Abstract
The persistence and infectivity of SARS-CoV-2 in different postmortem COVID-19 specimens remain unclear despite numerous published studies. This information is essential to improve corpses management related to clinical biosafety and viral transmission in medical staff and the public community. We aim to understand SARS-CoV-2 persistence and infectivity in COVID-19 corpses. We conducted a systematic review according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) protocols. A systematic literature search was performed in PubMed, Science Direct Scopus, and Google Scholar databases using specific keywords. We critically reviewed the collected studies and selected the articles that met the criteria. We included 33 scientific papers that involved 491 COVID-19 corpses. The persistence rate and maximum postmortem interval (PMI) range of the SARS-CoV-2 findings were reported in the lungs (138/155, 89.0%; 4 months), followed by the vitreous humor (7/37, 18.9%; 3 months), nasopharynx/oropharynx (156/248, 62.9%; 41 days), abdominal organs (67/110, 60.9%; 17 days), skin (14/24, 58.3%; 17 days), brain (14/31, 45.2%; 17 days), bone marrow (2/2, 100%; 12 days), heart (31/69, 44.9%; 6 days), muscle tissues (9/83, 10.8%; 6 days), trachea (9/20, 45.0%; 5 days), and perioral tissues (21/24, 87.5%; 3.5 days). SARS-CoV-2 infectivity rates in viral culture studies were detected in the lungs (9/15, 60%), trachea (2/4, 50%), oropharynx (1/4, 25%), and perioral (1/4, 25%) at a maximum PMI range of 17 days. The SARS-CoV-2 persists in the human body months after death and should be infectious for weeks. This data should be helpful for postmortem COVID-19 management and viral transmission preventive strategy.
Keywords: COVID-19, SARS-CoV-2, Postmortem, Persistence, Infectivity
Introduction
Coronavirus disease 2019 (COVID-19) pandemic due to the massive transmission of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in humans has led to an unexpected situation in global health. Compared to the SARS-CoV, the spike proteins of SARS-CoV-2 have a stronger binding affinity to the angiotensin-converting enzyme 2 (ACE2) receptors, resulting in a higher transmission rate [1]. SARS-CoV-2 persistency in human corpses also produced caution in postmortem disease management because of the possibility of transmission in medical staff or anyone involved during burial procedures [2]. However, the risk of infection from deceased COVID-19 patients may also be overestimated. Postmortem study is essential to establish the scientific basis for understanding SARS-CoV-2 infection [3].
The reverse-transcription quantitative polymerase chain reaction (RT-qPCR) test upon the respiratory swab specimens of the dead body has high sensitivity and specificity to detect SARS-CoV-2 RNA after COVID-19-related death [4]. The RT-qPCR method also evaluates viral persistency in patients’ lungs, heart, and abdominal organs [5]. This method has confirmed the viral persistency in COVID-19 decedents in autopsies with different times of specimen taken after death or postmortem intervals (PMI) [6]. However, the viral infectivity in the dead body only could be measured by viral culture method. Some reports showed that the infectivity of SARS-CoV-2 was detected after recent death but null after 12 h [7]. This systematic review collected information from international literature to clarify and understand SARS-CoV-2 persistence and infectivity in COVID-19 corpses.
Methods
We used Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocols. We conducted a systematic literature search from PubMed, Science Direct Scopus, and Google Scholar databases on March 2, 2022. The following terms were used: “COVID-19,” “SARS-CoV-2,” “postmortem,” “post-mortem,” “persistence,” “infectivity,” and “detection” in the title, abstract, and keywords. The references of all articles were screened, reviewed, and cross-checked to find relevant studies. Only papers in English were selected for this study. The inclusion criteria for eligibility were (1) postmortem findings on SARS-CoV-2 persistence or infectivity in the COVID-19 descendants, (2) stated the specimens and postmortem interval of viral detection, (3) original research articles, (4) case reports/series, and (5) research letters. One investigator (SPP) conducted the selection process and extracted the data independently with PRISMA standards of two times repetition to minimize study bias. Two investigators (TH and SPP) verified the data, and the other investigator (RTZ) validated the methodology for qualitative synthesis. This study did not involve the living subjects and thus was exempted from institutional review board approval.
Results
Review of the literature
We identified 187 articles. The duplicated articles were removed from the list, resulting in 94 scientific papers. The remaining 94 papers, including the titles and abstracts, were screened, resulting in 41 articles. A full-text review was carried out to exclude non-eligible articles, thus generating 33 references for qualitative analysis. These 33 eligible references were carefully assessed following the aim of the systematic review. From these studies, we collected 491 samples to analyze critically. We presented the PRISMA flowchart in Fig. 1 and the literature demography in Table 1.
Fig. 1.
