Abstract
Introduction: Staphylococcus aureus is the most common cause of orthopedic infections and can be challenging to treat, especially in the presence of a foreign body. The antistaphylococcal lysins exebacase and CF-296 have rapid bactericidal activity, a low propensity for resistance development, and synergize with some antibiotics. Methods: Rabbit implant-associated osteomyelitis was induced by drilling into the medial tibia followed by locally delivering exebacase, CF-296, or lysin carrier. A titanium screw colonized with methicillin-resistant S. aureus (MRSA) IDRL-6169 was inserted. Intravenous daptomycin or saline was administered and continued daily for 4 d. On day 5, rabbits were euthanized, and the tibiae and implants were collected for culture. Results were reported as log colony forming units (cfu) per gram of bone or log cfu per implant, and comparisons among the six groups were performed using the Wilcoxon rank sum test. Results: Based on implant and bone cultures, all treatments resulted in significantly lower bacterial counts than those of controls ( ). Exebacase alone or with daptomycin as well as CF-296 with daptomycin were more active than daptomycin alone ( ) or CF-296 alone ( ) based on implant cultures. CF-296 with daptomycin was more active than either CF-296 alone ( ) or daptomycin alone ( ) based on bone cultures. Conclusion: Local delivery of either exebacase or CF-296 offers a promising complement to conventional antibiotics in implant-associated infections.
1. Introduction
Staphylococcus aureus is the most common bacterium recovered in orthopedic infections (De Araujo et al., 2021; Urish and Cassat, 2020). S. aureus forms biofilms on implants and bone and can exist as small colony variants (SCVs) and persister cells; S. aureus can enter and survive in cells such as fibroblasts and osteoblasts, rendering it difficult for the immune system and antibiotics to come in to contact with the bacteria (Alder et al., 2020; Muthukrishnan et al., 2019; Nasser et al., 2020; Waters et al., 2016). Thus, there is an urgent, unmet need for improved antimicrobials that are fast acting and target biofilms to help manage associated infections.
Lysins are cell wall hydrolytic enzymes being developed for therapeutic use as direct lytic agents. They are recombinantly produced as purified proteins from genetic material derived from bacteriophages (Fischetti, 2008). Exebacase (previously known as CF-301) and CF-296 are antistaphylococcal lysins with activity against S. aureus. Exebacase has a similar domain arrangement to most phage lysins with cell wall-cleaving and -binding properties at the N-terminal and C-terminal domains, respectively (Schuch et al., 2014). CF-296 is an engineered variant of exebacase, developed to potentially maintain longer anti-biofilm activity and be administered in repeated and/or higher doses than exebacase. In vitro studies show activity of exebacase against a range of S. aureus strains with rapid bactericidal activity, low propensity for resistance development, enhancement in the presence of human serum components, synergy with several antibiotics, and lack of selection for SCVs or persister cells (Indiani et al., 2019; Kebriaei et al., 2021; Oh et al., 2019; Schuch et al., 2017, 2014; Swift et al., 2021; Watson et al., 2020). Animal studies have shown enhanced activity of exebacase and CF-296 in combination with conventional antibiotics in different models of biofilm-associated S. aureus infections (Asempa et al., 2019; Karau et al., 2021, 2019; Schuch et al., 2014; Shah et al., 2020; Swift et al., 2021). Recently, a phase 2 clinical trial (NCT03163446) was completed testing the efficacy and safety of exebacase as an addition to standard antibiotics for treatment of S. aureus bacteremia and endocarditis, showing improved clinical outcomes in the methicillin-resistant S. aureus (MRSA) subset (Fowler et al., 2020). Currently, exebacase is being studied in a phase 3 clinical trial (NCT04160468) for treatment of S. aureus bacteremia, including right-sided endocarditis (Clinicaltrials.Gov, 2021).
The referenced in vivo studies delivered lysin systemically; locally delivered lysin has been incompletely studied. Here, using a rabbit model of implant-associated osteomyelitis with bone-implanted screws colonized with MRSA, we tested the activity of exebacase or CF-296 delivered locally with and without systemic daptomycin therapy.
2. Methods
2.1. Materials
Exebacase and CF-296 were supplied as 10.64 and 10.16 mg mL , respectively, in a liquid carrier solution containing proprietary components (ContraFect Corporation, Yonkers, NY). Daptomycin (Xellia Pharmaceuticals USA Inc., North Wales, PA) was supplied as 500 mg of powder and was reconstituted in 10 mL of sterile 0.9 % sodium chloride. Implants were 1.5 mm mm titanium cortex screws (DePuy Synthes, Monument, CO). The study strain was MRSA IDRL-6169, an isolate recovered at the Mayo Clinic from a periprosthetic hip infection, which had minimum inhibitory concentrations (MICs) for daptomycin, exebacase, and CF-296 of 0.5 .
