Abstract
There is a notable lack of vaccine effectiveness studies using test-negative case-controlled approach in low- and middle-income countries which have different logistic, demographic and socio-economic conditions from high-income countries. We aimed to estimate the effectiveness of BNT162b2 vaccine against COVID-19 infection over time, intensive care unit admission, severe or critical disease and death due to COVID-19. This study was conducted in the resident population of Labuan aged ≥18 years who had been tested for SARS-CoV-2 by Reverse-Transcriptase Polymerase Chain Reaction between 1 March 2021 and 31 October 2021. We used a test-negative case-control design where 2644 pairs of cases and controls were matched by age, sex, testing date, nationality and testing reason. Analysis was stratified by age group to estimate age effect (<60 years and ≥60 years). Of 22217 individuals tested by Reverse-Transcriptase Polymerase Chain Reaction, 5100 were positive for SARS-CoV-2 and aged 18 years and above. Overall vaccine effectiveness ≥ 14 days after the second dose was 65.2% (95% CI: 59.8–69.9%) against COVID-19 infection, 92.5% (95% CI: 72.3–98.8%) against intensive care unit admission, and 96.5% (95% CI: 82.3–99.8%) against COVID-19 deaths. Among infected individuals, vaccine effectiveness was 79.2% (95% CI: 42.3–94.1%) in preventing severe or critical disease due to COVID-19. Vaccine effectiveness for ≥60 years was 72.3% (95% CI: 53.4–83.9%) in fully vaccinated individuals, higher than 64.8% (95% CI: 49.3–59.1%) for those <60 years. Two doses of BNT162b2 were highly effective against COVID-19 infection, severe or critical disease, intensive care unit admission and death due to COVID-19. This study addresses a gap in literature on BNT162b2 vaccine effectiveness in low- and middle-income populations and demonstrates the feasibility of such a study design in a resource limited setting while supporting evidence of waning immunity.
Keywords: BNT162b2, Case control, COVID-19, LMIC, Test-negative, Vaccine effectiveness
Abbreviations: LMIC, Low- and Middle-Income Countries
1. Introduction
The coronavirus disease-2019 (COVID-19) pandemic has evolved into a health, socioeconomic and humanitarian crises of unprecedented scale and impact has taken a significant toll globally. This resulted in rapid development of vaccines against SARS-CoV-2, the virus that causes COVID-19, and the subsequent authorisation of the vaccines for emergency use accelerated access to the vaccine for distribution to the population [1]. In Malaysia, the mass vaccination campaign against COVID-19 commenced in February 2021 with the mRNA-based BNT162b2 (Pfizer-BioNTech) vaccine being among the first to receive conditional registration approval by the local health authorities [2], [3].
Approval for BNT162b2 was granted on the basis of immunogenicity, efficacy, and safety data from clinical trials [4]. Monitoring of vaccine effectiveness (VE) post-licensure to confirm continued effectiveness among the population in the real-world setting is an essential component of vaccination programs. Several observational studies of BNT162b2 reported that the VE estimate against SARS-CoV-2 infection ranged from 57% to 92% following administration of one or two doses at various time points [5], [6], [7], [8], [9]. Nevertheless, a steady decline in antibody levels among vaccinated individuals have been documented in several studies, which suggest that the immune protection may diminish over time [10], [11], [12], [13], [14], [15]. Long-term follow-up of vaccine-trial participants has revealed a gradual and modest decline in efficacy [14], [16]. Furthermore, the emergence of variants of concern with mutation of the SARS-CoV-2 virus highlights the need for continuous monitoring of COVID-19 vaccine effectiveness [17].
Post-licensure evaluation of vaccine effectiveness is often associated with methodological challenges in countries with low resource settings [18]. For most low- and middle-income countries (LMICs), the data gap is an important consideration for selection of study design that is feasible to implement in their respective settings to conduct real-world VE studies [19]. The test-negative design has been routinely applied to estimate VE of various vaccines including COVID-19 vaccines. Despite its convenient and efficient approach, the use of test-negative design to estimate VE is subject to the availability of appropriate data elements to meet a clear and specific definitions of cases and controls, and ascertainment of exposure status. To date, there were only few studies from LMICs that has adopted this approach to study VE and waning immunity following COVID-19 vaccination [14], [15].
In this study, we aim to estimate the VE following administration of one or two doses of BNT162b2 vaccine in Labuan, a state in Malaysia. Labuan provides a unique setting where approximately 90% of the population were inoculated with BNT162b2 vaccine and being one of the first states within Malaysia that achieved 80% vaccination coverage within five months since the initiation of the Malaysia’s national COVID-19 immunisation program. The VE was measured against the following outcomes: SARS-CoV-2 infection, admission to intensive care unit (ICU), and death related to COVID-19.
2. Methods
This study is reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) checklist (Supplementary Table 1).
2.1. Study population, setting and design
This study was conducted in the resident population of Labuan aged ≥ 18 years who had been tested for SARS-CoV-2 by RT-PCR between 1 March 2021 and 31 October 2021. Details regarding Labuan population and setting are provided in Supplementary Data 1. Anyone in Labuan who underwent testing during the study period and had complete and consistent information between data sources on age, sex, vaccination status, reason for testing, testing status and date were included. ICU admissions and deaths not attributable to COVID-19 were excluded. Individuals with multiple positive test results more than 90 days apart were considered as reinfection cases [20]. Reinfection cases were not included the analysis as natural immunity provided by previous infection would bias VE. Individuals with previous infections were also excluded as controls. Individuals who had received COVID-19 vaccine other than BNT162b2 vaccine were excluded.
