Summary
Background
The estimated incidence of colorectal cancer is rising in Nigeria, where most patients present with advanced disease. Earlier detection of colorectal cancer is a goal of the Nigerian National Cancer Control Plan, but the utility of fecal-based screening is unclear. This study aimed to assess the fecal immunochemical test as a colorectal cancer screening modality in average-risk individuals in Nigeria.
Methods
A population-based, cross-sectional study of qualitative fecal immunochemical test-based colorectal cancer screening was done in asymptomatic, average-risk participants aged 45–75 years in three states in Nigeria (Osun, Kwara, and Lagos). Participants were invited to enrol using age-stratified and sex-stratified convenience sampling following community outreach. Exclusion criteria included a personal history of colorectal cancer or rectal bleeding in the previous 6 months, a first-degree relative with a known diagnosis of colorectal cancer, or a comorbidity that would preclude conscious sedation or general anesthesia. Participants with positive fecal immunochemical test results underwent colonoscopy, and the positive predictive value of fecal immunochemical testing for colorectal cancer and advanced adenomas (≥10 mm, tubulovillous or villous or high-grade dysplasia) was calculated. Data on demographics and acceptability of fecal immunochemical testing and colonoscopy were collected.
Findings
Between January and April 2021, 2330 participants were enrolled in the study and received a fecal immunochemical test, which was returned by 2109 participants. 1677 participants tested negative and 432 tested positive. Of these 432 participants, 285 underwent a colonoscopy (235 showed no polyps or cancer, 47 had polyps identified, and three had colorectal cancer identified). Of the 47 participants who had polyps identified, 20 had advanced adenomas diagnosed. The median age was 57 years (IQR 50–63), 958 (41%) were male and 1372 (59%) were female, and 68% had at least a secondary-level education. Participants were evenly spread across wealth quintiles. The positivity rate of the fecal immunochemical test was 21% overall (432 of 2109; 95% CI 20–21%), 11% (51 of 455; 95% CI 10–12) in Lagos, 20% (215 of 1052; 95% CI 20–21) in Osun, and 28% (166 of 597; 95% CI 27–29) in Kwara. Among the patients with a positive fecal immunochemical test who completed colonoscopy, the positive predictive value for invasive colorectal cancer was 1·1% (95% CI 0·3–3·3), and 7·0% (4·5–10·8) for advanced adenoma. The acceptability of fecal immunochemical screening among participants was very high.
Interpretation
Colorectal cancer screening with qualitative fecal immunochemical tests in Nigeria is feasible and acceptable to average-risk asymptomatic participants. However, the low positive predictive value for advanced neoplasia and high endoscopy burden investigating false positives suggests it might not be an appropriate screening tool in this setting.
Funding
Thompson Family Foundation, Prevent Cancer Foundation, National Institutes of Health/National Cancer Institute Program Cancer Center.
Introduction
Colorectal cancer is the third most common cancer and the fourth leading cause of deaths from cancer worldwide,1 although there is wide variation in colorectal cancer burden between countries.2 The incidence of colorectal cancer has been historically highest in economically developed, high-income countries.1 However, incidence has now stabilised and mortality is falling in most high-income countries,1,2 partially due to the introduction of population-based colorectal cancer screening programmes.3,4 By contrast, marked increases in the incidence of colorectal cancer are now being observed in many middle-income countries as they undergo major demographic, economic, and health transitions.2,5 In Nigeria, the incidence of colorectal cancer presentation at individual health facilities has increased substantially over the past three decades.6 Advanced stage of colorectal cancer presentation and poor survival outcomes are common.7
Evidence supporting colorectal cancer screening is drawn almost exclusively from high-income countries, where organised, population-based screening programmes have been shown to reduce the incidence and mortality of invasive colorectal cancer,3,4 and are cost-effective.8,9 WHO does not currently recommend organised or opportunistic screening for colorectal cancer outside of high-income countries.10,11 However, growing aware ness of the burden of this cancer in middle-income countries, including Nigeria, has led to rising public and political enthusiasm for cancer screening.6,12 The Nigerian National Cancer Strategy (2018–22) identifies colorectal cancer screening as a priority and endorses the establishment of a national screening programme.13 Stool-based screening is thought to be the most feasible method because endoscopic resources are scarce. The Society for Gastroenterology and Hepatology in Nigeria supports the use of the quantitative fecal immunochemical test while recognising the lack of context-specific evidence.14 Currently, colorectal cancer screening in Nigeria is opportunistic, and the target age range, most appropriate screening test, time interval for screening, health system readiness, and cost-effectiveness of this type of screening in the country have not been defined.6 Evidence from other sub-Saharan African countries on the role and performance characteristics of fecal-based colorectal cancer screening are scant,11 and limited to small, singleinstitution experiences in symptomatic or higher risk patients.15–17
The main objective was to evaluate the performance and role of community-based fecal immunochemical test colorectal cancer screening in average-risk, asymptomatic individuals in southwest and north central Nigeria. The study also aimed to identify factors that predict fecal immunochemical test positivity and colonoscopy uptake in those with a positive screen and understand the acceptability of colorectal cancer screening tests.
Methods
Study design and participants
This community-based, cross-sectional study was done in two states in southwest (Osun, Lagos) and one in north central (Kwara) Nigeria. Self-reported asymptomatic adults aged 45–75 years were eligible for enrolment. This age range was selected because it was in line with the 2018 American Society for Gastrointestinal Colonoscopy (ASGE) screening recommendations for African-ancestry populations living in the USA,18 and considers the earlier age of colorectal cancer onset in African-ancestry populations reported both in the USA and Nigeria.7,19,20 Exclusion criteria included a personal history of colorectal cancer, a history of rectal bleeding in the previous 6 months, a first-degree relative with a known colorectal cancer diagnosis, or severe co-morbidity that would preclude conscious sedation or general anesthesia. Female participants were asked to wait 3 days from the end of menstruation before providing a stool sample. Eligibility criteria were based on colorectal cancer screening recommendations endorsed by the Society of Gastroenterology and Hepatology in Nigeria.14 A population-based recruitment strategy was used with print media, radio, television, social media, and community mobilisers to advertise the study in each geographic catchment area. Mobilisers worked at the grassroots level, by political ward, with deliberate efforts to target both urban and rural populations and advertise across geographic and socioeconomic gradients. Stratified sampling for gender and age was done at the time of enrolment at each study site to ensure the gender and age ratios reflected the underlying population. Multiple study sites were available, located close to public transportation routes and open extended hours to maximise recruitment. Participants who met the study eligibility criteria were reimbursed for travel-related costs, including the travel costs of returning the fecal immunochemical test and having a colonoscopy. The study was approved by the institutional research boards at Obafemi Awolowo University Teaching Hospital, University of Ilorin Teaching Hospital, and the University of Lagos. All participants signed a consent form approved by the institutional research boards.
