Highlights
-
•
Two segregated networks for phonemic and semantic fluency were identified.
-
•
Disconnection of left FAT and frontal part of IFOF affects phonemic fluency.
-
•
Disconnection of left UF, AF, ILF and temporal IFOF fibers affects semantic fluency.
-
•
Proposal of a novel patient-tailored disconnection analysis following brain surgery.
Abbreviations: AF, arcuate fasciculus; CST, cortico-spinal tract; FAT, frontal aslant tract; FU, follow-up; HGG, high-grade glioma; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; LGG, low-grade glioma; SLF, superior longitudinal fasciculus; SLS, superior longitudinal system; UF, uncinate fasciculus; WM, white matter
Keywords: Phonemic fluency, Semantic fluency, White matter fibers, Disconnection analyses, Glioma resection, Neural correlates
Abstract
Phonemic and semantic fluency are neuropsychological tests widely used to assess patients’ language and executive abilities and are highly sensitive tests in detecting language deficits in glioma patients. However, the networks that are involved in these tasks could be distinct and suggesting either a frontal (phonemic) or temporal (semantic) involvement.
42 right-handed patients (26 male, mean age = 52.5 years, SD=±13.3) were included in this retrospective study. Patients underwent awake (54.8%) or asleep (45.2%) surgery for low-grade (16.7%) or high-grade-glioma (83.3%) in the frontal (64.3%) or temporal lobe (35.7%) of the left (50%) or right (50%) hemisphere. Pre-operative tractography was reconstructed for each patient, with segmentation of the inferior fronto-occipital fasciculus (IFOF), arcuate fasciculus (AF), uncinate fasciculus (UF), inferior longitudinal fasciculus (ILF), third branch of the superior longitudinal fasciculus (SLF-III), frontal aslant tract (FAT), and cortico-spinal tract (CST). Post-operative percentage of damage and disconnection of each tract, based on the patients’ surgical cavities, were correlated with verbal fluencies scores at one week and one month after surgery. Analyses of differences between fluency scores at these timepoints (before surgery, one week and one month after surgery) were performed; lesion-symptom mapping was used to identify the correlation between cortical areas and post-operative scores.
Immediately after surgery, a transient impairment of verbal fluency was observed, that improved within a month. Left hemisphere lesions were related to a worse verbal fluency performance, being a damage to the left superior frontal or temporal gyri associated with phonemic or semantic fluency deficit, respectively. At a subcortical level, disconnection analyses revealed that fluency scores were associated to the involvement of the left FAT and the left frontal part of the IFOF for phonemic fluency, and the association was still present one month after surgery. For semantic fluency, the correlation between post-surgery performance emerged for the left AF, UF, ILF and the temporal part of the IFOF, but disappeared at the follow-up.
This approach based on the patients’ pre-operative tractography, allowed to trace for the first time a dissociation between white matter pathways integrity and verbal fluency after surgery for glioma resection. Our results confirm the involvement of a frontal anterior pathway for phonemic fluency and a ventral temporal pathway for semantic fluency. Finally, our longitudinal results suggest that the frontal executive pathway requires a longer interval to recover compared to the semantic one.
1. Introduction
Verbal fluency tasks are widely adopted for cognitive function testing (Lezak et al., 2004) in oncological, vascular, or degenerative brain diseases, and are extremely sensitive in detecting language deficits in patients with glioma (Boone et al., 2016, Ng et al., 2018, Papagno et al., 2012, Rijnen et al., 2019). Patients are asked to generate in one minute as many words as possible starting from a given phonemic cue to assess phonemic fluency (e.g., “F”) or from a semantic category for semantic fluency (e.g., “animals”) (Novelli et al., 1986). Lexical retrieval based on phonemic or semantic cue does not only engage language abilities (Adams et al., 1989, Cattaneo et al., 2011), but is also linked to executive functions and semantic knowledge for phonemic and semantic fluency, respectively (Baldo et al., 2006, Jurado et al., 2000, Moscovitch, 1994, Raboutet et al., 2010, Robinson et al., 2012, Schmidt et al., 2019, Troyer et al., 1997, Unsworth et al., 2011, Zarino et al., 2014). Indeed, Baldo et al. (2006) reported that frontal regions are crucially involved in the strategic word retrieval following phonological cue, as confirmed also by other authors (Jurado et al., 2000, Robinson et al., 2012) who demonstrated that frontal lobe lesions severely affect phonemic fluency according to the hypothesis of Moscovitch (1994). Moreover, Troyer et al. (1998, 1997) proposed that both verbal fluencies are supported by two main mechanisms: clustering, intended as word retrieval within subcategories supported by the temporal lobe, and switching, namely the ability to shift between different subcategories and related to frontal lobe functioning.
Nevertheless, a more “frontal” or “executive” role in phonemic compared to semantic fluency is still largely debated, especially considering that several studies highlighted an involvement of the frontal lobe also in lexical retrieval based on semantic cue (Baldo et al., 2010, Baldo et al., 2001, Baldo and Shimamura, 1998, Biesbroek et al., 2016, Chouiter et al., 2016). For instance, Kavè et al. (Kavé et al., 2011) identified semantic fluency as the best indicator of executive deficit after traumatic brain injury, while Cattaneo et al. (2011) reported that anodal transcranial direct current stimulation (tDCS) of the left Broca’s area increases the number of words generated on both semantic or phonological cue. Similar results were obtained by Ghanavati et al. (2019) with anodal stimulation of the left dorso-lateral-prefrontal cortex. These results suggest that a frontal lesion impairs words search strategy, with a worst performance in both verbal fluencies tests, as reported also by Reverberi et al. (2006).
Still, several lesion studies identified distinct, although partially shared, neural correlates for the two tasks, with left frontal or temporal lobe lesions being associated with disrupted phonemic or semantic fluency, respectively (Baldo et al., 2010, Baldo et al., 2006, Biesbroek et al., 2021, Biesbroek et al., 2016, Billingsley et al., 2004, Chouiter et al., 2016, Henry and Crawford, 2004, Hirshorn and Thompson-Schill, 2006, Schmidt et al., 2019).
At the subcortical level, by using fibers diffusion coefficients (e.g., fractional anisotropy, FA), Catani et al. (2013) identified a higher involvement of the Frontal Aslant Tract (FAT) in non-fluent primary progressive aphasia with an impairment in phonemic fluency, and of the UF in the semantic variant of primary progressive aphasia, with a predominant impairment of semantic fluency. Similar results have been found in stroke patients and in people affected by multiple sclerosis, with an association between phonemic fluency and semantic processing and a reduction in diffusion coefficients of dorsal and ventral white matter (WM) fibers, respectively (Blecher et al., 2019, Li et al., 2017). In the last decade, thanks to intraoperative Direct Electrical Stimulation (DES) (Sarubbo et al., 2020) and fibers disconnection approach with the implementation of tractography and WM fibers atlases (Catani et al., 2012, Foulon et al., 2018, Rojkova et al., 2016), several studies on neurosurgical patients showed associations between stimulation or impairment of left IFOF, UF, ILF, SLF and FAT and both phonemic and semantic fluency, without a clear dissociation (Almairac et al., 2015, Chernoff et al., 2019, Diao et al., 2015, Dragoy et al., 2020, Kinoshita et al., 2015, Moritz-Gasser et al., 2013, Papagno et al., 2011b).
Therefore, given these contrasting results, we aimed at verifying whether phonemic and semantic fluencies depend on different networks located in both hemispheres, specific for each type of fluency, consequently clarifying the role of the left and/or right frontal and temporal cortical and subcortical structures in the two tasks. To do this, we investigated patients with gliomas located in the left and right frontal and temporal lobes, introducing a novel approach in disconnection analyses: indeed, considering fibers infiltration and displacement related to glioma (Abhinav et al., 2015, Kuhnt et al., 2012, Mandonnet et al., 2006, Nimsky et al., 2005), we performed a tractographic patient-based analysis, with the quantification of the bundles volume impacted by surgical resections and the resulting fibers disconnection, using the patient’s own pre-operative tractography reconstruction instead of WM fibers atlases (Catani et al., 2012, Foulon et al., 2018, Rojkova et al., 2016).
2. Materials and methods
2.1. Patients
Forty-two (n = 42, 26 males, mean age 52.5 years; SD = ±13.3) right-handed patients (according to the Edinburgh Handedness Inventory Test) (Oldfield, 1971) were included in this study. They underwent awake (54.8%) or asleep (45.2%) brain surgery at the Division of Neurosurgery of “S. Chiara Hospital” (Azienda Provinciale per i Servizi Sanitari, Provincia Autonoma di Trento, Italy). Patients had left (50%) or right (50%) low-grade (16.7%) or high-grade gliomas (83.3%) (LGGs and HGGs, respectively). Inclusion criteria were: 1) tumor location either in the frontal (64.3%) or temporal lobe (35.7%); 2) no previous resection of recurrent lesions, and no residual enhancing tissue at post-operative MRI; 3) no evidence of language deficits before surgery as revealed by spontaneous speech, naming and comprehension tests. Patients’ demographic and clinical characteristics are reported in Table 1. The imaging acquisition procedure and the neuropsychological assessment described below represent the standard management of glioma patients in this department, for which patients gave their informed consent after an accurate discussion of risks and benefits. This study was conducted following the ethical standards of the Declaration of Helsinki and was approved by the local ethical committee (authorization ID A734).
Table 1.
