Abstract
Purpose:
To survey urologic clinicians regarding interpretation of and practice patterns in relation to emerging aspects of prostate cancer grading, including quantification of high-grade disease, cribriform/intraductal carcinoma, and impact of MRI-targeted needle biopsy.
Materials and Methods:
The Genitourinary Pathology Society (GUPS) distributed a survey to urology and urologic oncology-focused societies and hospital departments. 834 responses were collected and analyzed using descriptive statistics.
Results:
80% of survey participants use quantity of Gleason pattern 4 on needle biopsy for clinical decisions, less frequently with higher Grade Groups. 50% interpret “tertiary” grade as a minor/<5% component. 70% of respondents would prefer per core grading as well as a global/overall score per set of biopsies, but 70% would consider highest Gleason score in any single core as the grade for management. 75% utilize Grade Group terminology in patient discussions. For 45%, cribriform pattern would affect management, while for 70% the presence of intraductal carcinoma would preclude active surveillance.
Conclusions:
This survey of practice patterns in relationship to prostate cancer grading highlights similarities and differences between contemporary pathology reporting and its clinical application. As utilization of Gleason pattern 4 quantification, minor tertiary pattern, cribriform/intraductal carcinoma, and the incorporation of MRI-based strategies evolve, these findings may serve as a basis for more nuanced communication and guide research efforts involving pathologists and clinicians.
Keywords: prostate cancer, grading, active surveillance, cribriform, MRI
1. Introduction
