Abstract
FarA of Streptomyces lavendulae FRI-5 is a specific receptor protein for IM-2, a butyrolactone autoregulator that controls the production of a blue pigment and the nucleoside antibiotics showdomycin and minimycin. Gel shift assays demonstrated that FarA binds to the farA upstream region and that this binding is abolished in the presence of IM-2. The FarA binding sequence was localized by DNase I footprinting to a 28-bp sequence located approximately 70 bp upstream of the farA translational start site. High-resolution S1 nuclease mapping of farA transcripts revealed a putative transcription start site, located at an A residue positioned 64 bp upstream from the farA translation start codon and 4 bp downstream from an Escherichia coli ς70-like −10 recognition region. The FarA-binding sequence overlaps this −10 region and contains the farA transcription initiation site, suggesting that FarA acts as a repressor that, in the absence of IM-2, represses transcription of farA.
IM-2 [(2R,3R,6R)-2-(hydroxybutyl)-3-(hydroxymethyl)butanolide] of Streptomyces lavendulae FRI-5 is one of many butyrolactone autoregulators found in Streptomyces species. These molecules appear to function as microbial hormones that, at nanomolar concentrations, control the production of secondary metabolites and/or morphological differentiation (8, 24). IM-2 itself triggers the production of the nucleoside antibiotics showdomycin and minimycin as well as an undefined blue pigment at a concentration of only 3 nM (5, 13, 24, 28). These autoregulators have a common 2,3-disubstituted γ-butyrolactone skeleton, and the 10 identified to date can be classified into three groups (IM-2 type [3], virginiae butanolide [VB] type [2, 11, 22, 27], and A-factor type [10, 14]) based on minor structural differences (12, 16, 21).
Receptor proteins corresponding to each of the three types of autoregulators have been purified, and the genes encoding them have been cloned and characterized (17, 18, 25). The IM-2-specific receptor protein (FarA) from S. lavendulae FRI-5 is a 221-amino-acid protein of 24,282 Da. The N-terminal region of FarA (amino acids 17 to 54) shows extensive homology with the other two types of autoregulator receptors, namely, the VB-specific receptor BarA of S. virginiae and the A-factor specific receptor ArpA of S. griseus. While this homologous region was predicted to encode a helix-turn-helix DNA-binding motif, and DNA-binding activities of both ArpA and BarA have been studied (9, 19), the precise function of FarA remains unclear. During early growth, cultures of S. lavendulae produce the antibiotic d-cycloserine (13, 28) but later switch to producing the nucleoside antibiotics showdomycin and minimycin (5). The predicted DNA-binding activity of FarA may regulate the transcription of genes involved in the synthesis of all three antibiotics. Consequently FarA, together with IM-2, may play an important role in the signal transduction mechanisms that trigger secondary metabolism in S. lavendulae.
Previous work analyzed the DNA-binding activities of the autoregulator receptor proteins BarA and ArpA. Although their DNA-binding sequences have been partially characterized (9, 19), the precise nature of these sequences and the abilities of different autoregulators to influence DNA binding have not been extensively studied. Moreover, nothing was known about the sequences recognized by FarA. In this study, we demonstrate the DNA-binding activity of FarA in gel shift assays and localize its target sequence by DNase I footprinting. The sequence to which FarA binds overlaps the −10 region and transcriptional start site of the farA promoter. These results suggest that FarA represses its own synthesis under the regulation of IM-2.
Strain, growth conditions, and plasmids.
Streptomyces sp. strain FRI-5 (MAFF10-06015; National Food Research Institute, Ministry of Agriculture, Forestry and Fisheries, Tsukuba, Japan), which was taxonomically identified as S. lavendulae by courtesy of S. Miyado (Meiji Seika Kaisha Ltd.), was used as a source of total RNA and grown at 28°C as described previously (5). DNA manipulations in Escherichia coli and in Streptomyces were performed as described by Sambrook et al. (23) and Hopwood et al. (6), respectively.
