Abstract
Hepatocellular carcinoma (HCC), the most common primary liver cancer has a high mortality in China, and it is usually diagnosed at a late stage, thereby leaving patients with few effective treatment options. Chimeric antigen receptor-T (CAR-T) cell therapy, a novel immunotherapy that has shown promising results in leukemia, lymphoma and multiple myeloma, is also expected to work well in solid tumors, including HCC. However, the ideal therapeutic efficacy has not yet been achieved, in part due to tumor antigen escape caused by antigen heterogeneity. To overcome such challenge, we screened a panel of biomarkers in HCC cell lines and found that GPC3 and B7H3 were highly expressed on HCC with expression heterogeneity. Then we developed a novel bispecific T cell engagers CAR-T (CAR.T-BiTEs) that drives the expression of a CAR specific for GPC3 and BiTEs against CD3 and B7H3, herein referred to as “GPC3-BiTE CAR.” We found that BiTEs promoted the increased activation of untransduced T cells and IFN-γ release. Moreover, BiTEs secreted by GPC3-BiTE CAR-HEK293T cells promoted increased cytotoxicity activity of untransduced T cells against GPC3+/B7H3+ (GPC3 positive/B7H3 positive) and GPC3-/B7H3+(GPC3 negative/B7H3 positive) HCC cell lines. In vitro function assays showed that GPC3-BiTE CAR-T cells exhibited greater cytotoxicity activity against GPC3+/B7H3+ HCC cell lines than GPC3 CAR-T cells (GPC3-targeted CAR-T cells) and B7H3 CAR-T cells (B7H3-targeted CAR-T cells). Furthermore, GPC3-BiTE CAR-T cells exhibited superior cytotoxicity against GPC3 negative HCC cell lines compared with GPC3 CAR T cells. In conclusion, our study showed that GPC3-BiTE CAR T cells exhibited superior antitumor activity than single-target CAR-T cells and can overcome tumor escape induced by antigen heterogeneity, suggesting that this could be a promising therapeutic strategy for HCC.
Keywords: Hepatocellular carcinoma, GPC3, B7H3-Targeted BiTE, CAR-T cell, Tumor relapse
Highlights
-
•
The expression of GPC3 and B7H3 was heterogeneous in HCC tissues.
-
•
BiTEs enhanced the cytotoxicity of untransduced T cells against HCC cell lines.
-
•
GPC3-BiTE CAR-T cells showed enhanced cytotoxicity against GPC3+/B7H3+ cells.
-
•
GPC3-BiTE CAR-T cells can resist GPC3 antigen escape in the in vitro assay.
Abbreviation
- BiTEs
bispecific T cell engagers
- B7H3
B7–H3
- CAR
chimeric antigen receptor
- GPC3
Glypican-3
- HCC
hepatocellular carcinoma
- PBMC
peripheral blood mononuclear cell
- ScFv
single-chain variable fragment
UTD untransduced T cells.
1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer in China, with a high mortality rate [1]. Besides, most of HCC patients are usually diagnosed at a late stage, and thus are ineligible for curative surgery/interventions [2]. Moreover, chemotherapy, radiotherapy, specific inhibitors and immune checkpoint inhibitors do not significantly improve the survival rates [3]. Thus, there is an urgent need to develop novel and effective treatment strategies for HCC.
Chimeric antigen receptor (CAR)-T cells (CAR-T) genetically engineered to express CAR molecules that targets surface antigens on tumor cells, allowing them to recognize and kill tumor cells more accurately and effectively. CAR-T cell therapy has made remarkable achievements in the treatment of hematological malignancies including acute lymphoblastic leukemia, lymphoma, and multiple myeloma [[4], [5], [6]]. Such breakthroughs in hematological malignancies sparked a surge in research into translating CAR-T therapy into the treatment of solid tumors, including HCC [7]. Glypican-3 (GPC3), a cell-surface glycophosphatidylinositol (GPI)-anchored protein that highly expressed in HCC, is considered as a promising immunotherapeutic target [8,9]. GPC3 CAR-T cells have been shown to be effective against HCC in preclinical and clinical trials [10,11]. However, the efficacy is not fully satisfactory in part due to antigen escape (antigen loss, antigen-shedding and antigen-low escape) [12,13], suggesting that multi-target strategies are expected to circumvent antigen escape thereby improve CAR-T efficacy. Bispecific T cell Engagers (BiTEs) are bispecific antibodies that redirect bystander T cells to target antigen-expressing tumors. Locally secreted BiTEs can complement CAR-T cells and were considered as potential strategy to address antigen escape [7,14]. As previously reported, CAR-T.BiTE cells that expressed EGFRVIII CAR and secreted EGFR-specific BiTEs successfully eliminated heterogeneous tumors in glioblastoma mouse models with no detectable toxicity [15]. However, it is unclear whether CAR-T.BiTEs can be used in HCC therapy, and if so, which target is most effective in combination, remains elusive.
