Abstract
Rationale & Objective
Focal segmental glomerulosclerosis (FSGS) is a rare condition that can lead to kidney function decline and chronic kidney failure. Immunosuppressants are used to treat primary FSGS. However, their efficacy and safety in FSGS are not clearly established. We assessed current knowledge on clinical effectiveness and safety of immunosuppressants for primary FSGS.
Study Design
Systematic review of randomized controlled trials, interventional nonrandomized controlled trials, observational studies, retrospective studies, and registries.
Setting & Participants
Patients with primary and genetic FSGS.
Selection Criteria for Studies
Medline, EMBASE, and the Cochrane Central Register of Controlled Trials were searched for English-language, primary-FSGS studies from inception to 2019. Clinical outcomes were changes from baseline in proteinuria, kidney function, and kidney survival.
Data Extraction
2 investigators independently screened studies and extracted data.
Analytical Approach
Study results were summarized using random-effects models either as ratios of means between follow-up and baseline measurements or as HRs.
Results
We included 98 articles. Substantial heterogeneity was observed in patient baseline characteristics and study designs. Most studies assessed treatment with corticosteroids alone or combined with other drugs, mainly immunosuppressants. Patients treated with immunosuppressants showed reduced proteinuria (14 studies; ratio of means, 0.36; 95% CI, 0.20-0.47), decreased creatinine clearance (mean difference, −25.03; 95% CI, −59.33 to −9.27) and (significantly) lower estimated glomerular filtration rates (mean difference, −7.61 mL/min/1.73 m2; 95% CI, −14.98 to 0.25 mL/min/1.73 m2). Immunosuppressant therapy had an uncertain effect on reducing the chronic kidney failure risk. Hypertension and infections were the most commonly reported adverse events.
Limitations
Heterogeneity in study designs, patient populations, and treatment regimens; no access to individual patient–level data.
Conclusions
This systematic review supports proteinuria reduction with immunosuppressant therapy in primary FSGS over varying follow-up periods. The effects of immunosuppressants on kidney survival remain uncertain. This review underscores the need for better-designed and adequately controlled studies to assess immunosuppressant therapy in patients with primary FSGS.
Index Words: Chronic kidney failure, end-stage kidney disease, end-stage renal disease, focal segmental glomerulosclerosis, immunosuppressive therapy, proteinuria
Plain-Language Summary.
Focal segmental glomerulosclerosis (FSGS) is a rare condition that damages the kidney and may result in chronic kidney failure. This systematic review examined treatment of primary FSGS with therapies that suppress the immune system (ie, immunosuppressants), including steroids, calcineurin inhibitors, and alkylating agents, in 98 publications (7 were randomized controlled trials). Treatment of FSGS targets the reduction of protein in urine to protect long-term kidney health. On average, protein in urine was reduced by >50% in patients treated with immunosuppressants. The effects on kidney function and kidney survival were uncertain. The most common adverse events during treatment were hypertension and infections. To better understand the effects of immunosuppressant therapies in FSGS, adequately controlled studies are needed.
Introduction
Focal segmental glomerulosclerosis (FSGS) is a rare condition affecting patients of any age, which can lead to decline in kidney function and progression to chronic kidney failure (CKF). FSGS arises as a consequence of multiple pathways either individually or collectively resulting in injury to the podocyte and can be classified into primary, secondary, and genetic forms.1 Primary FSGS, which is presumably caused by a putative circulating factor that leads to podocyte injury, typically presents with abrupt-onset, severe nephrotic syndrome.2 Patients with primary FSGS presenting with nephrotic syndrome are usually treated with corticosteroids and other immunosuppressive drugs. Although corticosteroids remain the mainstay of first-line treatment for primary FSGS, more aggressive therapeutic approaches may be taken in patients who relapse or remain persistently nephrotic despite conservative therapy: namely, additional use of or a shift to other immunosuppressive agents, such as calcineurin inhibitors (CNIs), mycophenolate mofetil, or rituximab.3,4
Despite general acceptance in clinical practice of their use in the management of primary FSGS, the efficacy and safety of immunosuppressive therapies are not yet clearly established. Several years have passed since the last attempts to qualitatively or quantitatively synthesize the available evidence on the effects of immunosuppressive therapies in patients with primary or idiopathic FSGS,3,5 highlighting the necessity for an update on this important subject. Therefore, the objective of this systematic literature review was to assess the current knowledge on the clinical effectiveness and safety of immunosuppressants in the treatment of primary FSGS.
Methods
Search Strategies
A comprehensive search of the peer-reviewed literature was conducted on April 5, 2019, using the Medline (PubMed), Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects), and EMBASE databases. The systematic literature review was structured using the Population, Intervention, Comparator, and Outcomes strategy. Keywords and the respective syntax used in each database are shown in Tables S1-S3. Systematic searches were further supplemented with manual searches to identify additional, relevant studies not retrieved with the systematic literature review protocol or published after the date of the systematic search.
Study Selection
Although randomized controlled trials (RCTs) provide the strongest level of evidence, very few randomized studies investigating the clinical efficacy of immunosuppressants in the treatment of patients with primary or idiopathic FSGS are currently available. Therefore, a broad range of study designs were included in this systematic literature review in addition to RCTs, including interventional non-RCTs (ie, single-arm clinical trials and nonrandomized comparative studies), observational studies, retrospective studies, and registries. Studies were required to meet the following additional criteria to be included in this investigation: (1) report treatment of patients with primary or idiopathic FSGS with any immunosuppressant agent, either with a single arm or in comparison with nonimmunosuppressive agents, placebo, or no treatment; (2) describe any of the following efficacy outcomes: proteinuria (as daily proteinuria or the urinary protein-creatinine ratio), kidney function (estimated glomerular filtration rate [eGFR] or creatinine clearance [CrCl]) and survival (CKF, doubling of creatinine, or author-reported survival), and adverse events; (3) be human studies; and (4) be published in English with the full text available. No time limit was applied in this literature search.
The exclusion criteria considered in this systematic literature review were as follows: (1) patients with secondary FSGS; (2) FSGS recurrence after transplantation; (3) immunosuppressive therapy with rituximab or other monoclonal antibodies; (4) animal and in vitro studies; and (5) economic evaluations, editorials, notes, comments or letters, narratives, articles without abstracts or nonsystematic literature reviews, case reports, or case series.
Data Extraction
Studies were independently screened by 2 investigators (BM/MF) who subsequently extracted pertinent data and analyzed the results. Any disagreements or discrepancies in study selection and data collection between the 2 authors were resolved by discussion with a third author (NP).
Daily proteinuria was recorded in g/day or the urinary protein-creatinine ratio. Kidney function was represented as the eGFR or CrCl. Kidney outcomes were recorded as the kidney survival rate, rate of patients reaching CKF, or time to CKF. Treatment-related changes at baseline and follow-up periods were reported as mean values and standard deviations, unless otherwise specified. Whenever the variation was represented as the standard error of the mean, the standard deviation was calculated using the following formula: standard error of the mean × square root of sample size.
Quality Assessment
Risks of bias of randomized clinical trials considered for inclusion in the systematic literature review were assessed independently by 2 authors (BM/MF) using the risk-of-bias checklist developed by the Cochrane Renal Group for RCTs. Discrepancies were resolved by discussion with a third author (AZ). The items assessed in the checklist were allocation concealment; blinding of investigators, participants, outcome assessors and data analysts; the intention-to-treat analysis; and completeness to follow-up. Each item was answered with yes, no, or unclear, in combination with a narrative response and an overall assessment of the risk of bias.
Statistical Analyses
Meta-analyses were performed with R (v. 3.6.0), using the dplyr (0.8.3), meta (4.9.5), and metaphor (2.1.0) packages. The ratio of mean (ROM) values at the last time point reported and of mean values at baseline, as well as their 95% confidence intervals (95% CIs), were calculated for the included studies and transformed into an estimated summary ROM. The ROM was computed using the last time point available for each study. Similarly, the mean differences (MDs) between mean values at the last time point reported and of mean values at baseline, as well as their 95% CIs, were calculated for the included studies and pooled into an estimated summary MD. The standardized MD was computed between treatment and control arms using the last time point reported for both arms. Summary effects of the ROM, MD, standardized MD, and hazard ratio (HR) were computed using the random-effects model.
Results
Study Selection and Characteristics of the Studies
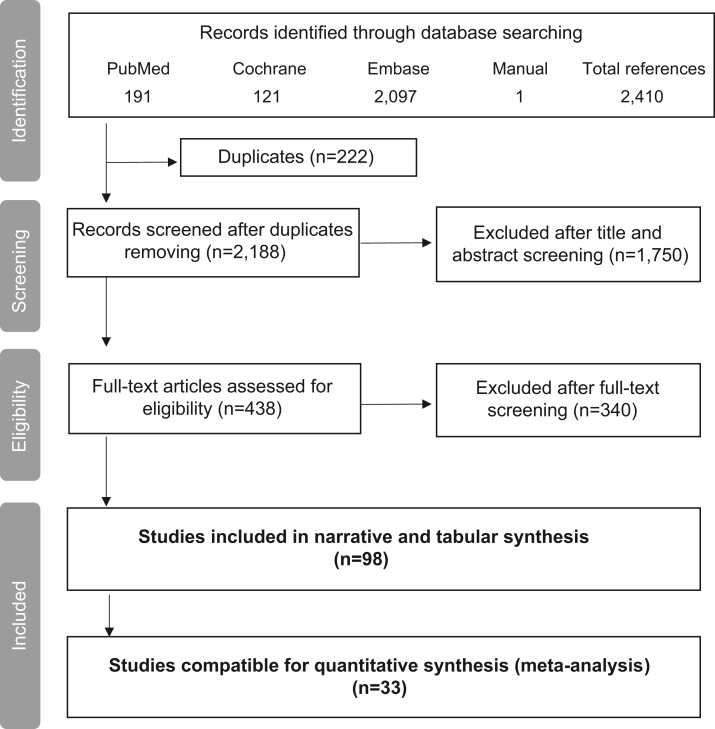
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses chart presented in Fig 1 displays the selection process of the articles included in this systematic literature review. The original systematic search retrieved 2,409 articles from the 5 databases, whereas the manual search identified only 1 paper. A total of 2,188 records were assessed for relevance after removal of duplicates. After title and abstract screening, 438 articles were considered for a full-text assessment, and a total of 98 articles were deemed relevant for inclusion in terms of the study population, intervention, and reported outcomes. Among these 98 publications, 33 were eligible for quantitative assessment synthesis and meta-analysis construction.
Figure 1.
Flowchart describing the study selection process, showing the numbers of studies identified, screened, assessed for eligibility, and included for narrative (tabular) or quantitative (meta-analysis) synthesis.
The 98 publications deemed relevant for inclusion corresponded to 97 independent studies. Most of the included articles were observational studies (n = 85 studies), followed by clinical trials (n = 12 studies), of which 7 were RCTs. A risk-of-bias assessment was performed on the latter studies and, overall, all the included RCTs demonstrated a risk of bias for at least 1 of the 4 potential sources of bias considered in the assessment (Table S4). All studies were conducted in patients with primary or idiopathic FSGS or reported results specifically for this target population. Only 1 study6 included a subpopulation analysis on patients with genetic FSGS. The age groups of the populations admitted in the studies were nearly equally divided between children or adolescents (n = 45) and adults (n = 43), with 8 studies reporting results for a mixed population of children and adults and 1 study not specifying the ages of the included patients. As expected, the majority of the studies were conducted on patients with nephrotic syndrome, with 4 studies incorporating <50% of patients with nephrotic syndrome and 11 studies in which the nephrotic status was not specified. Some studies evaluated the treatment of naïve patients, whereas others evaluated patients who were steroid resistant (or dependent).
Various types of immunosuppressive or immunomodulatory drugs were assessed in the studies, including steroids (eg, prednisone, methylprednisolone), CNIs (eg, cyclosporine A and tacrolimus), and alkylating agents (eg, cyclophosphamide). Individual classes of immunosuppressants were used alone (n = 9 studies), in combination with other types of immunosuppressants (n = 82 studies), or in combination with other nonimmunosuppressive agents (n = 55 studies). The majority of the extracted literature evaluated the efficacy of steroids in combination with other therapies (n = 78 publications), with only a handful as monotherapy (n = 6 publications). The preponderance of studies focusing on the use of steroids is in alignment with the Kidney Disease: Improving Global Outcome (KDIGO) guidelines,4 confirming that these agents are the preferred first line of treatment in primary FSGS management. Four studies evaluated the effects of nonsteroid immunosuppressants or immunomodulator agents as monotherapy, namely with cyclosporine (n = 3 studies) and cyclophosphamide (n = 1 study). Angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers were the most common control or concomitant therapies. Other nonimmunosuppressive concomitant treatments were also reported (eg, other antihypertensive agents, diuretics, statins, antiplatelets), although to a much lesser extent.
Considerable heterogeneity was found among the studies because of different baseline characteristics, patient populations, study designs, treatment regimens, investigated drugs, and time intervals between baseline and follow-up time measurements. The characteristics of the included studies are shown in Table 1.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103
Table 1.
