Highlights
-
•
Botulinum toxin is considered as the first-line therapy in oromandibular dystonia (OMD) by most experts and evidence-based literature.
-
•
Oromandibular dystonia (OMD) can be classified into several subtypes so primary muscle involvements can be identified for botulinum toxin (BoNT) injections.
-
•
This review article aims to provide a framework for practical clinical approaches in patients with OMD for BoNT injections.
-
•
Careful stepwise planning is recommended to identify primary muscles responsible and employ a conservative approach to dosing titration.
-
•
Treating physicians should be diligent to observe for adverse events as muscles involved in OMD are small, delicate and situated in close proximity.
Keywords: Oromandibular dystonia, Segmental craniocervical dystonia, Botulinum toxin, Meige’s syndrome, Jaw-opening, Jaw-closing, Lingual dystonia, Perioral, Abobotulinum toxin, Onabotulinum toxin
Abstract
Oromandibular dystonia (OMD) is a form of focal dystonia that involves the masticatory, lower facial, labial, and lingual musculature. It is a disabling disorder which had limited treatment options until the recent introduction of botulinum toxin (BoNT) as the recommended first-line therapy by most experts and evidence-based literature. Owing to the complex relationship between the muscles of mastication and surrounding muscles, there is a wide variety of dynamic clinical presentations, making clinical recognition and the corresponding approach to BoNT injection therapy difficult. In this review, the authors provide a framework for practical clinical approaches, beginning with the recognition of clinical subtypes of OMD (jaw-opening, jaw-closing, jaw-deviating, lingual, peri-oral, and/or pharyngeal dystonias), followed by patient selection and clinical evaluation to determine function interferences, with injection techniques illustrated for each subtype. Careful stepwise planning is recommended to identify the muscles that are primarily responsible and employ a conservative approach to dosing titration. Treating physicians should be diligent in checking for adverse events, especially for the first few injection cycles, as muscles involved in OMD are small, delicate, and situated in close proximity. It is recommended that future studies should aim to establish the clinical efficacy of each subtype, incorporating muscle targeting techniques and patient-centred outcome measures that are related to disturbed daily functions.
1. Introduction
Oromandibular dystonia (OMD) is a form of focal dystonia that involves the masticatory, lower facial, labial, and lingual musculature innervated by the trigeminal, facial, and hypoglossal cranial nerves [1]. While dystonic movements in OMD primarily affect orbicularis oculi and the lower facial or oromandibular muscles, the spasms may extend extracranially to the cervical region or the limbs, leading to a recent term ‘segmental craniocervical dystonia’ being proposed to accurately describe the combination of blepharospasm and dystonia of other head and neck muscles that are useful for phenotypic and aetiological classifications [2]. Different eponyms such as “Wood syndrome”. “Meige syndrome”, “Brueghel syndrome”, and “Blepharospasm plus syndrome” have been mentioned in the literature to characterise imprecise combinations of blepharospasm and lower face muscle and/or masticatory muscle dystonia, however, the use of these terms is currently not recommended [2], [3], [4]. Due to different combinations and severity of muscle involvement, clinical presentations of OMD are myriad and often dynamic, resulting in socially embarrassing, disfiguring, and disturbing daily functions [5]. The development of botulinum toxin (BoNT) has markedly expanded the treatment armamentarium of OMD with evidence-based reviews and expert opinions endorsing BoNT as the first-line treatment for OMD regardless of its clinical presentations, although recent well-controlled clinical trials are still lacking [6], [7], [8]. This review aims to provide a framework for practical clinical approaches, beginning with the recognition of clinical subtypes of OMD (jaw-opening, jaw-closing, jaw-deviating, lingual, peri-oral, and/or pharyngeal dystonias), followed by patient selection and clinical evaluation to determine function interferences with injection techniques illustrated for each subtype. Careful stepwise planning is recommended to identify the muscles that are primarily responsible and employ a conservative approach to dosing titration. It is the authors’ intention for this review article to be a foundation for early injectors to recognise clinical subtypes of OMD and gain therapeutic concepts with BoNT and a teaching tool for advanced injectors to demonstrate muscle identification and localising techniques, serving as practical guidance if they wish to train future injectors.
2. Epidemiology, clinical features and aetiologies
There is a wide variability in the prevalence estimates of OMD, influenced by race and ethnicity, but overall, OMD is estimated to affect approximately 70 per million people, frequently affecting women more than men (2:1) [1], [9]. Age appears to be an independent risk factor for OMD, with the mean age at onset between 50 and 60 years. OMD can be classified into various subtypes, which include jaw-opening, jaw-closing, jaw-deviating, lingual, perioral, and/or pharyngeal dystonia. Jaw-closing and jaw-opening OMD are amongst the two most frequent subtypes, with a mixed pattern identified in one-third of patients; jaw deviation is probably the least prevalent [10], [11].
Common symptoms experienced by OMD patients result from abnormal contractions of these muscles, resulting in involuntary biting of the tongue, cheek, or lips and difficulty with speaking and chewing. Its appearance is often socially embarrassing and disfiguring and may be triggered by mandibular activities such as talking, yawning, chewing, or swallowing. In patients with jaw-closing OMD, dystonic spasms of the temporalis and masseter muscles may result in clenching, or trismus, and grinding of the teeth, or bruxism. On the other hand, the lateral pterygoids, anterior belly of the digastric muscle, and other submental muscles are commonly involved in jaw-opening dystonia, and contractions of these muscles may also lead to some degree of anterocollis. OMD, especially the primary form, may be alleviated by introducing different proprioceptive sensory inputs (‘sensory tricks’), such as touching the lips or chin, chewing gum, or biting on a toothpick, with these strategies being most effective in jaw-opening dystonia compared to other forms [12], [13].