Procedures
Participants who met the study eligibility criteria and provided informed consent completed a baseline questionnaire, which included information on demographics, medical history, and health literacy with respect to cancer screening and cancer symptoms. Socioeconomic status was evaluated using an asset-based wealth index questionnaire validated in Nigeria.21 All questionnaires and study instructions (written and verbal) were offered in English or the local language, on the basis of participant preference.
Eligible participants underwent fecal immunochemical testing. A qualitative fecal immunochemical test with a manufacturer-set lower limit of haemoglobin detection of 50 ng/mL was used (Pinnacle Biolabs, Nashville, TN, USA). Participants were given a stool collection tube, provided with written and verbal instructions on how to provide an uncontaminated stool specimen, and asked to return it within 48 h of evacuation for processing by the research team. We have previously demonstrated a time-dependent and temperature-dependent degradation of stool-based haemoglobin in fecal immunochemical tests in Nigeria, which informed the return time of 48 h at an ambient median temperature of 27°C.16 Each specimen was processed per the manufacturer instructions, including verification of an activated internal control. The result of each test was interpreted and recorded by two members of the research team. While the test was being processed, returning participants completed a questionnaire to elicit their perceptions of the acceptability of stool-based colorectal cancer screening. Participants with a positive fecal immunochemical test result were counselled on the result, and arrangements were made for a follow-up colonoscopy. Written and verbal instructions for attending the colonoscopy procedure were provided.
Participant colonoscopies were done at one centre in each study state by trained endoscopists within 8 weeks of returning a positive fecal immunochemical test result. Participants undergoing colonoscopy completed a post-procedure questionnaire to ascertain the acceptability of the procedure in the context of colorectal cancer screening follow-up. Cecal intubation and quality of bowel preparation (poor, fair, good, or excellent) were recorded as per ASGE and American College of Gastroenterology Taskforce on Quality in Colonoscopy recommen dations.22 Any pathology identified was documented on a colonoscopy study proforma. Structural lesions were biopsied, removed, or tattooed for later identification as per surgeon or institutional preference and guidelines. Specimens underwent histopathological analysis at the laboratory typically used by each endoscopy site, as well as central review by a gastrointestinal pathologist based at Obafemi Awolowo University or Lagos University Teaching Hospital for confirmation. Colonic pathology identified at colonoscopy was managed according to the institution’s standard of care. Referral to a surgeon and surgical treatment as necessary was included for advanced neoplasia patients. Direct medical costs of care incurred by participants resulting from positive fecal immunochemical tests were covered in their entirety by the study (including bowel preparation, colonoscopy, and treatment arising from colonoscopy findings).
Outcomes
The primary outcome of interest was the positive predictive value of the fecal immunochemical test for the detection of colorectal cancer in an asymptomatic, average-risk population in Nigeria. Secondary outcomes were the positive predictive value of the fecal immunochemical test for the detection of advanced adenomas (defined as adenomas ≥10 mm or with high-grade dysplasia or with ≥25% villous histological features), the number needed to screen to detect one colorectal cancer, the advanced adenoma and neoplasia detection rate per 1000 screened, the programmatic cost per colorectal cancer and per advanced neoplasia case detected, the acceptability of stool-based colorectal cancer screening and follow-up colonoscopy in participants with a positive fecal immunochemical test, the effect of key demographic (age, gender, geographic residence, socioeconomic status, and education level) and clinical factors (history of gastrointestinal symptoms, pre-menopausal, sidedness of structural findings at endoscopy) on fecal immunichemical test positivity, colonoscopy attendance, and the presence of advanced neoplasia. Advanced neoplasia was defined as either invasive cancer or advanced adenoma. Only structural lesions for which a histopathological diagnosis was obtained were included in the analysis of histopathological polyp type and advanced adenoma and neoplasia detection rate. Although the location and size of all lesions identified at colonoscopy were recorded, for the purposes of calculating the positive predictive value of the fecal immunochemical test for advanced neoplasia detection, only the most advanced colorectal epithelial lesion (the index lesion) and its location were used. If two similarly advanced lesions were identified, the larger of the two was considered the index lesion, consistent with other colorectal cancer screening studies.23
Statistical analysis
Categorical baseline participant characteristics, including sociodemographic information, past medical history, family history, previous screening participation, and cancer perceptions are presented using frequencies and proportions. Percentages have been rounded to the nearest whole number. Univariate analysis was done to test for factors associated with fecal immunochemical test positivity, colonoscopy attendance, and advanced neoplasia. p values were calculated using Pearson’s χ2 test for binary or nominal variables with statistical significance considered at an α level of 0·05. 95% CIs are provided for cases in which they were appropriate. The positive predictive value following colonoscopy for a positive fecal immunochemical screening test was calculated for invasive adenocarcinoma, advanced adenoma, and the combined outcome advanced neoplasia. Colorectal cancer detection rate per 1000 individuals screened via fecal immunochemical test was also calculated. The number needed to screen to detect one colorectal cancer was calculated. Study outcomes were analysed as both as-screened populations and intentionto-screen. Participants who declined colonoscopy were included in the a priori analysis. Results are reported in concordance with the STARD Guidelines for Diagnostic Accuracy Studies.24 Programme costs were calculated using an ingredients-based costing approach. Personnel, equipment, supplies, and facility costs were included. Travel-related expenses for the research team were not included. Personnel costs were established using a timeand-motion study on the final day of enrolment at each center. All costs were collected in the local currency (Naira) and converted to US dollars using the Central Bank of Nigeria’s conversion rate on March 1, 2021 (Naira=379 per US$1).
