Summary
Excessive substance use and substance use disorders (SUDs) are common, serious and relapsing medical conditions. They frequently co-occur with other diseases that are leading contributors to disability worldwide. While heavy substance use may potentiate the course of some of these illnesses, there is accumulating evidence suggesting common genetic architectures. In this narrative review, we focus on four heritable medical conditions - cardiometabolic disease, chronic pain, depression and COVID-19, which are commonly overlapping with, but not necessarily a direct consequence of, SUDs. We find persuasive evidence of underlying genetic liability that predisposes to both SUDs and chronic pain, depression, and COVID-19. For cardiometabolic disease, there is greater support for a potential causal influence of problematic substance use. Our review encourages de-stigmatization of SUDs and the assessment of substance use in clinical settings. We assert that identifying shared pathways of risk has high translational potential, allowing tailoring of treatments for multiple medical conditions.
Funding
SSR acknowledges T29KT0526, T32IR5226 and DP1DA054394; RLK acknowledges AA028292; AA acknowledges DA054869 & K02DA032573. The funders had no role in the conceptualization or writing of the paper.
Keywords: Substance use, Comorbidity, Psychiatric, Medical, Genomics research
Introduction
Substance Use Disorders (SUDs) are serious and often treatable yet relapsing medical conditions that arise from prolonged use of psychoactive substances (either licit or illicit) that contribute to physiological and psychological impairment.1 They are associated with high morbidity and mortality, contributing directly or indirectly to the leading causes of disability in developing and developed nations.2 Excessive substance use and SUDs contribute directly to cancers, liver disease, respiratory diseases, infectious diseases (e.g., HIV, HCV), and prenatal exposure can be associated with infant health (e.g., fetal alcohol syndrome) - these feature among the leading contributors to Disability Adjusted Life Years (DALYs) worldwide.3 SUDs are also associated with other common health conditions, such as cardiometabolic disease,4, 5, 6 depression,7 and chronic pain,8 which are also leading contributors to DALYs, and also associated with COVID-19.9 These medical conditions may not result as a direct consequence of excessive substance use/SUDs; rather, their co-occurrence may arise from a common genetic basis. In this review, we examine the association between substance use, SUDs and these 4 health outcomes - all heritable conditions - from a genetic perspective. First, we introduce the classification of SUDs, their heritability and comorbidities. Second, we outline our strategy for selecting these 4 medical conditions, and terms for inclusion in this narrative review. Then, we present the epidemiological backdrop substantiating associations between SUDs and these medical conditions, including preliminary support for genetic mechanisms from family and twin studies. Our results outline evidence regarding the extent to which genetically-informed methodologies support correlational and causal mechanisms of association between SUDs and these conditions. We close by presenting a few research gaps and the overall implications of our observations.
SUDs are broadly characterized by the transition from occasional or even regular use for pleasure or as prescribed, to compulsive use directed at alleviating distress experienced when not using the substance. Clinically, SUDs are typically diagnosed using the Fifth Edition of the Diagnostic and Statistical Manual (DSM-5) when individuals meet 2 or more of 11 criteria that include aspects of physiological response (tolerance to and withdrawal from the substance), escalating use with loss of control, preoccupation with obtaining or using the substance, and continued use despite psychological, physical and social consequences.10 The DSM-5 also diagnoses a range of mild (2-3 criteria), moderate (4-5 criteria) and severe (6-11 criteria) SUDs. Relatedly, the 11th Edition of the International Classification of Diseases (ICD-11) diagnoses unhealthy substance use as hazardous, harmful (either episodically or chronically) or related to dependence, with the latter being classified by criteria representing impaired control over substance use, increasing prioritization of substance use over other aspects of life and physiological features.11 Harmful, hazardous or problematic patterns of substance use can also be evaluated in healthcare settings using short patient-reported screeners (e.g., the Alcohol Use Disorders Identification Test, or AUDIT12; Drug Use Disorder Identification Test (DUDIT)13; the Fagerström Test for Nicotine Dependence14) to provide rapid identification of at-risk individuals.
SUDs are heritable conditions (∼30-60%) and can present comorbidly with each other, with increasing evidence demonstrating that multiple SUDs may share overlapping genetic architectures.15,16 In addition, SUDs have neurobehavioral underpinnings that distinguish them from substance use.17, 18, 19 Different substances have different disorder liability (i.e., the proportion of individuals who develop a SUD when they use substances), yet overall, SUDs tend to be underdiagnosed, and therefore, are undertreated,20 further perpetuating the chronicity of the illness.
SUDs clinically present comorbidly with other psychiatric disorders,21, 22, 23 often further complicating the course of these illnesses. Beyond the brain, SUDs and problematic substance use also have widespread effects on other organ systems.24 Some of these medical consequences are substance-specific and a result of exposure toxicity. For instance, heavy alcohol use has been linked to liver disease25 and fetal alcohol syndrome26; cigarette smoking is a major contributor to lung cancer27; and injection drug use increases risk for HIV/AIDS and Hepatitis B and C.28 However, there are many other common medical conditions (e.g., cardiometabolic disease, chronic pain, depression, COVID-19) that may not be a direct consequence of a SUD; rather, their co-occurrence in individuals with SUDs or problematic substance use may arise from a common genetic liability to both disorders. For instance, excessive alcohol use has been linked to cardiometabolic disease, although the mechanisms underlying this association remain unclear.29 Likewise, a growing literature indicates that common reward-related processes may underlie the seemingly causal associations between opioid use and chronic pain.30 A more thorough epidemiological overview appears below. Notably, these medical conditions are frequently seen by health-care providers and pose a large economic burden.
The assumption that SUDs cause or complicate these medical conditions may have contributed to stigmatization of individuals with SUDs, including deprioritizing them for other medical care.31 However, the heritable nature of both SUDs and these individual medical conditions suggest alternative polygenic pleiotropic mechanisms (i.e., genetic loci affecting multiple traits) may underlie their associations. Evidence for pleiotropy or causation may have early implications for treatment and prevention. For instance, if purely correlational mechanisms underlie a pair of traits (e.g., genetic correlation between SUDs and depression), then enhanced vigilance towards both disorders in those with a family history of either may prove to be worthwhile. Furthermore, identification of these shared genetic pathways could provide insights into novel pharmacotherapeutics for those with multiple comorbid conditions. On the other hand, if one condition causes the other (e.g., SUDs cause cardiometabolic disease) then patient stratification by exposure may be beneficial.
Selection criteria and search strategy
Selection of medical conditions
We based our selection of medical conditions on the 2019 Global Health Estimates report identifying the top 20 leading contributors to DALYs globally.3 First, we excluded conditions for which there was limited to no evidence for the influence of SUDs, including diarrhoeal diseases, malaria , tuberculosis, other hearing loss and uncorrected refractive errors. Next, we identified those conditions that could be consequentially related to a specific substance or groups of substances (even if they were not linked to SUDs in the report). These included: neonatal conditions or congenital anomalies , which could partially include the impact of fetal exposure to alcohol or narcotics; road injury and falls, which could be associated with acute alcohol and drug intoxication (i.e., driving under the influence); chronic obstructive pulmonary disease, trachea, bronchus and lung cancers and lower respiratory tract infections, which tend to be elevated in tobacco smokers; and cirrhosis of the liver and kidney diseases, which have been linked to excessive alcohol use. We also excluded HIV/AIDs, because injection drug use has been directly linked to increased likelihood of HIV infection. The remaining 3 conditions we included broadly reflected cardiometabolic disease (ischaemic heart disease, stroke, diabetes mellitus), pain (back and neck), and depressive disorders. To this list, we included COVID-19 infection and severity as, since 2019, severe illness due to COVID-19 has been a major source of DALYs.32
General search strategy
To assess the role of common genetic variants in liability to substance use, SUDs and these four medical conditions, we relied only on large-scale (N>50,000) genome-wide association studies (GWAS) of substance use and SUDs, which captured a significant proportion of the heritability of these conditions. As this pool of GWAS is not vast, we used an open-ended search to identify relevant findings. We searched PubMed, MEDLINE, Web of Science, pre-print servers (bioRxiv, medRxiv) and Google. We included pre-prints because genetic approaches to the study of SUD comorbidities is a fairly new area of research and larger GWAS of SUDs are ever-arising. Studies that were included spanned 2017 to 2022. Only articles published in English were considered. We recognize that our search criteria and databases may miss relevant literature not included in these databases. To set the stage for the genetic studies, we briefly review epidemiological support for comorbidity between SUDs and these 4 medical conditions - we did not conduct a systematic review of all studies for this section, which is intended only to provide the impetus for the genetic studies.
