Abstract
Healthcare workers experienced high degree of stress during COVID-19. Purpose of the present article is to compare mental health (depressive and Post-Traumatic-Stress-Disorders—PTSD—symptoms) and epigenetics aspects (degree of methylation of stress-related genes) in front-line healthcare professionals versus healthcare working in non-COVID-19 wards. Sixty-eight healthcare workers were included in the study: 39 were working in COVID-19 wards (cases) and 29 in non-COVID wards (controls). From all participants, demographic and clinical information were collected by an ad-hoc questionnaire. Depressive and PTSD symptoms were evaluated by the Patient Health Questionnaire-9 (PHQ-9) and the Impact of Event Scale—Revised (IES-R), respectively. Methylation analyses of 9 promoter/regulatory regions of genes known to be implicated in depression/PTSD (ADCYAP1, BDNF, CRHR1, DRD2, IGF2, LSD1/KDM1A, NR3C1, OXTR, SLC6A4) were performed on DNA from blood samples by the MassARRAY EpiTYPER platform, with MassCleave settings. Controls showed more frequent lifetime history of anxiety/depression with respect to cases (χ2 = 5.72, p = 0.03). On the contrary, cases versus controls presented higher PHQ-9 (t = 2.13, p = 0.04), PHQ-9 sleep item (t = 2.26, p = 0.03), IES-R total (t = 2.17, p = 0.03), IES-R intrusion (t = 2.46, p = 0.02), IES-R avoidance (t = 1.99, p = 0.05) mean total scores. Methylation levels at CRHR1, DRD2 and LSD1 genes was significantly higher in cases with respect to controls (p < 0.01, p = 0.03 and p = 0.03, respectively). Frontline health professionals experienced more negative effects on mental health during COVID-19 pandemic than non-frontline healthcare workers. Methylation levels were increased in genes regulating HPA axis (CRHR1) and dopamine neurotransmission (DRD2 and LSD1), thus supporting the involvement of these biological processes in depression/PTSD and indicating that methylation of these genes can be modulated by stress conditions, such as working as healthcare front-line during COVID-19 pandemic.
Keywords: Health professionals, COVID-19, Mental health, Epigenetics, Stress-related genes
Introduction
COVID-19 pandemic resulted to be a highly traumatic and stressful event for general population [1]. Different studies reported an increased frequency of mental conditions and insomnia in previously healthy subjects [2] as a result of social isolation, restrictions and fear of contamination [3]. The effects of the pandemic were even more devastating for specific groups of subjects such as individuals with chronic medical conditions (e.g., diabetes or rheumatoid arthritis) [4, 5] or health professionals who had to face directly the sanitary emergency [6].
From the beginning of pandemic, healthcare workers experienced high rates of anxiety, depression and stress [6]. Being front-line, female gender and young age have been identified as the main factors associated with the development of affective symptoms in health professionals [6]. These aspects have been displayed by different studies conducted in various geographical areas including Italy [7], France [8] and Pakistan [9]. Of note, healthcare professionals working in COVID-19 wards were found to be more prone to develop depression, insomnia and Post-Traumatic Stress Disorder (PTSD) symptoms compared to those working in other wards [10, 11].
With regard to biological aspects, COVID-19 and mental disorders share abnormalities in inflammation and Hypothalamic–Pituitary–Adrenal (HPA) axis [12]. A recent study demonstrated that patients with lymphopenia had an increased risk to receive a psychotropic medication during SARS-CoV-2 infection [13]. On the other hand, prolonged stress associated to events such as COVID-19 is likely to modify different biological systems and predispose health care workers to develop psychiatric symptoms [14]. Of note, some authors reported that high levels of stress are associated with the onset of depressive symptoms together with prominent inflammation [15], cortisol dysregulation [16] and modifications in oxytocin-related pathways [17].
A possible link between exposure to stressing factors, such as looking after COVID-19 patients, and psychiatric conditions in front-line healthcare professionals is represented by epigenetic modifications, induced by an adverse environment and leading to changes in gene expression and neural circuit function [18]. Epigenetic modifications can be defined as changes occurring “above” the level of the DNA sequence, thus influencing gene expression without altering DNA sequence [19]. The most widely known and investigated epigenetic mechanism is DNA methylation, consisting in the addition of methyl groups to the cytosine at dinucleotides cytosine–guanine, termed “CpG” dinucleotides, located at DNA regions involved in gene expression regulation.
DNA methylation is a mechanism involved in the regulation of gene expression in the brain, as demonstrated by several studies [20, 21]. Of note, alterations in DNA methylation in the central nervous system may favor the development of neuropsychiatric disorders, such as major depression, schizophrenia or PTSD [22]. Indeed, individuals exposed to chronic stress conditions showed altered methylation levels of genes, such as BDNF and SLC6A4 [23–25]. In addition, early life stress events, such as the in utero exposure to adverse conditions, can result in the setting of altered DNA methylation patterns of genes controlling emotional aspects of behavior, such as NR3C1, SLC6A4, and OXTR [26]. Interestingly, alterations in methylation levels established during pregnancy could be stably maintained throughout life, and predispose individuals to psychiatric illness, such as major depression [27, 28]. Finally, available literature indicates epigenetic changes in glucocorticoid, serotonergic and neurotrophin signaling in subjects experiencing intense stress and, therefore, vulnerable to suffer from mood disorders [29].
Notably, a number of researches [30–34] demonstrated that alterations in DNA methylation in peripheral cells could reflect those occurring in brain cells, thus proving new opportunities for studying psychiatric disorders by investigating peripheral epigenetic markers of the illness [35, 36]. Purpose of the present article is to compare front-line healthcare professionals versus the other healthcare workers in terms of mental health (depression and PTSD symptoms) and methylation levels of a panel of stress-related genes.
