Abstract
Rising global temperatures are expected to increase reproductive costs for wildlife as greater thermoregulatory demands interfere with reproductive activities. However, predicting the temperatures at which reproductive performance is negatively impacted remains a significant hurdle. Using a thermoregulatory polygon approach, we derived a reproductive threshold temperature for an Arctic songbird—the snow bunting (Plectrophenax nivalis). We defined this threshold as the temperature at which individuals must reduce activity to suboptimal levels (i.e. less than four-time basal metabolic rate) to sustain nestling provisioning and avoid overheating. We then compared this threshold to operative temperatures recorded at high (82° N) and low (64° N) Arctic sites to estimate how heat constraints translate into site-specific impacts on sustained activity level. We predict buntings would become behaviourally constrained at operative temperatures above 11.7°C, whereupon they must reduce provisioning rates to avoid overheating. Low-Arctic sites had larger fluctuations in solar radiation, consistently producing daily periods when operative temperatures exceeded 11.7°C. However, high-latitude birds faced entire, consecutive days when parents would be unable to sustain required provisioning rates. These data indicate that Arctic warming is probably already disrupting the breeding performance of cold-specialist birds and suggests counterintuitive and severe negative impacts of warming at higher latitude breeding locations.
Keywords: thermoregulatory polygon, hyperthermia, snow bunting, climate change, heat dissipation limit theory, sustained performance
1. Introduction
Animals frequently experience life-history stages that demand significant increases in their sustained rate of energy expenditure [1–3]. In an era of rapid climate change that is impacting species and ecosystems worldwide [4], understanding energy expenditure limits and their causes is paramount for predicting an organisms response to rising global temperatures [5]. Historically, energetic limits among endotherms have been attributed to intrinsic physiological factors (central limitation hypothesis [6]) or constraints in the metabolic capacity of specific peripheral tissues (peripheral limitation hypothesis [1]). Recently, Speakman & Król [7–9] proposed an alternative hypothesis, termed the heat dissipation limit (HDL) theory, which contends that the maximal rate of energy expenditure for an endothermic animal is limited by physiological factors governing heat dissipation capacity and the consequent avoidance of lethal body temperatures. Importantly, whereas the peripheral limitation hypothesis argues that energetic constraints may act on a range of tissues and organs, the HDL theory proposes a universal constraint in the form of heat dissipation and provides a mechanistic link between an animal's physiological capacity to maximize energy expenditure with the interplay between heat dissipation and ambient temperature.
Despite the conceptual gains that the HDL theory has provided in linking heat dissipation capacity with energetic expenditure, our ability to predict the ambient temperatures that will constrain an animal's performance (i.e. sustained rate of energy expenditure) remains a major impediment to assessing species vulnerability to climate change [10]. Although several studies have reported threshold temperatures above which sustained activity and/or reproductive performance were compromised (see [11] and references therein), these studies derived threshold values from post hoc analyses on behavioural observations and are therefore not predictive by design. Recently, Rezende & Bacigalupe [12] proposed a predictive analytical tool—the thermoregulatory polygon—for estimating the dimensional space in which thermoregulation is possible given an animal's combined rate of energy expenditure and the environmental temperatures it is operating within. Thermoregulatory polygons are built from commonly measured physiological variables (basal and maximal metabolic rate, and minimum and maximum thermal conductance) to delineate the boundaries in which heat production and dissipation are balanced [12]. Thus, thermoregulatory polygons can help estimate responses to further warming by integrating concepts of the HDL theory to predict the ambient temperatures over which endothermic animals can sustain activity and avoid overheating. Surprisingly, despite their potential as a predictive tool, to our knowledge, only one study has applied thermoregulatory polygons, using them to predict the energetic consequences of activity time in nocturnal and diurnal mammals [5].
Among endotherms, birds are expected to be particularly sensitive to increasing environmental temperatures [13,14]. The offspring-rearing period for parents with dependent young requires substantial increases in sustained work effort, with adults performing at rates often reported between 4 and 6 times their basal metabolic rate (BMR) [2,3,6], although lower rates can also be observed depending on conditions ([15,16], see [17] for a discussion on this topic). Any excess heat generated as a by-product from foraging and provisioning must ultimately be dissipated, or birds risk overheating. Indeed, birds often decrease activity on days with warmer ambient temperatures, probably a thermoregulatory response to avoid heat stress [18,19]. When a bird's capacity to dissipate heat is increased (e.g. by experimentally removing insulative feathers), provisioning adults can sustain higher levels of activity and invest more in both their current and future reproductive efforts [20–23]. Thus, reproductive performance can be constrained by a bird's capacity to dissipate body heat produced during essential breeding activities, suggesting that increasing environmental temperatures could significantly impact reproductive success.
Here, we apply a thermoregulatory polygon to snow buntings (Plectrophenax nivalis; figure 1b), an Arctic-breeding songbird, to investigate how environmental temperature affects the interaction between thermoregulation and sustained energy expenditure. Applying thermoregulatory polygons to Arctic endotherms is pertinent and valuable for predicting how increasing temperatures under climate change will impact certain life-history stages via heat constraints on behaviour. Many Arctic animals are cold specialists and have evolved physiological adaptations for minimizing heat loss [24,25]. Consequently, high-latitude breeding species are probably vulnerable to even moderate increases in ambient temperature [26–29]; an alarming fact given that the Arctic has warmed faster than the global average and is expected to continue outpacing the global average over the twenty-first century [4]. Additionally, O'Connor et al. [26] recently showed that buntings become heat-stressed at moderate air temperatures and have an extremely limited evaporative cooling capacity. Consequently, highly active, breeding buntings exposed to constant solar radiation and modest rises in air temperature would be more likely to depend on behavioural thermoregulatory strategies (e.g. reducing provisioning effort) rather than physiological mechanisms (e.g. sustained increases in evaporate water loss rates) to dissipate body heat and avoid overheating.
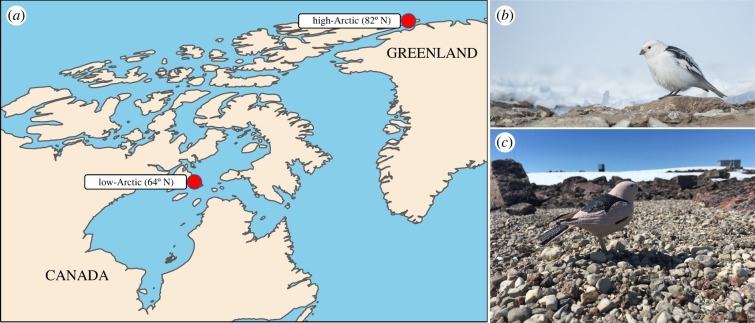
Figure 1.