Study data were collected and managed using REDCap electronic data capture tools hosted at Memorial Sloan Kettering Cancer Center (NY, USA).25 All analyses were done in Stata SE (version 14.0).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
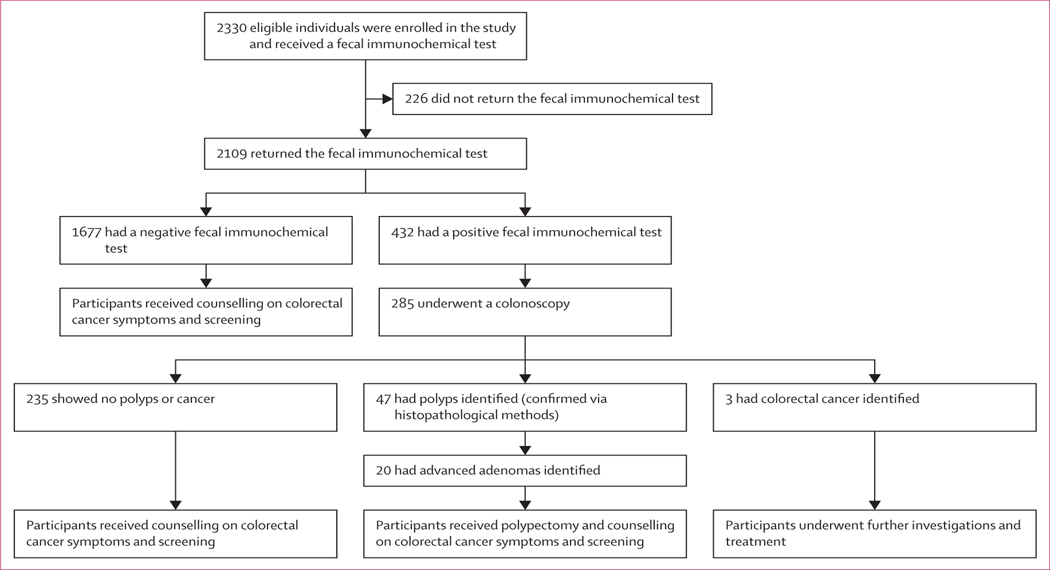
The study was done between January and April 2021. Overall, 2330 participants enrolled in the study and received a fecal immunochemical test, of which 1150 (49%) were from Osun state, 524 (22%) were from Lagos, and 656 (28%) were from Kwara state (figure). 2109 partici pants returned the test, and of these, 1677 participants tested negative and 432 participants tested positive. Of the 432 participants who had a positive fecal immunochemical test, 285 underwent a colonoscopy (235 participants showed no polyps or cancer, 47 had polyps identified, and three had colorectal cancer identified). 20 participants of the 47 who had polyps had advanced adenomas (figure). Data have been presented for the as-screened population, because no significant differences were found when compared with the intention-to-screen population (data not shown).
Figure:
Study profile
The median age was 57 years (IQR 50–63), 958 (41%) were male and 1372 (59%) were female, and 1087 (47%) had a college-level education. Participants from Lagos state were wealthier on a locally validated asset-based wealth index (table 1). Previous participation in opportunistic screening for any cancer type was reported by 20% (n=477) of participants overall, most commonly breast cancer (15%; n=204) and cervical cancer (161 [12%] of 1372) among women, and prostate cancer (75 [8%] of 958) among men (table 1; appendix p 1). Previous participation in opportunistic screening was highest among women in Lagos. Overall, 27 participants (1%) had discussed colorectal cancer screening with a health provider before and 15 (1%) were advised to undergo a colonoscopy. Baseline awareness among participants of cardinal colorectal cancer symptoms was low (appendix p 1).
Table 1:
Baseline characteristics
| Osun (n=1150) | Lagos(n=524) | Kwara (n=656) | Overall (n=2330) | |
|---|---|---|---|---|
| Age | ||||
| Median, years | 60 (53–63) | 55 (44–66) | 55 (47–62) | 57 (50–63) |
| Sex | ||||
| Male | 486 (42%) | 215 (41%) | 257 (39%) | 958 (41%) |
| Female | 664 (58%) | 309 (59%) | 399 (61%) | 1372 (59%) |
| Ethnicity | ||||
| Yoruba | 1067 (93%) | 479 (91%) | 627 (96%) | 2173 (93%) |
| Hausa | 42 (4%) | 9 (2%) | 1 (<1%) | 52 (2%) |
| Igbo | 22 (2%) | 19 (4%) | 7 (1%) | 48 (2%) |
| Other | 19 (2%) | 17 (3%) | 21 (3%) | 57 (2%) |
| Education level | ||||
| No formal education | 167 (15%) | 22 (4%) | 115 (18%) | 304 (13%) |
| Primary | 267 (23%) | 76 (15%) | 92 (14%) | 435 (19%) |
| Secondary | 247 (21%) | 135 (26%) | 111 (17%) | 493 (21%) |
| College or university | 464 (40%) | 290 (55%) | 333 (51%) | 1087(47%) |
| No response | 5 (<1%) | 1 (<1%) | 5 (1%) | 11 (<1%) |
| Health insurance status | ||||
| Insured | 231 (20%) | 176 (34%) | 154 (23%) | 561 (24%) |
| Not insured | 919 (80%) | 348 (66%) | 502 (77%) | 1769 (76%) |
| Occupation | ||||
| Unemployed | 39 (3%) | 2 (<1%) | 8 (1%) | 49 (2%) |
| Pensioner | 44 (4%) | 5 (1%) | 13 (2%) | 62 (3%) |
| Junior civil servant | 54 (5%) | 31 (6%) | 39 (6%) | 124 (5%) |
| Senior civil servant | 117 (10%) | 111 (21%) | 124 (19%) | 352 (15%) |
| Trader | 404 (35%) | 106 (20%) | 171 (26)) | 681 (29%) |
| Farmer | 84 (7%) | 1 (<1%) | 6 (1%) | 91 (4%) |
| Driver | 15 (1%) | 14 (3%) | 33 (5%) | 62 (3%) |
| Self-employed | 94 (8%) | 54 (10%) | 59 (9%) | 207 (9%) |
| Retired | 139 (12%) | 41 (8%) | 80 (12%) | 260 (11%) |
| Student | 0 | 0 | 0 | 0 |
| Professor or lecturer | 43 (4%) | 16 (3%) | 22 (3%) | 81 (3%) |
| Doctor | 7 (1%) | 5 (1%) | 10 (2%) | 22 (1%) |
| Nurse | 11 (1%) | 23 (4%) | 14 (2%) | 48 (2%) |
| Other | 99 (9%) | 115 (22%) | 76 (12%) | 290 (12%) |
| Home ownership | ||||
| Rental | 285 (25%) | 288 (55%) | 152 (23%) | 725 (31%) |
| Homeowner | 795 (69%) | 192 (37%) | 481 (73%) | 1468 (63%) |
| Government property | 45 (4%) | 22 (4%) | 8 (1%) | 75 (3%) |
| Other | 15 (1%) | 7 (1%) | 6 (1%) | 28 (1%) |
| Household income | ||||
| Median, Naira | 150 000 (50 000–430 000) |
200 000 (100 000–500 000) |
200 000 (50 000–450 000) |
180 000 (60 000–500 000) |
| Wealth index | ||||
| 1st quintile | 286 (25%) | 45 (9%) | 129 (20%) | 460 (20%) |
| 2nd quintile | 271 (24%) | 105 (20%) | 121 (18%) | 497 (21%) |
| 3rd quintile | 172 (15%) | 106 (20%) | 123 (19%) | 401 (17%) |
| 4th quintile | 252 (22%) | 104 (20%) | 152 (23%) | 508 (22%) |
| 5th quintile | 169 (15%) | 164 (31%) | 131 (20%) | 464 (20%) |
| Other cancer screening participation | ||||
| Previous participation in non-colorectal cancer screening | 148 (13%) | 188 (36%) | 141(21%) | 477 (20%) |
| Breast* | 61/664 (9%) | 80/309 (26%) | 63/399 (16%) | 204/1372 (15%) |
| Cervical* | 50/664 (8%) | 64/309 (21%) | 47/399 (12%) | 161/1372 (12%) |
| Prostate† | 24/486 (5%) | 26/215 (12%) | 25/257 (10%) | 75/958 (8%) |
| Other | 13 (1%) | 17 (3%) | 6 (1%) | 36 (2%) |
| Previously discussed colorectal cancer screening with health provider | ||||
| Yes | 12 (1%) | 8 (2%) | 7 (1%) | 27 (1%) |
| No | 1138 (99%) | 516 (98%) | 649 (99%) | 2303 (99%) |
Data are n (%), n/N (%), or median (IQR), unless otherwise specified. Percentages are reported to the nearest whole number.
