Summary
Background
The evidence of early treatment for radiation-induced brain necrosis (RN) in head and neck cancer survivors remains insufficient. This study aimed to determine whether early anti-RN treatment was associated with lower mortality.
Methods
In this cohort study, we utilized data from the Study in Radiotherapy-related Nervous System Complications (NCT03908502) and Hong Kong Cancer Registry. We included consecutive patients who had received radiotherapy (RT) for head and neck cancers and had subsequently developed RN between Jan 8, 2005 and Jan 19, 2020. Patients who had tumor progression before the diagnosis of RN, underwent surgical brain necrosis lesions resection before corticosteroids and/or bevacizumab treatment, had intracranial metastases before the diagnosis of RN, lacked follow-up data, or had a follow-up period of less than three months were excluded. Individual-level data were extracted from electronic medical records of the above-mentioned registries. The primary outcome was all-cause death. The vital status of each patient was confirmed through a standardized telephone interview. We compared patients who received early treatment (initiating bevacizumab or corticosteroids treatment within three months after RN diagnosis) with patients who did not (following a “watch-and-wait” policy).
Findings
Of 641 eligible patients, 451 patients (70·4%) received early treatment after RN diagnosis and 190 patients (29·6%) did not. Overall, 112 patients (17·5%) died, of whom 73 (16·2%) in the early treatment group and 39 (20·5%) in the watch-and-wait group, during a median follow-up of 3·87 years. The early treatment group showed a lower risk of all-cause death compared with the watch-and-wait group after adjusting for age, sex, absence or presence of neurological symptoms at baseline, RN lesion features on brain magnetic resonance imaging, history of stroke, prior tumor-related characteristics (TNM stage, RT dose and techniques, and chemotherapy), and the time interval from RT to RN (HR 0·48, 95%CI 0·30 to 0·77; p = 0·0027), and extensive sensitivity analyses yielded similar results. There was no significant difference in the effect of early treatment on post-RN survival among subgroups stratified by presence or absence of neurological symptoms at diagnosis (p for interaction=0·41).
Interpretation
Among head and neck cancer survivors with RN, initiating treatment early after RN diagnosis is associated with a lower risk of all-cause mortality as compared with following the watch-and-wait policy, irrespective of whether patients exhibit symptoms or not. Further prospective randomised studies would be needed to validate our findings since the observational study design might lead to some potential confounding. In the absence of data from randomised trials, our study will have an important implication for clinicians regarding the optimal timing of treatment for RN, and provides the foundation and supporting data for future trials on this topic.
Funding
National Natural Science Foundation of China (81925031, 81820108026, 81872549, 81801229, 82003389), the Science and Technology Program of Guangzhou (202007030001), Young Teacher Training Program of Sun Yat-sen University (20ykpy106), Key-Area Research and Development Program of Guangdong Province (2018B030340001), the National Medical Research Council Singapore Clinician Scientist Award (NMRC/CSA-INV/0027/2018, CSAINV20nov-0021), the Duke-NUS Oncology Academic Program Goh Foundation Proton Research Programme, NCCS Cancer Fund, the Kua Hong Pak Head and Neck Cancer Research Programme, and the National Research Foundation Clinical Research Programme Grant (NRF-CRP17-2017-05).
Keywords: Radiotherapy, Radiation-induced brain necrosis, Head and neck cancer, Mortality, Early treatment
Research in context.
Evidence before this study
We previously searched PubMed for articles published in English from database inception up to Jan 1, 2020, and only comparative studies, multicentre studies, observational studies, and randomised controlled trials were included. The search terms were “(radiation-induced brain injury OR radiation-induced brain necrosis OR radiation-induced temporal lobe necrosis) AND (therapy OR treatment OR corticosteroids OR Pulsed steroid OR steroid OR bevacizumab) AND (mortality OR survival OR prognosis OR efficacy OR effectiveness OR outcome)”. We found 75 articles, from which we identified no relevant study focusing on optimal treatment timing on long-term prognosis for radiation-induced brain necrosis (RN). Our previous head-to-head, randomised controlled trial recognized the efficacy and safety of bevacizumab and corticosteroids in reducing RN and improving clinical outcomes; another prediction model study, using a random forest model, revealed that the duration between radiotherapy (RT) and bevacizumab treatment and the duration between RT and RN diagnosis were highly ranked predictors for RN recurrence after bevacizumab treatment. The prior studies seem to suggest that earlier initiation of treatment is beneficial for patients with RN, however, there is no definitive answer to whether early treatment is associated with improved survival outcomes yet.
Added value of this study
To our knowledge, this study is the first, as well as the largest, cohort study of RN to investigate the survival outcomes of different treatment timing in patients with newly diagnosed RN, and it provides novel evidence about the long-term effectiveness of early treatment with corticosteroids or bevacizumab. Early treatment for patients with RN who met indications of these agents was associated with a lower risk of death, compared with a watch-and-wait strategy.
Implications of all the available evidence
The results of the present study suggest that early treatment, initiated within three months after the diagnosis of RN, significantly reduced the risk of all-cause death for patients with newly diagnosed RN. In the absence of randomised clinical trials, our study will have an important implication for clinicians regarding the optimal timing of treatment for RN, and provides the foundation and supporting data for future trials on this topic.
Alt-text: Unlabelled box
Introduction
Radiotherapy (RT) is an integral modality in the treatment of head and neck cancers, especially for nasopharyngeal carcinoma.1 However, radiation may lead to brain necrosis, which is usually a severe, irreversible or even life-threatening cerebral complication due to healthy brain tissue damage in or around the RT fields.2, 3, 4 Common neurological symptoms among patients with radiation-induced brain necrosis (RN) include headache, seizure, cognitive dysfunction, focal neurological deficits, and others.5,6
The incidence of RN varies with different primary tumor sites, RT techniques and dose to brain, duration of follow-up and detection methods (with magnetic resonance image [MRI] being more sensitive than computed tomography), ranging widely from 2·5 to 24%.5,7,8 With the continual improvement in cancer treatment and development of imaging technology, a higher incidence of RN may be anticipated due to improved RT treatment and earlier detection of brain lesions.9 Determining the best strategies to manage RN are needed for long-term cancer survivors.