B7H3 is a type I transmembrane protein that is overexpressed in multiple solid tumors and considered as a potential therapeutic target [16,17]. B7H3-targeted CAR-T cells, humanized antibodies, and BiTEs have shown potent antitumor activity in several solid tumors, [[18], [19], [20]]. Similarly, B7H3 is highly expressed in human HCC and is associated with tumor aggressiveness and postoperative recurrence [21,22], suggesting B7H3 as attractive target for HCC therapy.
In this study, we analyzed the expression of B7H3 and GPC3 in The Cancer Genome Atlas (TCGA) database and found that both are highly expressed in HCC but have little correlations, suggesting them as an ideal antigen pair for dual targeting to overcome antigen escape or heterogeneity. We then generated GPC3-BiTE CAR-T cells that expresses GPC3-specific CARs while also secreting BiTEs against B7H3, and tested their antitumor activity in vitro. As expected, GPC3-BiTE CAR-T cells secreted B7H3-specific BiTEs which recruited untransduced bystander T cells against GPC3-B7H3+ HCC cells and exhibited more potent antitumor activity in vitro. Thus, GPC3-BiTE CAR-T cells could be a promising therapeutic option for HCC patients with GPC3 heterogeneous expression.
2. Materials and methods
2.1. Cell lines
Human HCC cell line Huh7 was purchased from the Chinese Academy of Sciences Shanghai Cell Bank in China. Human embryonic kidney (HEK) 293T cell was purchased from the American Type Culture Collection (ATCC). SK-HEP-1 cell line was purchased from Guangzhou Cellcook Biotechnology Co., Ltd, China. Huh7 and HEK293T cell lines were cultured in DMEM medium (Gibco) supplemented with 10% FBS (Gibco) and 1% penicillin-streptomycin (PS) (Gibco). SK-HEP-1 and SK-HEP-1-B7H3 were generated to stably express B7H3 by lentivirus, GenBank: NM_001024736.2. and the stable cell lines were cultured in RMPI-1640 medium (Gibco) supplemented with 10% FBS and 1% PS. All cell lines were cultured at 37 °C with 5% CO2.
2.2. Flow cytometry
Cell surface expression levels of GPC3, B7H3 and CAR were assessed and analyzed by flow cytometry. In brief, cells were washed with ice-cold PBS, collected by centrifugation, and then incubated with antibodies specific to human GPC3 (APC-anti-Glypican3 Antibody, clone 024, Sino Biological) or B7H3 (APC anti-human CD276, clone MIH42, Biolegend) for 30 min at 4 °C in the dark. For CAR expression analyses, cells were labeled with primary antibodies of biotin-goat anti-Human IgG, F(ab')₂ fragment (Jackson) (corresponding to B7H3 CAR) or biotin-goat anti-Mouse IgG, F(ab')₂ fragment (Jackson) (corresponding to GPC3 CAR or GPC3-BiTE CAR) for 30min, followed by staining with APC-conjugated streptavidin (Biolegend). After washing once with ice-cold PBS, the cells were resuspended in 200 μL of PBS and analyzed using flow cytometer (CytoFLEX, Beckman). Data were analyzed using FlowJo 10.5.3. (https://www.flowjo.com).
2.3. Construction of CARs
One GPC3-BiTE CAR construct and two additional CAR constructs (anti-GPC3 and anti-B7H3) were synthesized and cloned into a lentiviral plasmid of pWPXLd. All CAR and GPC3-BiTE CAR constructs contained a human CD8a hinge and transmembrane domain, an intracellular 4-1BB costimulatory domain and a CD3ζ signaling domain. BiTEs against B7H3 and CD3 were designed, with both sequences flanked by an IGHV3-2 leader peptide (IGHV3-2-SP) and a His-tag element. Individual anti-GPC3 and anti-B7H3 scFv sequences were prepared described in these papers [10,23]. The anti-CD3 scFv sequences were obtained from publicly available Muromonab-CD3 (OKT3) sequences.
2.4. Production of lentivirus
For lentivirus production, HEK293T cells were transfected with each lentivirus vector (GPC3 CAR, B7H3 CAR, GPC3-BiTE CAR, and B7H3-EGFP, respectively) together with helper plasmids psPAX2 and pMD2G using Lipofectamine™ 3000 Reagent (Invitrogen). 48–72 h after transfection, virus-containing supernatant was collected, and centrifuged at 2000 rpm for 10min followed by filtration with a 0.45 μm filter.