Demographical data and distribution of clinical features in the total group of 42 patients and results of proportion test for the distribution of clinical features in the entire cohort. 95% of Confidence Interval (C.I.) is reported. HGG = high-grade glioma; LGG = low-grade glioma. SD = Standard Deviation.
| OVERALL (N = 42) | |||
|---|---|---|---|
| Mean age (SD) | 52.5 years (±13.3) | ||
| Mean surgical cavity volume (SD) | 20.7 cc (±13.3) | ||
| Features | Proportion | p-value | 95% C.I. |
| Tumor (%) | |||
| HGG | 35 (83.3%) | P < 0.001 | 0.686–0.93 |
| LGG | 7 (16.7%) | 0.07–0.314 | |
| IDH mutation (%) | |||
| No | 30 (71.4%) | P < 0.01 | 0.157–0.446 |
| Yes | 12 (28.6%) | 0.554–0.843 | |
| MGMT methylation (%) | |||
| No | 22 (52.4%) | P = 0.644 | 0.298–0.613 |
| Yes | 20 (47.6%) | 0.387–0.702 | |
| Side (%) | |||
| Left | 21 (50%) | P = 1 | 0.342–0.658 |
| Right | 21 (50%) | 0.342–0.658 | |
| Sex (%) | |||
| Female | 16 (38.1%) | P = 0.164 | 0.236–0.544 |
| Male | 26 (61.9%) | 0.456–0.764 | |
| Location (%) | |||
| Frontal | 27 (64.3%) | P = 0.088 | 0.48–0.784 |
| Temporal | 15 (35.7%) | 0.215–0.52 | |
| Surgery (%) | |||
| Awake | 19 (45.2%) | P = 0.644 | 0.387–0.702 |
| Asleep | 23 (54.8%) | 0.298–0.613 | |
2.2. Neuropsychological assessment
Each patient underwent an extensive neuropsychological assessment (see Dallabona et al., 2017, Papagno et al., 2012, Papagno et al., 2011b, Zigiotto et al., 2020 and Supplementary Material for details) at three time-points, namely one week before surgery (“pre”), one week after surgery (“post”) and one month after surgery (“follow-up”-FU), as a previously reported and current standard in the management of glioma patients (Dallabona et al., 2017, Papagno et al., 2012, Zigiotto et al., 2020). Mean scores, standard deviations, and percentage of impaired patients in each test at every neuropsychological assessment are reported in Supplementary Table 1. As expected, according to the lesion location, cognitive deficits were mostly found in memory and executive functions, especially after surgery. Adjuvant therapy (e.g., radio- and chemotherapy) for HGG started in all cases within 6 weeks after surgery according to the internal neuro-oncology guidelines of the APSS. The 1-month FU is therefore collected at this time-point, before starting therapies, to avoid possible negative effects on cognition. Our complete battery includes the assessment of Language, Memory, Constructional praxis, Attention, and Executive functions. For the purpose of this study we consider phonemic (letters “F”-“P”-“L”) and semantic (“fruits”-“animals”-“brands of cars” categories) fluency test, in the version validated in the Italian population (Novelli et al., 1986).
2.3. MRI acquisition, diffusion processing, and tractography
Each patient underwent an MRI examination as a standard protocol for glioma surgery at “Santa Chiara Hospital” in Trento, with the acquisition of structural and diffusion images for pre-operative planning of glioma resection on a clinical Optima MR450w GE 1.5T scanner (GE Healthcare, Milwaukee, WI, United States), equipped with an 8-channel receive head RF coil. A T1-weighted volumetric sequence (axial acquisition, TR/TI/TE = 10.64/450/4.23 ms, FA = 12°, square field of view (FOV) = 256 mm, voxel size = 1×1×1 mm3) was performed for each patient. For tractography, a DWI scheme with 60 directions was acquired (one acquisition) using a single-shot multislice spin echo–echo planar sequence with the following attributes: 50 slices; square FOV 240 mm; voxel size = 2.4×2.4×2.4 mm3; TR/TE = 13000/95.8 ms; FA = 90°; b values of 0 and 1,000 s/mm2. It must be noted that tractography with a similar (or lower) number of slices and directions in a 1.5T scanner is considered reliable and is widely adopted in clinical practice (see for instance Ashmore et al., 2020, Becker et al., 2022, Toselli et al., 2017, Zacà et al., 2018). A second early post-operative T1 with gadolinium acquired 24 h after surgery according to the protocol defined above was used for the characterization of the surgical cavity (Zacà et al., 2018).
The processing of diffusion MRI data was carried out concatenating a step of pre-processing, a step of diffusivity model reconstruction, and a step of probabilistic tracking. The pipeline of elaboration was implemented using FSL and Dipy, an open-source library for the analysis of diffusion MRI data (Garyfallidis et al., 2014, Jenkinson et al., 2002). After the conversion from DICOM to Nifti format, the DWI data were preprocessed to correct eddy current and head motion distortions. Brain mask was computed, and brain volumes were extracted. For each voxel the fiber orientation distribution function was computed using a constrained spherical deconvolution (CSD) model (Tournier et al., 2007). A final tracking step was carried out using a seed-based probabilistic strategy (Tournier et al., 2010) with a step size of 1.0 mm, maximum length 250 mm, and minimum length 10 mm. The fiber orientation distribution function (fODF) amplitude cut-off was set to 0.1, and the maximum angle of curvature to 30°.
2.4. Lesion symptom mapping
We performed region of interest (ROI)-based lesion symptom mapping (Bates et al., 2003) (RLSM) using NiiStat toolbox for MATLAB (https://www.nitrc.org/projects/niistat/): by comparing cortical ROI instead of individual voxel, this approach improves the statistical power by decreasing the number of statistical comparisons needed and the rate of family-wise error (Findlater et al., 2016, Smith et al., 2013). ROIs were defined using the Automated Anatomical Labeling (AAL) (Tzourio-Mazoyer et al., 2002) template. Each lesioned voxel of the entire patients’ cohort was mapped into a specific region and then the proportion of damage to a given region was entered into a general linear model (Findlater et al., 2016), comparing cognitive performance of patients with a lesion in a specific region (i.e., left or right, frontal or temporal region) and scores of patients having the same region spared. This procedure was adopted given the heterogeneity of our sample, in order to identify which lesioned area at cortical level, mainly impairs fluency tasks: the results, converted in Z-scores, statistically identify which region is associated with fluencies scores one week after surgery. We included only regions damaged in at least 6 patients (about 15% of the samples), as voxels that are rarely affected have lower statistical power and may affect the false discovery rate (Findlater et al., 2016, Puglisi et al., 2019a, Smith et al., 2013).
2.5. Tractography virtual dissection
WM fibers virtual dissection was manually performed in the tumor-affected hemisphere for each patient using Trackvis software (https://trackvis.org) on their pre-surgical probabilistic CSD tractography. A full description of the functional role of every bundle is reported in the Supplementary Material. A constrained ROI-based approach has been used on each patient’s tractogram. ROIs have been placed as follows:
-
-
Inferior fronto-occipital fasciculus (IFOF): according to the most recent and solid evidence, we included in the IFOF reconstruction the ventral (i.e. passing through the external capsule, or EC) fibers running from the occipital cortex, the pre-cuneus, the superior parietal lobule and the temporo-basal regions to the frontal area, specifically to the inferior frontal gyrus (IFG, in particular pars opercularis and pars triangularis) and the dorso-lateral prefrontal cortex (DLPFC) (De benedictis et al., 2021, Hau et al., 2016, Sarubbo et al., 2019, 2013); the presence of sparse direct monosynaptic connections in primates brain has also been reported from frontal to occipital (Markov et al., 2014) using viral axonal tracing, as well as a direct connection between frontal and occipital areas using autoradiography (Schmahmann et al., 2007) and Klinger micro-dissection (Sarubbo et al., 2019), even if this is still a controversial topic which needs further considerations. For the virtual dissection, a stem-based approach has been adopted, with an inclusion ROI placed on the coronal plane at the level of the limen insulae within the antero-ventral third of the EC (Bertò et al., 2021, De benedictis et al., 2021, Hau et al., 2016, Sarubbo et al., 2013) (i.e., where the streamlines converge into a compact bundle). Streamlines passing through the anterior temporal lobe, the cerebellum, and the brainstem as well as the callosal streamlines passing through the inter-hemispheric fissure were removed.
-
-
Uncinate fasciculus (UF): the UF, which connects the medial and lateral orbito-frontal cortex with the temporal pole (Catani et al., 2002, Catani and Thiebaut de Schotten, 2008, Hau et al., 2017, 2016), has been dissected by placing a single ROI on the coronal plane at the stem level, nearby the insula, where streamlines curve downward and gather into a compact bundle, before descending to the temporal cortex (Hau et al., 2017, 2016). Finally, a coronal slice posterior to the EC was specified to exclude the streamlines not belonging to this pathway.
-
-
Inferior longitudinal fasciculus (ILF): for the ILF dissection we included fibers running longitudinally from the occipital cortex to the anterior temporal lobe (ATL) (Catani et al., 2003, Catani and Thiebaut de Schotten, 2008, Zemmoura et al., 2021) by placing two ROIs on the coronal plane, the first one in the ATL and the second one encircling the WM of the occipital lobe; an exclusion ROI was drawn at the level of the IFOF stem to remove artifactual streamlines.
-
-
Arcuate fasciculus (AF): the AF connects the frontal (including part of the posterior thirds of the inferior and the middle frontal gyri, IFG and MFG respectively, and the ventral portion of the pre-central gyrus) and the temporal lobes (including superior, middle, and inferior temporal gyri, or STG, MTG and ITG respectively), with a typical half-moon shape (Catani et al., 2005, Catani and Mesulam, 2008, Catani and Thiebaut de Schotten, 2008). For the virtual dissection of the direct AF component, three ROIs were defined: a frontal inclusion ROI, drawn on the coronal plane just anterior to the central sulcus; a temporal inclusion ROI, drawn on the coronal and axial planes in the posterior part of the STG and MTG; an exclusion parietal ROI, drawn on the coronal plane at the level of the inferior parietal lobule (supramarginal and angular gyri, SMG and AG respectively) to exclude the indirect components belonging to the SLF (Catani et al., 2005, Catani and Mesulam, 2008, Forkel et al., 2014, Zacà et al., 2018).
-
-
Superior longitudinal fasciculus, third branch (SLF III): inclusion ROIs for the segmentation of the SLF III (Petrides and Pandya, 1984) were placed on the coronal plane in the IFG and within the SMG, excluding fibers running towards the temporal lobe, as this bundle connects the SMG with the IFG (De Benedictis et al., 2014, Forkel et al., 2014, Howells et al., 2020, Kamali et al., 2014, Martino et al., 2013, Vavassori et al., 2021, Zacà et al., 2018).
