Abstract
Objective:
Older women are at increased risk of developing Alzheimer’s disease compared to men. One proposed reason is that following menopause there is a decline in estrogens. Estrogens are important for cholinergic functioning and attenuate the impact of cholinergic antagonists on cognitive performance in postmenopausal women. Self-reported or subjective cognitive complaints in middle or older age may represent a harbinger of cognitive decline and those who endorse cognitive complaints appear more likely to develop future cognitive impairment. However, the response of individuals with cognitive complaints after menopause to estrogen and the relationship to cholinergic functioning has not been investigated. This study investigated the effect of estrogen treatment using 17β-estradiol on cognitive performance following anticholinergic blockade in postmenopausal women and the relationship of this interaction with the level of self-reported (subjective) postmenopausal cognitive complaints.
Methods:
Forty postmenopausal women (aged 50–60 years) completed a 3-month treatment regimen of either 1 mg oral estradiol or placebo. Participants then completed four challenge days in which they completed cognitive and behavioral tasks after one of four cholinergic antagonist drug conditions (oral mecamylamine (MECA), intravenous scopolamine, combined MECA and scopolamine, or PLC).
Results:
Compared to PLC, the estradiol treated group performed worse on attention tasks under cholinergic challenge including the choice reaction time task and the critical flicker fusion task. In addition, participants who endorsed greater cognitive complaints showed reduced performance on the N-back working memory task, regardless of whether they received estradiol treatment.
Conclusions:
The findings of this study indicate that estradiol treatment was unable to mitigate anticholinergic blockade in postmenopausal women with subjective cognitive complaints, and worsened performance on attention tasks. Moreover, the present study suggests that greater levels of cognitive complaints following menopause may be associated with an underlying decline in cholinergic function that may manifest as an inability to compensate during working memory tasks.
Keywords: cholinergic system, cognitive complaints, estrogen, mecamylamine, menopause, scopolamine, working memory
1 |. INTRODUCTION
Changes in mood and cognition are commonly reported by women during and following the menopause transition (Dumas et al., 2013; Halbreich et al., 1995; Weber et al., 2014; Woods et al., 2000). While in many cases these changes are not long-lasting, in other cases this transition may be the beginning of long-term concerns about memory and cognition. In some of these women, these self-reported changes may progress to the development of objective deficits in cognition or Mild Cognitive Impairment (MCI), which is associated with increased likelihood of later development of Alzheimer’s disease (AD; Buckley et al., 2016; Jessen, 2014; Mitchell et al., 2014; Reisberg et al., 2010). Postmenopausal women endorsing greater numbers of cognitive complaints have been shown to perform worse on objective measures of memory and attention compared to postmenopausal women who do not endorse these complaints (Schaafsma et al., 2010; Weber et al., 2012), indicating that there may be someveracity to these concerns. The above findings highlight the importance of investigation into the relationship of postmenopausal cognitive complaints, and what underlying biological processes may contribute to them as well as the potential for amelioration.
Alterations in mood and cognition after menopause are thought to be caused by the reduction of estrogens in the brain which may accelerate cognitive decline to a level beyond chronological age (Halbreich et al., 1995; Maki & Dumas, 2009). Several studies over the past 20 years have examined hormone therapy (HT) to enhance cognition both during and following the menopause transition with mixed success (Espeland et al., 2004; Henderson et al., 2016; MacLennan et al., 2006). The lack of a definitive improvement could be due to an underlying deficit that does not respond to HT. One of the more well-known systems affected by the depletion of estrogens is the cholinergic system, whose activity is modulated by 17β-estradiol (Newhouse & Dumas, 2015). The cholinergic system is involved in many cognitive processes, including attention and memory (Ballinger et al., 2016; Sarter et al., 2005). Additionally, the reduction of cholinergic system efficiency is associated with the development of pathological cognitive decline with aging (Dumas & Newhouse, 2011). There is evidence from both human and animal models that loss of estradiol (E2) directly impacts the integrity of the cholinergic system (Gibbs et al., 1994; Maki & Dumas, 2009; McMillan et al., 1996; Norbury et al., 2007; Smith et al., 2011). Following depletion, adding exogenous E2 can reverse the impact on cholinergic tone (Yamamoto et al., 2007). In humans, the length of time that postmenopausal women were on HT has been observed to be positively correlated with cholinergic transporter binding at the synapse in several cortical regions, including the hippocampus and frontal cortex (Smith et al., 2001). Moreover, women who started HT shortly after menopause displayed greater hippocampal and posterior cingulate acetylcholine compared to women who had not taken HT, which supports the proposal of a critical period of effectiveness in which HT is effective (Smith et al., 2011; Whitmer et al., 2011). Therefore, underlying changes in cholinergic functioning during the menopause transition are more detectable and potentially more modifiable within the first few years after menopause compared to many years later.
This cholinergic system is expressed through two types of receptors, namelyion-gated nicotinic acetylcholine receptors, and metabotropic muscarinic acetylcholine receptors. Nicotinic acetylcholine receptors are expressed throughout the cortex but have high concentrations in the ventral tegmental area, prefrontal cortex and hippocampus, as well as at neuromuscular junctions (Levin, 2002; Picciotto et al., 2012). Muscarinic acetylcholine receptors are expressed throughout cortical and subcortical areas but are highly expressed in the prefrontal cortex, hippocampus, striatum, and thalamus (Ballinger et al., 2016; Moran et al., 2019). Outside the brain, muscarinic receptors are located on organs in the cardiovascular, gastrointestinal systems and on sweat glands. Nicotinic receptor expression is faster compared to muscarinic receptor expression, as muscarinic receptors often act as the initial point of a complex cascade of expression using second messengers (Caulfield, 1993; Picciotto et al., 2012). Both nicotinic and muscarinic receptors are important in the encoding of new memories (Green et al., 2005) however in different ways. Nicotine receptors have been shown to boost excitatory transmission during the encoding of new information, while muscarinic receptors suppress feedback mechanisms that could interfere with the encoding process (Hasselmo, 2006; Hasselmo & Sarter, 2011). For attention processes, muscarinic receptors are particularly important for topdown control of attention (Gould et al., 2015; Parikh & Sarter, 2008), whereas nicotinic receptors are involved in both topdown control and also sensory-driven, bottom-up attentional streams (Guillem et al., 2011). As such, cholinergic receptors can influence higher-order cognitive processes in different ways, by controlling the transmission of information throughout the cortex.
One way to examine the influence of E2 on cholinergic system functioning is to use a cholinergic inactivation model, which uses cholinergic antagonists to induce temporary deficits in cholinergic function and task performance (Ellis et al., 2006; Newhouse, Sunderland, Tariot, Blumhardt, et al., 1988). Anticholinergic challenge using the muscarinic antagonist scopolamine and the nicotinic antagonist mecamylamine (MECA) has been observed to suppress performance on tasks of attention and memory in both healthy volunteers (Baakman et al., 2017; Green et al., 2005; Newhouse et al., 1992, 1994), and also in patient populations (Huff et al., 1988; Newhouse et al., 1996; Newhouse, Sunderland, Tariot, Weingartner, et al., 1988). This challenge model allows for the examination of group or treatment differences that may be masked by increased cholinergic activation in healthy adults. In studies of postmenopausal women, MECA and scopolamine have been utilized to demonstrate the impact of the loss of E2 on the cholinergic system following menopause (Newhouse & Dumas, 2015). A study by Dumas and colleagues (Dumas et al., 2006) showed that 3 months of 1 mg oral E2 treatment in postmenopausal women lessened the impact of both MECA or scopolamine on attention, psychomotor speed, and episodic memory tasks compared to placebo. A second study (Dumas et al., 2008) showed that recently postmenopausal women responded better following cholinergic blockade compared to older postmenopausal women, performing better on a verbal episodic memory task following 3-months of oral 2 mg E2 treatment.
The present study seeks to build on previous results by investigating the effects of E2 treatment on cognitive performance during anticholinergic challenge and investigating the relationship with the level of postmenopausal cognitive complaints. All participants were between the ages of 50–60 years, to target the relative age range of greatest effectiveness for E2 treatment, based on the timeline of the critical window hypothesis (Whitmer et al., 2011). Participants received either 3 months oral E2 or placebo treatment, then completed four anticholinergic challenge days, in which they received MECA, scopolamine (SCOP), a combination of MECA and scopolamine, or placebo. On these challenge days, participants were administered a series of cognitive tasks sensitive to alterations in cholinergic functioning. Based on the results of previous studies, we predicted that the 3 months of E2 treatment would be successful in boosting cholinergic system integrity and would enable the participants to be able to compensate more effectively for the reduced activation due to anticholinergic challenge from MECA or scopolamine. Therefore, we hypothesized that participants who received 3 months of E2 treatment would perform better on tasks of attention and memory following cholinergic blockade compared to those who received placebo. We also predicted that participants with greater endorsement of cognitive complaints would respond worse to E2 treatment, performing worse on tasks of attention and memory compared to those who endorsed fewer complaints.