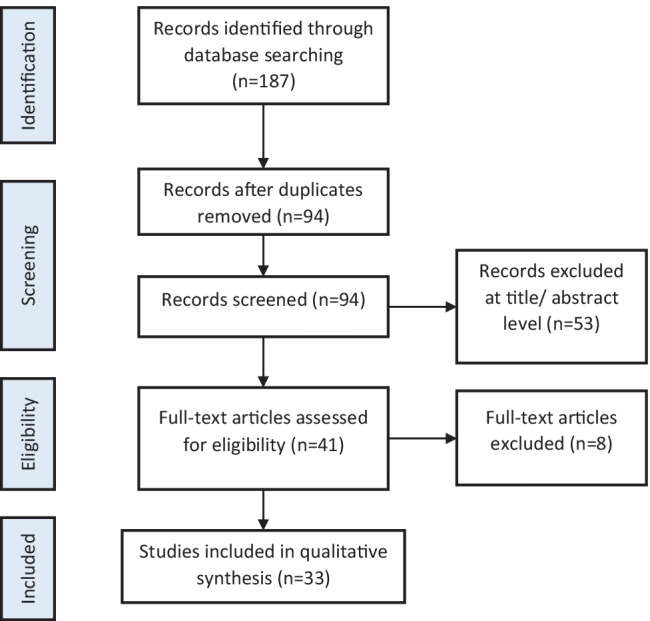
Postmortem SARS-CoV-2 persistence and infectivity search strategy using PRISMA flowchart
Table 1.
Demography of references included in the systematic review
| Article | Study country | Death cases (N) | Sex | Mean age (years) | Median age (years) | Age range (years) |
|---|---|---|---|---|---|---|
| Aschman et al. [8] | Germany | 43 | 31 males, 11 females | n/a | 72 | n/a |
| Beltempo et al. [9] | Italy | 1 | 1 male | 60 | n/a | n/a |
| Berezowska et al. [10] | Switzerland | 12 | 7 males, 5 females | n/a | 73 | 35–96 |
| Bogdanovic et al. [11] | Serbia | 1 | 1 male | 56 | n/a | n/a |
| Bonelli et al. [12] | Italy | 1 | 1 male | 41 | n/a | n/a |
| Casagrande et al. [13] | Germany | 11 | 5 males, 6 females | 68.5 | n/a | n/a |
| Dell'Aquila et al. [14] | Italy | 12 | 4 males, 8 females | 82.3 | n/a | 54–93 |
| Diao et al. [15] | China | 6 | n/a | n/a | n/a | n/a |
| Dorward et al. [16] | UK | 11 | 10 male, 1 female | 76.8 | n/a | n/a |
| Ducloyer et al. [17] | France | 1 | 1 male | 75 | n/a | n/a |
| Fuest et al. [18] | Germany | 23 | 16 males, 7 females | 72 | n/a | n/a |
| Grassi et al. [19] | Italy | 29 | n/a | 69 | n/a | n/a |
| Heinrich et al. [20] | Germany | 11 | 8 males, 3 females | n/a | n/a | 52–90 |
| Jurek et al. [21] | Poland | 2 | 1 male, 1 female | 90 | n/a | 89–91 |
| Kurabi et al. [22] | USA | 6 | 3 males, 3 females | n/a | 65 | 44–91 |
| List et al. [23] | Austria | 16 | 10 males, 6 females | n/a | 79 | 53–93 |
| Zito Marino et al. [24] | Italy | 27 | 15 males, 12 females | 66.7 | n/a | n/a |
| Matschke et al. [25] | Germany | 27 | n/a | n/a | n/a | n/a |
| Matsumoto et al. [26] | Japan | 12 | 7 males, 5 females | 71 | n/a | n/a |
| Musso et al. [27] | Italy | 16 | n/a | n/a | n/a | n/a |
| Mwananyanda et al. [28] | Zambia | 58 | 48 males, 10 females | n/a | 48 | n/a |
| Nagasawa et al. [29] | Japan | 5 | 3 males, 2 females | 70 | n/a | 58–78 |
| Penkava et al. [30] | Germany | 20 | 14 males, 6 females | n/a | 69 | 44–95 |
| Plenzig et al. [31] | Germany | 2 | 2 females | n/a | n/a | n/a |
| Plenzig et al. (2) [32] | Germany | 4 | 3 males, 1 female | 81 | n/a | 65–88 |
| Remmelink et al. [33] | Belgium | 17 | 12 males, 5 females | n/a | 72 | 62–77 |
| Sablone et al. [34] | Italy | 5 | 4 males, 1 female | 63.8 | n/a | 44–84 |
| Schröder et al. [35] | Germany | 33 | n/a | 79 | n/a | 55–99 |
| Sekulic et al. [36] | USA | 2 | 2 males | n/a | n/a | 54–81 |
| Servadei et al. [37] | Italy | 27 | 15 males, 12 females | 76.2 | n/a | n/a |
| Wong et al. [38] | Germany | 8 | 5 males, 3 females | 68 | n/a | n/a |
| Yang et al. [39] | China | 12 | n/a | 65 | n/a | 42–87 |
| Zacharias et al. [40] | Austria | 30 | 14 males, 16 females | n/a | 79 | 65–93 |
| Total | 491 |
n/a, data not available
SARS-CoV-2 persistence
We summarized the postmortem SARS-CoV-2 persistence findings of the 33 different studies in Table 2. The viral persistence in different postmortem specimens was generally evaluated by viral RNA detection with RT-qPCR or in situ hybridization (ISH). From the results, we classified the nucleic acid detection into several specimen sites: (1) lower respiratory tract, (2) upper respiratory tract, (3) nervous system, (4) abdominal organs, (5) heart, (6) skin, muscle, and skeletal tissue, and (7) other organs. We evaluated the PMI of each site and highlighted the most prolonged viral persistence observed. Significant findings such as viral protein and virion detection were also recorded.
