Abstract
Following the hugely successful application of deep learning methods to protein structure prediction, an increasing number of design methods seek to leverage generative models to design proteins with improved functionality over native proteins or novel structure and function. The inherent flexibility of proteins, from side-chain motion to larger conformational reshuffling, poses a challenge to design methods, where the ideal approach must consider both the spatial and temporal evolution of proteins in the context of their functional capacity. In this review, we highlight existing methods for protein design before discussing how methods at the forefront of deep learning-based design accommodate flexibility and where the field could evolve in the future.
Keywords: deep learning, protein design, generative models, protein flexibility, protein switches
Introduction
By interacting with substrates, performing precise chemical reactions, and transducing signals, proteins directly govern a wide range of regulatory functions in living cells. Consequently, in silico protein design, where a protein is either re-engineered from a native template or de novo designed, offers a direct route to addressing a wide range of complex bioengineering issues (Mahendran et al., 2020; Sterner and Sterner, 2021) without expensive and time-consuming experimental screening. De novo design can, in principle, facilitate the programming of any desired function, making it highly versatile over re-engineering, which is more restricted by the native protein fold. However, pure de novo protein design is often more challenging than re-engineering. It requires careful consideration of the optimal binding site that confers the desired function, and the active fold state of the designed protein must be both thermodynamically stable and kinetically accessible along a folding pathway. The inherent flexibility of proteins further exacerbates this complexity from a local side-chain to global scale, where multiple conformational states can be crucial for function. Switching between states can be triggered by external stimuli such as ligand binding. Thus, the design of any function that requires some internal motion such as molecular transport, allosteric regulation, and mechanotransduction, must carefully consider the coupling between the stimuli and switching of a protein’s occupied fold state and subsequent functional capacity.
Over the last 3 years, there has been a shift in the paradigm in the biophysical study of proteins, with the application of deep learning (DL) methods for structure prediction far outperforming traditional physics-based methods (Pakhrin et al., 2021). Broadly, DL is used to process unstructured data to learn underlying descriptors of that data (features) that can then be exploited for either generative or classification purposes. Some data, such as discrete variables, can be projected into a higher dimension (embedding) such that features that are more alike are closer in the embedding space, enabling more meaningful learning of relationships. By leveraging the many layers of a neural network, DL can learn complex and non-linear relationships to map the raw input into some low dimensional latent space that describes the data. The power of DL, and machine learning in general, is in backpropagation, where the error between the output of a network, such as in a classification task, is connected directly to the input of the network in an end-to-end fashion—with the weights connecting nodes between layers adjusted based on the overall gradient.
Protein structure prediction methods such as AlphaFold2 (Jumper et al., 2021) and RoseTTAfold (Baek et al., 2021), rely on a multiple sequence alignment (MSA) to learn an evolution-based history of residue contacts, working in harmony with a pairwise feature map that encodes information about residue relationships. Predicted structures represent the most likely state occupied by a protein given the distribution of structural states present in the PDB training data and input MSA. Therefore, while local flexibility is inherently accounted for within these networks, conformational switching is (usually, see later) not. This represents a significant limitation in current structure prediction methods. Thus most conformational state-based design studies continue to rely on re-engineering existing proteins known to occupy multiple states (Alberstein et al., 2022).
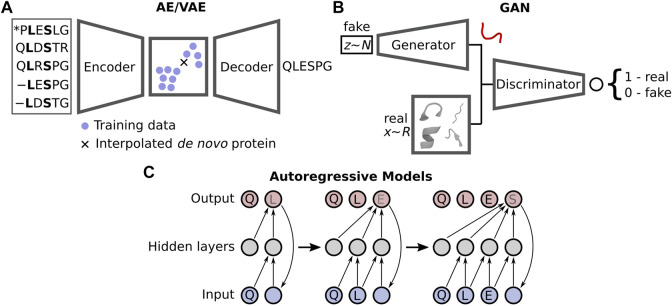
Despite the current limitations, the improvements gained by moving to DL-based prediction has motivated a similar change within the protein design community, with novel methods distancing themselves from the traditional design approaches such as Rosetta (Huang et al., 2011; Ollikainen et al., 2015; Bonet et al., 2018) and others (Röder and Wales, 2018) that rely on scoring functions describing physical energies. Numerous DL design strategies have recently emerged, broadly falling into two categories: sequence-(Wu et al., 2021) and structure-based design (Ovchinnikov and Huang, 2021). These employ what are known as generative neural networks, which create an underlying model that represents the distribution of the example training data. Interrogation of this model via interpolation in a constructed latent space yields plausible samples, i.e., non-native proteins. DL protein design chiefly uses one of three types of generative networks (Figure 1): autoencoders (AE) and closely related variational autoencoders (VAE) (Kingma and Welling, 2014), generative adversarial networks (GAN) (Goodfellow et al., 2014), and autoregressive likelihood models (Bengio et al., 2003). There are other generative networks yet to be directly applied to protein design (Bond-Taylor et al., 2021), but they have seen use in adjacent problems such as protein-protein interaction prediction (Gainza et al., 2019) and the modelling of protein dynamics (Noé et al., 2019). Both AEs and VAEs utilise an encoder to convert real features, e.g., coordinates, into a latent space representing either a transformation of the original data (AE) or a Gaussian distribution of the original data (VAE). A decoder is then used to sample this latent space, where interpolation between training samples yields plausible solutions, although this is more challenging for AEs as the latent space is non-regularised. GANs pit a generator network producing fake but realistic data against a discriminator, which attempts to decide if an input sample is real or not. The two compete, resulting in an iterative improvement of both the generator and discriminator. Autoregressive models, often used for Natural Language Processing, forecast future data samples based on historical context—such as the next amino acid in a sequence. We refer the reader to the recent review by Strokach and Kim (2022) where they discuss these models in extensive detail within the context of protein design.