The optimal timing of initiating medical treatment for RN is a challenging clinical decision for radiation oncologists, neurooncologists and neurologists. Patient symptom, performance status, co-morbidities, and the tempo of RN progression are important factors that need to be considered. The majority of RN lesions progress either clinically or radiologically, while others may remain stable for years.10 Thus, an important issue is whether patients with RN should receive early medical treatment following a diagnosis of RN. Some experts only recommended RN treatment in symptomatic patients, while conservatively observing asymptomatic patients by close radiological monitoring, since the goal of treating RN is mainly to alleviate symptoms and improve quality of life.11 However, considering that some patients may progress to major and life-threatening complications, early administration of medical therapy for RN may be useful to prolong survival. Although there are some studies focusing on the treatment of RN, most of which evaluated the short-term effects of medical intervention, little is known about the association between a medical intervention and mortality.12, 13, 14, 15
To address this question, we conducted a multicentre, retrospective, registry-based cohort study to assess the association of early treatment for RN with mortality in head and neck cancer survivors.
Methods
Study design and data source
For this multicentre, retrospective, registry-based cohort study, data were extracted from electronic medical records of the Study in Radiotherapy-related Nervous System Complications (an observational study of radiation injuries in Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China, NCT03908502),16 and Hong Kong Cancer Registry (the database contained records from six public oncology centres in Hong Kong, China).17
The baseline was defined as the date of RN being first diagnosed. Detailed baseline information from medical records, including demographic data (date of birth, sex, smoking and drinking history); prior tumor-related characteristics (TNM stage according to 7th edition of AJCC/UICC staging system, tumor progression, commencement of RT, RT dose and techniques, chemotherapy); medical history (neurological symptoms, co-existing illnesses, and brain surgery); laboratory testing for organ function; brain MRI assessments and anti-RN treatment details (corticosteroids, bevacizumab or none) were collected. Tumor progression was defined by the diagnosis of new metastatic disease or locoregional recurrence after the primary treatment of the patient's malignancy. Medical records reviewers were blinded to individual outcomes.
This study was approved by the Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (SYSEC-KY-KS-014), and the requirement for informed consent was waived due to the retrospective study design.
Patients
We screened all patients hospitalized in above-mentioned centres from January 2005 through January 2020. Patients were included if they met the following criteria: (1) aged 18 years or older; (2) received and completed RT (+/- chemotherapy if applicable) for histologically confirmed head and neck cancers; (3) had radiographic evidence to support the diagnosis of RN after RT without tumor recurrence or metastases.18 We excluded patients who: (1) had tumor progression before the diagnosis of RN; (2) underwent surgical brain necrosis lesions resection before corticosteroids and/or bevacizumab treatment; (3) had intracranial metastases before the diagnosis of RN; (4) had unavailable follow-up data; (5) had a follow-up period of less than three months (died within three months or lost to follow-up). The diagnosis of RN was based on opinions from both neurologists and radiologists, and diagnostic criteria were as follows: (1) history of RT for head and neck cancer; (2) typical radiographic change of a high-intensity lesion on fluid-attenuated inversion recovery (FLAIR) imaging and a lesion of enhancement on post-gadolinium imaging, especially “soap bubble” or “Swiss cheese” enhancement19; (3) a radiographic lesion occurring in the radiation field; (4) irrespective of whether the patients exhibit neurological symptoms or not. When necessary, the diagnosis was confirmed by positron emission tomography/computed tomography (PET/CT) imaging or biopsy.
Eligible patients were divided into two treatment groups in accordance to the time when medical therapy was first initiated: (1) Early treatment group, defined as anti-RN treatment (corticosteroids or bevacizumab) being initiated within three months after the diagnosis of RN; (2) Watch-and-wait group, the time to initiate anti-RN treatment was more than three months after the diagnosis of RN or continual conservative treatment without bevacizumab and corticosteroids.
Since only bevacizumab and corticosteroid are currently considered the first-line treatment for RN, we only defined the administration of these two therapeutic agents as anti-RN treatment. Bevacizumab was administered 5mg/kg intravenously every two weeks for up to four cycles. Patients would be recognized as receiving anti-RN treatment provided that they had received bevacizumab regardless of the total cycles of treatment they had completed. Corticosteroids administration had multiple initial doses: methylprednisolone intravenously 80-500mg per day for three to five consecutive days, and then gradually tapered until a maintenance dose of 10 mg oral prednisone per day was reached. The oral prednisone schedules could be adjusted at any time during the outpatient follow-up depending on disease conditions, thus the patients would be recognized as receiving anti-RN treatment provided that they had ever received intravenous corticosteroids treatment for RN, regardless of any taper or maintenance schedule adjustments. The time of initiating anti-RN treatment would be recorded. Patients who initiated anti-RN treatment within three months after the diagnosis of RN would be included in the early treatment group; otherwise, they would be included in the watch-and-wait group.
Outcomes
The primary outcome was all-cause death, defined as the time from baseline (the first time of the RN diagnosis) to death due to any cause. Patients who were still alive would be censored at their last follow-up date. Between July 2020 and March 2021, the vital status (date of death and cause of death if applicable) of each patient was confirmed through a standardized telephone interview. The secondary outcome was causes-specific death, including cancer-related death, RT complication-related death and others. Telephone interviewers were blinded to individual baseline profiles. All data were censored as of March 22, 2021.