SUD search terms
As many of the comorbidities that we selected may be evident in individuals with heavy, excessive or problem substance use (but not necessarily a SUD diagnosis), we included a broad range of terms for SUDs (use disorder OR abuse OR dependence OR addiction OR problem use OR excessive use). We used several terms for substances (alcohol, drinking, nicotine, tobacco, smoking, cannabis, marijuana, heroin, opioid, opiate, prescription opioid misuse, cocaine, stimulants, methamphetamine, drug, illicit, polysubstance).
Medical condition search terms
We report findings that considered cardiometabolic disease (obesity OR body mass index [BMI] OR cardiovascular OR stroke OR heart disease, diabetes OR blood pressure OR hypertension), chronic pain (pain - we did not restrict our search to neck and lower back), depression (major depressive disorder OR depression), COVID-19 OR SARS-CoV-2. The number of GWASs for these conditions is substantial but, for the purposes of this review, we were interested only in studies that linked them to substance use or SUDs; therefore we only included studies reporting genetic analyses of these 4 medical conditions with substance use or SUDs GWASs.
Epidemiologic support for comorbidity
Cardiometabolic disease
There are well documented associations between excessive substance use and the onset and course of heart disease, hypertension, type 2 diabetes and obesity.33,34
All levels of tobacco smoking are associated with increased risk for cardiometabolic disease. Post-combustion products from smoking have been shown to contribute to atherogenesis, and cigarette smoking is a leading contributor to cardiovascular disease (CVD)-related mortality.35 The role of acute and chronic use of cocaine on worsening CVD is indisputable36; however, the long-term consequences after cocaine cessation are less well-known. For example, Ritalin, a stimulant medication that was recently used to treat drowsiness, appetite loss and even depression in older adults, contributed to transient but significant increases in CVD.37
In contrast, the association between cardiometabolic disease and alcohol consumption is controversial. The hypothesized J-shaped distribution of risk of alcohol use on CVD suggests that light to moderate alcohol consumption exerts cardioprotective effects, lack of drinking (either lifetime or recent) slightly elevates risk, while excessive alcohol consumption dramatically increases risk for CVD. However, numerous studies, including meta-analyses,29 have failed to replicate the cardioprotective effects of moderate drinking or have not identified the risk-conferring aspects of lifetime or recent abstinence. This paradoxical association may reflect the reverse causal effects of drinking cessation for therapeutic reasons in individuals with CVD onset. A similar, inconclusive literature surrounds the association between alcohol use and type 2 diabetes, with some studies suggesting reduced risk in light to moderate drinkers (e.g.,38,39) and others indicating no association or elevated risk (e.g.,40,41). Thus, the most robustly replicated associations between alcohol and cardiometabolic disease are observed in heavy drinkers.
Studies of cannabis use provide mixed evidence, with some suggesting significant elevation in risk for coronary artery disease and stroke,42 and others indicating lower body mass index and improved metabolic outcomes, such as reduced likelihood of type 2 diabetes.43,44 Finally, exogenous and endogenous opioids can profoundly impact cardiovascular systems, and this has resulted in increasing scrutiny of opioid prescribing for pain in individuals with CVD.45 However, the role of opioid use in cardiometabolic health is still unclear.
Chronic pain
Chronic pain, which is broadly defined as the experience of pain for longer than 3 months, is a prevalent condition that tends to co-occur (40%) with SUDs.46 Individuals with chronic pain show worse response to SUD treatment,47 and experience of pain has been shown to predict heavy drinking relapses.48, 49, 50 Unlike cardiometabolic disease, many of the epidemiological studies between pain and substance use assume that pain is the initial event, followed by substance use (for instance, the consumption of alcohol to ameliorate pain). This is especially the case for opioids, which are one of the most commonly prescribed medications to treat chronic pain conditions. However, neurobiological studies suggest that common reward mechanisms may underlie both subjective experiences of pain and SUDs.30 Therefore, the reported phenotypic association between prescription opioids, SUDs, and pain is likely complex, and the possibility of pain arising from, or being exacerbated by SUDs, cannot be excluded.
Depression
Given the mood altering properties of alcohol and other substances, the comorbidity between SUDs and depression is expected. The elevated prevalence of depressive disorders in individuals with alcohol use disorders has been documented in numerous nationally-representative samples (e.g.,51, 52, 53, 54). Beyond alcohol, tobacco smoking and other SUDs also occur comorbidly with depressive disorders.21,55 For example, heavy cannabis use has been linked to depression particularly during adolescence.56 For opioids, comorbid depression is frequent, particularly in those using opioid medications for chronic pain.57,58 Although negative affect is a notable clinical characteristic of a majority of SUDs, not all of the comorbidity between depression and SUDs reflects substance-induced mood disorders. Similar to pain, the hypothesis of “self-medication” - the voluntary intake of substances to ameliorate dysphoria and anhedonia associated with depression - is also frequently posited as a contributor.59 These two disorder groups often complicate prognosis, with only modest benefits of antidepressant medications for patients with combined depressive- and substance-use disorders.60
COVID-19
The COVID-19 pandemic placed the vulnerabilities associated with SUDs into sharp focus.61 While anecdotal information indicated protective effects of smoking tobacco and cannabis (in contrast to cannabidiol62), clinical data overwhelmingly documented that individuals with SUDs were more likely to require hospitalization or die due to COVID-19 (Wang et al., 2021), those with opioid use disorders being particularly at high risk. Despite expectations that pandemic-related stress would promote heavier substance use, results were mixed for substances such as alcohol63,64 and indicative of escalating use for others, such as opioids and polysubstance use.65,66
Collectively, these epidemiologic studies have shown robust phenotypic correlations between SUDs and these four medical conditions. Whereas SUDs are posited to antedate some of these conditions (e.g., heavy drinking and CVD), be a consequence (e.g., pain and higher opioid use) or be the result of bidirectional effects (e.g., depression and substance use, and vice versa), there are limitations to these epidemiological observations. First, despite most epidemiological studies being correlational in nature, phenotypic findings can easily lend themselves to untested causal interpretations because studies might not have the necessary data structure or include methods to test for alternative hypotheses. For example, an association between alcohol use disorder and depression in older adults is confounded by exposure to both heavy alcohol and depressogenic effects of stressful life events, as well as third variables (e.g., socioeconomic status). The study of comorbidities in related individuals (e.g., siblings, twins) have, thus, played a role in establishing the role of genetic influences as an alternative mechanism of association.
Evidence from genetic epidemiology
Twin studies were foundational in demonstrating that SUDs share genetic underpinnings with each other and with numerous other traits, primarily of a psychiatric nature.67 Just as the heritability of a single trait can be calculated by comparing the twin pair correlation for the trait in identical twins (who share 100% of their segregating genes) and fraternal twins (who share 50% of their segregating genes), the contributions of genetic factors to the correlation between a pair of traits can be estimated by examining cross-trait correlations across identical and fraternal twin pairs. For example, twin studies demonstrated that a significant proportion of the genetic factors that influence SUDs and depression are correlated.68, 69, 70 Beyond psychiatric conditions, twin studies also demonstrated substantial correlations between high alcohol consumption and CVD-related mortality, although the magnitude of the association was no greater in identical than in fraternal twin pairs, suggesting that non-genetic factors (i.e., familial environment) were likely to be relevant.71 To our knowledge, the majority of twin studies that evaluated SUDs did not also assess pain (or COVID-19, although it is far too contemporary) in the same samples.
There are a few illustrations of the utility of twin data in addressing causal mechanisms for the comorbidities studied in this review. Utilizing the cotwin-as-control approach (i.e., comparing the risk of an outcome in an exposed twin compared to their genetically related unexposed co-twin),72,73 studies have shown that while SUDs and depression were genetically correlated, twins with alcohol or cannabis use disorder were more likely to also meet criteria for depression, even when compared to their genetically identical co-twin without these SUDs.70,74 This residual association in the twins with SUDs suggest that factors beyond shared genetics, including possibly causal effects of SUD, play a role. On the other hand, genetic factors explained most of the association between nicotine dependence and depression.73,75 Again, only a handful of studies have examined both SUDs and non-psychiatric health outcomes. For instance, a longitudinal study of veteran males found that the alcohol abstaining member of a discordant pair was at a two-fold increased relative risk of all-cause mortality, and also specifically for CVD-related death, when compared to their alcohol-consuming co-twin.76 Thus, cardioprotective effects of alcohol appeared to be causal in nature, although the study did not assess the origins of alcohol abstinence in this cohort. Furthermore, this decreased mortality was not evident in smokers.76
Statistical power is frequently a challenge in discordant twin studies - within-pair analyses necessitate a reasonable number of identical twin pairs where one twin engages in a behavior while the other does not. As SUDs are highly heritable, such discordance can be difficult to identify and therefore investigators have extended the study of discordance to pairs of relatives by leveraging large nationalized registries. In such analyses, instead of relying on identical twins (an infrequent relative type), co-relative comparisons are made in pairs of individuals with varying degrees of relatedness (e.g., cousins vs. half siblings vs. full siblings) and the pattern of associations is extrapolated to project an effect size in identical twin pairs. Using the Swedish national registries, investigators have found that individuals with an AUD are at increased risk for suicide death when compared to their relatives without an AUD, although the magnitude of this association decreased with increasing degree of relatedness, suggesting that genetic factors also played a role.77
Family, twin and registry-based studies of relatives have the advantage of being population-representative. However, these designs require that multiple traits are measured in closely related individuals. Complementary to these methods are larger scale GWAS in unrelated cohorts, where traits can be measured in independent samples. This is the methodology that we relied on for the current review.