FIGURE 1.
Three types of generative models are generally applied in DL-based protein design: (A) autoencoders/variational autoencoders (AE/VAEs), (B) generative adversarial networks (GANs), and (C) autoregressive models.
Sequence design (Figure 2A) relies on learning a distribution of protein family sequences to sample new sequences that offer similar or improved functionality. Structure design (Figure 2B) begins with a design objective—such as a binding site fold and aims to generate a structure that supports that objective before populating the structure with a sequence. Much like in structure prediction, dealing with flexibility in these networks remains a challenge. Herein, we will briefly overview these current methods, summarised briefly in Table 1, before discussing how innovative approaches are considering the question of protein flexibility in the design of proteins and how we could better harness MSA data in protein design. For a more detailed insight into the latest advances in sequence and structure design DL methods, we refer the reader to recent reviews by Wu et al. (2021) and Ovchinnikov and Huang (2021), respectively.
FIGURE 2.
Most DL protein design methods tackle design as either a (A) sequence generation or (B) structure generation problem, each accompanied by the general process outlined here. Examples of both methods used to assess the quality of generated samples and specific DL protein design examples are also indicated.
TABLE 1.
Summary of the key deep learning protein design methods discussed in this review, with their generation type and generative model type indicated by a *. ∼ in the structure design field suggests that some minor design coincides with sequence design. The design target of each method is also provided.
| Method | Generation type | Generative model | Design target | |||
|---|---|---|---|---|---|---|
| Sequence | Structure | VAE | GAN | Autoregressive | ||
| Grisoni et al. (2018) | * | * | Antimicrobial peptides | |||
| Müller et al. (2018) | * | * | Membranolytic anticancer peptides | |||
| PepCVAE | * | * | Antimicrobial peptides | |||
| Hawkins-Hooker et al. (2021) | * | * | Luciferase enzymes | |||
| ProteinGAN | * | * | MDH-like enzymes | |||
| Greener et al. (2018) | * | * | Metalloproteins | |||
| Gupta and Zou (2019) | * | * | Antimicrobial peptides | |||
| Amimeur et al. (2020) | * | * | Human antibodies | |||
| Ingraham et al. (2019) | * | * | Non-specific | |||
| ProteoGAN | * | * | Non-specific | |||
| ProteinSolver | * | * | Non-specific | |||
| Anand et al. (2022) | * | * | Non-specific | |||
| Ig-VAE | * | * | Immunoglobulins | |||
| Anand et al. (2019) | * | * | Non-specific | |||
| Tischer et al. (2020) | * | ∼ | Inverted structure prediction model | Non-specific | ||
| Anishchenko et al. (2021) | * | * | Inverted structure prediction model | Non-specific | ||
| Norn et al. (2021) | * | ∼ | Inverted structure prediction model | Non-specific | ||
Sequence versus structure in deep learning protein design
Sequence generation
Sequence design leverages available sequence data (Bateman et al., 2021) to learn statistical patterns that indicate function or folding stability (Wu et al., 2021). Networks are typically trained to learn the distribution of sequences in a desired protein family, from which new protein sequences can be extracted. Recurrent neural networks, a subclass architecture of autoregressive models, have been used to design antimicrobial and membranolytic anticancer peptides (Grisoni et al., 2018; Müller et al., 2018). PepCVAE constructs a latent space representing the distribution of known sequences for antimicrobial peptides, where interpolation within the space yields novel sequences (Das et al., 2018), and Hawkins-Hooker et al. (2021) recently included MSA data within VAE training to produce active luciferase enzymes. ProteinGAN has been similarly designed to produce active enzyme sequences (Repecka et al., 2021). While sequences generated through these methods have been identified as functional in silico (Alford et al., 2017; Müller et al., 2017; Sillitoe et al., 2019), they are not necessarily improvements on native proteins, and owing to the training method, any novel functionality is generally serendipitous. Attempts have been made to optimise sequences to improve functionality via biased training data in GANs (Gupta and Zou, 2019; Amimeur et al., 2020), and reinforcement learning (Angermüller et al., 2020), though these serve more as examples of functional optimisation than programming. Conditional learning, where data in pre-defined categories is used to train the network such that new samples can be generated based on those categories, is necessary to deliver fine-tuned programming. However, while sequence generative models can harness divergent sequences from the proteome to offer protein variants with novel functionality, conditional learning to control this functionality remains in its infancy. Greener et al. (2018)’s VAE was trained to produce sequences containing metal-binding sites based on the labelling of bound metal cofactors. Current efforts with a biased network training approach (Gupta and Zou, 2019; Amimeur et al., 2020) to introduce some programmability need to ensure a delicate balance between sequence diversity and the desired functional result (Linder et al., 2020). Ingraham et al. (2019) were able to design sequences using an autoregressive model conditioned on graphs of 3D structures, designing plausible sequences for protein folds outside the training data, providing an example of more targeted sequence functional design given the relationship between structure and function. Kucera et al. (2022)’s GAN offers one of the first examples of a function-specific conditional general sequence generation method. Trained on labels of the hierarchical Gene Ontology, their network was able to produce a wide variety of proteins with distinct functional properties based on the input label or labels, including mixed labels absent in the training data. Nevertheless, improving the functional specificity, e.g., activation from a specific ligand, is a considerably more difficult task given the niche training set size. All sequence-based methods require significant validation, most relying on in silico methods such as peptide classifiers (Müller et al., 2017) outside the gold standard of experimental testing. Kucera et al. introduced a novel in silico validation metric based on ensuring sequence diversity, conditional consistency with the labels, and distributional similarity to try and address the absence of reliable evaluation metrics. Arguably the most effective in silico validation method of structure prediction may prove challenging. Generated de novo sequences featuring high conformational entropy versus any native sequence may not be structurally verifiable with conventional or DL-based protein folding methods such as AlphaFold2, although new orphan-protein structure prediction DL methods are emerging that could address this (Chowdhury et al., 2021; Wang et al., 2022).