Methods
Study participants
Healthcare professionals working at the IRCCS Policlinico Foundation, Milan, Italy were recruited during the second wave of the COVID-19 pandemic in Italy. Health workers were divided into two groups, based on their role in the COVID emergency:
39 frontline health workers, directly involved in the care of COVID 19 patients (from emergency or internal medicine departments; hereinafter referred to as "cases");
29 healthcare professionals not directly involved in the care of patients affected by COVID-19 (staff from genetics laboratory or pathological anatomy; hereinafter referred to as "controls").
Exclusion criteria were: (1) subjects with current SARS-CoV-2 infection for the direct effects on mental health and epigenetics; (2) healthcare professional who did not work during the second wave; (3) individuals with a recent traumatic event (e.g., death of a relative or a recent diagnosis of a severe medical condition); (4) pregnancy. Cases and controls were age- and gender-matched.
Clinical assessment
Demographic (age, gender) and clinical (Body Mass Index-BMI, smoking status, number of cigarettes/day, number of coffee cups/day, number of alcohol units/month, presence of hypercholesterolemia, lifetime history of anxiety or depression, presence of a sedentary lifestyle, frequency and duration of physical activity, frequency of using screen-based media, history of COVID-19 infection) were collected by an anamnestic questionnaire. Depressive symptoms were assessed by the Patient Health Questionnaire-9 (PHQ-9), a self-reported 9-item scale frequently used to assess depression in contexts other than psychiatry [37]. A cutoff of 10 or higher identifies individuals with clinically significant depressive symptoms [38]. We used the third question on this scale to assess sleep problems (difficulty falling or staying asleep, or oversleeping). Concerning PTSD, symptoms were assessed using the Impact of Event Scale—Revised (IES-R), a self-assessment scale consisting of three subscales (intrusion, avoidance and hyper-excitation) whose sub-scores contribute to the final total score. The total score ranges from 0 to 88. A total score ≥ 33 indicates the probable presence of PTSD [39].
Epigenetic analyses
Genomic DNA was extracted from blood samples using the QiaSymphony automated platform (Qiagen, Hilden, Germany).
Six-hundred nanograms of DNA were bisulphite-treated, to convert unmethylated cytosines into thymines, using the EZ DNA Methylation-Gold kit (Zymo Research, Irvine, CA, USA), according to the user manual. We analysed promoters/regulatory regions of 9 genes, already known to be associated with psychiatric disorders, as detailed in Table 1 [29, 40–48].
Table 1.
Function and implication in human mental health of the selected genes
| Gene (s) | Location brain | Function | Involvement in depression/PTSD | References |
|---|---|---|---|---|
|
ADCYAP1 (Pituitary Adenylate Cyclase-activating polypeptide) |
Amygdala, hippocampus |
Stimulates adenylate cyclase and increases cyclic adenosine monophosphate (cAMP) levels, resulting in the transcriptional activation of target genes The products of this gene are key mediators of neuroendocrine stress responses |
Methylation changes associated with PTSD | Ressler et al. 2011 [46] |
|
BDNF (Brain Derived Neutrophic Factor) |
Prefrontal cortex | May play a role in the regulation of the stress response and in the biology of mood disorders | Increased promoter methylation associated with borderline personality disorder and PTSD | Kim et al. 2017 [43] |
|
CRHR1 (Corticotropin Releasing Hormone Receptor 1) |
HPA axis | Encodes a G-protein coupled receptor that binds neuropeptides of the corticotropin releasing hormone family that are major regulators of the hypothalamic–pituitary–adrenal pathway | Peripheral hypo-responsive HPA-system and elevated CRH concentrations in cerebrospinal fluid in PTSD |
Ströhle 2003 [48] Ding e Dai 2019 [42] |
|
DRD2 (Dopamine Type 2 Receptor) |
Midbrain | Dopamine receptor D2, adenylate cyclase inhibiting, G protein coupled receptor superfamily, expressed in the basal ganglia, regulated by DNA methylation, involved in the control of appetite and growth hormone | Association with depression, anxiety and social dysfunction | Lawford 2006 [44] |
|
IGF2 (Insulin-Like Growth Factor 2) |
Cerebral cortex | Encodes a member of the insulin family of polypeptide growth factors, which are involved in development and growth | Involved in PTSD, Schizophrenia, Bipolar disorder, Alcohol-related disorders, Alzheimer, memory impairment | Pardo 2019 [45] |
|
LSD1 (Lysine specific demethylase 1) |
Frontal cortex | Acts as a co-repressor by mediating demethylation of H3K4me, a specific tag for epigenetic transcriptional activation | Protective of brain functionality in cortical and hippocampus. Changes in its expression are associated with neuropsychiatric disorders (e.g., depression and PTSD) | Cristopher et al. 2017 [41] |
|
NR3C1 (Nuclear Receptor Subfamily 3 Group C Member 1) |
Prefrontal cortex | Encodes glucocorticoid receptor, which can function both as a transcription factor that binds to glucocorticoid response elements in the promoters of glucocorticoid responsive genes to activate their transcription, and as a regulator of other transcription factors | Increased activity or hyper-responsiveness of glucocorticoid receptor associated with PTSD |
Chourbaji et al. 2008 [40] Ding e Dai 2019 [42] |
|
OXTR (oxytocin and oxytocin receptor) |
Brain cortex, cerebellum | The protein encoded by this gene belongs to the G-protein coupled receptor family and acts as a receptor for oxytocin. Its activity is mediated by G proteins which activate a phosphatidylinositol–calcium second messenger system | Involved in mood and social behavior regulation | Serati et al. 2021 [47] |
|
SLC6A4 (Solute Carrier Family 6 Member 4) |
Amygdala |
Encodes an integral membrane protein that transports the neurotransmitter serotonin from synaptic spaces into presynaptic neurons; Serotonin transporter whose primary function in the central nervous system involves the regulation of serotonergic signaling via transport of serotonin molecules from the synaptic cleft back into the pre-synaptic terminal for re-utilization |
Involved in the etiology of mood and anxiety disorders | Park et al., 2019 [29] |
PTSD post-traumatic stress disorder
Target genes were: ADCYAP1, BDNF, CRHR1, DRD2, IGF2, LSD1/KDM1A, NR3C1, OXTR, SLC6A4. Genomic regions to investigate were determined based on previously published data and in silico prediction of promoter sequences (FirstEF, http://rulai.cshl.org/tools/FirstEF/). For each locus, T7 5 'promoter-tagged PCR primers were designed using the EpiDesigner software (https://www.epidesigner.com, Agena Bioscience, San Diego, CA, USA). Primer sequences and targeted genomic regions are described in Table 2.