A matched test-negative case control study design was used to estimate VE against outcomes of interest. This study design has been used extensively for assessment of VE including COVID-19 [21], [22]. Cases and controls from the study population were matched one-to-one by sex, age, nationality, reason for SARS-CoV-2 testing and week of testing. Matching of cases and controls was performed to control for known differences in risk of exposure to infection and health seeking behaviour. Age of cases and controls were matched in order to control for potential confounding arising due to age being a known determinant of COVID-19 outcomes. A total of four matched case-control sets were constructed for each outcome. Vaccination status of cases and controls was compared. Characteristics of matched case-control sets for COVID-19 infection are provided in Table 1 while characteristics of matched case-control sets for ICU admission, death and severe or critical disease are provided in Supplementary Table 2.
Table 1.
Demographic characteristics of (1) study population by SARS-CoV-2 status (2) RT-PCR cases (positive) and controls (negative).
| Characteristics |
SARS-CoV-2 status |
Matched sets for COVID-19 infection |
||
|---|---|---|---|---|
|
Test positive (n = 5100) (%) |
Test negative (n = 9099) (%) |
Cases (n = 2644) (%) |
Controls (n = 2644) (%) |
|
| Sex | ||||
| Female | 2418 (47.4) | 3550 (39.0) | 1193 (45.1) | 1193 (45.1) |
| Male | 2682 (52.6) | 5549 (61.0) | 1451 (54.9) | 1451 (54.9) |
| Age Groups | ||||
| 18–39 | 3283 (64.4) | 5897 (64.8) | 1851 (70.0) | 1851 (70.0) |
| 40–59 | 1389 (27.2) | 2368 (26.0) | 616 (23.3) | 616 (23.3) |
| >60 | 428 (8.4) | 834 (9.2) | 177 (6.7) | 177 (6.7) |
| Nationality | ||||
| Malaysian | 4661 (91.4) | 8713 (95.8) | 2554 (96.6) | 2554 (96.6) |
| Non-Malaysian | 439 (8.6) | 386 (4.2) | 90 (3.4) | 90 (3.4) |
| Reason for testing | ||||
| Contact tracing | 2226 (43.6) | 965 (10.6) | 790 (29.9) | 790 (29.9) |
| Mandatory testing | 2317 (45.4) | 7705 (84.7) | 1567 (59.3) | 1567 (59.3) |
| Symptomatic | 527 (10.3) | 429 (4.7) | 287 (10.9) | 287 (10.9) |
| Vaccination status* | ||||
| 1 dose BNT-162b2 vaccine | 4393 (86.1) | 8276 (91.0) | 2194 (83.0) | 2362 (89.3) |
| 2 doses BNT-162b2 vaccine | 3882 (76.1) | 7917 (87.0) | 1983 (75.0) | 2233 (84.5) |
| Unvaccinated | 707 (13.9) | 823 (9.0) | 450 (17.0) | 282 (10.7) |
| Clinical staging (Category) | ||||
| 1 | 4201 (82.4) | – | 1988 (75.2) | – |
| 2 | 276 (5.4) | – | 183 (6.9) | – |
| 3 | 65 (1.3) | – | 34 (1.3) | – |
| 4 | 28 (0.5) | – | 19 (0.7) | – |
| 5 | 139 (2.7) | – | 92 (3.5) | – |
| Missing | 391 (7.7) | – | – | – |
| Week of testing | ||||
| Week 1–5 | 86 | 693 | 75 | 75 |
| Week 6–10 | 50 | 493 | 45 | 45 |
| Week 11–15 | 2593 | 1759 | 1362 | 1362 |
| Week 16–20 | 2039 | 1351 | 876 | 876 |
| Week 21–25 | 168 | 1459 | 133 | 133 |
| Week 26–30 | 100 | 2193 | 96 | 96 |
| Week 31–35 | 64 | 1133 | 57 | 57 |
* Vaccination status does not take into account 14 days after second dose for fully vaccinated.
2.2. Data sources and definitions
Data on SARS-CoV-2 laboratory testing, vaccination, infection, clinical outcomes, and related demographic details were retrieved from state-level databases as described in Supplementary Table 3. Laboratory tests results, infections, and clinical admission related to COVID-19 are subject to clinical audit and is part of mandatory reporting in Malaysia within the national COVID-19 surveillance. The vaccination register of the Federal Territory of Labuan (hereinafter referred to as Labuan) was combined with the national vaccination register (MyVas) for determination of vaccination status of individuals who received vaccines outside the island.
A COVID-19 positive case was defined as a person with RT-PCR confirmation of infection with SARS-CoV-2 irrespective of clinical signs or symptoms. COVID-19 ICU admissions referred to confirmed COVID-19 cases that were admitted to ICU based on clinical judgement due to disease progression to severe disease, such as category 4 (requiring oxygen therapy) or category 5 (requiring mechanical ventilation). A COVID-19 death was defined as any “death attributable to COVID-19”. For COVID-19 cases that require ICU admission and fatal cases, the database contained the classification of cases into the following categories according to the clinical audit requirement: “ICU admission with COVID-19”, “ICU admission due to COVID-19”, “death with COVID-19”, and “death due to COVID-19”.
