Abstract
In accordance with Article 6 of Regulation (EC) No 396/2005, the applicant Nufarm Europe Gmbh submitted a request to the competent national authority in Austria to modify the existing maximum residue levels (MRLs) for the active substance acetamiprid in honey, linseeds, poppy seeds, mustard seeds and gold of pleasure seeds. The data submitted in support of the request were found to be sufficient to derive MRL proposals for linseeds, poppy seeds, mustard seeds and gold of pleasure seeds. For honey, however, data gaps were identified by EFSA and were not fully addressed by the justification provided by the applicant. Considering the remaining uncertainties, risk managers are given the option to either accept the justification provided and the related uncertainties or to merge the provided data with a data set from a previous application to derive an MRL proposal. Adequate analytical methods for enforcement are available to control the residues of acetamiprid in plant matrices and in honey at the validated limit of quantification (LOQ) of 0.01 mg/kg. Based on the risk assessment results, EFSA concluded that the short‐term and long‐term intake of residues resulting from the use of acetamiprid according to the reported agricultural practices is unlikely to present a risk to consumer health.
Keywords: acetamiprid, honey, various oilseed crops, pesticide, MRL, consumer risk assessment
Summary
In accordance with Article 6 of Regulation (EC) No 396/2005, Nufarm Europe Gmbh submitted an application to the competent national authority in Austria (evaluating Member State, EMS) to modify the existing maximum residue levels (MRLs) for the active substance acetamiprid in honey and various oilseed crops. The EMS drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA) on 3 June 2021. To accommodate for the intended uses of acetamiprid in oilseed crops and to set an MRL for honey, the EMS proposed for linseeds, poppy seeds, mustard seeds and gold of pleasure seeds to raise the existing MRLs from the limit of quantification (LOQ) of 0.01–0.06 mg/kg and for honey to raise the existing MRL from the limit of quantification (LOQ) of 0.05–2 mg/kg.
EFSA assessed the application and the evaluation report as required by Article 10 of the MRL regulation. EFSA identified data gaps, which were requested from the EMS. On 8 June 2022 the EMS submitted a revised evaluation report, which replaced the previously submitted evaluation report.
Based on the conclusions derived by EFSA in the framework (EC) No 1107/2009, the data evaluated under previous MRL assessments, and the additional data provided by the EMS in the framework of this application, the following conclusions are derived.
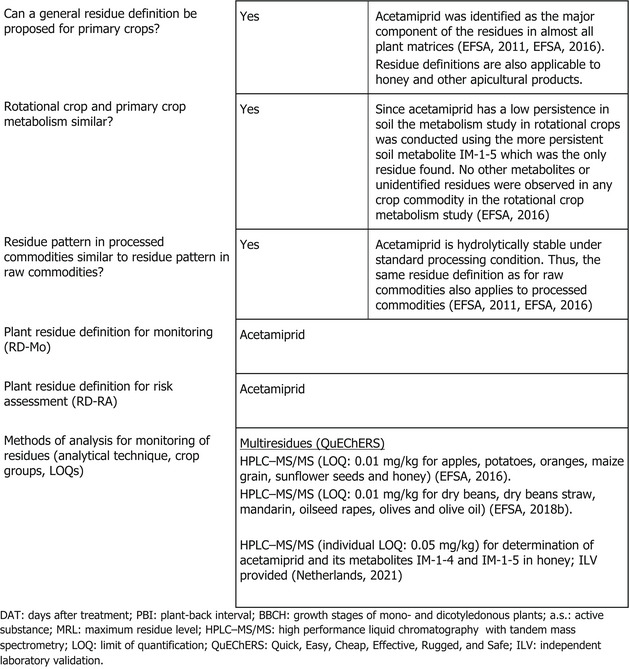
The metabolism of acetamiprid following foliar applications was investigated in crops belonging to the groups of fruit crops, root crops, leafy crops and pulses/oilseeds showing that acetamiprid is the main residue in primary crops.
Studies investigating the effect of processing on the nature of acetamiprid (hydrolysis studies) demonstrated that the active substance is stable.
In rotational crops, the major residue identified in metabolism studies was the soil metabolite IM‐1‐5, the presence of which was not confirmed in the rotational crop field studies.
It is also expected that residues in floral nectar resulting from the use of acetamiprid in primary crops consist mainly of acetamiprid. The nectar is processed by bees following a process of regurgitation and then the honey is stored under specific conditions in the beehives, before harvesting. Since there is limited information available on whether the enzymatic processes occurring in the bee gut or the storage in the beehive have an impact on the nature of residues in honey, it would be desirable to further investigate these aspects.
Based on the metabolic pattern identified in metabolism studies, hydrolysis studies, the toxicological significance of metabolites and the stability of acetamiprid under storage conditions, the residue definitions for plant products were proposed as ‘acetamiprid’ for both enforcement and risk assessment. These residue definitions are applicable to primary crops, rotational crops and processed products as well as honey. The current enforcement residue definition in Regulation (EC) No 396/2005 is also acetamiprid.
EFSA concluded that for the crops assessed in this application, the metabolism of acetamiprid in primary and in rotational crops, and the possible degradation in processed products have been sufficiently addressed and that the previously derived residue definitions are applicable and could be considered valid also for honey.
Sufficiently validated analytical methods based on HPLC‐MS/MS are available to quantify residues of acetamiprid at or above 0.01 mg/kg (LOQ) in the crops assessed in these applications as well as in honey according to the enforcement residue definition.
The available residue trials are sufficient to derive an MRL proposal of 0.06 mg/kg for linseeds, poppy seeds, mustard seeds and gold of pleasure seeds. For honey however, data gaps were identified by EFSA and were not fully addressed by the justification provided by the applicant. Considering the remaining uncertainties, Risk Managers are given the option to either accept the justification provided and the related uncertainties or to merge the provided data with a data set from a previous application to derive an MRL proposal. EFSA considered the second approach more robust since it is based on a higher number of residue trials which are all compliant with the criteria of the honey guidelines and since it is not leading to a possible overestimation of the MRL in honey as also indicated by the available monitoring data.
Specific studies investigating the magnitude of acetamiprid residues in processed commodities were assessed in the framework of the MRL review and the EU pesticides peer review. No new data were submitted in the framework of the current application. Nevertheless, further processing studies for the commodities under assessment are not required as they are not expected to affect the outcome of the risk assessment.
The occurrence of acetamiprid residues in rotational crops was investigated in the framework of the EU pesticides peer review and a confirmatory study was also provided with the current application. Based on the available information on the nature and magnitude of residues, it was concluded that significant residue levels are unlikely to occur in rotational crops, provided that the active substance is used on the primary crop according to the proposed Good Agricultural Practice (GAP).
As the crops under consideration and their by‐products are used as feed products, a potential carry‐over into the food of animal origin was assessed. The calculated livestock dietary burden exceeded the trigger value of 0.004 mg/kg body weight (bw) for all relevant animal species. However, the contribution of acetamiprid residues in the crops under consideration in this MRL application to the total livestock exposure was insignificant and therefore a modification of the existing MRLs for commodities of animal origin was considered unnecessary.
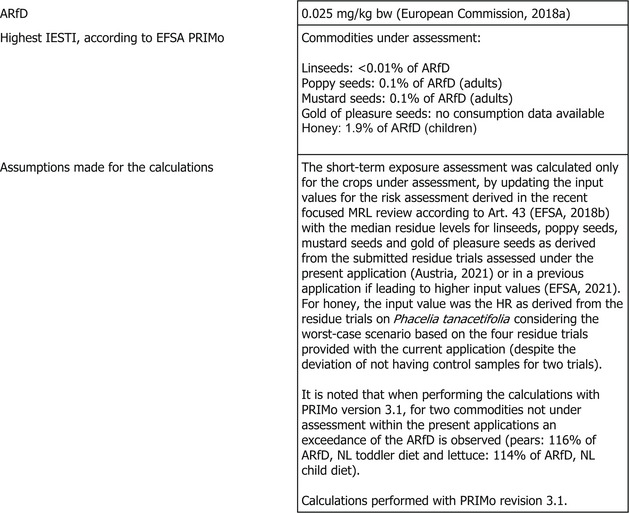
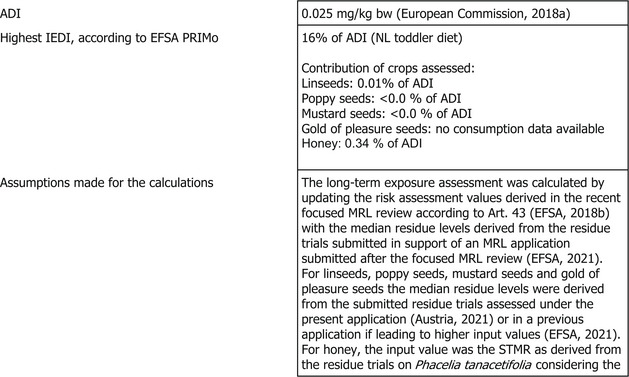
The toxicological profile of acetamiprid was assessed in the framework of the EU pesticides peer review under Regulation (EC) No 1107/2009 and the data were sufficient to derive an acceptable daily intake (ADI) value of 0.025 mg/kg bw per day and an acute reference dose (ARfD) of 0.025 mg/kg bw.
The consumer risk assessment was performed with revision 3.1 of the EFSA Pesticide Residues Intake Model (PRIMo).
The short‐term exposure assessment was performed only for the commodities assessed in the present MRL application and did not exceed the ARfD for any of the crops assessed. In the framework of the focused MRLs review according to Art. 43 of Regulation (EC) No 396/2005 a comprehensive long‐term exposure assessment was performed, taking into account the existing uses at EU level and the acceptable Codex maximum residue limits (CXLs). EFSA updated this calculation with the relevant STMR values derived from the residue trials submitted in support of an MRL application submitted after the focused MRL review and the STMR values derived from the residue trials submitted with the present MRL application. Additionally, the proposed CXL and STMR values from seed spices presented in the 2019 JMPR report for which EFSA expressed a positive reservation, have also been included in this updated calculation. Finally, the crops on which no uses were reported in the MRL review were excluded from the exposure calculation. The estimated long‐term dietary intake accounted for 16% of the ADI (NL toddler diet).
EFSA concluded that the proposed use of acetamiprid for linseeds, poppy seeds, mustard seeds and gold of pleasure seeds as well as the potential transfer of residues into honey will not result in a consumer exposure exceeding the toxicological reference values and therefore is unlikely to pose a risk to consumers' health.
It must be noted that the investigation of possible risk to honeybees related to the use of acetamiprid is outside the scope of this reasoned opinion. The evaluation of the risk to honeybees was evaluated in the framework of the peer review of acetamiprid at EU level. Additionally, national competent authorities at Member State level should pay attention to the bee health and bee protection when granting authorisations for plant protection products according to the provisions laid out in the Regulation (EU) 2018/113.
Moreover, Commission is discussing with EFSA a possible mandate on acetamiprid according to Art. 31. This mandate should address if new scientific evidence that has become available since the assessment conducted in the framework of the renewal in 2018 warrants a re‐evaluation of the toxicological properties of acetamiprid and its metabolites and a change in residue definition would be needed. Therefore, the conclusions reported in this reasoned opinion might need to be reconsidered in light of the outcome of this evaluation.
EFSA proposes to amend the existing MRLs as reported in the summary table below.