2.2. Implant colonization
To colonize individual implants with MRSA, 37 of a 10 colony forming units (cfu) per milliliter suspension of MRSA IDRL-6169 in trypticase soy broth (TSB) was added to microcentrifuge tubes containing individual titanium screws and placed on an orbital shaker at 37 C for h. On each surgical day, pre- and post-surgery, one screw was quantitatively cultured to determine representative log cfu per implant. Figure 1 shows a representative image of the seeded implant.
Figure 1.

Scanning electron microscopy of the MRSA-seeded implant.
2.3. Biofilm time–kill curve
Time–kill studies were performed to confirm lysin activity against seeded implants. Screws were seeded with bacteria and placed into either 0.1 mL of lysin carrier, exebacase, or CF-296 for 1, 2, 4, 8, 12, or 24 h at 37 C. Three screws per time point for each treatment were quantitatively cultured. Briefly, screws were removed from treatment, dipped into saline, placed into 1 mL saline, vortexed and sonicated, and quantitatively cultured. Results were reported as log cfu per screw.
Figure 2.
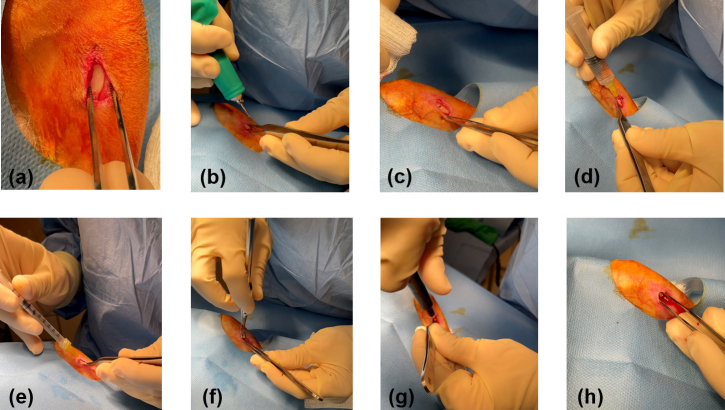
Surgical procedure: an incision was made over the left medial tibia, and the smooth flat portion was exposed (a); using a micro drill, a hole was created through the cortical bone into the medullary cavity (b, c); a total of 2 mL of water (d) followed by 0.6 mL of lysin or lysin carrier (e) was injected; and a seeded implant was inserted (f), tightened with a screwdriver (g), and confirmed to be secure (h).
2.4. Surgical methods
A total of 60 New Zealand male and female rabbits (10–12 weeks old, 2.5–3.0 kg) were studied. All animals tolerated procedures well with no unintentional loss of life throughout the study. Each surgical day, rabbits were randomly placed into one of six treatments groups (systemic/local) with 10 rabbits per group: saline/lysin carrier, daptomycin/lysin carrier, saline/exebacase, daptomycin/exebacase, saline/CF-296, or daptomycin/CF-296. Rabbits were administered anesthesia and analgesia. A 1.5 cm incision was made on the proximal portion of the medial left tibia. The fascia and muscle were cleared to expose a smooth flat portion of the tibia (Fig. 2a). Using micro drill with a 1.4 mm round burr, a hole was punched through the cortical bone into the medullary cavity (Fig. 2b, c). To remove bone fragments, 2 mL of sterile water was flushed into the tibia (Fig. 2d) followed by 0.6 mL of exebacase ( mg equivalent), CF-296 ( mg equivalent), or lysin carrier (Fig. 2e). Concurrently, systemic treatment of either daptomycin (6 mg kg ) or saline was delivered intravenously. An implant with 5.24 log cfu per implant was placed into the hole (Fig. 2f, g, h), and the site was closed. Figure 3 is a representative X-ray of the implant.
Figure 3.
