Abstract
Background
HER2-positive breast cancers are rare amongst BRCA mutation carriers. No data exist regarding clinicopathological characteristics and prognosis of this subgroup of patients.
Materials and methods
Using a retrospective matched cohort design, we collected data from 700 women who were diagnosed with operable invasive breast cancer from January 2006 to December 2016 and were screened for germline BRCA mutations. Clinicopathological features and survival rates were analyzed by BRCA and HER2 status.
Results
One hundred and fifteen HER2-positive/BRCA mutated cases were evaluated in comparison to the three control groups: HER2-positive/BRCA wild type (n = 129), HER2-negative/BRCA mutated (n = 222), HER2-negative/BRCA wild type (n = 234). HER2-positive breast cancers were more likely to have high histologic grade and high proliferation rate than HER2-negative neoplasms, regardless of BRCA mutation status. An interaction between BRCA mutations and HER2-positive status was found to correlate with worse survival after adjusting for prognostic variables (HR = 3.4; 95% CI: 1.3–16.7).
Conclusions
Co-occurrence of BRCA mutations and HER2-positive status is a poor prognostic factor in patients with early or locally advanced breast cancer. This finding may be a proof of concept that a combined pharmacological intervention directed to these targets could be synergistic.
Keywords: BRCA, HER2, Prognosis, Germline mutation, Breast cancer
Highlights
-
•
Co-occurrence of BRCA mutations and HER2-positive status is a poor prognostic factor in patients with breast cancer.
-
•
Consider an actionable role for BRCA mutations in HER2-positive disease.
-
•
Combinations of PARPi plus anti-HER2 therapies are warranted in this setting.
1. Introduction
HER2 (Her-2/neu, c-erbB-2) is a 185-kDa transmembrane tyrosine kinase protein giving higher aggressiveness in breast cancers. In humans, HER2 protein overexpression/gene amplification occurs in 15–20% of primary breast tumors and is associated with diminished disease-free and overall survival [1]. Anti-HER2 therapies are effective for all stages of HER2-positive breast cancer [[1], [2], [3], [4]]. However, intrinsic or acquired resistance to these drugs may occur in a significant number of patients and, except for HER2 status, no validated predictive factors of response/resistance have been identified to date [4,5].
Hereditary Breast and Ovarian Cancer (HBOC) syndrome accounts for about 5–10% of all breast cancers and has been associated, in about 50% of cases, with germline mutations in BRCA1 and BRCA2 genes [6,7]. Those genes are important tumor suppressor genes implicated in the homologous recombination DNA repair mechanism [8,9]. Women who inherit the mutated form of one of the BRCA genes have a lifetime risk to develop breast and ovarian cancer of 55–60% and 16–59%, respectively [10,11]. Poly (ADP-ribose) polymerase (PARP) inhibitors have been shown to be effective in patients with HER2-negative, BRCA-mutated metastatic breast cancer [12,13].
Previous reports have found a low frequency (ranging from 2.1% to 10%) of HER2-positive status in the breast cancers of BRCA1 mutation carriers, and a slightly higher rate (ranging from 6.8% to 13%) in those with mutations in BRCA2 [14]. So far, no data have been reported on the clinical outcome of HER2-positive, BRCA-mutated breast cancers.
Based on these considerations, the aim of the present, multicenter, observational study was to evaluate clinicopathological characteristics and prognosis of a population of women with breast cancer with known HER2 and BRCA status.
2. Material and methods
2.1. Study design and subject selection
We evaluated women who were diagnosed with breast cancer from January 2006 to December 2016 and received oncology care at three different University Hospitals in Italy (Parma, Modena, and Ancona) and one Cancer Center in France (Institute Gustave-Roussy, Villejuif). Only patients with operable, stage I to IIIA invasive breast cancers with known HER2 status, and who were screened for germline BRCA mutations, were accounted for. BRCA testing was offered to breast cancer patients at the study centers according to the following common criteria [6,15], which did not change over the observation period of the study: i) strong family history of breast, ovarian, pancreatic and/or high grade/metastatic prostate cancer; ii) diagnosis of breast cancer before the age of 45; iii) diagnosis of triple negative breast cancer before the age of 60; iv) personal history of ovarian cancer or second breast cancer or male sex. The starting time of data collection (January 1, 2006) was chosen to obtain an adequate number of patients who were homogeneous across the four study centers by the main indications for BRCA testing [6,15], by accuracy and reproducibility of the HER2 testing [[16], [17], [18]], and by indication of anti-HER2-based treatment in the early setting [19]. No significant changes of anti-HER2 therapies were observed over the study period except for the European Medicines Agency (EMA) indication for use of neoadjuvant pertuzumab in combination with trastuzumab and chemotherapy in July 2015 [2]. None of the study patients were treated with PARP inhibitors (PARPi). Using a retrospective matched
Cohort design, patients with HER2-positive/BRCA mutated breast cancers were matched by age ( ± 5 years) and year of diagnosis ( ± 2 years) to each of the following groups: i) HER2-positive/BRCA wild type breast cancers; ii) HER2-negative/BRCA mutated breast cancers; iii) HER2-negative/BRCA wild type breast cancers.
