SUMMARY
Background:
Men with grade group (GG) 2 or 3 prostate cancer are often considered ineligible for active surveillance; of select patients with GG2 prostate cancer who are managed with active surveillance, some will experience early disease progression requiring radical therapy. This study aimed to investigate whether targeted focal therapy can safely reduce treatment burden for patients with localized GG2/3 intermediate- or high-risk prostate cancer.
Methods:
Men aged ≥50 years with unilateral, magnetic resonance imaging (MRI)-visible, primary, intermediate-risk, previously untreated prostate adenocarcinoma (prostate-specific antigen [PSA] ≤20 ng/mL, GG2/3) confirmed on combined biopsy (combining MRI-targeted and systematic biopsies) were eligible for this phase 2b study. MR-guided focused ultrasound (MRgFUS) energy was delivered to the index lesion and planned ≥5-mm margin of normal tissue, using real-time MR thermometry for intraoperative monitoring. Co-primary outcomes were safety (adverse events up to 24 months) and oncologic outcomes (absence of ≥GG2 cancer in the treated area at 6- and 24-month combined biopsy); genitourinary functional outcomes were also assessed. This study is registered with ClinicalTrials.gov, NCT01657942 (no longer recruiting).
Findings:
At 8 U.S. healthcare centers, 101 men were treated with MRgFUS between May 4, 2017 to December 21, 2018. Median age was 63 (IQR 58–67) years; mean PSA was 5.7 (IQR 4.2–7.5) ng/mL. Most cancers were GG2 (78%; 78/101). No serious treatment-related adverse events were reported and only one adverse event >Grade 2 (Grade 3 UTI). At 24 months, 78/89 men (88%; 95% CI 79–94%) had no evidence of GG ≥2 prostate cancer in the treated area.
Interpretation:
MRgFUS focal therapy is safe and effectively treats GG2/3 prostate cancer based on 24-month biopsy outcomes. These results support focal therapy in select patients and support its use in comparative trials to determine if a tissue-preserving approach is effective for delaying or eliminating the need for radical whole-gland treatment long-term.
Funding:
Insightec and the National Cancer Institute.
Introduction
Although prostate cancer is the most common malignancy in men, its disease course varies dramatically. In men with low-risk prostate cancer, predominantly Grade Group 1 (GG1) disease, closely monitoring the cancer using an active surveillance strategy is recommended.1 Conversely, therapeutic strategies for men with intermediate-risk (GG 2/3) prostate cancer have been directed at the whole gland, despite substantial variation in cancer volume, location, and other risk factors within this category. 2Notably, radical prostatectomy or radiation therapy with or without systemic therapy is associated with significant erectile dysfunction in over half of men and up to 10% of men experience long-term stress urinary incontinence.2
In contrast to whole-gland approaches, focal therapy involves selective treatment of visible and biopsy-confirmed areas of malignancy within the prostate, with preservation of normal prostate tissues outside of the treatment margins and surrounding structures. The strategy is to reduce the risk of metastases and preserve quality of life (QoL) by treating only the index tumor; that is, the highest grade tumor with the highest risk of metastasis.3 The emergence of multiparametric magnetic resonance imaging (MRI) and the introduction of ultrasound-MR fusion devices to perform MRI-targeted prostate biopsies have raised the possibility of an organ-sparing, focal therapy approach.4
Novel technologies capable of focal ablation have emerged in recent years, using both thermal and non-thermal energy sources. Among these treatments, high-intensity focused ultrasound (HIFU) has demonstrated safety and feasibility to successfully thermally ablate malignant prostate tissue in early-phase clinical trials and retrospective case series.3,5–8 However, most HIFU trials to date have occurred in single centers, included predominantly low-risk prostate cancer patients, and were performed under ultrasound guidance where treatment areas cannot be directly monitored in real-time. More recently, the TACT study of MR-guided transurethral ultrasound whole-gland ablation, which enrolled men with both low and intermediate-risk prostate cancer, reported that 65% of patients had no evidence of cancer at 1 year.9 The MR-guided focused ultrasound (MRgFUS) system for the prostate used here combines a transrectal ultrasound device for energy delivery with MR imaging of the pelvis to visualize the tumor to be targeted, to monitor therapy with MR thermometry for real-time thermal feedback and control, and to evaluate the ablated tissue immediately after treatment.10 Here we describe the results of a multicenter phase 2b clinical trial of MRgFUS for the focal treatment of intermediate-risk prostate cancer.
Methods
Study design and participants
Men with unilateral, organ-confined, intermediate-risk prostate adenocarcinoma (prostate-specific antigen [PSA] ≤20 ng/mL, GG2 or GG3, tumor classification ≤T2) visible on MRI and confirmed on combined MRI-targeted and systematic biopsy,11 and with no previous treatment for prostate cancer, were eligible for this prospective single-arm multicenter phase 2b study (ClinicalTrials.gov, NCT01657942). Patients with findings suspicious for extracapsular extension on MRI or younger than 50 years were excluded; there was no upper age limit or minimum life expectancy requirement. Treatment was only directed to the GG2 or GG3 cancer focus. Concomitant GG1 prostate cancer elsewhere in the gland was allowed and observed. Independent institutional approval of the study was obtained by each participating research site, and all patients gave written informed consent.
Procedures
The protocol allowed for either transperineal or transrectal MRI-targeted biopsy at baseline, 6 months, and 24 months; however, the same technique used to assess eligibility was required for post-treatment biopsy. Systematic transperineal biopsy was based on a saturation-biopsy template using a 5-mm grid. Systematic transrectal biopsy comprised 14 cores, including two cores directed to the anterior of the prostate gland.11 Targeted sampling included at least two cores directed at the MRI-visible index lesion. All men had pre-therapy computed tomography (CT) scans; patients were excluded if a calcification measuring ≥2 mm was detected by CT within 5 mm of the rectal wall, or calcification measuring >5 mm was located between the target and the sonication array. In addition, patients were not enrolled if the anterior margin of an index lesion was >40 mm from the rectal wall or beyond the focal length of the transducer, as measured on MRI, or if they had a hip arthroplasty distorting the MR images. All participating sites used the same biopsy criteria.
