Abstract
Background
Venous thromboses are well‐established complications of hormonal therapy. Thrombosis risk is seen with both hormonal contraceptive agents and with hormone replacement therapy for menopause and gender transition. Over the past several decades, large epidemiological studies have helped better define these risks.
Objectives
To review and discuss the differences in thrombosis risk of the many of hormonal preparations available as well as their interaction with patient‐specific factors.
Methods
We conducted a narrative review of the available literature regarding venous thrombosis and hormonal therapies including for contraception, menopausal symptoms, and gender transition.
Results
Thrombosis risk with estrogen‐containing compounds increases with increasing systemic dose of estrogen. While progesterone‐only–containing products are not associated with thrombosis, when paired with estrogen in combined oral contraceptives, the formulation of progesterone does impact the risk. These components, along with patient‐specific factors, may influence the choice of hormonal preparation. For patients who develop thrombosis on hormonal treatment, anticoagulation is protective against future thrombosis. Duration of anticoagulation is dependent on ongoing and future hormone therapy choice. Finally, the optimal management of hormone therapy for individuals diagnosed with prothrombotic illnesses such as COVID‐19 remains unclear.
Conclusions
When contemplating hormonal contraception or hormone replacement therapy, clinicians must consider a variety of factors including hormone type, dose, route, personal and family history of thrombosis, and other prothrombotic risk factors to make informed, personalized decisions regarding the risk of venous thrombosis.
Keywords: estrogens; hormonal contraception; hormone replacement therapy; thrombosis, transgender people
Essentials.
Certain hormonal therapies increase the risk of developing venous blood clots.
This article reviews the various hormonal therapies and the factors that influence the risk.
The risk of blood clots increases as the dose of estrogen increases.
Those who develop a blood clot should stay on anticoagulation if unable to stop hormonal therapy.
1. INTRODUCTION
Hormone‐containing therapies are ubiquitous in modern health care. Combined oral contraceptive (COC) medications are the most frequently prescribed medications to young women with as many as 33%–40% of women in certain age groups using COCs at any one time. 1 Approximately 40% of postmenopausal women in the United States have been on hormone replacement therapy (HRT). The number of people on hormonal therapy (HT) will likely increase in the coming years with the growing population of individuals undergoing gender transition. 2 , 3 Due to the prevalence of these therapies, any complication – even rare ones – can have an impact on a large number of individuals. This article provides an updated review of the estimated venous thrombotic risks of hormone‐containing therapies along with a discussion of modifiers to this risk.
2. CHALLENGES IN DATA GATHERING
The ideal method for determining hormone‐related thrombosis risks is through prospective randomized trials of these agents. However, this is not always feasible. Instead, large patient populations are studied using a variety of epidemiological techniques, including reviews of large pharmacy databases, biomarker studies, centralized hospital registries, cohort studies, and case–control studies. The information below is integrated from these sources to better inform practice. Ranges for data, where included, represent the 95% confidence intervals (CIs). Findings regarding the various hormone‐containing products are summarized in Figure 1.
FIGURE 1.
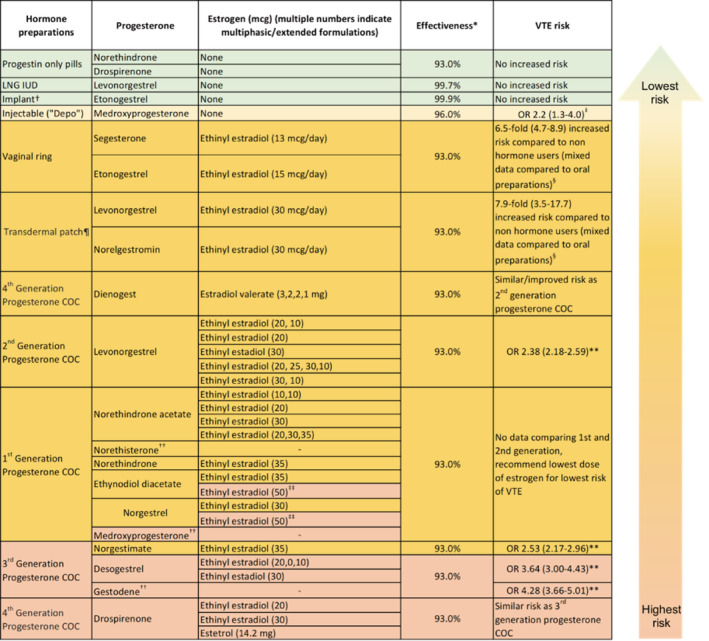
List of available hormone preparations for contraception in the United States. Formulations are grouped roughly in order of ascending risk of VTE based on the best available evidence. Note the individual exceptions in each of the different generations of COC. *Effectiveness as measured by prevention of unwanted pregnancy in the first year of typical use (Reference [33]) †Contraindicated in those with prior DVT, though this is based on data regarding oral preparations of etonogestrel ‡Reference [30] §Reference [34] ¶ Contraindicated in body mass index ≥30 kg/m2 **Reference [20] ††Not available in the United States for contraception but is included in this table for completion's sake ‡‡ All formulations with ethinyl estradiol dose of >50 μg are labeled as high risk of VTE. Abbreviations: COC, combined oral contraception; LNG IUD, levonorgestrel intrauterine device; OR, odds ratio; VTE, venous thromboembolism
3. HORMONAL CONTRACEPTION
The overall risk of thrombosis in COC users is anywhere from 2‐ to 9‐fold higher compared to nonusers. 4 , 5 , 6 , 7 Various manifestations of hormonal contraceptive–associated venous thromboembolism (VTE) have been reported and include deep vein thrombosis (DVT), pulmonary emboli (PE), and cerebral venous sinus thrombosis. 8
3.1. Estrogen
The earliest hormonal contraceptive pills contained 150 μg ethinyl estradiol (EE). Over the past 50 years, considerable attention has been directed to dose reduction of EE. Epidemiologic studies have demonstrated a decreased risk of venous thrombosis with a lower dose. The risk of VTE is reduced by 17%–32% with a decrease in estrogen dose from 50 μg to 40–30 μg, and further reduced by 18% with a 20‐μg dose, although the risk remains elevated compared to nonusers. 4 , 6 Notably, this dose reduction does not affect the efficacy of contraception; however, it can increase the incidence of breakthrough uterine bleeding because lower doses are less effective at maintaining endometrial integrity. 9 , 10
Traditionally, all COCs included EE as the estrogen component. Recently, estradiol valerate (E2V), which is hydrolyzed to 17‐estradiol and valerate, has been introduced into formulations. A prospective cohort study demonstrated a reduced incidence of VTE with E2V compared to EE, with a hazard ratio (HR) of 0.4 (95% CI, 0.2–1.0). The study did not clarify which estrogen doses were used for the EE COC group. 11 E2V has also been shown to have a lower metabolic impact. 12 , 13 , 14
3.2. Progesterone
Progestins in the first two generations of COC include levonorgestrel, norethisterone, and medroxyprogesterone. For the third‐generation pills, the progesterone was modified to reduce unwanted androgenic side effects, such as hirsutism and the development of an adverse lipid profile. The third‐generation progestins include desogestrel, gestodene, and norgestimate. Unexpectedly, multiple large epidemiological studies have demonstrated an increased risk of thrombosis in users of third‐generation pills compared to second‐generation pills. 15 In individual studies, the risk of VTE with third‐generation compared to second‐generation pills ranged from no increase to a 2.6‐fold increased risk of thrombosis 4 , 16 , 17 with a large meta‐analysis showing a 70% increased risk (95% CI, odds ratio [OR] 1.4–2.0). 15