Individual-level data of all SARS-CoV-2 test conducted in Labuan was linked to the vaccination database and clinical admission register to establish the status of COVID-19 vaccination, COVID-19 infection, ICU admission and death associated with COVID-19. Data linkage was performed using a unique, common patient identifier (citizen registration number) via deterministic matching.
2.3. Vaccination status
Exposure of interest was receipt of at least one dose of BNT162b2 vaccine. Patients were considered to be fully vaccinated 14 days after the second dose of the BNT162b2 vaccine, whereas partial vaccinated patients include those who had received their first dose of BNT162b2 vaccine and those who were in 1–13 days post second dose of vaccination [8]. Vaccination status was further narrowed down to fortnightly intervals after full vaccination to ascertain VE over elapsed time.
2.4. Outcomes
All individuals who developed outcomes of interests were considered as cases. The primary outcome was COVID-19 infection ascertained by RT-PCR tests for SARS-CoV-2 on respiratory specimens, comprising of samples from the nasopharynx and saliva. Secondary outcomes were COVID-19-related ICU admission and death. Controls were defined as individuals with negative RT-PCR test and without a positive test in the previous 90 days or the following 14 days. If an individual had multiple negative test results during the study period, only one test date was selected randomly.
For analysis among those who had COVID-19 infection, outcome was measured in terms of progression to severe or critical disease. Classification of COVID-19 case severity was in accordance with WHO guidelines [23], based on assessment during hospitalisation for COVID-19 where the worst outcome category was recorded. Severe or critical disease referred to those in category 3, 4, and 5 of COVID-19 infection clinical staging.
In addition, VE was measured against COVID-19 infection over time according to time elapsed between second dose of the vaccine and RT-PCR test.
2.5. Covariates
Details of age, sex, nationality, reason for testing and testing dates were obtained from the testing database. Nationality was used as a proxy for occupational risk as occupational details were not completely available. Reason for testing was categorised into three categories: symptomatic testing, contact tracing, and mandatory testing (i.e., testing is compulsory due to requirements for work, travel, education etc.).
2.6. Statistical analysis
Descriptive analyses were conducted to compare characteristics between test positive cases and test-negative controls. A 7-day moving average of COVID-19 infections was also plotted against the proportion of population vaccinated to give an overall view of the situation during the study period (Supplementary Data 1).
The data set was stratified by age groups to obtain: (i) the proportion of the population that was vaccinated, and (ii) the proportion of disease outcomes of interest occurring amongst the vaccinated. We then estimated odds ratios using conditional logistic regression models, which compares odds of vaccination in cases against controls. VE was calculated using the following formula:
VE against COVID-19 infection was estimated by vaccination status and stratified by age groups. We then repeated the analyses for ICU admission, death and severe or critical disease due to COVID-19. All tests were two-sided and used p < 0.05 as the level of statistical significance. All the data processing and analysis, including data cleaning and linking were performed using R version 4.0.5.
2.7. Additional analysis
Analysis was stratified by age group to estimate age effect (<60 years and ≥60 years). Age was analysed as an interaction term for all outcomes. In a sensitivity analysis, all eligible tested individuals were included in the analysis without matching of cases and controls. Logistic regression model was used to compute the adjusted odds ratio, adjusting for age, sex, nationality, reason for testing and testing dates.
3. Results
This test-negative case control vaccine effectiveness study looks into the case of Labuan, an island of the coast of East Malaysia. Infections went up to 1329 cases (1327 cases per 100,000) per week at the end of May 2021, but showed a consistent drastic decline in COVID-19 case incidence since the week of 7 to 13 June, from a 7-day moving average of 180 cases in early June 2021 to 23 cases in August 2021. Rate of infection was at its lowest in September 2021 but showed a slight increase in October (Table 1 & Supplementary Data 1).