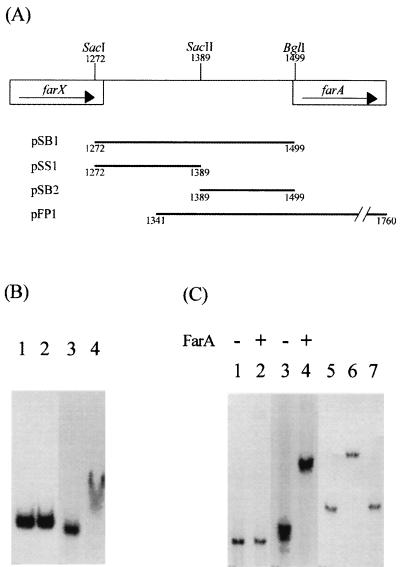
FarA binds specifically to the farX-farA intergenic region.
Northern blot hybridization analysis of farA transcripts had shown that the addition of IM-2 during the cultivation of FRI-5 resulted in enhanced (1.5- to 1.7-fold) transcription of farA (25). Promoter-probe analysis using the vector pIJ486 had identified promoter activity in the SacI-BglI fragment immediately upstream of farA (Fig. 1A and data not shown). It thus seemed possible that FarA regulated its own synthesis and might therefore bind to its own promoter region. To address this possibility, gel shift assays were performed with purified recombinant FarA (rFarA) (25) and the 227-bp SacI-BglI fragment (Fig. 1B) containing the farX-farA intergenic region as target DNA. As shown in Fig. 1B (lane 4), rFarA bound to this fragment, reducing its electrophoretic mobility, while the control fragment (rplK from S. virginiae) was not retarded (Fig. 1B, lane 2). To further localize the rFarA-binding sequence(s), the SacI-BglI segment was separated into two fragments, a 117-bp SacI-SacII fragment and a 110-bp SacII-BglI fragment. While the SacI-SacII fragment showed no sign of rFarA binding (Fig. 1C, lane 2), the SacII-BglI fragment was retarded (Fig. 1C, lane 4). Addition of the unlabeled SacII-BglI fragment to a molar level equivalent to that of rFarA abolished visible retardation of the labeled fragment (Fig. 1C, lanes 5 to 7). From these data, we conclude that there is a specific FarA-binding sequence present in the 110-bp SacII-BglI fragment.
FIG. 1.
Map of the farA upstream region and gel shift assays using rFarA. (A) Map of the fragments used for binding studies in the farA upstream region. Numbers indicate nucleotide positions. (B and C) Gel shift assays. The plasmids were digested with EcoRI and HindIII, and the desired fragments were labeled with [α-32P]dCTP. The fragments were incubated with 8 μg of purified rFarA in 15 μl of DNA binding buffer [20 mM triethanolamine-HCl containing 0.1 M KCl, 20% (vol/vol) glycerol, and 1 μg of poly(dI-dC) · (dI-dC), pH 7.0] at 25°C for 10 min. (B) Binding of rFarA to the farA upstream region. Lanes: 1, control DNA; 2, control DNA plus rFarA; 3, 227-bp SacI-BglI fragment containing the farX-farA intergenic region; 4, SacI-BglI fragment plus rFarA. (C) Further localization of the rFarA-binding region. Lanes: 1 and 2, 117-bp SacI-SacII fragment; 3 and 4, 110-bp SacII-BglI fragment; 5, 32P-labeled SacII-BglI fragment; 6, fragment plus rFarA; 7, fragment plus rFarA and unlabeled DNA.
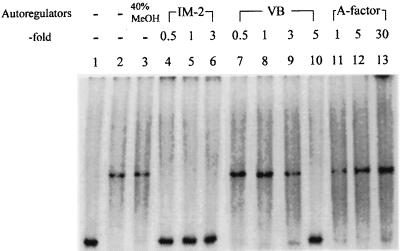
The DNA-binding activity of FarA is controlled by IM-2.