Table 2.
Primer sequences and targeted genomic regions
| Gene | Chromosome | Sequence ID | start—end | N° CpG sites | CpG coverage | PCR product size (bps) |
|---|---|---|---|---|---|---|
| ADCYAP1 | 18 | AP000894.6 | 110,108—110,333 | 10 | 9 | 226 |
| BDNF | 11 | NG_011794.1 | 4002—4,350 | 22 | 21 | 349 |
| CRHR1 | 17 | NG_009902.1 | 3971—4,263 | 24 | 21 | 291 |
| DRD2 | 11 | NG_008841.1 | 4581—4,789 | 12 | 12 | 209 |
| IGF2 | 11 | NG_008849.1 | 21,346—21,832 | 29 | 25 | 487 |
| LSD1/KDM1A | 1 | NG_047129.1 | 5217—5,464 | 25 | 18 | 248 |
| NR3C1 | 5 | NG_009062.1 | 36,173—36,575 | 47 | 27 | 403 |
| OXTR | 3 | KY798268.1 | 350—768 | 27 | 18 | 419 |
| SLC6A4 | 17 | NG_011747.2 | 4906—5,202 | 29 | 20 | 297 |
The methylation levels at the investigated loci were measured by the MassARRAY® EpiTYPER platform, with MassCleave settings (Agena Biosciences, San Diego, CA, USA), in accordance with manufacturer's recommendations and protocols, as previously described [49]. Mass spectra were acquired through a MassARRAY mass spectrometer (Agena Bioscience) and analyzed using the EpiTYPER® MassARRAY® software, which provided a quantification of methylated/unmethylated CpGs, with values ranging from 0 to 1.
Statistical methods
Descriptive analyses were performed on the total sample. The two groups (cases and controls) were compared by independent sample T tests for quantitative variables (including the mean methylation levels of the selected genes) and χ2 tests for qualitative ones.
Statistical significance was set at p ≤ 0.05 and SPSS 26 version was used to perform the analyses.
Results
Analyses of clinical variables
A total of 68 healthcare professionals were included in the study. Descriptive analysis of the total sample and the comparison between cases and controls are reported in Table 3. 24.2% and 15.7% of the total sample presented clinically significant depressive and PTSD symptoms, reporting, respectively, a total score ≥ 10 and ≥ 33 at PHQ-9 and IES-r. As detailed in Table 3, controls showed more frequent history of anxiety/depression with respect to cases (χ2 = 5.72, p = 0.03). With regard to this latter aspect, only 4 subjects among the total sample reported past mood and anxiety symptoms (before the pandemic). On the contrary, cases versus controls presented higher PHQ-9 (t = 2.13, p = 0.04), PHQ-9 sleep item (t = 2.26, p = 0.03), IES-R total (t = 2.17, p = 0.03), IES-R intrusion (t = 2.46, p = 0.02), IES-R avoidance (t = 1.99, p = 0.05) mean total scores.
Table 3.
Demographic and clinical variables of the total sample and of the two groups identified according to working in COVID or non-COVID wards
| Variables | Total Sample N = 68 | Non-COVID wards N = 29 (57.4%) | COVID wards N = 39 (42.6%) | p value | |
|---|---|---|---|---|---|
| Age | 38.06 (± 10.15) | 39.10 (± 10.57) | 37.28 (± 9.90) | 0.47 | |
| BMI | 23.33 (± 3.80) | 22.97 (± 3.06) | 23.60 (± 4.28) | 0.51 | |
| Gender | Male | 27 (39.7%) | 14 (48.3%) | 13 (33.3%) | 0.32 |
| Female | 41 (60.3%) | 15 (51.7%) | 26 (66.7%) | ||
| Smoking status | Non-smoker | 40 (58.8%) | 17 (58.6%) | 23 (59%) | 0.78 |
| Past smoker | 19 (28.0%) | 9 (31.0%) | 10 (25.6%) | ||
| Smoker | 9 (13.2%) | 3 (10.4%) | 6 (15.4%) | ||
| Number of cigarettes/day | 1.62 (± 3.94) | 1.83 (± 4.22) | 1.46 (± 3.76) | 0.71 | |
| Number of coffee cups/day | 2.65 (± 1.83) | 2.90 (± 1.86) | 2.47 (± 1.81) | 0.34 | |
| Number of alcohol units/month | 13.88 (± 12.76) | 16.93 (± 15.44) | 11.69 (± 10.07) | 0.10 | |
| Presence of hypercholesterolemia | No | 58 (85.3%) | 23 (79.3%) | 35 (89.7%) | 0.31 |
| Yes | 10 (14.7%) | 6 (20.7%) | 4 (10.3%) | ||
| Lifetime history of anxiety/depression | No | 64 (94.1%) | 25 (86.2%) | 39 (100.0%) | 0.03 |
| Yes | 4 (5.9%) | 4 (13.8%) | 0 (0.0%) | ||
| Presence of a sedentary lifestyle | Sedentary | 14 (20.6%) | 5 (17.2%) | 9 (23.1%) | 0.93 |
