Abstract
The β-glucoside cellobiose has been reported to specifically repress the PrfA-dependent virulence genes hly and plcA in Listeria monocytogenes NCTC 7973. This led to the hypothesis that β-glucosides, sugars of plant origin, may act as signal molecules, preventing the expression of virulence genes if L. monocytogenes is living in its natural habitat (soil). In three other laboratory strains (EGD, L028, and 10403S), however, the effect of cellobiose was not unique, and all fermentable carbohydrates repressed hly. This suggested that the downregulation of virulence genes by β-glucosides is not a specific phenomenon but, rather, an aspect of a global regulatory mechanism of catabolite repression (CR). We assessed the effect of carbohydrates on virulence gene expression in a panel of wild-type isolates of L. monocytogenes by using the PrfA-dependent phospholipase C gene plcB as a reporter. Utilization of any fermentable sugar caused plcB repression in wild-type L. monocytogenes. However, an EGD variant was identified in which, as in NCTC 7973, plcB was only repressed by β-glucosides. Thus, the regulation of L. monocytogenes virulence genes by sugars appears to be mediated by two separate mechanisms, one presumably involving a CR pathway and another specifically responding to β-glucosides. We have identified in L. monocytogenes a 4-kb operon, bvrABC, encoding an antiterminator of the BglG family (bvrA), a β-glucoside-specific enzyme II permease component of the phosphoenolpyruvate-sugar phosphotransferase system (bvrB), and a putative ADP-ribosylglycohydrolase (bvrC). Low-stringency Southern blots showed that this locus is absent from other Listeria spp. Transcription of bvrB was induced by cellobiose and salicin but not by arbutin. Disruption of the bvr operon by replacing part of bvrAB with an interposon abolished the repression by cellobiose and salicin but not that by arbutin. Our data indicate that the bvr locus encodes a β-glucoside-specific sensor that mediates virulence gene repression upon detection of cellobiose and salicin. Bvr is the first sensory system found in L. monocytogenes that is involved in environmental regulation of virulence genes.
The gram-positive, facultative intracellular bacterium Listeria monocytogenes causes listeriosis, a severe, often fatal infectious disease of humans and animals. L. monocytogenes is an opportunistic pathogen that affects predominantly debilitated individuals with a defective cell-mediated immune response. Its natural habitat is soil, from which it reaches the vertebrate host via the food chain (15, 45). As in many other bacterial pathogens that can live saprophytically in inanimate environments, virulence gene expression is tightly regulated in L. monocytogenes. Almost all known listerial virulence determinants are coordinately expressed under the positive control of the PrfA protein, a transcription factor structurally and functionally related to cyclic AMP receptor protein (CRP) (6, 24, 25, 51, 54). A model has been proposed for the regulatory mechanism of PrfA in which, as for CRP, the protein becomes transcriptionally active via an allosteric conformational transition brought about by a putative low-molecular-weight cofactor (41, 54). There is evidence that the PrfA regulon is subject to complex environmental control in L. monocytogenes. Virulence genes are expressed at 37°C (i.e., the body temperature of warm-blooded animals) but not at 26°C or below (i.e., environmental temperatures) (10, 27, 41), suggesting that temperature sensing may enable L. monocytogenes to detect its transition from the free environment to the animal host. However, an increase in temperature is not sufficient for the full activation of the PrfA regulon (40–42). L. monocytogenes produces little or no virulence factors in rich medium (e.g., brain heart infusion [BHI]) at 37°C, but a clear induction of virulence genes takes place at this temperature if BHI is either supplemented with the absorbent activated charcoal (40–43) or replaced by minimal essential medium (4). Clearly, therefore, chemical components of the extracellular environment also play a critical role in regulating the expression of the PrfA regulon in L. monocytogenes.
Park and Kroll reported in 1993 (35) that the presence in the growth medium of the β-glucoside cellobiose, but not of other common fermentable carbohydrates, strongly repressed the expression of two genes of the PrfA virulence regulon (hly, encoding the hemolysin, and plcA, encoding a phosphatidylinositol-specific phospholipase C) in L. monocytogenes NCTC 7973. Another β-glucoside, arbutin, was shown to have a similar effect in the same strain (34). In soil, L. monocytogenes is thought to utilize decaying vegetation as its primary growth substrate. This is perhaps most clearly illustrated by silage, a fermented vegetable fodder which, if incorrectly made, supports the growth of high numbers of L. monocytogenes bacteria and causes listeriosis outbreaks in domestic ruminants (52). Both cellobiose and arbutin are unique to the plant kingdom and are presumably abundant in decaying vegetation, so Park and Kroll reasoned that these plant-derived β-glucosides may act as specific “signature molecules,” allowing L. monocytogenes bacteria to sense that they are present in a soil environment and, consequently, to repress their virulence genes (35). This notion was recently refuted by Milenbachs et al. (32), who found that in L. monocytogenes strains other than NCTC 7973 not only cellobiose, but several other readily metabolized sugars, such as glucose or fructose, caused virulence gene downregulation. They observed that sugar-mediated inhibition of virulence gene expression only occurred in the presence of amounts of carbohydrates sufficient to significantly stimulate growth, indicating that the repression of virulence genes by sugar metabolism in L. monocytogenes might result from a general catabolite repression (CR) response (32). This view has been challenged by a recent report from the same group (3), showing that an L. monocytogenes homolog of the transcription factor CcpA, an important mediator of CR in low G+C content gram-positive bacteria (21, 46), appears not to be involved in the carbon source regulation of virulence genes. Nevertheless, CR in gram-positive bacteria is still poorly understood, and there are other possible CR mediators and pathways that may account for the repression of virulence genes in L. monocytogenes.
In this study we present evidence that reconciles the two hypotheses: that of Park and Kroll (35) suggesting that β-glucosides act as specific “environmental signature” repressor molecules and that of Milenbachs et al. (32) that the regulation of virulence genes by sugars is part of a global CR mechanism.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
Wild-type L. monocytogenes strains were from a previously described panel comprising recent clinical and environmental isolates and collection or laboratory strains of various serotypes (41, 42). The strains tested met with the phenotypic criteria of the wild-type prfA genotype (41, 42): they exhibited weak to undetectable hemolytic and lecithinase reactions in BHI at 37°C, and the production of the corresponding activities was strongly activated in charcoal-treated medium. Escherichia coli DH5α was used as cloning host. Bacteria were cultured at 37°C in BHI (Difco) or Luria-Bertani (LB) media, with antibiotics if required. Liquid cultures were shaken at 170 rpm. Fresh stock solutions of carbohydrates were filter sterilized and added to the culture medium to a final concentration of 10 to 25 mM. Charcoal-treated BHI was prepared as previously described (42).
DNA methods.
Restriction and modification enzymes were purchased from Pharmacia and were used according to the manufacturer’s instructions. Listeria chromosomal DNA was isolated as previously described (20). Specific DNA was amplified with the Expand High Fidelity PCR system (Boehringer Mannheim). PCR products were purified with the Quiaquick Gel Extraction Kit (Qiagen) and were cloned with the Sure Clone Kit (Pharmacia). Plasmid DNA was extracted from E. coli with the Plasmid Purification Kit (Qiagen). Sequences were determined from both strands of plasmid DNA with an Applied Biosystems 377 apparatus. For Southern blot analysis, DNA fragments were subjected to electrophoresis in 1% agarose in Tris-borate-EDTA, denatured, and transferred to nitrocellulose membrane (Schleicher & Schuell) according to standard protocols (1). DNA probes consisted of PCR or restriction fragments internal to the genes of interest. They were radiolabeled with the Boehringer Mannheim Random Primed DNA Labeling Kit and [α-32P]dATP (Amersham). Prehybridization (2 h) and hybridization (16 h) were performed in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 56°C. Filters were then washed twice for 30 min at 45°C each in 5× SSC–0.1% sodium dodecyl sulfate and autoradiographed.