During the MRgFUS procedure, patients underwent general anesthesia and were positioned in the lithotomy position on the MR table, and the transducer was placed. Multiplanar T2-weighted MR imaging was used for planning. The phased-array transducer configuration enabled the system to direct ultrasound energy to the desired location within the prostate based on real-time thermometry MR images acquired during sonication. Acoustic energy was sequentially titrated to temperatures sufficient for tissue ablation (approximately 60–70 °C) guided by real-time MR-based temperature feedback of the treated region. Between each sonication, updated anatomic imaging was acquired to allow for intra-operative modification of the treatment plan to account for treatment-induced changes in the gland volume. Sonications swept across the region of treatment slice-by-slice through the prostate gland, with sonication repeated on each axial slice until the user-defined tumor and treatment margin were covered by thermal dose. This MRgFUS acoustic energy was delivered to the MRI-visible GG2 or GG3 lesion including a planned ≥5-mm margin of surrounding, normal-appearing tissue. The MRI-visible lesion was defined as having Prostate Imaging Reporting and Data System (PIRADS) score ≥3.
All patients underwent combined MRI-targeted and systematic prostate biopsy at 6 and 24 months post-procedure; these biopsies also included at least two cores aimed at the ablated area. Safety of the MRgFUS therapy was assessed with standard adverse event reporting at each follow-up visit (at 1 week, 1,3, 6, 9, 12, 18, and 24 months). Adverse events were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Details of the ExAblate MRgFUS device (Insightec, Miami, FL) and treatment protocol are described in the appendix p. 1.
Outcomes
The primary endpoint for oncologic efficacy was defined as absence of GG ≥2 cancer in the treated area on prostate biopsy at 6 and 24 months post-treatment. Patients who met criteria for failure (GG≥2 on 6 or 24-month biopsy) and underwent radical prostatectomy or radiotherapy exited the study but were included in the final analysis. Where biopsy results were not available at 24 months and GG ≥2 cancer had been found in the treated area at 6 months, 24-month results were assumed to be GG ≥2. All biopsy results were reviewed by dedicated genitourinary pathologists. Pathology was also reviewed centrally at a core pathology lab, and a single pathologist at MSK was designated to confirm Gleason grading if a discrepancy was found between the treatment site and the core lab..
Statistical analysis
The primary endpoints of the trial were oncologic efficacy (6 and 24-month MRI-targeted and systematic biopsy in the treated region) and safety-based standard adverse event reporting at 24 months post-treatment. The secondary endpoints used validated surveys to assess genitourinary functional outcomes and overall QoL measured at baseline and periodically until 24 months after treatment. Urinary function was measured with the International Prostate Symptom Score (IPSS) and the International Consultation on Incontinence Questionnaire - Urinary Incontinence Short Form (ICIQ-UI SF).12,13 Erectile function was measured using the International Index of Erectile Function-15 (IIEF-15).14 Health-related QoL was evaluated using the Functional Assessment of Cancer Therapy - Prostate (FACT-P).15
To assess changes in genitourinary functional outcomes, QoL, and PSA, we used generalized estimating equations (GEE) regression to estimate the mean change from baseline scores along with 95% confidence intervals. We specified these models using exchangeable correlation structure. We described changes in erectile function post-treatment by stringently defining functional erections as IIEF score ≥24.16 We collected data on the use of medications or devices to support sexual function as recommended by the International Consortium for Health Outcomes Measurement; post-treatment erectile dysfunction (ED) was defined based on the CTCAE version 4.03. Grade 0 ED was defined as IIEF score ≥24 or ≤4-point decrease from baseline with no change in medication status. Grade 1 ED was defined as IIEF score ≥11 (moderate ED) without initiating medications or devices to support sexual function. Grade 2 ED was defined as moderate ED supported by medication initiated post-treatment. Grades 0, 1 and 2 ED were considered “good erectile function.” Grade 3 ED was defined as IIEF score <11 independent of whether medication was initiated post-treatment. Similarly, we report longitudinal GEE probability estimates for urinary continence, defined as ICIQ-UI score <10.
The original sample size called for 40 patients, based on a one-stage phase 2 design with null and alternative proportions of 60% and 80% free of GG ≥2 cancer in the treated area, and a decision rule of 30 responders at 6 months. After institutional review board approval at multiple institutions, the FDA’s 510K study guidance mandated expanding the sample size to 100 patients to adequately estimate the adverse event profile with clinically meaningful precision, including the incidence of infrequent device- or procedure-related complications. Therefore, the protocol was amended, adding a range of 100–103 subjects to allow any patients in the screening process and who met eligibility requirements to be treated, even if we were approaching our treatment limit. The null and alternative proportions of 60% and 80% free of GG ≥2 cancer in the treated area at 24 months were maintained. Statistical analyses were prepared using R version 4.0.4 with the geepack (v1.3.1), tidyverse (v1.3.1) and gtsummary (v1.5.2) packages.17–20
Role of the funding source
The funders of the study had no role in study design, data analysis, data interpretation, or writing of the report. Insightec played a limited role in the centralized collection and monitoring of data from the sites. The authors had full access to all the data in the study, take complete responsibility for the integrity of the data and the accuracy of the data analysis, and had final responsibility for the decision to submit for publication.
Results
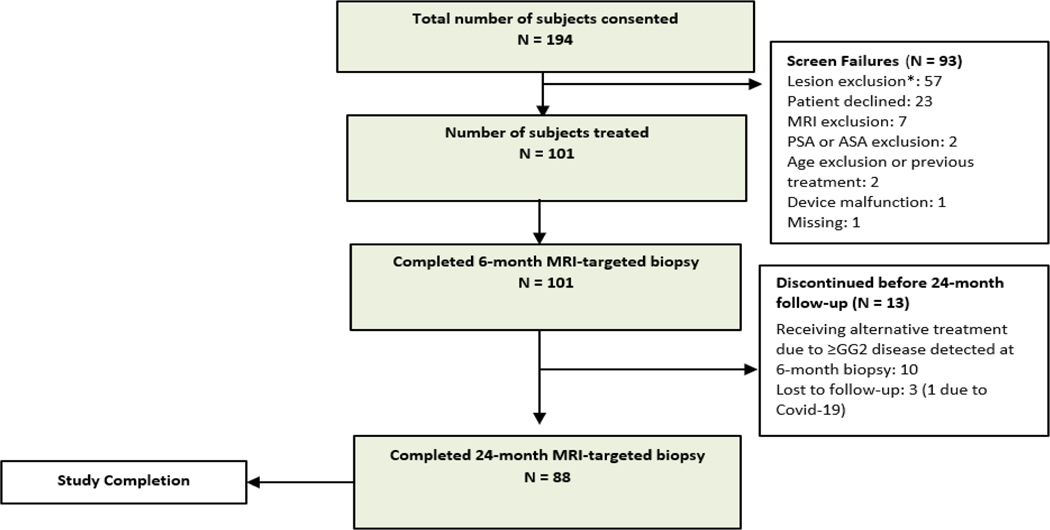
From May 4, 2017 to December 21, 2018, 101 men enrolled on this protocol were treated with MRgFUS at eight healthcare centers in the US (see Figure 1 and appendix p. 2). Baseline characteristics are shown in Table 1. Overall, 53/101 (52%) had treatment in the apex, 81/101 (80%) treatment in mid-gland, 44/101 (44%) treatment in base, and 26/101 (26%) treatment anteriorly directed in the transition zone. The median treatment duration was 110 minutes and includes the time after induction of anesthesia and the patient being positioned before the initial MRI scan until the final sonication before the patient is extubated.