Results
Evidence from genome-wide methods
Our search strategy identified 27 SUD GWAS, which pertained to problematic tobacco use and nicotine dependence (6), alcohol use, misuse and alcohol use disorders (12), cannabis use and cannabis use disorders (2), problematic opioid use and opioid use disorders (6), and general SUD (1).
Genome-wide association studies (GWASs; Figure 1) are conceptually straightforward. Given the abundance of common variation within the genome, researchers can readily identify genomic variants - represented by single base pair changes - that are more common in individuals with certain medical conditions. GWASs are unbiased because they do not prioritize specific genes or variants, allowing for genome-wide discovery.
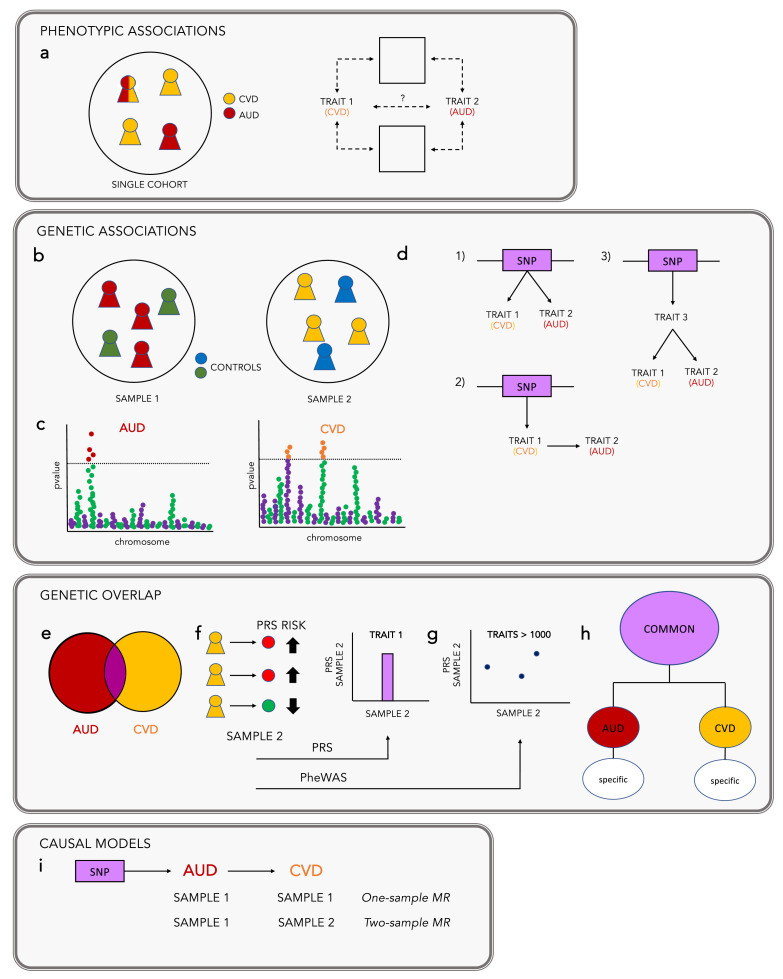
Figure 1.
Current methods to capture genetic comorbidity across traits and diseases. Substance use disorders frequently co-occur with other medical conditions. (a) Phenotypic associations are identified in epidemiologic studies as the increased instance of one trait (e.g., alcohol use disorder, AUD) in the presence of another trait (e.g., cardiovascular disease, CVD). However, the factors that cause these traits, and whether they share common causal factors, is not known. (b) Genetic associations can be identified through large scale genome-wide association studies (GWAS) of complex traits. Traits can be defined as either case/control (e.g., AUD) or as a quantitative measure (e.g., alcohol consumption). These provide trait-genotype associations from thousands to millions of genetic markers (also known as single nucleotide polymorphisms, or SNPs). (c) These associations can be visualized using a Manhattan plot, which simply documents the p-values of the association between a trait and these individual variants along the genome. Because results from GWAS do not contain personal identifiable information they can be shared and have become the basis for the development of methods aimed at comorbidity studies. (d) Some SNPs are associated with multiple traits (known as pleiotropy). An association between a SNP and two traits may mean: (1) that there is a direct genetic association between the SNP and trait 1 and trait 2; (2) that there is a genetic association between the SNP and trait 1, and a phenotypic association between trait 1 and trait 2; (3) that there is a genetic association between the SNP and an unidentified trait 3, which is then phenotypically associated with trait 1 and trait 2. (e) Genetic correlations estimate how much of the phenotypic correlation between two traits is due to common variants, and can be conducted across the genome or parsed into localized estimates.78,79 Unlike phenotypic correlations, genetic correlations are calculated between pairs of traits that are generally measured in independent samples. (f) Polygenic risk, or polygenic score (PRS/PGS) analyses aggregate the effects of multiple SNPs to predict individual risk for a given trait.80,81 From a large discovery GWAS, effect sizes for each SNP are used to weight genotypes in an independent sample. The aggregated effects of these weighted SNPs are summarized in a single score, commonly referred to as PRS/PGS. This approach can be used to assess the association of genetic liability for one trait (e.g., AUD) with a second trait (e.g., CVD). (g) Phenome-wide association studies (PheWAS) extend on PRS analyses by testing the association between a PRS against hundreds to thousands of traits. Because associations between genetic liability for the primary trait and secondary traits may be due to phenotypic correlation, a supplementary analysis can be performed where the first trait is included as a covariate. (h) Other methods82 use GWAS results to examine the effects of genetic variants that are shared across multiple correlated traits. This framework capitalizes on the genetic correlations between traits to identify the variants that are common across all, from those that are specific to each trait. One such approach is a form of structural equation modeling or factor analysis that models how different SUDs and medical conditions might coalesce based on their genetic correlations. (i) Mendelian randomization (MR) analyses were introduced to infer potentially genetically causal relationships using GWAS results. These analyses can be conducted in a single sample (one-sample MR), assuming both traits have been measured in the same sample, or using GWAS results from two different samples (two-sample MR). These approaches can help to examine evidence about putative causal relations between a single “exposure” (e.g., AUD) or multiple exposures or confounders (e.g., AUD and BMI, referred to as Multiple Variable MR) and “outcome” (e.g., CVD) by using genetic variants as instrumental variables.83 MR analyses are not affected by reverse causation because genetic variants are fixed at conception. They are also less biased by the environment, compared to traditional observational studies, because genetic instruments are assumed to affect the outcome only via the exposure, independent of confounders.
GWASs require large datasets and technical capabilities, both of which have increasingly become available via collaborative science. Well-powered GWASs of substance use and SUDs are now available for alcohol, nicotine, cannabis and opioids.84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95 Beyond identification of associated variants, well-powered GWAS have mobilized a suite of analytic paradigms aimed at studying genetic sources of comorbidity - Figure 1 illustrates these specific approaches.