PlcB (lecithinase) activity determinations.
The expression of plcB was quantified via the lecithinase activity of its protein product, the wide-substrate-range phospholipase C, PlcB (53), by using a previously described turbidimetric assay (42) and type IV phosphatidylcholine from egg yolk (Sigma) as the substrate. We use plcB as a reporter of PrfA-dependent expression (40, 41) because it has several advantages over the hly (hemolysin) gene used by others (2, 3, 32). First, unlike hly, which is expressed from PrfA-dependent and -independent promoters (12), the expression of plcB is driven uniquely by a PrfA-dependent promoter, PactA (53). This promoter also has a threshold for activation by PrfA that is higher than that of hly (41), which helps eliminate any constitutive background activity. Second, the PlcB assay gives more clearcut, linear results than do hemolysin determinations (41). PlcB activity was determined in the supernatant of cultures collected when the optical density at 600 nm (OD600) reached 2.0. PrfA-dependent virulence gene expression is normally silenced in wild-type L. monocytogenes growing in BHI at 37°C (see above) (40–42). Therefore, to enable the repressor effect exerted by sugars on plcB expression to be observed, wild-type strains were cultured in charcoal-treated BHI, in which the PrfA regulon is fully activated (40–43).
Cloning and sequencing of the bvr locus.
A 4.4-kb DNA fragment was amplified by PCR from EGDCR by using the oligonucleotide B35031 (5′-AGGTTGTGATGTTTATGGAAA), specific for parB, and the degenerate primer A35030 (5′-RTTNCCYTCYTCNGTRTATAAYTT; where R = purine, Y = pyrimidine, and N = any base), designed from the Listeria seeligeri kat-encoded oligopeptide KFYTEEGN (7) (see below and Fig. 2). Several attempts to clone the full-length fragment in E. coli by using pUC18 failed, suggesting that this DNA region was toxic for bacteria if present in multiple copies. However, a plasmid was isolated (pKLB3A) that harbored a deletion at the parB-proximal part of the 4.4-kb fragment. The 2.1-kb insert of pKBL3A was partially sequenced and two further oligonucleotides, 94C230 (5′-GTTGCTCCTGCAGCCGGTGT-3′) and 94C229 (5′-TAATTCCAAGCGCATGTCCT), were designed which, when used with B35031 and A35030, respectively, gave rise to 2.1- and 2.4-kb PCR products. These PCR fragments were inserted into pUC18, yielding the plasmids pKLB3B (B35031-94C230 fragment) and pKLB3C (A35030-94C229 fragment). The entire sequence of the bvr locus, encompassed by the parB and kat genes (see Fig. 2), was determined from the overlapping inserts of pKLB3B and pKLB3C.
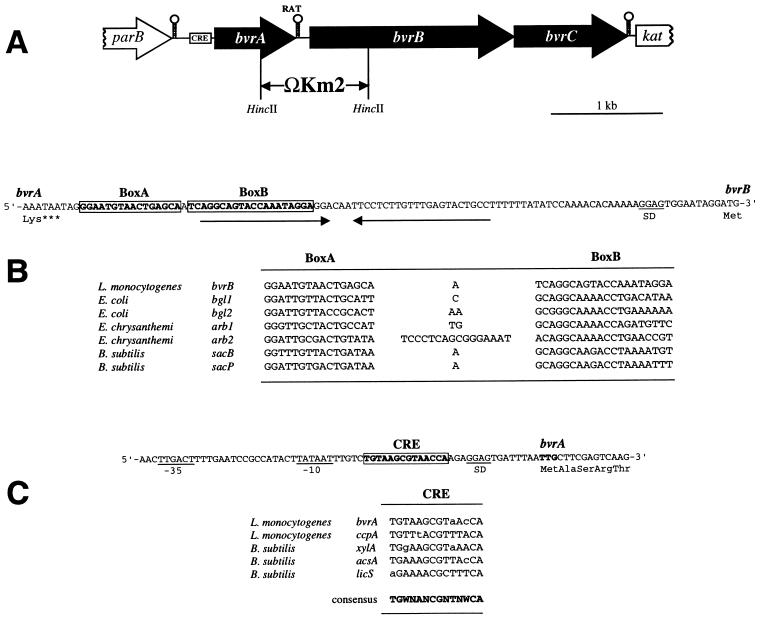
FIG. 2.
(A) Scheme of the genetic structure of the bvr locus of L. monocytogenes. The positions of the putative transcriptional terminators, CRE, and RAT sequences are shown. The bvr mutation was created by allele exchange by using a plasmid construct in which a HincII fragment encompassing the 3′-terminal third of bvrA and the 5′-proximal third of bvrB was replaced by the ΩKm2 interposon, as indicated. (B) Nucleotide sequence of the intergenic region between bvrA and bvrB (the respective last and first codons are shown) and position of the putative RAT sequence (boxed), which overlaps the 5′ part of a palindromic structure that may act as transcriptional terminator (indicated by inverted arrows). Below, comparison of the putative RAT sequence preceding bvrB with known RAT sequences (13). (C) Nucleotide sequence of the 3′ region upstream from bvrA (its TTG start codon is in boldface type) showing −10 and −35 putative promoter sequences and the CRE-like sequence (boxed). Below, comparison of the putative CRE preceding bvrA with the CRE-like element of the ccpA promoter region of L. monocytogenes (3) and known active CRE sites from gram-positive bacteria (21). Deviations from the CRE consensus sequence (in boldface) are shown in lowercase (N = any nucleotide; W = A or T).
Construction of the bvrAB::ΩKm mutant.
pKLB3B was digested with HincII and blunt-end ligated to the 2.1-kb SmaI fragment of pBR322WKm. This fragment contains the Ω-Km2 interposon, which bears the aphA-3 kanamycin resistance gene from Tn1545 and, at both ends, transcription and translation termination signals in each reading frame (36). In the resulting plasmid, pKLBM1, the 850-bp fragment between the two HincII sites encompassing the 3′ end of bvrA and the 5′-proximal region of bvrB was replaced by the Ω-Km2 element (see Fig. 2). The interposon-disrupted bvr locus was PCR amplified from pKLB1 by using the primers B35031 and 94C230 (see above). This PCR fragment was then blunt-end ligated into the SmaI site of the shuttle vector pLSV1 (55), giving rise to pKLBM2. pLSV1 has an erythromycin resistance gene and a thermosensitive origin of replication for gram-positive bacteria. pKLBM2 was introduced into L. monocytogenes EGDCR as previously described (41). Transformants were grown for 6 h at 30°C in BHI broth in the presence of 25 μg of kanamycin and 5 μg of erythromycin per ml, after which serial dilutions of the culture were plated onto BHI agar plates containing the same antibiotics. The plates were incubated for 48 h at 42°C to select for single crossover events between the chromosome and pKLBM2. One of the resulting colonies was grown overnight at 37°C in BHI broth containing 25 μg of kanamycin per ml, and appropriate dilutions of this culture were plated onto kanamycin BHI agar. Bacteria in which a second crossover event took place, leading to bvr allelic exchange, were selected by checking colonies for the loss of erythromycin resistance. The bvrAB::ΩKm mutation was confirmed by Southern blot and PCR with appropriate primers.
RNA procedures.