Full details of all end points and the consumer risk assessment can be found in Appendices Appendix B – List of end points, Appendix C – Pesticide Residue Intake Model (PRIMo)–D.
| Code (a) | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Acetamiprid | ||||
| 0401010 | Linseeds | 0.01* | 0.06 | Data on oilseed rape extrapolated to linseeds, poppy seeds, mustard seeds and gold of pleasure seeds. The submitted data are sufficient to derive a MRL proposal for the NEU use. Risk for consumers unlikely. |
| 0401030 | Poppy seeds | 0.01* |
Further risk management considerations required (0.3 or 0.06) |
Data on oilseed rape extrapolated to linseeds, poppy seeds, mustard seeds and gold of pleasure seeds. The submitted data are sufficient to derive a MRL proposal for the NEU use. EFSA notes that a higher MRL value (0.3 mg/kg) was proposed in a recent output (EFSA, 2021) but this MRL is not implemented yet in the EU Regulation. Risk for consumers unlikely for both MRLs proposed. |
| 0401080 | Mustard seeds | 0.01* |
Further risk management considerations required (0.15 or 0.06) |
Data on oilseed rape extrapolated to linseeds, poppy seeds, mustard seeds and gold of pleasure seeds. The submitted data are sufficient to derive a MRL proposal for the NEU use. EFSA notes that a higher MRL value (0.15 mg/kg) was proposed in a recent output (EFSA, 2021) but this MRL is not implemented yet in the EU Regulation. Risk for consumers unlikely for both MRLs proposed. |
| 0401130 | Gold of pleasure seeds | 0.01* | 0.06 | Data on oilseed rape extrapolated to linseeds, poppy seeds, mustard seeds and gold of pleasure seeds. The submitted data are sufficient to derive a MRL proposal for the NEU use. Risk for consumers unlikely. |
| 1040000 | Honey and other apiculture products | 0.05* |
Further risk management considerations required (2 or 0.3) |
Risk Managers are given the options to either set an MRL for honey of 2 mg/kg based on the four residue trials provided with the current application (despite the deviation of not having control samples for two trials) or merge two data sets to derive an MRL of 0.3 mg/kg based on six residue trials performed in accordance with the requirements of the honey guidelines. Risk for consumers unlikely for both MRLs proposed. |
MRL: maximum residue level; NEU: northern Europe; SEU: southern Europe; GAP: Good Agricultural Practice.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Assessment
The European Food Safety Authority (EFSA) received an application to modify the existing maximum residue levels (MRLs) for acetamiprid in honey and various oilseed crops. The detailed description of the intended uses of acetamiprid in honey, linseeds, poppy seeds, mustard seeds and gold of pleasure seeds, which are the basis for the current MRLs application, is reported in Appendix A.
Acetamiprid is the ISO common name for (E)‐N1‐[(6‐chloro‐3‐pyridyl)methyl]‐N2‐cyano‐N1‐methylacetamidine (IUPAC name). The chemical structures of the active substance and its main metabolites are reported in Appendix E.
Acetamiprid is an insecticide, which was evaluated for renewal of the approval in the framework of Regulation (EC) No 1107/2009 1 with the Netherlands designated as rapporteur Member State (RMS) for the representative uses as foliar treatments on pome fruits, tomatoes and potatoes. The renewal assessment report (RAR) prepared by the RMS has been peer reviewed by EFSA (2016). The decision on the renewal of acetamiprid entered into force on 1 March 2018. 2
The EU MRLs for acetamiprid are established in Annexes II of Regulation (EC) No 396/2005 3 . The review of existing MRLs according to Article 12 of Regulation (EC) No 396/2005 (MRL review) has been performed (EFSA, 2011) and the proposed modifications have been implemented in the MRL legislation. After completion of the MRL review, EFSA has issued several reasoned opinions on the modification of MRLs for acetamiprid. In addition, certain Codex maximum residue limits (CXLs) have been taken over in the EU MRL legislation 4 . Moreover, a focused MRL review according to Art. 43 of Regulation (EC) No 396/2005 and based on the new toxicological reference values agreed as part of the renewal of approval has been performed (EFSA, 2018b) and the proposed modifications have been implemented in the MRL legislation. Additionally, in a statement published in January 2022, the EFSA PPR Panel concluded that there is no conclusive evidence of higher hazards from acetamiprid compared to previous assessments with respect to genotoxicity, developmental toxicity, neurotoxicity including developmental neurotoxicity and immunotoxicity (EFSA, 2022). However, it was recommended that an assessment of endocrine disrupting properties for acetamiprid is conducted in line with EFSA/ECHA guidance document for the identification of endocrine disruptors. Those findings are currently under discussion at the Standing Committee on Plants, Animals, Food and Feed (SCPAFF), section Phytopharmaceuticals – Legislation with the view of possible regulatory action for acetamiprid. Moreover, Commission is discussing with EFSA a possible mandate on acetamiprid according to Art. 31. This mandate should address if new scientific evidence that has become available since the assessment conducted in the framework of the renewal in 2018 warrants a re‐evaluation of the toxicological properties of acetamiprid and its metabolites and a change in residue definition would be needed.
In accordance with Article 6 of Regulation (EC) No 396/2005, Nufarm Europe Gmbh submitted an application to the competent national authority in Austria (evaluating Member State, EMS) to modify the existing MRLs for the active substance acetamiprid in honey, linseeds, poppy seeds, mustard seeds and gold of pleasure seeds. The EMS drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to EFSA on 3 June 2021. To accommodate for the intended uses of acetamiprid in oilseed crops and to set an MRL in honey, the EMS proposed for linseeds, poppy seeds, mustard seeds and gold of pleasure seeds to raise the existing MRLs from the limit of quantification (LOQ) of 0.01–0.06 mg/kg and for honey to raise the existing MRL from the limit of quantification (LOQ) of 0.05–2 mg/kg.
EFSA assessed the application and the evaluation report as required by Article 10 of the MRL regulation. EFSA identified data gaps, which were requested from the EMS. On 8 June 2022, the EMS submitted a revised evaluation report (Austria, 2021), which replaced the previously submitted evaluation report.
EFSA based its assessment on the evaluation report submitted by the EMS (Austria, 2021), the renewal assessment report (RAR) and its addenda (Netherlands, 2015, 2016) prepared under Regulation (EC) 1107/2009, the Commission review report on acetamiprid (European Commission, 2018a), the conclusion on the peer review of the pesticide risk assessment of the active substance acetamiprid (EFSA, 2016), as well as the conclusions from previous EFSA opinions on acetamiprid (EFSA, 2021), including the reasoned opinion on the MRL review according to Article 12 of Regulation No 396/2005 (EFSA, 2011) and the focused MRL review according to Art. 43 of Regulation (EC) 396/2005 (EFSA, 2018b).
For this application, the data requirements established in Regulation (EU) No 283/2013 5 and the guidance documents applicable at the date of submission of the application to the EMS are applicable (European Commission, 2010, 2017; OECD, 2011, 2013). The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/2011 6 .
A selected list of end points of the studies assessed by EFSA in the framework of this MRL application including the end points of relevant studies assessed previously, is presented in Appendix B.
The evaluation report submitted by the EMS (Austria, 2021) and the exposure calculations using the EFSA Pesticide Residues Intake Model (PRIMo) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available as background documents to this reasoned opinion.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
The metabolism of acetamiprid in primary crops belonging to the group of fruit crops (eggplants, apples), root crops (carrots), leafy crops (cabbages) and pulses/oilseeds (cotton) has been investigated in the framework of the MRL review and the EU pesticides peer review (EFSA, 2011, 2016). No new metabolism studies were submitted with the present application.
In the crops tested, acetamiprid was identified as the major component of the TRRs accounting for ca. 30–90% TRR, 14–90 days after the last application. The only exceptions were head cabbages and cotton seeds where the 6‐chloronicotinic acid metabolite (IC‐0) was the sole component identified, representing 46% TRR (0.023 mg eq/kg) and 24% TRR (0.27 mg/kg) respectively. IC‐0 was also detected in carrot roots (26% TRR, 0.02 mg/kg). Other identified metabolites were observed but at low levels, accounting mostly for < 5% TRR, except metabolite IM‐1‐4 in immature carrot leaves (43% TRR). As acetamiprid was identified as the major component of the residues in almost all plant matrices and since the toxicity of the IC‐0 metabolite is covered by the toxicity of the parent acetamiprid, no further metabolism data were required.
Regarding honey, honey is a product originated from sugary secretions of plants (floral nectar mainly). Based on the similar results of metabolism studies in four different primary crop groups, EFSA expects that residues in floral nectar resulting from the use of acetamiprid in primary crops would also consist mainly of acetamiprid. The nectar is processed by bees following a process of regurgitation and then the honey is stored under specific conditions in the beehives before harvesting. Further information, on whether enzymatic processes occurring in the bee gut involved in the production of honey or the storage in the beehive have an impact on the nature of residues is not available, but in principle would be desirable (European Commission, 2018b).
Therefore, for the intended uses, the metabolic behaviour in primary crops is considered as sufficiently addressed.
1.1.2. Nature of residues in rotational crops
Linseed/flax, poppy seeds, mustard seeds and gold of pleasure seeds may be grown in rotation with other crops and therefore, residues in rotational crops need to be investigated.
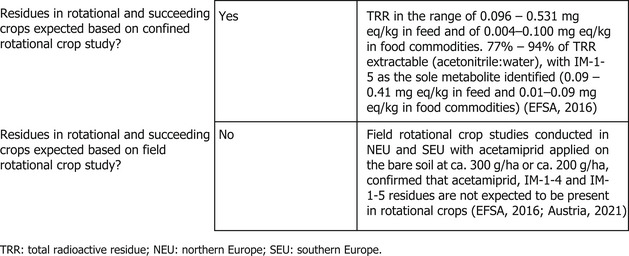
The nature of residues in rotational crops (confined studies) has been evaluated during the peer review (EFSA, 2016). Since acetamiprid has a low persistence in soil (highest field DT90 43 days and 20°C lab DT90 54 days), the metabolism study in rotational crops was not conducted with acetamiprid but using the more persistent soil metabolite IM‐1‐5 (DT50 ranging from 319 to 663 days). In the different rotational crops investigated (wheat, turnip, spinaches), the metabolite IM‐1‐5 was the main component of the radioactive residues accounting in mature plant at harvest for 77–94% TRR. No other metabolites or unidentified residues were observed in any crop commodity.
Moreover, a new metabolism study performed with [14C]‐IM‐1‐5 with the same succeeding crops was provided with the present application (Austria, 2021) confirming the finding of the previous study assessed during the peer‐review. In this new study, [14C]‐IM‐1‐5 was applied to the soil as a single spray application at a nominal rate of 160 g a.s/ha. The study was designed to only investigate the fate of this metabolite and therefore no ageing of the soil was required following application. IM‐1‐5 was confirmed as the major component of the total radioactive residue, accounting for 6.3–86.6% of the TRR. Only limited metabolism of IM‐1‐5 was observed in the rotational crops and no metabolic pathway was proposed for IM‐1‐5.
The metabolic behaviour of acetamiprid and its major soil metabolite (IM‐1‐5) in rotational crops is considered as sufficiently addressed. The GAPs under assessment are covered by the available studies.
1.1.3. Nature of residues in processed commodities
The effect of processing on the nature of acetamiprid was investigated in the framework of the MRL review and the EU pesticides peer review (EFSA, 2011, 2016). These studies showed that acetamiprid is hydrolytically stable under standard processing conditions representative of pasteurisation, baking/brewing/boiling and sterilisation.
The process of converting nectar to honey does not involve hydrolytic conditions at elevated temperature; however, honey may be used as an ingredient in processed products that are heat treated. Considering the available studies addressing the nature of residues in processed commodities, it is unlikely that in processed honey products residues of acetamiprid are degraded to other compounds.
1.1.4. Analytical methods for enforcement purposes in plant commodities and in honey
Analytical methods for the determination of acetamiprid residues in plant commodities were assessed during the EU MRL review, the pesticides peer review and in subsequent MRLs applications (EFSA, 2011, 2016, 2018b, 2021). These analytical methods are sufficiently validated to enforce acetamiprid residues with a LOQ of 0.01 mg/kg in all plant commodity groups as well as in honey. No new data were submitted with the present application.
Therefore, EFSA concludes that sufficiently validated analytical methods are available to monitor residues of acetamiprid in the plant commodities under consideration as well as in honey at or above the LOQ of 0.01 mg/kg. EFSA further notes that the extraction efficiency for the analytical methods applied for enforcement and used for the residue trials is not sufficiently proven for all commodities groups according to the requirements of the extraction efficiency Guidance, SANTE 2017/10632 (European Commission, 2017). Further investigation on this matter would in principle be required.