Denominator used for percentage calculation is the total number of women.
Denominator used for percentage calculation is the total number of men.
Among participants who received a fecal immunochemical test kit, 91% (2109 of 2330) completed and returned the test within 48 h of sample collection (figure). Younger (age 45–54 years), poorer, and less educated participants, and participants from Lagos were less likely to return their fecal immunochemical test following enrolment (table 2). Among all participants who completed the test, 21% (432 of 2109; 95% CI 20–21) had a positive screen result. There was no significant difference in fecal immunochemical test positivity rate by age group, gender, education level, or wealth index. The fecal immunochemical test positivity rate differed significantly across the three states at 11% (51 of 458; 95% CI 10–12) in Lagos, 20% (215 of 1053; 95% CI 19–21) in Osun, and 28% (166 of 598; 95% CI 27–29) in Kwara. A remote history (>6 months) of blood in the stool, changes in bowel habits, and unexplained weight loss were all significantly associated with fecal immunochemical test positive screen results (table 2).
Table 2:
Participant characteristics associated with fecal immunochemical test completion and positivity, colonoscopy attendance, and subsequent advanced neoplasia detection
| Enrolled in study | Completed fecal immunochemical test | p value | Positive fecal immunochemical test | p value | Colonoscopy attendance | p value | Advanced neoplasia | p value | |
|---|---|---|---|---|---|---|---|---|---|
| Summary | |||||||||
| Overall | 2330 | 2109/2330 (91%) | … | 432/2109 (21%) | … | 285/432 (66%) | … | 23/285 (8%) | … |
| Sex | |||||||||
| Male | 958(41%) | 854/958 (89%) | 0·059 | 183/854 (21%) | 0·387 | 111/183 (61%) | 0·134 | 11/111 (10%) | 0·36 |
| Female | 1372 (59%) | 1255/1372 (91%) | … | 249/1255 (20%) | … | 174/249 (70%) | … | 12/174 (7%) | … |
| Age, years | |||||||||
| 45–54 | 963 (41%) | 853/963 (89%) | 0·010 | 176/853 (21%) | 0·981 | 121/176 (69%) | 0·747 | 5/121 (4%) | 0·010 |
| 55–64 | 879 (38%) | 815/879 (93%) | … | 165/815 (20%) | … | 106/165 (64%) | … | 8/106 (8%) | … |
| 65–75 | 488 (21%) | 441/488 (90%) | … | 91/441 (21%) | … | 58/91 (64%) | … | 10/58 (17%) | … |
| Study site | |||||||||
| Osun | 1150 (49%) | 1053/1150 (92%) | 0·031 | 215/1053 (20%) | <0·0001 | 137/215 (64%) | 0·86 | 17/137 (12%) | 0·033 |
| Lagos | 524 (22%) | 458/524 (87%) | … | 51/458 (11%) | … | 37/51 (73%) | … | 2/37 (5%) | … |
| Kwara | 656 (28%) | 598/656 (91%) | … | 166/598 (28%) | … | 111/166 (67%) | … | 4/111 (4%) | … |
| Education level | |||||||||
| No formal education | 304 (13%) | 256/304 (84%) | <0·0001 | 48/256 (19%) | 0·86 | 23/48 (48%) | <0·0001 | 0 | 0·25 |
| Primary | 435 (19%) | 388/435 (89%) | … | 84/388 (22%) | … | 46/84 (55%) | … | 6/46 (13%) | … |
| Secondary | 493 (21%) | 432/493 (88%) | … | 89/432 (21%) | … | 55/89 (62%) | … | 3/55 (5%) | … |
| College or university | 1087 (47%) | 1019/1087 (94%) | … | 208/1019 (20%) | … | 161/208 (77%) | … | 14/161 (9%) | … |
| Wealth index | |||||||||
| Quintile 1 | 460 (20%) | 394/460 (86%) | 0·0007 | 83/394 (21%) | 0·66 | 49/83 (59%) | 0·381 | 3/49 (6%) | 0·96 |
| Quintile 2 | 497 (21%) | 454/497 (91%) | … | 82/454 (18%) | … | 50/82 (61%) | … | 4/50 (8%) | … |
| Quintile 3 | 401 (17%) | 363/401 (91%) | … | 80/363 (22%) | … | 55/80 (69%) | … | 4/55 (7%) | … |
| Quintile 4 | 508 (22%) | 476/508 (94%) | … | 101/476 (21%) | … | 72/101 (71%) | … | 6/72 (8%) | … |
| Quintile 5 | 464 (20%) | 422/464 (91%) | … | 86/422 (20%) | … | 60/86 (70%) | … | 6/60 (10%) | … |
| History of gastrointestinal symptoms | |||||||||
| Blood in stool (at least 6 months ago) | 174 (7%) | 163/174 (94%) | 0·08 | 47/163 (29%) | 0·0069 | 40/47 (85%) | 0·013 | 3/40 (8%) | 0·88 |
| Changes in bowel habit | 253 (11%) | 232/253 (92%) | 0·48 | 65/232 (28%) | 0·0026 | 51/65 (78%) | 0·038 | 7/51 (14%) | 0·11 |
| Unexplained weight loss | 82 (4%) | 77/82 (94%) | 0·29 | 26/77 (34%) | 0·0033 | 23/26 (88%) | 0·013 | 4/23 (17%) | 0·096 |
| Extended fatigue | 272 (12%) | 245/272 (90%) | 0·97 | 51/245 (21%) | 0·95 | 32/51 (63%) | 0·53 | 2/32 (6%) | 0·68 |
| Parasite infection | 189 (8%) | 171/189 (90%) | 0·99 | 33/171 (19%) | 0·67 | 26/33 (79%) | 0·12 | 3/33 (9%) | 0·51 |
| Anal fissure | 654 (28%) | 572/654 (87%) | 0·0068 | 129/572 (23%) | 0·21 | 81/129 (63%) | 0·38 | 7/81 (9%) | 0·83 |
| Menopausal status | |||||||||
| Pre-menopausal status | 193 (8%) | 170/193 (88%) | 0·074 | 29/170 (17%) | 0·34 | 24/29 (83%) | 0·20 | 1/24 (4%) | 0·55 |
Data are n (%) or n/N (%), unless otherwise specified. Records with missing values were excluded for all variables. Percentages are reported to the nearest whole number.