The Gleason grading system for prostate cancer (PCa) has evolved with several important modifications in the past two decades [1–2]. The Genitourinary Pathology Society (GUPS) is a global organization formed in 2018, to advance the care of patients with urologic diseases. GUPS has recently addressed emerging areas in PCa grading from a pathology perspective [3]. Herein, we report results of an international GUPS clinician survey to evaluate emerging PCa grading issues.
2. Materials and Methods
A GUPS survey on clinical PCa practice patterns was formulated using an online software (SurveyMonkey Inc.; San Mateo, California, USA) and circulated to PCa-focused clinicians through distribution lists of the American Society of Radiation Oncology, Chesapeake Urology, European Association of Urology, European Society of Radiotherapy & Oncology, NRG Oncology, Society of Urological Oncology, Urological Society of Australia and New Zealand, as well as to clinicians at GUPS members’ institutions. We attempted to enlist additional urological/radiation oncology societies, but some did not allow membership surveys. The 25 survey questions are listed in Supplemental Table 1. The survey assessed participant demographics and their interpretation of and practice patterns regarding PCa, related to: a) Gleason pattern 4 (GP4) quantification, b) “tertiary” grading patterns at radical prostatectomy (RP), c) global/overall grading, d) grading of magnetic resonance imaging (MRI)-targeted needle biopsy (NBx), and e) cribriform/intraductal carcinoma. Specialty-specific data is not presented from respondents other than urologists and radiation oncologists due to limited numbers; geographic differences are only presented when substantive. Results were reported using descriptive statistics.