Figure 1. Study CONSORT flow diagram.
*Under screen failures, reasons for “lesion exclusion” included Gleason score, lesion visibility, calcifications, tumor size, or contact with capsule/extracapsular extension.
Table 1.
Patient and treatment characteristics.Statistics presented are median (quartiles) or frequency (%).
|
|
|
|---|---|
| All patients (n = 101) | |
|
| |
| Age, years | 63 (58–67) |
|
| |
| PSA, ng/mL | 5.7 (4.2–7.5) |
|
| |
| Race | |
| White | 87 (86%) |
| Black | 7 (6.9%) |
| Asian, Hispanic, or other | 7 (6.9%) |
|
| |
| Clinical classification ≤T1C | 84 (83%) |
|
| |
| Grade group | |
| 2 | 79 (78%) |
| 3 | 22 (22%) |
|
| |
| Baseline patient-reported functional outcomes | |
| Functional erection: IIEF ≥24 | 58 (59%) |
| Urinary continence: ICIQ <10 | 98 (98%) |
|
| |
| Treatment parameters | |
| Duration (min) | 110 (79–141) |
| Number of sonications | 15 (12–18) |
No serious treatment-related adverse events were observed during the study period. Only one Grade 3 adverse event related to the device or procedure was reported, a urinary tract infection, and it resolved within 3 days. Common Grade <3 adverse events were hematuria, reported in 24% (24/101 patients) and urinary retention, experienced by 15% (15/101 patients). Urinary retention was observed immediately post-treatment and resolved within 7 days. One patient experienced a urethral stricture after 90 days that resolved after a single dilation (Table 2).
Table 2.
Adverse events (AEs) associated with MRgFUS procedure. All data shown as frequency (%).
| Adverse Event | N = 101 | |
|---|---|---|
| Grade 1–2 | Grade 3 | |
| Anal/rectal pain | 2 (2.0) | — |
| Bladder spasm | 3 (3.0) | — |
| Bullous dermatitis | 1 (1.0) | — |
| Constipation/bloating | 3 (3.0) | — |
| Deep vein thrombosis | 1 (1.0) | — |
| Diarrhea | 1 (1.0) | — |
| Edema limbs | 2 (2.0) | — |
| Ejaculation disorder | 9 (8.9) | — |
| Erectile dysfunction | 20 (20) | — |
| Fatigue | 8 (7.9) | — |
| Groin/pelvic/suprapubic pain | 3 (3.0) | — |
| Hematospermia | 13 (13) | — |
| Hematuria | 24 (24) | — |
| Hemorrhoidal hemorrhage | 1 (1.0) | — |
| Orchitis | 1 (1.0) | — |
| Paresthesia | 1 (1.0) | — |
| Penile/testicular pain | 13 (13) | — |
| Positional pain | 1 (1.0) | — |
| Proctitis | 1 (1.0) | — |
| Prostatic cyst | 1 (1.0) | — |
| Prostatic pain | 1 (1.0) | — |
| Testicular infection | 1 (1.0) | — |
| Urethral stricture | 1 (1.0) | — |
| Urinary frequency | 9 (8.9) | — |
| Urinary hesitancy | 6 (5.9) | — |
| Urinary incontinence | 18 (18) | — |
| Urinary retention | 15 (15) | — |
| Urinary tract infection | 1 (1.0) | 1 (1.0) |
| Urinary tract pain | 6 (5.9) | — |
| Urinary urgency | 6 (5.9) | — |
| Vertigo | 1 (1.0) | — |
No grade 4 or 5 events occurred in the study population.
Overall, 96/101 men (95%; 95% CI 89% to 98%) had no evidence of GG ≥2 prostate cancer on 6-month MRI-targeted and systematic biopsy in the treated area of the prostate gland and 78/89 men (88%; 95% CI 79% to 94%) had no evidence of GG ≥2 in the treated area on 24-month biopsy (Table 3). One case with GG ≥2 cancer in the treated area at 6 months and no 24-month result was assumed to be GG ≥2 at 24 months. Our findings met the original prespecified criteria to establish effectiveness: the lower bound of the 95% CI was greater than 60% for the proportion of biopsies negative for GG ≥2 at 24 months and the observed rate exceeded 80%. Among the 11 men with GG ≥2 cancer detected in the treatment area, 3 had GG ≥4 cancer. Overall, 59/98 men (60%; 95% CI 50% to 70%) had no evidence of GG ≥2 cancer anywhere in the prostate gland on 24-month combined MRI-targeted and systematic biopsy (Table 3 and appendix p. 3). Nine cases with GG ≥2 cancer outside of the treatment area at 6 months and no 24-month result were assumed to be GG ≥2 at 24 months.
Table 3.