1.1.5. Storage stability of residues in plants and in honey
The storage stability of acetamiprid residues in plants stored under frozen conditions was investigated in the framework of the MRL review and the EU pesticides peer review (EFSA, 2011, 2016). The stability of acetamiprid residues was demonstrated in plant matrices stored at ≤ −18°C for up to 12–15 months in high water, high acid, high oil and high protein content matrices and for up to 8–15 months in dry/high starch content matrices. Additionally, a study assessing the stability of acetamiprid residues in honey was submitted and assessed in the framework of a recent MRL application (EFSA, 2021) showing that acetamiprid and the two metabolites IM‐1‐4 and IM‐1‐5 are stable in honey for at least 4 months when stored at or below −18°C.
Moreover, as part of the present application, the applicant provided additional studies to demonstrate storage stability of acetamiprid residues at or below −18°C in high protein content (dry bean seed), high water content (apple fruit), high acid content (orange peel and pulp), high oil content (olives) and dry (dry bean straw) commodities for a period up to 12 months and in high starch content (wheat grain) commodities for up to 15 months (Austria, 2021). Linseed/flax, poppy seeds, mustard seeds and gold of pleasure seeds are of high oil content and all residue trials were performed in accordance with conditions ensuring the stability of acetamiprid residues. Finally, a new storage stability study of acetamiprid residues was also provided in honey, demonstrating stability for a period of 11 months at or below −18°C, which supports the stability of samples in the semi‐field tunnel trials performed to determine acetamiprid residues in honey.
1.1.6. Proposed residue definitions
Based on the metabolic pattern identified in metabolism studies, the results of hydrolysis studies, the toxicological significance of metabolites and the capabilities of enforcement analytical methods, the following residue definitions were proposed
residue definition for risk assessment: acetamiprid;
residue definition for enforcement: acetamiprid.
The same residue definitions are applicable to rotational crops and processed products.
The residue definition for enforcement set in Regulation (EC) No 396/2005 is identical with the above‐mentioned residue definition.
EFSA notes that similar to other food products, residue definitions need to be derived for honey which should cover the toxicologically relevant compounds occurring in honey following the use of acetamiprid on crops foraged by bees. Honey is produced by bees following sugary secretions of plants (mainly nectar) through regurgitation, enzymatic conversion and water evaporation followed by storage of honey in beehives. As indicated in the Technical Guidelines for determining the magnitude of pesticide residues in honey and setting MRLs in honey (European Commission, 2018b), in the absence of specific metabolism studies with honeybees, the residue definition for risk assessment needs to be derived taking into account other sources of information such as studies on the nature of residues in primary and rotational crops and degradation during pasteurisation. As the same residue definition (acetamiprid) applies both in primary and rotational crops, and acetamiprid is stable under pasteurisation conditions, EFSA considers that the above plant residue definitions could be considered valid also for honey and other apicultural products.
1.2. Magnitude of residues in plants and honey
1.2.1. Magnitude of residues in primary crops and honey
In support of the MRL application, the applicant submitted residue trials performed in oilseed rape which were extrapolated to linseeds/flax, poppy seeds, mustard seeds and gold of pleasure seeds. Moreover, semi‐field/tunnel trials performed with Phacelia tanacetifolia used as a surrogate crop were also submitted to determine acetamiprid residues in honey. The residue trial samples were analysed for the parent compound as indicated in the residue definitions for enforcement and risk assessment.
According to the assessment of the EMS, the methods used were sufficiently validated and fit for purpose and the samples of these residue trials were stored under conditions for which integrity of the samples has been demonstrated.
Linseeds/flax, poppy seeds, mustard seeds and gold of pleasure seeds
NEU outdoor GAP: Foliar treatment at 1 × 50 g a.s./ha, BBCH 70–71, PHI = 28 days
The applicant provided eight outdoor residue trials performed in NEU on oilseed rape. Out of these eight residue trials, only four were performed according to the intended number of applications for linseeds/flax, poppy seeds, mustard seeds and gold of pleasure seeds. Since all seeds in the scope of this application are identified as minor crops in NEU, the number of trials provided is considered sufficient. These four residue trials (two decline and two harvest trials) are considered independent as they were performed in different geographical locations and periods. Moreover, in line with the applicable EU guidance document on setting MRLs, comparability of residue trials and extrapolation (European Commission, 2020), the extrapolation from oilseed rape to linseeds/flax, poppy seeds, mustard seeds and gold of pleasure seeds is acceptable.
However, the application rate in the available oilseed rape trials was at 61–66 g a.s./ha, which is overdosed for more than 25% compared to the intended GAP for the various seeds in the scope of this application (up to ×1.32). Therefore, residue trial values were scaled down to match the intended GAP in line with the ALARA principle. EFSA notes that no other parameters than the application rate deviate from the intended GAP, therefore the application of extrapolation and proportionality principles is supported (EFSA, 2018c).
Therefore, an MRL of 0.06 mg/kg is derived for acetamiprid in linseeds/flax, poppy seeds, mustard seeds and gold of pleasure seeds.
Honey
Surrogate crop: Phacelia tanacetifolia, GAP1: 2 × 80 g a.s./ha, interval = 7–10 days, BBCH 61–65, PHI 5–19 days
Since the intended GAP may result in applications of acetamiprid on melliferous crops during flowering, residues in bee products need to be addressed in line with the requirements of the Technical Guideline SANTE/11956/2016 (European Commission, 2018b, hereafter refer to as ‘honey guidelines’). Therefore, to investigate the magnitude of acetamiprid residues in honey, the applicant provided four residue trials on P. tanacetifolia, used as a surrogate crop with high melliferous capacity under semi‐field conditions at four different locations in Germany and Northern France.
These trials were conducted with two applications of 80 g a.s./ha on P. tanacetifolia. The first application took place at the BBCH growth stages 61–63 while the second application was performed during full flowering of the crop (BBCH growth stages 63–65) and during bee‐flight activity, 7–10 days after the first application. Samples of honey were taken 5–19 days after the last application. The application rate was chosen, according to the honey guidelines, to correspond to the most critical scenario on a crop representing a worst case in terms of residues in honey. The relevant residues for honey were defined as parent acetamiprid (see Section 1.1.6).
EFSA agrees with the approach proposed by the applicant and supported by the EMS in relation to the use of P. tanacetifolia as a surrogate crop. Therefore, EFSA assessed the newly submitted semi‐field/tunnel trials in line with the requirements of the honey guidelines. These four submitted trials were performed with a correct design except for two trials where the applicant reported that no control samples were available (study field 2, Drusenheim, N‐France and study field 4 Brensbach, Germany). The applicant justified the absence of control samples in these two trials indicating that ‘most likely as a result of the high dryness of the soil during the entire growing season, no honey was available in the control colonies at study field 2 and study field 4’. However, EFSA notes that according to the honey guidelines, each trial site should consist of a control plot and one treated plot and replicates of control samples should be analysed together with treated samples. Therefore, EFSA considers these two trials as non‐compliant with the requirements of the honey guidelines and the remaining number of trials not sufficient to derive an MRL for honey. The applicant was therefore requested to provide two additional semi‐field/tunnel trials with acetamiprid in P. tanacetifolia to derive an MRL in honey.
As a response to this request, the applicant did not provide new residue trials but a justification why the absence of these two control samples should be considered as a minor deviation not invalidating these residue trials. According to the applicant, no residues are expected in control samples for the following reasons:
-
–
The pesticide history clearly indicates that study fields were not treated with acetamiprid in the recent years before the trials (2017–2019). The target analyte acetamiprid is not persistent and any uptake from soil is excluded.
-
–
Acetamiprid is not volatile (vapour pressure 1 × 10 −6 Pa at 20°C). Any translocation from the treated plot to untreated (control) plot as vapour is excluded.
-
–
Drift from spray application to untreated control plots is excluded for several reasons. Both treated and control tunnels are completely covered with gauze to keep the bees inside the tunnels. Wind speeds at applications account for a maximum of 1.5 m/s (5.4 km/h, wind speed 1, Brensbach) and 0 m/s (Drusenheim). Besides the low wind speeds, the vast majority of potentially drifting droplets would be trapped either inside the first gauze (treated tunnel) or outside the second gauze (control tunnel) so that no significant drift deposit is expected.
EFSA has still some reservations about the absence of control samples. When considering the four trials (including the ones with no control samples), the resulting MRL proposal might be much higher than necessary and this is mainly driven by one trial without a control sample (study field 2, Drusenheim, N‐France). Therefore, EFSA would be in favour of disregarding these two trials with no control samples.
Furthermore, it should be noted that another application for setting an MRL in honey for acetamiprid with P. tanacetifolia as a surrogate crop was recently assessed (EFSA, 2021). In this application, a higher application rate on the same surrogate crop was chosen (GAP 2: 2 × 100 g a.s./ha). Since the difference between the application rate of the newly submitted trials (2 × 80 g a.s/ha) and the previously assessed trials (2 × 100 g a.s./ha) is within the ± 25% rule, EFSA considers the two data sets can be combined. Therefore, as an alternative approach, EFSA proposed to merge the two data sets (excluding the trials without control samples) to derive a more robust MRL for honey based on six residue trials. EFSA considered this approach more robust since it is based on a higher number of residue trials which are all compliant with the criteria of the honey guidelines and since it is not leading to a possible over‐estimation of the MRL in honey as also indicated by the available monitoring data (see below).
However, Risk Managers are given the option to either set an MRL for honey based on the four residue trials provided with the current application (despite the deviation of not having control samples for two trials) or based on a more robust data set of six residue trials performed in accordance with the requirements of the honey guidelines.
It should be noted that currently, MRLs set for honey are not applicable to other apicultural products following Commission Regulation (EU) 2018/62 1 .
Magnitude of residues from EU national monitoring programme
In the framework of Article 32 of Regulation (EC) No 396/2005 (official national control programmes), monitoring data were submitted to EFSA. The majority of the honey samples analysed resulted in acetamiprid residue levels below the LOQ of 0.05 mg/kg. From the monitoring data of 2019, only 1 sample out of 49 exceeded the LOQ (detected value = 0.087 mg/kg). While from the monitoring data of 2020 5 samples out of 26 exceeded the LOQ (detected values in the range 0.053–0.15 mg/kg). These monitoring data are also supportive of the proposed approach above to merge the two data sets (excluding the trials without control samples) to derive a more robust MRL for the honey of 0.3 mg/kg based on six residue trials.
In addition, the applicant provided also an expert statement, which is included in the evaluation report, indicating that ‘the set up proposed in the honey guidelines results in unrealistic high residue levels and leads to a massive overestimation of MRL in honey’ and proposing that ‘MRL should be derived from the monitoring data’. EFSA agrees that Art. 16 of Reg. (EC) 396/2005 allows the setting of temporary MRL in honey based on monitoring data and the honey guidelines do not clearly state if data from monitoring studies should be preferred to data from residue trials or vice versa. According to EFSA, in presence of both monitoring and semi‐field trials, both should be assessed but preference should be given to semi‐field trials while monitoring data should be used as supporting information. This principle has been applied in previous Art. 10 reasoned opinions adopted by EFSA which included the setting of MRL in honey based on both monitoring data and semi‐field/tunnel trials.
1.2.2. Magnitude of residues in rotational crops
The possible transfer of acetamiprid residues to crops that are grown in crop rotation has been assessed in the EU pesticides peer review (EFSA, 2016). The available studies demonstrated that no significant residues (residues below 0.01 mg/kg) of acetamiprid or the metabolites IM‐1‐4 and IM‐1‐5 are expected in succeeding crops (turnip, spinaches and wheat) planted in soil treated at 300 g a.s./ha.
Moreover, a new field rotational study was provided in the context of the present application (Austria, 2021). In this new study, acetamiprid was applied to bare soil at a target rate of 200 g a.s./ha and crops were sown at different plant‐back intervals (29–32, 69–73, 119–132 and 363–410 days). Succeeding crops (radish, spinach and wheat) as well as soil were analysed for residues of acetamiprid and its soil persistent metabolites IM‐1‐4 and IM‐1‐5. Residue levels for acetamiprid were not detectable (< 0.003 mg/kg) and residues for its metabolites were below the LOQ (< 0.01 mg/kg) or also not detectable with only IM‐1‐5 at the LOQ level in radish leaves 160 days after application. The results of this new rotational field study are in line with the results of the previous study assessed in the framework of the EU pesticides peer review (EFSA, 2016) with no residues of acetamiprid and its soil persistent metabolites (IM‐1‐4 and IM‐1‐5) expected in rotational crops.
