Abstract
In the past 20 years, BRO β-lactamase-producing Moraxella catarrhalis strains have emerged. We show that BRO is expressed as a 33-kDa lipoprotein associated with the inner leaflet of the outer membrane. To our knowledge, this is the first description of a lipidated β-lactamase in a gram-negative species.
In recent years, Moraxella (Branhamella) catarrhalis has emerged as a significant cause of upper respiratory tract infections in children and lower respiratory tract infections in patients with underlying chest disease (2, 4, 14). In addition to being directly pathogenic, M. catarrhalis may be indirectly pathogenic, because it produces penicillin-degrading enzymes (β-lactamases), which may protect concomitantly infecting, more virulent bacteria such as Streptococcus pneumoniae or Haemophilus influenzae from antibiotic therapy (10, 23). While ampicillin-resistant M. catarrhalis strains were first described only 20 years ago (13, 17), over 90% of the M. catarrhalis strains isolated currently worldwide are β-lactamase positive (6, 9). The β-lactamases produced by M. catarrhalis—the BRO-1 type and the BRO-2 type—are distinguishable by their isoelectric-focusing patterns (22). BRO-1-producing strains are more resistant to β-lactam antibiotics than BRO-2 producers, most likely due to higher levels of BRO-1 (1, 9, 22).
In an earlier study, we reported the cloning and sequencing of the genes (bro) coding for M. catarrhalis BRO β-lactamases (1). The bro genes appeared to be located on the chromosome and coded for polypeptides that differed in only one amino acid. BRO production appeared to be constitutive, not affected by the presence of antibiotics (21). Interestingly, we observed that the G+C content of bro (31%) was quite different from that of the rest of the M. catarrhalis genome (41%), suggesting that BRO was derived from another, as-yet-unknown species. In support of this notion, bro was found to encode a signal sequence characteristic for lipoproteins (LTGC), a feature uncharacteristic of β-lactamases occurring in gram-negative bacteria. Here we determine whether BRO is indeed a lipoprotein.
Lipoproteins are synthesized as precursors with N-terminal signal peptides that are modified by lipidation (3). The first step in the lipid modification of such proteins is the addition of a diglyceride to a cysteine residue. In the second step, lipoprotein-specific signal peptidase II cleaves the signal peptide at the amino-terminal side of the modified cysteine, whereupon further fatty acylation of the cysteine residue takes place, and the resultant fully modified lipoprotein is assembled into the membrane (3).
Lipid-modified, membrane-bound β-lactamases have been described for a number of gram-positive organisms. The first membrane-bound enzyme was found in Bacillus cereus (18), and similar β-lactamases have been observed in Bacillus licheniformis (25) and Staphylococcus aureus (15). The membrane-bound forms of gram-positive β-lactamases have been proposed to be the precursors of extracellular enzymes, generated by proteolytic cleavage of the lipid anchor (16, 25). Several studies have indicated that BRO β-lactamases are, at least in part, produced as membrane-bound proteins (7, 20). We have shown that in both M. catarrhalis and Escherichia coli harboring bro, 10 and 45% of β-lactamase activity, respectively, was found in the membrane compartment (1), suggestive of a lipid anchor.
Identification of the bro product in M. catarrhalis and E. coli.
