Abstract
The Bradyrhizobium japonicum hupT gene was sequenced, and its gene product was found to be homologous to NtrB-like histidine kinases. A hupT mutant expresses higher levels of hydrogenase activity than the wild-type strain under hydrogenase-inducing conditions (i.e., microaerobiosis plus hydrogen, or symbiosis), whereas in noninduced hupT cells, hupSL expression is derepressed but does not lead to hydrogenase activity. We conclude that HupT is involved in the repression of HupSL synthesis at the transcriptional level but that enzymatic activation requires inducing conditions.
A remarkable feature of hydrogen oxidation in Bradyrhizobium japonicum is that induction of the hydrogen uptake system is triggered when the bacterium shifts to either of two extremely different lifestyles. Hydrogenase activity in Hup+ strains develops during chemoautotrophic growth in a microaerobic, hydrogen-containing environment and when the bacterium differentiates into a symbiotic, nitrogen-fixing bacteroid (reviewed in reference 20). Although the presence of the substrate hydrogen appears to be the common denominator in both lifestyles, it has become increasingly apparent that the regulatory networks that govern the formation of the uptake hydrogenase are as complex as the different lifestyles. The structural genes for the B. japonicum hydrogenase enzyme are encoded by the hupSL genes (28), and more than 20 other hup-related genes with various functions in structure, assembly, processing, maturation, nickel metabolism, and regulation have been identified (3, 10–12, 23, 30, 32). In free-living bradyrhizobia, hydrogenase expression is induced by hydrogen, low oxygen concentrations, and trace amounts of nickel (14, 15, 29) and hupSL expression requires RpoN, integration host factor (2), and the DNA-binding transcriptional activator HoxA (31). In bacteroids, HoxA is not essential for transcription and it has been proposed that nitrogenase and hydrogenase are coregulated through FixK2 (5). The pathway through which these inducing signals are perceived and transduced has not yet been elucidated in detail. A multicomponent system including a pseudohydrogenase (HupUV/HoxBC) and a repressor protein (HupT/HoxJ) has been proposed to be involved in hydrogen sensing and signal transduction in Rhodobacter capsulatus (6, 8) and in Alcaligenes hydrogenophilus (16, 17). The genes encoding the pseudohydrogenase (HupUV) in B. japonicum have been previously identified (3). Here, we present the nucleotide sequence and mutant analysis of a regulatory gene, hupT, showing that it has a repressor function in the regulatory cascade leading to hup gene expression under both free-living and symbiotic conditions. In addition, we studied the expression of the gene encoding the transcriptional regulator HoxA and demonstrated that hoxA is expressed under aerobic conditions and that expression is positively autoregulated under hydrogenase-inducing conditions (microaerobiosis plus hydrogen).
Bacterial strains, plasmids, and growth of cells.
B. japonicum CB1809 (CSIRO, St. Lucia, Australia) is routinely grown at 30°C in PSY medium (26). To measure hydrogenase activity under free-living conditions, cells were grown in minimal salts medium (described in reference 12), supplemented with 0.05% sodium gluconate and 0.05% sodium glutamate, under a gas atmosphere of 5% H2-5% CO2-1% O2-89% N2 for 48 h. Then the cells were lysed, and equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis for Western blotting (1). To obtain soybean bacteroids, surface-sterilized soybean seeds (cultivar Williams) were inoculated with B. japonicum cultures grown on YMB (33) and plants were grown in Leonard jar-type assemblies under bacteriologically controlled conditions in a growth chamber (18 h of daylight [25°C]-6 h of darkness [18°C] regime) as described previously (19). After 24 days, bacteroids were isolated as previously described (18) and hydrogenase activities of bacteroid suspensions were determined amperometrically (27).
