Summary
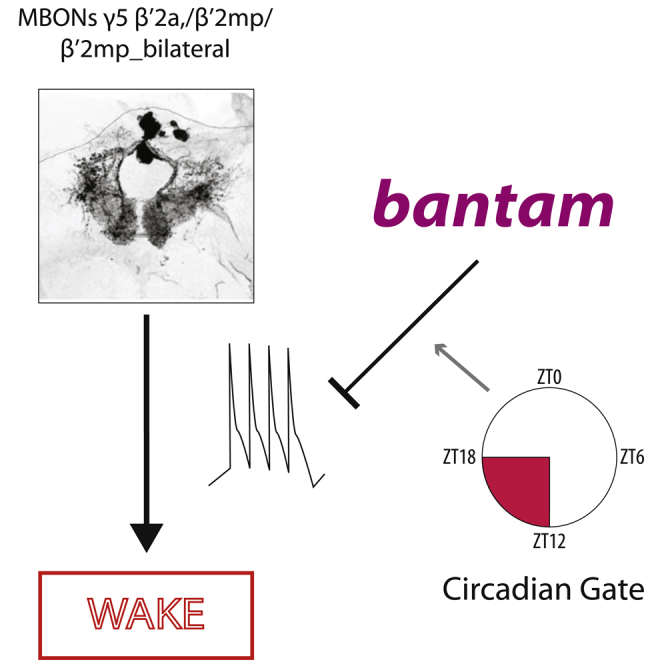
Sleep circuitry evolved to have both dedicated and context-dependent modulatory elements. Identifying modulatory subcircuits and understanding their molecular machinery is a major challenge for the sleep field. Previously, we identified 25 sleep-regulating microRNAs in Drosophila melanogaster, including the developmentally important microRNA bantam. Here we show that bantam acts in the adult to promote early nighttime sleep through a population of glutamatergic neurons that is intimately involved in applying contextual information to behaviors, the γ5β′2a/β′2mp/β′2mp_bilateral Mushroom Body Output Neurons (MBONs). Calcium imaging revealed that bantam inhibits the activity of these cells during the early night, but not the day. Blocking synaptic transmission in these MBONs rescued the effect of bantam knockdown. This suggests bantam promotes early night sleep via inhibition of the γ5β′2a/β′2mp/β′2mp_bilateral MBONs. RNAseq identifies Kelch and CCHamide-2 receptor as possible mediators, establishing a new role for bantam as an active regulator of sleep and neural activity in the adult fly.
Subject areas: Molecular physiology, Neuroscience, Cell biology
Graphical abstract
Highlights
-
•
The bantam microRNA has both developmental and adult roles in the regulation of sleep
-
•
Bantam promotes early nighttime sleep in adult 5β′2a/β′2mp/β′2mp_bilateral MBONs
-
•
Bantam suppresses MBON neuronal activity specifically in the early nighttime
-
•
Suppressing activity of 5β′2a/β′2mp/β′2mp_bilateral MBONs rescues ban knockdown
Molecular physiology; Neuroscience; Cell biology
Results
Bantam promotes nighttime sleep via the γ5β′2a/β′2mp/β′2mp_bilateral MBONs
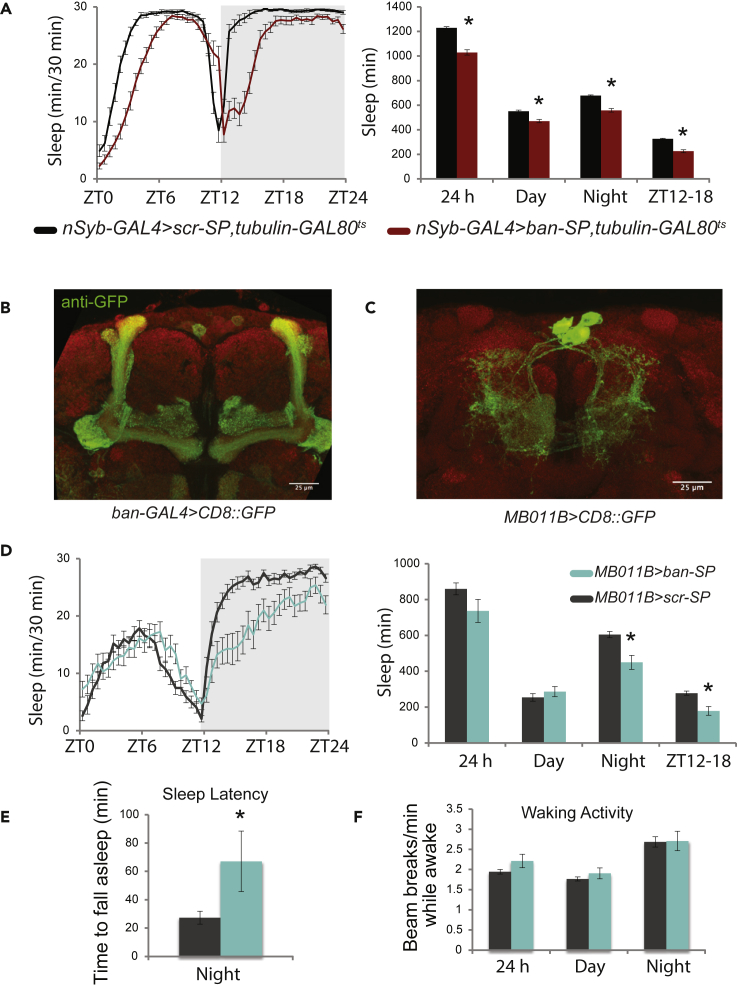
To investigate the roles of bantam (ban) in sleep, we utilized a previously validated (Becam et al., 2011) microRNA sponge transgene to reduce its levels. The technique allows spatially- and temporally-specific interrogation of the roles of microRNAs (Fulga et al., 2015). We had previously shown that pan-neuronal (with nSyb-GAL4) expression of the bantam sponge (ban-SP) led to a reduction in sleep (Goodwin et al., 2018), indicating that ban acts in the nervous system to promote sleep. Because sleep-regulating genes can exert their effects at different stages throughout the lifespan of an animal, as developmental regulators contributing to the formation of sleep circuits (Chakravarti Dilley et al., 2020; Gong et al., 1998; Xie et al., 2019) or as active regulators of adult sleep (for review see Crocker and Sehgal, 2010), it was important to determine the temporal window of ban action. We made use of an inducible expression system to control sponge expression. ban-SP was placed under the control of the pan-neuronal driver nSyb-GAL4 and a ubiquitously expressed temperature-sensitive inhibitor of GAL4 activity-tubulin-GAL80ts (McGuire et al., 2003). In this genotype, ban-SP is expressed at 29°C, but not at 17–18°C. Female nSyb>ban-SP, tubulin-GAL80ts flies were raised at 17°C and then shifted to 29°C after eclosion and for the duration of the sleep assay. Adult-specific pan-neuronal knockdown of ban led to a significant reduction in daytime and nighttime sleep compared to control animals expressing a scrambled sponge transgene (UAS-scr-SP) (Figure 1A). This reduction was most prominent in the early night, ZT12-18 (Figure 1A). In contrast, developmental knockdown of ban led to a significant reduction in daytime, but not nighttime, sleep (Figure S1A). These results indicate that in the adult brain, ban acts acutely to promote early night sleep. Consistent with this, pan-neuronal overexpression of ban increased sleep (Figure S1B).
Figure 1.
Bantam promotes nighttime sleep via the γ5β′2a/β′2mp/β′2mp_bilateral MBONs
(A) Adult-specific knockdown of ban. Sleep data for nSyb>ban-SP, tubulin-GAL80ts (N = 27) and scramble controls (N = 31) raised at 17°C and tested at 29°C (mean ± SEM). ∗ represents p ≤ 0.0001, Mann-Whitney-Wilcoxon test or unpaired t-test.