(a) The location of the low-Arctic and high-Arctic study sites examining thermal tolerance in snow buntings (Plectrophenax nivalis). (b) A snow bunting in the high-Arctic (photo: F. Vézina). (c) A three-dimensional printed model in the low-Arctic (photo: O.P. Love). (Online version in colour.)
Our goal was to estimate how sensitive buntings' performance may be to increasing Arctic temperatures, given their limited heat dissipation capacity. We first used thermal physiological data to construct a thermoregulatory polygon and predict the threshold temperatures at which sustainable performance would be expected to decline in buntings maintaining thermal balance (i.e. heat produced = heat dissipated). We then compared the thermoregulatory polygon prediction to operative and air temperatures measured in the field at two breeding sites separated by 18° of latitude, representing the general southern and northern limits of this species breeding range, to evaluate how heat constraints on bunting performance (i) differed between a low- and high-Arctic region, and (ii) could translate into site-specific impacts on reproductive performance and success.
2. Material and methods
(a) . Operative and air temperature measurements
We measured operative (Te) and air (Ta) temperatures during the bunting breeding period at two sites in northern Canada representing the low-Arctic (East Bay Island; 64°01′ N, 81°47′ W) and high-Arctic (Alert; 82°30′ N, 62°20′ W; figure 1a). Operative temperature represents the temperature of the thermal environment as perceived by an individual and integrates the physical properties of the animal with the thermal properties of the local environment [30]. To measure Te perceived by buntings at our two sites, we used three-dimensional-printed, hollow plastic bird models (hereafter three-dimensional models; [31,32]; figure 1c). We printed the three-dimensional models to match the size and shape of an adult bunting (see the electronic supplementary material, figure S1 in appendix S1). Additionally, we painted the three-dimensional models to match the spectral properties of male buntings in breeding plumage. We focused on males given their simplified monochromatic breeding plumage (figure 1b) [33] and because males actively provision offspring at similar rates to females [34]. We used a spectrophotometer (Ocean Optics Jaz spectrometer) to measure the spectra of the black (n = 16 birds) and white (n = 27 birds) feather regions of male buntings. We used the pavo package in R [35] to convert the spectra wavelengths to a red : green : blue (R : G : B) colour combination. We then used an R : G : B-to-paint converter (https://www.e-paint.co.uk/convert-rgb.asp) to acquire a paint that best matched the R : G : B colour combination of male bunting feathers. We opted to paint the three-dimensional models instead of placing the skin and plumage of a male bunting over the models as this optimized our experimental design by allowing us to record Te in numerous models simultaneously across a broader geographical area [36]. Additionally, multiple studies (e.g. [37,38]) suggest that rough approximations of the study animal can be adequate for estimating operative temperature, and therefore, we felt comfortable using numerous painted operative temperature models over a few models covered with the feathers and skin of a male bunting.
We measured the internal temperature of each three-dimensional model by placing a temperature logger in the centre of each model. At the high-Arctic site, we drilled a hole in the belly and secured an iButton (model DS1921G-F5, Maxim Integrated, San Jose, CA USA; resolution = 0.5°C) in the approximate centre (electronic supplementary material, figures S2 and S3 in appendix S1) by gluing it to the end of a wooden dowel surrounded by a rubber stopper, creating an airtight seal around the drill-hole (electronic supplementary material, figure S4 in appendix S1). At the low-Arctic site, models were similarly set up except for using Hobo data loggers (Pendant model, MX2201, Onset Inc., Bourne, MA USA; resolution = 0.1°C) instead of iButtons, which we secured with silicone caulking. At both sites, the three-dimensional-printed models were secured to a wooden plank by gluing a wooden dowel to a notch in the three-dimensional model (electronic supplementary material, figures S3 and S4 in appendix S1). We cut the wooden dowels to approximate the height of a standing bunting. We covered each plank in the field using the substrate beneath the models to mimic the thermal properties of buntings’ natural environment (electronic supplementary material, figure S5 in appendix S1).
At each site, we deployed three-dimensional models within representative breeding territories and across naturally occurring habitats to adequately capture the thermal heterogeneity experienced by buntings. In the high-Arctic, we deployed 68 three-dimensional models and recorded Te every 5 min from 22 May to 7 September 2019. Models were deployed over six separate periods, each lasting approximately 7 days (owing to iButton memory limitations). After 7 days, we downloaded the Te data and redeployed the three-dimensional models to a new location. In the low-Arctic, we deployed 13 three-dimensional models and recorded Te continuously from 11 June to 19 July 2019 at 2 min intervals.
At both study sites, we collected Ta data to compare against operative temperatures. In the high-Arctic, meteorological data was measured at the National Oceanic and Atmospheric Administration's (NOAA) broadband radiation station located adjacent to the Global Atmospheric Watch Observatory (82°28′ N, 62°30′ W). These data are 1 min averages of Ta obtained at a height of 3 m above the ground using an aspirated Vaisala HMP-235 (PT100 sensor). In the low-Arctic, we collected Ta values every 30 min from six Kestrel weather meters (model 5500, Boothwyn, PA, USA) placed 2–3 m above ground level at separate locations across the study site.
(b) . Thermoregulatory polygon parameters and construction
We calculated the BMR (n = 28 birds), minimum wet thermal conductance (Cmin; n = 20 birds) and maximum dry thermal conductance (Cmax; n = 21 birds) using physiological data collected on a wild population of buntings at our high-Arctic site from 2 June to 25 July 2018. All physiological data were derived from previously published research [26,39] approved by the animal care committee of the Université du Québec à Rimouski (CPA-71–17-194, CPA-54-13-130 and CPA-71-17-195) and conducted under scientific (NUN-SCI-15-05 and SC-48) and banding permits (10889 and 10889E) from Environment and Climate Change Canada. Information on gas analysers, experimental protocol, body and air temperature measurements, and equations used for calculating metabolic rates are described in detail in Le Pogam et al. [39–41] and O'Connor et al. [26]. Briefly, we measured BMR overnight on fasted individuals resting inside a darkened metabolic chamber at thermoneutral temperatures (mean Ta = 26.2 ± 0.8°C; note, Ta = Te inside metabolic chambers [30]). For Cmin, we measured metabolic rates on individuals at a constant Ta below their lower critical temperature of 10°C ([24]; mean Ta = −19.0 ± 1.8°C). We did not measure rates of evaporative water loss during our Cmin runs and therefore for each bird we calculated minimum wet thermal conductance as
| 2.1 |
where MR represents metabolic rate in Watts, and Tb and Ta are the mean body and air temperatures, respectively. At Ta below the lower critical temperature, evaporative heat loss is minimal and thus its inclusion has little influence on Cmin [42]. During metabolic measurements for Cmin, we measured Tb at the start and end of each run and used the mean value for our calculations.