There is some uncertainty regarding the findings of these due to concerns about sources of potential bias, including preferential use of newer agents in women with thrombotic risk factors, increased index of suspicion for thrombosis in users of newer products, and duration of use of older versus newer agents. 18 , 19 A large, nested case–control study of patients with first VTE on COCs controlled for confounding risk factors (obesity, smoking status) and revealed increased thrombosis risk for third‐generation progestins except for norgestimate. Specifically, preparations containing gestodene and desogestrel were associated with significantly higher risks of VTE (OR, 4.28 [95% CI, 3.66–5.01]; and 3.64 [95% CI, 3.00–4.43], respectively) compared to preparations containing either levonorgestrel or norgestimate (OR, 2.38 [95% CI, 2.18–2.59]; and 2.53 [95% CI, 2.17–2.96], respectively) with the reference variable being no exposure. 20 Similarly, the third‐generation progesterone cyproterone acetate, available outside of the United States and used traditionally for acne and polycystic ovarian disease (PCOS), has an increased relative risk of 2.04 (95% CI, 1.55–2.49) compared with levonorgestrel containing COCs. 21
The newer fourth‐generation progesterone drospirenone is a derivative of spironolactone and has mild antimineralocorticoid and antiandrogenic effects. 22 Currently, it is unclear if thrombosis risk is increased with its use compared to second‐generation pills due to conflicting evidence. Two large prospective studies showed no increase in thrombosis with drospirenone‐containing COCs compared to other progestin‐containing COCs, namely, the second‐generation progestin levonorgestrel, 23 , 24 , 25 while a systematic review and network meta‐analysis demonstrated an increased OR of up to 4.2. 4 , 6 , 20 , 26 , 27 A 2014 Cochrane Review on the topic concluded that the relative risk of venous thrombosis associated with COCs containing drospirenone was similar to those containing third‐generation progestin formulations. The review suggested that preparations with both the third‐ and fourth‐generation progestins had a 50%–80% increased risk of VTE compared to the second‐generation progestin levonorgestrel. 9 Because the thrombogenicity of drospirenone remains controversial, we recommend that the agent is limited to women who may benefit from its specific antiadrenergic properties, like patients with PCOS, and do not have other established risk factors for VTE, including family history. 28 , 29
Regarding progestin‐only contraceptives, evidence from a 2016 systematic review and 2012 meta‐analysis demonstrated no increased risk of venous thrombosis with progestin‐only formulations, including oral progesterone (the mini‐pill), intrauterine device (IUD), and subdermal implants (OR, 1.03 [95% CI, 0.76–1.39]). 30 , 31 Since the 2016 review, drospirenone has been incorporated into a progestin‐only pill. One study demonstrated that the drospirenone pill induced no significant changes in hemostatic laboratory values such as factor VIII levels. 32 While not equivalent to real‐world data, this suggests that like other progestin‐only pills, those containing drospirenone do not carry an increased risk for thrombosis. Of note, the progestin‐only pill has reduced efficacy overall and requires excellent compliance compared to the levonorgestrel (LNG) IUD and subdermal implant. 33
3.3. Method of delivery
For both compliance and convenience, estrogen‐containing patches and vaginal rings are becoming more popular as methods of hormonal contraception. The progesterone components – 17‐deacytylnorgestimate (metabolite of norgestimate) in patches and etonogestrel (metabolite of desogestrel) in implants and vaginal rings – are the same as those in third‐generation pills, raising concerns for increased thrombosis rates compared to second‐generation pills. In one study, the combined contraceptive patch and ring had a relative risk (RR) of 7.9 (95% CI, 3.5–17.7) and 6.5 (95% CI, 4.7–8.9) for VTE, respectively, compared to nonusers of any hormonal contraceptives. 34 Traditionally, data for the patch have been contradictory, with study results varying from improved safety profile to 2‐fold risk of thrombosis compared to oral COCs containing the third‐generation norgestimate. 35 , 36 , 37 , 38 , 39 , 40
The depot formulation contains medroxyprogesterone (second generation). 23 , 41 Two studies have shown an increased risk of thrombosis with injectable depot‐medroxyprogesterone (DMPA) compared to nonusers with an OR ranging from 2.2 to 3.6. 42 A 2016 systematic review also demonstrated an increased OR of VTE of 2.2 (95% CI, 1.3–4.0) with the use of injectable DMPA when compared to nonusers of hormones. 30
The IUD that releases LNG is another option. Several studies have shown no increased rate of thrombosis in users of the LNG IUD compared to nonusers of COC. 4 , 34 , 42 , 43 Therefore, the LNG IUD is an optimal choice for women with thrombophilia, previous thrombosis, or significant risk factors. 4 , 34 , 42 For patients who are intolerant of or averse to the IUD, the subdermal implant (which releases the progesterone etonogestrel) likely offers a similar balance of safety with high efficacy. While few studies offer a dedicated perspective regarding this, the few that have included the implant in analyses have either shown no increased risk or increased risk with wide CIs overlapping with odds of nonusers. 30 , 43 Both the LNG IUD and subdermal implant offer excellent efficacy for pregnancy prevention (<1% failure rate) and do not require daily dosing.
4. HORMONE REPLACEMENT THERAPY
The risk of venous thrombosis is increased in women taking HRT and was 2‐fold higher compared to nonusers in a large double‐blind randomized control trial. Older age, increased body mass index (BMI), and factor V Leiden (FVL; both homozygous and heterozygous mutations) further increased this risk. 44 Baseline biomarkers may also predict likelihood of developing VTE on HRT. In one large case–control study, baseline elevated D‐dimer was associated with a 6‐fold increased odds (95% CI, 3.6–9.8) of VTE compared to normal baseline D‐dimer for patients started on HRT. 45
4.1. Estrogen
Hormone replacement therapy has traditionally used one of two formulations—estradiol (E2) or conjugated equine estrogen (CEE). One observational study demonstrated an increased venous thrombosis risk with oral CEE compared to oral E2 (OR, 2.08 [95% CI, 1.02–4.27]). 46 This finding is supported by the results of a large, nested case–control study that demonstrated a lower risk of VTE with both E2‐only (OR, 0.85 [95% CI, 0.76–0.95]) and combined preparations (OR, 0.83 [95% CI, 0.76–0.91]) compared to CEE. 40 However, a 2021 large retrospective cohort study of HT users showed no increased risk of VTE when comparing CEE to oral E2 (HR, 0.96 [95% CI, 0.64–1.46]) and transdermal E2 (HR, 0.95 [95% CI, 0.60–1.49]). 47 Given the variability of results, it is unclear if the formulation of estrogen affects VTE in HRT.
4.2. Progesterone
In a large prospective study, estrogen‐only HRTs posed less risk than combination therapies. 40 However, most formulations of HRT contain progestin to prevent excessive buildup of the uterine lining and subsequent increased risk of endometrial cancer. For this reason, unopposed estrogen is contraindicated in postmenopausal women without a history of hysterectomy. Acknowledging that progestin plays a necessary role in these therapies, studies have focused on determining which progesterone is the safest. In a large prospective study, oral combined HRT with first‐generation progesterone medroxyprogesterone showed an increased risk of VTE compared to HRT with other progestins, with a RR of 2.67 (95% CI, 2.25–3.17) versus 1.19 (95% CI, 1.69–2.17). 41 For women who require combined HRT, we recommend formulations that contain second‐generation progestins due to the lower associated VTE risk in both HRT and COC studies.