-
-
Frontal aslant tract (FAT): the FAT is an oblique WM tract that connects the superior frontal gyrus (SFG, in particular SMA/pre-SMA) to the IFG (mainly pars opercularis and pars triangularis) (Burkhardt et al., 2021, Catani et al., 2013, Catani et al., 2012, Kinoshita et al., 2012). The FAT was dissected using two inclusion ROIs: the first one was placed on the axial plane within the SFG, and the second one on the sagittal plane, just over the IFG.
-
-
Cortico-spinal tract (CST): the CST, which controls body movements and connects the motor cortex and the spinal cord, has been traced as a control pathway since we expected no contribution of this bundle in fluency abilities. Two inclusion ROIs have been drawn on the axial plane, one within the WM of the precentral gyrus and the other in the ipsilateral cerebral pedunculus; finally, mid-sagittal and cerebellar exclusion ROIs have been placed to eliminate artifacts (Howells et al., 2020, Howells et al., 2018, Thiebaut de Schotten et al., 2011).
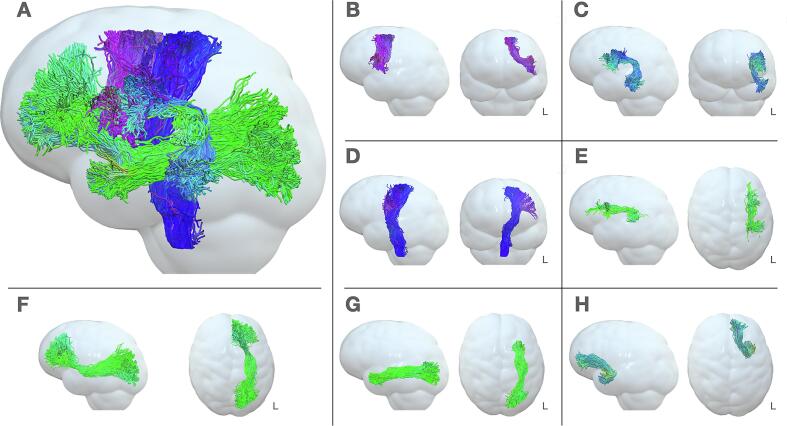
Virtual dissection was performed by a neuropsychologist (LZ) and revised by a neurosurgeon expert in brain anatomy, virtual tractography, and ex vivo micro-dissection (SS) (see Fig. 1 for an example of segmentation of the bundles of interest). From a total of 294 WM bundles (7 bundles per patient × 42 patients), we were able to identify 290 tracts, excluding two UF, one ILF and one IFOF due to tumor mass and absence of diffusion. These 4 tracts, belonging to 3 different patients, were not considered as damaged following surgery since they could not be segmented even before the procedure; indeed, no streamlines passing through the tumor were found. Bundles were finally refined using the interactive segmentation tool Tractome (https://tractome.org), to manually remove outlier and non-plausible streamlines (see Supplementary Fig. 1 for an example) as previously reported (Bertò et al., 2021, Porro-Muñoz et al., 2015), following visual inspection.
Fig. 1.
3D visualization of fibers virtual dissections. In this 3D graphical visualization of virtually dissected fibers, a Gaussian filter was applied to the anatomical image in order to smooth the brain surface. (A) Sagittal 3D view of the 7 left white matter fibers dissected; (B) Sagittal and coronal view of FAT; (C); Sagittal and coronal view of AF; (D) Sagittal and coronal view of PT; (E) Sagittal and axial view SLF-III; (F) Sagittal and axial view of IFOF; (G) Sagittal and axial view of ILF; (H) Sagittal and axial view of UF. L = left.
2.6. Surgical procedure
For each patient surgery was performed with the help of a neuronavigation system where the volumetric structural images (T1 with gadolinium for HGG and T2/Flair for LGG) were merged with the tractographic reconstructions of the critical pathways. During awake surgery, the cortical and subcortical functional mapping was performed using a bipolar probe with and electrical stimulation of 60 Hz, with 1 ms of duration and amplitude ranging between 2 and 4 mA, as previously reported (Dallabona et al., 2017, Zacà et al., 2018, Zigiotto et al., 2020). The amplitude threshold for both cortical and subcortical mapping was set when the electrical stimulation elicited speech arrest at the level of the ventral pre-motor cortex (VPMC), regardless of tumor laterality. Resection stopped when functional responses (e.g., linguistic or movement errors) were elicited from the cortical and subcortical stimulation of eloquent structures; to correctly monitor the cognitive performance during awake surgery, intraoperative neuropsychological assessment had been customized for each patient. Considering the frontal or temporal lesion, during awake surgery the following tasks were performed: counting (0–10) (Coello et al., 2013, Mandonnet et al., 2017, Sarubbo et al., 2015, Zigiotto et al., 2020) and motor task (Sarubbo et al., 2015, Zigiotto et al., 2020); object naming, verb generation, reading and comprehension (Coello et al., 2013, Duffau and Zalc, 2016, Sarubbo et al., 2020, Zigiotto et al., 2020); palm-pyramid-tree test (PPTT) (Coello et al., 2013, Sarubbo et al., 2020, Vilasboas et al., 2017, Zigiotto et al., 2020); Stroop test (Puglisi et al., 2019a, Puglisi et al., 2019b, Rudà et al., 2020, Zigiotto et al., 2020); the modified version of “reading the mind in the eyes” (namely, the classic “reading the mind in the eyes test” but suitable for intra-operative stimulation, with two choices instead of four, as reported in Herbet et al., 2015, Sarubbo et al., 2020). In the asleep procedure, total intravenous anesthesia with Remifentanil and Propofol infusion was administered, and oro-tracheal intubation was used.
2.7. Lesion mapping and disconnection analyses
A different neurosurgeon (LA) blind to the purpose of the study manually drew the surgical resection cavities in each patient’s native space of the early post-operative structural T1 MRI with gadolinium acquired 24 h after surgery (see Fig. 2 for left and right surgical cavities’ distribution and overlap): each 3DT1 MRI was converted into Nifti (Neuroimaging Informatics Technology Initiative) files and lesions were contoured by hand using MRIcroGL software (https://www.nitrc.org/projects/mricrogl/).
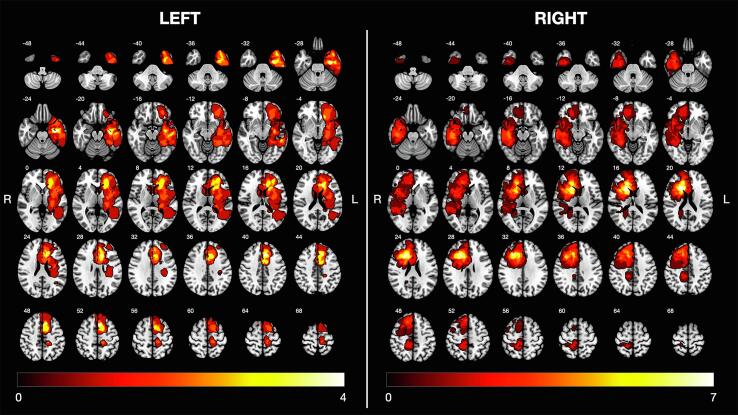
Fig. 2.
Overlap and distribution of surgical cavities. Left (N = 21, maximum overlap = 4) and right (N = 21, maximum overlap = 7) lesions. White numbers on top of each slice indicate Z coordinates of the MNI space.
The resection cavities, i.e., cavities after brain surgery, were computed by manual drawing of multiple ROIs, one for each structural MRI slice (as in Zigiotto et al., 2020). Resection cavities were then registered to the pre-operative T1 and tractography (Howells et al., 2020, Puglisi et al., 2019a) by applying six degrees of freedom rigid body transformation (Mang et al., 2008, Zacà et al., 2018) using FSL’s linear image registration tool (FLIRT) (Jenkinson et al., 2002, Sarubbo et al., 2020, Visser et al., 2020). The quality of registration was systematically visually inspected.
We quantified the effect of the surgical resection in terms of WM damage and consequent bundles disconnection, by way of two measurements already used in previous studies (Howells et al., 2020, Puglisi et al., 2019a). The volume (in cc) of each bundle intersecting with the resection cavity was expressed as a percentage of the pre-operative total tract volume (mean pre- and post-surgery volume and mean % of damage are reported in Supplementary Table 2, separately for the left and right hemisphere and in the entire cohort) and was taken as a direct index of tract damage (Cochereau et al., 2020, Nakajima et al., 2021, Nakajima et al., 2018, Puglisi et al., 2019a) (Fig. 3A and B). On the other hand, the tract disconnection was computed as the percentage of the number of streamlines of a tract intersecting with the resection cavity divided by the total number of tract streamlines (e.g., 200/600 streamlines passing in the resection cavity indicates 30% of tract disconnection) (Howells et al., 2020, Langen et al., 2018, Viganò et al., 2022) (Fig. 3A and C). Unlike previous studies that calculated bundles’ damage and disconnection by reporting the patient’s lesion into a WM atlas (Catani et al., 2012, Catani and Thiebaut de Schotten, 2008, Foulon et al., 2018, Rojkova et al., 2016), our innovative patient-tailored approach investigates the effect of the lesion on the patient’s own fibers’ reconstruction.
