Abstract
Background
Tumor grade determined by the Ki67 index is the best prognostic factor for pancreatic neuroendocrine tumors (PanNETs). However, we often observe that the grade of metastases differs from that of their primary tumors. This study aimed to investigate the frequency of grade changes between primary tumors and metastases, explore its association with clinical characteristics, and correlate the findings with the prognosis.
Methods
Six hundred forty-eight patients with pancreatic neuroendocrine neoplasms treated at Fudan University Shanghai Cancer Center were screened for inclusion, and 103 patients with PanNETs who had paired primary tumors and metastases with an available Ki67 index were included. Re-evaluation of Ki67 was performed on 98 available samples from 69 patients.
Results
Fifty cases (48.5%) had a Ki67 index variation, and 18 cases (17.5%) displayed a grade increase. Metachronous metastases showed significantly higher Ki67 index variation than synchronous metastases (P=0.028). Kaplan–Meier analyses showed that high-grade metastases compared to low-grade primary tumors were significantly associated with decreased progression-free survival (PFS, P=0.012) and overall survival (OS, P=0.027). Multivariable Cox regression analyses demonstrated that a low-grade increase to high-grade was an unfavorable and independent prognostic factor for PFS and OS (P=0.010, and P=0.041, respectively).
Conclusions
A high-grade increase in metastases was an unfavorable predictor of PanNETs, which emphasized the importance of accurate pathological grading and could provide a reference for clinical decision-making.
Keywords: pancreatic neuroendocrine tumors, Ki67 index variation, grade increase, preoperative neoadjuvant treatment, prognosis
Introduction
Pancreatic neuroendocrine tumors (PanNETs) are a rare and heterogeneous group of tumors arising from the neuroendocrine system, and their prevalence has markedly increased over the last four decades (1–3). The clinical courses of PanNETs are highly variable, and their prognosis differs widely, ranging from indolent tumors with reasonable survival to distant metastases of an aggressive nature with a poor prognosis. Up to 80% of patients with PanNETs present with metastases at the time of diagnosis, mainly to the liver (4–6), whose median survival is only 23 months, compared to 124 months for localized disease (7). PanNETs with metastases require a multidisciplinary treatment approach, including surgery (8), somatostatin analogs (SSAs), targeted therapy, namely, everolimus and sunitinib (9, 10), chemotherapy (11), and/or peptide receptor radionuclide therapy (12). Surgery remains the only curative treatment for these patients, and patients can benefit from neoadjuvant treatment (NAT).
For better prognostic stratification, the European Neuroendocrine Tumor Society (ENETS) originally proposed a three-tiered grading system for gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) in 2006, and the World Health Organization (WHO) adopted the classification in 2010, which was mainly based on the Ki67 index and mitotic index (13–15). The WHO modified the classification in 2017 and divided pancreatic neuroendocrine neoplasms (PanNENs) into well-differentiated neuroendocrine tumors (NETs) and poorly differentiated neuroendocrine carcinoma (NEC). Given that the Ki67 index has been proven to be the most reliable and best prognostic factor of PanNENs (16), the 2017 WHO classification requires its use and strongly recommends careful evaluation of the Ki67 index. Accordingly, well-differentiated NETs are further divided into the following grades by the Ki67 index: G1: <3%; G2: 3–20%; G3: >20% (17, 18).
Grade currently plays a pivotal role in the clinical management of patients with NETs, especially PanNETs (19). However, concerns exist regarding Ki67 index variation and grade differences between the primary tumors and metastases (20–22). We also observed that the grade of metastases differed from that of their primary tumors in PanNET patients in clinical setting. Here, we set out to investigate the frequency of grade changes between primary tumors and metastases in PanNETs, to explore the association between clinical characteristics and grade changes and to determine whether grade changes correlate with patient outcomes. A better understanding of grade changes could add to our understanding of the heterogeneity between primary tumors and metastases in PanNETs and contribute to clinical decision-making.
Methods
Patient population
A total of 648 patients with pathology-confirmed PanNENs who were treated at Fudan University Shanghai Cancer Center (FUSCC) between January 1, 2006, and February 1, 2020, were screened for inclusion. Exclusion criteria for the study cohort were familial syndromes, no metastatic disease, and no pathology reports available or consultation with our institution. Then, 169 patients with metastatic PanNETs were included for further eligibility evaluation. Next, 103 patients who had paired primary tumors and metastases with an available Ki67 index were identified as the study cohort. To assess the selection bias due to the inclusion of patients who had paired primary tumors and metastases with an available Ki67 index, the remaining 66 patients who did not have paired tumors with an available Ki67 index were identified as the bias-control group. Additionally, to explore whether NETs evolved to NEC, 15 patients with metastatic PanNEC were served as additional data.
The study cohort consisted of 91 patients who underwent resection and 12 patients who underwent needle biopsy or laparotomy with biopsy. In the resection cohort, 74 and 17 patients had synchronous and metachronous metastases, respectively. The biopsy cohort consisted of 12 patients with synchronous metastases. Metastatic PanNETs patients with high-risk factors, including relatively large tumors, blood vessels and adjacent organ invasion were routinely received preoperative NAT. And surgical resection was considered after multidisciplinary discussion if the tumors stabilized or regressed. Pathology reports, including Ki67 index, size of the tumor, perineural, lymphatic, and microvascular invasion, and clinical data, including sex, age, operative procedures, treatment information, and follow-up information, were retrospectively reviewed from the medical record database.
Patients were observed at 3- to 6-month intervals following resection or biopsy and underwent physical examination, laboratory investigation and contrast-enhanced computed tomography or magnetic resonance imaging of the chest/abdomen/pelvis. PFS was defined as the time from the date of resection or biopsy to the date of progression. OS was defined as the time from the date of resection or biopsy to the date of either death (event) or the last follow-up (censored).
Tumors were restaged and regraded based on the 8th edition American Joint Committee on Cancer (AJCC 8th) tumor-node-metastasis (TNM) staging system and 2019 WHO classification. Metastatic diseases were divided into locoregional (nodal or mesenteric involvement) and distant metastases (liver, peritoneum or other sites). Liver metastases (LM) were classified into type I, II and III (23). Type I LM were confined to one liver lobe or two adjacent segments that could be removed by a standard anatomical resection. Type II LM primarily affected one lobe, with smaller satellites contralaterally, and could be managed surgically, including ablative approaches. Type III LM were diffuse, multifocal liver metastases that could not be treated surgically.
This retrospective study was approved by the institutional research ethics committee of FUSCC, and informed consent was obtained from the patients included in this study.
Immunohistochemistry and re-evaluation
Formalin-fixed, paraffin-embedded (FFPE) samples of 69 patients were available, including 63 primary tumors, 5 involved lymph nodes, and 30 LM. Slides of these samples were subjected to Ki67 immunohistochemical staining (MIB-1, Dako Corporation, CA, USA) using a two-step method as previously described (24–27). Detailed experimental procedures were described in the Supplementary Materials [available in a digital data repository (28)]. Additionally, TP53 mutation was associated with Ki67 index variation and might be a possible biologic mechanism for high-grade transition (22, 29, 30). Therefore, we selected FFPE samples of 27 patients for p53 immunohistochemical staining (ab1101, Abcam, Cambridge, UK). Abnormal p53 expression was defined as complete absence or more than 10% of tumor cells presented with moderate to strong nuclear positivity (30–32).