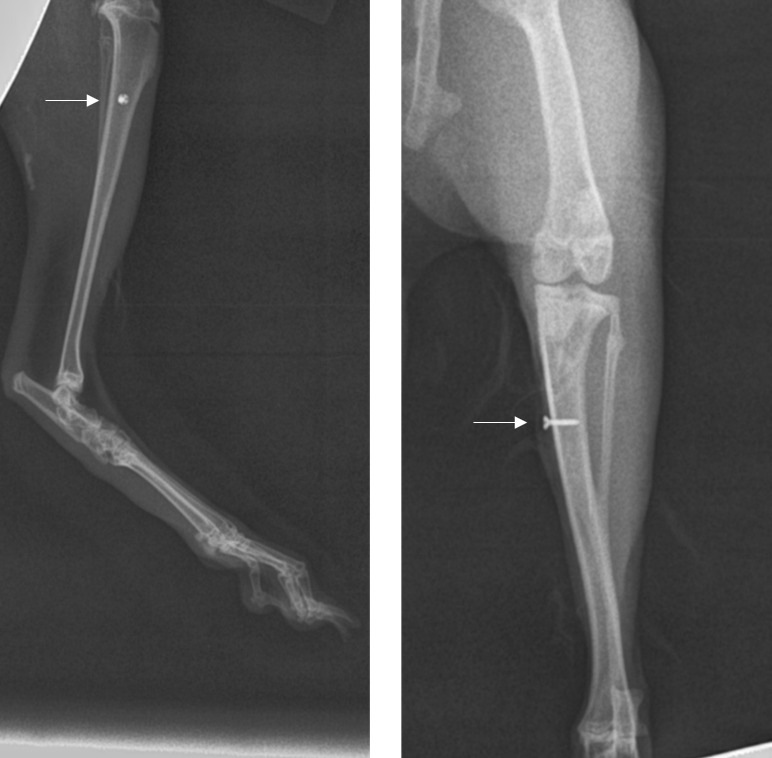
Medial and supine X-rays of the left rabbit tibia to show implant placement (arrow).
2.5. Post-operative management
Daptomycin (6 mg kg ) or saline was administered intravenously daily for 3 d. A rabbit dose of 12 mg kg of daptomycin results in a similar area under the plasma concentration curve to a human dosing of 6 mg kg (Chambers et al., 2009); however, a daptomycin dose of 6 mg kg was selected to assess the effects of combination activity. On day 5, rabbits were euthanized, and the tibiae and implants were aseptically collected and cultured separately.
Whole tibiae were cryopulverized with a Mixer Mill MM 400 (Retsch, Newtown, PA). The resultant bone powder was suspended in 10 mL saline, and the implants were placed in 1 mL saline. Tubes were vortexed and sonicated for 5 min and quantitatively cultured. Results were reported as log cfu g of bone or implant. MICs were performed on recovered MRSA to assess for the emergence of resistance.
2.6. Statistical analyses
Descriptive summaries are reported as medians. A sample size of 10 per group was chosen based on an 80 % power to detect a difference of 1.3 standard deviations or larger based on a two-sided two-sample test at an level of 0.05. Comparisons among the six groups were first performed using the Kruskal–Wallis test. Due to statistically significant differences between the groups, further comparisons between the groups were performed in a pairwise manner using the Wilcoxon rank sum test. Nonparametric tests were used due to the small sample size and non-normally distributed data. No adjustment was made for multiple comparisons. All tests were two sided, with values less than 0.05 considered statistically significant. Analysis was performed using SAS software version 9.4 (SAS Institute Inc, Cary, NC).
Figure 4.
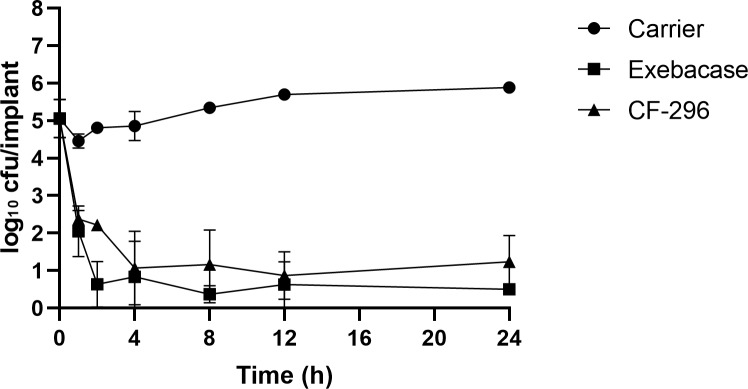
Implant time–kill study. Titanium screws grown overnight with MRSA IDRL-6169 were placed in 0.1 mL of lysin carrier, exebacase (10.64 mg mL ), or CF-296 (10.16 mg mL ). After 1, 2, 4, 8, 12, or 24 h, three screws each were processed for quantitative culture and results were reported as log cfu per implant.
Figure 5.