(B) Anti-GFP staining (green) for the central brain of a representative ban>CD8::GFP fly (63X). Anti-BRP (nc82) was used to stain neuropil (red). Scale bar measures 25μm.
(C) Anti-GFP (green) and anti-BRP (red) staining for the brain of a representative MB011B>UAS-CD8::GFP fly (63X). Scale bar measures 25μm.
(D) Sleep data for MB011B>ban-SP (N = 17) and scramble controls (N = 24) shown as mean± SEM ∗ represents p ≤ 0.0005, unpaired t-test.
(E) Sleep latency for same animals (minutes to fall asleep after lights out) shown as mean± SEM ∗ represents p ≤ 0.05, unpaired t-test.
(F) Beam breaks per active minute (general locomotor activity) for same animals (mean± SEM).
We next sought to localize the effect of ban on sleep to a specific population of neurons. We expressed CD8::GFP under the control of the ban-GAL4 driver to examine the expression pattern of ban in the adult brain (Figure 1B). Immunohistochemistry of ban>CD8::GFP brains revealed high GFP staining in both the intrinsic and extrinsic cells of the mushroom body (MB; Figure 1B), a centrally located neuropil with a well-characterized role in Drosophila sleep (Joiner et al., 2006; Pitman et al., 2006). The mushroom body scaffold is composed of the axonal projections of Kenyon cells (intrinsic cells), which communicate with several populations of extrinsic cells. The primarily input cell type is dopaminergic (Mao and Davis, 2009) whereas the primary output of Kenyon cells is via synapses onto the dendrites of Mushroom Body Output Neurons (MBONs) (Aso et al., 2014a). Given the high expression level of ban-GAL4 in the MB, we first examined whether ban expression in Kenyon cells was necessary for normal sleep. Expression of ban-SP under the powerful Kenyon cell driver OK107-GAL4 had no effect on sleep (Figure S1C), indicating that ban does not regulate sleep through MB intrinsic cells. We next tested the effect of ban knockdown on dopaminergic neurons. Expression of ban-SP under the dopaminergic driver TH-GAL4 also had no effect on nighttime sleep, although it did lead to a small increase in daytime sleep (Figure S1D). These negative results directed our attention to the third major component of the mushroom body circuit-the MBONs.
The MBONs consist of 21 subtypes divided into three classes determined by neurotransmitter identity (GABA, acetylcholine and glutamate) with each MBON named on the basis of the MB lobe section(s) which it innervates (Aso et al., 2014a). Several MBON subtypes have been implicated in sleep regulation, including the glutamatergic γ5β′2a/β′2mp/β′2mp_bilateral MBONs (Figure 1B) which have been previously shown to inhibit sleep when activated (Aso et al., 2014a; Sitaraman et al., 2015). To test if ban regulates sleep via this cell type, we drove ban-SP with the MB011B split-GAL4. MB011B>ban-SP flies exhibited a significant reduction in nighttime sleep (Figures 1D and S1E), in addition to increased sleep latency (Figure 1E) compared to animals expressing a control scrambled sponge (scr-SP). Nighttime sleep loss was most severe in the early night ZT12-18 period (Figure 1D). Driving ban-SP with an independent GAL4 line, GMR14C08, which has expression in this same subset of MBONs, also caused early night sleep loss (see Figure 3F, top panel). Interestingly, in contrast to the pan-neuronal adult knockdown results, ban knockdown in these MBONs had no effect on daytime sleep, indicating that the daytime sleep regulatory role of ban is likely mediated by different neurons. Importantly, knockdown of ban in the γ5β′2a/β′2mp/β′2mp_bilateral MBONs had no effect on waking motor activity (Figure 1F), demonstrating that the effect of this microRNA was specific to sleep rather than affecting general motor behavior.
Figure 3.
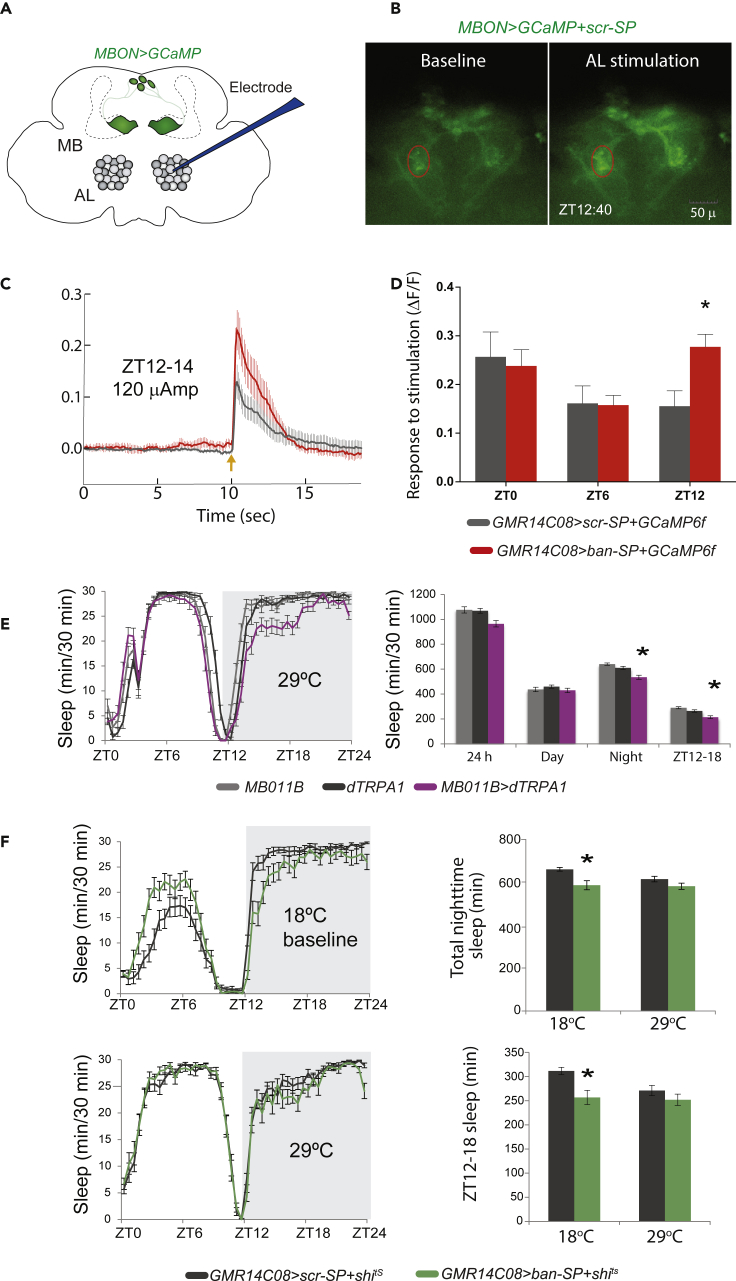
Bantam inhibits neural activity in the wake-promoting γ5β′2a/β′2mp/β′2mp_bilateral MBONs
(A) Schematic diagram of paradigm for antennal lobe (AL) microelectrode stimulation and γ5β′2a/β′2mp/β′2mp_bilateral MBON GCaMP6f recording. Dashed line represents mushroom body.
(B) GCaMP6f images showing representative dendritic Ca2+ responses of GMR14C08>scr-SP+GCaMP6f during baseline and after antennal lobe stimulation. Experiment was carried out at ZT12:40. 0.12mA stimulation was delivered. Scale bar measures 50μm.
(C) Average ΔF/F time course for Ca2+ responses of GMR14C08>scr-SP+GCaMP6f (gray) and GMR14C08>ban-SP+GCaMP6f (red) dendrites to antennal lobe stimulation (orange arrow) in the ZT12-14 time window (mean± SEM).