Statistical analysis
Continuous variables conforming to normal distribution were expressed by their means and standard deviations (SD), while those not conforming to normal distribution were described by medians and interquartile ranges (IQR). Categorical variables were described by counting numbers and calculating percentages. Univariate comparisons of baseline features between two treatment groups were performed using Student's t, Mann-Whitney or χ2 tests according to the type and distribution of variables. Multiple imputation was performed to handle missing values in the original dataset by using the “MICE” package in R software.20 All relevant variables were used to estimate missing values, including demographic features, medical history, and laboratory results as well as outcomes. A total of twenty datasets were imputed, then multivariable Cox regression analyses were performed and we pooled the results obtained from each dataset.
In the primary analysis, time-to-event analysis for all-cause death was conducted using Cox proportional hazard regression model, and multivariable-adjusted Kaplan-Meier curve was constructed as well. Covariates included in Cox model were age (continuous), sex (female or male), neurological symptoms at baseline (symptomatic or asymptomatic), bilateral necrosis (with or without), involving ≥2 brain regions (with or without), brain stem necrosis (with or without), history of stroke (with or without), time interval from RT to RN diagnosis (continuous), RT techniques (iMRT or non-iMRT), TNM stage, having received chemotherapy (with or without), nose RT dose (continuous) and neck RT dose (continuous). The sensitivity analyses were conducted to assess the influence of missing data by excluding samples with missing data or converting missing values to dummy variables, then adjusting for the same covariates in the Cox model. In the subgroup analysis, which was set out to explore whether the effects of early treatment varied in the subgroups defined according to the above covariates, the interaction p values were calculated by the tests of exposure-by-covariate interaction in the Cox model.
Furthermore, to assess the robustness of our findings, we estimated the individual propensities for receiving early treatment with the use of a multivariable logistic regression model that included the same covariates as the Cox model, then conducted additional propensity-score analyses, including the stabilized inverse probability of treatment weighting (IPTW), propensity score 1:1 matching, and additional adjustment for the propensity score in the Cox model.21
In the secondary analysis, cancer-related death and RT complication-related death were analysed using Fine-Gray methods, and the plots of cumulative incidence function were constructed.22
All p values were reported as two-sided tests with significance defined as p<0·05. Statistical analyses were performed in the R software (Version 4·0·3, R Core Team) and additional information about statistical details have been provided in supplementary materials (eText).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. DP and YT have directly accessed and verified the underlying data. YT has final responsibility for the decision to submit for publication.
Results
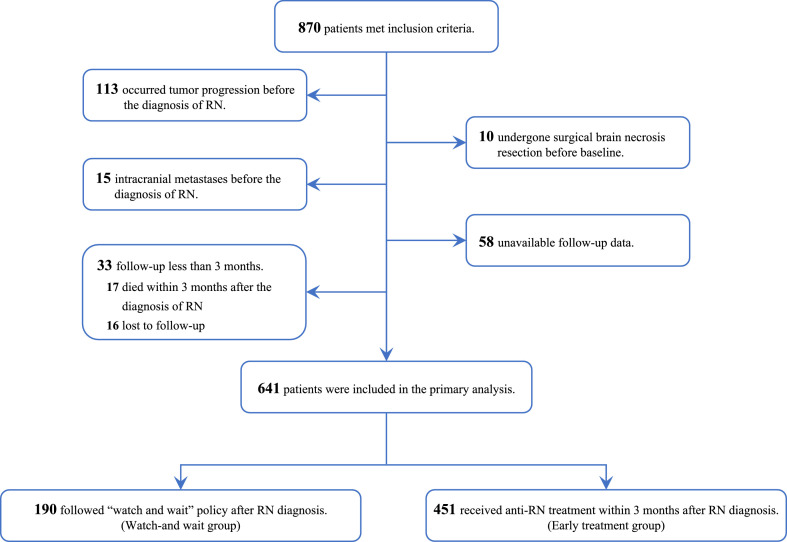
A total of 870 patients met the inclusion criteria, of whom 229 were excluded (113 had occurred tumor progression before the diagnosis of RN, 10 had undergone surgical brain necrosis resection before baseline, 15 had intracranial metastases before the diagnosis of RN, 58 had unavailable follow-up data, and 33 had a follow-up periods of less than three months). 641 patients were finally involved in this study (Figure 1).
Figure 1.
Study flowchart.
Abbreviation: RN, Radiation-induced brain necrosis.
Of 641 eligible patients, 451 (70·4%) received bevacizumab or corticosteroids treatment early after the diagnosis of RN and 190 (29·6%) did not. Baseline characteristics of all patients and groups categorized by treatment allocations were displayed in Table 1, and the actual sample size of each variable with missing data were noted. Patients who received early treatment (vs. watch-and-wait group) were younger (median age [IQR], 50·4 [43·9-57·4] years vs. 53·7 [46·1-61·1] years, p = 0·0012), less often with a history of stroke (21/451 [4·7%] vs. 17/190 [9·0%], p = 0·055), and less often presenting with metastasis status (TNM stage, p = 0·038) and receiving iMRT (121/311 [38·9%] vs. 72/121 [59·5%], p = 0·0002). Patients in the early group had more severe RN: more bilateral necrosis (281/451 [62·3%] vs. 96/190 [50·5%], p = 0·0074) and lesions involving ≥2 brain regions (71/451 [15·7%] vs. 18/190 [9·5%], p = 0·049). Baseline characteristics of 229 patients excluded from the study cohort were displayed in Table S1.
Table 1.