Statistical analyses
Statistical analyses were performed using SPSS (version 25.0, IBM, Armonk, NY, USA). Continuous variables were compared by nonparametric tests or Student’s t-test. Categorical variables were compared by Pearson’s chi-squared test or Fisher’s test. The Kaplan–Meier method and log-rank test were used to estimate significant differences in survival. Cox regression analyses were performed to identify independent prognostic factors of PFS and OS. Variables significantly associated with PFS or OS in the univariable analysis were included in the multivariable analysis. Logistic regression models were used to control confounding variables. Two-sided P values of <0.05 were considered statistically significant. Figures were drawn with GraphPad Prism (version 8.0, San Diego, CA, USA, www.graphpad.com).
Results
Patient characteristics
A total of 103 patients who had paired primary tumors and metastases with an available Ki67 index were identified as the study cohort. These patients were divided into a resection cohort and a biopsy cohort ( Figure 1 ). The clinicopathological characteristics and treatment information of the study cohort, resection cohort, and biopsy cohort were analyzed and are summarized in Table 1 . In the study cohort, 4 patients had functional tumors, and their specific information is shown in Supplementary Table S1 . The grade at first diagnosis was distributed as follows: G1 NET-10 (9.7%), G2 NET-76 (73.8%), and G3 NET-17 (16.5%). A total of 20 patients underwent pancreatoduodenectomy with or without LM resection, 66 patients underwent distal pancreatectomy with or without LM resection, 4 patients underwent total pancreatectomy with or without LM resection, and one patient underwent middle pancreatectomy with LM resection. Furthermore, 3 patients underwent laparotomy with biopsy, and 9 patients underwent needle biopsy. For sites of metastases, 92 patients (89.3%) had LM, 9 patients (8.7%) had nodal or mesenteric metastases, and 2 patients (1.9%) had peritoneum or other distant metastases. In addition, 86 patients (83.5%) were diagnosed with synchronous metastases, and 17 patients (16.5%) developed metachronous metastases.
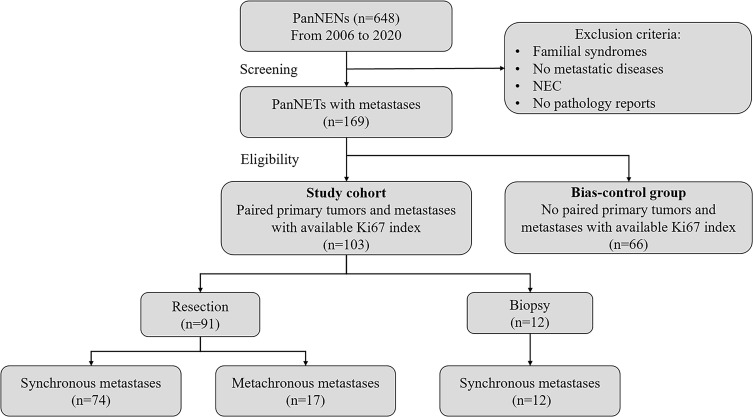
Figure 1.
Flow diagram of the study cohort. Out of the 648 patients with PanNENs, 169 patients with metastatic PanNETs were included for further eligibility evaluation. Then 103 patients had paired primary tumors and metastases with an available Ki67 index were identified as the study cohort, and the remaining 66 patients were identified as the bias-control group. The study cohort consisted of 91 patients underwent resection and 12 patients experienced biopsy. In resection cohort, 74 and 17 patients had synchronous and metachronous metastases, respectively. And biopsy cohort consisted of 12 patients with synchronous metastases.
Table 1. Demographics and clinical characteristics of the study cohort, resection and biopsy cohort.
| Characteristics | Study cohort (n=103) | Resection cohort (n=91) | Biopsy cohort(n=12) |
|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |
| Gender | |||
| Male | 50 (48.5) | 42 (46.2) | 8 (66.7) |
| Female | 53 (51.5) | 49 (53.8) | 4 (33.3) |
| Age, years, median | 51 | 50.5 | 49.5 |
| Tumor size, mm | |||
| Mean (SD) | 48.7 (3.0) | 47.7 (3.0) | 56.8 (3.4) |
| Median (range) | 40.0 (8.0-160.0) | 40.0 (8.0-160.0) | 47.0 (20.0-120.0) |
| Location | |||
| Head | 28 (27.2) | 22 (24.2) | 6 (50.0) |
| Neck | 6 (5.8) | 5 (5.5) | 1 (8.3) |
| Body | 15 (14.6) | 14 (15.4) | 1 (8.3) |
| Tail | 25 (24.3) | 23 (25.3) | 2 (16.7) |
| Body-Tail | 29 (28.2) | 27 (29.7) | 2 (16.7) |
| Functional | |||
| Yes | 4 (3.9) | 4 (4.4) | 0 (0.0) |
| No | 99 (96.1) | 87 (95.6) | 12 (100.0) |
| Lymph node positive | n=88 | n=88 | NA |
| Yes | 48 (54.5) | 48 (54.5) | |
| No | 40 (45.5) | 40 (45.5) | |
| Perineural invasion | n=84 | n=84 | NA |
| Yes | 41 (48.8) | 41 (48.8) | |
| No | 43 (51.2) | 43 (51.2) | |
| Microvascular invasion | n=84 | n=84 | NA |
| Yes | 57 (67.9) | 57 (67.9) | |
| No | 27 (32.1) | 27 (32.1) | |
| CgAa | n=102 | n=90 | n=12 |
| Positive | 97 (95.1) | 86 (95.6) | 11 (91.7) |
| Negative | 5 (4.9) | 4 (4.4) | 1 (8.3) |
| Syna | n=101 | n=89 | n=12 |
| Positive | 100 (99.0) | 88 (98.9) | 12 (100.0) |
| Negative | 1 (1.0) | 1 (1.1) | 0 (0.0) |
| DAXXa | n=38 | n=38 | NA |