Genetic underpinnings of SUD and medical diseases
Cardiometabolic disease
While genetic liability to tobacco smoking was reported to be associated with increased susceptibility to cardiometabolic diseases,91 alcohol, opioid and cannabis use disorders were not genetically correlated with these traits.88 On the other hand, alcohol consumption has shown negative genetic correlations with many cardiometabolic traits.88,93,96, 97, 98, 99 These paradoxical associations, particularly with drinking frequency, have been partially attributed to measurement error, or inaccurate self-reports, or changes in alcohol consumption over time (e.g., individuals who abstain from drinking due to medical reasons, or former drinkers100,101), as well as higher socioeconomic status that is frequently associated with drinking frequency in the population-based cohorts where the original GWASs were drawn from.89,102
Polygenic risk score (PRS) analyses for cannabis, alcohol and prescription opioid misuse have revealed widespread associations with cardiometabolic conditions (incl. ischemic heart disease, stroke, diabetes mellitus).86,89,92 With regards to alcohol, genetic liability to alcohol misuse was associated with increased risk for heart disease in a large hospital EHR database.89 Polygenic liability to how often someone drank alcohol was associated with decreased risk for metabolic conditions.89 Importantly, these associations did not persist in the absence of the clinical manifestations of AUD (i.e., when covarying for a diagnosis of AUD), suggesting that they may index peripheral effects putatively caused by alcohol, rather than an underlying common genetic architecture, and encouragingly, suggest that treating alcohol misuse could have widespread effects on CVD health. Mendelian randomization (MR) studies using a genetic instrument for alcohol consumption have shown either a causal risk-conferring effect,33 or a null effect, of alcohol consumption on cardiometabolic outcomes.103
With regards to nicotine, a tobacco smoking PRS was associated with circulatory system and metabolic phenotypes, including ischemic heart disease, obesity, and type 2 diabetes.104 As in the alcohol analyses, when a diagnosis of tobacco use disorder was added as a covariate, many of these associations became non-significant, suggesting that they were driven by the effects of tobacco use rather than an underlying genetic architecture. The genetic association between cardiometabolic disease (including type 2 diabetes) and tobacco and alcohol use disorders has been replicated in several other studies.105,106 Another study found that polygenic risk for prescription opioid misuse was associated with increased risk for cardiometabolic outcomes, with metabolic biomarkers as measured using laboratory values extracted from the EHR.92 As a measure of prescription opioid use was not available in the PheWAS dataset, the authors instead covaried for diagnoses of OUD and any SUD. Interestingly, the association with cardiometabolic outcomes persisted following correction for these diagnoses, suggesting that genetic liability, beyond its impact on OUD, might be influencing the likelihood of cardiometabolic disease. With regards to cannabis, one study identified that genetic liability for cannabis use disorders was associated with endocrine or metabolic conditions in a health-care system cohort.86 To date, MR studies of cannabis use or disorder on type 2 diabetes have not been conclusive.107
Chronic pain
Recent large-scale GWAS of SUDs have demonstrated positive genetic correlations between chronic pain and substance use. As expected, pain conditions have been associated with genetic liability for OUD and opioid cessation.84,85,87,108,109 However, these associations also extend to other substances (tobacco, alcohol, general SUD).85,89,110 A plausible mechanism underlying these associations may be explained by reward mechanisms that substances (particularly, opioids) act upon. To date, there are no studies using causal methods to explore these comorbidities and common pathways. Such approaches could potentially illuminate novel treatments for OUD that could target pain after conditioning on substance use liability; which, if identified, could have tremendous therapeutic value. For example, KDM4A, which is a gene that interacts with disulfiram, opioid anesthetics and antidepressants, was recently associated with problematic opioid use.92
Depression
There is robust support for genetic influences on the comorbidity between AUD and depression. Recent GWAS have reinforced that there are positive genetic correlations between both problematic alcohol use and AUD, and depression.89,94,95,97,111 PRS analyses have also consistently found that depression, as well as using substances to relieve negative affect, are amongst the top associations with polygenic liability to several SUDs derived from multiple populations,15,85,89,92,97,112,113 including studies using samples with detailed psychiatric interviews of major depression.114 Furthermore, some of these associations persisted even after covarying for a SUD diagnosis, suggesting a shared genetic basis. On the other hand, the association between tobacco use disorder and polygenic risk for major depression was attenuated when controlling for depression diagnoses,114 suggesting mediating effects of depression on tobacco use.
Genetic causal studies of SUDs and depression suggest some evidence for genetic causal effects of depression on AUD but not vice versa (e.g.,90,113), whereas a bidirectional causal association was found between major depression and OUD87 and prescription opioid use risk.115 While replication studies are necessary, these findings may inform prevention and intervention strategies directed toward the SUD epidemic and depression.
COVID-19
The role of shared genomic variants is far more nuanced when studying the elevated probability of severe COVID-19 in individuals with SUDs. Here, we consider evidence regarding the host genome (i.e., the static genome of affected individuals) - these studies explore whether variants within the host genome that modify an individual's COVID-19 susceptibility (to illness upon infection, severity and prognosis) include variants associated with SUDs. Studies report associations between genetic liability to COVID-19 severity and alcohol, tobacco smoking and cannabis use disorder.85,115,116 For instance, polygenic risk for severe COVID-19 (i.e., requiring hospitalization) has been associated with smoking and alcohol consumption, as well as with cannabis use disorder, even after controlling for covariates. Emerging insights also hint at causal mechanisms, with MR analyses suggesting that a proportion of the association between COVID-19 severity and alcohol and tobacco smoking may be due to causal effects of the latter on the former.117
Insights, caveats and outstanding questions
Collectively, genetic studies are providing persuasive evidence that there is underlying genetic liability that predisposes to both SUDs and chronic pain, depression, and COVID-19. For cardiometabolic disease, there is greater support for a potential causal influence of problematic substance use. Despite this impressive array of studies, the wish-list of advances that would facilitate greater resolution between causal and correlational mechanisms is extensive. Here, we highlight a few caveats, key priority areas and considerations.
Our review primarily focuses on SUDs with well-powered GWAS. Without a doubt, larger GWAS of SUDs will be needed to disentangle causal mechanisms from shared genetic influences. In particular, there are currently no well-powered GWAS of cocaine use disorder and other stimulants (e.g., methamphetamine), which dominate in some global regions. In general, the sample size burden is especially high for SUD GWASs because they are highly polygenic (i.e., the effects of individual genetic variants is exceedingly small and distributed across the genome).
Furthermore, data from individuals of European genetic ancestry are almost exclusively responsible for the findings that we have reviewed. Therefore, an urgent requirement is well-powered GWASs of both SUDs and these four medical conditions in other ancestries. The absence of such diverse studies limits our understanding of the global interplay between SUDs and these 4 medical conditions from a genetic perspective. While it is possible that potential ancestry-specific genetic effects will arise (e.g., different causal loci within the same gene, or novel genetic signals), we will have to be cognizant that certain ancestral differences in genetic contributions may reflect differences in ascertainment and environmental (e.g., diet), societal and cultural factors.118,119 As current methods do not account for the complex sociocultural experiences of individuals that may impact these medical conditions,120 future studies will need to ensure that these sociocultural factors are considered and that phenotypes in understudied groups are well characterized.
GWAS were intended to probe the effects of individual, commonly occurring variants in the genome. Other forms of genetic variation, such as rare single variants121 or structural polymorphisms122 may also be relevant. Furthermore, identifying aggregate genetic overlap or even individual loci that similarly associate with SUDs and these medical conditions provides only limited insight into the pathophysiology of these comorbidities. Downstream in silico analyses that leverage curated ‘omics data to outline networks of genes that underlie these conditions is a necessary next step before GWAS products can be brought forward to preclinical testing and subsequent drug development.123 Novel methods towards linking GWAS results to the action of drugs on cell transcriptomes offers one low-cost opportunity to probe the feasibility of repurposed drugs.124
In the literature, and in this review, individual medical conditions were conceptualized as independent SUD comorbidities, but the four medical conditions studied in this review also occur concomitantly. For instance, the comorbidity between chronic pain and SUDs likely contributes to the comorbidity between depression and SUDs.125 The comorbidity between SUDs and cardiometabolic disease is a necessary consideration when assessing the elevated likelihood of COVID-19 complications in individuals with SUDs.9,126 Thus, despite our attempt to disentangle the mechanisms underlying pairs of comorbidities, it is likely that risk is better represented by a matrix of comorbidities with many shared genetic and non-genetic pathways. Furthermore, this matrix of medical conditions is likely more extensive, including conditions that are developmentally salient (e.g., Late Onset Alzheimer's Disease or Dementias) or cross-cutting aspects of well being (e.g., sleep health). Future research may wish to explore the extent to which these comorbidities arise due to genetic influences that extend across multiple SUDs (i.e., a general addiction liability) or those that are substance-specific.85 Structural equation modeling or subset analyses of genomic data (e.g.,82,127) are a few of the approaches for categorizing this web of comorbidities. Such analyses allow for the construction of confirmatory factor models of multiple variables that are found to be genetically correlated in an attempt to identify loci that undergird all or subsets of traits.82, 89, 127, 128 Likewise, multiple variable causal modeling,129 which allows for the inclusion of heritable confounders, could be valuable in understanding whether the relationship between SUDs and these medical conditions could be attributed to, or mediated by other underlying factors (e.g., the extent to which causal effects of alcohol on cardiometabolic disease are confounded by tobacco smoking or depression).