Since the maximum annual application rate for the GAP under consideration (i.e. 50 g a.s./ha) is significantly lower than the application rates tested in these rotational crop studies, it is concluded that no residues are expected, provided that the active substance is applied according to the proposed GAP.
1.2.3. Magnitude of residues in processed commodities
Processing studies with several crops have been assessed in the EU pesticide peer review demonstrating a reduction of acetamiprid residues in different processed products (EFSA, 2016).
Specific processing studies for the crops under assessment are not available and are not required as they are not expected to affect the outcome of the risk assessment considering the extremely low contribution to the acceptable daily intake (ADI) of the crops under assessment (see Section B.3).
1.2.4. Proposed MRLs
The available data are considered sufficient to derive an MRL proposal as well as risk assessment values for linseed/flax, poppy seeds, mustard seeds and gold of pleasure seeds (see Appendix B.1.2.1). Regarding the proposed MRL for honey, Risk Managers are given the options to either set an MRL at 2 mg/kg based on the four residue trials provided with the current application (despite the deviation of not having control samples for two trials) or to set an MRL at 0.3 mg/kg by merging two different data sets as reported in Section 1.2.1 above.
In Section 3, EFSA assessed whether residues on these commodities resulting from the intended uses of acetamiprid are likely to pose a consumer health risk.
2. Residues in livestock
Linseed by‐products (flaxseed/Linseed meal) might be fed to livestock. Hence, it was necessary to update the previous dietary burden calculation for livestock performed during a focused MRL review (EFSA, 2018b) to estimate whether the intended use of acetamiprid would have an impact on the residues expected in food of animal origin.
Therefore, EFSA updated the most recent animal dietary burden for acetamiprid calculated using the feeding tables listed in the OECD guidance (OECD, 2013) by including the residues in flaxseed/linseed meal expected from the intended use of acetamiprid. The input values for the exposure calculation for livestock are presented in Appendix D.1. The calculated dietary burdens for all groups of livestock were found to exceed the trigger value of 0.004 mg/kg body weight (bw) with the main contributors being kale leaves (for cattle and swine diet) and wheat straw (for sheep and poultry diet). Further investigation of residues is therefore required in all commodities of animal origin. The calculated dietary burden was then compared to the intakes which were previously considered to derive the current MRLs for animal commodities (see Appendix B.2). Comparing the results of the revised dietary burden calculation with the dietary burden derived previously (EFSA, 2018b), it is evident that the residues in flaxseed/linseed meal have a negligible impact on the expected livestock exposure and a modification of the MRLs set for animal commodities is not required.
Regarding fish and fish products, according to the new data requirement of Regulation (EC) 283/2013, a feeding study may be triggered where the plant protection product is used in crops whose parts or products, also after processing, are fed to fish and where residues in the feed may occur from the intended application. Processed linseed and mustard seeds may be used as fish feed items according to the working document on the nature of pesticide residues in fish (SANCO/11187/2013, European Commission, 2013). As acetamiprid is not fat soluble (EFSA, 2016) investigation of the nature and magnitude of residues in fish in principle would not be required according to SANCO/11187/2013. The applicant nevertheless assessed the exposure of fish to acetamiprid residues via intake of feed containing treated linseed and mustard seeds. The fish dietary burden was calculated based on input values from relevant commodities assessed during the focused MRL review (EFSA, 2018b) and considering the intake of linseed and mustard products calculated with the STMR value of 0.01 mg/kg as derived from the submitted residue trials with the default processing factor of 2. The maximum dietary burden for common carp and rainbow trout was calculated to be 0.03 and 0.02 mg/kg dry matter (DM), respectively and the calculated worst‐case intakes for both fish species are below 0.1 mg/kg DM (Austria, 2021) thus demonstrating that further studies investigating the nature and magnitude of residues in fish are not required.
3. Consumer risk assessment
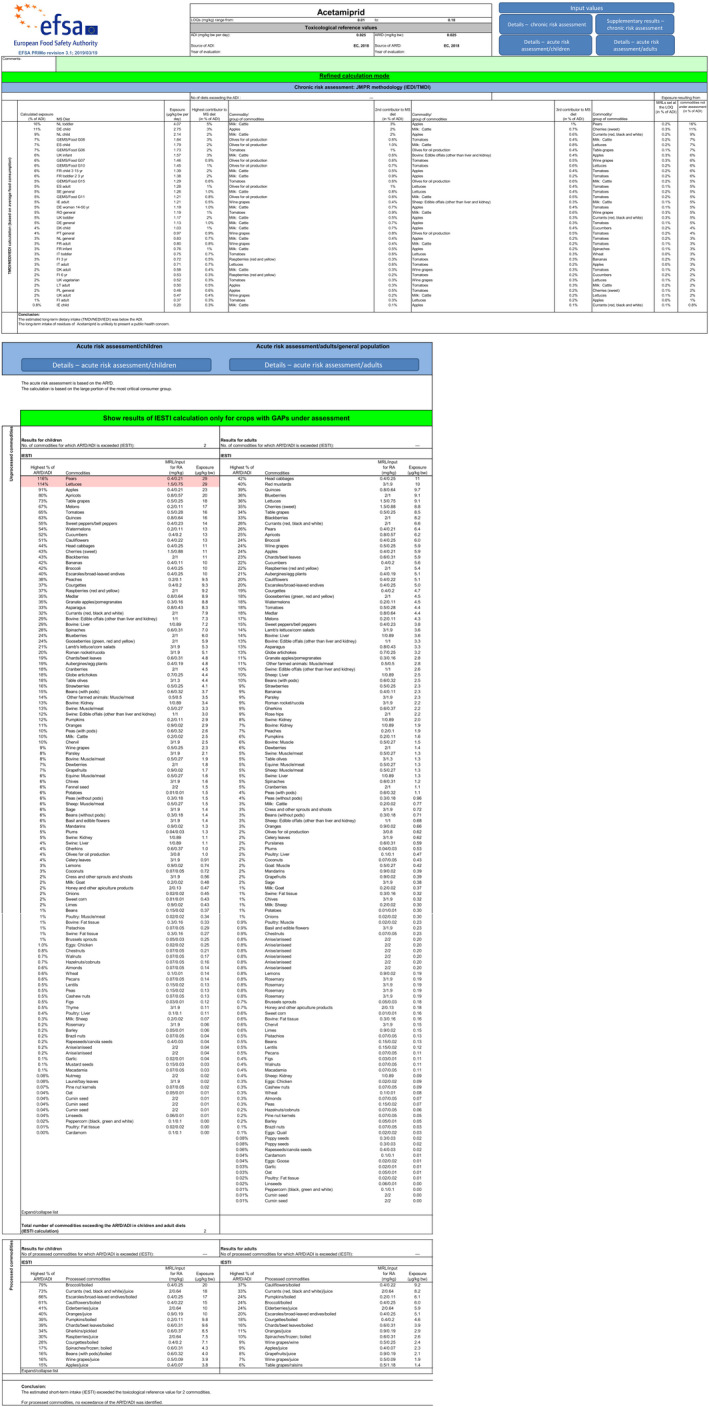
EFSA performed a dietary risk assessment using revision 3.1 of the EFSA PRIMo (EFSA, 2018a, 2019). This exposure assessment model contains food consumption data for different sub‐groups of the EU population and allows the acute and chronic exposure assessment to be performed in accordance with the internationally agreed methodology for pesticide residues (FAO, 2016).
The toxicological reference values for acetamiprid used in the risk assessment (i.e. ADI of 0.025 mg/kg bw per day and acute reference dose (ARfD) of 0.025 mg/kg bw) were derived in the framework of the EU pesticides peer review (European Commission, 2018a).
Short‐term (acute) dietary risk assessment
The short‐term exposure assessment was performed for the commodities assessed in this application. The calculations were based on the highest residue (HR) value for honey and median residue (STMR) values for linseeds/flax, poppy seeds, mustard seeds and gold of pleasure seeds as derived from the submitted supervised field trials and the complete list of input values can be found in Appendix D.2. When different MRLs proposal and input values were considered, the highest values were used for the consumer risk assessment as a worst‐case scenario.
The short‐term exposure did not exceed the ARfD for any of the commodities assessed in this application (see Appendix B.3).
Long‐term (chronic) dietary risk assessment
In the framework of the focused MRL review according to Art. 43 of Regulation (EC) 396/2005 a comprehensive long‐term exposure assessment was performed, taking into account the existing uses at the EU level and the acceptable CXLs (EFSA, 2018b). Reviewed MRLs were then implemented into Regulation (EU) 2019/88 7 .
EFSA updated this calculation with the relevant STMR values derived from the residue trials submitted in support of an MRL application submitted after the focused MRL review (EFSA, 2021) and the STMR values derived from the residue trials submitted with the present MRL application. Additionally, the proposed CXL and STMR values from seed spices presented in the 2019 JMPR report for which EFSA expressed a positive reservation, have also been included in this updated calculation. Finally, the crops on which no uses were reported in the MRL review were excluded from the exposure calculation. The input values used in the exposure calculations are summarised in Appendix D.2.
The estimated long‐term dietary intake accounted for 16% of the ADI (NL toddler diet). The contribution of residues expected in the commodities assessed in this application to the overall long‐term exposure is presented in more detail in Appendix B.3.
EFSA concluded that the long‐term intake of residues of acetamiprid resulting from the existing and the intended uses is unlikely to present a risk to consumer health.
For further details on the exposure calculations, a screenshot of the Report sheet of the PRIMo is presented in Appendix C.
4. Conclusion and Recommendations
The data submitted in support of this MRL application were found to be sufficient to derive an MRL proposal for linseeds, poppy seeds, mustard seeds and gold of pleasure seeds. For honey, however, data gaps were identified by EFSA and were not fully addressed by the justification provided by the applicant. Risk Managers are given the option to either accept the justification provided and the related uncertainties or to merge the provided data with a data set from a previous application to derive an MRL proposal. EFSA considered the second approach more robust since it is based on a higher number of residue trials which are all compliant with the criteria of the honey guidelines and since it is not leading to a possible overestimation of the MRL in honey as also indicated by the available monitoring data.
EFSA concluded that the proposed use of acetamiprid on honey, linseeds, poppy seeds, mustard seeds and gold of pleasure seeds will not result in a consumer exposure exceeding the toxicological reference values and therefore is unlikely to pose a risk to consumers’ health.
It must be also noted that the investigation of possible risk to bees related to the use of acetamiprid is outside the scope of this reasoned opinion. The evaluation of the risk to honeybees was evaluated in the framework of the peer review of the approval of acetamiprid at EU level. Additionally, national competent authorities at Member State level should pay attention to the bee health and bee protection when granting authorisations for plant protection products.