4.3. Method of delivery
While oral estrogen and progesterone are shown to have an increased risk of VTE, the risk is much lower for transdermal estrogen in HRT. For example, in one study, the RR for VTE was 1.42 (95% CI, 1.21–1.66) for oral estrogen and 0.82 (95% CI, 0.64–1.06) for transdermal estrogen‐only with nonusers as reference. 41 A 2019 nested case–control study of over 80 000 women with VTE demonstrated transdermal preparations to be the safest method of HRT with no associated increased risk of VTE. 40 Oral estrogen therapy has been shown to activate the coagulation cascade and result in acquired protein C resistance, while transdermal estrogen has minimal effects on hemostatic parameters. 48 The transdermal formulations studied in HRT did not contain a progesterone, which may explain the difference in risk of VTE with HRT versus COC patches. Notably, there is no clear association of thrombosis with topical vaginal estrogen administration for postmenopausal dryness and VTE. 49
5. TRANSGENDER MEDICINE
Recently, more individuals have had the opportunity to seek medical care for gender transitions. Data from 2011 has reported that up to 700 000 people identify as transgender in the United States alone. 50 The field of transgender medicine involves both hormonal therapies and surgical procedures that align with one's preferred gender identity. For many, these interventions are essential to the gender‐affirming process. The World Professional Association for Transgender Health and the Endocrine Society have developed guidelines to help clinicians and patients in these efforts. 51 , 52 , 53 Unfortunately, compelling data regarding the risks associated with these therapies is currently lacking. We have summarized the recommendations regarding thrombotic complications in this specific population from a collective of retrospective studies and extrapolations from the abundance of information regarding postmenopausal cisgender women receiving HRT and cisgender men receiving androgen deprivation or estrogen therapy in the setting of prostate cancer. 54 Additional prospective studies are needed to better understand the inherent risks of these therapies and to pursue appropriate risk reduction.
5.1. Estrogen
Estrogen is the mainstay intervention for transgender women (assigned male sex at birth).
Trans women on HT experience an increased rate of VTE with a prevalence as high as 6% on HT in one cross‐sectional study in which the average length of use was 10 years. 55 Similar to estrogen‐containing therapies in cis women, the risk of VTE is increased with a history of thrombosis, thrombophilia, smoking, or obesity. 56 The available retrospective data demonstrate that the risk of VTE is highest with oral estrogen, particularly EE, and during the first year of therapy. Therefore, we recommend that individuals with the aforementioned high‐risk features should be given formulations containing E2V or 17‐estradiol at the lowest possible dose. As noted above, transdermal preparations appear to have low to no risk of VTE, leading some experts to suggest it as first‐line therapy – particularly for patients aged >40 years. 57
5.2. Testosterone
Testosterone is the mainstay of HT for trans men (assigned female sex at birth) and enhances male secondary sexual characteristics. Additional agents, including medroxyprogesterone or gonadotropin‐releasing hormone analogs, can be employed for menses cessation.
The primary hematologic complication of testosterone therapy is an increased risk of secondary erythrocytosis. 58 Unlike the primary erythrocytosis seen in myeloproliferative malignancies, this side effect of exogenous testosterone has not been convincingly linked to an increased risk of thrombosis. 59 , 60 , 61 Regardless, current guidelines from the Endocrine Society recommend discontinuation of testosterone therapy in hematocrit levels >54%. 62 Efforts can be made to maintain adequate red cell indices by avoiding high‐dose testosterone and intramuscular testosterone, although transdermal testosterone has been shown to have this effect as well. 63 , 64
Reassuringly, in cis men, supplemental testosterone has not been associated with increased rates of VTE in large retrospective cohort studies or an increased risk of cardiovascular disease in a large prospective study. 65 , 66 , 67 , 68 One case–control study in cis men demonstrated an increased risk of VTE of 1.63 (95% CI, 1.12–2.37) within the first 6 months of use that was no longer significant at 1 year of use. 69 There are fewer studies for women on testosterone, including trans women. Limited data from case reports of women on testosterone and a retrospective observational series of trans men have yielded similar results to those of cis men regarding VTE risk. 65
6. THROMBOSIS RISK FACTORS
The risk of VTE in individuals depends on both medication‐related factors, such as length of exposure, and user‐related risk factors, including age, BMI, and inherited or acquired thrombophilia. A suggested approach regarding these is summarized in Figure 2A.
FIGURE 2.
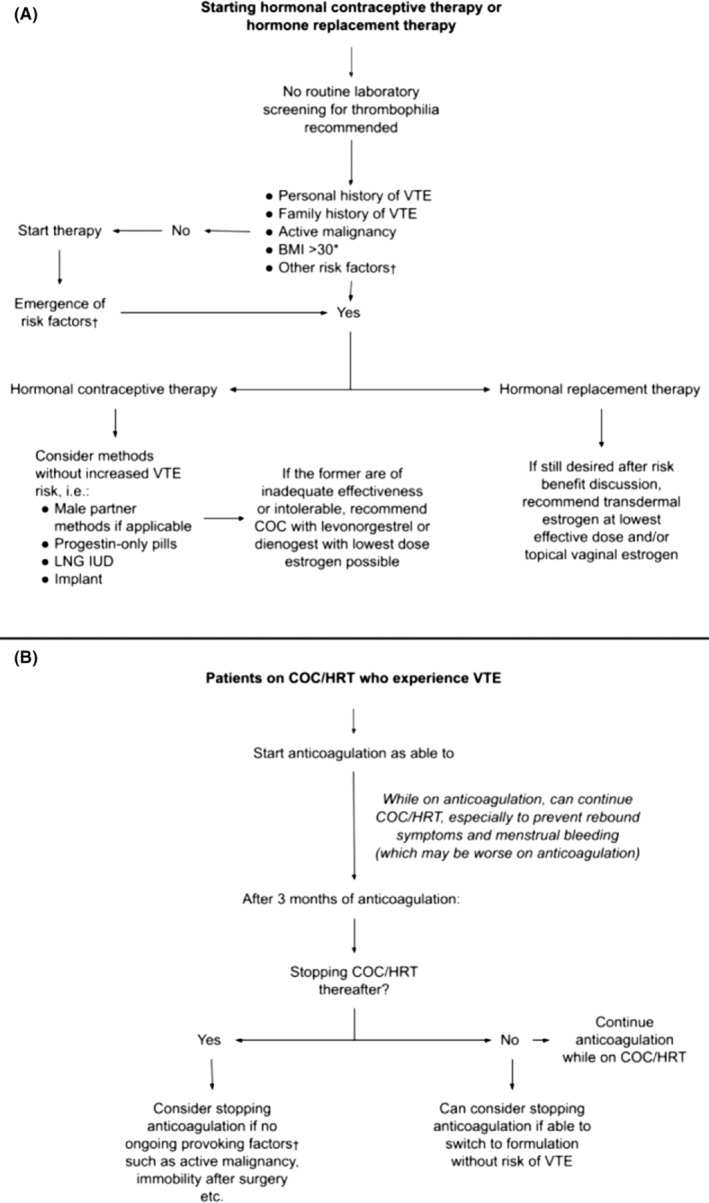
Proposed flowcharts for the consideration of initiating hormonal contraceptive therapy or hormone replacement therapy (A), and for the approach to the patient who develops a venous thromboembolic event while on the aforementioned therapy (B). *Risk factor for consideration but should undergo risk/benefit discussion with the patient †Expert advice may be needed to determine factors. Abbreviations: BMI, body mass index; COC, combined oral contraceptive; HRT, hormone replacement therapy; LNG IUD, levonorgestrel intrauterine device; VTE, venous thromboembolism
6.1. Length of exposure
All studies show a marked increase in the risk of thrombosis within the first months of use of hormonal therapies with an OR as high as 12 reported for the first 3 months and 9 within the first year. 4 , 6 , 7 This increased risk within the first year is most pronounced in women aged <30 years. 7 With time, the risk of thrombosis decreases, perhaps because higher‐risk patients stop using due to thrombosis, but always remains higher than nonusers. This risk normalizes 3 months after stopping the pill. 70 These data have not been replicated in trans women.