2.5. ELISA assay
Supernatants were collected from GPC3 CAR-HEK293T and GPC3-BiTE CAR-HEK293T cells 48 h after transfection. The concentration of BiTEs was measured using a His-Tag ELISA Detection Kit (GenScript) according to the manufacturer's instruction, and determined by measuring the OD450 value by Microplate Reader (Thermo Fisher Scientific). Detailed experimental procedures are shown in Supplementary Files.
2.6. T-cell activation and functional assays
Untransduced T cells were cocultured with Huh7 cells in the presence of supernatant from GPC3 CAR-HEK293T or GPC3-BiTE CAR-HEK293T cells. Cell pellets were obtained 20 h later for detection of CD69 by staining with APC anti-human CD3 Antibody (Biolegend) and FITC anti-human CD69 Antibody (Biolegend), then analyzed by flow cytometry. The supernatant was collected, and the IFN-γ level was determined using flow cytometry with the Cytometric Bead Array (CBA) Human Th1/Th2 Cytokine Kit (BD™). Detailed experimental procedures are shown in Supplementary Files.
2.7. Preparation of CAR-T cells
Fresh blood was obtained from healthy donors with informed consent, in compliance with Shenzhen Institutes of Advanced Technology's ethics committee and Institutional Review Board approval. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Paque PLUS (GE Healthcare). After being washed with D-PBS, CD3+ T cells were isolated and activated using Dynabeads™ CD3/CD28 (Thermo fisher Scientific) and cultured overnight in KBM581 medium (Hyclone) supplemented with 10% FBS and IL-2 (Thermo fisher Scientific) at 37 °C, and 5% CO2. The activated T cells were then transduced with lentiviral supernatants containing the different CAR constructs. 8ug/ml of Polybrene (Beyotime) was used when transducing GPC3-BiTE CAR-T cells. 2 days after transducing the cells with lentivirus, fresh media (KBM581 medium) were used as replacement. The transduction efficiency was determined after 3 days post-transduction by flow cytometry. GPC3-BiTE CAR-T cells were purified using a biotin goat Anti-Human IgG, F(ab')₂ fragment specific antibody (Jackson), followed by a MagniSort™ Streptavidin Positive Selection Bead (Invitrogen). CAR-T cells were cultured for another 2 or 3 days, followed by the removal of the CD3/CD28 Dynabeads™ before being tested for cytotoxicity assay.
2.8. In vitro cytotoxicity assay
In this study, the xCELLigence real-time cell analyzer (RTCA) (AECA Biosciences) was used for cytotoxicity assay as previously described [24,25] with slight modifications. Briefly, 50 μl complete cell culture medium was used to blank the 96-well E-Plates (ACEA Biosciences). 50ul target cell suspension with 5000 cells were seeded into the each well. The plate was kept in the incubator for 15min and then transferred to the RTCA SP Instrument (AECA Biosciences) inside a cell culture incubator. After about 24 h, the effector cells (untransduced T cells (UTD) or CAR-T cells) were collected, counted, and resuspended in 100 μl KBM581 medium supplemented with 10% FBS and added into the well at the corresponding effector/target ratio. To test BiTE cytotoxicity against HCC cell lines, the untransduced T cell were resuspended with 100 μl supernatants collected from GPC3 CAR-HEK293T cells and GPC3-BiTE CAR-HEK293T cells at effector/target ratios of 20:1, 10:1, and 5:1. The control group consisted of a well containing only cell culture medium and target cells, the RTCA was used to monitor the cell index (CI) of the cells for the indicated time (50–60 h).
The Cell Index (CI) was normalized using the RTCA software Pro (version 2.3.0) and percentage of cytotoxicity was calculated as described [24,25] at any given time:
| % cytotoxicity = ((CI control – CI effector)/ (CI control)) × | 100 |
2.9. Statistical analysis
All analyses were reported as mean ± SD, statistical analysis was performed using GraphPad Prism 9.0.0 (San Diego, CA).
