Abstract
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver a scientific opinion on the conversion of calcium‐l‐methylfolate and (6S)‐5‐methyltetrahydrofolic acid glucosamine salt (collectively called 5‐MTHF hereafter) into dietary folate equivalents (DFE). Following a systematic review, the conclusions of the opinion are based on one intervention study in adults for intakes < 400 μg/day and three intervention studies in adults for intakes ≥ 400 μg/day. At intakes below 400 μg/day, folic acid (FA) is assumed to be linearly related to responses of biomarkers of intake and status and is an appropriate comparator for deriving a DFE conversion factor for 5‐MTHF. It is proposed to use the same factor as for folic acid for conversion of 5‐MTHF into DFE for intakes < 400 μg/day. As such intake levels are unlikely to be exceeded through fortified food consumption, the conversion factor of 1.7 relative to natural food folate (NF) could be applied to 5‐MTHF added to foods and to food supplements providing < 400 μg/day. At 400 μg/day, 5‐MTHF was found to be more bioavailable than folic acid and a conversion factor of 2 is proposed for this intake level and for higher intakes. The derived DFE equations are DFE = NF + 1.7 × FA + 1.7 × 5‐MTHF for fortified foods and food supplements providing intakes < 400 μg/day; and DFE = NF + 1.7 × FA + 2.0 × 5‐MTHF for food supplements providing intakes ≥ 400 μg/day. Although this assessment applies to calcium‐L‐methylfolate and 5‐MTHF glucosamine salt, it is considered that the influence of the cation on bioavailability is likely to be within the margin of error of the proposed DFE equations. Therefore, the proposed equations can also be applied to 5‐MTHF associated with other cations.
Keywords: 5‐MTHF glucosamine, CaLMF, bioavailability, fortified food, food supplements, food for specific groups, DFE
1. Introduction
1.1. Background
Annex II to Directive 2002/46/EC 1 lists the chemical substances that may be used as sources of vitamins and minerals in the manufacture of food supplements.
On 12 March 2015, Commission Regulation (EU) No 2015/414 was adopted to allow the use of (6S)‐5‐methyltetrahydrofolic acid, glucosamine salt (5‐MTHF‐glucosamine) as a source of folate in food supplements, following EFSA’s favourable opinion on (6S)‐5‐methyltetrahydrofolic acid, glucosamine salt as a source of folate added for nutritional purposes to food supplements and the bioavailability of folate from this source. 2
The annex to Regulation (EU) No 609/2013 3 establishes a Union list of substances that may be added for nutritional purposes to one or more categories of food covered by the scope of the regulation. According to the Union list, calcium‐L‐methylfolate (CaLMF) may be used as a source of folate in food for special medical purposes and total diet replacement for weight control.
Following requests from the Commission, EFSA adopted Scientific Opinions on CaLMF as a source of folate (i) in foods for particular nutritional uses, food supplements and foods intended for the general population on 28 October 2004, 4 and (ii) added for nutritional purposes to infant and follow‐on formula, baby food and processed cereal‐based food on 27 November 2019. 5
In the latter Scientific Opinion, EFSA concluded that CaLMF is a source from which folate is bioavailable and that CaLMF is safe under the proposed uses and use levels for infants and young children. It was further noted that the bioavailability of CaLMF is expected to be comparable to that of folic acid in infants and young children.
Delegated Regulations 6 , 7 , 8 adopted under the scope of Regulation (EU) No 609/2013 as well as Commission Directive 2006/125/EC 9 lay down specific requirements for the folate content of specific categories of food. In some cases, 2 , 3 , 4 such compositional requirements are expressed in dietary folate equivalent (DFE) for which a legal definition is provided for by the mentioned pieces of legislation. 10 Such legal definition, however, does not provide for a conversion factor that would allow to convert the amount of CaLMF into DFE. Concerns have been raised by some Member States that the absence of such a conversion factor might cause difficulties for the national competent authorities in enforcing compliance with the mentioned requirements on folate.
In addition, both Regulation (EU) No 1169/2011 11 and Directive 2002/46/EC foresee that the information on vitamins and minerals in a product shall be expressed as a percentage of the daily reference intakes. Annex XIII of Regulation (EU) No 1169/2011 lists these daily reference intakes, including that for folic acid, without providing for a conversion factor that would allow to convert the amount of CaLMF into DFE.
1.2. Terms of reference as provided by the requestor
In accordance with Article 29 of Regulation (EC) No 178/2002, the European Commission asks the EFSA to assess the extent to which folate is bioavailable from CaLMF and 5‐MTHF‐glucosamine, as well as to derive a conversion factor that allows to convert absolute amounts in μg of these nutrient sources into μg of DFE.
1.3. Interpretation of the terms of reference
The Panel understands that it is expected:
-
–
to provide an assessment of to which extent folate is bioavailable from CaLMF and 5‐MTHF‐glucosamine, considering all food categories and population groups (e.g. infants, children, adults including pregnant or lactating women; healthy subjects, patients with a disease);
-
–
to derive a conversion factor that allows to convert absolute amounts of these two nutrient sources in μg into μg of DFE.
Aspects such as the review/update of the conversion factor of μg folic acid to DFE (relative to food folate (NF)) set by the Institute of Medicine (IOM, 1998); the review of the possible metabolic/beneficial effect(s) of oral consumption of CaLMF or 5‐MTHF‐glucosamine; the update of the assessment of the safety of their consumption, the exposure assessment of CaLMF, of 5‐MTHF‐glucosamine or of all forms of folate in general in the European population are outside the scope of this mandate.
1.4. Previous assessments
Institute of Medicine (IOM, 1998)
DFE have been defined by IOM (1998) in order to take into account the difference in absorption efficiency of folic acid (i.e. synthetic) and NF. The values used to derive the conversion factor for folic acid to DFE were mainly based on two studies in humans (Sauberlich et al., 1987; Pfeiffer et al., 1997). Considering that (i) folic acid consumed under fasting conditions is almost 100% bioavailable, (ii) folic acid taken with food is 85% bioavailable compared with folic acid consumed without food and (iii) NF is 50% bioavailable compared with folic acid ingested without a meal, the IOM proposed that DFE is defined as follows:
1 μg DFE = 1 μg NF = 0.6 μg folic acid 12 from fortified food or as a supplement consumed with food = 0.5 μg of a folic acid supplement taken on an empty stomach.
EFSA NDA Panel (2014)
Dietary reference values (DRVs) for folate were set by the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) (EFSA NDA Panel, 2014). For adults, an average requirement (AR) of 250 μg DFE/day and a population reference intake (PRI) of 330 μg DFE/day were set. DRVs for other population groups include an adequate intake (AI) of 80 μg DFE/day for infants (7–11 months), PRIs for children ranging from 120 (1–3 years) to 330 μg DFE/day (15–17 years), an AI for pregnant women of 600 μg DFE/day and a PRI of 500 μg DFE/day for lactating women.
This previous opinion also provided an overview of data on folate chemistry, analytical methods, biochemical functions, bioavailability, transport in blood, storage, biomarkers and interaction with other nutrients or effects of genotypes. These considerations are taken into account in the present assessment. Additionally, in the assessment of the Scientific Committee on Food (SCF) on the tolerable upper intake level (UL) of folic acid (SCF, 2000), a lowest observed adverse effect level (LOAEL) of 5 mg/day and an UL of 1 mg/day of folic acid for adults were set based on safety concerns regarding its high intake by cobalamin‐deficient individuals.
EFSA ANS Panel (2013)
The EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS) 13 assessed (5‐MTHF‐glucosamine and proposed it as an alternative source of folate to be used in food supplements up to 1.8 mg/day (i.e. 1 mg 5‐MTHF and 0.8 mg glucosamine).
With respect to bioavailability, the ANS Panel concluded that the bioavailability of the two forms of 5‐MTHF, 5‐MTHF‐glucosamine and CaLMF is comparable after oral exposure in humans (EFSA ANS Panel, 2013).
EFSA AFC Panel (2004) and EFSA NDA Panel (2020)
The EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) and the EFSA NDA Panel assessed the safety of CaLMF for addition to foods. With respect to bioavailability, the AFC Panel concluded that in aqueous media, CaLMF dissociates readily and completely into Ca and L‐5‐MTHF ions and that its bioavailability is similar or even slightly higher than that of folic acid (EFSA AFC Panel, 2004), while the NDA Panel confirmed that CaLMF is a source from which folate is bioavailable (EFSA NDA Panel, 2020).
1.5. Regulatory context
Delegated Regulations adopted under the scope of Regulation (EU) No 609/2013, as well as Commission Directive 2006/125/EC, lay down specific requirements for the folate content of specific categories of food. For the folate content in both infant and follow‐on formula, the compositional requirements of Annexes I and II, respectively, of Delegated Regulation (EU) 2016/127 are 3.6–11.4 μg DFE per 100 kJ (i.e. 15–47.6 μg DFE per 100 kcal). For both processed cereal‐based foods for infants and young children, as well as baby foods for infants and young children, the maximum content for the product ready for use (Annexes I and II, respectively, of Commission Directive 2006/125/EC) is 12 μg folic acid per 100 kJ (i.e. 50 μg folic acid per 100 kcal). Total diet replacement for weight control products shall provide at least the following amount of folate per day: 330 μg DFE, laid down in Commission Delegated Regulation (EU) 2017/1798. 8 For food for special medical purposes, the folate content range (in DFE) for products developed for infants indicated in Table 1 of Annex 1 of Commission Delegated Regulation (EU) 2016/128 is in line with Delegated Regulation (EU) 2016/127, and the folic acid range indicated in Table 2 (products other than those developed for infants) of that Annex is 2.5–12.5 μg/100 kJ, i.e. 10–50 μg/100 kcal.
2. Data and methodologies
2.1. Data
This Scientific Opinion is based on data retrieved through dedicated systematic literature searches conducted by EFSA's information specialist, initially in four databases, i.e. Embase, PubMed, Scifinder‐n and Europe PMC on 30 September 2020. For better coverage, an additional search was made in two other databases (protocol amendment 1), i.e. CAB Abstracts and Scopus, on 14 January 2021.
The searches were designed to retrieve publications reporting on studies on:
-
–
humans (clinical trials on healthy subjects or subjects with a disease, either repeated dose or acute studies),
-
–
animals, i.e. non‐ruminant mammals (e.g. rats, mice, pigs, dogs, cats, guinea pigs, hamsters, primates, rabbits) and birds (e.g. hens),
-
–
in vitro gastrointestinal systems.
The searches focused on comparative bioavailability assessment, i.e. oral consumption of CaLMF and/or 5‐MTHF glucosamine (with 5‐MTHF in the (6S,αS) configuration) versus NF or folic acid. However, studies' retrieval was not limited to these two 5‐MTHF forms but considered 5‐MTHF in the (6S,αS) configuration independent on the cation present in the salt.
The searches were limited to EU languages and conducted without applying limits to the date of publication. The database searches were complemented by snowballing in the retrieved relevant publications and in reviews or statements from competent authorities or patents also found in the search.
The title and abstract screenings were carried out in parallel by two reviewers in Distiller SR®. The artificial intelligence (AI) module of Distiller SR® was used also for screening some of the references as a second ‘reviewer’. Conflicts between reviewers, including the AI reviewer, were solved by discussion among reviewers.
The eligibility criteria of the searches are reported in the protocol as Appendix A of this Scientific Opinion. The search strings are available in Appendix B.
The PRISMA flow chart is included in Appendix C. Briefly, a total of 4,559 titles and abstracts were screened, of which 39 publications were identified as pertinent after full‐text screening of 76 references. From snowballing, three additional articles were added while during the data extraction step, four studies were excluded (see PRISMA flow chart in Appendix C for reasons of exclusion). The total number of studies included in the review is 38.
Data extraction of aggregated data was performed in prespecified forms in Microsoft Excel® by an external contractor according to the protocol (Appendix A). The characteristics of the studies upon which the conclusions are based are presented in Appendix E.
Authors of 33 papers were contacted. Nine were contacted with a request to provide individual participant data, in particular to assess the influence of time on the results reported (protocol amendment 2). Two authors provided such data, one for a study performed in exclusively formula‐fed infants (Troesch et al., 2019) and one for a repeated dose study in adults (Green et al., 2013). The remaining requests aimed at gathering missing information.
The Panel also took note of several previous EFSA opinions and reports from other scientific bodies (see Section 1.4), from which information was used for the present assessment on the conversion of CaLMF and 5‐MTHF glucosamine into DFE.
To provide the opportunity for stakeholders and other interested parties to submit studies relevant for this mandate, EFSA released a call for data from 9 July to 4 October 2021. Information on this can be found in Annex A.
2.2. Methodologies
For this scientific assessment, a protocol (Appendix A) has been developed in line with existing methodology (EFSA, 2020).
The present assessment is in line with the principles of the EFSA guidance on bioavailability (EFSA ANS Panel, 2018), i.e. comparative bioavailability assessment and adaptation of the methodological choices for such an assessment on a case‐by‐case basis.
Appraisal of studies was performed according to the protocol using the risk of bias (RoB) tool of the Office of Health Assessment and Translation (OHAT) of the US National Toxicology Program (NTP) Approach for Systematic Reviews (NTP, 2019) (Appendix D).
Data analysis of the individual and of the aggregated data was undertaken using R version 4.0.3 and RStudio version 1.3.1093. The statistical report is provided in Annex B.
In line with EFSA's policy on openness and transparency, and for EFSA to receive comments from the scientific community and stakeholders, the draft Scientific Opinion was released for public consultation from 11 May 2022 to 8 June 2022. 14 The outcome of the public consultation is described in a technical report published as Annex C to this Scientific Opinion.
2.3. Protocol amendments
-
1)
Systematic literature searches were initially conducted by EFSA's information specialist in four databases, i.e. in Embase, Pubmed, Scifinder‐n and Europe PMC. For better coverage, an additional search was conducted in two other databases, i.e. CAB Abstracts and Scopus.
-
2)
Initial data analysis was foreseen on aggregated data only. Authors were also contacted to invite them to share individual data from their published repeated dose studies in humans.
-
3)
Data were analysed separately for adults and infants, while such a separation was not initially foreseen. The data on infants (one study for which individual data were available to EFSA) were subsequently excluded because of an error in the provided data set.
3. Assessment
3.1. Identity of folate, CaLMF and 5‐MTHF glucosamine
Folate is a generic term used for a group of compounds with a core structure consisting of a pterin moiety linked through a methylene bridge to p‐aminobenzoic acid, to which one or more glutamate residues are bound by peptide bonds. The pterin moiety can be substituted at the N‐5 or N‐10 position by different one‐carbon units. NF are reduced vitamers which are usually polyglutamates containing five to seven glutamate residues. NF are unstable (EFSA NDA Panel, 2014) (Figure 1).
Figure 1.
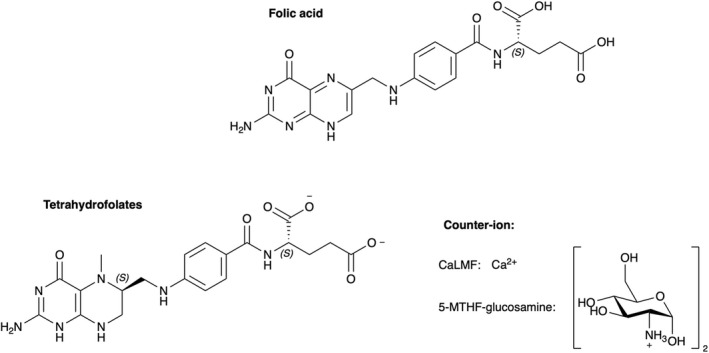
- glucosamine5‐MTHF‐glucosamine: (6S)‐5‐methyltetrahydrofolic acid glucosamine salt, Ca: calcium.
Several synthetic forms of folates are available. The key information on the chemistry of folic acid and on the two salts of 5‐MTHF, i.e. 5‐MTHF‐glucosamine and CaLMF, are displayed in Table 1, based on two Scientific Opinions by EFSA ANS Panel and the EFSA NDA Panel (EFSA ANS Panel, 2013; EFSA NDA Panel, 2014). It is noted that 5‐MTHF has two chiral carbon atoms, i.e. the C‐atom in position 6 of the pteroyl moiety and the α‐C atom in the glutamic acid moiety. The major natural form of the reduced folates is the (6S,αS) diastereoisomer, which has a greater biological activity than the (6R,αS) isomer (EFSA ANS Panel, 2013).
Table 1.
Identity of folic acid, 5‐MTHF‐glucosamine and CaLMF
| Folic acid | 5‐MTHF‐glucosamine | CaLMF | |
|---|---|---|---|
| Synonyms | Pteroylmonoglutamic acid (PGA) | (6S)‐L‐5‐methyltetrahydrofolic acid, glucosamine salt; (6S)‐5‐methylfolate, glucosamine salt |
N5‐Methyl‐tetrahydrofolic acid, calcium salt; 5‐Methyltetrahydropteroylglutamate, calcium salt |
| Chemical name (IUPAC) | L‐Glutamic acid, N‐[4‐[[(2‐amino‐3,4‐dihydro‐4‐oxo‐6‐pteridinyl)methyl]amino]benzoyl]‐ | L‐Glutamic acid, N‐[4‐[[[(6S)‐2‐amino‐3,4,5,6,7,8‐hexahydro‐5‐methyl‐4‐oxo‐6‐pteridinyl]methyl]amino]benzoyl]‐, compound with 2‐amino‐2‐deoxy‐D‐glucose (1:2) | L‐Glutamic acid, N‐[4‐[[[(6S)‐2‐amino‐3,4,5,6,7,8‐hexahydro‐5‐methyl‐4‐oxo‐6‐pteridinyl]methyl]amino]benzoyl]‐, calcium salt |
| Molecular formula | C19H19N7O6 | C20H25N7O6.2C6H13NO5 | C20H25N7O6.Ca |
| Molecular weight | 441.4 g/mol | 817.80 g/mol | 497.5 g/mol |
| CAS Registry number | 59–30‐3 | 1181972–37‐1 | 129025–21‐4 |
The production process of 5‐MTHF‐glucosamine and CaLMF has been described elsewhere (EFSA AFC Panel, 2004; EFSA ANS Panel, 2013).
3.2. Analytical methodology
Analytical methods used for the assessment of total folate and individual folate derivatives in plasma/serum, whole blood, tissues and food have been described in the Scientific Opinion on DRVs for folate (EFSA NDA Panel, 2014).
Protein‐binding assays are the most commonly used folate assays in clinical laboratories. However, they have the disadvantage that the binding protein has a different affinity to various folate derivatives (EFSA NDA Panel, 2014). Thus, they are suitable only for analysis of samples containing predominantly a single folate derivative such as serum or plasma.
The microbiological assay using the cryopreserved chloramphenicol‐resistant L. casei subsp. rhamnosus (ATCC 7469) is considered a sensitive, robust and accurate method for the measurement of total folate in biological samples (EFSA NDA Panel, 2014).
The chromatographic assays and especially the isotope dilution–liquid chromatography–tandem mass spectrometry (ID/LC/MS/MS) methods have a high sensitivity and specificity and are able to detect individual folate derivatives at very low concentrations (EFSA NDA Panel, 2014).
The Panel notes that considerable analytical variability has been shown between different laboratories using similar assays and between various methods analysing common sets of serum/plasma and RBC folate samples.
Good agreement between LC/MS/MS methods and the microbiological assay has been reported, but considerable differences have been found between LC/MS/MS methods and some protein‐binding assays (EFSA NDA Panel, 2014).
3.3. Absorption, distribution, metabolism and excretion (ADME) of various folate forms in healthy individuals
The involvement of folate in one‐carbon metabolism is summarised in Figure 2. Interactions of folate with cobalamin (vitamin B12) and vitamin B6 were discussed by the Panel in the previous Scientific Opinion on DRVs for folate (EFSA NDA Panel, 2014) (Figure 2).
Figure 2.
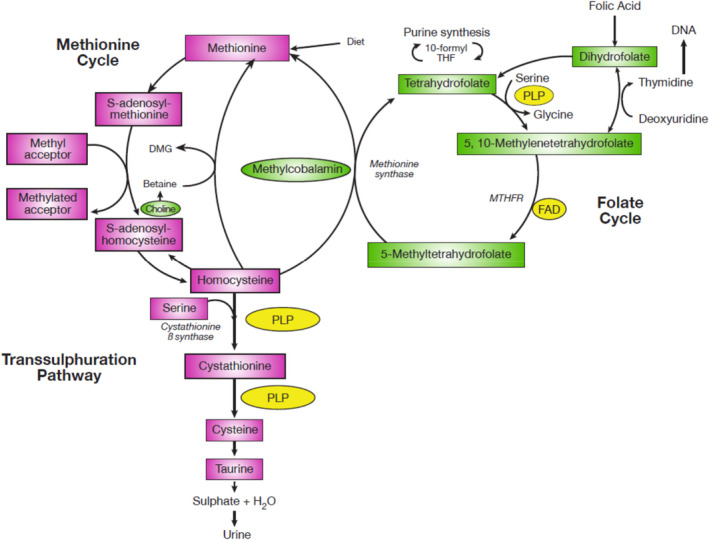
- DMG: dimethylglycine, DNA: deoxyribonucleic acid, FAD: flavin adenine dinucleotide, MTHFR: methylene tetrahydrofolate reductase, PLP: pyroxidal 5′‐phosphate, THF: tetrahydrofolate.
The intestinal absorption of folate follows two different pathways: One is active and saturable (primarily in the jejunum) and the second one passive and unsaturable (primarily in the ileum). Some folate is absorbed also in the large intestine. Upon ingestion, polyglutamated folate forms (i.e. NF) are subjected to hydrolysis to their monoglutamates by γ‐glutamyl carboxypeptidase (also called folate conjugase, γ‐glutamyl hydrolase or glutamate carboxypeptidase II). Monoglutamates are then transported across the brush border membrane by reduced folate carrier (RFC) and proton‐coupled folate transporter (PCFT); however, PCFT has a more important role than RFC in this process due to the slightly acidic environment in the proximal jejunum. In the intestinal cells, they are usually reduced and methylated and exported into the blood stream as 5‐MTHF, again through a carrier‐mediated process involving multidrug resistance protein 3 (MRP3) (McNulty and Pentieva, 2010; EFSA NDA Panel, 2014).
While the affinity of the folate carrier in the brush border membrane is similar for NF and folic acid, the capability of the body to convert folic acid in the intestinal cells into reduced folate derivatives with the involvement of dihydrofolate reductase is limited. When the capacity for reduction and methylation of folic acid is exceeded, unmetabolised folic acid may appear in the blood stream. There is also evidence that folic acid enters the portal vein unchanged, with reduction and methylation taking place only once it reaches the liver (Wright et al., 2003; EFSA NDA Panel, 2014; Patanwala et al., 2014).
Data discussed in previous EFSA assessments suggested that CaLMF and 5‐MTHF glucosamine readily dissociate in the gastrointestinal tract (EFSA AFC Panel, 2004; EFSA ANS Panel, 2013; EFSA NDA Panel, 2014). The difference between ‘added’ and ‘naturally present’ 5‐MTHF is not only that the naturally present 5‐MTHF is in a polyglutamate form but also that it is an integral part of the food and is bound by strong covalent bonds to macromolecules (proteins and carbohydrates). By this bonding, it is trapped in the food matrix and requires enzymatic digestion by protease and amylase in order to be released for absorption (Gregory 3rd, 1989). In contrast, 5‐MTHF added to food is in a monoglutamate form and although interactions have been observed with the food matrix or binding biomolecules (e.g. folate‐binding proteins), the bioaccessibility is less dependent on the composition of the food, which renders 5‐MTHF added to food more readily available for absorption than NF (Arkbåge et al., 2003; Ringling and Rychlik, 2017).
Folate in the human body is mainly present as 5‐MTHF monoglutamate in circulation and stored as folate polyglutamates in tissues, predominantly in the liver (EFSA NDA Panel, 2014).