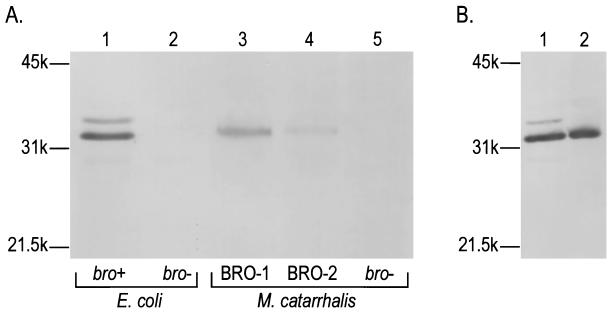
To obtain specific antibodies, BRO-1 was produced in excess as a maltose-binding protein (MBP) fusion protein by using the pMAL-cRI vector (New England Biolabs, Beverly, Mass.). Five monoclonal antibodies (MAbs) directed against BRO were produced after immunization with 20 μg of MBP-BRO according to standard protocols. Enzyme-linked immunosorbent assay identified MAbs PA1 to PA5 as immunoglobulin G type 1 (IgG1) antibodies specific for BRO and not MBP. A mixture of PA1 to PA5 was used in immunoblotting to identify the bro product in cell lysates of both M. catarrhalis and recombinant E. coli. In M. catarrhalis, BRO β-lactamase was identified as a protein with an apparent molecular mass of 33 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Fig. 1A, lanes 3 and 4), in good agreement with the calculated molecular size of 32 kDa (1). No β-lactamase could be detected in M. catarrhalis lacking the bro gene (Fig. 1A, lane 5). The expected higher expression level of BRO-1, based on enzymatic assays, was confirmed by immunoblots, which revealed a more intense band in BRO-1 strains than in BRO-2 strains (Fig. 1A, lanes 3 and 4, respectively). Two protein bands were detected on immunoblots of E. coli harboring bro (Fig. 1A, lane 1) that were absent in E. coli strains lacking bro (Fig. 1A, lane 2). The most intense band had the same mobility as the 33-kDa band detected in M. catarrhalis and was associated with the membrane fraction of E. coli (Fig. 1B, lane 2), indicating that this protein band represented the mature, lipid-modified BRO. The second, minor band may represent a nonlipidated precursor protein including a signal peptide.
FIG. 1.
Immunoblot analysis of BRO β-lactamase expressed by M. catarrhalis and recombinant E. coli strains. (A) Whole-cell lysates of E. coli (1 × 108/lane) and M. catarrhalis (5 × 108/lane). Lane 1, E. coli pMC100 (bro+); lane 2, E. coli pUK21 (vector); lane 3, M. catarrhalis 43618 (BRO-1); lane 4, M. catarrhalis 43617 (BRO-2); lane 5, M. catarrhalis 3.21S (BRO negative). (B) Subcellular localization of BRO in E. coli pMC100. Lane 1, whole-cell fraction; lane 2, outer- plus inner-membrane fractions. The outer- and inner-membrane protein fractions of E. coli harboring bro were obtained from cell-free extracts by centrifugation at 150,000 × g for 60 min at 4°C. All samples were separated on an SDS–12.5% PAGE gel, blotted, and incubated with a mixture of 5 BRO-specific MAbs. Positions of molecular size markers are shown on the left.
Lipid modification of M. catarrhalis BRO β-lactamase.
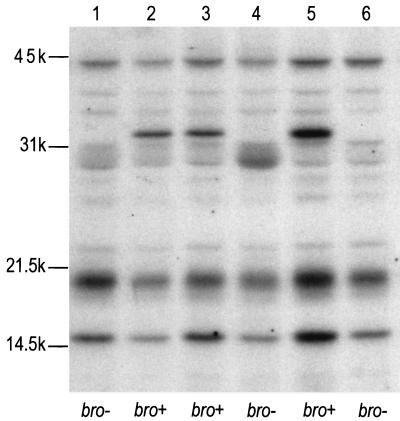
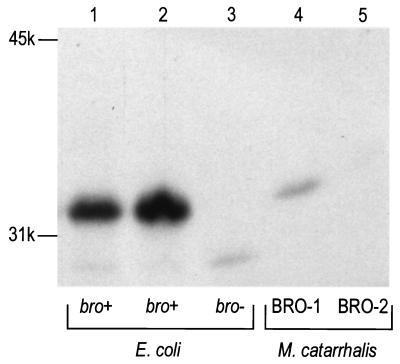