Characteristics of eligible patients.
| Characteristics | All patients | Watch-and-wait group | Early treatment group | p values |
|---|---|---|---|---|
| N = 641 | N = 190 | N = 451 | ||
| Sex – no. (%) | 0·55 | |||
| Females | 167 (26·1%) | 46 (24·2%) | 121 (26·8%) | |
| Males | 474 (73·9%) | 144 (75·8%) | 330 (73·2%) | |
| Age – years, median (IQR) | 51·2 (44·6-58·3) | 53·7 (46·1-61·1) | 50·4 (43·9-57·4) | 0·0012 |
| Symptomatic at diagnosis – no. (%) | 400 (62·4%) | 115 (60·5%) | 285 (63·2%) | 0·58 |
| Co-existing disorders – no. (%) | ||||
| Hypertension | 83 (12·9%) | 25 (13·2%) | 58 (12·9%) | 1·0 |
| Ischemic or hemorrhagic stroke | 38 (5·9%) | 17 (9·0%) | 21 (4·7%) | 0·055 |
| Diabetes | 25 (3·9%) | 11 (5·8%) | 14 (3·1%) | 0·17 |
| Cigarette smoking | 81 (12·6%) | 23 (12·1%) | 58 (12·9%) | 0·89 |
| Alcohol consumption | 35 (5·5%) | 12 (6·3%) | 23 (5·1%) | 0·67 |
| RT features | ||||
| Time from the commencement of RT to diagnosis of RN – years, median (IQR) | 3·7 (2·6-6·4) (n = 432) | 3·7 (2·9-7·8) (n = 121) | 3·7 (2·5-6·2) (n = 311) | 0·56 |
| TNM stage – no. (%) | 0·038 | |||
| I | 7/432 (1·6%) | 3/121 (2·5%) | 4/311 (1·3%) | |
| II | 47/432 (10·9%) | 9/121 (7·4%) | 38/311 (12·2%) | |
| III | 208/432 (48·1%) | 50/121 (41·3%) | 158/311 (50·8%) | |
| IV | 170/432 (39·4%) | 59/121 (48·8%) | 111/311 (35·7%) | |
| RT techniques – with iMRT, no. (%) | 193 (44·7%) (n = 432) | 72 (59·5%) (n = 121) | 121 (38·9%) (n = 311) | 0·0002 |
| Nose dose-Gy, mean (SD) | 69·7 (6·3) (n = 431) | 69·2 (6·7) (n = 120) | 69·9 (6·2) (n = 311) | 0·30 |
| Neck dose-Gy, mean (SD) | 57·7 (17·3) (n = 431) | 56·1 (20·5) (n = 120) | 58·3 (15·9) (n = 311) | 0·30 |
| Received chemotherapy – no. (%) | 339 (78·5%) (n = 432) | 100 (82·6%) (n = 121) | 239 (76·8%) (n = 311) | 0·24 |
| Brain MRI findings – no. (%) | ||||
| Bilateral lesions | 377 (58·8%) | 96 (50·5%) | 281 (62·3%) | 0·0074 |
| Involving ≥ 2 brain regions | 89 (13·9%) | 18 (9·5%) | 71 (15·7%) | 0·049 |
| With brain stem lesions | 20 (3·1%) | 4 (2·1%) | 16 (3·6%) | 0·48 |
| Anti-RN treatment schedules | ||||
| Agents – no. (%) | .. | |||
| Bevacizumab | 97 (15·1%) | 12 (6·3%) | 85 (18·8%) | |
| Corticosteroids | 399 (62·2%) | 33 (17·4%) | 366 (81·2%) | |
| None of above | 145 (22·6%) | 145 (76·3%) | 0 (0·0%) | |
| Time from diagnosis to administration – days, median (IQR) | 1 (0-4) | 366 (212-807) | 1 (0-3) | .. |
| All-cause death – no. (%) | 112 (17·5%) | 39 (20·5%) | 73 (16·2%) | .. |
| Cause-specific death – no. (%) | .. | |||
| Cancer-related death | 40 (6·2%) | 16 (8·4%) | 24 (5·3%) | |
| RT complication-related death | 43 (6·7%) | 19 (10·0%) | 24 (5·3%) | |
| Others | 29 (4·5%) | 4 (2·1%) | 25 (5·5%) |
Abbreviations: no., Numbers; SD, Standard Deviation; IQR, Interquartile Range; RN, Radiation-induced brain necrosis; RT, Radiotherapy; iMRT, Intensity-modulated radiation therapy; MRI, Magnetic resonance imaging.
Primary endpoint
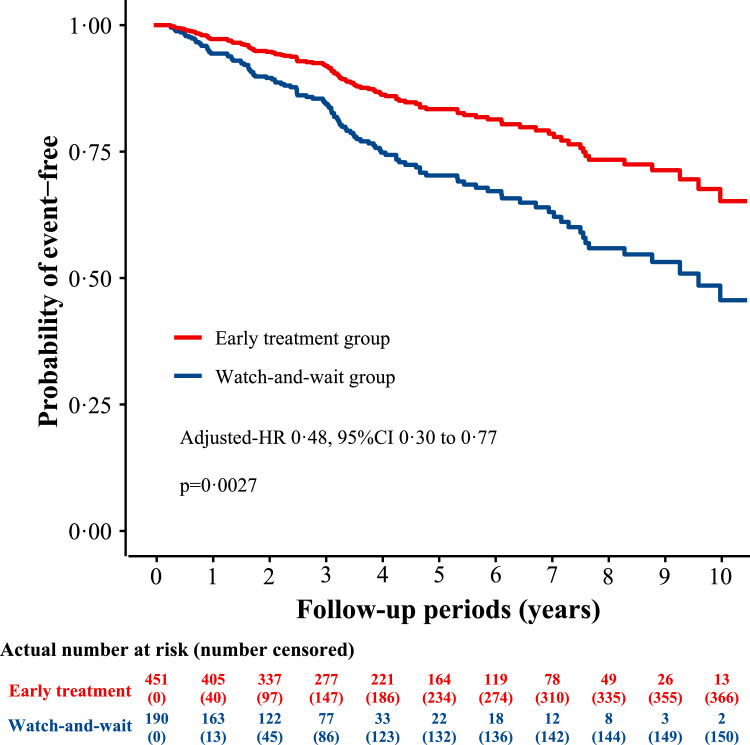
Over a median follow-up period of 3·87 years, 112 patients had died of any cause (17·5%), of whom 73 (16·2%) and 39 (20·5%) were in the early treatment group and the watch-and-wait group, respectively. The early treatment group showed a lower risk of all-cause death compared with the watch-and-wait group (unadjusted-HR 0·48, 95% CI 0·32 to 0·71, p = 0·0004; Table 2). After adjusting for confounders, the risk of all-cause death in the early treatment group remained significantly lower than that in the watch-and-wait group (adjusted-HR 0·48, 95% CI 0·30 to 0·77, p = 0·0027; Table 2, Figure 2). In addition, sensitivity analyses, both the analysis that excluded samples with missing data (adjusted-HR 0·36, 95% CI 0·21 to 0·64, p = 0·0004; Table 2) and the analysis that converted missing values to dummy variables then adjusting in the same Cox model (adjusted-HR 0·43, 95% CI 0·27 to 0·67, p = 0·0003; Table 2), showed similar results.