Colonoscopy attendance among those with a fecal immunochemical test positive screen was 66% overall (285 of 432) and was highest in Lagos (73%; 37 of 51); however, there was no significant difference in colonoscopy attendance rates among study sites. A higher education level was significantly associated with higher rates of colonoscopy attendance, as was a history of gastrointestinal symptoms (blood in the stool, changes in bowel habits, and unexplained weight loss; table 2).
Of the 285 participants with a positive fecal immunochemical test who underwent colonoscopy, 20 showed advanced adenomas, and three had invasive adenocarcinoma (table 3). The number needed to screen with fecal immunochemical test to detect one colonoscopy-confirmed case of colorectal cancer was 777. Benign tubular adenomas were found in 25 (9%) of participants who underwent a colonoscopy, and inflammatory polyps were found in 19 (7%). More than half (13 of 23) of advanced neoplastic lesions were on the left side, and ten (43%) of 23 were in the rectosigmoid. Haemorrhoids were found in 146 (51%) participants who underwent a colonoscopy (table 3). Older age (65–75 years) and living in the Osun state were associated with a significantly higher rate of advanced adenomas at colonoscopy than were younger age or residence in Lagos or Kwara (table 2). The positive predictive value for advanced adenoma at colonoscopy was 7·0% (95% CI 4·5–10·8) and 1·1% (0·3–3·3) for colorectal cancer. The colorectal cancer detection rate was 1·4 cases per 1000 fecal immunochemical test-screened participants, and the advanced neoplasia detection rate was 10·9 cases per 1000 fecal immunochemical test-screened partici pants (table 3).
Table 3:
Endoscopic and histopathology findings on colonoscopy
| Colonoscopy findings (n=285) | |
|---|---|
| Benign structural findings | |
| Haemorrhoids | 146 (51%) |
| Diverticulosis | 25 (9%) |
| Histopathology | |
| Adenocarcinoma | 3 (1%) |
| Signet ring adenocarcinoma | 1 (04%) |
| Participants with histopathology-confirmed polyps | 47 (16%) |
| Histopathology of highest-grade polyp | |
| Traditional adenoma | 27 (9%) |
| Tubular | 25 (9%) |
| Tubulovillous | 1 (<1%) |
| Villous | 2 (1%) |
| High-grade dysplasia | 3 (1%) |
| Serrated adenoma | 0 |
| Inflammatory polyp | 19 (7%) |
| Advanced lesions | |
| Advanced adenomas | 20 (7%) |
| Advanced neoplasia | 23 (8%) |
| Location of advanced neoplasia | |
| Rectosigmoid | 10/23 (44%) |
| Left | 3/23 (13%) |
| Transverse | 6/23 (26%) |
| Right | 4/23 (17%) |
| Positive predictive values at colonoscopy (95% CI) | |
| Advanced neoplasia | 8·1% (5·3–12·0) |
| Advanced adenoma | 7·0% (4·5–10·8) |
| Colorectal cancer | 1·1% (0·3–3·3) |
| Detection rate, per 1000 participants screened with a fecal immunochemical test | |
| Colorectal cancer | 1·4 per 1000 participants |
| Advanced neoplasia | 10·9 per 1000 participants |
Data are n (%) or n/N (%), unless otherwise specified. Positive predictive values were based on participants with a positive fecal immunochemical test who underwent colonoscopy. Detection rate is expressed per 1000 participants who completed fecal immunochemical screening. Records with missing values were excluded for all variables. Percentages are reported to nearest whole number. Eight participants had <1 cm structural lesions considered low risk for adenoma on the basis of endoscopic appearance, which are not included in the analysis. These were not biopsied and therefore histopathological diagnosis is not available. For cases in which a participant had more than one histopathology-confirmed polyp, the highest grade polyp is reported.
Most study participants rated their overall experience of the fecal immunochemical test screening as favourable (good or very good: n=2102, 90%), thought the test kit was easy to use (n=2082, 89%), and would recommend the screening test to family and friends (n=2093, 90%) (appendix p 2). Overall, 73% (n=1700) of participants would pay to undergo colorectal cancer screening with a fecal immunochemical test, for a median of 1500 Naira ($3·65), and 89% (n=2080) would participate in fecal immunochemical test-based colorectal cancer screening if it were free.
The overall study cost per participant was $28·50, with a further $225·85 incurred for each participant who tested positive with fecal immunochemical testing who then underwent colonoscopy. The cost per colorectal cancer case detected was $43 591, and per advanced neoplasia detected was $5686.