3. Results
3.1. Expression analysis of GPC3 and B7H3 in HCC
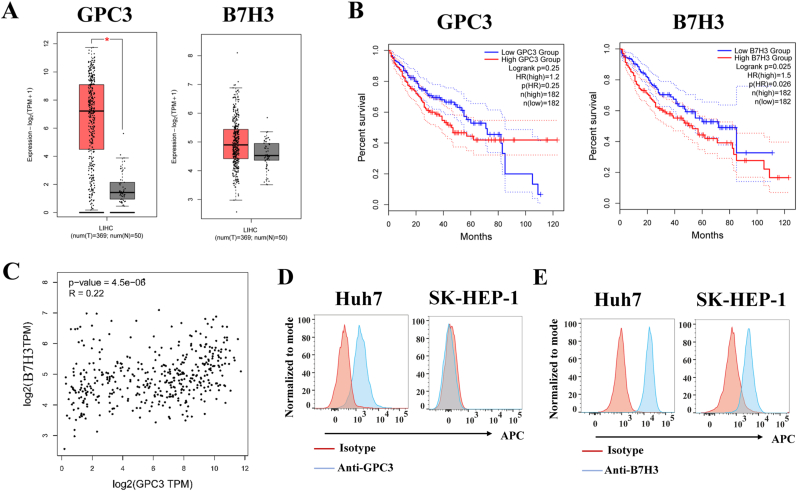
To assess the expression pattern of GPC3 and B7H3 in HCC, we analyzed their expression in HCC tissues. The results showed that the mRNA of GPC3 and B7H3 was highly expressed in the HCC tissue based on the TCGA datasets using the web server of Gene Expression Profiling Interactive Analysis 2 (GEPIA2) [26] (Fig. 1A). The correlation between the expression of GPC3 or B7H3 and survival was also assessed using GEPIA 2. According to the data, higher levels of GPC3 or B7H3 expression in HCC patients were associated with a five-year shorter life expectancy (Fig. 1B); however, the expression correlation between GPC3 and B7H3 is low, with an index of 0.22 (Fig. 1C). Next, we assessed GPC3 and B7H3 expression pattern on HCC cell lines Huh7 and SK-HEP-1 by flow cytometry. The results showed that GPC3 and B7H3 were highly expressed on Huh7 cell lines (Fig. 1D and E). However, GPC3 was not expressed in SK-HEP-1 cell lines, whereas B7H3 was expressed at low level (Fig. 1D and E).
Fig. 1.
Analysiss of expression and survival of GPC3 and B7H3 in HCC. (A) mRNA expression analysis of GPC3 and B7H3 in HCC based on the TCGA datasets using GEPIA2. (B) Correlational analysis between overall survival time and GPC3 or B7H3 expression level in HCC using GEPIA2. (C) Correlation analysis of GPC3 and B7H3 expression using GEPIA 2. (D, E) Analysis of GPC3 and B7H3 expression on HCC cell lines Huh7 and SK-HEP-1 by flow cytometry. LIHC: Liver hepatocellular carcinoma.
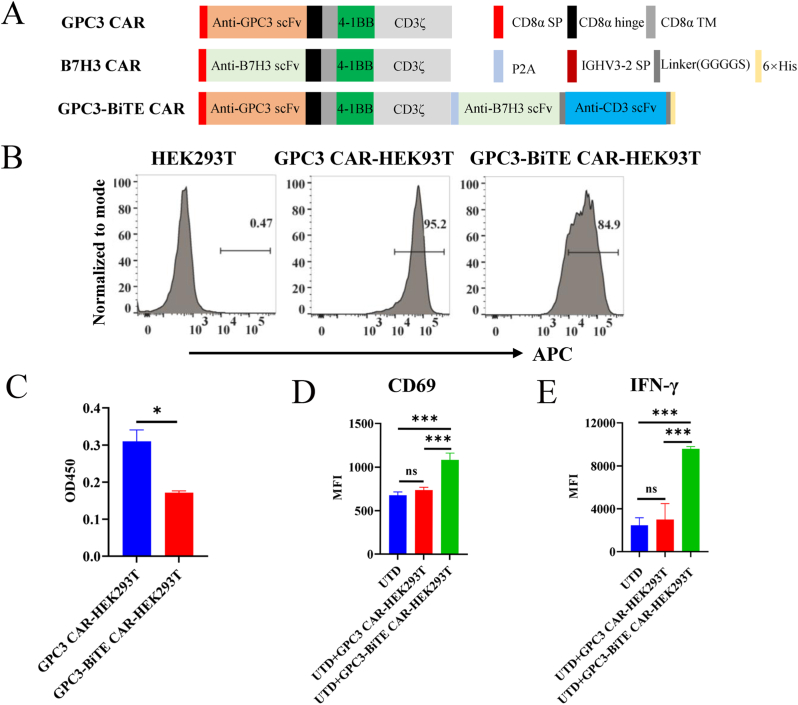
3.2. Construction of CAR
In this study, we constructed three different CARs, as shown in Fig. 2A. The GPC3 CAR and B7H3 CAR had the same backbone, which included a CD8a hinge and transmembrane domain, a 4-1BB costimulatory domain, and a CD3ζ signaling domain, excepting for the scFv targeting GPC3 and B7H3, respectively. The GPC3-BiTE CAR constructs consisted of the GPC3 CAR, P2A, IGHV3-2-SP, B7H3 scFv targeting the antigen of B7H3, glycine-serine linker (GGGGS), CD3 scFv targeting the antigen of CD3, glycine-serine linker (GGGGS) and a polyhistidine-tag (His-tag). Additionally, the BiTEs were designed against the B7H3 on the HCC cell and CD3 on T cells.