The study was conducted based on the ethical standards prescribed by the Helsinki Declaration of the World Medical Association [20] and with the approval of local independent ethics committee (IEC). Clinical information was collected, and patients were de-identified by removing all identifiers that could be linked back to the patient.
2.2. Clinical and pathological data
Diagnosis date, pathological stage, nodal involvement, histological grade, hormone receptor status, proliferation rate, BRCA/HER2 status, neo/adjuvant treatment performed, disease relapse and vital status of the selected cases were obtained from patient clinical records and collected into a pseudo-anonymous electronic registration template.
According to the American Society of Clinical Oncology-College of American Pathologists (ASCO-CAP) guidelines [[16], [17], [18]], patients were considered to have HER2-positive disease if the primary lesion showed immunohistochemistry (IHC) staining of 3+ (uniform, intense membrane staining of >30% of invasive tumor cells) or had gene amplification (ratio of HER2 to chromosome 17 copy number greater than 2.2) by fluorescence in situ hybridization (FISH). Patients were considered to have HER2-negative disease if they either had negative expression by IHC (0, 1+) or did not have gene amplification by FISH.
Classification of BRCA1/2 variants was ascertained using the ClinVar variation report and interpretation [21]. Study patients were categorized by BRCA status as follows: i) BRCA mutated: harboring ‘pathogenic’ or ‘likely pathogenic’ BRCA1/2 variants in germline DNA; ii) BRCA wild type: harboring wild-type BRCA1/2 and benign variants. Patients with BRCA1/2 genetic variants of ‘unknown significance’ (insufficient data to be considered pathogenic mutations) were excluded from study analysis.
2.3. Statistical analysis
Differences between study cases and controls were assessed by the chi-square test (or Fisher exact test if needed) for categorical variables [22]. By setting the HER2-positive/BRCA mutated case group as the reference category, separate unadjusted odds ratios (ORs) and their 95% confidence intervals (CIs) were provided for categorical outcomes of each control group compared to the reference group. The prognostic significance of HER2 and BRCA status was evaluated on invasive disease-free survival (iDFS) and overall survival (OS). iDFS was defined as the date of diagnosis of primary breast cancer to the date of ipsilateral local or regional, contralateral, distant invasive recurrence, second non-breast primary cancer, death of any cause, or date of last medical record entry, whichever came first (all in situ cancer events were excluded) [23]. OS was defined as the time between the date of diagnosis and the date of death from any cause or the last date the patient was known to be alive. Survival distributions will be estimated by the Kaplan–Meier method and compared using the log-rank test [24,25]. Differences were considered significant if the log-rank P-value was <0.05. Cox regression analysis was used to identify independent prognostic variables influencing survival. An interaction term between BRCA mutations and HER2-positive status was included in the Cox regression to test for a significant interaction. Variables were included in the multivariate model using forward selection if P-value <0.1 [26]. The hazard ratio (HR) and 95% CI were also estimated.