| Positive | 34 (89.5) | 34 (89.5) | |
| Negative | 4 (10.5) | 4 (10.5) | |
| ATRXa | n=36 | n=36 | NA |
| Positive | 32 (88.9) | 32 (88.9) | |
| Negative | 4 (11.1) | 4 (11.1) | |
| SSTRa | n=57 | n=55 | n=2 |
| Positive | 53 (93.0) | 51 (92.7) | 2 (100.0) |
| SSTR2 | 38 (66.7) | 36 (65.4) | 2 (100.0) |
| SSTR2+SSTR5 | 15 (26.3) | 15 (27.3) | 0 (0.0) |
| Negative | 4 (7.0) | 4 (7.3) | 0 (0.0) |
| NSE classification | n=84 | n=76 | n=8 |
| High | 26 (31.0) | 22 (28.9) | 4 (50.0) |
| Low | 58 (69.0) | 54 (71.1) | 4 (50.0) |
| PROGRP classification | n=46 | n=43 | n=3 |
| High | 8 (17.4) | 7 (16.3) | 1 (33.3) |
| Low | 38 (82.6) | 36 (83.7) | 2 (66.7) |
| Metastases | |||
| Site | |||
| Liver | 92 (89.3) | 81 (89.0) | 11 (91.7) |
| Nodal/mesenteric | 9 (8.7) | 9 (9.9) | 0 (0.0) |
| Peritoneum/others | 2 (1.9) | 1 (1.1) | 1 (8.3) |
| Type | |||
| Synchronous | 86 (83.5) | 74 (81.3) | 12 (100.0) |
| Metachronous | 17 (16.5) | 17 (18.7) | 0 (0.0) |
| AJCC 8th TNM stage | |||
| I | 1 (1.0) | 1 (1.1) | 0 (0.0) |
| II | 12 (11.7) | 12 (13.2) | 0 (0.0) |
| III | 10 (9.7) | 10 (11.0) | 0 (0.0) |
| IV | 80 (77.7) | 68 (74.7) | 12 (100.0) |
| WHO classificationb | |||
| G1 | 10 (9.7) | 9 (9.9) | 1 (8.3) |
| G2 | 76 (73.8) | 68 (74.7) | 8 (66.7) |
| G3 | 17 (16.5) | 14 (15.4) | 3 (25.0) |
| Operating methods | |||
| Pancreatoduodenectomy Distal Pancreatectomy Total pancreatectomy Total pancreatectomy with LM resection Middle pancreatectomy with LM resection Pancreatoduodenectomy with LM resection Distal Pancreatectomy with LM resection Laparotomy with biopsy. Needle biopsy |
6 (5.8) 15 (14.6) 2 (1.9) 2 (1.9) 1 (1.0) 14 (13.6) 51 (49.5) 3 (2.9) 9 (8.7) |
6 (6.6) 15 (16.5) 2 (2.2) 2 (2.2) 1 (1.1) 14 (15.4) 51 (56.0) 0 (0.0) 0 (0.0) |
0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 3 (25.0) 9 (75.0) |
| Treatment | |||
| Neoadjuvant treatment SSAs with or without targeted therapy CAPTEM with or without targeted therapy SSAs+CAPTEM with or without targeted therapy Locoregional treatment Other chemotherapy Targeted therapy Others |
n=33 9 (27.3) 11 (33.3) 7 (21.2) 1 (3.0) 3 (9.1) 1 (2.0) 1 (3.0) |
n=33 9 (27.3) 11 (33.3) 7 (21.2) 1 (3.0) 3 (9.1) 1 (3.0) 1 (2.0) |
NA |
| Adjuvant treatment SSAs with or without targeted therapy CAPTEM with or without targeted therapy SSAs+CAPTEM with or without targeted therapy Locoregional treatment Other chemotherapy Targeted therapy Others |
n=78 35 (44.9) 6 (7.7) 6 (7.7) 12 (15.4) 4 (5.1) 15 (19.2) 0 (0.0) |
n=78 35 (44.9) 6 (7.7) 6 (7.7) 12 (15.4) 4 (5.1) 15 (19.2) 0 (0.0) |
NA |
| Treatment for patients with biopsy SSAs with or without targeted therapy CAPTEM with or without targeted therapy SSAs+CAPTEM with or without targeted therapy Locoregional treatment Other chemotherapy Targeted therapy Others |
n=12 4 (33.3) 2 (16.7) 3 (25.0) 1 (8.3) 0 (0.0) 2 (16.7) 0 (0.0) |
NA | n=12 4 (33.3) 2 (16.7) 3 (25.0) 1 (8.3) 0 (0.0) 2 (16.7) 0 (0.0) |
CgA, chromogranin; Syn, synaptophysin; DAXX, death domain associated protein; ATRX, alpha-thalassemia/mental retardation X-linked; SSTR, somatostatin receptor; NSE, neuron specific enolase; PROGRP, progastrin releasing peptide; AJCC, American Joint Committee on Cancer; WHO, World Health Organization; a The expression in primary tumors; b Grade at first diagnosis; LM, liver metastases; SSAs, somatostatin analogs; CAPTEM, capecitabine and temozolomide; NA, not available.
Ki67 index variation and grade changes between primary tumors and metastases
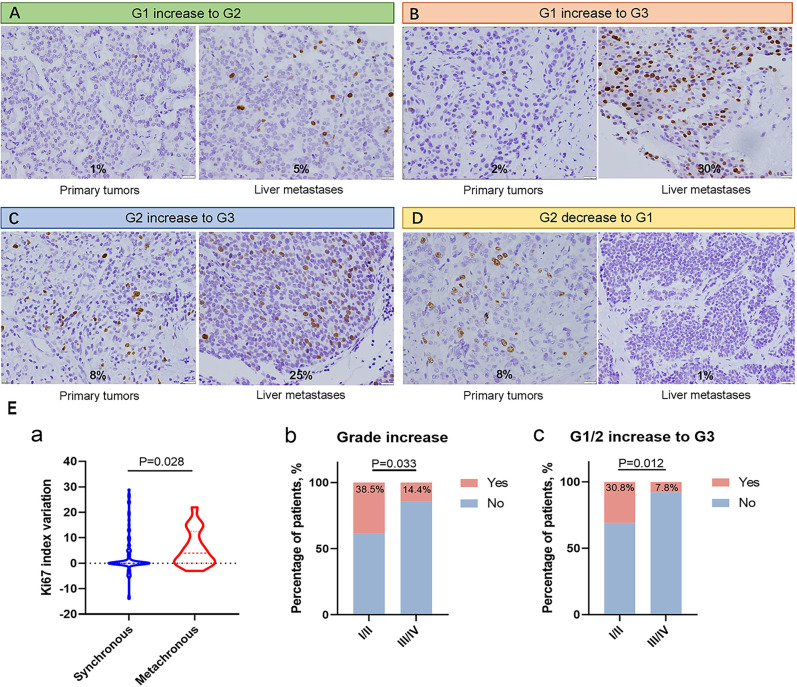
In the study cohort, 50 cases (48.5%) showed variance in the Ki67 index between primary tumors and metastases, and the median variation in the Ki67 index in metastases compared to primary tumors was 0%, ranging from -14% to +29% ( Table 2 ). The Ki67 variation led to grade changes between the primary tumors and metastases; 18 patients (17.5%) had a grade increase, and a grade decrease was observed in 6 patients (5.8%). Among those with grade increases, 7 patients (6.8%) changed from G1 to G2, 4 patients (3.9%) changed from G1 to G3, and 7 patients (6.8%) changed from G2 to G3 ( Figures 2A–D ). The characteristics of the eleven patients with G1 or G2 (G1/G2) increase to G3 are shown in Supplementary Table S2 . In addition, grade changes between the primary tumors and metastases were found in 21 cases (23.1%) in the resection cohort and 3 cases (25.0%) in the biopsy cohort.