We also contemplated various study designs that could be particularly well-suited to further research on SUD comorbidities. Current genetic analyses largely rely on cross-sectional data, particularly from health system biobanks, or data with limited information on timing of onset. Longitudinal studies of within-person change, with data collection spanning the period prior and subsequent to substance use and disorder onset, are widely hailed as a gold-standard approach for disentangling causation from correlational findings. However, such studies, especially with large enough sample sizes to examine the role of varying genetic propensity, can be expensive and resource intensive. Attrition or loss to follow-up in such studies may be correlated with heavy substance use and SUDs, posing another challenge. In particular, large national registries such as those in Denmark130 and Sweden131 provide an opportunity for population-based longitudinal analyses. Even within cross-sectional data, tests of causal hypotheses would benefit from access to information on the temporal ordering of onsets, whether through self-recall or EHR registrations and the application of time-variant methodologies, such as survival analysis. However, across all of these large-scale repositories, SUD comorbidity research is disadvantaged by the notoriously low rate of diagnoses and the absence of access to self-reported substance use information.
The need in the field of SUD comorbidity, therefore, is the systematic assessment of substance use in a majority of research and clinical settings, regardless of presenting conditions. Many studies and most physicians gather some information on substance use (e.g., ever using an illicit drug, how much and how often someone drinks or smokes). While such assessments of recent use can identify at-risk individuals, research suggests that they do not fully capture liability to SUDs, especially from a genetic perspective.89 We suggest the use of short screeners [e.g.,12,13,132, 133, 134, 135, 136, 137], which provide researchers and clinicians the opportunity to further document an individual participant's or patient's experiences with substances. Most screeners can be administered rapidly (<1 min), and regularly (e.g., at annual well visits), can assess both substance use (e.g., how much, how often) and problematic use (e.g., impairment due to substance use), and have been culturally and linguistically adapted.
Similarly, we encourage the assessment of family history of SUDs in a majority of research and healthcare settings. Many physicians evaluate whether the patient's family members have a history of cardiometabolic disease, pain-related illness, and even depression. If shared genetic pathways link SUDs to medical conditions commonly confronted in clinical settings, then family history of SUDs could serve as an early risk monitoring and preventative tool, not only for SUDs but also for other comorbid medical conditions.
Conclusion
It is undeniable that problematic substance use and SUDs co-occur with cardiometabolic disease, chronic pain, depression and COVID-19 - all leading causes of worldwide disability. Approaching SUDs as medical conditions, similar to cardiometabolic disorders or pain, rather than moral inadequacies or indicators of lack of interest in personal health, will ensure that at-risk individuals are prioritized for treatment of all of their medical comorbidities rather than penalized for their substance use history. A multi-faceted view of the origins of SUDs, and the use of destigmatizing language,138 could also promote disclosure of substance use behaviors allowing a more accurate assessment of an individual's overall health.
We have shown that rapid advances in genomic studies have allowed researchers to estimate the extent to which comorbidities are due to shared genetic mechanisms or are causally related, which has implications for prevention, intervention and treatment. From a clinical standpoint, if one condition causes another, patient stratification by exposure may be beneficial. However, if comorbidities are due to common genetic factors, then identifying shared pathways of risk has high translational potential. For instance, one could repurpose existing drugs that act on the intersection of genetic pathways for multiple conditions.139 Similarly, tailoring treatment for multiple medical conditions could be informed by genetics (e.g., in the form of polygenic risk scores) thereby enabling precision medicine. Prior to these advances, GWASs and polygenic risk scores, especially for SUDs, will need to explain a greater amount of variability in genetic risk and be equally informative across ancestries.
Contributors
S.S.R., R.L.K. and A.A. jointly researched, wrote and edited the manuscript. All authors have read and approved the final version of the manuscript.
Declaration of interests
S.S.R. and A.A. report consulting fees and speaker honoraria from NIH. R.L.K. reports honoraria and editorial service fees, outside the submitted work.
Acknowledgment
SSR acknowledges T29KT0526, T32IR5226 and DP1DA054394; RLK acknowledges AA028292; AA acknowledges DA054869 & K02DA032573. The funders had no role in the conceptualization or writing of the paper.
References
- 1.Hasin DS, O'Brien CP, Auriacombe M, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170(8):834–851. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Degenhardt L, Charlson F, Ferrari A, et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5(12):987–1012. doi: 10.1016/S2215-0366(18)30337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO; 2020. Global Health Estimates 2020: Disease burden by Cause, Age, Sex, by Country and by Region, 2000-2019.https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates Available from: [Google Scholar]
- 4.Eddie D, Greene MC, White WL, Kelly JF. Medical burden of disease among individuals in recovery from alcohol and other drug problems in the United States: findings From the National Recovery Survey. J Addict Med. 2019;13(5):385–395. doi: 10.1097/ADM.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eddie D, Bates ME, Buckman JF. Closing the brain–heart loop: Towards more holistic models of addiction and addiction recovery. Addict Biol. 2022;27(1) doi: 10.1111/adb.12958. https://onlinelibrary.wiley.com/doi/10.1111/adb.12958 [cited 2022 Jul 1]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner JD, Mouton AJ. In: Comprehensive Physiology. 1st ed. Terjung R, editor. Wiley; 2015. Alcohol effects on cardiac function; pp. 791–802.https://onlinelibrary.wiley.com/doi/10.1002/cphy.c140046 [cited 2022 Jul 1]. Available from: [DOI] [PubMed] [Google Scholar]
- 7.Swendsen JD, Merikengas KR. The comorbidity of depression and substance use disorders. Clin Psychol Rev. 2000;20(2):173–189. doi: 10.1016/s0272-7358(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 8.Martel MO, Shir Y, Ware MA. Substance-related disorders: A review of prevalence and correlates among patients with chronic pain. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87:245–254. doi: 10.1016/j.pnpbp.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 9.Wang QQ, Kaelber DC, Xu R, Volkow ND. COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry. 2021;26(1):30–39. doi: 10.1038/s41380-020-00880-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Psychiatric Association . 5th ed. American Psychiatric Association; Washington DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 11.First MB, Gaebel W, Maj M, et al. An organization- and category-level comparison of diagnostic requirements for mental disorders in ICD -11 and DSM -5. World Psychiatry. 2021;20(1):34–51. doi: 10.1002/wps.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 13.Berman AH, Bergman H, Palmstierna T, Schlyter F. Evaluation of the drug use disorders identification test (DUDIT) in criminal justice and detoxification settings and in a swedish population sample. Eur Addict Res. 2005;11(1):22–31. doi: 10.1159/000081413. [DOI] [PubMed] [Google Scholar]
- 14.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The fagerstrom test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 15.Abdellaoui A, Smit DJA, van den Brink W, Denys D, Verweij KJH. Genomic relationships across psychiatric disorders including substance use disorders. Drug Alcohol Depend. 2021;220 doi: 10.1016/j.drugalcdep.2021.108535. [DOI] [PubMed] [Google Scholar]
- 16.Hatoum AS, Johnson EC, Colbert SMC, et al. The addiction risk factor: a unitary genetic vulnerability characterizes substance use disorders and their associations with common correlates. Neuropsychopharmacology. 2021 doi: 10.1038/s41386-021-01209-w. https://www.nature.com/articles/s41386-021-01209-w epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pando-Naude V, Toxto S, Fernandez-Lozano S, Parsons CE, Alcauter S, Garza-Villarreal EA. Gray and white matter morphology in substance use disorders: a neuroimaging systematic review and meta-analysis. Transl Psychiatry. 2021;11(1):29. doi: 10.1038/s41398-020-01128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose EJ, Picci G, Fishbein DH. Neurocognitive precursors of substance misuse corresponding to risk, resistance, and resilience pathways: implications for prevention science. Front Psychiatry. 2019;10:399. doi: 10.3389/fpsyt.2019.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Boekel LC, Brouwers EPM, van Weeghel J, Garretsen HFL. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1–2):23–35. doi: 10.1016/j.drugalcdep.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Calarco CA, Lobo MK. International Review of Neurobiology. Elsevier; 2021. Depression and substance use disorders: Clinical comorbidity and shared neurobiology; pp. 245–309.https://linkinghub.elsevier.com/retrieve/pii/S0074774220301422 [cited 2022 Mar 30]. Available from: [DOI] [PubMed] [Google Scholar]