Fig. 2.
BiTEs activated untransduced T cells in HEK293T cells. (A) Schematic diagram of three CARs used in this study. (B) Transduction efficiency of GPC3 CAR and GPC3-BiTE CAR on HEK293T cells was detected by flow cytometry. (C) Expression of BiTEs in HEK293T cells were detected by ELISA. (D) Expression analysis of CD69 was detected by flow cytometry. (E) Expression of IFN-γ was detected by flow cytometry using CBA kit. MFI: Mean Fluorescence Intensity. Statistical significance was assessed by t-test (C) and one-way ANOVA (D and E). Not significant ns, P > 0.05, *P ≤ 0.05, ***P ≤ 0.001.
3.3. BiTEs enhanced activation of untransduced T cells
The lentivirus of GPC3 CAR and GPC3-BiTE CAR was used to transduced HEK293T cells, and flow cytometry analysis showed that the transduction efficiency of HEK293T cells expressing GPC3 CAR and GPC3-BiTE CAR was 95% and 84%, respectively (Fig. 2B). The supernatants from the GPC3 CAR-HEK293T and GPC3-BiTE CAR-HEK293T cells were then used in the competitive ELISA to detect BiTEs expression. The results showed that supernatant from the GPC3-BiTE CAR-HEK293T had lower OD450 compared to those from the GPC3 CAR-HEK293T (Fig. 2C). Next, we also investigated whether the activation of untransduced T cells was promoted by BiTEs. The results showed that untransduced T cells cocultured with the Huh7 cells and supernatant from GPC3-BiTE CAR-HEK293T showed higher CD69 expression (Fig. 2D). Furthermore, untransduced T cells cocultured with Huh7 cells and supernatant from GPC3-BiTE CAR-HEK293T cells generated more higher IFN-γ (Fig. 2E). These findings suggest that BiTEs enhanced the activation of untransduced T cells.
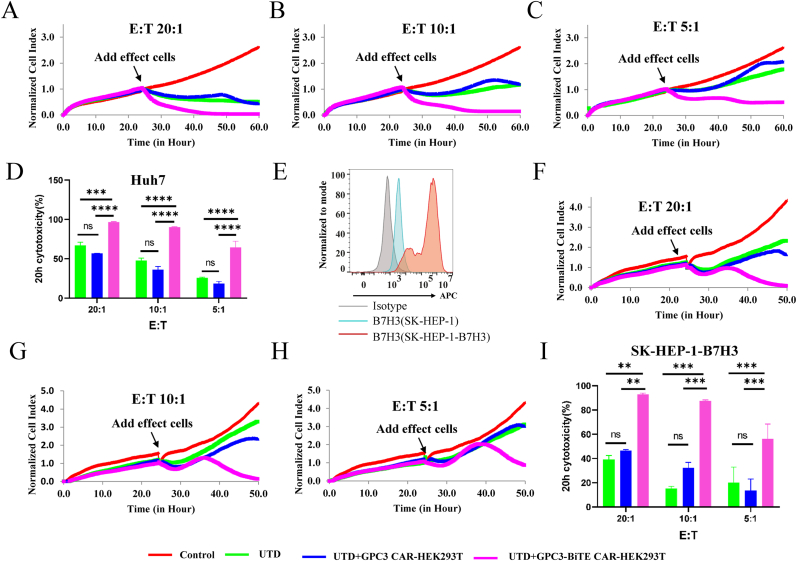
3.4. BiTEs enhanced cytotoxicity activity of untransduced T cells against HCC cell lines
Next, we assessed whether the BiTEs from GPC3-BiTE CAR-HEK293T cells could enhance untransduced T cell cytotoxicity against HCC cell lines. To investigate this, supernatant was collected from HEK293T, GPC3 CAR-HEK293T, and GPC3-BiTE CAR-HEK293T and cocultured with the untransduced T cell and Huh7 cells respectively using the xCELLigence RTCA system. Our findings show that when untransduced T cells were cocultured with supernatant from the GPC3-BiTE CAR-HEK293T cells, they displayed more potent cytotoxicity against Huh7 cells than the supernatant from GPC3 CAR-HEK293T cells at the different E:T ratios (Fig. 3A–D). We used SK-HEP-1 cell lines stably expressing B7H3 (SK-HEP-1-B7H3) to test BiTE cytotoxicity against GPC3-/B7H3+ cell lines (Fig. 3E). The results showed that when untransduced T cells were cocultured with supernatant from GPC3-BiTE CAR HEK293T cells, they produced more potent cytotoxicity against SK-HEP-1-B7H3 cells than supernatant from GPC3 CAR-HEK293T cells at the different E:T ratios (Fig. 3F–I).