Table 2.
Association between the early treatment and the mortality.
| Analyses | Results |
|---|---|
| The primary analysis | |
| All-cause death | |
| No. of events/no. of patients (%) | |
| Early treatment group | 73/451 (16·2) |
| Watch-and-wait group | 39/190 (20·5) |
| Crude analysis –HR (95% CI) | 0·48 (0·32–0·71), p = 0·0004 |
| Multivariable-adjusted analyses –HR (95% CI) | |
| the main analysisa | 0·48 (0·30–0·77), p = 0·0027 |
| sensitivity analysis 1b | 0·36 (0·21–0·64), p = 0·0004 |
| sensitivity analysis 2c | 0·43 (0·27–0·67), p = 0·0003 |
| Propensity-score analysesd–HR (95% CI) | |
| with stabilized IPTWe | 0·52 (0·35-0·79), p = 0·0023 |
| with 1:1 matchingf | 0·46 (0·25-0·83), p = 0·011 |
| adjusted for propensity scoreg | 0·48 (0·31-0·74), p = 0·0009 |
| The secondary analysis | |
| Cancer-related death | |
| No. of events/no. of patients (%) | |
| Early treatment group | 24/451 (5·3) |
| Watch-and-wait group | 16/190 (8·4) |
| Competing risk analysish–HR (95% CI) | 0·52 (0·27-0·99), p = 0·045 |
| RT complication-related death | |
| No. of events/no. of patients (%) | |
| Early treatment group | 24/451 (5·3) |
| Watch-and-wait group | 19/190 (10·0) |
| Competing risk analysish–HR (95% CI) | 0·34 (0·17-0·65), p = 0·0012 |
Abbreviations: No., Numbers; HR, hazard ratio; CI, confidence interval; IPTW, inverse probability of treatment weighting; RT, Radiotherapy; RN, Radiation-induced brain necrosis.
Shown is the pooled result of 20 imputation datasets, using a multivariable Cox proportional hazard regression model with adjusting for age (continuous), sex (female or male), neurological symptoms at baseline (symptomatic or asymptomatic), lesion sites (unilateral or bilateral), lesion regions (involving only one brain region or ≥ 2 brain regions), brain stem lesions (with or without), history of stroke (with or without), time from the commencement of RT to RN diagnosis, RT techniques (iMRT or non-iMRT), TNM stage, having received chemotherapy (with or without), nose RT dose (continuous) and neck RT dose (continuous). All 641 patients were included in the analysis.
The analysis only included 432 patients who had no missing data and repeated the Cox regression analysis with adjusting for the same covariates.
The analysis included all 641 patients, with missing values being converted to dummy variables and then adjusted for the same covariates.
The propensity-score analyses were performed in the first imputed dataset and included all 641 patients.
The hazard ratio was from the multivariable Cox regression model adjusting for the same covariates with stabilized inverse probability of treatment weighting according to propensity score.
The hazard ratio was from the multivariable Cox regression model adjusting for the same covariates with 1:1 propensity-score matching (173 patients who received early treatment and 173 patients who did not).
The hazard ratio was from the multivariable Cox regression model adjusting for the above covariates with additional adjustment for the propensity score.
Multivariable competing risk analyses adjusted for the same covariates.
Figure 2.
Multivariable-adjusted probability of post-RN survival.
The survival curve was adjusted for age (continuous), sex (female or male), neurological symptoms at baseline (symptomatic or asymptomatic), lesion sites (unilateral or bilateral), lesion regions (involving only one brain region or ≥ 2 brain regions), brain stem lesions (with or without), history of stroke (with or without), time from the commencement of RT to RN diagnosis, RT techniques (iMRT or non-iMRT), TNM stage, having received chemotherapy (with or without), nose RT dose (continuous) and neck RT dose (continuous), using Cox proportional hazard regression model.
Abbreviations: RT, Radiotherapy; RN, Radiation-induced brain necrosis; iMRT, intensity-modulated radiotherapy; TNM, tumor-node-metastasis.
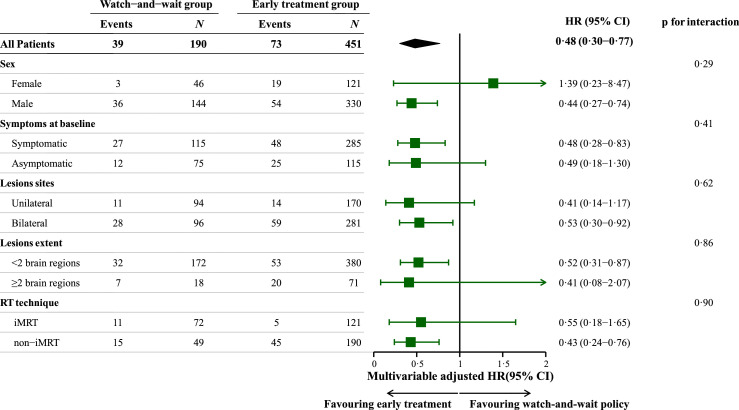
We also studied the effects of early treatment among subgroups of patients who were symptomatic or asymptomatic at the RN diagnosis as preplanned covariates, since the presence of neurological symptoms at baseline is usually one of the major determinants of treatment timing. In the subgroup analyses, however, there was no significant difference in the effect of early treatment on post-RN survival among subgroups stratified by presence or absence of neurological symptoms at diagnosis (Symptomatic subgroup: adjusted-HR 0·48, 95% CI 0·28 to 0·83; Asymptomatic subgroup: adjusted-HR 0·49, 95% CI 0·18 to 1·30; p for interaction=0·41, Figure 3).