We determined Cmax by exposing birds to gradually increasing Ta [26]. We only included birds that tolerated Ta above 31.5°C, representing the mean Ta minus the s.d. at which buntings started panting [26], as we assumed that birds that had initiated panting had reached their Cmax [43]. This resulted in the removal of one bird from the dataset. At higher Ta, evaporative heat loss becomes significant and must be accounted for in the calculation of Cmax [42]. We thus calculated maximum dry thermal conductance for each bird as
| 2.2 |
where EHL represents evaporative heat loss measured during respirometry trials [26]. During Cmax experiments, we measured Tb continuously and therefore could calculate an average Tb over the same 5 min time window that metabolic rates were calculated [26].
To build the thermoregulatory polygon, we calculated a combined mean across birds for each parameter (i.e. BMR, Cmin, Cmax and Tb). The BMR mean became the bottom boundary of the thermoregulatory polygon. The Cmin and Cmax means became the slopes of the left and right boundaries, respectively. We calculated the y-intercepts for the Cmin and Cmax slopes using the equation:
| 2.3 |
where C represents the combined Cmin or Cmax mean across birds and b is the y-intercept. We assumed Ta = Tb when MR = 0 [42] and used the combined Tb mean across birds during Cmin (41.0 ± 0.4°C) and Cmax (42.6 ± 0.7°C) measurements.
(c) . Estimating sustainable performance in the high-Arctic and low-Arctic
We conducted all analyses in R v. 4.0.4 [44]. In the high-Arctic, we recorded a total of 843 773 individual Te values from 68 three-dimensional models and a total of 107 092 Ta values. In the low-Arctic, we recorded 405 000 individual Te values from 13 models and a total of 10 803 Ta values. We used these raw temperature data to create a time series of Te and Ta for each site averaged at 1 h intervals using the timeAverage function in the openair package [45].
The discontinuous sampling protocol in the high-Arctic (e.g. downloading data and redeploying models) resulted in 643 1 h gaps in our Te time series. To estimate the percentage of time on a given day that buntings would have been behaviourally constrained from heat (see below), it was necessary to fill these gaps. We filled the Te gaps by fitting an artificial neural network [46] with the neuralnet package [47] to predict Te based on seven radiative and meteorological variables observed at the NOAA broadband radiation station (see the electronic supplementary material, appendix S2 for details). The neural network predicted hourly operative temperatures with an average mean square error of 1.8°C (range = 1.2 to 2.7°C).
We used the Cmax slope to estimate the maximum sustainable energy expenditure of buntings maintaining thermal balance under either Ta or Te. As the provisioning period is one of the most energetically expensive life-history stages for birds [3], we focused on the maximal sustainable performance possible for buntings during this period. At the high-Arctic site, adult buntings are typically observed provisioning from 4 July to 25 July (A. Le Pogam 2016-2019, personal observations) and at the low-Arctic site from 3 July to 24 July [33,34]. We thus used these respective periods to represent the typical provisioning period at each site. We defined performance as a multiple of BMR and assumed that four-time BMR is the minimum sustainable performance required for adult buntings to adequately provision nestlings [2,3]. Although lower levels of daily energy expenditure during provisioning have been reported for other species [17], we believe four-time BMR to be a plausible minimum sustainable performance requirement for snow buntings given that: (i) they produce a single clutch during the breeding season, (ii) they have a very short-time window for breeding, and (iii) nestlings grow fast and have a short growth period (approx. 13 days) for a passerine of their size [48]. Therefore, we defined four-time BMR as the energetic threshold for ‘optimal performance’, and we calculated the percentage of time on a given day that buntings could work at either optimal (greater than or equal to four-time BMR) or suboptimal (less than four-time BMR) performance levels based on either Te or Ta. However, we did include a continuous colour scheme into our figures to illustrate the discrepancy around our four-time BMR threshold value, thereby introducing a gradual transition into a darker red zone representing a more serious impact on sustainable performance. Lastly, we assumed buntings rested and reduced provisioning rates for 3 h a day [49], and we therefore only used temperature values measured between 01.00 and 22.00 when calculating the daily percentage of time that buntings could work at optimal or suboptimal performance levels.
3. Results
(a) . Thermoregulatory polygon
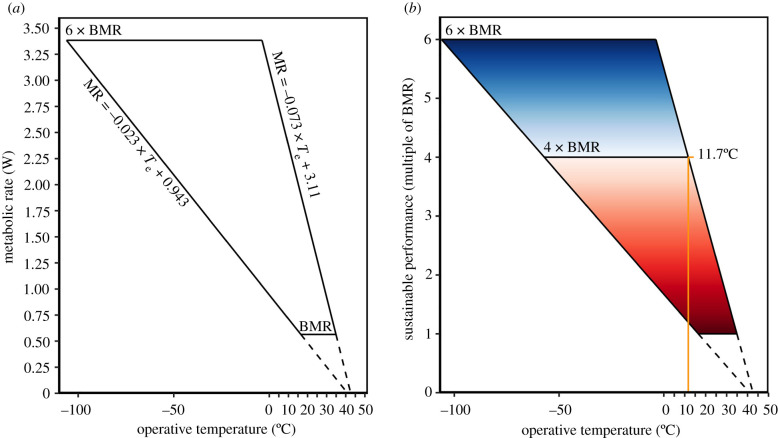
All values reported are mean ± s.d. The mean BMR of buntings was 0.564 ± 0.076 W. Mean thermal conductance varied threefold, with a calculated minimum wet thermal conductance of 0.023 ± 0.005 W/°C and a maximum dry thermal conductance of 0.073 ± 0.023 W/°C (figure 2a). The thermoregulatory polygon bounded by these parameters predicted that buntings could maintain thermal balance and sustain optimal performance (i.e. greater than four-time BMR) at operative temperatures (Te) of up to 11.7°C (figure 2b). Once Te exceeds 11.7°C, we expect buntings to become behaviourally constrained by heat and forced to perform at suboptimal levels to avoid overheating.
Figure 2.
(a) Snow bunting (Plectrophenax nivalis) thermoregulatory polygon bounded by BMR (0.564 W), minimum wet thermal conductance (0.023 W/°C), maximum dry thermal conductance (0.073 W/°C) and maximal sustained metabolic rate set at 6 × BMR. (b) Sustainable performance (expressed as a multiple of BMR) is possible for buntings under thermal balance. At operative temperatures below 11.7°C, buntings can maintain thermal balance and sustain optimal performance (i.e. performance ≥ 4 × BMR). As operative temperatures increase, buntings must reduce activity, and concomitantly metabolic rate, to maintain thermal balance, resulting in a suboptimal performance (i.e. performance less than 4 x BMR). Optimal performance is defined as the sustained level of work required by adults to sufficiently rear nestlings. The continuous colour scheme signifies the transition into more detrimental impacts on sustainable performance at higher temperatures (i.e. dark red zone). The black dashed lines are the extrapolation of the minimum and maximum thermal conductance slopes to the average body temperature recorded during laboratory measurements. (Online version in colour.)