Table 2.
SARS-CoV-2 persistence findings in postmortem studies
| Article | Postmortem specimen | Mean PMI | Median PMI | PMI | Diagnostic method | Positive postmortem cases | Other findings |
|---|---|---|---|---|---|---|---|
| Aschman et al. [8] | Lung tissue | n/a | 45 h | < 6 days | RT-qPCR | 38/43 (88%) | RT-qPCR detected viral RNA in the heart (10/42), quadriceps (7/41), and deltoid (2/42) tissues |
| Beltempo et al. [9] | Nasopharyngeal and oropharyngeal swab | n/a | n/a | 35 days | RT-qPCR | 1/1 (100%) | |
| Berezowska et al. [10] | FFPE tissue of lung | 27 h | 14 h | 6.5–70 h | RT-qPCR | 12/12 (100%) | IHC detected viral spike protein in lung tissue (6/12). ISH detected viral mRNA in lung tissue (3/5) |
| Bogdanovic et al. [11] | Vitreous humor | n/a | n/a | 3 months | RT-qPCR | 1/1 (100%) | |
| Bonelli et al. [12] | Nasopharyngeal swab | n/a | n/a | 41 days | RT-qPCR | 1/1 (100%) | |
| Casagrande et al. [13] | Corneal disc | 2.7 days | n/a | n/a | RT-qPCR | 6/11 (67%) | |
| Dell'Aquila et al. [14] | Nasopharyngeal swab | 43.92 h | n/a | 12–120 h | RT-qPCR | 7/12 (58%) | RT-qPCR detected viral RNA in tracheal swab (3/12) and lung swab (5/12) |
| Diao et al. [15] | Kidney tissue | n/a | n/a | 0–24 h | ISH | 6/6 (100%) | IHC detected viral nucleocapsid and spike protein (6/6) |
| Dorward et al. [16] | Lung tissue | n/a | 19.3 h | n/a | RT-qPCR | 11/11 (100%) | |
| Ducloyer et al. [17] | Nasopharyngeal swab | n/a | n/a | 48 h | RT-qPCR | 1/1 (100%) | |
| Fuest et al. [18] | Nasopharyngeal swab | 34.5 h | n/a | n/a | RT-qPCR | 4/23 (17%) | 12/23 deceased patients tested negative in the last RT-qPCR test |
| Grassi et al. [19] | Nasopharyngeal swab | 4.7 days | n/a | n/a | RT-qPCR | 13/29 (45%) | |
| Heinrich et al. [20] | Nasopharyngeal swab | n/a | 5.7 h | 2.9–32 h | RT-qPCR | 11/11 (100%) | RT-qPCR detected viral RNA after death within 0 h (9/11), 12 h (10/11), 24 h (10/11), 36 h (11/11), 60 h (11/11), 72 h (11/11), 96 h (11/11) and 168 h (11/11) |
| Jurek et al. [21] | Bone marrow | n/a | n/a | 12 days | RT-qPCR | 2/2 (100%) | |
| Kurabi et al. [22] | Middle ear | n/a | n/a | 3 h | RT-qPCR | 3/6 (50%) | RT-qPCR detected viral RNA in nasal septal mucosa (3/6) |
| List et al. [23] | Aqueous humor and vitreous humor | n/a | 21.5 h | 4–53 h | RT-qPCR | 0/16 (0%) | |
| Zito Marino et al. [24] | FFPE tissue of lung | n/a | n/a | 3–5 days | RT-qPCR | 27/27 (100%) | ISH detected viral RNA in lung FFPE tissue (12/27) |
| Matschke et al. [25] | FFPE tissue from the frontal lobe | 3.3 days | n/a | n/a | RT-qPCR | 13/27 (48%) | IHC detected viral spike (14/40) and nucleocapsid (8/40) proteins in the brain |
| Matsumoto et al. [26] | Nasopharyngeal swab | 67.67 h | n/a | < 240 h | RT-qPCR | 7/7 (100%) | |
| Musso et al. [27] | Lung tissue | n/a | n/a | 24–78 days | RT-qPCR | 12/16 (75%) | |
| Mwananyanda et al. [28] | Nasopharyngeal swab | n/a | n/a | < 48 h | RT-qPCR | 58/58 (100%) | |
| Nagasawa et al. [29] | Nasopharyngeal swab | n/a | n/a | 2–11 days | RT-qPCR | 5/5 (100%) | |
| Penkava et al. [30] | Conjunctival swabs | n/a | n/a | 19–78 h | RT-qPCR | 10/20 (50%) | RT-qPCR detected viral RNA in vitreous swab (6/20) |
| Plenzig et al. [31] | Lung tissue | n/a | n/a | 4 months | RT-qPCR | 2/2 (100%) | |
| Plenzig et al. (2) [32] | Lung tissue | n/a | n/a | 1–17 days | RT-qPCR | 4/4(100%) | RT-qPCR detected viral RNA from the kidney (1/2), small intestine (1/4), and brain (1/4) tissue |
| Remmelink et al. [33] | Lung tissue | n/a | n/a | < 5 days | RT-qPCR | 16/17 (94%) | RT-qPCR detected viral RNA in the heart (14/17), liver (14/17), bowel (14/17), spleen (11/17), and kidney (10/17). IHC detected viral protein in the lung (11/17) |
| Sablone et al. [34] | Nasopharyngeal swab | n/a | n/a | 22–27 days | RT-qPCR | 5/5 (100%) | RT-qPCR detected viral RNA in respiratory tract swabs and multiorgan biopsies (5/5) |
| Schröder et al. [35] | Nasopharyngeal swab | 3.5 days | n/a | 0–17 days | RT-qPCR | 24/33 (72%) | RT-qPCR detected viral RNA in perioral swabs (21/24), body bag (6/24), hands (14/24), wrists (14/24), shoulder & hips (13/24) |