- 22.Conrod PJ, Nikolaou K. Annual research review: On the developmental neuropsychology of substance use disorders. J Child Psychol Psychiatry. 2016;57(3):371–394. doi: 10.1111/jcpp.12516. [DOI] [PubMed] [Google Scholar]
- 23.Wilson L, Szigeti A, Kearney A, Clarke M. Clinical characteristics of primary psychotic disorders with concurrent substance abuse and substance-induced psychotic disorders: a systematic review. Schizophr Res. 2018;197:78–86. doi: 10.1016/j.schres.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Schulte MT, Hser YI. Substance Use and Associated Health Conditions throughout the Lifespan. Public Health Rev. 2013;35(2):3. doi: 10.1007/BF03391702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehm J, Gmel GE, Gmel G, et al. The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction. 2017;112(6):968–1001. doi: 10.1111/add.13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Institutes of Health . NIH; Washington, D.C.: 2010. Fetal Alcohol Spectrum Disorders Fact Sheet. [Google Scholar]
- 27.Pezzuto A, Citarella F, Croghan I, Tonini G. The effects of cigarette smoking extracts on cell cycle and tumor spread: novel evidence. Future Sci OA. 2019;5(5):FSO394. doi: 10.2144/fsoa-2019-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization . WHO; 2022. Global HIV, Hepatitis and STIs Programmes- People who inject drugs.https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/populations/people-who-inject-drugs Available from: [Google Scholar]
- 29.Yoon SJ, Jung JG, Lee S, et al. The protective effect of alcohol consumption on the incidence of cardiovascular diseases: is it real? A systematic review and meta-analysis of studies conducted in community settings. BMC Public Health. 2020;20(1):90. doi: 10.1186/s12889-019-7820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11–36. doi: 10.1016/j.neuron.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 31.Zwick J, Appleseth H, Arndt S. Stigma: how it affects the substance use disorder patient. Subst Abuse Treat Prev Policy. 2020;15(1):50. doi: 10.1186/s13011-020-00288-0. s13011-020-00288–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan CY, Fann JCY, Yang MC, et al. Estimating global burden of COVID-19 with disability-adjusted life years and value of statistical life metrics. J Formos Med Assoc. 2021;120:S106–S117. doi: 10.1016/j.jfma.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biddinger KJ, Emdin CA, Haas ME, et al. Association of habitual alcohol intake with risk of cardiovascular disease. JAMA Netw Open. 2022;5(3) doi: 10.1001/jamanetworkopen.2022.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piano MR. Alcohol's effects on the cardiovascular system. Alcohol Res Curr Rev. 2017;38(2):219–241. doi: 10.35946/arcr.v38.2.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy A, Rawal I, Jabbour S. Cardiovascular, Respiratory, and Related Disorders. 3rd ed. The International Bank for Reconstruction and Development /The World Bank; Washington (DC): 2017. Tobacco and cardiovascular disease: a summary of evidence. [PubMed] [Google Scholar]
- 36.Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010;122(24):2558–2569. doi: 10.1161/CIRCULATIONAHA.110.940569. [DOI] [PubMed] [Google Scholar]
- 37.Tadrous M, Shakeri A, Chu C, et al. Assessment of stimulant use and cardiovascular event risks among older adults. JAMA Netw Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.30795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rimm EB, Chan J, Stampfer MJ, Colditz GA, Willett WC. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ. 1995;310(6979):555–559. doi: 10.1136/bmj.310.6979.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stampfer MJ, Colditz GA, Willett WC, Speizer FE, Hennekens CH. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. N Engl J Med. 1988;319:267–273. doi: 10.1056/NEJM198808043190503. [DOI] [PubMed] [Google Scholar]
- 40.Balkau B, Randrianjohany A, Papoz L, Eschwege E. A prospective population-based study of alcohol use and non-insulin-dependent diabetes mellitus. Am J Epidemiol. 1991;134(12):1469–1470. doi: 10.1093/oxfordjournals.aje.a116056. [DOI] [PubMed] [Google Scholar]
- 41.Shinchi K, Kono S, Imanishi K. Lifestyle and glucose tolerance: a crossectional study of Japanese men. Ann Epidemiol. 1994;4(5):363–368. doi: 10.1016/1047-2797(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 42.Subramaniam VN, Menezes AR, DeSchutter A, Lavie CJ. The cardiovascular effects of marijuana: are the potential adverse effects worth the high? Mo Med. 2019;116(2):146–153. [PMC free article] [PubMed] [Google Scholar]
- 43.Alshaarawy O, Anthony JC. Brief report: cannabis smoking and diabetes mellitus. Epidemiology. 2015;26(4):597–600. doi: 10.1097/EDE.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ravi D, Ghasemiesfe M, Korenstein D, Cascino T, Keyhani S. Associations between marijuana use and cardiovascular risk factors and outcomes: a systematic review. Ann Intern Med. 2018;168(3):187. doi: 10.7326/M17-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chow SL, Sasson C, Benjamin IJ, et al. Opioid use and its relationship to cardiovascular disease and brain health: a presidential advisory from the American Heart Association. Circulation. 2021;144(13) doi: 10.1161/CIR.0000000000001007. https://www.ahajournals.org/doi/10.1161/CIR.0000000000001007 [cited 2022 Mar 31]. Available from: [DOI] [PubMed] [Google Scholar]
- 46.Tetsunaga T, Tetsunaga T, Nishida K, et al. Drug dependence in patients with chronic pain: a retrospective study. Medicine. 2018;97(40):e12748. doi: 10.1097/MD.0000000000012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.John WS, Wu LT. Chronic non-cancer pain among adults with substance use disorders: prevalence, characteristics, and association with opioid overdose and healthcare utilization. Drug Alcohol Depend. 2020;209 doi: 10.1016/j.drugalcdep.2020.107902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witkiewitz K, Vowles KE, McCallion E, Frohe T, Kirouac M, Maisto SA. Pain as a predictor of heavy drinking and any drinking lapses in the COMBINE study and the UK Alcohol Treatment Trial: physical pain and alcohol treatment outcomes. Addiction. 2015;110(8):1262–1271. doi: 10.1111/add.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeung EW, Craggs JG, Gizer IR. Comorbidity of alcohol use disorder and chronic pain: genetic influences on brain reward and stress systems. Alcohol Clin Exp Res. 2017;41(11):1831–1848. doi: 10.1111/acer.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeung EW, Lee MR, McDowell Y, Sher KJ, Gizer IR. The association between alcohol consumption and pain interference in a nationally representative sample: the moderating roles of gender and alcohol use disorder symptomatology. Alcohol Clin Exp Res. 2020;44(3):645–659. doi: 10.1111/acer.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balhara YS, Gupta P, Elwadhi D. Co-occurring depression and alcohol-use disorders in South-East Asia: a narrative review. WHO South-East Asia J Public Health. 2017;6(1):50. doi: 10.4103/2224-3151.206166. [DOI] [PubMed] [Google Scholar]
- 52.Boden JM, Fergusson DM. Alcohol and depression. Addiction. 2011;106(5):906–914. doi: 10.1111/j.1360-0443.2010.03351.x. [DOI] [PubMed] [Google Scholar]
- 53.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64(7):830. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 54.Huang H, Shen H, Ning K, et al. Quality of life and its correlates in alcohol use disorder patients with and without depression in China. Front Psychiatry. 2021;11 doi: 10.3389/fpsyt.2020.627338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathew AR, Hogarth L, Leventhal AM, Cook JW, Hitsman B. Cigarette smoking and depression comorbidity: systematic review and proposed theoretical model: Smoking and depression. Addiction. 2017;112(3):401–412. doi: 10.1111/add.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horwood LJ, Fergusson DM, Coffey C, et al. Cannabis and depression: an integrative data analysis of four Australasian cohorts. Drug Alcohol Depend. 2012;126(3):369–378. doi: 10.1016/j.drugalcdep.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Emery MA, Akil H. Endogenous opioids at the intersection of opioid addiction, pain, and depression: the search for a precision medicine approach. Annu Rev Neurosci. 2020;43(1):355–374. doi: 10.1146/annurev-neuro-110719-095912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazereeuw G, Sullivan MD, Juurlink DN. Depression in chronic pain: might opioids be responsible? Pain. 2018;159(11):2142–2145. doi: 10.1097/j.pain.0000000000001305. [DOI] [PubMed] [Google Scholar]