(D) ΔF/F for Ca2+ responses of GMR14C08>scr-SP+GCaMP6f (gray) and GMR14C08>ban-SP+GCaMP6f (red) dendrites to antennal lobe stimulation (0.12mA) in the ZT0-2, ZT6-8 and ZT12-14 time windows (mean± SEM). ∗ represents p ≤ 0.05, two-way ANOVA and Sidak post hoc comparison. n = 8 for all groups.
(E) Sleep data for MB011B>dTrpA1 (n = 31), MB011B control (n = 27) and UAS-dTrpA1 control (n = 30) tested for sleep behavior at 29°C (mean± SEM). ∗ represents p ≤ 0.01, Kruskal-Wallis test with Dunn’s multiple comparison test.
(F) Sleep data for GMR14C08>ban-SP+shits (n = 30) and GMR14C08>scr-SP+shits (n = 31) tested at 18°C and 29°C (mean± SEM). ∗ represents p ≤ 0.01, Mann-Whitney-Wilcoxon test.
Bantam negatively regulates wake-promoting genes in the γ5β′2a/β′2mp/β′2mp_bilateral MBONs
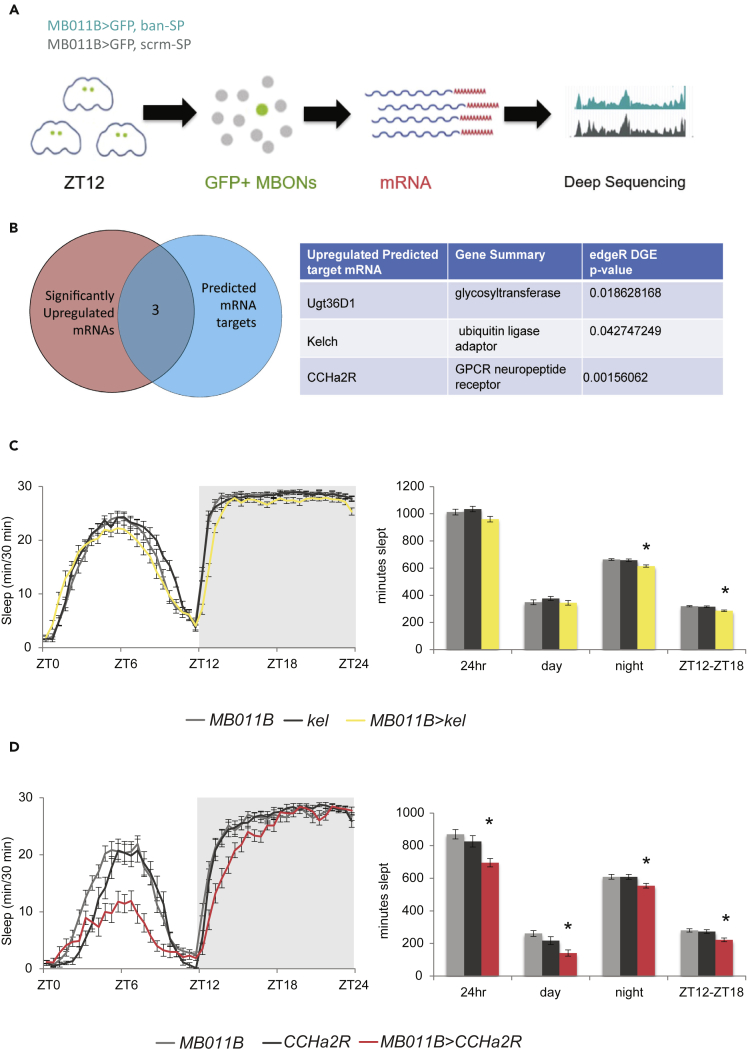
MicroRNAs exert their effects on cellular functions via inhibition of target mRNAs either through transcript degradation or translational silencing (Jonas and Izaurralde, 2015). MicroRNA knockdown leads to increased expression of its target mRNAs, either through increased RNA stability, translation or both. Knockdown of ban in the γ5β′2a/β′2mp/β′2mp_bilateral MBONs should therefore lead to upregulation of ban target mRNAs. To identify candidate targets of ban, we performed fluorescence-activated cell sorting (FACS) and deep sequencing on γ5β′2a/β′2mp/β′2mp_bilateral MBONs expressing GFP and ban-SP or scr-SP. Adult brains were dissected in the ZT12-13:30 time window (the time of maximum sleep-loss- Figure 1D). GFP+ MBONs were isolated by FACS and total mRNA was sequenced using a modified SMART-seq2 protocol (Liu et al., 2017; Picelli et al., 2014). RNA sequencing and Differential Gene Expression edgeR analysis (Robinson et al., 2010) for three biological replications was then performed to identify mRNAs that were significantly upregulated in the ban knockdown condition (Figure 2A). The list of statistically significant upregulated genes was compared to lists of putative target mRNAs generated by two microRNA-mRNA target prediction algorithms, TargetscanFly7.2 (Agarwal et al., 2018; Ruby et al., 2007) and DIANA-microT (Paraskevopoulou et al., 2013; Reczko et al., 2012). This pipeline produced 3 candidate mRNAs that were both significantly upregulated in the ban knockdown condition and were predicted direct targets- UDP-glycosyltransferase family 36 member D1 (Ugt36D1), Kelch (kel) and CCHamide-2 receptor (CCHa2-R) (Figure 2B).
Figure 2.
Bantam negatively regulates wake-promoting genes in the γ5β′2a/β′2mp/β′2mp_bilateral MBONs
(A) Schematic diagram of the MBON purification and sequencing paradigm.
(B) Venn diagram representing selection criteria for putative ban microRNA targets in the γ5β′2a/β′2mp/β′2mp_bilateral MBONs. Table representing the three “hits”, their gene summarizations and their p-value significance for edgeR differential gene expression analysis.
(C) Sleep data for MB011B>kel (n = 31), MB011B control (n = 32) and UAS-kel control (n = 28) (mean± SEM). ∗ represents p ≤ 0.001, Kruskal-Wallis test with Dunn’s multiple comparison test.
(D) Sleep data for MB011B>CCHa2-R (n = 23), MB011B control (n = 27) and UAS-CCHa2-R control (n = 22) (mean± SEM). ∗ represents p ≤ 0.05, Kruskal-Wallis test with Dunn’s multiple comparison test or one-way ANOVA with Tukey’s multiple comparisons test.
To determine if upregulation of these mRNAs contributes to the ban sleep loss phenotype, we overexpressed these genes within the γ5β′2a/β′2mp/β′2mp_bilateral MBONs and measured their effect on sleep. MB011B>Ugt36D1 flies exhibited normal sleep (Figure S2A), but both MB011B>kel and MB011B>CCHa2-R flies exhibited small but statistically significant reductions in early nighttime sleep (Figures 2C and 2D). qPCR on nsyb-GAL4>CCHa2-R fly heads and parental line controls was used to verify the efficacy of the UAS-CCHa2-R transgene in driving GAL-4 dependent overexpression (Figure S2B); the UAS-kelch overexpression transgene was previously validated (Hudson et al., 2015). These results suggest that the negative regulation of these target mRNAs may contribute to the nighttime sleep-promoting effect of ban in the γ5β′2a/β′2mp/β′2mp_bilateral MBONs.