The MRL recommendations are summarised in Appendix B.4.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CF
conversion factor for enforcement to risk assessment residue definition
- cGAP
critical GAP
- CXL
Codex maximum residue limit
- DAR
draft assessment report
- DAT
days after treatment
- DM
dry matter
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- EMS
evaluating Member State
- eq
residue expressed as a.s. equivalent
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- HPLC–MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- ISO
International Organisation for Standardisation
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint FAO/WHO Meeting on Pesticide Residues
- LOQ
limit of quantification
- MRL
maximum residue level
- MS
Member States
- NEU
northern Europe
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant‐back interval
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged, and Safe (analytical method)
- RA
risk assessment
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- SCPAFF
Standing Committee on Plants, Animals, Food and Feed (formerly: Standing Committee on the Food Chain and Animal Health; SCFCAH)
- SL
soluble concentrate
- STMR
supervised trials median residue
- TAR
total applied radioactivity
- TRR
total radioactive residue
- WHO
World Health Organization
Appendix A – Summary of intended GAP triggering the amendment of existing EU MRLs
| Crop and/or situation | NEU, SEU, MS or country | F G or I (a) | Pests or group of pests controlled | Preparation | Application | Application rate per treatment | PHI (days) (d) | Remarks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type (b) | Conc. a.s. (g/kg) | Method kind | Range of growth stages & season (c) | Number min–max | Interval between application (days) min‐max | g a.s./hL min–max | Water (L/ha) min–max | Rate min–max | Unit | ||||||
| Linseeds | NEU | F | Brassica pod midge (DASYBR, Dasineura brassicae) Cabbage seed/shoot weevil (CEUTAS, Ceutorhynchus obstrictus syn assimilis), Rape flee beetle/cabbage stem flea beetle (PSYICH, Psylliodes chrysocephala) Aphididae (1APHIF) Ceutorhynchus assimilis (CEUTPL) Ceutorhynchus pallidactylus (CEUTQU) Athalia rosae (ATALCO) | SL | 200 g/L | Foliar treatment ‐ broadcast spraying | BBCH 70–71 | 1 | 25 | 200 | 50 | g a.i./ha | 28 | Critical GAP | |
| Poppy seeds | NEU | F | Brassica pod midge (DASYBR, Dasineura brassicae) Cabbage seed/shoot weevil (CEUTAS, Ceutorhynchus obstrictus syn assimilis), Rape flee beetle/cabbage stem flea beetle (PSYICH, Psylliodes chrysocephala) Aphididae (1APHIF) Ceutorhynchus assimilis (CEUTPL) Ceutorhynchus pallidactylus (CEUTQU) Athalia rosae (ATALCO) | SL | 200 g/L | Foliar treatment ‐ broadcast spraying | BBCH 70–71 | 1 | 25 | 200 | 50 | g a.i./ha | 28 | Critical GAP | |
| Mustard seeds | NEU | F | Brassica pod midge (DASYBR, Dasineura brassicae) Cabbage seed/shoot weevil (CEUTAS, Ceutorhynchus obstrictus syn assimilis), Rape flee beetle/cabbage stem flea beetle (PSYICH, Psylliodes chrysocephala) Aphididae (1APHIF) Ceutorhynchus assiimilis (CEUTPL) Ceutorhynchus pallidactylus (CEUTQU) Athalia rosae (ATALCO) | SL | 200 g/L | Foliar treatment ‐ broadcast spraying | BBCH 70–71 | 1 | 25 | 200 | 50 | g a.i./ha | 28 | Critical GAP | |
| Gold of pleasure seeds | NEU | F | Brassica pod midge (DASYBR, Dasineura brassicae) Cabbage seed/shoot weevil (CEUTAS, Ceutorhynchus obstrictus syn assimilis), Rape flee beetle/cabbage stem flea beetle (PSYICH, Psylliodes chrysocephala) Aphididae (1APHIF) Ceutorhynchus assimilis (CEUTPL) Ceutorhynchus pallidactylus (CEUTQU) Athalia rosae (ATALCO) | SL | 200 g/L | Foliar treatment ‐ broadcast spraying | BBCH 70–71 | 1 | 25 | 200 | 50 | g a.i./ha | 28 | Critical GAP | |
MRL: maximum residue level; GAP: Good Agricultural Practice; NEU: northern European Union; SEU: southern European Union; MS: Member State; a.s.: active substance; SL: soluble liquid.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 7th Edition. Revised March 2017. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI – minimum preharvest interval.
In the framework of the review of existing MRLs according to Art. 12 of EU Regulation 396/2005 (EFSA, 2011), subsequent MRL applications and the focused assessment of certain existing MRLs under Art. 43 (EFSA, 2018b), numerous GAPs were reported for crops that might be attractive to bees for food foraging and that might contribute to the final residues of acetamiprid in honey. However, since the MRL application in honey is not linked to one specific GAP and applies to honey as food item for consumers, the use pattern in Phacelia tanacetifolia as surrogate crop with high melliferous capacity is not included in this Appendix but described in the Section 1.2 of the reasoned opinion.
Appendix B – List of end points
B.1 Residues in plants
B.1.1. Nature of residues and analytical methods for enforcement purposes in plant commodities
B.1.1.1. Metabolism studies, analytical methods and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Fruit crops | Eggplants | Dotting on leave and fruit surface, 1 × 9.5 g a.s./hl | 7, 14 | Radiolabelled active substance: pyridine‐2,6‐14C acetamiprid (EFSA, 2011, 2016) | |
| Apples | Foliar, 1 × 208 g/ha | 0, 7, 14, 28, 62, 90 | Radiolabelled active substance: pyridine‐2,6‐14C acetamiprid (EFSA, 2011, 2016) | ||
| Fruit dotting, 1 × 104 g/ha | 0, 14, 28, 62 | ||||
| Root crops | Carrots | Foliar, 2 × 100 g/ha | 14 | Radiolabelled active substance: pyridine‐2,6‐14C acetamiprid (EFSA, 2011, 2016) | |
| Leafy crops | Cabbages | Foliar, 1 × 302 g/ha | 0, 7, 14, 21, 28, 63 | Radiolabelled active substance: pyridine‐2,6‐14C acetamiprid (EFSA, 2011, 2016) | |
| Soil treatment, 1 × 5,940 g/ha | 7, 14, 28 | ||||
| Foliar, 1 × 299 g/ha | 0, 7, 14, 28, 63 | Radiolabelled active substance: cyano‐14C acetamiprid (EFSA, 2011, 2016) | |||
| Pulses/oilseeds | Cotton | Foliar, 4 × 123 g/ha Foliar, 4 × 1,230 g/ha | 14, 28 DAT | Radiolabelled active substance: pyridine‐2,6‐14C acetamiprid (EFSA, 2011, 2016) |
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Root/tuber crops | Turnips | Bare soil, 266 g a.s./ha | 0 | Radiolabelled active substance: the study was conducted with the most persistent acetamiprid soil metabolite IM‐1‐5 (DT50 319–663 days). (EFSA, 2016) | |
| Leafy crops | Spinaches | Bare soil, 266 g a.s./ha | 0 | ||
| Cereal (small grain) | Wheat | Bare soil, 266 g a.s./ha | 0 | ||
| Root/tuber crops | Turnips | Bare soil, 160 g a.s./ha | 0 | Radiolabelled active substance: the study was conducted with the most persistent acetamiprid soil metabolite IM‐1‐5 (DT50 319–663 days). (Austria, 2021) | |
| Leafy crops | Spinaches | Bare soil, 160 g a.s./ha | 0 | ||
| Cereal (small grain) | Wheat | Bare soil, 160 g a.s./ha | 0 |
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/Source |
|---|---|---|---|
| Pasteurisation (20 min, 90°C, pH 4) | Yes | Acetamiprid was stable under standard hydrolysis conditions. Pasteurisation, baking/brewing/boiling and sterilisation are unlikely to result in any significant metabolites (EFSA, 2011, 2016) | |
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | ||
| Sterilisation (20 min, 120°C, pH 6) | Yes | ||
| Other processing conditions |
B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High water content | Cabbage, cucumber | −18 | 12 | Months | Acetamiprid | EFSA (2016) | |
| Apple, tomato | −18 | ≤ 13 | Months | Acetamiprid | EFSA (2016) | ||
| Apple | −18 | 12 | Months | Acetamiprid | Austria (2021) | ||
| lettuce | −18 | 15 | Months | Acetamiprid | EFSA (2016) | ||
| High oil content | Cotton seed, cotton oil, orange oil | −18 | 12 | Months | Acetamiprid | EFSA (2016) | |
| Olive whole fruits | −18 | 12 | Months | Acetamiprid | Austria (2021) | ||
| High protein content | Fodder peas | −18 | 12 | Months | Acetamiprid | EFSA (2016) | |
| Dry bean seed | −18 | 12 | Months | Acetamiprid | Austria (2021) | ||
| Dry/high starch | Potato tuber | −18 | 8 | Months | Acetamiprid | EFSA (2016) | |
| Dry bean straw | −18 | 12 | Months | Acetamiprid | EFSA (2018b), Austria (2021) | ||
| Wheat (grain) | −18 | 15 | Months | Acetamiprid | Austria (2021) | ||
| High acid content | Orange, orange juice | −18 | 12 | Months | Acetamiprid | EFSA (2016) | |
| Orange peel and pulp | −18 | 12 | Months | Acetamiprid | Austria (2021) | ||
| Specific matrices | Honey | −18 | 4 | Months | Acetamiprid, IM‐1‐4 and IM‐1‐5 | EFSA (2021) | |
| Honey | −18 | 11 | Months | Acetamiprid | Austria (2021) | ||
| Processed products | Apple juice/wet pomace Cotton gin trash/hulls/meal Orange dried pulp, orange juice | −18 | 12 | Months | Acetamiprid | EFSA (2016) | |
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials
| Commodity | Region (a) | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) | HR (b) (mg/kg) | STMR (c) (mg/kg) | CF (d) |
|---|---|---|---|---|---|---|---|
| Linseed/flax, poppy seeds, mustard and gold of pleasure seeds | NEU |
Oilseed rape trials (unscaled): 2 × < 0.01; 0.01; 0.037 Oilseed rape trials scaled to cGAP rate: 3 × < 0.01; 0.029 |
Residue trials on oilseed rape are overdosed compared to the cGAP, all other parameters are compliant. Residue levels are scaled down according to proportionality principle and extrapolated to Linseed/flax, poppy seeds, mustard and gold of pleasure seeds | 0.06 | 0.03 | 0.01 | n/a |
| Honey | NEU |
GAP1: 2 × 80 g a.s./ha, interval = 7–10 days, BBCH 61–65, PHI 5–19 days ( Austria, 2021 ): 0.03, 0.09, 0.16, 0.85 (Underline: no control sample available) GAP2: 2 × 100 g a.s./ha, interval = 10–13 days, BBCH 61–67, PHI 4–24 days (EFSA, 2021): 2 × < 0.05; 0.051, 0.162 Combined data sets (excluding the two trials with no control samples): 0.03, 2 × < 0.05, 0.051, 0.09, 0.162 |
Residue levels determined in honey from different sets of residue trials performed in semi‐field/tunnels using Phacelia tanacetifolia as surrogate crops with melliferous properties (Austria, 2021; EFSA, 2021). |
2.0 0.3 0.3 |
0.85 0.16 0.16 |
0.13 0.05 0.05 |
n/a n/a n/a |
MRL: maximum residue level; cGAP: critical Good Agricultural Practice; Mo: monitoring; RA: risk assessment.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, EU: indoor EU trials or Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment refers to the whole commodity and not to the edible portion.
Conversion factor to recalculate residues according to the residue definition for monitoring to the residue definition for risk assessment.
B.1.2.2. Residues in rotational crops
B.1.2.3. Processing factors
No processing studies were submitted in the framework of the present MRL application.
B.2 Residues in livestock
Dietary burden calculation according to OECD (2013).
| Relevant groups | Dietary burden expressed in | Most critical diet (a) | Most critical commodity (b) | Trigger exceeded (Yes/No) | Previous assessment (EFSA, 2018b) | |||
|---|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | 0.004 | Max burden | |||||
| Median | Maximum | Median | Maximum | mg/kg bw | mg/kg DM | |||
| Cattle (all diets) | 0.022 | 0.054 | 0.58 | 1.42 | Cattle (dairy) | Kale, leaves | Yes | 1.42 |
| Cattle (dairy only) | 0.022 | 0.054 | 0.58 | 1.42 | Cattle (dairy) | Kale, leaves | Yes | 1.42 |
| Sheep (all diets) | 0.009 | 0.035 | 0.22 | 0.82 | Sheep (lamb) | Wheat, straw | Yes | 0.82 |
| Sheep (ewe only) | 0.007 | 0.027 | 0.22 | 0.82 | Sheep (ram/ewe) | Wheat, straw | Yes | 0.82 |
| Swine (all diets) | 0.009 | 0.019 | 0.41 | 0.83 | Swine (breeding) | Kale, leaves | Yes | 0.83 |
| Poultry (all diets) | 0.004 | 0.014 | 0.06 | 0.21 | Poultry (layer) | Wheat, straw | Yes | 0.21 |
| Poultry (layer only) | 0.004 | 0.014 | 0.06 | 0.21 | Poultry (layer) | Wheat, straw | Yes | 0.21 |
bw: body weight; DM: dry matter.