Both the membrane association and the signal peptide sequence of bro suggested that BRO could be a lipoprotein. Lipid modification of M. catarrhalis β-lactamase was initially investigated in E. coli. Logarithmic cultures of E. coli harboring different (sub)-clones of bro were labeled with 50 μCi of [3H]palmitic acid per ml for 1 h. Labeling was stopped by the addition of trichloroacetic acid (TCA) to a final concentration of 10%. Precipitates were then collected by centrifugation, washed with acetone and chloroform-methanol (2:1 [vol/vol]), dried, and dissolved in 50 mM Tris–1% SDS (pH 6.8) by heating at 100°C for 2 min. Subsequently, 20-μl aliquots were analyzed by SDS-PAGE and fluorography (Fig. 2). Since bro was present on a 4-kb HindIII insert in E. coli that contained two other potential open reading frames (orf1 and orf3), a set of subclones was used in which orf1 or orf3 was deleted to confirm that the lipid-modified band was indeed encoded by bro. Whenever an intact bro gene was present in E. coli, [3H]palmitic acid was found to be incorporated into a 33-kDa protein (Fig. 2, lanes 2, 3, and 5). This labeled protein band disappeared when the bro gene was disrupted (Fig. 2, lanes 1, 4, and 6). We could not, however, detect lipid-modified BRO in M. catarrhalis directly, most probably because it was masked by other lipidated proteins of a similar size. Definite proof that lipids were also incorporated in BRO in M. catarrhalis was obtained by immunoprecipitation. TCA precipitates were suspended in 200 μl of 25 mM Tris-HCl (pH 8.0) supplemented with 50 mM glucose and 10 mM EDTA, after which β-lactamase was isolated by using polyclonal antiserum raised against MBP-BRO, coupled to protein G-Sepharose beads (Pharmacia, Uppsala, Sweden) at a concentration of 30 mg of protein G/ml. By this approach, the 33-kDa protein was isolated from both E. coli and M. catarrhalis producing BRO-1 (Fig. 3). Although the lipoprotein signal sequence of the BRO-2 enzyme is identical to that of BRO-1 (1), we did not succeed in isolating [3H]palmitate-labeled BRO-2. Considering the lower level of BRO-2 production, this lack of success was probably due to the limited sensitivity of the detection method.
FIG. 2.
Incorporation of [3H]palmitic acid into BRO β-lactamase. Cells harboring different (sub)clones of bro were labeled with [3H]palmitate for 1 h, collected, and analyzed by SDS-PAGE and fluorography. Lane 1, E. coli pUK21 (vector); lane 2, E. coli pMC100 (orf1+ bro+ orf3+); lane 3, E. coli pMC105 (bro+ orf3+); lane 4, E. coli pMC106 (orf3+); lane 5, E. coli pMC097 (orf1+ bro+); lane 6, E. coli pMC096 (orf1+ orf3). Positions of molecular size markers are indicated on the left.
FIG. 3.
Immunoprecipitation of lipid-modified β-lactamase. BRO β-lactamase was isolated by using polyclonal anti-BRO antiserum and analyzed by SDS-PAGE and fluorography. Lane 1, E. coli pMC100 (orf1+ bro+ orf3+); lane 2, E. coli pMC105 (bro+ orf3+); lane 3, E. coli pMC106 (orf3+); lane 4, M. catarrhalis 43618 (BRO-1); lane 5, M. catarrhalis 43617 (BRO-2). Positions of molecular size markers are shown on the left.
Subcellular localization of M. catarrhalis BRO β-lactamase.