As with most forms of dystonia, the majority patients with OMD belong to the idiopathic category, accounting for 60–80 % of the reported cases [5], [14]. Tardive dystonia represents the most common cause of secondary OMD, frequently associated with lingual dystonia and stereotypic movements in the limbs, and occasionally with akathisia and respiratory dyskinesias. [5], [15]. Less commonly, OMD can occur as an accompanying manifestation of neurodegenerative disorders (e.g., Wilson’s disease, neurodegeneration with brain iron accumulation), post-anoxic states, and focal brain or brainstem lesions.
3. Treatment options of oromandibular dystonia
Proper treatment of OMD requires multidisciplinary evaluation [16], [17]. All patients with OMD should have an oral and dental evaluation and those with dysphagia should receive a swallowing and nutritional assessment to determine oropharyngeal function and nutritional status. Thus, patients with disabling symptoms, or daily function interference, should be evaluated for therapeutic interventions. Treatments of OMD should not rely on responses to sensory tricks alone as they are often unable to provide meaningful functional benefits and interfere with normal functional activities [18]. Indeed, therapeutic options of OMD are multimodal, ranging from oral medications, BoNT, muscle afferent block, occlusal splint, and surgical therapies. Amongst the different classes of oral medications, clonazepam and anticholinergics benzhexol seem to be the most effective, with both typical and atypical antipsychotics, dopamine depletors, levodopa, and baclofen also utilised. However, none of these agents has been tested in rigorously controlled clinical trials [19]. When evaluated in patients with segmental cranio-cervical dystonia, the blepharospasm component was found to be more responsive than the oromandibular region although this clinical impression is only supported by limited evidence involving a small number of patients and short follow-up visits [20], [21]. Tetrabenazine was also found to be efficacious in some patients with OMD, particularly those with tardive OMD or when used in combination with lithium and trihexyphenidyl [22], [23]. Other options include sodium valproate and zolpidem, but benefits are only demonstrated in a few single studies, and the evidence for levetiracetam as a treatment of OMD is conflicting [24], [25], [26], [27], [28]. Muscle afferent block by intramuscular injection of lidocaine and alcohol was proposed as a treatment of drug-resistant OMD, but again the evidence is limited to a small study demonstrating a 57 % improvement in a self-rating scale and the measurement of maximal mouth opening [29]. When the authors claimed that this muscle afferent block caused less muscle weakness compared to BoNT injection, the duration of effect was shorter and further study is needed to determine the long-term efficacy and benefit [29]. Evidence is also emerging for the use of deep brain stimulation of the globus pallidus interna and subthalamic nucleus with a recent meta-analysis demonstrating efficacy in patients with refractory craniocervical dystonia [30].
Despite a lack of well-controlled clinical trials, recent systematic reviews, evidence-based reviews and clinical experience strongly regards BoNT as the first-line treatment for OMD regardless of its clinical presentation [1], [6], [7], [8], [18], [19], [31]. When the evidence was classified by the American Academy of Neurology on its quality for the treatment of OMD, abobotulinumtoxinA (Abo-BoNT) and onabotulinumtoxinA (Ona-BoNT) were given a level C recommendation (possibly effective in the specified population) while incobotulinumtoxinA (Inco-BoNT) and rimabotulinumtoxinB (Rima-BoNT) had a level U recommendation (data is inadequate and treatment is unproven) (Table 1) [32]. As a result, BoNT dosages provided in this article are limited to Ona-BoNT and Abo-BoNT, with additional recommendations, where available, from consensus guidelines [33] and the author’s own clinical experience. However, these tables do not take into account the published literature suggesting dose equivalence of Ona-BoNT (Botox®) and Abo-BoNT (Dysport®), which is reported to vary between 1:2.5 and 1:6 and 1:1 for Ona-BoNT (Botox®) and Inco-BoNT (Xeomin®) [34]. Dosages for Rima-BoNT were only included when the specific references were available. Table 2 provides details of studies with BoNT in patients with OMD [11], [14], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]. In the largest prospective long-term study, a mean total duration of response of Ona-BoNT was demonstrated to last up to 16.4 (±7.1) weeks with the best response obtained in patients with jaw-closing OMD.
Table 1.
Levels of Evidence for the treatment of oromandibular dystonia with botulinum toxin according to the American Academy of Neurology classification of evidence.
| Botulinum Toxin (Strain) | Level of Evidence |
|---|---|
| Ona-BoNT | C |
| Abo-BoNT | C |
| Inco-BoNT | U |
| Rima-BoNT | U |
C = Possibly effective, ineffective, or harmful (or possibly useful/predictive or not useful/predictive) for the given condition in the specified population. Requires at least one Class II study or two consistent Class III studies.
U = Data inadequate or conflicting; given current knowledge, treatment (test, predictor) is unproven. Assigned in cases of only one Class III study, only Class IV studies, or evidence that is conflicting and cannot be reconciled.
Table 2.
Clinical trials of botulinum toxin in oromandibular dystonia.