Figure 3.
Subgroup analysis.
The HRs and their 95%CIs were calculated by the Cox model adjusted for the same covariates in the main analysis.
Abbreviations: HR, hazard ratio; CI, confidence interval; iMRT, intensity-modulated radiotherapy.
Furthermore, propensity-score analyses conducted in the first imputation dataset, with stabilized IPTW (adjusted-HR 0·52, 95% CI 0·35 to 0·79, p = 0·0023), 1:1 matching (173 patients in each group were successfully matched; adjusted-HR 0·46, 95% CI 0·25 to 0·83, p = 0·011), and additional adjustment for propensity score (adjusted-HR 0·48, 95% CI 0·31 to 0·74, p = 0·0009), yielded similar results as well (Table 2). The details of propensity-score analyses were available in supplementary materials (Table S4-S6, Figure S3, and eText). Results from other imputed datasets were basically consistent, which have been reported in Table S7.
Secondary endpoint
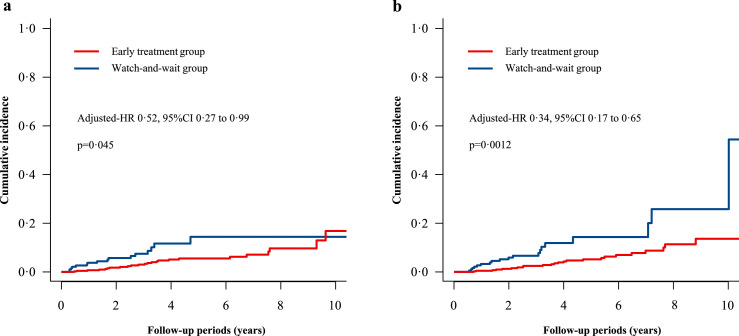
In the secondary analysis, we investigated the association between the early treatment and the cause-specific death using Fine-Gray models. Of 641 patients, 40 (6·2%) died of cancers, 43 (6·7%) died of RT complications and the rest 29 (4·5%) died of other causes. The early anti-RN treatment significantly reduced the risks of RT complication-related death (adjusted-HR 0·34, 95% CI 0·17 to 0·65, p = 0·0012) and cancer-related death (adjusted-HR 0·52, 95% CI 0·27 to 0·99, p = 0·045) in multivariable competing risk models (Table 2, Figure 4).
Figure 4.
Cumulative incidence of causes-specific death using Fine-Gray method.
(a) Cancer-related death; (b) Radiotherapy complication-related death.
In addition, we conducted an exploratory analysis to investigate whether there was a difference between bevacizumab and corticosteroids in improving post-RN survival, whereas the analysis showed no significant difference in the risk of all-cause death after confounders adjustment (Figure S4).
Discussion
In this multicentre, retrospective, registry-based cohort study, the first to investigate the mortality of treatment timing in head and neck cancer survivors with RN, we found that early treatment was associated with a lower risk of all-cause death compared with following the watch-and-wait policy.
A previous retrospective cohort study by Lam et al. in 2010 on 174 nasopharyngeal carcinoma patients with RN reported 2-year and 5-year overall survival of 57·5% and 35·4%, respectively.12 However, they did not investigate the timing of initiating treatment for RN on mortality, and bevacizumab, which had shown to improve clinical outcomes as compared with corticosteroids,14,15 were not widely used for patients with RN at that time. Since most prior studies have focused on short-term effects while little is known about long-term outcome, whether early medical treatment for newly diagnosed RN can be beneficial for survival, especially for those patients without clinical symptoms, is still unclear. Current views on the treatment goal for RN are to relieve clinical symptoms and improve quality of life. As such, asymptomatic patients are usually monitored without any medical intervention until presenting with RN-related neurological symptoms.8,11
The mechanisms underlying RN involve blood-brain barrier dysfunction, leakage of intravascular inflammatory cytokines, microglia overreaction and subsequent cellular damage6,23, 24, 25; thus RN on brain MRI typically presents as a contrast-material enhanced white matter lesion with adjacent cerebral parenchymal edema, which if untreated would ultimately lead to cyst formation or even cerebral hernia.10,18 Although some RN could be asymptomatic with mild brain edema at the early stage, the majority of them would progress and eventually become irreversible without effective interventions. Unfortunately, the risk factor leading to RN deterioration remains unclear. Hence, we hypothesized that the early anti-RN treatment for head and neck cancer survivors might prevent or retard the deleterious pathophysiological processes, leading to improved long-term clinical outcomes.
Findings of the present study support our hypothesis and showed a 52% decreased risk of all-cause death in the early treatment group compared with that in the watch-and-wait group. Multiple analysis models yielded similar results to the main analysis. The subgroup analyses in both symptomatic and asymptomatic patients also supported the benefit of early treatment for RN. In our study, although patients in the watch-and-wait group presented with milder RN conditions (less bilateral lesions and less lesions extent), they still showed poorer outcomes as compared with those in the early treatment group, either in crude or multivariable adjusted analyses. One possible explanation is that patients following the watch-and-wait policy may lead to less frequent follow-up and are less likely to be monitored as closely. Once they get worse and look for medical help, they may already have irreversible brain necrosis and other severe RT-related complications leading to poor response to treatment. Our previous study had found that the longer the interval between RT and RN treatment, the more refractory to the treatment, and the higher the RN recurrent rate.26 On the other hand, patients in the early treatment group are more likely to receive comprehensive medical intervention in addition to bevacizumab or corticosteroids, including nutrition counselling, rehabilitation training, and familial nursing care guidance, during the outpatient follow-up. Moreover, the patients in the early treatment group might have better physical condition (young age, less stroke history, less metastatic disease at presentation), which might probably lead to better response to anti-RN treatment. Whereas, our study excluded those patients who had occurred tumor progression before RN diagnosis or underwent surgical brain lesions resection, and those whose follow-up period was too short (to avoid immortal time bias), thus it must take caution to apply our findings to these populations. Overall, the findings of this study show that early treatment is crucial for patients with RN even if they are asymptomatic at the time of the RN diagnosis.