| Active (low intensity aerobic activities) | 37 (54.4%) | 16 (55.3%) | 21 (53.8%) | ||
| Sporty | 10 (14.7%) | 5 (17.2%) | 5 (12.8%) | ||
| Competitive sporty | 7 (10.3%) | 3 (10.3%) | 4 (10.3%) | ||
| Frequency of physical activity | Never | 21 (30.9%) | 11 (38.0%) | 10 (25.6%) | 0.38 |
| < 2 times in a week | 21 (30.9%) | 6 (20.7%) | 15 (38.5%) | ||
| 2–4 times in a week | 23 (33.8%) | 10 (34.4%) | 13 (33.3%) | ||
| > 4 times in a week | 3 (4.4%) | 2 (6.9%) | 1 (2.6%) | ||
| Duration of a single session of physical activity (hours) missing n = 4 | 0 | 21 (32.8%) | 11 (40.7%) | 10 (27.1%) | 0.35 |
| 1 | 24 (37.5%) | 11 (40.7%) | 13 (35.1%) | ||
| 2 | 17 (26.6%) | 4 (14.9%) | 13 (35.1%) | ||
| 3 | 2 (3.1%) | 1 (3.7%) | 1 (2.7%) | ||
| Frequency of using screen-based media | < 1 h/day | 20 (29.4%) | 7 (24.2%) | 13 (33.3%) | 0.53 |
| 1–2 h/day | 27 (39.7%) | 11 (37.9%) | 16 (41.1%) | ||
| > 2 h/day | 21 (30.9%) | 11 (37.9%) | 10 (25.6%) | ||
| History of COVID-19 missing n = 1 | No | 47 (69.1%) | 21 (72.4%) | 26 (66.7%) | 0.79 |
| Yes | 21 (30.9%) | 8 (27.6%) | 13 (33.3%) | ||
| PHQ-9 mean scores | 4.50 (± 3.94) | 3.38 (± 3.41) | 5.33 (± 4.14) | 0.04 | |
| PHQ-9 sleep item mean scores | 0.81 (± 0.83) | 0.55 (± 0.69) | 1.00 (± 0.89) | 0.03 | |
| IES-R mean total scores | 21.97 (± 20.58) | 15.93 (± 21.53) | 26.70 (± 18.75) | 0.03 | |
| IES-R intrusion mean scores | 8.45 (± 8.39) | 5.69 (± 8.52) | 10.62 (± 7.72) | 0.02 | |
| IES-R avoidance mean scores | 7.86 (± 7.92) | 5.72 (± 7.62) | 9.54 (± 7.84) | 0.05 | |
| IES-R hyperarousal mean scores | 5.74 (± 5.88) | 4.55 (± 6.23) | 6.68 (± 5.49) | 0.15 | |
Standard deviations for quantitative variables and percentages for qualitative variables are reported into brackets
In bold statistically significant p resulting from χ2 or unpaired student’s t tests ≤ 0.05
BMI body mass index, IES-R impact of event scale-revised, PHQ-9 patient health questionnaire-9
Methylation analyses
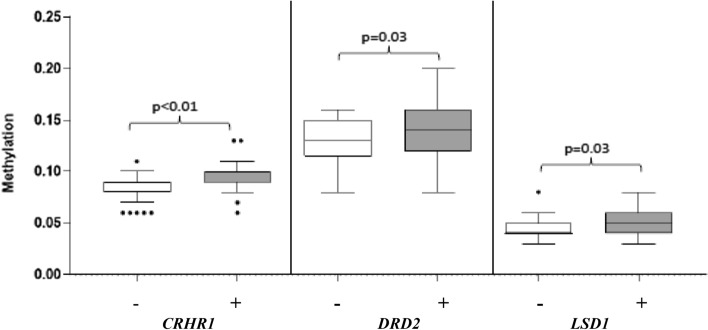
Methylation levels of 3 promoter regions, CRHR1, DRD2, LSD1 were significantly higher in cases versus controls. Indeed, as detailed in Table 4 and depicted in Fig. 1, mean methylation levels (± standard deviation) of cases versus controls were: 0.092 (± 0.01) versus 0.084 (± 0.01) (p < 0.01) for CRHR1; 0.143 (± 0.03) versus 0.128 (± 0.02) (p = 0.03) for DRD2; 0.050 (± 0.01) versus 0.044 (± 0.015) (p = 0.03) for LSD1, respectively. No significant differences were found in the other investigated genes (Table 4).
Table 4.
Methylation values of all investigated regions
| Gene | Methylation in controls | Methylation in cases | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Min | Max | Mean | SD | Median | Min | Max | ||
| ADCYAP1 | 0.140 | 0.044 | 0.128 | 0.093 | 0.313 | 0.141 | 0.043 | 0.133 | 0.095 | 0.320 | 0.89475 |
| BDNF | 0.095 | 0.035 | 0.090 | 0.044 | 0.212 | 0.091 | 0.018 | 0.089 | 0.039 | 0.128 | 0.51807 |
| CRHR1 | 0.084 | 0.013 | 0.085 | 0.061 | 0.109 | 0.092 | 0.010 | 0.091 | 0.061 | 0.128 | 0.00420 |
| DRD2 | 0.128 | 0.023 | 0.127 | 0.083 | 0.161 | 0.143 | 0.028 | 0.141 | 0.081 | 0.204 | 0.02718 |
| IGF2 | 0.497 | 0.059 | 0.486 | 0.343 | 0.612 | 0.501 | 0.060 | 0.494 | 0.369 | 0.705 | 0,78,694 |
| LSD1 | 0.044 | 0.010 | 0.042 | 0.029 | 0.076 | 0.050 | 0.011 | 0.048 | 0.028 | 0.077 | 0.03333 |
| NR3C1 | 0.047 | 0.015 | 0.045 | 0.028 | 0.090 | 0.047 | 0.013 | 0.045 | 0.021 | 0.087 | 0.93112 |
| SLC6A4 | 0.051 | 0.009 | 0.048 | 0.037 | 0.082 | 0.053 | 0.008 | 0.053 | 0.038 | 0.073 | 0.25221 |
| OXTR1 | 0.247 | 0.038 | 0.249 | 0.179 | 0.315 | 0.257 | 0.032 | 0.255 | 0.161 | 0.334 | 0.27992 |
p values of genes showing significant differences between cases and controls are highlighted in bold
Min minimum, Max maximum, SD standard deviation
Fig. 1.