Bantam inhibits neural activity in the wake-promoting γ5β′2a/β′2mp/β′2mp_bilateral MBONs
Our results suggest that ban expression in the γ5β′2a/β′2mp/β′2mp_bilateral MBONs is required for normal early nighttime sleep. However, the underlying physiological processes regulated by ban within these neurons were unknown. Given the previously characterized role of ban in the regulation of cellular proliferation and differentiation (Banerjee and Roy, 2017; Brennecke et al., 2003; Lerner et al., 2015; Weng and Cohen, 2015), we asked whether knockdown of ban in γ5β′2a/β′2mp/β′2mp_bilateral MBONs led to an alteration in cell number or structure. Comparison of MB011B>CD8::GFP+ban-SP to control MB011B>CD8::GFP+scr-SP brains revealed no differences in GFP-positive cell number nor gross morphology (Figures S3A and S3B). These results suggest an adult role for ban in the regulation of behavior and cellular processes in the γ5β′2a/β′2mp/β′2mp_bilateral MBONs.
Given the apparent normality of the structure of these MBONs, we hypothesized that ban had a role in regulation of their activity. It had been previously shown that dTrpA1-induced (Hamada et al., 2008) neural activation of the γ5β′2a/β′2mp/β′2mp_bilateral MBONs reduces sleep (Aso et al., 2014a; Sitaraman et al., 2015). In this same study, suppression of transmitter release using a temperature-sensitive dominant negative dynamin transgene (shibirets) (Kitamoto, 2001), did not affect sleep, suggesting a model in which MBONs modify baseline sleep levels only as a response to salient information presented to the MB. Given that neuronal activation of the γ5β′2a/β′2mp/β′2mp_bilateral MBONs phenocopied the effect of ban knockdown on sleep, this suggested that knockdown of ban may increase the ability of these neurons to respond to stimuli. Consistent with this, we noted an increase in arousal at night, as measured by P(wake) (Wiggin et al., 2020), in animals with reduced ban function in these MBONs (Figure S1F).
To test the idea that ban regulates neuronal activity, we drove GCaMP6f, a calcium sensor, with GMR14C08-GAL4, a stronger driver for γ5β′2a/β′2mp/β′2mp_bilateral MBONs. MBONs are downstream of an excitatory pathway that conveys olfactory information to the MB circuit; antennal lobe (AL) projection neurons send excitatory inputs to Kenyon cells which in turn make excitatory connections on MBONs (Aso et al., 2014a; Barnstedt et al., 2016; Ueno et al., 2013). This excitatory pathway was activated by a glass suction microelectrode placed on a subset of AL neurons while calcium dynamics in the dendrites of the ipsilateral group of γ5β′2a/β′2mp/β′2mp_bilateral MBONs were recorded (Figures 3A and 3B) (Ueno et al., 2013, 2017; Wang et al., 2008). Consistent with our hypothesis, GMR14C08>ban-SP+GCaMP6f MBONs exhibited a significantly higher mean dendritic ΔF/F calcium response than control GMR14C08>scr+GCaMP6f MBONs when the AL was stimulated in the ZT12-14 window, the time of maximal sleep loss (Figures 3C and 3D). Because ban knockdown reduces sleep in a time-of-day-dependent manner we asked whether the effect of ban knockdown on responsiveness exhibited a similar pattern. Mirroring our behavioral results, AL stimulation at ZT0 and ZT6 produced calcium responses which were statistically indistinguishable from scr-SP controls (Figure 3D). These results demonstrate that ban negatively regulates the activity of the γ5β′2a/β′2mp/β′2mp_bilateral MBONs during the early night, but not during the day, in line with its effects on sleep.
To ask if the increased neuronal activity associated with ban knockdown was causally related to the sleep loss phenotype we first confirmed that activation of the γ5β′2a/β′2mp/β′2mp_bilateral MBONs with the temperature-sensitive cation channel dTrpA1 decreased nighttime sleep (Figure 3E) and that inhibition of MBON synaptic release with shibirets had no effect on baseline sleep (Figure S3C). We then hypothesized that if the increased responsiveness associated with ban knockdown was causative for the sleep loss phenotype, inhibition of γ5β′2a/β′2mp/β′2mp_bilateral MBON activity in ban-SP-expressing flies should rescue early night sleep. To test this, we co-expressed ban-SP with shits. GMR14C08>ban-SP+shits flies exhibited decreased nighttime sleep when tested at the permissive temperature of 18°C (Figure 3F), consistent with loss of ban activity. However, when tested at the restrictive temperature of 29°C (which blocks MBON synaptic signaling), GMR14C08-GAL4>ban-SP+shits flies exhibited sleep levels statistically indistinguishable from scr-SP controls (Figure 3F). The ability of shits to rescue the sleep loss phenotype indicates that ban knockdown enhances neurotransmission by MBONs which in turn inhibits nighttime sleep. These results demonstrate that ban-mediated control of neuronal activity within the wake-promoting γ5β′2a/β′2mp/β′2mp_bilateral MBONs acts to promote early nighttime sleep. In addition, the fact that the alternative γ5β′2a/β′2mp/β′2mp_bilateral MBON driver, GMR14C08-GAL4, produced a sleep-loss phenotype when expressing the ban-SP at the permissive temperature (Figure 3F) supports the idea that ban acts in this neuronal sub-population to regulate sleep.
Discussion
MicroRNAs have emerged as key regulators of sleep and wake, but the cellular and physiological mechanisms via which individual microRNAs influence sleep remain largely uncharacterized. Here we show a new role for the microRNA bantam (ban) in promoting early nighttime sleep by decreasing the responsiveness of the wake-promoting γ5β′2a, β′2mp, β′2mp_bilateral MBONs. ban is a widely-expressed microRNA with well-characterized developmental roles in the specification of neuron number and morphology (Parrish et al., 2009; Song et al., 2012; Weng and Cohen, 2015). Known adult roles for ban have been largely limited to regulation of stem cells or other proliferative populations (Huang et al., 2014; Shcherbata et al., 2007) making the adult-specific role of ban in regulation of neuronal excitability a previously unrecognized function for this microRNA. It is important to note, however, that this is likely not the only way in which ban can affect sleep-its developmental functions contribute to adult daytime sleep generation and it appears to have a wake-promoting effect in dopaminergic cells (Figure S1).
Down-regulation of ban in the adult γ5β′2a, β′2mp, β′2mp_bilateral MBONs produces a sleep loss phenotype largely restricted to the early night ZT12-18 period as well as an increase in sleep latency. A number of sleep-regulating genes exhibit patterns of sleep loss that are largely confined to the early night period (Cong et al., 2015) or have large effects on latency (Agosto et al., 2008). Conversely, other sleep-regulating genes primarily contribute to late night sleep (Gmeiner et al., 2013; Kunst et al., 2014). This may reflect the fact that early and late-night sleep represent physiologically distinct states under differential genetic and neuroanatomical regulation and which likely serve different functions. Mammalian sleep varies across the night with slow-wave non-REM sleep predominant during the early night and REM sleep more frequent during the late night. Early and late night sleep has also been shown to facilitate different aspects of sleep-dependent memory processing (Plihal and Born, 1997; Yordanova et al., 2008). Our data show that bantam is important in allowing the initiation of early night sleep by suppressing activation of the γ5β′2a/β′2mp/β′2mp_bilateral MBONs.
Similar to mammalian sleep, Drosophila sleep is regulated by a widely distributed neural network (Shafer and Keene, 2021). The role of the mushroom body in the sculpting of responses to external conditions makes this structure uniquely suited to providing context-specific regulation of sleep. Consistent with this, synaptic silencing of the γ5β′2a/β′2mp/β′2mp_bilateral MBONs with shits (this study and Sitaraman et al., 2015), electrical silencing (with UAS-kir2.1) and induced apoptosis (with UAS-hid) of the γ5β′2a/β′2mp/β′2mp_bilateral MBONs (MH, data not shown) have no effect on basal sleep. A similar sleep regulation pattern was demonstrated for the wake-promoting C01+A05 neurons: knockdown of the calcium sensor Neurocalcin in these cells led to reduced sleep and increased neural activity, whereas electrical silencing of the cells had no effect on sleep (Chen et al., 2019). This supports a framework in which many of the identified sleep-regulating neurons may actually be conditionally-recruited loci that allow specific internal and external states to influence immediate sleep/wake probability but do not have major effects on sleep in normal conditions.