| Sekulic et al. [36] | FFPE tissue of lung | n/a | n/a | 29–39 h | RT-qPCR | 2/2 (100%) | RT-qPCR detected viral RNA in FFPE tissue of the lymph node (2/2), bronchus, spleen, and heart (1/2) |
| Servadei et al. [37] | Nasopharyngeal swab | n/a | n/a | 2 h | RT-qPCR | 19/27 (70%) | RT-qPCR detected viral RNA after death within 12 h (16/27) and 24 h (18/27) |
| Wong et al. [38] | FFPE tissue of the lung | n/a | 35.5 h | 20–48 h | RT-qPCR | 8/8 (100%) | RT-qPCR detected viral RNA in FFPE tissue of the salivary gland and tonsil (7/8), trachea (6/8), thyroid (6/8), heart (6/8), stomach (3/8), kidney, liver, spleen, and adrenal gland (2/8) |
| Yang et al. [39] | Testes | n/a | n/a | 1 h | RT-qPCR | 1/12 (8.3%) | |
| Zacharias et al. [40] | Nasopharyngeal swab | n/a | 23 h | 8–124 h | RT-qPCR | 11/11 (100%) |
n/a data not available
The SARS-CoV-2 RNA in the lower respiratory tract was observed in lung tissue (138/155, 89.0%) and tracheal swab (9/20, 45.0%). The maximum time of viral RNA persistence was detected with RT-qPCR after 4 months in lung tissue specimens of exhumed corpses (4/4, 100%) [31]. This finding is the longest PMI of all studies. The descendants’ burial in winter with constant low temperatures should be considered an influencing factor of persistence. Most studies that evaluated SARS-CoV-2 persistence in lung tissue (8/12) found the viral RNA in all samples (100%). The remaining studies (4/12) identified the viral RNA above 40% in the lungs. Viral spike and nucleocapsid protein also could be detected with immunohistochemistry (IHC) examination in lung tissue (37/75, 49.3%; max PMI range was 5 days). In the tracheal swab specimens, the viral persistence was found up to 120 h postmortem (9/20, 45.0%) in 2 studies available [14, 38].
The viral RNA was detected in the upper respiratory tract specimens in nasopharyngeal/ oropharyngeal (156/248, 62.9%) and nasal septal (3/6, 50.0%) swabs with RT-qPCR. In a case report study [12], the maximum PMI was 41 days, detected primarily from a nasopharyngeal swab. The case described a 41-year-old Ukrainian man who drowned after swimming in the sea. The patient showed no COVID-19 symptoms before death with no specific antemortem diagnosis. The body was stored in a cold room at 4 °C. Another case report study found the second most extended PMI in 35 days [9]. The descendent was a 60-year-old man with a confirmed COVID-19 as the primary cause of death. Before taking nasopharyngeal swabs, the patient was transported to a local crematorium and placed in a refrigerator (− 4 °C) for 35 days. The other studies [22, 38] examined the nasal septal specimens and found a maximum PMI range of 3 h.
The postmortem case series [25] investigated SARS-CoV-2 RNA in the brain, especially in the fresh frozen paraffin-embedded (FFPE) of the frontal lobe and medulla oblongata tissue. The study was conducted in Hamburg, Germany, between March 13 and April 24, 2020. We highlighted the 27 samples to be investigated with RT-qPCR and found the viral RNA in 13/27 samples (48.1%). However, the author did not mention the maximum time of PMI of the 27 RT-qPCR samples, instead of the median PMI of 3.3 days, including other corpses that had undergone different diagnostic procedures. IHC procedure of the brain also detected viral spike (14/40, 35%) and nucleocapsid (8/40, 20%) protein in this period. Another study [32] revealed viral RNA persistence up to 17 days after death (1/4, 25.0%) as the most extended PMI observed in the brain.
SARS-CoV-2 RNA had been detected in the abdominal organs, including the kidney (19/29, 65.5%), gastro-intestine (18/29, 62.1%), liver (16/25, 64.0%), and spleen (14/27, 51.9%) with a maximum PMI range of 17 days after death. In another original study [15], the viral spike and nucleocapsid protein were observed in kidney tissue (6/6, 100%) 24 h after death. The cases were COVID-19 deaths with acute kidney injury (AKI). In this study, six corpses underwent an ISH test, and the result showed that all kidney tissues expressed positive signals for Probe-V-nCoV2019-S and Probe-V-nCoV2019-S-sense. This finding confirmed that the virus directly infects human kidney tubules and replicates in vivo.