Box-plots representing minimum, first quartile, median, third quartile, and maximum methylation values of genes resulted significantly hypermethylated in cases ( +) versus controls (−)
Moreover, in cases stratified according to gender, higher methylation levels of OXTR were found in females (0.26 ± 0.03) versus males (0.24 ± 0.03) (p = 0.05).
Discussion
A large number of subjects included in our sample reported clinically significant depressive and PTSD symptoms with frequencies higher than those reported in general population [6]. Considering the two groups, identified according to the type of work, frontline health professionals resulted to be more affected by the pandemic as showed by the higher mean total scores at psychometric scales with respect to non-frontline workers. The findings are in agreement with those reported by the available literature indicating a negative effect of pandemic on mental health of most people [50] and in particular in frontline health professionals [6, 11]. It is not surprising that in our sample non-frontline workers presented more frequently a history of anxiety or depression (before the pandemic) than frontline ones, because they were likely to be excluded from more extenuating jobs in the light of their vulnerability to stress and consequently to clinically significant depressive and PTSD symptoms. However, this aspect even supports more the deleterious effect of frontline work on mental well-being of health workers as these subjects were basically less vulnerable to the development of psychiatric disorders than non-frontline professionals. Similar results were reported in a study conducted in Barcelona, where a significant number of frontline healthcare workers suffered from significant depression and anxiety during the pandemic, but few of them showed a history of anxiety and depression before COVID-19 outbreak [51].
With regard to epigenetic results, frontline workers (versus non-frontline ones) showed a higher degree of methylation of genes regulating HPA axis (CRHR1) or dopamine neurotransmission (DRD2 and LSD1). Available literature indicates that HPA axis is frequently involved in stress responses including the onset of cognitive or mood symptoms [52]. In agreement with our data, a recent study reported that during COVID-19 pandemic first-line health professionals, particularly physicians, presented higher hair cortisol concentrations with respect to workers not in direct contact with patients [14]. On the other hand, epigenetic modulation of genes involved in dopamine transmission may account for the development of behavioural and substance addictions, anhedonia and depressed mood, or psychotic symptoms. Of note, DRD2 promoter methylation was reported to be directly associated with the severity of alcohol misuse [53]. Stress is a well-known risk factor for alcohol misuse [54] and, although this aspect did not emerge in our sample, some authors reported an increase in alcohol use in health professionals assisting patients with COVID-19 [55]. According to our results, DRD2 would result less expressed in cases versus controls. This aspect is in agreement with results from animal models showing that DRD2 mRNA expression is reduced in case of chronic mild stress and in case of expression of depressive-like behaviours [56]. Furthermore, a lower expression of DRD2 (e.g., for the presence of Taq1 polymorphism) was reported to be associated with the onset of PTSD symptoms [57], and in our sample DRD2 resulted to have a higher degree of methylation in cases who in turn presented more severe PTSD symptoms than controls.
Finally, regarding the different methylation of OXTR in female cases than male ones, this is not surprising, because oxytocin is a neuropeptide that modulates the behavioural response to stress and has a different effect according to gender. While in males oxytocin facilitates the change of anxiety (in response to stress) in happiness, in females this neuropeptide favours relaxation [31].
It should be noted that the reported changes in DNA methylation levels are subtle (generally < 5%), and this is likely due to the fact that higher differences in methylation would not be tolerated by cells, reinforcing the role of epigenetic regulation in modulating the expression of the investigated gene, in one hand, and their role in psychiatric disorders, on the other hand.
Another issue, that would deserve perspective studies, is whether epigenetic changes observed in frontline health workers will be stable over time or sensitive to external interventions.
The study limitations include:
(1) pharmacological treatment for medical diseases (e.g., for dyslipidaemia) might have influenced some of our epigenetic results, although no statistically significant clinical differences (a part from the history of anxiety and depression) were detected between cases and controls;
(2) the recruitment in a single centre in a European country that limits the generalization of the present findings (with regard to this point, it is useful to highlight that Italy was the first European country to face the emergency of pandemic and it is one of the European countries with the most deaths due to the COVID-19 pandemic);
(3) the clinical information acquired by subjects might not be always accurate, although this limitation is mitigated by the fact that sample consisted of health professionals;
(4) the analysis of DNA methylation of peripheral blood lymphocytes (PBLs) instead of brain cells. Nevertheless, we assumed that methylation levels in PBLs could reflect those of the brain cells, based on previous evidences. Indeed, it has been demonstrated that DNA methylation levels at PBLs mirror those of brain cells [30, 31], at that for this reason PBLs can be used as reliable peripheral markers of psychiatric conditions, such as major depressive disorder [32, 33]. Recently, the finding that blood–brain barrier permeability is increased in individuals with central nervous system diseases reinforces the idea that it is possible to retrieve in peripheral blood molecules originating from brain and this, in turn, facilitates the diagnosis of psychiatric/neurological disorders by the analysis of PBLs [34].
The results of our study show that frontline health workers were more negatively affected by the pandemic than healthcare professionals not in direct contact with patients in the case of a single center in Italy (a single European Country). Future multi-centric studies with larger samples and investigating different biological systems are necessary to support the preliminary results of the present study and to have a more complete picture of the effects of acute stressful events (such as a pandemic) on mental health.
Acknowledgements
The study protocol was approved by the local Ethics Committee (OSMAMI-12/03/2021-0010501-U). A detailed study description was given to all interested participants, and an informed written consent was obtained. We want to thank all the participants to the time dedicated to the present research despite the great effort to face the pandemic.