- 59.Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4(5):231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- 60.Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291(15):1887. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- 61.Sohi I, Chrystoja BR, Rehm J, et al. Changes in alcohol use during the COVID-19 pandemic and previous pandemics: a systematic review. Alcohol Clin Exp Res. 2022;46(4):498–513. doi: 10.1111/acer.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen LC, Yang D, Nicolaescu V, et al. Cannabidiol inhibits SARS-CoV-2 replication through induction of the host ER stress and innate immune responses. Sci Adv. 2022;8(8):eabi6110. doi: 10.1126/sciadv.abi6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manthey J, Carr S, Anderson P, et al. Reduced alcohol consumption during the COVID-19 pandemic: analyses of 17 000 patients seeking primary health care in Colombia and Mexico. J Glob Health. 2022;12:05002. doi: 10.7189/jogh.12.05002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patrick ME, Terry-McElrath YM, Miech RA, Keyes KM, Jager J, Schulenberg JE. Alcohol use and the COVID-19 pandemic: historical trends in drinking, contexts, and reasons for use among U.S. adults. Soc Sci Med. 2022;301 doi: 10.1016/j.socscimed.2022.114887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Niles JK, Gudin J, Radcliff J, Kaufman HW. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020. Popul Health Manag. 2021;24(s1) doi: 10.1089/pop.2020.0230. S-43-S-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.The Lancet A time of crisis for the opioid epidemic in the USA. Lancet. 2021;398(10297):277. doi: 10.1016/S0140-6736(21)01653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ystrom E, Reichborn-Kjennerud T, MC Neale, Kendler KS. Genetic and environmental risk factors for illicit substance use and use disorders: joint analysis of self and co-twin ratings. Behav Genet. 2014;44(1):1–13. doi: 10.1007/s10519-013-9626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edwards AC, Kendler KS. A twin study of depression and nicotine dependence: Shared liability or causal relationship? J Affect Disord. 2012;142(1–3):90–97. doi: 10.1016/j.jad.2012.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kendler KS, Heath AC, Neale MC. Alcoholism and major depression in women: a twin study of the causes of comorbidity. Arch Gen Psychiatry. 1993;50(9):690–698. doi: 10.1001/archpsyc.1993.01820210024003. [DOI] [PubMed] [Google Scholar]
- 70.Smolkina M, Morley KI, Rijsdijk F, et al. Cannabis and depression: a twin model approach to co-morbidity. Behav Genet. 2017;47(4):394–404. doi: 10.1007/s10519-017-9848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dai J, Mukamal KJ, Krasnow RE, Swan GE, Reed T. Higher usual alcohol consumption was associated with a lower 41-y mortality risk from coronary artery disease in men independent of genetic and common environmental factors: the prospective NHLBI Twin Study. Am J Clin Nutr. 2015;102(1):31–39. doi: 10.3945/ajcn.114.106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McHugh R. Alcohol use disorder and depressive disorders. Alcohol Res Curr Rev. 2019;40(1) doi: 10.35946/arcr.v40.1.01. arcr.v40.1.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prescott CA, Aggen SH, Kendler KS. Sex-specific genetic influences on the comorbidity of alcoholism and major depression in a population-based sample of US twins. Arch Gen Psychiatry. 2000;57(8):803. doi: 10.1001/archpsyc.57.8.803. [DOI] [PubMed] [Google Scholar]
- 74.Lynskey MT, Agrawal A, Heath AC. Genetically informative research on adolescent substance use: methods, findings and challenges. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1202–1214. doi: 10.1016/j.jaac.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kendler KS. Smoking and major depression: a causal analysis. Arch Gen Psychiatry. 1993;50(1):36. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- 76.Carmelli D, Swan GE, Page WF, Christian JC. World War II-veteran male twins who are discordant for alcohol consumption: 24-year mortality. Am J Public Health. 1995;85(1):99–101. doi: 10.2105/ajph.85.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Edwards AC, Ohlsson H, Sundquist J, Sundquist K, Kendler KS. Alcohol use disorder and risk of suicide in a Swedish population-based cohort. Am J Psychiatry. 2020;177(7):627–634. doi: 10.1176/appi.ajp.2019.19070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bulik-Sullivan BK, Loh PR, Finucane HK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rietveld CA, Esko T, Davies G, et al. Common genetic variants associated with cognitive performance identified using the proxy-phenotype method. Proc Natl Acad Sci. 2014;111(38):13790–13794. doi: 10.1073/pnas.1404623111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wray NR, Lee SH, Mehta D, Vinkhuyzen AAE, Dudbridge F, Middeldorp CM. Research Review: Polygenic methods and their application to psychiatric traits. J Child Psychol Psychiatry. 2014;55(10):1068–1087. doi: 10.1111/jcpp.12295. [DOI] [PubMed] [Google Scholar]
- 82.Grotzinger AD, Rhemtulla M, de Vlaming R, et al. Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nat Hum Behav. 2019;3(5):513–525. doi: 10.1038/s41562-019-0566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362 doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deak JD, Zhou H, Galimberti M, et al. Genome-wide association study and multi-trait analysis of opioid use disorderidentifies novel associations in 639,709 individuals of European and African ancestry. Molecular Psychiatry. 2022 doi: 10.1038/s41380-022-01709-1. http://medrxiv.org/lookup/doi/10.1101/2021.12.04.21267094 epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hatoum AS, Colbert SMC, Johnson EC, et al. Multivariate genome-wide association meta-analysis of over 1 million subjects identifies loci underlying multiple substance use disorders. Preprint. 2022 doi: 10.1038/s44220-023-00034-y. http://medrxiv.org/lookup/doi/10.1101/2022.01.06.22268753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson EC, Demontis D, Thorgeirsson TE, et al. A large-scale genome-wide association study meta-analysis of cannabis use disorder. Lancet Psychiatry. 2020;7(12):1032–1045. doi: 10.1016/S2215-0366(20)30339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kember RL, Vickers-Smith R, Xu H, et al. Cross-ancestry meta-analysis of opioid use disorder uncovers novel loci with predominant effects on brain. Preprint; medRxiv. 2021. Available from: 10.1101/2021.12.13.21267480. [DOI] [PMC free article] [PubMed]
- 88.Liu M, Jiang Y, Wedow R, Li Y, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51(2):237–244. doi: 10.1038/s41588-018-0307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mallard TT, Savage JE, Johnson EC, et al. Item-level genome-wide association study of the alcohol use disorders identification test in three population-based cohorts. Am J Psychiatry. 2022;179:58–70. doi: 10.1176/appi.ajp.2020.20091390. appi.ajp.2020.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Polimanti R, Walters RK, Johnson EC, et al. Leveraging genome-wide data to investigate differences between opioid use vs. opioid dependence in 41,176 individuals from the Psychiatric Genomics Consortium. Mol Psychiatry. 2020;25(8):1673–1687. doi: 10.1038/s41380-020-0677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Quach BC, Bray MJ, Gaddis NC, et al. Expanding the genetic architecture of nicotine dependence and its shared genetics with multiple traits. Nat Commun. 2020;11(1):5562. doi: 10.1038/s41467-020-19265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanchez-Roige S, Fontanillas P, Jennings MV, et al. Genome-wide association study of problematic opioid prescription use in 132,113 23andMe research participants of European ancestry. Mol Psychiatry. 2021;26(11):6209–6217. doi: 10.1038/s41380-021-01335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanchez-Roige S, Palmer AA, Fontanillas P, et al. Genome-wide association study meta-analysis of the alcohol use disorders identification test (AUDIT) in two population-based cohorts. Am J Psychiatry. 2019;176(2):107–118. doi: 10.1176/appi.ajp.2018.18040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walters RK, Polimanti R, Johnson EC, et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci. 2018;21(12):1656–1669. doi: 10.1038/s41593-018-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou H, Sealock JM, Sanchez-Roige S, et al. Genome-wide meta-analysis of problematic alcohol use in 435,563 individuals yields insights into biology and relationships with other traits. Nat Neurosci. 2020;23(7):809–818. doi: 10.1038/s41593-020-0643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clarke TK, Adams M, Davies G, et al. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank. Mol Psychiatry. 2017;9 doi: 10.1038/mp.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kranzler HR, Zhou H, Kember RL, et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun. 2019;10(1):1499. doi: 10.1038/s41467-019-09480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mallard TT, Sanchez-Roige S. Dimensional phenotypes in psychiatric genetics: lessons from genome-wide association studies of alcohol use phenotypes. Complex Psychiatry. 2021;7(3–4):45–48. doi: 10.1159/000518863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sanchez-Roige S, Fontanillas P, Elson SL, et al. Genome-wide association study of alcohol use disorder identification test (AUDIT) scores in 20 328 research participants of European ancestry: GWAS of AUDIT. Addict Biol. 2019;24(1):121–131. doi: 10.1111/adb.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dao C, Zhou H, Small A, et al. The impact of removing former drinkers from genome-wide association studies of AUDIT-C. Addiction. 2021;116(11):3044–3054. doi: 10.1111/add.15511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xue A, Jiang L, Zhu Z, et al. Genome-wide analyses of behavioural traits are subject to bias by misreports and longitudinal changes. Nat Commun. 2021;12(1):20211. doi: 10.1038/s41467-020-20237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kranzler HR, Zhou H, Kember RL. Identifying and reducing bias in genome-wide association studies of alcohol-related traits. Am J Psychiatry. 2022;179(1):14–16. doi: 10.1176/appi.ajp.2021.21111107. [DOI] [PubMed] [Google Scholar]