| Reference |
No. patients (Diagnosis) |
Neurotoxin products and injection techniques | Reported outcomes | Duration of benefit |
|---|---|---|---|---|
| Randomized controlled trials | ||||
| Jankovic & Orman [35] |
3 (OMD) | Ona-BoNT No guidance technique |
20 % improvement on blinded examiner rating and videotape scoring. 6.7 % improvement on subjective self-assessment rating The duration of efficacy lasted 5.6 weeks. |
12.5 weeks (5–28) |
| Open-label trials | ||||
| Brin et al. [37] | 5 (4 Jaw-closing and 1 lingual OMD) | Ona-BoNT Direct laryngoscopy and Laryngeal electromyography |
3 out of 4 patients with jaw-closing OMD and one patient with lingual OMD reported motor improvement on a qualitative scale (0–3). | 2.5–3 months |
| Blitzer et al. [38] | 20 (OMD) | Ona-BoNT Laryngeal EMG |
19 patients reported benefit with an average 47 % improvement by patient self-assessment. | Not available |
| Hermanowicz and Truong [39] | 5 (4 jaw-closing OMD, 1 lingual OMD) | Ona-BoNT EMG guidance |
All patients reported mild-to-marked clinical improvement on a four-point scale. | Not available |
| Van den Bergh et al. [40] | 12 (5 OMD, 7 Meige’s syndrome) |
Abo-BoNT EMG guidance |
6 out of 12 patients showed marked improvement on a subjective rating scale (0–5). | 27.0 ± 4.5 weeks |
| Prospective, observational studies | ||||
| Tan and Jankovic [14] | 162 (jaw-opening, jaw-closing OMD) | Ona-BoNT No guidance technique |
67.9 % of patients reported definite functional improvement as shown by Global Rating Scale. | 16.4 ± 7.1 |
| Nastasi et al. [42] | 50 (jaw opening, jaw closing, jaw deviation, lingual) |
Ona-BoNT or Abo-BoNT EMG guidance |
Significant improvement in the OMDQ-25 at 1 and 2 months. | 5.9 ± 4.3 |
| Retrospective studies | ||||
| Gonzalez-Alegre et al. [11] | 23 (12 jaw-opening, 11 jaw-closing) |
Ona-BoNT No guidance technique |
All patient with jaw-closing OMD and 71 % of patient with jaw-opening OMD demonstrated improvement in global impression scale. |
Not Given |
| Sinclair et al. [41] | 59 (Jaw-opening, jaw-closing, jaw deviation) |
Ona-BoNT EMG guidance |
66 % of patients continued treatment. | Not available |
OMD: Oromandibular dystonia; Ona-BONT: OnabotulinumtoxinA, Abo-BoNT: AbobotulinumtoxinA; EMG: Electromyography; OMDQ-25: Oromandibular Dystonia Questionnaire-25.
4. Patient selection for botulinum toxin and examination techniques
Patients with OMD require careful examination to determine the patterns of muscle contractions to enable classification into various subtypes (Table 3). The examination should include both patient at rest with eyes open and eyes closed, and when patients are performing different actions to determine the effects of speaking, chewing, and any exacerbating activities. The tongue should be examined whilst at rest on the floor of the mouth as well as in protrusion, when moving side-to-side, and during speech. The effects of sensory tricks in alleviating dystonic activities should also be noted. In addition, patient’s self-reported observations should not be neglected as certain abnormalities may not be evident during clinical examination. In most OMD patients, substantial benefits can be achieved by limiting injections to the most affected muscles (Table 4). Although no controlled trials were available on which OMD subtype responds best to BoNT, most literature advocates those with jaw-closing OMD as better responders than those with jaw-opening OMD [1], [6], [14], [45]. BoNT therapy for lingual dystonia is more challenging due to the complexity of injecting the delicate underlying muscle fibres and their propensity for dysphagia and dysarthria. Additional injections may be needed for accompanying blepharospasm and cervical dystonia, details of which are available in separate chapters. Table 4 has been modified from the original publication to provide lists of muscles involved in different subtypes of OMD, their respective function, and the corresponding dosages of Ona-BoNT and Abo-BoNT [1].
Table 3.
Muscles in the oromandibular region and their respective functions.
| Muscle name | Function |
|---|---|
| Temporalis | Closes the jaw |
| Posterior fibres retract the mandible | |
| Moves the jaw to the ipsilateral side | |
| Masseter | Closes the jaw by elevating the mandible |
| Retraction of the jaw | |
| Medial Pterygoid | Closes the jaw |
| Protrudes the jaw | |
| Moves the jaw to the contralateral side | |
| Lateral Pterygoid | Opens the mouth |
| Protrudes the jaw | |
| Moves the jaw to the contralateral side | |
| Digastric | Opens the jaw |
| Elevates the hyoid bone | |
| Mylohyoid | Opens the jaw |
| Raises the floor of the mouth | |
| Geniohyoid | Opens the jaw |
| Elevates and draws hyoid bone forward | |
| Stylopharyngeus muscle | Pulls the nasopharyngeal wall dorsally |
| Salpingopharyngeus muscle | Raises the pharynx and larynx during swallowing |
| Laterally draws the pharynx walls up | |
| Palatopharyngeus muscle | Elevates the pharynx superiorly, anteriorly, and medially during swallowing |
| Platysma | Assists forced depression of the mandible, and depresses skin of the lower face with a minor role in lip depressor function |
Table 4.
Oromandibular dystonia subtypes, identification of primary muscles and botulinum toxin applications.
| Subtypes | Muscles involved | Dosage per side (Units) | |
|---|---|---|---|
| Ona-BoNT, Inco-BoNT | Abo-BoNT | ||
| Jaw closing | Temporalis | 20–30 | 100 |
| Masseter | 30 | 100 | |
| Medial pterygoid | 20 | 30 | |
| Jaw opening | Lateral pterygoid | 20–40 | 60 |
| Mylohyoid | 20 | 90 | |
| Digastric muscle | |||
| Geniohyoid | |||
| Jaw deviation | Contralateral lateral pterygoid |
20–40 | 60 |
| Ipsilateral temporalis | 20–30 | 100 | |
| Lingual dystonia | Genioglossus | 10 | 30 |
| Perioral dystonia | Orbicularis oris | 1.25–2.5/injection site |
5–10/injection site |
| Zygomaticus | |||
| Mentalis | |||
| Risorius | |||
| Levator anguli oris | |||
| Depressor anguli oris | |||
| Platysma | 20 | 60 | |
| Pharyngeal dystonia |
Stylopharyngeus muscle | 5–10 | 30 |
| Salpingopharyngeus muscle | |||
| Palatopharyngeus muscle | |||
Note: The dosage provided for Inco-BoNT was demonstrated as an equivalent to Ona-BoNT. However, the study using Inco-BoNT in OMD was not available.
Ona-BoNT: OnabotulinumtoxinA; Abo-BoNT: AbobotulinumtoxinA, Inco-BoNT: IncobotulinumtoxinA.