6.2. Age
Thrombosis rates have been shown to significantly increase with age. In women aged <30 years, venous thrombosis incidence is 1.2–3.7 events/10 000 person‐years with estrogen use, which increases to 2–10 events/10 000 person‐years for women aged 30–40 years. 6 In one study, the thrombosis risk with oral hormonal contraceptive use was 14.2/10 000 person‐years in 40‐year‐old women and 34/10 000 person‐years in women aged >45 years. 17
A case–control study of VTE risk in women aged >50 (range, 50–70; average, 59) years found that exposure to any HT significantly increased the risk of VTE 6.3‐fold compared to similarly aged nonhormone users. This risk was further increased to 10.2 with COCs containing desogestrel (third‐generation progesterone). Oral combined postmenopausal HRT use demonstrated a 3.9 to 4‐fold increased risk compared to nonusers. 71
6.3. Obesity
For any patient, obesity is a risk factor for thrombosis, and this holds true for users of COCs. 1 , 16 , 70 In several studies, the OR of thrombosis for women on COCs with BMI < 20 kg/m2 compared to >30 kg/m2 was 2.7–4.6. 25 , 70 In the MEGA study, women with BMI >30 on COCs had an increased risk of VTE with an OR of 24 (95% CI, 13.35–42.34), while nonusers with a BMI >30 had an OR of 3 (95% CI, 2.85–6.03). 72 In a separate study on postmenopausal women, the HR for VTE was 5.61 (95% CI, 3.12–10.11) in women with a BMI > 30 on combined HRT and 2.87 (95% CI, 1.52–5.4) in women with BMI > 30 on placebo, using nonusers with a BMI <25 as reference. 44 As obesity rises in the population, this interaction with hormonal therapies will become a more prevalent risk factor.
6.4. Inherited thrombophilia
The presence of inherited thrombophilia is a major modifier of thrombotic risk in users of hormonal therapies. The majority of the data come from women with FVL, as this is the most common inherited thrombophilia with a heterozygous prevalence of 5% and homozygous prevalence of 0.02% in the general population. Of note, FVL mutations are primarily observed in White people. The risk of first VTE with an FVL mutation is increased 3‐fold. Multiple studies show an increase in the risk of thrombosis ranging from 15‐ to 35‐fold in women with a FVL mutation who use COCs compared to nonusers without a prothrombotic mutation. 1 , 73 , 74 The risk of VTE with COC use is also significantly increased in women with a prothrombin gene mutation and rarer thrombophilias, such as protein C and S deficiency or antithrombin deficiency. A 2016 meta‐analysis on COC users showed that the RR of VTE was 6 (95% CI, 4.21–8.23) for those with “mild” thrombophilia (FVL or prothrombin mutation) and 7 (95% CI, 2.93–17.45) for those with “severe thrombophilia” (antithrombin deficiency, protein C and S deficiency, and homozygosity for FVL or prothrombin mutation), with COC users without thrombophilia as reference. 75
We recommend women with thrombophilia who require hormonal contraception be placed on the least thrombogenic formulations like the LNG IUD or implant. There may be some situations where the specific benefits of estrogen‐containing contraceptives outweigh the VTE risk. Examples include women with heavy menstrual bleeding, PCOS, endometriosis, or severe cycle‐related symptoms. In these situations, symptom management and treatment with COC use could significantly improve quality of life and may be worth the potential risk of thrombosis, even in the disease states that carry an independent risk for VTE, such as in PCOS. Although women with FVL mutations have an increased risk of thrombosis with contraception (33:10 000 people per year), the risk is even higher during pregnancy (1:1000 people per year). 76 Overall, we recommend providers have detailed risk–benefit discussions involving shared decision making with any patient with thrombophilia considering estrogen‐containing therapies. If they decide to initiate an estrogen or COC therapy, we recommend using the lowest dose of estrogen possible and a lower‐risk progesterone (second generation).
Currently, universal screening is not recommended before the initiation of COC. Screening may be considered in women with a family history of venous thrombosis (two or more episodes of VTE in first‐degree relatives); however, progesterone‐only therapy (such as the LNG IUD or subdermal implant) would be preferred for patients with a family history regardless of a laboratory diagnosis of thrombophilia. Notably, the PILGRIM study found that the prevalence of VTE in women on COCs was not statistically significant regardless of the presence of a first‐degree family history of VTE (29.3% with family history vs 23.9% without; p = 0.09). 77 The study also revealed that non‐O blood type was a predictor of VTE (OR, 1.98 [95% CI, 1.57–2.49]), potentially due to decreased von Willebrand factor levels in type O blood groups. 78 Despite this evidence, no formal recommendations have been made regarding blood type and COCs or HRT. Finally, there are no strong data to support the screening for thrombophilia in trans women undergoing hormonal transition.
6.5. History of superficial venous thrombosis
There is some evidence that a history of superficial venous thrombosis (SVT) may be associated with an increased risk of future DVT or VTE. The MEGA study has shown a 4‐ to 6‐fold increase of VTE after SVT. In women with a history of SVT, the use of COC, HT, and pregnancy were associated with a 34.9‐fold increased VTE risk. 6 , 79 Importantly, however, these data come from self‐reported prevalence of SVT, which were not confirmed, and no distinction was made between lower‐extremity SVT and catheter‐associated upper‐extremity SVT. No comment was made in this study regarding the formulation of contraception. While this analysis warrants confirmation of the findings, these data may still inform shared decision‐making conversations regarding the use of contraception, family planning, and hormonal therapies.
6.6. Hormone therapy in the era of COVID‐19
Coagulopathy has been recognized as a complication of COVID‐19, and the incidence of thrombotic events may be as high as 31% among critically ill patients with the disease. 80 , 81 There is a concern that hormone therapy may compound this thrombotic risk. The Spanish Menopause Society has released guidelines taking the stance that there would likely be a greater risk of thrombosis in users of estrogen with COVID‐19. Based on expert opinion, they advise patients with COVID‐19 on hormonal therapies to stop treatment or switch to lower‐risk therapy in most cases. 82 Epidemiologic data, however, have failed to reveal such an association, and the Italian Society of Contraception recommend against discontinuing contraception in cases of asymptomatic to moderate infection. 83 , 84 In fact, women with COVID‐19 are less likely to develop thrombosis compared to men, and there is suspicion that progesterone and certain estrogens may be protective against severe infection with some even proposing estradiol as a potential treatment. 85 , 86 , 87 This is a paradox to the fact that patients who are pregnant or recently pregnant (and thus with high levels of circulating estrogens/progesterones) and contract COVID‐19 are more likely to experience severe illness compared to women of reproductive age who are not pregnant, outlined in a couple of systemic reviews. The aforementioned studies do not comment on thrombotic events in this population. 88 The discrepancies in data and recommendations highlight the need for further research on the role of estrogen and progesterone in the complications, prevention, and perhaps the treatment of COVID‐19. Until research identifies a clear association between hormone therapy and thrombosis risk in COVID‐19 patients, we recommend continuing hormone therapy in non–critically ill patients.