Several polymorphisms have been described that lead to impaired folate metabolism, affecting biomarkers of folate status. The 677C → T polymorphism of the gene encoding methylene tetrahydrofolate reductase (MTHFR) has been reported to be the polymorphism with the highest impact, in particular, when folate intakes are low. Homozygosity for the T allele is associated with an up to 70% lower MTHFR enzyme activity, leading to around 20–25% lower serum folate and higher plasma total homocysteine concentrations compared with the 677CC genotype. Its prevalence is up to 12% in Northern European and up to 24% in Southern European populations (EFSA NDA Panel, 2014). Conditions that may also influence folate metabolism are gastrointestinal diseases (e.g. Crohn's disease), some haematological conditions (Bailey et al., 2015) as well as various liver diseases (Maruyama et al., 2001).
3.4. Bioavailability
Bioavailability of a nutrient can be defined as the availability of the nutrient from a specific nutrient source to be used by the body (EFSA ANS Panel, 2018). As such, bioavailability can be defined as the fraction of an ingested nutrient which is absorbed from the gastrointestinal tract and utilised for normal physiological function or storage (EFSA NDA Panel, 2010). Bioavailability is considered to be the result of the following processes: digestibility and solubilisation of the nutrient in the gastrointestinal tract; absorption of the nutrient by the intestinal cells and transport into the circulation; metabolism; excretion and elimination; and incorporation into the functional entity or target (Alegría et al., 2015). Host‐related factors may play a role, such as stomach acidity, single‐nucleotide polymorphisms related to uptake and transport as well as nutrient status, but food/diet composition and food preparation are generally the main determinants of nutrient bioavailability (EFSA NDA Panel, 2010). Bioavailability is assessed by in vivo studies and it requires a valid biomarker to be employed. In human studies, relative bioavailability can be assessed by comparing the area under the plasma concentration time curve (AUC) of the biomarker resulting from administration of the different forms (sources) of the nutrient (EFSA ANS Panel, 2018). It can also be assessed in repeated‐dose studies of sufficient duration using reliable biomarkers of status or intake.
Bioaccessibility of a nutrient refers to the amount of the compound that is released from the food matrix and is considered to be available for absorption through the gut wall (Minekus et al., 2014). This is particularly influenced by the chemical form in which the nutrient is present and the surrounding food matrix. Bioaccessibility can be assessed by in vitro methods simulating human oral and gastrointestinal digestion (Brodkorb et al., 2019).
3.5. Influence of the cation associated with 5‐MTHF on bioavailability
In the context of the call for data published in the framework of the present assessment (see Section 2.1), a report of an unpublished study (No authors listed, 2010, unpublished) was submitted in which the bioavailability of CaLMF and 5‐MTFH glucosamine was compared. This study had already been discussed by the ANS Panel in the Scientific Opinion published in 2013 (EFSA ANS Panel, 2013). This study was a randomised cross‐over trial in 24 human male and female volunteers aged 18–55 years, with a wash‐out period of 7 days. It compared the bioavailability of folate from 5‐MTHF‐glucosamine and from CaLMF after a single oral exposure (400 μg as free 5‐MTHF, both with co‐administration of 400 μg of folic acid). Serum folate concentrations were measured up to 12 h after each intervention by a protein‐binding assay. There were no significant differences in the serum concentrations of total folate between groups. After 5‐MTHF‐glucosamine administration, titre‐normalised area under the curve (AUCt) (mean ± SD) was 549 ± 143 nmol/L/h, compared to 501 ± 151 nmol/L/h after CaLMF administration in a post hoc analysis. The Panel notes that this study indicates no biologically relevant difference in bioavailability of CaLMF and 5‐MTFH glucosamine.
The Panel considers that the nature of the cation associated with 5‐MTHF is likely not to greatly affect its bioavailability even though differences in solubility may exist, depending on the cation.
3.6. Biomarkers of folate intake and status in humans
Biomarkers of folate intake and status in humans have been discussed in the previously published Scientific Opinion on DRVs for folate (EFSA NDA Panel, 2014) and elsewhere (Bailey et al., 2015).
Red blood cell folate concentration
RBC folate is the most reliable biomarker of folate status and reflects long‐term dietary intake. As folate is incorporated into RBCs only during their maturation in the bone marrow and folate concentration remains stable throughout the 120‐day lifespan of the cells, this biomarker responds only slowly to changes in folate intake (EFSA NDA Panel, 2014).
Serum/plasma folate concentration
Serum/plasma total folate (PTF) concentration is a sensitive marker of recent dietary intake. However, if used for the assessment of folate status, multiple measurements of serum/plasma folate would be needed taken over a period of several weeks or a single measurement combined with other biomarkers of folate status (EFSA NDA Panel, 2014).
Plasma total homocysteine concentration
Plasma total homocysteine (tHcy) concentration is a sensitive biomarker of folate status and function. However, it is not specific since it is influenced by various other factors (EFSA NDA Panel, 2014).
Other biomarkers of folate status or intake and folate adequacy
Neither urinary folate concentrations nor mean RBC volume is useful biomarkers of folate intake and/or status (EFSA NDA Panel, 2014).
Folate adequacy has been defined as RBC folate ≥ 340 nmol/L and PTF ≥ 10 nmol/L. These cut‐offs have been derived from data from the third National Health and Nutrition Examination Survey (NHANES) in the United States that showed a levelling off of plasma tHcy at these concentrations (Selhub et al., 2008; EFSA NDA Panel, 2014).
3.7. Comparative bioavailability of 5‐MTHF in adults
3.7.1. Repeated dose studies in adults comparing 5‐MTHF with folic acid
A total of 20 publications on 16 parallel human repeated‐dose intervention studies in adults were retrieved through the systematic search (Bostom et al., 2000; Venn et al., 2002, 2003; Lamers et al., 2004, 2006; de Meer et al., 2005; Houghton et al., 2006, 2009; Pietrzik et al., 2007; Akoglu et al., 2008; Khandanpour et al., 2009; Wright et al., 2010; Diefenbach et al., 2013; Green et al., 2013; Zappacosta et al., 2013; Hekmatdoost et al., 2015; Bailey and Ayling, 2018; Henderson et al., 2018; Sicińska et al., 2018; Bayes et al., 2019). Three of these studies were conducted in diseased individuals (Bostom et al., 2000; Akoglu et al., 2008; Khandanpour et al., 2009). One study reported in two publications (Houghton et al., 2006, 2009) was conducted in lactating women and one in prepregnant and pregnant women (Hekmatdoost et al., 2015). Most of these studies investigated the comparative bioavailability of 5‐MTHF vs. folic acid only, based on the responses of the biomarkers listed in Table 2. Only two (Wright et al., 2010; Zappacosta et al., 2013) compared the bioavailability of 5‐MTHF with NF. All studies were performed using CaLMF as 5‐MTHF form, except for three studies for which the form was not reported and no information was provided by the authors (Akoglu et al., 2008; Khandanpour et al., 2009; Hekmatdoost et al., 2015). The study by Khandanpour et al. (2009) was performed in the United Kingdom at a time when only the CaLMF was authorised for use in food supplements.
Table 2.
Potentially relevant repeated dose studies in humans retrieved through the systematic search
| Biomarker | No of studies | References |
|---|---|---|
| RBC folate | 8 | (Venn et al., 2002; Venn et al., 2003; Houghton et al., 2006; Lamers et al., 2006; Pietrzik et al., 2007; Khandanpour et al., 2009; Wright et al., 2010; Diefenbach et al., 2013; Green et al., 2013; Henderson et al., 2018) |
| PTF | 13 | (Venn et al., 2002; Venn et al., 2003; Lamers et al., 2004; de Meer et al., 2005; Houghton et al., 2006; Khandanpour et al., 2009; Wright et al., 2010; Diefenbach et al., 2013; Green et al., 2013; Hekmatdoost et al., 2015; Bailey and Ayling, 2018; Henderson et al., 2018; Sicińska et al., 2018; Bayes et al., 2019) |
| tHcy | 12 | (Bostom et al., 2000; Venn et al., 2003; Lamers et al., 2004; de Meer et al., 2005; Akoglu et al., 2008; Houghton et al., 2009; Khandanpour et al., 2009; Wright et al., 2010; Diefenbach et al., 2013; Hekmatdoost et al., 2015;Henderson et al., 2018 ; Sicińska et al., 2018) |
| Serum/plasma 5‐MTHF | 2 | (de Meer et al., 2005; Bailey and Ayling, 2018) |
| Serum/plasma vitamin B12 | 2 | (Henderson et al., 2018; Sicińska et al., 2018) |
| Plasma total cysteine | 1 | (Henderson et al., 2018) |
| Plasma tetrahydrofolate | 1 | (Diefenbach et al., 2013) |
| Plasma methionine | 1 | (Henderson et al., 2018) |
| Plasma choline | 1 | (Henderson et al., 2018) |
| Plasma betaine | 1 | (Henderson et al., 2018) |
| Plasma 5,10‐methenyl‐tetrahydrofolate | 1 | (Diefenbach et al., 2013) |
5‐MTHF: 5‐methyltetrahydrofolate; PTF: plasma total folate; RBC: red blood cell; tHCy: total homocysteine.
In the study by Zappacosta et al. (2013), the unit of measurement for RBC folate concentrations was missing. Upon request from EFSA, one of the authors of the study indicated that RBC folate concentrations were reported in nmol/L. Based on this information, the study would have been conducted in a population with severe folate deficiency. In this light, the results on RBC folate were inconsistent with those reported for total homocysteine, and in addition, they were outliers as compared with the results of the other included studies. Owing to the uncertainties that are associated with this study, the Panel decided not to consider this study further in the assessment.
In the repeated dose studies, a number of potentially relevant biomarkers (as laid down in the protocol developed for the current opinion) were investigated, as outlined in Table 2. However, only the assessment of data on RBC folate concentrations, PTF concentrations and tHcy concentrations was further pursued in line with the conclusions of the Panel in its Scientific Opinion on DRVs for folate (EFSA NDA Panel, 2014) on what constitute the most reliable biomarkers of folate intake or status.
Regarding plasma tHcy, when data were plotted, a decline of tHcy concentrations following 5‐MTHF or folic acid supplementation was observed without an obvious trend between the biomarker results and the dose of the folate form or the study duration. Therefore, data on tHcy could not be used to quantitatively assess a dose–response relationship and are not further described in the Scientific Opinion. Further details are found in the statistical report in Annex B. Consequently, studies that did not provide sufficient details to be included in the analysis of tHCy in Annex B will also not be addressed further.
Quantitative analyses were carried out on RBC folate concentrations and PTF concentrations in healthy adults. These are described in general terms in the following sections. A complete description can be found in the statistical report in Annex B.
Studies performed in diseased individuals are in the following addressed separately from studies in healthy adults.
3.7.1.1. RBC folate concentrations in healthy adults (5MTHF vs. folic acid)
Seven randomised repeated dose studies (reported in 11 publications) in healthy adults were retrieved through the systematic search that investigated the effect of consuming either 5‐MTHF or folic acid on RBC folate concentrations. Three studies (six publications) were classified as having a low RoB (Tier 1) (Venn et al., 2002, 2003; Lamers et al., 2004, 2006; Pietrzik et al., 2007; Green et al., 2013) and four studies (five publications) were of intermediate RoB (Tier 2) (Houghton et al., 2006, 2009; Wright et al., 2010; Diefenbach et al., 2013; Henderson et al., 2018). The publications by Lamers et al. (2004), Lamers et al. (2006) and Pietrzik et al. (2007) refer to the same study and will in the following be reported as Pietrzik et al. (2007). Equally, Houghton et al. (2006) and Houghton et al. (2009) report to the same study and will be referenced as Houghton et al. (2006). Venn et al. (2002) describe a subset of the population for which results on RBC folate concentrations are reported by Venn et al. (2003). Therefore, Venn et al. (2003) have been used in the assessment.
Houghton et al. (2006) (Tier 2) studied a population of lactating women who had been supplemented during pregnancy with 1 mg/day of folic acid and then consumed around 400 μg/day of 5‐MTHF or folic acid (non‐randomised) for 16 weeks post‐partum. As this population was substantially different from other study populations in terms of physiology and previous exposure to high folate, the study was not combined with the other studies in the statistical analysis.
Therefore, the final statistical analysis on RBC folate concentrations included six studies, three studies in Tier 1 (Venn et al., 2003; Pietrzik et al., 2007; Green et al., 2013) and three in Tier 2 (Wright et al., 2010; Diefenbach et al., 2013; Henderson et al., 2018).
The detailed approach towards the statistical analysis is described in the statistical report in Annex B.
All the studies included in the analysis were of a parallel design. The number of recruited subjects ranged from 15 (Green et al., 2013) to 86 (Diefenbach et al., 2013). The studies lasted between 12 (Henderson et al., 2018) and 24 weeks (Venn et al., 2003; Pietrzik et al., 2007; Diefenbach et al., 2013). Users of folic acid supplements/medicines or fortified foods were included in the study by Pietrzik et al. (2007), while in the other studies, they were either excluded or the information was not given. In all studies, supplements or the foods fortified with folate were given once per day (for doses see Figure 1). Supplements were taken on an empty stomach in the studies by Pietrzik et al. (2007) and Venn et al. (2003). In the study by Wright et al. (2010), participants had the choice whether supplements were taken with a meal or without. In the study by Green et al. (2013), participants consumed a wheat roll fortified with folate. For the studies by Diefenbach et al. (2013) and Henderson et al. (2018), it was not described whether supplemental folate was consumed with a meal or not.
Information on the baseline folate intake was available for the studies by Diefenbach et al. (2013) (mean ± SD 176 ± 70 μg/day folate), Venn et al. (2003) (geometric means (95%CI) 5‐MTHF group 244 (217–275) μg/day folate, folic acid group 211 (182–244) μg/day), Pietrzik et al. (2007) (mean ± SD 257 ± 108 μg/day DFE; data reported in Lamers et al. (2004)) and Wright et al. (2010) (mean ± SE 5‐MTHF group 293 ± 12 μg/day folate, folic acid group 313 ± 16 μg/day).
Modelling the dose–response for the ratio of mean changes from baseline in RBC folate concentrations in a linear fixed‐effect meta‐regression model showed a significant log‐linear relationship between the ratio of mean changes in RBC folate concentrations (after 5‐MTHF or folic acid supplementation) and the daily dose administered. The relationship is depicted in Figure 3.
Figure 3.

- The red dashed line corresponds to a ratio equal to one (i.e. similar effect of 5‐MTHF and folic acid on RBC folate concentrations). The size of the point is proportional to the weight of the study as defined by the inverse of its variance in the meta‐regression model.
In this meta‐regression model, three regions could be identified:
-
•
Region 1: For daily doses belonging to the range 227 nmol 15 to 382 nmol 16 (consumed once per day), the effect of 5‐MTHF on RBC folate concentration was slightly lower than the effect of folic acid;
-
•
Region 2: For daily doses belonging to the range 382 nmol to 614 nmol 17 (consumed once per day), the effect of 5‐MTHF on RBC folate concentration was similar to the effect of folic acid; this range is represented by the intercept of the confidence interval (CI) of the regression line with the red dashed line in Figure 1;
-
•
Region 3: For daily doses belonging to the range 614 nmol to 2,270 nmol 18 (consumed once per day), the effect of 5‐MTHF on RBC folate was higher than the effect of folic acid; however, with a wide CI.
At doses of 227 nmol/day, the effect of 5‐MTHF on RBC folate concentrations was 23% (95% CI: −14%; −32%) lower than the effect of folic acid; at 906 nmol/day (around 400 μg), the effect of 5‐MTHF on RBC folate concentrations was 23% (95% CI: 11%; 37%) higher compared with the effect of folic acid, and at 2,270 nmol/day, the effect was 69% higher (95% CI: 41%; 102%).
There was no significant heterogeneity between the studies, which was also supported by the non‐significant result of the test for residual heterogeneity. However, the limited number of observations included in the model does not allow a thorough diagnosis of the model. It is important to note that there is a high uncertainty in relation to the estimations as they are based on a limited number of studies.
The Panel notes that the meta‐regression model on RBC folate concentrations indicates dose‐dependent differences in bioavailability of 5‐MTHF and folic acid in healthy adults.
Results on RBC concentrations of studies not considered in the analysis
The results of the study in lactating women by Houghton et al. (2006), described above, are consistent with the findings of the statistical analysis performed using the other studies. The decline in RBC folate concentrations was around twice lower in the group consuming 5‐MTHF than in the folic acid group at doses of 906 nmol/day (around 400 μg/day folate) after 16 weeks of supplementation.
3.7.1.2. RBC folate concentrations in diseased adults (5‐MTHF vs. folic acid)
One study on the effect of 5‐MTHF vs. folic acid on RBC folate in diseased adults was available.
The study by Khandanpour et al. (2009), Tier 2, was conducted in 133 men and women (70.0 ± 8.2 years) suffering from peripheral arterial disease, who did not consume B‐vitamin supplements. The participants were randomly allocated to receive 400 μg/day folic acid or 400 μg/day 5‐MTHF or placebo for 16 weeks. Baseline folate intake was not reported. Analysis per protocol (PP) showed an increase of RBC folate in the two folate groups compared with placebo; however, there was no significant difference between the responses of folate biomarkers to folic acid and 5‐MTHF interventions [median difference (95% CI) 19 RBC folate 69.0 (−132.8, 290.2); p = 0.975, p‐value corrected for multiple testing]. Analysis according to intention to treat (ITT) was also performed, but it did not change the results.
The Panel notes that, based on results of RBC folate, 5‐MTHF and folic acid had a similar bioavailability at a dose of 400 μg/day (around 906 nmol/day) in individuals with peripheral arterial disease.
3.7.1.3. Serum/plasma total folate concentrations in healthy adults (5‐MTHF vs. folic acid)
Twelve randomised human repeated dose studies (reported in 14 publications) in apparently healthy adults were retrieved through the systematic search that investigated the effect of consuming either 5‐MTHF or folic acid on PTF concentrations. Three studies reported in five publications were in Tier 1 (Venn et al., 2002; Venn et al., 2003; Lamers et al., 2004; Lamers et al., 2006; Green et al., 2013), eight studies reported in nine publications were in Tier 2 (de Meer et al., 2005; Houghton et al., 2006, 2009; Wright et al., 2010; Diefenbach et al., 2013; Hekmatdoost et al., 2015; Bailey and Ayling, 2018; Sicińska et al., 2018; Bayes et al., 2019). The publications by Lamers et al. (2004) and Lamers et al. (2006) refer to the same study, the same cited above as Pietrzik et al., 2007. In the following, it will be referred to as Lamers et al. (2006). Venn et al. (2002) describe a subset of the population for which results on RBC folate concentrations are reported by Venn et al. (2003). Therefore, Venn et al. (2003) have been used in the assessment.
The study by Houghton et al. (2006) (Tier 2) has not been included in the analysis for the reasons outlined in Section 3.7.1.1. The study by Hekmatdoost et al. (2015) (Tier 2) was a study in women who had had three or more idiopathic abortions. Only women who became pregnant and did not have a further abortion were followed up. The duration of follow‐up differed (depending on when women became pregnant) and was planned up to gestational week 20. As this population was substantially different from other study populations in terms of physiology (women becoming pregnant during the study), the study was not combined with the other studies in the statistical analysis.
The study by Sicińska et al. (2018) (Tier 2) was also excluded from analysis as the daily dose of 5‐MTHF/folic acid was administered to the subjects in two equal daily doses and not once daily as for the other studies in adults included.
Bailey and Ayling (2018) (Tier 2) administered high doses of 5‐MTHF or folic acid (7.5 mg/day) for 3 or 5 days and relevant comparisons are only available for this time period in the study. As this was substantially different from the other studies in the pool, this study was not included in the analysis.
The results reported by Bayes et al. (2019) (Tier 3) were excluded, because they were implausible for the group consuming folic acid, as serum folate concentrations were reported to decline from week 2 to 4 after an initial increase from baseline to week 2, even though compliance was reported to have been satisfactory. In addition, no description of the analytical method used to analyse PTF concentrations has been provided in the publication.
The final statistical analysis on PTF concentrations included therefore seven studies, three in Tier 1 (Venn et al., 2003; Lamers et al., 2006; Green et al., 2013) and four in Tier 2 (de Meer et al., 2005; Wright et al., 2010; Diefenbach et al., 2013; Henderson et al., 2018). All but one parallel study (de Meer et al., 2005) have already been considered in the analyses on RBC folate concentrations.
The characteristics of the studies by Diefenbach et al. (2013), Green et al. (2013), Lamers et al. (2006) (cited above as Pietrzik et al. (2007)), Wright et al. (2010), Henderson et al. (2018) and Venn et al. (2003) have been described in Section 3.7.1.1 above. The study populations in the study by de Meer et al. (2005) consisted of a group of younger adults and one of older adults (12 each (6 per folate group) who were supplemented with 400 μg/day folic acid or an equimolar amount of 5‐MTHF (454 μg/day) consumed once per day for a duration of 5 weeks. Baseline folate intake was not reported.
A similar dose–response model to the one for RBC folate concentrations, but with a random component to account for the hierarchical structure introduced by the study by de Meer et al. (2005) on two population groups, was applied to the data on PTF concentrations, yielding similar results as for RBC folate concentrations, i.e. that three different regions could be identified. It has, however, to be recognised that the studies included in dose–response model for PTF concentrations were mostly the same as the ones that were already included in the model for RBC folate concentrations (see Figure 4).
Figure 4.
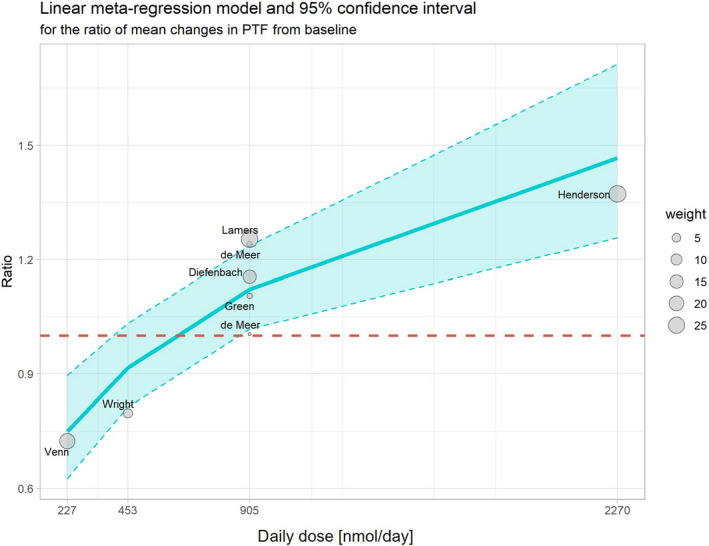
Dose–response meta‐regression of the ratio of mean changes in PTF concentrations (MTHF/folic acid supplementation) from baseline to end of supplementation and the daily dose (nmol, administered once per day)
- The red dashed line corresponds to a ratio equal to one (i.e. similar effect of 5‐MTHF and folic acid on PTF concentrations). The size of the point is proportional to the weight of the study as defined by the inverse of its variance in the meta‐regression model.
The regions identified in the model were:
-
•
Region 1: For daily doses belonging to the range 227 nmol 20 to 391 nmol 21 (consumed once per day), the effect of 5‐MTHF on PTF concentrations was slightly lower than the effect of folic acid;
-
•
Region 2: For daily doses belonging to the range 391 nmol to 852 nmol 22 (consumed once per day), the effect of 5‐MTHF on PTF concentrations was similar to the effect of folic acid. This range is represented by the intercept of the CI of the regression line with the red dashed line in Figure 2;
-
•
Region 3: For daily doses belonging to the range 852 nmol to 2,270 nmol/ 23 (consumed once per day), the effect of 5‐MTHF on PTF concentrations was higher than the effect of folic acid, however, with a wide CI.