Total RNA was extracted from mid-exponential-phase cultures of L. monocytogenes by using a hot-acid-phenol protocol as described elsewhere (43). The operon structure of the bvr locus was investigated by using 5 μg of total RNA and the Titan One Tube reverse transcription-PCR (RT-PCR) Kit from Boehringer Mannheim. The effect of β-glucosides on bvrB and plcB transcription was assessed by semiquantitative RT-PCR as previously described (14). Samples of total RNA (0.5 μg/μl) were serially diluted in diethyl-pyrocarbonate-treated water, and cDNA was synthesized from 2 μl of each dilution by using the First-Strand Synthesis Kit from Stratagene. Then, 2-μl volumes of each primer extension reaction were subject to 15, 20, 25, and 30 cycles of PCR, and amplified DNA fragments were detected after electrophoresis in 1% agarose by ethidium bromide staining. Appropriate oligonucleotides internal to the genes of interest were used for primer extension and PCR amplification of cDNA. As controls, primers specific for the 16S rRNA and the sod gene encoding superoxide dismutase (5) of L. monocytogenes were used.
RESULTS
Evidence that cellobiose downregulates virulence gene expression via a specific mechanism.
Milenbachs et al. (32) showed that NCTC 7973 has an anomalous pattern of carbon source regulation, with cellobiose being the only sugar, of a variety of fermentable carbohydrates tested, causing virulence gene repression. This behavior suggests that (i) NCTC 7973 is probably a deregulated mutant with a defect in an aspect of carbon source regulation (2) and that (ii) cellobiose represses virulence genes via a specific pathway that is different from that used by other common carbohydrates.
The observations made with NCTC 7973 should, however, be interpreted with caution. This strain belongs to a class of L. monocytogenes mutants, called prfA*, that constitutively overexpress the PrfA virulence regulon (42). This phenotype is due to a point mutation in prfA leading to the synthesis of a mutant form of the regulatory protein, PrfA* (Gly145Ser), which binds with an increased affinity to the specific target sequences in the promoter regions of PrfA-regulated genes (41, 54). We (40, 41) and others (2) have presented evidence that the constitutive overexpression of PrfA-dependent genes conferred by the prfA* allele overcomes carbon source regulation in L. monocytogenes. Therefore, it is difficult to discern in the NCTC 7973 background whether the sugar-insensitive phenotype results simply from a masking effect exerted by the prfA* mutation alone or from additional mutations in central CR pathways. There is evidence that NCTC 7973 has accumulated other mutations affecting carbohydrate catabolic pathways (2), which may indeed lead to a completely distorted pattern of sugar-mediated virulence gene repression.
The carbon source regulation of virulence genes, on the other hand, has been studied in only a restricted number of L. monocytogenes strains. Besides NCTC 7973, only three other laboratory strains of serogroup 1 have been analyzed: EGD, 10403S, and L028 (32). Strains kept in laboratory conditions for a long time may accumulate mutations and exhibit aberrant phenotypes, as illustrated by NCTC 7973 and by at least one other of the strains examined, L028, which also has an anomalous pattern of virulence gene expression (41, 42). We therefore examined additional L. monocytogenes strains with a confirmed wild-type PrfA phenotype (see Materials and Methods). The fermentable carbohydrates glucose, fructose, and mannose and the β-glucoside cellobiose were tested for their ability to repress virulence gene expression. Saccharose, which is not utilized by L. monocytogenes and consequently does not repress virulence genes (32, 40), was used as a control. The product of the plcB gene, the wide-substrate-range phospholipase C, PlcB (or lecithinase), was used to monitor PrfA-dependent virulence gene expression (see Materials and Methods).
Consistent with previous observations with hly as a reporter (32), a strong downregulation of plcB occurred in all strains with all of the fermentable carbohydrates tested (not shown). There was, however, an exception. The group of wild-type strains tested included two subcultures of L. monocytogenes EGD from different laboratories: one was that kept by one of us (J.K.), while the other came from T. Chakraborty (University of Giessen, Giessen, Germany). The latter, termed EGD-e, is the strain whose DNA is being sequenced by the European Listeria genome consortium. Interestingly, EGD-e exhibited the normal repression pattern of wild-type strains, but our EGD subculture resembled NCTC 7973 in that plcB expression was only downregulated by cellobiose (see Fig. 1). Since our EGD variant (which was otherwise of wild-type prfA background) was no longer repressed by glucose and other common fermentable sugars, we presumed it was affected in a central CR pathway and thus called it EGDCR.
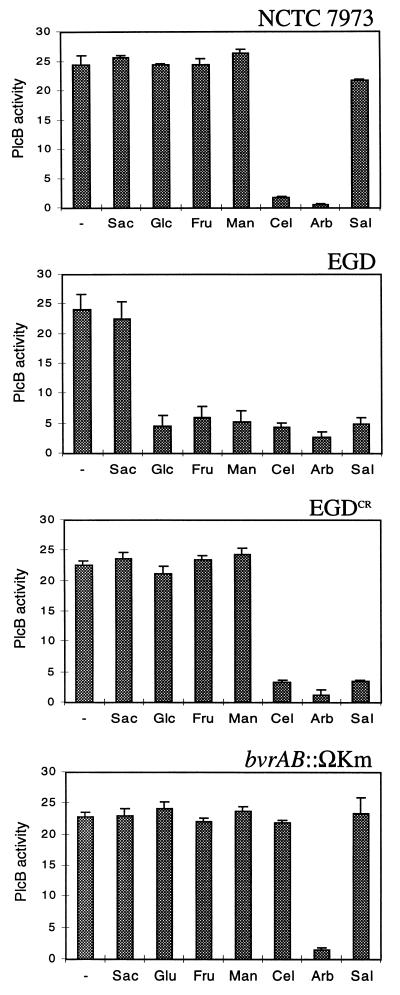
FIG. 1.
Patterns of virulence gene (plcB) repression in response to sugars in L. monocytogenes strains NCTC 7973, EGD, and EGDCR and the bvrAB::Km mutant (Sac, saccharose; Glc, glucose; Fru, fructose; Man, mannose; Cel, cellobiose; Arb, arbutin; Sal, salicin; –, no sugar). Means for three independent experiments ± the standard error are shown.
In contrast to cellobiose and arbutin, the phenolic β-glucoside salicin has been reported to have no downregulating effect on hly expression in NCTC 7973 (34). We thus analyzed in more detail whether NCTC 7973 and EGDCR had similar defects by assessing plcB expression in these two strains grown in the presence of each three β-glucosides. In NCTC 7973, as expected, plcB expression was repressed in the presence of cellobiose and arbutin but was not repressed by salicin (Fig. 1). EGDCR, however, differed from NCTC 7973 in that virulence gene expression was identically downregulated by the three natural β-glucosides (Fig. 1). An identical pattern was observed in EGD-e (Fig. 1), indicating that the EGDCR variant retained a wild-type response to β-glucoside sugars.
Overall, these observations suggest: (i) that β-glucosides cause virulence gene repression via a specific regulatory pathway, independent from the CR mechanism presumably used by glucose and other common utilizable sugars; and (ii) that there may also be various sugar-sensing mechanisms, with different substrate specificities, involved in the regulation of virulence genes by β-glucosides.
Cloning and sequence analysis of the bvr locus of L. monocytogenes.