When several diets are relevant (e.g. cattle, sheep and poultry ‘all diets’), the most critical diet is identified from the maximum dietary burdens expressed as ‘mg/kg bw per day’.
The most critical commodity is the major contributor identified from the maximum dietary burden expressed as ‘mg/kg bw per day’.
B.3 Consumer risk assessment
B.4 Recommended MRLs
| Code (a) | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Acetamiprid | ||||
| 0401010 | Linseeds | 0.01* | 0.06 | Data on oilseed rape extrapolated to linseeds, poppy seeds, mustard seeds and gold of pleasure seeds. The submitted data are sufficient to derive a MRL proposal for the NEU use. Risk for consumers unlikely. |
| 0401030 | Poppy seeds | 0.01* | Further risk management considerations required (0.3 or 0.06) | Data on oilseed rape extrapolated to linseeds, poppy seeds, mustard seeds and gold of pleasure seeds. The submitted data are sufficient to derive a MRL proposal for the NEU use. EFSA notes that a higher MRL value (0.3 mg/kg) was proposed in a recent output (EFSA, 2021) but this MRL is not implemented yet in the EU Regulation. Risk for consumers unlikely for both MRLs proposed. |
| 0401080 | Mustard seeds | 0.01* | Further risk management considerations required (0.15 or 0.06) | Data on oilseed rape extrapolated to linseeds, poppy seeds, mustard seeds and gold of pleasure seeds. The submitted data are sufficient to derive a MRL proposal for the NEU use. EFSA notes that a higher MRL value (0.15 mg/kg) was proposed in a recent output (EFSA, 2021) but this MRL is not implemented yet in the EU Regulation. Risk for consumers unlikely for both MRLs proposed. |
| 0401130 | Gold of pleasure seeds | 0.01* | 0.06 | Data on oilseed rape extrapolated to linseeds, poppy seeds, mustard seeds and gold of pleasure seeds. The submitted data are sufficient to derive a MRL proposal for the NEU use. Risk for consumers unlikely. |
| 1040000 | Honey and other apiculture products | 0.05* | Further risk management considerations required (2 or 0.3) | Risk Managers are given the options to either set an MRL for honey of 2 mg/kg based on the four residue trials provided with the current application (despite the deviation of not having control samples for two trials) or merge two data sets to derive an MRL of 0.3 mg/kg based on six residue trials performed in accordance with the requirements of the honey guidelines. Risk for consumers unlikely for both MRLs proposed. |
| Further risk management considerations required | For the NEU use a MRL proposal of 1 mg/kg was calculated. | |||
| No MRL proposal | ||||
MRL: maximum residue level; NEU: northern Europe; SEU: southern Europe; GAP: Good Agricultural Practice.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Appendix C – Pesticide Residue Intake Model (PRIMo)
Appendix D – Input values for the exposure calculations
D.1 Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: acetamiprid | ||||
| Alfalfa, forage (green) | 0.09 | STMR (EFSA, 2011) | 0.41 | HR (EFSA, 2011) |
| Alfalfa, hay (fodder) | 0.23 | STMR × 2.5 (a) (EFSA, 2011) | 1.03 | HR × 2.5 (a) (EFSA, 2011) |
| Alfalfa, meal | 0.23 | STMR × 2.5 (a) (EFSA, 2011) | 1.03 | HR × 2.5 (a) (EFSA, 2011) |
| Alfalfa, silage | 0.10 | STMR × 1.1 (a) (EFSA, 2011) | 0.45 | STMR × 1.1 (a) (EFSA, 2011) |
| Barley, straw Oat, straw | 0.18 | STMR (EFSA, 2018) | 0.32 | HR (EFSA, 2018) |
| Cabbage, heads leaves | 0.10 | STMR (EFSA, 2011) | 0.50 | HR (EFSA, 2011) |
| Kale, leaves (forage) | 0.10 | STMR (EFSA, 2015) | 0.73 | HR (EFSA, 2015) |
| Triticale, straw Wheat, straw | 0.27 | STMR (EFSA, 2011) | 1.6 | HR (EFSA, 2011) |
| Potato, culls | 0.01* | STMR (EFSA, 2011) | 0.01* | STMR (EFSA, 2011) |
| Barley, grain Oat, grain | 0.01 | STMR (EFSA, 2018) | 0.01 | STMR (EFSA, 2018) |
| Bean, seed (dry) Cowpea, seed Lupin, seed Pea (Field pea), seed (dry) | 0.02 | STMR (EFSA, 2016a) | 0.02 | STMR (EFSA, 2016a) |
| Cotton, undelinted seed | 0.09 | STMR (EFSA, 2011) | 0.09 | STMR (EFSA, 2011) |
| Triticale, grain Wheat, grain | 0.01 | STMR (EFSA, 2016a) | 0.01 | STMR (EFSA, 2016a) |
| Apple, pomace, wet | 0.30 | STMR × PF (1.3) (EFSA, 2011) | 0.30 | STMR × PF (1.3) (EFSA, 2011) |
| Brewer's grain, dried Wheat, distiller's grain (dry) | 0.03 | STMR × 3.3 (a) (EFSA, 2016a) | 0.03 | STMR × 3.3 (a) (EFSA, 2016a) |
| Canola (Rape seed), meal | 0.06 | STMR × 2 (a) (EFSA, 2016a) | 0.06 | STMR × 2 (a) (EFSA, 2016a) |
| Citrus fruits, dried pulp | 1.90 | STMR × 10 (a) (EFSA, 2011) | 1.90 | STMR × 10 (a) (EFSA, 2011) |
| Coconut, meal | 0.02 | STMR × 1.5 (a) (EFSA, 2011) | 0.02 | STMR × 1.5 (a) (EFSA, 2011) |
| Cotton, meal | 0.04 | STMR × PF (0.4) (EFSA, 2011) | 0.04 | STMR × PF (0.4) (EFSA, 2011) |
| Lupin seed, meal | 0.02 | STMR × 1.1 (a) (EFSA, 2016a) | 0.02 | STMR × 1.1 (a) (EFSA, 2016a) |
| Potato, process waste | 0.01* | STMR (b) (EFSA, 2011) | 0.01* | STMR (b) (EFSA, 2011) |
| Potato, dried pulp | 0.01* | STMR (b) (EFSA, 2011) | 0.01* | STMR (b) (EFSA, 2011) |
| Rape, meal | 0.06 | STMR × 2 (a) (EFSA, 2016a) | 0.06 | STMR × 2 (a) (EFSA, 2016a) |
| Wheat gluten, meal | 0.02 | STMR × 1.8 (a) (EFSA, 2016a) | 0.02 | STMR × 1.8 (a) (EFSA, 2016a) |
| Wheat, milled by‐pdts | 0.07 | STMR × 7 (a) (EFSA, 2016a) | 0.07 | STMR × 7 (a) (EFSA, 2016a) |
| Flaxseed/Linseed, meal | 0.02 | STMR × 2 (a) (intended use) | 0.02 | STMR × 2 (a) (intended use) |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor.
Indicates that the input value is proposed at the limit of quantification.
In the absence of processing factors supported by data, default processing factors (in bracket) were respectively included in the calculation to consider the potential concentration of residues in these commodities.
For potatoes process waste and dried pulp, no default processing factor was applied because residues in the raw commodities were below the LOQ. Concentration of residues in these commodities is therefore not expected.
D.2 Consumer risk assessment
| Commodity | Existing/proposed MRL (mg/kg) | Source | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment (a) | |||
| Risk assessment residue definition: acetamiprid | ||||||
| Grapefruits | 0.9 | EFSA (2018b) | 0.00494 | STMR‐RAC × PeF | 0.02158 | HR‐RAC × PeF |
| Oranges | 0.9 |
EFSA (2018b) |
0.00494 | STMR‐RAC × PeF | 0.02158 | HR‐RAC × PeF |
| Lemons | 0.9 |
EFSA (2018b) |
0.00494 | STMR‐RAC × PeF | 0.02158 | HR‐RAC × PeF |
| Limes | 0.9 |
EFSA (2018b) |
0.00494 | STMR‐RAC × PeF | 0.02158 | HR‐RAC × PeF |
| Mandarins | 0.9 |
EFSA (2018b) |
0.00494 | STMR‐RAC × PeF | 0.02158 | HR‐RAC × PeF |
| Other citrus fruit | 0.9 |
EFSA (2018b) |
0.00494 | STMR‐RAC × PeF | ||
| Almonds | 0.07 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.05 | HR‐RAC |
| Brazil nuts | 0.07 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.05 | HR‐RAC |
| Cashew nuts | 0.07 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.05 | HR‐RAC |
| Chestnuts | 0.07 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.05 | HR‐RAC |
| Coconuts | 0.07 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.05 | HR‐RAC |
| Hazelnuts/cobnuts | 0.07 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.05 | HR‐RAC |
| Macadamia | 0.07 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.05 | HR‐RAC |
| Pecans | 0.07 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.05 | HR‐RAC |
| Pine nut kernels | 0.07 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.05 | HR‐RAC |
| Pistachios | 0.07 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.05 | HR‐RAC |
| Walnuts | 0.07 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.05 | HR‐RAC |
| Other tree nuts | 0.07 |
EFSA (2018b) |
0.01 | STMR‐RAC | ||
| Apples | 0.4 |
EFSA (2018b) |
0.07 | STMR‐RAC | 0.21 | HR‐RAC |
| Pears | 0.4 |
EFSA (2018b) |
0.07 | STMR‐RAC | 0.21 | HR‐RAC |
| Quinces | 0.8 |
EFSA (2018b) |
0.23 | STMR‐RAC | 0.64 | HR‐RAC |
| Medlar | 0.8 |
EFSA (2018b) |
0.23 | STMR‐RAC | 0.64 | HR‐RAC |
| Loquats/Japanese medlars | 0.8 |
EFSA (2018b) |
0.23 | STMR‐RAC | 0.64 | HR‐RAC |
| Other pome fruit | 0.8 |
EFSA (2018b) |
0.23 | STMR‐RAC | ||
| Apricots | 0.8 |
EFSA (2018b) |
0.22 | STMR‐RAC | 0.57 | HR‐RAC |
| Cherries (sweet) | 1.5 |
EFSA (2018b) |
0.45 | STMR‐RAC | 0.88 | HR‐RAC |
| Peaches | 0.2 |
EFSA (2018b) |
0.06 | STMR‐RAC | 0.1 | HR‐RAC |
| Plums | 0.04 | EFSA (2021) | 0.01 | STMR‐RAC | 0.03 | HR‐RAC |
| Table grapes | 0.5 |
EFSA (2018b) |
0.09 | STMR‐RAC | 0.25 | HR‐RAC |
| Wine grapes | 0.5 |
EFSA (2018b) |
0.09 | STMR‐RAC | 0.25 | HR‐RAC |
| Strawberries | 0.5 |
EFSA (2018b) |
0.1 | STMR‐RAC | 0.25 | HR‐RAC |
| Blackberries | 2 |
EFSA (2018b) |
0.64 | STMR‐RAC | 1 | HR‐RAC |
| Dewberries | 2 |
EFSA (2018b) |
0.64 | STMR‐RAC | 1 | HR‐RAC |
| Raspberries (red and yellow) | 2 |
EFSA (2018b) |
0.64 | STMR‐RAC | 1 | HR‐RAC |
| Other cane fruit | 2 |
EFSA (2018b) |
0.64 | STMR‐RAC | ||
| Blueberries | 2 |
EFSA (2018b) |
0.64 | STMR‐RAC | 1 | HR‐RAC |
| Cranberries | 2 |
EFSA (2018b) |
0.64 | STMR‐RAC | 1 | HR‐RAC |
| Currants (red, black and white) | 2 |
EFSA (2018b) |
0.64 | STMR‐RAC | 1 | HR‐RAC |
| Gooseberries (green, red and yellow) | 2 |
EFSA (2018b) |
0.64 | STMR‐RAC | 1 | HR‐RAC |
| Rose hips | 2 |
EFSA (2018b) |
0.64 | STMR‐RAC | 1 | HR‐RAC |
| Mulberries (black and white) | 2 |
EFSA (2018b) |
0.64 | STMR‐RAC | 1 | HR‐RAC |
| Elderberries | 2 |
EFSA (2018b) |
0.64 | STMR‐RAC | 1 | HR‐RAC |