Discussion
There is growing interest in the role of organised colorectal cancer screening in Nigeria,6 where the incidence and mortality of this disease are rising.2 Evidence in support of fecal-based screening for colorectal cancer is drawn almost exclusively from high-income countries,26 and the performance of this type of testing in sub-Saharan African populations has not been studied. In this pragmatic, cross-sectional study of colorectal cancer screening in 2330 average-risk participants in Nigeria, a fecal immunochemical testing-based screening strategy yielded a colorectal cancer detection rate of 1·4 cases per 1000 screened participants, and a positive predictive value for colorectal cancer among participants testing positive completing colonoscopy of 1·1% (95% CI 0·3–3·3) and 7·0% (4·5–10·8) for advanced adenoma. Comparatively, the reported positive predictive value for colorectal cancer following first-round fecal immunochemical screening (at haemoglobin concentration threshold ≥20 μg/g) in high-income countries is at least 5%, while for advanced adenoma detection positive predictive value ranges from 33% to 54%.27 In high-income countries, the colorectal cancer detection rate in average-risk populations completing fecal immunochemical test screening is also three to five times higher than shown in Nigeria.28 The high number of false positive tests in this study generated a large endoscopic burden in a country with a paucity of endoscopic resources, particularly outside large urban centers (Alatise O, Society for Gastroenterology and Hepatology, Nigeria, personal communication). These findings suggest that the introduction of qualitative fecal immunochemical test-based colorectal cancer screening with a 50 ng/mL haemoglobin detection threshold might not be appropriate in Nigeria at present.
Benign, non-polyp related causes of occult bleeding, especially haemorrhoids, were common among participants who tested positive with the fecal immunochemical testing. Haemorrhoids were found at colonoscopy in more than half of participants who tested positive in this study, and are a common finding on colonoscopy in Nigeria and other parts of sub-Saharan Africa in average-risk populations.29 There are mixed reports in the literature on the prevalence and contribution of haemorrhoids to false-positive findings in fecal immunochemical testing in high-income countries.30–32 Anal fissure and perianal eczema have been strongly associated with false-positives in other fecal immunochemical testing studies,30,33 and were reported by just under a third of participants overall, but were not associated with fecal immunochemical testing positivity. Diverticulosis was an uncommon finding and has not previously been shown to affect fecal immunochemical testing positivity.31
The threshold for haemoglobin detection at which a fecal immunochemical screening test is considered positive has a direct effect on the positive predictive value of the test. There is no universally accepted cut-off, and thresholds vary across screening programmes.27,34,35 Threshold choice affects the advanced neoplasia detection rate, the probability of missing an invasive cancer, and the proportion of screen-positive and false-positive tests. Threshold choice is especially important in countries such as Nigeria where there is a practical need to balance maximising cancer detection rates against the resource constraints and cost required to investigate a positive screen.6,36 In this study, we chose the highest existing threshold (50 ng/mL) for a commercially available qualitative fecal immunochemical testing, recognising the need to balance test sensitivity with endoscopic resources in Nigeria. We also chose a point-of-care qualitative test rather than a laboratory-based quantitative test to address concerns regarding scalability. Considering the high number of false positive fecal immunochemical test results in our study, higher haemoglobin detection thresholds in qualitative tests might need to be developed and evaluated for use in Nigeria. Thailand introduced a national colorectal cancer screening programme using quantitative fecal immunochemical test with a 150 ng/mL threshold.36 This threshold offered both high positive predictive value and negative predictive value for advanced neoplasia detection in the Thai population without overwhelming the country’s limited endoscopy resources.
A three to five times lower event rate for invasive colorectal cancer was observed in this study than observed for other colorectal cancer screening series from high-income countries in average-risk populations of similar age range.27 Of 2330 total participants, 285 tested positive with the fecal immunochemical test and completed a colonoscopy. Of these 285, three invasive cancers were identified. This result probably reflects a lower prevalence of colorectal cancer in Nigeria than in high-income countries implementing colorectal cancer screening. 1 The true prevalence and incidence of colorectal cancer in Nigeria are not known.6 Data from the Nigerian National System of Cancer Registries, which are drawn predominantly from hospital-based registries in urban regions, suggest age-standardised rates for colorectal cancer of six to seven incident cases per 100 000 (vs about 40–65 incident cases per 100 000 in high-income countries).3 Screening only becomes feasible and costeffective when the prevalence of a condition reaches a certain threshold.12
The positive predictive value of fecal immunochemical testing for advanced adenoma detection was much lower than in comparable studies outside of sub-Saharan Africa.27 This result might reflect a lower burden of high-risk adenomatous pathology, which has been reported in average-risk populations across sub-Saharan Africa undergoing colonoscopy.15 Endoscopic findings from our study also showed a low rate of high-risk tubulovillous or villous adenomas, large adenomas (≥10 mm), and high-grade dysplasia. Colorectal cancer screening programmes assume that most colorectal cancer cases develop via the classic adenoma-carcinoma sequence of chromosomal instability,37 and identification and removal of pre malignant adenomas can prevent progression to carcinoma.38 However, whether the adenoma-carcinoma sequence is the dominant pathway in the development of colorectal cancer outside of the high-income, mostly European populations in which it has been studied remains unclear. There is some evidence to suggest that alternative pathways driving colorectal cancer tumorigenesis, including microsatellite instability and the CpG island methylation pathway, might be more frequent in Nigeria and other west African countries.39 If the adenoma-carcinoma sequence is not the dominant pathway in Nigeria, this might have implications for determining the most appropriate colorectal cancer screening modalities and intervals, which is an area of ongoing investigation.
Despite the low positive predictive value of fecal immunochemical testing as a colorectal cancer screening test in Nigeria, most participants in this study reported having a positive experience. Additionally, two thirds of participants who tested positive completed follow-up colonoscopies, which is comparable to rates reported in the literature.27 This is the first time that the uptake of colonoscopies has been evaluated in Nigeria and is important because uptake for screening and any follow-up test must be high for the intervention to be considered effective.
Health system and individual out-of-pocket costs are important considerations in introducing a screening programme.11 Most participants were willing to pay a median of USD$3·65 for the fecal immunochemical screening test, substantially less than the per-participant cost of administering the test in this study. The cost of a colonoscopy to investigate a positive result was equivalent to half of the median monthly household income reported. These aspects will require consideration in the design, delivery, and financing of any formal screening programme in Nigeria. Formal cost-effectiveness analyses of organised and opportunistic colorectal cancer screening in Nigeria, as well as comparative cost-effectiveness analysis for programmes aimed at earlier detection of symptomatic cases of colorectal cancer are important and require future investigation.