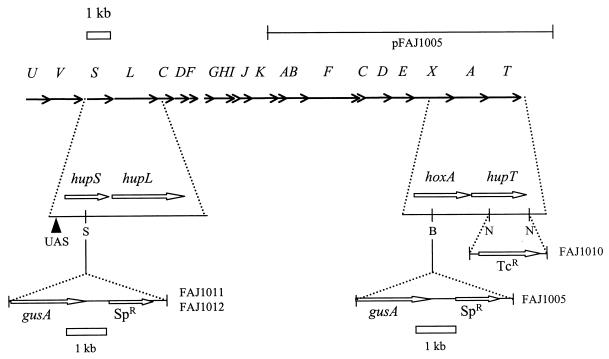
The hupT mutant FAJ1010 was constructed by replacing an internal 900-bp NotI fragment of the B. japonicum CB1809 genome with a tetracycline resistance gene cassette (Fig. 1). To this end, hupT was cloned as a 4.8-kbp BamHI fragment in the conditionally lethal suicide vector pJQ200mp18 (24). After NotI digestion, the ends were blunted with the Klenow fragment of DNA polymerase and a 2-kb tetracycline resistance gene cartridge, isolated from SmaI-digested pHP45-TcR (9), was ligated into the coding sequence of hupT. This construct, pFAJ1029, was mobilized to CB1809, and selection on PSY medium with 5% sucrose and 100 μg of tetracycline/ml forced replacement of the wild-type hupT copy with the truncated allele. The structure of the mutated region in the hupT mutant, FAJ1010, was confirmed by Southern hybridization (data not shown). To measure hupSL expression, a promoterless gusA gene was inserted into the hupS coding sequence in the wild-type and FAJ1010 strains as follows: the hupS promoter region, encompassing the entire hupS gene and the 5′ part of hupL on a 3-kb BamHI fragment, was blunted with the Klenow fragment of DNA polymerase and ligated in SmaI-digested pJQ200UC1.
FIG. 1.
Physical and genetic map of the hup region of B. japonicum. Horizontal arrows indicate the positions and sizes of the B. japonicum hup (U to K and T), hyp (A to E), and hox (X and A) genes. The horizontal line at the top of the figure represents the DNA region present in cosmid pFAJ1005. Shown in details are the regions relevant to the construction of the reporter and mutant strains, including the relevant restriction sites (S = SacI, B = BamHI, and N = NotI), as well as the locations of the tetracycline interposon (TcR) in mutant FAJ1010 and of the promoterless gusA-spectinomycin cassettes (SpR) used to construct genomic reporter fusions in hupS (present in strains FAJ1011 and FAJ1012) and hoxA (strain FAJ1005). UAS, HoxA-binding site.
A 4-kb SacI fragment from pWM5 (21), containing a promoterless gusA cassette preceded by a ribosome binding site and a spectinomycin resistance gene, was cloned in the SacI site of hupS, thus putting the gusA gene under the transcriptional control of the hupS promoter region. This construct, called pFAJ1050, was mobilized to B. japonicum, and replacement of the wild-type hupS copy on the chromosome was forced by selecting for double homologous recombination as described above. The resulting reporter strains, FAJ1011 (CB1809 hupS::gusA) and FAJ1012 (FAJ1010 hupS::gusA), allow the measurement of hupS expression in the presence and in the absence of HupT, respectively (Fig. 1). The structures of the mutated regions in FAJ1011 and FAJ1012 were confirmed as correct by means of Southern hybridization (data not shown).
To induce cells to undergo hupSL transcription, cells were grown in HUM medium (4), washed twice in modified minimal salts medium (as described above), and diluted in this medium to an optical density of 0.1 at 600 nm. One-milliliter aliquots of these cell suspensions were transferred to 80-ml test tubes at 30°C (time zero) and cultured under air (aerobic growth conditions) or under a defined gas atmosphere (1% O2-99% N2 for microaerobic growth conditions, 5% H2-5% CO2-1% O2-89% N2 for inducing conditions). The tubes used for growth under a defined atmosphere were tightly capped with rubber stoppers and flushed twice a day with the gas mixture. β-Glucuronidase activities of the cell suspensions were quantified after 48 h of growth by using the substrate p-nitrophenyl-β-d-glucuronide (13) and expressed in Miller units (22).