Structure generation
The workflow of structure generation typically follows four stages: 1) formulation of a design objective (e.g., a fold that confers desired binding), 2) the generation of coordinates that support the fold, 3) sequence design to stabilise any generated structure, and 4) evaluation of generated designs, typically via Molecular Dynamics or Rosetta energy checks (Ovchinnikov and Huang, 2021). By considering the design objective from the first stage, structure generation already addresses one of the key limitations of sequence generation in that the specific functional outcome is used as a constraint in design. Stage 2 can be achieved with 1-3D data. 1D data typically describes local bond lengths, angles etc., and non-local features such as interaction energies between residues (O’Connell et al., 2018; Wang et al., 2018); recurrent networks have already been applied in protein forcefield development (Greener and Jones, 2021) and could be extended to design. 2D pairwise matrices can leverage popular image classifiers or “deepfake” methods (Eguchi and Huang, 2020). Finally, the most challenging is 3D coordinate data, which is always unique to the input protein, unlike 1D or 2D basic descriptors such as contact maps, which share many common attributes (e.g., bond lengths) across the proteome, although there are examples of 3D DL structure generation (Eguchi et al., 2020). Exacerbating the complexity, unlike 1D and 2D data, 3D data is not rotationally invariant, necessitating careful treatment in design (Renaud et al., 2021). However, direct 3D design is end-to-end, i.e., the conditions and objectives are fully connected to the direct 3D output, meaning backpropagation occurs directly from a proposed structural solution to the input. In contrast, 1D and 2D data must be converted to 3D coordinate data in a separate stage outside the network. This is analogous to sequence design, where further validation is often required in silico through structure prediction. Therefore, while more challenging, direct 3D structure generation must approximately learn protein physics to produce reasonable structures, a highly generalisable property. Numerous approaches exist to tackle converting 1-2D maps to 3D (Anand and Huang, 2018), such as a decoder network featuring two discriminators able to handle GAN generated output without a ground truth and produce coordinates with the correct chirality (Anand et al., 2019). Stage 3 of structure generation commonly wield pre-existing sequence design methods to stabilise the backbone. For example, structural designs generated by the aforementioned GAN (Anand et al., 2019) and VAE have produced immunoglobulin specific backbones and SARS-CoV-2 binders (Eguchi et al., 2020) using standard Rosetta FastDesign (Bhardwaj et al., 2016) to fill the backbone. Thus, a limitation of current structure generation lies in its inability to include sequence and by extension side-chain interactions that stabilise protein structures during design. While not explicitly considering side-chain interactions, sequence generation, particularly those that leverage powerful transformer-based language models (Ferruz and Höcker, 2022), can identify potential relationships between individual amino acids that confer stability. ProteinSolver (Strokach et al., 2020) is a DL example of a backbone sequence populator, leveraging a graphical neural network that splits individual amino acids into nodes connected by edges that represent distance constraints to predict masked residue positions. Aside from an expanded training dataset, it improves on Ingraem et al. (2019)’s approach by considering both the successive and preceding residue identities during design. Trained on 72 million sequences corresponding to 80,000 unique structures, the network learnt the relationship between common structural and sequence motifs, ultimately providing de novo sequences for four stable protein folds absent in the training set. However, side-chain reconstruction was neglected within the network, which is crucial for determining thermodynamic stability. The authors instead relied on homology modelling of large, generated datasets for validation. In contrast, Anand et al. (2022) aimed to explicitly build side-chain conformers given a structural template and evaluate a full atomistic model using a conditional convolutional autoregressive neural network. Their approach iteratively samples amino acid types and rotamers at specific residue positions conditioned on the local chemical environment, producing sequences that satisfied the fold of a de novo TIM-barrel backbone (Huang et al., 2016b), indicating that their network had learnt something of the underlying physics that guides folding.
Directly comparing the two general strategies for protein design purposes, structure generation appears more versatile than sequence generation as the inclusion of functional objectives such as binding site folds enhances functional programmability (Gao et al., 2020). Indeed, the increased variety of features allows one to profit from more advanced techniques in Machine Learning. Furthermore, structure design is more generalisable, as demonstrated by Table 1, with most sequence generation methods requiring some specific protein family design target. However, structure generation methods must still undergo subsequent sequence design. This disconnect between structure and sequence is inherently problematic from a switchable state perspective, as to perform multi-state design on an ensemble of generated backbones is computationally expensive. The same disconnect is true in reverse of course, with the lack of explicit modelling in sequence generation hindering our ability to design conformational flexibility.