| Figs | 0.03 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Table olives | 3 |
EFSA (2018b) |
0.8 | STMR‐RAC | 1.3 | HR‐RAC |
| Bananas | 0.4 |
EFSA (2018b) |
0.04949 | STMR‐RAC × PeF | 0.1078 | HR‐RAC × PeF |
| Granate apples/pomegranates | 0.3 |
EFSA (2021) |
0.09 | STMR‐RAC | 0.16 | HR‐RAC |
| Potatoes | 0.01 |
EFSA (2018b) |
0.01 | LOQ | 0.01 | LOQ |
| Garlic | 0.02 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Onions | 0.02 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.02 | HR‐RAC |
| Tomatoes | 0.5 |
EFSA (2018b) |
0.13 | STMR‐RAC | 0.28 | HR‐RAC |
| Sweet peppers/bell peppers | 0.4 |
EFSA (2021) |
0.05 | STMR‐RAC | 0.23 | HR‐RAC |
| Aubergines/egg plants | 0.4 |
EFSA (2021) |
0.12 | STMR‐RAC | 0.19 | HR‐RAC |
| Okra/lady's fingers | 0.2 |
EFSA (2018b) |
0.04 | STMR‐RAC | 0.14 | HR‐RAC |
| Other solanacea | 0.2 | Reg. (EU) 2019/88 | 0.2 | MRL | ||
| Cucumbers | 0.4 |
EFSA (2021) |
0.06 | STMR‐RAC | 0.2 | HR‐RAC |
| Gherkins | 0.6 |
EFSA (2018b) |
0.14 | STMR‐RAC | 0.37 | HR‐RAC |
| Courgettes | 0.4 |
EFSA (2021) |
0.06 | STMR‐RAC | 0.2 | HR‐RAC |
| Other cucurbits ‐ edible peel | 0.4 |
EFSA (2021) |
0.06 | STMR‐RAC | ||
| Melons | 0.2 |
EFSA (2018b) |
0.05 | STMR‐RAC | 0.11 | HR‐RAC |
| Pumpkins | 0.2 |
EFSA (2018b) |
0.05 | STMR‐RAC | 0.11 | HR‐RAC |
| Watermelons | 0.2 |
EFSA (2018b) |
0.05 | STMR‐RAC | 0.11 | HR‐RAC |
| Other cucurbits ‐ inedible peel | 0.2 |
EFSA (2018b) |
0.05 | STMR‐RAC | ||
| Sweet corn | 0.01 |
EFSA (2018b) |
0.01 | LOQ | 0.01 | LOQ |
| Broccoli | 0.4 |
EFSA (2018b) |
0.03 | STMR‐RAC | 0.25 | HR‐RAC |
| Cauliflowers | 0.4 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.22 | HR‐RAC |
| Other flowering brassica | 0.4 |
EFSA (2018b) |
0.03 | STMR‐RAC | ||
| Brussels sprouts | 0.05 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.03 | HR‐RAC |
| Head cabbages | 0.4 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.25 | HR‐RAC |
| Lamb's lettuce/corn salads | 3 |
EFSA (2018b) |
0.83 | STMR‐RAC | 1.9 | HR‐RAC |
| Lettuces | 1.5 |
EFSA (2018b) |
0.49 | STMR‐RAC | 0.75 | HR‐RAC |
| Escaroles/broad‐leaved endives | 0.4 |
EFSA (2018b) |
0.1 | STMR‐RAC | 0.25 | HR‐RAC |
| Cress and other sprouts and shoots | 3 |
EFSA (2018b) |
0.83 | STMR‐RAC | 1.9 | HR‐RAC |
| Land cress | 3 |
EFSA (2018b) |
0.81 | STMR‐RAC | 1.9 | HR‐RAC |
| Roman rocket/rucola | 3 |
EFSA (2018b) |
0.83 | STMR‐RAC | 1.9 | HR‐RAC |
| Red mustards | 3 |
EFSA (2018b) |
0.81 | STMR‐RAC | 1.9 | HR‐RAC |
| Baby leaf crops (including brassica species) | 3 |
EFSA (2018b) |
0.83 | STMR‐RAC | 1.9 | HR‐RAC |
| Spinaches | 0.6 |
EFSA (2018b) |
0.2 | STMR‐RAC | 0.31 | HR‐RAC |
| Purslanes | 0.6 |
EFSA (2018b) |
0.2 | STMR‐RAC | 0.31 | HR‐RAC |
| Chards/beet leaves | 0.6 |
EFSA (2018b) |
0.2 | STMR‐RAC | 0.31 | HR‐RAC |
| Other spinach and similar | 0.6 |
EFSA (2018b) |
0.2 | STMR‐RAC | ||
| Chervil | 3 |
EFSA (2018b) |
0.83 | STMR‐RAC | 1.9 | HR‐RAC |
| Chives | 3 |
EFSA (2018b) |
0.83 | STMR‐RAC | 1.9 | HR‐RAC |
| Celery leaves | 3 |
EFSA (2018b) |
0.83 | STMR‐RAC | 1.9 | HR‐RAC |
| Parsley | 3 |
EFSA (2018b) |
0.83 | STMR‐RAC | 1.9 | HR‐RAC |
| Sage | 3 |
EFSA (2018b) |
0.83 | STMR‐RAC | 1.9 | HR‐RAC |
| Rosemary | 3 |
EFSA (2018b) |
0.83 | STMR‐RAC | 1.9 | HR‐RAC |
| Thyme | 3 |
EFSA (2018b) |
0.83 | STMR‐RAC | 1.9 | HR‐RAC |
| Basil and edible flowers | 3 |
EFSA (2018b) |
0.83 | STMR‐RAC | 1.9 | HR‐RAC |
| Laurel/bay leaves | 3 |
EFSA (2018b) |
0.83 | STMR‐RAC | 1.9 | HR‐RAC |
| Tarragon | 3 |
EFSA (2018b) |
0.83 | STMR‐RAC | 1.9 | HR‐RAC |
| Other herbs | 3 |
EFSA (2018b) |
0.83 | STMR‐RAC | ||
| Beans (with pods) | 0.6 |
EFSA (2018b) |
0.06 | STMR‐RAC | 0.32 | HR‐RAC |
| Beans (without pods) | 0.3 |
EFSA (2018b) |
0.03 | STMR‐RAC | 0.18 | HR‐RAC |
| Peas (with pods) | 0.6 |
EFSA (2018b) |
0.06 | STMR‐RAC | 0.32 | HR‐RAC |
| Peas (without pods) | 0.3 |
EFSA (2018b) |
0.03 | STMR‐RAC | 0.18 | HR‐RAC |
| Asparagus | 0.8 |
EFSA (2018b) |
0.26 | STMR‐RAC | 0.43 | HR‐RAC |
| Globe artichokes | 0.7 |
EFSA (2018b) |
0.11 | STMR‐RAC | 0.25 | HR‐RAC |
| Beans | 0.15 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.02 | STMR‐RAC |
| Lentils | 0.15 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.02 | STMR‐RAC |
| Peas | 0.15 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.02 | STMR‐RAC |
| Lupins/lupini beans | 0.15 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.02 | STMR‐RAC |
| Other pulses | 0.15 |
EFSA (2018b) |
0.02 | STMR‐RAC | ||
| Linseeds | 0.06 | Proposed | 0.01 | STMR‐RAC | 0.01 | STMR‐RAC |
| Poppy seeds | 0.3 | EFSA ( 2021 ) | 0.03 | STMR‐RAC | 0.03 | STMR‐RAC |
| Rapeseeds/canola seeds | 0.4 |
EFSA (2018b) |
0.03 | STMR‐RAC | 0.03 | STMR‐RAC |
| Mustard seeds | 0.15 | EFSA ( 2021 ) | 0.03 | STMR‐RAC | 0.03 | STMR‐RAC |
| Cotton seeds | 0.7 |
EFSA (2018b) |
0.09 | STMR‐RAC | 0.09 | STMR‐RAC |
| Gold of pleasure seeds | 0.06 | Proposed | 0.01 | STMR‐RAC | 0.01 | STMR‐RAC |
| Olives for oil production | 3 |
EFSA (2018b) |
0.8 | STMR‐RAC | 0.8 | STMR‐RAC |
| Barley | 0.05 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.01 | STMR‐RAC |
| Oat | 0.05 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.01 | STMR‐RAC |
| Wheat | 0.1 |
EFSA (2018b) |
0.01 | STMR‐RAC | 0.01 | STMR‐RAC |
| Anise/aniseed | 2 | CXL (FAO, 2019) | 0.57 | STMR‐RAC | 2 | HR‐RAC |
| Black caraway/black cumin | 2 | CXL (FAO, 2019) | 0.57 | STMR‐RAC | 2 | HR‐RAC |
| Celery seed | 2 | CXL (FAO, 2019) | 0.57 | STMR‐RAC | 2 | HR‐RAC |
| Coriander seed | 2 | CXL (FAO, 2019) | 0.57 | STMR‐RAC | 2 | HR‐RAC |
| Cumin seed | 2 | CXL (FAO, 2019) | 0.57 | STMR‐RAC | 2 | HR‐RAC |
| Dill seed | 2 | CXL (FAO, 2019) | 0.57 | STMR‐RAC | 2 | HR‐RAC |
| Fennel seed | 2 | CXL (FAO, 2019) | 0.57 | STMR‐RAC | 2 | HR‐RAC |
| Fenugreek | 2 | CXL (FAO, 2019) | 0.57 | STMR‐RAC | 2 | HR‐RAC |
| Nutmeg | 2 | CXL (FAO, 2019) | 0.57 | STMR‐RAC | 2 | HR‐RAC |
| Other spices (seeds) | 2 | CXL (FAO, 2019) | 0.57 | STMR‐RAC | ||
| Cardamom | 0.1 | Reg. (EU) 2019/88 | 0.1 | MRL | 0.1 | MRL |
| Peppercorn (black, green and white) | 0.1 | Reg. (EU) 2019/88 | 0.1 | MRL | 0.1 | MRL |
| Horseradish, root spices | 0.07 | Reg. (EU) 2019/88 | 0.07 | MRL | 0.07 | MRL |
| Swine: Muscle/meat | 0.5 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.27 | HR‐RAC |
| Swine: Fat tissue | 0.3 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.16 | HR‐RAC |
| Swine: Liver | 1 |
EFSA (2018b) |
0.11 | STMR‐RAC | 0.89 | HR‐RAC |
| Swine: Kidney | 1 |
EFSA (2018b) |
0.11 | STMR‐RAC | 0.89 | HR‐RAC |
| Swine: Edible offals (other than liver and kidney) | 1 | Reg. (EU) 2019/88 | 1 | MRL | 1 | MRL |
| Bovine: Muscle/meat | 0.5 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.27 | HR‐RAC |
| Bovine: Fat tissue | 0.3 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.16 | HR‐RAC |
| Bovine: Liver | 1 |
EFSA (2018b) |
0.11 | STMR‐RAC | 0.89 | HR‐RAC |
| Bovine: Kidney | 1 |
EFSA (2018b) |
0.11 | STMR‐RAC | 0.89 | HR‐RAC |
| Bovine: Edible offals (other than liver and kidney) | 1 | Reg. (EU) 2019/88 | 1 | MRL | 1 | MRL |
| Sheep: Muscle/meat | 0.5 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.27 | HR‐RAC |
| Sheep: Fat tissue | 0.3 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.16 | HR‐RAC |
| Sheep: Liver | 1 |
EFSA (2018b) |
0.11 | STMR‐RAC | 0.89 | HR‐RAC |
| Sheep: Kidney | 1 |
EFSA (2018b) |
0.11 | STMR‐RAC | 0.89 | HR‐RAC |
| Sheep: Edible offals (other than liver and kidney) | 1 | Reg. (EU) 2019/88 | 1 | MRL | 1 | MRL |
| Goat: Muscle/meat | 0.5 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.27 | HR‐RAC |
| Goat: Fat tissue | 0.3 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.16 | HR‐RAC |
| Goat: Liver | 1 |
EFSA (2018b) |
0.11 | STMR‐RAC | 0.89 | HR‐RAC |
| Goat: Kidney | 1 |
EFSA (2018b) |
0.11 | STMR‐RAC | 0.89 | HR‐RAC |
| Goat: Edible offals (other than liver and kidney) | 1 | Reg. (EU) 2019/88 | 1 | MRL | 1 | MRL |
| Equine: Muscle/meat | 0.5 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.27 | HR‐RAC |
| Equine: Fat tissue | 0.3 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.16 | HR‐RAC |
| Equine: Liver | 1 |
EFSA (2018b) |
0.11 | STMR‐RAC | 0.89 | HR‐RAC |
| Equine: Kidney | 1 |
EFSA (2018b) |
0.11 | STMR‐RAC | 0.89 | HR‐RAC |
| Equine: Edible offals (other than liver and kidney) | 1 | Reg. (EU) 2019/88 | 1 | MRL | 1 | MRL |
| Poultry: Muscle/meat | 0.02 |
EFSA (2018b) |
0.02 | LOQ | 0.02 | LOQ |
| Poultry: Fat tissue | 0.02 |
EFSA (2018b) |
0.02 | LOQ | 0.02 | LOQ |
| Poultry: Liver | 0.1 |
EFSA (2018b) |
0.1 | LOQ | 0.1 | LOQ |
| Other farmed animals: Muscle/meat | 0.5 | Reg. (EU) 2019/88 | 0.3 | MRL | 0.5 | MRL |
| Other farmed animals: Fat tissue | 0.3 | Reg. (EU) 2019/88 | 0.3 | MRL | 0.3 | MRL |
| Other farmed animals: Liver | 1 | Reg. (EU) 2019/88 | 1 | MRL | 1 | MRL |
| Other farmed animals: Kidney | 1 | Reg. (EU) 2019/88 | 1 | MRL | 1 | MRL |
| Other farmed animals: Fat tissue | 0.3 | Reg. (EU) 2019/88 | 0.3 | MRL | 0.3 | MRL |
| Other farmed animals: Liver | 1 | Reg. (EU) 2019/88 | 1 | MRL | 1 | MRL |
| Other farmed animals: Kidney | 1 | Reg. (EU) 2019/88 | 1 | MRL | 1 | MRL |
| Other farmed animals: Edible offals (other than liver and kidney) | 1 | Reg. (EU) 2019/88 | 1 | MRL | 1 | MRL |
| Milk: Cattle | 0.2 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.02 | STMR‐RAC |
| Milk: Sheep | 0.2 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.02 | STMR‐RAC |
| Milk: Goat | 0.2 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.02 | STMR‐RAC |