5. Injection techniques
The oromandibular region is small with several muscle groups and other delicate anatomical structures, therefore, a targeting technique is generally recommended to enable precise localisation of affected muscles in OMD. Although these techniques (EMG and ultrasonography) are not an absolute requirement for all muscles involved, they were utilised in most reported BoNT studies in OMD, and in certain muscles (such as pterygoid muscles), the use of targeting technique is strongly recommended and considered to be mandatory by most experts [7]. As there is a lack of controlled trials and a significant heterogeneity of clinical presentation of OMD, the discussion that follows, which is subdivided according to main clinical types, is primarily based on the clinical experience of the authors, supplemented by literature review.
5.1. Jaw-closing oromandibular dystonia
For jaw-closing OMD, the masseter is often the initial muscle selected for denervation. If the response is not adequate, other muscles can be considered, including the temporalis and medial pterygoid muscles. Injection is individualized for each patient and EMG guidance is left as a last resort to identify deep muscles which are not available to manual palpation [1].
The masseter is a thick quadrilateral muscle consisting of three parts—superficial, intermediate and deep—which arise from the zygomatic arch and insert into the angle and the lateral surface of the ramus of the mandible [46] (Fig. 1). It is easily palpable by instructing the patient to clench their teeth. The masseter can be approached by using a Teflon-coated needle connected to an EMG machine at 1 cm anterior to the posterior border of the ramus. The muscle discharge, when the patient clenches their teeth, also helps to localize the insertion and avoid the parotid gland, which extends from the ear to the masseter and partially covers the posterior part of this muscle. A good starting dose is 30 units of Ona-BoNT (Botox®) or 100 units of Abo-BoNT (Dyport®). The recommended dosage from the consensus guidelines is between 20 and 60 units of Ona-BoNT (Botox®) on each side. Dosage recommendations with Rima-BoNT (Myobloc®) are only available from two non-English journals, suggesting 2500 units of Rima-BoNT for each masseter muscle [1].
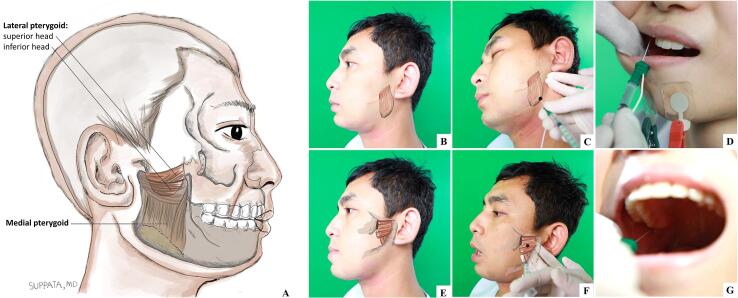
Fig. 1.
(A) View of the medial and lateral pterygoid muscles, showing its origin and insertion; (B-D) Surface anatomy (B), mandibular approach (C), and intraoral approach (D) for botulinum toxin injection of medial pterygoid muscle; (E-G) Surface anatomy (E), lateral approach (F), and intraoral approach (G) for botulinum toxin injection of lateral pterygoid muscle. Black dots denote approximate injection sites of medial (C) and lateral (F) pterygoid muscles.
The medial pterygoid occupies the inner aspect of the ramus of the mandible opposite that of the masseter. It arises from the lateral pterygoid plate and the pyramidal process of the palatine bone and inserts into the lower and back part of the medial surface of the ramus and angle of the mandible [46] (Fig. 1B-D). The medial pterygoid can be very active in jaw-closing OMD as a result of the so-called “whack-a-mole phenomenon” after repeated BoNT injections into the masseter and temporalis muscles [47]. Due to its deep location, its injection often requires EMG guidance to avoid the risk of complications, such as haematoma or arterial bleeding. The medial pterygoid can be approached either intraorally or extraorally. For an intraoral approach, the muscle can be palpated at the inner aspect of the mandibular ramus with a mouth opening position. The needle should be inserted at the medial aspect of the mandibular ramus and angulated posteriorly and superiorly, about 20 degrees to the occlusal plane. The EMG activity should confirm the corrected insertion area during the mouth closing (Fig. 1D) [47].
For extraoral or mandibular approaches, the needle is pointed from below and inserted about 0.5–1 cm anterior to the angle of the mandible along the interior aspect of the mandible and angled perpendicularly to the mandible until it can be verified by EMG with the patient clenching their teeth (Fig. 1C). Care should be taken to avoid the facial artery, which lies anteriorly. A good starting dose (per side) is 20 units of Ona-BoNT (Botox®) or 30 units of Abo-BoNT (Dysport®), or 1000 units of Rima-BoNT (Myobloc®) [1].
The third muscle involved the jaw-closing OMD is the temporalis muscle (Fig. 1). This broad, radiating muscle arises from the temporal fossa. Its tendon inserts into the medial surface, apex and anterior border of the coronoid process and the anterior border of the ramus of the mandible [46]. The temporalis closes the jaw, and its posterior fibers retract the mandible. The temporalis is approached perpendicular to its plane and as high as possible in the temporal fossa as the lower part of the temporalis is mostly tendon where injection can be painful. Due to its wide radiation pattern, 3–4 injections should be given. The recommended dosage (per side) by the consensus guideline is between 20 and 80 units of Ona-BoNT (Botox®) [33]. The authors, however, opt for around 20 to 30 units of Ona-BoNT (Botox®) per side. Starting dose for Abo-BoNT (Dysport®) is about 100 units per side and adjusted according to patient’s response [40].
5.2. Jaw-opening oromandibular dystonia
The muscles involved in jaw-opening include the lateral pterygoid, mylohyoid, digastric, geniohyoid and platysma. Opening of the jaws is performed primarily by the lateral pterygoid muscle. However, at the start of opening, it receives assistance from the submentalis complex which includes the mylohyoid, digastric and geniohyoid [46]. The platysma may also play a minor role in the opening of the jaw. Most investigators reported injections of the lateral pterygoid in jaw-opening OMD, although others claim success with injection of the submentalis complexes only [1].