Characteristics of All the Studies Included in the Systematic Literature Review for Qualitative Analysis
| Baseline Characteristics |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Study Type | Study Arm (N) | Patient |
Proteinuria, g/day | UP/C | eGFR, mL/min/1.73 m2 | CrCl, mL/min/1.73 m2 | Follow-Up and/or Therapy Duration | Clinical Outcomes Reported | Main Conclusion of the Study | ||
| Disease | Age, y | Nephrotic | ||||||||||
| Abeyagunawardena et al, 200719 | Retrospective | CYC and/or CsA ± chlorambucil ± vincristine (66) | SRNS idiopathic FSGS | <16 | 100% | — | — | — | — | Follow-up: 10 y | Response to therapy; kidney survival; progression or time to CKF; AEs and/or death | Prolonged treatment with corticosteroids increases chances of remission and preserves kidney function in patients with idiopathic FSGS; chance of remission may be increased with addition of CYC or vincristine and with prolonged use of low-dose CsA |
| Abrantes et al, 2006 (Brazil)20 | Retrospective | Pred ± CYC ± MP ± CsA ± AH (110) | Primary FSGS | <15 | 100% | — | — | — | — | Follow-up: 10 y | Kidney survival; progression/ time to CKF; HR | At presentation, 3 factors were predictive of CKD: age, creatinine level, and nonresponse to Pred; at kidney biopsy, 4 factors were predictive of CKD: age, percentage of global sclerosis, creatinine level, and presence of hematuria |
| Abrantes et al, 2006 (Brazil)21 | Retrospective | Pred ± CYC ± MP ± CsA ± AH (110) | Primary FSGS | <15 | 100% | — | — | — | — | Follow-up: 10 y | Response to therapy; kidney survival; progression or time to CKF; AEs and/or death | Long-term overall kidney survival seems to be more favorable in this cohort of FSGS than others |
| Adhikari et al, 1997 (South Africa)22 | Prospective | Arm 1: CYC + MP + Pred (7); Arm 2: Shorter CYC + MP + Pred (4) |
SR FSGS | <10 | 83% | — | 2.6 ± 1.2; 3.6 + 3.7 |
63.1 ± 50.9; 97.3 ± 76.9 |
— |
Follow-up: arm 1: mean, 38.1 ± 8.8 mo; arm 2: mean 14.6 ± 11.7 mo; Therapy duration: arm 1: up to 18 mo; arm 2: up to 6 mo |
Response to therapy; proteinuria or UP/C; SrCr; eGFR; kidney survival; progression or time to CKF; AEs and/or death | Compared with regimen A, regimen B is 6 times less costly, with a quarter of the number of hospital visits |
| Adhikari et al, 2001 (South Africa)23 | Retrospective | CYC ± MP ± CsA ± diuretics ± AH (75) | NS FSGS | <18 | 100% | — | — | — | — | Follow-up: 2 y or more | Response to therapy; kidney survival; progression or time to CKF; AEs and/or death | Similar proportion of NS between Black and Indian patients, as well as in kidney outcomes |
| Agarwal et al, 1993 (India)24 | Retrospective | Pred (42) | Idiopathic FSGS | ≥14 | 90.5% | — | — | — | — | Follow-up: 32 ± 7.6 mo | Response to therapy; kidney survival; progression or time to CKF | Steroid responders have better prognoses than nonresponders; Pred should be given an average of 8-12 weeks before categorizing the response |
| Agrawal et al, 2019 (India)7 | Retrospective | Arm 1: Tac responsive (Tac + steroids; 7); Arm 2: Tac resistant (Tac + steroids; 7) |
SR primary FSGS | ≥18 | 93% | 5.5 ± 6.1; 4.9 ± 3.8 |
— | 102.8 ± 25.8 mL/min; 91.6 ± 24.4 mL/min |
— | Follow-up: 60 wk; Therapy duration: 48 wk | Proteinuria or UP/C; SrCr; eGFR | Subpodocytic space was preserved in patients on Tac with complete remission and lost in patients with partial response and Tac-resistant cases |
| Al Salloum, 2004 (Saudi Arabia)25 | Prospective | Pred + CYC (15) | SR primary FSGS | <12 | 33% | — | — | — | — | Follow-up: 4 y | Response to therapy; kidney survival; progression or time to CKF | Beneficial therapy for initial SR FSGS remains to be determined, but CYC side effects were negligible |
| Alexopoulos et al, 2000 (Greece)26 | Retrospective | Arm 1: Pred ± CsA or CsA ± ACEi (18); Arm 2: Supportive therapy ± ACEi (15) |
SR primary FSGS | >15 | 51% | — | — | — | — | Follow-up: mean, 55 mo (range, 8-142 mo); Therapy duration: mean, 9 mo (range, 6-12 mo) | Response to therapy; SrCr; kidney survival; progression or time to CKF; AEs and/or death |
Patients with NS with FSGS may benefit from a more prolonged course of Pred; patients with NS responding to treatment have significantly better kidney survival than nonresponders |
| Arbus et al, 1982 (Canada)27 | Retrospective | Steroids ± CYC (51) | NS idiopathic FSGS | <16 | 100% | — | — | — | — | Follow-up: mean, 10.6 y (range, 2.4-24.0 y) | Response to therapy; kidney survival; progression or time to CKF |
Patients with FSGS and NS who go into remission, apparently as a result of steroid or CYC therapy, may become SR within 18 mo and ultimately experience progressive kidney failure |
| Arias et al, 2011 (Colombia)16 | Retrospective | Arm 1: FSGS NOS (Steroids, or Steroids + AZA, or CYC, or MMF, or CsA; 196); Arm 2: Tip variant (Steroids, or Steroids + AZA, or CYC, or MMF, or CsA; 37) |
Primary FSGS | 1-65 | 97% | — | — | — | — | Follow-up: arm 1: 40 mo (range, 24-132 mo); arm 2: 49 mo (range, 24-87 mo) | Kidney survival; progression or time to CKF; AEs and/or death | The study does not demonstrate a clearly favorable prognosis in a group of patients with FSGS tip variant |
| Ayar et al, 2016 (Turkey)28 | Retrospective | CsA or steroids or CYC or AZA or MMF or ACEi or ARBs (68) | FSGS | ≥18 | 41% | 3.62 (range, 0.78-46) | — | 65.91 mL/min (range, 15.05-217.13 mL/min) | — | Follow-up: mean, 22 mo (range, 8-76 mo) | Proteinuria or UP/C; eGFR; kidney survival; progression or time to CKF; AEs and/or death | Early diagnosis, follow-up and appropriate immunosuppressive medications affect mortality and clinical progress in primary GN |
| Bagchi et al, 2016 (India)29 | Retrospective | Steroids ± CsA or Tac ± CYC or MMF or Rituximab ± ACEi and/or ARBs (116) | Primary FSGS | ≥18 | 100% | 5.1 ± 2.6 | — | 96.9 ± 35.1 | — | Follow-up: 23.6 mo (range, 6-65.1 mo) | Response to therapy; kidney survival; progression or time to CKF; AEs and/or death; HR | Patients with steroid resistance have reasonable kidney survival, if proteinuria is reduced with timely use of alternate immunosuppression; CNI resistance is major hurdle in management with limited treatment options |
| Beşbaş et al, 2010 (Turkey)30 | Retrospective | Pred ± CYC or CsA ± MP (222) | Primary FSGS | ≤16 | 89.4% | — | 12.9 ± 10.9 | — | — | Follow-up: 47.9 mo (range, 0.26-270.5 mo) | Response to therapy; kidney survival; progression or time to CKF | Use of immunosuppressive treatment in conjunction with prolonged steroid seems beneficial in children with primary FSGS |
| Bhimma et al, 2006 (South Africa)31 | Prospective | Pred + Tac + ACEi + diuretics + CCB (20) | SR idiopathic FSGS | ≤16 | 100% | — | 12.5 ± 3.2 | 117.4 ± 74.0 mL/m2/min | — | Follow-up after stop of Tac therapy mean, 27.5 mo (range, 13.7-43.7 mo); Tac therapy duration: 12 mo |
Response to therapy;proteinuria or UP/C; SrCr; eGFR; AEs and/or death | Tac is a safe and effective treatment for SR FSGS; however, like CsA, some children tend to relapse following cessation of treatment |
| Brodehl et al, 1988 (Germany)32 | Prospective | CsA (13) | SRNS FSGS | ≤16 | 100% | 10.5 ± 6.8 | — | — | — | Follow-up: up to 45 mo; therapy duration: at least 6 mo | Response to therapy; kidney survival; progression or time to CKF; AEs and/or death | Only half of patients benefited from CsA, so possibly a combination of immunosuppressive drugs could be more effective than CsA alone |
| Cattran & Rao, 1998 (Canada)33 | Retrospective | Arm 1: Adults (Pred ± CYC ± AH; 55); Arm 2: Children (Pred ± CYC ± AH; 38) |
FSGS | 35; 6.0 |
55%; 76% |
— | — | — | 90 ± 66; 84 ± 30 |
Follow-up: 11 y | Response to therapy;Proteinuria or UP/C; CrCl; kidney survival; progression or time to CKF | Steroid treatment beyond 6 months does not appear to be beneficial; CR confers excellent long-term prognosis in children and adults |
| Cattran et al, 1999 (USA)34 | Prospective, single-blind, randomized trial | Arm 1: CsA + Pred ± ACEi and/or ARBs (26); Arm 2: Placebo + Pred ± ACEi and/or ARBs (23) |
SRNS FSGS | >18 | 100% | 6.9 ± 3.3; 8.7 ± 4.7 |
— | — | 86 ± 27; 86 ± 31 |
Follow-up: mean 200 wk | Response to therapy; kidney survival; progression or time to CKF | CsA does not work in every case of FSGS, but a short-term remission to subnephrotic proteinuria was observed in 69% of cases |
| Cattran et al, 2003 (USA)13 | Prospective, single-blind, randomized trial | Arm 1: CsA + Pred ± ACEi and/or ARBs (18); Arm 2: Placebo + Pred ± ACEi and/or ARBs (9) |
SRNS FSGS | >18 | 100% | 7.2 (range, 3.6-14.4); 9.5 (range, 4-22.4) |
— | — | 72 ± 24; 66 ± 30 |
Follow-up: up to 208 wk; therapy duration: 26 wk | Response to therapy; proteinuria or UP/C; SrCr; CrCl | There is no relationship between Palb and outcome, as the antiproteinuric effect of CsA appeared independent of changes in Palb |
| Cattran et al, 2004 (USA)35 | Open-label trial | Pred + MMF ± ACEi and/or ARBs (18) | SRNS primary FSGS | ≥18 | 100% | — | 9.1 ± 5.2 | — | — | Therapy duration mean, 4 mo (range, 3-19 mo) | Response to therapy; proteinuria or UP/C; SrCr; kidney survival; progression or time to CKF | MMF appears safe to use in this group of patients and did lower proteinuria in 44% of this cohort resistant to other forms of treatment |
| Chávez-Mendoza et al, 2019 (Mexico)36 | Retrospective | Arm 1: High-dose Pred ± ACEi ± statins (39) Arm 2: First-line CNI (first-line CNI + low-dose Pred ± ACEi ± statins; 11); Arm 3: Rescue CNI first-line high-dose Pred + added CNI rescue for SR disease ± ACEi ± statins; 16) |
Primary FSGS | ≥18 | 100% | — | 3.7 (IQR, 3.2-7.3); 4.9 (IQR, 3.7-7.6); 5.8 (IQR, 3.6-8.7) |
60 (IQR, 41-115 mL/min/y); 58 (IQR, 48-122 mL/min/y); 79 (IQR, 66-112 mL/min/y) |
— | Follow-up: 51 mo (IQR, 30-77 mo); CNI therapy duration: 12 mo (range, 6-16 mo) |
Response to therapy; eGFR; kidney survival; progression or time to CKF; AEs and/or death | An initial CNI plus low-dose corticosteroid approach in primary FSGS reduces corticosteroid exposure, with a response-to-therapy rate similar to that of the currently recommended high-dose corticosteroid regimen |
| Chishti et al, 2001 (USA)12 | Retrospective | Pred + CsA ± ACEi (21) | SR and SD NS FSGS | ≤16 | 100% | — | — | 115 ± 36.5 | — | Follow-up: mean, 32 mo (range, 12-72 mo); Therapy duration: mean, 20.6 ± 13.7 mo | Response to therapy; eGFR; kidney survival; progression or time to CKF | Single, daily, low-dose CsA appears to be effective for long-term treatment of children with FSGS and NS, with fewer side effects than twice-daily dosing |
| Choi et al, 2002 (USA)37 | Retrospective | MMF ± steroids or CsA or AZA + ACEi and/or ARBs (18) | Primary FSGS | ≥16 | 52.9% | — | 4.7 ± 5.1 | — | — | MMF therapy duration: 8 mo (range, 3-26 mo) | Proteinuria or UP/C; SrCr; eGFR | Empirical MMF therapy in majority of patients with primary GN was well tolerated and achieved the goal of steroid withdrawal, improvement of NS, and stabilization of kidney function |
| Chun et al, 2004 (USA)38 | Retrospective | Pred ± CsA or CYC (51) | Primary FSGS | >15 | 75% | 7.6 ± 3.7 | — | — | — | Follow-up: 73 ± 94 mo; Pred therapy duration: 3.18 ± 1.49 mo |
Response to therapy; kidney survival; progression or time to CKF | Patients with NS with primary FSGS should receive a trial of therapy, irrespective of the histologic lesion, when not contraindicated |
| Crook et al, 2005 (USA)39 | Retrospective | Steroids ± ACEi and/or ARBs (43) | Primary FSGS | >18 | 73.6% | 8.85 ± 7.2 | — | — | — | Follow-up: 46.7 ± 6.6 mo; Steroid therapy duration: 11.7 ± 2.4 mo |
Kidney survival; progression or time to CKF; HR | A beneficial effect of steroids was not observed in this predominantly African American adult cohort, as there was no significant association between steroid status and achieving remission of proteinuria |
| Dumas De La Roque et al, 2018 (France)40 | Retrospective | Steroids ± CsA ± MMF ± CYC ± ACEi ± ARB ± ACEi + ARB (68) | NS idiopathic FSGS | ≥15 | 100% | 6 (IQR, 3.9-9) | — | 42.5 (IQR, 29-58) | — | Follow-up: 66 mo (IQR, 30-92 mo); Therapy duration: 15 mo (IQR, 8-36 mo) |
Response to therapy; kidney survival; progression or time to CKF; AEs and/or death | No predictive factor for relapse was identified by multivariate analysis |
| Deegens et al, 2005 (Netherlands)41 | Retrospective | Arm 1: Initially untreated (± ACEi then Pred ± CYC; 20); Arm 2: Treated (Pred; 8) |
Primary FSGS | ≥18 | 100% | 10.0 ± 5.5; 9.8 ± 3.1 |
— | — | 101 ± 34 mL/min; 123 ± 35 mL/min |
Follow-up: Arm 1: 9.4 y (IQR, 2.1-18.6 y); Arm 2: 9.2 y (IQR, 4.0-11.2 y); Therapy duration: Pred: 2.5 ± 0.9 mo CYC: 3 mo |
Response to therapy; kidney survival; progression or time to CKF; AEs and/or death | Case definition using strict clinical criteria identifies a subgroup of patients with idiopathic FSGS who have a good prognosis; in the majority of these patients, immunosuppressive therapy is not warranted unless kidney function deteriorates |
| Deegens et al, 2008 (the Netherlands)42 | Retrospective | Arm 1: Treated (Pred or Pred + CYC ± ACEi and/or ARBs; 40); Arm 2: Untreated (± ACEi and/or ARBs; 53) |
Primary FSGS | ≥18 | 83%; 49% |
10.4 ± 5.8; 5.8 ± 3.7 |
— | — | — | Follow-up: 66 mo (range, 1-273 mo); Therapy duration: Pred: 5.6 mo (range, 1.3-55.3 mo); CYC: 3 mo (range, 2-12 mo) |
Response to therapy; kidney survival; progression or time to CKF; HR | Kidney survival and remission rates were higher in patients with the tip variant |
| Dhanapriya et al, 2016 (India)43 | Retrospective | Pred ± CYC ± CsA ± Tac ± MMF (170) | Primary FSGS | ≥13 | 79% | 4.26 ± 1.9 | — | — | 85.8 ± 34 mL/min | Follow-up: 4.32 ± 1.2 y; Pred therapy duration: 6 mo |