At 227 nmol/day 5‐MTHF, the effect of 5‐MTHF on PTF concentrations was 25% (95% CI: −10%; −43%) lower than the effect of folic acid, and at 2,270 nmol/day, its effect on PTF concentrations was 46% (95% CI: 25%; 71%) higher than the effect of folic acid.
The Panel notes that the meta‐regression model on PTF concentrations indicates dose‐dependent differences in bioavailability of 5‐MTHF and folic acid in healthy adults.
Similar to the outcomes of the RBC folate model, there is a high uncertainty in relation to these estimations as they are based on a limited number of studies (only one study belongs to the first and one to the second region, respectively). Five studies belong to the third region, but not all of them are positioned in the area delineated by the CI of the model.
The scarcity in the number of observations included in the model does not allow a thorough diagnosis of the model. Considering the positioning of the data within the confidence interval of the model, it can be concluded that the model is fitting reasonably well the data. However, in contrast to the RBC folate model, the heterogeneity of the PTF model is much higher, with the test for residual heterogeneity being significant.
The Panel notes that the meta‐regression model on PTF concentrations indicates dose‐dependent differences in bioavailability of 5‐MTHF and folic acid in healthy adults.
Results of studies on PTF concentrations not considered in the analysis
The studies by Houghton et al. (2006), Hekmatdoost et al. (2015) and Bailey et al. (2015) showed a higher effect of 5‐MTFH on PTF concentrations than folic acid at folate doses of around 400 μg/day (906 nmol/day), 1 mg (around 2,270 nmol/day) and 7.5 mg/day (around 17,000 nmol/day), respectively. In the study by Houghton et al. (2006), the decline in PTF concentrations was around 1.4 times lower in the group consuming 5‐MTHF than in the folic acid group. In the study by Hekmatdoost et al. (2015), 5‐MTHF supplementation led to around 1.2 times higher PTF concentrations than supplementation with folic acid, and in the study by Bailey et al. (2015), PTF concentrations were around 1.6 times higher following 5‐MTHF supplementation than following folic acid supplementation.
In the study by Sicińska et al. (2018), volunteers consumed either 5‐MTHF or folic acid in a dose of 2 × 454 nmol/day (or around 2 × 200 μg/day folate) for 4 weeks. The study showed that the effect of 5‐MTHF on PTF concentrations was around 37% lower than the effect of folic acid.
The Panel notes that the results of three of the four studies are in line with the results of studies included in the statistical analyses, i.e. that at doses of 400 μg/day (906 nmol/day, consumed once per day) and above, 5‐MTHF has a greater effect on PTF concentrations than folic acid.
3.7.1.4. Serum/plasma total folate concentrations in diseased adults (5‐MTHF vs folic acid)
The study by Khandanpour et al. (2009), Tier 2, in individuals with peripheral arterial disease described in Section 3.7.1.2 also reported results on PTF concentrations. The PP analysis showed an increase of PTF in the two folate groups compared with placebo; however, there was no significant difference between the responses of folate biomarkers to folic acid and 5‐MTHF treatments [median difference (95% CI)19 PTF: −5.1 (−16.4, 5.9); p = 0.823, p‐value corrected for multiple comparisons]. Analysis according to ITT was also performed, but it did not change the results. The Panel notes that 5‐MTHF and folic acid had similar effects on PTF concentrations at a dose of 400 μg/day (around 906 nmol/day).
3.7.1.5. Conclusions regarding repeated dose studies in adults comparing 5‐MTHF with folic acid
The Panel notes that the available studies for the analysis of RBC folate (n = 6) and PTF (n = 7) concentrations are at a low and intermediate RoB. There is similarity between the results obtained from the RBC folate and PTF regression models, i.e. that the bioavailability of 5‐MTHF compared to folic acid depends on the level of the intervention dose and that, across the investigated range of folate doses (227–2,270 nmol consumed once per day), three different bioavailability regions could be identified, which are generally similar between the two models. However, there is a higher heterogeneity between the studies included in the PTF model, whereas the heterogeneity of the RBC folate model appeared to be low.
The responses of RBC folate and PTF concentrations to supplementation with either 5‐MTHF or folic acid are consistent within each study, except for the study by Wright et al. (2010) in which a higher bioavailability of 5‐MTHF compared with folic acid could be derived from the results on RBC folate, but a lower one when the results of PTF concentrations are taken as a basis. This is an observation that cannot be explained and might have been due to short‐term changes in folate intakes to which PTF has responded.
The obtained results from both regression models for differential bioavailability of 5‐MTHF and folic acid depending on the level of folate dose could be explained to some extent by the differences in pharmacokinetics and metabolism of the two folate forms.
The lower bioavailability of 5‐MTHF than folic acid in the region of low folate intake (227–382 nmol consumed once per day, derived from the model on the ratio of change in RBC folate concentrations) was a finding which originates from only one study (Venn et al., 2003) in this dose region. The study by Venn et al. (2003) is a randomised controlled trial (RCT) with a low RoB (Tier 1) and contains one of the largest sample sizes and the longest intervention periods (168 days) when compared with the rest of the studies included in the RBC folate model. The authors reported no significant difference in bioavailability of 5‐MTHF and folic acid based on both RBC and PTF changes, but the current analysis of the ratios of the aggregated data showed a significantly lower effect of 5‐MTHF on biomarkers of folate status and intake than folic acid. However, it could be plausible that this is a true biological effect. Proton‐coupled folate transporter (PCFT) situated at the apical brush‐border membrane of the jejunum is considered to be the primary high‐affinity folate transporter at the intestinal pH and reviews on the topic describe similar high affinities (i.e. between 1 and 5 μM) for folic acid, 5‐MTHF and 5‐formyl THF (5‐FTHF) at pH 5.5 (Zhao et al., 2011), although folate absorption through the PCFT is affected by conditions that alter the pH in the intestinal environment. A study by Qiu et al. (2007) investigated transport of mouse and rat PCFT protein in stably transfected HepG2 cells and found that the antifolate pemetrexed has the highest affinity for the PCFT, but within a narrow range, the observed folate affinities are pemetrexed > 5‐FTHF > folic acid > 5‐MTHF > the antifolate methotrexate. Thus, under ideal conditions, with optimal absorption rates for both folate forms, folic acid might be slightly favoured over 5‐MTHF. However, the difference, if any, is likely to be small and dependent on the pH of the intestinal lumen. On the other hand, the mechanism of folate transport out of the mucosal cells into the portal circulation is less clear. There is some evidence that MRP3, which is expressed at the basolateral membrane, is involved in this process, but MRP3 is less efficient for transporting folic acid than reduced folate forms (Zeng et al., 2001). However, the lower bioavailability of 5‐MTHF compared to folic acid in the region of low folate intake (227–382 nmol consumed once per day) found in the current analysis could be a chance finding, as the effect sizes observed in the study by Venn et al. (2003) after treatment are small and experimental bias could account for these differences. For example, the group on folic acid had a higher number of men and higher RBC folate concentrations at baseline. Also, the conversion from geometric mean and associated 95% CI into arithmetic mean and standard error for the inclusion of the study into the analysis may have contributed to the differences in results between the original publication and the present analysis.
In the region of high folate doses (614–2,270 nmol consumed once per day; based on the model on RBC folate concentration), the model showed higher bioavailability of 5‐MTHF compared with folic acid, which is probably a consequence of the fact that, after absorption, 5‐MTHF could be used directly in the transfer of one carbon units, whereas folic acid is required to be reduced in a two‐step process by the enzyme dihydrofolate reductase to tetrahydrofolate in order to enter one‐carbon metabolism. However, dihydrofolate reductase in humans has low and highly variable activity, which limits its ability to metabolise efficiently folic acid (Bailey and Ayling, 2009). It would be expected that under the conditions of high folic acid intake, dihydrofolate reductase would be quickly saturated and this would lead to appearance of unmetabolised folic acid in the circulation with an increase of urinary folic acid excretion (West et al., 2012); this would result in reduction of the normal metabolic utilisation of this folate form that in turn could impact RBC folate concentrations. Moreover, there is mechanistic evidence that haematopoietic cells more efficiently internalise 5‐MTHF than folic acid (Henderson et al., 1987).
Sweeney et al. (2007) attempted to identify threshold doses above which unmetabolised folic acid appears in plasma of healthy folate‐replete individuals. Twenty individuals consumed 400 μg/day as folic acid supplements for 14 weeks. This pre‐saturation period was followed by three 7‐day periods in which bread fortified with folic acid was provided. In the first period, two slices of bread containing 200 μg of folic acid were eaten on two separate occasions 4 h apart. In the second and third period, the slices contained 100 and 50 μg of folic acid, respectively. All bread periods were separated by periods in which subjects were supplemented with 400 μg/day of folic acid. Blood was drawn at the end of each bread consumption period in the morning in fasting state ~ 20 h after the last bread consumption occasion, 1 and 2 h after the first slice and 1 and 2 h after the second slice of bread. Unmetabolised folic acid appeared in blood of all individuals after having consumed 400 μg/day folic acid as food supplement for 14 weeks and at the end of the bread period after the consumption of the first and second slice of bread containing 200 μg folic acid each. There was an indication that unmetabolised folic acid started to accumulate in plasma after the consumption of the second slice of bread. The Panel notes that this study shows that after doses of 200–400 μg/day folic acid, unmetabolised folic acid starts to appear in blood. The Panel also notes that this may also be related to changes in bioavailability, although there is no evidence for that.
A non‐linear relationship between folic acid intakes and biomarker responses has been observed by Duffy et al. (2014) in a meta‐analysis of RCTs. In this analysis, a linear dose–response relationship between intake and RBC folate and PTF concentrations was found among studies providing < 400 μg/day folic acid, while the relationship was non‐linear for studies administering > 400 μg/day.
The Panel notes that there is evidence that at folic acid intakes > 400 μg/day responses of biomarkers of intake and status are not linear. The observation that unmetabolised folic acid appears in blood at doses between 200 and 400 μg/day might indicate that folic acid bioavailability starts levelling off already in this dose range. This observation of non‐linearity in the biomarker response to folic acid intake can explain the non‐linear relationship between the bioavailability of 5‐MTHF and folic acid that was observed in the dose–response models described in Sections 3.7.1.1 and 3.7.1.3.
The Panel considers that folic acid, in the linear dose range, is a suitable comparator for the derivation of a conversion factor for 5‐MTHF into DFE. However, because of its non‐linear relationship with biomarker responses at higher intakes, it is not a suitable comparator at these doses, possibly > 400 μg/day.
3.7.2. Repeated dose studies in adults comparing 5‐MTHF with natural folate
One non‐randomised repeated‐dose study in humans (Tier 2), also included in the analysis on RBC folate and PTF concentrations, used NF as a comparator to assess the bioavailability of CaLMF (Wright et al., 2010).
It was a 16‐week open‐label placebo‐controlled study in 163 healthy males and females (18–65 years), in which 453 nmol/day (208 μg/day) 5‐MTHF or folic acid was intended to be compared with an equimolar dose of NF, provided by folate‐rich foods. Participants received a list of folate‐containing foods to which a unit was assigned based on their folate content (1 unit = 111.25 nmol folate). Participants were asked to consume four units per day. Folate intake was assessed using 7‐day weighed food records during the month before the intervention and the last month of the intervention. Supplement compliance was 96%. The average increase in folate intake of the group consuming NF was of 370 nmol/day, equivalent to 82% of the target dose. In the 5‐MTHF group, natural folate intakes decreased by around 19 nmol/day and in the folic acid group by around 44 nmol/day. The authors report that, in the NF group, the mean increase in RBC folate concentrations was 40% of that in 5‐MTHF group and 43% of that of the folic acid group. The mean increase in PTF concentrations in the NF group was 39% and 31% of the increase in 5‐MTHF and folic acid groups, respectively. Correcting for differences in folate intakes, the effect of 5‐MTHF was around two times and the effect of folic acid around 1.9 higher than the effect of NF on RBC folate concentrations.
The Panel notes that in the study by Wright et al. (2010), taking into consideration the lower folate intake in the NF group, at a dose of 453 nmol/day (208 μg/day), the effect of 5‐MTHF on RBC folate concentrations was about twice higher than the effect of NF and similar to the one of folic acid when compared with NF.
3.7.2.1. Conclusions regarding repeated dose studies in adults comparing 5‐MTHF with food folate
The Panel notes that only one human repeated dose intervention study with limitations (i.e. study not randomised, differences in baseline RBC folate concentrations, even though not statistically significant and intake targets in the NF group not reached) is available. Wright et al. (2010) show a similar effect of 5‐MTHF on RBC folate concentrations as folic acid when compared to NF. These results with respect to the differences in bioavailability between folic acid and NF are similar to the findings by Sauberlich et al. (1987) which is one of the studies on which the original DFE conversion factor for folic acid is based, i.e. that folic acid is around twice more bioavailable than NF.
The Panel considers that this study indicates that the same conversion factor as for folic acid could be used for 5‐MTHF at a dose of around 200 μg/day.
3.7.3. Acute dose studies in adults comparing 5‐MTHF with folic acid
According to the protocol, in the acute dose studies, potentially relevant biomarkers for assessment of relative folate bioavailability of 5‐MTHF and folic acid were investigated, including the AUC of PTF or urinary total folate concentrations. In addition, folate content in stomal effluent was also considered in studies in ileostomy subjects. In some of the studies, also kinetic parameters (e.g. Cmax, Tmax) were investigated. However, given that the kinetic variables provide information on the response to intervention at a particular time point only, whereas AUC indicates the overall response of plasma or urinary folate, typically in the course of 8–10 h post‐intervention, the results with respect to kinetic parameters are in the following not described.
Most of the acute studies used CaLMF as a form of 5‐MTHF (Pentieva et al., 2004; Prinz‐Langenohl et al., 2009; Obeid et al., 2020), except for one study in which sodium 5‐MTHF (Obeid et al., 2020) was used and two that used the free form, i.e. 5‐MTHF acid (Tamura and Stokstad, 1973; Gregory et al., 1992). The number of subjects ranged from 6 to 24.
Studies on PTF or plasma 5‐MTHF in healthy adults
For three acute randomised cross‐over studies, the AUC (PTF or (6S)‐5‐MTHF) was calculated. Their RoB was assessed by the panel to be low (Pentieva et al., 2004), intermediate (for AUC for (6S)‐5‐MTHF) (Obeid et al., 2020) or high (Prinz‐Langenohl et al., 2009; Obeid et al., 2020). Single doses of 5‐MTHF tested were 416 μg (Prinz‐Langenohl et al., 2009), 436 μg (Obeid et al., 2020) and 500 μg (Pentieva et al., 2004). In one study (Pentieva et al., 2004), the tested substances were given with a meal, while they were consumed on an empty stomach in the others (Prinz‐Langenohl et al., 2009; Obeid et al., 2020).
One study (Pentieva et al., 2004) (Tier 1), using the highest dose of 5‐MTHF consumed with a meal, found that the AUCs for plasma folate were comparable between 5‐MTHF and folic acid. On the contrary, the studies of intermediate or high RoB (Tiers 2 and 3) and using slightly smaller doses consumed on an empty stomach found that 5‐MTHF administration led to a higher AUC than folic acid (Prinz‐Langenohl et al., 2009; Obeid et al., 2020).
Study on urinary folate excretion in healthy adults
The acute cross‐over study by Tamura and Stokstad (1973) (Tier 2), the bioavailability of 5‐MTHF compared to an equivalent dose of folic acid was investigated by measuring urinary folate excretion. A free form of L‐5‐MTHF acid (500 μg) was consumed as a supplement on an empty stomach. The bioavailability of 5‐MTHF was estimated in urine samples by reference to the response curve previously derived from administration of varying doses of folic acid given to the same subjects. The results indicated around 21% higher bioavailability of 5‐MTHF than folic acid, although this result was not statistically significant.
Studies using stable isotopes in healthy adults
An acute cross‐over study by Gregory et al. (1992), assessed to be of low RoB (Tier 2), investigated the bioavailability of 5‐MTHF and folic acid by using stable isotopes. Subjects were given a single oral dose of d2‐5‐MTHF (517 nmol = 237 μg) or d2‐folic acid (677 nmol = 299 μg) and an intravenous injection of d4‐PteGlu as a control. Bioavailability was assessed by the isotope excretion ratios of urinary folates (d2/d4). The results showed significantly lower bioavailability of 5‐MTHF compared with folic acid (1.13 vs. 1.53).
Studies in diseased individuals
Two acute cross‐over studies assessed the bioavailability of 5‐MTHF against folic acid in ileostomy subjects who previously had ulcerative colitis. One of the studies was assessed as having a low RoB (Witthöft et al., 2006) (Tier 1) and the other an intermediate RoB (Ohrvik et al., 2010) (Tier 2). Both studies used CaLMF, at doses of 192 μg in a supplement (Witthöft et al., 2006) or 213 μg stable isotope of CaLMF in fortified wholemeal bread (Ohrvik et al., 2010). Absorbed folate was estimated by AUC based on PTF concentrations (corrected for the dose administered) and a kinetic model, and non‐absorbed folate was determined by ileostomal folate content. Both studies found similar folate content in stomal effluent after ingestion of 5‐MTHF and folic acid. The study by Witthöft et al. (2006) found that the AUCs were comparable between 5‐MTHF and folic acid, indicating similar bioavailability of the two folate forms, whereas the AUC results of Ohrvik et al. (2010) showed more than twice higher bioavailability of 5‐MTHF compared with folic acid.
3.7.3.1. Conclusions regarding acute dose studies in adults comparing 5‐MTHF with folic acid
The panel notes that various parameters including AUC of plasma folate response or continuous monitoring of urinary folate excretion have been used in acute dose studies in adults. The conclusions are based on the overall plasma or urinary folate response. The Panel notes that the available seven acute cross‐over studies investigating the bioavailability of 5‐MTHF by using as a comparator an equivalent dose of folic acid (two studies in Tier 1, three studies in Tier 2 and one study in Tier 3) showed inconsistent AUC results irrespective of whether labelled or non‐labelled folates were administered, even within a close dose range. The Panel also notes that the AUC results obtained from acute studies in both healthy people and ileostomy subjects were similarly inconsistent. The Panel considers that these inconsistencies between AUC results could be related, as suggested by Wright et al. (2003), to the differential kinetic behaviour and hepatic first‐pass effect of folic acid and reduced folates, which might be a potential limitation for the interpretation of AUC results of acute studies that use folic acid as a reference substance. Therefore, the Panel concludes that the results from the acute studies using as a comparator folic acid cannot be considered for the conversion of 5‐MTHF added to foods into dietary folate equivalents (contrary to those that compare the bioavailability of different salts of 5‐MTHF with each other).
3.7.4. Acute dose studies in adults comparing 5‐MTHF with natural folate
The available data originate from one pilot cross‐over study by Witthöft et al. (2003) in two male ileostomy patients. The Panel considers that no conclusions can be drawn from this study on the comparative bioavailability of 5‐MTHF with NF.
3.7.5. Conclusions on the bioavailability of 5‐MTHF in adults
The Panel concludes that based on the study by Wright et al. (2010) the same conversion factor as for folic acid could be used for 5‐MTHF in the dose range in which biomarker responses are assumed to respond linearly to intakes. There are insufficient data to establish at which dose precisely folic acid bioavailability starts levelling off, but it is expected that there is a high interindividual variability. In the absence of such data, the Panel assumes linearity at intakes below 400 μg/day folic acid, based on the meta‐analysis by Duffy et al. (2014). For setting a DFE conversion factor for higher doses, the panel proposes to use the observation that at intakes of ~ 400 μg/day, 5‐MTHF led to about 1.2 times higher RBC folate concentrations than folic acid, based on the three studies investigating that dose (Pietrzik et al., 2007; Diefenbach et al., 2013; Green et al., 2013) (see Section 3.7.1.1).
3.8. Comparative bioavailability of 5‐MTHF in infants
3.8.1. Repeated dose study in healthy infants comparing 5‐MTHF with folic acid
The panel initially had decided to conduct separate analyses on data available for infants and those for adults, because of the differences in physiology, the different consumption pattern between infant and adult populations and the potential impact the presence of folate‐binding protein (FBP) in milk‐based infant formulae could have on folate absorption (see Section 3.3).
Individual data for infants were provided to EFSA by the study by Troesch et al. (2019), which assessed, in an RCT‐design, the suitability of 5‐MTHF to be used as a source of folate for infant and follow‐on formula. According to the publication and to the raw data made available to EFSA, this study investigated the effect of 5‐MTHF vs. folic acid supplementation as an ingredient in infant formula on RBC folate concentrations. During the public consultation on the draft of this Scientific Opinion, EFSA was informed that the values originally denominated as RBC folate concentrations were in fact whole blood folate concentrations. As EFSA did not have access to the individual data on haematocrit, which would have allowed to calculate RBC folate concentrations from whole blood folate concentrations, the study by Troesch et al. (2019) could not be used in the assessment any longer.
3.8.2. Conclusions on the bioavailability of 5‐MTHF in infants
The Panel notes that there is no study available to EFSA which would have allowed investigating the effect of 5‐MTHF vs. folic acid intake on RBC folate concentrations in infants. The Panel considers it unlikely that the bioavailability of 5‐MTHF in infants is lower than the one of folic acid. Therefore, the Panel proposes to assume a similar bioavailability between 5‐MTHF and folic acid in infants which ensures that folate from 5‐MTHF is provided to infants in at least the labelled amounts and which is considered a conservative approach.
The Panel considers that there is no evidence available to derive a separate DFE equation for infants.
3.9. Comparative bioavailability of 5‐MTHF in animal models
3.9.1. Repeated dose studies in rats and hens
Three subacute animal studies were available, one in hens and assessed as having a low RoB (Tier 1) (Tactacan et al., 2010), one in weanling rats and assessed as having an intermediate RoB (Tier 2) (Pérez‐Conesa et al., 2009) and one in pregnant rats and assessed as having a high RoB (Tier 3) (Pannia et al., 2021). Two studies used an unspecified form of 5‐MTHF (Pérez‐Conesa et al., 2009; Tactacan et al., 2010) and one used CaLMF (Pannia et al., 2021). The number of animals per group ranged between 6 (Pérez‐Conesa et al., 2009) and 16–18 (Pannia et al., 2021).
Parameters investigated were concentrations of RBC folate (Pérez‐Conesa et al., 2009), PTF (Pérez‐Conesa et al., 2009; Tactacan et al., 2010), plasma 5‐MTHF (Pannia et al., 2021), egg folate (Tactacan et al., 2010), liver folate (Pérez‐Conesa et al., 2009; Tactacan et al., 2010) and liver 5‐MTHF (Pérez‐Conesa et al., 2009; Pannia et al., 2021).
Two studies provided only the final measurements (Tactacan et al., 2010; Pannia et al., 2021), while Pérez‐Conesa et al. (2009)) reported measurements at different time points.
In the study by Tactacan et al. (2010), Shaver White and Shaver Brown laying hens were provided with diets either without supplemental folate containing 1.49 mg/kg diet of folate or with 10 mg/kg diet of added folic acid or with 11.3 mg/kg diet of added 5‐MTHF (equimolar to folic acid). The dietary folate requirement for laying hens is 0.25 mg/kg diet of folic acid, according to the authors of the publication. The diets were fed for a 2‐week run‐in period and a 1‐week egg collection period. Blood samples were taken at the end of the intervention. There were no statistically significant differences between folate groups in egg folate, PTF and liver folate concentrations.
In the study by Pannia et al. (2021), pregnant Wistar rats received either a diet containing the recommended amount of folate, i.e. 2 mg/kg diet or five times this quantity of folic acid or 5‐MTHF. The diet was consumed until birth, for around 3 weeks. There were no statistically significant differences in plasma or liver 5‐MTHF concentrations.