3.1. Study population
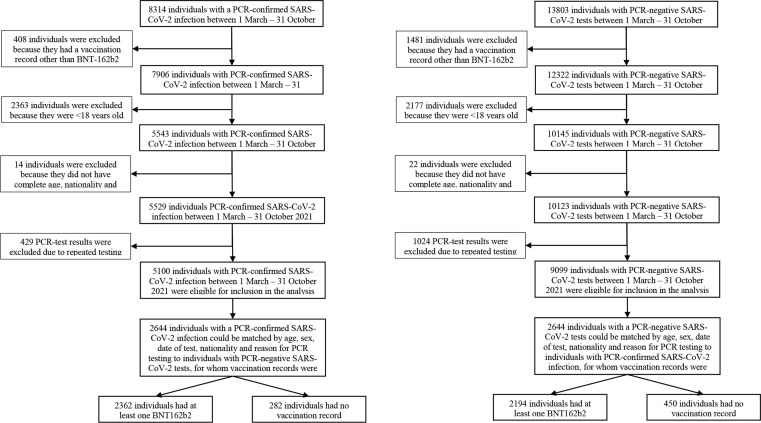
Overall, 22,217 (case = 8314; control = 13803) individuals were tested for SARS-CoV-2 from 1 March 2021 to 31 October 2021 of which 1889 (case = 408; control = 1481) individuals with vaccination records other than BNT162b2 were excluded. A total of 4540 (case = 2363; control = 2177) individuals < 18 years old were excluded, while 36 (case = 14; control = 22) individuals were excluded as they did not have complete age, nationality and gender records. After excluding those who had multiple test results, there were 14,199 unique individuals with test results, of which 5100 tested positive for SARS-CoV-2 (35.9%). A total of 12,669 (89.2%) individuals had received at least one dose of BNT-162b2 vaccine, and 11,799 (83.0%) had received two doses. The study population had a median age of 34 years, and comprised of 5.8% non-Malaysians. Mandatory testing was the common reason for testing (n = 10022), followed by contact tracing (n = 3191) and symptomatic testing (n = 956). Throughout the study period, there were 55 cases of reinfection (tested positive within 90 days of last positive result), 81 ICU admissions and 118 deaths (Table 1). Flowcharts describing the population selection process for investigation and estimation of VE are presented in Fig. 1 .
Fig. 1.
Flowcharts describing the population selection process to assess vaccine effectiveness.
RT-PCR results were matched for case and control sets in 1:1 ratio resulting in 2644 matched pairs. Table 1 shows the characteristics of eligible participants with positive and negative RT-PCR test results, and selected cases and matched controls. The matched sets in Table 1 describe the demographics of the cases and controls for the primary outcome of SARS-CoV-2 infection. Matched characteristics were balanced for all matched covariates i.e., sex, age, nationality, reason for testing and week of testing. There were more controls who had received at least one dose of vaccine (n = 2362; 89.3%) compared to cases (n = 2194; 83.0%). Likewise, there were more controls who had received two doses of vaccine (n = 2233; 84.5%) compared to cases (n = 1983; 75.0%).
There were 79 matched pairs with a median age of 51 years and only one non-Malaysian pair included in the analysis for COVID-19 associated ICU admission (Supplementary Table 3). For COVID-19 deaths, there were 105 matched pairs with a median age of 57.5 years. Matched pairs for death outcomes were more concentrated in the months of May - July while matched pairs for ICU admissions were spread out over the study period. There were 179 matched pairs in testing for COVID-19 severity, with a median age of 48.5 years.
3.2. Vaccine effectiveness against COVID-19 infection
The median age of cases and controls was 32 years, where 54.9% (n = 1451) of each arm were male. Mandatory testing comprised of the largest number of matches, followed by contact tracing and symptomatic testing. Majority of the cases were asymptomatic i.e., Category 1 (n = 1988; 75.2%). The adjusted effectiveness against COVID-19 infection was 29.2% (95% confidence interval 17.5–39.3%) for partially vaccinated individuals and 65.2% (59.8–69.9%) for fully vaccinated individuals (Table 2 ).
Table 2.
Odds ratio and vaccine effectiveness against COVID-19 infection, ICU admission, or death due to COVID-19.
|
Odds ratio (95% CI) |
Vaccine effectiveness, % (95% CI) |
|
|---|---|---|
| COVID-19 Infection | ||
| Unvaccinated | Reference | – |
| Fully Vaccinated | 0.35 (0.30 to 0.40) | 65.2 (59.8 to 69.9) ^ |
| Partially Vaccinated | 0.71 (0.61 to 0.83) | 29.2 (17.5 to 39.3) ^ |
| ICU Admission due to COVID-19 | ||
| Unvaccinated | Reference | – |
| Fully Vaccinated | 0.08 (0.01 to 0.28) | 92.5 (72.3 to 98.8) ^ |
| Partially Vaccinated | 0.41 (0.16 to 0.99) | 59.4 (1.0 to 84.2) ^ |
| Death due to COVID-19 | ||
| Unvaccinated | Reference | – |
| Fully Vaccinated | 0.04 (0.00 to 0.18) | 96.5 (82.3 to 99.8) ^ |
| Partially Vaccinated | 0.20 (0.07 to 0.50) | 79.8 (49.8 to 93.9) ^ |
| Severe or critical disease due to COVID-19 (Category 3–5) a | ||
| Unvaccinated | Reference | – |
| Fully Vaccinated | 0.21 (0.06 to 0.58) | 79.2 (42.3 to 94.1) ^ |
| Partially Vaccinated | 0.71 (0.38 to 1.32) | 28.7 (–32.3 to 61.9) |
Abbreviations: CI, confidence interval.
p ≤ 0.05. a Compared to Category 1-2
3.3. Vaccine effectiveness against COVID-19 associated ICU admissions, deaths and severe COVID-19
The adjusted effectiveness against COVID-19 associated ICU admission was 92.5% (72.3–98.8%) for fully vaccinated individuals (Table 2). The adjusted effectiveness against death due to COVID-19 was 79.8% (49.8–93.9%) for partially vaccinated individuals and 96.5% (82.3–99.8%) for fully vaccinated individuals. Among individuals who were tested positive, full vaccination gave an adjusted effectiveness of 79.2% (42.3–94.1%) in preventing severe or critical disease due to COVID-19 (i.e., developing Category 3–5 COVID-19) (Table 3).