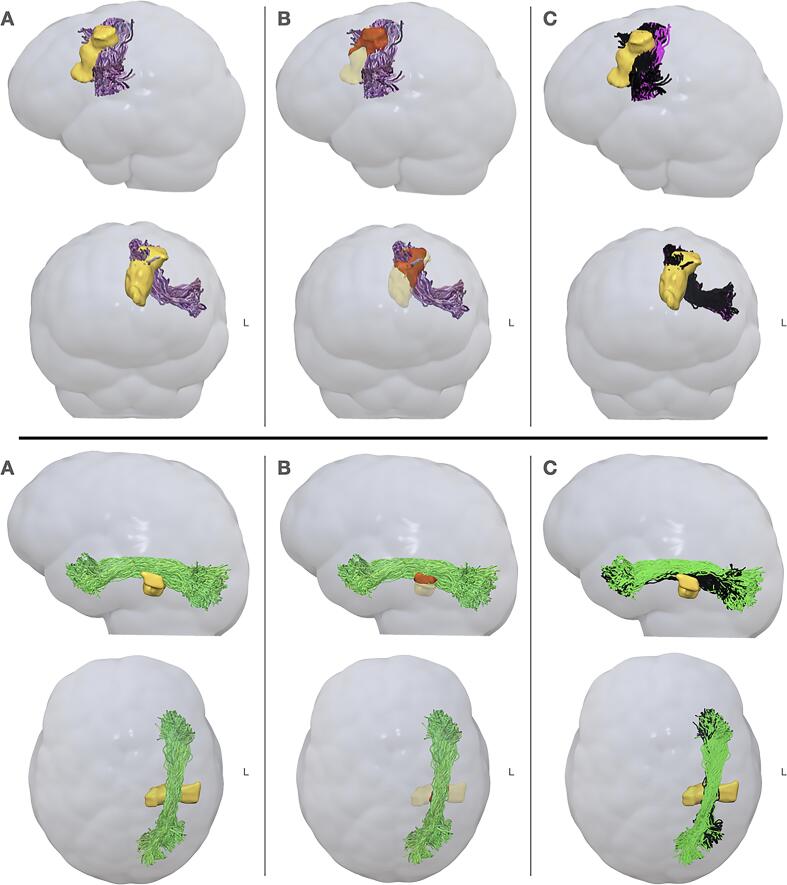
Fig. 3.
Illustration of the definition of tracts damage and disconnection scores. Here are reported bundles damage (B) and disconnection (C) in two patients presenting a frontal (top row) and a temporal (bottom row) glioma, with the reconstruction of the FAT and the ILF respectively. Column A depicts the relationship between the FAT (light violet, in sagittal and coronal view) and the ILF (light green, in sagittal and axial view) and the surgical cavities (in yellow): while the quantification of the bundle damage (B) consists in the identification of its local intersection (in red) with the surgical cavity (in light yellow), the disconnection (C) considers all the fibers that were impacted by the surgical resection (in black). L = left. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.8. Statistical analysis
Analyses were performed using SPSS v26.0 software. Considering the non-normal distribution (Shapiro-Wilk test < 0.05), non-parametric analyses were used. To assess the distribution of demographic and clinical features such as tumor grade, sex, surgery (awake or asleep), lesion side and location in our cohort, we performed a binomial proportion test; 95% of Confidence Interval (C.I.) was also reported. Friedman repeated measures ANOVA (rmANOVA), distinct for both phonemic and semantic fluency scores, adjusted for age and education, following Capitani and Laiacona’s procedure (Capitani and Laiacona, 2009), were run with pre-, post- and FU assessments as factors. Post-hoc analyses were carried out using Wilcoxon rank paired T-test; for significant results the effect size (r) was reported (Rosenthal, 1994).
The potential effect of sex, low or high tumor grade, type of surgery, lesion side and site on both phonemic and semantic fluency outcome (i.e., “post” and “FU” adjusted score) were tested with a Mann-Whitney U non-parametric independent samples t-test; for significant results the effect size (r) was reported (Rosenthal, 1994) and level of significance was set at P < 0.05. Even for RLSM, the statistical significance was set at P < 0.05, using permutation thresholding (10,000 permutations) to control for multiple comparisons (Karnath et al., 2018). The lesion volume of each patient was used as a regression coefficient to improve the precision of this analysis (Karnath et al., 2018, Sperber and Karnath, 2017).
Finally, a two-tailed Spearman correlation was conducted between verbal fluency scores and percentage of both damage volume and disconnection for each tract. For correlation results, p-values were adjusted for multiple comparison using a False Discovery Rate (FDR) correction, considering statistically significant associations with FDR below 5% (i.e., corrected p-value < 0.05) (Benjamini and Hochberg, 1995).
3. Results
3.1. Demographic and clinical data
Our cohort was statistically balanced for left or right hemisphere tumor (P = 1), gender (P = 0.164), awake or asleep surgery (P = 0.644), frontal or temporal lobe tumor site (P = 0.088) and tumor MGMT (O6-methylguanine-DNA methyltransferase) methylation (P = 0.644). A significant difference emerged for what concerns the higher number of HGG compared to LGG (P < 0.001), as well as for IDH (Isocitrate dehydrogenase) mutations (P < 0.01), with few patients presenting the mutation; however, neither the tumor grade nor the possible presence of IDH mutation influenced the patients’ post-surgery verbal fluency, as demonstrated by the Mann-Whitney test (see further). All the results, including 95% C.I., are reported in Table 1. Besides the distinction between Low-Grade and High-Grade Gliomas, nowadays the biomolecular patterns such as IDH mutation and MGMT methylation are identified as the best indicators of a favorable or unfavorable prognosis (Louis et al., 2016): for this reason, in order to control the effect of every variable, these indicators were also considered in the further analyses.
3.2. Phonemic and semantic fluency outcome
The Friedman ANOVA showed a significant difference in phonemic fluency (χ2 = 7.045, P < 0.05), with improvement at FU (mean number of words = 29) compared to post-surgery assessment (mean words number = 23.4, P < 0.001, r = −0.532). Differences between pre- (mean number of words = 25.83) and post-surgery (P = 0.439) or FU (P = 0.133) did not reach significance. A similar result was observed for semantic fluency (χ2 = 8.787, P < 0.05), where patients produced a lower score after surgery (mean words number = 31.55) as compared to pre-surgery assessment (mean words number = 36.9, P < 0.05, r = −0.316), with a significant improvement at FU (mean words number = 36.83, P < 0.01, r = −0.482). No other differences were found (pre vs. FU P = 0.883). Full results are reported in Table 2 and Supplementary Fig. 2.
Table 2.
Results of Friedman rm ANOVA, comparing the general trend of each test, and results of Wilcoxon test, comparing each assessment time-point. Results are reported for the entire sample (TOTAL), and then separated for left-hemisphere lesion (LEFT) and right-hemisphere lesion (RIGHT). For significative result of this last test r is reported, as a measure of the effect size. SD = Standard Deviation.
| TOTAL | ||||
|---|---|---|---|---|
| Test | Time | Mean score (SD) | % of deficit | p-value Friedman (p-value Wilcoxon) |
| Phonemic fluency | Pre | 25.83 (±10.55) | 23.81 % | χ2 = 7.045; P = 0.03 (pre vs. post Z = −0.773; P = 0.439) (post vs. FU Z = −3.449; P = 0.001, r = −0.376) (pre vs. FU Z = −1.501; P = 0.133) |
| Post | 23.4 (±14.24) | 33.33 % | ||
| FU | 29 (±11.34) | 14.29 % | ||
| Semantic fluency | Pre | 36.9 (±10.59) | 19.05 % |
χ2 = 8.787;P = 0.012 (pre vs. post Z = −2.05;P = 0.04, r = −0.224) (post vs. FU Z = −3.128;P = 0.002, r = −0.341) (pre vs. FU Z = −0.147; P = 0.883) |
| Post | 31.55 (±14.5) | 26.19 % | ||
| FU | 36.83 (±10.21) | 11.9 % | ||
| LEFT | ||||
| Phonemic fluency | Pre | 22.95 (±10.83) | 33.33 % |
χ2 = 7.407;P = 0.025 (pre vs. post Z = −1.961;P = 0.049, r = −0.303) (post vs. FU Z = −2.840;P = 0.001, r = −0.438) (pre vs. FU Z = −0.112; P = 0.911) |
| Post | 16.00 (±13.74) | 57.14 % | ||
| FU | 24.52 (±19.05) | 19.05 % | ||
| Semantic fluency | Pre | 32.76 (±10.55) | 28.57 % |
χ2 = 14.0;P = 0.001 (pre vs. post Z = −2.465;P = 0.014, r = −0.380) (post vs. FU Z = −3.671;P < 0.001, r = −0.566) (pre vs. FU Z = −0.081; P = 0.936) |
| Post | 22.67 (±13.95) | 52.38 % | ||
| FU | 32.62 (±11.02) | 23.81 % | ||
| RIGHT | ||||
| Phonemic fluency | Pre | 28.71 (±9.67) | 14.29 % |
χ2 = 4.289; P = 0.117 (pre vs. post Z = −1.421; P = 0.155) (post vs. FU Z = −1.920; P = 0.055) (pre vs. FU Z = −1.913; P = 0.056) |
| Post | 30.81 (±10.56) | 9.52 % | ||
| FU | 33.48 (±12.06) | 9.52 % | ||
| Semantic fluency | Pre | 41.05 (±9.09) | 9.52 % |
χ2 = 0.481; P = 0.786 (pre vs. post Z = −0.070; P = 0.944) (post vs. FU Z = −0.427; P = 0.669) (pre vs. FU Z = −0.580; P = 0.562) |
| Post | 40.43 (±8.41) | 0 % | ||
| FU | 41.05 (±7.41) | 0 % | ||
The same analysis was also performed separately for patients with a left tumor and for patients with a right tumor. In the case of left-hemisphere lesions, significant results emerged for both phonemic (χ2 = 7.407, P < 0.05) and semantic fluency (χ2 = 14.0, P < 0.01): in particular, in the phonemic task, patients produced less words after surgery (mean words number = 16) as compared to pre-surgery assessment (mean words number = 22.95, P < 0.05, r = −0.303), improving at FU (mean words number = 24.52, P < 0.01, r = −0.438), while no differences emerged between pre-surgery and FU assessment (P = 0.911). In a similar way, considering the semantic task, patients showed a significant decrease in scores after surgery (mean words number = 22.67) compared both to pre-surgery assessment (mean words number = 32.76, P < 0.05, r = −0.380) and FU (mean words number = 32.62, P < 0.001, r = −0.566), with a similar performance between pre-surgery and FU assessment (P = 0.936). For patients with a right-hemisphere tumor no significant differences emerged between the three time-points (all Ps > 0.055). Complete results are reported in Table 2.
Moreover, no effects of sex, type of surgery, tumor grade, IDH mutation, MGMT methylation and location were found on fluency scores (all Ps > 0.136), except for lesion side: indeed, lower scores were observed for left-hemisphere lesions on both phonemic (post P < 0.01, r = −0.519; FU P < 0.05, r = −0.361) and semantic fluency (post P < 0.001, r = −0.612; FU P < 0.05, r = −0.395) and at both post-surgery and FU assessment as compared to right-sided lesions. See Supplementary Table 3 for results.