Fig. 3.
Cytotoxicity of BiTEs against HCC cell lines Huh7 and SK-HEP-1-B7H3 was assessed by RTCA. The supernatant was collected from HEK293T cells transduced with lentivirus of GPC3 CAR and GPC-BiTE CAR and cocultured with untransduced T cell and HCC cell lines. (A–D) The cell index curve and cytotoxicity statistical analysis of BiTEs against Huh7 cell lines. (E) Expression of B7H3 on SK-HEP-1 and SK-HEP-1-B7H3 cell lines was detected by flow cytometry. (F–I) The cell index curve and cytotoxicity statistical analysis of BiTEs against SK-HEP-1-B7H3 cell lines. Statistical significance was assessed by two-way ANOVA (D and I). Not significant ns, P > 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
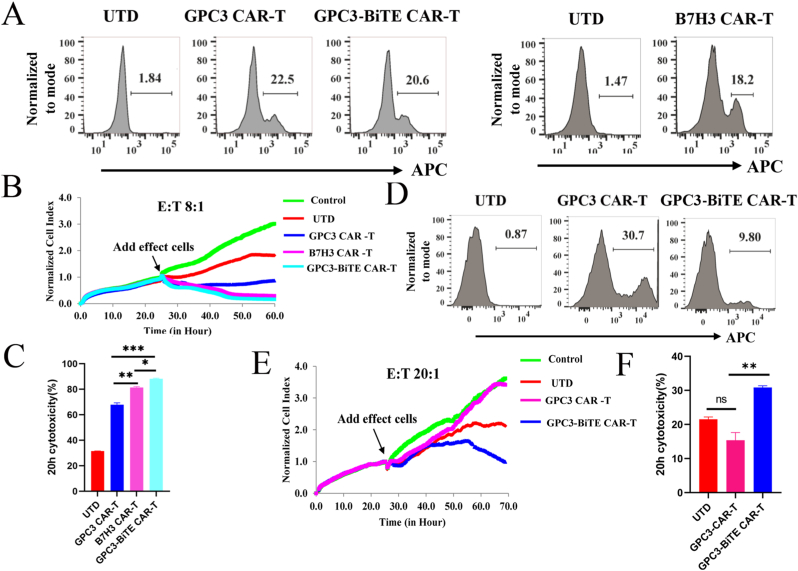
3.5. GPC3-BiTE CAR-T cells exhibit enhanced antitumor activity than GPC3 CAR-T or B7H3 CAR-T cells in vitro assay
The antitumor activity of GPC3-BiTE CAR-T cells were assessed against HCC cell lines, we used the GPC3 CAR-T cells, B7H3 CAR-T cells and GPC3-BiTE CAR-T cells that were successfully constructed (Fig. 4A). The results of the cytotoxicity assay showed that GPC3-BiTE CAR-T cells were more potent against the Huh7 cells compared to the GPC3 CAR-T cells and B7H3 CAR-T cells at the E:T ratio of 8:1 (Fig. 4B and C). Moreover, we also found that the B7H3 CAR-T cells showed more potent cytotoxicity against Huh7 cells than GPC3 CAR-T cells (Fig. 4B and C). To investigate whether the GPC3-BiTE CAR-T cells had more potent cytotoxicity against GPC3-/B7H3+ cell lines than GPC3 CAR-T cells in vitro, the SK-HEP-1-B7H3 cells were used as the target cells. Using PBMC from another healthy donor, we generated GPC3 CAR-T cells and GPC3-BiTE CAR-T cells (Fig. 4D). Cytotoxicity analysis revealed that GPC3 CAR-T cells exhibited similar cytotoxicity to UTD; however, GPC3-BiTE CAR-T cells exhibited a superior cytotoxicity against the SK-HEP-1-B7H3 cell compared with the GPC3 CAR-T cells (Fig. 4E and F).