Author contributions
ST and MB designed the research, performed the statistical analyses and wrote the first draft of the manuscript. LT collected the clinical data. CP collected the blood samples. ST, MGC, GB, GG, MF and PC performed the laboratory analyses. The other authors supervised the research and contributed to the final draft.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. No funding sources supported the present research.
Declarations
Conflict of interest
The authors have not conflicts of interest to disclose in relation to the present article.
Ethical approval
This study was performed in line with the principles of 1964 Helsinki Declaration. All procedures have been approved by the Institutional Review Boards.
References
- 1.Gloster AT, Lamnisos D, Lubenko J, Presti G, Squatrito V, Constantinou M, Nicolaou C, Papacostas S, Aydın G, Chong YY, Chien WT, Cheng HY, Ruiz FJ, Garcia-Martin MB, Obando-Posada DP, Segura-Vargas MA, Vasiliou VS, McHugh L, Höfer S, Baban A, Dias Neto D, Nunes da Silva A, Monestès JL, Alvarez-Galvez J, Paez-Blarrina M, Montesinos F, Valdivia-Salas S, Ori D, Kleszcz B, Lappalainen R, Ivanović I, Gosar D, Dionne F, Merwin RM, Kassianos AP, Karekla M. Impact of COVID-19 pandemic on mental health: an international study. PLoS ONE. 2020;15:e0244809. doi: 10.1371/journal.pone.0244809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hossain MM, Tasnim S, Sultana A, Faizah F, Mazumder H, Zou L, McKyer ELJ, Ahmed HU, Ma P. Epidemiology of mental health problems in COVID-19: a review. F1000Res. 2020;9:636. doi: 10.12688/f1000research.24457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capuzzi E, Caldiroli A, Di Brita C, Colmegna F, Nava R, Colzani LC, Sibilla M, Prodi T, Buoli M. Clerici M (2021) Profile of patients attending psychiatric emergency care during the coronavirus 2019 (COVID 19) pandemic: a comparative cross-sectional study between lockdown and post-lockdown periods in Lombardy, Italy. Int J Psychiatry Clin Pract. 2021 doi: 10.1080/136515011939385. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty C, Sharma AR, Bhattacharya M, Sharma G, Agoramoorthy G, Lee SS. Diabetes and COVID-19: a major challenge in pandemic period? Eur Rev Med Pharmacol Sci. 2020;24:11409–11420. doi: 10.26355/eurrev_202011_23634. [DOI] [PubMed] [Google Scholar]
- 5.Ingegnoli F, Buoli M, Posio C, Di Taranto R, Lo Muscio A, Cumbo E, Ostuzzi S, Caporali R. Covid-19 related poor mental health and sleep disorders in rheumatic patients: a citizen science project. BMC Psychiatry. 2021;21:385. doi: 10.21203/rs.3.rs-381954/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vizheh M, Qorbani M, Arzaghi SM, Muhidin S, Javanmard Z, Esmaeili M. The mental health of healthcare workers in the COVID-19 pandemic: a systematic review. J Diabetes Metab Disord. 2020;19:1–12. doi: 10.1007/s40200-020-00643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conti C, Fontanesi L, Lanzara R, Rosa I, Porcelli P. Fragile heroes. The psychological impact of the COVID-19 pandemic on health-care workers in Italy. PLoS ONE. 2020;15:0242538. doi: 10.1371/journal.pone.0242538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurent A, Fournier A, Lheureux F, Louis G, Nseir S, Jacq G, Goulenok C, Muller G, Badie J, Bouhemad B, Georges M, Mertes PM, Merdji H, Castelain V, Abdulmalak C, Lesieur O, Plantefeve G, Lacherade JC, Rigaud JP, Sedillot N, Roux D, Terzi N, Beuret P, Monsel A, Poujol AL, Kuteifan K, Vanderlinden T, Renault A, Vivet B, Vinsonneau C, Barbar SD, Capellier G, Dellamonica J, Ehrmann S, Rimmelé T, Bohé J, Bouju P, Gibot S, Lévy B, Temime J, Pichot C, Schnell D, Friedman D, Asfar P, Lebas E, Mateu P, Klouche K, Audibert J, Ecarnot F, Meunier-Beillard N, Loiseau M, François-Pursell I, Binquet C, Quenot JP, PsyCOVID-ICU Trial Investigators and the CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) Mental health and stress among ICU healthcare professionals in France according to intensity of the COVID-19 epidemic. Ann Intensive Care. 2021;11:90. doi: 10.1186/s13613-021-00880-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin F, Sharif S, Saeed R, Durrani N, Jilani D. COVID-19 pandemic- knowledge, perception, anxiety and depression among frontline doctors of Pakistan. BMC Psychiatry. 2020;20:459. doi: 10.1186/s12888-020-02864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Tella M, Romeo A, Benfante A, Castelli L. Mental health of healthcare workers during the COVID-19 pandemic in Italy. J Eval Clin Pract. 2020;26:1583–1587. doi: 10.1111/jep.13444. [DOI] [PubMed] [Google Scholar]
- 11.Wańkowicz P, Szylińska A, Rotter I. Assessment of mental health factors among health professionals depending on their contact with COVID-19 patients. Int J Environ Res Public Health. 2020;17:5849. doi: 10.3390/ijerph17165849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramezani M, Simani L, Karimialavijeh E, Rezaei O, Hajiesmaeili M, Pakdaman H. The role of anxiety and cortisol in outcomes of patients with Covid-19. Basic Clin Neurosci. 2020;11:179–184. doi: 10.32598/bcn.11.covid19.1168.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capuzzi E, Caldiroli A, Leo S, Buoli M, Clerici M. Initiation of psychotropic medication in hospitalized patients with COVID-19: association with clinical and biological characteristics. Hum Psychopharmacol. 2021;36:e278. doi: 10.1002/hup.