We have previously reported the cloning and characterization of the kat gene encoding the catalase of the nonpathogenic species L. seeligeri (20). Upstream from kat, separated by a 370-bp intergenic region, we identified in L. seeligeri the parB locus (7). Like kat, parB was present in a highly conserved form in all Listeria species (7). While attempting to isolate the L. monocytogenes homolog of kat, we found that, in this species, there was an insertion of about 4 kb between this gene and parB. As such genomic differences between pathogenic and nonpathogenic species of the same genus may correspond to virulence-associated chromosomal islands (18, 19), we were interested in characterizing the DNA region between parB and kat in L. monocytogenes. A 4.4-kb fragment comprising the entire parB-kat intergenic region of L. monocytogenes was amplified by PCR, subcloned, and sequenced (see Materials and Methods for details). It comprised three open reading frames (ORF) of 810, 1,919, and 980 bp arranged in the same orientation (Fig. 2A). The locus was called bvrABC (for β-glucoside-mediated virulence gene repression [see below]).
The largest ORF, bvrB, encoded a 640-residue protein with extensive similarity to various permease components (enzyme II complex) of the phosphoenolpyruvate-sugar phosphotransferase system (PTS). The highest degree of similarity of the bvrB product, BvrB, was with ArbF from Erwinia chrysanthemi (13), BglF from E. coli (50), and BglP from Bacillus subtilis (26) (Fig. 3B), all of which are involved in the uptake of β-glucosides. BvrB also had the multidomain structure IIBCA, which is characteristic of enzymes II of the subfamily of β-glucoside PTS permeases (8, 28). These permeases are regulated by transcriptional antitermination, brought about by a family of structurally related antiterminator proteins (AT), the prototype of which is BglG from E. coli (44, 47). In the β-glucoside PTS operons of the enteric bacteria E. coli and E. chrysanthemi, the AT genes (bglG and arbG, respectively) are immediately upstream from the genes encoding the β-glucoside-specific enzymes II. In B. subtilis, in contrast, the two genetic determinants are not located in the same chromosomal region (44). Although L. monocytogenes is phylogenetically very closely related to B. subtilis, the genetic structure of the bvrAB locus was virtually identical to that of the enterobacterial loci bglGF and arbGF: the predicted bvrA product, BvrA (270 amino acids), was very similar to BglG (50) and related ATs from the E. chrysanthemi arb operon (ArbG) (13) and the B. subtilis bgl and sac regulons (LicT, SacY, and SacT) (9, 11, 48) (Fig. 3A). ATs bind specifically to conserved target sequences called “RAT” (for ribonucleic antiterminator), which overlap the 5′ part of stem-loop transcriptional terminator structures present in the leader sequences of the controlled genes or operons, thereby preventing the formation of the terminator and allowing readthrough transcription to the downstream (PTS permease) genes (44). In the 110-bp region between bvrA and bvrB, we identified a putative RAT sequence for BvrA overlapping a potential rho-independent transcriptional terminator (Fig. 2B).
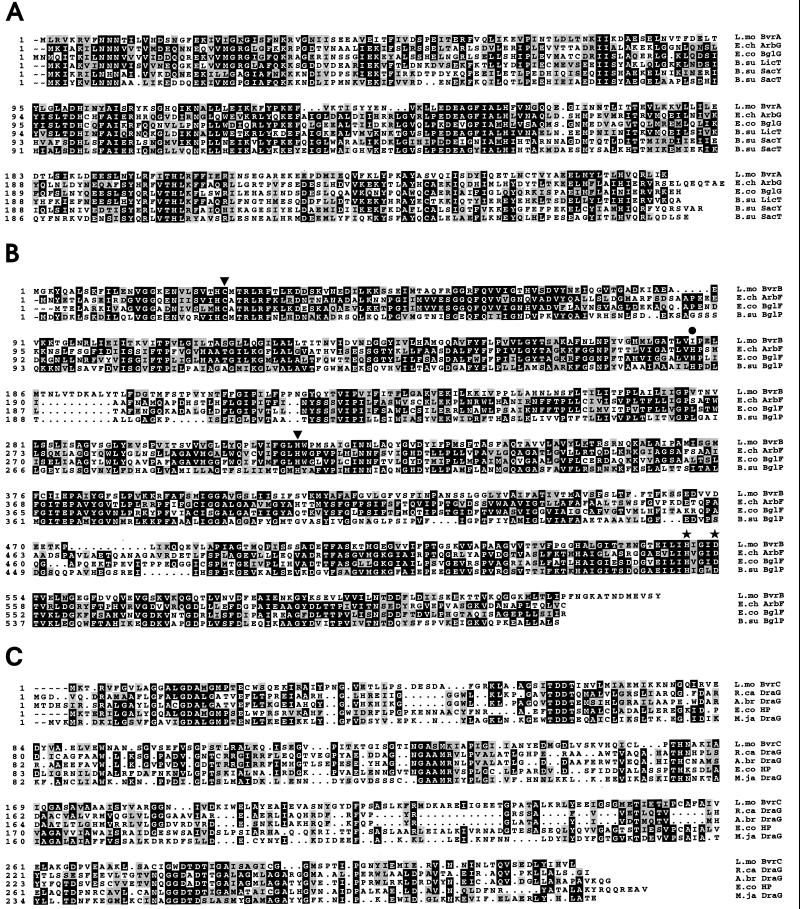
FIG. 3.
Multiple alignments of the deduced sequences of the polypeptides encoded by the bvr locus with their corresponding protein homologs from various bacteria (A.br, Azospirillum brasilense; B.su, B. subtilis; E.ch, E. chrysanthemi; E.co, E. coli; L.mo., L. monocytogenes; M.ja, M. jannaschii; R.ca, Rhodobacter capsulatus). The Bvr primary structures shown are those predicted from DNA sequences determined from EGDCR (accession number of the bvr locus: AG007877). (A) Alignment of BvrA with known ATs (accession numbers: ArbG, P26211; BglG, P11989; LicT, P39805; SacY, P15401; and SacT, P26212; identities with BvrA: 33, 28, 35, 30, and 29%, respectively). (B) Alignment of BvrB with known β-glucoside-specific enzyme II PTS permease components (accession numbers: ArbF, P26207; BglF, P08722; and BglP, P40739; identities with BvrB: 37, 34, and 32%, respectively). The positions of residues involved in the catalytic function of the E. coli BglF permease, as determined by site-specific mutagenesis (49), are indicated by black symbols: inverted triangles, Cys-24 and His-306 residues essential for the transfer of the phosphoryl group to the sugar; stars, His-547 and Asp-551 residues involved in the phosphorylation of the permease by HPr; and circle, His-183 residue important for substrate specificity. Note that almost all of these catalytically relevant residues are conserved in BvrB and related permeases. The only exception is the histidyl residue that aligns with position 183 of BglF, which is absent from the BvrB sequence. This sequence divergence may account for the differences in substrate specificity between BvrB and other β-glucoside permeases (see the text). (C) Alignment of BvrC with DraG proteins from R. capsulatus (accession number X71131) and A. brasilense (accession number I39752) and DraG homologs encoded in the genomes of M. jannaschii (accession number C64448) and E. coli (hypothetical protein b2099; accession number B64977). In the nitrogen-fixing bacteria R. rubrum, A. brasiliense, and R. capsulatus, dinitrogenase reductase (an enzyme essential for nitrogen fixation) is inhibited by ADP-ribosylation catalyzed by DraT (dinitrogenase reductase ADP-ribosyltransferase) and activated by the removal of the ADP-ribosyl group catalyzed by the ADP-ribosylglycohydrolase DraG (dinitrogenase reductase activating glycohydrolase) (30, 37, 56). Posttranslational modification via ADP-ribosylation has also been shown to be important in the regulation of glutamine synthetase in R. rubrum and Rhizobium meliloti and eventually also of sporulation in B. subtilis (22).