Response to therapy; kidney survival; progression or time to CKF; AEs and/or death | Attaining CR significantly improves kidney survival |
| Dimkovic et al, 2009 (Serbia & Montenegro)44 | Retrospective | Steroids + MMF + ACEi ± ARBs or statins (13) | Primary FSGS | ≥18 | Not specified | 5.1 (IQR, 2.7-8.9) | 3.26 (IQR, 1.52-7.92) | 56.2 ± 33.2 mL/min | — | Follow-up: 12 mo | Response to therapy; proteinuria or UP/C; eGFR | MMF proved to be efficient in 70% of high-risk patients with primary GN, who reached either complete or partial remission without safety concern after 12 months of treatment |
| Ehrich et al, 2007 (Germany)6 | Retrospective | MP + CsA + Pred ± MMF or Tac ± ACEis (52) | SRNS idiopathic FSGS | <18 | 100% | — | — | — | — | Follow-up: 5 ± 3.6 y; Therapy duration: Pred: 24 wk CsA: at least 36 wk |
Response to therapy; kidney survival; progression or time to CKF | Combined Pred +CsA therapy including IV-MP pulses resulted in a higher rate of remission when compared with previous reports on using CsA monotherapy or other immunosuppressive combination therapies |
| El-Husseini et al, 2004 (Egypt)45 | Retrospective | Arm 1: CsA + ketoconazole ± ACEi (88); Arm 2: CsA ± ACEi (28) |
SR or SD primary FSGS | <18 | Not specified | 6.53; 6.73 |
— | — | 123; 126 |
Follow-up: 33 mo (range 14-84 mo); Therapy duration: 1-2 y |
Response to therapy; proteinuria or UP/C; CrCl; kidney survival; progression or time to CKF; side effects | Coadministration of ketoconazole and CsA in children with idiopathic FSGS is safe; this combination not only reduces the costs but also may improve the response to CsA and stabilize the kidney function |
| El-Refaey et al, 2007 (USA)46 | Retrospective | Arm 1: Collapsing FSGS (Pred ± CsA ± MMF + ACEi; 11); Arm 2: Noncollapsing FSGS (Pred ± CsA ± MMF + ACEi; 28) |
Idiopathic FSGS | ≤17 | 63.6%; 36% |
— | 10.8 ± 5.7; 8.6 ± 9.4 |
96 ± 49; 136.6 ± 65 |
— | Follow-up: 31.5 ± 22.3 mo; 18.7 ± 12.9 mo |
Response to therapy; proteinuria or UP/C; SrCr; eGFR; kidney survival; progression or time to CKF | The outcome of patients with collapsing FSGS at 30 months was better than in previous reports because of stronger immunosuppression or the use of ACEi |
| El-Refaey et al, 2010 (Egypt)47 | Retrospective | Pred ± MP ± CsA ± MMF (72) | NS primary FSGS | ≤16 | 100% | 3.3 ± 2.8 | — | — | — | Follow-up: 76.3 ± 42 mo (range, 9-156 mo) | Response to therapy; kidney survival; progression or time to CKF | Kidney survival is better than in other cohorts |
| Futrakul et al, 2004 (Thailand)48 | Retrospective | ACEi + CCB + AP + vitamins E and C ± ARB + Pred (10) | FSGS | Not reported | 100% | 3.1 ± 4.4 | — | 35 ± 53.8 | 34 ± 37.9 | Follow-up: ≥10 y | Proteinuria or UP/C; eGFR; CrCl | Therapeutic intervention with multidrug regimens relaxes the arteriolar constriction, thus impacting on the pathogenetic mechanism of kidney disease progression |
| Futrakul et al, 2004 (Thailand)49 | Prospective | Arm 1: Pred + CYC + AH (11); Arm 2: ACEi, ARB, CCB + AP + baby aspirin ± heparin (18) |
FSGS | <21 | 100% | 3.2 ± 0.7; 3 ± 0.8 |
— | 60 ± 21; 51 ± 23 |
47 ± 25; 50 ± 19 |
Follow-up: 77 ± 24 mo; 97 ± 33 mo |
eGFR; BP; kidney survival; progression or time to CKF | In contrast to the therapeutic failure with conventional therapy in Arm 1, clinical improvement in response to the combined formula has been substantiated in all 18 patients in Arm 2 |
| Gellermann et al, 2012 (Germany)50 | Retrospective, uncontrolled | MP + Pred + CsA ± ACEi and/or ARBs ± diuretics, then MMF (23) | SRNS primary FSGS | <18 | 100% | — | — | 106.2 ± 26.3 (n = 16) | — | Follow-up: 7.0 y (range, 1.7-16.5 y); MMF therapy duration: 3.6 y (range, 0.8-10 y) |
Response to therapy; eGFR; AEs and/or death | In children with SRNS or FSGS achieving initial remission, a sequential steroid-free therapy consisting of a combination of CsA and MMF, followed by MMF alone (with the addition of ACEi and ARBs), can provide sustained long-term remission, preservation of kidney function, and better control of BP |
| Gheissari et al, 2018 (Iran)51 | Prospective, uncontrolled | Pred + CsA + CYC (26) | FSGS | ≤16 | Not specified | 1.09 ± 1.47 | — | — | — | Follow-up: 6 mo | Kidney survival; progression or time to CKF | TRPC6 may be useful for genetic screening in Iranian children with FSGS |
| Ghiggeri et al, 2004 (Italy)52 | Open-label, nonrandomized, retrospective | Arm 1: CsA responsive (steroids ± CYC and/or CsA ± MP; 20); Arm 2: CsA intolerant or resistant (steroids ± CYC and/or CsA ± MP; 35); Arm 3: No CsA treatment (84) |
SRNS FSGS | <45 | 100% | — | — | — | — | Follow-up: >2 y Arm 1: 81 (IQR, 4-115); Arm 2: 41 (IQR, 23-92); Arm 3: 48 (IQR, 28-106) |
Response to therapy; kidney survival; progression or time to CKF | Long-term CsA (>2 years) has a persistent antiproteinuric effect in the absence of kidney fibrosis |
| Gipson et al, 2011 (USA)53 | Open-label, randomized controlled trial | Arm 1: MMF and DEX + Pred + ACEi and/or ARB ± additional AH (66); Arm 2: CsA + Pred + ACEi and/or ARB ± additional AH (72) |
SR primary FSGS | 2-40 | Not specified | — | — | — | — | Follow-up: 78 wk | BP; AEs and/or death | The study did not find a difference in rates of proteinuria remission following 12 months of CsA compared to MMF and DEX in patients with SR FSGS |
| Gorsane et al, 2016 (Tunisia)17 | Retrospective | CsA (23) | Idiopathic FSGS | ≥16 | 100% | 7.01 ± 4.5 | — | — | — | Follow-up: 6.8 ± 3.7 y | Response to therapy; kidney survival; progression or time to CKF; AEs and/or death | CsA is effective in treatment of patients with idiopathic FSGS; initial kidney function and CsA resistance are predictive factors of CKF in SR or SD FSGS |
| Goumenos et al, 2006 (UK & Greece)54 | Retrospective | Arm 1: treated (Pred or Pred + AZA or Pred + CsA; 25); Arm 2: untreated (ACEi + CCB + beta-blockers; 26) |
Idiopathic FSGS | >18 | 80%; 50% |
5.8 ± 6.6; 4.4 ± 2 |
— | — | — | Follow-up: 5 y; Therapy duration: 20 ± 6 mo |
Response to therapy; kidney survival; progression or time to CKF | Combination of low Pred dose with CsA could be used as initial treatment in patients with higher risk for side effects from the usual Pred dose |
| Greenwood et al, 2017 (Australia)55 | Retrospective | Pred ± ACEi and/or ARBs or diuretics (98) | Primary and secondary FSGS | >18 | 51% | — | — | — | — | Follow-up: 4.32 y (range, 0-17 y) | Response to therapy; kidney survival; progression or time to CKF; AEs and/or death; HR | Concomitant diabetic nephropathy, higher serum creatinine, and lower eGFR at kidney biopsy were associated with poorer kidney prognosis |
| Gulati et al, 2000 (India)56 | Prospective | Arm 1: early onset (Pred or Pred + CYC; 36); Arm 2: late onset (Pred or Pred + CYC; 36) |
Idiopathic FSGS | ≤12 and >12 | 100% | — | — | 92 ± 11; 94 ± 14 |
— | Follow-up: 14.6 ± 13 mo; 17.8 ± 13 mo |
Response to therapy; SrCr; eGFR; kidney survival; progression or time to CKF | Early onset is more often steroid responsive and has a better prognosis than late-onset FSGS |
| Hari et al, 2001 (India)18 | Prospective | Arm 1: initial resistance (DEX or MP + CYC; 31); Arm 2: late resistance (DEX or MP + CYC; 34) |
SRNS FSGS | ≤14 | 100% | 2.6; 3.5 |
9.7; 10.0 |
— | — | Follow-up after stoppage of pulse therapy: 25.6 mo (range, 20 mo to 7.3 y); Therapy duration: up to 52 wk |
Response to therapy; proteinuria or UP/C; kidney survival; progression or time to CKF; AEs and/or death | Prolonged treatment with IV corticosteroids and oral CYC is beneficial in patients with SR FSGS; although this regimen has considerable side effects, it may prevent chronic kidney failure in a number of patients |
| Heering et al, 2004 (Germany)57 | Prospective, randomized | Arm 1: Pred + acetylsalicylic acid ± CsA (34); Arm 2: Pred ± chloroambucil or CsA (23) |
Idiopathic FSGS | ≥18 | 100% | 5.2 ± 1.1; 4.6 ± 3.1 |
— | — | — | Follow-up: 4 y; Therapy duration: 2 mo to several y CsA therapy duration: 23 ± 16.5 mo |
Response to therapy; proteinuria or UP/C; kidney survival; progression or time to CKF | Additional treatment with chlorambucil was found to be ineffective in FSGS; patients responded to treatment with steroids or CsA |
| Hogg et al, 2013 (USA)58 | Post hoc analysis | Arm 1: MMF and DEX + Pred + ACEi and/or ARB ± additional AH (20); Arm 2: CsA + Pred + ACEi and/or ARB (losartan) ± additional AH (22) |
SR primary FSGS | 7-34 | Not specified | — | 3.6 (10th-90th percentile, 1.1-9.6); 2.7 (10th-90th percentile, 1.1-5.1) |
122.6 ± 50.7; 126.8 ± 50.5 |
— | Follow-up; CsA or MMF and DEX therapy duration 78 wk; 52 wk |
Proteinuria or UP/C; eGFR | The improvement in UP/C after CsA or MMF and DEX was largely sustained for 6 months after therapy; reduction in eGFR in the CsA group was improved 6 months after CsA was stopped, although the levels were lower than baseline in 7 patients who entered the study with decreased eGFR |
| Hoseini et al, 2012 (Iran)59 | Retrospective | CYC ± CsA ± MMF (58) | Primary FSGS | ≤18 | Not specified | — | — | — | — | Follow-up: 5.7 y (range, 3-20 y) | Kidney survival; progression or time to CKF; AEs and/or death | The study demonstrates an increasing trend in the FSGS incidence in Iranian children; however, kidney survival rates were similar to those reported by others in different countries |
| Huang et al, 2018 (China)11 | Prospective, open-label, controlled trial | Arm 1: Pred + ARBs or ACEi (52); Arm 2: ARBs or ACEi (50) |
Primary FSGS | >18 | No | 1.67 (range, 1.04-3.26); 1.58 (range, 1.09-3.43) |
— | 72.94 ± 28.52 mL/min; 71.33 ± 30.82 mL/min |
— | Follow-up: 36 mo (range, 12-101); 37.5 mo (range, 12-117) |
Response to therapy; proteinuria or UP/C; AEs and/or death | Additional glucocorticoid therapy is more efficacious compared with ACEi and/or ARBs alone in the treatment of patients with primary FSGS and moderate proteinuria |
| Inaba et al, 2016 (Japan)60 | Retrospective | Arm 1: CsA (39); Arm 2: CYC (24) |
SRNS idiopathic FSGS | <18 | 100% | — | — | — | — | Follow-up: Arm 1: 9.0 y (range, 6.3-16.1 y); Arm 2: 14.6 y (range, 10.4-19.0 y); CsA therapy duration until toxicity was found: 4.8 y (range, 2.0-5.8 y) |
Response to therapy; kidney survival; progression or time to CKF, AEs and/or death; HR | Therapeutic regimen with CsA could considerably improve both the initial remission rate and the long-term kidney survival rate of children with idiopathic SRNS |
| Ingulli et al, 1995 (USA)61 | Prospective | CsA + Pred ± furosemide (21) | SR FSGS | <18 | Not specified | 6.2 ± 0.2 | — | — | — | Follow-up: 8.5 ± 4.7 y; CsA therapy duration: 27.5 ± 22 mo |
Proteinuria or UP/C; SrCr; kidney survival; progression or time to CKF; AEs and/or death | Long-term CsA therapy successfully reduces the proteinuria in Black and Hispanic children with SR FSGS and blunts the progression to kidney failure |
| Jafry et al, 2012 (Pakistan)62 | Retrospective | Pred ± CYC ± AH (124) | Idiopathic or primary FSGS | ≥17 | 72% | 6.0 ± 4.5 | — | — | — | Follow-up: 113.6 ± 118.6 wk; Steroid therapy duration: 20.2 ± 7.4 wk |
Response to therapy; proteinuria or UP/C; SrCr | Half of adults with primary FSGS achieve sustained remission with steroids and, consequently, exhibit an excellent prognosis for long-term outcome |
| Jellouli et al, 2016 (Tunisia)63 | Retrospective | Pred + MP ± CsA or MMF ± CYC (30) | Primary FSGS | ≤18 | 87% | 113 ± 87 mg/kg | — | 110.5 ± 46 | — | Follow-up: at least 1 y; Pred tx duration: 5 mo | Response to therapy; kidney survival; progression or time to ESKD | Children treated with CsA had a high response rate |
| Kallash & Aviles, 2014 (USA)64 | Retrospective | Pred + Tac (22) | SRNS primary FSGS | ≤17 | 100% | — | mean, 12.0 (range, 2.2-48.64) | mean, 92.30 (range, 31.3-142) | — | Follow-up: 2.9 y (average, 0.5-7 y) | Response to therapy; eGFR | Tac is a viable option in the treatment of children with idiopathic, SR FSGS; FSGS with nephrotic-range proteinuria should be treated aggressively because even partial remission improves kidney survival |
| Kambham et al, 2001 (USA)65 | Retrospective | Steroids ± cytotoxic agents (50, idiopathic FSGS) | Idiopathic FSGS | Mean, 32.6 y | 66% | — | — | — | — | Follow-up: 39 mo (range, 1-140 mo) | Kidney survival; progression or time to ESKD | Clinically, ORG is distinguished from idiopathic FSGS by its lower incidence of NS, more benign course, and slower progression to kidney failure |
| Kangovi et al, 2012 (USA)66 | Retrospective | Arm 1: CNI, alkilating agents ± ACEi and/or ARBs (32); Arm 2: ACEi and/or ARBs (35) |
Primary FSGS | <21 | Not specified | — | 14.4 ± 11.5; 4.5 ± 6.3 |
132.9 ± 56.1; 100.8 ± 43.1 |
— | Follow-up: 70.2 ± 49.5 mo 53.9 ± 28.9 mo; Therapy duration: 11.0 mo; 80.5 mo |
Response to therapy; kidney survival; progression or time to CKF; HR | Patients treated initially with RAASi monotherapy may have outcomes that are comparable to or better than those of patients treated with immunosuppression |
| Kirpekar et al, 2002 (USA)67 | Retrospective | Arm 1: FSGS with or without mesangial hypercellularity (Pred + MP ± alkylating agent; 10); Arm 2: FSGS plus mesangial proliferation (Pred + MP ± alkylating agent; 15) |
SRNS idiopathic FSGS | <18 | 100% | — | 6.1 ± 4.3; 14.9 ± 14.7 |
— | 122.7 ± 100.0; 149.6 ± 79.3 |
Follow-up: 91.8 ± 55.9 mo; 51.2 ± 33.6 mo; Therapy duration: 17.4 ± 19.4 mo; 9.6 ± 5.8 mo |
Response to therapy; kidney survival; progression/ time to CKF | The cause of SRNS cannot be determined by analysis of clinical variables, including age, sex, ethnicity, UP/C ratio, and initial serum creatinine and albumin levels |