- 103.Lankester J, Zanetti D, Ingelsson E, Assimes TL. Alcohol use and cardiometabolic risk in the UK Biobank: a Mendelian randomization study. Taniyama Y, editor. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0255801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hartwell EE, Merikangas AK, Verma SS, et al. Genetic liability for substance use associated with medical comorbidities in electronic health records of African- and European-ancestry individuals. Addict Biol. 2022;27(1) doi: 10.1111/adb.13099. https://onlinelibrary.wiley.com/doi/10.1111/adb.13099 [cited 2022 Apr 11]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sanchez-Roige S, Palmer AA. Emerging phenotyping strategies will advance our understanding of psychiatric genetics. Nat Neurosci. 2020;23(4):475–480. doi: 10.1038/s41593-020-0609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vujkovic M, Keaton JM, Lynch JA, et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet. 2020;52(7):680–691. doi: 10.1038/s41588-020-0637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baumeister SE, Baurecht H, Nolde M, et al. Cannabis use, pulmonary function, and lung cancer susceptibility: a Mendelian randomization study. J Thorac Oncol. 2021;16(7):1127–1135. doi: 10.1016/j.jtho.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 108.Cox JW, Sherva RM, Lunetta KL, et al. Genome-wide association study of opioid cessation. J Clin Med. 2020;9(1):180. doi: 10.3390/jcm9010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Song W, Kossowsky J, Torous J, et al. Genome-wide association analysis of opioid use disorder: a novel approach using clinical data. Drug Alcohol Depend. 2020;217 doi: 10.1016/j.drugalcdep.2020.108276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sanchez-Roige S, Cox NJ, Johnson EO, Hancock DB, Davis LK. Alcohol and cigarette smoking consumption as genetic proxies for alcohol misuse and nicotine dependence. Drug Alcohol Depend. 2021;221 doi: 10.1016/j.drugalcdep.2021.108612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Colbert SMC, Funkhouser SA, Johnson EC, et al. Novel characterization of the multivariate genetic architecture of internalizing psychopathology and alcohol use. Am J Med Genet B. 2021;186(6):353–366. doi: 10.1002/ajmg.b.32874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hartwell M, Greiner B, Dunn K, Croff J, Beaman J. Prescription opioid use and laboratory value derangements: a cross-sectional analysis of NHANES data. Pain Physician. 2021;24(1):E95–E100. [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou H, Rentsch CT, Cheng Z, et al. Association of OPRM1 functional coding variant with opioid use disorder: a genome-wide association study. JAMA Psychiatry. 2020;77(10):1072. doi: 10.1001/jamapsychiatry.2020.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kember RL, Hartwell EE, Xu H, et al. Phenome-wide association analysis of substance use disorders in a deeply phenotyped sample. Preprint medRxiv. 2022 doi: 10.1101/2022.02.09.22270737. http://medrxiv.org/lookup/doi/10.1101/2022.02.09.22270737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rosoff DB, Yoo J, Lohoff FW. Smoking is significantly associated with increased risk of COVID-19 and other respiratory infections. Commun Biol. 2021;4(1):1230. doi: 10.1038/s42003-021-02685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wendt FR, De Lillo A, Pathak GA, De Angelis F. COVID-19 host genetics initiative, Polimanti R. host genetic liability for severe COVID-19 associates with alcohol drinking behavior and diabetic outcomes in participants of European descent. Front Genet. 2021;12 doi: 10.3389/fgene.2021.765247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu H, Xin J, Cai S, Jiang X. Mendelian randomization analysis provides causality of smoking on the expression of ACE2, a putative SARS-CoV-2 receptor. eLife. 2021;10:e64188. doi: 10.7554/eLife.64188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harpak A, Przeworski M. The evolution of group differences in changing environments. PLoS Biol. 2021;19(1) doi: 10.1371/journal.pbio.3001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McAllister K, Mechanic LE, Amos C, et al. Current challenges and new opportunities for gene-environment interaction studies of complex diseases. Am J Epidemiol. 2017;186(7):753–761. doi: 10.1093/aje/kwx227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Atkinson EG, Bianchi SB, Ye GY, et al. Cross-ancestry genomic research: time to close the gap. Neuropsychopharmacology. 2022 doi: 10.1101/2022.02.09.22270737. https://www.nature.com/articles/s41386-022-01365-7 epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cirulli ET, White S, Read RW, et al. Genome-wide rare variant analysis for thousands of phenotypes in over 70,000 exomes from two cohorts. Nat Commun. 2020;11(1):542. doi: 10.1038/s41467-020-14288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mukamel RE, Handsaker RE, Sherman MA, et al. Protein-coding repeat polymorphisms strongly shape diverse human phenotypes. Science. 2021;373(6562):1499–1505. doi: 10.1126/science.abg8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.So HC, Chau CKL, Chiu WT, et al. Analysis of genome-wide association data highlights candidates for drug repositioning in psychiatry. Nat Neurosci. 2017;20(10):1342–1349. doi: 10.1038/nn.4618. [DOI] [PubMed] [Google Scholar]
- 124.Reay WR, Cairns MJ. Advancing the use of genome-wide association studies for drug repurposing. Nat Rev Genet. 2021;22(10):658–671. doi: 10.1038/s41576-021-00387-z. [DOI] [PubMed] [Google Scholar]
- 125.Morasco BJ, Corson K, Turk DC, Dobscha SK. Association between substance use disorder status and pain-related function following 12 months of treatment in primary care patients with musculoskeletal pain. J Pain. 2011;12(3):352–359. doi: 10.1016/j.jpain.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jemberie WB, Stewart Williams J, Eriksson M, et al. Substance use disorders and COVID-19: multi-faceted problems which require multi-pronged solutions. Front Psychiatry. 2020;11:714. doi: 10.3389/fpsyt.2020.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bhattacharjee S, Rajaraman P, Jacobs KB, et al. A subset-based approach improves power and interpretation for the combined analysis of genetic association studies of heterogeneous traits. Am J Hum Genet. 2012;90(5):821–835. doi: 10.1016/j.ajhg.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu Y, Xia L, Lee S, Zhou X, et al. Subset-based analysis using gene-environment interactions for discovery of genetic associations across multiple studies or phenotypes. Hum Hered. 2018;83(6):283–314. doi: 10.1159/000496867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sanderson E. Multivariable Mendelian randomization and mediation. Cold Spring Harb Perspect Med. 2021;11(2) doi: 10.1101/cshperspect.a038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;17(7):449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Laugesen K, Ludvigsson JF, Schmidt M, et al. Nordic health registry-based research: a review of health care systems and key registries. Clin Epidemiol. 2021;13:533–554. doi: 10.2147/CLEP.S314959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Adamson SJ, Kay-Lambkin FJ, Baker AL, et al. An improved brief measure of cannabis misuse: the Cannabis Use Disorders Identification Test-Revised (CUDIT-R)☆. Drug Alcohol Depend. 2010;110(1–2):137–143. doi: 10.1016/j.drugalcdep.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 133.Bruehl S, Stone A, Palmer C, et al. Self-reported cumulative medical opioid exposure and subjective responses on first use of opioids predict analgesic and subjective responses to placebo-controlled opioid administration. Reg Anesth Pain Med. 2019;44(1):92–99. doi: 10.1136/rapm-2018-000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the current opioid misuse measure. Pain. 2007;130(1):144–156. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Coyne KS, Barsdorf AI, Currie BM, et al. Construct validity and reproducibility of the prescription opioid misuse and abuse questionnaire (POMAQ) Curr Med Res Opin. 2021;37(3):493–503. doi: 10.1080/03007995.2020.1865890. [DOI] [PubMed] [Google Scholar]
- 136.Knisely JS, Wunsch MJ, Cropsey KL, Campbell ED. Prescription Opioid Misuse Index: a brief questionnaire to assess misuse. J Subst Abuse Treat. 2008;35(4):380–386. doi: 10.1016/j.jsat.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 137.Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med. 2005;6(6):432–442. doi: 10.1111/j.1526-4637.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 138.Volkow ND, Gordon JA, Koob GF. Choosing appropriate language to reduce the stigma around mental illness and substance use disorders. Neuropsychopharmacology. 2021;46(13):2230–2232. doi: 10.1038/s41386-021-01069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77. doi: 10.1016/S2215-0366(19)30416-X. [DOI] [PMC free article] [PubMed] [Google Scholar]