Detection of prostate cancer from the combined MRI-targeted and systematic biopsy at 6 months and 24 months. (A) Targeted area; (B) whole prostate gland. All data shown as frequency (%).
| A. | |||
|---|---|---|---|
|
|
|||
| 6 months, N=101 | 24 months, N=891 | ||
|
|
|||
| Oncologic efficacy | |||
| No evidence of grade group ≥2 | 96 (95%) | 78 (88%) | |
| Biopsy outcome | |||
| No evidence of cancer | 92 (91%) | 71 (80%) | |
| Grade group 1 | 4 (4.0%) | 7 (7.9%) | |
| Grade group 2 | 4 (4.0%) | 6 (6.7%) | |
| Grade group 3 | 1 (1.0%) | 2 (2.2%) | |
| Grade group 4 | 0 | 1 (1.1%) | |
| Grade group 5 | 0 | 2 (2.2%) | |
|
|
|||
| B. | 6 months, N=101 | 24 months, N=981 | |
|
|
|||
| Oncologic efficacy | |||
| No evidence of grade group ≥2 | 77 (76%) | 59 (60%) | |
| Biopsy outcome | |||
| No evidence of cancer | 41 (41%) | 39 (40%) | |
| Grade group 1 | 36 (36%) | 20 (20%) | |
| Grade group 2 | 18 (18%) | 24 (24%) | |
| Grade group 3 | 3 (3.0%) | 9 (9.2%) | |
| Grade group 4 | 2 (2.0%) | 3 (3.1%) | |
| Grade group 5 | 1 (1.0%) | (3.1%) | |
There was one case with a GG ≥2 result in the treatment region at 6 months and missing data at 24 months; this case was assumed to be GG ≥2 at 24 months.
There were nine cases with GG ≥2 results outside of the treated area at 6 months and missing data at 24 months; these cases were assumed to be GG ≥2 at 24 months.
Serum PSA measurements decreased after treatment and stabilized at six months (n=100) before rising slightly at 24 months (n=60) (appendix p. 4); the mean decrease in PSA measured 6 months after treatment was −3.0 ng/mL (95% CI −3.6 to −2.4) and at 24 months the mean decrease from baseline was −2.6 ng/mL (95% CI −3.3 to −2.0).
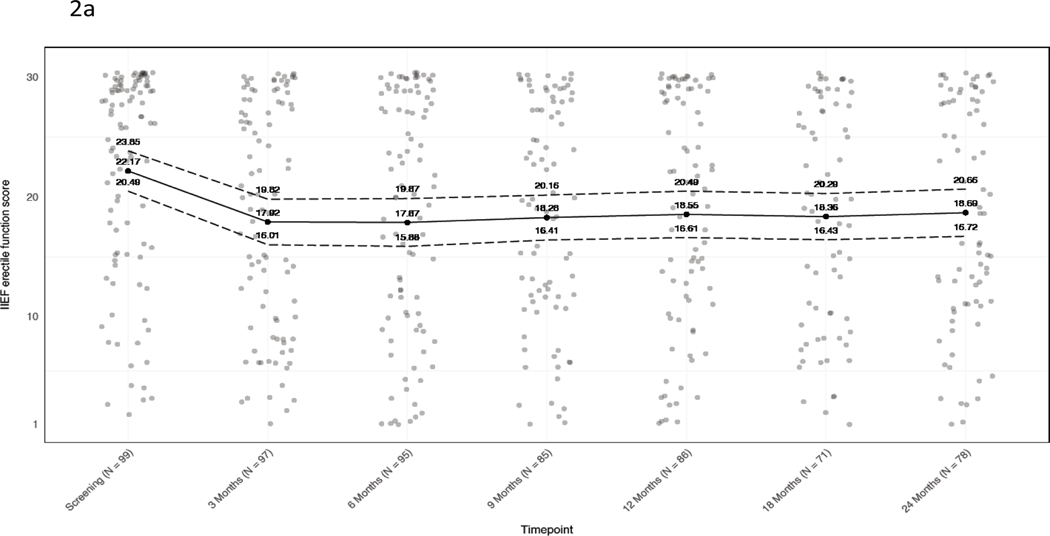
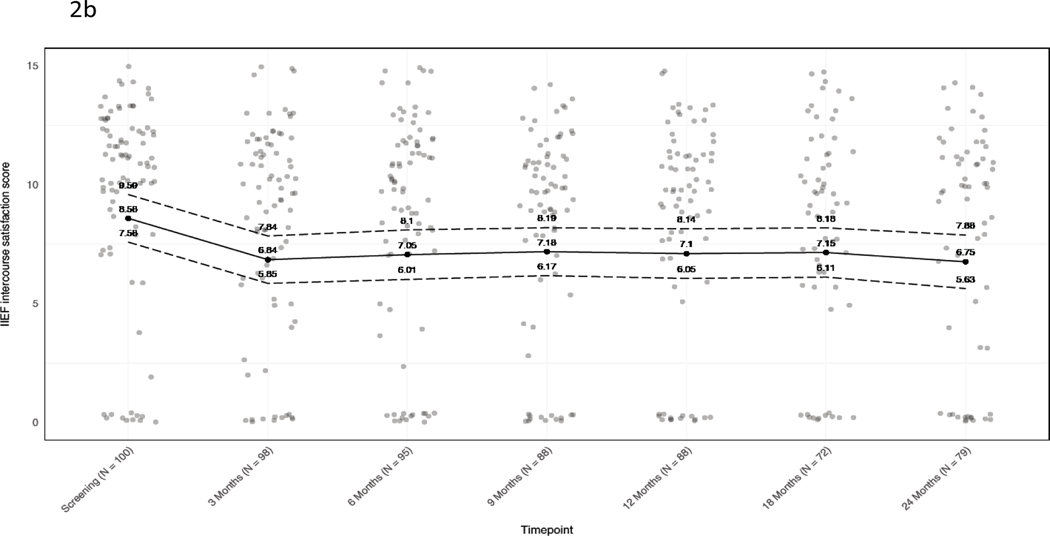
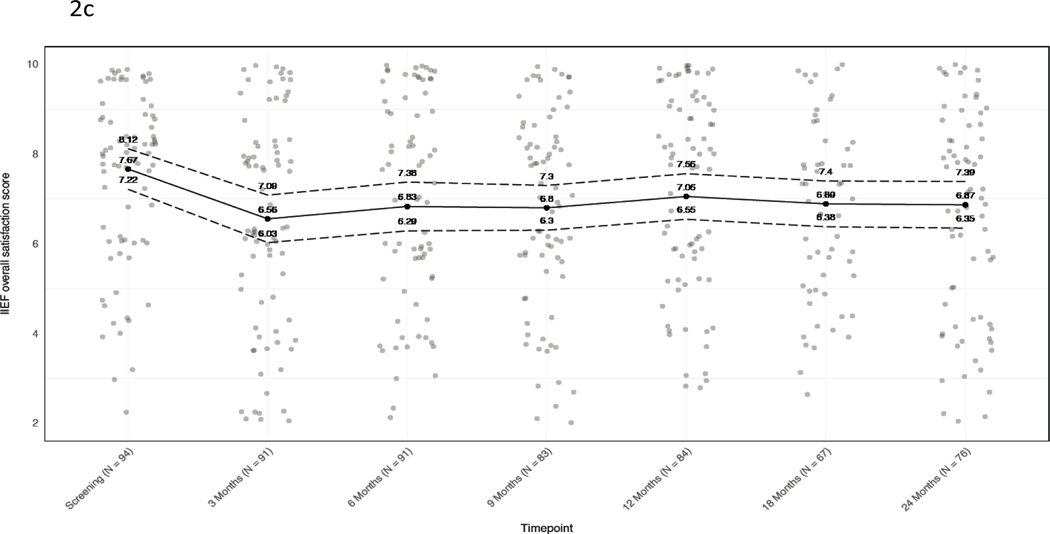
International Index of Erectile Function-15 (IIEF-15) erectile function scores were slightly worse at 24 months (n=40) than at baseline (n=56) (mean score difference of −3.5; 95% CI −5.4 to −1.6), as were mean intercourse satisfaction (−1.8; 95% CI −2.9 to −0.80) and overall satisfaction scores (−0.80; 95% CI −1.3 to −0.26) (Figure 2). Based on a commonly used alternative definition of adequate erections (IIEF question 2 score ≥2), among 91 patients who met that definition at baseline, 69/91 responded on 24-month follow-up survey and 58/69 (84%) achieved erections adequate for intercourse.