Accounting for flexibility in deep learning protein design
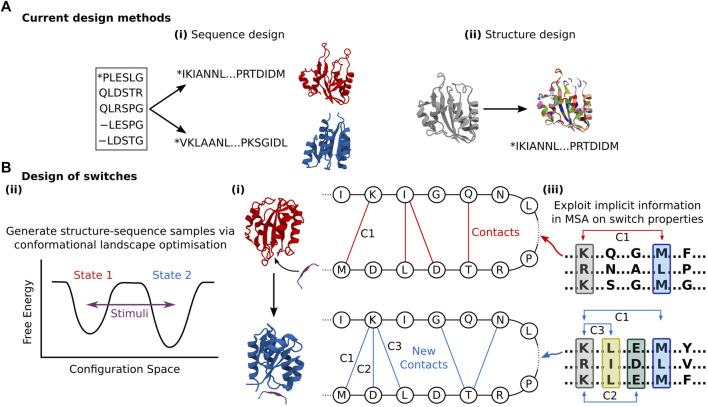
Sequence generation effectively avoids the question of flexibility entirely by relying on pure bioinformatics, and thus cannot explicitly consider the flexibility problem. Yet, sequence generation methods can yield novel structural folds representative of hybridisation between homologous sequences that represent a spectrum of structural states. For instance, ProteinGAN produced distinct structural sequence motifs, validated by CATH (Sillitoe et al., 2019), suggesting that the network can learn generalised relationships between residues and produce sequences with increased structural diversity (Repecka et al., 2021). However, these sequences still only represent a single structural fold. Ultimately, even if proteins with purpose-built conformational flexibility or switchable state can be constructed, any functionality must be verified through further in silico validation (Nivedha et al., 2018; Chen et al., 2020), meaning programming function from sequence generation is not end-to-end differentiable. Analogous to structure prediction, structure generation methods inherently account for local side-chain flexibility owing to the ensemble of rotamer positions examined per residue during construction (Defresne et al., 2021). Yet there remains a conceptual gap to more extensive conformational flexibility. The sequence-structure design problem is still treated as a one-to-one mapping (Figure 3A), when in fact conformational selection requires concurrent exploration of sequence and structure design space given that a sequence can be connected to multiple fold states. Instead, during the sequence population stage of structure generation, the goal is primarily to stabilise the identified fold, and not offer perturbed structures which deviate from those produced by the network. Some exceptions exist; Tischer et al. (2020) created binding motifs within a discontinuous scaffold through the trRosetta structure prediction network (Yang et al., 2020) to tolerate larger backbone flexibility. They applied a loss function that rewarded both recapitulation of the input motif template and global structure stability, the latter facilitating some deviation from the inserted motif to ensure fold stability, thereby providing sequence and minor structure design. Norn et al. (2021) demonstrated that they could backpropagate gradients through trRosetta to generate sequences, exploring the sequence and structure space via optimisation of the conformational energy landscape towards one smooth funnelled state, thereby considering the global fold state and ensuring thermodynamic stability. Anishchenko et al. (2021) were able to generate completely de novo structure-sequence pairs by feeding random sequences into the trRosetta network, and performing an iterative Monte Carlo simulated annealing process to substitute individual amino acids randomly. By then re-predicting the distance and orientation maps from the network and accepting the substitution based on an increased Kullback–Leibler divergence, they transformed initially homogenous residue contact maps to ones with distinct structural features. Intriguingly, the similarity of the produced “hallucinated” sequences with native ones was very low, indicating the design of true de novo proteins. However, this process tended to neglect non-idealised structures, producing well-defined α-helices and ß-sheets connected by short loops. Long loops can be critical to function, from substrate binding, catalysis, and allosteric regulation. While it is noted that the loss function could be modified to retain specific sites such as binding interfaces (Tischer et al., 2020) or catalytic sites (Wang et al., 2021), whether this can be used to stabilise motifs such as binding loops remains to be seen. All three of these approaches that facilitate some structure design leverage inverted structure prediction models, as opposed to the direct generative models discussed above. While this makes intuitive sense given the inverted relationship between protein design and structure prediction, the consequence of this is that there is less control over the designed outputs, with the network acting as a black box. In addition to function conditional inputs, applying purpose-built generative models would allow for the specific application of powerful methods from the DL community.
FIGURE 3.
(A) Current design methods either: (i) Produce new sequences corresponding to some structure with limited design objective conditioning that could be leveraged for conformational flexibility design. (ii) Produce novel folds that confer some function that must be stabilised through sequence design. Both these approaches are inherently negligent of conformational flexibility. (B) (i) The general goal of DL-based protein switch design is to connect multiple structures to one sequence, with conformational perturbation triggered by some controlled signal. I.e., Given some stimuli (e.g., palatinate peptide), the contacts of a designed sequence in one state (red) shift given some new fold (blue), providing novel functional capacity. This could be achieved through (ii) Conformational landscape optimisation of multiple states given some design objective, similar to Norn et al., (iii) Harnessing of implicit relationships between sequence and multiple structures contained within MSA data, as demonstrated by del Alamo et al. (2022). Here, co-evolving residues (denoted in the coloured blocks) in two different low-depth MSAs make distinct contacts (shown as C1, C2, etc.,) that change the overall fold state.
While the networks by Norn et al. and Anishchenko et al. were able to learn something of the intimate relationship between structure and sequence, the adaptability of these methods towards purposeful conformational flexibility or switch design, where dual or even multiple conformational states are accessible by the same sequence is more challenging. Norn et al. optimised towards one clear funnelled state in the conformational landscape, while Anishchenko et al.’s network favoured selecting secondary structure elements that delivered global stability of a singular ground state, where any reshuffling into a second state would be energetically challenging. Yet, one of Anishchenko et al.’s designs did appear to adopt multiple monomeric conformations when tested experimentally, the authors attributing this switching behaviour to the lack of explicit side-chain representations in the modelling. While this is non-ideal for a monomeric protein without stimulus, the network has returned structure-sequence topologies able to adopt multiple conformations, albeit unintentionally. Adapting this approach to deliberately consider multiple states connected by a coherent path in the conformational landscape, while ambitious, could provide the means for switch design. Another potential solution lies in the greater exploitation of the known numerous states native proteins can occupy. In principle, multiple conformational landscapes should be connected to identical or evolutionary related sequences, the difference between them being perturbation by external stimuli (Figure 3B). AlphaFold2 recently demonstrated this via quaternary structure prediction (Ghani et al., 2021; Tsaban et al., 2022), where MSAs that included bound substrates returned different and accurate structural predictions versus the unbound MSA. In a peptide docking case, no MSA was necessary for the peptide, and the network could still predict conformational changes depending on the bound peptide (Tsaban et al., 2022). del Alamo et al. (2022) recently demonstrated they were able to predict multiple conformational states of transporters and GPCRs not present in the AlphaFold2 training data by reducing the depth of input MSA to AlphaFold2, indicating that while deep MSAs tend to relate to one fixed structure, stochastically sampled shallower MSAs are associated with a diversity of structural states. These works reveal that MSAs contain crucial underlying relationships that couple sequences with numerous plausible conformations (Wang and Barth, 2015), which could be leveraged for expanded functional capacity design. A sequence generation approach that also harnesses MSA data could recognise the divergence of structural states from phylogenetic trees of extensive protein families constructed using existing modern methods (Azouri et al., 2021). Here, the goal would be to learn the general motif changes in key sites that lead to the adoption of multiple states, and exploit that in design.