Table 2. Ki67 index variation and grade changes in metastases compared to primary tumors in PanNETs.
| Ki67 index variation | Study cohort (n=103) | Resection cohort (n=91) | Biopsy cohort (n=12) |
|---|---|---|---|
| Patient number (%) | 50 (48.5) | 43 (47.3) | 7 (58.3) |
| Delta Ki67 index, median (range) | 0 (-14 to +29) | 0 (-14 to +29) | 0.5 (-5 to +27) |
| Tumor grade | |||
| Stable, n (%) | 79 (76.7) | 70 (76.9) | 9 (75.0) |
| Grade increase, n (%) | 18 (17.5) | 15 (16.5) | 3 (25.0) |
| G1 increase to G2 | 7 (6.8) | 6 (6.6) | 1 (8.3) |
| G1 increase to G3 | 4 (3.9) | 4 (4.4) | 0 (0) |
| G2 increase to G3 | 7 (6.8) | 5 (5.5) | 2 (16.7) |
| Grade decrease, n (%) | |||
| G2 decrease to G1 | 6 (5.8) | 6 (6.6) | 0 (0) |
Figure 2.
(A–D) Immunohistochemistry staining of Ki67 in available paired primary tumors and metastases, showing G1 increase to G2, G1 increase to G3, G2 increase to G3, and G2 decrease to G1, respectively. Magnification: 400×. (E) Association among clinicopathological characteristics, Ki67 index variation and grade changes. (a) Metachronous metastases showed higher Ki67 index variation than synchronous metastases. (b, c) Patients with AJCC 8th stage I/II showed higher frequency of grade increase, and G1/G2 increase to G3 than patients with AJCC 8th stage III/IV.
Association among clinical characteristics, Ki67 index variation, and grade changes
Metachronous metastases showed significantly higher Ki67 index variation than synchronous metastases (P=0.028, Figures 2Ea ). Meanwhile, metachronous metastases had a higher proportion of G1/G2 increase to G3 than synchronous metastases, although the difference was not significant (P=0.060). Patients with AJCC 8th stage I/II showed a higher frequency of grade increase and G1/G2 increase to G3 than patients with AJCC 8th stage III/IV (P=0.033 and P=0.012, respectively, Figures 2Eb, c ), which was consistent with the result that metachronous metastases showed higher Ki67 index variation. In the eleven patients with a G1/G2 increase to G3, all 4 patients with metachronous metastases and AJCC 8th stage II at first diagnosis showed a G2 increase to G3; among the 7 patients with synchronous metastases and AJCC 8th stage IV at first diagnosis, 4 patients had a G1 increase to G3, and the remaining 3 patients had a G2 increase to G3, indicating that patients with stage IV were more likely to have a more severe grade increase ( Supplementary Table S2 ).
Ki67 index variation and grade changes in advanced patients who received NAT
Increasing evidence supports the application of NAT in advanced PanNETs (33, 34). Among the 68 advanced patients (AJCC 8th stage IV) who underwent surgical resection, 31 patients received NAT, and 37 cases did not receive NAT. The clinicopathological characteristics and treatment information of these patients are summarized in Supplementary Table S3 . Of the 31 patients receiving NAT, 9 patients (29.0%) received SSAs with or without targeted therapy, 11 patients (35.5%) received capecitabine and temozolomide (CAPTEM) with or without targeted therapy, 7 patients (22.6%) received SSAs+CAPTEM with or without targeted therapy, a case (3.2%) received locoregional treatment, 2 patients (6.5%) received other chemotherapeutic regimens, and one patient (3.2%) received targeted therapy. All patients treated with NAT had LM, including 2 patients (6.5%) with type I LM, 2 patients (6.5%) with type II LM, and 27 patients (87.1%) with type III LM. Patients who received NAT presented a significantly higher proportion of type III LM than those who did not receive NAT (87.1% vs. 50.0%, P=0.009).
These patients achieved the effect of tumor stabilization or regression after receiving NAT and could be considered for surgery after multidisciplinary discussion. Among them, 8 patients underwent pancreaticoduodenal resection with LM, and 23 cases underwent distal pancreaticoduodenal resection with LM. Additionally, advanced patients who received NAT presented a significantly higher proportion of grade increase (25.8% vs. 5.4%, P=0.018) than those who did not receive NAT ( Table S3 ).
Survival analyses
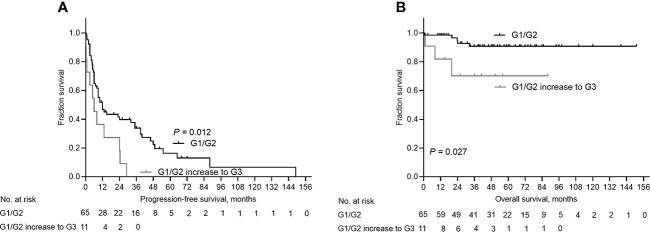
Next, we performed survival analyses to determine whether grade changes affect the patient prognosis. In the study cohort, the median follow-up time was 48.0 months [95% confidence interval (CI): 39.70-56.30], and the median PFS was 10.0 months (95% CI: 6.33-13.67). The Kaplan–Meier survival probability estimates of 1-year, 3-year, and 5-year progression-free survival were 41.7%, 18.4%, and 6.8%, respectively. The Kaplan–Meier survival probability estimates of 1-year, 3-year, and 5-year overall survival were 85.4%, 52.4%, and 28.2%, respectively ( Figure S1 ). Of importance, G1/G2 increase to G3 was significantly associated with shorter PFS and OS than stable G1/G2 (P=0.012 and P=0.027, respectively, Figure 3 ). G2 increase to G3 was associated with shorter PFS and OS, although it was not statistically significant (P=0.115, and P=0.064, respectively, Supplementary Figures S2A, B ). In addition, G2 decrease to G1 was associated with a longer OS, although the difference was not statistically significant (P=0.445, Supplementary Figure S2D ).
Figure 3.
Kaplan-Meier curves depicting progression-free survival (PFS) and overall survival (OS) for patients G1/G2 increase to G3. (A) Patients with G1/G2 increase to G3 had decreased PFS than patients with stable G1/2 (P=0.012). (B) Patients with G1/G2 increase to G3 had decreased OS than patients with stable G1/2 (P=0.027).
Univariable analyses demonstrated that biopsy, NAT, and a low-grade increase to high-grade were associated with a shorter PFS (HR=3.443, 95% CI: 1.809–6.552, P=0.000; HR=2.186, 95% CI: 1.284-3.720, P=0.004, and HR=2.281, 95% CI: 1.162-4.479, P=0.017, respectively, Table 3 ). Biopsy and a low-grade increase to high-grade were associated with shorter OS (HR=6.862, 95% CI: 1.969-23.916, P=0.002; and HR=4.418, 95% CI: 1.051-18.578, P=0.043, respectively). Multivariable survival analyses indicated that NAT and a low-grade increase to high-grade were independent prognostic factors for PFS (HR=2.756, 95% CI: 1.474-5.153, P=0.001; and HR=2.695, 95% CI: 1.273-5.706, P=0.010, respectively). In addition, a low-grade increase to high-grade was an independent prognostic factor for OS (HR= 4.565, 95% CI: 1.063-19.612, P=0.041).