The lateral pterygoid is a short conical muscle which arises by two heads, a superior from the great wing of the sphenoid bone and an inferior from the lateral surface of the pterygoid plate of the sphenoid (Fig. 1E-G) [46]. The inferior heads pull the mandibular condyle anteriorly, which results in clenching, contralateral, ipsilateral, protrusive, retrusive and open-close jaw movements. On the contrary, the superior heads pull the articular capsule anteriorly which results in contralateral, protrusive and opening jaw movements [48]. The lateral pterygoid muscle can be approached intraorally or laterally through the mandibular incisure. In the intraoral approach, patients were asked to open the mouth and deviate to the opposite side, and the needle was inserted at a point above the mucobuccal fold above the second molar (Fig. 1G) [47]. However, the intraoral approach can be challenging in patients with high-frequency movement or widely displacing movements of the mandible [44]. For the extraoral or lateral approach under an EMG guidance, the needle is angled upward at about 15 degrees to reach the inferior head of the lateral pterygoid with an entry point about 35 mm from the external auditory canal and 10 mm from the inferior margin of the zygomatic arch (Fig. 1F). In close proximity, but more rostral, is the pterygoid branch of the maxillary artery. The amount of toxin reported to be effective in the literature ranges from 20 units to 40 units of Ona-BoNT (Botox®) per side [38], [49]. There are limited experiences reported with Abo-BoNT (Dysport®), and we recommend a starting dose of about 60 units per side, which can be titrated up if needed.
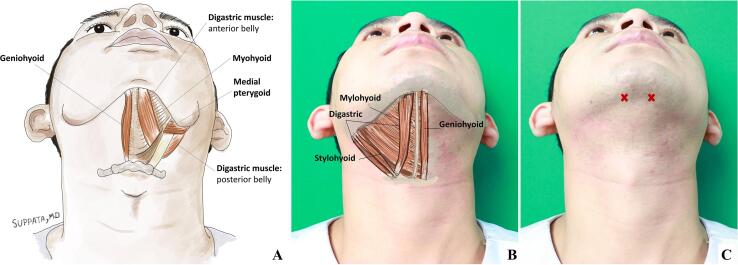
The digastric muscle is part of the submentalis complex. It arises from the mastoid notch of the temporal bone and attaches to digastric fossa of the mandible (Fig. 2). It is divided into the anterior and posterior belly by the middle tendon, which is attached to the hyoid bone [46]. Besides elevating the hyoid bone, the digastric pulls the chin backwards and downwards during opening of the mouth in conjunction with the lateral pterygoid. In contrast to the posterior belly, which is crowded with many nerves, sympathetic trunks, arteries and veins, the anterior belly is open to intervention. The geniohyoid arises from the hyoid bone and inserts into the inferior genial tubercle of the mandible. It elevates the hyoid bone and base of the tongue. With the hyoid bone fixed, it depresses the mandible and opens the mouth. The mylohyoid arises from the hyoid bone as well and attaches to the mylohyoid line on the mandible (Fig. 2). It raises the floor of the mouth during swallowing. The mylohyoid elevates the hyoid bone, thereby pushing the tongue upward or causing protrusion of the tongue [46]. It also assists in opening of the mouth. Muscles of the submentalis complex may be fused together rendering them difficult to be separated from one another [46]. This muscle group can be palpated when the patient opens their mouth. It is approached about 1 cm from the mandible tip and injected slightly lateral from the midline (Fig. 2B-C). A good starting dose of Ona-BoNT (Botox®) is 20 units per side. These units are divided and injected into two locations on each side. Higher doses of up to 200 units for the submentalis complex have been reported, but the risk of severe dysphagia is considerable. For Abo-BoNT (Dysport®), 90 units per side is a good starting dose and for Rima-BoNT (Myobloc®) about 500 units.
Fig. 2.
(A) Inferior view of the floor of the mouth, showing the submentalis complex; (B) Surface anatomy of submentalis complex; (C) The sign X denotes approximate injection sites of digastric muscles.
In some patients, injection of the platysma can give additional improvement. This muscle depresses the mandible and soft tissues of the lower face as well as tensing the skin of the neck. The platysma fascicles can be easily identified with visual inspection. Often each platysma is injected with 20 units of Ona-BoNT (Botox®), 60 units of Abo-BoNT (Dysport®), or 1000 units of Rima-BoNT (Myobloc®). The recommended dosage from the consensus guidelines is between 20 and 40 units of Ona-BoNT per side [33].
5.3. Jaw-deviating oromandibular dystonia
The contralateral lateral pterygoid works in conjunction with the contralateral medial pterygoid to deviate the mouth from one side to the other. The temporalis pulls the jaw to the same side. The injections follow the above-mentioned techniques.
5.4. Lingual oromandibular dystonia
In a large series of 172 patients with lingual OMD, four subtypes were identified based on patterns of involuntary movements, including protrusion (thrusting, 68.6 %), retraction (16.9 %), curling (rolling, 7.6 %), and laterotrusion (7.0 %) [50]. Movements of the tongue are delicate, requiring the coordinated function of various extrinsic muscles of the tongue, consisting of the genioglossus, hyoglossus, chondroglossus, styloglossus and palatoglossus, so careful examination of the tongue is essential for successful BoNT treatment. Tongue thrusting, one of the movements often encountered in oromandibular dystonia, is due to the action of the posterior fibers of the genioglossus, whereas the anterior fibers draw the tongue back into the mouth [45], [46]. The genioglossus can be accessed via the submandibular route. Under EMG guidance, the genioglossus can be identified through the digastric muscle at 2 cm in depth. Suggested initial doses, per side, are 10 units of Ona-BoNT (Botox®), or 30 units of Abo-BoNT (Dysport®) respectively. There is no known experience of Rima-BoNT (Myobloc®) with this muscle. Experiences vary on what subtype was the most responsive to BoNT injections. One note of caution when injecting lingual muscles is that the therapeutic window is quite narrow, meaning that doses slightly above the therapeutic level can induce disabling weakness associated with severe dysphagia.