2 |. METHODS
2.1 |. Participants
A total of 56 healthy, postmenopausal women aged between 50 and 60 years were recruited and screened. Of this sample, 40 women (mean 55.95 ± 2.7 years) completed all study visits; and data were analyzed on these participants. All participants were without menses for at least 1 year with a follicle-stimulating hormone level greater than 30 mIU/ml. Participants had to have not used any HT for at least 1 year before participating in the study. All participants had to have gone through natural rather than surgical menopause, however having undergone a hysterectomy was allowed if participants did not have a bilateral oophorectomy. Recruitment of participants was targeted to capture postmenopausal women with varying levels of cognitive complaints that they reported began after the menopause transition. Following screening, all participants were randomized to receive either 1 mg oral 17β-E2 or placebo (n = 20 for each treatment condition). The current study was conducted at the University of Vermont (UVM) and the Vanderbilt University Medical Center (VUMC). Both Institutional Review Boards approved all study protocols, and all participants gave written informed consent in accordance with the Declaration of Helsinki. Exclusion criteria for all participants included: (1) any active neurologic and/or psychiatric disease, history of significant head trauma followed by persistent neurologic deficits, or known structural brain abnormalities, (2) current major depression or another major psychiatric disorder as described in the Diagnostic and Statistical Manual of Mental Disorders version IV including use of psychotropic medications (e.g., antidepressants), (3) any history of alcohol or substance abuse or dependence, (4) any significant systemic illness or unstable medical condition which could lead to difficulty complying with the protocol including, (4a) history of myocardial infarction in the past year or unstable, severe cardiovascular disease including angina or congestive heart failure with symptoms at rest, or clinically significant abnormalities on the ECG (4b) clinically significant and/or unstable pulmonary, gastrointestinal, hepatic, or renal disease (4c) insulin-requiring diabetes or uncontrolled diabetes mellitus, (4d) uncontrolled hypertension (systolic BP > 160 or diastolic BP > 100), (5) use of HT during the last year, (6) a history of breast cancer, and (7) and a history or presence of severe menopausal symptoms. Exclusion criteria for MRI scanning included: (1) non-removable ferromagnetic material on or in the body and (2) claustrophobia. All participants who passed inclusion and exclusion criteria were screened to ensure a normal level of cognitive performance for age, and exclude MCI or dementia using the Mini-Mental State Exam (MMSE; Folstein et al., 1975), Brief Cognitive Rating Scale, and the Mattis Dementia Rating Scale to establish a Global Deterioration Scale score (Reisberg et al., 1988). Participants were required to have a Global Deterioration Scale score of 1–2 and an MMSE score of greater than 26. Participants were required to be euthymic as assessed by a partial Structured Clinical Interview (SCID) for DSM disorders (Nichols et al., 1976) and the Beck Depression Inventory (BDI; score <13 Beck et al., 1961).
2.2 |. Subjective measures of cognitive and symptomatic complaints
Following screening, participants completed the Cognitive Complaint Index (CCI) battery (Saykin et al., 2006) to determine their level of endorsed subjective cognitive complaints. The CCI battery included the Memory Functioning Questionnaire (Gilewski et al., 1990), Memory Self-Rating Questionnaire (Squire et al., 1979), Neurobehavioral Function and Activities of Daily Living Rating Scale (Saykin, 1992), Informant Questionnaire on Cognitive Decline in the Elderly (Jorm et al., 1994), 4 items related to cognition from the Geriatric Depression Scale (Yesavage et al., 1982), 12 items from a telephone-based screening for MCI, and 20 items from the Memory Assessment Questionnaire adapted in part from the Functional Activities Questionnaire. Responses to 114 questions were dichotomized as representing an endorsed or unendorsed complaint. The CCI score was expressed as the percent of all items endorsed. This baseline CCI was used as the marker of endorsed cognitive complaints in all future analyses. The severity of postmenopausal symptoms was assessed using the Menopause Symptom Checklist (MSC; Newhouse et al., 2010), which includes 60 items measured on a Likert scale from 0 to 4 that describe the severity of physiological symptoms over the past 4 weeks. The MSC was completed prior to and following the 3-month treatment period.
2.3 |. Procedure
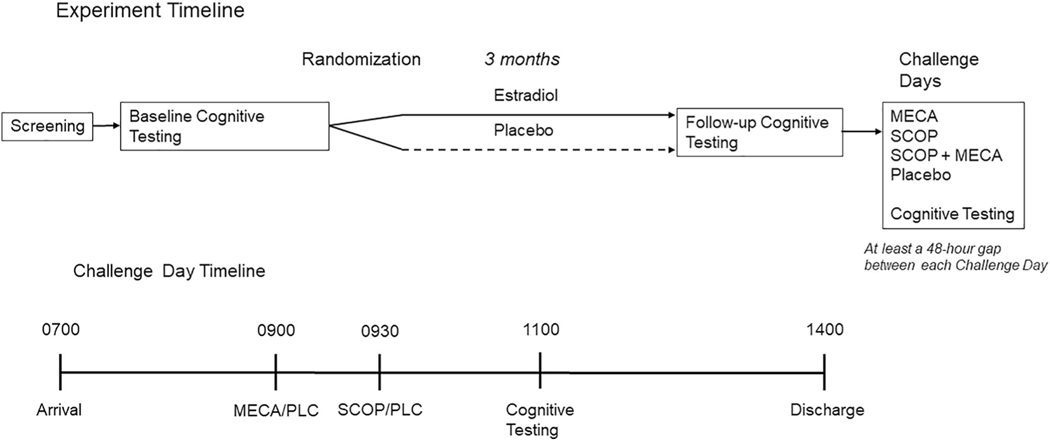
Figure 1 outlines the overall procedure of the study, as well as the procedure during anticholinergic challenge days. After the initial screening session, participants completed baseline cognitive and neuroimaging assessments. The cognitive battery used at baseline was the same used on each study day and consisted of tasks designed to measure arousal, attention, and memory. The participants then completed 3 months of treatment (1 mg oral E2 or placebo) before undertaking a post-treatment cognitive and neuroimaging assessment. At the end of the treatment phase and after all challenges are completed, all participants who had not undergone a previous hysterectomy were administered 10 mg medroxyprogesterone acetate per day for 12 days to produce endometrial shedding. The participants who had previously undergone hysterectomy did not receive the medroxyprogesterone. Compliance was verified by pill counts during biweekly telephone calls, and at in-person study visits. Following the 3-month treatment period, the participants completed 4 days of testing in which they were given one of four different cholinergic challenge treatments: 20 mg oral MECA + intravenous (IV) placebo, 2.5 μg/kg IV scopolamine (SCOP) + oral placebo, 10 mg oral MECA plus 2.5 μg/kg IV scopolamine in combination (SCOP + MECA), or an oral and IV placebo. The order of challenge days was randomized across participants, and investigators were blinded to the drugs being administered. The procedure for the testing days was as follows. Participants were admitted to the clinical research center at UVM or VUMC in the morning at 0700. At this time, an IV was started with saline continuously delivered for the next 7 hours to reduce peripheral effects of the antagonists. Participants received an oral dose of MECA or placebo at 0900 and at 0930 an intravenous dose of scopolamine or placebo depending on treatment assignment as indicated above. Cognitive testing began at 1100, at the estimated peak drug effect time (based on prior work) for the cholinergic antagonists taken 1.5 and 2 h prior. The order of the cognitive tasks was counterbalanced across the challenge days. Participants were discharged at approximately 1400, or at 7 hours following the beginning of the saline administration. All participants were fasting on the day of testing and had no caffeine on the day before admission. The four challenge days were completed across a 3-week period in which all challenge days were separated by at least 48 h. This was to ensure that enough time had passed for the drugs to dissipate before completion of the next challenge day (Baakman et al., 2017; Ebert et al., 2001; Newhouse et al., 1992; 1994; Newhouse, Sunderland, Tariot, Weingartner, et al., 1988; Putcha et al., 1989; Young et al., 2001).
FIGURE 1.
Experimental design of the treatment period and challenge days
2.4 |. Cognitive battery
2.4.1 |. Measures of attention and arousal
The critical flicker fusion task (CFF; Kupke & Lewis, 1989) is a test of attention/vigilance using the frequency of a flickering LED (between 12 and 50 Hz). The outcome variable for CFF is the frequency (Hz) for ascending and descending trials. In an ascending trial, the participant presses a button indicating when the light is flickering to a speed that the lights appear to be continuously on or fused. In a descending trial, the participant presses a button when the frequency of apparently fused lights is decreased such that lights begin to appear to be flashing.
The choice reaction time (CRT; Hindmarch, 1984) task is a measure of attention and psychomotor speed. In this task, the participant must press one of six buttons in an arc when a light corresponding to the button turns on. She then must press and hold a “home” button, which sits closest to her on the box. Outcome variables on the CRT include the mean and median total reaction time, which can be separated into recognition time (time from stimulus onset to initiation of movement) and motor time (time from initiation of movement to stimulus termination).
2.4.2 |. Measures of episodic and working memory
A visually presented N-back sequential letter task was used to assess working memory performance (Jonides et al., 1997; Saykin et al., 2004). Four conditions were presented: 0-back, 1-back, 2-back, and 3-back. The 0-, 1-, 2-, and 3-back conditions were performed in two blocks of 27 trials each for a total of 216 trials. The main outcome variable of the n-back task was sensitivity (d′) for each of the four conditions, calculated as Z (Hit) – Z (False Alarms).
Immediate and delayed episodic memory was assessed using The Selective Reminding Task (SRT; Buschke, 1973). This task involves word list learning and selective reminding over 8 trials and a delayed recall trial after a 20-min delay. SRT total immediate recall was the number of correctly recalled words across trials 1–8, recall consistency was the number of words correctly on two consecutive trials across trials 1–8, recall failure was the number of words not recalled on two consecutive trials across trials 1–8, and delayed recall was the number of words correctly recalled after a 20-min delay.
2.5 |. Statistical analyses
Demographic information for the two treatment groups was compared by independent samples t-tests for continuous variables, and by Chi-square likelihood ratios for categorical variables. Analysis of the MSC scores was performed using a repeated-measures analysis of variance (ANOVA) with one within-subject factor of time (screening vs. post-treatment) and one between-subjects factor of treatment (E2 vs. placebo). To examine differences in the length of time since menopause, participants were categorized according to the Stages of Reproductive Aging Workshop criteria (STRAW+10 model; Harlow et al., 2012). To examine whether there was any difference in the E2 treatment response between participants in the two STRAW conditions, we ran a repeated-measures ANOVA within-subject factor of time (screening vs. post-treatment) and one between subject’s factor of STRAW (Stage 1 vs. Stage 2) on the participants who received 3-months E2 treatment. Analyses for each cognitive task used the outcome variables specified above. The analyses on the outcome variables were performed as follows: To examine the impact of the 3-months E2 or placebo treatment, as well as to ensure that objective performance of the two treatment groups was similar without cholinergic blockade, we used the cognitive data from both baseline and post-treatment sessions to perform a repeated-measures ANOVA with one within subject’s factor of time (baseline vs. post-treatment) and one between subject’s factor of treatment (E2 vs. placebo). The next step was to examine the cholinergic blockade effects, and to do this we evaluated the effect of anticholinergic challenge day performance versus placebo challenge day performance for the participants who received 3-months placebo treatment. This analysis was performed as a 4-level (Challenge Day) repeated measures ANOVA. Post-hoc contrasts compared individual challenge day performance against the placebo challenge day performance.