The persistence of viral RNA was reported generally in postmortem heart tissue (31/69, 44.9%) with the PMI up to 6 days. However, a case–control study [8] found no viral protein in heart muscles with the IHC test during this period. In the same study, the viral RNA was found in skeletal muscle of quadriceps (7/41, 17.1%) and deltoid (2/42, 4.8%) tissue with RT-qPCR. The maximum PMI range was 6 days. Another study [21] detected viral RNA in the bone marrow (2/2, 100%) at 12 days postmortem using the real-time loop-mediated isothermal amplification (RT-LAMP) method and confirmed with agarose gel electrophoresis. At skin level, a study of deceased bodies of COVID-19 decedents reported the RNA persisted in swab specimens of hands (14/24, 58.3%), wrists (14/24, 58.3%), shoulders and hips (13/24, 54.16%), and body bag surface (6/24, 25%) with PMI of 0–17 days [35]. The longest PMI observed in skin swabs was 9 days.
The SARS-CoV-2 RNA also had been identified in other organs. The sites (persistence rate; maximum PMI range) were the vitreous humor (7/37, 18.9; 3 months), perioral (21/24, 87.5%; 17 days), conjunctiva (10/20, 50%; 78 h), tonsils (7/8, 87.5%; 48 h), adrenal glands (2/8, 25%; 48 h), thyroid glands (6/8, 75%; 48 h), hilar and peribronchial lymph node (2/2, 100%; 39 h), middle ear (3/6, 50.0%; 3 h), and testes (1/12, 8.3%; 1 h). An immersive long PMI (up to 3 months) was reported in a study of vitreous humor sample [11]. The case was a 56-year-old man who died after having fever, cough, and shortness of breath with no history of travel abroad for a long time, and the autopsy was conducted 3 months later. No additional information was available about the environmental condition in this case.
SARS-CoV-2 infectivity
Four of 33 references in the systematic review investigated the viability and infectivity of SARS-CoV-2 in postmortem specimens using the viral culture method (Table 3). The first study [40] found that the cultivation was positive in 7 of 11 lung tissue swabs at a PMI range of 14–68 h (median 25 h). The cases were Caucasians, with a median age of 79 years (range 65–93 years), and 56% were male. The median disease duration (the interval between the first RT-qPCR confirmation and death) was 9 days (range 3–34 days).
Table 3.
SARS-CoV-2 infectivity findings in postmortem studies
| Article | Postmortem specimen | Mean PMI | Median PMI | PMI | Diagnostic method | Positive postmortem cases | Other findings |
|---|---|---|---|---|---|---|---|
| Zacharias et al. [40] | Lung tissue | n/a | 25 h | 14–68 h | Viral culture | 7/11 (64%) | |
| Plenzig et al. (2) [32] | Lung tissue | n/a | n/a | 1–17 days | Viral culture | 2/4 (50%) | Positive culture in trachea (2/4), perioral (1/4), and oropharynx (1/4) |
| Plenzig et al. [31] | Lung tissue | n/a | n/a | 4 months | Viral culture | 0/2 (0%) | |
| Schröder et al. [35] | Perioral swab | n/a | n/a | 0–5 days | Viral culture | 0/11 (0%) | No positive culture in the hands (0/5), wrist (0/6), shoulder and hips (0/6), and body bag (0/2) |
n/a data not available
The second study [32] on cell culture was conducted from four COVID-19 corpses. The research detected the cytopathogenic effect (CPE) as the sign of SARS-CoV-2 infectivity. The CPE was observed in the lungs (2/4, 50%), trachea (2/4, 50%), oropharynx (1/4, 25%), and perioral (1/4, 25%) tissue. The PMI of the two cases was 4 days (case 1: positive for the lungs, trachea, and oropharynx) and 17 days (case 2: positive for the lungs, trachea, and perioral). In case 2, where the longest PMI of infectious SARS-CoV-2 was found, the patient was an 82-year-old woman contacted by relatives who showed flu-like symptoms. Then, she developed fever, dyspnea, and cough 2 days before death. Her husband found she was dead on the floor in the living room. The RT-qPCR diagnosis was made 2 days after death from a tracheal swab. An autopsy was taken on day 17, and the cause of death was COVID-19 with a severe pre-existing medical condition.
The third study [31] was conducted on two long-buried exhumed corpses. The cases were patients who confirmed positive COVID-19 antemortem by PCR (case 1) and rapid antigen test (case 2). The postmortem viral RNA was found positive in lung tissue exhumation after 4 months (case 1) and 3 months 26 days (case 2). However, no viable virus was found in the cell culture observation of both cases, so the infectivity was not measurable. The viability of SARS-CoV-2 was also undetected from another cell culture study [35] of the body surface and the body environment swab specimens at PMI of 5 days.
Discussion
Investigating SARS-CoV-2 persistence and infectivity on postmortem COVID-19 corpses is essential to ensure a preferable strategy for safely handling deceased patients. Despite a widely reported viral persistence [41], there is still limited evidence of viral infectivity after death, while some strict protocols have been established in medical and community settings to prevent viral transmission from the dead body [42–45]. This systematic review critically collects and observes viral persistence and infectivity in different postmortem COVID-19 specimens from published scientific papers. We highlight the most extended postmortem interval and some demographical information of the selected cases to present the updated condition.