| Klaassen et al, 2015 (Germany)68 | Retrospective chart review | CsA (23) | SRNS FSGS | ≤15 | 100% | 3.4 g/m2/day (range, 1-55.8 g/m2/day) | — | — | — | Follow-up: 15.5 y (range, 1.8-27.7 y); CsA therapy duration: 3.1 y (range, 0.5-14 y) |
Response to therapy; kidney survival; progression or time to CKF | CNI seems to be justified as a first-line treatment in SRNS; it is successful in many patients and, in our experience, can be stopped successfully in individual patients with CR after treatment |
| Korbet et al, 1986 (USA)69 | Retrospective chart review | Arm 1: Patients with NS (± Pred ± chlorambucil; 29); Arm 2: Patients without NS (no Pred ± chlorambucil; 17) |
Primary FSGS | >18 | 63% | 9.47 ± 1.09; 1.64 ± 0.19 |
— | — | — | Follow-up: 48.0 ± 8.9 mo; 79.9 ± 17.8 mo |
SrCr; kidney survival; progression or time to CKF; AEs and/or death | Primary FSGS and nephrotic proteinuria in adults have the same ominous prognostic significance as the similar syndrome previously observed in children; a therapeutic response appears to identify a subgroup among the patients with NS with a better prognosis; when the same glomerular lesion is seen in a patient with nonnephrotic proteinuria, the disease follows a more indolent course |
| Laurin et al, 2016 (USA)70 | Retrospective analysis of inception cohort study | Arm 1: Collapsing FSGS (ACEi, ARB, or selective aldosterone blocker ± steroids or CNI; 61); Arm 2: FSGS (ACEi, ARB, or selective aldosterone blocker ± steroids or CNI; 126) |
Collapsing and NOS FSGS | Children: ≤15; Adults: >18 |
58.3% |
12.2 (IQR, 5.6-14.8); 4.4 (IQR, 2.3-8.1) |
8.9 (IQR, 6.4-15.5); 6.6 (IQR, 2.7-10.1) |
48 (IQR, 26-73); 60 (IQR, 42-92) |
— | Follow-up: 61 mo (IQR, 17-117 mo); 73 mo (IQR, 24-148 mo); Steroids therapy duration 3.9 mo (IQR, 1.4-6.7 mo); 2.5 mo (IQR, 1.9-6.2 mo); CNI therapy duration 18.2 mo (IQR, 6.1-26.8 mo); 11.5 mo (IQR, 2.9-31.7 mo) |
SrCr; kidney survival; progression or time to CKF; HR | Patients with idiopathic, collapsing FSGS present with more severe proteinuria and kidney dysfunction and have overall worse kidney outcomes compared with patients with NOS FSGS; difference in outcomes may be attributable to the baseline severity of disease and possible differences in the decision to treat with immunosuppressive therapy |
| Laurin et al, 2016 (USA)71 | Retrospective | Arm 1: no immunosuppressants (183); Arm 2: glucocorticoids alone (173), CNIs ± glucocorticoids (90) other immunosuppressants (AZA, MMF, and CYC; 12) |
Primary FSGS | Children: <18; Adults: ≥18 |
35.6% | 3.8 (IQR, 3.5-12); 2.4 (IQR, 1.7-3.3) |
— | 43.8 (IQR, 27.2-69.9); 62.8 (IQR, 41.7-85.7) |
— | Steroids therapy duration: 3.0 mo (IQR, 1.5-5.9 mo); CNI therapy duration: 19.6 mo (IQR, 6.5-34.8 mo) |
Kidney survival; progression or time to CKF; HR | Significant association between treatment with immunosuppressive therapy and better kidney survival, despite the fact that patients treated with immunosuppressives tended to have evidence of more severe NS than those who were untreated |
| Li et al, 2014 (China)72 | Retrospective | Pred ± Tac ± ACEi and/or ARBs (109) | Primary FSGS | ≥14 | 100% | 7.52 ± 2.95 | — | 100 ± 31 | — | Follow-up: 12 mo (range, 6-24 mo) | Proteinuria or UP/C | suPAR is specifically elevated in some patients with FSGS, which differs from the finding in patients with MCD and MN; a suPAR assay may help predict steroid responses in patients with primary FSGS |
| Lieberman & Tejani, 1996 (USA)73 | Double blinded, prospectively randomized, placebo controlled | Arm 1: CsA ± CCB (15); Arm 2: Placebo (15) |
SR idiopathic FSGS | ≤19 | Not specified | 151.7 ± 162.4 mg/kg/24h; 166.9 ± 137.1 mg/kg/24h |
— | 103.4 ± 36.7; 86.0 ± 31.3 |
— | Follow-up: 6 mo | Proteinuria or UP/C; eGFR; kidney survival; progression or time to CKF; AEs and/or death | For patients with SR FSGS, CsA is an additional therapeutic approach for a disease with limited options and a virtually certain progression to CKF |
| Loeffler et al, 2004 (USA)15 | Retrospective case series | Tac ± Pred ± ACEi and/or ARBs ± other AH (16; 13 with FSGS) | Treatment-resistant NS FSGS | ≤18 | 100% | — | 729.2 ± 419.8 | — | 217.2 ± 152.3 | Follow-up: mean, 6.5 mo (range, 2.5-18 mo); Therapy duration: mean range, 0.4-13 y | Response to therapy; proteinuria or UP/C; CrCl; BP; AEs and/or death | Tac is an effective, well-tolerated medication for treatment-resistant forms of NS in children |
| Mahmoud et al, 2005 (Egypt)10 | Retrospective | Arm 1: SDNS FSGS (CsA + Pred ± ketoconazole; 61); Arm 2: SRNS FSGS (CsA + Pred ± ketoconazole; 45) |
SDNS + SRNS primary FSGS | ≤16 | 100% | 6.7 ± 2.2; 6.6 ± 2 |
— | — | — | Follow-up; CsA tx duration 12.4 ± 7 mo; 22.6 ± 10 mo; 21 ± 12 mo |
Proteinuria or UP/C; SrCr kidney survival; progression or time to CKF; AEs and/or death | Treatment with CsA can be a good therapeutic option in both children with steroid-sensitive or SR FSGS, which may save the toxic effects of long-term, large doses of steroids and improve the control of cases and possibly ameliorate the natural progressive course of the disease |
| Martinelli et al, 2001 (Brazil)74 | Prospective | Pred ± CYC (39) | Primary FSGS | ≤18 | 100% | — | — | — | — | Follow-up: 84.6 ± 79.8 mo | Response to therapy; kidney survival; progression or time to CKF; AEs and/or death | Remission of the proteinuria predicts a good long-term outcome in children with NS and FSGS; the use of immunosuppressive medication in conjunction with Pred seems beneficial in the treatment of SR FSGS |
| Martinelli et al, 2004 (Brazil)75 | Open-label, nonrandomized, long-term trial | Arm 1: Pred ± ACEi (30); Arm 2: Pred + CYC ± ACEi (24) |
NS idiopathic FSGS | 24.0 ± 12.0; 14.2 ± 12.1 |
100% | — | — | — | — | Follow-up: 51.5 ± 59.1 mo; 113.8 ± 88.6 mo |
Response to therapy; kidney survival; progression or time to CKF; AEs and/or death | CYC in combination with steroids could be a reasonable choice in the treatment of SR FSGS |
| Mendoza et al, 1990 (USA)76 | Retrospective | Pred + MP ± CYC or chlorambucil (23) | SRNS FSGS | ≤15 | 100% | — | 11.6 ± 7.1 | 133.5 ± 63.5 | — | Follow-up: 45.7 ± 26.6 mo | Proteinuria or UP/C; eGFR; kidney survival; progression or time to CKF; AEs and/or death | This group of children with FSGS has done extremely well following treatment with intravenous MP; the outcome in this retrospective study is significantly better than those in all series we reviewed of children with SR NS and FSGS |
| Meyrier et al, 1994 (France)77 | Prospective | CsA ± Pred (36; 14 with FSGS) | NS idiopathic FSGS | ≥15 | 100% | — | — | — | — | Follow-up: 78 mo; CsA therapy duration: 78 mo |
Response to therapy; SrCr | FSGS with preexisting, incipient kidney insufficiency and tubulointerstitial lesions should be considered relatively hazardous; a repeat biopsy after a year of treatment is necessary to determine whether treatment can be safely continued or should be stopped; when prolonged remission has been obtained with CsA treatment of more than a year, progressing tapering of the drug to a stop is often followed by stable remission |
| Mubarak et al, 2010 (Pakistan)78 | Retrospective | Steroids ± CsA (10) | Idiopathic, collapsing FSGS | ≥18 | 100% | — | — | — | — | Follow-up: 28.7 ± 16.7 mo | Response to therapy; SrCr; kidney survival; progression or time to CKF; AEs and/or death | These preliminary results show that CsA may be effective in the treatment of patients with NS that resist every other form of treatment and especially in the treatment of those with minimal change to lipoid nephrosis |
| Mungan et al, 2015 (Turkey)79 | Retrospective | Steroids + ACEi and/or ARBs ± cytotoxic agents (20) | Primary FSGS-tip variant | >18 | Not specified | 5167.35 ± 3975.1 | — | — | — | Follow-up: 46 mo (range, 3-137 mo) | Response to therapy; proteinuria or UP/C; SrCr | FSGS-tip variant is a favorable subtype among other FSGS forms; immunosuppressive treatment with steroids that may or may not include cytotoxic agents will resolve proteinuria in the majority of cases |
| Naseri et al, 2009 (Iran)80 | Prospective | Pred ± CYC ± CsA (62) | Idiopathic FSGS | <15 | Not specified | — | — | — | — | Follow-up mean, 7 y and 2 mo (range, 3 mo-16 mo and 4 mo) | Response to therapy; kidney survival; progression or time to CKF; AEs and/or death | Favorable response to steroid and CYC treatment is a protective factor against disease progression to CKF, and resistance to immunosuppressants implies a poor prognosis |
| Newman et al, 1976 (USA)81 | Retrospective | Steroids ± CYC or AZA (33) | FSGS | Children: ≤16 Adults: ≥17 |
57.6% | — | — | — | — | Not specified | Response to therapy; SrCr; BP; AEs and/or death | FGS in adults represents a more severe and progressive disease process and is less responsive to therapy |
| Niaudet, 1994 (France)82 | Prospective | CsA + Pred ± nifedipine and/or acebutolol (20) | SRNS idiopathic FSGS | ≤15 | 100% | — | — | — | — | Follow-up: 45 mo | Response to therapy; kidney survival; progression or time to CKF | CsA, in combination with steroids, is effective in a significant proportion of patients with SR, idiopathic nephrosis |
| Paik et al, 2007 (South Korea)83 | Retrospective | Pred ± CYC ± CsA ± MP (92) | SR primary FSGS | ≤15 | 92.4% | — | 24.4 ± 25.3 | — | — | Follow-up: 98.2 ± 63.3 mo; Therapy duration: MP: 14.2 ± 10.1 mo; CYC and Pred: 2.8 ± 0.8 mo; CsA and Pred: 9.3 ± 6.8 mo |
Response to therapy; kidney survival; progression or time to CKF; HR | A more prolonged use of corticosteroid therapy and early introduction of CsA may improve the prognosis for primary FSGS in patients with initial steroid nonresponsiveness |
| Pei et al, 1987 (Canada)84 | Prospective | Arm 1: Adults - steroids ± CYC or AZA (55); Arm 2: Children - steroids ± CYC or AZA (38) |
Idiopathic FSGS | Children: ≤16; Adults: >16 |
Not specified | — | — | — | 1.5 ± 1.11 mL/second/1.73 m2; 1.4 ± 0.511 mL/second/1.73 m2 |
Follow-up: 59 mo; 64 mo |
Kidney survival; progression or time to CKF | All patients with the “typical” lesion of FSGS and idiopathic NS should be treated with a course of steroids, with or without a cytotoxic drug, for up to 6 mo; a significant response rate for both adults and children |
| Ponticelli et al, 1999 (Italy)85 | Retrospective | Arm 1: Pred ± MP (53); Arm 2: CYC ± AZA ± MP ± Pred ± CsA ± diuretics ± ACEi ± hypolipemics (27) |
Primary FSGS | >18 | 100% | 6.96 ± 4.36; 6.58 ± 4.42 |
— | — | — | Follow-up: 86 mo (range, 12-342 mo); Therapy duration: Pred: 24.5 ± 25.68 wk MP: 19.1 ± 12.34 wk; Other immunosuppressants: 87.1 ± 84.23 wk |
Response to therapy; kidney survival; progression or time to CKF; AEs and/or death; HR | ∼70% of adults with FSGS and NS given prolonged treatment with steroids, immunosuppressive agents, or CsA may still have normal kidney function after 10 y, with a high probability of being without NS |
| Raja et al, 2016 (India)8 | Prospective, observational | Arm 1: Collapsing FSGS (Pred ± CYC ± Tac ± rituximab + ARBs; 22); Arm 2: Resistant FSGS (Pred ± CYC ± Tac ± rituximab + ARBs; 19) |
Collapsing primary FSGS | ≥14 | 50%; 57.9% |
4.6 ± 3.0; 4.5 ± 3.8 |
— | — | — | Follow-up: 6 to 24 mo | Response to therapy; proteinuria or UP/C; SrCr; kidney survival; progression or time to CKF; AEs and/or death | Cases with collapsing FSGS have a dismal outcome; the prognosis of therapy-resistant, noncollapsing FSGS and collapsing FSGS are similar and lend support to the hypothesis of therapy response being among the most important predictors of progression to CKF |
| Ramachandran et al, 2014 (India)86 | Prospective, observational study | Arm 1: Tac responsive (Tac + Pred + ARBs + atorvastatin; 23); Arm 2: Tac resistant (Tac + Pred + ARBs + atorvastatin; 21); Total: All patients (Tac + Pred + ARBs + atorvastatin; 44) |
SRNS primary FSGS | ≥18 | 100% | 4.57 ± 4.16; 4.57 ± 2.98; 4.57 ± 3.6 |
— | 98.42 ± 22.37; 105.1 ± 21.56; 101.6 ± 24.4 |
— | Follow-up; Tac tx duration, all patients: 76.64 ± 16.86 wk; 48 wk | Response to therapy; proteinuria or UP/C; SrCr; eGFR; AEs and/or death | Tac is just as effective as any other CNI, but this effectiveness comes at the cost of increased infections, impaired and fasting glucose and diabetes mellitus |
| Ren et al, 2013 (China)87 | Prospective, open-label, randomized controlled trial | Arm 1: Pulse CYC + Pred ± ACEi and/or ARBs ± CCB (18); Arm 2: Tac + Pred ± ACEi and/or ARBs ± CCB (15) |
SR or SD primary FSGS | >18 | 100% | 3.7 ± 0.5; 4.1 ± 0.5 |
— | — | — | Follow-up: 12 mo; Therapy duration: 12 mo |
Response to therapy; proteinuria or UP/C; SrCr; kidney survival; progression or time to CKF; AEs and/or death | Combination of steroids with either CYC or Tac has similar beneficial effect in treatment of patients with refractory (SD or SR primary) FSGS, as indicated by declined proteinuria, an elevated albumin level in plasma, and stable kidney function; the efficacious effect of Tac is similar to the effect of CYC in a short-term treatment |
| Rennert et al, 1999 (South Africa)88 | Prospective | CYC + Pred (10) | SRNS FSGS | 6.2 years; ± 3.8 years | 100% | — | 2.3 ± 2.81 | — | — | Follow-up after IVCP: 26 ± 9 mo; IVCP therapy duration: 6 mo |
eGFR; AEs and/or death | IV CYC is a valuable adjunctive therapy for children with SRNS and FSGS; remission can be achieved and sustained in the majority of cases, with minimal risk of drug-related side effects |
| Risler et al, 1996 (Germany)89 | Prospective | Arm 1: Pred + acetylsalicylate, then CsA (23); Arm 2: Pred ± chlorambucil if no response to corticosteroids, then CsA (24) |
Primary GN | >16 | 100% | 5.4 ± 5.2; 3.4 ± 4.9 |