3.3. Lesion analysis
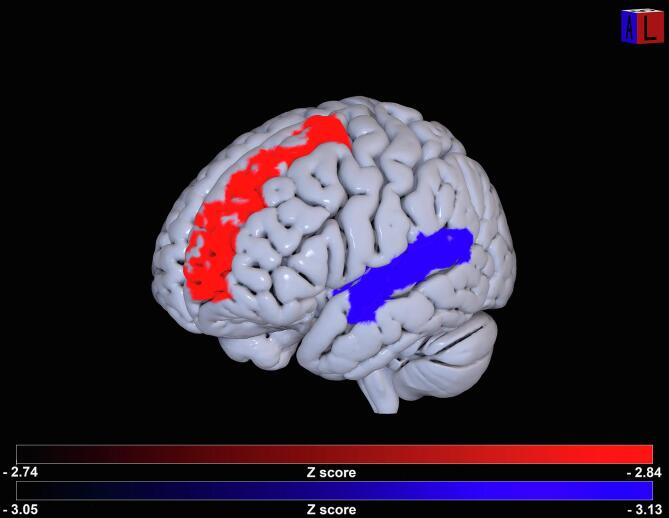
To control for the effect of cortical lesions on performance one week after surgery, a lesion symptom mapping analysis using a ROI-based approach, defined on the AAL template, was performed (RLSM). Results were thresholded for 10,000 permutations, including regions damaged in at least 6 patients (about 15% of the samples), with a regression for the lesion volume. RLSM showed a positive association between the right middle frontal gyrus and both phonemic (Z = 2.95) and semantic (Z = 3.47) fluency. However, as we expected that lesions would produce a performance impairment but not improvement, only significant results with negative Z-scores (Z < −2.74 for phonemic and Z < −3.05 for semantic fluency, P < 0.05 using permutation correction) were considered and further reported for this analysis, as described in literature (Shahid et al., 2017). Indeed, positive Z-scores in this analysis (i.e., higher fluency scores following lesion of specific brain region) mean that brain injury in that location predicts the absence of impairment compared to damage to other regions, and they do not indicate a better performance (Shahid et al., 2017). This confirms that a lesion to the right hemisphere does not affect verbal fluency as a left hemisphere lesion does. Interestingly, this analysis showed a significant negative association between lesions of the left superior frontal gyrus and phonemic fluency (Z = −2.84, P < 0.05 using permutation correction), and between left superior temporal gyrus lesions and semantic fluency (Z = −3.13, P < 0.05 using permutation correction) (Fig. 4). The same analysis was performed with fluency scores of the follow-up assessment; no significative negative Z-scores emerged.
Fig. 4.
Results of RLSM. Statistical significant association emerged between damage on superior frontal gyrus (in red) and phonemic scores and superior temporal gyrus (in blue) and semantic fluency scores. Z-scores are reported in the color-bars. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Association between tracts damage/disconnection and fluency outcome
Considering the effect of lesion side, correlations were performed separately for left and right lesions: for each patient with a left-hemisphere lesion, a 0.0% of damage was attributed to tracts of the right contralesional hemisphere, while the opposite pattern was calculated for patients with right-hemisphere lesions. Correlation results were thresholded at an FDR adjusted p-value of 0.05.
Considering the volume of the tract damage, a significant negative correlation was observed between the left IFOF (PFDR < 0.05) and the FAT (PFDR < 0.05) and post-surgery phonemic fluency scores, as higher volume damage corresponded to lower fluency abilities. Interestingly, regarding semantic fluency, a negative correlation was reported for the left IFOF, UF, ILF and AF (all PsFDR < 0.05); higher volume damage of those tracts was associated with lower score in semantic fluency. No FDR corrected significant results were found for SLF III or CST or tracts of the right hemisphere (see Table 3 for full results).
Table 3.
Results of Spearman correlation with fluency scores immediately after surgery (post) and 1 month after surgery (FU) and left and right tracts volume damage or tracts disconnection. IFOF = Inferior Fronto-Occipital Fasciculus; UF = Uncinate Fasciculus; AF = Arcuate Fasciculus; ILF = Inferior Longitudinal Fasciculus; FAT = Frontal Aslant Tract; CST = Cortico-Spinal Tract; SLF III = Superior Longitudinal Fasciculus III. Phon. = phonemic fluency; Sem. = semantic fluency. pFDR = p-value adjusted for False Discovery Rate (FDR) at 0.05. *= p < 0.05.
| Spearman correlation |
White matter fibers (% of volume damage) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test scores | Left |
Right |
|||||||||||||
| IFOF | UF | AF | ILF | FAT | CST | SLF III | IFOF | UF | AF | ILF | FAT | CST | SLF III | ||
| Phon. post |
rho = P= PFDR= |
−0.425 0.005 0.035* |
−0.208 0.186 0.261 |
−0.249 0.111 0.222 |
−0.239 0.127 0.223 |
−0.483 0.001 0.014* |
−0.175 0.268 0.341 |
−0.239 0.128 0.199 |
0.294 0.059 0.207 |
0.142 0.368 0.429 |
0.349 0.024 0.112 |
0.108 0.497 0.535 |
0.256 0.102 0.238 |
0.290 0.062 0.174 |
0.079 0.617 0.617 |
| Sem. Post |
rho= P= pFDR= |
−0.511 0.001 0.014* |
−0.389 0.011 0.038* |
−0.4 0.009 0.042* |
−0.461 0.002 0.014* |
−0.358 0.02 0.057 |
−0.082 0.607 0.607 |
−0.251 0.108 0.216 |
0.226 0.151 0.211 |
0.169 0.283 0.361 |
0.287 0.066 0.154 |
0.160 0.312 0.336 |
0.245 0.117 0.182 |
0.250 0.111 0.194 |
0.167 0.289 0.337 |
| Phon. FU |
rho= P= PFDR= |
−0.525 0.0001 0.001* |
−0.29 0.062 0.289 |
−0.199 0.207 0.483 |
−0.211 0.181 0.507 |
−0.472 0.002 0.014* |
−0.193 0.22 0.44 |
−0.187 0.236 0.413 |
0.154 0.331 0.515 |
0.023 0.884 0.952 |
0.244 0.12 0.42 |
0.008 0.96 0.96 |
0.087 0.584 0.743 |
0.111 0.484 0.677 |
−0.046 0.773 0.902 |
| Sem. FU |
rho= P= PFDR= |
−0.319 0.04 0.187 |
−0.372 0.015 0.21 |
−0.169 0.284 0.568 |
−0.32 0.039 0.273 |
−0.132 0.405 0.515 |
0.132 0.404 0.566 |
0.123 0.439 0.473 |
0.204 0.194 0.543 |
0.139 0.381 0.593 |
0.218 0.166 0.581 |
0.174 0.27 0.63 |
0.148 0.351 0.614 |
−0.130 0.413 0.482 |
0.083 0.603 0.603 |
| Spearman correlation | White matter fibers (% of tracts disconnection) | ||||||||||||||
| Phon. Post |
rho= P= PFDR= |
−0.409 0.007 0.049* |
−0.213 0.175 0.272 |
−0.256 0.102 0.286 |
−0.241 0.124 0.248 |
−0.482 0.001 0.014* |
−0.098 0.539 0.581 |
−0.239 0.128 0.224 |
0.212 0.177 0.248 |
0.044 0.783 0.783 |
0.349 0.024 0.112 |
0.134 0.397 0.463 |
0.247 0.114 0.266 |
0.343 0.026 0.091 |
0.143 0.367 0.467 |
| Sem. Post |
rho= P= PFDR= |
−0.499 0.001 0.014* |
−0.393 0.01 0.035* |
−0.404 0.008 0.037* |
−0.461 0.002 0.014* |
−0.351 0.023 0.064 |
−0.05 0.752 0.752 |
−0.251 0.108 0.189 |
0.198 0.231 0.294 |
0.083 0.602 0.648 |
0.287 0.066 0.132 |
0.199 0.453 0.528 |
0.241 0.124 0.193 |
0.292 0.061 0.142 |
0.218 0.165 0.231 |
| Phon. FU |
rho= P= PFDR= |
−0.524 0.0001 0.001* |
−0.294 0.058 0.271 |
−0.199 0.207 0.483 |
−0.209 0.184 0.515 |
−0.467 0.002 0.014* |
−0.03 0.85 0.915 |
−0.187 0.236 0.413 |
0.075 0.639 0.895 |
−0.054 0.736 0.937 |
0.244 0.12 0.42 |
0.018 0.912 0.912 |
0.078 0.622 0.967 |
0.197 0.211 0.422 |
0.037 0.818 0.954 |
| Sem. FU |
rho= P= PFDR= |
−0.301 0.053 0.247 |
−0.368 0.017 0.238 |
−0.159 0.315 0.49 |
−0.306 0.049 0.343 |
−0.122 0.443 0.477 |
0.167 0.292 0.584 |
0.123 0.439 0.512 |
0.18 0.253 0.708 |
0.149 0.345 0.483 |
0.218 0.166 0.581 |
0.173 0.273 0.637 |
0.115 0.47 0.47 |
−0.137 0.387 0.492 |
0.159 0.314 0.549 |
The same results emerged considering the percentage of tract disconnection, with a significant negative correlation between phonemic fluency scores and left IFOF and FAT disconnections (PsFDR < 0.05) and between semantic fluency scores and left IFOF, UF, ILF and AF (all PsFDR < 0.05) (see Table 3).
Moreover, as left IFOF damage correlated with both fluency tasks, we distinguished the percentage of damage and disconnection for the temporal (posterior to the IFOF stem at the level of insula) or frontal (anterior to the IFOF stem at the level of insula) tract and correlated each component with phonemic and semantic post-surgery scores: these results (see Table 4) confirmed a higher involvement of the left frontal IFOF component for phonemic fluency (PFDR < 0.05) and of the left temporal IFOF component for semantic fluency (PFDR < 0.05) with both indices.
Table 4.