SARS-CoV-2 RNA and structural protein persistence are observed in most COVID-19 dead bodies. The leading site is the respiratory tract, particularly the lungs and the pharynx. RT-qPCR might still detect the viral RNA in the lung months after the death, and the RNA is generally persistent for hours to weeks in other organs. This feature extends the 9-day persistence duration of other coronaviruses, including severe acute respiratory syndrome coronavirus (SARS-CoV) and middle east respiratory syndrome coronavirus (MERS-CoV) in the inanimate surface [46]. However, the viral RNA or protein solely cannot infect another human cell. The detection of the remaining postmortem RNA is explained by the rates of residual RNA replication, its catabolic degradation, and the cytotoxic activity of formerly living infected cells [47]. There is also evidence that lower temperature and humidity may prolong viral RNA degradation in the human body [48]. This finding may explain the long PMI of detectable RNA in the long-stored or buried corpses in such conditions.
The SARS-CoV-2 infectivity is still detected until 17 days after the death, particularly in the lung, trachea, and perioral of one postmortem case that was immediately sent to the cooling chamber [32]. This finding should be explained by the increased stability of SARS-CoV-2 at a lower temperature [48]. The Ct value below 34 in the RT-qPCR test (in this case, 20.88) may also increase the probability of positive cell culture [49]. However, the evidence of viral infection from specimens taken at the body surface is null, but postmortem management’s safety procedures are still very considered [50]. A higher precaution is needed in handling pre-cooled corpse autopsies.
The limitation of this study is primarily due to the design of the selected studies. The data might have biases because of the studies’ non-uniformity included in the review and the minimal samples per specimen targets. The cases may also not represent the general population, mainly drawing specific study populations. Here we describe the updated findings as preferable as a precaution in handling COVID-19 corpses in the fields.
Conclusion
This systematic review explains the SARS-CoV-2 persistence and infectivity in corpses. The virus may persist months after death and remain viable and infectious for weeks postmortem under certain conditions. This study helps to give a clearer image of SARS-CoV-2 in the dead body and improves the importance of using a precaution safety procedure for medical staff in different postmortem settings. We recommend further study focusing on the access of external and internal factors that increase the viral transmission and infection rate from COVID-19 corpses in different environmental conditions.
Key points
The SARS-CoV-2 is still detected four months after death and remains viable and infectious for 17 days postmortem.
Acknowledgements
SPP is involved in study design, data collection and analyses, and manuscript drafting. TH verified the data and validated the concept. RTZ validated the methodology for qualitative synthesis. All authors read and approved the final manuscript.
Declarations
Ethics approval
This study did not involve the living subjects and thus exempted from institutional ethical review board approval.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yesudhas D, Srivastava A, Gromiha MM. COVID-19 outbreak: history, mechanism, transmission, structural studies and therapeutics. Infection. 2021;49(2):199–213. doi: 10.1007/s15010-020-01516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aquila I, Ricci P, Bonetta CF, Sacco MA, Longhini F, Torti C, et al. Analysis of the persistence time of the SARS-CoV-2 virus in the cadaver and the risk of passing infection to autopsy staff. Med Leg J. 2021;89(1):40–53. doi: 10.1177/0025817220980601. [DOI] [PubMed] [Google Scholar]
- 3.Sperhake J-P. Autopsies of COVID-19 deceased? Absolutely! Leg Med. 2020;47. [DOI] [PMC free article] [PubMed]
- 4.Hall JA, Harris RJ, Emmett HE, Lowe B, Singanayagam A, Twohig KA, et al. On the sensitivity and specificity of postmortem upper respiratory tract testing for SARS-CoV-2. J Infect Dis. 2021;224(3):389–394. doi: 10.1093/infdis/jiab270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brook OR, Piper KG, Mercado NB, Gebre MS, Barouch DH, Busman-Sahay K, et al. Feasibility and safety of ultrasound-guided minimally invasive autopsy in COVID-19 patients. Abdom Radiol. 2021;46(3):1263–1271. doi: 10.1007/s00261-020-02753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pomara C, Salerno M, Sessa F, Esposito M, Barchitta M, Ledda C, et al. Safe management strategies in clinical forensic autopsies of confirmed COVID-19 cases. Diagnostics. 2021;11(3). [DOI] [PMC free article] [PubMed]
- 7.Pomara C, Sessa F, Galante D, Pace L, Fasanella A, Di Nunno N, et al. Do we really need hazard prevention at the expense of safeguarding death dignity in covid-19? Diagnostics. 2021;11(10). [DOI] [PMC free article] [PubMed]