(b) . Estimated sustainable performance in the high-Arctic and low-Arctic
At the high-Arctic site, Te and air temperatures (Ta) increased steadily from the beginning of the breeding period until peaking during the nestling-provisioning period and then gradually declined towards the post-fledging period (electronic supplementary material, figure S1a in appendix S3). Operative temperatures experienced by buntings frequently exceeded Ta, and on average were 3.5 ± 3.1°C warmer (range of differences between Te and Ta = −4.9°C to 14.5°C; electronic supplementary material, figure S1b in appendix S3).
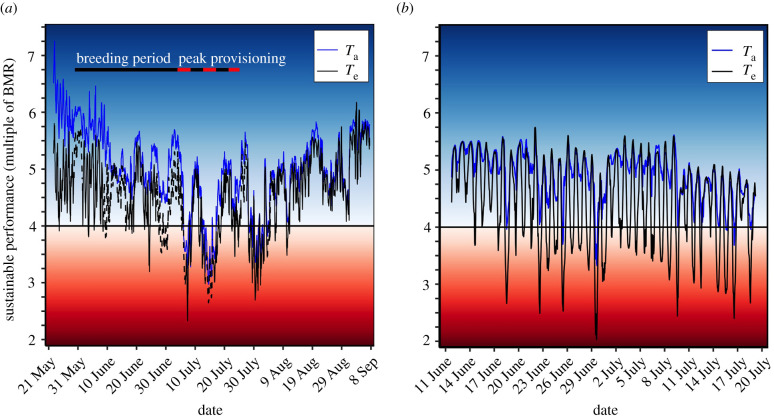
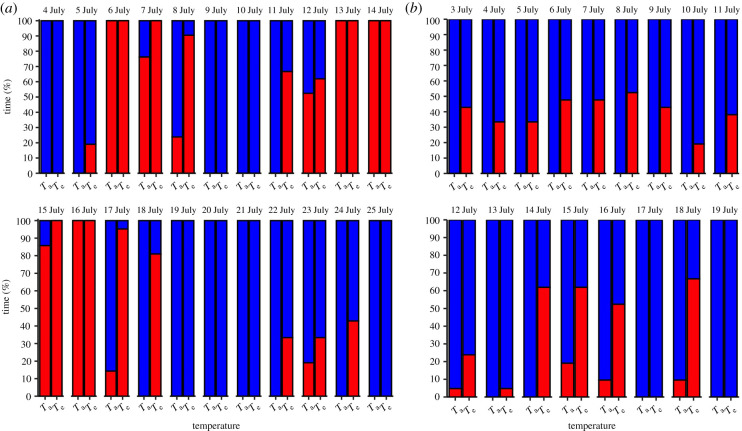
At the high-Arctic site, Te, but not Ta, exceeded the predicted thermoregulatory polygon threshold value of 11.7°C before 5 July (figure 3a). However, from 5 July to 5 August, both Ta and Te periodically exceeded 11.7°C (figure 3a), suggesting that buntings would have had to regularly perform at suboptimal levels below four-time BMR during this period. Within the nestling-provisioning period at the high-latitude site (i.e. 4 July–25 July), buntings experienced multi-day periods where they could have either performed at optimal levels for their entire active period (i.e. 01.00–22.00) or they would have been heat constrained and forced to work at suboptimal levels (figure 4a). For example, under Te, there were two periods of consecutive days (9–11 July and 19–22 July) where we predict that buntings could have worked at optimal performance levels for their entire active period (figure 4a). However, there were two periods of consecutive days (6–8 July and 13–17 July) when Te exceeded 11.7°C for their entire active period, and we predict that buntings would have had to reduce their provisioning rates to lower metabolic heat production and avoid overheating. From 13 to 19 July, buntings experienced only 5 h with Te that we predict allowed them to both maintain thermal balance and sustain a performance level greater than or equal to four-time BMR. Overall, under Te at the high-Arctic site, the percentage of time each day that buntings would have been behaviourally constrained from heat during their active period ranged from a minimum of 19% (4 h) to a maximum of 100% (21 h; figure 4a).
Figure 3.
Estimated sustainable performance possible for snow buntings (Plectrophenax nivalis) maintaining thermal balance at a (a) high-Arctic and (b) low-Arctic breeding site. The transition into the blue zone represents the times when average hourly operative (Te) or air (Ta) temperature was below the thermoregulatory polygon threshold temperature of 11.7°C, predicting that buntings could sustain performance levels ≥ four-time BMR without altering behaviour. The transition into the red zone represents the times when Te or Ta exceeded 11.7°C, predicting that buntings would be required to reduce their provisioning behaviour and work below four-time BMR to limit heat production and avoid overheating. Note that the darker the blue colour, the colder the recorded operative temperatures and the darker the red colour, the hotter the operative temperatures. The dashed black lines in (a) represent the predicted Te values from the artificial neural network (see methods for details). (Online version in colour.)
Figure 4.
The daily percentage of time during their active period (01.00–22.00) when snow buntings (Plectrophenax nivalis) at a (a) high-Arctic and (b) low-Arctic breeding site could either sustain an optimal performance level (blue region) or forced to work at suboptimal performance levels (red region) based on either operative (Te) or air (Ta) temperature recordings. Optimal and suboptimal performance is defined as the periods when buntings could sustain levels of work ≥ four-time BMR or less than four-time BMR, respectively, while maintaining thermal balance. (Online version in colour.)
At the low-Arctic site, average hourly temperatures were relatively consistent across the study period (electronic supplementary material, figure S2a in appendix S3). The overall mean difference between Te and Ta was 4.0 ± 4.1 (range = −2.7°C to 15.5°C; electronic supplementary material, figure S2b in appendix S3). In contrast with the high-Arctic site, where both Te and Ta exceeded the threshold temperature of 11.7°C, only Te at the low-Arctic site consistently placed a heat constraint on buntings' sustainable performance (figure 3b). For example, during the typical nestling-provisioning period in the low-Arctic (i.e. 3 July–24 July), we predict that buntings would have been behaviourally constrained on just 4 days under Ta, whereas Te values suggest that bunting performance would have been constrained to some degree on 15 out of 17 days (figure 4b). Furthermore, unlike the high-Arctic birds, we predict buntings at the low-Arctic site would not be forced to perform at suboptimal levels for their entire active period, but would instead be forced to alter performance for a portion of each day (figure 4b). Overall, under Te at the low-Arctic site, the percentage of time that buntings would have been behaviourally constrained from heat on a given day during their active period ranged from a minimum of 5% (1 h) to a maximum of 67% (14 h; figure 4b).