This study has several limitations. To power the study for a sensitivity of 80% to detect colorectal cancer, we would have required about 27 000 participants. This high number of participants would have generated an endoscopic and resource burden that was untenable in Nigeria. To address the colorectal cancer screening recommendations in the current Nigerian National Cancer Strategy (2018–22) a pragmatic study design that evaluated fecal immunochemical testing on the basis of positive predictive value alone was required. This approached has recently been employed in fecal immunochemical testing-based colorectal cancer screening studies in Thailand and Mexico.40,41 We did not include a specific urban-rural stratum in our sampling strategy. Differences in colorectal cancer risk factors and prevalence of benign causes of gastrointestinal bleeding between urban and rural populations have been reported and might have contributed to observed differences in fecal immunochemical testing positivity across the study catchments, most notably between Lagos and the other regions. Overall, the study cohort had higher levels of post-secondary education than the general population in Nigeria.42 This higher level of education might reflect bias in the recruitment and sampling strategy, which might favour those able to access the study sites or patients with higher health literacy. A further limitation is that age inclusion criteria were selected according to screening recommendations for African-American populations living in the USA, and reported earlier age of colorectal cancer onset among Nigerians. The optimal age at which to start colorectal cancer screening in Nigeria remains unclear.
Colonoscopy attendance after a positive fecal immunochemical test result was reduced among patients with lower educational attainment compared with those of high educational attainment (but was not affected by other demographic factors including wealth), which might reflect differential drop-out owing to lower health literacy.
This study provides new insights into the utility and acceptability of fecal immunochemical test-based colorectal cancer screening in average-risk, clinically asymptomatic individuals in Nigeria, with relevance to other middle-income countries considering colorectal cancer screening. At a haemoglobin concentration threshold of 50 ng/mL, one in five participants returned a positive result for the fecal immunochemical test, generating a large endoscopic burden that would exceed national capacity if rolled out at a national level, with a low positive predictive value for invasive cancer or advanced adenoma at colonoscopy compared with that reported in other countries. These findings suggest that country-level and context-specific data on fecal-based colorectal cancer screening modalities are required, because key performance characteristics of fecal immunochemical testing might not translate across diverse populations.
Supplementary Material
Research in context.
Evidence before this study
We searched for evidence evaluating colorectal cancer screening in low-income and middle-income countries. We searched PubMed, MEDLINE, and ClinicalTrials.gov for articles published between Jan 1, 1990, and Aug 1, 2021, using the terms “colorectal cancer” OR “bowel cancer” AND “screening” AND “middle-income” OR “low-income” OR “low and middle-income (LMIC)”, without language restrictions. We applied an additional filter for geographical region using “Nigeria” OR “West Africa” OR “sub-Saharan Africa”. We found that the role and performance of fecal-based colorectal cancer screening is not well characterised outside of high-income settings. Identified articles were mostly narrative reviews or policy analyses, which extrapolated evidence on colorectal cancer screening from high-income settings. Studies from two upper-middle income countries, Thailand and Mexico, reported on the feasibility of introducing organised, population-based screening using quantitative fecal immunochemical testing. These studies showed acceptable positive predictive values with single-round fecal immunochemical screening for advanced neoplasia detection. One single-centre study in Ibadan, Nigeria, evaluated this type of screening among patients presenting to a primary care clinic for other concerns. This study demonstrated a 10% fecal immunochemical test positivity rate. The high rate of non-completion of colonoscopy (71%) among participants who tested positive in this study precluded meaningful evaluation of fecal immunochemical test performance characteristics for advanced neoplasia detection. The pilot study we did before undertaking the present study was also retrieved by the search. No other prospective studies of community-based colorectal cancer screening in sub-Saharan Africa were identified.
Added value of this study
To our knowledge, our study is the first to provide data on the positive predictive value, utility, and acceptability of fecal-based colorectal cancer screening in average-risk individuals in Nigeria, and sub-Saharan Africa. Fecal-based screening has been recommended for low-income and middle-income countries that are considering introducing screening, and improved access to fecal-based screening is a goal of Nigeria’s National Cancer Control Plan. Our findings demonstrate that a fecal-based colorectal cancer screening strategy performed poorly in Nigeria, with low positive predictive value for colorectal cancer and advanced adenoma. Caution should be taken in adopting fecal-based screening strategies developed and validated in high-income populations, which might not be generalisable to other settings.
Implications of all the available evidence
Colorectal cancer incidence appears to be rising in many middle-income countries, including in Nigeria, prompting interest in the role of population-based colorectal cancer screening. Population-specific data on fecal-based colorectal cancer screening modalities are required, particularly in sub-Saharan African countries, because test characteristics might not translate across diverse populations. Further research is needed to understand the performance, feasibility, and cost-effectiveness of screening strategies for colorectal cancer outside of high-income countries.
Acknowledgments
This study was funded by the Thompson Family Foundation, Prevent Cancer Foundation, and National Institutes of Health/National Cancer Institute Cancer Center Support (grant P30-CA008748).
Footnotes
Declaration of interests
TPK reports personal fees from Olympus outside of the submitted work. AJD reports consulting fees from Memorial Sloan Kettering Cancer Center during the conduct of this study. All other authors declare no competing interests.
Data sharing
Memorial Sloan Kettering Cancer Center and Obafemi Awolowo University Teaching Hospital support the international committee of medical journal editors and the ethical obligation of responsible sharing of data from prospective clinical studies. The protocol summary, a statistical summary, and informed consent forms are available upon request at any time. Requests for deidentified individual participant data can be made beginning 12 months after publication and for up to 36 months post-publication. De-identified individual participant data reported in the manuscript will be shared under the terms of a Data Use Agreement and can only be used for approved proposals. Requests should be made to: crdatashare@mskcc.org.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017; 66: 683–91. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso R, Guo F, Heisser T, et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol 2021; 22: 1002–13. [DOI] [PubMed] [Google Scholar]
- 4.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013; 369: 1106–14. [DOI] [PubMed] [Google Scholar]
- 5.Bray F, Soerjomataram I. The changing global burden of cancer: transitions in human development and implications for cancer prevention and control. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, eds. Cancer: disease control priorities. Third edn (volume 3). Washington (DC: ): The World Bank, 2015. [PubMed] [Google Scholar]
- 6.Knapp GC, Alatise OI, Olasehinde OO, et al. Is colorectal cancer screening appropriate in Nigeria? J Glob Oncol 2019; 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gullickson C, Goodman M, Joko-Fru YW, et al. Colorectal cancer survival in sub-Saharan Africa by age, stage at diagnosis, and human development index: a population-based registry study. Int J Cancer 2021; 149: 1553–63. [DOI] [PubMed] [Google Scholar]
- 8.Sekiguchi M, Igarashi A, Sakamoto T, Saito Y, Esaki M, Matsuda T. Cost-effectiveness analysis of colorectal cancer screening using colonoscopy, fecal immunochemical test, and risk score. J Gastroenterol Hepatol 2020; 35: 1555–61. [DOI] [PubMed] [Google Scholar]
- 9.Patel SS, Kilgore ML. Cost effectiveness of colorectal cancer screening strategies. Cancer Control 2015; 22: 248–58. [DOI] [PubMed] [Google Scholar]
- 10.WHO. WHO report on cancer: setting priorities, investing wisely and providing care for all. Geneva: World Health Organization, 2020. [Google Scholar]
- 11.Ralaidovy AH, Gopalappa C, Ilbawi A, Pretorius C, Lauer JA. Cost-effective interventions for breast cancer, cervical cancer, and colorectal cancer: new results from WHO-CHOICE. Cost Eff Resour Alloc 2018; 16: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan T, Sullivan R, Ginsburg OM. Screening for cancer: considerations for low- and middle-income countries. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, eds. Cancer: disease control priorities. Third edn (vol 3). Washington (DC: ): The World Bank, 2015. [PubMed] [Google Scholar]
- 13.Nigeria National Cancer Control Plan 2018–2022. https://www.iccpportal.org/system/files/plans/NCCP_Final%5B1%5D.pdf (accessed June 2, 2021).