Following this, we examined the impact of the treatment and endorsed complaints on cognitive performance under cholinergic blockade. To do this we first calculated difference scores for each of the outcome variables. Each difference score was the anticholinergic challenge performance minus placebo challenge performance. To examine the impact of the blockade of nicotinic, muscarinic or both nicotinic and muscarinic receptors on cognitive performance these differences scores from both treatment groups were included in a one-way analysis of covariance (ANCOVA) for each anticholinergic challenge day separately, with a between participants’ factor of hormone treatment (E2 vs. placebo), and CCI score used as a covariate. Due to technical scoring issues, data from some of the 40 participants were not analyzed on each task. The analysis of the pre/post-treatment MSC was run on 32 participants, with the final sample having 15 participants who received E2 and 17 participants who received placebo. For each of the cognitive tasks on the challenge days, the statistical analysis was run on 38 participants. For the CRT and CFF, N-back tasks, the final sample had 19 participants who received E2 and 19 participants who received placebo. For the SRT the final sample was 20 participants who received E2 and 18 participants who received placebo. All statistical analyses were run using International Business Machines Corporation SPSS version 20 (Armonk, NY).
3 |. RESULTS
Table 1 summarizes participant demographic information for each group. The two groups were similar in all characteristics, including age, years since menopause, as well as the levels of cognitive complaints. There were more women in the placebo treatment group that had previously used HT (7 vs. 2) however, this difference was not statistically significant (p > 0.05). The average duration of HT use at least 12 months prior to the study was 3.1 ± 2.2 years, and the most common form of HT reported was oral estrogen medication (n = 5). The mean period post menopause before participation in the study was 6.9 ± 4.06 years. According to the most recent STRAW+10 criteria (Harlow et al., 2012), 22 of the 40 participants were in Stage +1 and 18 participants were in Stage +2. The STRAW+10 stages did not differ between treatment groups (p = 0.3). In the reporting of analyses following anticholinergic challenge, the term “treatment” always refers to E2 or placebo treatment and “challenge” refers to anticholinergic challenge (MECA, SCOP, SCOP + MECA) or the placebo challenge.
TABLE 1.
Demographic information
| Estradiol |
Placebo |
|||
|---|---|---|---|---|
| Measure | Mean (SD) | Range | Mean (SD) | Range |
| Age | 55.35 (2.68) | 50–59 | 56.55 (2.67) | 52–60 |
| CCI (% complaints endorsed) | 18.71 (12.2) | 1.8–47 | 21.36 (13.5) | 0–51 |
| BDI | 3.1 (3.1) | 0–10 | 2.95 (3.2) | 0–12 |
| BMI | 24.49 (3.1) | 20–29 | 24.5 (2.8) | 20.3–30.1 |
| Years since menopause | 6.55 (4.3) | 2–16 | 7.2 (3.9) | 2–15 |
| STRAW+10 score (1/2) | 13/7 | 10/10 | ||
| MSC at screening | 14.0 (8.2) | 2–30 | 16.2 (8.0) | 6–37 |
| MSC post-treatment a | 17.27 (10.4) | 2–36 | 22.2 (12.3) | 6–50 |
| MMSE | 29.05 (1.05) | 26–30 | 28.9 (1.41) | 26–30 |
| Education (years) | 16.1 (1.9) | 13–20 | 15.8 (2.1) | 12–20 |
| Prior HT use (yes/no) | 2/18 | 7/13 | ||
Note: All numbers are displayed as mean (standard deviation) unless stated.
Abbreviations: BDI, Beck Depression Inventory; BMI, Body Mass Index; CCI, Cognitive Complaint Index; HT, Hormone Replacement Therapy; MMSE, Mini-Mental State Exam; MSC, Menopause Symptom Checklist; STRAW+10, Stages of Reproductive Aging, 10-year revision.
p < 0.05.
n = 32, not 40.
3.1 |. Estrogen treatment effects on cognition and symptomatology
The analysis of the cognitive performance of the two treatment groups following 3-months E2 or placebo treatment showed no significant interaction between time and treatment, nor was there a main effect of treatment observed for any of the cognitive tasks. For the SRT, there was a main effect of time for both delayed recall and recall consistency in which performance improved following treatment for both groups (Delayed Recall: F [1, 35] = 5.6, p = 0.024, η2p = 0.14; Recall Consistency: F [1, 35] = 4.15, p = 0.049, η2p= 0.11). No other effects of time were observed. These comparisons are reported in further detail in the Figure S1, S2. The analysis of the MSC scores from screening to post-treatment did not show an interaction between time and treatment or a main effect of treatment (both p > 0.1). There was a main effect of time (F [1, 30] = 4.36, p = 0.045, η2p = 0.13), with both treatment groups showing an increase in the number of endorsed symptoms over the 3-month treatment period (see Table 1). While the MSC scores at both baseline and post-treatment being positively associated with CCI scores (Baseline: r = 0.63, p < 0.001; Post-treatment: r = 0.38, p = 0.03), there was no relationship between the CCI and the change in endorsed symptoms over the treatment period (r =−0.07, p > 0.7), indicating that the participants with higher CCI scores did not have the largest increase in the endorsement of somatic symptoms over the treatment period.
3.2 |. Effect of anticholinergic challenge alone on cognitive performance
Reported below are the results of the participants who received 3 months of placebo treatment alone on each anticholinergic challenge day (reported in further detail in Supporting Information S1). The placebo treatment group is presented alone initially to demonstrate that the anticholinergic challenge days were effective in blunting performance in comparison to the placebo challenge days.
3.2.1 |. Measures of arousal and attention
Critical Flicker Fusion Task:
The performance of the participants who received placebo treatment on the CFF was significantly different following anticholinergic challenge for both trial types (Ascending: F [3, 54] = 3.05, p = 0.036, η2p = 0.145; Descending: F [3, 54] = 12.2, p < 0.001, η2p = 0.4); however, the pattern of results differed between ascending and descending trials. Compared to the placebo challenge day, performance following MECA improved on the ascending trial (mean frequency, Placebo: 35.5 ± 2.8; MECA: 36.7 ± 2.9 Hz; p < 0.01); whereas SCOP and SCOP + MECA performance were similar to placebo performance (SCOP: 35.8 ± 3.2; SCOP + MECA: 35.6 ± 3.6 Hz). On the descending trial, performance on the placebo and MECA challenge days were similar, but performance decreased following SCOP or SCOP + MECA (mean frequency, Placebo: 37.6 ± 3.6; MECA: 37.4 ± 2.95; SCOP: 35.6 ± 3.7; SCOP + MECA: 34.4 ± 2.96 Hz).
Choice Reaction Time Task:
The results of the placebo treatment group showed that compared to placebo challenge performance, participants were slower at responding across all three anticholinergic challenge days (Total RT: F (3, 54) = 7.3, p < 0.001, η2p = 0.29; Recognition RT: F (3, 54) = 5.6, p < 0.01, η2p = 0.24; Motor RT: F (3, 54) = 3.75, p = 0.016, η2p = 0.17). Total reaction time for all four challenge days was slowest following SCOP + MECA (mean RT, Placebo: 758 ± 105.3; MECA: 810.1 ± 109.6; SCOP: 809.8 ± 145.2; SCOP + MECA: 836.8 ± 120.6 ms), while the recognition and motor components revealed more selective anticholinergic challenge drug effects. The recognition component was increased by SCOP (mean RT, Placebo: 398.4 ± 47.7; MECA: 407.9 ± 41.3; SCOP: 418.6 ± 55.2; SCOP + MECA: 430.2 ± 57.1 ms), whereas the motor component was more affected by MECA (mean RT, Placebo: 353.3 ± 78; MECA: 389 ± 79; SCOP: 380.7 ± 102.2; SCOP + MECA: 393.7 ± 80.3 ms).
3.2.2 |. Measures of episodic and working memory
N-back:
The performance of the placebo group on the 0-back across the challenge days showed decreasing accuracy following SCOP and SCOP + MECA challenge days compared to placebo (mean d′: Placebo: 5.58 ± 1.0; MECA: 5.36 ± 0.82; SCOP: 4.37 ± 2.0; SCOP + MECA: 4.52 ± 1.5; F [3, 54] = 2.87, p = 0.024; η2p = 0.16). There was a significant effect of cholinergic blockade for the 1-back block (F [3, 54] = 3.6, p < 0.02; η2p = 0.17), with decreased performance for both SCOP and SCOP + MECA challenge days (mean d’: Placebo: 5.0 ± 1.5; MECA: 4.6 ± 1.5; SCOP: 4.2 ± 1.2; SCOP + MECA: 3.8 ± 1.4). However, performance on both two- and 3-back blocks did not differ across challenge days (both p > 0.2).
Selective Reminding Task:
For the placebo treatment group, anticholinergic challenge was associated with lower performance on all 4 outcome measures of the SRT, with progressively worse performance from MECA to SCOP, with SCOP + MECA resulting in the lowest performance (Immediate Recall: F [3, 54] = 6.95, p < 0.001, η2p= 0.3; Delayed Recall: F [3, 54] = 4.8, p < 0.01, η2p= 0.22; Recall Consistency: F [3, 54] = 5.98, p < 0.001, η2p = 0.26; Recall Failure: F [3, 54] = 5.4, p < 0.01, η2p= 0.24).
3.3 |. Estrogen-anticholinergic interaction effects on cognitive performance
Reported below are the comparisons of both treatment groups across the four anticholinergic challenge days and with their baseline levels of endorsed cognitive complaints. Each of the treatment and CCI comparisons with the anticholinergic challenge days are represented as difference scores (Active drug – Placebo performance). Table 2 provides the average performance of participants on each outcome variable across the three anticholinergic challenge days. The term “treatment” always refers to E2 or placebo treatment and “challenge” refers to anticholinergic challenge (MECA, SCOP, SCOP + MECA) or the placebo challenge.
TABLE 2.
