 2012;3:229–233. doi: 10.1007/s13204-012-0125-5. [DOI] [Google Scholar]
- Rahdar A, Hasanein P, Bilal M, Beyzaei H, Kyzas GZ. Quercetin-loaded F127 nanomicelles: Antioxidant activity and protection against renal injury induced by gentamicin in rats. Life Sci. 2021;276:119420. doi: 10.1016/j.lfs.2021.119420. [DOI] [PubMed] [Google Scholar]
- Rajakumar G, Gomathi T, Thiruvengadam M, Rajeswari VD, Kalpana VN, Chung IM. Evaluation of anti-cholinesterase, antibacterial and cytotoxic activities of green synthesized silver nanoparticles using from Millettia pinnata flower extract. Microb Pathog. 2017;103:123–128. doi: 10.1016/j.micpath.2016.12.019. [DOI] [PubMed] [Google Scholar]
- Rajaram K, Aiswarya DC, Sureshkumar P. Green synthesis of silver nanoparticle using Tephrosia tinctoria and its antidiabetic activity. Mater Lett. 2015;138:251–254. doi: 10.1016/j.matlet.2014.10.017. [DOI] [Google Scholar]
- Rajendran P, Ammar RB, Al-Saeedi FJ, Mohamed ME, ElNaggar MA, Al-Ramadan SY..., Soliman AM Kaempferol Inhibits Zearalenone-Induced Oxidative Stress and Apoptosis via the PI3K/Akt-Mediated Nrf2 Signaling Pathway: In vitro and in vivo studies. Int J Mol Sci. 2021;22(1):217. doi: 10.3390/ijms22010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman S, Kandula MP, Jacob JA, Soundararajan K, Ramar T, Palani G, Muthukalingan K, Shanmugam A. Cytotoxic effect of green synthesized silver nanoparticles using Melia azedarach against in-vitro HeLa cell lines and lymphoma mice model. Process Biochem. 2012;47:273–279. doi: 10.1016/j.procbio.2011.11.003. [DOI] [Google Scholar]
- Ramkumar VS, Pugazhendhi A, Kumar G, Sivagurunathan P, Saratale GD, Dung TNB, Kannapiran E. Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnology Reports. 2017;14:1–7. doi: 10.1016/j.btre.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao B, Boominathan M. Antibacterial activity of silver nanoparticles of seaweeds. American Journal of Advanced Drug Delivery. 2015;3:296–307. [Google Scholar]
- Rao NH, Lakshmidevi N, Pammi SVN, Kollu P, Ganapaty S, Lakshmi P. Green synthesis of silver nanoparticles using methanolic root extracts of Diospyros paniculataand their antimicrobial activities. Material Science Engineering C. 2016;62:553–557. doi: 10.1016/j.msec.2016.01.072. [DOI] [PubMed] [Google Scholar]
- Robert EL (1989) ״ Phycology ״ second Edition. Cambridge University press, PP. 3, 41, 115
- Rosenzweig N, Dvir-Szternfeld R, Tsitsou-Kampeli A, Keren-Shaul H, Ben-Yehuda H, Weill-Raynal P, ..., Weiner A (2019) PD-1/PD-L1 checkpoint blockade harnesses monocyte-derived macrophages to combat cognitive impairment in a tauopathy mouse model. Nat Commun 10(1), 1-15 [DOI] [PMC free article] [PubMed]
- Sakat S, Juvekar AR, Gambhire MN. In-vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int J Pharma Pharmacol Sci. 2010;2(1):146–155. [Google Scholar]
- Salem SS, Ali OM, Reyad AM, Abd-Elsalam KA, Hashem AH. Pseudomonas indica-Mediated Silver Nanoparticles: Antifungal and Antioxidant Biogenic Tool for Suppressing Mucormycosis Fungi. Hournal of Fungi. 2022;8(2):126. doi: 10.3390/jof8020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saratale RG, Benelli G, Kumar G, Kim DS, Saratale GD. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ Sci Pollut Res. 2018;25(11):10392–10406. doi: 10.1007/s11356-017-9581-5. [DOI] [PubMed] [Google Scholar]
- Saratale RG, Shin HS, Kumar G, Benelli G, Ghodake GS, Jiang YY, Kim DS, Saratale GD. Exploiting fruit byproducts for eco-friendly nanosynthesis: Citrus clementinapeel extract mediated fabrication of silver nanoparticles with high efficacy against microbial pathogens and rat glial tumor C6 cells. Environ Sci Pollut Res. 2018;25(11):10250–10263. doi: 10.1007/s11356-017-8724-z. [DOI] [PubMed] [Google Scholar]
- Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275(2):496–502. doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Sousa A, Ferreira IC, Barros L, Bento A, Pereira JA. Effect of solvent and extraction temperatures on the antioxidant potential of traditional stoned table olives “alcaparras”. LWT-Food Science and Technology. 2008;41(4):739–745. doi: 10.1016/j.lwt.2007.04.003. [DOI] [Google Scholar]
- Sriranjani R, Srinithya B, Vellingiri V, Brindha P, Anthony SP, Sivasubramanian A, Muthuraman MS. Silver nanoparticle synthesis using Clerodendrum phlomidis leaf extract and preliminary investigation of its antioxidant and anticancer activities. J Mol Liq. 2016;220:926–930. doi: 10.1016/j.molliq.2016.05.042. [DOI] [Google Scholar]
- Subhasree B, Baskar R, Keerthana RL, Susan RL, Rajasekaran P. Evaluation of antioxidant potential in selected green leafy vegetables. Food Chem. 2009;115(4):1213–1220. doi: 10.1016/j.foodchem.2009.01.029. [DOI] [Google Scholar]
- Sudha A, Jeyakanthan J, Srinivasan P. Green synthesis of silver nanoparticles using Lippia nodiflora aerial extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Resource-Efficient Technologies. 2017;3(4):506–515. doi: 10.1016/j.reffit.2017.07.002. [DOI] [Google Scholar]
- Suganthy N, Pandima Devi K. In-vitro antioxidant and anti-cholinesterase activities of Rhizophora mucronata. Pharm Biol. 2016;54(1):118–129. doi: 10.3109/13880209.2015.1017886. [DOI] [PubMed] [Google Scholar]
- Velmurugan P, Jaehong S, Kim K, Oh BT. Prunus yedoensis tree gum mediated synthesis of platinum nanoparticles with antifungal activity against phytopathogens. Mater Lett. 2016;174:61–65. doi: 10.1016/j.matlet.2016.03.069. [DOI] [Google Scholar]
- Wang Z, Zhao J, Li F, Gao D, Xing B. Adsorption and inhibition of acetyl cholinesterase by different nanoparticles. Chemosphere. 2009;77(1):67–73. doi: 10.1016/j.chemosphere.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Watkins R, Wu L, Zhang C, Davis RM, Xu B. Natural product-based nanomedicine: recent advances and issues. Int J Nanomed. 2015;10:6055. doi: 10.2147/IJN.S92162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadi M, Rahimi Z, Ghafori V. The green synthesis, characterization and antimicrobial activities of silver nanoparticles synthesized from green alga Enteromorpha flexuosa (wulfen) J Agardh Mater Lett. 2014;137:1–4. doi: 10.1016/j.matlet.2014.08.110. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supporting data presented in this paper are available on request from the corresponding author.