Another important finding was that in the competing risk analysis of the secondary outcome, the early treatment group, compared with the watch-and-wait group, showed a significantly decreased risk of RT complication-related death, whereas there was a marginally significant difference in cancer-related death (p = 0·045). In our institutional practice, the treatment being administered is meant to treat RN and other RT-related complications but not cancer. When newly diagnosed patients with RN showed uncontrolled tumors or lesions that were difficult to differentiate from RN, we usually recommended that they should first seek medical help from cancer experts because anti-cancer treatment always took priority over anti-RN treatment. It is reasonable for anti-RN treatment to significantly reduce the risk of RT complication-related death while the protective effect of early treatment on improving cancer-related survival may be explained by that early anti-RN treatment could contribute to closer patient follow-up, which helps early detection of tumor recurrence hence anti-cancer treatment could be timely administered. We think, however, that it is difficult to draw a conclusion that early anti-RN treatment could lower the risk of cancer-related death, since the current evidence is not robust enough. Therefore, future prospective studies would be needed to validate this finding.
This study has several limitations. Firstly, some covariates had about 30% missing data (mainly RT-related variables). Although we had performed multiple imputation and then conducted several sensitivity analyses to assess the robustness of our findings, it could still lead to some unknown confounding. Secondly, due to the observational design, the allocation of individuals was not random but rather depended on actual treatment schedules. Various factors could affect clinical decision-making, such as the severity of clinical symptoms and brain lesions, co-existing disorders, and socioeconomic factors. Although we did perform multivariable regression models for adjusting confounders and estimated the individual propensities for intervention, we were unable to correct for all potential biases, especially for those unknown or unmeasured. Thirdly, our study only included head and neck cancer survivors, which could lead to selection bias, hence our conclusions may not be applicable to other cancer patients who suffer from RT injuries, such as patients with glioma or brain metastases. Furthermore, despite superiority in alleviating clinical symptoms and improving RN lesions, patients receiving bevacizumab did not show better post-RN survival than those receiving corticosteroids in the current study due to the insufficient sample size of the bevacizumab subgroup and the short follow-up time. Finally, we were unable to compare the effects of the two different treatment strategies on quality of life and neurological function.
In conclusion, among head and neck cancer survivors with RN, early treatment with bevacizumab or corticosteroids is associated with a lower risk of all-cause death as compared with following the watch-and-wait policy, irrespective of whether patients exhibit symptoms or not. In the absence of randomised clinical trials, our study will have an important implication for clinicians and provides the foundation and supporting data for future trials on this topic.
Contributors
Conceptualization: DP, XR, and YT.
Data curation: DP, XR, DC, WTN, HM, YL, HL, JJ, JCa, JCh, and YX.
Analysis and interpretation of data: DP, XR, DC, and YT. writing – original draft: DP, XR, DC, and YT.
Writing, review and/or revision of the manuscript: DP, XR, DC, JJ, WTN, HM, YL, HL, JCa, JCh, YX, MC, CS, SL, and YT.
Study supervision: YT.
DP, XR, and DC contributed equally to the study.
DP and YT have directly accessed and verified the underlying data.
Data sharing statement
Data supporting the findings of the study are available on reasonable request after approval of a proposal from the corresponding author (YT; tangym@mail.sysu.edu.cn).
Declaration of interests
YT declares funding from the National Natural Science Foundation of China (81925031, 81820108026) and the Science and Technology Program of Guangzhou (202007030001). XR declares funding from the Young Teacher Training Program of Sun Yat-sen University (20ykpy106). YL declares funding from the National Natural Science Foundation of China (81872549) and Key-Area Research and Development Program of Guangdong Province (2018B030340001). HL declares funding from the National Natural Science Foundation of China (82003389). YX declares funding from the Youth Program of National Natural Science Foundation of China (81801229). MC reports consulting fees from ImmunoSCAPE, Telix Pharmaceuticals, and IQVIA; personal fees from Astellas, Bayer, MSD, Janssen, Pfizer, and Varian; being a co-inventor of the patent of a High Sensitivity Lateral Flow Immunoassay For Detection of Analyte in Sample (10202107837T), Singapore; participation on a data safety monitoring board or advisory board for AstraZeneca, Astellas, Bayer, and Janssen; owning stocks in Digital Life Line; and non-financial interests in AstraZeneca, Decipher Bioscience, MedLever, and Varian. All other authors declare no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81925031, 81820108026), and the Science and Technology Program of Guangzhou (202007030001) to YT. Young Teacher Training Program of Sun Yat-sen University (20ykpy106) to XR. National Natural Science Foundation of China (81872549), and Key-Area Research and Development Program of Guangdong Province (2018B030340001) to YL. National Natural Science Foundation of China (82003389) to HL. Youth Program of National Natural Science Foundation of China (81801229) to YX. MC is supported by the National Medical Research Council Singapore Clinician Scientist Award (NMRC/CSA-INV/0027/2018, CSAINV20nov-0021), the Duke-NUS Oncology Academic Program Goh Foundation Proton Research Programme, NCCS Cancer Fund, and the Kua Hong Pak Head and Neck Cancer Research Programme. This research is also supported by the National Research Foundation Clinical Research Programme Grant (NRF-CRP17-2017-05).
We are thankful to the staff of registries for data collection and patient follow-up.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101618.