Discussion
Over the last 3 years, deep learning has revolutionised protein structure prediction (Baek et al., 2021; Jumper et al., 2021). Given the mantra that protein design is effectively the reverse folding problem, it stands to reason that DL methods should also impact protein design. However, while we have witnessed the rapid growth of DL based methods, much like in protein structure, the question of how to accommodate protein flexibility, particularly their ability to adopt multiple conformational states for function, remains. The explicit inclusion of conformational flexibility and switching properties in protein design has a wide range of biomedical applications. For example, the development of synthetic light-activated ion channels for studying neurological disorders (Beck et al., 2018), the engineering of GPCR biomarkers that trigger on diagnostic ligand association (Adeniran et al., 2018), and the design of highly ligand specific molecular on-switches that mediate CAR T-cell activity (Zajc et al., 2020).
Most existing design methods pivot towards either sequence (Wu et al., 2021) or structure generation (Ovchinnikov and Huang, 2021), with significant strides having been made with both approaches. Some design methods have even pioneered the design of both structure and sequence simultaneously (Tischer et al., 2020; Yang et al., 2020; Anishchenko et al., 2021), which is necessary when designing proteins with multiple fold states. However, it is worth noting that, with few exceptions (Grisoni et al., 2018; Amimeur et al., 2020; Linder et al., 2020; Strokach et al., 2020; Anishchenko et al., 2021; Hawkins-Hooker et al., 2021; Repecka et al., 2021; Anand et al., 2022) most DL design methods lack any experimental validation, relying instead on pure in silico examination. Experimental validation is essential to truly validate a network and examine whether they are transferable to other systems.
Coupling the loss of generated structure-sequence topologies to the dynamic fold state of a protein is beyond the capabilities of current generative modelling design algorithms. Nevertheless, we have already observed successful de novo design of proteins via DL methods and the harnessing of MSA data within the structure prediction field to extract multiple conformations landscapes of a protein from a single sequence given contextual information. Given that conformational landscape optimisation is increasingly employed in design, and the generalisability of MSA-based networks have demonstrated that multiple conformational landscapes can be intimately linked to the same sequence, greater exploitation of MSA in DL-based protein design could yield de novo topologies able to adopt multiple conformations based on some stimulus. However, it has been indicated that AlphaFold2 is unable to accurately learn the underlying energy landscape that describes protein folding and function (Saldaño et al., 2021). Thus, future design methods could be assisted by DL work in orthogonal fields, which have shown their ability to predict ensembles of biophysically related states (Jin et al., 2021; Ramaswamy et al., 2021; Tian et al., 2021). Of particular interest are networks where the loss includes explicit physics-based terms (Ramaswamy et al., 2021), which could be seen as an alternative to the MSA bioinformatics approach, offering a more intimate understanding of a protein’s folding landscape during design while ensuring that kinetic pathways are accessible between states.
Over the last few decades, protein design has been based on re-engineering native proteins to alter their functionality. Yet, this restricts our programming of novel function. While there have been pure de novo successes, protein design remains a highly complex optimisation problem with the vast space of all possible sequences and structures far outside the known proteome (Huang et al., 2016a) inaccessible to traditional approaches. DL is well suited for these complex tasks, having already revolutionised the structure prediction field. AlphaFold2 and RoseTTAfold present a general solution to the protein folding problem. Protein design, considered the inverse problem, contributes an additional layer of complexity. Rather than just predicting a plausible structure from sequence, comprehensive programmability requires an appreciation of how the sequence, structure and dynamic conformational state of a protein underpin its function. DL is already expanding our design capabilities and knowledge of a dynamic proteome, while the machine learning field itself is undergoing significant and continuous innovation. Leveraging these evolving techniques while improving the exploitation of MSA data or physics-based descriptors could prove key to designing proteins with significant conformational flexibility and thus more advanced functional capacity.
Acknowledgments
The authors would like to thank Matthieu Marfoglia and Chloe A. Fuller for critically reviewing the manuscript and the Barth Lab for thought-provoking discussion.
Author contributions
LR and PB devised the scope of the review. LR performed the literature review. LR and MH designed the figures. LR and PB wrote and edited the manuscript. All authors discussed and provided critical feedback to the manuscript.
Funding
This work was supported by Swiss National Science Foundation grants (31003A_182263 and 310030_208179), Swiss Cancer Research (KFS-4687-02-2019), Novartis Foundation for medical-biological Research (21C195), funds from EPFL and the Ludwig Institute for Cancer Research to PB. Open access funding provided by École Polytechnique Fédérale de Lausanne.