— | — | — | Follow-up: 36 mo | Proteinuria or UP/C; kidney survival; progression or time to CKF | In some but not all patients with FSGS, CsA can reduce proteinuria |
| Roberti & Vyas, 2010 (USA)90 | Retrospective chart review | Tac ± CsA or ACEi, ARBs, or statins (19; 10 with FSGS) | SRNS FSGS | <15 | 100% | — | 7.3 ± 3.7 | — | — | Follow-up: mean: 55 mo (range, 17-111 mo) | Response to therapy; proteinuria or UP/C; kidney survival; progression or time to CKF; AEs and/or death |
Tac therapy in children with severe NS is well tolerated and initially quite efficacious in achieving remission |
| Rydel et al, 1995 (USA)91 | Retrospective | Pred ± cytotoxic agents (81 patients; 30 treated out of 60 patients with NS) | Primary FSGS | 35 ± 16 | 74% | 8.1 ± 8.1 | — | — | — | Follow-up: 62 ± 75 mo | Response to therapy; kidney survival; progression or time to CKF | Patients with NS with primary FSGS may benefit from a more prolonged course of therapy with Pred |
| Schwartz et al, 1999 (USA)92 | Retrospective, clinicopathologic analysis | Arm 1: FSGS-classical segmental scars (Pred; 57); Arm 2: FSGS-cellular lesion (Pred; 43) |
Primary FSGS | >18 | 63%; 91% |
4.8 ± 3.6; 12.2 ± 9.8 |
— | — | — | Follow-up: 75 ± 85 mo; 39 ± 37 mo |
Kidney survival; progression or time to CKF; HR | The favorable outcome associated with a remission of proteinuria strongly supports therapeutic intervention in patients with NS with primary FSGS, regardless of the presence of the cellular lesion |
| Segarra et al, 2002 (Spain)93 | Noncomparative, open, uncontrolled (prospective) | Pred + Tac + ACEi and/or ARBs ± other AH ± statins (25) | SR idiopathic FSGS | >18 | 56% | 10.3 ± 9.5 | — | 73 ± 15 | — | Follow-up: up to 2 y | Response to therapy; proteinuria or UP/C SrCr; eGFR; CrCl; kidney survival; progression or time to CKF; AEs and/or death |
Combined therapy of Tac and steroids induced sustained remission of proteinuria in a significant number of patients with idiopathic focal glomerulosclerosis whose disease was not controlled by the standard therapy of steroids and CsA |
| Segarra et al, 2007 (Spain)94 | Observational clinical trial | MMF + ACEi and/or ARBs ± other AH ± statins (98; 22 with FSGS) | SR primary FSGS | >18 | 100% | 7.2 ± 3.2 | — | 67.1 ± 6.3 | — | Follow-up: 12 mo; Therapy duration: 12 mo |
Response to therapy; proteinuria or UP/C; eGFR; BP | The data do not allow us either to quantify definitively the efficacy of MMF in primary glomerulonephritis that is resistant to other, conventional therapies or to establish solid treatment rules; however, when MMF is prescribed as a rescue treatment, it can cause a moderate reduction of proteinuria in more than half of the patients who do not have other treatment options |
| Segarra Medrano et al, 2011 (Spain)95 | Observational study with prospective follow-up | CsA + MMF + ACEi and/or ARBs ± amlodipine and/or furosamide ± statins (27) | Steroid- and CsA-resistant primary FSGS | >18 | 100% | 7.74 ± 3.9 | — | 88.6 ± 16.5 | — | Follow-up: 5 y | Response to therapy; proteinuria or UP/C; eGFR; BP; kidney survival; progression or time to CKF; AEs and/or death |
The data from this pilot study show that for patients with CsA-resistant FSGS, treatment combination of CsA and MMF for 12 mo does not significantly modify the evolution of kidney function, although it may induce partial reductions in proteinuria in some patients |
| Senthil Nayagam et al, 2008 (India)14 | Randomized, open-label study | Arm 1: MMF + Pred + ACEi and/or ARBs ± other AH ± diuretics ± statins (28; 17 with FSGS); Arm 2: Pred + ACEi and/or ARBs ± other AH ± diuretics ± statins (26; 16 with FSGS) |
FSGS | ≥18 | 100% | 4.68 ± 1.82 (all patients); 4.95 ± 1.65 (all patients) |
— | 87 ± 14.2 (FSGS specific); 84 ± 10.1 (FSGS specific) |
— | Follow-up: 15.3 mo (range, 12.8-18.2 mo); 16.2 mo (range, 14.5-19.6 mo) |
Response to therapy; proteinuria or UP/C; eGFR; AEs and/or death |
In the short term, MMF is as effective as a first-line agent as conventional forms of therapy in the management of adults with NS due to FSGS and MN and is well tolerated. MMF-based therapy seems to induce remission faster and reduces exposure to steroids in FSGS |
| Shatat et al, 2007 (USA)96 | Retrospective review of clinical and biochemical data | CsA ± ACEi (16) | SRNS or SDNS primary FSGS | <18 | 100% | — | — | 130.5 (95% CI, 111.7-144.8) | — | Follow-up while on CsA: 21 mo (range, 8-43.5 mo) | Response to therapy; eGFR | In primary FSGS, steroid resistance may predict CsA resistance; genetic testing for known mutations associated with resistance to immunosuppression may be advisable before treatment of patients with SR FSGS with CsA |
| Sherali et al, 2010 (Pakistan)97 | Prospective | CsA + Pred (30) | SDNS + SRNS primary FSGS | <18 | 100% | — | — | — | — | Follow-up: 12 mo; CsA therapy duration: 12 mo |
Response to therapy; SrCr; kidney survival; progression or time to CKF; AEs and/or death | CsA in combination with low-dose, oral Pred was effective in majority of the patients with primary FSGS |
| Silverstein & Craver, 2007 (USA)98 | Retrospective analysis | Steroids ± Tac or CsA ± MMF ± ACEi and/or ARBs (41) | SRNS or SSNS primary FSGS | <18 | 63.4% | — | — | 105.4 ± 4.6 | — | Follow-up: 3.9 ± 0.5 y (range, 1-17 y) | Response to therapy; eGFR; BP; kidney survival; progression or time to CKF | Impression that more aggressive treatment with CNI, in combination with ACEi and/or ARB or with MMF, may provide a more favorable prognosis for kidney survival in pediatric FSGS |
| Singh et al, 1999 (USA)9 | Retrospective | CsA (83; 42 with FSGS) | SRNS FSGS | <18 | 100% | 7.10 ± 7.13 | — | — | — | Follow-up: 22.8 mo; CsA therapy duration: at least 8 wk |
Response to therapy; proteinuria or UP/C; SrCr; kidney survival; progression or time to CKF; AEs and/or death |
CsA showed efficacy in inducing remission in many children with SRNS; whether CsA can prevent progression to CKF in patients with FSGS can only be answered in a large, multicenter trial |
| Stirling et al, 2005 (UK)99 | Retrospective record review | Arm 1: Pred ± CYC ± CsA ± AZA (76); Arm 2: untreated (60) |
NS primary FSGS | >14 | 100% | 10 (IQR, 6-15); 6.2 (IQR, 5-10) |
— | — | — | Pred therapy duration: up to 20 y | Response to therapy; HR | Patients with primary FSGS and nephrotic-range proteinuria, who are treated with corticosteroids, are more likely to enter remission than those who are not treated; remission rates of up to 80% can be achieved with prolonged treatment, and remission is an independent predictor of survival off dialysis; patients who do not achieve remission have a poor prognosis |
| Tarshish et al, 1996 (USA)100 | Prospective, randomized trial | Arm 1: Pred (25); Arm 2: Pred + CYC (35) |
SRNS idiopathic FSGS | ≤19 | 100% | 161 ± 145 mg/h per m2; 227 ± 207.06 mg/h per m2 |
— | 118 ± 42; 109 ± 51.47 |
— | Follow-up: mean, 44.5 mo; mean, 42.4 mo; range, 3-102 mo |
Response to therapy; AEs and/or death | Although 1/4 of the patients underwent CR and another quarter had a decrease in proteinuria, they could not differentiate between spontaneous resolution of the disease and an effect of alternate-day Pred; CYC therapy for children with SR FSGS is not recommended |
| Troyanov et al, 2005 (Canada)101 | Retrospective | Pred ± AZA or CsA ± CYC or MMF ± ACEi and/or ARBs (281) | Primary FSGS | >18 | 68% | — | — | — | 73 ± 31 | Follow-up: 64 mo (range, 12-346 mo) | Response to therapy; eGFR; BP; kidney survival; progression or time to CKF; AEs and/or death; HR | Achievement and maintenance of PR strongly correlated with both reduction in the rate of kidney disease progression and better kidney survival |
| Wasilewska & Zoch-Zwierz, 2004 (Poland)102 | Prospective | Pred + CsA + ACEi (24) | SDNS FSGS | <12 | 100% | 32 ± 19 | — | — | 133 ± 28 | Follow-up: 12 mo; CsA therapy duration: 12 mo |
Proteinuria or UP/C; SrCr; CrCl |
The urinary TGFβ1 level increases in proportion to proteinuria during relapse of the NS; CsA administration, in combination with an ACEi, causes a systematic reduction in urinary TGFβ1, although the concentration is still higher after 12 mo of therapy than in healthy children |
| Zagury et al, 2013 (Brazil)103 | Retrospective | CYC ± CsA + Pred ± MMF (136; 87 with FSGS) | SRNS idiopathic FSGS | <18 | 100% | — | — | — | — | Follow-up: 6.1 y (0.25-30.83 y) | Kidney survival; progression or time to CKF; AEs and/or death; HR | CsA resistance and FSGS were risk factors for CKF |
Note: Unless otherwise specified, data are reported as means ± standard deviations or medians (95% CIs, IQRs, or ranges).
Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; AE, adverse event; AH, antihypertensives; AP, antiplatelets; ARB, angiotensin receptor blockers; AZA, azathioprine; BP, blood pressure; CCB, calcium channel blockers or inhibitors; CI, confidence interval; CKD, chronic kidney disease; CKF, chronic kidney failure; CNI, calcineurin inhibitors; CrCl, creatinine clearance; CR, complete remission; CsA, cyclosporine or cyclosporine A; CYC, cyclophosphamide; DEX, dexamethasone; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; FSGS, focal segmental glomerulosclerosis; GN, glomerulonephropathy; HR, hazard ratio; IQR, interquartile range; IV, intravenous; IVCP, intravenous pulse cyclophosphamide; MCD, minimal change disease; MMF, mycophenolate mofetil; MN, membranous nephropathy; MP, methylprednisone; NOS, nitric oxide synthase; NS, nephrotic syndrome; ORG, obesity-related glomerulopathy; Palb, albumin permeability; Pred, prednisone or prednisolone; SD, steroid dependent; SDNS, steroid-dependent nephrotic syndrome; SR, steroid resistant; SrCr, serum creatinine; SRNS, steroid-resistant nephrotic syndrome; SSNS, steroid sensitive nephrotic syndrome; suPAR, soluble urokinase plasminogen activator receptor; Tac, Tacrolimus; TGFβ1, transforming growth factor β; TRPC6, transient receptor potential cation channel subfamily C member 6; UP/C, urinary protein-creatinine.
Effect on Proteinuria
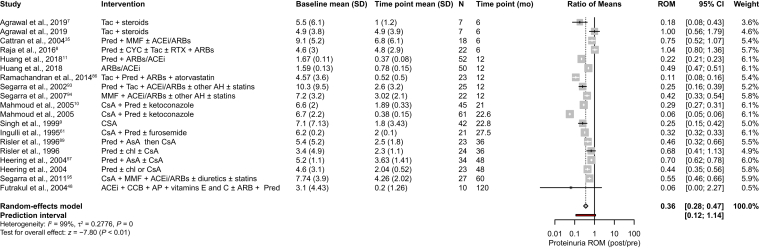
Twenty-three studies assessed daily proteinuria after treatment with immunosuppressants, of which 9 studies were excluded in the meta-analysis because of incompatibility of the reported data with this type of analysis (eg, only median values of urine protein were available; variance results were lacking and/or calculation of the standard deviation from the presented data was not possible; or results were only associated with the type of response after immunosuppressive treatment). A ROM meta-analysis was performed with the remaining 14 studies and is depicted in Fig 2.
Figure 2.
Changes in daily proteinuria outcomes in patients treated with immunosuppressants. Changes in daily proteinuria are expressed as the ratio of means (response ratio) between measurements from the last time point reported and baseline. Study arms with the same treatment within 1 study were included if they corresponded to different patient populations. In Agrawal et al7 the patients were tacrolimus responsive (first arm in the figure) and tacrolimus resistant (second arm in the figure); in Mahmoud et al10 the patients had SRNS (first arm in the figure, arm 2 in Table 1) and SDNS (second arm in the figure, arm 1 in Table 1). The summary effect of all studies, regardless of the type of immunosuppressant therapy, is highlighted in bold. Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; AH, antihypertensives; AP, antiplatelets; ARB, angiotensin receptor blockers; AsA, acetylsalicylate; CCB, calcium channel blockers; chl, chloramphenicol; CI, confidence interval; CsA, cyclosporine A; CYC, cyclophosphamide; MMF, mycophenolate mofetil; Pred, prednisone; ROM, ratio of means; RTX, rituximab; SD, standard deviation; SDNS, steroid-dependent nephrotic syndrome; SRNS, steroid-resistant nephrotic syndrome; Tac, tacrolimus.
In patients treated with immunosuppressants, independently of their class, a statistically significant reduction in daily proteinuria of >50% was observed from baseline to the last follow-up measurement (ROM, 0.36; 95% CI, 0.20-0.47; Fig 2). Although the majority of the pooled studies showed a reduction in daily proteinuria, in 2 studies,7,8 in which patients with primary FSGS received a combination of different immunosuppressants, daily proteinuria values remained unchanged after a follow-up period of 6 months.
Of the 14 pooled studies, only 1 retrospective study9 evaluated daily proteinuria in patients treated with cyclosporine as a monotherapy, and a 75% reduction in this outcome was reported after a follow-up period of 22.8 months (ROM, 0.25; 95% CI, 0.15-0.42; Fig 2).
A retrospective study10 assessed the effect on daily proteinuria when children with steroid-dependent or steroid-resistant primary FSGS received cyclosporine in combination with prednisone, in addition to ketoconazole for some patients. Both population subgroups experienced a reduction in daily proteinuria of >50%. This decrease was more pronounced in patients that were steroid dependent (Fig 2).10
The majority of the studies included in the meta-analysis had a retrospective design, making it difficult to estimate the effects on daily proteinuria at specific periods of time. However, there were sufficient eligible studies to stratify the results by durations of 6 months (n = 3 studies) and 12 months (n = 4 studies). A more prominent and significant decrease in daily proteinuria was observed at 12 months (ROM, 0.27; 95% CI, 0.16-0.44) when compared with daily proteinuria evaluated at 6 months (ROM, 0.69; 95% CI, 0.41-1.16; Fig S1).