3. Results
The survey generated 834 responses, with varying tallies for individual questions. Figure 1 summarizes the demographic information. More than 90% of respondents chose Urology (70%) or Radiation Oncology (22%) as their medical specialty, and most respondents practiced in Europe (40%) or the United States (31%). The distribution of years in practice ranged from 14% with <5 years to 35% with >20 years; 90% practiced either in university (academic) hospitals (62%) or in private practices (28%). Select clinical survey responses are summarized in Tables 1–2.
Figure 1.
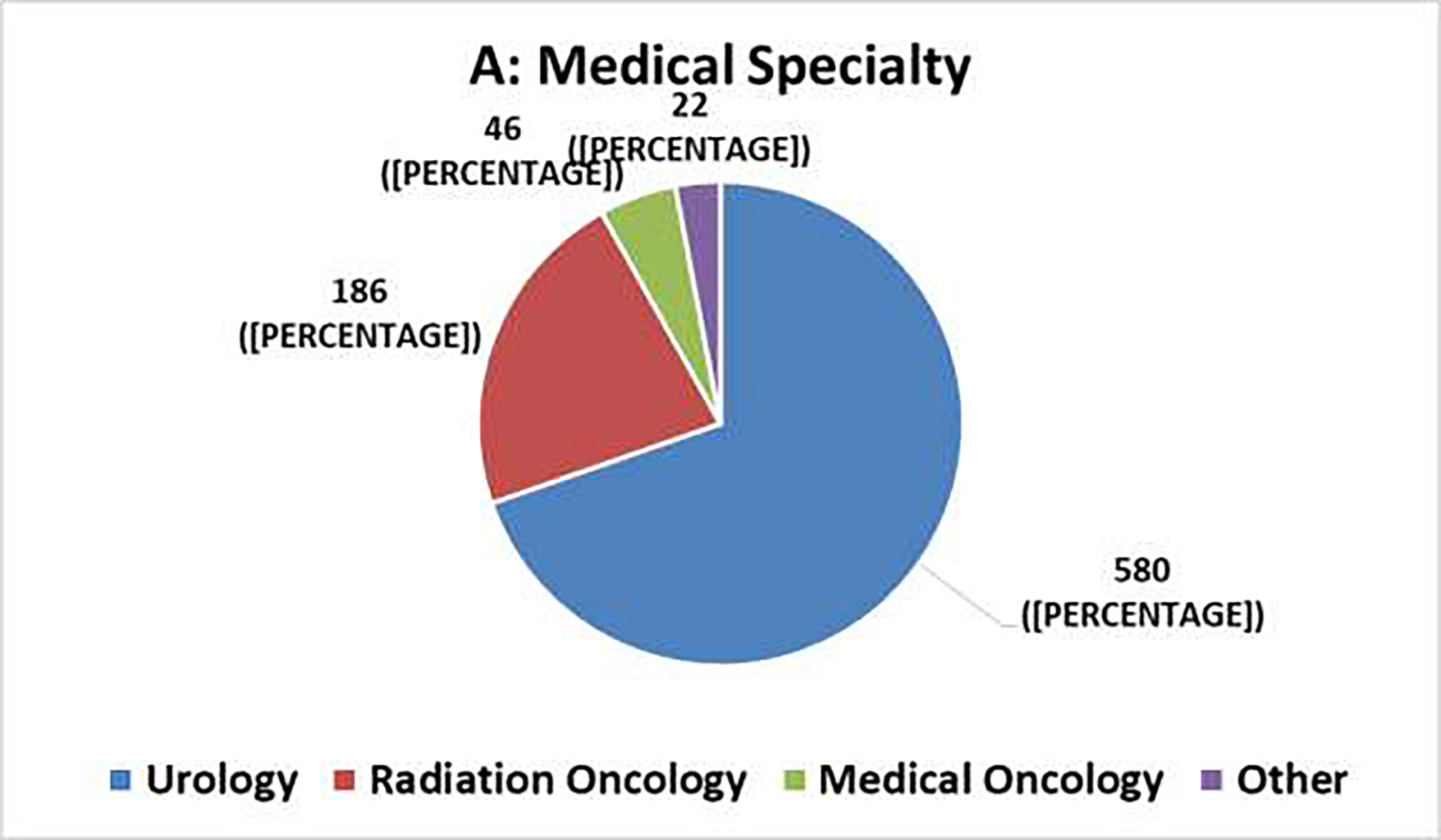
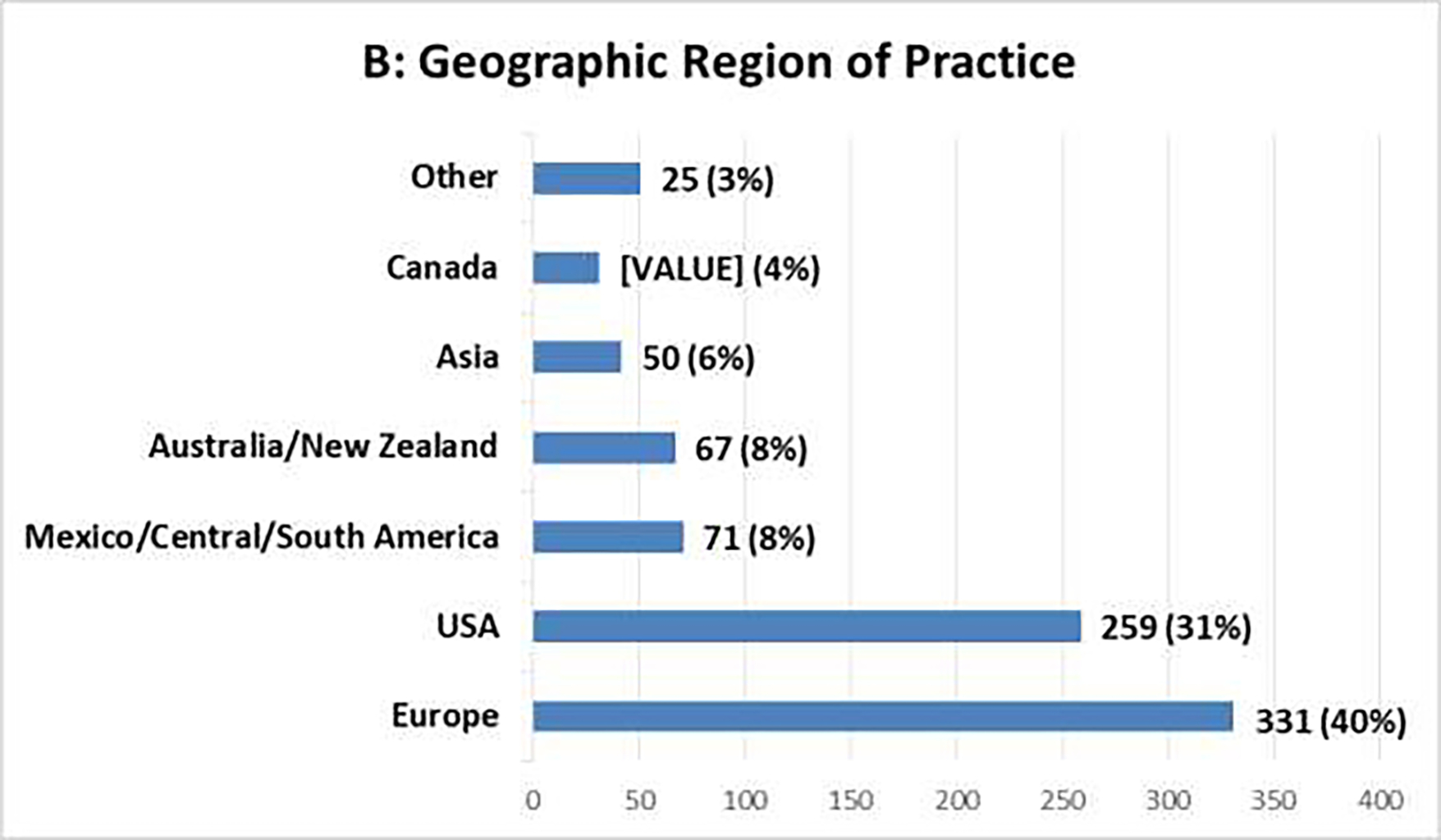
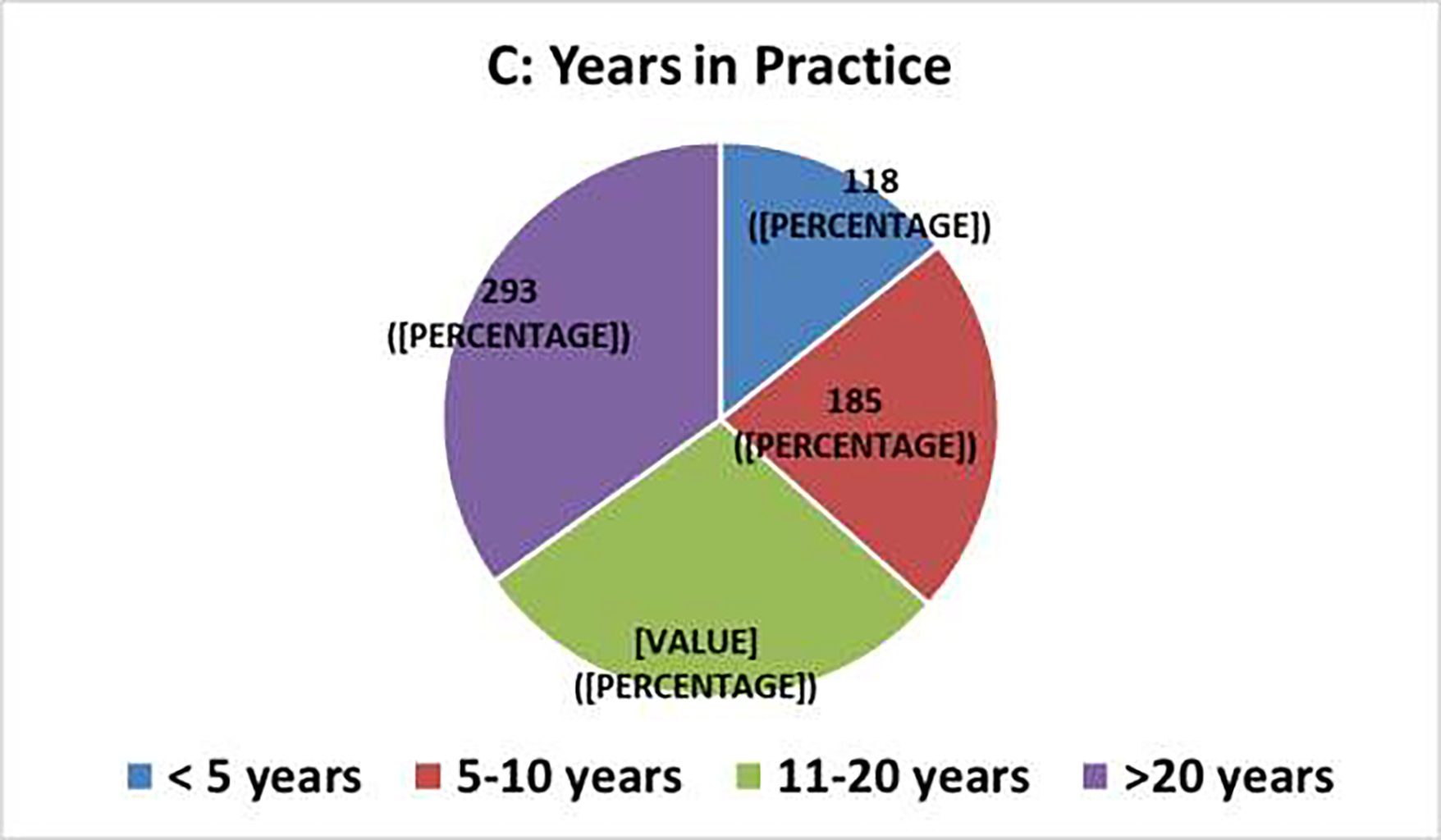
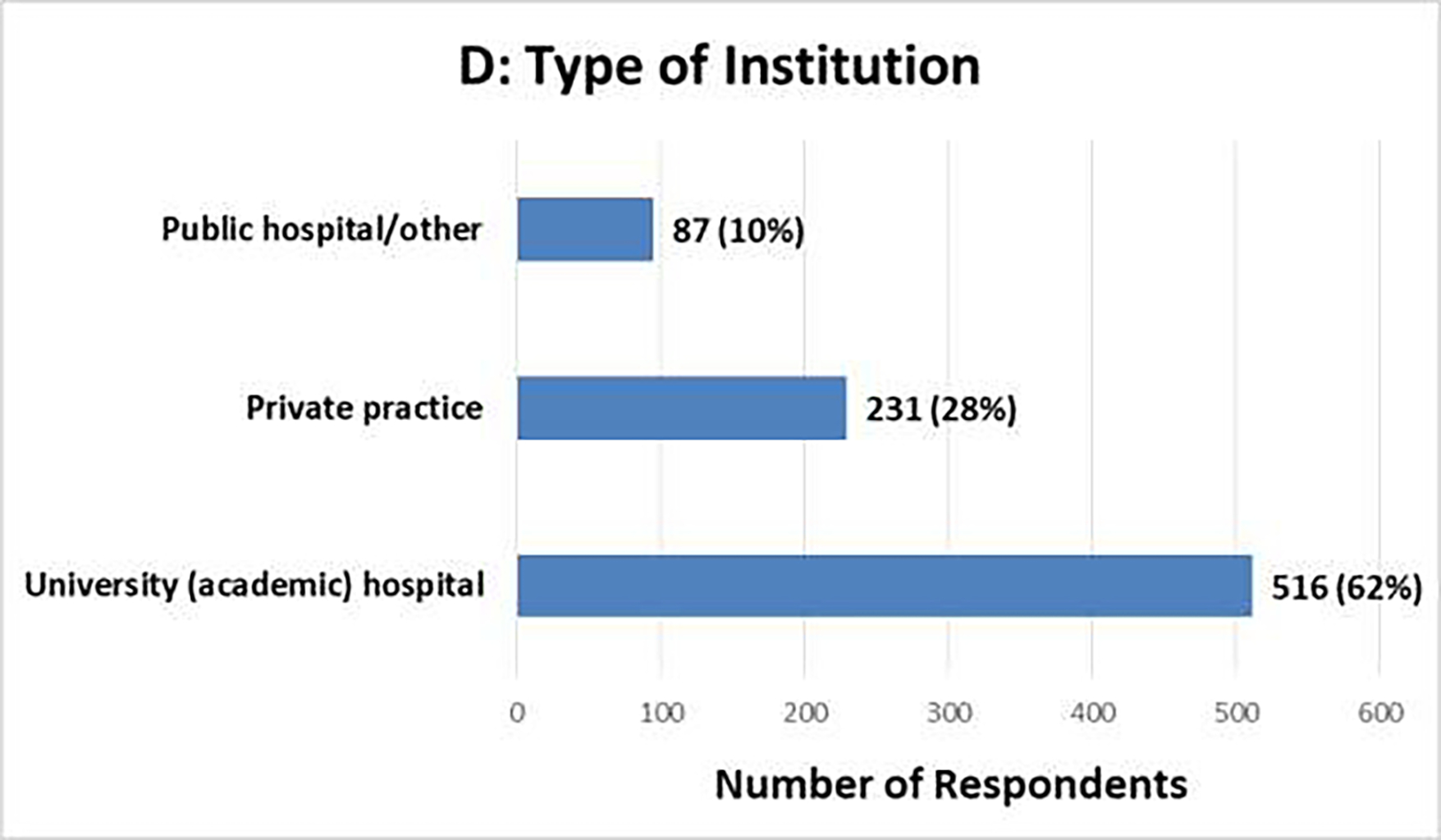
Clinical Survey Demographics [N=834]: (A) Medical Specialty, (B) Geographic Region of Practice, (C) Years in Practice, (D) Type of Institution
Table 1.
Clinical survey responses regarding quantification of pattern 4 in a variety of needle biopsy and radical prostatectomy scenarios
| Question | All Responses (N=834)* | Urology (N=580) | Radiation Oncology (N=186) |
|---|---|---|---|
| Q5: Do you EVER utilize the quantity of pattern 4 on NB in clinical decision making? | YES: 80% (N=661) NO: 20% (N=168) [Responses: 829] |
YES: 82% (N=470) NO: 18% (N=106) [Responses: 576] |
YES: 76% (N=141) NO: 24% (N=45) [Responses: 186] |
| Q7: In NB with highest Grade group 3 prostate cancer, would it be valuable to know the quantity of pattern 4 (e.g. 60% vs. 90% pattern 4)? | YES: 62% (N=284) NO: 38% (N=176) [Responses: 460] |
YES: 60% (N=202) NO: 40% (N=134) [Responses: 336] |