PTS genes are frequently clustered with the corresponding catabolic enzymes in operons (38, 44). In the case of the E. coli, E. chrysanthemi, and B. subtilis β-glucoside-specific PTS operons and regulons, the enzyme II gene is immediately followed by the structural gene of phospho-(P)-β-glycosidase (bglC, arbC, and bglH, respectively), which is involved in the further metabolization of the incorporated sugar (13, 26, 50). In the L. monocytogenes bvr locus, however, no such P-β-glucosidase gene was identified downstream from bvrB. FASTA searches detected a significant degree of similarity (21.3% identity, 51.5% similarity) between the bvrC product, BvrC, and the ADP-ribosylglycohydrolase DraG from Rhodospirillum rubrum (16). BvrC (327 amino acids) was also very similar to the proteins encoded by two ORFs present in the genomes of E. coli (hypothetical protein b2099: 33.3% identity, 65.2% similarity) and Methanococcus jannaschii (DraG-homolog: 25.8% identity, 59.3% similarity). The predicted primary structure of these two polypeptides also showed significant similarity to that of ADP-ribosylglycohydrolases (Fig. 3C).
bvrB expression is induced by β-glucosides.
ATs act as positive regulators of bacterial catabolic operons via a regulatory process induced by the substrate. For example, in the absence of β-glucosides, the BglF PTS permease of E. coli inactivates BglG by phosphorylating it. Thus, constitutive readthrough transcription of the operon is prevented by terminators preceding the bglG and bglF genes. However, in the presence of inducer β-glucosides, the phosphate groups are transferred from BglF to the sugar during uptake, creating a backward flow that dephosphorylates BglG. Active BglG prevents termination by binding to RAT sequences and induces the expression of the operon (38, 44, 47). The structure of the L. monocytogenes bvrAB locus is very similar to that of the E. coli bglGF locus (see above), suggesting functional similarity. If a similar antitermination mechanism of positive regulation operates in bvrAB, then the expression of the putative permease gene bvrB should be induced if L. monocytogenes is grown in the presence of β-glucosides.
We studied bvrB transcription in L. monocytogenes in the presence of glucose (control) or the β-glucosides cellobiose, salicin, and arbutin (Fig. 4). In the 5′ region immediately upstream from bvrA, overlapping a putative promoter, we identified a 14-bp sequence that matched the reported consensus sequence of catabolite responsive elements (CRE) (Fig. 2C), the cis-acting binding sites for the repressor transcription factors (presumably, the CcpA/HPr[Ser-46-P] complex) involved in CR in low-G+C-content, gram-positive bacteria (21, 46). This suggests that the bvr locus may be subject to CR if glucose or other readily metabolizable carbon sources are present in the culture medium. Therefore, to avoid the effects of any possible expressional crosstalk between the bvr locus and the putative global CR mechanism involved in carbon source regulation, we performed the experiments with EGDCR. bvrB transcription was semiquantitatively determined by RT-PCR. The level of bvrB expression was significantly higher in the presence of cellobiose and salicin (Fig. 4A). These results are consistent with the structural data, which predict that BvrAB is a β-glucoside-specific, substrate-inducible PTS permease system functionally similar to BglEF from E. coli. Arbutin, however, did not activate the transcription of bvrB (Fig. 4A).
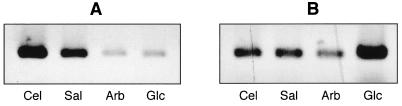
FIG. 4.
RT-PCR transcription analysis of bvrB (A) and plcB (B) expression in EGDCR grown in the presence of cellobiose (Cel), salicin (Sal), arbutin (Arb), and glucose (Glc) (control). Note that in panel A, cellobiose and salicin, but not arbutin, upregulate bvrB. In panel B, the three β-glucosides repress plcB, a finding consistent with the data obtained by using PlcB activity as reporter (Fig. 1).
The TGA stop codon of bvrB overlapped with the ATG start codon of bvrC, suggesting that the two genes are cotranscribed and that the bvr locus constitutes an operon. We confirmed this by RT-PCR with total RNA from L. monocytogenes grown on cellobiose and oligonucleotide primers specific for bvrA and bvrC (not shown).
The bvr locus is L. monocytogenes specific.
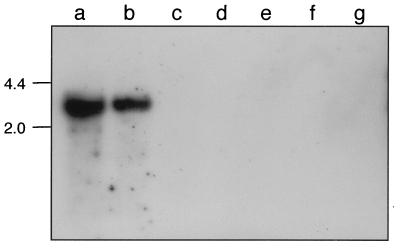
We were interested in determining whether bvr-homologous sequences were present elsewhere on the L. seeligeri chromosome or if the locus was present in other Listeria species. Southern blot analyses were performed with chromosomal DNA from all known Listeria species, hybridizing at low stringency with a bvr probe. Positive hybridization signals were only obtained with L. monocytogenes (Fig. 5). This is consistent with the fact that we have only observed bvr-positive PCR reactions with DNA from L. monocytogenes isolates.
FIG. 5.
Detection of bvr sequences in Listeria spp. by low-stringency Southern blot. Lanes: a, L. monocytogenes EGDCR; b, L. monocytogenes NCTC 7973; c, L. ivanovii ATCC 19119; d, L. seeligeri SLCC 5921; e, L. welshimeri SLCC 5334; f, L. grayi. Chromosomal DNA was digested with EcoRI. Numbers on the left indicate the size in kilobases.
The bvr locus is involved in β-glucoside-mediated virulence gene repression.
Our structural and functional data suggest that BvrB is a β-glucoside-specific PTS permease. It was therefore possible that the bvr locus was somehow involved in β-glucoside-mediated virulence gene repression in L. monocytogenes. To test this, we constructed a bvr mutant in EGDCR by replacing a HincII fragment spanning the 3′ terminal part of bvrA, the bvrAB intergenic region, and the 5′ part of bvrB with the Ω-Km2 interposon (Fig. 2A) (see Materials and Methods). RT-PCR analyses indicated that bvrB expression was abolished in the bvrAB::ΩKm mutant (not shown). No transcripts were detected for bvrC, demonstrating that the bvr locus constitutes an operon.
We studied the effect of the bvrAB::ΩKm mutation on virulence gene repression by β-glucosides by using plcB as a reporter. The repression exerted by cellobiose and salicin was totally abolished in bvrAB::ΩKm. Arbutin, however, still downregulated plcB expression to a level similar to that of the parent strain (Fig. 1). These results were entirely consistent with the fact that bvrB expression is induced by cellobiose and salicin but not by arbutin (Fig. 4) and demonstrate that the BvrB PTS permease is involved in virulence gene repression by β-glucosides.
PTS permeases mediate the transport into the bacterial cell of specific carbohydrate substrates, thus initiating their catabolism (28, 38, 46). We therefore investigated whether the bvr locus was important for cellobiose and salicin utilization by L. monocytogenes. The growth characteristics of bvrAB::ΩKm and its parent strain were determined in LB broth supplemented with glucose (control), cellobiose, salicin, or arbutin as the only carbon source. LB is a poor growth medium, in which L. monocytogenes can hardly grow in the absence of an additional carbon source (maximum OD578 of 0.5 versus 2.0 in LB plus 10 mM fermentable carbohydrate) (40). Virtually identical growth curves and final bacterial yields were observed for both strains in LB medium with glucose or any one of the three β-glucosides (not shown). No differences were observed when the sugar utilization profiles of the parent and mutant strains were investigated with the API 50 CH system.