Table 3. Univariable and multivariable analysis of factors for progression-free survival and overall survival in the study cohort.
| Factors | Progression-free Survival | Overall Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |||||
| Gender: male vs female | 0.787 (0.505-1.227) | 0.291 | 1.844 (0.563-6.040) | 0.312 | ||||||||
| Age: <51 vs ≥51 years | 0.745 (0.480-1.156) | 0.189 | 0.474 (0.146-1.543) | 0.215 | ||||||||
| Tumor size: <40 vs ≥ 40mm | 1.135 (0.720-1.790) | 0.584 | 0.785 (0.262-2.350) | 0.665 | ||||||||
| Tumor location: head vs neck, body and tail | 1.582 (0.967-2.590) | 0.068 | 1.058 (0.287-3.892) | 0.933 | ||||||||
| Functional: no vs yes | 0.408 (0.126-1.321) | 0.135 | 1.443 (0.185-11.283) | 0.726 | ||||||||
| Lymph node positive: no vs yes | 1.128 (0.692-1.838) | 0.628 | 1.892 (0.472-7.592) | 0.368 | ||||||||
| Perineural invasion: no vs yes | 1.103 (0.668-1.823) | 0.701 | 1.599 (0.376-6.798) | 0.525 | ||||||||
| Microvascular invasion: no vs yes | 0.738 (0.431-1.263) | 0.268 | 3.938 (0.483-32.072) | 0.200 | ||||||||
| CgA: negative vs positive | 0.511 (0.206-1.271) | 0.149 | 0.659 (0.084-5.158) | 0.691 | ||||||||
| Syn: negative vs positive | 0.351 (0.048-2.575) | 0.303 | 0.167 (0.021-1.320) | 0.090 | ||||||||
| DAXX: negative vs positive | 1.411 (0.332-6.007) | 0.641 | 23.321 (0-3.78E+10) | 0.771 | ||||||||
| ATRX: negative vs positive | 2.470 (0.705-8.652) | 0.157 | 31.539 (0-1.10E+7) | 0.596 | ||||||||
| SSTR: negative vs positive | 0.512 (0.180-1.456) | 0.210 | 22.359 (0-1.26E+10) | 0.762 | ||||||||
| Metastases site: liver vs nodal/mesenteric vs peritoneum/others | 0.821 (0.479-1.409) | 0.475 | 0.608 (0.096-3.864) | 0.598 | ||||||||
| Metastases type: synchronous vs metachronous | 0.816 (0.462-1.443) | 0.485 | 0.270 (0.034-2.125) | 0.214 | ||||||||
| Surgery: yes vs biopsy | 3.443 (1.809-6.552) | 0.000 | NA | NA | 6.862 (1.969-23.916) | 0.002 | 4.254 (0.804-22.507) | 0.088 | ||||
| Neoadjuvant treatment: no vs yes | 2.186 (1.284-3.720) | 0.004 | 2.756 (1.474-5.153) | 0.001 | 0.743 (0.151-3.669) | 0.716 | ||||||
| Grade changes: G1/G2 vs G1/G2 increase to G3 | 2.281 (1.162-4.479) | 0.017 | 2.695 (1.273-5.706) | 0.010 | 4.418 (1.051-18.578) | 0.043 | 4.565 (1.063-19.612) | 0.041 | ||||
HR, hazard ratio; CI, confidence interval; CgA, chromogranin; Syn, synaptophysin; DAXX, death domain associated protein; ATRX, alpha-thalassemia/mental retardation X-linked; SSTR, somatostatin receptor; NSE, neuron specific enolase; PROGRP, progastrin releasing peptide; TNM, tumor–node–metastasis; NA, not available; bold values indicate statistical significance.
Association between p53 expression and Ki67 index variation
To further explore the association between high-grade transition and TP53 mutation, we performed p53 immunohistochemistry staining. Four out of 27 patients (14.8%) showed different p53 expression pattern between primary tumors and liver metastases, which was correlated with higher Ki67 index variation (P=0.031, Supplementary Figure S5 )
Discussion
In this retrospective study, 648 patients with PanNENs were screened, and 103 patients with advanced PanNETs who had paired primary tumors and metastases were identified as the study cohort. We described the Ki67 index variation and tumor grade changes in PanNETs, explored the association between the clinical characteristics and grade changes, and determined whether the grade changes predicted the clinical prognosis. The findings of this study could help improve our understanding of the heterogeneity of PanNETs and contribute to clinical decision-making.
In this study, 48.5% of cases had Ki67 index variation, 23.3% displayed grade changes, and 17.5% showed a grade increase. Of note, 10.7% of cases had high-grade metastases compared to low-grade primary tumors. For the associations among clinical characteristics, Ki67 index variation, and grade changes, metachronous metastases presented with higher Ki67 index variation than synchronous metastases. Patients with early-stage disease showed a higher frequency of grade increase and G1/G2 increase to G3 than patients with advanced-stage disease, indicating that early-stage tumors had more time to evolve, which was consistent with previous observations that NETs dedifferentiated over time. Additionally, G1 increase to G3 only presented in patients with stage IV and synchronous metastases, indicating that advanced tumors had a worse evolution than less advanced tumors. In the study cohort, all patients had only one primary tumor except one patient with two primary tumors, and the Ki67 values of the two primary tumors were both 5%. Therefore, the Ki67 index heterogeneity among primary tumors was not analyzed.
Previous studies reported that 35.3%-63% of metastatic GEP-NENs showed higher Ki-67 index at LM than primary site (35–37), and 7.5%-39% presented a grade increase (20, 21, 38–40). As for longitudinal increase, about 58.6%-65.1% of NEN patients showed an increase in Ki67 index, and nearly 28% showed upgrade when progression (41–44). Shi et al. reported that nearly two-thirds of small intestinal NETs had grade increase in LM (45). As for PanNENs, several studies described that 62.5%-63.6% patients with PanNETs had an increased grade when the disease evolved over time (22, 46). Alexandraki et al. analyzed 264 PanNENs and showed that 15 patients (5.7%) developed an increase in Ki67 during disease course (31). However, this study also included patients with multiple endocrine neoplasia type 1, and did not provide information about the 264-patient cohort. Given the strong heterogeneity of NENs, the results obtained in relatively homogeneous tumors would be more powerful. Therefore, this work aimed to explore the heterogeneity between primary tumors and metastases in a homogeneous and large-scale patient cohort of PanNETs, mainly to characterize the grade changes and correlate the findings with patient prognosis, also providing real-life data from the clinical treatment.
The “transformed” NETs that would evolve to either NET G3 or NEC has aroused interest. Several studies investigated this phenomenon (47–51) and proposed that the boundary between NETs and NEC was blurred. In this study, we observed that NET G1/G2 could evolve to NET G3, but did not find the transition from NETs to NEC. The concept that well-differentiated NETs would develop to poor-differentiated NEC probably needed further investigation.