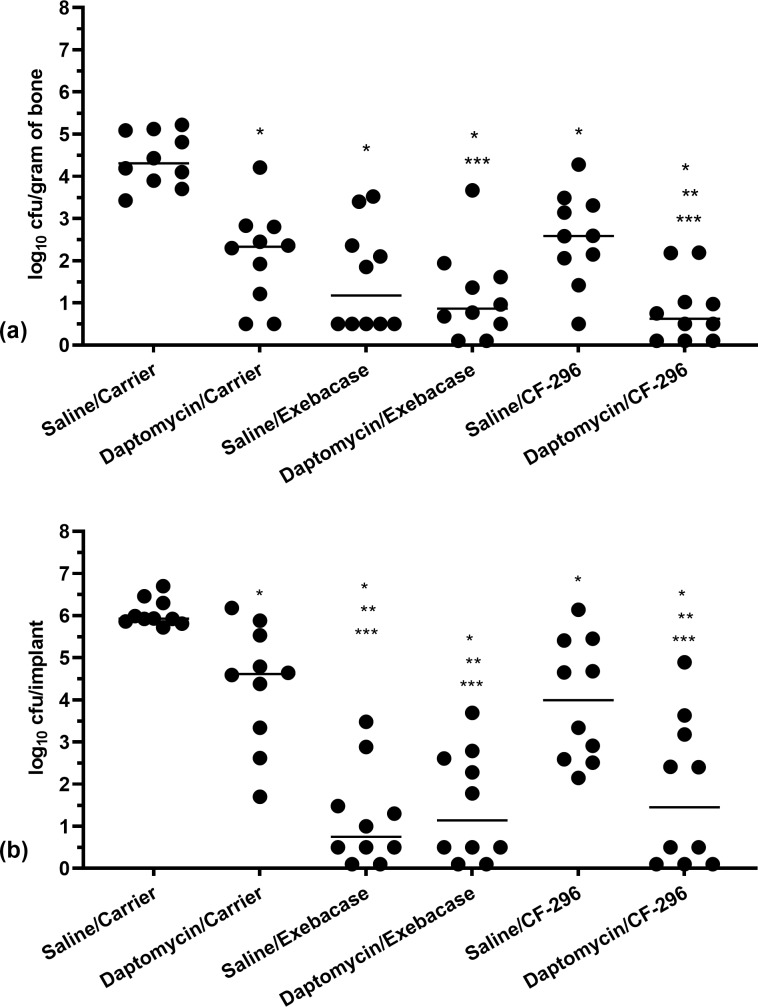
Quantities of MRSA recovered from bones (a) and implants (b) on day 5. Dots represent values from individual animals; per group; horizontal lines represent median values. Asterisks indicate significant reductions compared to * saline/carrier ( ), ** daptomycin/carrier ( ), or *** saline/CF-296 ( ). (Note that slashes depict the treatment grouping, with the top being the systemic treatment given daily and the bottom being the local treatment administered at the time of surgery.)
3. Results
3.1. In vitro results
In an in vitro biofilm time–kill experiment (Fig. 4), the mean bacterial load prior to treatment was 5.06 log cfu per implant. Compared with the lysin carrier, there was a mean reduction of 2.41 and 2.08 log cfu per implant after 1 h and 5.38 and 4.65 log cfu per implant after 24 h for exebacase and CF-296, respectively, indicating bactericidal activity (Pankey and Sabath, 2004).
3.2. In vivo results
Results of the bone and screw cultures are shown in Fig. 5a and b, respectively. All treatments (systemic intravenous treatment/local delivery) studied were active compared with controls (saline/lysin carrier), whether based on bone or implant cultures ( ). For bone and implant cultures, there were and log cfu reductions, respectively, for saline/exebacase, daptomycin/exebacase, and daptomycin/CF-296, compared with saline/carrier. Compared with bones of daptomycin/carrier, there were 1.15, 1.46, and 1.70 log cfu reductions in bones of animals receiving saline/exebacase, daptomycin/exebacase, and daptomycin/CF-296, respectively. In addition, bacterial densities in bones were less than 1 log cfu in 50 %, 60 %, and 70 % of animals in those same groups, respectively. Daptomycin/CF-296 resulted in significant reductions in S. aureus recovered from bones and implants compared with daptomycin/carrier ( and , respectively) or CF-296 alone ( and , respectively). Bacterial densities on implants were reduced by 3.87 ( ), 3.48 ( ), and 3.17 ( ) log cfu for animals treated with saline/exebacase, daptomycin/exebacase, and daptomycin/CF-296, respectively, compared with daptomycin/carrier-treated animals. In these respective groups, 60 %, 50 %, and 50 % of the animals had log cfu recovered from their implant. Exebacase alone resulted in greater cfu reductions on implants than locally delivered CF-296 alone ( ); there was no difference between the two lysins' activity when delivered locally in conjunction with systemic daptomycin, whether based on bone or implant cultures. Overall, locally delivered exebacase alone or with systemic daptomycin and locally delivered CF-296 with systemically delivered daptomycin showed the most activity. No emergence of resistance was found to daptomycin nor to either lysin.