Figure 2. Baseline and longitudinal mean IIEF-15 scores.
A) Erectile function. B) Intercourse satisfaction. C) Overall satisfaction. Jitter has been added to mitigate point overlap. Black dots and solid line, mean; dotted lines, 95% confidence intervals. At each timepoint, values are given for the mean and 95% CI.
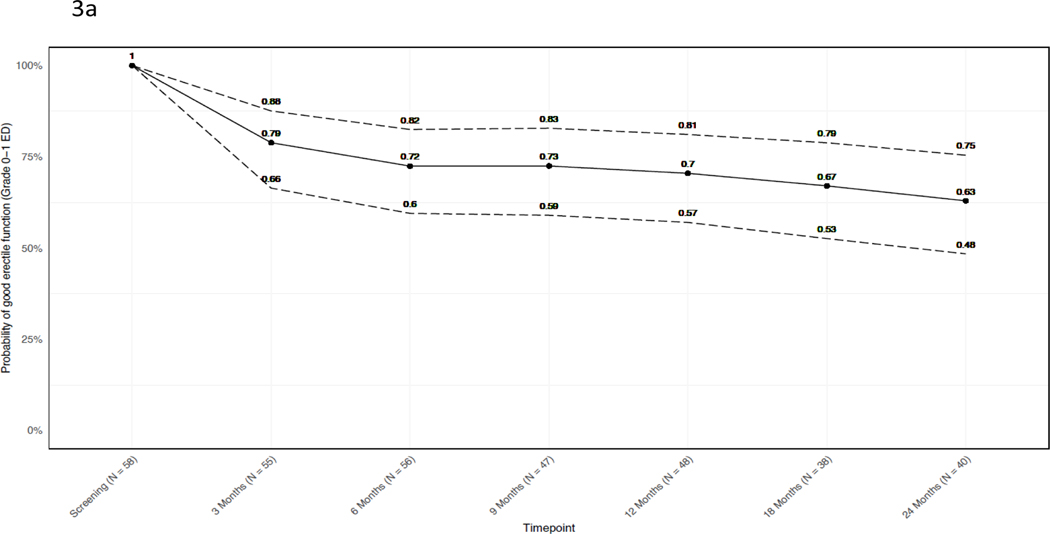
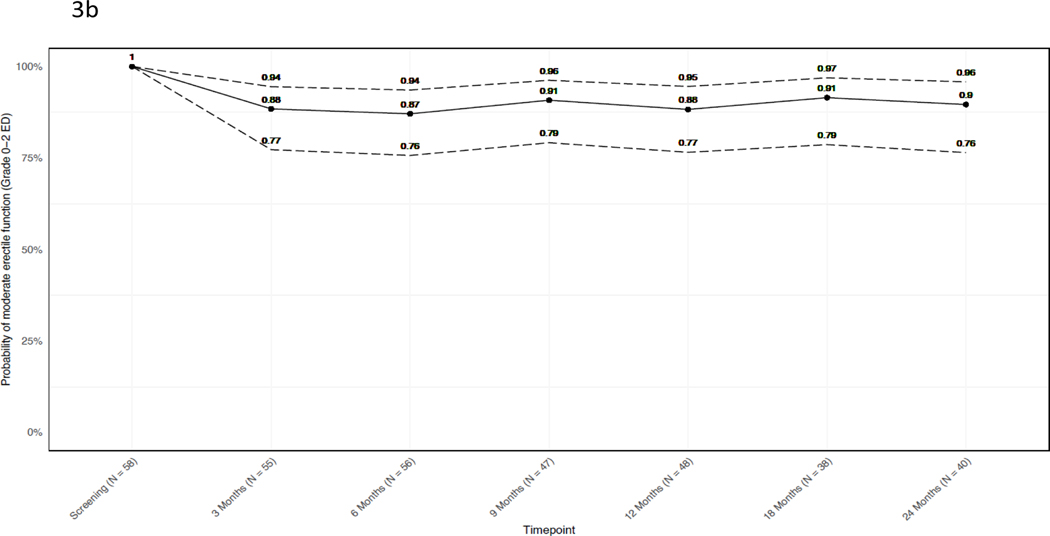
The probability of functional erections decreased slightly over the follow-up period. At 24 months, only 10% (4/40) of patients with functional erections at baseline reported severe or Grade 3 ED. Of the 58 men who reported functional erections at baseline (IIEF ≥24), 40 responded at 24-month follow-up, of whom 18/40 (45%; 95% CI 29 to 62%) reported Grade 0 ED, 7/40 (18%; 95% CI 7% to 33%) reported Grade 1 ED (no change in erectile medications), 11/40 (28%; 95% CI 15% to 44%) reported Grade 2 ED (initiation of erectile medications) (Figure 3), and 4/40 (10%; 95% CI 3% to 24%) reported Grade 3 ED (IIEF <11 regardless of medication). The mean IIEF score difference between baseline and 24 months among subjects with IIEF < 24 at baseline was −0.16 (95% CI −3.2 to 2.8) (appendix p. 5).