Additionally, a suspicion of a grade increase in metastatic sites compared to primary tumors might constitute a negative prognostic factor (52). Therefore, we performed survival analyses and found that high-grade metastases compared to low-grade primary tumors were significantly associated with decreased PFS and OS. Multivariable survival analyses indicated that a low-grade increase to high-grade was an independent prognostic factor for PFS and OS. Based on the above results, we concluded that PanNET patients with metastases, whether synchronous or metachronous, would have a grade increase in metastases compared to their primary tumors. Because of the longer clinical course of metachronous metastases, the probability of a grade increase was higher than among those with synchronous metastases. In addition, G1 increase to G3, a progression of two grades, but only in AJCC 8th stage IV tumors, suggesting that advanced tumors had a worse evolution than less advanced tumors.
Furthermore, Cox regression analysis showed that patients who received NAT had a shorter PFS, whereas there was no significant difference in OS. It should be noted that patients with high-risk factors, were routinely received preoperative NAT. Surgical resection was considered after multidisciplinary discussion if the tumors stabilized or regressed. It was obvious that patients with high-risk factors had a worse prognosis, but the results did not show any significant difference in OS compared to those without high-risk factors, indicating the potential effectiveness of preoperative NAT in patients with metastatic PanNETs.
Our results revealed the poor survival of patients with a high-grade increase in metastases, indicating the necessity of close follow-up and early intervention. Those patients with high-grade increase warrant different therapeutic strategies. In addition, this study also supported the use of multiple point puncture and accurate pathological grading for PanNET patients in metastatic lesions or when disease progression.
The possible underlying mechanism of Ki67 index heterogeneity and high-grade transition might due to the polyclonal tumor origin of NENs, with different mutational events, microenvironmental context, and/or epigenetic divergence between and within tumors (45). During the progression course, the selection of subclonal populations with a higher proliferative index (20), and de-reprograming would happen. In this study, higher Ki67 index variation was correlated with changes in p53 expression pattern, implying the TP53 mutation was probably involved in the high-grade progression. Additionally, treatment effects and therapy resistance might also contribute to the transition towards higher grade (44). The above hypotheses warrant further research.
Several limitations existed in the current study. First, all retrospective studies have inherent limitations. Second, the prognosis of most patients with PanNETs is good (53), but the median follow-up time of this study was relatively short. The follow-up data were available up until February 1, 2022, by which time only 13 cases (12.6%) had died. Those who had not died were considered right-censored, which might underestimate the overall survival time. Third, this study had selection bias of low to intermediate degrees due to the nonsignificant difference in survival between the study cohort and the bias control group. In addition, we did not explore the molecular mechanism of the clinical phenomenon that low-grade primary tumors would increase to high-grade metastases. To address this limitation, we are conducting another study to detect paired low-grade primary tumors and high-grade metastases using whole-exome sequencing to identify the mutant genes driving the grade increase of metastases and to investigate its molecular mechanism.
Overall, this study found that 17.5% of patients with metastatic PanNETs had a grade increase in metastases compared to their primary tumors. A high-grade increase in metastases was an unfavorable prognostic factor for PFS and OS, which could provide a useful reference for clinical decision-making.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by institutional research ethics committee of Fudan University Shanghai Cancer Center. The patients/participants provided their written informed consent to participate in this study.
Author contributions
W-HZ, JC, X-JY, X-WX and S-RJ conceived the original idea of the study. H-LG, W-SL, and YQ contributed to sample preparation and data collection. WH-Z, ZY, XL, and FW conducted all statistical analyses. WH-Z, Q-FZ, ZY, YZ, and X-MC contributed to the implementation of the research and interpretation of data. All authors discussed the results, helped prepare and shape the research and gave final approval of the manuscript to be published.
Funding
This work was jointly supported by the Rare Tumor Research Special Project of the National Natural Science Foundation of China (82141104), National Natural Science Foundation of China (U21A20374), Shanghai Municipal Science and Technology Major Project (21JC1401500), Scientific Innovation Project of Shanghai Education Committee (2019-01-07-00-07-E00057), Clinical Research Plan of Shanghai Hospital Development Center (SHDC2020CR1006A), Xuhui District Artificial Intelligence Medical Hospital Cooperation Project (2021–011), Shanghai Municipal Science and Technology Commission (20ZR1471100), National Natural Science Foundation of China (No. 82141129, 82173281, 82173282, 82172577, 82172948, 81972725, 81972250, and 81871950), Commission of Health and Family Planning (2018YQ06), and Shanghai Municipal Science and Technology Commission (19QA1402100).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.941210/full#supplementary-material
References
- 1. Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (Pnets): Incidence, prognosis and recent trend toward improved survival. Ann Oncol (2008) 19(10):1727–33. doi: 10.1093/annonc/mdn351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fraenkel M, Kim M, Faggiano A, de Herder WW, Valk GD, Knowledge N. Incidence of gastroenteropancreatic neuroendocrine tumours: A systematic review of the literature. Endocr Relat Cancer (2014) 21(3):R153–63. doi: 10.1530/ERC-13-0125 [DOI] [PubMed] [Google Scholar]
- 3. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the united states. JAMA Oncol (2017) 3(10):1335–42. doi: 10.1001/jamaoncol.2017.0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cives M, Strosberg JR. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin (2018) 68(6):471–87. doi: 10.3322/caac.21493 [DOI] [PubMed] [Google Scholar]
- 5. Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: The current incidence and staging based on the who and European neuroendocrine tumour society classification: An analysis based on prospectively collected parameters. Endocr Relat Cancer (2010) 17(4):909–18. doi: 10.1677/ERC-10-0152 [DOI] [PubMed] [Google Scholar]