We speculate that he γ5β′2a/β′2mp/β′2mp_bilateral MBONs are activated by physiological or environmental factors that reduce sleep in response to competing motivational drives, with ban expression acting as the switch to turn neural activity off and on. These MBON neurons are known to be required for behaviors that might compete with sleep, including avoidance of aversive stimuli, aversion to pathogen-infected food, ingestion, startle-induced locomotion, and memory for visual and olfactory cues (Al-Anzi and Zinn, 2018; Aso et al., 2014b; Kobler et al., 2020; Lewis et al., 2015; Owald et al., 2015; Sun et al., 2018; Yamazaki et al., 2018).
The sleep-promoting effect of ban in the γ5β′2a/β′2mp/β′2mp_bilateral MBONs appears to be the result of regulation of several mRNA targets, likely including CCHamide-2 receptor and kelch. Kelch is a BTB-domain adaptor protein for the Cullin-3 ubiquitin E3 ligase that is involved in actin regulation (Hudson and Cooley, 2010; Kelso et al., 2002) and dendritic branching (Djagaeva and Doronkin, 2009). Cullin-3 and Insomniac, another BTB-domain adaptor protein, have been previously implicated in sleep (Pfeiffenberger and Allada, 2012). CCha2-R is a GPCR for the gut-derived peptide hormone CCHamide-2 which has a critical role in feeding and growth (Ren et al., 2015; Sano et al., 2015). Activation of the CCHa2-R receptor enhances calcium responses in these neuroendocrine cells (Sano et al., 2015), consistent with a role for CCHa2-R in promoting neural activity of MBONs.
Limitations of the study
This study identifies a very small group of adult neurons as regulators of early night sleep. Our use of a validated microRNA sponge strongly suggests expression of ban in γ5β′2a/β′2mp/β′2mp_bilateral MBONs but does not directly demonstrate it. In addition, these neurons likely act as context-dependent modulators of sleep rather than required elements for the behavior, so the phenotypes we demonstrate are by their nature small. Because microRNAs typically act on suites of genes, the identification of single ban targets as major mediators of its effects on sleep may be difficult if groups of genes are acting synergistically. Future work, examining synergy between targets and carrying out epistasis experiments will likely yield a better understanding of how modulation of the ban gene network regulates sleep in these MBONs.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-GFP | Invitrogen | Cat# A-11122 RRID: AB_221569 |
| anti-BRP | DSHB | nc82; RRID: AB_2314866 |
| Alexa Fluor 488 (goat anti-rabbit) | Invitrogen | Cat# A-11008 RRID: AB_143165 |
| Alexa Fluor 633 (goat anti-mouse) | Invitrogen | Cat# A-21052 RRID:AB_2535719 |
| Vectashield | Vector Laboratories, Inc. | Cat# H-1000 RRID: AB_2336789 |
| Chemicals, peptides, and recombinant proteins | ||
| Tetrodotoxin (TTX) | Tocris Bioscience | Cat# 1078 |
| Papain | Worthington | Cat# LK003176 |
| Agencourt AMPure XP beads | Beckman Coulter | Cat# A63880 |
| Critical commercial assays | ||
| Dynabead mRNA Direct Purification kit | Invitrogen | Cat# 61,011 |
| Nextera XT DNA Library Preparation Kit | Illumina | Cat# FC-131-1096 |
| Experimental models: Organisms/strains | ||
| UAS-ban-SP | Becam et al., 2011 | N/A |
| UAS-scamble-SP | BDSC | #61501 |
| UAS-CD8::GFP | Lee and Luo, 1999 | N/A |
| 20XUAS-GCaMP6f | BDSC | #52869 |
| pJFRC124-20XUAS-IVS-dTrpA1 | Gift from Janelia | N/A |
| UAS-kelch | Hudson et al., 2015 | N/A |
| 20XUAS-GCaMP6f | BDSC | #52869 |
| 20xUAS-IVS-Syn21-Shits | Pfeiffer et al., 2012 | N/A |
| UAS-Ugt36D1 | This paper | N/A |
| UAS-CCHa2-R | This paper | N/A |
| nsyb-GAL4 | BDSC | #51941 |
| bantam-GAL4 | Gift from Sebastian Kadener | N/A |
| OK107-GAL4 | BDSC | #854 |
| TH-GAL4 | BDSC | #8848 |
| GMR14C08-GAL4 | BDSC | #48606 |
| MB011B Split-GAL4 | BDSC | #68294 |
| tubulin-GAL80ts | BDSC | #7019 |
| RNA seq data set | GEO | GSE208649 |
| Oligonucleotides | ||
| CCHa2-R mRNA forward primer for cloning | AGTCCTGTGCACGGATTC | N/A |
| CCHa2-R mRNA reverse primer for cloning | ACATTTATGGAATCCATTTATTTACATTAA | N/A |
| Ugt36D1 mRNA forward primer for cloning | TTTCATTCGGATGTAGTCTCC | N/A |
| Ugt36D1 mRNA reverse primer for cloning | ATTTTAAAAACTTTATTTATTTCCGTACTAG | N/A |
| Recombinant DNA | ||
| pUASTattB | Addgene | GenBank: EF362409.1 |
| Software and algorithms | ||
| Fiji | http://fiji.sc | RRID: SCR_002285 |
| GraphPad Prism | GraphPad Software | RRID: SCR_002798 |
| MATLAB R2012b | MathWorks | RRID: SCR_001622 |
| Leica TCS SP5 | SP5 confocal microscope | RRID: SCR_002140 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Leslie C. Griffith (griffith@brandeis.edu).
Materials availability
Newly generated transgenic lines available on request to lead contact.
Experimental model and subject details
Adult mated female Drosophila melanogaster (∼4–10 days old) were employed in all studies except where noted.
Method details
Drosophila lines
UAS lines include UAS-ban-SP and UAS-scramble-SP (Fulga et al., 2015), UAS-CD8::GFP (Lee and Luo, 1999), UAS-kelch (Hudson et al., 2015), 20XUAS-GCaMP6f (Chen et al., 2013), 20XUAS-IVS-dTrpA1 and 20xUAS-IVS-Syn21-Shits (Pfeiffer et al., 2012). UAS-Ugt36D1 and UAS-CCha2-R lines were generated by cloning their cDNA-derived mRNA sequences into the Drosophila expression vector pUASTattB which was then integrated into the attp40 PhiC31 integration site on chromosome 2 (Bischof et al., 2007). Injections were performed by Rainbow Transgenic Flies Inc (Camarillo, CA).GAL4s include nSyb-GAL4, ban-Gal4 (generous gift of Sebastian Kadener, Brandeis University), OK107-GAL4 (Aso et al., 2009), TH-GAL4 (Friggi-Grelin et al., 2003) and GMR14C08-GAL4 (Jenett et al., 2012). Split-GAL4s include MB011B (Aso et al., 2014a). GAL80 lines include tubulin-GAL80ts (McGuire et al., 2003).