FarA shows high ligand specificity, readily discriminating IM-2 from the structurally similar VBs and A-factor. While FarA binds IM-2 with a Kd of 1.3 nM (21), the VBs show at least 10-fold less activity, and A-factor shows almost no affinity toward FarA. However, the ability of each ligand to influence the DNA-binding activity of FarA was not assessed. To address this issue, the gel shift assays were repeated with increasing concentrations of the autoregulators (Fig. 2). With IM-2, addition of a 0.5 molar equivalent of IM-2 with respect to rFarA completely abolished DNA-binding activity (lane 4). Since Bmax (binding maximum) of purified rFarA for IM-2 is 0.38 mol of [3H]IM-2-C5/mol of rFarA monomer (25), this was not surprising (the low Bmax presumably indicates that only approximately 38% of the rFarA was capable of binding IM-2 due to inactivation during purification, and we suspect that this 38% of rFarA can bind DNA). VB-C was also able to inhibit the DNA-binding activity of rFarA (lane 10) but was required at much higher concentrations than IM-2. The presence of a fivefold molar excess of VB-C over rFarA, 10 times the concentration of IM-2 eliciting the same effect, completely eliminated the mobility shift. In contrast, a 30-fold molar excess of A-factor had no effect. These results agreed well with the earlier biochemical studies of the ligand specificity of rFarA and indicated that rFarA in its unligated form possesses DNA-binding activity that is lost upon binding of IM-2 (25). All three autoregulator receptors characterized thus far (FarA, BarA, and ArpA) were found to exist as dimers under native conditions (17, 18, 25). Since the dimeric form of rFarA obtained by TSK G-2000SWXL gel filtration high-pressure liquid chromatography showed clear DNA-binding activity (data not shown), and since rFarA remains as a dimer after binding IM-2, it seems likely that it is the dimeric form of rFarA that binds to DNA in vivo.
FIG. 2.
Effects of different autoregulators on DNA-binding activity of rFarA. When IM-2, VB-C, or A-factor was added to the reaction mixture, the mixture was incubated at 25°C for 5 min. Lane: 1, 110-bp SacII-BglI fragment only; 2, DNA plus rFarA; 3, DNA plus rFarA and 40% methanol (MeOH); 4 to 6, 0.5, 1, and 3 molar equivalents of IM-2; 7 to 10, 0.5, 1, 3, and 5 molar equivalents of VB-C; 11 to 13, 1, 5, and 30 molar equivalents of A-factor.
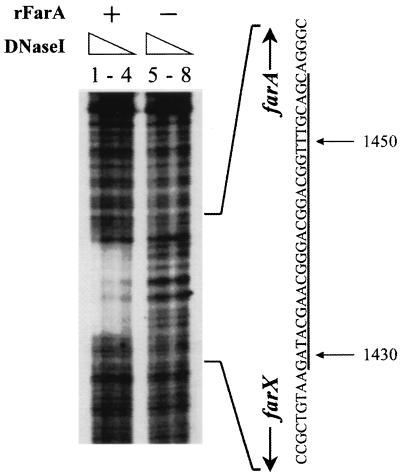
Analysis of the FarA-binding site by DNase I footprinting.
To further localize the FarA-binding sequence in the SacII-BglI fragment, DNase I footprinting experiments were performed with the 420-bp DNA fragment (positions 1341 to 1760) labeled on the coding strand by PCR amplification (Fig. 3). The 32P-end-labeled fragment was incubated with DNase I in the presence and absence of rFarA. After incubation at 25°C for 10 min, DNase I (Boehringer Mannheim) in 100 mM MgCl2–50 mM CaCl2 was added to each reaction mixture to final concentrations of 40, 20, 10, and 5 μg per ml and incubated at 25°C for 1 min. The region protected by rFarA contains 28 bp of the farX-farA intergenic region, extending from nucleotides 1429 to 1456. This sequence, named FARE (FarA-responsive element), is located approximately 70 bp upstream of the farA translational initiation site and shows significant homology to one of the artificial ArpA-binding sequences (Fig. 4). Onaka and Horinouchi (19) concluded that a palindromic sequence was necessary for ArpA binding and deduced the half-site sequence 5′-GG(T/C)CGGT(A/T)(T/C)G(T/G)-3′ as a consensus ArpA-binding site. Although the homology of FARE to this ArpA consensus half-site is high (81.8% identity), FARE is only partially palindromic. Since no native ArpA-binding site has been cloned or sequenced, the proposal that autoregulator receptor proteins generally recognize binding sites with dyad symmetry remains to be resolved.
FIG. 3.
DNase I footprinting analysis of the rFarA-binding site. The 458-bp insert of pFP1 containing upstream sequences and part of the farA coding sequence (positions 1341 to 1760) was amplified by PCR using 32P-labeled FP1 (5′-AACTGCAGCTCATCGGCACACCACGGCC-3′) and unlabeled M13 primer M3, and the PCR product was used in DNase I footprinting. Dideoxy DNA sequence ladders were derived from primer FP1. Lanes: 1 to 4, 14 μg of purified rFarA incubated with 40, 20, 10, and 5 μg of DNase I per ml, respectively; 5 to 8, no rFarA incubated with 40, 20, 10, and 5 μg of DNase I per ml, respectively. Protected bases are underlined.