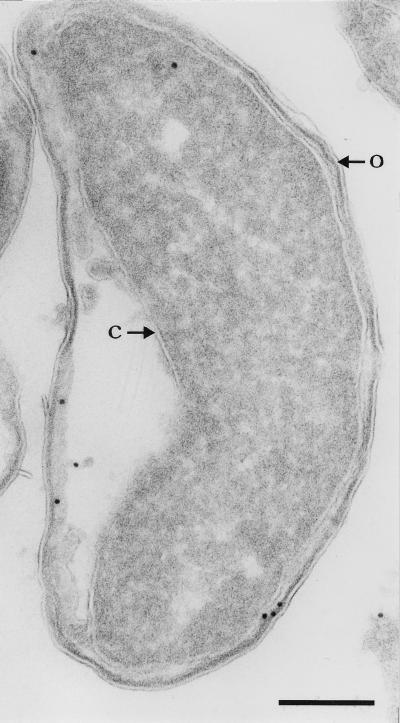
Whether lipoproteins are targeted to the inner or outer membrane is determined mainly by the residue next to the modified cysteine residue (24). Lipoproteins possessing aspartic acid at this position are destined for the inner membrane, whereas an amino acid other than aspartic acid results in outer-membrane localization. In the BRO-1 signal sequence, a lysine is present at the position following the cysteine (1), thus predicting outer-membrane localization of the enzyme. We used immunogold electron microscopy to investigate the subcellular localization of M. catarrhalis β-lactamase in E. coli. To separate the cytoplasmic membrane from the outer membrane, we subjected the bacteria to plasmolysis by incubating them in 0.35 M sucrose (5). Then, the bacteria were pelleted at 1,000 × g and suspended in 2% paraformaldehyde (PFA) and 0.2% glutaraldehyde in 0.1 M phosphate buffer (PB) (pH 7.4). After 2 h, the fixative was removed, and pelleted bacteria were stored in 2% PFA in PB for 18 h. The bacteria were then washed three times with phosphate-buffered saline containing 0.15 M glycine and finally embedded in 10% gelatin in PB. Small cubes were cut at 4°C and infused with 2.3 M sucrose in PB at 4°C for at least 2 h, after which they were frozen in liquid nitrogen. Ultrathin cryosections were prepared at −120°C on an Ultracut S (Leica, Vienna, Austria) by using a diamond knife (Drukker International, Cuick, The Netherlands) according to Liou et al. (11). The sections were immunolabeled as described previously (19) with a mixture of PA1 to PA5 (20 μg of each/ml in PBS-bovine serum albumin [1%]), followed by incubation with rabbit anti-mouse IgG (DAKO, Glostrup, Denmark), and 10-nm protein A-gold (Department of Cell Biology, Utrecht University, Utrecht, The Netherlands). No specific labeling was detected in M. catarrhalis, presumably due to the low level of expression of BRO β-lactamase. However, specific labeling was obtained in E. coli harboring the bro gene (Fig. 4). Over a total of 200 bacteria, the percentages of gold particles associated with the inner and outer membranes were 5 and 60%, respectively. This is highly significantly different from an equal distribution of label over the two membranes (chi-square by Mantel-Haenszel procedure, P < 0.0001). Thus, BRO β-lactamase was found to be largely associated with the outer membrane rather than the cytoplasmic membrane in E. coli. Moreover, the enzyme appeared to be located on the inside of the outer membrane, facing the periplasm. The relatively high labeling of the cytoplasm (35%) may be due to the presence of (uncompleted) polypeptide chains still associated with ribosomes. No labeling was observed in E. coli without bro (not shown).
FIG. 4.
Electron micrograph of immunogold-labeled E. coli harboring the BRO-1 β-lactamase gene. Cells were subjected to sucrose shock and labeled with a mixture of MAbs recognizing BRO-1 β-lactamase. Arrows indicate outer (O) and cytoplasmic (C) membranes. Bar, 0.2 μm. The image is representative.
Concluding remarks.