6.7. Pregnancy
In discussing contraceptives, it is worthwhile to consider pregnancy and its thrombotic risks. Pregnancy itself induces a prothrombotic state, which leads to a significantly increased risk of thrombosis, with an incidence of 1–2:1000 and a death rate from thrombosis of 1–4:100 000. 89 , 90 , 91 In one study, the baseline risk for thrombosis in nonusers of COC was 4.4/10 000 person‐years, which increased to 8/10 000 person‐years with levonorgestrel‐containing COCs and 29.1/10 000 person‐years during pregnancy. 25 , 92 Therefore, it is important to factor in the high thrombosis risk in pregnancy when discussing the risks and benefits of hormonal contraception with patients. Clinicians and patients should also consider male partner contraceptive options, including vasectomy or barrier contraception, which impart no increased risk for thrombosis for either partner.
7. MANAGEMENT OF VENOUS THROMBOSIS WHILE ON HORMONAL THERAPY
7.1. Immediate management
The approach to the patient who develops thrombosis while on therapy is summarized in Figure 2B. Estrogen is the most common risk factor for thrombosis in young women. 93 Abundant data have shown that the risk of future thrombosis is low after 3 months of anticoagulation, and therefore, women with any thrombosis on estrogen‐only medication may require only short‐term anticoagulation for 3 months. This recommendation does not necessarily apply to those with independent provocative factors including active malignancy and inflammatory bowel disease, and situations such as these require careful consideration for anticoagulation duration. Although the presence of thrombophilia predicts the first thrombosis, it is unclear if it predicts an increased risk of recurrent thrombosis.
Among women who require anticoagulation for the treatment of VTE or other conditions, special consideration must be given to those of childbearing age, owing to possible adverse effects of direct oral anticoagulants (DOACs) and vitamin K antagonists (VKAs) on fetal well‐being. Also, anticoagulation is associated with an increase in heavy or abnormal uterine bleeding, which can be mitigated by hormonal contraceptive use. Various organizations including the World Health Organization have developed recommendations around the necessity of contraception while on anticoagulation, with considerable variation between societies. Analysis of data from the EINSTEIN DVT and PE trials demonstrated no increased association between recurrent DVT for women on VKAs or DOACs and combination or progestin‐only hormonal therapies. 94 Therefore, thrombosis experts tend to agree on continuing effective contraceptive use while undergoing anticoagulation and that for those who remain on hormonal contraception going forward, continued anticoagulation appears effective in reducing recurrent VTE risk. 95 For women on warfarin and COCs, additional dose adjustments and monitoring may be required when starting, discontinuing, or holding therapy (placebo days) due to altered hepatic metabolism of warfarin. 96
7.2. Implications for future estrogen use
Patients with a history of thrombosis are at an increased risk of another clot if they resume estrogen therapy. In one prospective study, women who had an estrogen‐related thrombosis had a long‐term recurrence rate of 9.7/1000 patient‐years if they remained off of estrogen, which increased to 27.3/1000 patient‐years if they had any COC use after the first VTE. 93 Of note, the majority of the women in the study received ≤1 year of anticoagulant therapy. In women with a history of non–estrogen‐related VTE who later used COC, the thrombosis rate was even higher, at 35/1000 patient‐years. While the risk of recurrence in women using COCs after a diagnosis of VTE has never been studied in a randomized placebo‐controlled trial, the risk has been studied in women on HRT. This study, however, was stopped early due to a dramatically increased recurrence rate in the estrogen group (8.5%) compared to the placebo group (1%). In women with a history of thrombosis, estrogen‐containing therapies should be avoided if possible. If avoidance is not possible, anticoagulation should be strongly considered for as long as the woman requires this therapy, with regular discussion of risks, benefits, and alternatives. 97
7.3. Management considerations for transgender women
Trans women who develop a VTE while on HT should be treated with therapeutic anticoagulation to decrease the risk of recurrent thrombosis. Risk reduction can be achieved by changing oral estrogen to a transdermal formulation. 57 While the risk of recurrent VTE is lower with transdermal estrogen, there is insufficient evidence to determine if the risk is mitigated with this therapy adjustment. As such, barring other contraindications to anticoagulation, we recommend continuing anticoagulation for trans women with a history of VTE while on HT, which is presumed indefinite in the setting of gender transition, following careful discussion of risks and benefits. Of note, there are no data to support the use of prophylactic aspirin or anticoagulation in trans women.
A trans woman on HT undergoing elective surgery is at an increased risk of thrombosis due to both exogenous estrogen and the inherent risk of VTE in the perioperative period. Guidance is based on extrapolations from expert opinion in perioperative management of HRT in postmenopausal cis women. In this population, formal consensus recommends holding HRT for 2–4 weeks before surgery. 98 Studies to support this are lacking, and the recommendation relies on the notion of the relatively low risk of holding the medication. A recent single‐center retrospective study in 2021 showed no significant risk of VTE associated with continuing HT throughout the perioperative period; however, the study was limited to gender‐affirming surgeries. 99 Acknowledging the lack of comprehensive data, we agree with previous recommendations that advise holding HT for 2 weeks before surgery with resumption once mobility is regained, in addition to routine postoperative DVT prophylaxis.
8. SUMMARY
Hormone therapy is indicated in many clinical contexts including contraception, postmenopausal HRT, and transgender medicine. With knowledge of the established thrombotic risks in mind, providers should consider the following when pursuing HTs with patients.
8.1. Hormonal contraception
The risk of venous thrombosis can be decreased by using the lowest possible dose of estrogen in combination with lower‐risk progestins (second generation) or progesterone‐only contraceptives (Figure 1).
Rings, patches, implants, and oral delivery methods of estrogen‐containing contraception all carry an elevated risk of venous thrombosis, as does the use of DMPA.
Intrauterine progestin‐only devices are the safest choice for hormonal contraception in patients with an increased risk of VTE, including those with a history of thrombosis, certain thrombophilias, and obesity. Subdermal implants have a similar safety profile.
The RR of venous thrombosis while on hormonal contraception should be considered in the context of the higher RR of pregnancy‐associated venous thrombosis.
With regards to contraception, the risk of venous thrombosis can be mitigated by using nonhormonal forms of birth control.
8.2. Hormone replacement therapy
Hormone replacement therapy is associated with higher risk of thrombosis and is further elevated with certain patient characteristics including increased age, BMI, and elevated baseline biomarkers such as D‐dimer.
It is uncertain if the formulation of estrogen influences thrombotic risk.
Topical and transdermal routes of administration of estrogen have no associated increased risk of VTE.
8.3. Transgender care
Male‐to‐female (MTF) HT increases the risk of thrombosis and, similar to cisgender individuals, this risk is further increased by an underlying history of thrombosis, thrombophilia, obesity, or other risk factors.
For MTF patients pursuing HT, risk reduction can be achieved by using lower‐risk estrogen formulations (estradiol valerate), the lowest possible dose to achieve desired effects, and the transdermal route of administration.
There are no data to support prophylactic aspirin or anticoagulation, nor screening for thrombophilia in the MTF population seeking HT.
8.4. Patient care
There is no recommendation to screen women for inherited thrombophilias before initiating hormonal contraceptives, particularly in the absence of a known family history of inheritable thrombophilia.
For those who develop a VTE on HT, HT does not need to be stopped while on anticoagulation. The duration of anticoagulation is dependent on risk factors present including any ongoing and future HT use.
There is no clear consensus regarding the handling of HT during active COVID‐19 infection.