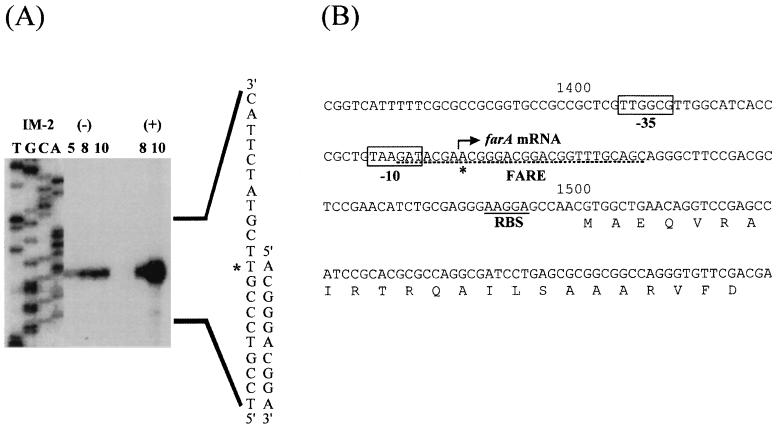
FIG. 4.
Comparison of binding sequences of FarA and ArpA (19). Asterisks indicate nucleotides conserved between FARE and one of the ArpA-binding sites. In the ArpA-binding consensus sequence, positions 3, 8, 9, and 11 are more highly conserved.
Analysis of the farA promoter region by high-resolution S1 nuclease mapping.
The location of FARE relative to the farA translational initiation codon suggested that FarA might control the transcription of farA. To address this possibility, the transcriptional start site of farA was determined by high-resolution S1 nuclease protection analysis performed as described by Janssen et al. (7) and White and Bibb (26) (Fig. 5A). Forty micrograms of RNA which was hybridized with 30,000 cpm of probe in sodium trichloroacetate buffer (15) was extracted essentially as described previously (6) except that the mycelium was scraped directly into modified Kirby mixture. A protected fragment corresponding to a putative transcription start site at an A residue at position 1436, 64 bp upstream of the farA translational start codon, was observed. Five base pairs upstream of this putative start site lies the hexameric sequence TAAGAT, and 16 bp upstream lies the motif TTGGCG; these sequences may act as the −10 and −35 regions, respectively, of the farA promoter (Fig. 5B) (1). The binding sites of most transcriptional regulators (activators or repressors) either overlap the RNA polymerase-binding site or are located immediately upstream of this sequence. FARE covers the putative farA transcription initiation site and overlaps the probable −10 region of farA, suggesting that FarA acts as transcriptional repressor of its own synthesis by preventing RNA polymerase binding. Binding of IM-2 to FarA would then result in the dissociation of the receptor from the DNA, allowing transcription of farA to occur. IM-2 addition during cultivation clearly induced farA transcription, as detected by S1 nuclease mapping, with some basal level transcription in the absence of IM-2 (Fig. 5A). We propose that in the early stages of cell growth, before IM-2 production can be detected, low-level synthesis of farA mRNA generates sufficient FarA to repress its own transcription. During later stages of growth, when IM-2 can be detected in the culture medium, transcription of farA is derepressed and increases dramatically. The resultant FarA could then act to regulate the expression of genes other than farA.
FIG. 5.
(A) High-resolution S1 nuclease mapping of the farA transcription start site and time course of farA expression in the presence and absence of IM-2. For RNA isolation, strain FRI-5 was grown in liquid medium (5) and total RNA was prepared from the mycelium of the indicated cultivation time (hours). (+), IM-2 was added to a final concentration of 100 nM at 5 h of cultivation, and cultivation continued for a further 3 or 5 h; (−), IM-2 was not added at 5 h. The probe (331 bp) for S1 nuclease mapping was generated by PCR using pFP1 as the template and 32P-labeled SN1 (5′-TCCGAGATGGTGGCCGCCTGGTAGC-3′) and unlabeled M13 primer RV as primers. Lanes T, C, G, and A represent sequence ladders generated with the primer that was used to derive the end-labeled probe. Asterisks indicate the apparent transcription start site. (B) Nucleotide sequence of the farA upstream region. The putative −35 and −10 regions of farA are boxed, the apparent transcription start site is denoted by an asterisk, and the putative ribosome-binding site (RBS) is underlined. The region (FARE) protected from DNase I digestion by purified rFarA is underlined with a broken line.