Drosophila husbandry and sleep assay
Flies were grown on standard cornmeal/agar food supplemented with yeast. Drosophila Activity Monitor (DAM) system (TriKinetics, Waltham) was used to measure sleep (Donelson et al., 2012). Female flies were loaded into glass sleep tubes containing a food mixture of 5% sucrose and 2% agar. Young female flies were housed with w1118 males >24 h before loading in sleep tubes in order to ensure that flies were mated. Temperature was kept constant at 25°C throughout sleep recording unless otherwise noted. Flies were considered sleeping if they were inactive for 5 min or more (Hendricks et al., 2000; Shaw et al., 2000). Sleep was averaged across 2–5 days unless otherwise noted. DAM data was analyzed using a custom MATLAB program called Sleep and Circadian Analysis MATLAB Program (SCAMP) (Donelson et al., 2012). The NAMEAN measure (beam breaks per active minute) was used to assess basal locomotor behavior. For all sleep duration data, a D’Agostino-Pearson test was used to test for normality. If normally distributed, data was analyzed with ANOVA or T-test depending on the number of groups. If not normally distributed, data was analyzed with a Kruskal-Wallis ANOVA with Dunn’s multiple comparison test or with a Mann-Whitney test. Statistics were performed using GraphPad Prism.
Immunohistochemistry
Fly brains were dissected in ice cold Schneider’s Insect Medium (S2) and fixed in 2% paraformaldehyde for 55 min at room temperature. Brains were then washed 4X in PBS with .5% Triton X-100 (PBS-T) and then placed in blocking solution (PBS-T with 5% normal goat serum (NGS; Invitrogen)) for 90 min at room temperature. Incubation in primary and secondary antibodies was performed for 2–3 days at 4°C. Anti-GFP (raised in rabbit; Invitrogen) and anti-BRP (raised in mouse; monoclonal Nc82) were used at concentrations 1:1000 and 1:25, respectively. Anti-rabbit Alexa Flour 488 and anti-mouse Alexa Flour 633 (both Invitrogen) were used at 1:500. Brains were then washed 4X times in PBS-T and placed in 2% paraformaldehyde for 4 h at room temperature. Brains were washed 4X times in PBS-T and then mounted using Vectashield Mounting Medium (Vector Laboratories). Slides were imaged on a Leica SP5 confocal microscope with a 63x objective. Maximum intensity Z projections were generated using FIJI software.
FACS sorting and RNA-seq of MBONs
FACS was performed using a previously described protocol (Ma et al., 2021). The brains of MB011B>UAS-CD8::GFP+UAS-ban-SP and control MB011B>UAS-CD8::GFP+UAS-scr-SP mated female flies (3–4 days after eclosion) were dissected between ZT12 and ZT13:30 in artificial hemolymph (AHL- 108 mM NaCl, 5 mM KCl, 2 mM CaCl2, 8.2 mM MgCl2, 4 mM NaHCO3, 1 mM NaH2PO4-H2O, 5 mM trehalose, 10 mM sucrose, 5 mM HEPES; pH 7.5) and 0.1 μM tetrodotoxin (TTX). Brains were collected in SM medium (active Schneider’s medium) and 0.1 μM TTX. Brains were then digested in Papain (Worthington PAP2, 50 unit/mL, with approximately 2 μL per brain) for 30 min and then quenched with 5X volume of SM media+TTX. Trituration was performed with flame-rounded 1000 μl and 200 μL pipette tips. Filtration was performed with a 100 μm sieve. Cell sorting was performed using a BD FACS Melody using the GFP channel. Sorted cells were collected in an Eppendorf tube with 50μL lysis buffer (Dynabead mRNA Direct Purification kit, Invitrogen) and stored at −80°C.
RNA sequencing was performed using a modified SMART-seq2 protocol (Li et al., 2017; Picelli et al., 2014). Briefly, mRNA was isolated from cells using a Dynabead mRNA purification kit (Invitrogen). Poly(A)-tailed RNA was reverse transcribed and PCR-amplified with 25 cycles. cDNA libraries were cleaned using AMPure beads (Agencourt). The resulting full-length cDNA was used as the input to the tagmentation based Nextera XT (Illumina) protocol to generate sequencing libraries. Two biological replications were sequenced on a Next-seq 500 platform with paired-end 75bp reads and one biological replication was sequenced on a Hi-seq platform with paired-end 150bp reads. Reads were aligned to the Drosophila genome (dm6) using STAR (Dobin et al., 2013). EdgeR was used to perform DGE on the aligned reads of the three biological replicates (Robinson et al., 2010). The list of predicted ban targets was generated by TargetscanFly7.2 (Agarwal et al., 2018; Ruby et al., 2007) and DIANA-microT (Paraskevopoulou et al., 2013; Reczko et al., 2012) using an miTG threshold of .5.
GCaMP6f imaging and antennal lobe stimulation
Calcium imaging was performed using a modified version of a previously published protocol (Ueno et al., 2013). All imaged flies were mated females, 4–5 days after eclosion. GMR14C08>UAS-ban-SP+GCaMP6f and GMR14C08>UAS-scr-SP+GCaMP6f brains were dissected in ice cold HL3.1 (Feng et al., 2004) and loaded into a recording chamber submerged in HL3.1. A glass microelectrode was used to stimulate one antennal lobe. The microelectrode was approximately one fourth the size of the antennal lobe. The microelectrode delivered a stimulation train of 20 0.12mA pulses at 100 Hz with a pulse width of 1 millisecond (ms) and an inter-pulse interval of 9 ms.
Simultaneously, GCaMP signals were recorded from the dendritic arborizations of the β′2a/β′2mp/β′2mp_bilateral MBONs (identifiable by their distinct morphology). Imaging data was collected at an acquisition rate of 10 Hz (100 millisecond exposure) at a 512x512 resolution using an Olympus microscope with a 40x objective. Only MBON dendrites ipsilateral to the stimulated Antennal Lobe were included in analysis. A ΔF/F0 was calculated for the baseline period (5 s before stimulus onset) and the max calcium response during antennal lobe stimulation. Statistical analysis (two-way ANOVA with Sidak post hoc test) was performed using GraphPad Prism.
Quantification and statistical analysis
Statistical analysis was performed using GraphPad Prism. Information about statistical analysis (statistical tests, quantifications and n-values) for individual experiments may be found in their respective figure legends and method details. n-value refers to the number of flies employed per condition per experiment.
Acknowledgments
This work was supported by P01NS090994 and R01MH067284 to LCG. MH and EJRR were supported by T32MH019929. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40 OD018537) were used in this study. We thank Michael Rosbash for support and critical comments on this work.
Author contributions
M.H. and L.C.G. designed the study. M.H., K.D., M.A., E.J.R.R., E.A.K., and D.M. performed experiments and analyzed data. M.H. wrote the original draft and M.H. and L.C.G. edited with input from all authors.
Declaration of interests
The authors declare no competing interests.
Published: September 16, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104874.
Supplemental information
Data and code availability
-
•
The datasets supporting this manuscript are available on request directed to the lead contact.
-
•
This paper does not report original code.
-
•
The RNA seq data have been deposited at GEO (accession number GEO: GSE208649). Any additional information required to reanalyze the data reported in this paper is available from the lead contact on request.