3. Results
3.1. Clinicopathological characteristics of the study population
One hundred and fifteen HER2-positive/BRCA mutated cases were evaluated in comparison to the three control groups: HER2-positive/BRCA wild type (n = 129), HER2-negative/BRCA-mutated (n = 222), HER2-negative/BRCA wild type (n = 234). Table 1 shows the comparison of selected clinicopathological characteristics between cases and controls. Tumor stage distribution did not differ between the groups. Patients with HER2-positive breast cancers were more likely to have tumors with high histologic grade and high proliferation rate than patients with HER2-negative neoplasms, regardless of BRCA mutation status. Of interest, HER2-positive/BRCA mutated cases were more frequently BRCA2-positive, while HER2-negative/BRCA mutated controls carried more commonly a BRCA1 mutation. This difference almost reached statistical significance (P = 0.06; Table 2). Eighty-seven percent of HER2-positive/BRCA mutated cases and 81% of HER2-positive/BRCA wild type controls were treated with trastuzumab-based neoadjuvant and/or adjuvant therapy, while 39% of HER2-negative/BRCA mutated and 41% of HER2-negative/BRCA wild type subjects received either neo- or adjuvant chemotherapy (Table 1). Neoadjuvant pertuzumab in combination with trastuzumab and chemotherapy was administered in 12 (10%) out of the 115 HER2-positive/BRCA mutated cases and in 12 (9%) of the 129 HER2-positive/BRCA wild type controls. No imbalances in the rates of patients treated with adjuvant endocrine therapy were observed between cases and controls (Table 1). None of the HER2-positive/BRCA mutated cases, none of the HER2-positive/BRCA wild type, 20 (9%) out of the 222 HER2-negative/BRCA mutated, and 10 (4%) out of the 234 HER2-negative/BRCA wild type controls received platinum salts in the neo-/adjuvant setting. Only 199 (28%) out of the 700 study patients received genetic test results before surgery. Contralateral prophylactic mastectomy was performed in 10 (9%) out of the 115 HER2-positive/BRCA mutated cases and in 22 (10%) out of the 222 HER2-negative/BRCA mutated controls. Prophylactic salphingo-oophorectomy was performed in 29 (25%) and 55 (25%) HER2-positive/BRCA mutated cases and HER2-negative/BRCA mutated controls, respectively.
Table 1.
Clinicopathological characteristics of study groups.
| Characteristic | HER2-positive/ BRCA mutated (total, n = 115) n (%) |
HER2-positive/ BRCA wild type (total, n = 129) n (%) |
HER2-negative/ BRCA mutated (total, n = 222) n (%) |
HER2-negative/ BRCA wild type (total, n = 234) n (%) |
OR∗ (95% CI) |
P |
|---|---|---|---|---|---|---|
| Age | 1.4 (0.8-2.3); | 0.21 | ||||
| 18-49 | 68 (59) | 66 (51) | 115 (52) | 131 (56) | 1.3 (0.8-2.1); | 0.20 |
| ≥ 50 | 47 (41) | 63 (49) | 107 (48) | 103 (44) | 1.1 (0.7-1.8) | 0.57 |
| Ax lymph nodesa | 1.5 (0.9-2.5); | 0.14 | ||||
| Negative | 47 (47) | 69 (57) | 90 (50) | 110 (52) | 1.1 (0.7-1.8); | 0.71 |
| Positive | 52 (53) | 51 (43) | 91 (50) | 100 (48) | 1.2 (0.7-1.9) | 0.42 |