- 6. Nigri G, Petrucciani N, Debs T, Mangogna LM, Crovetto A, Moschetta G, et al. Treatment options for pnet liver metastases: A systematic review. World J Surg Oncol (2018) 16(1):142. doi: 10.1186/s12957-018-1446-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yao JC, Eisner MP, Leary C, Dagohoy C, Phan A, Rashid A, et al. Population-based study of islet cell carcinoma. Ann Surg Oncol (2007) 14(12):3492–500. doi: 10.1245/s10434-007-9566-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rossi RE, Massironi S, Conte D, Peracchi M. Therapy for metastatic pancreatic neuroendocrine tumors. Ann Transl Med (2014) 2(1):8. doi: 10.3978/j.issn.2305-5839.2013.03.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Voi M, Brandt U, et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: Overall survival and circulating biomarkers from the randomized, phase iii radiant-3 study. J Clin Oncol (2016) 34(32):3906–13. doi: 10.1200/JCO.2016.68.0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faivre S, Niccoli P, Castellano D, Valle JW, Hammel P, Raoul JL, et al. Sunitinib in pancreatic neuroendocrine tumors: Updated progression-free survival and final overall survival from a phase iii randomized study. Ann Oncol (2017) 28(2):339–43. doi: 10.1093/annonc/mdw561 [DOI] [PubMed] [Google Scholar]
- 11. Ma ZY, Gong YF, Zhuang HK, Zhou ZX, Huang SZ, Zou YP, et al. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J Gastroenterol (2020) 26(19):2305–22. doi: 10.3748/wjg.v26.i19.2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW, et al. Long-term efficacy, survival, and safety of [(177)Lu-Dota(0),Tyr(3)]Octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res (2017) 23(16):4617–24. doi: 10.1158/1078-0432.CCR-16-2743 [DOI] [PubMed] [Google Scholar]
- 13. Kloppel G, La Rosa S. Ki67 labeling index: Assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch (2018) 472(3):341–9. doi: 10.1007/s00428-017-2258-0 [DOI] [PubMed] [Google Scholar]
- 14. Rindi G, Kloppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al. Tnm staging of foregut (Neuro)Endocrine tumors: A consensus proposal including a grading system. Virchows Arch (2006) 449(4):395–401. doi: 10.1007/s00428-006-0250-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rindi G, Wiedenmann B. Neuroendocrine neoplasms of the gut and pancreas: New insights. Nat Rev Endocrinol (2011) 8(1):54–64. doi: 10.1038/nrendo.2011.120 [DOI] [PubMed] [Google Scholar]
- 16. Khan MS, Luong TV, Watkins J, Toumpanakis C, Caplin ME, Meyer T. A comparison of ki-67 and mitotic count as prognostic markers for metastatic pancreatic and midgut neuroendocrine neoplasms. Br J Cancer (2013) 108(9):1838–45. doi: 10.1038/bjc.2013.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scoazec J-Y, Couvelard A, Reseau T. Classification of pancreatic neuroendocrine tumours: Changes made in the 2017 who classification of tumours of endocrine organs and perspectives for the future. ANNALES PATHOLOGIE (2017) 37(6):444–56. doi: 10.1016/j.annpat.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 18. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 who classification of tumours of the digestive system. Histopathology (2020) 76(2):182–8. doi: 10.1111/his.13975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oberg K, Knigge U, Kwekkeboom D, Perren A, Group EGW. Neuroendocrine gastro-Entero-Pancreatic tumors: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2012) 23 Suppl 7:vii124–30. doi: 10.1093/annonc/mds295 [DOI] [PubMed] [Google Scholar]
- 20. Grillo F, Albertelli M, Brisigotti MP, Borra T, Boschetti M, Fiocca R, et al. Grade increases in gastroenteropancreatic neuroendocrine tumor metastases compared to the primary tumor. NEUROENDOCRINOLOGY (2016) 103(5):452–9. doi: 10.1159/000439434 [DOI] [PubMed] [Google Scholar]
- 21. Keck KJ, Choi A, Maxwell JE, Li G, O'Dorisio TM, Breheny P, et al. Increased grade in neuroendocrine tumor metastases negatively impacts survival. Ann OF Surg Oncol (2017) 24(8):2206–12. doi: 10.1245/s10434-017-5899-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Botling J, Lamarca A, Bajic D, Norlen O, Lonngren V, Kjaer J, et al. High-grade progression confers poor survival in pancreatic neuroendocrine tumors. NEUROENDOCRINOLOGY (2020) 110(11-12):891–8. doi: 10.1159/000504392 [DOI] [PubMed] [Google Scholar]
- 23. Pavel M, Baudin E, Couvelard A, Krenning E, Oberg K, Steinmueller T, et al. Enets consensus guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. NEUROENDOCRINOLOGY (2012) 95(2):157–76. doi: 10.1159/000335597 [DOI] [PubMed] [Google Scholar]
- 24. Wang WQ, Liu L, Xu HX, Wu CT, Xiang JF, Xu J, et al. Infiltrating immune cells and gene mutations in pancreatic ductal adenocarcinoma. Br J Surg (2016) 103(9):1189–99. doi: 10.1002/bjs.10187 [DOI] [PubMed] [Google Scholar]
- 25. Zhang W-H, Wang W-Q, Han X, Gao H-L, Xu S-S, Li S, et al. Infiltrating pattern and prognostic value of tertiary lymphoid structures in resected non-functional pancreatic neuroendocrine tumors. J FOR IMMUNOTHERAPY OF Cancer (2020) 8(2):e001188. doi: 10.1136/jitc-2020-001188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang WH, Wang WQ, Gao HL, Xu SS, Li S, Li TJ, et al. Tumor-infiltrating neutrophils predict poor survival of non-functional pancreatic neuroendocrine tumor. J Clin Endocrinol Metab (2020) 105(7):dgaa196. doi: 10.1210/clinem/dgaa196 [DOI] [PubMed] [Google Scholar]
- 27. Wang WQ, Zhang WH, Gao HL, Huang D, Xu HX, Li S, et al. A novel risk factor panel predicts early recurrence in resected pancreatic neuroendocrine tumors. J Gastroenterol (2021) 56(4):395–405. doi: 10.1007/s00535-021-01777-0 [DOI] [PubMed] [Google Scholar]
- 28. Zhang WH, Gao HL, Liu WS, Qin Y, Ye Z, Lou X, et al. Supplementary material for the manuscript “A real-life treatment cohort of pancreatic neuroendocrine tumors: High-grade increase in metastases confers poor survival. (2002) figshare. doi: 10.6084/m9.figshare.19350545.v8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tanaka M, Shinozaki-Ushiku A, Kunita A, Yasunaga Y, Akamatsu N, Hasegawa K, et al. High-grade transformation of pancreatic neuroendocrine tumor associated with Tp53 mutations: A diagnostic pitfall mimicking neuroendocrine carcinoma. Pathol Int (2022). doi: 10.1111/pin.13252 [DOI] [PubMed] [Google Scholar]
- 30. Ali AS, Gronberg M, Federspiel B, Scoazec J-Y, Hjortland GO, Gronbaek H, et al. Expression of P53 protein in high-grade gastroenteropancreatic neuroendocrine carcinoma. PloS One (2017) 12(11):e0187667. doi: 10.1371/journal.pone.0187667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alexandraki KI, Kaltsatou M, Kyriakopoulos G, Mavroeidi V, Kostopoulou A, Atlan K, et al. Distinctive features of pancreatic neuroendocrine neoplasms exhibiting an increment in proliferative activity during the course of the disease. Endocrine (2021) 72(1):279–86. doi: 10.1007/s12020-020-02540-w [DOI] [PubMed] [Google Scholar]