YES: 70% (N=64) NO: 30% (N=28) [Responses: 92] |
| Q8: One NB has Grade group 3 involving 10% of a core; 3 other cores show Grade group 2. Is it valuable to know if the 3 cores had 10% vs. 40% pattern 4? | YES: 51% (N=237) NO: 49% (N=223) [Responses: 460] |
YES: 54% (N=180) NO: 46% (N=156) [Responses: 336] |
YES: 51% (N=47) NO: 49% (N=45) [Responses: 92] |
| Q9: One NB has Grade group 4 involving 10% of a core; 3 other cores show Grade group 3. Is it valuable to know if the 3 cores had 60% vs. 90% pattern 4? | YES: 30% (N=137) NO: 70% (N=323) [Responses: 460] |
YES: 30% (N=100) NO: 70% (N=236) [Responses: 336] |
YES: 35% (N=32) NO: 65% (N=60) [Responses: 92] |
| Q11: Would it be valuable to know the % pattern 4 for GS 7 (Grade groups 2–3) on RP? | YES: 53% (N=316) NO: 47% (N=280) [Responses: 596] |
YES: 56% (N=235) NO: 44% (N=188) [Responses: 423] |
YES: 50% (N=64) NO: 50% (N=64) [Responses: 128] |
| Q12: In RP specimens with GS 7 (Grade groups 2–3) and a reported “tertiary” Gleason pattern 5, do you assume that the “tertiary” pattern is minor (<5%) or could be any amount (as long as 3rd most common)? | MINOR: 52% (N=307) ANY: 48% (N=289) [Responses: 596] |
MINOR: 53% (N=224) ANY: 47% (N=199) [Responses: 423] |
MINOR: 48% (N=62) ANY: 52% (N=66) [Responses: 128] |
| Q13: In RP specimens with GS 7 (Grade groups 2–3), does a minor (<5%) component of Gleason pattern 5 affect further therapy? | YES: 43% (N=259) NO: 57% (N=337) [Responses: 596] |
YES: 43% (N=183) NO: 57% (N=239) [Responses: 422] |
YES: 45% (N=58) NO: 55% (N=70) [Responses: 128] |
Also includes responses from Medical Oncologists (N=46) and ‘Other’ (N=22)
NB: needle biopsy; GS: Gleason score; RP: radical prostatectomy
Table 2.