AUTHOR CONTRIBUTIONS
TGD, SN, and BSB created the initial concept and writing for this review. CL, TK, and JS contributed further extensive writing, revisions, and figures. All authors reviewed and edited the manuscript prior to review.
RELATIONSHIP DISCLOSURES
JS receives consulting fees from Aronora, Inc. He also receives salary support from the National Institutes of Health via an RO1 award focusing on the prevention of device associated thrombosis. TGD receives funding for his contributions in developing lectures for Hippo Medical Education. The other authors declare no conflicts of interest.
LaVasseur C, Neukam S, Kartika T, Samuelson Bannow B, Shatzel J, DeLoughery TG. Hormonal therapies and venous thrombosis: Considerations for prevention and management. Res Pract Thromb Haemost. 2022;6:e12763. doi: 10.1002/rth2.12763
Funding information
The authors received no financial support for the preparation and writing of this manuscript.
Handling Editor: Dr Suzanne Cannegieter
Contributor Information
Thomas Kartika, Email: kartika@ohsu.edu.
Bethany Samuelson Bannow, @bsamuelson_md.
Joseph Shatzel, @clotmaster.
Thomas G. DeLoughery, @bloodman.
REFERENCES
- 1. Rosendaal FR, Van Hylckama VA, Tanis BC, Helmerhorst FM. Estrogens, progestogens and thrombosis. J Thromb Haemost. 2003;1(7):1371‐1380. [DOI] [PubMed] [Google Scholar]
- 2. Ness J, Aronow WS. Prevalence and causes of persistent use of hormone replacement therapy among postmenopausal women: a follow‐up study. Am J Ther. 2006;13(2):109‐112. [DOI] [PubMed] [Google Scholar]
- 3. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lidegaard O, Lokkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboembolism: national follow‐up study. BMJ. 2009;339:b2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Battaglioli T, Martinelli I. Hormone therapy and thromboembolic disease. Curr Opin Hematol. 2007;14(5):488‐493. [DOI] [PubMed] [Google Scholar]
- 6. van Hylckama VA, Helmerhorst FM, Vandenbroucke JP, Doggen CJ, Rosendaal FR. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case‐control study. BMJ. 2009;339:b2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martinelli I, Maino A, Abbattista M, et al. Duration of oral contraceptive use and the risk of venous thromboembolism. A case‐control study. Thromb Res. 2016;141:153‐157. [DOI] [PubMed] [Google Scholar]
- 8. Amoozegar F, Ronksley PE, Sauve R, Menon BK. Hormonal contraceptives and cerebral venous thrombosis risk: a systematic review and meta‐analysis. Front Neurol. 2015;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;(3):Cd010813. doi: 10.1002/14651858.CD010813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaunitz AM. Oral contraceptive estrogen dose considerations. Contraception. 1998;58(3 Suppl):15S‐21S. quiz 66S. [DOI] [PubMed] [Google Scholar]
- 11. Dinger J, Do Minh T, Heinemann K. Impact of estrogen type on cardiovascular safety of combined oral contraceptives. Contraception. 2016;94(4):328‐339. [DOI] [PubMed] [Google Scholar]
- 12. Jensen JT. Evaluation of a new estradiol oral contraceptive: estradiol valerate and dienogest. Expert Opin Pharmacother. 2010;11(7):1147‐1157. [DOI] [PubMed] [Google Scholar]
- 13. Haverinen A, Kangasniemi M, Luiro K, Piltonen T, Heikinheimo O, Tapanainen JS. Ethinyl estradiol vs estradiol valerate in combined oral contraceptives ‐ effect on glucose tolerance: a randomized, controlled clinical trial. Contraception. 2021;103(1):53‐59. [DOI] [PubMed] [Google Scholar]
- 14. Kangasniemi MH, Haverinen A, Luiro K, et al. Estradiol valerate in COC has more favorable inflammatory profile than synthetic ethinyl estradiol: a randomized trial. J Clin Endocrinol Metab. 2020;105(7):e2483‐e2490. [DOI] [PubMed] [Google Scholar]
- 15. Kemmeren JM, Algra A, Grobbee DE. Third generation oral contraceptives and risk of venous thrombosis: meta‐analysis. BMJ. 2001;323(7305):131‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Effect of different progestagens in low oestrogen oral contraceptives on venous thromboembolic disease. World Health Organization collaborative study of cardiovascular disease and steroid hormone contraception. Lancet (London, England). 1995;346(8990):1582‐1588. [PubMed] [Google Scholar]
- 17. Farmer RD, Lawrenson RA, Thompson CR, Kennedy JG, Hambleton IR. Population‐based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet (London, England). 1997;349(9045):83‐88. [DOI] [PubMed] [Google Scholar]
- 18. Hannaford PC. Epidemiology of the contraceptive pill and venous thromboembolism. Thromb Res. 2011;127(suppl 3):S30‐S34. [DOI] [PubMed] [Google Scholar]
- 19. Han L, Jensen JT. Does the progestogen used in combined hormonal contraception affect venous thrombosis risk? Obstet Gynecol Clin North Am. 2015;42(4):683‐698. [DOI] [PubMed] [Google Scholar]
- 20. Vinogradova Y, Coupland C, Hippisley‐Cox J. Use of combined oral contraceptives and risk of venous thromboembolism: nested case‐control studies using the QResearch and CPRD databases. BMJ. 2015;350:h2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dragoman MV, Tepper NK, Fu R, Curtis KM, Chou R, Gaffield ME. A systematic review and meta‐analysis of venous thrombosis risk among users of combined oral contraception. Int J Gynaecol Obstet. 2018;141(3):287‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sitruk‐Ware R, Nath A. The use of newer progestins for contraception. Contraception. 2010;82(5):410‐417. [DOI] [PubMed] [Google Scholar]
- 23. Seeger JD, Loughlin J, Eng PM, Clifford CR, Cutone J, Walker AM. Risk of thromboembolism in women taking ethinylestradiol/drospirenone and other oral contraceptives. Obstet Gynecol. 2007;110(3):587‐593. [DOI] [PubMed] [Google Scholar]
- 24. Sehovic N, Smith KP. Risk of venous thromboembolism with drospirenone in combined oral contraceptive products. Ann Pharmacother. 2010;44(5):898‐903. [DOI] [PubMed] [Google Scholar]
- 25. Dinger JC, Heinemann LA, Kuhl‐Habich D. The safety of a drospirenone‐containing oral contraceptive: final results from the European active surveillance study on oral contraceptives based on 142,475 women‐years of observation. Contraception. 2007;75(5):344‐354. [DOI] [PubMed] [Google Scholar]
- 26. Pearce HM, Layton D, Wilton LV, Shakir SA. Deep vein thrombosis and pulmonary embolism reported in the prescription event monitoring study of Yasmin. Br J Clin Pharmacol. 2005;60(1):98‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta‐analysis. BMJ. 2013;347:f5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dunn N. The risk of deep venous thrombosis with oral contraceptives containing drospirenone. BMJ. 2011;342:d2519. [DOI] [PubMed] [Google Scholar]
- 29. Guido M, Romualdi D, Giuliani M, et al. Drospirenone for the treatment of hirsute women with polycystic ovary syndrome: a clinical, endocrinological, metabolic pilot study. J Clin Endocrinol Metab. 2004;89(6):2817‐2823. [DOI] [PubMed] [Google Scholar]
- 30. Tepper NK, Whiteman MK, Marchbanks PA, James AH, Curtis KM. Progestin‐only contraception and thromboembolism: a systematic review. Contraception. 2016;94(6):678‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mantha S, Karp R, Raghavan V, Terrin N, Bauer KA, Zwicker JI. Assessing the risk of venous thromboembolic events in women taking progestin‐only contraception: a meta‐analysis. BMJ. 2012;345:e4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Regidor PA, Colli E, Schindler AE. Drospirenone as estrogen‐free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24 + 4 cycle with desogestrel 75 μg per day. Gynecol Endocrinol. 2016;32(9):749‐751. [DOI] [PubMed] [Google Scholar]
- 33. Trussell J AACeICT, 21st ed, Hatcher RA, Trussell J, Nelson AL, et al (Eds), Ayer Company Publishers, Inc., New York 2018. p. 844. Copyright © 2018 Contraceptive Technology Communications, Inc.