4. Discussion
(a) . Using the thermoregulatory polygon to predict thermal constraints
The HDL theory postulates that an animal's maximum sustained energy expenditure scales with its capacity to dissipate body heat [8]. Many factors influence an animal's thermoregulatory ability, including BMR and thermal conductance [50,51]. Given buntings' BMR and maximum dry thermal conductance, the thermoregulatory polygon predicts that at operative temperatures above 11.7°C, snow buntings cannot maintain thermal balance and sustain activity at optimal expenditure rates of four-time BMR. Consequently, when operative temperature exceeds the threshold temperature for extended periods, we would expect to observe a slower growth rate in nestlings, prolonged breeding period and potentially reduced fledging mass as adults reduce provisioning rates to maintain thermal balance [52,53]. Supporting a temperature dependence on provisioning rates among buntings, Hoset et al. [54] reported lower parental feeding rates during periods when air temperatures were high (the study did not measure operative temperatures), even though the range of air temperatures was small (e.g. 0–5°C). Similarly, Cunningham et al. [55] reported lower provisioning rates at higher ambient temperatures in common fiscals (Lanius collaris) and that fledglings were significantly lighter when maximum air temperature frequently exceeded 33°C. The comparatively low-threshold temperature for buntings (11.7°C) probably stems from their physiological adaptions for life in the cold [41]. Consequently, snow buntings’ cold specialization appears to come at the cost of not being able to adequately dissipate heat through increases in maximum thermal conductance at even moderate operative temperatures.
Because the thermoregulatory polygon boundaries are set by the thermal conductance of the animal, they represent the space in which an animal can balance heat loss and gain through non-evaporative pathways. Theoretically, an animal could maintain thermal balance and sustain a high rate of energy expenditure outside its thermoregulatory polygon by continuously dissipating body heat evaporatively. However, O'Connor et al. [26] recently showed that the evaporative cooling capacity of buntings is extremely limited, with most birds unable to evaporatively shed an amount of heat equivalent to their metabolic heat production. Therefore, it is unlikely that snow buntings can rely on evaporative cooling for prolonged periods to sustain activity outside their thermoregulatory polygon limits and, instead, will be highly dependent on behavioural thermoregulation.
(b) . Site-specific impacts of thermal constraints on breeding performance and success
Solar radiation is a major driving force of operative temperature and can vary by time of day, year or geographical location [56,57]. Our two sites represent the general southern and northern breeding limits for Arctic-breeding snow bunting populations in Canada [49] and are separated by approximately 18° latitude. This difference leads to distinct amounts of solar radiation reaching the Earth's surface [56], probably producing the significant differences observed in the duration and frequency that operative temperature exceeded the predicted threshold temperature. For example, during the peak nestling-provisioning period, buntings at the high-Arctic site were predicted to frequently experience consecutive days where they would not be able to perform at four times their BMR. By contrast, buntings in the low-Arctic were predicted to experience shorter, but more consistent heat constraints on provisioning activity almost every day. Given that snow bunting nestlings have some of the highest recorded growth rates of any passerine (11–13% of adult body mass per day; [48]), these latitudinal differences in constraints suggest that warming will produce different impacts on provisioning behaviour, offspring growth and survival in different populations. For instance, lower latitude breeding birds could possibly make up for reduced provisioning opportunities each day by adjusting their activity budget; working harder during the cooler periods to counteract overheating risks during warmer periods [5]. Indeed, under identical heat loads, Tapper et al. [23] observed higher feeding rates in wild female tree swallows (Tachycineta bicolor) that had their ventral feathers clipped to experimentally increase heat dissipation rates relative to unclipped females. Alternatively, parents breeding at lower latitudes could provision growing nestlings at lower rates per day and possibly extend the developmental period of the growing young. However, this could nonetheless impose survival constraints on nestlings and fledglings given that ground-nesting songbird species have evolved rapid growth rates and shorter in-nest development periods owing to high rates of nest predation [58], as well as the short, ephemeral nature of productivity in insects required for offspring growth [59].
For higher latitude populations, the accumulation of reduced provisioning opportunities over consecutive days could impose substantial developmental costs on nestlings that may be too high for parents to overcome on cooler days. Chick provisioning in buntings typically lasts 13 days; lowering provisioning rates for 3–4 consecutive days could have major impacts on chick condition at fledging and, consequently, post-fledging survival [11,60,61]. Therefore, as rapid Arctic warming continues [4], the temperature-dependent costs on reproductive performance may be more strongly felt at higher latitudes where climatic and meteorological patterns subject individuals to unique operative temperature cycles, with above threshold temperatures potentially lasting for days at the peak of breeding activities. It is worth noting, however, that our three-dimensional models were painted to match the male colour morph and therefore represent operative temperatures perceived by male snow buntings. During the provisioning period, both male and female buntings feed young and thus the operative temperatures experienced by females may differ from males leading to different sex constraints on performance. For instance, females lack the full dark back of male buntings and hence may experience lower operative temperatures allowing them to maintain higher provisioning rates than males. Nevertheless, under such a scenario, we would still predict negative impacts on nestling condition and fledgling success as both parents cannot adequately feed young at optimal rates.
5. Conclusion
A growing body of evidence suggests that increasing environmental temperatures associated with climate change will impose reproductive costs on birds via trade-offs between essential breeding behaviours and the need to dissipate body heat and avoid lethal body temperatures [10,11,14,21]. To date, predicting the threshold temperatures that will adversely affect breeding activity has been a limiting factor in forecasting the impacts of anthropogenic climate change on birds. Additionally, studies on how thermoregulatory demands will negatively impact breeding behaviour within birds are overwhelmingly focused on hot, arid climates while studies on Arctic birds are severely lacking.
Using a thermoregulatory polygon approach, we estimated the maximal sustained energy expenditure in an Arctic songbird maintaining thermal balance across a range of environmental temperatures at two field locations representing the southern and northern breeding limits of its breeding populations in Canada. Assuming an optimal performance level of four-time BMR [3], our findings predict that buntings will become heat constrained at operative temperatures above 11.7°C. Above this threshold, buntings would need to reduce their maximal sustained energy expenditure and provision their offspring at suboptimal performance levels to balance heat loads and avoid overheating. Importantly, our conclusions would remain unchanged even if buntings were found to maintain lower sustained performance rates as reported in other species [17]. For example, assuming buntings operated at three-time BMR, our threshold value would rise to 19.4°C for reduced performance. Indeed, Alert has already been experiencing short periods of air temperatures above 20°C for several years, suggesting even higher levels of operative temperature. However, we acknowledge that the actual sustained working level of provisioning snow buntings remains to be measured empirically.