Clinical survey responses regarding cribriform and intraductal carcinoma in needle biopsy scenarios
| Question | All Responses (N=834)* | Urology (N=580) | Radiation Oncology (N=186) |
|---|---|---|---|
| Q19: For cases with GS 7 (Grade groups 2–3), would knowing if the pattern 4 component was cribriform vs. not cribriform affect patient counseling or management? | YES: 44% (N=241) NO: 56% (N=311) [Responses: 552] |
YES: 50% (N=196) NO: 50% (N=196) [Responses: 392] |
YES: 24% (N=29) NO: 76% (N=92) [Responses: 121] |
| Q21: If you would consider active surveillance in men with GS 3+4=7 (Grade group 2) who have over 10-year life expectancy, does whether the pattern 4 component is cribriform vs. not cribriform impact the decision? | YES: 63% (N=197) NO: 37% (N=114) [Responses: 311] |
YES: 71% (N=159) NO: 29% (N=65) [Responses: 224] |
YES: 34% (N=20) NO: 66% (N=39) [Responses: 59] |
| Q22: Would you recommend active surveillance for a man with cancer who is otherwise a candidate if their NB also shows intraductal carcinoma? | YES: 29% (N=153) NO: 71% (N=381) [Responses: 534] |
YES: 24% (N=89) NO: 76% (N=289) [Responses: 378] |
YES: 41% (N=49) NO: 59% (N=70) [Responses: 119] |
| Q24: If a biopsy shows GS 6 and intraductal carcinoma, do you routinely perform repeat biopsy to look for higher grade cancer? | YES: 35% (N=188) NO: 65% (N=344) [Responses: 532] |
YES: 33% (N=125) NO: 67% (N=253) [Responses: 378] |
YES: 38% (N=45) NO: 62% (N=73) [Responses: 118] |
| Q25: If a biopsy report indicates that there is intraductal carcinoma in addition to invasive cancer with GS 7–10 (Grade groups 2–5), would it affect therapy selection? | YES: 31% (N=165) NO: 69% (N=369) [Responses: 534] |
YES: 29% (N=108) NO: 71% (N=270) [Responses: 378] |
YES: 34% (N=41) NO: 66% (N=78) [Responses: 119] |
Also includes responses from Medical Oncologists (N=46) and ‘Other’ (N=22)
GS: Gleason score; NB: needle biopsy
3.1. Quantification of Gleason Pattern 4 on Needle Biopsy
The vast majority (80%) utilized extent of GP4 on NBx for clinical decisions, although they were equally divided on their preferred metric: maximum extent GP4 in any one part vs. overall extent GP4 in all parts combined [44% each; 204/460 vs. 202/460, respectively]. 44% of respondents found reporting GP4 in 10% increments most useful for patient counseling or management, while 49% favored reporting percent GP4 in quartiles. When at least one NBx part had Grade Group 3 or 4 PCa, 51% and 30%, respectively, found it valuable to know the extent of GP4 in lower grade parts. These reported practice patterns were similar among medical subspecialties (Table 1).
3.2. Quantification of Gleason Pattern 4 and “Tertiary” Grading Patterns in RP Specimens
Approximately one-half of respondents (53%) found it valuable to know the extent of GP4 in RP specimens with Grade Groups 2–3. Clinicians were equally divided as to whether the 3rd most common pattern (tertiary) had to be “minor” (i.e., <5%) or could be “any amount”. In RP specimens with Grade Groups 2–3, 43% responded that a minor (<5%) component of Gleason pattern 5, along with other clinicopathologic parameters, may determine timing and nature of postoperative therapy. Practice patterns regarding quantification of GP4 and “tertiary” grading patterns in RP specimens were similar among medical subspecialties (Table 1).
3.3. Global/Overall Grading
In systematic NBx with different Grade Groups in different biopsy parts, 68% (373/552) preferred – in addition to individual grade per part – a global/overall score for the entire case (Europe/Canada/Australia/New Zealand: 75%; USA: 54%). Nonetheless, 69% (380/552) considered highest Grade Group in any part to be the overall Grade Group/Gleason score (GS) for treatment purposes. A minority wanted a global/overall grade based on averaging all parts (14%) or derived by adding the most common and highest-grade patterns in the case (14%).
3.4. Grading of MRI-targeted Needle Biopsy
In MRI-targeted NBx showing multiple positive cores with different grades, 76% (418/552) felt that pathologists should grade each individual positive core in each MRI-targeted area separately, while the remainder (24%) favored a grade averaged from all positive cores from a given target. For cases with positive cores in both systemic and MRI-targeted NBx, approximately one-third favored each of the following: a) grade each part separately and give a global/overall score for the case; b) grade each part separately and give separate global/overall scores for systematic and MRI-targeted NBx; and c) grade each part separately and do not assign a global/overall score.
3.5. Terminology of Grading
Respondents utilize the following terminology with patients: GS only: 25% (141/552), Grade Groups only: 7% (38/552), or both GS and Grade Groups: 68% (373/552).
3.6. Cribriform and Intraductal Carcinoma and Their Impact on Active Surveillance (AS)
The biggest differences among medical specialties were in dealing with cribriform carcinoma (Table 2). In NBx Grade Groups 2–3, 44% considered cribriform pattern 4 to affect patient counseling/management (Urology: 50%, Radiation Oncology: 24%). 57% (315/552) of respondents would consider AS in men with Grade Group 2 with a >10-year life expectancy (Urology: 66%, Radiation Oncology: 50%); however, for 63% of these clinicians, cribriform pattern 4 would impact this decision (Urology: 71%, Radiation Oncology: 34%).