To our knowledge, BRO β-lactamase is the first-described lipidated and, therefore, membrane-associated β-lactamase found in gram-negative bacteria. Two other gram-negative bacteria, Pseudomonas pseudomallei and Capnocytophaga sp., have been reported to produce membrane-bound β-lactamases, but no molecular proof was provided that these enzymes were lipoproteins (8, 12). It does not seem likely that a gram-negative bacterium such as M. catarrhalis would benefit much from a membrane-bound β-lactamase. For gram-positive organisms, lipoprotein modification of β-lactamases efficiently retains a portion of the secreted enzyme close to the cell. Gram-negative bacteria do not depend on such a mechanism, since their enzymes are retained in a periplasmic compartment. As we observed by electron microscopy, the membrane-bound enzyme appeared to be facing the periplasmic space. Therefore, it may function as a periplasmic enzyme, similar to other β-lactamases produced by gram-negative bacteria. The distinct G+C content of bro compared to those of other M. catarrhalis genes is strong evidence for a relatively recent acquisition. Taken together, the present data suggest that BRO β-lactamase originates from a gram-positive bacterium and that its lipidation is a remnant of its origin.
Acknowledgments
We are grateful to Joen Luirink for advice on labeling of lipoproteins.
This work was supported by grant no. 32.96.67 from the Dutch Asthma Foundation.
REFERENCES
- 1.Bootsma H J, van Dijk H, Verhoef J, Fleer A, Mooi F R. Molecular characterization of the BRO β-lactamase of Moraxella (Branhamella) catarrhalis. Antimicrob Agents Chemother. 1996;40:966–972. doi: 10.1128/aac.40.4.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle F M, Georgiou P R, Tilse M H, McCormack J G. Branhamella (Moraxella) catarrhalis: pathogenic significance in respiratory infections. Med J Aust. 1991;154:592–596. doi: 10.5694/j.1326-5377.1991.tb121219.x. [DOI] [PubMed] [Google Scholar]
- 3.Braun V, Wu H C. Elsevier Science, Amsterdam, The Netherlands. 1994. Lipoproteins: structure, function, biosynthesis and model for protein export, p. 319–341. In J.-M. Ghuysen and R. Hakenbeck (ed.), New comprehensive biochemistry, vol. 27: bacterial cell wall. [Google Scholar]
- 4.Catlin B W. Branhamella catarrhalis: an organism gaining respect as a pathogen. Clin Microbiol Rev. 1990;3:293–330. doi: 10.1128/cmr.3.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cota-Robles E H. Electron microscopy of plasmolysis in Escherichia coli. J Bacteriol. 1963;85:499–503. doi: 10.1128/jb.85.3.499-503.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doern G V, Brueggemann A B, Pierce G, Hogan T, Preston Holley H, Jr, Rauch A. Prevalence of antimicrobial resistance among 723 outpatient clinical isolates of Moraxella catarrhalis in the United States in 1994 and 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:2884–2886. doi: 10.1128/aac.40.12.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliasson I, Kamme C, Vang M, Waley S G. Characterization of cell-bound papain-soluble beta-lactamases in BRO-1 and BRO-2 producing strains of Moraxella (Branhamella) catarrhalis and Moraxella nonliquefaciens. Eur J Clin Microbiol Infect Dis. 1992;11:313–321. doi: 10.1007/BF01962070. [DOI] [PubMed] [Google Scholar]
- 8.Foweraker J E, Hawkey P M, Heritage J, Van Landuyt H W. Novel β-lactamase from Capnocytophaga sp. Antimicrob Agents Chemother. 1990;34:1501–1504. doi: 10.1128/aac.34.8.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung C-P, Yeo S-F, Livermore D M. Susceptibility of Moraxella catarrhalis isolates to β-lactam antibiotics in relation to β-lactamase pattern. J Antimicrob Chemother. 1994;33:215–222. doi: 10.1093/jac/33.2.215. [DOI] [PubMed] [Google Scholar]