Results of Spearman correlation with fluency scores immediately after surgery and frontal and temporal part of left/right IFOF volume damage and tract disconnection. IFOF = Inferior Fronto-Occipital Fasciculus. Phon. = phonemic fluency; Sem. = semantic fluency. pFDR = p-value adjusted for False Discovery Rate (FDR) at 0.05. *= p < 0.05.
| Spearman correlation |
White matter fibers (% of volume damage) |
||||
|---|---|---|---|---|---|
| Test scores | Left |
Right |
|||
| IFOF frontal | IFOF temporal | IFOF frontal | IFOF temporal | ||
| Phon. post |
rho= P= PFDR= |
−0.435 0.004 0.016* |
−0.219 0.164 0.164 |
0.276 0.077 0.154 |
0.231 0.141 0.188 |
| Sem. post |
rho= P= PFDR= |
−0.33 0.033 0.066 |
−0.448 0.003 0.012* |
0.265 0.09 0.12 |
0.065 0.682 0.682 |
| Spearman correlation | White matter fibers (% of tract disconnection) | ||||
| Phon. post |
rho= P= PFDR= |
−0.456 0.002 0.008* |
−0.194 0.217 0.29 |
0.221 0.16 0.32 |
0.161 0.307 0.307 |
| Sem. post |
rho= P= PFDR= |
−0.332 0.032 0.064 |
−0.436 0.004 0.016* |
0.222 0.157 0.21 |
0.058 0.715 0.715 |
Finally, we correlated the percentage of tracts damage and disconnection with the FU scores to investigate the longitudinal outcome: in the case of phonemic fluency, lower scores still correlated with higher left IFOF (PFDR < 0.01) and FAT (PFDR < 0.05) damage and disconnection. The results of this longitudinal analysis are reported in Table 3, while no significant correlation at the FU emerged for semantic fluency with both indices.
In order to test the specificity of the relationship between the bundles damage or disconnection and the impaired performance in fluency tasks- therefore, to determine whether a surgical resection produces a significant impairment per se – we also performed a correlation analysis using a non-linguistic executive test (Trial Making Test part B) (Giovagnoli et al., 1996) and a sentence comprehension test (Capasso and Miceli, 2001) as control. Crucially, no significant correlations were found after FDR correction between both control tests and WM fibers damage and/or disconnection (all PsFDR > 0.084).
As last analysis, considering the results of the RLSM, we performed a partial correlation between tracts percentage of damage/disconnection and phonemic fluency post-surgery scores using the proportion of lesioned left superior frontal gyrus as covariate; the same analysis, for what concern semantic fluency post-surgery scores, was performed using the proportion of lesioned left superior temporal gyrus as covariate. No significant results emerged after FDR correction (all PsFDR > 0.056, see Supplementary Table 4 for full results).
4. Discussion
The role of frontal and/or temporal cortices and subcortical connections in phonemic and semantic fluency are still debated. To better understand the involvement of frontal and temporal cortical regions and subcortical connecting pathways in these tasks we performed an original disconnection analysis, based on the patients’ own pre-operative tractography, correlating the volume and percentage of fiber damage and disconnection due to surgical resection with the post-operative cognitive scores in LGG and HGG patients. Lexical retrieval on phonemic and semantic cues are similar tasks (Lezak et al., 2004), but they rely on different networks (Billingsley et al., 2004, Birn et al., 2010, Chouiter et al., 2016), involving different cortical areas (Baldo et al., 2010, Baldo et al., 2006) and different WM pathways as revealed by the present study. Our cohort was balanced for sex, awake or asleep surgery, presence of MGMT methylation, lesion side and site, while it differed in the proportion of HGG and LGG and in the presence of IDH mutation; these features, however, did not affect the outcome. A significant effect on post-operative and FU results was observed only when considering lesion side, with left-hemisphere lesions being associated to a worse performance compared to right-brain damage. This is in accordance with previous studies, that identified a major involvement of the left hemisphere compared to the right hemisphere in verbal fluency (Baldo et al., 2006, Biesbroek et al., 2021, Biesbroek et al., 2016, Cipolotti et al., 2021, Schmidt et al., 2019).
With respect to both phonemic and semantic fluency, a transient impairment was observed immediately after surgery, systematically followed by a significant improvement after one month, with scores comparable to the pre-surgical values. Similar results, with a transient impairment immediately after surgery followed by a general recovery, are reported in the literature both for LGGs and HGGs (see for instance Dallabona et al., 2017, Duffau et al., 2003, Ng et al., 2020, Papagno et al., 2012, Zigiotto et al., 2020). Moreover, the analyses performed separately for patients with left or right tumors confirmed a worse performance in both fluency tests after a lesion of the left hemisphere. While surgery may potentially reduce white matter fibers compression (Lazar et al., 2006), the transient impairment observed immediately after surgery, for resection of left-hemisphere tumors, is related to a partial damage of cortical and subcortical structures.
Our analysis of the relationship between cortical lesions and impairment in verbal fluency one week after surgery confirmed the involvement of the left superior frontal gyrus in phonemic fluency and of the left superior temporal gyrus in semantic fluency, in accordance with previous results that identified a cortical dissociation (Baldo et al., 2006, Billingsley et al., 2004, Henry and Crawford, 2004, Hirshorn and Thompson-Schill, 2006, Schmidt et al., 2019). Interestingly, in the FU assessment no negative relationships were found between cortical lesions and fluencies: this result reinforces the idea of a functional reorganization, thanks to the cortical plasticity, that may occur after glioma resection, in particular for motor function and language (Cirillo et al., 2019, Conway et al., 2017, Duffau, 2020, Duffau, 2021, Duffau, 2020, Duffau, 2014, Duffau et al., 2003, Saviola et al., 2022). Indeed, cortical areas nearby the tumor may have plastically reorganized their functions, in order to compensate the brain lesion (Duffau, 2020, Duffau, 2021, Duffau, 2014, Duffau et al., 2003).
Disconnection analyses revealed that lower scores in phonemic fluency were related to left FAT and IFOF partial damage/disconnection, specifically in its frontal part. The left FAT plays a pivotal role in speech production and initiation (Chernoff et al., 2019, Dragoy et al., 2020, Kemerdere et al., 2016, Kinoshita et al., 2015), and implications in working memory and executive functions (in particular, inhibition) have been reported (see Burkhardt et al., 2021, for a review). Interestingly, these last cognitive processes are also involved in lexical retrieval on phonemic cue (Robinson et al., 2012, Shao et al., 2014, Troyer et al., 1997, Unsworth et al., 2011). The involvement of the FAT in verbal fluency on phonemic cue is in line with previous results (Catani et al., 2013) on neurodegenerative diseases. Moreover, superior cortical terminations of this tract are found in the Supplementary Motor Area (SMA and preSMA) (Burkhardt et al., 2021, Catani et al., 2013): a lesion in this part of the superior frontal gyrus, anterior to the precentral gyrus, may lead to the so-called SMA syndrome, implying transient akinesia, mutism and dysexecutive deficits (Bannur and Rajshekhar, 2000, Sjöberg et al., 2019, Vergani et al., 2014). At the same time, the SMA is connected by the FAT to the inferior frontal gyrus, in particular to Broca’s area (pars opercularis and pars triangularis) (Burkhardt et al., 2021, Catani et al., 2013), which is involved in speech articulation and phonemic fluency (Birn et al., 2010, Cattaneo et al., 2011, Heim et al., 2008, Sarubbo et al., 2020, Zacà et al., 2018). This same pattern of frontal cortical connectivity was confirmed for the outer layer of the IFOF (De benedictis et al., 2021, Duffau, 2015, Duffau, 2020, Sarubbo et al., 2013): therefore, the association between damage to the frontal part of this tract (despite its classical association with the semantic system (De Benedictis et al., 2021) and difficulties in phonemic fluency is not surprising, especially considering the cognitive load requested by this not purely linguistic test, and the role of the IFOF in lexical retrieval and in verbal perseveration (Khan et al., 2014). Interestingly, no association with resection of the classic dorsal phonological pathway (i.e., SLF and AF) was observed: this confirms that phonemic fluency is more affected by executive and lexical impairment (Baldo and Shimamura, 1998, Robinson et al., 2012, Shao et al., 2014, Troyer et al., 1997) rather than by a disruption of the phonological pathway (Hickok and Poeppel, 2004). However, considering for instance the small percentage of damage/disconnection of the CST (see Supplementary Table 2) and the lack of motor deficits in our cohort, it must also be considered that at least a certain amount of tracts damage/disconnection may be necessary in order to produce a specific deficit.
Concerning semantic fluency, an association was observed with lesions of the left UF, ILF and of the temporal part of the IFOF. These tracts represent the ventral stream of language and are crucial for semantic association and processing (Duffau et al., 2013). Stimulating the IFOF along its entire course during awake surgery systematically induces semantic paraphasias and errors in semantic processing and pictures category association (Almairac et al., 2015, De benedictis et al., 2021, Duffau, 2015, Duffau, 2020, Moritz-Gasser et al., 2013, Sarubbo et al., 2016). Considering the multiple components of the IFOF (Sarubbo et al., 2013, Wu et al., 2016), which connects the frontal, the temporal, the parietal and the occipital lobe and its role in several cognitive domains (De Benedictis et al., 2021), the association of a frontal or a temporal cortical lesion with a lesion of a frontal or temporal part of this bundle may explain the different role of the IFOF in phonemic or semantic fluency.
The left ILF is involved in word retrieval on visual cue, semantic autobiographic memory, learning and processing (Herbet et al., 2019, Herbet et al., 2018, Herbet et al., 2016b, Zemmoura et al., 2021). However, the exact role of this fiber tract in semantic knowledge is still debated (Herbet et al., 2018, Mandonnet et al., 2007, Zemmoura et al., 2021). Both ILF and UF end in the anterior temporal lobe: indeed, the left UF is considered to play a role in semantic processing and access (Han et al., 2013, Papagno et al., 2016, Papagno et al., 2011b, Sarubbo et al., 2016). The involvement of the UF in semantic fluency is also in line with the literature on neurodegenerative diseases (Catani et al., 2013) showing that the UF is impaired in semantic dementia, and its damage correlates with semantic fluency.