4. Discussion
Local delivery of antimicrobials directly to the site of infection in addition to systemic therapy has been an area of interest with the potential to overcome some hurdles associated with traditional therapies (e.g., low biofilm activity, tissue penetration, and intracellular activity). Applying a treatment locally to an affected area allows immediate, direct contact with the infecting agent(s), compared with delayed time of delivery and variable low concentrations of available drug with systemic dosing. Over the past 20 years, vancomycin powder has been used in spine surgeries to prevent surgical site infections (Dodson et al., 2019). It is also used in fractures and traumatic injuries (O'Toole et al., 2021). More recently it has been used in primary joint arthroplasties and revisions, albeit with variable results (Movassaghi et al., 2022; Saidahmed et al., 2021). We recently showed vancomycin powder to be active against Staphylococcus epidermidis biofilms on spinal implants in vivo (Karau et al., 2020).
Exebacase has shown excellent in vitro activity against S. aureus which hypothetically indicates that activity could be leveraged with locally delivered lysin. When planktonic cells were exposed to exebacase, Schuch et al. (2014) observed the loss of cytoplasmic components within 15 s when viewed by electron microscopy, with logs of killing in 30 min as assessed by time–kill curves. There was faster binding of daptomycin or vancomycin to bacterial cell walls in the presence of exebacase (Schuch et al., 2014). Exebacase has been shown to disrupt S. aureus biofilms grown on several surface types. Exebacase disrupted biofilms grown on catheters within 1 h and killed bacteria within 6 h; exebacase has also shown activity against SCVs and persister cells (Schuch et al., 2017). Additionally, synergy has been observed in time–kill assays with log reductions when exebacase was used in addition to either daptomycin or vancomycin, compared with exebacase or antibiotic alone (Schuch et al., 2014; Watson et al., 2020).
The current model used orthopedic screws colonized with MRSA and treated with locally delivered lysin; results were reproducible, and rabbits tolerated the procedures well. Given the limited activity of many traditional antibiotics against implant-associated infections, these results indicate that locally delivered lysins can reduce bacterial loads in vivo. When used with systemic daptomycin, there were marked reductions in bacterial loads. Bactericidal activity was shown based on both implant and bone cultures, with a greater than 3 log reduction in bacterial counts with systemic daptomycin combined with locally delivered lysin, with three animals having no detectable bacteria in either sample type studied.
In a mouse periprosthetic knee S. aureus infection model incorporating debridement and implant retention (DAIR), Sosa et al. (2020) recently showed that the intra-articular and systemic administration of exebacase in combination with vancomycin reduced bacterial loads on implants and in surrounding tissues, although not in bone, when compared with vancomycin alone. There was no evaluation of exclusive local delivery, as was done herein. In a rat model of implant-associated osteomyelitis using a tibial screw implant, a CRISPR-Cas9 modified phage was administered into the bone with and without fosfomycin administered locally in an alginate hydrogel and compared to the alginate hydrogel or fosfomycin alone after resection the implant (Cobb et al., 2019). After 1 d, there was a reduction in MRSA in soft tissues compared with no treatment, but there was no difference in activity between fosfomycin, phage, nor the combination thereof. Although combination therapy was not more effective than the other treatments studied, fosfomycin was more active than alginate hydrogel or phage alone based on bone cultures.
In France, where exebacase has been administered on a compassionate-use basis into knees of elderly patients with recurrent methicillin-resistant S. epidermidis periprosthetic joint infection undergoing DAIR who had limited therapeutic options (Ferry et al., 2021), two of the four patients studied had favorable outcomes after 1 year, supporting our findings and suggesting that local administration of lysins should be further pursued.
These studies, along with ours, suggest that the local administration of lysins in addition to systemic antibiotics could be an option in treating implant-associated S. aureus infection. In our study, using such an approach, reductions in MRSA in bone and on implants in all treatment groups were shown compared with control animals. Moreover, a combination of CF-296 and daptomycin was more active than either daptomycin or CF-296 alone. We demonstrated excellent activity against MRSA with exebacase, a combination of exebacase and daptomycin, and a combination of CF-296 and daptomycin, with all being more active than daptomycin alone on implants.