Similarly, 71% would not recommend AS for an otherwise suitable candidate, if the NBx showed intraductal carcinoma. In NBx with coexisting intraductal carcinoma and Grade Group 1 carcinoma, 65% would not repeat NBx to look for higher grade PCa. In biopsies with intraductal carcinoma and Grade Groups 2–5 cancer, 69% responded that intraductal carcinoma would not affect therapy selection. With only intraductal carcinoma on NBx, respondents were evenly divided (45% each; 242/534 and 239/534) on whether to proceed with definitive therapy vs. perform repeat NBx to look for invasive PCa.
4. Discussion
A recent GUPS manuscript provided recommendations for pathologists dealing with emerging areas in PCa grading [3]; understanding the clinical perspective is equally important. In contrast to prior pathology-generated clinician surveys, which were narrow in scope and/or targeted clinicians in limited geographies [4–6], the current international survey evaluated clinician viewpoints regarding evolving PCa grading issues. This work aimed to: 1) better understand the use of PCa grading in clinical practice, and 2) serve as a baseline for improved communication between pathologists and clinicians, and hence, improved patient management. While a significant number of participants did not answer all questions, a limitation observed in surveys with > 10 questions, this report reflects the largest survey of diverse urological clinicians relating to contemporary PCa pathology issues.
4.1. Quantification of Gleason Pattern 4 in Needle Biopsy Scenarios
There are several important clinical rationales for quantifying extent GP4 on NBx. AS is now the preferred management for men with very low risk/low risk PCa, with some consideration of AS in select favorable intermediate risk patients (e.g. low-volume Grade Group 2) [7]. Emerging evidence demonstrates similar [low] rates of adverse RP pathology for NBx Grade Group 1 vs. Grade Group 2 with limited GP4 [8] and quantifying increased amounts of GP4 in Grade Group 2 adds value in predicting adverse RP pathology [9]. Accordingly, reporting extent GP4 in Grade Group 2 PCa has become standard practice (90% of GUPS pathology survey respondents) [3]. The current study reveals that 80% of clinician respondents also use extent GP4 for clinical decision-making.
Quantifying percent GP4 in NBx Grade Group 3 (i.e., 60% vs. 90%) may also be beneficial, as the former may represent oversampled GP4 in Grade Group 2 tumor. This is clinically relevant as Grade Group 2 vs. 3 can impact the use of androgen deprivation therapy in radiation therapy patients [7, 10]. There is conflicting literature on the association between extent GP4 in Grade Group 3 and higher grade/stage at RP [9, 11], which may be reflected in the lower number of GUPS pathologists who report GP4 (74%) [3], and clinicians (62%) who utilize extent GP4 in Grade Group 3 PCa management.
In cases with highest NBx Grade Group 4, the value of quantifying extent of GP4 for other parts with lower Grade Groups has not been studied. However, this information may predict downgrading at RP, seen in up to 50% of cases [12]. It is possible that patients with highest NBx Grade Group 4, yet low extent of GP4 in other cores, represent a subset suited for less aggressive therapy, with implications for those electing radiotherapy [7, 10]. Nonetheless, in the presence of Grade Group 4, only 30% of surveyed clinicians found extent of GP4 useful for management.
As opposed to the vast majority (95%) of GUPS pathologists who report percent GP4 on NBx as <5% or <10% with 10% increments thereafter [3], clinicians were equally divided as to whether a smaller increment or quartile approach (e.g., <25%, 25–49%, 50–74%, >75%) is most useful. Although this has not been extensively studied, there is concern that a quartile approach risks lumping dissimilar patients together (e.g., those with 5–10% vs. ~25% pattern 4). In limited studies, inter-observer variability for NBx using smaller increments was found to be relatively reproducible [13].
4.2. Quantification of Gleason Pattern 4 and “Tertiary” Grading Patterns in RP Specimens
In patients treated with RP, some studies demonstrated association between increasing percent GP4 and decreased rates of biochemical recurrence-free survival, while models controlled for standard parameters beyond Grade Group found no added value for outcome prediction [14]. While ~75% of GUPS pathologists assign percent GP4 for RP Grade Groups 2–3 [3], only half of clinical respondents found it valuable.
In RP with Grade Groups 2–3, a “tertiary grade” refers to a third, least common component of pattern 5. Most studies have used a 5% cutoff, while others use a higher/no cut-off (i.e., tertiary = 3rd most common of any amount) [15–17]. Whereas 78% of GUPS pathologists defined “tertiary” as <5% [3], only half of clinicians presumed this was the case. While some studies show PCa with tertiary pattern 5 behave similarly to those in the next highest Grade Group, others find a prognosis intermediate between Grade Groups [16–17]. GUPS has recommended replacing “tertiary grade” with “minor tertiary pattern 5”, to be used only in RP Grade Groups 2 or 3 with <5% pattern 5; if pattern 5 is 3rd most common, but >5%, it is included as the secondary grade [3]. With greater consistency in reporting, more comparable studies could determine the true clinical impact of minor tertiary pattern 5.