A chromosomal insertion of a promoterless gusA cassette in hoxA was obtained by insertion of the same 4-kb gusA-Spr cassette described above into the blunted BamHI site of pFAJ1020 (31) followed by mobilization to B. japonicum CB1809 and forced double homologous recombination. The structure of the mutated region was confirmed by Southern hybridization (data not shown), and the resulting hoxA mutant was called FAJ1005 (Fig. 1). An intact hoxA gene was supplied in trans by introduction of cosmid pFAJ1005 (Fig. 1) (32).
Sequencing of the B. japonicum hupT gene.
Double-stranded DNA sequencing was carried out on DNA located downstream of the previously identified hoxA gene (31, 32), on both strands on overlapping fragments subcloned in pUC19, with an automated sequencer (A.L.F.; Pharmacia Biotech). Nucleotide sequences were compiled and analyzed with PC/GENE software (IntelliGenetics, Inc., Mountain View, Calif.). Analysis of codon usage (25) showed a 1,368-bp open reading frame with a codon profile characteristic for B. japonicum and partially overlapping the 3′ end of the hoxA gene, which suggests translational coupling (see below). The deduced amino acid sequence consists of 455 amino acid residues and encodes a putative protein with a predicted molecular mass of 50,059 Da. When the amino acid sequence was compared with sequences present in databases, the highest degrees of similarity were found to be with the hoxJ gene of A. hydrogenophilus and A. eutrophus (38.7 and 38.5% identity, respectively) (16, 17) and the hupT gene of R. capsulatus (36% identity) (8). These gene products are homologous to NtrB-like sensor histidine kinases, and in the central and C-terminal regions, functional domains for autophosphorylation, kinase or phosphatase activity, and nucleotide binding are highly conserved. The N-terminal parts of the proteins are much less conserved and are probably involved in sensing a signal. In R. capsulatus, the hupT gene product, together with the hupU and hupV gene products, is part of a repression system of hydrogen uptake gene expression (6). In A. hydrogenophilus, HoxJ is proposed to be involved in signal transduction from the hydrogen-sensing HoxBC complex to HoxA (17).
hupT mutant analysis.
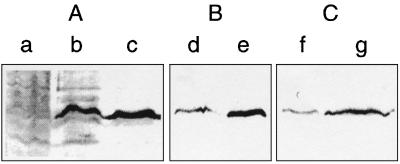
In FAJ1010, HupT is truncated at amino acid 163 and thus lacks the conserved central and C-terminal domains containing the typical histidine kinase motifs. To study the function of hupT in B. japonicum, we measured hydrogenase activity amperometrically both in free-living and in symbiotic B. japonicum CB1809 (wild-type) and FAJ1010 (hupT mutant) cells. Table 1 shows that hydrogenase activity in the hupT mutant is approximately twofold higher than that in the wild type, both in free-living induced cells and in bacteroids. In free-living cells grown aerobically in the presence or absence of hydrogen or microaerobically in the absence of hydrogen, no hydrogenase activity could be detected (Table 1), indicating that the simultaneous presence of low oxygen concentrations and hydrogen is required to obtain an active hydrogenase enzyme. When equal amounts of crude protein of free-living induced cells were probed for the presence of HupL protein by means of Western blotting with anti-HupL serum, extracts of the hupT mutant clearly gave a stronger signal than wild-type cells, indicating that relatively more hydrogenase protein is formed in the absence of HupT (Fig. 2, lanes d and e). A similar increase in HupL levels was observed in bacteroids of the HupT mutant (Fig. 2, compare lanes f and g). These results are consistent with the observed increase in hydrogenase activity associated with the absence of HupT (Table 1). In protein extracts prepared from aerobically grown cells, no signal was present for wild-type cells but a clear signal could be observed for the hupT mutant (Fig. 2, compare lanes a and b). Since the hupT mutant grown aerobically had no hydrogenase activity, the observed immunoreactive band presumably corresponds to inactive (i.e., unprocessed) HupL protein. In fact, the band observed in aerobic cells had a slower mobility (Fig. 2, compare lanes b and c).
TABLE 1.