Figure 3. Probability of functional erections over time.
A) Patients reporting functional erections (IIEF ≥24 or ≤4-point decrease) or moderate erectile function (IIEF ≥11) without a change in medication status. B) Patients reporting moderate erectile function whether or not medication was initiated. Patients who did not report IIEF ≥24 (n=43) at baseline are excluded. Black dots and solid line, mean; dotted lines, 95% confidence intervals. At each timepoint, values are given for the mean and 95% CI.
Lower urinary tract symptoms (LUTS), assessed by IPSS, were similar at baseline (n=99) and at 24 months (n=79) (mean score difference of 1.1; 95% CI 0.33 to 1.8), as were mean IPSS QoL scores (0.07; 95% CI −0.12 to 0.27). Overall, most patients reported moderate or mild LUTS at baseline and throughout the study period (appendix p. 6). While 18/101 (18%) patients reported Grade ≤2 incontinence, no patient reported stress urinary incontinence requiring pad use throughout the study period. The reported probability of excellent urinary continence, defined as ICIQ-UI score <10, was 100% (n/N) at 24 months post-treatment for those who reported continence by this definition at baseline (appendix p. 7).
FACT-P overall scores were similar at 24 months (n=80) (mean change −2.6; 95% CI −5.6 to 0.4) compared to baseline (n=97).
Discussion
The results of this multicenter, open-label, phase 2 study establish that MRgFUS focal therapy targeting an MRI-visible index lesion using real-time MR thermometry has a low rate of genitourinary adverse events and, based on 6 and 24-month biopsy outcomes, can be used to treat GG2 and GG3 index lesions with a high degree of success. These data support the effectiveness of MRgFUS focal therapy for targeting prostate cancer tissue in adequately selected patients with intermediate-risk prostate cancer seeking to avoid radical whole-gland treatment. We report no serious adverse events associated with MRgFUS treatment, demonstrating its safety as a minimally invasive approach to selectively treat cancer within the prostate gland and preserve adjacent structures critical for urinary and bowel continence as well as erectile function.
By 24 months, no patient had reported urinary incontinence requiring pad use. The probability of functional erections decreased slightly over the follow-up period.Further, although the difference in mean erectile function scores was statistically significant, the small difference should be interpreted across the range of the overall score and considered across the time range of 2 years, in which small decreases in erectile function score are expected without treatment—making this change statistically significant but not clinically significant. These functional outcomes compare very favorably to patient-reported outcomes after whole-gland treatments such as radical prostatectomy and radiation therapy, which, while effective, are associated with significant and persistent side effects that impact QoL.2 In an observational study including men with favorable-risk localized prostate cancer enrolled in population-based registries in the United States, only 28% of these men reported erections sufficient for intercourse 1 year after nerve-sparing radical prostatectomy and only 51% after external beam radiation therapy.2 Fifty percent of men in that study with favorable-risk prostate cancer reported urinary leakage requiring pad use 1 year after radical prostatectomy.
The oncologic outcomes of focal therapy targeting prostate cancer using ultrasound-guided HIFU have been studied in a few single-arm trials and retrospective case reports. A single-arm study involving 42 men with low- and intermediate-risk prostate cancer demonstrated no evidence of cancer after HIFU in 77%, while 92% were free of GG ≥2 cancer at 6 months post-treatment.5 Another single-arm clinical trial that used HIFU to focally treat index tumors in 56 patients with low- and intermediate-risk prostate cancer demonstrated 65% of men had no evidence of cancer in the treated area.3 A single-institution registry enrolling 72 patients with low- and intermediate-risk prostate cancer who underwent hemi-gland HIFU treatment reported 84% of patients had no evidence of cancer in the targeted area.6 A multicenter registration study of 625 men treated with HIFU, with median follow-up of 56 months, reported 98% of patients were metastasis-free at 5 years. This cohort described by Guillaumier et al. differed from our study because it included approximately 30% of patients with low-risk prostate cancer and allowed retreatment with HIFU for clinically significant prostate cancer detected in biopsies within or outside of the original treatment area. Also, unlike our study in which every patient underwent post-treatment biopsy to assess disease recurrence, Guillaumier et al. relied on imaging and clinical characteristics to trigger biopsy, such that only 36% (222/625) of men in their study underwent post-treatment prostate biopsy.7 Similarly, in a retrospective study of 1032 prostate cancer patients treated with either focal or hemi-ablation, Stabile et al. reported that only 41% (424/1032) of patients underwent post-treatment biopsy and 49% (208/424) of these patients had GG ≥2 prostate cancer post-treatment.8
In comparison to these prior clinical trials and observational studies, our study had a higher rate of success in treating cancer in the targeted region. Several factors may explain this difference. First, the previous studies were conducted using an ultrasound-guided device, which lacks the ability of MRI both to delineate the tumor target accurately and to provide precise real-time monitoring of the treatment effect by MR thermometry. The MRgFUS device is a closed-loop system that combines a transrectal phased-array transducer to guide ultrasound waves using high-resolution anatomic MR imaging and real-time MR thermometry for intra-operative treatment verification. Second, as part of patient selection, the men enrolled in our study underwent systematic biopsy and either MRI-targeted prostate biopsy or in-gantry MRI-guided prostate biopsy for selection, and treatment was imaging-guided to a region of interest on MRI confirmed to be the index cancer. Third, studies comparing three-dimensional software-based registration of MRI and whole-mount pathology specimens after radical prostatectomy report that MRI underestimates histologically determined tumor boundaries.21 Our treatment planning included a treatment margin around the tumor of at least 5 mm and up to 10 mm—confirmed during real-time MR treatment planning—to enhance the probability of treating the entire histological tumor volume during focal ablative therapy.