3.4. Vaccine effectiveness against COVID-19 infection over time
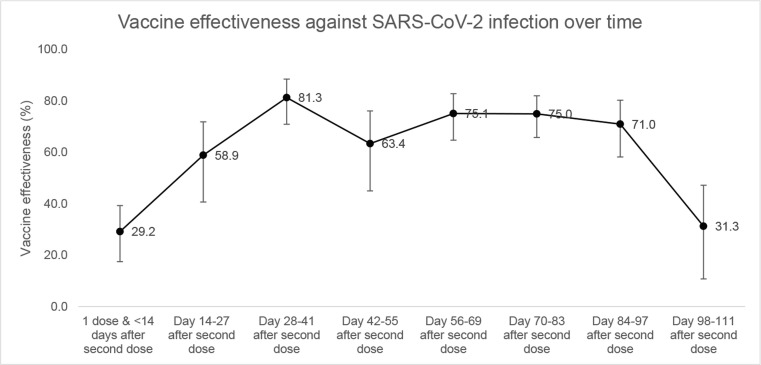
Fig. 2 shows the estimates for VE 14 or more days after the second dose over the specified intervals. VE was the highest at 81.3% (70.9–88.5%) 28–41 days after the second dose. This was followed by a general decline over time with VE at 31.3% (10.8–47.2%) 98–111 days after second dose. Notably, there was a dip in VE at day 42–55 after second dose (Fig. 2 & Supplementary Table 4).
Fig. 2.
Vaccine effectiveness against COVID-19 infection over time.
3.5. Sensitivity analysis
VE for ≥ 60 years was 72.3% (53.4–83.9%) in fully vaccinated individuals, higher than 64.8% for those < 60 years. Partially vaccinated individuals ≥ 60 years also had higher VE at 50.4% (12.9–71.2%) than VE of 27.4% (17.5–39.3%) in partially vaccinated individuals < 60 years. Adjusted effectiveness against death due to COVID-19 was 90.1% (40.4–99.5%) for partially vaccinated individuals and 92.3% (55.9–99.6%) for fully vaccinated individuals ≥ 60 years. The vaccine was also shown to be 92.6% (57.9–99.6%) effective in preventing Category 3–5 COVID-19 in individuals aged ≥ 60 years who tested positive. There were insufficient samples to conduct a sensitivity analysis for VE against ICU admission (Supplementary Table 5). However, when analysed as an interaction term, age was not found to be statistically significant with vaccination status.
A second form of sensitivity analysis was carried out using a cohort study design that compares infection incidence in those vaccinated with that in the cohort of those who underwent RT-PCR testing (Supplementary Table 6). VE against COVID-19 infection was 43.0% (36.0–49.3%) for partially vaccinated individuals and 87.9% (86.3–89.4%) in fully vaccinated individuals. Meanwhile, for fully vaccinated individuals, VE was 97.5% (82.1–99.6%) against COVID-19 associated ICU admission and 99.3% (96.8–100%) against COVID-19 associated death, which was similar, albeit slightly higher than the results in the main analysis.
4. Discussion
This test-negative matched case-control study estimated that two doses of BNT162b2 vaccine had an effectiveness of 65% against COVID-19 infection in adults 18 years and older in Labuan, Malaysia. The VE against ICU admission and death associated with COVID-19 was higher at 93% and 97%, respectively. Although the vaccine may not completely prevent COVID-19 infection, it greatly diminishes the risk of severe disease and mortality. There appears to be a trend towards decreasing effectiveness with increase in the time that elapsed after second dose.
Our results indicate that fully vaccinated individuals are 79.2% protected against developing Category 3, 4 or 5 COVID-19 disease, should they develop an infection. This is an important point for healthcare capacity as Category 3 and above COVID-19 infections are more likely to require hospitalization. In this case, 4 out 5 fully vaccinated individuals infected with SARS-CoV-2 would not require hospitalization. As such, a reduction in the severity to this extent will reduce the short-term burden on the healthcare system.
The quality of VE studies depends on the data available, particularly in LMIC where disease burdens may be high with suboptimal systems for routine data collection [24]. For our study, the setting within Labuan allowed for robust data collection as it had one centralised healthcare facility and only two RT-PCR testing centres servicing the entire population. Testing strategy employed was sufficiently accurate where only 8.1% of individuals with multiple tests within 7 days had RT-PCR negative results developing into positive cases. RT-PCR tests were readily available to the public for free at the two government-run testing centres. We also limited bias due to false negative results from testing by removing individuals who were classified as cases from the pool of possible controls. This study establishes the feasibility of proper and necessary data collection for test-negative case-control study design in a LMICs. Previous studies have indicated difficulty in recruiting sufficient controls [25], especially in the case of using PCR-testing in LMIC where large numbers of testing may be costly.