By examining impacts at both a low- and high-Arctic breeding site, our data reveal site-specific differences in operative temperature, probably linked to latitude and the consequential differences in available sunlight and radiative flux, culminating in site-specific patterns in the heat constraints placed on an animal's maximal sustained energy expenditure. It appears that synoptic-scale (i.e. weather-scale, 2–4 days) influences on local temperature dominate in modulating operative temperatures in the high-Arctic, whereas the diurnal cycle is the dominant factor in the low-Arctic. We also argue that intraspecific differences among bunting populations in heat tolerance may be minimal given that recent genetic evidence suggests mixing between our two study populations [62]. Additionally, recent data show comparable metabolic responses to cold in buntings from Alert before breeding and wintering buntings in eastern Québec, two populations known to breed at different locations [41]. Taken together, a thermoregulatory polygon built upon physiological parameters from our East Bay population may not significantly differ from that presented currently.
Collectively, our results indicate that while Arctic warming will expose all snow bunting populations to more periods above their threshold temperature for sustained optimal performance, high-Arctic birds will probably face greater increases in the duration and magnitude of these periods owing to the suppressed amplitude of the diurnal cycle where the sun is above the horizon continuously from early April through to early September. The expectation then will be that high-Arctic populations will face greater downstream costs to reproductive performance, and ultimately breeding success, compared to low-Arctic populations.
Acknowledgements
We sincerely thank the 2019 East Bay and Alert field teams for their assistance. We are grateful to Dr Christina Semeniuk for the use of spectrophotometer measurement equipment and to Kevyn Gammie-Janisse and Chris Harris for their assistance with spectrophotometer measurements and analysis. We are also indebted to Lincoln Savi for providing essential help with the three-dimensional models.
Ethics
All physiological data were derived from previously published research [26,39] approved by the animal care committee of the Université du Québec à Rimouski (CPA-71–17-194, CPA-54-13-130 and CPA-71-17-195) and conducted under scientific (NUN-SCI-15-05 and SC-48) and banding permits (10889 and 10889E) from Environment and Climate Change Canada.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.vmcvdnctr [63].
The data are also provided in the electronic supplementary material [64].
Authors' contributions
R.S.O.: conceptualization, data curation, formal analysis, methodology and writing—original draft; A.L.P.: investigation, writing—review and editing; K.G.Y.: investigation, writing—review and editing; O.P.L.: conceptualization, funding acquisition, methodology, writing—review and editing; C.J.C.: data curation, writing—review and editing; G.R.: investigation, writing—review and editing; F.R.: investigation, writing—review and editing; K.H.E.: conceptualization, funding acquisition, writing—review and editing; A.L.H.: conceptualization, funding acquisition, writing—review and editing; E.S.C.: writing—review and editing; H.G.G.: writing—review and editing; D.B.: writing—review and editing; A.T.: funding acquisition, writing—review and editing; F.V.: conceptualization, funding acquisition, methodology, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
O.P.L. is supported by an NSERC Discovery Grant (grant no. 06724/05507), Canada Research Chairs (grant no. 34387) and Polar Knowledge (grant no. 622-18/648-19). F.V. is supported by an NSERC Discovery Grant (grant no. 05244/05628). F.V., K.H.E. and A.L.H. are supported by a FRQNT Team Grant (grant no. 253477). F.V. and D.B. received financial and logistical support from the Department of National Defence of Canada. C.J.C and the Alert radiation station receive support from the NOAA Arctic Research Program.
References
- 1.Peterson CC, Nagy KA, Diamond J. 1990. Sustained metabolic scope. Proc. Natl Acad. Sci. USA 87, 2324-2328. ( 10.1073/pnas.87.6.2324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piersma T. 2011. Why marathon migrants get away with high metabolic ceilings: towards an ecology of physiological restraint. J. Exp. Biol. 214, 295-302. ( 10.1242/jeb.046748) [DOI] [PubMed] [Google Scholar]
- 3.Drent RH, Daan S. 1980. The prudent parent: energetic adjustments in avian breeding. Ardea 68, 225-252. ( 10.5253/arde.v68.p225) [DOI] [Google Scholar]
- 4.IPCC. 2021. Climate change 2021: the physical science basis. Contribution of working group I to the sixth assessment report of the Intergovernmental Panel on Climate Change. Masson-Delmotte V et al. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Bonebrake TC, Rezende EL, Bozinovic F. 2020. Climate change and thermoregulatory consequences of activity time in mammals. Am. Nat. 196, 45-56. ( 10.1086/709010) [DOI] [PubMed] [Google Scholar]
- 6.Weiner J. 1992. Physiological limits to sustainable energy budgets in birds and mammals: ecological implications. Trends Ecol. Evol. 7, 384-388. ( 10.1016/0169-5347(92)90009-Z) [DOI] [PubMed] [Google Scholar]
- 7.Speakman JR, Król E. 2010. Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. J. Anim. Ecol. 79, 726-746. ( 10.1111/j.1365-2656.2010.01689.x) [DOI] [PubMed] [Google Scholar]
- 8.Speakman JR, Król E. 2011. Limits to sustained energy intake. XIII. Recent progress and future perspectives. J. Exp. Biol. 214, 230-241. ( 10.1242/jeb.048603) [DOI] [PubMed] [Google Scholar]
- 9.Speakman JR, Król E. 2010. The heat dissipation limit theory and evolution of life histories in endotherms—time to dispose of the disposable soma theory? Integr. Comp. Biol. 50, 793-807. ( 10.1093/icb/icq049) [DOI] [PubMed] [Google Scholar]
- 10.Smit B, Zietsman G, Martin RO, Cunningham SJ, McKechnie AE, Hockey PAR. 2016. Behavioural responses to heat in desert birds: implications for predicting vulnerability to climate warming. Clim. Chang. Responses 3, 9. ( 10.1186/s40665-016-0023-2) [DOI] [Google Scholar]
- 11.Cunningham SJ, Gardner JL, Martin RO. 2021. Opportunity costs and the response of birds and mammals to climate warming. Front. Ecol. Environ. 19, 300-307. ( 10.1002/fee.2324) [DOI] [Google Scholar]
- 12.Rezende EL, Bacigalupe LD. 2015. Thermoregulation in endotherms: physiological principles and ecological consequences. J. Comp. Physiol. B 185, 709-727. ( 10.1007/s00360-015-0909-5) [DOI] [PubMed] [Google Scholar]
- 13.Albright TP, Mutiibwa D, Gerson AR, Smith EK, Talbot WA, O'Neill JJ, McKechnie AE, Wolf BO. 2017. Mapping evaporative water loss in desert passerines reveals an expanding threat of lethal dehydration. Proc. Natl Acad. Sci. USA 114, 2283-2288. ( 10.1073/pnas.1613625114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conradie SR, Woodborne SM, Cunningham SJ, McKechnie AE. 2019. Chronic, sublethal effects of high temperatures will cause severe declines in southern African arid-zone birds during the 21st century. Proc. Natl Acad. Sci. USA 116, 14 065-14 070. ( 10.1073/pnas.1821312116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryant DM, Tatner P. 1991. Intraspecies variation in avian energy expenditure: correlates and constraints. Ibis (Lond. 1859) 133, 236-245. ( 10.1111/j.1474-919X.1991.tb04565.x) [DOI] [Google Scholar]