5.5. Perioral oromandibular dystonia
There is no standardised approach for BoNT injections in perioral dystonia. The muscle involved, the orbicularis oris, is an intricate facial expression muscle that consists of fibres from various facial muscles including the buccinator, superior levator labii, inferior depressor labii, zygomaticus major, mentalis, and inferior incisivus labii. As these muscles are superficial and attached to the skin, a subcutaneous approach should be used similar to the standard procedure for blepharospasm. Only limited evidence is available from two small studies employing different BoNT techniques and injection sites which demonstrated significant reduction of the symptoms.
5.6. Pharyngeal oromandibular dystonia
The pharyngeal muscles consist of the three constrictor muscles and the stylo-, salpingo-, and palatopharyngeus. The three constrictors are superior, middle, and inferior constrictors and exercise general sphincteric and peristaltic actions in swallowing. Pharyngeal OMD often involves the constrictor pharynges, with patients often complaining of choking and swallowing difficulties. In addition, pharyngeal OMD often occurs with spasmodic dysphonia. We have noted that sometimes, after treatment of spasmodic dysphonia, there is also unexpected improvement of pharyngeal dystonia. As treatments of constrictor pharynges are almost invariably associated with dysphagia, injections of these muscles are seldom performed. Dosage used per side is 5–10 units of Ona-BoNT (Botox®) or 30 units of Abo-BoNT (Dysport®).
5.7. Adverse events
In a recent meta-analysis, adverse events were identified in 27 % of patients with dysphagia with the most common attributed to BoNT spread to contiguous muscles involved in swallowing [7]. Other commonly reported adverse events were chewing weakness, dysarthria (especially with tongue injections), pain, lip numbness, and dry mouth, caused by diffusion into salivary glands [7], [8], [51]. When muscle selection is done carefully and dosing made conservatively, excessive weakness can be minimized. As BoNT labeling of all formulations includes a box warning on potential spreading of toxin effect, remote side effects are always possible, but observed to be very rare, and include generalized weakness, allergic reactions, flu-like symptoms, or even documentation of EMG abnormalities distant to the site of toxin injection. To reduce the risk of xerostomia, good knowledge of anatomy is essential to avoid unintended injections to the parotid glands that overlie the posterior border of masseter muscles. Another adverse event that is probably not frequently mentioned in the literature, but probably occurs more frequently in author’s experience, is lower facial asymmetry and an asymmetric smile, which occur when BoNT diffuses to the risorius and levator anguli oris muscles. Therefore, injections should be targeted at sites that are far from the anterior borders of these muscles.
6. Conclusions
The development of botulinum toxin has markedly altered the treatment of OMD in recent years, with proposed classification of subtyping, clinical evaluations, muscle targeting techniques, and evidence-based literature published. Even though BoNT is still not officially approved by the US Food and Drug Administration for OMD, it is recommended as the first-line therapy by most experts and evidence-based literature. However, this chapter highlights the need for further controlled studies of various BoNT formulations in OMD to establish robust efficacy and to document potential adverse events associated with such injections. Treating physicians should be diligent when evaluating patients with OMD, performing careful clinical observation and examination as well as evaluating functional interferences (e.g., chewing, speaking), affected by dystonic movements. Once a patient’s candidacy for BoNT is established, careful stepwise planning should begin by selecting the muscles that are primarily responsible and employing a conservative approach to dosing titration. As muscles involved in OMD are small, delicate, and situated in closed proximity, adverse events due to BoNT spreading may occur and close follow-up visits are advisable, especially for the first few injection cycles. Patients should be involved in outcome selections and priority should be given to outcomes that are directly related to disturbed daily functions. Future studies in OMD should aim to harmonize candidate selections, injection protocols and outcome assessments to deliver robust efficacy to gain therapeutic approval for this much needed disorder.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Roongroj Bhidayasiri is supported by Senior Research Scholar Grant (RTA6280016) of the Thailand Science Research and Innovation (TSRI), International Research Network Grant of the Thailand Research Fund (IRN59W0005), Chulalongkorn Academic Advancement Fund into Its 2nd Century Project of Chulalongkorn University, and Centre of Excellence grant of Chulalongkorn University (GCE 6100930004-1), Bangkok, Thailand
References
- 1.Bhidayasiri R., Cardoso F., Truong D.D. Botulinum toxin in blepharospasm and oromandibular dystonia: comparing different botulinum toxin preparations. Eur. J. Neurol. 2006;13(Suppl 1):21–29. doi: 10.1111/j.1468-1331.2006.01441.x. [DOI] [PubMed] [Google Scholar]
- 2.LeDoux M.S. Meige syndrome: what's in a name? Parkinsonism Relat. Disord. 2009;15(7):483–489. doi: 10.1016/j.parkreldis.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bereau M., Tatu L. Brueghel Syndrome or Meige Syndrome? Two Sides of a Same Disease. Front. Neurol. Neurosci. 2018;41:98–103. doi: 10.1159/000475701. [DOI] [PubMed] [Google Scholar]