In the last study (Pérez‐Conesa et al., 2009), weanling rats were folate‐depleted for 28 days (folate content of the diet under the detection limit by high‐performance liquid chromatography (HPLC)). Thereafter, they received for 4 weeks either folic acid or 5‐MTHF within a young‐child formula (around 1,000 μg/L). PTF, RBC folate, liver folate and liver 5‐MTHF concentrations in each group were measured at the end of the study. RBC folate and liver 5‐MTHF concentrations were statistically significantly higher in the 5‐MTHF group. The point estimate for PTF concentrations was higher and the one for liver folate lower in the group consuming 5‐MTHF compared with the folic acid group. These comparisons did not reach statistical significance.
3.9.2. Acute studies in rats
Three acute studies in rats have been identified in the literature, all assessed as having an intermediate RoB (Tier 2) (Bhandari and Gregory, 1992) or a high RoB (Tier 3) (Fernandez‐Borrachero et al., 1996; Miraglia et al., 2016). One of the studies compared CaLMF and 5‐MTHF glucosamine with folic acid (Miraglia et al., 2016), another used the barium salt of 5‐MTHF (Fernandez‐Borrachero et al., 1996) and the last one used the free form, i.e. 5‐MTHF acid (Bhandari and Gregory, 1992). The number of animals per group ranged from 6 (Miraglia et al., 2016) to 16 (Fernandez‐Borrachero et al., 1996).
Bhandari and Gregory (1992) administered intragastrically a small dose (0.22–0.23 μg/kg body weight) of radiolabelled tritiated 5‐MTHF and folic acid to male Sprague–Dawley rats (Crl:CDR BR) after overnight food deprivation. Both compounds underwent nearly complete absorption within 8 h and no significant difference in the excretion kinetics was detected. Isotopic distributions and the pattern of labelled folates in urine and tissues were similar regardless of the form administered. According to the authors, the two compounds exhibited equivalent bioavailability.
Fernandez‐Borrachero et al. (1996) studied the jejunal folate absorption in male Wistar rats submitted to 24 h starvation via 60 min perfusion of either the barium salt of 5‐MTHF (dose range 0.88–4.4 mg/kg body weight) or folic acid (dose range 0.45–4.2 mg/kg body weight). Absorption values, expressed as serum folate (nmol/20 cm), were higher for folic acid than for 5‐MTHF at the four time points considered (15, 30, 45 and 60 min), with an average ratio of 1.7, 1.9 and 2.0 at the three dose levels tested; the difference was generally statistically significant.
Miraglia et al. (2016) administered a single dose (70 μg/kg body weight, as glucosamine salt) of either 5‐MTHF glucosamine or CaLMF or folic acid via capsules in the feed to male Sprague Dawley rats. The AUC of plasma 5‐MTHF for 5‐MTHF glucosamine was found to be similar (i.e. 1.12 times) to that of CaLMF and 9.7 times higher than that of folic acid.
3.9.3. Conclusions on the bioavailability data derived from animal studies
The Panel notes that, as already noted by the ANS Panel (EFSA ANS Panel, 2013) in its assessment of 5‐MTHF glucosamine, the interpretation of studies on folate bioavailability in animals may be complicated by the influence of coprophagy and dietary constituents on the outcome and, without controlling for these factors, results from animal studies may not be relevant for humans. These issues were considered in the RoB assessment (Tier allocation).
The results from the only study in Tier 1 (Tactacan et al., 2010), a repeated dose study in hens, did not indicate a different bioavailability between 5‐MTHF and folic acid. In the repeated dose study in Tier 2 (Pérez‐Conesa et al., 2009), carried out in folate depleted rats, the results indicated a higher bioavailability of 5‐MTHF compared to folic acid. However, folate deficiency may have had an influence on the effect. Therefore, it is difficult to extrapolate its findings to non‐folate‐deficient humans. In the acute study in rats in Tier 2 by Bhandari and Gregory (1992), no significant differences in bioavailability between 5‐MTHF and folic acid were observed. All remaining studies were in the highest RoB Tier.
The Panel considers that these animal studies, in particular in view of their limitations, are of limited use in establishing the comparative human bioavailability of the different folate forms under assessment.
3.10. Bioaccessibility and intestinal translocation of 5‐MTHF in in vitro gastrointestinal systems
Some in vitro gastrointestinal models deal with the release of the test substance from the matrix after simulated gastrointestinal digestion and provide information on the amount solubilised for potential absorption in the intestine (bioaccessibility). Other models entail the use of cell lines, representative of the human intestinal epithelium and address the potential uptake and translocation into the basolateral compartment. Such models may give some insight into absorption, which is prerequisite for bioavailability.
Only three relevant in vitro studies have been identified in the literature, two with an intermediate RoB (Tier 2) (Verwei et al., 2003; Chandra‐Hioe et al., 2013) and one having a high RoB (Tier 3) (Arkbåge et al., 2003). These studies investigated CaLMF (Chandra‐Hioe et al., 2013) or sodium 5‐MTHF (Arkbåge et al., 2003; Verwei et al., 2003).
Two studies investigated the in vitro bioaccessibility of folic acid and 5‐MTHF in milk and yoghurt and the effect of added FBP on bioaccessibility via a dynamic in vitro gastrointestinal model (Arkbåge et al., 2003; Verwei et al., 2003). The bioaccessible fraction was defined as the folate fraction released in the small intestinal compartment relative to the content in the test items and was obtained by summing the folate content of jejunal and ileal dialysates (membrane cut‐off of 5 kDa). The non‐bioaccessible folate fraction was estimated by analysing the ileal delivery. Collectively, these studies show that the bioaccessibility of folic acid in milk is lower than that of 5‐MTHF and it is reduced by the addition of FBP, whereas this is not the case for 5‐MTHF. In the study where both folic acid and 5‐MTHF‐fortified milks and yoghurts were compared, the bioaccessibility was found to be 70% and 81% for folic acid, and 77% and 83% for 5‐MTHF, in milk and yoghurt, respectively (Arkbåge et al., 2003). In yoghurt, the addition of FBP reduced the bioaccessibility of folic acid (to 34%) more than that of 5‐MTHF (i.e. to 57%); results were mirrored by relative variations in the non‐bioaccessible folate fraction. In milk, the effect of FBP was less pronounced for folic acid compared with yoghurt (bioaccessibility of 61% vs. 34% with FBP added); for 5‐MTHF, no detrimental effect of FBP on bioaccessibility was detected (89% vs. 77%). The panel notes that FBP present in milk and dairy products may negatively affect the bioaccessibility, and thus the bioavailability, of folic acid, whereas no such effect is observed for 5‐MTHF.
The third study investigated in vitro bioaccessibility and transport across a Caco‐2 monolayer of folic acid and 5‐MTHF in fortified bread (Chandra‐Hioe et al., 2013). Bioaccessibility, measured as the folate fraction solubilised after in vitro simulated gastric and small intestinal digestion using a static model, was found to be 100% for both folic acid and 5‐MTHF. On the other hand, folic acid showed a higher transport across the Caco‐2 monolayer than 5‐MTHF, i.e. 13.7% (from white bread) and 14.0% (from wholemeal bread) compared to 1.3% (from white bread) or 2.1% (from wholemeal bread). The authors put forward that in the selected cell line, intracellular folate metabolism gives preference to 5‐MTHF over folic acid and, following uptake, monoglutamate 5‐MTHF is converted to polyglutamate forms to be retained within the cells and used for intracellular folate metabolism. The Panel notes that, at the tested concentrations and in the experimental conditions of the study, folic acid is more efficiently translocated across a cell model of the human intestinal epithelium, although caution has to be exercised in extrapolating this finding to a prediction of comparative bioavailability in vivo in humans.
3.10.1. Conclusions on the data derived from in vitro gastrointestinal models
The panel notes that there is evidence from in vitro gastrointestinal models that the presence of FBP in milk products reduces the absorption mostly of folic acid, while the absorption of 5‐MTHF is much less affected. This observation is supported by results from a study on the binding kinetics of different forms of folate to FBP (Nygren‐Babol and Jägerstad, 2012). In this study, it was observed that folic acid had the fastest association rate and the slowest dissociation constant, and that the affinity of 5‐MTHF to FBP was 100 times lower than that of folic acid.
This may be of particular relevance for infants who are exclusively fed a formula derived from milk proteins, even though infants are able to absorb the folate–FBP complex intact for weeks or months postnatally because of the not yet mature gut barrier, which also ensures that immunoglobulins can be absorbed intact (Nygren‐Babol and Jägerstad, 2012).
It should be noted that active FBP has only be detected in raw and pasteurised milk or freeze‐dried or spray‐dried milk powder, but not in milk products treated with ultra‐high temperature. FBP is mainly found in the whey fraction of milk. Therefore, hard cheese contains very little FBP (Nygren‐Babol and Jägerstad, 2012).
3.11. DFE conversion for CaLMF and 5‐MTHF glucosamine
The panel considers that based on the study by Wright et al. (2010) on adults, the same factor as for folic acid, i.e. 1.7, can be proposed for conversion of 5‐MTHF into DFE in foods that are fortified with 5‐MTHF for all population groups. This also applies to food supplements providing intakes below 400 μg/day 5‐MTHF. Beyond that intake there is evidence that the bioavailability of folic acid levels off and folic acid is no longer a suitable comparator for the derivation of a conversion factor for 5‐MTHF into DFE. For food supplements containing ≥ 400 μg/day 5‐MTHF, the Panel proposes to take into account the observation that at a supplemental dose of 400 μg/day, the effect of 5‐MTHF on RBC folate concentrations was on average about 1.2 times higher than the effect of folic acid, based on the three studies investigating this dose (Pietrzik et al., 2007; Diefenbach et al., 2013; Green et al., 2013) (see Section 3.7.1.1). Multiplied by 1.7, a conversion factor of 5‐MTHF of 2 can be derived. The Panel notes that this proposal is based on expert judgement for doses > 400 μg/day. The Panel suggests not to make this distinction for fortified foods based on the assumption that fortified foods are usually not fortified with amounts of 5‐MTHF that lead to intakes exceeding 400 μg/day. This assumption is supported by data on folic acid intake from fortified foods. In the Dutch national food consumption survey, the highest P95 (among various adult population groups) of daily folic acid intake from fortified foods was 146 μg (van Rossum et al., 2011), and in the Irish National Adult Nutrition Survey, the highest P75 was 180 μg (Hopkins et al., 2015).
Therefore, the Panel proposes the following updated DFE equations:
For fortified foods and food supplements providing intakes < 400 μg/day:
For food supplements providing intakes ≥ 400 μg/day.
The Panel notes that this assessment applies to CaLMF and 5‐MTHF glucosamine salt only. However, the Panel considers that the influence of the cation on bioavailability is likely to be within the margin of error of the proposed DFE equations. Therefore, the proposed equations can also be applicable to 5‐MTHF associated with other cations.
4. Discussion and uncertainties
The basis for the derivation of an equation to convert 5‐MTHF as μg of substance into μg DFE for foods fortified with 5‐MTHF and food supplements providing intakes < 400 μg/day is based on one study on adults (Wright et al., 2010). The proposed conversion factor for food supplements supplying ≥ 400 μg/day 5‐MTHF originates from three intervention studies (Pietrzik et al., 2007; Diefenbach et al., 2013; Green et al., 2013) that investigated the comparative bioavailability of 5‐MTHF and folic acid at a dose of ~ 400 μg/day. The conclusion to use the conversion factor derived from these three studies also for intakes > 400 μg/day is based on expert judgement.
The main uncertainties that are associated with the proposed DFE equations are described below:
-
•
The bioavailability of folic acid is non‐linear at high intakes. There is evidence that this is the case for intakes > 400 μg/day. It has, however, also been observed that unmetabolised folic acid appears in blood at intakes between 200 and 400 μg/day. This might indicate that folic acid bioavailability starts levelling off already in this dose range. In the non‐linear range, folic acid is not a suitable comparator for deriving a DFE conversion factor. Despite the uncertainty whether at a dose of 400 μg/day, a linear relationship between folic acid intake and its bioavailability still exists, the Panel decided to derive the DFE conversion factor for 5‐MTHF in food supplements providing ≥ 400 μg/day on studies in which the effects of 5‐MTHF versus folic acid supplementation on RBC folate concentrations was investigated at doses of ~ 400 μg/day. The Panel considers that even if the linearity assumption does not hold for this dose, the deviation from linearity at intakes of 400 μg/day is likely not to be substantial. In addition, reflecting in the DFE equation that 5‐MTHF is more bioavailable than folic acid at higher intakes better represents the true relationship between 5‐MTHF and folic acid at these intakes.
-
•
The proposed conversion factors are based on a limited number of studies that investigated only a limited number of doses.
-
•
The limited number of studies did not allow the influence of the background folate intake to be studied.
-
•
There is insufficient information to study the influence of the food matrix on the comparative bioavailability of 5‐MTHF and folic acid. There is also insufficient information whether the consumption with a meal or on an empty stomach or the formulation in which food supplements are consumed (e.g. liquids vs. solid forms, prolonged release forms vs. standard forms) alters the relationship. There is indeed evidence from acute studies in humans and in vitro studies that the presence of a food matrix might change the bioavailability of 5‐MTHF/folic acid.
-
•
The extrapolation of the results from studies providing ~ 400 μg/day 5‐MTHF/folic acid to doses beyond 400 μg/day is not based on data, but on expert judgement.
-
•
There is insufficient information to assess the impact of genetic and/or non‐genetic factors on folate bioavailability, including the dose–response for the appearance of unmetabolised folic acid.
-
•
No reliable data on the effect of 5‐MTHF versus folic acid supplementation on RBC folate concentrations in infants were available to EFSA. Therefore, it could not be assessed whether the differences in physiology between infants and adults alter the bioavailability of 5‐MTHF compared with folic acid.
5. Conclusions
The panel concludes that the following DFE equations best reflect the currently available evidence on the bioavailability of CaLMF and 5‐MTHF glucosamine salt added to foods:
-
•
For fortified foods and food supplements providing intakes < 400 μg/day
-
•
For food supplements providing intakes ≥ 400 μg/day
This assessment applies to CaLMF and 5‐MTHF glucosamine salt only. However, the influence of the cation on bioavailability is likely to be within the margin of error of the proposed DFE equations. Therefore, the proposed equations can also be applied to 5‐MTHF associated with other cations.
6. Recommendation
-
•
Further experiments would be needed to clarify whether the relationship between 5‐MTHF intake and responses of biomarkers of status and/or intake is linear or not and whether it is influenced by the food matrix or the way these folate compounds are consumed (i.e. with or without a meal).
-
•
Also, further studies would be needed to elucidate a more precise dose at which folic acid bioavailability levels off.
-
•
Dose–response studies on the comparative bioavailability of 5‐MTHF and NF would add precision to the DFE conversion factor in the lower dose range (at which it is feasible to compare 5‐MTHF with NF).
-
•
Studies in infants, children, pregnant and lactating women, older adults as well as individuals with genetic polymorphisms affecting one‐carbon metabolism would also add precision to the DFE conversion factor.
7. Documentation as provided to EFSA
Answer regarding EFSA's Call for data relevant to the assessment of the conversion factor of calcium‐L‐methylfolate and (6S)‐5‐methyltetrahydrofolic acid glucosamine salt into dietary folate equivalent. October 2021. Submitted by Gnosis be Lesaffre.
Individual participant data for the studies by Green et al. (2013) and Troesch et al. (2019).
Abbreviations
- 5‐FTHF
5‐formyl tetrahydrofolate
- 5‐MTHF
(6S)‐5‐methyltetrahydrofolate
- 5‐MTHF‐glucosamine
(6S)‐5‐methyltetrahydrofolic acid, glucosamine salt
- ADME
absorption, distribution, metabolism and excretion
- AFC Panel
EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food
- AI
adequate intake
- AI
artificial intelligence
- ANCOVA
analysis of covariance
- ANS Panel
EFSA Panel on Food Additives and Nutrient Sources Added to Food
- AR
average requirement
- AUC
area under curve
- BMI
body mass index
- CaLMF
calcium‐L‐methylfolate
- CI
confidence interval
- Da
Dalton
- DFE
Dietary Folate Equivalents
- DRV
dietary reference value
- FBP
folate‐binding protein
- FDA
Food and Drug Administration (US)
- HPLC
high‐performance liquid chromatography
- ID/LC/MS/MS
isotope dilution–liquid chromatography–tandem mass spectrometry
- IOM
Institute of Medicine
- ITT
intention‐to‐treat
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LOAEL
lowest observed adverse effect level
- MRP3
multidrug resistance protein 3
- MTHFR
methylene tetrahydrofolate reductase
- NDA Panel
EFSA Panel on Nutrition, Novel Foods and Food Allergens
- NF
Natural Folate
- NHANES
National Health and Nutrition Examination Survey
- NTP
US National Toxicology Program
- OHAT
Office of Health Assessment and Translation
- P
percentile
- PCFT
proton coupled folate transporter
- PP
per protocol
- PRI
population reference intake
- PTF
serum/plasma total folate
- RBC
red blood cell
- RCT
randomised control trial
- RFC‐1
reduced‐folate carrier
- RoB
risk of bias
- SCF
Scientific Committee on Food
- TEER
transepithelial electrical resistance
- tHcy
total homocysteine
- UL
tolerable upper intake level
Appendix A – Protocol for the assessment of the scientific evidence on the conversion factor of calcium‐L‐methylfolate and (6S)‐5‐methyltetrahydrofolic acid glucosamine salt into dietary folate equivalent
A.1. Problem formulation (assessment questions and subquestions)
The following protocol has been developed in line with existing methodology (EFSA, 2020).
In order to reply to the terms of reference (ToR), the Panel considers that the following questions and subquestions need to be answered (Table A.1).
Table A.1.
List of questions and subquestions to be answered for this assessment
| Questions and subquestions | Method to answer questions/possible source of information |
|---|---|
| 1. Identity of CaLMF and (6S)‐5‐methyltetrahydrofolic acid, glucosamine salt (5‐MTHF glucosamine)? | Narrative review |
| 2. Absorption, distribution, metabolism and excretion (ADME) of CaLMF and 5‐MTHF glucosamine in healthy subjects compared to NF and folic acid? What are relevant biomarkers of intake and status? | Narrative review |
| 3. ADME of folate in non‐healthy state: what diseases/conditions could influence folate absorption/metabolism compared to healthy subjects? | Narrative review |
| 3.1. For which diseases is CaLMF used in foods for special medical purposes? | Call for data |
| 4. Investigating bioavailability of CaLMF and 5‐MTHF glucosamine compared to NF or folic acid in humans, in (i) repeated‐dose studies and (ii) acute studies (single dose): | |
| 4.1. What are the relevant assessment methods (analytical methods)? | Narrative review |
| 4.2. Are there special considerations regarding specific population groups e.g. infants, subjects with diseases? | Narrative review |
| 4.3. How does bioavailability of these two forms compare with NF and folic acid? Does bioavailability of these two forms vary? (if enough data are available) | Systematic literature search and meta‐regression analysis |
| 5. Investigating bioavailability of CaLMF and 5‐MTHF glucosamine in animal models, compared to NF or folic acid: | |
| 6.1. How does folate absorption/metabolism in animal models differ from humans? | Narrative review |
| 6.2. What are the relevant animal models from which data could be extrapolated to humans? | See Section 1.5 |
| 6.3. What is the dose‐range in animal models that would be relevant for human intake? | Narrative review |
| 6.4. What is the relationship between the bioavailability of CaLMF and 5‐MTHF glucosamine compared to NF or folic acid in animal models? | Systematic literature search |
| 7. Investigating bioaccessibility in in vitro gastrointestinal systems, compared to NF or folic acid: | |
| 7.1. What are the differences in bioaccessibility in foods, if any, between added CaLMF or 5‐MTHF glucosamine, folic acid and NF | Systematic literature search |
| 8. To what extent could bioavailability data from bioavailability studies using 5‐MTHF associated with other cations than Ca and glucosamine be extrapolated to CaLMF and 5‐MTHF glucosamine? | Expert judgement |
To answer these questions, the steps shown in Figure A.1 and described in the following sections will be followed.
Figure A.1.

Methodological steps followed for the scientific assessment
A.2. Planned approach towards the evidence retrieval from the scientific literature
A.2.1. Method for the study selection for inclusion/exclusion
Questions in Section A.1 will be answered as indicated in Table A.1 described in detail in the following sections. The searches will be conducted by EFSA's information specialist, in Embase, Pubmed, Scifinder‐n and Europe PMC.
No limitation on the date of publication will be applied but the search will be limited to EU languages. The search strings can be found in Appendix B.
The screening at title, abstract and full‐text levels will be done using DistillerSR® (Evidence Partners, Ottawa, Canada), where at least two reviewers will do the screening in parallel. Conflicts that might arise will be discussed among the reviewers of that study. At the level of the title and abstract screening, the artificial intelligence tool of Distiller SR® may be used as the second screener. The references will then be exported to EndNote®.
Additional searches will be performed on the websites of relevant institutions and authorities, such as Health Canada, Australia or New Zealand authorities (a document from the US Food and Drug Administration (FDA) was already provided by the European Commission to EFSA to retrieve published reports on how to convert the amounts of CaLMF or 5‐MTHF glucosamine into μg DFE). The search strategy will be set up by EFSA's information specialist and is reported in Appendix B.
Reference lists of included papers, and possibly of relevant systematic or narrative reviews and meta‐analyses, patents and statements/assessments from other scientific bodies identified during the screening, will be searched (snowballing).
Authors of publications not reporting, in the full text, the form of 5‐MTHF (counter‐ion calcium, sodium, glucosamine of (6S)‐5‐MTHF, etc.) used in the relevant studies selected after the screening process will be contacted for further clarifications.
Regarding grey literature, a call for data will be set up to collect information from interested parties. The aim will be to collect information, mainly full study reports, on any unpublished studies (in vitro gastrointestinal models, studies on animals and/or humans):
-
•
Comparing the bioavailability of the two compounds of interest against a similar amount of folic acid or NF (ideally equimolar dose).
-
•
Studies on added 5‐MTHF associated with different cations.
-
•
Information on labelling practices and rationale for possible conversion factor used for CaLMF or 5‐MTHF glucosamine.
-
•
Information on conditions or diseases for which foods for special medical purposes containing CaLMF are produced.
A.2.2. Definition of the eligibility criteria for study selection
For questions for which a systematic literature search will be undertaken (see Table A.1), the inclusion and exclusion criteria that will be applied at all screening levels are described below. Reasons for exclusion of articles at full text level will be recorded in DistillerSR®.
Inclusion criteria
-
a
Intervention studies in an acute or repeated‐dose design, in all age and population groups, on comparative bioavailability assessment, i.e. oral consumption of CaLMF and/or 5‐MTHF glucosamine salt versus NF or folic acid (i.e. at least two arms needed in a study with parallel design or two distinct periods in a cross‐over study).
-
•
Intervention studies on another 5‐MTHF salt added to food, will be also considered, with the aim to possibly test the hypothesis that the cation would have a minimal effect on the bioavailability. Data discussed in previous EFSA assessments suggest that CaLMF or 5‐MTHF glucosamine salt readily dissociate in the gastrointestinal tract: the Panel will consider all possible cations present with (6S)‐5‐MTHF (i.e. no exclusion), but identify the cation and assess, if possible, if the results on comparative bioavailability of 5‐MTHF with folic acid or NF change according to the associated cation.
-
○
The study should provide similar, i.e. ideally equimolar, amount of added 5‐MTHF or folic acid/NF to the two different groups (e.g. for a two‐arm parallel study, if one arm receives twice the amount of the other arm, such a study should be excluded).
-
○
Relevant biomarkers that may be investigated in these intervention studies may be PTF concentration, RBC folate, possibly serum/plasma or RBC concentration of other forms of folate, urinary folate excretion, plasma tHCy, mean cell volume. tHCy and mean RBC volume are not specific to folate as they are influenced also by other nutrients (EFSA NDA Panel, 2014).
-
○
CaLMF or 5‐MTHF glucosamine are authorised for use in a variety of foods including foods for special medical purposes i.e. in foods that may be consumed by people with diseases who may also follow a medical treatment for their diseases. Hence, intervention studies, e.g. in populations with a disease, comparing CaLMF combined with a drug or 5‐MTHF glucosamine combined with a drug vs folic acid or NF combined with the same drug (same dose) should be included.