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibar C, Fortuna F, Gonzalez D, Jamardo J, Jacobsen D, Pugliese L, Giraudo L, Ceres V, Mendoza C, Repetto EM, Reboredo G, Iglesias S, Azzara S, Berg G, Zopatti D, Fabre B. Evaluation of stress, burnout and hair cortisol levels in health workers at a university hospital during COVID-19 pandemic. Psychoneuroendocrinology. 2021;128:105213. doi: 10.1016/j.psyneuen.2021.105213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slavich GM, Sacher J. Stress, sex hormones, inflammation, and major depressive disorder: extending social signal transduction theory of depression to account for sex differences in mood disorders. Psychopharmacology. 2019;236:3063–3079. doi: 10.1007/s00213-019-05326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2017;1391:20–34. doi: 10.1111/nyas.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushita H, Latt HM, Koga Y, Nishiki T, Matsui H. Oxytocin and stress: neural mechanisms, stress-related disorders, and therapeutic approaches. Neuroscience. 2019;417:1–10. doi: 10.1016/j.neuroscience.2019.07.046. [DOI] [PubMed] [Google Scholar]
- 18.Dalton VS, Kolshus E, McLoughlin DM. Epigenetics and depression: return of the repressed. J Affect Disord. 2014;155:1–12. doi: 10.1016/j.jad.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg MVC, Bourc’his D. The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol. 2019;20:590–607. doi: 10.1038/s41580-019-0159-6. [DOI] [PubMed] [Google Scholar]
- 20.Rasheed M, Liang J, Wang C, Deng Y, Chen Z. Epigenetic regulation of neuroinflammation in Parkinson's disease. Int J Mol Sci. 2021;22:4956. doi: 10.3390/ijms22094956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saw G, Tang FR. Epigenetic regulation of the hippocampus, with special reference to radiation exposure. Int J Mol Sci. 2020;21:9514. doi: 10.3390/ijms21249514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saad L, Zwiller J, Kalsbeek A, Anglard P. Epigenetic regulation of circadian clocks and its involvement in drug addiction. Genes. 2021;12:1263. doi: 10.3390/genes12081263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alasaari JS, Lagus M, Ollila HM, Toivola A, Kivimäki M, Vahtera J, Kronholm E, Härmä M, Puttonen S, Paunio T. Environmental stress affects DNA methylation of a CpG rich promoter region of serotonin transporter gene in a nurse cohort. PLoS ONE. 2012;7:e45813. doi: 10.1371/journal.pone.0045813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duman EA, Canli T. Influence of life stress, 5-HTTLPR genotype, and SLC6A4 methylation on gene expression and stress response in healthy Caucasian males. Biol Mood Anxiety Disord. 2015;5:2. doi: 10.1186/s13587-015-0017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirvani-Farsani Z, Maloum Z, Bagheri-Hosseinabadi Z, Vilor-Tejedor N, Sadeghi I. DNA methylation signature as a biomarker of major neuropsychiatric disorders. J Psychiatr Res. 2021;141:34–49. doi: 10.1016/j.jpsychires.2021.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Cv B, Martins CM, Tofoli SM, Juruena MF. Early life stress in depressive patients: HPA axis response to GR and MR agonist. Front Psychiatry. 2014;5:2. doi: 10.3389/fpsyt.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bekdash RA. Early life nutrition and mental health: the role of DNA methylation. Nutrients. 2021;13:3111. doi: 10.3390/nu13093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jawaid A, Jehle KL, Mansuy IM. Impact of parental exposure on offspring health in humans. Trends Genet. 2021;37:373–388. doi: 10.1016/j.tig.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Park C, Rosenblat JD, Brietzke E, Pan Z, Lee Y, Cao B, Zuckerman H, Kalantarova A, McIntyre RS. Stress, epigenetics and depression: a systematic review. Neurosci Biobehav Rev. 2019;102:139–152. doi: 10.1016/j.neubiorev.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Braun PR, Han S, Hing B, Nagahama Y, Gaul LN, Heinzman JT, Grossbach AJ, Close L, Dlouhy BJ, Howard MA, 3rd, Kawasaki H, Potash JB, Shinozaki G. Genome-wide DNA methylation comparison between live human brain and peripheral tissues within individuals. Transl Psychiatry. 2019;9:47. doi: 10.1038/s41398-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Q, Hu S. Sex differences of oxytocin and vasopressin in social behaviors. Handb Clin Neurol. 2021;180:65–88. doi: 10.1016/B978-0-12-820107-7.00005-7. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y, Strachan E, Fowler E, Bacus T, Roy-Byrne P, Zhao J. Genome-wide profiling of DNA methylome and transcriptome in peripheral blood monocytes for major depression: a monozygotic discordant twin study. Transl Psychiatry. 2019;9:215. doi: 10.1016/j.bbr.2011.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu C, Wang D, Niu K, Feng Q, Chen H, Zhu H, Xiang H. Gene expression profiling in peripheral blood lymphocytes for major depression: preliminary cues from Chinese discordant sib-pair study. Transl Psychiatry. 2021;11:540. doi: 10.1038/s41398-021-01665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janigro D, Bailey DM, Lehmann S, Badaut J, O'Flynn R, Hirtz C, Marchi N. Peripheral blood and salivary biomarkers of blood-brain barrier permeability and neuronal damage: clinical and applied concepts. Front Neurol. 2021;11:577312. doi: 10.3389/fneur.2020.577312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chouliaras L, Pishva E, Haapakoski R, Zsoldos E, Mahmood A, Filippini N, Burrage J, Mill J, Kivimäki M, Lunnon K, Ebmeier KP. Peripheral DNA methylation, cognitive decline and brain aging: pilot findings from the Whitehall II imaging study. Epigenomics. 