Hydrogenase activity in B. japonicum wild-type and hupT mutant strains grown under free-living and symbiotic conditions
| Strain | Genotype | Hydrogenase activity ina:
|
||
|---|---|---|---|---|
| Vegetative cells
|
Bacteroids | |||
| Aerobic growth | Hydrogenase-induced growth | |||
| CB1809 | Wild type | <100 | 10,454 | 14,724 |
| FAJ1010 | hupT::Tcr | <100 | 25,163 | 27,163 |
Measured by the amperometric method and expressed in nanomoles of H2 consumed per hour per milligram of protein. Values are means of two replicates.
FIG. 2.
Immunodetection of HupL in cell extracts. Western blots containing crude cellular extracts were incubated with HupL antiserum at a dilution of 1:1,000. Cell extracts were obtained from wild-type CB1809 (lanes a, d, and f) or hupT mutant strain (lanes b, c, e, and g) aerobically grown vegetative cells (panel A, lanes a and b), induced vegetative cells (panel A, lane c, and panel B), or soybean bacteroids (panel C).
To study the effect of HupT on expression of the hydrogenase structural genes under free-living conditions, a promoterless gusA gene was inserted in the hupS coding sequence in the wild type and the hupT mutant, as described above. β-Glucuronidase activities of the reporter strains were measured after 48 h of growth under aerobic, microaerobic, or inducing conditions (Table 2). In the wild-type FAJ1011 background, hupSL expression is blocked under aerobic and microaerobic conditions and is induced under conditions of microaerobiosis plus hydrogen (inducing conditions). When hupT is knocked out (in FAJ1012), hupSL expression is derepressed (although at low levels) under aerobic and microaerobic conditions. This low level of expression does not increase over time (data not shown) and is consistent with the presence of HupL protein in these cells (Fig. 2). In induced cells, expression is highly increased as a function of time and is twice the expression level seen in wild-type induced cells after 48 h (Table 2). These results suggest that HupT somehow represses hupSL expression under both noninducing and inducing conditions, since the level of hupSL expression is consistently higher in the absence of HupT. The effect of HupT on hydrogenase activity is observed only in induced cells (free-living as well as symbiotic).
TABLE 2.
Effect of hupT on hupSL expression in vegetative B. japonicum cells under aerobic, microaerobic, or hydrogenase-inducing growth conditions
| Strain | Genotype | β-Glucuronidase activitya
|
||
|---|---|---|---|---|
| Aerobic growth | Microaerobic growth | Hydrogenase-induced growth | ||
| FAJ1011 | hupS::gusA | 4 ± 3 | 0 | 225 ± 34 |
| FAJ1012 | hupS::gusA hupT::Tcr | 35 ± 5 | 42 ± 2 | 445 ± 127 |
Numbers are average values (in Miller units) of five replicates, assayed in duplicate, ± standard deviations.
We conclude from these results that HupT blocks transcription of the hupSL promoter under conditions in which the expression of a hydrogenase enzyme would be futile (i.e., under aerobic conditions or in the absence of the substrate hydrogen) and somehow modulates the level of expression under inducing conditions. Under these conditions, HupT might be inactivated by a process dependent on HupUV, as has been proposed for A. eutrophus (17). The observed increase in hupSL expression in the absence of HupT may indicate that the inactivation of HupT is not complete under inducing conditions.
The mechanism by which HupT affects transcription of the hupSL promoter in free-living cells is likely mediated by HoxA, since it has been shown that under these conditions HoxA is essential for the induction of hupSL expression and binds to a defined region of this promoter (31). Therefore, we propose that in free-living B. japonicum cells, HupT interacts with HoxA, modifying its transcription-inducing activity. HoxA is homologous to transcriptional activators of the NtrC family, whose transcriptional activities are dependent on phosphorylation by partner histidine kinases. Although the phosphorylated form of an NtrC-like regulator usually has transcriptional activation activity, in the case of A. eutrophus it has been proposed that HoxA is transcriptionally active in the nonphosphorylated form (17). HupT autophosphorylation has been demonstrated in A. eutrophus (17) and R. capsulatus (7). The structural similarities of B. japonicum HupT and these homologous proteins, together with the observed effect of HupT on hupSL expression, suggest that in B. japonicum HupT also modulates (represses) the transcriptional activity of HoxA via phosphorylation. Taking into account these observations, we hypothesize that the active form of B. japonicum HoxA is the nonphosphorylated form.