- 8.Aschman T, Schneider J, Greuel S, Meinhardt J, Streit S, Goebel HH, et al. Association between SARS-CoV-2 infection and immune-mediated myopathy in patients who have died. JAMA Neurol. 2021;78(8):948–960. doi: 10.1001/jamaneurol.2021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beltempo P, Curti SM, Maserati R, Gherardi M, Castelli M. Persistence of SARS-CoV-2 RNA in postmortem swab 35 days after death: a case report. Forensic Sci Int. 2021;319:110653. doi: 10.1016/j.forsciint.2020.110653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berezowska S, Lefort K, Ioannidou K, Ndiaye D-R, Maison D, Petrovas C, et al. Postmortem cardiopulmonary pathology in patients with covid-19 infection: single-center report of 12 autopsies from lausanne, switzerland. Diagnostics. 2021;11(8). [DOI] [PMC free article] [PubMed]
- 11.Bogdanović M, Skadrić I, Atanasijević T, Stojković O, Popović V, Savić S, et al. Case report: postmortem histopathological and molecular analyses of the very first documented COVID-19-related death in Europe. Front Med. 2021;8(2):1–5. doi: 10.3389/fmed.2021.612758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonelli M, Rosato E, Locatelli M, Tartaglia A, Falco P, Petrarca C, et al. Long persistence of severe acute respiratory syndrome coronavirus 2 swab positivity in a drowned corpse: a case report. J Med Case Rep. 2022;16(1). [DOI] [PMC free article] [PubMed]
- 13.Casagrande M, Fitzek A, Spitzer MS, Püschel K, Glatzel M, Krasemann S, et al. Presence of SARS-CoV-2 RNA in the cornea of viremic patients with COVID-19. JAMA Ophthalmol. 2021;139(4):383–388. doi: 10.1001/jamaophthalmol.2020.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dell’Aquila M, Cattani P, Fantoni M, Marchetti S, Aquila I, Stigliano E, et al. Postmortem swabs in the severe acute respiratory syndrome coronavirus 2 pandemic. Arch Pathol Lab Med. 2020;144(11):1298–1302. doi: 10.5858/arpa.2020-0362-SA. [DOI] [PubMed] [Google Scholar]
- 15.Diao B, Wang C, Wang R, Feng Z, Zhang J, Yang H, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun. 2021;12(1):1–9. doi: 10.1038/s41467-021-22781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorward DA, Russell CD, Um IH, Elshani M, Armstrong SD, Penrice-Randal R, et al. Tissue-specific immunopathology in fatal COVID-19. Am J Respir Crit Care Med. 2021;203(2):192–201. doi: 10.1164/rccm.202008-3265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ducloyer M, Gaborit B, Toquet C, Castain L, Bal A, Arrigoni PP, et al. Complete postmortem data in a fatal case of COVID-19: clinical, radiological and pathological correlations. Int J Legal Med. 2020;134(6):2209–2214. doi: 10.1007/s00414-020-02390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuest M, Boor P, Knuechel R, Walter P, Salla S. Postmortem conjunctival and nasopharyngeal swabs in SARS‐CoV‐2 infected and uninfected patients. Acta Ophthalmol. 2021;99(4). [DOI] [PMC free article] [PubMed]
- 19.Grassi S, Arena V, Cattani P, Dell’Aquila M, Liotti FM, Sanguinetti M, et al. SARS-CoV-2 viral load and replication in postmortem examinations. Int J Legal Med. 2022. [DOI] [PMC free article] [PubMed]
- 20.Heinrich F, Meißner K, Langenwalder F, Püschel K, Nörz D, Hoffmann A, et al. Postmortem stability of SARS-CoV-2 in nasopharyngeal mucosa. Emerg Infect Dis. 2021;27(1):329–331. doi: 10.3201/eid2701.203112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurek T, Rorat M, Szleszkowski Ł, Tokarski M, Pielka I, Małodobra-Mazur M. SARS-CoV-2 viral RNA is detected in the bone marrow in post-mortem samples using RT-LAMP. Diagnostics. 2022;12(2):515. doi: 10.3390/diagnostics12020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurabi A, Pak K, DeConde AS, Ryan AF, Yan CH. Immunohistochemical and qPCR detection of SARS-CoV-2 in the human middle ear versus the nasal cavity: case series. Head Neck Pathol. 2021. [DOI] [PMC free article] [PubMed]
- 23.List W, Regitnig P, Kashofer K, Gorkiewicz G, Zacharias M, Wedrich A, et al. Occurrence of SARS-CoV-2 in the intraocular milieu. Exp Eye Res. 2020;201(8):108273. doi: 10.1016/j.exer.2020.108273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zito Marino F, De Cristofaro T, Varriale M, Zannini G, Ronchi A, La Mantia E, et al. Variable levels of spike and ORF1ab RNA in postmortem lung samples of SARS-CoV-2-positive subjects: comparison between ISH and RT-PCR. Virchows Arch. 2022. [DOI] [PMC free article] [PubMed]
- 25.Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto S, Takasu S, Shimmura S, Sakai A, Kanto Y, Kanuka H, et al. Comprehensive severe acute respiratory syndrome coronavirus 2 detection using polymerase chain reaction and rapid antigen testing in postmortem specimens. Am J Forensic Med Pathol. 2022;00:1–5. doi: 10.1097/PAF.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 27.Musso N, Falzone L, Stracquadanio S, Bongiorno D, Salerno M, Esposito M, et al. Post-mortem detection of sars-cov-2 RNA in long-buried lung samples. Diagnostics. 2021;11(7):1–12. doi: 10.3390/diagnostics11071158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mwananyanda L, Gill CJ, Macleod W, Kwenda G, Pieciak R, Mupila Z, et al. Covid-19 deaths in Africa: prospective systematic postmortem surveillance study. BMJ. 2021;372:9–13. doi: 10.1136/bmj.n334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagasawa S, Mori A, Hirata Y, Motomura A, Ishii N, Okaba K, et al. SmartAmp method can rapidly detect SARS-CoV-2 in dead bodies. Forensic Sci Int. 2022;331:111168. doi: 10.1016/j.forsciint.2021.111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penkava J, Muenchhoff M, Badell I, Osterman A, Delbridge C, Niederbuchner F, et al. Detection of SARS-CoV-2-RNA in post-mortem samples of human eyes. Graefe’s Arch Clin Exp Ophthalmol. 2021. [DOI] [PMC free article] [PubMed]