| Stage at diagnosisa | 0.6 (0.4-1.1); | 0.10 | ||||
| I-II | 62 (63) | 62 (52) | 122 (67) | 113 (54) | 1.2 (0.7-2.1); | 0.42 |
| IIIA | 37 (37) | 58 (48) | 59 (33) | 97 (46) | 0.7 (0.4-1.1) | 0.14 |
| Histologic gradea | 1.4 (0.8-2.5); 2.6 (1.6-4.2); 2.5 (1.5-4.1) |
0.26 | ||||
| 1-2 | 33 (29) | 45 (36) | 111 (52) | 117 (51) | 0.0001 | |
| 3 | 80 (71) | 80 (64) | 104 (48) | 113 (49) | 0.0002 | |
| Ki-67 indexa | 1.6 (0.9-2.7); | 0.11 | ||||
| Low | 30 (27) | 46 (37) | 113 (53) | 119 (52) | 3.0 (1.8-4.5); | <0.0001 |
| High | 80 (73) | 78 (63) | 100 (47) | 109 (48) | 2.9 (1.8-4.8) | <0.0001 |
| Hormone receptora | 1.0 (0.6-1.7); | 0.96 | ||||
| Positive | 46 (40) | 52 (41) | 111 (50) | 119 (51) | 1.5 (0.9-2.3); | 0.09 |
| Negative | 68 (60) | 76 (59) | 111 (50) | 114 (49) | 1.5 (0.9-2.4) | 0.06 |
| Type of surgerya | 0.8 (0.5-1.4); | 0.51 | ||||
| Quadrantectomy | 63 (58) | 68 (54) | 121 (55) | 104 (45) | 0.9 (0.6-1.4); | 0.63 |
| Mastectomy | 46 (42) | 59 (46) | 99 (45) | 128 (55) | 0.6 (0.4-0.9) | 0.02 |
| NACT/ACTa | 1.6 (0.8-3.2); | 0.21 | ||||
| Performed | 95 (87)b,c | 103 (81)b,c | 86 (39)c | 95 (41)c | 10.6 (5.7-19.7); | <0.0001 |
| Not performed | 14 (13) | 24 (19) | 134 (61) | 137 (59) | 9.8 (5.3-18.2) | <0.0001 |
| AETa | 1.1 (0.5-1.6); | 0.94 | ||||
| Performed | 44 (40) | 52 (41) | 109 (49) | 119 (51) | 1.4 (0.8-2.4); | 0.10 |
| Not performed | 65 (60) | 75 (59) | 111 (51) | 113 (49) | 1.5 (0.8-2.3) | 0.07 |
| iDFS eventsd ─ n | 14 | 18 | 23 | 15 |
5-year iDFS rates (95% CI)e 88% (80-95%); 86% (78-93%); 89% (84-94%); 93% (90-97%) |
|
| Death eventsd ─ n | 7 | 4 | 12 | 3 |
5-year OS rates (95% CI)e 93% (88-99%); 97% (93-100%); 94% (90-97%); 99% (97-100%) |
|
Abbreviations: n, number; OR, odds ratio; %, percentage; CI, confidence interval; Ax, axillary; NACT, neoadjuvant chemotherapy; ACT adjuvant chemotherapy; AET, adjuvant endocrine therapy; iDFS, invasive disease-free survival; OS, overall survival.
Unadjusted ORs and their 95% CIs were provided for categorical variables (positive [bad] outcomes: early age at onset, positive axillary lymph node status, stage III at diagnosis, histologic grade 3, high ki-67 index, negative hormone receptor status, mastectomy surgery, neo/adjuvant chemotherapy and endocrine therapy) of the reference group (HER2-positive/BRCA mutated cases) compared to each of the three control groups (HER2-positive/BRCA wild type; HER2-negative/BRCA mutated; HER2-negative/BRCA wild type).
Numbers in these categories do not sum to the total because of missing data.
All HER2-positive cases treated with neoadjuvant and/or adjuvant chemotherapy also received trastuzumab.
None of the HER2-positive/BRCA mutated cases, none of the HER2-positive/BRCA wild type, 20 of the HER2-negative/BRCA mutated, and 10 out of the HER2-negative/BRCA wild type controls received platinum salts in the neo-/adjuvant setting.
Median follow-up of 83.7 months.