- 4.Tolosa E., Marti M.J. Blepharospasm-oromandibular dystonia syndrome (Meige's syndrome): clinical aspects. Adv. Neurol. 1988;49:73–84. [PubMed] [Google Scholar]
- 5.Scorr L.M., Factor S.A., Parra S.P., Kaye R., Paniello R.C., Norris S.A., Perlmutter J.S., Baumer T., Usnich T., Berman B.D., Mailly M., Roze E., Vidailhet M., Jankovic J., LeDoux M.S., Barbano R., Chang F.C.F., Fung V.S.C., Pirio Richardson S., Blitzer A., Jinnah H.A. Oromandibular Dystonia: A Clinical Examination of 2,020 Cases. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.700714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comella C.L. Systematic review of botulinum toxin treatment for oromandibular dystonia. Toxicon. 2018;147:96–99. doi: 10.1016/j.toxicon.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Dadgardoust P.D., Rosales R.L., Asuncion R.M., Dressler D. Botulinum neurotoxin a therapy efficacy and safety for oromandibular dystonia: a meta-analysis. J. Neural. Transm. (Vienna). 2019;126(2):141–148. doi: 10.1007/s00702-018-1960-7. [DOI] [PubMed] [Google Scholar]
- 8.Hassell T.J.W., Charles D. Treatment of Blepharospasm and Oromandibular Dystonia with Botulinum Toxins. Toxins (Basel). 2020;12(4):269. doi: 10.3390/toxins12040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nutt J.G., Muenter M.D., Aronson A., Kurland L.T., Melton L.J., 3rd Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov. Disord. 1988;3:188–194. doi: 10.1002/mds.870030302. [DOI] [PubMed] [Google Scholar]
- 10.Clark G.T., Ram S. Orofacial Movement Disorders, Oral Maxillofac. Surg. Clin. North Am. 2016;28(3):397–407. doi: 10.1016/j.coms.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 11.P. Gonzalez-Alegre, R.L. Schneider, H. Hoffman, Clinical, Etiological, and Therapeutic Features of Jaw-opening and Jaw-closing Oromandibular Dystonias: A Decade of Experience at a Single Treatment Center, Tremor Other Hyperkinet. Mov. (N. Y.). 4 (2014) 231. [DOI] [PMC free article] [PubMed]
- 12.Schramm A., Classen J., Reiners K., Naumann M. Characteristics of sensory trick-like manoeuvres in jaw-opening dystonia. Mov. Disord. 2007;22(3):430–433. doi: 10.1002/mds.21354. [DOI] [PubMed] [Google Scholar]
- 13.Lo S.E., Gelb M., Frucht S.J. Geste antagonistes in idiopathic lower cranial dystonia. Mov. Disord. 2007;22(7):1012–1017. doi: 10.1002/mds.21149. [DOI] [PubMed] [Google Scholar]
- 14.Tan E.K., Jankovic J. Botulinum toxin A in patients with oromandibular dystonia: long-term follow-up. Neurology. 1999;53:2102–2107. doi: 10.1212/wnl.53.9.2102. [DOI] [PubMed] [Google Scholar]
- 15.Tan E.K., Jankovic J. Tardive and idiopathic oromandibular dystonia: a clinical comparison. J. Neurol. Neurosurg. Psychiat. 2000;68:186–190. doi: 10.1136/jnnp.68.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida K. Botulinum Toxin Therapy for Oromandibular Dystonia and Other Movement Disorders in the Stomatognathic System. Toxins (Basel). 2022;14(4):282. doi: 10.3390/toxins14040282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maestre-Ferrin L., Burguera J.A., Penarrocha-Diago M., Penarrocha-Diago M. Oromandibular dystonia: a dental approach. Med. Oral Patol. Oral Cir. Bucal. 2010;15:e25–e27. [PubMed] [Google Scholar]
- 18.Saraf U., Chandarana M., Divya K.P., Krishnan S. Oromandibular Dystonia - A Systematic Review. Ann. Indian Acad. Neurol. 2022;25:26–34. doi: 10.4103/aian.aian_242_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma H., Qu J., Ye L., Shu Y., Qu Q. Blepharospasm, Oromandibular Dystonia, and Meige Syndrome: Clinical and Genetic Update. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.630221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hipola D., Mateo D., Gimenez-Roldan S. Meige's syndrome: acute and chronic responses to clonazepan and anticholinergics. Eur. Neurol. 1984;23:474–478. doi: 10.1159/000115731. [DOI] [PubMed] [Google Scholar]
- 21.Tanner C.M., Glantz R.H., Klawans H.L. Meige disease: acute and chronic cholinergic effects. Neurology. 1982;32:783–785. doi: 10.1212/wnl.32.7.783. [DOI] [PubMed] [Google Scholar]
- 22.Jankovic J., Ford J. Blepharospasm and orofacial-cervical dystonia: clinical and pharmacological findings in 100 patients. Ann. Neurol. 1983;13:402–411. doi: 10.1002/ana.410130406. [DOI] [PubMed] [Google Scholar]
- 23.Miguel R., Mendonça M.D., Barbosa R., Ladeira F., Lampreia T., Vale J., Bugalho P. Tetrabenazine in treatment of hyperkinetic movement disorders: an observational study. Ther. Adv. Neurol. Disord. 2017;10(2):81–90. doi: 10.1177/1756285616677004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An J.Y., Kim J.-S., Kim Y.-I., Lee K.S. Successful treatment of the Meige syndrome with oral zolpidem monotherapy. Mov. Disord. 2008;23(11):1619–1621. doi: 10.1002/mds.22179. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki Y., Sako W., Asanuma K., Izumi Y., Miki T., Kaji R. Efficacy of zolpidem for dystonia: a study among different subtypes. Front. Neurol. 2012;3:58. doi: 10.3389/fneur.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park J.E., Srivanitchapoom P., Maurer C.W., Mathew P., Sackett J., Paine R., Ramos V.L., Hallett M. Lack of efficacy of levetiracetam in oromandibular and cranial dystonia. Acta Neurol. Scand. 2017;136(2):103–108. doi: 10.1111/ane.12701. [DOI] [PubMed] [Google Scholar]