As observed with daily proteinuria, pooling of studies assessing the urinary protein-creatinine ratio after immunosuppressive treatment (n = 7 studies) also demonstrated a decrease of >50% in this outcome from baseline to the follow-up time point (ROM, 0.27; 95% CI, 0.20-0.38; follow-up period ranging from 8-62 months; Fig S2).
Only 1 controlled study11 estimated the effects of immunosuppressive versus nonimmunosuppressive therapies (prednisone + ACEi and/or angiotensin receptor blockers vs ACEi and/or angiotensin receptor blockers) on daily proteinuria. The last follow-up measurement (12 months) showed that adding immunosuppressants to the treatment regimen led to a stronger reduction in daily proteinuria than ACEi and/or angiotensin receptor blocker monotherapy (MD, −0.41; 95% CI, −0.46 to −0.36; Fig 3). However, in contrast to most primary FSGS studies that look at patients with nephrotic syndrome, this study was conducted in patients with subnephrotic proteinuria (1-3.5 g/24 h).
Figure 3.
Comparison of immunosuppressive treatment versus nonimmunosuppressive treatment on daily proteinuria. The treatment effect is expressed as the MD between the intervention and control arms at the last time point (prednisone + ACEi and/or ARBs vs ACEi and/or ARBs alone). Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; CI, confidence interval; Ctrl, control; MD, mean difference; SD, standard deviation; Tx, therapy.
Effect on Kidney Function
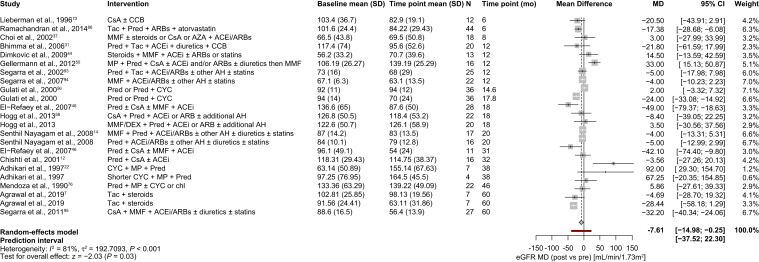
The glomerular filtration rate is the key indicator of kidney function and can be determined with several methods, such as eGFR and CrCl.104 In this systematic literature review, 20 studies were considered eligible to estimate the MD in the glomerular filtration rate between various follow-up and baseline measurements, using eGFR in 18 studies and CrCl in 2 studies. All studies evaluated the effects of concomitant immunosuppressive therapies, composed of different types of immunosuppressants and/or nonimmunosuppressive medicines.
In studies reporting eGFR, the mean eGFR at baseline was 96.8 mL/min/1.73 m2, and a statistically significant decrease of 7.61 mL/min/1.73 m2 was observed after follow-up at any time point (MD, −7.61; 95% CI, −14.98 to −0.25; Fig 4). When the pooled studies were stratified by length of the follow-up period or time point, statistically significant reductions in the eGFR were observed after various months of follow-up (eg, 6, 18, 31, 38, and 60 months; Figs S3 and S4). However, there was no clear correlation between the length of follow-up and the observed effect size.
Figure 4.
Changes in eGFR in patients treated with immunosuppressants. Changes in eGFR are expressed as MDs between measurements at the last time point and baseline. Study arms with the same treatment within 1 study were included if they corresponded to different patient populations. In Agrawal et al7 the patients were tacrolimus responsive (first arm in the figure) and tacrolimus resistant (second arm in the figure); in Gulati et al,56 there were patients with early onset (first arm in the figure) and late-onset (second arm in the figure) of idiopathic FSGS. The summary effect of all studies, regardless of the type of immunosuppressant therapy, is highlighted in bold. Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; AH, antihypertensives; ARB, angiotensin receptor blockers; AZA, azathioprine; CCB, calcium channel blockers; chl, chloramphenicol; CI, confidence interval; CsA, cyclosporine A; CYC, cyclophosphamide; DEX, dexamethasone; FSGS, focal segmental glomerulosclerosis; eGFR, estimated glomerular filtration rate; MD, mean difference; MMF, mycophenolate mofetil; MP, methylprednisolone; Pred, prednisone; SD, standard deviation; Tac, tacrolimus.
One study12 measured eGFR values from baseline to follow-up in patients with primary FSGS that were both steroid-dependent and steroid-resistant, after they received low-dose cyclosporine, prednisone, and, in certain patients, ACEi. Although a statistical comparison of these 2 patient subgroups was not performed by the authors, an increase in eGFR was observed in patients that were steroid dependent, whereas those that were steroid resistant experienced a reduction in kidney function from baseline to follow-up.
Only 2 controlled studies were eligible for inclusion in a meta-analysis to estimate the effects on eGFR of immunosuppressive treatments versus control.13,14 Both trials demonstrated suboptimal quality, because no allocation concealment and blinding of investigators was practiced. However, in both trials the investigated arms were comparable for treatment completion and were followed for an equal length of time, and in Cattran et al13 participants were blinded to treatment allocation. The standardized MD meta-analysis results showed that concomitant use of nonsteroid (mycophenolate mofetil in Senthil Nayagam et al14 or cyclosporine in Cattran et al13) and steroid (prednisolone) drugs as immunosuppressant treatment had uncertain or inconclusive effects on the glomerular filtration rate when compared with the use of steroids alone (Fig 5).
Figure 5.
Comparison of the effects of combinations of immunosuppressive treatments (nonsteroid + prednisone vs prednisone alone) on GFR (any measurement). The treatment effect is expressed as the SMD between the intervention and control arms at the last time point. Abbreviations: CI, confidence interval; Ctrl, control; GFR, glomerular filtration rate; MD, mean difference; SMD, standardized mean difference; Tx, therapy.
In the 2 studies assessing the effect of immunosuppressive treatment on CrCl,13,15 a statistically significant reduction of 25.0 mL/min/1.73 m2 (MD, −25.03; 95% CI, −59.33 to −9.27) was observed at the last follow-up in relation to a mean baseline value of 144.6 mL/min/1.73 m2 (Fig 6). The significant decline in CrCl should be interpreted with caution because of the high mean CrCl value at baseline, suggesting possible hyperfiltration in some patients, as well as the possibility of natural disease progression.
Figure 6.
Changes in CrCl in patients treated with immunosuppressants. Changes in CrCl are expressed as the MD between measurements at the last time point and baseline. The summary effect of all studies, regardless of the type of immunosuppressant therapy, is highlighted in bold. Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; AH, antihypertensives; ARB, angiotensin receptor blockers; CI, confidence interval; CrCl, creatinine clearance; CsA, cyclosporine A; MD, mean difference; Pred, prednisone; SD, standard deviation; Tac, tacrolimus.
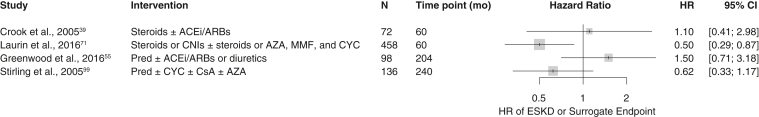
Kidney Survival
Only 4 retrospective studies reported the HRs of reaching CKF for the use of immunosuppressive medications as a categorical variable (exposure to immunosuppressant treatment vs no exposure). Although these studies used the same measure to determine the impacts of immunosuppressants on kidney survival outcomes, they also showed considerable degrees of heterogeneity in patient populations and treatment regimens. Therefore, the pooled effect of immunosuppressive treatment on kidney survival, when defined as the risk of reaching CKF, was judged to be inconclusive (Fig 7).
Figure 7.
Effects of immunosuppressive treatments on the risk of reaching CKF (or a surrogate end point), assessed using the univariate HR. The risk of reaching CKF (or surrogate end point) is expressed as the HR. Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; AZA, azathioprine; CI, confidence interval; CKF, chronic kidney failure; CNI, calcineurin inhibitor; CsA, cyclosporine A; CYC, cyclophosphamide; ESKD, end-stage kidney disease; HR, hazard ratio; MMF, mycophenolate mofetil; Pred, prednisone.
Safety and Tolerability
Forty-six out of the 98 included studies showed safety and tolerability outcomes potentially related to the use of immunosuppressants, either as monotherapy or in combination with other immunosuppressant or nonimmunosuppressant medications. Hypertension (n = 21 studies) and infections (n = 20 studies) were the most frequently described adverse events in all patient cohorts. When these events were considered by the authors as treatment related, a higher association was observed with CNI monotherapy treatment than with other classes of immunosuppressants. Death was reported in 20 studies evaluating a variety of immunosuppressant treatment regimens, with only 1 study reporting steroid-related deaths due to sepsis in 2 patients,16 and another study reporting the death of 1 patient when receiving cyclosporine monotherapy.17 Other mortality events were not associated with treatment. Hyperkalemia was only reported in 1 study,18 in 18.6% of patients that were steroid resistant, nephrotic, and had primary FSGS, who were treated with dexamethasone or methylprednisolone in combination with cyclophosphamide. Other side effects, such as hospitalization, were not common among the different treatment cohorts, and could not be associated with a specific immunosuppressive therapy.
Discussion
FSGS is a histologic pattern of glomerular injury resulting from heterogeneous clinicopathology entities, and can lead to declines in kidney function and progression to CKF. Steroids and other immunosuppressive drugs are often used to treat patients with primary FSGS suffering from nephrotic syndrome. We have performed a systematic literature review and meta-analysis aimed at assessing the clinical effectiveness and safety of immunosuppressants in the treatment of primary FSGS.
This systematic literature review demonstrates that patients treated with immunosuppressants experienced, on average, decreases in proteinuria from baseline to varying follow-up time points. The effect was more pronounced with longer treatment periods, suggesting that immunosuppressive therapy should be given for at least 6-12 months. Multiple immunosuppressant regimens were evaluated, with the majority of studies (8 of 14) assessing the combination of CNI and corticosteroids. The studies in this analysis include pediatric (n = 3 studies), adult (n = 8 studies), and mixed adult and pediatric (n = 3 studies) populations. Only 1 study (Huang et al11) compared renin-angiotensin system blockade alone to corticosteroid therapy. Interpretation of this study is limited, as it only included patients with subnephrotic levels of proteinuria, which is uncommon in primary FSGS. Because of the significant heterogeneity observed among studies and the lack of properly controlled studies, it is difficult to determine how much of the observed effect can be attributed to immunosuppressive treatment. There is a need for more data on potential therapies for primary FSGS, including rituximab and other monoclonal antibodies, which have not been studied in controlled trials in this population.
The effects of immunosuppression on eGFR, CrCl, and kidney survival are uncertain or inconclusive because of high degrees of variability among available studies. The impacts of immunosuppressive treatment on kidney function may require administration of the drugs for longer periods of time than the durations of the studies included in this systematic literature review.
In addition, the safety and tolerability data were limited in the available studies. The most common adverse events included infection and hypertension. Surprisingly, there was very limited reporting of hyperkalemia, which is a well-known side effect of CNIs, especially when combined with renin-angiotensin-aldosterone system blockade. Given the variability in study designs, it is unclear whether hyperkalemia did not occur or whether it was underreported.
Finally, all evaluated studies purportedly included patients with “idiopathic” or “primary” FSGS. The determination of primary FSGS is not well defined, in part because of the lack of measurable biomarkers to distinguish primary from secondary FSGS. The 2021 KDIGO glomerular disease guidelines use more stringent criteria for primary FSGS in adults (proteinuria >3.5 g/day plus serum albumin <30 g/L; with or without edema; the presence of diffuse foot process effacement on biopsy; and no secondary cause identified).4 Evaluation of immunosuppressive therapy in RCTs with enrollment based on delineation of the underlying pathophysiologic mechanism of disease may have a higher likelihood of demonstrating beneficial outcomes. In addition to distinguishing primary FSGS and secondary FSGS, screening for and identification of genetic causes of FSGS are needed for accurate evaluation of beneficial treatments for patients with different forms of FSGS and optimal clinical management.
Knowledge of a patient’s form of genetic FSGS has implications for individualized treatment, as responses to therapy, such as glucocorticoid sensitivity, resistance to immunosuppression, and potential response to CNIs, have been associated with some genes.1,105 Genetic testing using the most recent, large gene panels or whole-exon sequencing can be used to identify pathogenic mutations in genes that result in high-penetrance genetic FSGS, as well as APOL1 risk allele–associated FSGS in patients with sub-Saharan African ancestry.1,105 In the studies reviewed in the current systematic literature review, just 1 study examined a subpopulation of genetic FSGS. In the absence of genetic screening, it is likely that some patients with unrecognized genetic FSGS were included among the intended patients with primary FSGS, which may have led to misinterpretation of treatment responses in primary FSGS. Further controlled studies are needed to systematically examine different types of genetic FSGS and responses to therapies.
In conclusion, this systematic literature review highlights the limited evidence currently available in the literature and the need for better-designed, adequately controlled studies to reliably assess the effects of immunosuppressants on patients with primary FSGS and to evaluate the safety and tolerability of these treatments. It also underscores the need for clarity in the definition of “primary” FSGS. RCTs with clear entry criteria to identify patients with primary FSGS are needed to adequately evaluate immunosuppressive regimens in the treatment of primary FSGS.
Article Information
Authors’ Full Names and Academic Degrees
Dawn J. Caster, MD, Barbara Magalhaes, PhD, Natali Pennese, PhD, Andrea Zaffalon, PhD, Marina Faiella, PhD, Kirk N. Campbell, MD, Jai Radhakrishnan, MD, Vladmir Tesar, MD, and Howard Trachtman, MD.
Authors’ Contributions
Research idea and study design: BM, NP, AZ, MF; data extraction and analysis: BM, NP, AZ, MF; data interpretation: DJC, KNC, JR, VT, HT. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This systematic literature review was funded by Travere Therapeutics, Inc. Travere Therapeutics did not have a role in the study design; collection, analysis, and interpretation of the data; writing of the report; or the decision to submit the report for publication.
Financial Disclosure
Dr Caster has received consultancy fees from Aurinia, Calliditas, GlaxoSmithKline, and Travere Therapeutics. Drs Magalhaes, Pennese, Zaffalon, Faiella, have received consultancy fees from Travere Therapeutics. Dr Campbell has received consultancy fees from Aurinia, Goldfinch, Mallinckrodt, and Travere Therapeutics. Dr Radhakrishnan has no competing interests to declare. Dr Tesar has received consultancy fees from AbbVie, Amgen, Bayer, Boehringer-Ingelheim, ChemoCentryx, and Fresenius Medical Care. Dr Trachtman has received consultancy fees from ChemoCentryx, Kaneka, and Otsuka; was previously a consultant to Genzyme and Optherion; and has consultancy agreements with Goldfinch Biopharma and Travere Therapeutics through New York University.
Acknowledgements
Editorial support was provided by Courtney Breuel, ELS, of MedVal Scientific Information Services, LLC (Princeton, NJ).
Peer Review
Received May 11, 2022. Evaluated by 2 external peer reviewers, with direct editorial input by an Associate Editor and the Editor-in-Chief. Accepted in revised form June 2, 2022.
Footnotes
Complete author and article information provided before references.
Figure S1. Change in daily proteinuria in patients treated with immunosuppressants, stratified by timepoint
Figure S2. Change in UP/C ratio in patients treated with immunosuppressants
Figure S3. Change in eGFR in patients treated with immunosuppressants stratified by timepoint
Figure S4. Correlation between the last reported follow-up timepoint and the eGFR mean difference from baseline to last reported follow-up timepoint
Table S1. Systematic literature review protocol for PubMed search
Table S2. Systematic literature review protocol for EMBASE search
Table S3. Systematic literature review protocol for Cochrane search
Table S4. Bias assessment for RCTs included in the SLR.