iDFS and OS rates of the reference group (HER2-positive/BRCA mutated cases) and of the three control groups (HER2-positive/BRCA wild type; HER2-negative/BRCA mutated; HER2-negative/BRCA wild type). Three events of second non-breast primary cancer (ovarian cancer) were observed in the HER2-negative/BRCA mutated controls.
Table 2.
BRCA1 and BRCA2 gene mutations by HER2 status.
| HER2-positive/BRCA mutated (total, n = 115) n (%) | HER2-negative/BRCA mutated (total, n = 222) n (%) | P | |
|---|---|---|---|
| BRCA1 mutation | 52 (45) | 124 (56) | 0.06 |
| BRCA2 mutation | 63 (55) | 98 (44) |
Abbreviations: n, number; %, percentage.
3.2. Survival analysis between cases and controls
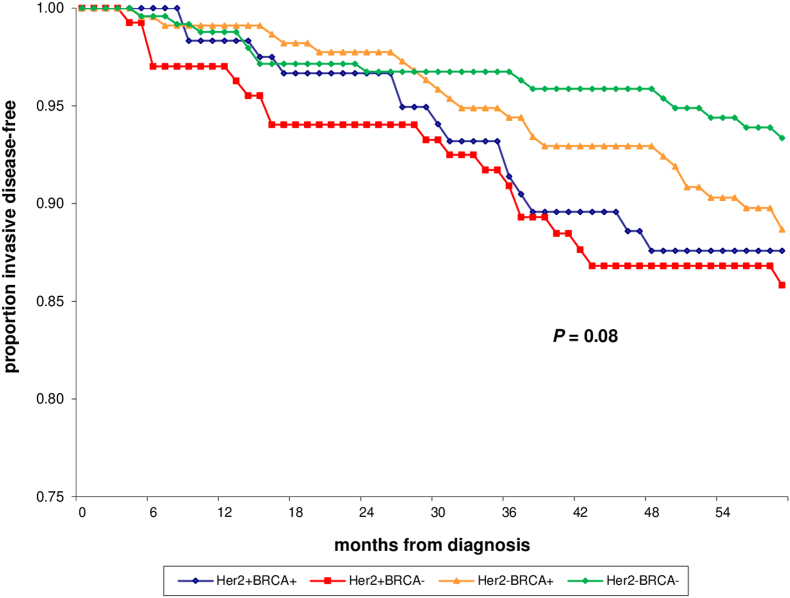
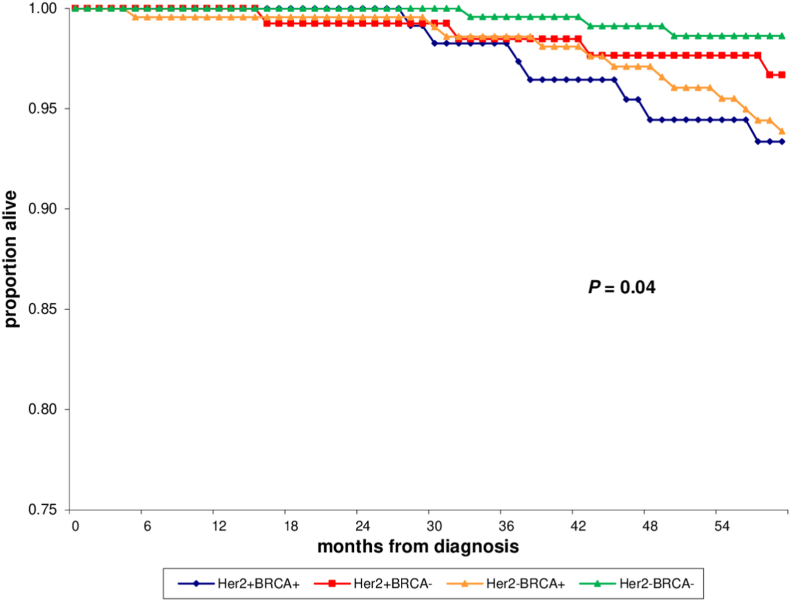
With a median follow-up of 83.7 months, the 5-year iDFS rate was 88% (95% CI, 80–95%) in HER2-positive/BRCA mutated cases and 86% (95% CI, 78–93%), 89% (95% CI, 84–94%) and 93% (95% CI, 90–97%) in HER2-positive/BRCA wild type, HER2-negative/BRCA mutated, and HER2-negative/BRCA wild type controls, respectively (P =0.08; Table 1, Fig. 1). Only 3 events of second non-breast primary cancer (ovarian cancer) were observed in HER2-negative/BRCA mutated controls. Most significantly, the OS rate at 5 years was 93% (95% CI, 88–99%) in HER2-positive/BRCA mutated cases and 97% (95% CI, 93–100%), 94% (95% CI, 90–97%) and 99% (95% CI, 97–100%) in HER2-positive/BRCA wild type, HER2-negative/BRCA mutated, and HER2-negative/BRCA wild type controls, respectively (P =0.04; Table 1, Fig. 2).
Fig. 1.
Invasive disease-free survival (iDFS) of HER2-positive/BRCA mutated (HER2+BRCA+) cases vs. HER2-positive/BRCA wild type (HER2+BRCA-), HER2-negative/BRCA mutated (HER2-BRCA+), and HER2-negative/BRCA wild type (HER2-BRCA-) controls.
Fig. 2.
Overall survival (OS) of HER2-positive/BRCA mutated (HER2+BRCA+) cases vs. HER2-positive/BRCA wild type (HER2+BRCA-), HER2-negative/BRCA mutated (HER2-BRCA+), and HER2-negative/BRCA wild type (HER2-BRCA-) controls.
An interaction between BRCA mutations and HER2-positive status was found to correlate with worse survival after adjusting for prognostic variables (HR = 3.4; 95% CI, 1.3–16.7).
Considering the crosstalk between estrogen receptor (ER) and HER2 pathways and the emerging data demonstrating considerable heterogeneity in breast cancers that express both hormone receptors and HER2 [[2], [3], [4],14], an exploratory survival analysis was performed in HER2-positive patients by ER status. The 5-year iDFS rate was 90% (95% CI, 85–95%) in HER2-positive/ER-positive subjects and 83% (95% CI, 75–90%) in HER2-positive/ER-negative ones (P = 0.09). The OS rate at 5 years was 97% (95% CI, 94%–99%) in HER2-positive/ER-positive subjects and 92% (95% CI, 86–98%) in HER2-positive/ER-negative patients (P = 0.13).
4. Discussion
This study provides evidence for the first time that co-occurrence of BRCA mutations and HER2-positive status is a poor prognostic factor in patients with early or locally advanced breast cancer.