- 32. Konukiewitz B, Schlitter AM, Jesinghaus M, Pfister D, Steiger K, Segler A, et al. Somatostatin receptor expression related to Tp53 and Rb1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20%. MODERN Pathol (2017) 30(4):587–98. doi: 10.1038/modpathol.2016.217 [DOI] [PubMed] [Google Scholar]
- 33. Perysinakis I, Aggeli C, Kaltsas G, Zografos GN. Neoadjuvant therapy for advanced pancreatic neuroendocrine tumors: An emerging treatment modality? Hormones (Athens) (2016) 15(1):15–22. doi: 10.14310/horm.2002.1636 [DOI] [PubMed] [Google Scholar]
- 34. Lania A, Ferrau F, Rubino M, Modica R, Colao A, Faggiano A. Neoadjuvant therapy for neuroendocrine neoplasms: Recent progresses and future approaches. Front Endocrinol (Lausanne) (2021) 12:651438. doi: 10.3389/fendo.2021.651438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shi H, Zhang Q, Han C, Zhen D, Lin R. Variability of the ki-67 proliferation index in gastroenteropancreatic neuroendocrine neoplasms - a single-center retrospective study. BMC ENDOCRINE Disord (2018) 18:51–7. doi: 10.1186/s12902-018-0274-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Furukawa T, Ozaka M, Takamatsu M, Takazawa Y, Inamura K, Inoue Y, et al. Ki-67 labeling index variability between surgically resected primary and metastatic hepatic lesions of gastroenteropancreatic neuroendocrine neoplasms. Int J OF Surg Pathol (2021) 29(5):475–81. doi: 10.1177/1066896921990715 [DOI] [PubMed] [Google Scholar]
- 37. Miller HC, Drymousis P, Flora R, Goldin R, Spalding D, Frilling A. Role of ki-67 proliferation index in the assessment of patients with neuroendocrine neoplasias regarding the stage of disease. World J OF Surg (2014) 38(6):1353–61. doi: 10.1007/s00268-014-2451-0 [DOI] [PubMed] [Google Scholar]
- 38. Dumars C, Foubert F, Touchefeu Y, Regenet N, Senellart H, Matysiak-Budnik T, et al. Can Pph3 be helpful to assess the discordant grade in primary and metastatic enteropancreatic neuroendocrine tumors? ENDOCRINE (2016) 53(2):395–401. doi: 10.1007/s12020-016-0944-3 [DOI] [PubMed] [Google Scholar]
- 39. Adesoye T, Daleo MA, Loeffler AG, Winslow ER, Weber SM, Cho CS. Discordance of histologic grade between primary and metastatic neuroendocrine carcinomas. Ann OF Surg Oncol (2015) 22:S817–S21. doi: 10.1245/s10434-015-4733-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zen Y, Heaton N. Elevated ki-67 labeling index in 'Synchronous liver metastases' of well differentiated enteropancreatic neuroendocrine tumor. Pathol Int (2013) 63(11):532–8. doi: 10.1111/pin.12108 [DOI] [PubMed] [Google Scholar]
- 41. Holmager P, Langer SW, Federspiel B, Willemoe GL, Garbyal RS, Melchior L, et al. Increase of ki-67 index and influence on mortality in patients with neuroendocrine neoplasms. J OF Neuroendocrinol (2021) 33(9):e13018. doi: 10.1111/jne.13018 [DOI] [PubMed] [Google Scholar]
- 42. Panzuto F, Cicchese N, Partelli S, Rinzivillo M, Capurso G, Merola E, et al. Impact of Ki67 re-assessmentat time of disease progression in patients with pancreatic neuroendocrine neoplasms. PloS One (2017) 12(6):e0179445. doi: 10.1371/journal.pone.0179445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cicchese N, Panzuto F, Rinzivillo M, Capurso G, Merola E, Pucci E, et al. Reassessment of proliferative activity at disease progression in neuroendocrine neoplasms. GASTROENTEROLOGY (2016) 150(4):S301–S. doi: 10.1016/S0016-5085(16)31054-X [DOI] [Google Scholar]
- 44. Singh S, Hallet J, Rowsell C, Law CHL. Variability of Ki67 labeling index in multiple neuroendocrine tumors specimens over the course of the disease. EJSO (2014) 40(11):1517–22. doi: 10.1016/j.ejso.2014.06.016 [DOI] [PubMed] [Google Scholar]
- 45. Shi CJ, Gonzalez RS, Zhao ZG, Koyama T, Cornish TC, Hande KR, et al. Liver metastases of small intestine neuroendocrine tumors ki-67 heterogeneity and world health organization grade discordance with primary tumors. Am J OF Clin Pathol (2015) 143(3):398–404. doi: 10.1309/AJCPQ55SKOCYFZHN [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Richards-Taylor S, Tilley C, Jaynes E, Hu H, Armstrong T, Pearce NW, et al. Clinically significant differences in ki-67 proliferation index between primary and metastases in resected pancreatic neuroendocrine tumors. PANCREAS (2017) 46(10):1354–8. doi: 10.1097/MPA.0000000000000933 [DOI] [PubMed] [Google Scholar]
- 47. Alcala N, Leblay N, Gabriel AAG, Mangiante L, Hervas D, Giffon T, et al. Integrative and comparative genomic analyses identify clinically relevant pulmonary carcinoid groups and unveil the supra-carcinoids. Nat Commun (2019) 10:3407–27. doi: 10.1038/s41467-019-11276-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dinter H, Bohnenberger H, Beck J, Bornemann-Kolatzki K, Schutz E, Kuffer S, et al. Molecular classification of neuroendocrine tumors of the thymus. J OF Thorac Oncol (2019) 14(8):1472–83. doi: 10.1016/j.jtho.2019.04.015 [DOI] [PubMed] [Google Scholar]
- 49. Pelosi G, Bianchi F, Dama E, Metovic J, Barella M, Sonzogni A, et al. A subset of Large cell neuroendocrine carcinomas in the gastroenteropancreatic tract may evolve from pre-existing well-differentiated neuroendocrine tumors. ENDOCRINE Pathol (2021) 32(3):396–407. doi: 10.1007/s12022-020-09659-6 [DOI] [PubMed] [Google Scholar]
- 50. Pelosi G, Bianchi F, Hofman P, Pattini L, Strobel P, Calabrese F, et al. Recent advances in the molecular landscape of lung neuroendocrine tumors. Expert Rev OF Mol DIAGNOSTICS (2019) 19(4):281–97. doi: 10.1080/14737159.2019.1595593 [DOI] [PubMed] [Google Scholar]
- 51. Tang LH, Untch BR, Reidy DL, O'Reilly E, Dhall D, Jih L, et al. Well-differentiated neuroendocrine tumors with a morphologically apparent high-grade component: A pathway distinct from poorly differentiated neuroendocrine carcinomas. Clin Cancer Res (2016) 22(4):1011–7. doi: 10.1158/1078-0432.CCR-15-0548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pelosi G, Bresaola E, Bogina G, Pasini F, Rodella S, Castelli P, et al. Endocrine tumors of the pancreas: Ki-67 immunoreactivity on paraffin sections is an independent predictor for malignancy: A comparative study with proliferating-cell nuclear antigen and progesterone receptor protein immunostaining, mitotic index, and other clinicopathologic variables. Hum Pathol (1996) 27(11):1124–34. doi: 10.1016/S0046-8177(96)90303-2 [DOI] [PubMed] [Google Scholar]
- 53. Partelli S, Inama M, Rinke A, Begum N, Valente R, Fendrich V, et al. Long-term outcomes of surgical management of pancreatic neuroendocrine tumors with synchronous liver metastases. Neuroendocrinology (2015) 102(1-2):68–76. doi: 10.1159/000431379 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.