- 16.Williams TD, Vézina F. 2001. Reproductive energy expenditure, intraspecific variation and fitness in birds. Curr. Ornithol. 16, 355-406. ( 10.1007/978-1-4615-1211-0_7) [DOI] [Google Scholar]
- 17.Williams TD. 2018. Physiology, activity and costs of parental care in birds. J. Exp. Biol. 221, jeb169433. ( 10.1242/jeb.169433) [DOI] [PubMed] [Google Scholar]
- 18.Clark L. 1987. Thermal constraints on foraging in adult European starlings. Oecologia 71, 233-238. ( 10.1007/BF00377289) [DOI] [PubMed] [Google Scholar]
- 19.Silva JP, Catry I, Palmeirim JM, Moreira F. 2015. Freezing heat: thermally imposed constraints on the daily activity patterns of a free-ranging grassland bird. Ecosphere 6, 1-13 ( 10.1890/ES14-00454.1) [DOI] [Google Scholar]
- 20.Nord A, Nilsson J. 2019. Heat dissipation rate constrains reproductive investment in a wild bird. Funct. Ecol. 33, 250-259. ( 10.1111/1365-2435.13243) [DOI] [Google Scholar]
- 21.Andreasson F, Nilsson JÅ, Nord A. 2020. Avian reproduction in a warming world. Front. Ecol. Evol. 8, 576331. ( 10.3389/fevo.2020.576331) [DOI] [Google Scholar]
- 22.Tapper S, Nocera JJ, Burness G. 2020. Experimental evidence that hyperthermia limits offspring provisioning in a temperate-breeding bird. R. Soc. Open Sci. 7, 201589. ( 10.1098/rsos.201589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tapper S, Nocera JJ, Burness G. 2020. Heat dissipation capacity influences reproductive performance in an aerial insectivore. J. Exp. Biol. 223, jeb222232. ( 10.1242/jeb.222232) [DOI] [PubMed] [Google Scholar]
- 24.Scholander PF, Hock R, Walters V, Johnson F, Irving L. 1950. Heat regulation in some Arctic and tropical mammals and birds. Biol. Bull. 99, 237-258. ( 10.2307/1538741) [DOI] [PubMed] [Google Scholar]
- 25.Blix AS. 2016. Adaptations to polar life in mammals and birds. J. Exp. Biol. 219, 1093-1105. ( 10.1242/jeb.120477) [DOI] [PubMed] [Google Scholar]
- 26.O'Connor RS, et al. 2021. Limited heat tolerance in an Arctic passerine: thermoregulatory implications for cold-specialized birds in a rapidly warming world. Ecol. Evol. 11, 1609-1619. ( 10.1002/ece3.7141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oswald SA, Arnold JM. 2012. Direct impacts of climatic warming on heat stress in endothermic species: seabirds as bioindicators of changing thermoregulatory constraints. Integr. Zool. 7, 121-136. ( 10.1111/j.1749-4877.2012.00287.x) [DOI] [PubMed] [Google Scholar]
- 28.Oswald SA, Bearhop S, Furness RW, Huntley B, Hamer KC. 2008. Heat stress in a high-latitude seabird: effects of temperature and food supply on bathing and nest attendance of great skuas Catharacta skua. J. Avian Biol. 39, 163-169. ( 10.1111/j.2008.0908-8857.04187.x) [DOI] [Google Scholar]
- 29.Choy ES, O'Connor RS, Gilchrist HG, Hargreaves AL, Love OP, Vézina F, Elliott KH. 2021. Limited heat tolerance in a cold-adapted seabird: implications of a warming Arctic. J. Exp. Biol. 224, jeb.242168. ( 10.1242/jeb.242168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakken GS. 1976. A heat transfer analysis of animals: unifying concepts and the application of metabolism chamber data to field ecology. J. Theor. Biol. 60, 337-384. ( 10.1016/0022-5193(76)90063-1) [DOI] [PubMed] [Google Scholar]
- 31.Watson CM, Francis GR. 2015. Three dimensional printing as an effective method of producing anatomically accurate models for studies in thermal ecology. J. Therm. Biol. 51, 42-46. ( 10.1016/j.jtherbio.2015.03.004) [DOI] [PubMed] [Google Scholar]
- 32.O'Connor RS, Brigham RM, McKechnie AE. 2018. Extreme operative temperatures in exposed microsites used by roosting rufous-cheeked nightjars (Caprimulgus rufigena): implications for water balance under current and future climate conditions. Can. J. Zool. 96, 1122-1129. ( 10.1139/cjz-2017-0310) [DOI] [Google Scholar]
- 33.Guindre-Parker S, Gilchrist HG, Baldo S, Doucet SM, Love OP. 2013. Multiple achromatic plumage ornaments signal to multiple receivers. Behav. Ecol. 24, 672-682. ( 10.1093/beheco/ars215) [DOI] [Google Scholar]
- 34.Guindre-Parker S, Gilchrist HG, Baldo S, Love OP. 2013. Alula size signals male condition and predicts reproductive performance in an Arctic-breeding passerine. J. Avian Biol. 44, 209-215. ( 10.1111/j.1600-048X.2012.05817.x) [DOI] [Google Scholar]
- 35.Maia R, Gruson H, Endler JA, White TE. 2019. pavo 2: New tools for the spectral and spatial analysis of colour in r. Methods Ecol. Evol. 10, 1097-1107. ( 10.1111/2041-210X.13174) [DOI] [Google Scholar]
- 36.Dzialowski EM. 2005. Use of operative temperature and standard operative temperature models in thermal biology. J. Therm. Biol. 30, 317-334. ( 10.1016/j.jtherbio.2005.01.005) [DOI] [Google Scholar]
- 37.Bakken GS. 1992. Measurement and application of operative and standard operative temperatures in ecology. Am. Zool. 32, 194-216. ( 10.1093/icb/32.2.194) [DOI] [Google Scholar]
- 38.Walsberg GE, Weathers WW. 1986. A simple technique for estimating operative environmental temperature. J. Therm. Biol. 11, 67-72. ( 10.1016/0306-4565(86)90020-3) [DOI] [Google Scholar]
- 39.Le Pogam A, O'Connor RS, Love OP, Petit M, Régimbald L, Vézina F. 2021. Coping with the worst of both worlds: phenotypic adjustments for cold acclimatization benefit northward migration and arrival in the cold in an Arctic-breeding songbird. Funct. Ecol. 35, 1240-1254. ( 10.1111/1365-2435.13793) [DOI] [Google Scholar]
- 40.Le Pogam A, Love OP, Régimbald L, Dubois K, Hallot F, Milbergue M, Petit M, O'Connor RS, Vézina F. 2020. Wintering snow buntings elevate cold hardiness to extreme levels but show no changes in maintenance costs. Physiol. Biochem. Zool. 93, 417-433. ( 10.1086/711370) [DOI] [PubMed] [Google Scholar]
- 41.Le Pogam A, et al. 2021. Snow buntings maintain winter-level cold endurance while migrating to the high Arctic. Front. Ecol. Evol. 9, 724876. ( 10.3389/fevo.2021.724876) [DOI] [Google Scholar]
- 42.McNab BK. 1980. On estimating thermal conductance in endotherms. Physiol. Zool. 53, 145-156. ( 10.1086/physzool.53.2.30152577) [DOI] [Google Scholar]
- 43.Tieleman BI, Williams JB, LaCroix F, Paillat P. 2002. Physiological responses of Houbara bustards to high ambient temperatures. J. Exp. Biol. 205, 503-511. ( 10.1242/jeb.205.4.503) [DOI] [PubMed] [Google Scholar]
- 44.R Core Team. 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org. [Google Scholar]
- 45.Carslaw DC, Ropkins K. 2012. openair—An R package for air quality data analysis. Environ. Model. Softw . 27–28, 52-61. ( 10.1016/j.envsoft.2011.09.008) [DOI] [Google Scholar]
- 46.Lek S, Guégan JF. 1999. Artificial neural networks as a tool in ecological modelling, an introduction. Ecol. Model. 120, 65-73. ( 10.1016/S0304-3800(99)00092-7) [DOI] [Google Scholar]
- 47.Fritsch S, Guenther F, Wright MN. 2019. neuralnet: training of neural networks. R package version 1.44.2. See https://CRAN.R-project.org/package=neuralnet.