Performance differences for cognitive tasks for each drug challenge by treatment type
| Cognitive construct | Task | Dependent variable | Treatment | MECA 20 mg + PLC | SCOP 2.5 μg/kg + PLC | SCOP 2.5 μg/kg + MECA 10 mg |
|---|---|---|---|---|---|---|
| Arousal & attention | CFF | Ascending (Hz)a | E2 | −0.337 (2.5) | −0.91 (3.75) | −3.48 (7.6) |
| PLC | 1.237 (1.8) | 0.32 (2.29) | 0.12 (2.0) | |||
| Descending (Hz) | E2 | −0.642 (2.5) | −2.1 (3.54) | −3.7 (4.3) | ||
| PLC | −0.2 (2.8) | −1.96 (3.1) | −3.2 (2.7) | |||
| CRT | Total RT (ms) | E2 | 51.26 (53.1) | 62.6 (81.98) | 110.47 (90.57) | |
| PLC | 51.02 (40.6) | 51.7 (87.1) | 78.8 (68.6) | |||
| Recognition RT (ms)b | E2 | 15 (24.27) | 36.4 (46.5) | 63.11 (46.7) | ||
| PLC | 9.5 (36.5) | 20.16 (41.9) | 31.8 (41.01) | |||
| Motor RT (ms) | E2 | 37.76 (43.7) | 26.6 (49.4) | 47.37 (54.2) | ||
| PLC | 35.66 (41.8) | 27.4 (55.6) | 40.4 (48.9) | |||
| Memory | N-back | 0-Back accuracy (d’) | E2 | 0.32 (1.1) | −0.47 (1.31) | −0.995 (1.76) |
| PLC | −0.24 (1.56) | −1.21 (2.14) | −0.927 (1.75) | |||
| 1-Back accuracy (d’) | E2 | −0.601 (1.57) | −1.34 (1.45) | −1.25 (1.28) | ||
| PLC | −0.22 (1.43) | −0.64 (1.71) | −1.13 (1.6) | |||
| 2-Back accuracy (d’) | E2 | −0.164 (1.41) | −0.596 (1.45) | −0.67 (1.43) | ||
| PLC | −0.065 (1.35) | −0.37 (1.64) | −0.355 (1.33) | |||
| 3- Back accuracy (d’) | E2 | −0.136 (1.37) | −0.38 (1.41) | −0.78 (1.24) | ||
| PLC | 0.148 (1.08) | −0.28 (1.03) | −0.27 (1.26) | |||
| SRT | Immediate recall (number | E2 | −4.45 (14.77) | −10.4 (14.38) | −12.5 (15.5) | |
| correct) | PLC | −5 (10.81) | −9.17 (13.9) | −12.67 (9.77) | ||
| Delayed recall (number | E2 | −0.4 (3.2) | −1.45 (3.17) | −2.3 (2.3) | ||
| correct) | PLC | −0.67 (2.7) | −1.44 (3.03) | −2.39 (2.85) | ||
| Recall consistency | E2 | −6 (15.13) | −15.35 (14.94) | −16.6 (16.24) | ||
| PLC | −4.6 (13.16) | −10.22 (18.05) | −15.06 (13.37) | |||
| Recall failure | E2 | 1.4 (12.4) | 3.55 (12.25) | 5.25 (13.81) | ||
| PLC | 4.67 (8.3) | 5.94 (7.92) | 7.28 (5.89) |
Note: Numbers represent difference scores of challenge day (active drug - placebo), with standard deviations in the parentheses. Significant effects of estrogen treatment are indicated. See text for challenge drug effects.
Abbreviations: CFF, Critical Flicker Fusion; CRT, Choice Reaction Time task; E2, Estradiol; MECA, Mecamylamine; PLC, Placebo; SCOP, Scopolamine; SRT, Selective Reminding Test.
p < 0.05
Main effect of estrogen treatment in the MECA analysis for Ascending (Hz) on the CFF, p = 0.05.
Main effect of estrogen treatment in the SCOP + MECA analysis for recognition RT on the CRT.
3.3.1 |. Mecamylamine challenge
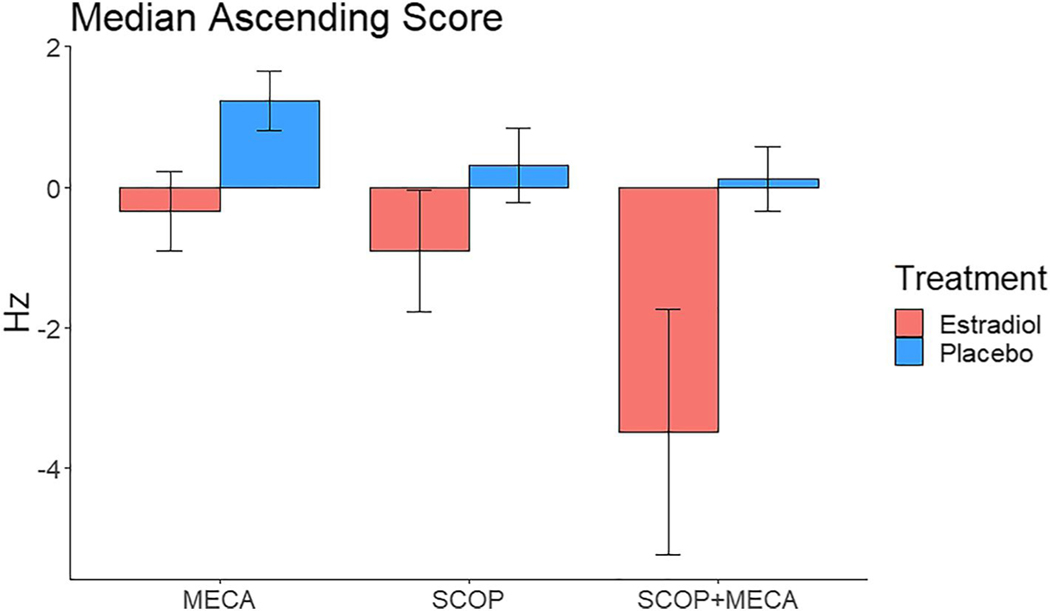
Following MECA administration participants’ performance differed significantly compared to the placebo challenge day on the CFF and the N-back tasks. The result of the ANCOVAs on the CFF data showed a significant effect of E2/placebo treatment following MECA challenge in the ascending condition, with participants who received E2 treatment having reduced performance following MECA, and participants who received placebo showing improved performance (Figure 2; mean frequency E2: −0.34 ± 2.5; Placebo: 1.2 ± 1.8 Hz; F [1, 35] = 4.1, p = 0.05, η2p = 0.104). Results of the CRT showed that participants responded slower following MECA compared to the placebo challenge day, though this difference was not significantly affected by the treatment group or CCI score (all p > 0.05).
FIGURE 2.
Median ascending frequency difference scores on the Critical Flicker Fusion task for the cholinergic challenge days (Challenge – Placebo) for participants who received estradiol or placebo (PLC) treatment
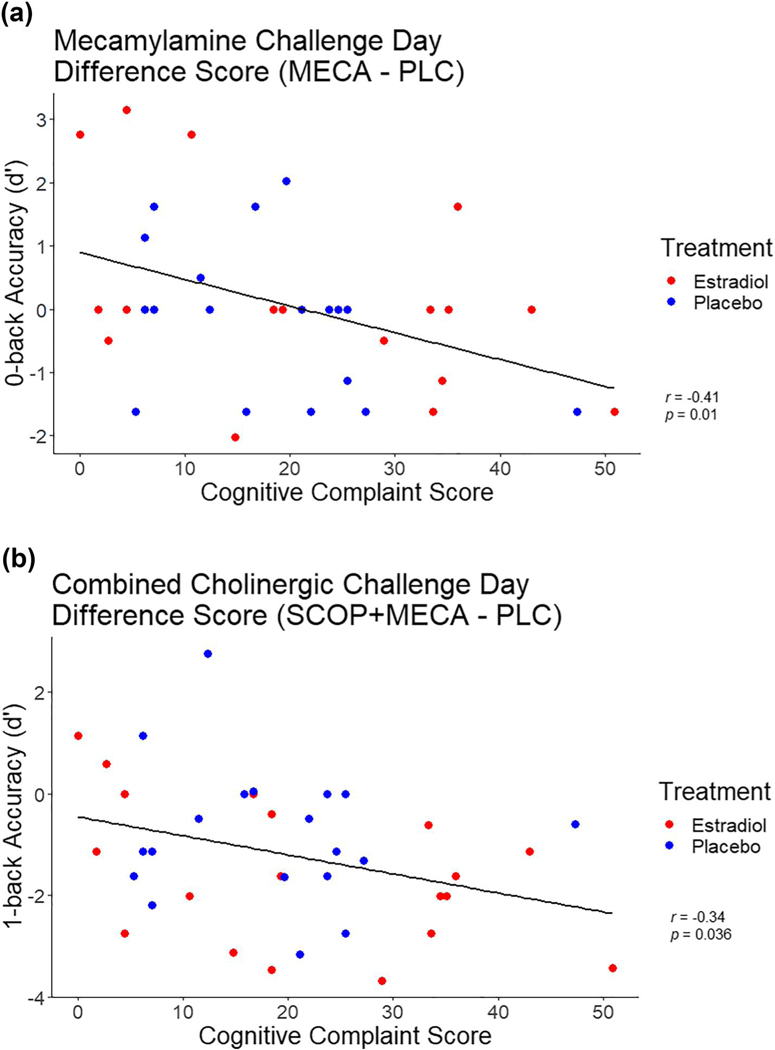
On the N-back task there was a significant effect of the CCI score on participants 0-back performance following MECA (Figure 3a, F [1, 35] = 6.7, p = 0.012, η2p = 0.17). Participants who endorsed more complaints were less accurate on the 0-back blocks of the task following MECA (r =−0.41, p = 0.01). There was no impact of CCI or treatment group on performance on the 1- two- or 3-back conditions following MECA. Analysis of the SRT showed that participants who had higher CCI scores had more recall failures following MECA compared to those that had lower CCI scores, however, this relationship was not statistically significant. Performance on the other SRT scores of immediate and delayed recall, and recall consistency were not significantly different from the placebo challenge day following MECA administration in either treatment group, or due to changes in CCI score (all p > 0.1).
FIGURE 3.
Relationships between Cognitive Complaint Index score (CCI) and difference in N-back accuracy for (a) 0-back mecamylamine (MECA) and placebo challenge; and (b) 1-back combined cholinergic and placebo challenge days. Correlation coefficients and p-values are Attached
3.3.2 |. Scopolamine challenge
Following SCOP administration participants performed worse on both attention and memory tasks compared to either the placebo or the MECA challenge days (see Table 2). However, these deficits in performance were consistent across both groups, as the analyses showed no significant effect of E2 treatment or CCI score on any of the four tasks (all p > 0.1).