4.3. Global/Overall Grading and Grading of MRI-targeted Needle Biopsy
Some pathologists assign a “case-level” GS on NBx, which can be: 1) highest GS in any part; 2) average GS of all parts; or 3) derived GS - most common pattern + highest pattern; the latter two approaches are termed “global/overall” GS [18]. While 40% of the non-United States based GUPS pathologists (mostly from Canada, Europe and Australia) separately report a global/overall GS, only 6% of United States pathologists do so [3]. Preference for highest GS in any part as the case-level score has been demonstrated in urologist surveys in several countries [4–6] and by ~70% of the respondents in this survey. Nearly 70% of the clinicians would prefer pathologists provide a separate global/overall score, in addition to GS for individual biopsy parts. However, only 28% of clinicians would utilize the global/overall grade based on either average or derived GS for management. A confounding factor with determining global/overall GS is that most PCa is multifocal and heterogeneous [19]. Adding grades of potentially multifocal cancers grade together in NBx may result in a different grade than that of the dominant tumor nodule.
When multiple cores are taken from a single MRI-targeted lesion, assessing each core from that location individually, as opposed to in aggregate, may result in a different GS and cancer extent, with only limited data available as to which method is superior [20]. In the current survey, ~75% of respondents felt that pathologists should grade each individual core from each MRI-targeted lesion separately, whereas GUPS pathologists were split on this issue [3]. The challenge of reporting MRI-targeted biopsies is compounded in cases with positive cores in both systematic and targeted NBx. This complexity is highlighted by the nearly equal number of respondents who preferred one of the three provided reporting schemes (see Q17 in Supplemental Table 1 and results above). With increasing prevalence of MRI-targeted approaches, studies are urgently needed to determine how to best characterize cancer grade and extent on MRI-targeted biopsies.
4.4. Terminology of Grading
Since its initial proposal in 2013 by the group from Johns Hopkins Hospital [21], the patient-centric Grade Group system has been validated in large multi-institutional cohorts [22–23] and “Grade Group” nomenclature has been accepted by international associations and organizations [24–25]. Reflecting these developments, 95% of GUPS respondents currently report both GS and Grade Group [3], with 75% clinician respondents using Grade Group terminology in discussions with patients, typically together with GS.
4.5. Cribriform and Intraductal Carcinoma and Their Impact on Active Surveillance
Multiple studies have demonstrated that cribriform morphology on NBx is associated with upgrading and upstaging at RP and adverse outcomes after RP or radiation therapy [26–28]. Intraductal carcinoma is most often considered a late event in the setting of high-grade disease and is associated with adverse outcomes [29–30]. Likewise, cribriform and intraductal carcinoma have been associated with molecular features typically found in aggressive, lethal prostate cancers [28]. However, these studies are confounded by: a) different definitions of what caliber cribriform glands are associated with adverse features, b) use of cohorts with sextant (6 core) NBx, not reflecting contemporary practice, c) lack of distinction between cribriform and intraductal carcinoma, such that the independent effect of each is unclear, and d) variable results regarding added predictive value of these features when controlling for common clinicopathologic parameters [26–30]. Nevertheless, considering their overwhelming associations, GUPS has recommended reporting presence of cribriform and/or intraductal carcinoma in both NBx and RP specimens [3]. Currently however, only 49% of GUPS pathologists report presence of cribriform morphology in NBx Grade Groups 2–3 [3] and only 44% of clinicians responded that a cribriform pattern 4 component would affect patient counseling/management, highlighting both emerging awareness and challenges regarding this parameter. Interestingly, two times as many urologists than radiation oncologists felt that cribriform carcinoma would impact clinical decision making in NBx Grade Groups 2–3 and consideration of AS in Grade Group 2 patients with >10-year life expectancy.
Almost 70% of respondents would not recommend AS for an otherwise suitable candidate if NBx showed intraductal carcinoma, reflecting recognition of its typical association with aggressive disease. Similarly, nearly 2/3 would proceed to definitive therapy if NBx showed Grade Group 1 cancer and intraductal carcinoma, and nearly 50% would do so even in the unusual scenario of intraductal carcinoma on NBx without invasive carcinoma.
5. Conclusions
This GUPS survey of practice patterns in relationship to prostate cancer grading highlights similarities and differences between contemporary pathology reporting and its clinical application. As utilization of newer pathology parameters evolve, this study establishes a basis for better communication between pathologists and clinicians and identifies several key areas that should be the subject of future research.
Supplementary Material
Highlights.