2018;10:585–595. doi: 10.2217/epi-2017-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi-Takagi A, Vawter MP, Iwamoto K. Peripheral biomarkers revisited: integrative profiling of peripheral samples for psychiatric research. Biol Psychiatry. 2014;75:920–928. doi: 10.1016/j.biopsych.2013.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ingegnoli F, Schioppo T, Ubiali T, Ostuzzi S, Bollati V, Buoli M, Caporali R. Patient perception of depressive symptoms in rheumatic diseases: a cross-sectional survey. J Clin Rheumatol. 2022;28:e18–e22. doi: 10.1097/RHU.0000000000001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Creamer M, Bell R, Failla S. Psychometric properties of the impact of event scale: revised. Behav Res Ther. 2003;41:1489–1496. doi: 10.1016/j.brat.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Chourbaji S, Vogt MA, Gass P. Mice that under- or overexpress glucocorticoid receptors as models for depression or posttraumatic stress disorder. Prog Brain Res. 2008;167:65–77. doi: 10.1016/S0079-6123(07)67005-8. [DOI] [PubMed] [Google Scholar]
- 41.Christopher MA, Myrick DA, Barwick BG, Engstrom AK, Porter-Stransky KA, Boss JM, Weinshenker D, Levey AI, Katz DJ. LSD1 protects against hippocampal and cortical neurodegeneration. Nat Commun. 2017;8:805. doi: 10.1038/s41467-017-00922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding Y, Dai J. Advance in stress for depressive disorder. Adv Exp Med Biol. 2019;1180:147–178. doi: 10.1007/978-981-32-9271-0_8. [DOI] [PubMed] [Google Scholar]
- 43.Kim TY, Kim SJ, Chung HG, Choi JH, Kim SH, Kang JI. Epigenetic alterations of the BDNF gene in combat-related post-traumatic stress disorder. Acta Psychiatr Scand. 2017;135:170–179. doi: 10.1111/acps.12675. [DOI] [PubMed] [Google Scholar]
- 44.Lawford BR, Young R, Noble EP, Kann B, Ritchie T. The D2 dopamine receptor (DRD2) gene is associated with co-morbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. Eur Psychiatry. 2006;21:180–185. doi: 10.1016/j.eurpsy.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Pardo M, Cheng Y, Sitbon YH, Lowell JA, Grieco SF, Worthen RJ, Desse S, Barreda-Diaz A. Insulin growth factor 2 (IGF2) as an emergent target in psychiatric and neurological disorders. Review Neurosci Res. 2019;149:1–13. doi: 10.1016/j.neures.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serati M, Grassi S, Redaelli M, Pergoli L, Cantone L, La Vecchia A, Barkin JL, Colombo E, Tiso G, Abbiati C, Bollati V, Buoli M. Is there an association between oxytocin levels in plasma and pregnant women's mental health? J Am Psychiatr Nurses Assoc. 2021;27(3):222–230. doi: 10.1177/1078390319890400. [DOI] [PubMed] [Google Scholar]
- 48.Ströhle A, Holsboer F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 2003;36:S207–S214. doi: 10.1055/s-2003-45132. [DOI] [PubMed] [Google Scholar]
- 49.Azzollini J, Pesenti C, Pizzamiglio S, Fontana L, Guarino C, Peissel B, Plebani M, Tabano S, Sirchia SM, Colapietro P, Villa R, Paolini B, Verderio P, Miozzo M, Manoukian S. Constitutive BRCA1 promoter hypermethylation can be a predisposing event in isolated early-onset breast cancer. Cancers (Basel) 2019;11:58. doi: 10.3390/cancers11010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pieh C, Budimir S, Delgadillo J, Barkham M, Fontaine JRJ, Probst T. Mental health during COVID-19 lockdown in the United Kingdom. Psychosom Med. 2021;83:328–337. doi: 10.1097/PSY.0000000000000871. [DOI] [PubMed] [Google Scholar]
- 51.SobregrauSangrà P, Aguiló Mir S, Castro Ribeiro T, Esteban-Sepúlveda S, García Pagès E, López Barbeito B, Pomar Moya-Prats JL, Pintor Pérez L, AguilóLlobet J. Mental health assessment of Spanish healthcare workers during the SARS-CoV-2 pandemic. A cross-sectional study. Compr Psychiatry. 2022;112:152278. doi: 10.1016/j.comppsych.2021.152278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morelli V, Ghielmetti A, Caldiroli A, Grassi S, Siri FM, Caletti E, Mucci F, Aresta C, Passeri E, Pugliese F, Di Giorgio A, Corbetta S, Scillitani A, Arosio M, Buoli M, Chiodini I. Mental health in patients with adrenal incidentalomas: is there a relation with different degrees of cortisol secretion? J Clin Endocrinol Metab. 2021;106:e130–e139. doi: 10.1210/clinem/dgaa695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bidwell LC, Karoly HC, Thayer RE, Claus ED, Bryan AD, Weiland BJ, YorkWilliams S, Hutchison KE. DRD2 promoter methylation and measures of alcohol reward: functional activation of reward circuits and clinical severity. Addict Biol. 2019;24:539–548. doi: 10.1111/adb.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clay JM, Parker MO. Alcohol use and misuse during the COVID-19 pandemic: a potential public health crisis? Lancet Public Health. 2020;5:e259. doi: 10.1016/S2468-2667(20)30088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stuijfzand S, Deforges C, Sandoz V, Sajin CT, Jaques C, Elmers J, Horsch A. Psychological impact of an epidemic/pandemic on the mental health of healthcare professionals: a rapid review. BMC Public Health. 2020;20:1230. doi: 10.1186/s12889-020-09322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu X, Peng S, Zhang S, Zhang X. Stress-induced depressive behaviors are correlated with Par-4 and DRD2 expression in rat striatum. Behav Brain Res. 2011;223:329–335. doi: 10.1016/j.bbr.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 57.Xiao Y, Liu D, Liu K, Wu C, Zhang H, Niu Y, Jiang X. Association of DRD2, 5-HTTLPR, and 5-HTTVNTR gene polymorphisms with posttraumatic stress disorder in Tibetan adolescents: a case-control study. Biol Res Nurs. 2019;21:286–295. doi: 10.1177/1099800419838325. [DOI] [PubMed] [Google Scholar]