Fig. 4.
Cytotoxicity of CAR-T cells against HCC cell lines was assessed by RTCA. (A) Transduction efficiency of GPC3 CAR, B7H3 CAR, GPC3-BiTE CAR on T cells was detected by flow cytometry. The right three T cells were stained with the Biotin-Goat Anti-Mouse IgG, F(ab') ₂ fragment specific and the right two T cells were stained with Biotin-Goat Anti-Human IgG, F(ab') ₂ fragment specific. (B and C) The cell index curve of RTCA and cytotoxicity statistical analysis of CAR-T cells against Huh7 cell lines. (D) Transduction efficiency of GPC3 CAR, GPC3-BiTE CAR on T cells was detected by flow cytometry. The T cells were isolated from another donor PBMC. (E and F) The cell index curve and cytotoxicity statistical analysis of CAR-T cells against SK-HEP-1-B7H3 cell lines. Statistical significance was assessed by two-way ANOVA (C and F). Not significant ns, P > 0.05, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
4. Conclusion
In this study, we found that GPC3-BiTE CAR-T cells exhibited a superior cytotoxicity against the HCC cells than GPC3 CAR-T cells and B7H3 CAR-T cells, and that they can overcome the problem of antigen escape induced by GPC3 heterogenous expression in the in vitro assay.
In clinical trials, antigen escape-mediated tumor recurrence has been reported in both hematological and solid tumors [27,28]. Antigen escape in CAR-T cell therapy can be overcome by increasing antigen expression on the cell membrane of tumor cells and genetically engineering CAR-T cells to target two or more antigens [14]. Surprisingly, clinical trials of GPC3 CAR-T cells therapy for HCC have not demonstrated significant efficacy [11]. The poor efficacy of CAR-T cell therapy in hepatocellular carcinoma may be due to antigen heterogeneity expression or antigen shedding [12,14], thus, avoiding potential antigen-escape mediated tumor relapse may improve the efficacy of CAR-T cells therapy for HCC treatment. The CAR.T-BiTE strategy has been reported have multiple advantages and is applicable in overcoming antigen escape in glioblastoma mouse model [15]. In this study, we combined the target antigen of GPC3 and B7H3 for the first time through the CAR.T-BiTE strategy to further confirm the feasibility of the CAR.T-BiTE strategy for hepatocellular carcinoma treatment. GPC3 or B7H3 were found to be highly expressed in HCC tissue, and their expression was heterogeneous. This suggests that designing CAR structures that targets these two antigens may produce improved efficacy. In addition, the HEK293T system, which we used to verify that BiTE was successfully secreted by GPC3-BiTE CAR and that it promoted stronger T cells activation and more IFN-γ secretion by untransduced T cells. We also demonstrated that BiTE promoted T cell killing activity against GPC3+/B7H3+ and GPC3-/B7H3+ HCC cell lines by collecting the supernatant of GPC3-BiTE CAR in HEK293T cells. Furthermore, we confirmed that GPC3-BiTE CAR-T cells showed higher cytotoxicity activity against GPC3+/B7H3+ HCC cell lines than GPC3 CAR-T cells or B7H3 CAR-T cells. Finally, by recruiting untransduced T cells via the BiTE system, GPC3-BiTE CAR-T cells exhibited greater cytotoxicity activity against GPC3-/B7H3+ HCC cell lines compared to GPC3 CAR-T cells, suggesting that GPC3-BiTE CAR-T cells can overcome the problem of tumor cell escape induced by heterogeneous expression of GPC3.
However, this study has several shortcomings: it is unclear whether the GPC3-BiTE CAR-T cell can generate more cytokines than GPC3 CAR-T cells and B7H3 CAR-T cells when cultured with target cells. Besides, this study found that GPC3-BiTE CAR-T cells had higher in vitro cytotoxicity than single target CAR-T cells, indicating that there is an urgent need to test the antitumor activity of GPC3-BiTE CAR-T cells in xenograft mouse models of hepatocellular carcinoma.
In conclusion, GPC3-BiTE CAR-T cells have superior cytotoxicity effect against HCC cell lines than single CAR-T cells and can overcome tumor cell escape caused by the heterogeneous GPC3 expression in vitro assay. This research opens up a new avenue for the use of CAR-T cell therapy in hepatocellular carcinoma.