GUPS survey: 834 clinician responses on practice patterns related to prostate cancer grading
Includes % high-grade disease, cribriform/intraductal carcinoma, MRI-targeted biopsy
Highlights similarities/differences between pathology reporting and clinical application
ABBREVIATION KEY
- PCa
prostate cancer
- GUPS
Genitourinary Pathology Society
- GP4
Gleason pattern 4
- RP
radical prostatectomy
- MRI
magnetic resonance imaging
- NBx
needle biopsy
- GS
Gleason score
- AS
active surveillance
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Epstein JI, Allsbrook WC Jr., Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol 2005;29:1228. [DOI] [PubMed] [Google Scholar]
- [2].Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 2016;40:244. [DOI] [PubMed] [Google Scholar]
- [3].Epstein JI, Amin MB, Fine SW, et al. The 2019 Genitourinary Pathology Society (GUPS) white paper on contemporary grading of prostate cancer. Arch Pathol Lab Med 2020. Jun 26; Online ahead of print. [DOI] [PubMed] [Google Scholar]
- [4].Rubin MA, Bismar TA, Curtis S, et al. Prostate needle biopsy reporting: how are the surgical members of the society of urologic oncology using pathology reports to guide treatment of prostate cancer patients? Am J Surg Pathol 2004;28:946. [DOI] [PubMed] [Google Scholar]
- [5].Descazeaud A, Rubin MA, Allory Y, et al. What information are urologists extracting from prostate needle biopsy reports and what do they need for clinical management of prostate cancer? Eur Urol 2005;48:911. [DOI] [PubMed] [Google Scholar]
- [6].Varma M, Narahari K, Mason M, et al. Contemporary prostate biopsy reporting: Insights from a survey of clinicians’ use of pathology data. J Clin Pathol 2018;71:874. [DOI] [PubMed] [Google Scholar]
- [7].Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2019;17:479. [DOI] [PubMed] [Google Scholar]
- [8].Huang CC, Kong MX, Zhou M, et al. Gleason score 3+4=7 prostate cancer with minimal quantity of Gleason pattern 4 on needle biopsy is associated with low-risk tumor in radical prostatectomy specimen. Am J Surg Pathol 2014;38:1096. [DOI] [PubMed] [Google Scholar]
- [9].Cole AI, Morgan TM, Spratt DE, et al. Prognostic value of percent Gleason grade 4 at prostate biopsy in predicting prostatectomy pathology and recurrence. J Urol 2016;196:405. [DOI] [PubMed] [Google Scholar]
- [10].Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol 2013;64:895. [DOI] [PubMed] [Google Scholar]
- [11].Sauter G, Steurer S, Clauditz TS, et al. Clinical utility of quantitative Gleason grading in prostate biopsies and prostatectomy specimens. Eur Urol 2016;69:592. [DOI] [PubMed] [Google Scholar]
- [12].Ranasinghe W, Reichard CA, Nyame YA, et al. Downgrading from biopsy grade group 4 prostate cancer in patients undergoing radical prostatectomy for high or very high risk prostate cancer. J Urol 2020. Apr 7; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [13].Sadimin ET, Khani F, Diolombi M, et al. Interobserver reproducibility of percent Gleason pattern 4 in prostatic adenocarcinoma on prostate biopsies. Am J Surg Pathol 2016;40:1686. [DOI] [PubMed] [Google Scholar]
- [14].Deng FM, Donin NM, Pe Benito R, et al. Size-adjusted quantitative Gleason score as a predictor of biochemical recurrence after radical prostatectomy. Eur Urol 2016;70:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mosse CA, Magi-Galluzzi C, Tsuzuki T, et al. The prognostic significance of tertiary Gleason pattern 5 in radical prostatectomy specimens. Am J Surg Pathol 2004;28:394. [DOI] [PubMed] [Google Scholar]
- [16].Baras AS, Nelson JB, Han M, et al. The effect of limited (tertiary) Gleason pattern 5 on the new prostate cancer grade groups. Hum Pathol 2017;63:27. [DOI] [PubMed] [Google Scholar]
- [17].Kato M, Hirakawa A, Kobayashi Y, et al. Integrating tertiary Gleason pattern 5 into the ISUP grading system improves prediction of biochemical recurrence in radical prostatectomy patients. Mod Pathol 2019;32:122. [DOI] [PubMed] [Google Scholar]
- [18].Trpkov K, Sangkhamanon S, Yilmaz A, et al. Concordance of “case level” global, highest, and largest volume cancer grade group on needle biopsy versus grade group on radical prostatectomy. Am J Surg Pathol 2018;42:1522. [DOI] [PubMed] [Google Scholar]
- [19].Andreoiu M, Cheng L. Multifocal prostate cancer: biologic, prognostic, and therapeutic implications. Hum Pathol 2010;41:781. [DOI] [PubMed] [Google Scholar]
- [20].Gordetsky JB, Schultz L, Porter KK, et al. Defining the optimal method for reporting prostate cancer grade and tumor extent on magnetic resonance/ultrasound fusion-targeted biopsies. Hum Pathol 2018;76:68. [DOI] [PubMed] [Google Scholar]
- [21].Pierorazio PM, Walsh PC, Partin AW, et al. Prognostic Gleason grade grouping: Data based on the modified Gleason scoring system. BJU Int 2013;111:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Epstein JI, Zelefsky MJ, Sjoberg DD, et al. A contemporary prostate cancer grading system: A validated alternative to the Gleason score. Eur Urol 2016;69:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Berney DM, Beltran L, Fisher G, et al. Validation of a contemporary prostate cancer grading system using prostate cancer death as outcome. Br J Cancer 2016;114:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. American Joint Committee on Cancer. Switzerland: Springer; 2017. [Google Scholar]
- [25].Moch H, Humphrey PA, Ulbright TM, et al. WHO Classification of Tumours of the Urinary System and Male Genital Organs. Geneva, Switzerland: WHO Press; 2016. [Google Scholar]
- [26].Kweldam CF, Kummerlin IP, Nieboer D, et al. Disease-specific survival of patients with invasive cribriform and intraductal prostate cancer at diagnostic biopsy. Mod Pathol 2016;29:630. [DOI] [PubMed] [Google Scholar]
- [27].Hollemans E, Verhoef EI, Bangma CH, et al. Large cribriform growth pattern identifies ISUP grade 2 prostate cancer at high risk for recurrence and metastasis. Mod Pathol 2019;32:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chua MLK, Lo W, Pintilie M, et al. A prostate cancer “nimbosus”: Genomic instability and SChLAP1 dysregulation underpin aggression of intraductal and cribriform subpathologies. Eur Urol 2017;72:665. [DOI] [PubMed] [Google Scholar]
- [29].Guo CC, Epstein JI. Intraductal carcinoma of the prostate on needle biopsy: Histologic features and clinical significance. Mod Pathol 2006;19:1528. [DOI] [PubMed] [Google Scholar]
- [30].van der Kwast T, Al Daoud N, Collette L, et al. Biopsy diagnosis of intraductal carcinoma is prognostic in intermediate and high-risk prostate cancer patients treated by radiotherapy. Eur J Cancer 2012;48:1318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