3.3.3 |. Combined anticholinergic challenge
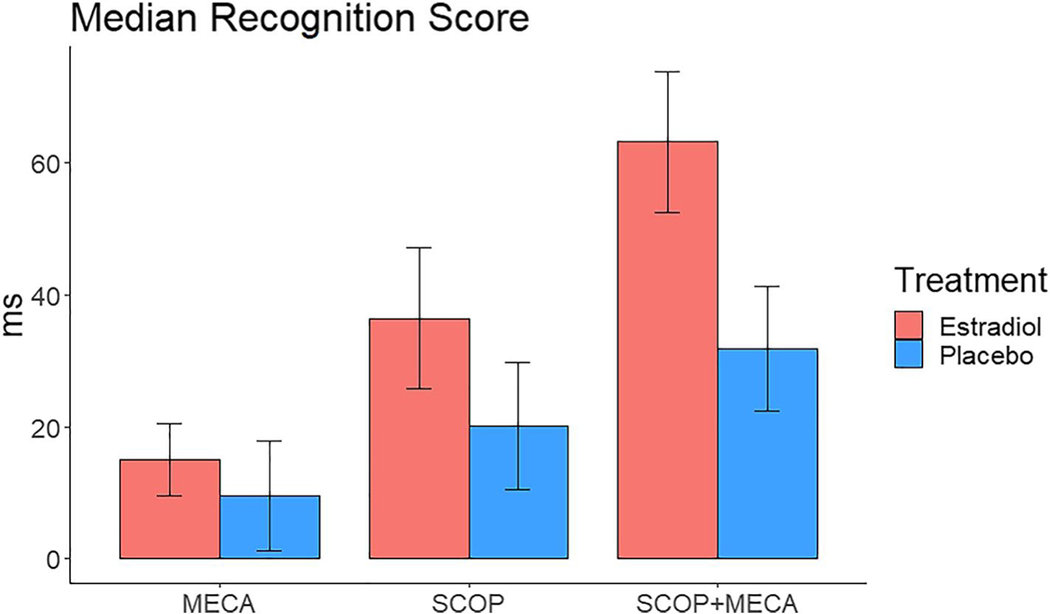
Following combined anticholinergic challenge participants performed worse on both attention and memory tasks compared to all three other challenge days (Table 2). Analysis of the CRT, following combined anticholinergic challenge day there was a significant effect of treatment on recognition time, with participants who received E2 treatment reacting slower than those who received placebo treatment (Figure 4; mean RT, E2: 63.11 ± 46.7; Placebo: 31.8 ± 41 ms; F [1, 35] = 5.06, p = 0.03, η2p= 0.122). There was no impact of CCI on any of the three performance scores on the CRT (all p > 0.05). On the N-back task there was a significant effect of the CCI score on participants 1-back performance following the combined anticholinergic challenge (Figure 3b, F [1, 35] = 4.76, p = 0.046, η2p= 0.11). This effect showed that increasing complaints were associated with less accurate performance on the 1-back block of the task following the combined challenge (r =−0.34, p = 0.036). There were no significant effects of on 0-, two- or 3-back blocks (all p > 0.05). Analysis of the CFF and SRT showed that while participants performed substantially worse compared to the placebo challenge day, there was no significant impact of E2 treatment or CCI score on performance following the combined anticholinergic challenge (all p > 0.1).
FIGURE 4.
Median recognition time difference scores on the Choice Reaction Time task for the cholinergic challenge days (Challenge – Placebo) for participants who received estradiol or placebo (PLC) treatment
4 |. DISCUSSION
We investigated the effect of 3 months of 1 mg E2 treatment or placebo on the cognitive performance of postmenopausal women who endorsed differing levels of cognitive complaints while undergoing anticholinergic challenge. Following 3-months of E2/placebo treatment, there was a modest improvement on the SRT for recall consistency and delayed recall performance across both groups. The anticholinergic challenge was effective at blunting performance on cognitive tasks, as shown by the performance of the placebo treatment group under anticholinergic versus placebo challenge. Contrary to our hypothesis, 3 months of E2 treatment did not mitigate the effect of cholinergic antagonists on task performance. Indeed, E2 treatment had a negative effect on attention task performance during the anticholinergic challenge, resulting in reduced performance compared to placebo treatment. On memory tasks, E2 treatment did not reduce performance, however there was no improvement compared to the placebo treatment. The associations of CCI score with performance were consistent with our predictions, with participants endorsing a greater number of complaints showing lower performance compared to those endorsing fewer complaints, regardless of treatment group, on the N-back task.
The present study extends our previous work in that it examines the relationship of differing levels of cognitive complaints to cognitive performance and cholinergic functioning of recently postmenopausal women. The presence of cognitive complaints may be associated with increased risk for neurodegeneration and may be associated with reduced performance on cognitive tasks. Changes in cholinergic system functioning may be relevant as postmenopausal women in this study with greater CCI scores demonstrated a reduced ability to compensate in response to the cholinergic blockade. This was evident on the N-back task, where a higher CCI score was related to reduced performance during 0- and 1-back blocks while under cholinergic blockade. The results of the 0-back task show that nicotinic blockade by MECA was effective at reducing attention to the target for participants with higher CCI scores. The results of the 1-back task showed a decline in working memory performance under combined anticholinergic challenge. However, these effects were not seen in the two- and 3-back blocks, the more difficult conditions of the task. This may be because participants’ accuracy on these blocks during the placebo challenge day was much lower than on the 0- and 1-back blocks, thus the effect of the anticholinergic challenge, either MECA alone or combined with SCOP, did not add significantly to this load-related decline in performance. Thus, despite performing worse on the harder conditions of the N-back following anticholinergic challenge compared to the placebo challenge day, this deficit was not significant. In contrast to the effects of the CCI score, the impact of E2 treatment was only seen on the performance on attention tasks. Participants who had received 3-month of E2 treatment displayed worse recognition time on the CRT while under combined cholinergic blockade. There was also a decrement in performance for E2-treated participants on the ascending trial of the CFF following MECA administration. Interestingly, the participants who received a 3-months placebo showed slightly improved performance on the CFF following MECA but decreased performance following muscarinic blockade by SCOP. This result is broadly consistent with our previous results which showed that postmenopausal women showed slightly improved performance on ascending trials following MECA but decreased performance following SCOP following either E2 or placebo treatment (Dumas et al., 2008). In contrast to the attention tasks, there was no impact of E2 treatment on memory task performance under cholinergic blockade.
The findings of the present study are interesting in comparing the effects of the different cholinergic antagonists. While the greatest deterioration of performance for all tasks was observed for the combined cholinergic blockade, when antagonists were administered alone, participants generally performed worse following muscarinic blockade by SCOP compared to nicotinic blockade by MECA regardless of treatment group. This difference in performance was driven by the effects that blocking nicotinic or muscarinic receptors in the cortex have on cortical processes. In attention processes, nicotinic receptors are in mediating attention in the medial prefrontal cortex, specifically the β2 subunit (Bloem et al., 2014; Guillem et al., 2011; Poorthuis & Mansvelder, 2013). Blocking nicotinic receptors by MECA may limit this mediation, reducing the effectiveness of the medial prefrontal cortex to direct attention. For attention tasks, muscarinic receptors are important in cue-detection and also in the filtering of task-relevant information (topdown control) by the prefrontal cortex (Ballinger et al., 2016; Gould et al., 2015; Parikh & Sarter, 2008). Thus, the blocking of the muscarinic receptors would reduce the transfer of goal-directed signals from the prefrontal cortex to the basal forebrain. In the present study, this deterioration in attention following SCOP was evident in the performance of participants on the recognition score of the CRT. In contrast, the effect of MECA was smaller on recognition performance, which indicates that these β2 receptors may be less important during recognition processes. On memory tasks, muscarinic receptors are important for suppressing feedback mechanisms during encoding (Green et al., 2005; Hasselmo, 2006). Under muscarinic blockade by SCOP, these feedback mechanisms interfere with the encoding of information at the hippocampus and the striatum, leading to decreased accuracy on the N-back task and poorer recall on the SRT. The impact of MECA on memory performance was important in the relationship between endorsed cognitive complaints and participants’ accuracy on the 0- and 1-back tasks. As nicotinic receptors are important at boosting the signal of sensory information during encoding (Hasselmo, 2006), the blockade of nicotinic receptors by MECA may be limiting this transfer of information, which would reduce working memory performance. This response to MECA was stronger in women endorsing more cognitive complaints, which indicates that these women may have less intact cholinergic activity in the cortex.
Previously we have demonstrated that E2 treatment in postmenopausal women was effective at mitigating or blunting the effects of the cholinergic blockade (Dumas et al., 2006, 2008). In comparison, the results of the present study suggest that in women with cognitive complaints, E2 may not enhance cholinergic system activity. Indeed, the women who received E2 treatment performed generally worse than the placebo group, especially on the attention tasks. This finding is contrary to our predictions, as we had believed that E2 treatment would stimulate cholinergic neurotransmission in the brain, and generally be supportive of the critical window hypothesis of estrogen enhancement in the first few years following menopause (Guo et al., 2020; MacLennan et al., 2006; Maki, 2013). Indeed, in previous research, E2 treatment has been shown to increase muscarinic receptor density in women (Norbury et al., 2007), and in animal models has been shown to modulate muscarinic activation of visuospatial attention (Tinkler & Voytko, 2005). E2 has also been observed to enhance attention processes through the potentiation of the α4β2 nicotinic receptors (Curtis et al., 2002; Howe et al., 2010; Jin & Steinbach, 2011). Therefore, why the E2 treated group was more affected by cholinergic blockade is puzzling. It is possible that the answer may lie in studies of aged animals. In older, ovariectomized rodents, E2 has only been shown to improve performance in combination with an anticholinesterase inhibitor, either donepezil or galantamine (Gibbs et al., 2009, 2011). Therefore, the addition of a cholinergic antagonist may have had the opposite effect on the E2 treated women in the present study, not only blocking cholinergic terminals, but also interfering with the E2 cholinergic interactions, which had a greater impact on performance than the antagonists alone. Another explanation may be related to the healthy cell bias of estrogen hypothesis (Brinton, 2008), which indicates that while E2 is beneficial in healthy women, individuals who already possess some underlying dysfunction may not benefit from increased E2 levels, in fact the additional E2 levels may exacerbate this dysfunction. Thus, in the present study, the impact of E2 in postmenopausal women who endorsed more cognitive complaints may have been detrimental to cognitive performance, particularly in the presence of cholinergic antagonists.