- 34. Lidegaard O, Nielsen LH, Skovlund CW, Lokkegaard E. Venous thrombosis in users of non‐oral hormonal contraception: follow‐up study, Denmark 2001‐10. BMJ. 2012;344:e2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mohammed K, Abu Dabrh AM, Benkhadra K, et al. Oral vs transdermal estrogen therapy and vascular events: a systematic review and meta‐analysis. J Clin Endocrinol Metab. 2015;100(11):4012‐4020. [DOI] [PubMed] [Google Scholar]
- 36. Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception. 2007;76(1):4‐7. [DOI] [PubMed] [Google Scholar]
- 37. Dore DD, Norman H, Loughlin J, Seeger JD. Extended case‐control study results on thromboembolic outcomes among transdermal contraceptive users. Contraception. 2010;81(5):408‐413. [DOI] [PubMed] [Google Scholar]
- 38. Fleischer K, van Vliet HA, Rosendaal FR, Rosing J, Tchaikovski S, Helmerhorst FM. Effects of the contraceptive patch, the vaginal ring and an oral contraceptive on APC resistance and SHBG: a cross‐over study. Thromb Res. 2009;123(3):429‐435. [DOI] [PubMed] [Google Scholar]
- 39. Jick SS, Hagberg KW, Kaye JA. ORTHO EVRA and venous thromboembolism: an update. Contraception. 2010;81(5):452‐453. [DOI] [PubMed] [Google Scholar]
- 40. Vinogradova Y, Coupland C, Hippisley‐Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case‐control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sweetland S, Beral V, Balkwill A, et al. Venous thromboembolism risk in relation to use of different types of postmenopausal hormone therapy in a large prospective study. J Thromb Haemost. 2012;10(11):2277‐2286. [DOI] [PubMed] [Google Scholar]
- 42. van Hylckama VA, Helmerhorst FM, Rosendaal FR. The risk of deep venous thrombosis associated with injectable depot‐medroxyprogesterone acetate contraceptives or a levonorgestrel intrauterine device. Arterioscler Thromb Vasc Biol. 2010;30(11):2297‐2300. [DOI] [PubMed] [Google Scholar]
- 43. Meirik O, Farley TM, Sivin I. Safety and efficacy of levonorgestrel implant, intrauterine device, and sterilization. Obstet Gynecol. 2001;97(4):539‐547. [DOI] [PubMed] [Google Scholar]
- 44. Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA. 2004;292(13):1573‐1580. [DOI] [PubMed] [Google Scholar]
- 45. Cushman M, Larson JC, Rosendaal FR, et al. Biomarkers, menopausal hormone therapy and risk of venous thrombosis: the Women's Health Initiative. Res Pract Thromb Haemost. 2018;2(2):310‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith NL, Blondon M, Wiggins KL, et al. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. 2014;174(1):25‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blondon M, Timmons AK, Baraff AJ, et al. Comparative venous thromboembolic safety of oral and transdermal postmenopausal hormone therapies among women veterans. Menopause. 2021;28:1125‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Olie V, Canonico M, Scarabin PY. Risk of venous thrombosis with oral versus transdermal estrogen therapy among postmenopausal women. Curr Opin Hematol. 2010;17(5):457‐463. [DOI] [PubMed] [Google Scholar]
- 49. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative observational study. Menopause (New York, NY). 2018;25(1):11‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. G. G. How many people are lesbian, gay,bisexual, and transgender. Los Angeles, CA: The Williams Institute; 2011.
- 51. Hembree WC, Cohen‐Kettenis P, Delemarre‐van de Waal HA, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(9):3132‐3154. [DOI] [PubMed] [Google Scholar]
- 52. Standards of care for the health of transsexual, transgender, and gender nonconforming people. World Professional Association for Transgender Health; 2011. 2011.
- 53. Connors JM, Middeldorp S. Transgender patients and the role of the coagulation clinician. J Thromb Haemost. 2019;17(11):1790‐1797. [DOI] [PubMed] [Google Scholar]
- 54. Shatzel JJ, Connelly KJ, DeLoughery TG. Thrombotic issues in transgender medicine: a review. Am J Hematol. 2017;92(2):204‐208. [DOI] [PubMed] [Google Scholar]
- 55. Wierckx K, Mueller S, Weyers S, et al. Long‐term evaluation of cross‐sex hormone treatment in transsexual persons. J Sex Med. 2012;9(10):2641‐2651. [DOI] [PubMed] [Google Scholar]
- 56. Nota NM, Wiepjes CM, de Blok CJM, Gooren LJG, Kreukels BPC, den Heijer M. The occurrence of acute cardiovascular events in transgender individuals receiving hormone therapy: results from a large cohort study. Circulation. 2019;139:1461‐1462. [DOI] [PubMed] [Google Scholar]
- 57. Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long‐term follow‐up study of mortality in transsexuals receiving treatment with cross‐sex hormones. Eur J Endocrinol. 2011;164(4):635‐642. [DOI] [PubMed] [Google Scholar]
- 58. Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle‐aged and older men: a meta‐analysis of randomized, placebo‐controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60(11):1451‐1457. [DOI] [PubMed] [Google Scholar]
- 59. Bhatt VR. Secondary polycythemia and the risk of venous thromboembolism. J Clin Med Res. 2014;6(5):395‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jones SD Jr, Dukovac T, Sangkum P, Yafi FA, Hellstrom WJ. Erythrocytosis and polycythemia secondary to testosterone replacement therapy in the aging male. Sex Med Rev. 2015;3(2):101‐112. [DOI] [PubMed] [Google Scholar]
- 61. Oakes M, Arastu A, Kato C, et al. Erythrocytosis and thromboembolic events in transgender individuals undergoing masculinizing therapy with testosterone [abstract]. Res Pract Thromb Haemost. 2021;5:PB1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536‐2559. [DOI] [PubMed] [Google Scholar]
- 63. Volpi RCV, Chiodera P, Chiodera P, Saccani‐Jotti G, Delsignore R. Extraprostatic complications of testosterone replacement therapy. J Endocrinol Invest. 2005;28:75‐77. [PubMed] [Google Scholar]
- 64. Fernandez‐Balsells MM, Murad MH, Lane M, et al. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta‐analysis. J Clin Endocrinol Metab. 2010;95(6):2560‐2575. [DOI] [PubMed] [Google Scholar]
- 65. Glueck CJ, Bowe D, Valdez A, Wang P. Thrombosis in three postmenopausal women receiving testosterone therapy for low libido. Womens Health (Lond Engl). 2013;9(4):405‐410. [DOI] [PubMed] [Google Scholar]
- 66. Glueck CJ, Richardson‐Royer C, Schultz R, et al. Testosterone therapy, thrombophilia‐hypofibrinolysis, and hospitalization for deep venous thrombosis‐pulmonary embolus: an exploratory, hypothesis‐generating study. Clin Appl Thromb Hemost. 2014;20(3):244‐249. [DOI] [PubMed] [Google Scholar]
- 67. Baillargeon J, Urban RJ, Morgentaler A, et al. Risk of venous thromboembolism in men receiving testosterone therapy. Mayo Clin Proc. 2015;90(8):1038‐1045. [DOI] [PubMed] [Google Scholar]