- 31.Plenzig S, Holz F, Bojkova D, Kettner M, Cinatl J, Verhoff MA, et al. Detection and infectivity of SARS-CoV-2 in exhumated corpses. Int J Legal Med. 2021;135(6):2531–2536. doi: 10.1007/s00414-021-02670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plenzig S, Bojkova D, Held H, Berger A, Holz F, Cinatl J, et al. Infectivity of deceased COVID-19 patients. Int J Legal Med. 2021;135(5):2055–2060. doi: 10.1007/s00414-021-02546-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remmelink M, De Mendonça R, D’Haene N, De Clercq S, Verocq C, Lebrun L, et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24(1):1–10. doi: 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sablone S, Solarino B, Ferorelli D, Benevento M, Chironna M, Loconsole D, et al. Postmortem persistence of SARS-CoV-2: a preliminary study. Forensic Sci Med Pathol. 2021;17(3):403–410. doi: 10.1007/s12024-021-00375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schröder AS, Edler C, Ondruschka B, Püschel K, Schädler J, Heinemann A, et al. The handling of SARS-CoV-2 associated deaths - infectivity of the body. Forensic Sci Med Pathol. 2021;17(3):411–418. doi: 10.1007/s12024-021-00379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekulic M, Harper H, Nezami BG, Shen DL, Sekulic SP, Koeth AT, et al. Molecular detection of SARS-CoV-2 infection in FFPE samples and histopathologic findings in fatal SARS-CoV-2 cases. Am J Clin Pathol. 2020;154(2):190–200. doi: 10.1093/ajcp/aqaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Servadei F, Mauriello S, Scimeca M, Caggiano B, Ciotti M, Anemona L, et al. Persistence of SARS-CoV-2 viral RNA in nasopharyngeal swabs after death: an observational study. Microorganisms. 2021;9(4). [DOI] [PMC free article] [PubMed]
- 38.Wong DWL, Klinkhammer BM, Djudjaj S, Villwock S, Timm MC, Buhl EM, et al. Multisystemic cellular tropism of sars-cov-2 in autopsies of covid-19 patients. Cells. 2021;10(8). [DOI] [PMC free article] [PubMed]
- 39.Yang M, Chen S, Huang B, Zhong J-M, Su H, Chen Y-J, et al. Pathological findings in the testes of COVID-19 patients: clinical implications. Eur Urol Focus. 2020;6(5):1124–1129. doi: 10.1016/j.euf.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zacharias M, Stangl V, Thüringer A, Loibner M, Wurm P, Wolfgruber S, et al. Rapid antigen test for postmortem evaluation of SARS-CoV-2 carriage. Emerg Infect Dis. 2021;27(6):1734–1737. doi: 10.3201/eid2706.210226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bussani R, Schneider E, Zentilin L, Collesi C, Ali H, Braga L, et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine. 2020;61. [DOI] [PMC free article] [PubMed]
- 42.Lacy JM, Brooks EG, Akers J, Armstrong D, Decker L, Gonzalez A, et al. COVID-19: postmortem diagnostic and biosafety considerations. Am J Forensic Med Pathol. 2020;41(3). [DOI] [PMC free article] [PubMed]
- 43.Centers for Disease Control and Prevention (CDC). Collection and submission of postmortem specimens from deceased persons with confirmed or suspected COVID-19. 2020 [cited 2022 Mar 12]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-postmortem-specimens.html
- 44.Yadav J, Patel B, S M, Js S. COVID-19 autopsy in India: protocols, procedures, and experiences. Cureus. 2021;13(10):e18984. [DOI] [PMC free article] [PubMed]
- 45.Skok K, Vander K, Setaffy L, Kessler HH, Aberle S, Bargfrieder U, et al. COVID-19 autopsies: procedure, technical aspects and cause of fatal course. Experiences from a single-center. Pathol Res Pract. 2021;217. [DOI] [PMC free article] [PubMed]
- 46.Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaspar-Rodríguez A, Padilla-González A, Rivera-Toledo E. Coronavirus persistence in human respiratory tract and cell culture: an overview. Brazilian J Infect Dis. 2021;25(5):101632. doi: 10.1016/j.bjid.2021.101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matson MJ, Yinda CK, Seifert SN, Bushmaker T, Fischer RJ, van Doremalen N, et al. Effect of environmental conditions on SARS-CoV-2 stability in human nasal mucus and sputum. Emerg Infect Dis. 2020;26(9):2276–2278. doi: 10.3201/eid2609.202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.La Scola B, Le Bideau M, Andreani J, Hoang VT, Grimaldier C, Colson P, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2020;39(6):1059–1061. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finegan O, Abboud D, Fonseca S, Malgrati I, Morcillo Mendez MD, Burri J-M, et al. International Committee of the Red Cross (ICRC): Cemetery planning, preparation and management during COVID-19: a quick guide to proper documentation and disposition of the dead. Forensic Sci Int. 2020;316. [DOI] [PMC free article] [PubMed]