We believe that FarA plays an important role in the regulation of secondary metabolism in S. lavendulae and that it does so in conjunction with IM-2. While the production of the nucleoside antibiotics showdomycin and minimycin is induced by addition of IM-2, the production of d-cycloserine is terminated. Whether the biosynthetic genes for the production of these compounds are regulated by FarA and, if so, whether directly or indirectly remain to be determined. If they are directly controlled by FarA, then many of them should be preceded by sequences similar to FARE. We are currently in the process of isolating additional FarA binding sites that may be helpful in answering this question.
Nucleotide sequence accession number.
The sequence shown in Fig. 1A has been assigned Genbank/EMBL accession no AB001683.
Acknowledgments
We thank Mervyn J. Bibb (John Innes Centre) for critical reading of the manuscript and helpful comments on the manuscript and Sinji Miyado (Meiji Seika Kaisha Ltd.) for taxonomically identifying strain FRI-5.
This study was supported in part by the Proposal-Based Advanced Industrial Technology R&D Program of the New Energy and Industrial Technology Development Organization of Japan and by the Research for the Future Program of the Japan Society for the Promotion of Science.
REFERENCES
- 1.Bourn W, Babb B. Computer assisted identification and classification of Streptomyces promoters. Nucleic Acids Res. 1995;23:3696–3703. doi: 10.1093/nar/23.18.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gräfe U, Reinhardt F, Schade W, Eritt I, Fleck W F, Radics L. Interspecific inducers of cytodifferentiation and anthracycline biosynthesis from Streptomyces bikiniensis and S. cyaneofuscatus. Biotechnol Lett. 1983;5:591–596. [Google Scholar]
- 3.Gräfe U, Schade W, Eritt I, Fleck W F, Radics L. A new inducer of anthracycline biosynthesis from Streptomyces viridochromogenes. J Antibiot. 1982;35:1722–1723. doi: 10.7164/antibiotics.35.1722. [DOI] [PubMed] [Google Scholar]
- 4.Guilfoile P G, Hutchinson C R. The Streptomyces glaucescens TcmR protein represses transcription of the divergently oriented tcmR and tcmA genes by binding to an intergenic operator region. J Bacteriol. 1992;174:3659–3666. doi: 10.1128/jb.174.11.3659-3666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashimoto K, Nihira T, Sakuda S, Yamada Y. IM-2, a butyrolactone autoregulator, induces production of several antibiotics in Streptomyces sp. FRI-5. J Ferment Bioeng. 1992;73:449–455. [Google Scholar]
- 6.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, United Kingdom: The John Innes Foundation; 1985. [Google Scholar]
- 7.Janssen G R, Ward J M, Bibb M J. Unusual transcriptional and translational features of the aminoglycoside phosphotransferase gene (aph) from Streptomyces fradiae. Genes Dev. 1989;3:415–429. doi: 10.1101/gad.3.3.415. [DOI] [PubMed] [Google Scholar]
- 8.Khokhlow A S. Problems of studies of specific cell autoregulators (on the example of substances produced by some actinomycetes. In: Ananchenko S N, editor. Frontiers of bioorganic chemistry and molecular biology. Oxford, United Kingdom: Pergamon Press; 1980. pp. 201–210. [Google Scholar]
- 9.Kinoshita H, Ipposhi H, Okamoto S, Nakano H, Nihira T, Yamada Y. Butyrolactone autoregulator receptor protein (BarA) as a transcriptional regulator in Streptomyces virginiae. J Bacteriol. 1997;179:6986–6993. doi: 10.1128/jb.179.22.6986-6993.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleiner E M, Pliner S A, Soifer V S, Onoprienko V V, Blashova T A, Rosynov B V, Khokhlov A S. The structure of A-factor, a bioregulator from Streptomyces griseus. Bioorg Khim. 1976;2:1142–1147. [Google Scholar]
- 11.Kondo K, Higuchi Y, Sakuda S, Nihira T, Yamada Y. New virginiae butanolides from Streptomyces virginiae. J Antibiot. 1989;42:1873–1876. doi: 10.7164/antibiotics.42.1873. [DOI] [PubMed] [Google Scholar]