There are several limitations to this study. First, only one strain of MRSA, one concentration of lysin, and one type of antibiotic were studied. Second, daptomycin was intentionally underdosed with reference to typical human dosing, to address activity in addition to the lysins; further work with varying concentrations should be performed to address synergy. Third, this novel model used colonized screws treated at time of insertion, a somewhat contrived scenario designed to allow assessment of the activity of locally delivered lysins against colonizing bacteria in vivo. Finally, there was less activity in bone than on implant surfaces; a possible explanation for the lesser degree of activity in bone could be the inability of lysins to reach bacteria residing within bone matrices and in cells. The combination of a bone-penetrating systemic antibiotic and local delivery of a fast-acting lysin to target biofilms on implants may provide a treatment option for these complicated infections.
In summary, an acute MRSA implant-associated osteomyelitis model was used to test local delivery of antistaphylococcal lysin in additional to systemic delivery of daptomycin. All treatment groups had significantly reduced amounts of MRSA recovered from both bone and implants, with the most active treatments being locally delivered exebacase alone and either lysin, exebacase, or CF-296, delivered locally in addition to systemic daptomycin. There was no difference between the activity of the two lysins delivered locally when administered with systemic daptomycin. Lysins administered locally, in combination with traditional therapies, may offer a potential strategy for combatting S. aureus implant-associated infections.
Acknowledgements
We thank Kerryl Greenwood-Quaintance and Scott Cunningham for their input and thoughtful review. We thank Scott Gamb for performing scanning electron microscopy on the colonized implants. We also thank the rabbit support staff; without their assistance, this project would not have been possible.
Disclaimer
Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. The U.S. Army Medical Research Acquisition Activity, 839 Chandler Street, Fort Detrick, MD 21702-5014, is the awarding and administering acquisition office. This work was presented, in part, at ASM Microbe 2022, Washington, D.C. Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data availability
Data are available upon request.
Author contributions
MJK contributed to study design; model development; study oversight; experimental procedures; data analysis; and manuscript preparation, review, and revisions. SMSM contributed to model development, experimental procedures, and manuscript preparation and review. JM contributed to data analysis and manuscript review. RS, CC, and DL contributed to study design and manuscript review and revisions. RP contributed to study design, study oversight, and manuscript review and revisions.
Competing interests
Robin Patel declares financial support (grants) from ContraFect Corporation, TenNor Therapeutics Limited, and BioFire Diagnostics. Robin Patel is a consultant to Curetis, Next Gen Diagnostics, PathoQuest, Selux Diagnostics, 1928 Diagnostics, PhAST, Torus Biosystems, Day Zero Diagnostics, Mammoth Biosciences, and Qvella Corporation; monies are paid to Mayo Clinic. Mayo Clinic and Robin Patel have a relationship with Pathogenomix. Robin Patel has research supported by Adaptive Phage Therapeutics. Mayo Clinic has a royalty-bearing know-how agreement and equity in Adaptive Phage Therapeutics. Robin Patel is also a consultant to Netflix and CARB-X. In addition, Robin Patel has a patent on PCR issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an anti-biofilm substance issued. Robin Patel receives honoraria from the NBME, Up-to-Date, and the Infectious Diseases Board Review Course.
Ethical statement
In the conduct of research involving hazardous organisms or toxins, the investigators adhered to the CDC-NIH guide for Biosafety in Microbiological and Biomedical Laboratories. All animal procedures performed were approved by the Institutional Animal Care and Use Committee (IACUC) at Mayo Clinic in Rochester as well as by the Animal Care and Use Review Office of the United States Army Medical Research and Development Command Office of Research Protections.
Review statement
This paper was edited by Parham Sendi and reviewed by three anonymous referees.