Conflict of interest
PB holds patents and provisional patent applications in the field of engineered T cell therapies and protein design.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Adeniran A., Stainbrook S., Bostick J. W., Tyo K. E. J. (2018). Detection of a peptide biomarker by engineered yeast receptors. ACS Synth. Biol. 7, 696–705. 10.1021/ACSSYNBIO.7B00410/ASSET/IMAGES/SB-2017-004103_M007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberstein R. G., Guo A. B., Kortemme T. (2022). Design principles of protein switches. Curr. Opin. Struct. Biol. 72, 71–78. 10.1016/j.sbi.2021.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford R. F., Leaver-Fay A., Jeliazkov J. R., O’Meara M. J., DiMaio F. P., Park H., et al. (2017). The Rosetta all-atom energy function for macromolecular modeling and design. J. Chem. Theory Comput. 13, 3031–3048. 10.1021/acs.jctc.7b00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amimeur T., Shaver J. M., Ketchem R. R., Taylor J. A., Clark R. H., Smith J., et al. (2020). Designing feature-controlled humanoid antibody discovery libraries using generative adversarial networks. bioRxiv. 10.1101/2020.04.12.024844 [DOI] [Google Scholar]
- Anand N., Eguchi R., Huang P. S. (2019). “Fully differentiable full-atom protein backbone generation,” in Deep generative models for highly structured data, ICLR 2019 Workshop, May 6–9, 2019 (New Orleans, LA: ICLR 2019; ). [Google Scholar]
- Anand N., Eguchi R., Mathews I. I., Perez C. P., Derry A., Altman R. B., et al. (2022). Protein sequence design with a learned potential. Nat. Commun. 13, 746. 10.1038/s41467-022-28313-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand N., Huang P.-S. (2018). “Generative modeling for protein structues,” in 6th International Conference on Learning Representations, ICLR 2018 - Workshop Track Proceedings, April 30 - May 3, 2018 (Vancouver, BC, Canada: ICLR 2018; ). [Google Scholar]
- Angermüller C., Dohan D., Belanger D., Deshpande R., Murphy K., Colwell L. J. (2020). Model-based reinforcement learning for biological sequence design. Available at: https://openreview.net/forum?id=HklxbgBKvr (Accessed March 16, 2022).
- Anishchenko I., Pellock S. J., Chidyausiku T. M., Ramelot T. A., Ovchinnikov S., Hao J., et al. (2021). De novo protein design by deep network hallucination. Nature 600, 547–552. 10.1038/s41586-021-04184-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouri D., Abadi S., Mansour Y., Mayrose I., Pupko T. (2021). Harnessing machine learning to guide phylogenetic-tree search algorithms. Nat. Commun. 12, 1983–1989. 10.1038/s41467-021-22073-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek M., DiMaio F., Anishchenko I., Dauparas J., Ovchinnikov S., Lee G. R., et al. (2021). Accurate prediction of protein structures and interactions using a three-track neural network. Science 373, 871–876. 10.1126/science.abj8754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A., Martin M. J., Orchard S., Magrane M., Agivetova R., Ahmad S., et al. (2021). UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 49, D480–D489. 10.1093/nar/gkaa1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S., Yu-Strzelczyk J., Pauls D., Constantin O. M., Gee C. E., Ehmann N., et al. (2018). Synthetic light-activated ion channels for optogenetic activation and inhibition. Front. Neurosci. 12, 643. 10.3389/fnins.2018.00643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengio Y., Ducharme R., Vincent P., Jauvin C. (2003). A neural probabilistic language model. JMLR 3, 1137–1155. [Google Scholar]
- Bhardwaj G., Mulligan V. K., Bahl C. D., Gilmore J. M., Harvey P. J., Cheneval O., et al. (2016). Accurate de novo design of hyperstable constrained peptides. Nature 538, 329–335. 10.1038/nature19791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond-Taylor S., Leach A., Long Y., Willcocks C. G. (2021). Deep generative modelling: A comparative review of VAEs, GANs, normalizing flows, energy-based and autoregressive models, IEEE Trans. Pattern Anal. Mach. Intell., 1. 10.1109/TPAMI.2021.3116668 [DOI] [PubMed] [Google Scholar]
- Bonet J., Wehrle S., Schriever K., Yang C., Billet A., Sesterhenn F., et al. (2018). Rosetta FunFolDes - a general framework for the computational design of functional proteins. PLoS Comput. Biol. 14, e1006623. 10.1371/journal.pcbi.1006623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. Y. M., Keri D., Barth P. (2020). Computational design of G Protein-Coupled Receptor allosteric signal transductions. Nat. Chem. Biol. 16, 77–86. 10.1038/s41589-019-0407-2 [DOI] [PubMed] [Google Scholar]
- Chowdhury R., Bouatta N., Biswas S., Rochereau C., Church G. M., Sorger P. K., et al. (2021). Single-sequence protein structure prediction using language models from deep learning. bioRxiv. 10.1101/2021.08.02.454840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P., Wadhawan K., Chang O., Sercu T., Santos C. D., Riemer M., et al. (2018). PepCVAE: Semi-Supervised targeted design of antimicrobial peptide sequences. arXiv. 10.48550/arxiv.1810.07743 [DOI] [Google Scholar]
- Defresne M., Barbe S., Schiex T. (2021). Protein design with deep learning. Ijms 22, 11741. 10.3390/IJMS222111741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Alamo D., Sala D., Mchaourab H. S., Meiler J. (2022). Sampling alternative conformational states of transporters and receptors with AlphaFold2. Elife 11, e75751. 10.7554/ELIFE.75751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi R. R., Choe C. A., Huang P.-S. (2020). Ig-VAE: Generative modeling of protein structure by direct 3D coordinate generation. bioRxiv. 10.1101/2020.08.07.242347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi R. R., Huang P. S. (2020). Multi-scale structural analysis of proteins by deep semantic segmentation. Bioinformatics 36, 1740–1749. 10.1093/bioinformatics/btz650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferruz N., Höcker B. (2022). Towards controllable protein design with conditional transformers. arXiv. 10.48550/arxiv.2201.07338 [DOI] [Google Scholar]