In contrast to showing a benefit of E2 treatment, our results support evidence of a model of cholinergic compensation following menopause (Dumas & Newhouse, 2011; Newhouse & Dumas, 2015). In the present study, the women with higher levels of cognitive complaints were not able to compensate as well during the cholinergic blockade compared to the women with lower endorsement of complaints. This suggests that cognitive complaints may be related to a decline in cholinergic function that may explain the inability of exogenous E2 to boost cholinergic tone. This susceptibility was most noticeable on the N-back task, which is consistent with previous research showing that postmenopausal women endorsing greater cognitive complaints having to engage in greater cortical activation during working memory tasks to compensate for the decreased efficiency in processing (Dumas et al., 2013). While the statistically significant effects were shown only for the 0- and 1-back blocks, there was a pattern of decrement following cholinergic blockade of all blocks of the N-back task. Thus, in the absence of cholinergic blockade, the women endorsing greater complaints may be able to perform adequately on less demanding tasks, but on more effortful tasks, they need to activate the cholinergic system to compensate for the reduction in cortical efficiency. In the presence of a cholinergic blockade, this ability to compensate is reduced, resulting in decreased performance.
The results of the present study are also consistent with several studies examining AD biomarkers in women at the different stages of the menopause transition and age-matched men. Compared to men and premenopausal women, perimenopausal and postmenopausal women showed reduced performance on paragraph recall tasks, which has been thought of as a measure of estrogen-dependent memory (Kampen & Sherwin, 1994; Mosconi et al., 2018). These studies have also observed that compared to men and premenopausal women, women in the perimenopause and postmenopausal groups possessed a greater number of AD risk factors including hypometabolism, reduction in cortical gray and white matter, a reduction in cerebral metabolism as measured by fluorodeoxyglucose positron emission tomography. Additionally, a number of recent studies show that the relative burden of AD-biomarkers in older women is higher compared to older men (Buckley et al., 2019; Lin et al., 2015; Mosconi et al., 2017, 2018; Oveisgharan et al., 2018). Analysis of the baseline neuroimaging data of the participants in the present study showed that higher levels of cognitive complaints were associated both with reduced medial temporal graymatter volume and increased resting-state connectivity in the executive control network, indicating underlying cortical dysfunction in postmenopausal women with subjective cognitive complaints (Conley et al., 2020; Vega et al., 2016). The age of menopause may also be a factor in the long-term cognitive health of women. Recent epidemiological evidence has shown that women who undergo the menopause transition at younger (Gilsanz et al., 2019) or older (Najar et al., 2020) ages of the population distribution are at higher risks of developing dementia later in life. These studies offer accumulating evidence that menopause may create a vulnerability to cognitive decline in at-risk women, which may contribute to women’s overall increased risk for AD (Beam et al., 2018; Paganini-hill & Henderson, 1994; Pike, 2017). In this vein, cognitive complaints may be how this phenotype is expressed.
The finding that increased levels of cognitive complaints were associated with decreased working memory performance could be argued as an effect of healthy aging, rather than a sex-specific effect of menopause. However, in the recent study in which we examined the baseline data of this sample (Conley et al., 2020), we found that the level of endorsed complaints was not associated with age, education or the number of years since menopause. In contrast, the number of cognitive complaints endorsed was highly associated with the number of somatic symptoms that these women reported. The evidence that postmenopausal women with higher baseline cognitive complaints had a smaller graymatter volume of the right medial temporal lobe is reinforced by the results of the current study, showing that these participants also had decreased working memory performance under cholinergic blockade. Interestingly, there was no impact of the E2 treatment on the number of somatic symptoms endorsed by participants, as across the treatment period, the MSC scores for both treatment groups increased. Whether this increase was due to the dosage of the E2 treatment (discussed more below), is unclear, however it may be due to the fact that these women were endorsing more subjective cognitive complaints. Indeed, the level of endorsed somatic symptoms was associated with more cognitive complaint endorsement both at baseline and following the treatment period. Therefore, based on these results, we believe that the impact of menopause is to diminish or remove the compensatory mechanism in these at-risk women. We propose that the reduction in cortical E2 levels that reduces both cholinergic and hippocampal activity unmasks the vulnerability of these women to future cognitive decline. While a definitive answer to this hypothesis is beyond the scope of the current article, an approach for future studies would be to examine the cholinergic blockade effects in combination with an examination of other biomarkers of neurodegenerative pathology, for instance, cortical amyloid-β and phosphorylated tau levels.
It is possible that for this age group, the dose of E2 treatment was not large enough to provide a beneficial effect on cholinergic functioning. Additionally, using oral capsules may have lessened the absorption of E2 compared to using a transdermal administration. E2 has been hypothesized to provide neuroprotective effects against cognitive decline if given at the critical time during the menopause transition (Petrovska et al., 2012; Sherwin, 2009). This idea is encapsulated in the healthy cell bias of estrogen hypothesis (Brinton, 2008). In a previous study, recently postmenopausal women were more responsive to E2 given at 2 mg compared to older postmenopausal women (Dumas et al., 2008). Therefore, it could be that a higher dose of E2 may have been able to blunt the cholinergic antagonists compared to placebo. However, in another study, Dumas et al. (2006) found that 3 months of 1 mg treatment was effective at mitigating some effects of the cholinergic antagonists MECA and scopolamine on cognitive performance. But, unlike the present study, in the prior studies, participants were not recruited based on levels of postmenopausal cognitive complaints. We hypothesized that the current sample of participants may represent a higher risk group than in prior studies and therefore were not receptive to E2 treatment effects on cholinergic functioning. Additionally, while the participants in the present study did not endorse subjective cognitive impairment to the level of preclinical MCI (Jessen, 2014), we believe that the impact of these complaints on participants represents a risk factor for future cognitive impairment. A limitation of the paper is the relatively small samples of the two treatment groups. Due to the difficult nature of the study with multiple challenge days, some participants withdrew prior to completing the full study. As such it is possible that some of the comparisons were statistically underpowered. Another limitation of the present study is that the majority of the participants were white, and this does limit the generalizability of the results. While these limitations do reduce the strength of conclusions that can be drawn from these comparisons, we still believe that the effects that are found in this paper represent the impact of self-reported cognitive complaints on memory performance under anticholinergic challenge.
In conclusion, the present study found that 3 months of E2 treatment did not mitigate cholinergic blockade effects on the performance of recently postmenopausal women with varying levels of cognitive symptoms. The results of the present study also show that postmenopausal women with greater endorsement of cognitive complaints performed worse on working memory tasks during cholinergic blockade regardless of receiving E2 treatment or placebo. The findings of the present study highlight that cognitive complaints in postmenopausal women might be a manifestation of cortical dysfunction modulated by changes in cholinergic system functioning, which is revealed as reduced performance in the presence of cholinergic antagonists. Future studies should examine whether the level of cognitive complaints may be indicative of early biomarker evidence of neurodegenerative disorders such as AD. It will also be important to investigate the relationship of cholinergic blockade on cognitive performance with brain activity and the presence or absence of early biomarker evidence of developing neuropathology.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by R01 AG021476 to PN, a Clinical and Translational Science Award 2UL1TR000445 for the Vanderbilt Institute for Clinical and Translational Research, and a 1S10OD021771-01 grant for the 3T MRI, housed in the Vanderbilt Center for Human Imaging. Xuewen Gong, Samantha Heller, and Caroline Perlman assisted with data processing.