References
- Alder KD, Lee I, Munger AM, Kwon H-K, Morris MT, Cahill SV, Back J, Kristin EY, Lee FY. Intracellular Staphylococcus aureus in bone and joint infections: A mechanism of disease recurrence, inflammation, and bone and cartilage destruction. Bone. 2020;141:115568. doi: 10.1016/j.bone.2020.115568. [DOI] [PubMed] [Google Scholar]
- Asempa TE, Abdelraouf K, Carabeo T, Schuch R, Nicolau DP. Synergistic activity of exebacase (CF-301) in addition to daptomycin against Staphylococcus aureus in a neutropenic murine thigh infection model. Antimicrob Agents Chemother. 2019;64:e02176–02119. doi: 10.1128/AAC.02176-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers HF, Basuino L, Diep B, Steenbergen J, Zhang S, Tattevin P, Alder J. Relationship between susceptibility to daptomycin in vitro and activity in vivo in a rabbit model of aortic valve endocarditis. Antimicrob Agents Chemother. 2009;53:1463–1467. doi: 10.1128/AAC.01307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direct lysis of resistant pathogen trial of exebacase (DISRUPT), NCT04160468 [Internet] Clinicaltrials.gov; [last access: 24 September 2021]. 2021. http://clinicaltrials.gov. [Google Scholar]
- Cobb LH, Park J, Swanson EA, Beard MC, McCabe EM, Rourke AS, Seo KS, Olivier AK, Priddy LB. CRISPR-Cas9 modified bacteriophage for treatment of Staphylococcus aureus induced osteomyelitis and soft tissue infection. PLoS One. 2019;14:e0220421. doi: 10.1371/journal.pone.0220421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Araujo FP, Monaco M, Del Grosso M, Pirolo M, Visca P, Pantosti A. Staphylococcus aureus clones causing osteomyelitis; a literature review (2000–2020) J Global Antimicrob Res. 2021;26:29–36. doi: 10.1016/j.jgar.2021.03.030. [DOI] [PubMed] [Google Scholar]
- Dodson V, Majmundar N, Swantic V, Assina R. The effect of prophylactic vancomycin powder on infections following spinal surgeries: A systematic review. Neurosurgical focus. 2019;46:E11. doi: 10.3171/2018.10.FOCUS18470. [DOI] [PubMed] [Google Scholar]
- Ferry T, Batailler C, Souche A, Cassino C, Chidiac C, Perpoint T, Le Corvaisier C, Josse J, Gaillard R, Roger J. Arthroscopic “debridement and implant retention” with local administration of exebacase (lysin CF-301) followed by suppressive tedizolid as salvage therapy in elderly patients for relapsing multidrug-resistant S. epidermidis prosthetic knee infection. Front Med. 2021;8:550853. doi: 10.3389/fmed.2021.550853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti VA. Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol. 2008;11:393–400. doi: 10.1016/j.mib.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler VG, Das AF, Lipka-Diamond J, Schuch R, Pomerantz R, Jáuregui-Peredo L, Bressler A, Evans D, Moran GJ, Rupp ME. Exebacase for patients with Staphylococcus aureus bloodstream infection and endocarditis. J Clin Invest. 2020;130:3750–3760. doi: 10.1172/JCI136577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indiani C, Sauve K, Raz A, Abdelhady W, Xiong YQ, Cassino C, Bayer AS, Schuch R. The antistaphylococcal lysin, CF-301, activates key host factors in human blood to potentiate methicillin-resistant Staphylococcus aureus bacteriolysis. Antimicrob Agents Chemother. 2019;63:e02291–02218. doi: 10.1128/AAC.02291-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karau MJ, Schmidt-Malan SM, Yan Q, Greenwood-Quaintance KE, Mandrekar J, Lehoux D, Schuch R, Cassino C, Patel R. Exebacase in addition to daptomycin is more active than daptomycin or exebacase alone in methicillin-resistant Staphylococcus aureus osteomyelitis in rats. Antimicrob Agents Chemother. 2019;63:e01235–01219. doi: 10.1128/AAC.01235-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karau MJ, Zhang C, Mandrekar JN, Kohrs NJ, Puleo DA, van Wijnen AJ, Patel R, Boyce TG, Larson AN, Milbrandt TA. Topical vancomycin for treatment of methicillin-resistant Staphylococcus epidermidis infection in a rat spinal implant model. Spine Deform. 2020;8:553–559. doi: 10.1007/s43390-020-00087-4. [DOI] [PubMed] [Google Scholar]