- 48.Hussell DJT. 1972. Factors affecting clutch size in Arctic passerines. Ecol. Monogr. 42, 317-364. ( 10.2307/1942213) [DOI] [Google Scholar]
- 49.Montgomerie R, Lyon B. 2020. Snow bunting (Plectrophenax nivalis). In Birds of the world (eds Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS). Ithaca, NY: Cornell; Lab of Ornithology. [Google Scholar]
- 50.Tattersall GJ, Sinclair BJ, Withers PC, Fields PA, Seebacher F, Cooper CE, Maloney SK. 2012. Coping with thermal challenges: physiological adaptations to environmental temperatures. Comp. Physiol. 2, 2151-2202. ( 10.1002/cphy.c110055) [DOI] [PubMed] [Google Scholar]
- 51.Fristoe TS, Burger JR, Balk MA, Khaliq I, Hof C, Brown JH. 2015. Metabolic heat production and thermal conductance are mass-independent adaptations to thermal environment in birds and mammals. Proc. Natl Acad. Sci. USA 112, 15 934-15 939. ( 10.1073/pnas.1521662112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van de Ven TMFN, McKechnie AE, Er S, Cunningham SJ. 2020. High temperatures are associated with substantial reductions in breeding success and offspring quality in an arid-zone bird. Oecologia 193, 225-235. ( 10.1007/s00442-020-04644-6) [DOI] [PubMed] [Google Scholar]
- 53.Lyon BE, Montgomerie RD. 1985. Incubation feeding in snow buntings: female manipulation or indirect male parental care? Behav. Ecol. Sociobiol. 17, 279-284. ( 10.1007/BF00300147) [DOI] [Google Scholar]
- 54.Hoset KS, Espmark Y, Moksnes A, Haugan T, Ingebrigtsen M, Lier M. 2004. Effect of ambient temperature on food provisioning and reproductive success in snow buntings Plectrophenax nivalis in the high Arctic. Ardea 92, 239-246. ( 10.1007/s00300-009-0664-8) [DOI] [Google Scholar]
- 55.Cunningham SJ, Martin RO, Hojem CL, Hockey PAR. 2013. Temperatures in excess of critical thresholds threaten nestling growth and survival in a rapidly-warming arid savanna: a study of common fiscals. PLoS ONE 8, e74613. ( 10.1371/journal.pone.0074613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angilletta MJ Jr. 2009. Thermal adaptation. Oxford, UK: Oxford University Press. [Google Scholar]
- 57.McCullough EC, Porter WP. 1971. Computing clear day solar radiation spectra for the terrestrial ecological environment. Ecology 52, 1008-1015. ( 10.2307/1933806) [DOI] [Google Scholar]
- 58.Callan LM, La Sorte FA, Martin TE, Rohwer VG. 2019. Higher nest predation favors rapid fledging at the cost of plumage quality in nestling birds. Am. Nat. 193, 717-724. ( 10.1086/702856) [DOI] [PubMed] [Google Scholar]
- 59.Bolduc E, et al. 2013. Terrestrial arthropod abundance and phenology in the Canadian Arctic: modelling resource availability for Arctic-nesting insectivorous birds. Can. Entomol. 145, 155-170. ( 10.4039/tce.2013.4) [DOI] [Google Scholar]
- 60.Bourne AR, Cunningham SJ, Spottiswoode CN, Ridley AR. 2020. High temperatures drive offspring mortality in a cooperatively breeding bird. Proc. R. Soc. B 287, 20201140. ( 10.1098/rspb.2020.1140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greño JL, Belda EJ, Barba E. 2008. Influence of temperatures during the nestling period on post-fledging survival of great tit Parus major in a Mediterranean habitat. J. Avian Biol. 39, 41-49. ( 10.1111/j.0908-8857.2008.04120.x) [DOI] [Google Scholar]
- 62.Patel KK. 2022. Using genetic approaches to study local adaptation and reproductive success in snow buntings (Plectrophenax nivalis). Windsor, Canada: University of Windsor. [Google Scholar]
- 63.O'Connor R, et al. 2022. Data from: Warming in the land of the midnight sun: breeding birds may suffer greater heat stress at high- versus low-Arctic sites. Dryad Digital Repository. ( 10.5061/dryad.vmcvdnctr) [DOI] [PMC free article] [PubMed]
- 64.O'Connor R, et al. 2022. Warming in the land of the midnight sun: breeding birds may suffer greater heat stress at high- versus low-Arctic sites. FigShare. ( 10.6084/m9.figshare.c.6135577) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- O'Connor R, et al. 2022. Data from: Warming in the land of the midnight sun: breeding birds may suffer greater heat stress at high- versus low-Arctic sites. Dryad Digital Repository. ( 10.5061/dryad.vmcvdnctr) [DOI] [PMC free article] [PubMed]
- O'Connor R, et al. 2022. Warming in the land of the midnight sun: breeding birds may suffer greater heat stress at high- versus low-Arctic sites. FigShare. ( 10.6084/m9.figshare.c.6135577) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.vmcvdnctr [63].
The data are also provided in the electronic supplementary material [64].