Acknowledgements
This work was supported by Key-Area Research and Development Program of Guangdong Province [2019B020201014].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2022.101324.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Llovet J.M., Kelley R.K., Villanueva A., et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse S.F., McGlynn K.A., Reichman M.E. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2009;27:1485–1491. doi: 10.1200/jco.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giraud J., Chalopin D., Blanc J.F., et al. Hepatocellular carcinoma immune landscape and the potential of immunotherapies. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.655697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maude S.L., Frey N., Shaw P.A., et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neelapu S.S., Locke F.L., Bartlett N.L., et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raje N., Berdeja J., Lin Y., et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl. J. Med. 2019;380:1726–1737. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rafiq S., Hackett C.S., Brentjens R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy, Nature reviews. Clin. Oncol. 2020;17:147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumhoer D., Tornillo L., Stadlmann S., et al. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am. J. Clin. Pathol. 2008;129:899–906. doi: 10.1309/HCQWPWD50XHD2DW6. [DOI] [PubMed] [Google Scholar]
- 9.Nishida T., Kataoka H. Glypican 3-targeted therapy in hepatocellular carcinoma. Cancers (Basel) 2019;11 doi: 10.3390/cancers11091339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao H., Li K., Tu H., et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin. Cancer Res. : Off. J. Am. Assoc. Cancer Res. 2014;20:6418–6428. doi: 10.1158/1078-0432.Ccr-14-1170. [DOI] [PubMed] [Google Scholar]
- 11.Shi D., Shi Y., Kaseb A.O., et al. Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: results of phase I trials. Clin. Cancer Res. : Off. J. Am. Assoc. Cancer Res. 2020;26:3979–3989. doi: 10.1158/1078-0432.Ccr-19-3259. [DOI] [PubMed] [Google Scholar]
- 12.Sun L., Gao F., Gao Z., et al. Shed antigen-induced blocking effect on CAR-T cells targeting Glypican-3 in Hepatocellular Carcinoma. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2020-001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majzner R.G., Mackall C.L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8:1219–1226. doi: 10.1158/2159-8290.Cd-18-0442. [DOI] [PubMed] [Google Scholar]
- 14.Kailayangiri S., Altvater B., Wiebel M., et al. Overcoming heterogeneity of antigen expression for effective CAR T cell targeting of cancers. Cancers (Basel) 2020;12 doi: 10.3390/cancers12051075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi B.D., Yu X., Castano A.P., et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 2019;37:1049–1058. doi: 10.1038/s41587-019-0192-1. [DOI] [PubMed] [Google Scholar]
- 16.Chapoval A.I., Ni J., Lau J.S., et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat. Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 17.Yang S., Wei W., Zhao Q. B7-H3, a checkpoint molecule, as a target for cancer immunotherapy. Int. J. Biol. Sci. 2020;16:1767–1773. doi: 10.7150/ijbs.41105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma W., Ma J., Ma P., et al. Targeting immunotherapy for bladder cancer using anti-CD3× B7-H3 bispecific antibody. Cancer Med. 2018;7:5167–5177. doi: 10.1002/cam4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loo D., Alderson R.F., Chen F.Z., et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin. Cancer Res. : Off. J. Am. Assoc. Cancer Res. 2012;18:3834–3845. doi: 10.1158/1078-0432.Ccr-12-0715. [DOI] [PubMed] [Google Scholar]
- 20.Du H., Hirabayashi K., Ahn S., et al. Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T cells. Cancer Cell. 2019;35:221–237. doi: 10.1016/j.ccell.2019.01.002. e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang F.B., Wang L., Jia H.C., et al. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15:45. doi: 10.1186/s12935-015-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun T.W., Gao Q., Qiu S.J., et al. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunol. Immunother. : CII. 2012;61:2171–2182. doi: 10.1007/s00262-012-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majzner R.G., Theruvath J.L., Nellan A., et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin. Cancer Res. : Off. J. Am. Assoc. Cancer Res. 2019;25:2560–2574. doi: 10.1158/1078-0432.Ccr-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Afolabi L.O., Bi J., Li X., et al. Synergistic tumor cytolysis by NK cells in combination with a pan-HDAC inhibitor, panobinostat. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.701671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afolabi L.O., Bi J., Chen L., et al. A natural product, Piperlongumine (PL), increases tumor cells sensitivity to NK cell killing. Int. Immunopharm. 2021;96 doi: 10.1016/j.intimp.2021.107658. [DOI] [PubMed] [Google Scholar]
- 26.Li C., Tang Z., Zhang W., et al. GEPIA2021: integrating multiple deconvolution-based analysis into GEPIA. Nucleic Acids Res. 2021;49:W242–w246. doi: 10.1093/nar/gkab418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sotillo E., Barrett D.M., Black K.L., et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.Cd-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Rourke D.M., Nasrallah M.P., Desai A., et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.