The overall VE estimated in our study is slightly lower compared to the earlier study from Malaysia with larger, nationwide population (RECoVaM study) [2], which reported effectiveness of 88% against COVID-19 infection in fully vaccinated. The difference is likely due to the different study designs employed to measure VE estimate. While RECoVaM used a mixed-method of screening method and cohort study design, our study used the matched test-negative case control design to measure VE for all outcomes. All observational studies are subject to bias and the different study designs have its own strengths and limitations in measuring VE [22]. Besides, our study was restricted to BNT162b2 vaccine recipients whereas the VE estimate for COVID-19 infection from the RECoVaM study was for population fully vaccinated with any COVID-19 vaccine. Thus, our findings complement the current evidence in demonstrating the effectiveness of the vaccine in the local population by using an approach widely used in evaluation of VE. The longer study period of our study (8 months) compared to the RECoVaM study (5.5 months) may be another reason for the lower overall VE in our study as we have demonstrated that VE decreases over time (Fig. 2).
A test-negative case-control study by Lopez Bernal et al. reported VE of 61% against the Delta variant in England [7] which is similar to the results of our main analysis. Our study was conducted during a time when a variant of concern (Delta) was present and rampant on the island [26]. Comparing with VE studies against the Delta variant, VE of 65.2% against COVID-19 infection observed in fully vaccinated individuals was in the middle of the range of VE by other studies [8], [15], [27]. Our results for VE of 92.6% against ICU admission was also similar to that reported at 90% using data from seven network hospitals [5]. This, in addition to 96.5% VE against death due to COVID-19, is in line with 93.4% VE against Delta-induced severe, critical or fatal disease found in a previous study [8]. A study in Canada also reported similar VE of 98% against hospital admission or death [6]. Our results indicate that fully vaccinated individuals are 79.2% protected against developing Category 3, 4 or 5 COVID-19 disease, should they develop an infection. This is an important point for healthcare capacity as Category 3 and above COVID-19 infections would require hospitalization. In this case, 4 out 5 fully vaccinated individuals infected with SARS-CoV-2 would not require hospitalization. As such, a reduction in the severity to this extent will reduce the short-term burden of COVID-19 on the healthcare system.
The decrease in VE over time supports emerging evidence of waning immunity [9], [12], [13]. Although our results showed lower VE at each time point compared to studies is Qatar [9] and the United Kingdom [13], the rate of decrease was similar. These findings demonstrate the need for more follow-up of vaccinated cohorts to investigate waning of vaccine immunity and for studies on the need for booster doses. The dip in VE on days 42–55 after second dose is likely due to higher infectivity rate (Rt) during the time when matched pairs were tested [28] as it occurred during the height of the Delta wave in Labuan.
The use of test-negative design for VE have been evaluated in several studies that support its use as an appropriate epidemiologic study design to measure VE in low-income settings as well as confirm the accuracy and precision of its estimates compared to the gold standard of classic per-protocol randomised control trials analysis [29], [30], [31]. This has policy implications as there are increasingly more COVID-19 VE studies with various study designs. Policymakers should take into account the study design of VE when evaluating the need for subsequent or booster doses. The key strength of test-negative case-control design is the control of bias arising from misclassification of infection and differences in healthcare-seeking behaviour between vaccinated and unvaccinated individuals [32], [33]. A case control study design would be less ideal in the case of COVID-19 VE studies due to difficulty in selection of correct controls [34]. The lack of test-negative subjects may result in possible misclassification and biased control group [35]. Furthermore, only 36 individuals were excluded due to incomplete records. Our study also demonstrated a difference in VE when using different methods i.e. test-negative case control vs retrospective cohort of tested individuals. This difference is due to the lack of matching of test cases and controls in the retrospective cohort where the matching criteria reduces bias arising from differences in health seeking behaviour.
Confounding variables that influence both the receipt of vaccine and the occurrence of medically attended COVID-19 (e.g. exposure to the virus, risk of severe disease associated with infection, and access to or uptake to care) were addressed by matching of five covariates i.e., sex, age, nationality, reason for testing, and date of testing. It is essential to consider that non-Malaysians, illegal immigrants, and undocumented workers will have higher thresholds for seeking health care and will generally be at higher risk for serious illness than other persons, regardless of vaccination status [36], [37]. As such, we attempted to address this by matching for citizenship status to reduce possible bias. Nevertheless, we also acknowledge the possible residual confounding due to differential exposure or susceptibility to infection and symptoms among vaccinated and unvaccinated individuals [38].
This study presents results on VE on an island with containment measures in place. As such, this study presents real world VE in an ideal situation with non-pharmaceutical interventions in place. Although generalizability beyond that population cannot be assessed with the study data alone, severe medical outcomes (e.g., hospitalization and ICU admission) are considered to be less sensitive to differences in care seeking [16]. The rapid mutation of the SARS-CoV-2 virus may require further VE estimates as these results may not be applicable to variants other than Delta. In addressing policy concerns, new VE estimates for the current dominant variant, Omicron.
One of the limitations of this study is the lack of complete data on comorbidities of the individuals tested. Data on comorbidities were only available for those who tested positive or were vaccinated, leaving those who tested negative or were unvaccinated with missing comorbidities data. The use of comorbidities in the analysis will give rise to bias and overestimate the effect of comorbidities on VE. Another limitation is possible misclassification of cases and controls. Imperfect specificity substantially affects test-negative and case-control studies [39]. Analytic validation studies for SARS-CoV-2 RT-PCR assays indicate that among persons who may have severe COVID-19, sensitivity has ranged from 33% to 80% due to factors such as delayed presentation when virus shedding is lower, inadequate swabbing, and site of sampling (nasal, oral, nasopharyngeal, tracheal) [40].