Funding information
National Institute on Aging
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Baakman AC, Alvarez-Jimenez R, Rissmann R, Klaassen ES, Stevens J, Goulooze SC, den Burger JCG, Swart EL, van Gerven JMA, & Groeneveld GJ (2017). An anti-nicotinic cognitive challenge model using mecamylamine in comparison with the anti-muscarinic cognitive challenge using scopolamine. British Journal of Clinical Pharmacology, 83(8), 1676–1687. 10.1111/bcp.13268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger EC, Ananth M, Talmage DA, & Role LW (2016). Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron, 91(6), 1199–1218. 10.1016/j.neuron.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam CR, Kaneshiro C, Jang JY, Reynolds CA, Pedersen NL, & Gatz M.(2018). Differences between women and men in incidence rates of dementia and alzheimer’s disease. Journal of Alzheimer’s Disease, 64(4), 1077–1083. 10.3233/JAD-180141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, & Erbaugh J.(1961). An inventory for measuring depression. Archives of General Psychiatry, 4(6), 561–571. 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- Bloem B, Poorthuis R, & Mansvelder H.(2014). Cholinergic modulation of the medial prefrontal cortex: The role of nicotinic receptors in attention and regulation of neuronal activity. Frontiers in Neural Circuits, 8. 17. 10.3389/fncir.2014.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD (2008). The healthy cell bias of estrogen action: Mitochondrial bioenergetics and neurological implications. Trends in Neurosciences, 31(10), 529–537. 10.1016/j.tins.2008.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RF, Maruff P, Ames D, Bourgeat P, Martins RN, Masters CL, Rainey-Smith S, Lautenschlager N, Rowe CC, Savage G, Villemagne VL, & Ellis KA (2016). Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer’s disease. Alzheimer’s and Dementia, 12(7), 796–804. 10.1016/j.jalz.2015.12.013 [DOI] [PubMed] [Google Scholar]
- Buckley RF, Mormino EC, Rabin JS, Hohman TJ, Landau S, Hanseeuw BJ, Jacobs HIL, Papp KV, Amariglio RE, Properzi MJ, Schultz AP, Kirn D, Scott MR, Hedden T, Farrell M, Price J, Chhatwal J, Rentz DM, Villemagne VL, …… Sperling, R. A. (2019). Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurology, 76(5), 542–551. 10.1001/jamaneurol.2018.4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschke H.(1973). Selective reminding for analyses of memory and learning. Journal of Verbal Learning and Verbal Behavior, 12, 543–550. [Google Scholar]
- Caulfield MP (1993). Muscarinic receptors—Characterization, coupling and function. Pharmacology & Therapeutics, 58(3), 319–379. 10.1016/0163-7258(93)90027-B [DOI] [PubMed] [Google Scholar]
- Conley AC, Albert KM, Boyd BD, Kim S-G, Shokouhi S, McDonald BC, Saykin AJ, Dumas JA, & Newhouse PA (2020). Cognitive complaints are associated with smaller right medial temporal graymatter volume in younger postmenopausal women. Menopause, 27, 1220–1227. 10.1097/gme.0000000000001613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis L, Buisson B, Bertrand S, & Bertrand D.(2002). Potentiation of human α4β2 neuronal nicotinic acetylcholine receptor by estradiol. Molecular Pharmacology, 61(1), 127–135. 10.1124/mol.61.1.127 [DOI] [PubMed] [Google Scholar]
- Dumas J, Hancur-Bucci C, Naylor M, Sites C, & Newhouse P.(2006). Estrogen treatment effects on anticholinergic-induced cognitive dysfunction in normal postmenopausal women. Neuropsychopharmacology, 31(9), 2065–2078. 10.1038/sj.npp.1301042 [DOI] [PubMed] [Google Scholar]
- Dumas J, Hancur-Bucci C, Naylor M, Sites C, & Newhouse P.(2008). Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: Evidence for the critical period hypothesis. Hormones and Behavior, 53(1), 159–169. 10.1016/j.yhbeh.2007.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, McDonald BC, Naylor MR, Pfaff AC, Saykin AJ, & Newhouse PA (2013). Increased working memory-related brain activity in middle-aged women with cognitive complaints. Neurobiology of Aging, 34(4), 1145–1147. 10.1016/j.neurobiolaging.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, & Newhouse PA (2011). The cholinergic hypothesis of cognitive aging revisited again: Cholinergic functional compensation. Pharmacology Biochemistry and Behavior, 99(2), 254–261. 10.1016/j.pbb.2011.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert U, Grossmann M, Oertel R, Gramatté T, & Kirch W.(2001). Pharmacokinetic-pharmacodynamic modeling of the electroencephalogram effects of scopolamine in healthy volunteers. The Journal of Clinical Pharmacology, 41(1), 51–60. 10.1177/00912700122009836 [DOI] [PubMed] [Google Scholar]
- Ellis JR, Ellis KA, Bartholomeusz CF, Harrison BJ, Wesnes KA, Erskine FF, Vitetta L, & Nathan PJ (2006). Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. International Journal of Neuropsychopharmacology, 9(2), 175–189. 10.1017/S1461145705005407 [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JAE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, & Hays J.(2004). Conjugated equine estrogens and global cognitive funtion in postmenopausal women: Women’s health initiative memory study. Journal of the American Medical Association, 291(24), 2959–2968. 10.1001/jama.291.24.2959 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). Mini-mental state: A practice method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Chipman AM, Hammond R, & Nelson D.(2011). Galanthamine plus estradiol treatment enhances cognitive performance in aged ovariectomized rats. Hormones and Behavior, 60(5), 607–616. 10.1016/j.yhbeh.2011.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Mauk R, Nelson D, & Johnson DA (2009). Donepezil treatment restores the ability of estradiol to enhance cognitive performance in aged rats: Evidence for the cholinergic basis of the critical period hypothesis. Hormones and Behavior, 56(1), 73–83. 10.1016/j.yhbeh.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Wu D, Hersh LB, & Pfaff DW (1994). Effects of estrogen replacement on the relative levels of choline acetyltransferase, trkA, and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Experimental Neurology, 129(1), 70–80. 10.1006/exnr.1994.1148 [DOI] [PubMed] [Google Scholar]
- Gilewski MJ, Zelinski EM, & Schaie KW (1990). The memory functioning questionnaire for assessment of memory complaints in adulthood and old age. Psychology and Aging, 5(4), 482–490. 10.1037//0882-7974.5.4.482 [DOI] [PubMed] [Google Scholar]
- Gilsanz P, Lee C, Corrada MM, Kawas CH, Quesenberry CP, & Whitmer RA (2019). Reproductive period and risk of dementia in a diverse cohort of health care members. Neurology, 92(17), e2005–e2014. 10.1212/WNL.0000000000007326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RW, Dencker D, Grannan M, Bubser M, Zhan X, Wess J, Xiang Z, Locuson C, Lindsley CW, Conn PJ, & Jones CK (2015). Role for the M1 muscarinic acetylcholine receptor in topdown cognitive processing using a touchscreen visual discrimination task in mice. ACS Chemical Neuroscience, 6, 1683–1695. 10.1021/acschemneuro.5b00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A, Ellis KA, Ellis J, Bartholomeusz CF, Ilic S, Croft RJ, Phan KL, & Nathan PJ (2005). Muscarinic and nicotinic receptor modulation of object and spatial n-back working memory in humans. Pharmacology Biochemistry and Behavior, 81(3), 575–584. 10.1016/j.pbb.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Guillem K, Bloem B, Poorthuis RB, Loos M, Smit AB, Maskos U, Spijker S, & Mansvelder HD (2011). Nicotinic acetylcholine receptor β2 subunits in the medial prefrontal cortex control attention. Science, 333(6044), 888–891. 10.1126/science.1207079 [DOI] [PubMed] [Google Scholar]
- Guo H, Liu M, Zhang L, Wang L, Hou W, Ma Y, & Ma Y.(2020). The critical period for neuroprotection by estrogen replacement therapy and the potential underlying mechanisms. Current Neuropharmacology, 18(6), 485–500. 10.2174/1570159X18666200123165652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Lumley LA, Palter S, Manning C, Gengo F, & Joe SH (1995). Possible acceleration of age effects on cognition following menopause. Journal of Psychiatric Research, 29(3), 153–163. 10.1016/0022-3956(95)00005-P [DOI] [PubMed] [Google Scholar]
- Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, & De Villiers TJ (2012). Executive summary of the stages of reproductive aging workshop + 10: Addressing the unfinished agenda of staging reproductive aging. Journal of Clinical Endocrinology and Metabolism, 97(4), 1159–1168. 10.1210/jc.2011-3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME (2006). The role of acetylcholine in learning and memory. Current Opinion in Neurobiology, 16(6), 710–715. 10.1016/j.conb.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, & Sarter M.(2011). Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology, 36(1), 52–73. 10.1038/npp.2010.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Jan St John MA, Howard Hodis MN, McCleary CA, Stanczyk FZ, Shoupe D, Kono N, Laurie Dustin M, Hooman Allayee M, & Mack WJ (2016). Cognitive effects of estradiol after menopause, A randomized trial of the timing hypothesis. Neurology, 87, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarch I.(1984). Psychological performance models as indicators of the effects of hypnotic drugs on sleep. Psychopharmacology - Supplementum, 1, 58–68. [DOI] [PubMed] [Google Scholar]
- Howe WM, Ji J, Parikh V, Williams S, Mocaër E, Trocmé-Thibierge C, & Sarter M.(2010). Enhancement of attentional performance by selective stimulation of α4β2* nAChRs: Underlying cholinergic mechanisms. Neuropsychopharmacology, 35(6), 1391–1401. 10.1038/npp.2010.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff JF, Mickel SF, Corkin S, & Growdon JH (1988). Cognitive functions affected by scopolamine in alzheimer’s disease and normal aging. Drug Development Research, 12(3–4), 271–278. 10.1002/ddr.430120310 [DOI] [Google Scholar]
- Jessen F.(2014). Subjective and objective cognitive decline at the predementia stage of alzheimer’s disease. European Archives of Psychiatry and Clinical Neuroscience, 264(1), 3–7. 10.1007/s00406-014-0539-z [DOI] [PubMed] [Google Scholar]
- Jin X, & Steinbach JH (2011). A portable site: A binding element for 17β-estradiol can be placed on any subunit of a nicotinic α4β2 receptor. Journal of Neuroscience, 31(13), 5045–5054. 10.1523/JNEUROSCI.4802-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, & Koeppe RA (1997). Verbal working memory load affects regional brain activation as measured by PET. Journal of Cognitive Neuroscience, 9(4), 462–475. 10.1162/jocn.1997.9.4.462 [DOI] [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Henderson AS, Korten AE, Mackinnon AJ, & Scott R.(1994). Complaints of cognitive decline in the elderly: A comparison of reports by subjects and informants in a community survey. Psychological Medicine, 24(2), 365–374. 10.1017/S0033291700027343 [DOI] [PubMed] [Google Scholar]
- Kampen DL, & Sherwin BB (1994). Estrogen use and verbal memory in healthy postmenopausal women. Obstetrics and Gynecology, 83(6), 979–983. 10.1097/00006250-199406000-00017199406000-00017 [DOI] [PubMed] [Google Scholar]
- Kupke T, & Lewis R.(1989). Relative influence of subject variables and neurological parameters on neuropsychological performance of adult seizure patients. Archives of Clinical Neuropsychology, 4(4), 351–363. 10.1016/0887-6177(89)90025-5 [DOI] [PubMed] [Google Scholar]
- Levin ED (2002). Nicotinic receptor subtypes and cognitive function. Journal of Neurobiology, 53(4), 633–640. 10.1002/neu.10151 [DOI] [PubMed] [Google Scholar]
- Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, & Doraiswamy PM (2015). Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimer’s and Dementia: Translational Research and Clinical Interventions, 1(2), 103–110. 10.1016/j.trci.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan AH, Henderson VW, Paine BJ, Mathias J, Ramsay EN, Ryan P, Stocks NP, & Taylor AW (2006). Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: The REMEMBER pilot study. Menopause, 13(1), 28–36. 10.1097/01.gme.0000191204.38664.61 [DOI] [PubMed] [Google Scholar]
- Maki PM (2013). Critical window hypothesis of hormone therapy and cognition: A scientific update on clinical studies. Menopause, 20, 695–709. 10.1097/gme.0b013e3182960cf8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, & Dumas J.(2009). Mechanisms of action of estrogen in the brain: Insights from human neuroimaging and psychopharmacologic studies. Seminars in Reproductive Medicine, 27(3), 250–259. 10.1055/s-0029-1216278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan PJ, Singer C. a, & Dorsa DM (1996). The effects of ovariectomy and estrogen replacement on trkA and choline acetyltransferase mRNA expression in the basal forebrain of the adult female Sprague-Dawley rat. The Journal of Neuroscience, 16(5), 1860–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, & Stubbs B.(2014). Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatrica Scandinavica, 130(6), 439–451. 10.1111/acps.12336 [DOI] [PubMed] [Google Scholar]
- Moran SP, Maksymetz J, & Conn PJ (2019). Targeting muscarinic acetylcholine receptors for the treatment of psychiatric and neurological disorders. Trends in Pharmacological Sciences, 40(12), 1006–1020. 10.1016/j.tips.2019.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Berti V, Quinn C, McHugh P, Petrongolo G, Varsavsky I, Osorio RS, Pupi A, Vallabhajosula S, Isaacson RS, De Leon MJ, & Brinton RD (2017). Sex differences in alzheimer risk: Brain imaging of endocrine vs chronologic aging. Neurology, 89(13), 1382–1390. 10.1212/WNL.0000000000004425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Rahman A, Diaz I, Wu X, Scheyer O, Hristov HW, Vallabhajosula S, Isaacson RS, de Leon MJ, & Brinton RD (2018). Increased alzheimer’s risk during the menopause transition: A 3-year longitudinal brain imaging study. PLoS ONE, 13(12), e0207885. 10.1371/journal.pone.0207885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najar J, Östling S, Waern M, Zettergren A, Kern S, Wetterberg H, Hällström T, & Skoog I.(2020). Reproductive period and dementia: A 44-year longitudinal population study of Swedish women. Alzheimer’s and Dementia, 16, 1153–1163. 10.1002/alz.12118 [DOI] [PubMed] [Google Scholar]
- Newhouse P, & Dumas J.(2015). Estrogen-cholinergic interactions: Implications for cognitive aging. Hormones and Behavior, 74, 173–185. 10.1016/j.yhbeh.2015.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse P, Potter A, & Corwin J.(1996). Effects of nicotinic cholinergic agents on cognitive functioning in alzheimer’s and parkinson’s disease. Drug Development Research, 38(3–4), 278–289. [DOI] [Google Scholar]
- Newhouse PA, Dumas J, Wilkins H, Coderre E, Sites CK, Naylor M, Benkelfat C, & Young SN (2010). Estrogen treatment impairs cognitive performance after psychosocial stress and monoamine depletion in postmenopausal women. Menopause, 17(4), 860–873. 10.1097/gme.0b013e3181e15df4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Corwin J, & Lenox R.(1992). Acute nicotinic blockade produces cognitive impairment in normal humans. Psychopharmacology, 108(4), 480–484. 10.1007/BF02247425 [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Corwin J, & Lenox R.(1994). Age-related effects of the nicotinic antagonist mecamylamine on cognition and behavior. Neuropsychopharmacology, 10(2), 93–107. 10.1038/npp.1994.11 [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Sunderland T, Tariot PN, Blumhardt CL, Weingartner H, Mellow A, & Murphy DL (1988). Intravenous nicotine in alzheimer’s disease: A pilot study. Psychopharmacology (Berl), 95(0033–3158), 171–175. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Sunderland T, Tariot PN, Weingartner H, Thompson K, Mellow AM, Cohen RM, & Murphy DL (1988). The effects of acute scopolamine in geriatric depression. Archives of General Psychiatry, 45(10), 906–912. 10.1001/archpsyc.1988.01800340028004 [DOI] [PubMed] [Google Scholar]
- Nichols N, Sköld R, Spink C, & Wadsö I.(1976). Thermochemistry of solutions of biochemical model compounds 6. α,ω-dicarboxylic acids, -diamines, and -diols in aqueous solution. The Journal of Chemical Thermodynamics, 8(10), 993–999. 10.1016/0021-9614(76)90115-49614(76)90115-4 [DOI] [Google Scholar]
- Norbury R, Travis MJ, Erlandsson K, Waddington W, Ell PJ, & Murphy DGM (2007). Estrogen therapy and brain muscarinic receptor density in healthy females: A SPET study. Hormones and Behavior, 51, 249–257. 10.1016/j.yhbeh.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, & Bennett DA (2018). Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathologica, 136(6), 887–900. 10.1007/s00401-018-1920-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganini-hill A, & Henderson VW (1994). Estrogen deficiency and risk of alzheimer’s disease in women. American Journal of Epidemiology, 140(3), 256–261. 10.1093/oxfordjournals.aje.a117244 [DOI] [PubMed] [Google Scholar]
- Parikh V, & Sarter M.(2008). Cholinergic mediation of attention. Annals of the New York Academy of Sciences, 1129(1), 225–235. 10.1196/annals.1417.021 [DOI] [PubMed] [Google Scholar]
- Petrovska S, Dejanova B, & Jurisic V.(2012). Estrogens: Mechanisms of neuroprotective effects. Journal of Physiology and Biochemistry, 68(3), 455–460. 10.1007/s13105-012-0159-x [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ, & Mineur YS (2012). Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron, 76(1), 116–129. 10.1016/j.neuron.2012.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ (2017). Sex and the development of Alzheimer’s disease. Journal of Neuroscience Research, 95(1–2), 671–680. 10.1002/jnr.23827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorthuis RB, & Mansvelder HD (2013). Nicotinic acetylcholine receptors controlling attention: Behavior, circuits and sensitivity to disruption by nicotine. Biochemical Pharmacology, 86(8), 1089–1098. 10.1016/j.bcp.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Putcha L, Cintrón NM, Tsui J, Vanderploeg JM, & Kramer WG (1989). Pharmacokinetics and oral bioavailability of scopolamine in normal subjects. Pharmaceutical Research: An Official Journal of the American Association of Pharmaceutical Scientists, 6(6), 481–485. 10.1023/A:1015916423156 [DOI] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, de Leon MJ, Franssen ESE, Kluger A, Mir P, Borenstein J, George AE, Shulman E, Steinberg G, & Cohen J.(1988). Stage-specific behavioral, cognitive, and in vivo changes in community residing subjects with age-associated memory impairment and primary degenerative dementia of the alzheimer type. Drug Development Research, 15(2–3), 101–114. 10.1002/ddr.430150203 [DOI] [Google Scholar]
- Reisberg B, Shulman MB, Torossian C, Leng L, & Zhu W.(2010). Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimer’s and Dementia, 6(1), 11–24. 10.1016/j.jalz.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, & Givens B.(2005). Unraveling the attentional functions of cortical cholinergic inputs: Interactions between signal-driven and cognitive modulation of signal detection. Brain Research Reviews, 48(1), 98–111. 10.1016/j.brainresrev.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Saykin AJ (1992). Neurobehavioral function and activities of daily living rating scale (NBFADL-63 item version). [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Flashman LA, McHugh TL, Mamourian AC, & Santulli RB (2004). Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain, 127, 1574–1583. 10.1093/brain/awh177 [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, & Mamourian AC (2006). Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology, 67(5), 834–842. 10.1212/01.wnl.0000234032.77541.a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma M, Homewood J, & Taylor A.(2010). Subjective cognitive complaints at menopause associated with declines in performance of verbal memory and attentional processes. Climacteric, 13(1), 84–98. 10.3109/13697130903009187 [DOI] [PubMed] [Google Scholar]
- Sherwin BB (2009). Estrogen therapy: Is time of initiation critical for neuroprotection? Nature Reviews Endocrinology, 5(11), 620–627. 10.1038/nrendo.2009.193 [DOI] [PubMed] [Google Scholar]
- Smith YR, Bowen L, Love TM, Berent-Spillson A, Frey KA, Persad CC, Reame NK, Koeppe RA, & Zubieta JK (2011). Early initiation of hormone therapy in menopausal women is associated with increased hippocampal and posterior cingulate cholinergic activity. Journal of Clinical Endocrinology and Metabolism, 96(11), E1761–E1770. 10.1210/jc.2011-0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YR, Minoshima S, Kuhl DE, & Zubieta JK (2001). Effects of long-term hormone therapy on cholinergic synaptic concentrations in healthy postmenopausal women. Journal of Clinical Endocrinology and Metabolism, 86(2), 679–684. 10.1210/jc.86.2.679 [DOI] [PubMed] [Google Scholar]
- Squire LR, Wetzel CD, & Slater PC (1979). Memory complaint after electroconvulsive therapy: Assessment with a new self-rating instrument. Biological Psychiatry, 14(5), 791–801. [PubMed] [Google Scholar]
- Tinkler GP, & Voytko ML (2005). Estrogen modulates cognitive and cholinergic processes in surgically menopausal monkeys. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 29(3), 423–431. 10.1016/j.pnpbp.2004.12.016 [DOI] [PubMed] [Google Scholar]
- Vega JN, Zurkovsky L, Albert K, Melo A, Boyd B, Dumas J, Woodward N, McDonald BC, Saykin AJ, Park JH, Naylor M, & Newhouse PA (2016). Altered brain connectivity in early postmenopausal women with subjective cognitive impairment. Frontiers in Neuroscience, 10, 1–11. 10.3389/fnins.2016.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MT, Maki PM, & McDermott MP (2014). Cognition and mood in perimenopause: A systematic review and meta-analysis. The Journal of Steroid Biochemistry and Molecular Biology, 142, 90–98. 10.1016/j.jsbmb.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MT, Mapstone M, Staskiewicz J, & Maki PM (2012). Reconciling subjective memory complaints with objective memory performance in the menopausal transition. Menopause, 19(7), 735–741. 10.1097/gme.0b013e318241fd22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Quesenberry CP, Zhou J, & Yaffe K.(2011). Timing of hormone therapy and dementia: The critical window theory revisited. Annals of Neurology, 69, 163–169. 10.1002/ana.22239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods NF, Mitchell ES, & Adams C.(2000). Memory functioning among midlife women: Observations from the seattle midlife women’s health study. Menopause, 7(4), 257–265. 10.1097/00042192-200007040-00008 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kitawaki J, Kikuchi N, Okubo T, Iwasa K, Kawata M, & Honjo H.(2007). Effects of estrogens on cholinergic neurons in the rat basal nucleus. The Journal of Steroid Biochemistry and Molecular Biology, 107(1–2), 70–79. 10.1016/j.jsbmb.2007.03.035 [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, & Leirer VO (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17(1), 37–49. 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- Young JM, Douglas Shytle R, Sanberg PR, & George TP (2001). Mecamylamine: New therapeutic uses and toxicity/risk profile. Clinical Therapeutics, 23(4), 532–565. 10.1016/S0149-2918(01)80059-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.