DISCUSSION
The regulatory effect of fermentable sugars on virulence genes of L. monocytogenes has recently attracted considerable interest (2, 3, 32, 34, 35). This issue has been investigated in only four laboratory strains, at least two of which (L028 and NCTC 7973) have anomalous patterns of virulence gene expression and even regulatory mutations in prfA (41, 42). To unambiguously determine the normal pattern of this type of carbon source regulation in L. monocytogenes, we assessed the effect of a selection of carbohydrates on the expression of the PrfA-dependent gene plcB by using a panel of well-characterized wild-type strains. Our results confirmed that the repressibility of PrfA-dependent virulence genes is a general characteristic of wild-type L. monocytogenes upon growth on any fermentable sugar.
Among the strains tested, we identified a phenotypic variant of strain EGD in which virulence gene expression was still repressed by the three natural β-glucosides (cellobiose, salicin, and arbutin) but not by other common utilizable sugars such as glucose. We concluded that the repression of virulence genes by sugars is mediated in L. monocytogenes by at least two different regulatory mechanisms: one presumably involving a general CR repression pathway, as suggested by Milenbachs et al. (32), which can be eliminated by a spontaneous mutation, and another responding specifically to β-glucosides. In support of this conclusion, we report here the identification of the bvr locus of L. monocytogenes, which specifically mediates virulence gene repression by β-glucoside sugars.
The bvr locus comprises three genes, bvrABC, which are expressed as an operon. bvrAB code for a putative β-glucoside-specific, substrate-inducible PTS permease system similar to that of the bgl and arb operons of E. coli and E. chrysamthemi, respectively, and the bgl regulon of B. subtilis. Transcription of the enzyme II gene, bvrB, was induced by cellobiose and salicin, but not by arbutin, suggesting that the BvrB permease was specific for the two first β-glucosides. Moreover, a knockout mutation of the bvr operon totally abolished the repression exerted by cellobiose and salicin, whereas arbutin downregulated virulence genes to the same extent as in the parent strain. These results demonstrate the involvement of the bvr locus in virulence gene regulation by β-glucosides, suggesting at the same time that various regulatory mechanisms, with different substrate specificities, may be involved in β-glucoside-mediated virulence gene regulation in L. monocytogenes. Arbutin is a major substrate for other bacterial β-glucoside PTS permeases (BglF, ArbF, and BglP from E. coli, E. chrysanthemi, and B. subtilis, respectively) (13, 26, 50) to which BvrB is homologous. Primary structure differences in certain conserved domains may account for the altered substrate specificity of BvrB (Fig. 3B).
It has been previously shown that cellobiose reduces virulence gene expression without affecting the levels of the PrfA protein (32, 39). The only PrfA-dependent virulence genes used to date to assess the effect of cellobiose are hly and plcA (2, 3, 23, 32, 34, 35). These genes are physically linked, divergent transcriptional units with overlapping promoter regions (31), so the possibility existed that the repressor effect exerted over them by cellobiose was completely unrelated to PrfA and involved a specific interaction with the common promoter region. Here we show that cellobiose and other β-glucosides also repress plcB, another gene of the virulence regulon, the expression of which is strictly dependent on PrfA (41). As shown in Fig. 4B, plcB is downregulated at the transcriptional level, as are hly and plcA (23, 32, 35). Thus, the regulatory mechanism triggered by β-glucosides appears to somehow interfere with PrfA.
How does the bvr locus brings about β-glucoside-mediated virulence gene regulation? It is well known that sugar transport by PTS permeases initiates a regulatory cascade of CR via PEP-dependent, enzyme I-catalyzed phosphorylation of the His-15 residue of the general PTS protein HPr both in gram-negative and gram-positive bacteria (21, 38, 46). However, our observations with EGDCR would rule out the possibility that a general CR pathway is involved in virulence gene repression by β-glucosides. There is extensive functional crosstalk between the various ATs of the bgl-sac family, of which there are three well-characterized members in B. subtilis (see above) (44). Therefore, one possibility is that BvrB-mediated dephosphorylation and activation of BvrA upon the transport of cellobiose and salicin positively regulate, by transcriptional antitermination, a putative effector system responsible for the inhibition of the PrfA regulon. Another possibility involves the bvrC-encoded putative ADP-ribosylglycohydrolase (Fig. 3C). In prokaryotes, these enzymes participate in posttranslational regulatory networks involving reversible ADP-ribosylation of target proteins (29) (see the legend to Fig. 3C). Thus, BvrC may well provide the link between the BvrAB β-glucoside-specific PTS permease system and the virulence regulon by posttranslationally controlling, directly or indirectly, PrfA function.
Mutation of the bvr locus totally eliminated the repressor effect exerted by cellobiose and salicin without affecting the utilization of these sugars. Clearly, therefore, other sugar transport systems mediating β-glucoside utilization should be present in L. monocytogenes. These systems are likely to be conserved in Listeria because all species in this genus are able to ferment β-glucosides (reference 33 and unpublished observations). Thus, any role of the bvr locus in the uptake of β-glucosides by L. monocytogenes would be purely accessory or redundant. bvr is present exclusively in L. monocytogenes, as a 4-kb chromosomal island inserted between two housekeeping genes, parB and kat, which are contiguous in the nonpathogenic species L. seeligeri (6). This may indicate that the function associated with this locus is relevant only to the biology of the pathogenic species L. monocytogenes. Virulence factors are primarily required during infection, and their synthesis outside the host would be a waste of energy, reducing the fitness of the bacterium to successfully compete in the free environment and thereby limiting its potential for transmission. Our results suggest that the bvr locus is responsible for a regulatory mechanism that, like thermoregulation, would be aimed at ensuring that virulence genes are shut off outside the vertebrate host. The PTS acts as a signal transduction system in positive chemotaxis in response to the presence of PTS-transported carbohydrates in the extracellular medium (17, 38). It is therefore possible that the Bvr system functions as a sensor mechanism triggering virulence gene silencing upon detection of β-glucosides, sugars specific to the plant kingdom which, as previously suggested by Park and Kroll (35), might be used by L. monocytogenes as signal molecules of the soil habitat. It must be noted that the virulence gene regulatory function of the bvr locus would have remained cryptic if L. monocytogenes were not cultured in PrfA-activating conditions (i.e., culture at 37°C in charcoal-treated BHI). This shows that bvr genes are responsible for a repressor mechanism that is superimposed on the positive control pathway that activates the PrfA virulence regulon. The environmental context sensed by L. monocytogenes in soil may sometimes become ambiguous, for example, if the ambient temperature rises above 30°C. We believe that in such situations the Bvr system may play a key role as a fail-safe mechanism to avoid any deleterious leaky expression of virulence genes.
ACKNOWLEDGMENTS
We thank T. Chakraborty for the gift of the EGD strain kept in his laboratory. N. Montero is acknowledged for excellent technical assistance.
This work was supported by grants from the European Commission (HRCX-CT94-451 and BMH4-CT96-659), the Fondo de Investigación Sanitaria (FIS 94/0043-02), the Dirección General de Investigación de la Comunidad de Madrid (29/97), the Deutsche Forschungsgemeinschaft (SFB 165), and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1991. [Google Scholar]
- 2.Behari J, Youngman P. Regulation of hly expression in Listeria monocytogenes by carbon sources and pH occurs through separate mechanisms mediated by PrfA. Infect Immun. 1998;66:3635–3642. doi: 10.1128/iai.66.8.3635-3642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behari J, Youngman P. A homolog of CcpA mediates catabolite control in Listeria monocytogenes but not carbon source regulation of virulence genes. J Bacteriol. 1998;180:6316–6324. doi: 10.1128/jb.180.23.6316-6324.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohne J, Sokolovic Z, Goebel W. Transcriptional regulation of prfA and PrfA-regulated virulence genes in Listeria monocytogenes. Mol Microbiol. 1994;11:1141–1150. doi: 10.1111/j.1365-2958.1994.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 5.Brehm K, Haas A, Goebel W, Kreft J. A gene encoding a superoxide dismutase of the facultative intracellular bacterium Listeria monocytogenes. Gene. 1992;118:121–125. doi: 10.1016/0378-1119(92)90258-q. [DOI] [PubMed] [Google Scholar]
- 6.Brehm K, Kreft J, Ripio M-T, Vázquez-Boland J-A. Regulation of virulence gene expression in pathogenic Listeria. Microbiología. 1996;12:219–236. [PubMed] [Google Scholar]
- 7.Brehm, K., and J. Kreft. Unpublished data.