-
○
Intervention studies comparing CaLMF combined with (a) nutrient(s) or 5‐MTHF glucosamine combined with (a) nutrient(s) vs folic acid or NF combined with the same nutrient(s) (same dose), should be included.
-
b
Animal models on comparative bioavailability assessment, i.e. oral consumption of CaLMF and/or 5‐MTHF glucosamine (or another added 5‐MTHF salt added to feed) versus NF or folic acid (i.e. at least two arms needed in a study with parallel design or two distinct periods in a cross‐over study).
-
•
Test animals include birds (e.g. hens) and non‐ruminant mammals (as the behaviour of folates in the gastrointestinal tract of ruminant animals is different from that in humans), e.g. rats, mice, pigs, dogs, cats, guinea pigs, hamsters, primates and rabbits.
-
c
All in vitro gastrointestinal models (either dynamic or static models) comparing CaLMF and/or 5‐MTHF glucosamine (or another added 5‐MTHF salt) versus NF or folic acid.
-
•
In vitro models usually designed to mimic digestion in the gastrointestinal tract can give insight into assessing the bioaccessibility and/or bioavailability.
Exclusion criteria
Studies not respecting the criteria for inclusion, e.g.
-
•
Studies not on CaLMF, 5‐MTHF glucosamine or another added 5‐MTHF salt, e.g. studies on folic acid vs placebo or food folate, studies on administration of folinic acid (5‐formyl‐tetrahydrofolate, i.e. a natural constituent of food) vs other NF or folic acid or placebo, studies on the racemic mixture, 24 i.e. (6RS)‐5‐MTHF, studies on natural 5‐MTHF present in food as polyglutamate;
-
•
The Panel decided to exclude data from studies on the ‘racemic compound’ (6RS), as it is a compound different from CaLMF or 5‐MTHF glucosamine (with different biological properties, i.e. The ‘R' part of 5‐MTHF may interact with/block the folate receptor and RFC‐1 and the racemic compound potentially has different biological properties (Sirotnak and Tolner, 1999).
-
•
Clinical trials on CaLMF or 5‐MTHF glucosamine compared only with placebo;
-
•
Studies in heavy alcohol consumers, as alcohol could interfere with folate absorption (Halsted, 1990);
-
•
Studies on ruminants (see ‘animal models’ above) or fish, as absorption of folates may occur in fish through a simple diffusion (Casirola et al., 1995) while folates are predominantly absorbed by active transport in humans;
-
•
Studies on cells (e.g. hepatocytes, cultures cells), except if used in an in vitro gastrointestinal system for assessing bioavailability);
-
•
Studies in humans or animals on another administration route than oral consumption (e.g. injection);
-
•
Single‐arm clinical studies;
-
•
All types of observational studies;
-
•
Other publication types e.g. protocols, abstracts of conferences/congress/symposia, editorials, commentaries; reviews (systematic or narrative)/meta‐analyses; statements/opinions from competent authorities on oral consumption of CaLMF or 5‐MTHF glucosamine e.g. US FDA, Health Canada, Food Standards of New Zealand or Australia, the Joint FAO/WHO Expert Committee on Food Additives (JECFA), etc.; patents on CalMF or 5‐MTHF glucosamine /preparations with these folate forms.
-
○
If relevant to the subject, reviews/statements from competent authorities/patents will be excluded from the following steps of the literature search. However, they will be labelled as to be ‘kept as background’ in DistillerSR®, so that they could still be easily identified, retrieved and used for the questions that will be answered through a narrative description (and not a systematic search).
-
○
Follow‐up of clinical trials;
-
○
Studies on stability of CaLMF or 5‐MTHF glucosamine (i.e. thermic stability).
Intervention studies undertaken in subjects with diseases/health conditions (or animal models of human diseases if any) will not be excluded but will be identified as such, to be able to separate them from studies undertaken in healthy subjects, for further analysis and discussion (see Section 1.5 on data synthesis). Conditions that may influence folate metabolism (Maruyama et al., 2001; Bailey et al., 2015) include:
-
–
gastrointestinal conditions (e.g. Crohn's disease),
-
–
liver diseases (e.g. hepatitis, patients that had liver transplant), 25
-
–
haematological conditions (e.g. anaemia because this could be folate‐related and would have impact on the biomarkers (mainly serum and RBC folate) for assessment of bioavailability).
A.3. Method for data extraction from included studies
Data will be extracted in pre‐specified forms in Microsoft Excel® by one reviewer (an external contractor) and a second reviewer will be used as validator. Wherever possible, drop‐down menus with agreed terminology will be set‐up. Five different data extraction forms will be set up for:
-
–
repeated‐dose studies in healthy subjects,
-
–
repeated‐dose studies in individuals with a disease,
-
–
acute studies (single dose) in humans,
-
–
animal data,
-
–
in vitro gastrointestinal system.
If different experiments are described in the same paper, data of each experiment will be extracted separately under the respective design. Also, if bioavailability of CaLMF or 5‐MTHF glucosamine (relative to NF or folic acid) is studied in different population groups in the same paper (e.g. young adults vs. older adults, serum tHCy reported by MTHFR genotype, 26 two different species of animals), each population will be extracted separately.
Data may also be extracted from figures using the online tool WebPlotDigitizer. 27
Data extraction will be performed in a stepwise approach: priority will be given to extract data from repeated‐dose studies.
Information received after requesting authors for possible missing data will be included in the data extraction sheets.
The parameters to be extracted for human studies of different designs include standard study characteristics (author, year of publication, study name, location, design, randomisation, power calculation, blinding, duration/condition of consumption and compliance among others and where applicable); subject characteristics including number of subjects with certain polymorphisms and comparability between population groups by age, gender and body mass index (BMI) among others; intervention and comparator information (form of 5‐MTHF, daily dose, vehicle and frequency of consumption of 5‐MTHF and/or folic acid, dietary intake data, completeness by the subjects) and biomarker information (tHcy (μmol/L), RBC folate (nmol/L), PTF (nmol/L), RBC 5‐MTHF (nmol/L), serum/plasma 5‐MTHF (nmol/L), serum/plasma B12 (pmol/L), breast milk folate (nmol/L) and analytical and assay methods).
For animal studies, the parameters to be extracted include study characteristics, intervention and biomarker information as described earlier for human studies and where applicable, and animal characteristics including animal model, species, strain or breed. For in vitro gastrointestinal studies similar parameters will be extracted as for animal studies and where applicable and will also include static/dynamic characterisation of the in vitro system, activity of added enzymes, meal size, membrane cut‐off, frequency of total dialysate sample collection during digestion and endogenous folate content.
A.4. Method for appraising evidence
An appraisal will be scheduled for all included studies. The purpose of the appraisal will be the assessment of their internal validity, i.e. their RoB, and not to further exclude papers.
The appraisal for the studies in humans and animals will be based on the tool proposed by the US National Toxicology Program (NTP) Office of Health Assessment and Translation (OHAT) for conducting a literature‐based health assessment (NTP, 2019). However, the original set of questions proposed in the tool was adapted as deemed most appropriate to the present assessment, as envisaged in the OHAT handbook. The appraisal for the studies on in vitro gastrointestinal systems will be based on questions specific to this study design and some questions in line with those used for the human and animal studies. The integration into the overall RoB tier followed the approach proposed by OHAT (NTP, 2019).
The appraisal will be done by two reviewers and will be discussed collegially in case of disagreement. For each of the questions below, a two‐level‐assessment (low/high RoB) will be applied. Key question (i.e. priority questions) and non‐key questions will be identified.
A.4.1. Appraisal of repeated dose studies in humans
Compliance/exposure, outcome assessment and randomisation will be considered as key questions, and attrition and blinding as non‐key questions.
The Panel estimated that statistical analysis applied by the authors of the studies will not be a parameter relevant for the appraisal of the included studies for this assessment, as the focus of this assessment is relative/comparative bioavailability between the two forms (ratios of biomarkers) and an in‐house statistical analysis will be undertaken, if feasible.
-
1)
Compliance/exposure
-
○Low RoB: All four below criteria present, or at least the first three if the study duration is 16 weeks or more.
-
□assessment of the compliance with the intervention,
-
□analytical check of the dose given,
-
□exclusion of usual consumers of folic acid supplements/fortified foods/medicine/contraceptives, if feasible, 28
-
□assessment of baseline folate intake, in particular for the repeated‐dose studies of a duration shorter than 16 weeks.
-
□
-
○High RoB:
-
□The first three criteria listed above present (with the fourth not present) but the study duration is less than 16 weeks,
-
□or one or two of the first three criteria missing (with the fourth not present),
-
□or all four criteria missing/not reported.
-
□
-
2)
Outcome assessment (based on the analytical method)
The focus of the assessment will be the ratio of investigated biomarkers between the group receiving 5‐MTHF vs the group(s) receiving folic acid or NF (see Section 1.5). The Panel estimated that, even focusing on a ratio, the analytical method is a relevant point for appraisal, as it needs to be checked if the analytical methods applied for each of the parameters investigated have a linear error or not, and if they are precise.
The confidence in the outcome assessment will be assessed by two reviewers with expertise in analytical methods.
To assess whether the analytical methods and approaches of the included studies were associated with a low or high RoB, considerations on analytical methods described in the previous Scientific Opinion on DRVs for folate (EFSA NDA Panel, 2014) were taken into account. Therefore, the use of microbiological assay as well as LC/MS/MS method for PTF was considered to be associated with a low RoB and the protein binding method is associated with a high RoB.
LC/MS/MS is the only available method to assess individual folate derivatives. Regarding the LC/MS/MS method, the processes entailing the use of isotopically labelled internal standards (ideally 13C‐labelled), will be associated with the lowest RoB. For the chromatographic determination of individual folate compounds, when sufficient description of the analytical method or appropriate references are provided, it will be considered as low RoB. In addition, aspects such as, sufficiently detailed description of the method, evidence of quality control, validation of the method or inter‐assay coefficient of variation, will be taken into account by the reviewers. The use of certified reference materials will be associated with low RoB, while if the method is insufficiently described or not described, the analytical methodology would be associated with a high RoB.
-
3)
Randomisation
In view of the expected low number of included studies and the particular setting of the assessment (i.e. comparative bioavailability assessment), the following criteria will be applied:
-
○
Low RoB: randomised studies, whatever the method, even if not described,
-
○
High RoB: not randomised.
-
4
Attrition
The number and percentage of persons lost will be calculated, for each group of each study included, from the extracted information on the number of subjects included in the study and the number of completers. A guidance that will be born in mind to decide on whether the percentage of drop‐outs is low or high will be 10% as suggested in the OHAT Risk of Bias Rating Tool for Human and Animal Studies (NTP, 2019).
-
○Low RoB:
-
□No drop‐out,
-
□Or few drop‐outs (reasons for drop‐outs given or not, numbers of drop‐outs balanced or not between groups),
-
□Or high number/percentage of drop‐outs (if the numbers are balanced or not between groups), provided the reasons for drop‐outs are given and they do not seem related to the tested substances.
-
□
-
○High RoB:
-
•Not enough information to assess attrition,
-
•Or high number/percentage of drop‐outs (no reasons provided, numbers not balanced between groups).
-
•
-
5)
Blinding
The following criteria will be applied for the question on blinding, in view of the expected low number of included studies, the setting of the assessment (i.e. comparative bioavailability assessment), and the expected low level/lack of details on the method to ensure blinding:
-
○Low RoB.
-
□Double‐blind studies (even with no information on how blinding was ensured).
-
□
-
○High RoB.
-
□Single blind studies,
-
□Open label studies.
-
□
The individual assessment of the RoB will be combined in an overall assessment according to the algorithm as presented in the OHAT Risk of Bias Rating Tool for Human and Animal Studies (NTP, 2019).
A.4.2. Appraisal of acute studies (single dose) in humans
Compliance/exposure and outcome assessment will be considered as key questions, and randomisation a non‐key question.
The Panel estimated that attrition and blinding, considered for the appraisal of the repeated‐dose human studies, will not be parameters considered for the appraisal of the acute human studies in this assessment.
-
1)
Exposure/baseline status
The following six criteria will be considered. However, the absence of criteria vi will not be a sufficient reason to downgrade.
-
i)
Analytical check of the dose given,
-
ii)
Exclusion of usual consumers of folic acid supplements/fortified foods/medicine/contraceptives,
-
iii)
If criteria ii is missing/NR: this will not be considered a reason to downgrade if the study encompassed a pre‐saturation regimen (iii).
-
iv)
Pre‐saturation regimen with folic acid: in case of absence of pre‐saturation regimen, it is expected that the administered folate goes directly to the tissue and does not circulate in the blood (Pietrzik et al., 1990; Gregory 3rd, 2001; McNulty and Pentieva, 2010), while measurements of blood responses are the key outcome of interest for this assessment.
-
v)
Adequate length of wash‐out period in case of cross‐over studies. As a guideline for assessing whether the length was adequate, it will be kept in mind that metabolic studies showed that, with limited intake, serum folate decreases rapidly within 1–3 weeks before any changes in tissue folate start to occur (Herbert, 1962; Kauwell et al., 2000).
-
vi)
Standardisation of meal for folate during test days,
-
vii)
Assessment of baseline folate intake.
-
○
Low RoB
A low RoB will be attributed in case all criteria will be present or criterion vi will be missing.
-
○
High RoB
A high RoB will be attributed in case one or more of the other criteria will be missing.
-
2)
Outcome assessment (based on the analytical method)
As the objective of the present assessment is an evaluation of comparative bioavailability, which will be assessed by calculating ratios, the number of times the biological samples were collected, as well as the duration of monitoring the response, will not be used for the appraisal.
Based on expert's judgement, it is expected that Cmax would be reached after around 90 min of administration and it was decided that duration of monitoring would need to be at least 6 h. It was also noted that the use of labelled compounds is usually considered as the gold standard (Pietrzik et al., 1990; Gregory 3rd, 2001; McNulty and Pentieva, 2010), but very few studies are expected to use such a technique for CaLMF or 5‐MTHF glucosamine. Hence, both studies using labelled or unlabelled doses will be considered.
The general considerations for outcome assessment discussed in relation to the repeated dose studies in humans will also apply for the acute studies.
-
3)
Randomisation
This criterion for acute human studies is considered by the Panel to be less important than for the repeated‐dose studies in humans, as in acute studies (single dose), only the order of administration of the two compounds is randomised and in such a setting (laboratory setting, hence controlled), few confounding factors are expected.
-
○
Low RoB: tests substance given in random order, whatever the method of randomisation, even if not described,
-
○
High RoB: not randomised.
The individual assessment of the RoB will be combined in an overall assessment according to the algorithm as presented in the OHAT Risk of Bias Rating Tool for Human and Animal Studies (NTP, 2019).
A.4.3. Appraisal of studies in animals
Exposure and outcome assessment will be considered as key questions, and randomisation and animal care/housing conditions non‐key questions.
-
1)
Exposure
The following three criteria will be considered for the appraisal of exposure.
-
iConfidence in the dose administered:
-
○If a radioactive dose was administered to the animals, this will be considered as presenting a low RoB.
-
○If no radioactive dose was administered to the animals, it will be assessed if the dose was analytical checked.
-
○
-
iiWhether measures to prevent coprophagy in rodents (e.g. mice, rats) and in rabbits have been reported (criterion not applicable to other animal species).
-
○This is based on a previous opinion of the ANS Panel that noted that ‘studies on folate bioavailability in animals may be complicated by the effects of both coprophagy and dietary constituents (Abad and Gregory 3rd, 1987 ), and therefore, without controlling for these factors, results from animal studies with folates may not be relevant for humans’ (EFSA ANS Panel, 2013).
-
○
-
iiiAnalytical check of the NF in the feed and amount of feed reported.
-
○Acute studies (single dose): the studies will be downgraded to high RoB in case no information will be reported on the NF content and amount of feed consumed before the intervention.
-
○Repeated‐dose studies: the studies will be downgraded to high RoB in case no information will be reported on the NF content of the feed and the amount of feed consumed during the intervention.
-
○
-
○
Low RoB:
The reviewers will conclude on a low RoB if the studies presented all three criteria above.
-
○
High RoB.
The reviewers will conclude on a high RoB if one or more of the three criteria above was/were missing.
-
2)
Outcome assessment
The general considerations for outcome assessment discussed for repeated dose studies in humans will also apply to animal studies. For repeated‐dose studies in animals it may also be discussed whether the studies were of adequate duration in relation to the biomarkers investigated.
-
3)
Randomisation/body weight
The parameter ‘randomisation’ was considered by the Panel as less important for animal studies compared with human studies, as the variability in laboratory animals from genetically selected breeds is lower than in humans.
It is noted that that body weight is correlated with age in experimental animals. It will be checked whether groups were balanced at baseline regarding the information on body weight or the average weight.
-
○
Low RoB:
The reviewers will conclude on a low RoB if the studies were randomised and the animals/groups of animals were balance at baseline regarding body weights.
-
○
High RoB.
The reviewers will conclude on a high RoB if the studies were not randomised or if no information on body weight of the animals was reported.
-
4)
Animal care/housing conditions
Whether the papers mention a reference/guideline for animal care or refer to good laboratory practice will be checked.
It is expected that most of the studies in animals on added CaLMF or 5‐MTHF glucosamine will present a low level of details in the reporting of housing conditions of the animals.
The following criteria will be considered.
-
i)
Are the animals caged individually?
-
ii)Other housing conditions: e.g. control of temperature, light, humidity and air‐change.
-
○Low RoB.
-
○
Studies reporting on criterion i. and some of the questions mentioned in criterion ii will be considered as of low RoB.
-
○
High RoB.
Studies not reporting on any of the conditions above will be considered of high RoB.
The individual assessment of the RoB will be combined in an overall assessment according to the algorithm as presented in the OHAT Risk of Bias Rating Tool for Human and Animal Studies (NTP, 2019).
A.4.4. Appraisal of studies on in vitro gastrointestinal systems
All three questions below will be considered as key questions.
-
1)
Exposure
Dynamic models or static models may be used in the studies, and static models may also use cell lines to assess uptake in cells and translocation. The Panel considered that exposure is particularly important when cell lines are used.
Common criteria for appraisal will be applied for all models:
-
○Whether endogenous folate was measured in added enzyme solutions/added bile:
-
□If so, the study will be considered as having a low RoB.
-
□Otherwise, the study will be considered as having a high RoB.
-
□
-
○Folate compounds tested for purity/analytical check of folate compounds:
-
□If so, the study will be considered as having a low RoB.
-
□Otherwise, the study will be considered as having a high RoB.
-
□
-
2)
System settings/enzyme activity check
Considerations regarding adequate settings and protocols of the in vitro gastrointestinal systems (static or dynamic, systems using or not cell lines) as described elsewhere (Minekus et al., 2014; Verhoeckx et al., 2015; Brodkorb et al., 2019) will be taken into account. Particularly, duration of growth of the cells and measurement of the transepithelial electrical resistance (TEER) will be taken into account for epithelial cell models.
The following two main criteria (a) and (b) will be considered for appraisal.
-
○Were the systems set up correctly and are the settings adequately described?
-
□If so, the study will be considered as having a low RoB.
-
□Otherwise, the study will be considered as having a high RoB.
-
□
-
○Were the activities of the added enzymes checked (as theoretically, one of the folate compounds investigated may react preferentially or less with the enzymes used compared to the other folate compound(s) investigated?
-
□If so, the study will be considered as having a low RoB.
-
□Otherwise, the study will be considered as having a high RoB.
-
□
-
2)
Outcome assessment (based on the analytical method)
The general considerations for outcome assessment related to the analytical method discussed for repeated dose studies in humans will also to studies using in vitro gastrointestinal systems.
The individual assessment of the RoB will be combined in an overall assessment according to the algorithm as presented in the OHAT Risk of Bias Rating Tool for Human and Animal Studies (NTP, 2019).
A.5. Methods for synthesising the evidence
The method for synthesising the evidence will be qualitative for the questions to be addressed narratively as specified in Table A.1. For the questions to be addressed in a systematic manner the approach described below will be followed.
Three groups of study subjects may need to be identified, if data are available:
-
i)
Healthy subjects.
-
ii)
Subjects with a disease and consuming a medicine not known to interfere with folate metabolism (considering CaLMF has been authorised to be added in foods for special medical purposes assuming various pathological conditions), studies on subjects with diseases will be included if relevant for the present assessment and will be identified as such. Whether the diseases are related to a disturbance of folate metabolism will need to be discussed.
-
iii)
Subjects with a disease and consuming a medicine known to interfere with folate metabolism/absorption in the relevant studies included will be considered.
Regarding studies in humans, repeated‐dose studies will constitute the main focus of the assessment, as steady state is expected to be reached with longer duration of study. Acute studies (single dose) in humans will have secondary importance.
Respective baseline mean/median values of biomarkers in each study group will need to be considered whenever possible, as a larger response is expected when supplementation will be given to subjects with lower baseline status.
A grading of the biomarkers in humans will be considered, based on the previous opinion on DRVs for folate (EFSA NDA Panel, 2014), i.e. from the highest weight to the lowest weight:
-
1)
RBC folate concentrations will be considered as the most reliable biomarker of status and indicator of long‐term dietary intake,
-
2)
PTF concentrations will be considered as a sensitive marker of recent dietary intake.
Data on the other biomarkers will be considered as supportive evidence, e.g.:
-
3)
Serum concentration of other forms of folate,
-
4)
Plasma tHcy (sensitive but not a specific biomarker of folate status and function). Available data on the baseline status of the other B‐vitamins and, in particular, cobalamin (vitamin B12) will be recorded.
-
5)
Urinary concentrations (not a sensitive indicator of folate intake and status),
-
6)
Mean RBC volume (detected in only advance stages of folate deficiency and lacks specificity).
Different approaches for data synthesis may be considered depending on the type and amount of data collected:
-
a)
A quantitative data synthesis, e.g. by meta‐analysis if enough data are available;
-
b)
A qualitative data synthesis if a quantitative one is not possible due to insufficient number of relevant papers, e.g. discussing the results of the papers narratively and in a harmonised way, by group of RoB;
-
c)
A qualitative data analysis and synthesis with visual data synthesis if feasible.
If enough data is available, priority for data analysis will be given to repeated‐dose studies in humans. In that case, data from repeated‐dose human studies will be considered separately by biomarker.
Whenever data allow for a meaningful quantitative synthesis of the evidence, a statistical analysis will be performed with the aim to compare the bioavailability of 5‐MTHF group vs folic acid or NF group.
Data quality checks will be performed for each study. For each variable, the proportion of missing observations will be assessed; range checks will be carried out for all included variables to ensure that all values are biologically plausible. In the case of missing data, flexible and transparent strategies will be pursued, such as requesting missing data from the authors or placing the original results in adequate context, according to feasibility and on a per‐study basis (e.g. transforming medians or geometric means into arithmetic means by making statistical assumptions).
An exploratory analysis will be done in order to provide summary statistics and visualisation plots (e.g. profile plots, summary table). The suitable mixed statistical model will be selected to perform the analysis, using fixed‐ and random‐effects appropriately in order to account for both within and across study variability (Neter et al., 1996).
All the potential factors identified in the exploratory analysis, suspected to influence the bioavailability of added 5‐MTHF or the comparators, i.e. folic acid or NF, will be assessed (whenever data is available).
The possibility of publication bias may be investigated taking also into account whether relevant data will be obtained through the call for data. If publication bias is investigated, this will be done using visual inspection of funnel plots to investigate the association between study size and effect size.
Regarding animal studies, the administered dose will be harmonised in μg/kg body weight per day, if feasible from the data available in the papers. In case of missing values, default values to calculate doses in mg/kg body weight per day from feed concentrations in mg/kg feed or mg/L of drinking water according to study design (i.e. acute, sub‐acute, sub‐chronic, chronic) as proposed by EFSA Scientific Committee (2012) may be used.