- Karau MJ, Schmidt-Malan SM, Mandrekar J, Lehoux D, Schuch R, Cassino C, Patel R. Activity of lysin CF-296 alone and in addition to daptomycin in a rat model of experimental methicillin-resistant Staphylococcus aureus osteomyelitis. Antimicrob Agents Chemother. 2021;65:e00117–00121. doi: 10.1128/aac.00117-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebriaei R, Stamper KC, Lev KL, Morrisette T, Abdul-Mutakabbir JC, Schuch R, Lehoux D, Rybak MJ. Exebacase, in addition to daptomycin against MRSA. Antimicrob Agents Chemother. 2021;65:00128–00121. doi: 10.1128/AAC.00128-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movassaghi K, Wang JC, Gettleman BS, Mayfield CK, Oakes DA, Lieberman JR, Heckmann ND. Systematic review and meta-analysis of intrawound vancomycin in total hip and total knee arthroplasty: A continued call for a prospective randomized trial. J Arthrop. 2022;37:1405–1415.e1. doi: 10.1016/j.arth.2022.03.047. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan G, Masters EA, Daiss JL, Schwarz EM. Mechanisms of immune evasion and bone tissue colonization that make Staphylococcus aureus the primary pathogen in osteomyelitis. Curr Osteopor Rep. 2019;17:395–404. doi: 10.1007/s11914-019-00548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser A, Azimi T, Ostadmohammadi S, Ostadmohammadi S. A comprehensive review of bacterial osteomyelitis with emphasis on Staphylococcus aureus. Microb Patho. 2020;148:104431. doi: 10.1016/j.micpath.2020.104431. [DOI] [PubMed] [Google Scholar]
- Oh JT, Cassino C, Schuch R. Postantibiotic and sub-MIC effects of exebacase (lysin CF-301) enhance antimicrobial activity against Staphylococcus aureus. Antimicrob Agents Chemother. 2019;63:e02616–02618. doi: 10.1128/AAC.02616-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole RV, Joshi M, Carlini AR, Murray CK, Allen LE, Huang Y, Scharfstein DO, O'Hara NN, Gary JL, Bosse MJ. Effect of intrawound vancomycin powder in operatively treated high-risk tibia fractures: A randomized clinical trial. JAMA Surg. 2021;156:e207259–e207259. doi: 10.1001/jamasurg.2020.7259. [DOI] [PubMed] [Google Scholar]
- Pankey GA, Sabath L. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis. 2004;38:864–870. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- Saidahmed A, Sarraj M, Ekhtiari S, Mundi R, Tushinski D, Wood TJ, Bhandari M. Local antibiotics in primary hip and knee arthroplasty: a systematic review and meta-analysis. European J Orthop Surg Trauma. 2021;31:669–681. doi: 10.1007/s00590-020-02809-w. [DOI] [PubMed] [Google Scholar]
- Schuch R, Lee HM, Schneider BC, Sauve KL, Law C, Khan BK, Rotolo JA, Horiuchi Y, Couto DE, Raz A, Fischetti V, Huang DB, Nowinski RC, Wittekind M. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J Infect Dis. 2014;209:1469–1478. doi: 10.1093/infdis/jit637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch R, Khan BK, Raz A, Rotolo JA, Wittekind M. Bacteriophage lysin CF-301: A potent anti-staphylococcal biofilm agent. Antimicrob Agents Chemother. 2017;61:02666–02616. doi: 10.1128/AAC.02666-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SU, Xiong YQ, Abdelhady W, Iwaz J, Pak Y, Schuch R, Cassino C, Lehoux D, Bayer AS. Effect of the lysin exebacase on cardiac vegetation progression in a rabbit model of methicillin-resistant Staphylococcus aureus endocarditis as determined by echocardiography. Antimicrob Agents Chemother. 2020;64:e00482–00420. doi: 10.1128/AAC.00482-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa BR, Niu Y, Turajane K, Staats K, Suhardi V, Carli A, Fischetti V, Bostrom M, Yang X. 2020 John Charnley Award: The antimicrobial potential of bacteriophage-derived lysin in a murine debridement, antibiotics, and implant retention model of prosthetic joint infection. Bone Joint J. 2020;102-B:3–10. doi: 10.1302/0301-620X.102B7.BJJ-2019-1590.R1. [DOI] [PubMed] [Google Scholar]
- Swift SM, Sauve K, Cassino C, Schuch R. Exebacase is active in vitro in pulmonary surfactant and is efficacious alone and synergistic with daptomycin in a mouse model of lethal Staphylococcus aureus lung infection. Antimicrob Agents Chemother. 2021;65:e02723–02720. doi: 10.1128/AAC.02723-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urish KL, Cassat JE. Staphylococcus aureus osteomyelitis: Bone, bugs, and surgery. Infect Immun. 2020;88:e00932–00919. doi: 10.1128/IAI.00932-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Rowe SE, O'Gara JP, Conlon BP. Convergence of Staphylococcus aureus persister and biofilm research: Can biofilms be defined as communities of adherent persister cells? PLoS Pathog. 2016;12:e1006012. doi: 10.1371/journal.ppat.1006012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A, Sauve K, Cassino C, Schuch R. Exebacase demonstrates in vitro synergy with a broad range of antibiotics against both methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2020;64:e01885–01819. doi: 10.1128/AAC.01885-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request.