- 8.Chen Q, Amster-Choder O. BglF, the Escherichia coli β-glucoside permease and sensor of the bgl system: domain requirement of the different catalytic activities. J Bacteriol. 1999;181:462–468. doi: 10.1128/jb.181.2.462-468.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crutz A-M, Steinmetz M, Aymerich S, Richter R, Le Coq D. Induction of levansucrase in Bacillus subtilis: an antitermination mechanism negatively controlled by the phosphotransferase system. J Bacteriol. 1990;172:1043–1050. doi: 10.1128/jb.172.2.1043-1050.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datta A R, Kothary M H. Effects of glucose, growth temperature, and pH on listeriolysin O production in Listeria monocytogenes. Appl Environ Microbiol. 1993;59:3495–3497. doi: 10.1128/aem.59.10.3495-3497.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Débarbouillé M, Arnaud M, Fouet A, Klier A, Rapoport G. The sacT gene regulating the sacPA operon in Bacillus subtilis shares strong homology with transcription antiterminators. J Bacteriol. 1990;172:3966–3973. doi: 10.1128/jb.172.7.3966-3973.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domann E, Wehland J, Niebuhr K, Haffner C, Leimeister-Wächter M, Chakraborty T. Detection of a prfA-independent promoter responsible for listeriolysin gene expression in mutant Listeria monocytogenes strains lacking the PrfA regulator. Infect Immun. 1993;61:3072–3075. doi: 10.1128/iai.61.7.3073-3075.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Hassouni M, Henrissat B, Chippaux M, Barras F. Nucleotide sequence of the arb genes, which control β-glucoside utilization in Erwinia chrysanthemi: comparison with the Escherichia coli bgl operon and evidence for a new β-glycohydrolase family including enzymes from eubacteria, archaebacteria, and humans. J Bacteriol. 1992;174:765–777. doi: 10.1128/jb.174.3.765-777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelbrecht F, Chun S-K, Ochs C, Hess J, Lottspeich F, Goebel W, Sokolovich Z. A new PrfA-regulated gene of Listeria monocytogenes encoding a small, secreted protein which belongs to the family of internalins. Mol Microbiol. 1996;21:823–837. doi: 10.1046/j.1365-2958.1996.541414.x. [DOI] [PubMed] [Google Scholar]
- 15.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzmaurice W P, Saari L L, Lowery R G, Ludden P W, Roberts G P. Genes coding for the reversible ADP-ribosylation system of dinitrogenase reductase from Rhodospirillum rubrum. Mol Gen Genet. 1989;218:340–347. doi: 10.1007/BF00331287. [DOI] [PubMed] [Google Scholar]
- 17.Garrity L F, Schiel S L, Merrill R, Reizer J, Saier M H, Ordall G W. Unique regulation of carbohydrate chemotaxis in Bacillus subtilis by the phosphoenolpyruvate-dependent phosphotransferase system and the methyl-accepting chemotaxis protein McpC. J Bacteriol. 1998;180:4475–4480. doi: 10.1128/jb.180.17.4475-4480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groisman E A, Ochman H. Pathogenicity islands: bacterial evolution in quantum leaps. Cell. 1996;87:791–794. doi: 10.1016/s0092-8674(00)81985-6. [DOI] [PubMed] [Google Scholar]
- 19.Hacker J, Blum-Oehler G, Mühldorfer I, Schäpe T. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 20.Haas A, Brehm K, Kreft J, Goebel W. Cloning, characterization, and expression in Escherichia coli of a gene encoding Listeria seeligeri catalase, a bacterial enzyme highly homologous to mammalian catalases. J Bacteriol. 1991;173:5159–5167. doi: 10.1128/jb.173.16.5159-5167.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hueck C J, Hillen W. Catabolite repression in Bacillus subtilis: a global regulatory mechanism for the gram-positive bacteria? Mol Microbiol. 1995;15:395–401. doi: 10.1111/j.1365-2958.1995.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 22.Huh J-W, Shima J, Ochi K. ADP-ribosylation of proteins in Bacillus subtilis and its possible role in sporulation. J Bacteriol. 1996;178:4935–4941. doi: 10.1128/jb.178.16.4935-4941.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klarsfeld A, Goossens P L, Cossart P. Five Listeria monocytogenes genes preferentially expressed in infected mammalian cells: plcA, purH, purD, pyrE and an arginine ABC transporter gene, arpJ. Mol Microbiol. 1994;13:585–597. doi: 10.1111/j.1365-2958.1994.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 24.Kreft J, Bohne J, Gross R, Kestler H, Sokolovic Z, Goebel W. Control of Listeria monocytogenes virulence genes by the transcriptional regulator PrfA. In: Rappuoli R, Scarlato V, Arico B, editors. Signal transduction and bacterial virulence. R. G. Austin, Tex: Landes Company; 1995. pp. 129–142. [Google Scholar]
- 25.Lampidis R, Gross R, Sokolovic Z, Goebel W, Kreft J. The virulence regulator protein of Listeria ivanovii is highly homologous to PrfA from Listeria monocytogenes and both belong to the Crp-Fnr family of transcriptional regulators. Mol Microbiol. 1994;13:141–151. doi: 10.1111/j.1365-2958.1994.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 26.Le Coq D, Lindner C, Krüger S, Steinmetz M, Stülke J. New β-glucoside (bgl) genes in Bacillus subtilis: the bglP gene product has both transport and regulatory functions similar to those of BglF, its Escherichia coli homolog. J Bacteriol. 1995;177:1527–1535. doi: 10.1128/jb.177.6.1527-1535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leimeister-Wächter M, Domann E, Chakraborty T. The expression of virulence genes in Listeria monocytogenes is thermoregulated. J Bacteriol. 1992;174:947–952. doi: 10.1128/jb.174.3.947-952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lengeler J W, Jahreis K, Wehmeier U F. Enzymes II of the phosphoenolpyruvate-dependent phosphotransferase systems: their structure and function in carbohydrate transport. Biochim Biophys Acta. 1994;1188:1–28. doi: 10.1016/0005-2728(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 29.Ludden P W. Reversible ADP-ribosylation as a mechanism of enzyme regulation in procaryotes. Mol Cell Biochem. 1994;138:123–129. doi: 10.1007/BF00928453. [DOI] [PubMed] [Google Scholar]
- 30.Masepohl B, Krey R, Klipp W. The draTG region of Rhodobacter capsulatus is required for post-translational regulation of both the molybdenum and the alternative nitrogenase. J Gen Microbiol. 1993;139:2667–2675. doi: 10.1099/00221287-139-11-2667. [DOI] [PubMed] [Google Scholar]
- 31.Mengaud J, Dramsi S, Gouin E, Vázquez-Boland J A, Milon G, Cossart P. Pleiotropic control of Listeria monocytogenes virulence factors by a gene that is autoregulated. Mol Microbiol. 1991;5:2273–2283. doi: 10.1111/j.1365-2958.1991.tb02158.x. [DOI] [PubMed] [Google Scholar]
- 32.Milenbachs A A, Brown D P, Moors M, Youngman P. Carbon source regulation of virulence gene expression in Listeria monocytogenes. Mol Microbiol. 1997;23:1075–1085. doi: 10.1046/j.1365-2958.1997.2711634.x. [DOI] [PubMed] [Google Scholar]