Expression of hoxA is positively autoregulated.
In aerobically grown wild-type cells, there is no hupSL transcription and, consequently, no hydrogenase activity. This lack of hupSL transcription could be due to either the absence of hoxA transcription under aerobic conditions or the presence of inactive HoxA. To discriminate between these two possibilities, we studied the expression pattern of hoxA under aerobic and inducing conditions. The similar genetic organizations of hypD, hypE, hoxX, and hoxA suggest that the genes are translationally coupled (32). Moreover, no obvious consensus promoter sequences could be identified upstream of hoxA or hoxX, except for one putative RpoN-dependent promoter 100 bp upstream of hoxX (32). Since a reporter plasmid containing 400 bp of DNA upstream of hoxX coupled to a promoterless gusA gene failed to show any change in expression level under inducing conditions compared to the level observed during aerobiosis (results not shown), it was concluded that this sequence does not correspond to a functional promoter. To monitor hoxA expression driven from any promoter further upstream, we constructed the reporter strain FAJ1005, in which the chromosomal hoxA coding sequence is interrupted by a promoterless gusA gene. An intact hoxA gene was supplied in trans on cosmid pFAJ1005, in which the insert encompasses the hypABFCDE, hoxXA, and hupT genes (Fig. 1). Expression was measured under aerobic and inducing conditions, as shown in Table 3. Under aerobic conditions, hoxA is expressed at a low basal level in the cells, presumably from a housekeeping promoter. Expression is induced 10-fold when cells are shifted to hydrogenase-inducing conditions, and this expression is under positive autoregulatory control since no induction can be seen in the absence of a functional hoxA gene. These data strongly suggest that under aerobic conditions, HoxA is present in the cells, although at a low level.
TABLE 3.
hoxA expression in vegetative B. japonicum cells grown under aerobic or hydrogenase-inducing conditions in the presence of HoxA
| Strain | Genotype | β-Glucuronidase activitya
|
|
|---|---|---|---|
| Aerobic growth | Hydrogenase-induced growth | ||
| FAJ1005(pFAJ1005) | Wild type | 17 ± 1 | 171 ± 8 |
| FAJ1005 | hoxA | 33 ± 2 | 31 ± 1 |
Numbers are average values (in Miller units) of five replicates, assayed in duplicate, ± standard deviations.
In this view, the observed lack of hupSL transcription in wild-type cells under conditions of aerobiosis indicates that HoxA is present in the inactive form and is unable to activate transcription. Since HoxA is the central transcriptional regulator of hydrogen oxidation in free-living cells, the above-presented data suggest that, at least for this type of cells, HupT modulates the transcriptional activity of HoxA, most probably through phosphorylation. Analogous to the situation in A. eutrophus, we hypothesize that under noninducing conditions, HupT represses hupSL transcription by keeping the HoxA pool in a phosphorylated state, for when hupT is knocked out, hupSL is expressed. In the absence of HupT, the available HoxA would be nonphosphorylated, leading to hupSL transcription and translation. It is clear that levels of transcription of hupSL and the content of HupL protein are still lower in aerobically grown cells than in induced cells of the hupT mutant. Full activation of inactive HoxA still seems to require the perception of inducing conditions by a not-yet-elucidated mechanism in which HupUV should play a major role.
Further research must focus on the identification of the additional factors required to obtain an active hydrogenase.
Nucleotide sequence accession number.
The nucleotide sequence of the hupT gene has been deposited in the GenBank database (accession no. AF132935).
Acknowledgments
This work was supported by the Belgian Fund for Scientific Research (FWO)—Flanders, by a postdoctoral fellowship to C.V.S., by a grant from Spain’s DGICYT (project PB95-0232) to T.R.-A., and by the IMPACT2 Project (Biotec Program; reference no. CT960027).