A.6. Preliminary identification of sources of uncertainty and methods for prioritising them
Uncertainty analysis of the scientific assessment, i.e. identifying possible limitations in scientific knowledge and assessing their implications for scientific conclusions, will be discussed briefly, based on the EFSA guidance on uncertainty (EFSA Scientific Committee, 2018). This implies identifying the sources of uncertainty affecting the assessment, prioritising these sources based on their expected influence on the outcome/results and final overall discussions and planning how the uncertainty analysis will be handled. It is expected that the following identified sources of uncertainties will be present:
-
○
Lack of exhaustivity of the data;
-
○
Heterogeneity of the data set;
-
○
Uncertainty about the amount of substance consumed, the background intake and the way in which the substance is consumed (e.g. empty stomach or with a meal);
-
○
Uncertainty about the effect of possible microencapsulation of the individual folate derivative;
-
○
Uncertainty about the exact form of 5‐MTHF used in the studies and the effect of cation;
-
○
Uncertainties related to the measurement of the biomarkers (e.g. methods, precision of measurements);
-
○
Uncertainties related to the extrapolation of results from animal and in vitro studies;
-
○
Limited information (or non‐harmonised information) on the genetic susceptibility (MTHFR polymorphism(s)), representativity/relevance for the EU population;
-
○
Publication bias.
A.7. Methods for analysing uncertainties individually and combined
Uncertainties will be identified at each step of the assessment, but no formal uncertainty assessment is foreseen.
Appendix B – Search strategy for retrieving evidence on the comparative bioavailability of 5‐MTHF
Embase
Date of the search 30‐09‐2020
| Set | Query | Results |
|---|---|---|
| #12 | #11 AND ([albanian]/lim OR [basque]/lim OR [bosnian]/lim OR [bulgarian]/lim OR [catalan]/lim OR [croatian]/lim OR [czech]/lim OR [danish]/lim OR [dutch]/lim OR [english]/lim OR [esperanto]/lim OR [estonian]/lim OR [finnish]/lim OR [french]/lim OR [german]/lim OR [greek]/lim OR [hungarian]/lim OR [irish gaelic]/lim OR [italian]/lim OR [latvian]/lim OR [lithuanian]/lim OR [macedonian]/lim OR [norwegian]/lim OR [polish]/lim OR [polyglot]/lim OR [portuguese]/lim OR [romanian]/lim OR [scottish gaelic]/lim OR [serbian]/lim OR [slovak]/lim OR [slovenian]/lim OR [spanish]/lim OR [swedish]/lim) | 1,579 |
| #11 | #6 OR #7 OR #10 | 1,594 |
| #10 | #8 AND #9 | 856 |
| #9 | ‘absorption’/exp OR ‘bioavailability’/exp OR ‘biological marker'/exp OR ‘blood level’/de OR ‘dietary intake’/de OR ‘drug administration’/de OR ‘erythrocyte count’/exp OR ‘fortified food’/exp OR ‘mean corpuscular volume’/exp OR ‘megalocytosis’/exp OR ‘nutritional support’/exp OR ‘oral intake’/exp OR ‘pharmacokinetics’/exp OR ‘supplementation’/de OR ‘urinary excretion’/de OR ‘vitamin supplementation’/exp | 1,560,360 |
| #8 | ‘5 methyltetrahydrofolic acid’/exp OR 5mthf:ti,ab,kw OR ‘5 methylthf’:ti,ab,kw OR ‘5 mthf’:ti,ab,kw OR l5methylthf:ti,ab,kw OR ‘l5mthf’:ti,ab,kw OR ‘l5 mthf’:ti,ab,kw OR ‘l5 methylthf’:ti,ab,kw OR 6s5mthf:ti,ab,kw OR ‘6s5methylthf’:ti,ab,kw OR ‘6 s5 mthf’:ti,ab,kw OR ‘6 s5 methylthf’:ti,ab,kw OR l5methyltetrahydrofolic:ti,ab,kw OR ‘l5methyl tetrahydrofolic’:ti,ab,kw OR ‘l5methyl tetra hydro folic’:ti,ab,kw OR ‘5methyl tetrahydrofolic’:ti,ab,kw OR methylfolate:ti,ab,kw OR ‘methyl folate’:ti,ab,kw OR methyltetrahydrofolate:ti,ab,kw OR ‘methyl tetrahydrofolate’:ti,ab,kw OR ‘methyl tetra hydrofolate’:ti,ab,kw OR ‘methyl tetra hydro folate’:ti,ab,kw OR lmethylfolate:ti,ab,kw OR ‘lmethyl folate’:ti,ab,kw OR l5methyltetrahydrofolate:ti,ab,kw OR ‘l5methyl tetrahydrofolate’:ti,ab,kw OR ‘l5methyl tetra hydrofolate’:ti,ab,kw OR ‘l5methyl tetra hydro folate’:ti,ab,kw OR 6s5methyltetrahydrofolate:ti,ab,kw OR ‘6s5methyl tetra hydrofolate’:ti,ab,kw OR ‘6s5methy tetra hydro folate’:ti,ab,kw OR methyltetrahydrofolic:ti,ab,kw OR ‘methyl tetrahydrofolic’:ti,ab,kw OR ‘methyl tetra hydro folic’:ti,ab,kw OR 5methyltetrahydrofolic:ti,ab,kw OR ‘5methyl tertrahydrofolic’:ti,ab,kw OR ‘5methyl tetra hydrofolic’:ti,ab,kw OR ‘5methyl tetra hydro folic’:ti,ab,kw OR 6s5methyltetrahydrofolic:ti,ab,kw OR ‘6s5methyl tetrahydrofolic’:ti,ab,kw OR ‘6s5methyl tetra hydrofolic’:ti,ab,kw OR ‘6s5methyl tetra hydro folic’:ti,ab,kw | 3,471 |
| #7 | ((5mthf OR ‘5 methylthf’ OR ‘5 mthf’ OR l5methylthf OR ‘l5mthf’ OR ‘l5 mthf’ OR ‘l5 methylthf’ OR 6s5mthf OR ‘6s5methylthf’ OR ‘6 s5 mthf’ OR ‘6 s5 methylthf’ OR methyltetrahydrofolic OR ‘methyl tetrahydrofolic’ OR ‘methyl tetra hydro folic’ OR l5methyltetrahydrofolic OR ‘l5methyl tetrahydrofolic'OR ‘l5methyl tetra hydro folic’ OR 5methyltetrahydrofolic OR ‘5methyl tetrahydrofolic’ OR ‘5methyl tetra hydro folic’ OR ‘6s5methyl tetrahydrofolic’ OR ‘6s5methyl tetra hydrofolic’ OR ‘6s5methyl tetra hydro folic’ OR methylfolate OR ‘methyl folate’ OR methyltetrahydrofolate OR ‘methyl tetrahydrofolate'OR ‘methyl tetra hydrofolate’ OR ‘methyl tetra hydro folate’ OR lmethylfolate OR ‘lmethyl folate’ OR l5methyltetrahydrofolate OR ‘l5methyl tetrahydrofolate’ OR ‘l5methyl tetra hydrofolate’ OR ‘l5methyl tetra hydro folate’ OR 6s5methyltetrahydrofolate OR ‘6s5methyl tetra hydrofolate’ OR ‘6s5methy tetra hydro folate’ OR methyltetrahydrofolic OR ‘methyl tetrahydrofolic’ OR ‘methyl tetra hydro folic’ OR 5methyltetrahydrofolic OR ‘5methyl tertrahydrofolic’ OR ‘5methyl tetra hydrofolic’ OR ‘5methyl tetra hydro folic’ OR 6s5methyltetrahydrofolic OR ‘6s5methyl tetrahydrofolic’ OR ‘6s5methyl tetra hydrofolic’ OR ‘6s5methyl tetra hydro folic’) NEAR/7 (absor* OR adme OR bioavailab* OR ‘biological availability’ OR bioequivalen* OR ‘biological equivalen*’ OR biomarker* OR ‘biological marker*’ OR consum* OR distribut* OR eliminat* OR erythrocyte* OR ‘folate concentrat*’ OR ‘folate excret*’ OR fortif* OR ‘homocysteine concentrat*’ OR intak* OR kinetic* OR macrocyt* OR ‘mean cell volume’ OR ‘mean cells volume’ OR ‘mean corpuscular volume'OR megalocytos* OR metabolism* OR oral OR pharmacokinectic* OR plasma OR ‘rbc concentr*’ OR ‘rbc count’ OR ‘red blood cell’ OR ‘red blood cells'OR erythrocyte* OR ‘red blood cell count’ OR ‘red blood cell concentration’ OR ‘red blood cells count’ OR ‘red blood cells concentration’ OR serum OR status OR supplement* OR urinary OR urine)):ti,ab,kw | 643 |
| #6 | #1 OR #2 OR #3 OR #4 OR #5 | 662 |
| #5 | metafolin:ti,ab,kw OR ‘levomefolate calcium’:ti,ab,kw OR ‘levomefolate ca’:ti,ab,kw OR mefolinate:ti,ab,kw | 39 |
| #4 | ((5mthf OR ‘5 methylthf’ OR ‘5 mthf’ OR l5methylthf OR ‘l5mthf’ OR ‘l5 mthf’ OR ‘l5 methylthf’ OR 6s5mthf OR ‘6s5methylthf’ OR ‘6 s5 mthf’ OR ‘6 s5 methylthf’) NEAR/5 (acid OR acids OR ca OR calcium OR glucosamine)):ti,ab,kw | 125 |
| #3 | ((methyltetrahydrofolic OR ‘methyl tetrahydrofolic’ OR ‘methyl tetra hydro folic’ OR l5methyltetrahydrofolic OR ‘l5methyl tetrahydrofolic’ OR ‘l5methyl tetra hydro folic’ OR 5methyltetrahydrofolic OR ‘5methyl tetrahydrofolic’ OR ‘5methyl tetra hydro folic’ OR ‘6s5methyl tetrahydrofolic’ OR ‘6s5methyl tetra hydrofolic’ OR ‘6s5methyl tetra hydro folic’) NEAR/5 (acid OR acids)):ti,ab,kw | 322 |
| #2 | ((methylfolate OR ‘methyl folate’ OR methyltetrahydrofolate OR ‘methyl tetrahydrofolate’ OR ‘methyl tetra hydrofolate’ OR ‘methyl tetra hydro folate’ OR lmethylfolate OR ‘lmethyl folate’ OR l5methyltetrahydrofolate OR ‘l5methyl tetrahydrofolate’ OR ‘l5methyl tetra hydrofolate’ OR ‘l5methyl tetra hydro folate’ OR 6s5methyltetrahydrofolate OR ‘6s5methyl tetra hydrofolate’ OR ‘6s5methy tetra hydro folate’ OR methyltetrahydrofolic OR ‘methyl tetrahydrofolic’ OR ‘methyl tetra hydro folic’ OR 5methyltetrahydrofolic OR ‘5methyl tertrahydrofolic’ OR ‘5methyl tetra hydrofolic’ OR ‘5methyl tetra hydro folic’ OR 6s5methyltetrahydrofolic OR ‘6s5methyl tetrahydrofolic’ OR ‘6s5methyl tetra hydrofolic’ OR ‘6s5methyl tetra hydro folic’) NEAR/5 (acidOR acids OR ca OR calcium)):ti,ab,kw | 593 |
| #1 | ‘151,533‐22‐1’:ti,ab,kw OR ‘1,181,972–37‐1’:ti,ab,kw OR 151533221:ti,ab,kw OR 1181972371:ti,ab,kw OR ‘5 methyl 5 6 7 8 tetrahydropteroyl l glutamic acid’:ti,ab,kw | 3 |
PubMed
Date of the search 30‐09‐2020
| Search | Query | Results |
|---|---|---|
| #13 | Search: #12 AND (“bulgarian”[Language] OR “catalan”[Language] OR “croatian”[Language] OR “czech”[Language] OR “danish”[Language] OR “dutch”[Language] OR “english”[Language] OR “estonian”[Language] OR “finnish”[Language] OR “french”[Language] OR “german”[Language] OR “greek modern”[Language] OR “hungarian”[Language] OR “italian”[Language] OR “latvian”[Language] OR “lithuanian”[Language] OR “multiple languages”[Language] OR “norwegian”[Language] OR “polish”[Language] OR “portuguese”[Language] OR “romanian”[Language] OR “scottish gaelic”[Language] OR “serbian”[Language] OR “slovak”[Language] OR “slovenian”[Language] OR “spanish”[Language] OR “swedish”[Language] OR “undetermined”[Language] OR “welsh”[Language]) Sort by: Most Recent | 1,845 |
| #12 | Search: #11 OR #6 Sort by: Most Recent | 1,858 |
| #11 | Search: #10 AND #8 Sort by: Most Recent | 1,640 |
| #10 | Search: “5‐methyltetrahydrofolate” [Supplementary Concept] OR 5MTHF[tiab] OR “5 methylTHF”[tiab] OR “5 MTHF”[tiab] OR l5methylthf[tiab] OR “l5mthf”[tiab] OR “l5 mthf”[tiab] OR “l5 methylthf”[tiab] OR 6S5mthf[tiab] OR “6s5methylthf”[tiab] OR “6 s5 mthf”[tiab] OR “6 s5 methylthf”[tiab] OR methyltetrahydrofolic[tiab] OR “methyl tetrahydrofolic”[tiab] OR “methyl tetra hydro folic”[tiab] OR l5methyltetrahydrofolic[tiab] OR “l5methyl tetrahydrofolic”[tiab] OR “l5methyl tetra hydro folic”[tiab] OR 5methyltetrahydrofolic[tiab] OR “5methyl tetrahydrofolic”[tiab] OR “5methyl tetra hydro folic”[tiab] OR “6s5methyl tetrahydrofolic”[tiab] OR “6s5methyl tetra hydrofolic”[tiab] OR “6s5methyl tetra hydro folic”[tiab] OR methylfolate[tiab] OR “methyl folate”[tiab] OR methyltetrahydrofolate[tiab] OR “methyl tetrahydrofolate”[tiab] OR “methyl tetra hydrofolate”[tiab] OR “methyl tetra hydro folate”[tiab] OR lmethylfolate[tiab] OR “lmethyl folate”[tiab] OR l5methyltetrahydrofolate[tiab] OR “l5methyl tetrahydrofolate”[tiab] OR “l5methyl tetra hydrofolate”[tiab] OR “l5methyl tetra hydro folate”[tiab] OR 6s5methyltetrahydrofolate[tiab] OR “6s5methyl tetra hydrofolate”[tiab] OR “6s5methy tetra hydro folate”[tiab] OR methyltetrahydrofolic[tiab] OR “methyl tetrahydrofolic”[tiab] OR “methyl tetra hydro folic”[tiab] OR 5methyltetrahydrofolic[tiab] OR “5methyl tertrahydrofolic”[tiab] OR “5methyl tetra hydrofolic”[tiab] OR “5methyl tetra hydro folic”[tiab] OR 6s5methyltetrahydrofolic[tiab] OR “6s5methyl tetrahydrofolic”[tiab] OR “6s5methyl tetra hydrofolic”[tiab] OR “6s5methyl tetra hydro folic”[tiab] Sort by: Most Recent | 2,255 |
| #8 | Search: “Administration, Oral”[Mesh] OR “Biomarkers”[Mesh] OR “Diet”[Mesh:NoExp] OR “Dietary Supplements”[Mesh] OR “Erythrocyte Count”[Mesh] OR “Erythrocyte Indices”[Mesh] OR “Food, Fortified”[Mesh] OR “Kinetics”[Mesh] OR “Pharmacokinetics”[Mesh] OR absor*[tiab] OR bioavailab*[tiab] OR “biological availab*”[tiab] OR biomarker*[tiab] OR “biological marker”[tiab] OR “biological markers”[tiab] OR consum*[tiab] OR distribut*[tiab] OR eliminat*[tiab] OR erythrocyte*[tiab] OR “folate concentrat*”[tiab] OR “folate excret*”[tiab] OR fortif*[tiab] OR “homocysteine concentrat*”[tiab] OR intak*[tiab] OR kinetic*[tiab] OR macrocyt*[tiab] OR “mean cell volume*”[tiab] OR “mean cells volume*”[tiab] OR “mean corpuscular volume*” OR megalocytos*[tiab] OR metabolism*[tiab] OR oral[tiab] OR pharmacokinectic*[tiab] OR plasma[tiab] OR “rbc concentr*”[tiab] OR “rbc count”[tiab] OR “red blood cell”[tiab] OR “red blood cells”[tiab] OR serum[tiab] OR status[tiab] OR supplement*[tiab] OR urinary[tiab] OR urine[tiab] Sort by: Most Recent | 7,453,865 |
| #6 | Search: #1 OR #2 OR #3 OR #4 Sort by: Most Recent | 1,114 |
| #4 | Search: (5MTHF[tiab] OR “5 methylTHF”[tiab] OR “5 MTHF”[tiab] OR l5methylthf[tiab] OR “l5mthf”[tiab] OR “l5 mthf”[tiab] OR “l5 methylthf”[tiab] OR 6S5mthf[tiab] OR “6s5methylthf”[tiab] OR “6 s5 mthf”[tiab] OR “6 s5 methylthf”[tiab]) AND (acid[tiab] OR acids[tiab] OR CA[tiab] OR calcium[tiab] OR glucosamine[tiab]) Sort by: Most Recent | 204 |
| #3 | Search: ((methyltetrahydrofolic[tiab] OR “methyl tetrahydrofolic”[tiab] OR “methyl tetra hydro folic”[tiab] OR l5methyltetrahydrofolic[tiab] OR “l5methyl tetrahydrofolic”[tiab] OR “l5methyl tetra hydro folic”[tiab] OR 5methyltetrahydrofolic[tiab] OR “5methyl tetrahydrofolic”[tiab] OR “5methyl tetra hydro folic”[tiab] OR “6s5methyl tetrahydrofolic”[tiab] OR “6s5methyl tetra hydrofolic”[tiab] OR “6s5methyl tetra hydro folic”[tiab]) AND (acid[tiab] OR acids[tiab])) Sort by: Most Recent | 247 |
| #2 | Search: Metafolin[tiab] OR “Levomefolate calcium”[tiab] OR “Levomefolate ca”[tiab] OR “levomefolate calcium” [Supplementary Concept] OR Mefolinate[tiab] Sort by: Most Recent | 24 |
| #1 | Search: “151,533–22‐1”[tiab] OR “1,181,972–37‐1”[tiab] OR 151533221[tiab] OR 1181972371[tiab] OR ((methylfolate[tiab] OR “methyl folate”[tiab] OR methyltetrahydrofolate[tiab] OR “methyl tetrahydrofolate”[tiab] OR “methyl tetra hydrofolate”[tiab] OR “methyl tetra hydro folate”[tiab] OR lmethylfolate[tiab] OR “lmethyl folate”[tiab] OR l5methyltetrahydrofolate[tiab] OR “l5methyl tetrahydrofolate”[tiab] OR “l5methyl tetra hydrofolate”[tiab] OR “l5methyl tetra hydro folate”[tiab] OR 6s5methyltetrahydrofolate[tiab] OR “6s5methyl tetra hydrofolate”[tiab] OR “6s5methy tetra hydro folate”[tiab] OR methyltetrahydrofolic[tiab] OR “methyl tetrahydrofolic”[tiab] OR “methyl tetra hydro folic”[tiab] OR 5methyltetrahydrofolic[tiab] OR “5methyl tertrahydrofolic”[tiab] OR “5methyl tetra hydrofolic”[tiab] OR “5methyl tetra hydro folic”[tiab] OR 6s5methyltetrahydrofolic[tiab] OR “6s5methyl tetrahydrofolic”[tiab] OR “6s5methyl tetra hydrofolic”[tiab] OR “6s5methyl tetra hydro folic”[tiab]) AND (acid[tiab] OR acids[tiab] OR Ca[tiab] OR calcium[tiab])) OR “5 Methyl 5 6 7 8 tetrahydropteroyl l glutamic acid”[tiab] Sort by: Most Recent | 1,088 |
Scifinder‐n
Date of the search 30‐09‐2020
| Search | References |
|---|---|
| Search by substance: 151533–22‐1 | 110 |
| Search by substance: 1181972–37‐1 | 11 |
| Search by substance: 129025–21‐4 | 15 |
| After de‐duplication: | 133 |
Europe PMC
Date of the search: 20‐09‐2020
| Search | Results |
|---|---|
| (BODY:”6s5methyl tetrahydrofolic” OR BODY:”6s5methyl tetra hydrofolic” OR BODY:”6s5methyl tetra hydro folic” OR BODY:methylfolate OR BODY:”methyl folate” OR BODY:methyltetrahydrofolate OR BODY:”methyl tetrahydrofolate” OR BODY:”methyl tetra hydrofolate” OR BODY:”methyl tetra hydro folate” OR BODY:lmethylfolate OR BODY:”lmethyl folate” OR BODY:l5methyltetrahydrofolate OR BODY:”l5methyl tetrahydrofolate” OR BODY:”l5methyl tetra hydrofolate” OR BODY:”l5methyl tetra hydro folate” OR BODY:6s5methyltetrahydrofolate OR BODY:”6s5methyl tetra hydrofolate” OR BODY:”6s5methy tetra hydro folate” OR BODY:methyltetrahydrofolic OR BODY:”methyl tetrahydrofolic” OR BODY:”methyl tetra hydro folic” OR BODY:5methyltetrahydrofolic OR BODY:”5methyl tertrahydrofolic” OR BODY:”5methyl tetra hydrofolic” OR BODY:”5methyl tetra hydro folic” OR BODY:6s5methyltetrahydrofolic OR BODY:”6s5methyl tetrahydrofolic” OR BODY:”6s5methyl tetra hydrofolic” OR BODY:”6s5methyl tetra hydro folic”) AND (BODY:metabolism* OR BODY:oral OR BODY:pharmacokinectic* OR BODY:plasma OR BODY:”rbc concentr*” OR BODY:”rbc count” OR BODY:”red blood cell” OR BODY:”red blood cells” OR BODY:serum OR BODY:status OR BODY:supplement* OR BODY:urinary OR BODY:urine) AND (TITLE:folate OR TITLE:”folic acid” OR TITLE:”folic acids” OR ABSTRACT:folate OR ABSTRACT:”folic acid” OR ABSTRACT:”folic acids”) | 1,407 |