| Milk: Horse | 0.2 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.02 | STMR‐RAC |
| Milk: Others | 0.2 |
EFSA (2018b) |
0.02 | STMR‐RAC | 0.02 | STMR‐RAC |
| Eggs: Chicken | 0.02 |
EFSA (2018b) |
0.02 | LOQ | 0.02 | LOQ |
| Eggs: Duck | 0.02 |
EFSA (2018b) |
0.02 | LOQ | 0.02 | LOQ |
| Eggs: Goose | 0.02 |
EFSA (2018b) |
0.02 | LOQ | 0.02 | LOQ |
| Eggs: Quail | 0.02 |
EFSA (2018b) |
0.02 | LOQ | 0.02 | LOQ |
| Eggs: Others | 0.02 |
EFSA (2018b) |
0.02 | LOQ | ||
| Honey and other apiculture products | 0.3/2 (b) | Proposed | 0.13 | STMR‐RAC | 0.85 | HR‐RAC |
STMR‐RAC: supervised trials median residue in raw agricultural commodity; HR‐RAC: highest residue in raw agricultural commodity; PeF: Peeling factor.
Input values for the commodities which are not under consideration for the acute risk assessment are reported in grey.
As explained in Section 1.2.1, 0.3 mg/kg referred to the MRL proposal based on merging two different data sets while 2 mg/kg ‐ and the corresponding input values (STMR and HR) used as worst‐case for the consumer risk assessment ‐ referred to the proposal derived based on the four residue trials provided in the present application.
Appendix E – Used compound codes
| Code/trivial name (a) | IUPAC name/SMILES notation/InChiKey (b) | Structural formula (c) |
|---|---|---|
| Acetamiprid |
(E)‐N1‐[(6‐chloro‐3‐pyridyl)methyl]‐N2‐cyano‐N1‐ methylacetamidine Clc1ccc(CN(C)C(\C)=N\C#N)cn1 WCXDHFDTOYPNIE‐RIYZIHGNSA‐N |
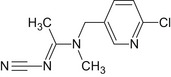
|
| N‐desmethyl‐acetamiprid (IM‐2‐1) |
(E)‐N‐[(6‐chloro‐3‐pyridyl)methyl]‐N′‐cyanoacetamidine Clc1ccc(CNC(\C)=N\C#N)cn1 AYEAUPRZTZWBBF‐UHFFFAOYSA‐N AYEAUPRZTZWBBF‐UHFFFAOYSA‐N |
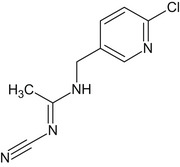
|
| IM‐1‐4 |
1‐(6‐chloro‐3‐pyridyl)‐N‐ methylmethanamine Clc1ccc(CNC)cn1 XALCOJXGWJXWBL‐UHFFFAOYSA‐N |
|
| IM‐1‐5 |
N‐[(6‐chloro‐3‐pyridyl)methyl]‐N‐methylacetamidine Clc1ccc(CN(C)C(C)=N)cn1 JHZWQGRBAHJYIZ‐UHFFFAOYSA‐N |
|
| 6‐chloronicotinic acid (IC‐0) |
6‐chloronicotinic acid OC(=O)c1cnc(Cl)cc1 UAWMVMPAYRWUFX‐UHFFFAOYSA‐N |
|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system; InChiKey: International Chemical Identifier Key.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2020.2.1 ACD/Labs 2020 Release (File version N15E41, Build 116563, 15 June 2020).
ACD/ChemSketch 2020.2.1 ACD/Labs 2020 Release (File version C25H41, Build 121153, 22 March 2021).
Suggested citation: EFSA (European Food Safety Authority) , Bellisai G, Bernasconi G, Brancato A, Cabrera LC, Castellan I, Ferreira L, Giner G, Greco L, Jarrah S, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Robinson T, Ruocco S, Santos M, Scarlato AP, Theobald A and Verani A, 2022. Reasoned Opinion on the modification of the existing maximum residue levels for acetamiprid in honey and various oilseed crops. EFSA Journal 2022;20(8):7535, 40 pp. 10.2903/j.efsa.2022.7535
Requestor European Commission
Question number EFSA‐Q‐2021‐00323
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements EFSA wishes to thank Stathis Anagnos, Laszlo Bura, Andrea Mioč, Marta Szot, Aikaterini Vlachou for the support provided to this scientific output.
Adopted: 20 July 2022
Notes
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) 2018/113 of 24 January 2018 renewing the approval of the active substance acetamiprid in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. OJ L 20, 25.1.2018, p. 7–10.
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
For an overview of all MRL Regulations on this active substance, please consult: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/active-substances/?event=search.as
Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 93, 3.4.2013, p. 1–84.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
Commission Regulation (EU) 2019/88 of 18 January 2019 amending Annex II to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for acetamiprid in certain products. C/2019/140. OJ L 22, 24.1.2019, p. 1–12.
References
- Austria , 2021. Evaluation report on the modification of MRLs for acetamiprid in honey, linseeds, poppy seeds, mustard seeds and gold of pleasure seeds. April 2021, revised in June 2022, 107 pp. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority) , 2011. Review of the existing maximum residue levels (MRLs) for acetamiprid according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2011;9(7):2328, 59 pp. 10.2903/j.efsa.2011.2328. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2015. Reasoned opinion on the modification of the existing maximumresidue levels for acetamiprid in leafy brassicas. EFSA Journal 2015;13(9):4229, 20 pp. 10.2903/j.efsa.2015.4229 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2016a. Reasoned opinion on the modification of the existing maximum residue levels for acetamiprid in various crops. EFSA Journal 2016;14(2):4385, 25 pp. 10.2903/j.efsa.2016.4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2016b. Conclusion on the peer review of the pesticide risk assessment of the active substance acetamiprid. EFSA Journal 2016;14(11):4610, 26 pp. 10.2903/j.efsa.2016.4610 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschner R, Medina P, Miron I, Nougadere A, Pedersen R, Reich H, Santos M, Stanek A, Tarazona J, Theobald A and Villamar‐Bouza L, 2018a. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2018b. Focussed assessment of certain existing MRLs of concern for acetamiprid and modification of the existing MRLs for table olives, olives for oil production, barley and oats. EFSA Journal 2018;16(5):5262, 39 pp. 10.2903/j.efsa.2018.5262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2018c. Recommendations on the use of the proportionality approach in the framework of risk assessment for pesticide residues. EFSA supporting publication 2018:EN‐1503, 18 pp. 10.2903/sp.efsa.2017.EN-1503 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou M, Brancato A, Carrasco Cabrera L, Ferreira L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Tarazona J, Theobald A and Verani A, 2019. Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1 (update of EFSA PRIMo revision 3). EFSA supporting publication 2019:EN‐1605, 15 pp. 10.2903/sp.efsa.2019.EN-1605 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Bellisai G, Bernasconi G, Brancato A, Carrasco Cabrera L, Ferreira L, Giner G, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Ruocco S, Santos M, Scarlato AP, Theobald A, Vagenende B and Verani A, 2021. Reasoned Opinion on the modification of the existing maximum residue levels foracetamiprid in various crops. EFSA Journal 2021;19(9):6830, 38 pp. 10.2903/j.efsa.2021.6830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Panel on Plant Protection Products and their Residues (PPR) , Hernandez Jerez A, Adriaanse P, Berny P, Coja T, Duquesne S, Focks A, Marinovich M, Millet M, Pelkonen O, Pieper S, Tiktak A, Topping C, Widenfalk A, Wilks M, Wolterink G, Rundlöf M, Ippolito A, Linguadoca A, Martino L, Panzarea M, Terron A and Aldrich A, 2022. Statement on the active substance acetamiprid. EFSA Journal 2022;24;20(1):7031, 71 pp. 10.2903/j.efsa.2022.7031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2010. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2013. Working document on the nature of pesticide residues in fish. SANCO/11187/2013‐rev. 3, 31 January 2013.
- European Commission , 2017. Technical guideline on the evaluation of extraction efficiency of residue analytical methods. SANTE 2017/10632, Rev. 4, 23 February 2022.
- European Commission , 2018a. Final renewal report for the active substance acetamiprid. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 13 December 2017 in view of the inclusion of active substance acetamiprid in Annex I of Council Directive 91/414/EEC. SANTE/10502/2017 Rev 4, 13 December 2017.
- European Commission , 2018b. Technical guidelines for determining the magnitude of pesticide residues in honey and setting Maximum Residue Levels in honey. SANTE/11956/2016 rev. 9. 14 September 2018.
- European Commission , 2020. Technical guidelines on data requirements for setting maximum residue levels,comparability of residue trials and extrapolation on residue data on products from plant and animal origin. SANTE/2019/12752, 23 November 2020.
- FAO (Food and Agriculture Organization of the United Nations) , 2016. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 3rd Edition. FAO Plant Production and Protection Paper 225, 298 pp. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) , 2019. Report of the extra Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group onPesticide Residues. Ottawa, Canada, 7–17 May 2019. Pesticide residue in food 2019, 360 pp. [Google Scholar]
- Netherlands , 2015. Renewal Assessment Report (RAR) and proposed decision of the Netherlands prepared in the context of the possible renewal of acetamiprid under Regulation (EC) 1107/2009. November 2015.
- Netherlands , 2016. Re‐Assessment Report and proposed decision of the Netherlands prepared in the context of the possible renewal of acetamiprid under Regulation (EC) 1107/2009. June 2016.
- OECD (Organisation for Economic Co‐operation and Development) , 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues.
- OECD (Organisation for Economic Co‐operation and Development) , 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 04 September 2013.