We thank C. Verreth and P. de Wilde for sequencing and L. Sayavedra-Soto for kindly providing the anti-HupL serum.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Short protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1992. [Google Scholar]
- 2.Black L K, Maier R J. IHF- and RpoN-dependent regulation of hydrogenase expression in Bradyrhizobium japonicum. Mol Microbiol. 1995;16:405–413. doi: 10.1111/j.1365-2958.1995.tb02406.x. [DOI] [PubMed] [Google Scholar]
- 3.Black L K, Fu C, Maier R J. Sequences and characterization of hupU and hupV genes of Bradyrhizobium japonicum encoding a possible nickel-sensing complex involved in hydrogenase expression. J Bacteriol. 1994;176:7102–7106. doi: 10.1128/jb.176.22.7102-7106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantrell M A, Haugland R A, Evans H J. Construction of a Rhizobium japonicum gene bank and use in isolation of a hydrogenase uptake gene. Proc Natl Acad Sci USA. 1983;80:181–185. doi: 10.1073/pnas.80.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durmowicz M C, Maier R J. The FixK2 protein is involved in regulation of symbiotic hydrogenase expression in Bradyrhizobium japonicum. J Bacteriol. 1998;180:3253–3256. doi: 10.1128/jb.180.12.3253-3256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsen S, Colbeau A, Chabert J, Vignais P M. The hupTUV operon is involved in negative control of hydrogenase synthesis in Rhodobacter capsulatus. J Bacteriol. 1996;178:5174–5181. doi: 10.1128/jb.178.17.5174-5181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsen S, Colbeau A, Vignais P M. Purification and in vitro phosphorylation of HupT, a regulatory protein controlling hydrogenase gene expression in Rhodobacter capsulatus. J Bacteriol. 1997;179:968–971. doi: 10.1128/jb.179.3.968-971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsen S, Richaud P, Colbeau A, Vignais P M. Sequence analysis and interposon mutagenesis of the hupT gene, which encodes a sensor protein involved in repression of hydrogenase biosynthesis in Rhodobacter capsulatus. J Bacteriol. 1993;175:7404–7412. doi: 10.1128/jb.175.22.7404-7412.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 10.Fu C, Maier R J. Nucleotide sequences of two hydrogenase related genes (hypA and hypB) from Bradyrhizobium japonicum, one of which (hypB) encodes an extremely histidine-rich region and guanine nucleotide-binding domains. Biochim Biophys Acta. 1994;1184:135–138. doi: 10.1016/0005-2728(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 11.Fu C, Maier R J. Organization of the hydrogenase gene cluster from Bradyrhizobium japonicum: sequences and analysis of five more hydrogenase-related genes. Gene. 1994;145:91–96. doi: 10.1016/0378-1119(94)90328-x. [DOI] [PubMed] [Google Scholar]
- 12.Hanus F J, Maier R J, Evans H J. Autotrophic growth of H2-uptake-positive strains of Rhizobium japonicum in an atmosphere supplied with hydrogen gas. Proc Natl Acad Sci USA. 1979;76:1788–1792. doi: 10.1073/pnas.76.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jefferson R A. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–405. [Google Scholar]
- 14.Kim H, Maier R J. Transcriptional regulation of hydrogenase synthesis by nickel in Bradyrhizobium japonicum. J Biol Chem. 1990;265:18729–18732. [PubMed] [Google Scholar]