Supplementary Material
Figures S1-S4. Tables S1-S3.
References
- 1.Rosenberg A.Z., Kopp J.B. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12(3):502–517. doi: 10.2215/CJN.05960616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Vriese A.S., Sethi S., Nath K.A., Glassock R.J., Fervenza F.C. Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J Am Soc Nephrol. 2018;29(3):759–774. doi: 10.1681/ASN.2017090958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun N., Schmutzler F., Lange C., et al. Immunosuppressive treatment for focal segmental glomerulosclerosis in adults. Cochrane Database Syst Rev. 2008;2008(3):CD003233. doi: 10.1002/14651858.CD003233.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4s):S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Beer A., Mayer G., Kronbichler A. Treatment strategies of adult primary focal segmental glomerulosclerosis: a systematic review focusing on the last two decades. BioMed Res Int. 2016;2016 doi: 10.1155/2016/4192578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrich J.H., Geerlings C., Zivicnjak M., Franke D., Geerlings H., Gellermann J. Steroid-resistant idiopathic childhood nephrosis: overdiagnosed and undertreated. Nephrol Dial Transplant. 2007;22(8):2183–2193. doi: 10.1093/ndt/gfm092. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal P., Nada R., Ramachandran R., Rayat C.S., Kumar A., Kohli H.S. Loss of subpodocytic space predicts poor response to tacrolimus in steroid-resistant calcineurin inhibitor-naïve adult-onset primary focal segmental glomerulosclerosis. Indian J Nephrol. 2019;29(2):90–94. doi: 10.4103/ijn.IJN_422_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raja R., Nada R., Yadav A.K., et al. A prospective study of collapsing focal segmental glomerulosclerosis. Ren Fail. 2016;38(6):894–898. doi: 10.3109/0886022X.2016.1164063. [DOI] [PubMed] [Google Scholar]
- 9.Singh A., Tejani C., Tejani A. One-center experience with cyclosporine in refractory nephrotic syndrome in children. Pediatr Nephrol. 1999;13(1):26–32. doi: 10.1007/s004670050557. [DOI] [PubMed] [Google Scholar]
- 10.Mahmoud I., Basuni F., Sabry A., et al. Single-centre experience with cyclosporin in 106 children with idiopathic focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2005;20(4):735–742. doi: 10.1093/ndt/gfh766. [DOI] [PubMed] [Google Scholar]
- 11.Huang J., Lin L., Xie J., et al. Glucocorticoids in the treatment of patients with primary focal segmental glomerulosclerosis and moderate proteinuria. Clin Exp Nephrol. 2018;22(6):1315–1323. doi: 10.1007/s10157-018-1585-z. [DOI] [PubMed] [Google Scholar]
- 12.Chishti A.S., Sorof J.M., Brewer E.D., Kale A.S. Long-term treatment of focal segmental glomerulosclerosis in children with cyclosporine given as a single daily dose. Am J Kidney Dis. 2001;38(4):754–760. doi: 10.1053/ajkd.2001.27692. [DOI] [PubMed] [Google Scholar]
- 13.Cattran D., Neogi T., Sharma R., McCarthy E.T., Savin V.J. Serial estimates of serum permeability activity and clinical correlates in patients with native kidney focal segmental glomerulosclerosis. J Am Soc Nephrol. 2003;14(2):448–453. doi: 10.1097/01.asn.0000046960.57614.17. [DOI] [PubMed] [Google Scholar]
- 14.Senthil Nayagam L., Ganguli A., Rathi M., et al. Mycophenolate mofetil or standard therapy for membranous nephropathy and focal segmental glomerulosclerosis: a pilot study. Nephrol Dial Transplant. 2008;23(6):1926–1930. doi: 10.1093/ndt/gfm538. [DOI] [PubMed] [Google Scholar]
- 15.Loeffler K., Gowrishankar M., Yiu V. Tacrolimus therapy in pediatric patients with treatment-resistant nephrotic syndrome. Pediatr Nephrol. 2004;19(3):281–287. doi: 10.1007/s00467-003-1370-3. [DOI] [PubMed] [Google Scholar]
- 16.Arias L.F., Franco-Alzate C., Rojas S.L. Tip variant of focal segmental glomerulosclerosis: outcome and comparison to “not otherwise specified” variant. Nephrol Dial Transplant. 2011;26(7):2215–2221. doi: 10.1093/ndt/gfq668. [DOI] [PubMed] [Google Scholar]
- 17.Gorsane I., Helal I., Yacoub I., Hamida F.B., Abderrahim E., Abdallah T.B. Cyclosporine therapy in steroid-dependent or steroid-resistant idiopathic focal and segmental glomerulosclerosis. Saudi J Kidney Dis Transpl. 2016;27(5):958–965. doi: 10.4103/1319-2442.190864. [DOI] [PubMed] [Google Scholar]
- 18.Hari P., Bagga A., Jindal N., Srivastava R.N. Treatment of focal glomerulosclerosis with pulse steroids and oral cyclophosphamide. Pediatr Nephrol. 2001;16(11):901–905. doi: 10.1007/s004670100680. [DOI] [PubMed] [Google Scholar]
- 19.Abeyagunawardena A.S., Sebire N.J., Risdon R.A., et al. Predictors of long-term outcome of children with idiopathic focal segmental glomerulosclerosis. Pediatr Nephrol. 2007;22(2):215–221. doi: 10.1007/s00467-006-0264-6. [DOI] [PubMed] [Google Scholar]
- 20.Abrantes M.M., Cardoso L.S., Lima E.M., et al. Predictive factors of chronic kidney disease in primary focal segmental glomerulosclerosis. Pediatr Nephrol. 2006;21(7):1003–1012. doi: 10.1007/s00467-006-0138-y. [DOI] [PubMed] [Google Scholar]
- 21.Abrantes M.M., Cardoso L.S., Lima E.M., et al. Clinical course of 110 children and adolescents with primary focal segmental glomerulosclerosis. Pediatr Nephrol. 2006;21(4):482–489. doi: 10.1007/s00467-006-0019-4. [DOI] [PubMed] [Google Scholar]
- 22.Adhikari M., Bhimma R., Coovadia H.M. Intensive pulse therapies for focal glomerulosclerosis in South African children. Pediatr Nephrol. 1997;11(4):423–428. doi: 10.1007/s004670050309. [DOI] [PubMed] [Google Scholar]
- 23.Adhikari M., Bhimma R., Coovadia H.M. Focal segmental glomerulosclerosis in children from KwaZulu/Natal, South Africa. Clin Nephrol. 2001;55(1):16–24. [PubMed] [Google Scholar]
- 24.Agarwal S.K., Dash S.C., Tiwari S.C., Bhuyan U.N. Idiopathic adult focal segmental glomerulosclerosis: a clinicopathological study and response to steroid. Nephron. 1993;63(2):168–171. doi: 10.1159/000187177. [DOI] [PubMed] [Google Scholar]
- 25.Al Salloum A.A. Pulse cyclophosphamide therapy for steroid-resistant focal segmental glomerulosclerosis in children. Ann Saudi Med. 2004;24(1):27–30. doi: 10.5144/0256-4947.2004.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexopoulos E., Stangou M., Papagianni A., Pantzaki A., Papadimitriou M. Factors influencing the course and the response to treatment in primary focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2000;15(9):1348–1356. doi: 10.1093/ndt/15.9.1348. [DOI] [PubMed] [Google Scholar]
- 27.Arbus G.S., Poucell S., Bacheyie G.S., Baumal R. Focal segmental glomerulosclerosis with idiopathic nephrotic syndrome: three types of clinical response. J Pediatr. 1982;101(1):40–45. doi: 10.1016/s0022-3476(82)80177-7. [DOI] [PubMed] [Google Scholar]
- 28.Ayar Y., Ersoy A. Can FE. Primary glomerulonephritis: a single-center retrospective experience. Acta Med Mediterr. 2016;32(5):1723–1727. [Google Scholar]
- 29.Bagchi S., Agarwal S., Kalaivani M., et al. Primary FSGS in nephrotic adults: clinical profile, response to immunosuppression and outcome. Nephron. 2016;132(2):81–85. doi: 10.1159/000442999. [DOI] [PubMed] [Google Scholar]
- 30.Beşbaş N., Ozaltin F., Emre S., et al. Clinical course of primary focal segmental glomerulosclerosis (FSGS) in Turkish children: a report from the Turkish Pediatric Nephrology FSGS Study Group. Turk J Pediatr. 2010;52(3):255–261. [PubMed] [Google Scholar]
- 31.Bhimma R., Adhikari M., Asharam K., Connolly C. Management of steroid-resistant focal segmental glomerulosclerosis in children using tacrolimus. Am J Nephrol. 2006;26(6):544–551. doi: 10.1159/000097864. [DOI] [PubMed] [Google Scholar]
- 32.Brodehl J., Brandis M., Helmchen U., et al. Cyclosporin A treatment in children with minimal change nephrotic syndrome and focal segmental glomerulosclerosis. Klin Wochenschr. 1988;66(22):1126–1137. doi: 10.1007/BF01727848. [DOI] [PubMed] [Google Scholar]
- 33.Cattran D.C., Rao P. Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis. 1998;32(1):72–79. doi: 10.1053/ajkd.1998.v32.pm9669427. [DOI] [PubMed] [Google Scholar]
- 34.Cattran D.C., Appel G.B., Hebert L.A., et al. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int. 1999;56(6):2220–2226. doi: 10.1046/j.1523-1755.1999.00778.x. [DOI] [PubMed] [Google Scholar]
- 35.Cattran D.C., Wang M.M., Appel G., Matalon A., Briggs W. Mycophenolate mofetil in the treatment of focal segmental glomerulosclerosis. Clin Nephrol. 2004;62(6):405–411. doi: 10.5414/cnp62405. [DOI] [PubMed] [Google Scholar]
- 36.Chávez-Mendoza C.A., Niño-Cruz J.A., Correa-Rotter R., Uribe-Uribe N.O., Mejía-Vilet J.M. Calcineurin inhibitors with reduced-dose steroids as first-line therapy for focal segmental glomerulosclerosis. Kidney Int Rep. 2019;4(1):40–47. doi: 10.1016/j.ekir.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi M.J., Eustace J.A., Gimenez L.F., et al. Mycophenolate mofetil treatment for primary glomerular diseases. Kidney Int. 2002;61(3):1098–1114. doi: 10.1046/j.1523-1755.2002.00214.x. [DOI] [PubMed] [Google Scholar]
- 38.Chun M.J., Korbet S.M., Schwartz M.M., Lewis E.J. Focal segmental glomerulosclerosis in nephrotic adults: presentation, prognosis, and response to therapy of the histologic variants. J Am Soc Nephrol. 2004;15(8):2169–2177. doi: 10.1097/01.ASN.0000135051.62500.97. [DOI] [PubMed] [Google Scholar]
- 39.Crook E.D., Habeeb D., Gowdy O., Nimmagadda S., Salem M. Effects of steroids in focal segmental glomerulosclerosis in a predominantly African-American population. Am J Med Sci. 2005;330(1):19–24. doi: 10.1097/00000441-200507000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Dumas De La Roque C., Prezelin-Reydit M., Vermorel A., et al. Idiopathic nephrotic syndrome: characteristics and identification of prognostic factors. J Clin Med. 2018;7(9):265. doi: 10.3390/jcm7090265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deegens J.K., Assmann K.J., Steenbergen E.J., et al. Idiopathic focal segmental glomerulosclerosis: a favourable prognosis in untreated patients? Neth J Med. 2005;63(10):393–398. [PubMed] [Google Scholar]
- 42.Deegens J.K., Steenbergen E.J., Borm G.F., Wetzels J.F. Pathological variants of focal segmental glomerulosclerosis in an adult Dutch population–epidemiology and outcome. Nephrol Dial Transplant. 2008;23(1):186–192. doi: 10.1093/ndt/gfm523. [DOI] [PubMed] [Google Scholar]
- 43.Dhanapriya J., Dineshkumar T., Gopalakrishnan N., Sakthirajan R., Balasubramaniyan T. Clinicopathological correlation and treatment response of primary focal segmental glomerulosclerosis in adults and adolescents. Indian J Nephrol. 2016;26(5):347–351. doi: 10.4103/0971-4065.167283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dimkovic N., Jovanovic D., Kovacevic Z., et al. Mycophenolate mofetil in high-risk patients with primary glomerulonephritis: results of a 1-year prospective study. Nephron Clin Pract. 2009;111(3):c189–c196. doi: 10.1159/000199459. [DOI] [PubMed] [Google Scholar]
- 45.El-Husseini A., El-Basuony F., Mahmoud I., et al. Effect of concomitant administration of cyclosporine and ketoconazole in children with focal segmental glomerulosclerosis. Am J Nephrol. 2004;24(3):301–306. doi: 10.1159/000078395. [DOI] [PubMed] [Google Scholar]
- 46.El-Refaey A.M., Kapur G., Jain A., et al. Idiopathic collapsing focal segmental glomerulosclerosis in pediatric patients. Pediatr Nephrol. 2007;22(3):396–402. doi: 10.1007/s00467-006-0312-2. [DOI] [PubMed] [Google Scholar]
- 47.El-Refaey A.M., Bakr A., Hammad A., et al. Primary focal segmental glomerulosclerosis in Egyptian children: a 10-year single-centre experience. Pediatr Nephrol. 2010;25(7):1369–1373. doi: 10.1007/s00467-010-1448-7. [DOI] [PubMed] [Google Scholar]
- 48.Futrakul N., Siriviriyakul P., Deekajorndej T., Futrakul P. Hemodynamic maladjustment and disease progression in nephrosis with FSGS. Ren Fail. 2004;26(3):231–236. doi: 10.1081/jdi-120039520. [DOI] [PubMed] [Google Scholar]
- 49.Futrakul N., Futrakul P., Siriviriyakul P. Correction of peritubular capillary flow reduction with vasodilators restores function in focal segmental glomerulosclerotic nephrosis. Clin Hemorheol Microcirc. 2004;31(3):197–205. [PubMed] [Google Scholar]
- 50.Gellermann J., Ehrich J.H., Querfeld U. Sequential maintenance therapy with cyclosporin A and mycophenolate mofetil for sustained remission of childhood steroid-resistant nephrotic syndrome. Nephrol Dial Transplant. 2012;27(5):1970–1978. doi: 10.1093/ndt/gfr572. [DOI] [PubMed] [Google Scholar]
- 51.Gheissari A., Meamar R., Kheirollahi M., et al. TRPC6 mutational analysis in Iranian children with focal segmental glomerulosclerosis. Iran J Kidney Dis. 2018;12(6):341–349. [PubMed] [Google Scholar]
- 52.Ghiggeri G.M., Catarsi P., Scolari F., et al. Cyclosporine in patients with steroid-resistant nephrotic syndrome: an open-label, nonrandomized, retrospective study. Clin Ther. 2004;26(9):1411–1418. doi: 10.1016/j.clinthera.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 53.Gipson D.S., Trachtman H., Kaskel F.J., et al. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 2011;80(8):868–878. doi: 10.1038/ki.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goumenos D.S., Tsagalis G., El Nahas A.M., et al. Immunosuppressive treatment of idiopathic focal segmental glomerulosclerosis: a five-year follow-up study. Nephron Clin Pract. 2006;104(2):c75–c82. doi: 10.1159/000093993. [DOI] [PubMed] [Google Scholar]