- Gainza P., Sverrisson F., Monti F., Rodolà E., Boscaini D., Bronstein M. M., et al. (2019). Deciphering interaction fingerprints from protein molecular surfaces using geometric deep learning. Nat. Methods 17 (17), 184–192. 10.1038/s41592-019-0666-6 [DOI] [PubMed] [Google Scholar]
- Gao W., Mahajan S. P., Sulam J., Gray J. J. (2020). Deep learning in protein structural modeling and design. Patterns 1, 100142. 10.1016/J.PATTER.2020.100142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghani U., Desta I., Jindal A., Khan O., Jones G., Kotelnikov S., et al. (2021). Improved docking of protein models by a combination of Alphafold2 and ClusPro. bioRxiv. 10.1101/2021.09.07.459290 [DOI] [Google Scholar]
- Goodfellow I. J., Pouget-Abadie J., Mirza M., Xu B., Warde-Farley D., Ozair S., et al. (2014). Generative adversarial nets. Adv. Neural Inf. Process. Syst. 27, 2672–2680. 10.3156/jsoft.29.5_177_2 [DOI] [Google Scholar]
- Greener J. G., Jones D. T. (2021). Differentiable molecular simulation can learn all the parameters in a coarse-grained force field for proteins. PLoS One 16, e0256990. 10.1371/journal.pone.0256990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greener J. G., Moffat L., Jones D. T. (2018). Design of metalloproteins and novel protein folds using variational autoencoders. Sci. Rep. 8, 16189. 10.1038/s41598-018-34533-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisoni F., Neuhaus C. S., Gabernet G., Müller A. T., Hiss J. A., Schneider G., et al. (2018). Designing anticancer peptides by constructive machine learning. ChemMedChem 13, 1300–1302. 10.1002/cmdc.201800204 [DOI] [PubMed] [Google Scholar]
- Gupta A., Zou J. (2019). Feedback GAN for DNA optimizes protein functions. Nat. Mach. Intell. 1, 105–111. 10.1038/s42256-019-0017-4 [DOI] [Google Scholar]
- Hawkins-Hooker A., Depardieu F., Baur S., Couairon G., Chen A., Bikard D., et al. (2021). Generating functional protein variants with variational autoencoders. PLoS Comput. Biol. 17, e1008736. 10.1371/JOURNAL.PCBI.1008736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P. S., Ban Y. E. A., Richter F., Andre I., Vernon R., Schief W. R., et al. (2011). RosettaRemodel: A generalized framework for flexible backbone protein design. PLoS One 6, e24109. 10.1371/JOURNAL.PONE.0024109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P. S., Boyken S. E., Baker D. (2016a). The coming of age of de novo protein design. Nature 537, 320–327. 10.1038/nature19946 [DOI] [PubMed] [Google Scholar]
- Huang P. S., Feldmeier K., Parmeggiani F., Fernandez Velasco D. F., Höcker B., Baker D., et al. (2016b). De novo design of a four-fold symmetric TIM-barrel protein with atomic-level accuracy. Nat. Chem. Biol. 12, 29–34. 10.1038/nchembio.1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham J., Garg V. K., Barzilay R., Jaakkola T. (2019). Generative models for graph-based protein design, Adv. Neural Inf. Process. Syst. 32, 15820–15831. Available at: https://papers.nips.cc/paper/2019/hash/f3a4ff4839c56a5f460c88cce3666a2b-Abstract.html (Accessed March 16, 2022). [Google Scholar]
- Jin Y., Johannissen L. O., Hay S. (2021). Predicting new protein conformations from molecular dynamics simulation conformational landscapes and machine learning. Proteins. 89, 915–921. 10.1002/prot.26068 [DOI] [PubMed] [Google Scholar]
- Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589. 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingma D. P., Welling M. (2014). “Auto-encoding variational bayes,” in Conference proceedings: papers accepted to the International Conference on Learning Representations (ICLR) 2014, March 16, 2022 (Ithaca, NY: arXiv.org; ). Available at: https://openreview.net/forum?id=33X9fd2-9FyZd (Accessed March 16, 2022). [Google Scholar]
- Kucera T., Togninalli M., Meng-Papaxanthos L. (2022). Conditional generative modeling for de novo protein design with hierarchical functions. Bioinformatics 38, 3454–3461. btac353. 10.1093/BIOINFORMATICS/BTAC353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder J., Bogard N., Rosenberg A. B., Seelig G. (2020). A generative neural network for maximizing fitness and diversity of synthetic DNA and protein sequences. Cell. Syst. 11, 49–62. e16. 10.1016/j.cels.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahendran A. S. K., Lim Y. S., Fang C. M., Loh H. S., Le C. F. (2020). The potential of antiviral peptides as COVID-19 therapeutics. Front. Pharmacol. 11, 575444. 10.3389/fphar.2020.575444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A. T., Gabernet G., Hiss J. A., Schneider G. (2017). modlAMP: Python for antimicrobial peptides. Bioinformatics 33, 2753–2755. 10.1093/bioinformatics/btx285 [DOI] [PubMed] [Google Scholar]
- Müller A. T., Hiss J. A., Schneider G. (2018). Recurrent neural network model for constructive peptide design. J. Chem. Inf. Model. 58, 472–479. 10.1021/acs.jcim.7b00414 [DOI] [PubMed] [Google Scholar]
- Nivedha A. K., Tautermann C. S., Bhattacharya S., Lee S., Casarosa P., Kollak I., et al. (2018). Identifying functional hotspot residues for biased ligand design in G-protein-coupled receptors. Mol. Pharmacol. 93, 288–296. 10.1124/mol.117.110395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noé F., Olsson S., Köhler J., Wu H. (2019). Boltzmann generators: Sampling equilibrium states of many-body systems with deep learning. Science 365, 365. 10.1126/science.aaw1147 [DOI] [PubMed] [Google Scholar]