- 14.Alatise O, Olasehinde O, Olokoba A, et al. Colorectal cancer screening guidelines for Nigeria in 2019. Nigerian J Gastro Hepatol 2019; 11: 42–55. [Google Scholar]
- 15.Ray-Offer E, Abdulkareem F. Screening colonoscopy in Port Harcourt, Nigeria. Gastroenterology Insights; 2019; 10: 1. [Google Scholar]
- 16.Knapp GC, Alatise O, Olopade B, et al. Feasibility and performance of the fecal immunochemical test (FIT) for average-risk colorectal cancer screening in Nigeria. PLoS One 2021; 16: e0243587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lussiez A, Dualeh SHA, Dally CK, et al. Colorectal cancer screening in Ghana: physicians’ practices and perceived barriers. World J Surg 2021; 45: 390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the US multi-society task force on colorectal cancer. Gastroenterology 2017; 153: 307–23. [DOI] [PubMed] [Google Scholar]
- 19.Paquette IM, Ying J, Shah SA, Abbott DE, Ho SM. African Americans should be screened at an earlier age for colorectal cancer. Gastrointest Endosc 2015; 82: 878–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, et al. Individualizing colonoscopy screening by sex and race. Gastrointest Endosc 2009; 70: 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssens W, Goedecke J, de Bree GJ, Aderibigbe SA, Akande TM, Mesnard A. The financial burden of non-communicable chronic diseases in rural Nigeria: wealth and gender heterogeneity in health care utilization and health expenditures. PLoS One 2016; 11: e0166121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015; 81: 31–53. [DOI] [PubMed] [Google Scholar]
- 23.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014; 370: 1287–97. [DOI] [PubMed] [Google Scholar]
- 24.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015; 351: h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012; 366: 697–706. [DOI] [PubMed] [Google Scholar]
- 27.Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2017; 112: 37–53. [DOI] [PubMed] [Google Scholar]
- 28.Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med 2014; 160: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kayamba V, Nicholls K, Morgan C, Kelly P. A seven-year retrospective review of colonoscopy records from a single centre in Zambia. Malawi Med J 2018; 30: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Klerk CM, Vendrig LM, Bossuyt PM, Dekker E. Participant-related risk factors for false-positive and false-negative fecal immunochemical tests in colorectal cancer screening: systematic review and meta-analysis. Am J Gastroenterol 2018; 113: 1778–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim NH, Park JH, Park DI, Sohn CI, Choi K, Jung YS. Risk factors for false fecal immunochemical test results in colorectal cancer screening. J Clin Gastroenterol 2017; 51: 151–59. [DOI] [PubMed] [Google Scholar]
- 32.van Turenhout ST, Oort FA, Terhaar sive Droste JS, et al. Hemorrhoids detected at colonoscopy: an infrequent cause of false-positive fecal immunochemical test results. Gastrointest Endosc 2012; 76: 136–43. [DOI] [PubMed] [Google Scholar]
- 33.Amitay EL, Cuk K, Niedermaier T, Weigl K, Brenner H. Factors associated with false-positive fecal immunochemical tests in a large German colorectal cancer screening study. Int J Cancer 2019; 144: 2419–27. [DOI] [PubMed] [Google Scholar]
- 34.Gibson DJ, Mooney T, Mooney J, Mulcahy HE, O’Donoghue D. Impact of a higher fecal immunochemistry test cut-off on pathology detected in subsequent rounds of a colorectal screening program. Gastrointest Endosc 2019; 89: 518–22. [DOI] [PubMed] [Google Scholar]
- 35.Ribbing Wilén H, Blom J, Höijer J, Hultcrantz R. Fecal immunochemical test in colorectal cancer screening: colonoscopy findings by different cut-off levels. J Gastroenterol Hepatol 2019; 34: 103–12. [DOI] [PubMed] [Google Scholar]
- 36.Aniwan S, Ratanachu Ek T, Pongprasobchai S, et al. The optimal cut-off level of the fecal immunochemical test for colorectal cancer screening in a country with limited colonoscopy resources: a multicenter study from Thailand. Asian Pac J Cancer Prev 2017; 18: 405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015; 21: 1350–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315: 2595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irabor DO, Oluwasola OA, Ogunbiyi OJ, et al. Microsatellite instability is common in colorectal cancer in native Nigerians. Anticancer Res 2017; 37: 2649–54. [DOI] [PubMed] [Google Scholar]
- 40.Khuhaprema T, Sangrajrang S, Lalitwongsa S, et al. Organised colorectal cancer screening in Lampang Province, Thailand: preliminary resultsfrom a pilot implementation programme. BMJ Open 2014; 4: e003671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manzano-Robleda MDC, Espinosa-Tamez P, Potter MB, et al. Fecal immunologic test results and diagnostic colonoscopy in a Mexican population at average risk for colorectal cancer. Cancer Prev Res (Phila) 2020; 13: 959–66. [DOI] [PubMed] [Google Scholar]
- 42.The World Bank. Education statistics—all indicators. Nigeria. https://databank.worldbank.org/reports.aspx?source=EducationStatistics (accessed Sept 9, 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.