Appendix. Supplementary materials
References
- 1.Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 2.Garden AS, Lewin JS, Chambers MS. How to reduce radiation-related toxicity in patients with cancer of the head and neck. Curr Oncol Rep. 2006;8(2):140–145. doi: 10.1007/s11912-006-0049-x. [DOI] [PubMed] [Google Scholar]
- 3.Coleman CN, Stone HB, Moulder JE, Pellmar TC. Medicine. Modulation of radiation injury. Science. 2004;304(5671):693–694. doi: 10.1126/science.1095956. [DOI] [PubMed] [Google Scholar]
- 4.Lee AW, Ng SH, Ho JH, et al. Clinical diagnosis of late temporal lobe necrosis following radiation therapy for nasopharyngeal carcinoma. Cancer. 1988;61(8):1535–1542. doi: 10.1002/1097-0142(19880415)61:8<1535::aid-cncr2820610809>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Na A, Haghigi N, Drummond KJ. Cerebral radiation necrosis. Asia Pac J Clin Oncol. 2014;10(1):11–21. doi: 10.1111/ajco.12124. [DOI] [PubMed] [Google Scholar]
- 6.Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: a review of the pathobiology, diagnosis and management considerations. J Clin Neurosci. 2013;20(4):485–502. doi: 10.1016/j.jocn.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Lee AW, Foo W, Chappell R, et al. Effect of time, dose, and fractionation on temporal lobe necrosis following radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1998;40(1):35–42. doi: 10.1016/s0360-3016(97)00580-4. [DOI] [PubMed] [Google Scholar]
- 8.Ali FS, Arevalo O, Zorofchian S, et al. Cerebral radiation necrosis: incidence, pathogenesis, diagnostic challenges, and future opportunities. Curr Oncol Rep. 2019;21(8):66. doi: 10.1007/s11912-019-0818-y. [DOI] [PubMed] [Google Scholar]
- 9.Kitpanit S, Lee A, Pitter KL, et al. Temporal lobe necrosis in head and neck cancer patients after proton therapy to the skull base. Int J Part Ther. 2020;6(4):17–28. doi: 10.14338/IJPT-20-00014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang YX, King AD, Zhou H, et al. Evolution of radiation-induced brain injury: MR imaging-based study. Radiology. 2010;254(1):210–218. doi: 10.1148/radiol.09090428. [DOI] [PubMed] [Google Scholar]
- 11.Zhuang H, Shi S, Yuan Z, Chang JY. Bevacizumab treatment for radiation brain necrosis: mechanism, efficacy and issues. Mol Cancer. 2019;18(1):21. doi: 10.1186/s12943-019-0950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam TC, Wong FC, Leung TW, Ng SH, Tung SY. Clinical outcomes of 174 nasopharyngeal carcinoma patients with radiation-induced temporal lobe necrosis. Int J Radiat Oncol Biol Phys. 2012;82(1):e57–e65. doi: 10.1016/j.ijrobp.2010.11.070. [DOI] [PubMed] [Google Scholar]
- 13.Zhuo X, Huang X, Yan M, et al. Comparison between high-dose and low-dose intravenous methylprednisolone therapy in patients with brain necrosis after radiotherapy for nasopharyngeal carcinoma. Radiother Oncol. 2019;137:16–23. doi: 10.1016/j.radonc.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Wong ET, Huberman M, Lu XQ, Mahadevan A. Bevacizumab reverses cerebral radiation necrosis. J Clin Oncol. 2008;26(34):5649–5650. doi: 10.1200/JCO.2008.19.1866. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Rong X, Hu W, et al. Bevacizumab monotherapy reduces radiation-induced brain necrosis in nasopharyngeal carcinoma patients: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2018;101(5):1087–1095. doi: 10.1016/j.ijrobp.2018.04.068. [DOI] [PubMed] [Google Scholar]
- 16.A Study in Radiotherapy-related Nervous System Complications. Available at: https://clinicaltrials.gov/ct2/show/NCT03908502. Accessed 23 July 2022.
- 17.Hong Kong Cancer Registry (HKCaR). Available at: https://www3.ha.org.hk/cancereg. Accessed 23 July 2022.
- 18.Martino A, Krainik A, Pasteris C, et al. Neurological imaging of brain damages after radiotherapy and/or chimiotherapy. J Neuroradiol. 2014;41(1):52–70. doi: 10.1016/j.neurad.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Carr CM, Benson JC, DeLone DR, et al. Intracranial long-term complications of radiation therapy: an image-based review. Neuroradiology. 2021;63(4):471–482. doi: 10.1007/s00234-020-02621-7. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. 2016;4(2):30. doi: 10.3978/j.issn.2305-5839.2015.12.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuss O, Blettner M, Borgermann J. Propensity score: an alternative method of analyzing treatment effects. Dtsch Arztebl Int. 2016;113(35-36):597–603. doi: 10.3238/arztebl.2016.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 23.Lee ST, Seo Y, Bae JY, et al. Loss of pericytes in radiation necrosis after glioblastoma treatments. Mol Neurobiol. 2018;55(6):4918–4926. doi: 10.1007/s12035-017-0695-z. [DOI] [PubMed] [Google Scholar]
- 24.He B, Wang X, He Y, et al. Gamma ray-induced glial activation and neuronal loss occur before the delayed onset of brain necrosis. FASEB J. 2020;34(10):13361–13375. doi: 10.1096/fj.202000365RR. [DOI] [PubMed] [Google Scholar]
- 25.Cheng J, Korte N, Nortley R, Sethi H, Tang Y, Attwell D. Targeting pericytes for therapeutic approaches to neurological disorders. Acta Neuropathol. 2018;136(4):507–523. doi: 10.1007/s00401-018-1893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Huang X, Jiang J, et al. Clinical variables for prediction of the therapeutic effects of bevacizumab monotherapy in nasopharyngeal carcinoma patients with radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2018;100(3):621–629. doi: 10.1016/j.ijrobp.2017.11.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.