- 68. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Martinez C, Suissa S, Rietbrock S, et al. Testosterone treatment and risk of venous thromboembolism: population based case‐control study. BMJ. 2016;355:i5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Venous thromboembolic disease and combined oral contraceptives: results of international multicentre case‐control study. World Health Organization collaborative study of cardiovascular disease and steroid hormone contraception. Lancet (London, England). 1995;346(8990):1575‐1582. [PubMed] [Google Scholar]
- 71. Roach RE, Lijfering WM, Helmerhorst FM, Cannegieter SC, Rosendaal FR, van Hylckama VA. The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy. J Thromb Haemost. 2013;11(1):124‐131. [DOI] [PubMed] [Google Scholar]
- 72. Pomp ER, le Cessie S, Rosendaal FR, Doggen CJ. Risk of venous thrombosis: obesity and its joint effect with oral contraceptive use and prothrombotic mutations. Br J Haematol. 2007;139(2):289‐296. [DOI] [PubMed] [Google Scholar]
- 73. Vandenbroucke JP, Rosing J, Bloemenkamp KW, et al. Oral contraceptives and the risk of venous thrombosis. N Engl J Med. 2001;344(20):1527‐1535. [DOI] [PubMed] [Google Scholar]
- 74. Khialani D, le Cessie S, Lijfering WM, Cannegieter SC, Rosendaal FR, van Hylckama VA. The joint effect of genetic risk factors and different types of combined oral contraceptives on venous thrombosis risk. Br J Haematol. 2020;191(1):90‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. van Vlijmen EF, Wiewel‐Verschueren S, Monster TB, Meijer K. Combined oral contraceptives, thrombophilia and the risk of venous thromboembolism: a systematic review and meta‐analysis. J Thromb Haemost. 2016;14(7):1393‐1403. [DOI] [PubMed] [Google Scholar]
- 76. Bird ST, Hartzema AG, Brophy JM, Etminan M, Delaney JA. Risk of venous thromboembolism in women with polycystic ovary syndrome: a population‐based matched cohort analysis. CMAJ. 2013;185(2):E115‐E120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Suchon P, Al Frouh F, Henneuse A, et al. Risk factors for venous thromboembolism in women under combined oral contraceptive. The PILl genetic RIsk monitoring (PILGRIM) study. Thromb Haemost. 2016;115(1):135‐142. [DOI] [PubMed] [Google Scholar]
- 78. Gallinaro L, Cattini MG, Sztukowska M, et al. A shorter von Willebrand factor survival in O blood group subjects explains how ABO determinants influence plasma von Willebrand factor. Blood. 2008;111(7):3540‐3545. [DOI] [PubMed] [Google Scholar]
- 79. van Langevelde K, Lijfering WM, Rosendaal FR, Cannegieter SC. Increased risk of venous thrombosis in persons with clinically diagnosed superficial vein thrombosis: results from the MEGA study. Blood. 2011;118(15):4239‐4241. [DOI] [PubMed] [Google Scholar]
- 80. Becker RC. COVID‐19 update: COVID‐19‐associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID‐19: a systematic review and meta‐analysis. Res Pract Thromb Haemost. 2020;4:1178‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ramírez I, De La Viuda E, Baquedano L, et al. Managing thromboembolic risk with menopausal hormone therapy and hormonal contraception in the COVID‐19 pandemic: recommendations from the Spanish Menopause Society, Sociedad Española de Ginecología y Obstetricia and Sociedad Española de Trombosis y Hemos. Maturitas. 2020;137:57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Spratt DI, Buchsbaum RJ. COVID‐19 and hypercoagulability: potential impact on management with oral contraceptives, estrogen therapy and pregnancy. Endocrinology. 2020;161(12):bqaa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fruzzetti F, Cagnacci A, Primiero F, et al. Contraception during coronavirus‐COVID 19 pandemia. Recommendations of the Board of the Italian Society of contraception. Eur J Contracept Reprod Health Care. 2020;25(3):231‐232. [DOI] [PubMed] [Google Scholar]
- 85. Miesbach W, Makris M. COVID‐19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost. 2020;26:107602962093814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mauvais‐Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID‐19 outcomes. Endocrinology. 2020;161(9):bqaa127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cattrini C, Bersanelli M, Latocca MM, Conte B, Vallome G, Boccardo F. Sex hormones and hormone therapy during COVID‐19 pandemic: implications for patients with cancer. Cancer. 2020;12(8):2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fidecicchi T, Fruzzetti F, Lete Lasa LI, Calaf J. COVID‐19, gender and estroprogestins, what do we know? Eur J Contracept Reprod Health Care. 2022;27(1):67‐74. [DOI] [PubMed] [Google Scholar]
- 89. Greer IA. Thrombosis in pregnancy: maternal and fetal issues. Lancet (London, England). 1999;353(9160):1258‐1265. [DOI] [PubMed] [Google Scholar]
- 90. James AH. Venous thromboembolism in pregnancy. Arterioscler Thromb Vasc Biol. 2009;29(3):326‐331. [DOI] [PubMed] [Google Scholar]
- 91. Martineau M, Nelson‐Piercy C. Venous thromboembolic disease and pregnancy. Postgrad Med J. 2009;85(1007):489‐494. [DOI] [PubMed] [Google Scholar]
- 92. Venous thromboembolism and hormonal contraception, Green‐Top Guideline [Press Release] 2010.
- 93. Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352‐2361. [DOI] [PubMed] [Google Scholar]
- 94. Martinelli I, Lensing AW, Middeldorp S, et al. Recurrent venous thromboembolism and abnormal uterine bleeding with anticoagulant and hormone therapy use. Blood. 2016;127(11):1417‐1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Klok FA, Schreiber K, Stach K, et al. Oral contraception and menstrual bleeding during treatment of venous thromboembolism: expert opinion versus current practice: combined results of a systematic review, expert panel opinion and an international survey. Thromb Res. 2017;153:101‐107. [DOI] [PubMed] [Google Scholar]
- 96. Zingone MM, Guirguis AB, Airee A, Cobb D. Probable drug interaction between warfarin and hormonal contraceptives. Ann Pharmacother. 2009;43(12):2096‐2102. [DOI] [PubMed] [Google Scholar]
- 97. Hoibraaten E, Qvigstad E, Arnesen H, Larsen S, Wickstrom E, Sandset PM. Increased risk of recurrent venous thromboembolism during hormone replacement therapy‐‐results of the randomized, double‐blind, placebo‐controlled estrogen in venous thromboembolism trial (EVTET). Thromb Haemost. 2000;84(6):961‐967. [PubMed] [Google Scholar]
- 98. Castanheira L, Fresco P, Macedo AF. Guidelines for the management of chronic medication in the perioperative period: systematic review and formal consensus. J Clin Pharm Ther. 2011;36(4):446‐467. [DOI] [PubMed] [Google Scholar]
- 99. Kozato A, Fox GWC, Yong PC, et al. No venous thromboembolism increase among transgender female patients remaining on estrogen for gender‐affirming surgery. J Clin Endocrinol Metab. 2021;106(4):e1586‐e1590. [DOI] [PubMed] [Google Scholar]