The breast tumor phenotype differs according to the germline BRCA1 or BRCA2 mutation status. BRCA1 mutation carriers mainly develop triple negative breast cancers, whereas BRCA2 carriers are more likely to develop hormone receptor-positive tumors [15,27,28]. Although the prevalence of HER2-positive breast cancers is generally considered low amongst BRCA1 and BRCA2 mutation carriers, the retrospective case series reported so far show wide variations in HER2 positivity rates, ranging from 2.1% to 10% in BRCA1 mutation carriers, and from 6.8% to 13%, in those with mutations in BRCA2 [14,29]. Our study aimed to address lack of data on clinical characteristics and outcomes of HER2-positive/BRCA mutated breast cancers. Using a retrospective matched cohort design, stage I to IIIA HER2-positive/BRCA mutated cases were evaluated in comparison to the three control groups: HER2-positive/BRCA wild type, HER2-negative/BRCA mutated, and HER2-negative/BRCA wild type ones. According to previous reports [14,[27], [28], [29]], HER2-positive/BRCA mutated cases were more frequently BRCA2-positive, while HER2-negative/BRCA mutated controls carried more commonly a BRCA1 mutation. Patients with HER2-positive breast cancers were more likely to have tumors with high histologic grade and high proliferation rate than patients with HER2-negative neoplasms, regardless of BRCA mutation status. Most patients with HER2-positive disease were treated with trastuzumab-based neoadjuvant and/or adjuvant treatment, while less than half of subjects with HER2-negative breast cancer received either neo- or adjuvant chemotherapy. Notably, we observed that HER2-positive/BRCA mutated cases had a poorer 5-year OS rate than controls.
Considering the known association between BRCA1/2 gene mutations and platinum sensitivity in HER2-negative breast cancer [30,31], we looked at the percentage of study patients who were treated with platinum salts: no patient with HER2-positive disease, either BRCA mutated or wild-type, was treated with platinum salts in the early setting, while neo-/adjuvant carboplatin was administered in 9% of HER2-negative/BRCA mutated controls.
Timing of BRCA testing relative to surgical treatment was highly variable; only 29% of study patients received genetic test results before surgery. This finding may in part explain the low rates of contralateral prophylactic mastectomy (9% and 10%) observed in HER2-positive/BRCA mutated cases and in HER2-negative/BRCA mutated controls [32].
Several limitations of the present study lie in its retrospective nature. BRCA-screened women were assessed as the source population from which study subjects were drawn. Therefore, only subjects with specific eligibility criteria for BRCA1/2 testing were accounted for, which may have resulted in sample selection bias. According to these considerations, HER2-negative controls were enriched for triple negative breast cancers than hormone receptor-positive tumors (Table 1).
HER2-positive breast cancer occurring in a germline BRCA1/2 mutated context is such an unusual phenotype/genotype association that arises the question of whether HER2-positive tumor is really driven by the underlying constitutive BRCA mutation or whether it should be considered a cancer phenocopy [29]. In a patient with constitutive BRCA1 mutation, the occurrence of HER2-positive breast cancer with loss of the mutated copy of BRCA1 and no inactivation of the wild type allele was observed [33]. This has implications for treatment with targeted therapy such PARPi since these would be inactive against ‘sporadic’ breast tumors that are not driven by BRCA mutations [[34], [35], [36]]. Nevertheless, our study findings indicating that co-occurrence of BRCA mutations and HER2-positive status is a poor prognostic factor in patients with early or locally advanced breast cancer, suggests an actionable role for BRCA mutations in HER2-positive disease. In preclinical models, inactivating BRCA2 mutations correlated with response to the HER2 tyrosine kinase inhibitors tucatinib and neratinib [37]. Furthermore, the addition of olaparib (PARPi) enhanced the effect of neratinib in breast cancer cell lines and niraparib (PARPi) enhanced neratinib effectiveness in ovarian cancers [37].
In conclusion, our study provides evidence for the first time that co-occurrence of BRCA mutations and HER2-positive status is associated with worse OS in patients with early or locally advanced breast cancer. This finding may be a proof of concept that a combined pharmacological intervention directed to these targets could be synergistic. Clinical trials evaluating novel combinations of PARPi plus anti-HER2 therapies ( ± platinum salts) are warranted in this setting.
Funding
B. P. was supported by ESMO with a Clinical Translational Fellowship aid supported by Roche. Any views, opinions, findings, conclusions, or recommendations expressed in this material are those solely of the authors and do not necessarily reflect those of ESMO or Roche.