| (BODY:”6s5methyl tetrahydrofolic” OR BODY:”6s5methyl tetra hydrofolic” OR BODY:”6s5methyl tetra hydro folic” OR BODY:methylfolate OR BODY:”methyl folate” OR BODY:methyltetrahydrofolate OR BODY:”methyl tetrahydrofolate” OR BODY:”methyl tetra hydrofolate” OR BODY:”methyl tetra hydro folate” OR BODY:lmethylfolate OR BODY:”lmethyl folate” OR BODY:l5methyltetrahydrofolate OR BODY:”l5methyl tetrahydrofolate” OR BODY:”l5methyl tetra hydrofolate” OR BODY:”l5methyl tetra hydro folate” OR BODY:6s5methyltetrahydrofolate OR BODY:”6s5methyl tetra hydrofolate” OR BODY:”6s5methy tetra hydro folate” OR BODY:methyltetrahydrofolic OR BODY:”methyl tetrahydrofolic” OR BODY:”methyl tetra hydro folic” OR BODY:5methyltetrahydrofolic OR BODY:”5methyl tertrahydrofolic” OR BODY:”5methyl tetra hydrofolic” OR BODY:”5methyl tetra hydro folic” OR BODY:6s5methyltetrahydrofolic OR BODY:”6s5methyl tetrahydrofolic” OR BODY:”6s5methyl tetra hydrofolic” OR BODY:”6s5methyl tetra hydro folic”) AND (BODY:eliminat* OR BODY:erythrocyte* OR BODY:”folate concentrat*” OR BODY:”folate excret*” OR BODY:fortif* OR BODY:”homocysteine concentrat*” OR BODY:intak* OR BODY:kinetic* OR BODY:macrocyt* OR BODY:”mean cell volume*” OR BODY:”mean cells volume*” OR BODY:”mean corpuscular volume*” OR BODY:megalocytos*) AND (TITLE:folate OR TITLE:”folic acid” OR TITLE:”folic acids” OR ABSTRACT:folate OR ABSTRACT:”folic acid” OR ABSTRACT:”folic acids”) | 1,115 |
| (BODY:”6s5methyl tetrahydrofolic” OR BODY:”6s5methyl tetra hydrofolic” OR BODY:”6s5methyl tetra hydro folic” OR BODY:methylfolate OR BODY:”methyl folate” OR BODY:methyltetrahydrofolate OR BODY:”methyl tetrahydrofolate” OR BODY:”methyl tetra hydrofolate” OR BODY:”methyl tetra hydro folate” OR BODY:lmethylfolate OR BODY:”lmethyl folate” OR BODY:l5methyltetrahydrofolate OR BODY:”l5methyl tetrahydrofolate” OR BODY:”l5methyl tetra hydrofolate” OR BODY:”l5methyl tetra hydro folate” OR BODY:6s5methyltetrahydrofolate OR BODY:”6s5methyl tetra hydrofolate” OR BODY:”6s5methy tetra hydro folate” OR BODY:methyltetrahydrofolic OR BODY:”methyl tetrahydrofolic” OR BODY:”methyl tetra hydro folic” OR BODY:5methyltetrahydrofolic OR BODY:”5methyl tertrahydrofolic” OR BODY:”5methyl tetra hydrofolic” OR BODY:”5methyl tetra hydro folic” OR BODY:6s5methyltetrahydrofolic OR BODY:”6s5methyl tetrahydrofolic” OR BODY:”6s5methyl tetra hydrofolic” OR BODY:”6s5methyl tetra hydro folic”) AND (BODY:absor* OR BODY:adme OR BODY:bioavailab* OR BODY:”biological availability” OR BODY:bioequivalen* OR BODY:”biological equivalen*” OR BODY:biomarker* OR BODY:”biological marker*” OR BODY:consum* OR BODY:distribut*) AND (TITLE:folate OR TITLE:”folic acid” OR TITLE:”folic acids” OR ABSTRACT:folate OR ABSTRACT:”folic acid” OR ABSTRACT:”folic acids”) | 1,225 |
| (BODY:5MTHF OR BODY:”5 methylTHF” OR BODY:”5 MTHF” OR BODY:l5methylthf OR BODY:”l5mthf” OR BODY:”l5 mthf” OR BODY:”l5 methylthf” OR BODY:6S5mthf OR BODY:”6s5methylthf” OR BODY:”6 s5 mthf” OR BODY:”6 s5 methylthf” OR BODY:methyltetrahydrofolic OR BODY:”methyl tetrahydrofolic” OR BODY:”methyl tetra hydro folic” OR BODY:l5methyltetrahydrofolic OR BODY:”l5methyl tetrahydrofolic” OR BODY:”l5methyl tetra hydro folic” OR BODY:5methyltetrahydrofolic OR BODY:”5methyl tetrahydrofolic” OR BODY:”5methyl tetra hydro folic”) AND (BODY:absor* OR BODY:bioavailab* OR BODY:”biological availab*” OR BODY:consum* OR BODY:fortif* OR BODY:intak* OR BODY:oral OR BODY:status OR BODY:supplement*)AND (TITLE:”Vitamin B9” OR TITLE:folate OR TITLE:”folic acid” OR TITLE:”folic acids” OR ABSTRACT:”Vitamin B9” OR ABSTRACT:folate OR ABSTRACT:”folic acid” OR ABSTRACT:”folic acids”) | 457 |
| After de‐duplication: | 1,502 |
Appendix C – PRISMA 29 flow chart for the systematic literature search
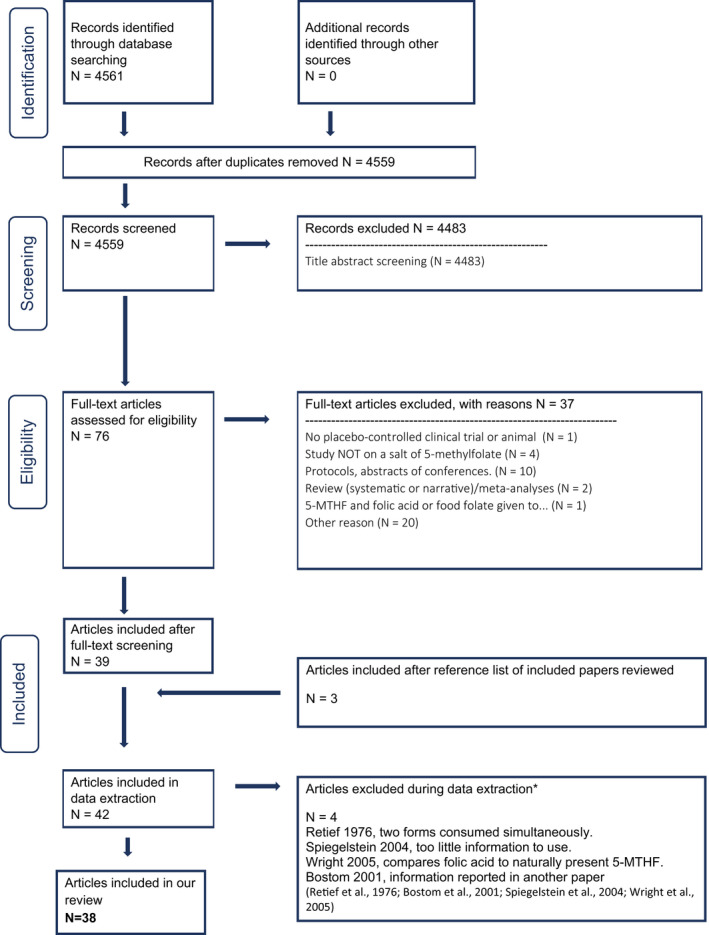
Appendix D – Outcome of the appraisal
Repeated dose studies in humans on RBC folate concentrations
| First author and year | Outcome | Exposure & Compliance 30 | Randomisation 29 | Outcome 29 | Attrition | Blinding | Tier |
|---|---|---|---|---|---|---|---|
| Diefenbach et al., 2013 | RBC folate | − | + | + | + | + | Tier 2 |
| Green et al., 2013 | RBC folate | + | + | + | + | + | Tier 1 |
| Henderson et al., 2018 | RBC folate | − | + | + | − | + | Tier 2 |
| Houghton et al., 2006 | RBC folate | + | − | + | + | + | Tier 2 |
| Pietrzik et al., 2007 | RBC folate | + | + | + | + | + | Tier 1 |
| Troesch et al., 2019 | RBC folate | + | + | + | + | + | Tier 1 |
| Venn et al., 2003 | RBC folate | + | + | + | + | + | Tier 1 |
| Wright et al., 2010 | RBC folate | − | − | + | + | + | Tier 2 |
| Khandanpour et al., 2009 | RBC folate | − | + | + | + | + | Tier 2 |
Repeated dose studies in humans on PTF
| First author and year | Outcome | Exposure & Compliance 29 | Randomisation 29 | Outcome 29 | Attrition | Blinding | Tier |
|---|---|---|---|---|---|---|---|
| Bailey and Ayling, 2018 | PTF | − | − | + | + | − | Tier 2 |
| de Meer et al., 2005 | PTF | − | + | − | + | + | Tier 2 |
| Diefenbach et al., 2013 | PTF | − | + | + | + | + | Tier 2 |
| Green et al., 2013 | PTF | + | + | + | + | + | Tier 1 |
| Hekmatdoost et al., 2015 | PTF | − | + | − | − | + | Tier 2 |
| Henderson et al., 2018 | PTF | − | + | + | − | + | Tier 2 |
| Houghton et al., 2006 | PTF | + | − | + | + | + | Tier 2 |
| Lamers et al., 2006 | PTF | + | + | + | + | + | Tier 1 |
| Sicińska et al., 2018 | PTF | − | + | − | + | + | Tier 2 |
| Venn et al., 2003 | PTF | + | + | + | + | + | Tier 1 |
| Wright et al., 2010 | PTF | − | − | + | + | + | Tier 2 |
| Bayes et al., 2019 | PTF | − | + | − | + | − | Tier 2 |
| Khandanpour et al., 2009 | PTF | − | + | + | + | + | Tier 2 |
Acute dose studies in humans
| First author and year | Parameters | Exposure 29 | Outcome 29 | Randomisation | Tier |
|---|---|---|---|---|---|
| Pentieva et al., 2004 | AUC | + | + | + | Tier 1 |
| Prinz‐Langenohl et al., 2009 | AUC | − | − | + | Tier 3 |
| Witthöft et al., 2006 | AUC dose corrected ratio | + | + | − | Tier 2 |
| Obeid et al., 2020 | AUC for plasma folate | − | − | + | Tier 3 |
| Obeid et al., 2020 | AUC for plasma 5‐MTHF | − | + | + | Tier 2 |
| Obeid et al., 2020 | AUC dose corrected | − | + | + | Tier 2 |
| Obeid et al., 2020 | Stomal content | − | + | + | Tier 2 |
| Gregory et al., 1992 | Isotope excretion ratio | + | + | − | Tier 2 |
| Tamura and Stokstad, 1973 | Folate availability | + | + | − | Tier 2 |
Animal studies
| First author and year | Parameters | Exposure 29 | Outcome 29 | Housing/animal care | Randomisation/body weight | Tier |
|---|---|---|---|---|---|---|
| Bhandari and Gregory, 1992 | Radioactivity in intestinal content | − | + | − | − | Tier 2 |
| Bhandari and Gregory, 1992 | % of retention | − | + | − | − | Tier 2 |
| Bhandari and Gregory, 1992 | % hepatic radioactivity | − | + | − | − | Tier 2 |
| Fernandez‐Borrachero et al., 1996 | jejunal absorption | − | − | − | + | Tier 3 |
| Miraglia et al., 2016 | AUC for plasma 5MTHF | − | − | + | + | Tier 3 |
| Pérez‐Conesa et al., 2009 | PTF | + | − | + | + | Tier 2 |
| Pérez‐Conesa et al., 2009 | RBC folate | + | − | + | + | Tier 2 |
| Pérez‐Conesa et al., 2009 | Liver folate | + | − | + | + | Tier 2 |
| Pérez‐Conesa et al., 2009 | Liver 5‐MTHF | + | − | + | + | Tier 2 |
| Tactacan et al., 2010 | Plasma/serum folate | + | + | + | + | Tier 1 |
| Tactacan et al., 2010 | Liver folate | + | + | + | + | Tier 1 |
| Pannia et al., 2021 | Plasma /serum 5‐MTHF | − | − | + | + | Tier 3 |
| Pannia et al., 2021 | Liver 5‐MTHF | − | − | + | + | Tier 3 |
In vitro studies
Appendix E – Characteristics of the human repeated dose studies upon which conclusions with respect to the update of DFE equation for adults are based
| Reference | Design (duration) | Subject characteristics | Intervention/compliance | Outcome assessment |
|---|---|---|---|---|
| Diefenbach et al., 2013 | Double‐blind, randomised controlled trial (24 weeks) |
n = 172 Healthy women, aged 18–40 y Baseline folate intake: mean 176 ± 70 μg |
Ethinylestradiol (EE)‐drospirenone + levomefolate calcium (CaLMF, 451 μg/day = 416 μg/day 5‐MTHF) or EE‐drospirenone and folic acid (400 μg/day) (invasion phase), and EE‐drospirenone for an additional 20 weeks (folate elimination phase). Consumption method: NR Compliance: tablet intake reported in diaries and return of unused study medication and empty blister packs at each visit. |
|
| Green et al., 2013 | Double‐blind, randomised, controlled trial (16 weeks) |
n = 45 (3 groups of 15) Healthy men and women, aged 18–45 year Baseline folate intake: NR |
Wheat rolls fortified with microencapsulated CaLMF (452 μg/day) or equimolar folic acid (400 μg/day) compared with wheat rolls containing no added folate (placebo) Participants were given 7 wheat rolls each week and were asked to consume 1 roll/day at their convenience. Consumption method: with a meal (wheat rolls) |
|
| Pietrzik et al., 2007 | Double‐blind, randomised, controlled, trial (24 weeks) |
n = 69 Healthy women of childbearing age 18–35 years Baseline folate intake: NR |
416 μg/day 5‐MTHF as Ca salt and equimolar 400 μg/day folic acid (hard gelatine capsules) Consumption: one capsule before breakfast Compliance: Pill count at each follow‐up visit |
|
| Wright et al., 2010 | Double‐blind, non‐randomised controlled trial (16 weeks) |
n = 163 healthy males and females aged 18–65 years Baseline folate intake: NR |
Volunteers consumed, in addition to their normal diets either (a) an additional 453 nmol/day natural food folate (from a selection of folate‐rich foods) or (b) a gelatine capsule containing supplemental 5‐MTHF (453 nmol) as Ca salt or (c) a gelatine capsule containing supplemental folic acid (453 nmol; 200 μg) or (d) a gelatine placebo capsule. Consumption: one capsule within each 24 h period Compliance: 7‐day weighed food record for food folate and pill count for 5‐MTHF and folic acid supplementation |
|
Annex A – Call for data on a draft scientific opinion on the conversion factor of calcium‐L‐methylfolate and (6S)‐5‐methyltetrahydrofolic acid glucosamine salt into dietary folate equivalent
The Annex can be found in the online version of this output, under the section ‘Supporting information’, at: https://doi.org/10.2903/j.efsa.2022.7452
Annex B – Statistical report
The Annex can be found in the online version of this output, under the section ‘Supporting information’, at: https://doi.org/10.2903/j.efsa.2022.7452
Annex C – Technical report: outcome of the public consultation on the draft scientific opinion on the conversion of calcium‐L‐methylfolate and (6S)‐5‐methyltetrahydrofolic acid glucosamine salt into dietary folate equivalents
The Annex can be found in the online version of this output, under the section ‘Supporting information’, at: https://doi.org/10.2903/j.efsa.2022.7452
Supporting information
Call for data on a draft scientific opinion on the conversion factor of calcium‐L‐methylfolate and (6S)‐5‐methyltetrahydrofolic acid glucosamine salt into dietary folate equivalent
Statistical report
Technical report: outcome of the public consultation on the draft scientific opinion on the conversion of calcium‐L‐methylfolate and (6S)‐5‐methyltetrahydrofolic acid glucosamine salt into dietary folate equivalents
Suggested citation: EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , Turck D, Bohn T, Castenmiller J, De Henauw S, Hirsch‐Ernst KI, Knutsen HK, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Peláez C, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Abrahantes JC, Dumas C, Ercolano V, Titz A and Pentieva K, 2022. Scientific Opinion on the conversion of calcium‐l‐methylfolate and (6S)‐5‐methyltetrahydrofolic acid glucosamine salt into dietary folate equivalents. EFSA Journal 2022;20(8):7452, 56 pp. 10.2903/j.efsa.2022.7452
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00542
Panel members: Dominique Turck, Torsten Bohn, Jacqueline Castenmiller, Stefaan De Henauw, Karen Ildico Hirsch‐Ernst, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Carmen Peláez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri and Marco Vinceti.
Declarations of interest: If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements: The Panel wishes to thank for their contribution to this output: the Working Group on Dietary Folate Equivalents: Francesco Cubadda and Kristina Pentieva; and EFSA staff members: Ester Artau Cortacans, Fulvio Barizzone, Angelo Cafaro, Janusz Ciok, Ionut Craciun, Irene Muñoz Guajardo, Estefania Noriega Fernandez, Charlotte Salgaard Nielsen and Angeliki Sofroniou. The Panel also wishes to acknowledge the contribution of Anne Molloy as hearing expert and all European competent institutions, Member State bodies and other organisations that provided data for this scientific output and the authors of published articles on folate who provided individual data or additional information upon request.
Adopted: 30 June 2022
Notes
OJ L 183, 12.7.2002, p. 51.
EFSA Journal 2013;11(10):3358.
OJ L 181, 29.6.2013, p. 35.
EFSA Journal 2004;2(11);135.
EFSA Journal 2020;18(1):5947.
Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow‐on formula and as regards requirements on information relating to infant and young child feeding, OJ L 25, 2.2.2016, p. 1.
Commission Delegated Regulation (EU) 2016/128 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for food for special medical purposes, OJ L 25, 2.2.2016, p. 30.
Commission Delegated Regulation (EU) 2017/1798 of 2 June 2017 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for total diet replacement for weight control, OJ L 259, 7.10.2017, p. 2.
OJ L 339, 6.12.2006, p. 16.
Dietary folate equivalent: 1 μg DFE = 1 μg food folate = 0.6 μg folic acid.
OJ L 304, 22.11.2011, p. 18.
i.e. 1 μg folic acid = 1.7 μg DFE.
The Panel on Food Additives and Flavourings (FAF) took over the work in most of the areas previously covered by the former Panel on Food Additives and Nutrient Sources Added to Food (ANS).
i.e. daily dose of about 100 μg folic acid or 104 μg 5‐MTHF, considering a molecular weight of folic acid of 441.4 g/mol and of 5‐MTHF of 459.5 g/mol.
i.e. daily dose of about 168 μg folic acid or 175 μg 5‐MTHF.
i.e. daily dose of about 271 μg folic acid or 282 μg 5‐MTHF.
i.e. daily dose of about 1.001 μg folic acid or 1.043 μg 5‐MTHF.
As reported in the publication.
i.e. daily dose of about 100 μg folic acid or 104 μg 5‐MTHF, considering a molecular weight of folic acid of 441.4 g/mol and of 5MTHF of 459.5 g/mol.
i.e. daily dose of about 173 μg folic acid or 180 μg 5‐MTHF.
i.e. daily dose of about 376 μg folic acid or 391 μg 5‐MTHF.
i.e. daily dose of about 1,002 mg folic acid or 1,043 mg 5‐MTHF.
Both the natural L‐isomer and the D‐isomer of 5‐MTHF have two chiral centres, with the consequence that four possible stereoisomers exist. However, by convention, both chiral centres in L‐MTHF have the natural L‐configuration (6S, αS) whereas in the D‐isomer (D‐5‐MTHF) the configuration of the chiral carbons is (6R, αS) i.e. the α‐C being the same as in the L‐ form. Thus, the difference between the D and L forms occurs as a function of a difference at only one chiral centre and this is the reason why the mixture of D and L forms is being referred to as a ‘racemic mixture’.
Liver transplants recipients take specific medications, including medicines which are protective for the liver and medicines blocking immunological reactions, and the results cannot be extrapolated to the general population.
MTHFR is a key enzyme in folate metabolism. In the opinion on DRV for folate (EFSA NDA Panel, 2014), it is explained that individuals with MTHFR 677TT have reduced MTHFR activity and it is recognised that they have higher dietary requirements for folate. Studies possibly only in people with MTHFR 677TT genotype will be considered separately.
It is considered difficult to exclude supplement consumers in some populations frequently supplemented, such as pregnant women that are generally supplemented in industrialised countries.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta‐Analyses.
Key question.