Achieving successful oncologic outcomes for patients treated with focal therapy is dependent not just on expertise in the technique used for treatment, but also on appropriate patient selection. At 6-month biopsy, 19/101 men (19%; 95% CI 12 to 28%) had newly detected GG ≥2 cancer outside of the treatment area only. Given the short interval between biopsies, rather than representing new sites of cancer, these men most likely harbored these additional undetected cancers prior to treatment. This is consistent with prior retrospective data demonstrating that up to 20% of prostate cancer foci measuring less than 1 cm can be missed on MRI-guided targeted and systematic template biopsy.4 Although the long-term clinical significance of these newly detected low-volume GG2 or GG3 tumors is unknown, a role for saturation systematic-template prostate biopsy combined with MR-targeted biopsy cores may exist to minimize short-term treatment failure after focal therapy.11
Our study had three key strengths. First, we conducted a prospective clinical trial with oncologic outcomes based on protocol-mandated imaging-guided prostate biopsy and longitudinal data collection assessing QoL. The participation of multiple institutions, including both academic centers and a private health system, improved the generalizability of these results. Second, our study only enrolled patients with intermediate-risk prostate cancer for whom treatment is considered necessary but avoidance of radical prostatectomy or radiation therapy would reduce morbidity. Third, our results compare favorably to other prospective focal therapy trials, as 88% of patients had no clinically significant cancer (GG ≥2) after treatment in the targeted area and 60% overall were observed to not have clinically significant prostate cancer detected anywhere within the prostate gland; thereby avoiding whole-gland treatment for at least 24 months post-MRgFUS treatment.
Our study had two important limitations. First, 24-month biopsy is not a sufficient surrogate endpoint for metastases or cancer-specific death. However, the aim of the study was to evaluate if using MRgFUS focal therapy can avoid whole-gland treatment based on biopsy outcomes post-treatment; the detection of metastases is unlikely in an intermediate-risk prostate cancer cohort during the 2-year study period. Second, in the absence of a comparative group of patients with intermediate-risk prostate cancer randomized to active surveillance, we cannot estimate the long-term clinical benefit of treating these men rather than following them on an active surveillance protocol. However, among patients with GG2 prostate cancer managed with active surveillance, observational studies report that approximately 40% experience disease progression to higher-grade cancer requiring definitive treatment after a median follow-up of 3–4 years.22,23 Based on this and contemporary treatment trends, most of these men would have undergone treatment with surgery or radiation if they had not been treated with MRgFUS. In our study, GG3 or higher-grade prostate cancer was detected in only 15% of patients by 24 months after treatment.
In conclusion, MRgFUS focal therapy targeting an MRI-visible index lesion using real-time MR thermometry has a low rate of genitourinary adverse events and, based on 24-month biopsy outcomes, can be used to treat GG2 and GG3 index lesions with a high degree of success. These data support the effectiveness of MRgFUS focal therapy for targeting prostate cancer tissue in adequately selected patients with intermediate-risk prostate cancer seeking to avoid radical whole-gland treatment.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed, with no language or date restrictions, on March 23, 2022, for all prior observational studies or clinical trials using the terms ([“prostate cancer”] AND [“focal” OR “partial gland ablation”] AND [“therapy” OR “treatment”]). Studies typically reported single-institutional retrospective data or observational registries. Overall, studies were highly heterogeneous in design and findings, including the cancer risk eligibility, follow-up duration, and outcome assessment. Most prospective trials were single-institution, had small sample sizes, and did not mandate post-treatment biopsy to assess efficacy. Further, most prospective studies included men with low-risk cancer for whom contemporary guidelines recommend active surveillance. Notably, the TACT study enrolled men with intermediate-risk prostate cancer treated with whole-gland ablation, using an MR-guided transurethral ultrasound device capable of focal therapy.
Added value of this study
To our knowledge, this is the first phase 2 study evaluating outcomes of imaging-guided focal therapy to treat intermediate-risk prostate cancer. Our study provides longitudinal self-reported quality-of-life data across multiple domains, including sexual/erectile function, urinary continence, and bothersome symptoms to better inform physicians to counsel patients. We provide complete post-treatment prostate biopsy results in all patients to enable more accurate estimates of the durability of focal therapy after 6-month biopsy results. In addition, we evaluate the outcomes of an imaging-guided treatment, MR-guided focused ultrasound (MRgFUS), that incorporates real-time MR thermometry to continually monitor treatment effect, which is lacking from other technologies used to treat prostate cancer.
Implications of all the available evidence
Our study demonstrates that MRgFUS focal therapy of intermediate-risk prostate cancer is safe and that quality-of-life outcomes compare favorably to studies of whole-gland treatments such as radical prostatectomy and radiation therapy. We provide longitudinal estimates of sexual and urinary function post-treatment to help physicians counsel patients. Our findings met the study’s prespecified criteria to establish effectiveness based on the proportion of negative biopsy outcomes at 24 months. These results support MRgFUS focal therapy in select patients and pursuit of a comparative phase 3 trial to determine if a tissue-preserving approach that maintains quality of life will delay or eliminate the need for radical whole-gland treatment long-term for patients with intermediate-risk prostate cancer.
Acknowledgments
This study was primarily supported by Insightec, with additional support to NCI-designated cancer center sites provided by NIH/NCI Cancer Center Support Grants P30 CA006516 (Dana-Farber/Brigham and Women’s), P30 CA015083 (Mayo Clinic), P30 CA008748 (MSK), P30 CA124435 (Stanford), P30 CA016042 (UCLA), and P30 CA044579 (University of Virginia). In addition, the Memorial Sloan Kettering (MSK) authors acknowledge the support of MSK’s Sidney Kimmel Center for Prostate and Urologic Cancers, and P.T. Scardino acknowledges the support of the David Koch Foundation and MSK’s Department of Surgery.Drs. James Eastham and Hedvig Hricak provided leadership during the planning phase of the study. Dr. Samson Fine helped define pathologic endpoints. All individuals who need to be acknowledged in this paper are listed above.