Declaration of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: A.V. received research grants from Pfizer, honoraria from Seagen, AstraZeneca, Daiichi Sankyo, and travel expenses from EISAI. B.P. received honoraria from Novartis and BMS. M. P. received honoraria from Pfizer, Istituto Gentili, Lilly, Novartis, Roche, pierreFabre, Gilead. A.M. received research grants from Roche and honoraria from Lilly, Novartis, EISAI, Seagen, Daiichi-Sankyo.
Acknowledgements
None.
References
- 1.Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Musolino A., Boggiani D., Pellegrino B., Zanoni D., Sikokis A., Missale G., Silini E.M., Maglietta G., Frassoldati A., Michiara M. Role of innate and adaptive immunity in the efficacy of anti-HER2 monoclonal antibodies for HER2-positive breast cancer. Crit Rev Oncol Hematol. 2020;149 doi: 10.1016/j.critrevonc.2020.102927. [DOI] [PubMed] [Google Scholar]
- 3.Cameron D., Piccart-Gebhart M.J., Gelber R.D., Procter M., Goldhirsch A., de Azambuja E., Castro G., Jr., Untch M., Smith I., Gianni L., Baselga J., Al-Sakaff N., Lauer S., McFadden E., Leyland-Jones B., Bell R., Dowsett M., Jackisch C. Herceptin Adjuvant (HERA) Trial Study Team. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative group (EBCTCG) Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021;22(8):1139–1150. doi: 10.1016/S1470-2045(21)00288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musolino A., Gradishar W.J., Rugo H.S., Nordstrom J.L., Rock E.P., Arnaldez F., Pegram M.D. Role of Fcγ receptors in HER2-targeted breast cancer therapy. J Immunother Cancer. 2022;10(1) doi: 10.1136/jitc-2021-003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malone K.E., Daling J.R., Doody D.R., Hsu L., Bernstein L., Coates R.J., Marchbanks P.A., Simon M.S., McDonald J.A., Norman S.A., et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res. 2006;66:8297–8308. doi: 10.1158/0008-5472.CAN-06-0503. [DOI] [PubMed] [Google Scholar]
- 7.Rennert G., Bisland-Naggan S., Barnett-Griness O., Bar-Joseph N., Zhang S., Rennert H.S., Narod S.A. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357(2):115–123. doi: 10.1056/NEJMoa070608. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor M.J. Targeting the DNA damage response in cancer. Mol Cell. 2015;60:547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrino B., Tommasi C., Solinas C., Campanini N., Silini E.M., Musolino A. The future potential of genome-wide mutational profiles in HRD detection in breast cancer. Expert Rev Mol Diagn. 2021;Dec;20:1–3. doi: 10.1080/14737159.2022.2015328. [DOI] [PubMed] [Google Scholar]
- 10.Antoniou A., Pharoah P.D.P., Narod S., Risch H.A., Eyfjord J.E., Hopper J.L., Loman N., Olsson H., Johannsson O., Borg Å., et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S., Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robson M., Im S.A., Senkus E., Xu B., Domchek S.M., Masuda N., Delaloge S., Li W., Tung N., Armstrong A., Wu W., Goessl C., Runswick S., Conte P. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 13.Litton J.K., Rugo H.S., Ettl J., Hurvitz S.A., Gonçalves A., Lee K.H., Fehrenbacher L., Yerushalmi R., Mina L.A., Martin M., Roché H., Im Y.H., Quek R.G.W., Markova D., Tudor I.C., Hannah A.L., Eiermann W., Blum J.L. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans D.G., Lalloo F., Howell S., Verhoef S., Woodward E.R., Howell A. Low prevalence of HER2 positivity amongst BRCA1 and BRCA2 mutation carriers and in primary BRCA screens. Breast Cancer Res Treat. 2016;155(3):597–601. doi: 10.1007/s10549-016-3697-z. Epub 2016 Feb 18. [DOI] [PubMed] [Google Scholar]
- 15.Musolino A., Bella M.A., Bortesi B., Michiara M., Naldi N., Zanelli P., Capelletti M., Pezzuolo D., Camisa R., Savi M., Neri T.M., Ardizzoni A. BRCA mutations, molecular markers, and clinical variables in early-onset breast cancer: a population-based study. Breast. 2007;16(3):280–292. doi: 10.1016/j.breast.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Wolff A.C., Hammond M.E., Schwartz J.N., Hagerty K.L., Allred D.C., Cote R.J., Dowsett M., Fitzgibbons P.L., Hanna W.M., Langer A., McShane L.M., Paik S., Pegram M.D., Perez E.A., Press M.F., Rhodes A., Sturgeon C., Taube S.E., Tubbs R., Vance G.H., van de Vijver M., Wheeler T.M., Hayes D.F. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 17.Wolff A.C., Hammond M.E., Hicks D.G., Dowsett M., McShane L.M., Allison K.H., et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.5858/arpa.2013-0953-SA. [DOI] [PubMed] [Google Scholar]