- 15.Kim H, Yu C, Maier R J. Common cis-acting region responsible for transcriptional regulation of Bradyrhizobium japonicum hydrogenase by nickel, oxygen, and hydrogen. J Bacteriol. 1991;173:3993–3999. doi: 10.1128/jb.173.13.3993-3999.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenz O, Strack A, Tran-Betcke A, Friedrich B. A hydrogen-sensing system in transcriptional regulation of hydrogenase gene expression in Alcaligenes species. J Bacteriol. 1997;179:1655–1663. doi: 10.1128/jb.179.5.1655-1663.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenz O, Friedrich B. A novel multicomponent regulatory system mediates H2 sensing in Alcaligenes eutrophus. Proc Natl Acad Sci USA. 1998;95:12474–12479. doi: 10.1073/pnas.95.21.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leyva A, Palacios J M, Murillo J, Ruiz-Argüeso T. Genetic organization of the hydrogen uptake (hup) cluster from Rhizobium leguminosarum. J Bacteriol. 1990;172:1647–1655. doi: 10.1128/jb.172.3.1647-1655.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leyva A, Palacios J M, Ruiz-Argüeso T. Conserved plasmid hydrogen-uptake (hup)-specific sequences within Hup+Rhizobium leguminosarum strains. Appl Environ Microbiol. 1987;53:2539–2543. doi: 10.1128/aem.53.10.2539-2543.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maier R J, Triplett E W. Toward more productive, efficient, and competitive nitrogen-fixing symbiotic bacteria. Crit Rev Plant Sci. 1996;15:191–234. [Google Scholar]
- 21.Metcalf W W, Wanner B L. Construction of new β-glucuronidase cassettes for making transcriptional fusions and their use with new methods for allele replacement. Gene. 1993;129:17–25. doi: 10.1016/0378-1119(93)90691-u. [DOI] [PubMed] [Google Scholar]
- 22.Miller J. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 23.Olson J W, Maier R J. The sequences of hypF, hypC, and hypD complete the hyp gene cluster required for hydrogenase activity in Bradyrhizobium japonicum. Gene. 1997;199:93–99. doi: 10.1016/s0378-1119(97)00352-1. [DOI] [PubMed] [Google Scholar]
- 24.Quandt J, Hynes M F. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene. 1993;127:15–21. doi: 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]
- 25.Ramseier T M, Göttfert M. Codon usage and G+C content in Bradyrhizobium japonicum genes are not uniform. Arch Microbiol. 1991;156:270–276. doi: 10.1007/BF00262997. [DOI] [PubMed] [Google Scholar]
- 26.Regensburger B, Hennecke H. RNA polymerase from Rhizobium japonicum. Arch Microbiol. 1983;135:103–109. doi: 10.1007/BF00408017. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz-Argüeso T, Hanus F J, Evans H J. Hydrogen production and uptake by pea nodules as affected by strains of Rhizobium leguminosarum. Arch Microbiol. 1978;116:113–118. [Google Scholar]
- 28.Sayavedra-Soto L A, Powell G K, Evans H J, Morris R O. Nucleotide sequence of the genetic loci encoding subunits of Bradyrhizobium japonicum uptake hydrogenase. Proc Natl Acad Sci USA. 1988;85:8395–8399. doi: 10.1073/pnas.85.22.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stults L W, Sray W A, Maier R J. Regulation of hydrogenase biosynthesis by nickel in Bradyrhizobium japonicum. Arch Microbiol. 1986;146:280–283. [Google Scholar]
- 30.Van Soom C, Browaeys J, Verreth C, Vanderleyden J. Nucleotide sequence analysis of four genes, hupC, hupD, hupF, and hupG, downstream of the hydrogenase structural genes in Bradyrhizobium japonicum. J Mol Biol. 1993;234:508–512. doi: 10.1006/jmbi.1993.1605. [DOI] [PubMed] [Google Scholar]
- 31.Van Soom C, de Wilde P, Vanderleyden J. HoxA is a transcriptional regulator for hydrogenase expression in free-living Bradyrhizobium japonicum CB1809. Mol Microbiol. 1997;23:967–977. doi: 10.1046/j.1365-2958.1997.2781648.x. [DOI] [PubMed] [Google Scholar]
- 32.Van Soom C, Verreth C, Sampaio M-J, Vanderleyden J. Identification of a potential transcriptional regulator of hydrogenase activity in free-living Bradyrhizobium japonicum strains. Mol Gen Genet. 1993;239:235–240. doi: 10.1007/BF00281623. [DOI] [PubMed] [Google Scholar]
- 33.Vincent J M. A manual for the practical study of the root-nodule bacteria. Oxford, United Kingdom: Blackwell Scientific Publications; 1970. [Google Scholar]