- 33.Mira-Gutiérrez J, Pérez de Lara C, Rodriguez-Iglesias M A. Identification of species of the genus Listeria by fermentation of carbohydrates and enzymatic patterns. Acta Microbiol Hung. 1990;37:123–129. [PubMed] [Google Scholar]
- 34.Park S F. The repression of listeriolysin O expression in Listeria monocytogenes by the phenolic β-d-glucoside arbutin. Lett Appl Microbiol. 1994;19:258–260. doi: 10.1111/j.1472-765x.1994.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 35.Park S F, Kroll R G. Expression of listeriolysin and phosphatidylinositol-specific phospholipase C is repressed by the plant-derived molecule cellobiose in Listeria monocytogenes. Mol Microbiol. 1993;8:653–661. doi: 10.1111/j.1365-2958.1993.tb01609.x. [DOI] [PubMed] [Google Scholar]
- 36.Pérez-Casal J, Caparon M G, Scott J R. Mry, a trans-acting positive regulator of the M-protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two component regulatory systems. J Bacteriol. 1991;173:2617–2624. doi: 10.1128/jb.173.8.2617-2624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierrard J, Ludden P W, Roberts G P. Posttranslational regulation of nitrogenase in Rhodobacter capsulatus: existence of two independent regulatory effects of ammonium. J Bacteriol. 1993;175:1358–1366. doi: 10.1128/jb.175.5.1358-1366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renzoni A, Klarsfeld A, Dramsi S, Cossart P. Evidence that PrfA, the pleiotropic activator of virulence genes in Listeria monocytogenes, can be present but inactive. Infect Immun. 1997;65:1515–1518. doi: 10.1128/iai.65.4.1515-1518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ripio M T, Brehm K, Lara M, Suárez M, Vázquez-Boland J A. Glucose-1-phosphate utilization by Listeria monocytogenes is PrfA dependent and coordinately expressed with virulence factors. J Bacteriol. 1997;179:7174–7180. doi: 10.1128/jb.179.22.7174-7180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ripio M-T, Domínguez-Bernal G, Lara M, Suárez M, Vázquez-Boland J A. A Gly145Ser substitution in the transcriptional activator PrfA causes constitutive overexpression of virulence factors in Listeria monocytogenes. J Bacteriol. 1997;179:1533–1540. doi: 10.1128/jb.179.5.1533-1540.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ripio M-T, Domínguez-Bernal G, Suárez M, Brehm K, Berche P, Vázquez-Boland J-A. Transcriptional activation of virulence genes in wild-type strains of Listeria monocytogenes in response to a change in the extracellular medium composition. Res Microbiol. 1996;147:371–384. doi: 10.1016/0923-2508(96)84712-7. [DOI] [PubMed] [Google Scholar]
- 43.Ripio M-T, Vázquez-Boland J A, Vega Y, Nair S, Berche P. Evidence for expressional crosstalk between the central virulence regulator PrfA and the stress response mediator ClpC in Listeria monocytogenes. FEMS Microbiol Lett. 1998;158:45–50. doi: 10.1111/j.1574-6968.1998.tb12798.x. [DOI] [PubMed] [Google Scholar]
- 44.Rutberg B. Antitermination of transcription of catabolic operons. Mol Microbiol. 1997;23:413–421. doi: 10.1046/j.1365-2958.1997.d01-1867.x. [DOI] [PubMed] [Google Scholar]
- 45.Ryser E T, Marth E H. Listeria, listeriosis and food safety. New York, N.Y: Marcel Dekker Inc.; 1991. Occurrence and survival of Listeria monocytogenes in natural environments; pp. 22–33. [Google Scholar]
- 46.Saier M H, Jr, Chauvaux S, Deutscher J, Reizer J, Ye J-J. Protein phosphorylation and regulation of carbon metabolism in gram-negative versus gram-positive bacteria. Trends Biochem Sci. 1995;20:267–271. doi: 10.1016/s0968-0004(00)89041-6. [DOI] [PubMed] [Google Scholar]
- 47.Schnetz K, Rak B. β-Glucoside permease represses the bgl operon of Escherichia coli by phosphorylation of the antiterminator protein and also interacts with glucose-specific enzyme II, the key element in catabolic control. Proc Natl Acad Sci USA. 1990;87:5074–5078. doi: 10.1073/pnas.87.13.5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schnetz K, Stuelke J, Gertz S, Kruger S, Krieg M, Hecker M, Rak B. LicT, a Bacillus subtilis transcriptional antiterminator protein of the BglG family. J Bacteriol. 1996;178:1971–1979. doi: 10.1128/jb.178.7.1971-1979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schnetz K, Sutrina S L, Saier M H, Rak B. Identification of catalytic residues in the β-glucoside permease of Escherichia coli by site-specific mutagenesis and demonstration of interdomain cross-reactivity between the β-glucoside and glucose systems. J Biol Chem. 1990;265:13464–13471. [PubMed] [Google Scholar]
- 50.Schnetz K, Toloczyki C, Rak B. β-Glucoside (bgl) operon of Escherichia coli K-12: nucleotide sequence, genetic organization, and possible evolutionary relationship to regulatory components of two Bacillus subtilis genes. J Bacteriol. 1987;169:2579–2590. doi: 10.1128/jb.169.6.2579-2590.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheehan B, Klarsfeld A, Ebright R, Cossart P. A single substitution in the putative helix-turn-helix motif of the pleiotropic activator PrfA attenuates Listeria monocytogenes virulence. Mol Microbiol. 1996;20:785–797. doi: 10.1111/j.1365-2958.1996.tb02517.x. [DOI] [PubMed] [Google Scholar]
- 52.Vázquez-Boland J A, Domínguez L, Blanco M, Rocourt J, Fernández-Garayzábal J F, Gutiérrez C B, Tascón R I, Rodríguez-Ferri E F. Epidemiologic investigation of a silage-associated epizootic of ovine listeric encephalitis, using a new Listeria-selective enumeration medium and phage typing. Am J Vet Res. 1992;53:368–371. [PubMed] [Google Scholar]
- 53.Vázquez-Boland J A, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud C, Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vega Y, Dickneite C, Ripio M-T, Böckmann R, González-Zorn B, Novella S, Domínguez-Bernal G, Goebel W, Vázquez-Boland J A. Functional similarities between the Listeria monocytogenes virulence regulator PrfA and cyclic AMP receptor protein: the PrfA* (Gly145Ser) mutation increases binding affinity for target DNA. J Bacteriol. 1998;180:6655–6660. doi: 10.1128/jb.180.24.6655-6660.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wuenscher M D, Köhler S, Goebel W, Chakraborty T. Gene disruption by plasmid integration in Listeria monocytogenes: insertional inactivation of the listeriolysin determinant lisA. Mol Gen Genet. 1991;228:177–182. doi: 10.1007/BF00282463. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Burris R H, Ludden P W, Roberts G P. Comparison studies of dinitrogenase reductase ADP-ribosyl transferase/dinitrogenase reductase activating glycohydrolase regulatory systems in Rhodospirillum rubrum and Azospirillum brasiliense. J Bacteriol. 1995;177:2354–2359. doi: 10.1128/jb.177.9.2354-2359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]