References
- Agarwal V., Subtelny A.O., Thiru P., Ulitsky I., Bartel D.P. Predicting microRNA targeting efficacy in Drosophila. Genome Biol. 2018;19:152. doi: 10.1186/s13059-018-1504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosto J., Choi J.C., Parisky K.M., Stilwell G., Rosbash M., Griffith L.C. Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat. Neurosci. 2008;11:354–359. doi: 10.1038/nn2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Anzi B., Zinn K. Identification and characterization of mushroom body neurons that regulate fat storage in Drosophila. Neural Dev. 2018;13:18. doi: 10.1186/s13064-018-0116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y., Grübel K., Busch S., Friedrich A.B., Siwanowicz I., Tanimoto H. The mushroom body of adult Drosophila characterized by GAL4 drivers. J. Neurogenet. 2009;23:156–172. doi: 10.1080/01677060802471718. [DOI] [PubMed] [Google Scholar]
- Aso Y., Hattori D., Yu Y., Johnston R.M., Iyer N.A., Ngo T.T.B., Dionne H., Abbott L.F., Axel R., Tanimoto H., Rubin G.M. The neuronal architecture of the mushroom body provides a logic for associative learning. Elife. 2014;3:e04577. doi: 10.7554/eLife.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y., Sitaraman D., Ichinose T., Kaun K.R., Vogt K., Belliart-Guérin G., Plaçais P.Y., Robie A.A., Yamagata N., Schnaitmann C., et al. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. Elife. 2014;3:e04580. doi: 10.7554/eLife.04580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Roy J.K. Dicer-1 regulates proliferative potential of Drosophila larval neural stem cells through bantam miRNA based down-regulation of the G1/S inhibitor Dacapo. Dev. Biol. 2017;423:57–65. doi: 10.1016/j.ydbio.2017.01.011. [DOI] [PubMed] [Google Scholar]
- Barnstedt O., Owald D., Felsenberg J., Brain R., Moszynski J.P., Talbot C.B., Perrat P.N., Waddell S. Memory-relevant mushroom body output synapses are cholinergic. Neuron. 2016;89:1237–1247. doi: 10.1016/j.neuron.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becam I., Rafel N., Hong X., Cohen S.M., Milán M. Notch-mediated repression of bantam miRNA contributes to boundary formation in the Drosophila wing. Development. 2011;138:3781–3789. doi: 10.1242/dev.064774. [DOI] [PubMed] [Google Scholar]
- Bischof J., Maeda R.K., Hediger M., Karch F., Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J., Hipfner D.R., Stark A., Russell R.B., Cohen S.M. Bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- Chakravarti Dilley L., Szuperak M., Gong N.N., Williams C.E., Saldana R.L., Garbe D.S., Syed M.H., Jain R., Kayser M.S. Identification of a molecular basis for the juvenile sleep state. Elife. 2020;9:e52676. doi: 10.7554/eLife.52676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.F., Lowe S., Lamaze A., Krätschmer P., Jepson J. Neurocalcin regulates nighttime sleep and arousal in Drosophila. Elife. 2019;8:e38114. doi: 10.7554/eLife.38114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.W., Wardill T.J., Sun Y., Pulver S.R., Renninger S.L., Baohan A., Schreiter E.R., Kerr R.A., Orger M.B., Jayaraman V., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong X., Wang H., Liu Z., He C., An C., Zhao Z. Regulation of sleep by insulin-like peptide system in Drosophila melanogaster. Sleep. 2015;38:1075–1083. doi: 10.5665/sleep.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A., Sehgal A. Genetic analysis of sleep. Genes Dev. 2010;24:1220–1235. doi: 10.1101/gad.1913110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djagaeva I., Doronkin S. COP9 limits dendritic branching via Cullin3-dependent degradation of the actin-crosslinking BTB-domain protein Kelch. PLoS One. 2009;4:e7598. doi: 10.1371/journal.pone.0007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelson N.C., Kim E.Z., Slawson J.B., Vecsey C.G., Huber R., Griffith L.C. High-resolution positional tracking for long-term analysis of Drosophila sleep and locomotion using the "tracker" program. PLoS One. 2012;7:e37250. doi: 10.1371/journal.pone.0037250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Ueda A., Wu C.F. A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J. Neurogenet. 2004;18:377–402. doi: 10.1080/01677060490894522. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin F., Coulom H., Meller M., Gomez D., Hirsh J., Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Fulga T.A., McNeill E.M., Binari R., Yelick J., Blanche A., Booker M., Steinkraus B.R., Schnall-Levin M., Zhao Y., DeLuca T., et al. A transgenic resource for conditional competitive inhibition of conserved Drosophila microRNAs. Nat. Commun. 2015;6:7279. doi: 10.1038/ncomms8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner F., Kołodziejczyk A., Yoshii T., Rieger D., Nässel D.R., Helfrich-Förster C. GABA(B) receptors play an essential role in maintaining sleep during the second half of the night in Drosophila melanogaster. J. Exp. Biol. 2013;216:3837–3843. doi: 10.1242/jeb.085563. [DOI] [PubMed] [Google Scholar]
- Gong Z.F., Xia S.Z., Liu L., Feng C.H., Guo A.K. Operant visual learning and memory in Drosophila mutants dunce, amnesiac and radish. J. Insect Physiol. 1998;44:1149–1158. doi: 10.1016/s0022-1910(98)00076-6. [DOI] [PubMed] [Google Scholar]
- Goodwin P.R., Meng A., Moore J., Hobin M., Fulga T.A., Van Vactor D., Griffith L.C. MicroRNAs regulate sleep and sleep homeostasis in Drosophila. Cell Rep. 2018;23:3776–3786. doi: 10.1016/j.celrep.2018.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada F.N., Rosenzweig M., Kang K., Pulver S.R., Ghezzi A., Jegla T.J., Garrity P.A. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks J.C., Finn S.M., Panckeri K.A., Chavkin J., Williams J.A., Sehgal A., Pack A.I. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Huang X., Shi L., Cao J., He F., Li R., Zhang Y., Miao S., Jin L., Qu J., Li Z., Lin X. The sterile 20-like kinase tao controls tissue homeostasis by regulating the hippo pathway in Drosophila adult midgut. J. Genet. Genomics. 2014;41:429–438. doi: 10.1016/j.jgg.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Hudson A.M., Cooley L. Drosophila Kelch functions with Cullin-3 to organize the ring canal actin cytoskeleton. J. Cell Biol. 2010;188:29–37. doi: 10.1083/jcb.200909017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A.M., Mannix K.M., Cooley L. Actin cytoskeletal organization in Drosophila germline ring canals depends on kelch function in a cullin-RING E3 ligase. Genetics. 2015;201:1117–1131. doi: 10.1534/genetics.115.181289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenett A., Rubin G.M., Ngo T.T.B., Shepherd D., Murphy C., Dionne H., Pfeiffer B.D., Cavallaro A., Hall D., Jeter J., et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner W.J., Crocker A., White B.H., Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- Jonas S., Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015;16:421–433. doi: 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- Kelso R.J., Hudson A.M., Cooley L. Drosophila Kelch regulates actin organization via Src64-dependent tyrosine phosphorylation. J. Cell Biol. 2002;156:703–713. doi: 10.1083/jcb.200110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- Kobler J.M., Rodriguez Jimenez F.J., Petcu I., Grunwald Kadow I.C. Immune receptor signaling and the mushroom body mediate post-ingestion pathogen avoidance. Curr. Biol. 2020;30:4693–4709e3. doi: 10.1016/j.cub.2020.09.022. [DOI] [PubMed] [Google Scholar]