- 55.Greenwood A.M., Gunnarsson R., Neuen B.L., Oliver K., Green S.J., Baer R.A. Clinical presentation, treatment and outcome of focal segmental glomerulosclerosis in Far North Queensland Australian adults. Nephrology (Carlton) 2017;22(7):520–530. doi: 10.1111/nep.12816. [DOI] [PubMed] [Google Scholar]
- 56.Gulati S., Elhence R., Kher V., et al. Early versus late-onset idiopathic focal segmental glomerulosclerosis. Pediatr Nephrol. 2000;14(10-11):960–964. doi: 10.1007/pl00013419. [DOI] [PubMed] [Google Scholar]
- 57.Heering P., Braun N., Müllejans R., et al. Cyclosporine A and chlorambucil in the treatment of idiopathic focal segmental glomerulosclerosis. Am J Kidney Dis. 2004;43(1):10–18. doi: 10.1053/j.ajkd.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 58.Hogg R.J., Friedman A., Greene T., et al. Renal function and proteinuria after successful immunosuppressive therapies in patients with FSGS. Clin J Am Soc Nephrol. 2013;8(2):211–218. doi: 10.2215/CJN.08330812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoseini R., Otukesh H., Fereshtehnejad S.M., et al. Prevalence and outcome of focal segmental glomerulosclerosis in Iranian children with nephrotic syndrome. Iran J Kidney Dis. 2012;6(1):18–24. [PubMed] [Google Scholar]
- 60.Inaba A., Hamasaki Y., Ishikura K., et al. Long-term outcome of idiopathic steroid-resistant nephrotic syndrome in children. Pediatr Nephrol. 2016;31(3):425–434. doi: 10.1007/s00467-015-3174-7. [DOI] [PubMed] [Google Scholar]
- 61.Ingulli E., Singh A., Baqi N., Ahmad H., Moazami S., Tejani A. Aggressive, long-term cyclosporine therapy for steroid-resistant focal segmental glomerulosclerosis. J Am Soc Nephrol. 1995;5(10):1820–1825. doi: 10.1681/ASN.V5101820. [DOI] [PubMed] [Google Scholar]
- 62.Jafry N., Ahmed E., Mubarak M., Kazi J., Akhter F. Raised serum creatinine at presentation does not adversely affect steroid response in primary focal segmental glomerulosclerosis in adults. Nephrol Dial Transplant. 2012;27(3):1101–1106. doi: 10.1093/ndt/gfr430. [DOI] [PubMed] [Google Scholar]
- 63.Jellouli M., Abidi K., Askri M., et al. Focal segmental glomerulosclerosis in children. Tunis Med. 2016;94(5):356–359. [PubMed] [Google Scholar]
- 64.Kallash M., Aviles D. Efficacy of tacrolimus in the treatment of children with focal segmental glomerulosclerosis. World J Pediatr. 2014;10(2):151–154. doi: 10.1007/s12519-014-0484-y. [DOI] [PubMed] [Google Scholar]
- 65.Kambham N., Markowitz G.S., Valeri A.M., Lin J., D'Agati V.D. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59(4):1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 66.Kangovi S., Edwards M., Woloszynek S., et al. Renin-angiotensin-aldosterone system inhibitors in pediatric focal segmental glomerulosclerosis. Pediatr Nephrol. 2012;27(5):813–819. doi: 10.1007/s00467-011-2056-x. [DOI] [PubMed] [Google Scholar]
- 67.Kirpekar R., Yorgin P.D., Tune B.M., Kim M.K., Sibley R.K. Clinicopathologic correlates predict the outcome in children with steroid-resistant idiopathic nephrotic syndrome treated with pulse methylprednisolone therapy. Am J Kidney Dis. 2002;39(6):1143–1152. doi: 10.1053/ajkd.2002.33382. [DOI] [PubMed] [Google Scholar]
- 68.Klaassen I., Özgören B., Sadowski C.E., et al. Response to cyclosporine in steroid-resistant nephrotic syndrome: discontinuation is possible. Pediatr Nephrol. 2015;30(9):1477–1483. doi: 10.1007/s00467-015-3109-3. [DOI] [PubMed] [Google Scholar]
- 69.Korbet S.M., Schwartz M.M., Lewis E.J. The prognosis of focal segmental glomerular sclerosis of adulthood. Medicine. 1986;65(5):304–311. doi: 10.1097/00005792-198609000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Laurin L.P., Gasim A.M., Derebail V.K., et al. Renal survival in patients with collapsing compared with not otherwise specified FSGS. Clin J Am Soc Nephrol. 2016;11(10):1752–1759. doi: 10.2215/CJN.13091215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laurin L.P., Gasim A.M., Poulton C.J., et al. Treatment with glucocorticoids or calcineurin inhibitors in primary FSGS. Clin J Am Soc Nephrol. 2016;11(3):386–394. doi: 10.2215/CJN.07110615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li F., Zheng C., Zhong Y., et al. Relationship between serum soluble urokinase plasminogen activator receptor level and steroid responsiveness in FSGS. Clin J Am Soc Nephrol. 2014;9(11):1903–1911. doi: 10.2215/CJN.02370314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lieberman K.V., Tejani A. A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J Am Soc Nephrol. 1996;7(1):56–63. doi: 10.1681/ASN.V7156. [DOI] [PubMed] [Google Scholar]
- 74.Martinelli R., Okumura A.S., Pereira L.J., Rocha H. Primary focal segmental glomerulosclerosis in children: prognostic factors. Pediatr Nephrol. 2001;16(8):658–661. doi: 10.1007/s004670100639. [DOI] [PubMed] [Google Scholar]
- 75.Martinelli R., Pereira L.J., Silva O.M., Okumura A.S., Rocha H. Cyclophosphamide in the treatment of focal segmental glomerulosclerosis. Braz J Med Biol Res. 2004;37(9):1365–1372. doi: 10.1590/s0100-879x2004000900011. [DOI] [PubMed] [Google Scholar]
- 76.Mendoza S.A., Reznik V.M., Griswold W.R., Krensky A.M., Yorgin P.D., Tune B.M. Treatment of steroid-resistant focal segmental glomerulosclerosis with pulse methylprednisolone and alkylating agents. Pediatr Nephrol. 1990;4(4):303–307. doi: 10.1007/BF00862503. [DOI] [PubMed] [Google Scholar]
- 77.Meyrier A., Noël L.H., Auriche P., Callard P. Long-term renal tolerance of cyclosporin A treatment in adult idiopathic nephrotic syndrome. Collaborative Group of the Société de Néphrologie. Kidney Int. 1994;45(5):1446–1456. doi: 10.1038/ki.1994.189. [DOI] [PubMed] [Google Scholar]
- 78.Mubarak M., Kazi J.I. Collapsing FSGS: a clinicopathologic study of 10 cases from Pakistan. Clin Exp Nephrol. 2010;14(3):222–227. doi: 10.1007/s10157-010-0275-2. [DOI] [PubMed] [Google Scholar]
- 79.Mungan S., Turkmen E., Aydin M.C., Saglam A.E., Baydar D.E. Tip lesion variant of primary focal and segmental glomerulosclerosis: clinicopathological analysis of 20 cases. Ren Fail. 2015;37(5):858–865. doi: 10.3109/0886022X.2015.1033635. [DOI] [PubMed] [Google Scholar]
- 80.Naseri M., Madani A., Ataei N. Correlation between prognosis and response to treatment in children with FSGS. Acta Med Iran. 2009;47(2):93–96. [Google Scholar]
- 81.Newman W.J., Tisher C.C., McCoy R.C., et al. Focal glomerular sclerosis: contrasting clinical patterns in children and adults. Medicine. 1976;55(1):67–87. doi: 10.1097/00005792-197601000-00004. [DOI] [PubMed] [Google Scholar]
- 82.Niaudet P. Treatment of childhood steroid-resistant idiopathic nephrosis with a combination of cyclosporine and prednisone. French Society of Pediatric Nephrology. J Pediatr. 1994;125(6 Pt 1):981–986. doi: 10.1016/s0022-3476(05)82020-7. [DOI] [PubMed] [Google Scholar]
- 83.Paik K.H., Lee B.H., Cho H.Y., et al. Primary focal segmental glomerular sclerosis in children: clinical course and prognosis. Pediatr Nephrol. 2007;22(3):389–395. doi: 10.1007/s00467-006-0301-5. [DOI] [PubMed] [Google Scholar]
- 84.Pei Y., Cattran D., Delmore T., Katz A., Lang A., Rance P. Evidence suggesting under-treatment in adults with idiopathic focal segmental glomerulosclerosis. Regional Glomerulonephritis Registry Study. Am J Med. 1987;82(5):938–944. doi: 10.1016/0002-9343(87)90155-0. [DOI] [PubMed] [Google Scholar]
- 85.Ponticelli C., Villa M., Banfi G., et al. Can prolonged treatment improve the prognosis in adults with focal segmental glomerulosclerosis? Am J Kidney Dis. 1999;34(4):618–625. doi: 10.1016/S0272-6386(99)70384-7. [DOI] [PubMed] [Google Scholar]
- 86.Ramachandran R., Kumar V., Rathi M., et al. Tacrolimus therapy in adult-onset steroid-resistant nephrotic syndrome due to a focal segmental glomerulosclerosis single-center experience. Nephrol Dial Transplant. 2014;29(10):1918–1924. doi: 10.1093/ndt/gfu097. [DOI] [PubMed] [Google Scholar]
- 87.Ren H., Shen P., Li X., Pan X., Zhang W., Chen N. Tacrolimus versus cyclophosphamide in steroid-dependent or steroid-resistant focal segmental glomerulosclerosis: a randomized controlled trial. Am J Nephrol. 2013;37(1):84–90. doi: 10.1159/000346256. [DOI] [PubMed] [Google Scholar]
- 88.Rennert W.P., Kala U.K., Jacobs D., Goetsch S., Verhaart S. Pulse cyclophosphamide for steroid-resistant focal segmental glomerulosclerosis. Pediatr Nephrol. 1999;13(2):113–116. doi: 10.1007/s004670050574. [DOI] [PubMed] [Google Scholar]
- 89.Risler T., Braun N., Bach D., et al. The German Glomerulonephritis Therapy Study: 10 years of controlled randomized trials for the treatment of idiopathic glomerulonephritis. Kidney Blood Press Res. 1996;19(3-4):196–200. doi: 10.1159/000174073. [DOI] [PubMed] [Google Scholar]
- 90.Roberti I., Vyas S. Long-term outcome of children with steroid-resistant nephrotic syndrome treated with tacrolimus. Pediatr Nephrol. 2010;25(6):1117–1124. doi: 10.1007/s00467-010-1471-8. [DOI] [PubMed] [Google Scholar]
- 91.Rydel J.J., Korbet S.M., Borok R.Z., Schwartz M.M. Focal segmental glomerular sclerosis in adults: presentation, course, and response to treatment. Am J Kidney Dis. 1995;25(4):534–542. doi: 10.1016/0272-6386(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 92.Schwartz M.M., Evans J., Bain R., Korbet S.M. Focal segmental glomerulosclerosis: prognostic implications of the cellular lesion. J Am Soc Nephrol. 1999;10(9):1900–1907. doi: 10.1681/ASN.V1091900. [DOI] [PubMed] [Google Scholar]
- 93.Segarra A., Vila J., Pou L., et al. Combined therapy of tacrolimus and corticosteroids in cyclosporin-resistant or -dependent idiopathic focal glomerulosclerosis: a preliminary uncontrolled study with prospective follow-up. Nephrol Dial Transplant. 2002;17(4):655–662. doi: 10.1093/ndt/17.4.655. [DOI] [PubMed] [Google Scholar]
- 94.Segarra A., Amoedo M.L., Martinez Garcia J.M., et al. Efficacy and safety of “rescue therapy” with mycophenolate mofetil in resistant primary glomerulonephritis–a multicenter study. Nephrol Dial Transplant. 2007;22(5):1351–1360. doi: 10.1093/ndt/gfl805. [DOI] [PubMed] [Google Scholar]
- 95.Segarra Medrano A., Vila Presas J., Pou Clavé L., Majó Masferrer J., Camps Doménech J. Efficacy and safety of combined cyclosporin A and mycophenolate mofetil therapy in patients with cyclosporin-resistant focal segmental glomerulosclerosis. Nefrologia. 2011;31(3):286–291. doi: 10.3265/Nefrologia.pre2011.Feb.10870. [DOI] [PubMed] [Google Scholar]
- 96.Shatat I.F., Schoeneman M., Flynn J.T., Woroniecki R.P. Association of steroid and cyclosporin resistance in focal segmental glomerulosclerosis. Pediatr Nephrol. 2007;22(6):834–839. doi: 10.1007/s00467-006-0413-y. [DOI] [PubMed] [Google Scholar]
- 97.Sherali A.R., Moorani K.N., Chishty S.H. Response to cyclosporin in children with primary focal segmental glomerulosclerosis. Pak Pediatr J. 2010;34(1):10–14. [Google Scholar]
- 98.Silverstein D.M., Craver R. Presenting features and short-term outcome according to pathologic variant in childhood primary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2007;2(4):700–707. doi: 10.2215/CJN.00230107. [DOI] [PubMed] [Google Scholar]
- 99.Stirling C.M., Mathieson P., Boulton-Jones J.M., et al. Treatment and outcome of adult patients with primary focal segmental glomerulosclerosis in five UK renal units. QJM. 2005;98(6):443–449. doi: 10.1093/qjmed/hci072. [DOI] [PubMed] [Google Scholar]
- 100.Tarshish P., Tobin J.N., Bernstein J., Edelmann C.M., Jr. Cyclophosphamide does not benefit patients with focal segmental glomerulosclerosis. A report of the International Study of Kidney Disease in Children. Pediatr Nephrol. 1996;10(5):590–593. doi: 10.1007/s004670050167. [DOI] [PubMed] [Google Scholar]
- 101.Troyanov S., Wall C.A., Miller J.A., Scholey J.W., Cattran D.C. Toronto Glomerulonephritis Registry Group. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16(4):1061–1068. doi: 10.1681/ASN.2004070593. [DOI] [PubMed] [Google Scholar]
- 102.Wasilewska A.M., Zoch-Zwierz W.M. Transforming growth factor-beta1 in nephrotic syndrome treated with cyclosporine and ACE inhibitors. Pediatr Nephrol. 2004;19(12):1349–1353. doi: 10.1007/s00467-004-1619-5. [DOI] [PubMed] [Google Scholar]
- 103.Zagury A., Oliveira A.L., Montalvão J.A., et al. Steroid-resistant idiopathic nephrotic syndrome in children: long-term follow-up and risk factors for end-stage renal disease. J Bras Nefrol. 2013;35(3):191–199. doi: 10.5935/0101-2800.20130031. [DOI] [PubMed] [Google Scholar]
- 104.Nankivell B.J. Abnormal laboratory results: cCreatinine clearance and the assessment of renal function. Aust Prescr. 2001;24(1):15–17. [Google Scholar]
- 105.De Vriese A.S., Wetzels J.F., Glassock R.J., Sethi S., Fervenza F.C. Therapeutic trials in adult FSGS: lessons learned and the road forward. Nat Rev Nephrol. 2021;17(9):619–630. doi: 10.1038/s41581-021-00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S4. Tables S1-S3.