- 10.Hol C, Van Dijke E E M, Verduin C M, Verhoef J, Van Dijk H. Experimental evidence for Moraxella-induced penicillin neutralization in pneumococcal pneumonia. J Infect Dis. 1994;170:1613–1616. doi: 10.1093/infdis/170.6.1613. [DOI] [PubMed] [Google Scholar]
- 11.Liou W, Geuze H J, Slot J W. Improving structural integrity of cryosections for immunogold labeling. Histochem Cell Biol. 1996;106:41–58. doi: 10.1007/BF02473201. [DOI] [PubMed] [Google Scholar]
- 12.Livermore D M, Chau P Y, Wong A I W, Leung Y K. β-Lactamase of Pseudomonas pseudomallei and its contribution to antibiotic resistance. J Antimicrob Chemother. 1987;20:313–321. doi: 10.1093/jac/20.3.313. [DOI] [PubMed] [Google Scholar]
- 13.Malmvall B E, Brorsson J E, Johnsson J. In vitro sensitivity to penicillin V and β-lactamase production of Branhamella catarrhalis. J Antimicrob Chemother. 1977;3:374–375. doi: 10.1093/jac/3.4.374. [DOI] [PubMed] [Google Scholar]
- 14.Murphy T F, Sethi S. Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;146:1067–1083. doi: 10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen J B K, Lampen J O. Membrane-bound penicillinases in Gram-positive bacteria. J Biol Chem. 1982;257:4490–4495. [PubMed] [Google Scholar]
- 16.Nielsen J B K, Lampen J O. β-Lactamase III of Bacillus cereus 569: membrane lipoprotein and secreted protein. Biochemistry. 1983;22:4652–4656. doi: 10.1021/bi00289a007. [DOI] [PubMed] [Google Scholar]
- 17.Percival A, Corkill J E, Rowlands J, Sykes R B. Pathogenicity of and β-lactamase production by Branhamella (Neisseria) catarrhalis. Lancet. 1977;ii:1175. doi: 10.1016/s0140-6736(77)91562-8. [DOI] [PubMed] [Google Scholar]
- 18.Pollock M R. The cell-bound penicillinase of Bacillus cereus. J Gen Microbiol. 1956;15:154–169. doi: 10.1099/00221287-15-1-154. [DOI] [PubMed] [Google Scholar]
- 19.Slot J W, Geuze H J, Gigengack S, Lienhard G E, James D E. Immuno localisation of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steingrube V A, Wallace R J, Jr, Beaulieu D. A membrane-bound precursor β-lactamase in strains of Moraxella catarrhalis and Moraxella nonliquefaciens that produce periplasmic BRO-1 and BRO-2 β-lactamases. J Antimicrob Chemother. 1993;31:237–244. doi: 10.1093/jac/31.2.237. [DOI] [PubMed] [Google Scholar]
- 21.Stobberingh E E, van Eck H J, Houben A W, van Boven C P. Analysis of the relationship between ampicillin resistance and β-lactamase production in Branhamella catarrhalis. Drugs. 1986;31(Suppl. 3):23–27. doi: 10.2165/00003495-198600313-00007. [DOI] [PubMed] [Google Scholar]
- 22.Wallace R J, Jr, Steingrube V A, Nash D R, Hollis D G, Flanagan C, Brown B A, Labidi A, Weaver R E. BRO β-lactamases of Branhamella catarrhalis and Moraxella subgenus Moraxella, including evidence for chromosomal β-lactamase transfer by conjugation in B. catarrhalis, M. nonliquefaciens, and M. lacunata. Antimicrob Agents Chemother. 1989;33:1845–1854. doi: 10.1128/aac.33.11.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wardle J R. Branhamella catarrhalis as an indirect pathogen. Drugs. 1986;31(Suppl. 3):93–96. doi: 10.2165/00003495-198600313-00020. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi K, Yu F, Innouye M. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell. 1988;53:423–432. doi: 10.1016/0092-8674(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto S, Lampen J O. Membrane penicillinase of Bacillus licheniformis 749/C: sequence and possible repeated tetrapeptide structure of the phospholipopeptide region. Proc Natl Acad Sci USA. 1976;73:1457–1461. doi: 10.1073/pnas.73.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]