Finally, in our series, semantic fluency also negatively correlated with volume of damage and percentage of disconnection of the left AF: this result is not in line with the distinction between dorsal (phonemic) and ventral (semantic) streams, as the AF is usually associated with phonological deficit (Duffau, 2015, Duffau, 2020, Sarubbo et al., 2020, Shinoura et al., 2013). Nevertheless, an involvement of the AF in semantic dementia (Agosta et al., 2010), in verbal memory tasks (Catani et al., 2007, Catani and Mesulam, 2008) and in semantic association (Dick and Tremblay, 2012) has been reported. Moreover, virtual and ex vivo dissection studies revealed a double sub-component of the left AF, and the “dorsal” component of the AF is demonstrated to subserve lexical and semantic language processing (Fernández-Miranda et al., 2015, Glasser and Rilling, 2008, Vavassori et al., 2021, Yagmurlu et al., 2016); thus, we cannot exclude a possible “multimodal” functional role of this large fibers pathways with so distributed cortical terminations. In this context, it has been recently pointed out that AF fibers project beyond the superior temporal gyrus, also reaching the middle and the inferior temporal gyrus, as well the temporo-basal region, which are considered a part of the classical ventral stream network; indeed, these regions may represent an integration between the dorsal and the ventral networks (Giampiccolo and Duffau, 2022). Alternatively, this result could be explained by taking into account brain plasticity: in the presence of a lesion in a cortical area that is critical for a specific network, the brain can reorganize (Desmurget et al., 2007, Duffau, 2008, Duffau, 2006). For instance, studies on aphasic patients showed the inclusion in the language network of quiescent areas of the right hemisphere or unaffected regions that are usually involved in different domains (Forkel et al., 2014, Stefaniak et al., 2020, Ueno et al., 2011). Indeed, a recent study concerning fluency abilities based on 1231 stroke patients revealed the involvement of the left superior temporal gyrus and of the temporal pole also in phonemic fluency (Biesbroek et al., 2021). Regarding subcortical connections, a few studies revealed that in the dual stream language model, plasticity-related changes may modify the normal networks even immediately after damage, engaging the undamaged pathway in order to compensate the damaged one (Stefaniak et al., 2020, Ueno et al., 2011). Also, Hula et al. (Hula et al., 2020) recently suggested the role of this pathway in semantic processing in post-stroke aphasia. Importantly, those results on language network and pathways contribution and functions were also found in glioma patients: for instance, Papagno et al. (Papagno et al., 2011a) reported the involvement of the posterior part of the AF in semantic tasks during awake surgery for LGG resection.
Taken together, our results depict two distinct cortical and subcortical pathways for verbal fluency, both located in the left hemisphere: 1) a ventral pathway for semantic fluency, involving the ILF, the UF, and the temporal part of the IFOF, as well as the superior temporal gyrus, partially corresponding to the semantic language stream, confirming the cortical temporal involvement in this task (Baldo et al., 2010, Baldo et al., 2006, Biesbroek et al., 2016, Billingsley et al., 2004, Chouiter et al., 2016) with a possible supporting role of AF fibers (direct fronto-temporal connections of the superior longitudinal system, SLS (Mandonnet et al., 2018)); 2) an anterior pathway for phonemic fluency, involving the FAT, the frontal part of the IFOF and the superior frontal gyrus, reflecting at least a part of the executive and motor programming circuits related to SMA/pre-SMA areas and the inferior frontal gyrus (Baldo et al., 2010, Baldo et al., 2006, Baldo et al., 2001, Baldo and Shimamura, 1998, Hirshorn and Thompson-Schill, 2006, Robinson et al., 2012). Moreover, the results of the partial correlation (see Supplementary Table 4) confirm that phonemic and semantic fluency are mediated by networks that involve both cortical and subcortical structures; indeed, removing the effects of cortical lesions in the SFG (for phonemic fluency) and in the STG (for semantic fluency), the amounts of damage/disconnection of white matter tracts was no longer associated with the performance in verbal fluencies.
To the best of our knowledge, for the first time two distinct and segregated cortical and subcortical networks emerged for these tasks: a lesion of the frontal lobe and of its subcortical structures affects only phonological retrieval, while a temporal cortical and subcortical lesion affects semantic fluency.
In fact, phonemic and semantic fluency also differ in terms of underlying processes: while for phonemic fluency it is crucial to adopt a strategic plan (for example, in the case of fluency starting with letter “F”, one has to avoid word repetition and proper name but can produce all words starting with FA, then switching to FE, etc., or one can look around the room for objects starting with F), in lexical retrieval based on semantic cue, it is crucial to access the semantic system (for example, for “animals”, one may retrieve every word inside the same “animals with four legs” cluster).
Moving to the methodology, our results were obtained by using each patient’s pre-surgical tractography and not by means of a WM atlas, which appear suboptimal considering the fibers displacement occurring in gliomas (Abhinav et al., 2015, Kuhnt et al., 2012, Mandonnet et al., 2006, Nimsky et al., 2005). To test the validity of this method we also considered the CST as a control tract, as we expected no effect on verbal fluency, and indeed no correlations approached significance. From a clinical perspective, this novel approach represents a crucial improvement in disconnection analyses after tumor resection, to better characterize the different role of WM fibers in neurocognitive performance and to identify a more exact percentage of damage and disconnection (e.g. 40%, 60% or 80% of disconnection of a specific tract) and, as a consequence, of spared fibers: indeed, even if the manual segmentation of each tracts is operator dependent, using patients’ pre-operative tractography it is possible to perform a more accurate disconnection analyses considering that a) automated and semi-automated methods for tracts segmentation not always reach accurate results, especially in a clinical set, and need to be supervised (Bertò et al., 2021) and b) the use of atlases allows identifying a probability of disconnection which may be less accurate than manual segmentation, especially in the case of tracts dislocation nearby a tumor (Abhinav et al., 2015, Kuhnt et al., 2012, Mandonnet et al., 2006, Nimsky et al., 2005). Moreover, we obtained the same results using either damage or disconnection indices. In addition, to ensure that significant results were not caused by an unspecific effect of surgical procedures, we also analyzed two control tests: a non-linguistic test of divided attention (TMT-B) and a sentence comprehension test. Importantly, no significant correlation emerged. The TMT-B test was adopted for this correlation to avoid possible linguistic effects on the performance (such as word reading, which is for instance necessary in the Stroop test). With respect to the lack of association between damage to tracts that are reported to be involved in sentence comprehension, such as IFOF and UF (Friederici, 2015), this may depend on the complexity of this function and the nature of the test used for its assessment. Indeed, the testing of such a highly-integrated and multimodal process would require a test whit a high sensibility to all its components. Moreover, as shown in Supplementary Table 1, a high percentage of accuracy in sentence comprehension emerged in our cohort. Nevertheless, even if the lack of a significant correlation with those tests may depend on the limited variance of the score in our cohort, this result may also prove that the observed associations with tracts damage/disconnection and phonemic and semantic fluency are not related to the effects of surgery itself.
Finally, in a longitudinal perspective, no further correlations emerged for semantic fluency one month after surgery; crucially, in this longitudinal analysis, associations between the IFOF and the FAT volume disconnection and lexical retrieval on phonemic cue persisted. White matter fibers have low neuroplastic potential and functional compensation (Duffau, 2021, Duffau, 2020, Duffau et al., 2022, Herbet et al., 2016a, Ius et al., 2011), and previous studies demonstrated that cognitive deficits before or after surgery may be explained by the degree of damage to specific subcortical tracts (Almairac et al., 2015, Herbet et al., 2016a, Herbet et al., 2014). In a similar perspective, in our study we showed a relationship between a certain degree of tracts damage or disconnection, which does not correspond to a tract complete functional and structural disruption (see Supplementary Table 2), and a higher or lower performance in phonemic and semantic fluency. In this light, our longitudinal results indicate that semantic fluency has a fast recovery, strengthening the possibility that WM fibers usually deputed to phonological tasks (i.e., the AF) or to visually-based semantic association (i.e., the ILF) can support a network involved in lexical-semantic retrieval in the presence of a growing tumor (Herbet et al., 2016a); at the same time, the spared connections of the executive and motor network take longer to allow recovering and compensation of this function: indeed, even if fluency scores in FU were similar to scores at the pre-surgery assessment, higher degrees of tracts damage were still correlated with lower performance in phonemic fluency. In the clinical context, this result points out that caution should be taken in the treatment of left frontal gliomas involving the FAT or the frontal terminations of the parietal, occipital and temporal connection of the IFOF.
4.1. Limitations
As it often occurs in clinical studies, the main limitation is the small, heterogeneous sample size, including both HGG and LGG; nevertheless, the tumor grade did not affect the outcome. A second limitation is represented by the manual virtual dissection of each patient’s tracts and by the lack of post-operative diffusion data: this procedure is time-consuming and operator-dependent, leading to possible errors in dissection and to possible mis-registration bias considering the alignment of post-operative surgical cavity in the pre-operative diffusion space. However, this method based on the single patient’s diffusion images provides more detailed and focused results compared to those achievable with the use of atlases, which do not take into account the WM dislocation produced by the tumor; nevertheless, even if using a WM atlas, the registration of the surgical cavity in MNI coordinates must be performed, which can also lead to errors in alignment. A third limitation is represented by the lesion symptom mapping analysis upon a small sample size, which should be carefully interpreted. At the same time, this analysis is not the focus of our study, as it was used to verify the cortical damage association in our cohort; nevertheless, our result confirmed previous studies with a larger sample. Moreover, the use of this analysis in a similar sample size is often reported in the literature (see for instance Martínez-Molina et al., 2022, Viganò et al., 2022, Zhang et al., 2021). Another limitation is represented by the quality of diffusion images acquired in a 1.5T scanner (60 directions, 50 slices, 2.4 mm of voxel size); however, it must be considered that similar parameters, or also lower number of directions, are often used in clinical studies and that this protocol is considered reliable and with a good balance between quality of data and acquisition time (see for instance Ashmore et al., 2020, Becker et al., 2022, Toselli et al., 2017, Zacà et al., 2018), which is crucial in clinical practice. Parallel forms of fluency tests were not used, which is another limitation; however, it must be noted that the different stimuli and cut-off of the validated existing parallel forms would have made results of different assessment hardly comparable. Moreover, the patients’ impaired performance after surgery in both fluency tests, compared to the first assessment, indicates that results were not influenced by practice effects. Finally, a total of 4 tracts out of 294 (i.e., 1.4% of the entire samples) could not be dissected, due to the tumor mass and the absence of diffusion. However, these tracts were not considered as damaged or disconnected after surgery.