- 12.Miyake K, Horinouchi S, Yoshida M, Chiba N, Mori K, Nogawa N, Morikawa N, Beppu T. Detection and properties of A-factor-binding protein from Streptomyces griseus. J Bacteriol. 1989;171:4298–4302. doi: 10.1128/jb.171.8.4298-4302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizuno K, Sakuda S, Nihira T, Yamada Y. Enzymatic resolution of 2-acyl-3-hydroxymethyl-4-butanolide and preparation of optically active IM-2, the autoregulator from Streptomyces sp. FRI-5. Tetrahedron. 1994;50:10849–10858. [Google Scholar]
- 14.Mori K. Revision of the absolute configuration A-factor. Tetrahedron. 1983;39:3107–3109. [Google Scholar]
- 15.Murray M G. Use of sodium trichloroacetate and mung bean nuclease to increase sensitivity and precision during transcript mapping. Anal Biochem. 1986;158:165–170. doi: 10.1016/0003-2697(86)90605-6. [DOI] [PubMed] [Google Scholar]
- 16.Nihira T, Shimizu Y, Kim H S, Yamada Y. Structure-activity relationship of virginiae butanolide C, an inducer of virginiamycin production in Streptomyces virginiae. J Antibiot. 1988;41:1828–1837. doi: 10.7164/antibiotics.41.1828. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto S, Nakamura K, Nihira T, Yamada Y. Virginiae butanolide binding protein from Streptomyces virginiae: evidence that VbrA is not the virginiae butanolide binding protein and reidentification of the true binding protein. J Biol Chem. 1995;270:12319–12326. doi: 10.1074/jbc.270.20.12319. [DOI] [PubMed] [Google Scholar]
- 18.Onaka H, Ando N, Nihira T, Yamada Y, Beppu T, Horinouchi S. Cloning and characterization of the A-factor receptor gene from Streptomyces griseus. J Bacteriol. 1995;177:6083–6092. doi: 10.1128/jb.177.21.6083-6092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onaka H, Horinouchi S. DNA-binding activity of the A-factor receptor protein and its recognition DNA sequences. Mol Microbiol. 1997;24:991–1000. doi: 10.1046/j.1365-2958.1997.4081772.x. [DOI] [PubMed] [Google Scholar]
- 20.Retzlaff L, Distler J. The regulator of streptomycin gene expression, StrR, of Streptomyces griseus is a DNA binding activator protein with multiple recognition sites. Mol Microbiol. 1995;18:151–162. doi: 10.1111/j.1365-2958.1995.mmi_18010151.x. [DOI] [PubMed] [Google Scholar]
- 21.Ruengjitchatchawalya M, Nihira T, Yamada Y. Purification and characterization of the IM-2-binding protein from Streptomyces sp. strain FRI-5. J Bacteriol. 1995;177:551–557. doi: 10.1128/jb.177.3.551-557.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakuda S, Yamada Y. Streochemistry of butyrolactone autoregulators from Streptomyces. Tetrahedron Lett. 1991;32:1817–1820. [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 24.Sato K, Nihira T, Sakuda S, Yanagimoto M, Yamada Y. Isolation and structure of a new butyrolactone autoregulator from Streptomyces sp. FRI-5. J Ferment Bioeng. 1989;68:170–173. [Google Scholar]
- 25.Waki M, Nihira T, Yamada Y. Cloning and characterization of the gene (farA) encoding the receptor for an extracellular regulatory factor (IM-2) from Streptomyces sp. strain FRI-5. J Bacteriol. 1997;179:5131–5137. doi: 10.1128/jb.179.16.5131-5137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White J, Bibb M J. bldA dependence of undecylprodigiosin production in Streptomyces coelicolor A3(2) involves a pathway-specific regulatory cascade. J Bacteriol. 1997;179:627–633. doi: 10.1128/jb.179.3.627-633.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada Y, Sugamura K, Kondo K, Yanagimoto M, Okada H. The structure of inducing factors for virginiamycin production in Streptomyces virginiae. J Antibiot. 1987;40:496–504. doi: 10.7164/antibiotics.40.496. [DOI] [PubMed] [Google Scholar]
- 28.Yanagimoto M, Enatsu T. Regulation of a blue pigment production by γ-nonalactone in Streptomyces sp. J Ferment Technol. 1983;61:545–550. [Google Scholar]