- 18.Wolff A.C., Hammond M.E.H., Allison K.H., Harvey B.E., Mangu P.B., Bartlett J.M.S., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36(20):2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 19.https://www.ema.europa.eu/en/documents/product-information/herceptin-epar-product-information_en.pdf
- 20.World Medical Association World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 21.https://www.ncbi.nlm.nih.gov/clinvar/
- 22.Freeman D.H. Marcel Dekker, Inc.; New York: 1987. Applied categorical data analysis. [Google Scholar]
- 23.Hudis C.A., Barlow W.E., Costantino J.P., Gray R.J., Pritchard K.I., et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 26.Lunn M., McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]
- 27.Palacios J., Honrado E., Osorio A., Cazorla A., Sarrió D., Barroso A., Rodríguez S., Cigudosa J.C., Diez O., Alonso C., Lerma E., Sánchez L., Rivas C., Benítez J. Immunohistochemical characteristics defined by tissue microarray of hereditary breast cancer not attributable to BRCA1 or BRCA2 mutations differences from breast carcinomas arising in BRCA1 and BRCA2 mutation carriers. Clin Cancer Res. 2003;9:3606–3614. [PubMed] [Google Scholar]
- 28.Foulkes W.D., Stefansson I.M., Chappuis P.O., Bégin L.R., Goffin J.R., Wong N., Trudel M., Akslen L.A. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–1485. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 29.Grushko T.A., Blackwood M.A., Schumm P.L., Hagos F.G., Adeyanju M.O., Feldman M.D., Sanders M.O., Weber B.L., Olopade O.I. Molecular-cytogenetic analysis of HER-2/neu gene in BRCA1-associated breast cancers. Cancer Res. 2002;62:1481–1488. [PubMed] [Google Scholar]
- 30.Graeser M., McCarthy A., Lord C.J., et al. A marker of homologous recombination predicts pathologic complete response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2010;16(24):6159–6168. doi: 10.1158/1078-0432.CCR-10-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Telli M.L., Timms K.M., Reid J., et al. Homologous Recombination Deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res. 2016;22(15):3764–3773. doi: 10.1158/1078-0432.CCR-15-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong J., Lynch K., Virgo K.S., Schwartz M.D., Friedman S., Dean M., Andrews J.E., Bourquardez Clark E., Clasen J., Conaty J., Parrillo O., Sutphen R. Utilization, timing, and outcomes of BRCA genetic testing among women with newly diagnosed breast cancer from a national commercially insured population: the ABOARD Study. JCO Oncol Pract. 2021;17(2):e226–e235. doi: 10.1200/OP.20.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curtit E., Benhamo V., Gruel N., Popova T., Manie E., Cottu P., Mariani O., Stoppa-Lyonnet D., Pivot X., Stern M.H., Vincent-Salomon A. First description of a sporadic breast cancer in a woman with BRCA1 germline mutation. Oncotarget. 2015;6(34):35616–35624. doi: 10.18632/oncotarget.5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaspers J.E., Kersbergen A., Boon U., et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors. Cancer Discov. 2013;3(1):68–81. doi: 10.1158/2159-8290.CD-12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y., Bernhardy A.J., Nacson J., et al. BRCA1 intronic Alu elements drive gene rearrangements and PARP inhibitor resistance. Nat Commun. 2019;10:1–12. doi: 10.1038/s41467-019-13530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruz C., Castroviejo-Bermejo M., Gutiérrez-Enriquez S., et al. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA mutated breast cancer. Ann Oncol. 2018;29:1203–1210. doi: 10.1093/annonc/mdy099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conlon N.T., Kooijman J.J., van Gerwen S.J.C., Mulder W.R., Zaman G.J.R., Diala I., Eli L.D., Lalani A.S., Crown J., Collins D.M. Comparative analysis of drug response and gene profiling of HER2-targeted tyrosine kinase inhibitors. Br J Cancer. 2021;124(7):1249–1259. doi: 10.1038/s41416-020-01257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]