Moreover, clinical findings of severe COVID-19 overlap with common causes of severe respiratory illness, such as influenza, pulmonary oedema, and asthma exacerbation. Patients with these conditions may coincidentally have test results positive for SARS-CoV-2 due to a concurrent mild infection or recovered infection with prolonged RNA shedding. This scenario is more common when community prevalence of SARS-CoV-2 is high, which was the case during our study period. Although misclassifying such individuals is problematic for any study design when vaccines attenuate disease severity, bias may potentially be greater in test-negative studies [41]. However, we minimised this risk of bias by only including the first positive test result for individuals with multiple positive results.
5. Conclusion
In conclusion, the BNT162b2 vaccine is highly effective in preventing COVID-19 infection, ICU admission and death due to COVID-19 and severe or critical disease in infected individuals. However, VE was seen to decline over time, indicating waning of vaccine protection. This study establishes the feasibility of test-negative case-control design for VE studies in LMIC.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We would like to thank the Director General of Health Malaysia for his permission to publish this article. We would also like to acknowledge Mr. Suah Jing Lian for his assistance in reviewing the manuscript draft.
Contributors’ Statement
KMP, SS, AHL and OSM conceptualized and designed the study. Data collection was conducted by JP, RR, IS, SND, TSS and IB. Data cleaning and merging was conducted by AHL. Data analysis was carried out by AHL, OSM and NAR. AHL and NAR prepared the first draft of the manuscript, which was circulated for comments before further editing by SS and KMP. All authors contributed to data interpretation, revised the draft critically for important intellectual content and agreed to the final submission.
Competing interest
The authors declared that no conflict of interest.
Data availability
Deidentified analysis data sets generated from the testing and vaccine registry databases are available from the corresponding author upon reasonable request.
Code availability
Code used to conduct statistical analysis and produce figures within this paper are available from the corresponding author upon reasonable request.
Ethics approval
This study was registered in the National Medical Research Register (NMRR-21-1865-61496) and approved by the Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.08.032.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Dellit T.H., Owens R.C., McGowan J.E., Gerding D.N., Weinstein R.A., Burke J.P., et al. Infectious diseases society of america and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 2.Suah J.L., Tok P.S.K., Ong S.M., Husin M., Tng B.H., Sivasampu S., et al. PICK-ing Malaysia’s epidemic apart: effectiveness of a diverse COVID-19 vaccine portfolio. Vaccines. 2021;9(12):1381. doi: 10.3390/vaccines9121381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang S.L., Mat Ripen A., Leong C.T., Lee J.V., Yen C.H., Chand A.K., et al. COVID-19 breakthrough infections and humoral immune response among BNT162b2 vaccinated healthcare workers in Malaysia. Emerging Microbes Infect. 2022;11(1):1262–1271. doi: 10.1080/22221751.2022.2065936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CodeBlue. Malaysia’s NPRA Approves Pfizer Covid-19 Vaccine. 2021.
- 5.Thompson M.G., Stenehjem E., Grannis S., Ball S.W., Naleway A.L., Ong T.C., et al. Effectiveness of Covid-19 vaccines in ambulatory and inpatient care settings. N Engl J Med. 2021;385(15):1355–1371. doi: 10.1056/NEJMoa2110362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung H, He S, Nasreen S, Sundaram ME, Buchan SA, Wilson SE, et al. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ. 2021;374:n1943. [DOI] [PMC free article] [PubMed]
- 7.Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. [DOI] [PMC free article] [PubMed]
- 8.Tang P., Hasan M.R., Chemaitelly H., Yassine H.M., Benslimane F.M., Al Khatib H.A., et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021 doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 9.Chemaitelly H., Tang P., Hasan M.R., AlMukdad S., Yassine H.M., Benslimane F.M., et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in qatar. N Engl J Med. 2021 doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naaber P., Tserel L., Kangro K., Sepp E., Jürjenson V., Adamson A., et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. The Lancet Regional Health - Europe. 2021;10 doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(24):e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Israel A, Merzon E, Schäffer AA, Shenhar Y, Green I, Golan-Cohen A, et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: test negative design study. BMJ. 2021;375:e067873. [DOI] [PMC free article] [PubMed]
- 13.Andrews N., Tessier E., Stowe J., Gower C., Kirsebom F., Simmons R., et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med. 2022;386(4):340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feikin D.R., Higdon M.M., Abu-Raddad L.J., Andrews N., Araos R., Goldberg Y., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. The Lancet. 2022;399(10328):924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Vaccine Access Center (IVAC). Johns Hopkins Bloomberg School of Public Health; 2022.