- Norn C., Wicky B. I. M., Juergens D., Liu S., Kim D., Tischer D., et al. (2021). Protein sequence design by conformational landscape optimization. Proc. Natl. Acad. Sci. U. S. A. 118, e2017228118. 10.1073/PNAS.2017228118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell J., Li Z., Hanson J., Heffernan R., Lyons J., Paliwal K., et al. (2018). SPIN2: Predicting sequence profiles from protein structures using deep neural networks. Proteins 86, 629–633. 10.1002/prot.25489 [DOI] [PubMed] [Google Scholar]
- Ollikainen N., de Jong R. M., Kortemme T. (2015). Coupling protein side-chain and backbone flexibility improves the Re-design of protein-ligand specificity. PLoS Comput. Biol. 11, e1004335. 10.1371/journal.pcbi.1004335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov S., Huang P. S. (2021). Structure-based protein design with deep learning. Curr. Opin. Chem. Biol. 65, 136–144. 10.1016/j.cbpa.2021.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakhrin S. C., Shrestha B., Adhikari B., Kc D. B. (2021). Deep learning-based advances in protein structure prediction. Ijms 22, 5553. 10.3390/ijms22115553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy V. K., Musson S. C., Willcocks C. G., Degiacomi M. T. (2021). Deep learning protein conformational space with convolutions and latent interpolations. Phys. Rev. X 11, 011052. 10.1103/PhysRevX.11.011052 [DOI] [Google Scholar]
- Renaud N., Geng C., Georgievska S., Ambrosetti F., Ridder L., Marzella D. F., et al. (2021). DeepRank: A deep learning framework for data mining 3D protein-protein interfaces. Nat. Commun. 12, 7068. 10.1038/s41467-021-27396-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repecka D., Jauniskis V., Karpus L., Rembeza E., Rokaitis I., Zrimec J., et al. (2021). Expanding functional protein sequence spaces using generative adversarial networks. Nat. Mach. Intell. 3, 324–333. 10.1038/s42256-021-00310-5 [DOI] [Google Scholar]
- Röder K., Wales D. J. (2018). Mutational basin-hopping: Combined structure and sequence optimization for biomolecules. J. Phys. Chem. Lett. 9, 6169–6173. 10.1021/acs.jpclett.8b02839 [DOI] [PubMed] [Google Scholar]
- Saldaño T., Escobedo N., Marchetti J., Zea D. J., Mac Donagh J. Mac, Velez Rueda A. J. V., et al. (2021). Impact of protein conformational diversity on AlphaFold predictions. bioRxiv. 10.1101/2021.10.27.466189 [DOI] [PubMed] [Google Scholar]
- Sillitoe I., Dawson N., Lewis T. E., Das S., Lees J. G., Ashford P., et al. (2019). Cath: Expanding the horizons of structure-based functional annotations for genome sequences. Nucleic Acids Res. 47, D280–D284. 10.1093/nar/gky1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner R. C., Sterner R. M. (2021). CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 11, 69. 10.1038/s41408-021-00459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strokach A., Becerra D., Corbi-Verge C., Perez-Riba A., Kim P. M. (2020). Fast and flexible protein design using deep graph neural networks. Cell. Syst. 11, 402–411. e4. 10.1016/j.cels.2020.08.016 [DOI] [PubMed] [Google Scholar]
- Strokach A., Kim P. M. (2022). Deep generative modeling for protein design. Curr. Opin. Struct. Biol. 72, 226–236. 10.1016/j.sbi.2021.11.008 [DOI] [PubMed] [Google Scholar]
- Tian H., Jiang X., Trozzi F., Xiao S., Larson E. C., Tao P., et al. (2021). Explore protein conformational space with variational autoencoder. Front. Mol. Biosci. 8, 781635. 10.3389/fmolb.2021.781635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer D., Lisanza S., Wang J., Dong R., Anishchenko I., Milles L. F., et al. (2020). Design of proteins presenting discontinuous functional sites using deep learning. bioRxiv. 10.1101/2020.11.29.402743 [DOI] [Google Scholar]
- Tsaban T., Varga J. K., Avraham O., Ben-Aharon Z., Khramushin A., Schueler-Furman O., et al. (2022). Harnessing protein folding neural networks for peptide-protein docking. Nat. Commun. 13 (13), 176–212. 10.1038/s41467-021-27838-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Cao H., Zhang J. Z. H., Qi Y. (2018). Computational protein design with deep learning neural networks. Sci. Rep. 8, 6349. 10.1038/s41598-018-24760-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Lisanza S., Juergens D., Tischer D., Anishchenko I., Baek M., et al. (2021). Deep learning methods for designing proteins scaffolding functional sites. bioRxiv. 10.1101/2021.11.10.468128 [DOI] [Google Scholar]
- Wang W., Peng Z., Yang J. (2022). Single-sequence protein structure prediction using supervised transformer protein language models. bioRxiv, 1–19. 10.1101/2022.01.15.476476 [DOI] [PubMed] [Google Scholar]
- Wang Y., Barth P. (2015). Evolutionary-guided de novo structure prediction of self-associated transmembrane helical proteins with near-atomic accuracy. Nat. Commun. 6, 7196. 10.1038/ncomms8196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Johnston K. E., Arnold F. H., Yang K. K. (2021). Protein sequence design with deep generative models. Curr. Opin. Chem. Biol. 65, 18–27. 10.1016/j.cbpa.2021.04.004 [DOI] [PubMed] [Google Scholar]
- Yang J., Anishchenko I., Park H., Peng Z., Ovchinnikov S., Baker D., et al. (2020). Improved protein structure prediction using predicted interresidue orientations. Proc. Natl. Acad. Sci. U.S.A. 117, 1496–1503. 10.1073/pnas.1914677117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajc C. U., Dobersberger M., Schaffner I., Mlynek G., Pühringer D., Salzer B., et al. (2020). A conformation-specific ON-switch for controlling CAR T cells with an orally available drug. Proc. Natl. Acad. Sci. U.S.A. 117, 14926–14935. 10.1073/pnas.1911154117 [DOI] [PMC free article] [PubMed] [Google Scholar]