- 27.Snoek J.W., van Weerden T.W., Teelken A.W., van den Burg W., Lakke J.P. Meige syndrome: double-blind crossover study of sodium valproate. J. Neurol. Neurosurg. Psychiat. 1987;50:1522–1525. doi: 10.1136/jnnp.50.11.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zesiewicz T.A., Louis E.D., Sullivan K.L., Menkin M., Dunne P.B., Hauser R.A. Substantial improvement in a Meige's syndrome patient with levetiracetam treatment. Mov. Disord. 2004;19(12):1518–1521. doi: 10.1002/mds.20233. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida K., Kaji R., Kubori T., Kohara N., Iizuka T., Kimura J. Muscle afferent block for the treatment of oromandibular dystonia. Mov. Disord. 1998;13(4):699–705. doi: 10.1002/mds.870130416. [DOI] [PubMed] [Google Scholar]
- 30.Wang X., Zhang Z., Mao Z., Yu X. Deep brain stimulation for Meige syndrome: a meta-analysis with individual patient data. J. Neurol. 2019;266:2646–2656. doi: 10.1007/s00415-019-09462-2. [DOI] [PubMed] [Google Scholar]
- 31.Pandey S., Sharma S. Meige's syndrome: History, epidemiology, clinical features, pathogenesis and treatment. J. Neurol. Sci. 2017;372:162–170. doi: 10.1016/j.jns.2016.11.053. [DOI] [PubMed] [Google Scholar]
- 32.Hallett M., Albanese A., Dressler D., Segal K.R., Simpson D.M., Truong D., Jankovic J. Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon. 2013;67:94–114. doi: 10.1016/j.toxicon.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Dressler D., Altavista M.C., Altenmueller E., Bhidayasiri R., Bohlega S., Chana P., Chung T.M., Colosimo C., Fheodoroff K., Garcia-Ruiz P.J. Consensus guidelines for botulinum toxin therapy: general algorithms and dosing tables for dystonia and spasticity. J. Neur. Transm. 2021;128:321–335. doi: 10.1007/s00702-021-02312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benecke R., Frei K., Comella C.L. In: Manual of Botulinum Toxin Therapy. Truong D., Dressler D., Hallett M., editors. Cambridge University Press; 2009. Treatment of cervical dystonia; pp. 29–42. [Google Scholar]
- 35.Jankovic J., Orman J. Botulinum A toxin for cranial-cervical dystonia: a double-blind, placebo-controlled study. Neurology. 1987;37:616–623. doi: 10.1212/wnl.37.4.616. [DOI] [PubMed] [Google Scholar]
- 36.Lee S.J., McCall W.D., Jr., Kim Y.K., Chung S.C., Chung J.W. Effect of botulinum toxin injection on nocturnal bruxism: a randomized controlled trial. Am J Phys Med Rehabil. 2010;89:16–23. doi: 10.1097/PHM.0b013e3181bc0c78. [DOI] [PubMed] [Google Scholar]
- 37.Brin M.F., Fahn S., Moskowitz C., Friedman A., Shale H.M., Greene P.E., Blitzer A., List T., Lange D., Lovelace R.E., McMahon D. Localized injections of botulinum toxin for the treatment of focal dystonia and hemifacial spasm. Mov. Disord. 1987;2(4):237–254. doi: 10.1002/mds.870020402. [DOI] [PubMed] [Google Scholar]
- 38.Blitzer A., Greene P.E., Brin M.F., Fahn S. Botulinum toxin injection for the treatment of oromandibular dystonia. Ann Otol Rhinol Laryngol. 1989;98(2):93–97. doi: 10.1177/000348948909800202. [DOI] [PubMed] [Google Scholar]
- 39.Hermanowicz N., Truong D.D. Treatment of oromandibular dystonia with botulinum toxin. The Laryngoscope. 1991;101:1216–1218. doi: 10.1288/00005537-199111000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Van den Bergh P., Francart J., Mourin S., Kollmann P., Laterre E.C. Five-year experience in the treatment of focal movement disorders with low-dose Dysport botulinum toxin. Muscle Nerve. 1995;18:720–729. doi: 10.1002/mus.880180708. [DOI] [PubMed] [Google Scholar]
- 41.Sinclair C.F., Gurey L.E., Blitzer A. Oromandibular dystonia: Long-term management with botulinum toxin. The Laryngoscope. 2013;123:3078–3083. doi: 10.1002/lary.23265. [DOI] [PubMed] [Google Scholar]
- 42.Nastasi L., Mostile G., Nicoletti A., Zappia M., Reggio E., Catania S. Effect of botulinum toxin treatment on quality of life in patients with isolated lingual dystonia and oromandibular dystonia affecting the tongue. J. Neurol. 2016;263(9):1702–1708. doi: 10.1007/s00415-016-8185-1. [DOI] [PubMed] [Google Scholar]
- 43.Singer C., Papapetropoulos S. A comparison of jaw-closing and jaw-opening idiopathic oromandibular dystonia. Parkinsonism Relat. Disord. 2006;12(2):115–118. doi: 10.1016/j.parkreldis.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Teemul T.A., Patel R., Kanatas A., Carter L.M. Management of oromandibular dystonia with botulinum A toxin: a series of cases. Br J Oral Maxillofac Surg. 2016;54(10):1080–1084. doi: 10.1016/j.bjoms.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 45.Yu G.L.T., Rosales R.L. Treatment of oromandibular dystonia using botulinum toxin injections–Case series and illustrative muscle targeting. Basal Ganglia. 2018;13:7–16. [Google Scholar]
- 46.Clemente C. In: Gray's Anatomy. Clemente C., editor. Lea & Feabiger; Philadelphia: 1984. Muscles and fasciae. [Google Scholar]
- 47.K. Yoshida, How do I inject botulinum toxin into the lateral and medial pterygoid muscles?, Movement Disorders Clinical Practice. 4 (2017) 285-285. [DOI] [PMC free article] [PubMed]
- 48.Bhutada M.K. Functions of the lateral pterygoid muscle. Ann R Australas Coll Dent Surg. 2004;17:68–69. [PubMed] [Google Scholar]
- 49.R. Laskawi, S. Rohrbach, [Oromandibular dystonia. Clinical forms, diagnosis and examples of therapy with botulinum toxin], Laryngorhinootologie. 80 (2001) 708-713. [DOI] [PubMed]
- 50.Yoshida K. Botulinum Neurotoxin Therapy for Lingual Dystonia Using an Individualized Injection Method Based on Clinical Features. Toxins (Basel). 2019;11(1):51. doi: 10.3390/toxins11010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dutton J.J. Botulinum-A toxin in the treatment of craniocervical muscle spasms: short- and long-term, local and systemic effects. Surv. Ophthalmol. 1996;41:51–65. doi: 10.1016/s0039-6257(97)81995-9. [DOI] [PubMed] [Google Scholar]