References
- Abad AR and Gregory 3rd JF, 1987. Determination of folate bioavailability with a rat bioassay. Journal of Nutrition, 117, 866–873. 10.1093/jn/117.5.866 [DOI] [PubMed] [Google Scholar]
- Akoglu B, Schrott M, Bolouri H, Jaffari A, Kutschera E, Caspary WF and Faust D, 2008. The folic acid metabolite L‐5‐methyltetrahydrofolate effectively reduces total serum homocysteine level in orthotopic liver transplant recipients: a double‐blind placebo‐controlled study. European Journal of Clinical Nutrition, 62, 796–801. 10.1038/sj.ejcn.1602778 [DOI] [PubMed] [Google Scholar]
- Alegría A, Garcia‐Llatas G and Cilla A, 2015. Static digestion models: general introduction. In: Verhoeckx K, Cotter P, López‐Expósito I, Kleiveland C, Lea T, Mackie A, Requena T, Swiatecka D and Wichers H (eds.). The impact of food bioactives on health. Cham, Heidelberg, New York, Dordrecht, London, Springer. pp. 3–12. [PubMed] [Google Scholar]
- Arkbåge K, Verwei M, Havenaar R and Witthöft C, 2003. Bioaccessibility of folic acid and (6S)‐5‐methyltetrahydrofolate decreases after the addition of folate‐binding protein to yogurt as studied in a dynamic in vitro gastrointestinal model. Journal of Nutrition, 133, 3678–3683. 10.1093/jn/133.11.3678 [DOI] [PubMed] [Google Scholar]
- Bailey SW and Ayling JE, 2009. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proceedings of the National Academy of Sciences of the United States of America, 106, 15424–15429. 10.1073/pnas.0902072106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SW and Ayling JE, 2018. The pharmacokinetic advantage of 5‐methyltetrahydrofolate for minimization of the risk for birth defects. Scientific Reports, 8, 4096. 10.1038/s41598-018-22191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory 3rd JF, Mills JL, Pfeiffer CM, Fazili Z, Zhang M, Ueland PM, Molloy AM, Caudill MA, Shane B, Berry RJ, Bailey RL, Hausman DB, Raghavan R and Raiten DJ, 2015. Biomarkers of nutrition for development‐folate review. Journal of Nutrition, 145, 1636S–1680S. 10.3945/jn.114.206599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayes J, Agrawal N and Schloss J, 2019. A pilot trial examining the absorption of oral forms of folate supplementation in a healthy population: a randomised control trial. Advances in Integrative Medicine, 6, 51–57. [Google Scholar]
- Bhandari SD and Gregory JF, 1992. Folic acid, 5‐methyl‐tetrahydrofolate and 5‐formyl‐tetrahydrofolate exhibit equivalent intestinal absorption, metabolism and in vivo kinetics in rats. Journal of Nutrition, 122, 1847–1854. 10.1093/jn/122.9.1847 [DOI] [PubMed] [Google Scholar]
- Bostom AG, Shemin D, Bagley P, Massy ZA, Zanabli A, Christopher K, Spiegel P, Jacques PF, Dworkin L and Selhub J, 2000. Controlled comparison of L‐5‐methyltetrahydrofolate versus folic acid for the treatment of hyperhomocysteinemia in hemodialysis patients. Circulation, 101, 2829–2832. 10.1161/01.cir.101.24.2829 [DOI] [PubMed] [Google Scholar]
- Bostom AG, Shemin D, Gohh RY, Beaulieu AJ, Bagley P, Massy ZA, Jacques PF, Dworkin L and Selhub J, 2001. Treatment of hyperhomocysteinemia in hemodialysis patients and renal transplant recipients. Kidney International. Supplement, 78, S246–S252. 10.1046/j.1523-1755.2001.59780246.x [DOI] [PubMed] [Google Scholar]
- Brodkorb A, Egger L, Alminger M, Alvito P, Assuncao R, Ballance S, Bohn T, Bourlieu‐Lacanal C, Boutrou R, Carriere F, Clemente A, Corredig M, Dupont D, Dufour C, Edwards C, Golding M, Karakaya S, Kirkhus B, Le Feunteun S, Lesmes U, Macierzanka A, Mackie AR, Martins C, Marze S, McClements DJ, Menard O, Minekus M, Portmann R, Santos CN, Souchon I, Singh RP, Vegarud GE, Wickham MSJ, Weitschies W and Recio I, 2019. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nature Protocols, 14, 991–1014. 10.1038/s41596-018-0119-1 [DOI] [PubMed] [Google Scholar]
- Casirola DM, Vinnakota RR and Ferraris RP, 1995. Intestinal absorption of water‐soluble vitamins in channel catfish (Ictalurus punctatus). American Journal of Physiology, 269, R490–R496. 10.1152/ajpregu.1995.269.3.R490 [DOI] [PubMed] [Google Scholar]
- Chandra‐Hioe MV, Addepalli R, Osborne SA, Slapetova I, Whan R, Bucknall MP and Arcot J, 2013. Transport of folic acid across Caco‐2 cells is more effective than 5‐methyltetrahydrofolate following the in vitro digestion of fortified bread. Food Research International, 53, 104–109. [Google Scholar]
- Diefenbach K, Trummer D, Ebert F, Lissy M, Koch M, Rohde B and Blode H, 2013. EE‐drospirenone‐levomefolate calcium versus EE‐drospirenone + folic acid: folate status during 24 weeks of treatment and over 20 weeks following treatment cessation. International Journal of Women's Health, 5, 149–163. 10.2147/IJWH.S37254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy ME, Hoey L, Hughes CF, Strain JJ, Rankin A, Souverein OW, Dullemeijer C, Collings R, Hooper L and McNulty H, 2014. Biomarker responses to folic acid intervention in healthy adults: a meta‐analysis of randomized controlled trials. American Journal of Clinical Nutrition, 99, 96–106. 10.3945/ajcn.113.062752 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2020. Draft framework for protocol development for EFSA's scientific assessments. EFSA Supporting Publication 2020;EN‐1843, 46 pp. 10.2903/sp.efsa.2020.EN-1843 [DOI]
- EFSA AFC Panel (Panel on food additives, flavourings, processing aids and materials in contact with food (AFC)) , 2004. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to calcium L‐methylfolate. EFSA Journal 2004;135, 20 pp. 10.2903/j.efsa.2004.135 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , 2013. Scientific Opinion on (6S)‐5‐methyltetrahydrofolic acid, glucosamine salt as a source of folate added for nutritional purposes to food supplements. EFSA Journal 2013;11(10):3358, 20 pp. 10.2903/j.efsa.2013.3358 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , 2018. Guidance on safety evaluation of sources of nutrients and bioavailability of nutrient from the sources. EFSA Journal 2018;16(6):5294, 35 pp. 10.2903/j.efsa.2018.5294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition, and Allergies) , 2010. Scientific Opinion on principles for deriving and applying Dietary Reference Values. EFSA Journal 2010;8(3):1458, 30 pp. 10.2903/j.efsa.2010.1458 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , 2014. Scientific Opinion on Dietary Reference Values for folate. EFSA Journal 2014;12(11):3893, 59 pp. 10.2903/j.efsa.2014.3893 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods Food Allergens) , 2020. Calcium L‐methylfolate as a source of folate added for nutritional purposes to infant and follow‐on formula, baby food and processed cereal‐based food. EFSA Journal 2020;18(1):5947, 17 pp. 10.2903/j.efsa.2020.5947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2018. Guidance on uncertainty analysis in scientific assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Borrachero O, Rubio JM, Delgado MJ, Carreras Sánchez O and Murillo ML, 1996. Folate absorption in the jejunum of chronic ethanol‐fed rats: in vivo studies. Annals of Nutrition and Metabolism, 40, 277–282. 10.1159/000177968 [DOI] [PubMed] [Google Scholar]
- Green TJ, Liu Y, Dadgar S, Li W, Böhni R and Kitts DD, 2013. Wheat rolls fortified with microencapsulated L‐5‐methyltetrahydrofolic acid or equimolar folic acid increase blood folate concentrations to a similar extent in healthy men and women. Journal of Nutrition, 143, 867–871. 10.3945/jn.113.174268 [DOI] [PubMed] [Google Scholar]
- Gregory 3rd JF, 1989. Chemical and nutritional aspects of folate research: analytical procedures, methods of folate synthesis, stability, and bioavailability of dietary folates. Advances in Food and Nutrition Research, 33, 1–101. 10.1016/s1043-4526(08)60126-6 [DOI] [PubMed] [Google Scholar]
- Gregory 3rd JF, 2001. Case study: folate bioavailability. Journal of Nutrition, 131, 1376S–1382S. 10.1093/jn/131.4.1376S [DOI] [PubMed] [Google Scholar]
- Gregory JF, Bhandari S, Bailey L, Toth J, Baumgartner T and Cerda J, 1992. Relative bioavailability of deuterium‐labeled monoglutamyl tetrahydrofolates and folic acid in human subjects. American Journal of Clinical Nutrition, 55, 1147–1153. 10.1093/ajcn/55.6.1147 [DOI] [PubMed] [Google Scholar]
- Halsted C, 1990. Intestinal absorption of dietary folates. In: Picciano M, Stockstad E and Gregory J (eds.). Folic acid metabolism in health and disease. Wiley‐Liss, New York. pp. 23–45. [Google Scholar]
- Hekmatdoost A, Vahid F, Yari Z, Sadeghi M, Eini‐Zinab H, Lakpour N and Arefi S, 2015. Methyltetrahydrofolate vs folic acid supplementation in idiopathic recurrent miscarriage with respect to methylenetetrahydrofolate reductase C677T and A1298C polymorphisms: a randomized controlled trial. PloS One, 10, e0143569. 10.1371/journal.pone.0143569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson GB, Tsuji JM and Kumar HP, 1987. Transport of folate compounds by leukemic cells. Evidence for a single influx carrier for methotrexate, 5‐methyltetrahydrofolate, and folate in CCRF‐CEM human lymphoblasts. Biochemical Pharmacology, 36, 3007–3014. 10.1016/0006-2952(87)90216-4 [DOI] [PubMed] [Google Scholar]
- Henderson AM, Aleliunas RE, Loh SP, Khor GL, Harvey‐Leeson S, Glier MB, Kitts DD, Green TJ and Devlin AM, 2018. L‐5‐Methyltetrahydrofolate supplementation increases blood folate concentrations to a greater extent than folic acid supplementation in Malaysian women. Journal of Nutrition, 148, 885–890. 10.1093/jn/nxy057 [DOI] [PubMed] [Google Scholar]
- Herbert V, 1962. Experimental nutritional folate deficiency in man. Transactions of the Association of American Physicians, 75, 307–320. [PubMed] [Google Scholar]
- Hopkins SM, Gibney MJ, Nugent AP, McNulty H, Molloy AM, Scott JM, Flynn A, Strain JJ, Ward M, Walton J and McNulty BA, 2015. Impact of voluntary fortification and supplement use on dietary intakes and biomarker status of folate and vitamin B‐12 in Irish adults. American Journal of Clinical Nutrition, 101, 1163–1172. 10.3945/ajcn.115.107151 [DOI] [PubMed] [Google Scholar]
- Houghton LA, Sherwood KL, Pawlosky R, Ito S and O'Connor DL, 2006. [6S]‐5‐Methyltetrahydrofolate is at least as effective as folic acid in preventing a decline in blood folate concentrations during lactation. American Journal of Clinical Nutrition, 83, 842–850. 10.1093/ajcn/83.4.842 [DOI] [PubMed] [Google Scholar]
- Houghton LA, Yang J and O'Connor DL, 2009. Unmetabolized folic acid and total folate concentrations in breast milk are unaffected by low‐dose folate supplements. American Journal of Clinical Nutrition, 89, 216–220. 10.3945/ajcn.2008.26564 [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) , 1998. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. 591 pp. [PubMed]
- Kauwell GP, Lippert BL, Wilsky CE, Herrlinger‐Garcia K, Hutson AD, Theriaque DW, Rampersaud GC, Cerda JJ and Bailey LB, 2000. Folate status of elderly women following moderate folate depletion responds only to a higher folate intake. The Journal of nutrition, 130, 1584–1590. [DOI] [PubMed] [Google Scholar]
- Khandanpour N, Armon MP, Jennings B, Finglas PM, Willis G, Clark A and Meyer FJ, 2009. Randomized clinical trial of folate supplementation in patients with peripheral arterial disease. British Journal of Surgery, 96, 990–998. 10.1002/bjs.6670 [DOI] [PubMed] [Google Scholar]
- Lamers Y, Prinz‐Langenohl R, Moser R and Pietrzik K, 2004. Supplementation with [6S]‐5‐methyltetrahydrofolate or folic acid equally reduces plasma total homocysteine concentrations in healthy women. American Journal of Clinical Nutrition, 79, 473–478. 10.1093/ajcn/79.3.473 [DOI] [PubMed] [Google Scholar]
- Lamers Y, Prinz‐Langenohl R, Brämswig S and Pietrzik K, 2006. Red blood cell folate concentrations increase more after supplementation with [6S]‐5‐methyltetrahydrofolate than with folic acid in women of childbearing age. American Journal of Clinical Nutrition, 84, 156–161. 10.1093/ajcn/84.1.156 [DOI] [PubMed] [Google Scholar]
- Maruyama S, Hirayama C, Yamamoto S, Koda M, Udagawa A, Kadowaki Y, Inoue M, Sagayama A and Umeki K, 2001. Red blood cell status in alcoholic and non‐alcoholic liver disease. Journal of Laboratory and Clinical Medicine, 138, 332–337. 10.1067/mlc.2001.119106 [DOI] [PubMed] [Google Scholar]
- McNulty H and Pentieva K, 2010. Folate bioavailability. In: Bailey L (ed.). Folate in health and disease. 2nd edn. CRC Press, Boca Raton, USA. pp. 25–48. [Google Scholar]
- de Meer K, Smulders YM, Dainty JR, Smith DE, Kok RM, Stehouwer CD, Finglas PM and Jakobs C, 2005. [6S]5‐methyltetrahydrofolate or folic acid supplementation and absorption and initial elimination of folate in young and middle‐aged adults. European Journal of Clinical Nutrition, 59, 1409–1416. 10.1038/sj.ejcn.1602254 [DOI] [PubMed] [Google Scholar]
- Minekus M, Alminger M, Alvito P, Ballance S, Bohn T, Bourlieu C, Carriere F, Boutrou R, Corredig M, Dupont D, Dufour C, Egger L, Golding M, Karakaya S, Kirkhus B, Le Feunteun S, Lesmes U, Macierzanka A, Mackie A, Marze S, McClements DJ, Menard O, Recio I, Santos CN, Singh RP, Vegarud GE, Wickham MS, Weitschies W and Brodkorb A, 2014. A standardised static in vitro digestion method suitable for food ‐ an international consensus. Food & Function, 5, 1113–1124. 10.1039/c3fo60702j [DOI] [PubMed] [Google Scholar]
- Miraglia N, Agostinetto M, Bianchi D and Valoti E, 2016. Enhanced oral bioavailability of a novel folate salt: comparison with folic acid and a calcium folate salt in a pharmacokinetic study in rats. Minerva Ginecologica, 68, 99–105. [PubMed] [Google Scholar]
- Neter J, Kutner MH, Nachtsheim CJ and Wasserman W, 1996. Applied linear statistical models. Chicago, USA, Irwin. 1408 pp. [Google Scholar]
- No authors listed , 2010, unpublished. Crossover comparative bioavailability study of 5‐methyltetrahydrofolate glucosamine salt compared to the reference metafolin® in healthy volunteers. 322 pp.
- NTP (National Institute of Environmental Health science) , 2019. Handbook for conducting a literature‐based health assessment using OHAT approach for systematic review and evidence integration. Available online: https://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookmarch2019_508.pdf [Google Scholar]
- Nygren‐Babol L and Jägerstad M, 2012. Folate‐binding protein in milk: a review of biochemistry, physiology, and analytical methods. Critical Reviews in Food Science and Nutrition, 52, 410–425. 10.1080/10408398.2010.500499 [DOI] [PubMed] [Google Scholar]
- Obeid R, Schön C, Pietrzik K, Menzel D, Wilhelm M, Smulders Y, Knapp JP and Böhni R, 2020. Pharmacokinetics of sodium and calcium salts of (6S)‐5‐methyltetrahydrofolic acid compared to folic acid and indirect comparison of the two salts. Nutrients, 12, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrvik V, Büttner B, Rychlik M, Lundin E and Witthöft C, 2010. Folate bioavailability from breads and a meal assessed with a human stable‐isotope area under the curve and ileostomy model. American Journal of Clinical Nutrition, 92, 532–538. 10.3945/ajcn.2009.29031 [DOI] [PubMed] [Google Scholar]
- Pannia E, Hammoud R, Simonian R, Arning E, Ashcraft P, Wasek B, Bottiglieri T, Pausova Z, Kubant R and Anderson GH, 2021. [6 s]‐5‐methyltetrahydrofolic acid and folic acid pregnancy diets differentially program metabolic phenotype and hypothalamic gene expression of wistar rat dams post‐birth. Nutrients, 13, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanwala I, King MJ, Barrett DA, Rose J, Jackson R, Hudson M, Philo M, Dainty JR, Wright AJ, Finglas PM and Jones DE, 2014. Folic acid handling by the human gut: implications for food fortification and supplementation. American Journal of Clinical Nutrition, 100, 593–599. 10.3945/ajcn.113.080507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentieva K, McNulty H, Reichert R, Ward M, Strain JJ, McKillop DJ, McPartlin JM, Connolly E, Molloy A, Krämer K and Scott JM, 2004. The short‐term bioavailabilities of [6S]‐5‐methyltetrahydrofolate and folic acid are equivalent in men. Journal of Nutrition, 134, 580–585. 10.1093/jn/134.3.580 [DOI] [PubMed] [Google Scholar]
- Pérez‐Conesa D, Haro‐Vicente JF, Braquehais FR and Ros G, 2009. [6S]‐5‐Methyltetrahydrofolate enhances folate status in rats fed growing‐up milk. European Journal of Nutrition, 48, 365–371. 10.1007/s00394-009-0022-1 [DOI] [PubMed] [Google Scholar]
- Pfeiffer CM, Rogers LM, Bailey LB and Gregory 3rd JF, 1997. Absorption of folate from fortified cereal‐grain products and of supplemental folate consumed with or without food determined by using a dual‐label stable‐isotope protocol. American Journal of Clinical Nutrition, 66, 1388–1397. [DOI] [PubMed] [Google Scholar]
- Pietrzik K, Hages M and Remer T, 1990. Methodological aspects in vitamin bioavailability testing. Journal of Micronutrient Analysis, 7, 207–222. [Google Scholar]
- Pietrzik K, Lamers Y, Brämswig S and Prinz‐Langenohl R, 2007. Calculation of red blood cell folate steady state conditions and elimination kinetics after daily supplementation with various folate forms and doses in women of childbearing age. American Journal of Clinical Nutrition, 86, 1414–1419. 10.1093/ajcn/86.5.1414 [DOI] [PubMed] [Google Scholar]
- Prinz‐Langenohl R, Brämswig S, Tobolski O, Smulders YM, Smith DE, Finglas PM and Pietrzik K, 2009. [6S]‐5‐methyltetrahydrofolate increases plasma folate more effectively than folic acid in women with the homozygous or wild‐type 677C‐‐ > T polymorphism of methylenetetrahydrofolate reductase. British Journal of Pharmacology, 158, 2014–2021. 10.1111/j.1476-5381.2009.00492.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Min SH, Jansen M, Malhotra U, Tsai E, Cabelof DC, Matherly LH, Zhao R, Akabas MH and Goldman ID, 2007. Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. American Journal of Physiology: Cell Physiology, 293, C1669–C1678. 10.1152/ajpcell.00202.2007 [DOI] [PubMed] [Google Scholar]
- Retief FP, Du PHA, Oosthuizen M and Van Reenen OR, 1976. In vivo plasma and urine folate binding after ingestation of 3H‐folic acid and 14C‐methyl‐folate. British Journal of Haematology, 33, 415–424. 10.1111/j.1365-2141.1976.tb03559.x [DOI] [PubMed] [Google Scholar]
- Ringling C and Rychlik M, 2017. Origins of the difference between food folate analysis results obtained by LC–MS/MS and microbiological assays. Analytical and Bioanalytical Chemistry, 409, 1815–1825. 10.1007/s00216-016-0126-4 [DOI] [PubMed] [Google Scholar]
- van Rossum CTM, Fransen HP, Verkaik‐Kloosterman J, Buurma‐Rethans EJM and Ocké MC, 2011. Dutch National Food Consumption Survey 2007–2010 ‐ Diet of children and adults aged 7 to 69 years. National Institute for Public Health and the Environment, Report number: 350050006/2011, 148 pp.
- Sauberlich HE, Kretsch MJ, Skala JH, Johnson HL and Taylor PC, 1987. Folate requirement and metabolism in nonpregnant women. American Journal of Clinical Nutrition, 46, 1016–1028. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee on Food) , 2000. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of folate. SCF/CS/NUT/UPPLEV/18 Final, 9. [Google Scholar]
- Selhub J, Jacques PF, Dallal G, Choumenkovitch S and Rogers G, 2008. The use of blood concentrations of vitamins and their respective functional indicators to define folate and vitamin B12 status. Food and Nutrition Bulletin, 29, S67–S73. 10.1177/15648265080292s110 [DOI] [PubMed] [Google Scholar]
- Sicińska E, Brzozowska A, Roszkowski W and Finglas PM, 2018. Supplementation with [6S]‐5‐methyltetrahydrofolate or folic acid equally reduces serum homocysteine concentrations in older adults. International Journal of Food Sciences and Nutrition, 69, 64–73. 10.1080/09637486.2017.1320536 [DOI] [PubMed] [Google Scholar]
- Sirotnak FM and Tolner B, 1999. Carrier‐mediated membrane transport of folates in mammalian cells. Annual Review of Nutrition, 19, 91–122. 10.1146/annurev.nutr.19.1.91 [DOI] [PubMed] [Google Scholar]
- Spiegelstein O, Mitchell LE, Merriweather MY, Wicker NJ, Zhang Q, Lammer EJ and Finnell RH, 2004. Embryonic development of folate binding protein‐1 (Folbp1) knockout mice: effects of the chemical form, dose, and timing of maternal folate supplementation. Developmental Dynamics, 231, 221–231. 10.1002/dvdy.20107 [DOI] [PubMed] [Google Scholar]
- Sweeney MR, McPartlin J and Scott J, 2007. Folic acid fortification and public health: report on threshold doses above which unmetabolised folic acid appear in serum. BMC Public Health, 7, 41. 10.1186/1471-2458-7-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tactacan GB, Jing M, Thiessen S, Rodriguez‐Lecompte JC, O'Connor DL, Guenter W and House JD, 2010. Characterization of folate‐dependent enzymes and indices of folate status in laying hens supplemented with folic acid or 5‐methyltetrahydrofolate. Poultry Science, 89, 688–696. 10.3382/ps.2009-00417 [DOI] [PubMed] [Google Scholar]
- Tamura T and Stokstad E, 1973. The availability of food folate in man. British Journal of Haematology, 25, 513–532. 10.1111/j.1365-2141.1973.tb01763.x [DOI] [PubMed] [Google Scholar]
- Troesch B, Demmelmair J, Gimpfl M, Hecht C, Lakovic G, Roehle R, Sipka L, Trisic B, Vusurovic M, Schoop R, Zdjelar S and Koletzko B, 2019. Suitability and safety of L‐5‐methyltetrahydrofolate as a folate source in infant formula: a randomized‐controlled trial. PloS One, 14, e0216790. 10.1371/journal.pone.0216790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn BJ, Green TJ, Moser R, McKenzie JE, Skeaff CM and Mann J, 2002. Increases in blood folate indices are similar in women of childbearing age supplemented with [6S]‐5‐methyltetrahydrofolate and folic acid. Journal of Nutrition, 132, 3353–3355. 10.1093/jn/132.11.3353 [DOI] [PubMed] [Google Scholar]
- Venn BJ, Green TJ, Moser R and Mann JI, 2003. Comparison of the effect of low‐dose supplementation with L‐5‐methyltetrahydrofolate or folic acid on plasma homocysteine: a randomized placebo‐controlled study. American Journal of Clinical Nutrition, 77, 658–662. 10.1093/ajcn/77.3.658 [DOI] [PubMed] [Google Scholar]
- Verhoeckx K, Cotter P, López‐Expósito I, Kleiveland C, Lea T, Mackie A, Requena T, Swiatecka D and Wichers H, 2015. The impact of food bioactives on health: in vitro and ex vivo models. Cham, CH, Springer. 355 p. [PubMed] [Google Scholar]
- Verwei M, Arkbåge K, Havenaar R, van den Berg H, Witthöft C and Schaafsma G, 2003. Folic acid and 5‐methyltetrahydrofolate in fortified milk are bioaccessible as determined in a dynamic in vitro gastrointestinal model. Journal of Nutrition, 133, 2377–2383. 10.1093/jn/133.7.2377 [DOI] [PubMed] [Google Scholar]
- West AA, Yan J, Perry CA, Jiang X, Malysheva OV and Caudill MA, 2012. Folate‐status response to a controlled folate intake in nonpregnant, pregnant, and lactating women. American Journal of Clinical Nutrition, 96, 789–800. 10.3945/ajcn.112.037523 [DOI] [PubMed] [Google Scholar]
- Witthöft CM, Strålsjö L, Berglund G and Lundin E, 2003. A human model to determine folate bioavailability from food: a pilot study for evaluation. Scandinavian Journal of Nutrition/Naringsforskning, 47, 6–18. [Google Scholar]
- Witthöft CM, Arkbåge K, Johansson M, Lundin E, Berglund G, Zhang JX, Lennernäs H and Dainty JR, 2006. Folate absorption from folate‐fortified and processed foods using a human ileostomy model. British Journal of Nutrition, 95, 181–187. 10.1079/bjn20051620 [DOI] [PubMed] [Google Scholar]
- Wright AJ, Finglas PM, Dainty JR, Hart DJ, Wolfe CA, Southon S and Gregory JF, 2003. Single oral doses of 13C forms of pteroylmonoglutamic acid and 5‐formyltetrahydrofolic acid elicit differences in short‐term kinetics of labelled and unlabelled folates in plasma: potential problems in interpretation of folate bioavailability studies. British Journal of Nutrition, 90, 363–371. 10.1079/bjn2003908 [DOI] [PubMed] [Google Scholar]
- Wright AJ, Finglas PM, Dainty JR, Wolfe CA, Hart DJ, Wright DM and Gregory JF, 2005. Differential kinetic behavior and distribution for pteroylglutamic acid and reduced folates: a revised hypothesis of the primary site of PteGlu metabolism in humans. Journal of Nutrition, 135, 619–623. 10.1093/jn/135.3.619 [DOI] [PubMed] [Google Scholar]
- Wright AJ, King MJ, Wolfe CA, Powers HJ and Finglas PM, 2010. Comparison of (6 S)‐5‐methyltetrahydrofolic acid v. folic acid as the reference folate in longer‐term human dietary intervention studies assessing the relative bioavailability of natural food folates: comparative changes in folate status following a 16‐week placebo‐controlled study in healthy adults. British Journal of Nutrition, 103, 724–729. 10.1017/S0007114509992339 [DOI] [PubMed] [Google Scholar]
- Zappacosta B, Mastroiacovo P, Persichilli S, Pounis G, Ruggeri S, Minucci A, Carnovale E, Andria G, Ricci R, Scala I, Genovese O, Turrini A, Mistura L, Giardina B and Iacoviello L, 2013. Homocysteine lowering by folate‐rich diet or pharmacological supplementations in subjects with moderate hyperhomocysteinemia. Nutrients, 5, 1531–1543. 10.3390/nu5051531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Chen ZS, Belinsky MG, Rea PA and Kruh GD, 2001. Transport of methotrexate (MTX) and folates by multidrug resistance protein (MRP) 3 and MRP1: effect of polyglutamylation on MTX transport. Cancer Research, 61, 7225–7232. [PubMed] [Google Scholar]
- Zhao R, Diop‐Bove N, Visentin M and Goldman ID, 2011. Mechanisms of membrane transport of folates into cells and across epithelia. Annual Review of Nutrition, 31, 177–201. 10.1146/annurev-nutr-072610-145133 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Call for data on a draft scientific opinion on the conversion factor of calcium‐L‐methylfolate and (6S)‐5‐methyltetrahydrofolic acid glucosamine salt into dietary folate equivalent
Statistical report
Technical report: outcome of the public consultation on the draft scientific opinion on the conversion of calcium‐L‐methylfolate and (6S)‐5‐methyltetrahydrofolic acid glucosamine salt into dietary folate equivalents