- Kunst M., Hughes M.E., Raccuglia D., Felix M., Li M., Barnett G., Duah J., Nitabach M.N. Calcitonin gene-related peptide neurons mediate sleep-specific circadian output in Drosophila. Curr. Biol. 2014;24:2652–2664. doi: 10.1016/j.cub.2014.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lerner I., Bartok O., Wolfson V., Menet J.S., Weissbein U., Afik S., Haimovich D., Gafni C., Friedman N., Rosbash M., Kadener S. Clk post-transcriptional control denoises circadian transcription both temporally and spatially. Nat. Commun. 2015;6:7056. doi: 10.1038/ncomms8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis L.P.C., Siju K.P., Aso Y., Friedrich A.B., Bulteel A.J.B., Rubin G.M., Grunwald Kadow I.C. A higher brain circuit for immediate integration of conflicting sensory information in Drosophila. Curr. Biol. 2015;25:2203–2214. doi: 10.1016/j.cub.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Li H., Horns F., Wu B., Xie Q., Li J., Li T., Luginbuhl D.J., Quake S.R., Luo L. Classifying Drosophila olfactory projection neuron subtypes by single-cell RNA sequencing. Cell. 2017;171:1206–1220.e22. doi: 10.1016/j.cell.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Li Y., He J., Guan Q., Chen R., Yan H., Zheng W., Song K., Cai H., Guo Y., et al. Robust transcriptional signatures for low-input RNA samples based on relative expression orderings. BMC Genom. 2017;18:913. doi: 10.1186/s12864-017-4280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D., Przybylski D., Abruzzi K.C., Schlichting M., Li Q., Long X., Rosbash M. A transcriptomic taxonomy of Drosophila circadian neurons around the clock. Elife. 2021;10:e63056. doi: 10.7554/eLife.63056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z., Davis R.L. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front. Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S.E., Le P.T., Osborn A.J., Matsumoto K., Davis R.L. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Owald D., Felsenberg J., Talbot C.B., Das G., Perisse E., Huetteroth W., Waddell S. Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron. 2015;86:417–427. doi: 10.1016/j.neuron.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevopoulou M.D., Georgakilas G., Kostoulas N., Vlachos I.S., Vergoulis T., Reczko M., Filippidis C., Dalamagas T., Hatzigeorgiou A.G. DIANA-microT web server v5.0: service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013;41:W169–W173. doi: 10.1093/nar/gkt393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish J.Z., Xu P., Kim C.C., Jan L.Y., Jan Y.N. The microRNA bantam functions in epithelial cells to regulate scaling growth of dendrite arbors in drosophila sensory neurons. Neuron. 2009;63:788–802. doi: 10.1016/j.neuron.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C., Allada R. Cul3 and the BTB adaptor insomniac are key regulators of sleep homeostasis and a dopamine arousal pathway in Drosophila. PLoS Genet. 2012;8:e1003003. doi: 10.1371/journal.pgen.1003003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer B.D., Truman J.W., Rubin G.M. Using translational enhancers to increase transgene expression in Drosophila. Proc. Natl. Acad. Sci. USA. 2012;109:6626–6631. doi: 10.1073/pnas.1204520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picelli S., Faridani O.R., Björklund A.K., Winberg G., Sagasser S., Sandberg R. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 2014;9:171–181. doi: 10.1038/nprot.2014.006. [DOI] [PubMed] [Google Scholar]
- Pitman J.L., McGill J.J., Keegan K.P., Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441:753–756. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- Plihal W., Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J. Cogn. Neurosci. 1997;9:534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- Reczko M., Maragkakis M., Alexiou P., Grosse I., Hatzigeorgiou A.G. Functional microRNA targets in protein coding sequences. Bioinformatics. 2012;28:771–776. doi: 10.1093/bioinformatics/bts043. [DOI] [PubMed] [Google Scholar]
- Ren G.R., Hauser F., Rewitz K.F., Kondo S., Engelbrecht A.F., Didriksen A.K., Schjøtt S.R., Sembach F.E., Li S., Søgaard K.C., et al. CCHamide-2 is an orexigenic brain-gut peptide in Drosophila. PLoS One. 2015;10:e0133017. doi: 10.1371/journal.pone.0133017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby J.G., Stark A., Johnston W.K., Kellis M., Bartel D.P., Lai E.C. Evolution, biogenesis, expression, and target predictions of a substantially expanded set of Drosophila microRNAs. Genome Res. 2007;17:1850–1864. doi: 10.1101/gr.6597907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Nakamura A., Texada M.J., Truman J.W., Ishimoto H., Kamikouchi A., Nibu Y., Kume K., Ida T., Kojima M. The nutrient-responsive hormone CCHamide-2 controls growth by regulating insulin-like peptides in the brain of Drosophila melanogaster. PLoS Genet. 2015;11:e1005209. doi: 10.1371/journal.pgen.1005209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer O.T., Keene A.C. The regulation of Drosophila sleep. Curr. Biol. 2021;31:R38–R49. doi: 10.1016/j.cub.2020.10.082. [DOI] [PubMed] [Google Scholar]
- Shaw P.J., Cirelli C., Greenspan R.J., Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Shcherbata H.R., Ward E.J., Fischer K.A., Yu J.Y., Reynolds S.H., Chen C.H., Xu P., Hay B.A., Ruohola-Baker H. Stage-specific differences in the requirements for germline stem cell maintenance in the Drosophila ovary. Cell Stem Cell. 2007;1:698–709. doi: 10.1016/j.stem.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman D., Aso Y., Jin X., Chen N., Felix M., Rubin G.M., Nitabach M.N. Propagation of homeostatic sleep signals by segregated synaptic microcircuits of the Drosophila mushroom body. Curr. Biol. 2015;25:2915–2927. doi: 10.1016/j.cub.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Ori-McKenney K.M., Zheng Y., Han C., Jan L.Y., Jan Y.N. Regeneration of Drosophila sensory neuron axons and dendrites is regulated by the Akt pathway involving Pten and microRNA bantam. Genes Dev. 2012;26:1612–1625. doi: 10.1101/gad.193243.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Xu A.Q., Giraud J., Poppinga H., Riemensperger T., Fiala A., Birman S. Neural control of startle-induced locomotion by the mushroom bodies and associated neurons in Drosophila. Front. Syst. Neurosci. 2018;12:6. doi: 10.3389/fnsys.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K., Naganos S., Hirano Y., Horiuchi J., Saitoe M. Long-term enhancement of synaptic transmission between antennal lobe and mushroom body in cultured Drosophila brain. J. Physiol. 2013;591:287–302. doi: 10.1113/jphysiol.2012.242909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K., Suzuki E., Naganos S., Ofusa K., Horiuchi J., Saitoe M. Coincident postsynaptic activity gates presynaptic dopamine release to induce plasticity in Drosophila mushroom bodies. Elife. 2017;6:e21076. doi: 10.7554/eLife.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Mamiya A., Chiang A.S., Zhong Y. Imaging of an early memory trace in the Drosophila mushroom body. J. Neurosci. 2008;28:4368–4376. doi: 10.1523/JNEUROSCI.2958-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng R., Cohen S.M. Control of Drosophila Type I and Type II central brain neuroblast proliferation by bantam microRNA. Development. 2015;142:3713–3720. doi: 10.1242/dev.127209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggin T.D., Goodwin P.R., Donelson N.C., Liu C., Trinh K., Sanyal S., Griffith L.C. Covert sleep-related biological processes are revealed by probabilistic analysis in Drosophila. Proc. Natl. Acad. Sci. USA. 2020;117:10024–10034. doi: 10.1073/pnas.1917573117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Tabuchi M., Corver A., Duan G., Wu M.N., Kolodkin A.L. Semaphorin 2b regulates sleep-circuit formation in the Drosophila central brain. Neuron. 2019;104:322–337e14. doi: 10.1016/j.neuron.2019.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki D., Hiroi M., Abe T., Shimizu K., Minami-Ohtsubo M., Maeyama Y., Horiuchi J., Tabata T. Two parallel pathways assign opposing odor valences during Drosophila memory formation. Cell Rep. 2018;22:2346–2358. doi: 10.1016/j.celrep.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Yordanova J., Kolev V., Verleger R., Bataghva Z., Born J., Wagner U. Shifting from implicit to explicit knowledge: different roles of early- and late-night sleep. Learn. Mem. 2008;15:508–515. doi: 10.1101/lm.897908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The datasets supporting this manuscript are available on request directed to the lead contact.
-
•
This paper does not report original code.
-
•
The RNA seq data have been deposited at GEO (accession number GEO: GSE208649). Any additional information required to reanalyze the data reported in this paper is available from the lead contact on request.