Footnotes
Declaration of interests
BE attends the medical advisory board of Insightec as an unpaid consultant, and has previously received consulting funds from Myriad Genetics. CMT has received consulting funds from Profound. DDS has received consulting funds from OPKO Health and Steba. ASK is on the medical advisory board of Insightec, Profound, and Janssen, and has received consulting funds from Advantagene DSMC, Bristol Myers Squibb, Merck, Bayer, and General Electric. QT has received consulting funds from Astellas, Bayer, Intuitive Surgical, and Janssen. JCD is the Chief Clinical Officer for Ajax Health and Cordis Accelco and has equity interests in Cordis; he is on the advisory board and has ownership/equity interests in Serpex Health and Adient Medical, and serves as the past chair of the Society of Interventional Radiology Foundation. OA has ownership/equity interests in Ezra AI. AJV is named on a patent for a statistical method to detect prostate cancer that has been commercialized by OPKO Health (from which he receives royalties and stock options) and has received consulting funds from Insightec and Steba. PTS is named on a patent for a statistical method to detect prostate cancer that has been commercialized by OPKO Health (from which he receives royalties and stock options) and chairs the Medical Advisory board of Insightec as an unpaid consultant. DS is the medical director and founder of Sperling Prostate Center, a private facility for prostate cancer treatment in Delray Beach, FL. LAM has collaborative and research agreements with Philips Healthcare, Inc. and Biobot Surgical, Ltd. GAS is on the medical advisory board of miR Scientific. PG is on the medical advisory boards of Insightec and SonALASense and has ownership/equity interests in SonALASense.
All other authors (FH, JYCW, BY, DAW, SSR, AJP, MHS, TDM) declare that they have no financial relationships to disclose.
Data sharing statement
All proposals for data sharing should be submitted to the corresponding author for consideration. Access to deidentified participant data that underlie the results reported in this article will be granted if the proposal is approved, i.e., found to be methodologically sound; use of the data is intended only for the aims in the approved proposal. These data will be made available immediately after publication, with no end date. The study protocol is available to all as part of the supplemental material for this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen RC, Rumble RB, Loblaw DA, Finelli A, Ehdaie B, Cooperberg MR, et al. Active surveillance for the management of localized prostate cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2016;34(18):2182–90. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman KE, Penson DF, Zhao Z, Huang LC, Conwill R, Laviana AA, et al. Patient-reported outcomes through 5 years for active surveillance, surgery, brachytherapy, or external beam radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA. 2020;323(2):149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed HU, Dickinson L, Charman S, Weir S, McCartan N, Hindley RG, et al. Focal ablation targeted to the index lesion in multifocal localised prostate cancer: a prospective development study. Eur Urol. 2015;68(6):927–36. [DOI] [PubMed] [Google Scholar]
- 4.Tan N, Margolis DJ, Lu DY, King KG, Huang J, Reiter RE, et al. Characteristics of detected and missed prostate cancer foci on 3-T multiparametric MRI using an endorectal coil correlated with whole-mount thin-section histopathology. AJR Am J Roentgenol. 2015;205(1):W87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed HU, Hindley RG, Dickinson L, Freeman A, Kirkham AP, Sahu M, et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol. 2012;13(6):622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feijoo ER, Sivaraman A, Barret E, Sanchez-Salas R, Galiano M, Rozet F, et al. Focal high-intensity focused ultrasound targeted hemiablation for unilateral prostate cancer: a prospective evaluation of oncologic and functional outcomes. Eur Urol. 2016;69(2):214–20. [DOI] [PubMed] [Google Scholar]
- 7.Guillaumier S, Peters M, Arya M, Afzal N, Charman S, Dudderidge T, et al. A multicentre study of 5-year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol. 2018;74(4):422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stabile A, Orczyk C, Hosking-Jervis F, Giganti F, Arya M, Hindley RG, et al. Medium-term oncological outcomes in a large cohort of men treated with either focal or hemi-ablation using high-intensity focused ultrasonography for primary localized prostate cancer. BJU Int. 2019;124(3):431–40. [DOI] [PubMed] [Google Scholar]
- 9.Klotz L, Pavlovich CP, Chin J, Hatiboglu G, Koch M, Penson D, et al. Magnetic resonance imaging-guided transurethral ultrasound ablation of prostate cancer. J Urol. 2021;205(3):769–79. [DOI] [PubMed] [Google Scholar]
- 10.Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 1995;34(6):814–23. [DOI] [PubMed] [Google Scholar]
- 11.Norris JM, Carmona Echeverria LM, Bott SRJ, Brown LC, Burns-Cox N, Dudderidge T, et al. What type of prostate cancer is systematically overlooked by multiparametric magnetic resonance imaging? An analysis from the PROMIS cohort. Eur Urol. 2020;78(2):163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry MJ, Fowler FJ Jr, ., O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–57; discussion 64. [DOI] [PubMed] [Google Scholar]
- 13.Lim R, Liong ML, Lim KK, Leong WS, Yuen KH. The minimum clinically important difference of the International Consultation on Incontinence Questionnaires (ICIQ-UI SF and ICIQ-LUTSqol). Urology. 2019;133:91–5. [DOI] [PubMed] [Google Scholar]
- 14.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822–30. [DOI] [PubMed] [Google Scholar]
- 15.Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50(6):920–8. [DOI] [PubMed] [Google Scholar]
- 16.Terrier JE, Mulhall JP, Nelson CJ. Exploring the optimal erectile function domain score cutoff that defines sexual satisfaction after radical prostatectomy. J Sex Med. 2017;14(6):804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halekoh U, Højsgaard S, Yan J. The r package geepack for generalized estimating equations. J Stat Softw. 2006;15(2):1–11. [Google Scholar]
- 18.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. [Google Scholar]
- 19.Sjoberg DD, Whiting K, Curry M, Lavery JA, Larmarange J. Reproducible summary tables with the gtsummary package. The R Journal. 2021;13(1):570–80. [Google Scholar]
- 20.Wickham H, Averick M, Bryan J, Chang W, D’Agostino McGowan L, Francois R, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. [Google Scholar]
- 21.Le Nobin J, Rosenkrantz AB, Villers A, Orczyk C, Deng FM, Melamed J, et al. Image guided focal therapy for magnetic resonance imaging visible prostate cancer: defining a 3-dimensional treatment margin based on magnetic resonance imaging histology co-registration analysis. J Urol. 2015;194(2):364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlsson S, Benfante N, Alvim R, Sjoberg DD, Vickers A, Reuter VE, et al. Risk of metastasis in men with Grade Group 2 prostate cancer managed with active surveillance at a tertiary cancer center. J Urol. 2020;203(6):1117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooperberg MR, Cowan JE, Hilton JF, Reese AC, Zaid HB, Porten SP, et al. Outcomes of active surveillance for men with intermediate-risk prostate cancer. J Clin Oncol. 2011;29(2):228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.