- 16.Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. The Lancet. 2021;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King C., Beard J., Crampin A.C., Costello A., Mwansambo C., Cunliffe N.A., et al. Methodological challenges in measuring vaccine effectiveness using population cohorts in low resource settings. Vaccine. 2015;33(38):4748–4755. doi: 10.1016/j.vaccine.2015.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soon M.P.S.C., Juan Z. Retrospective analysis of antibiotic prescribing pattern for upper respiratory tract infection (URTI) in Seremban 2 health clinic. Int J Adv Life Sci Res. 2020;3:29–39. [Google Scholar]
- 20.Yahav D., Yelin D., Eckerle I., Eberhardt C.S., Wang J., Cao B., et al. Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clin Microbiol Infect: Off Publicat Eur Soc Clin Microbiol Infect Diseases. 2021;27(3):315–318. doi: 10.1016/j.cmi.2020.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandenbroucke J.P., Pearce N. Test-negative designs: differences and commonalities with other case-control studies with “Other Patient” controls. Epidemiology. 2019;30:838–844. doi: 10.1097/EDE.0000000000001088. [DOI] [PubMed] [Google Scholar]
- 22.Patel M.K., Bergeri I., Bresee J.S., Cowling B.J., Crowcroft N.S., Fahmy K., et al. Evaluation of post-introduction COVID-19 vaccine effectiveness: summary of interim guidance of the World Health Organization. Vaccine. 2021;39(30):4013–4024. doi: 10.1016/j.vaccine.2021.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization (2021). COVID-19 clinical management: living guidance, 18 November 2021.
- 24.Hungerford D., Cunliffe N.A. Real world effectiveness of covid-19 vaccines. BMJ. 2021;374 [Google Scholar]
- 25.Nealon J., Lim W.-Y., Moureau A., Linus Lojikip S., Junus S., Kumar S., et al. Feasibility of case-control and test-negative designs to evaluate dengue vaccine effectiveness in Malaysia. Vaccine. 2019;37(39):5891–5898. doi: 10.1016/j.vaccine.2019.07.083. [DOI] [PubMed] [Google Scholar]
- 26.Nuradzimmah Daim T.A. Boost in vaccination helps Labuan stem Delta surge. New Straits. Times. 2021 [Google Scholar]
- 27.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achaiah N.C., Subbarajasetty S.B., Shetty R.M. R(0) and R(e) of COVID-19: can we predict when the pandemic outbreak will be contained? Ind J Crit Care Med: Peer-reviewed, Official Publicat Ind Soc Critical Care Med. 2020;24:1125–1127. doi: 10.5005/jp-journals-10071-23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Serres G., Skowronski D.M., Wu X.W., Ambrose C.S. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill. 2013;18 doi: 10.2807/1560-7917.es2013.18.37.20585. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz L.M., Halloran M.E., Rowhani-Rahbar A., Neuzil K.M., Victor J.C. Rotavirus vaccine effectiveness in low-income settings: An evaluation of the test-negative design. Vaccine. 2017;35(1):184–190. doi: 10.1016/j.vaccine.2016.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L.i., Wei M., Jin P., Li J., Zhu F. An evaluation of a test-negative design for EV-71 vaccine from a randomized controlled trial. Hum Vaccin Immunother. 2021;17(7):2101–2106. doi: 10.1080/21645515.2020.1859900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandenbroucke J.P., Brickley E.B., Pearce N., Vandenbroucke-Grauls C. The evolving usefulness of the test-negative design in studying risk factors for COVID-19. Epidemiology. 2022;33:e7–e8. doi: 10.1097/EDE.0000000000001438. [DOI] [PubMed] [Google Scholar]
- 33.Vandenbroucke J.P., Brickley E.B., Vandenbroucke-Grauls C.M.J.E., Pearce N. A test-negative design with additional population controls can be used to rapidly study causes of the SARS-CoV-2 epidemic. Epidemiology. 2020;31:836–843. doi: 10.1097/EDE.0000000000001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angulo F.J., Finelli L., Swerdlow D.L. Estimation of US SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths using seroprevalence surveys. JAMA Netw Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.33706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teerawattananon Y., Anothaisintawee T., Pheerapanyawaranun C., Botwright S., Akksilp K., Sirichumroonwit N., et al. A systematic review of methodological approaches for evaluating real-world effectiveness of COVID-19 vaccines: advising resource-constrained settings. PLoS ONE. 2022;17(1) doi: 10.1371/journal.pone.0261930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ab Rahman N., Sivasampu S., Mohamad Noh K., Khoo E.M. Health profiles of foreigners attending primary care clinics in Malaysia. BMC Health Serv Res. 2016;16(1) doi: 10.1186/s12913-016-1444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loganathan T, Rui D, Ng C-W, Pocock NS. Breaking down the barriers: Understanding migrant workers' access to healthcare in Malaysia. PLoS One. 2019;14:e0218669-e. [DOI] [PMC free article] [PubMed]
- 38.Lewnard J.A., Tedijanto C., Cowling B.J., Lipsitch M. Measurement of vaccine direct effects under the test-negative design. Am J Epidemiol. 2018;187:2686–2697. doi: 10.1093/aje/kwy163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson M.L., Rothman K.J. Effects of imperfect test sensitivity and specificity on observational studies of influenza vaccine effectiveness. Vaccine. 2015;33(11):1313–1316. doi: 10.1016/j.vaccine.2015.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 41.Patel M.M., Jackson M.L., Ferdinands J. Postlicensure evaluation of COVID-19 vaccines. JAMA. 2020;324:1939–1940. doi: 10.1001/jama.2020.19328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified analysis data sets generated from the testing and vaccine registry databases are available from the corresponding author upon reasonable request.