5. Conclusions
We demonstrated dissociations between frontal and temporal cortical and subcortical WM pathways for phonemic and semantic fluency, using a disconnection method based on the patient’s pre-operative tractography. More specifically, a higher damage and disconnection of the left superior frontal gyrus, of the left IFOF frontal part and of the FAT lead to impairment of phonemic fluency, while lesions of the left superior temporal gyrus and of the left IFOF temporal part, the UF, the ILF and the AF correlate with lower scores in semantic fluency immediately after surgery. Interestingly, one month after surgery the percentage of disconnection of the IFOF and of the FAT still correlated with lower scores on phonemic fluency, indicating that this frontal pathway probably needs more time for recovery.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors would like to thank the Health Department of Provincia Autonoma di Trento, the Direction Team of the Azienda Provinciale per i Servizi Sanitari and the Cassa di Risparmio di Trento e Rovereto foundation for their support to clinical and research activities of the Neurosurgical Department.
Funding
This study was supported by the Provincia Autonoma di Trento through “NeuSurPlan: an integrated approach to neurosurgery planning based on multimodal data” funding (ID 2021-D337-00094) to Azienda Provinciale per i Servizi Sanitari.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103149.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abhinav K., Yeh F.C., Mansouri A., Zadeh G., Fernandez-Miranda J.C. High-definition fiber tractography for the evaluation of perilesional white matter tracts in high-grade glioma surgery. Neuro-Oncology. 2015 doi: 10.1093/neuonc/nov113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M.L., Reich A.R., Flowers C.R. Verbal fluency characteristics of normal and aphasic speakers. Journal of Speech and Hearing Research. 1989;32:871–879. doi: 10.1044/jshr.3204.871. [DOI] [PubMed] [Google Scholar]
- Agosta F., Henry R.G., Migliaccio R., Neuhaus J., Miller B.L., Dronkers N.F., Brambati S.M., Filippi M., Ogar J.M., Wilson S.M., Gorno-Tempini M.L. Language networks in semantic dementia. Brain. 2010;133:286–299. doi: 10.1093/brain/awp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almairac F., Herbet G., Moritz-Gasser S., de Champfleur N.M., Duffau H. The left inferior fronto-occipital fasciculus subserves language semantics: a multilevel lesion study. Brain Structure and Function. 2015;220:1983–1995. doi: 10.1007/s00429-014-0773-1. [DOI] [PubMed] [Google Scholar]
- Ashmore, J., Pemberton, H.G., Crum, W.D., Jarosz, J., Barker, G.J. 2020. Implementation of clinical tractography for pre-surgical planning of space occupying lesions: An investigation of common acquisition and post-processing methods compared to dissection studies. PLOS ONE 15. e0231440. https://doi.org/10.1371/JOURNAL.PONE.0231440. [DOI] [PMC free article] [PubMed]
- Baldo J.V., Schwartz SOPHIE, Wilkins DAVID, Dronkers N.F. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. Journal of the International Neuropsychological Society. 2006;12(06) doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- Baldo J.V., Schwartz S., Wilkins D.P., Dronkers N.F. Double dissociation of letter and category fluency following left frontal and temporal lobe lesions. Aphasiology. 2010;24(12):1593–1604. [Google Scholar]
- Baldo J.v., Shimamura A.P. Letter and category fluency in patients with frontal lobe lesions. Neuropsychology. 1998;12:259–267. doi: 10.1037//0894-4105.12.2.259. [DOI] [PubMed] [Google Scholar]
- Baldo J.V., Shimamura A.P., Delis D.C., Kramer JOEL, Kaplan EDITH. Verbal and design fluency in patients with frontal lobe lesions. Journal of the International Neuropsychological Society. 2001;7(5):586–596. doi: 10.1017/s1355617701755063. [DOI] [PubMed] [Google Scholar]
- Bannur U., Rajshekhar V. Post operative supplementary motor area syndrome: Clinical features and outcome. British Journal of Neurosurgery. 2000;14:204–210. doi: 10.1080/026886900408379. [DOI] [PubMed] [Google Scholar]
- Bates E., Wilson S.M., Saygin A.P., Dick F., Sereno M.I., Knight R.T., Dronkers N.F. Voxel-based lesion–symptom mapping. Nat Neurosci. 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Becker, D., Neher, P., Jungk, C., Jesser, J., Pflüger, I., Brinster, R., Bendszus, M., Bruckner, T., Maier-Hein, K., Scherer, M., Unterberg, A., 2022. Comparison of Diffusion Signal Models for Fiber Tractography in Eloquent Glioma Surgery–Determination of Accuracy Under Awake Craniotomy Conditions. World Neurosurgery 158, e429–e440. https://doi.org/10.1016/J.WNEU.2021.11.006. [DOI] [PubMed]
- Benjamini Y., Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological) 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Bertò G., Bullock D., Astolfi P., Hayashi S., Zigiotto L., Annicchiarico L., Corsini F., De Benedictis A., Sarubbo S., Pestilli F., Avesani P., Olivetti E. Classifyber, a robust streamline-based linear classifier for white matter bundle segmentation. Neuroimage. 2021;224 doi: 10.1016/j.neuroimage.2020.117402. [DOI] [PubMed] [Google Scholar]
- Biesbroek J.M., Lim J.-S., Weaver N.A., Arikan G., Kang Y., Kim B.J., Kuijf H.J., Postma A., Lee B.-C., Lee K.-J., Yu K.-H., Bae H.-J., Biessels G.J. Anatomy of phonemic and semantic fluency: A lesion and disconnectome study in 1231 stroke patients. Cortex. 2021;143:148–163. doi: 10.1016/J.CORTEX.2021.06.019. [DOI] [PubMed] [Google Scholar]
- Biesbroek J.M., van Zandvoort M.J.E., Kappelle L.J., Velthuis B.K., Biessels G.J., Postma A. Shared and distinct anatomical correlates of semantic and phonemic fluency revealed by lesion-symptom mapping in patients with ischemic stroke. Brain Structure and Function. 2016;221:2123–2134. doi: 10.1007/s00429-015-1033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingsley R.L., Simos P.G., Castillo E.M., Sarkari S., Breier J.I., Pataraia E., Papanicolaou A.C. Spatio-temporal cortical dynamics of phonemic and semantic fluency. Journal of Clinical and Experimental Neuropsychology. 2004;26:1031–1043. doi: 10.1080/13803390490515333. [DOI] [PubMed] [Google Scholar]
- Birn R.M., Kenworthy L., Case L., Caravella R., Jones T.B., Bandettini P.A., Martin A. Neural systems supporting lexical search guided by letter and semantic category cues: A self-paced overt response fMRI study of verbal fluency. Neuroimage. 2010;49:1099–1107. doi: 10.1016/j.neuroimage.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecher T., Miron S., Schneider G.G., Achiron A., Ben-Shachar M. Association between white matter microstructure and verbal fluency in patients with multiple sclerosis. Frontiers in Psychology. 2019;10:1607. doi: 10.3389/fpsyg.2019.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone M., Roussel M., Chauffert B., Le Gars D., Godefroy O. Prevalence and profile of cognitive impairment in adult glioma: a sensitivity analysis. J Neurooncol. 2016;129(1):123–130. doi: 10.1007/s11060-016-2152-7. [DOI] [PubMed] [Google Scholar]
- Burkhardt E., Kinoshita M., Herbet G. Functional anatomy of the frontal aslant tract and surgical perspectives. J Neurosurg Sci. 2021;65(6) doi: 10.23736/S0390-5616.21.05344-3. [DOI] [PubMed] [Google Scholar]
- Capasso R., Miceli G. Springer Science & Business Media; 2001. Esame Neuropsicologico per l’Afasia: ENPA. [Google Scholar]
- Capitani, E., Laiacona, M., 2009. Aging and psychometric diagnosis of intellectual impairment: Some considerations on test scores and their use. http://dx.doi.org.proxy.unimib.it/10.1080/87565648809540416 4, 325–330. https://doi.org/10.1080/87565648809540416.
- Catani M., Allin M.P.G., Husain M., Pugliese L., Mesulam M.M., Murray R.M., Jones D.K. Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci U S A. 2007;104:17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Dell’Acqua F., Vergani F., Malik F., Hodge H., Roy P., Valabregue R., Thiebaut de Schotten M. Short frontal lobe connections of the human brain. Cortex. 2012;48:273–291. doi: 10.1016/j.cortex.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Catani M., Howard R.J., Pajevic S., Jones D.K. Virtual in Vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M., Jones D.K., Donato R., Ffytche D.H. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Catani M., Jones D.K., Ffytche D.H. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catani M., Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex. 2008;44:953–961. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Mesulam M.M., Jakobsen E., Malik F., Martersteck A., Wieneke C., Thompson C.K., Thiebaut de Schotten M., Dell’Acqua F., Weintraub S., Rogalski E. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain. 2013;136:2619–2628. doi: 10.1093/brain/awt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z., Pisoni A., Papagno C. Transcranial direct current stimulation over Broca’s region improves phonemic and semantic fluency in healthy individuals. Neuroscience. 2011;183:64–70. doi: 10.1016/j.neuroscience.2011.03.058. [DOI] [PubMed] [Google Scholar]
- Chernoff B.L., Sims M.H., Smith S.O., Pilcher W.H., Mahon B.Z. Direct electrical stimulation of the left frontal aslant tract disrupts sentence planning without affecting articulation. Cognitive Neuropsychology. 2019;36:178–192. doi: 10.1080/02643294.2019.1619544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouiter L., Holmberg J., Manuel A.L., Colombo F., Clarke S., Annoni J.M., Spierer L. Partly segregated cortico-subcortical pathways support phonologic and semantic verbal fluency: A lesion study. Neuroscience. 2016;329:275–283. doi: 10.1016/j.neuroscience.2016.05.029. [DOI] [PubMed] [Google Scholar]
- Cipolotti L., Xu T., Harry B., Mole J., Lakey G., Shallice T., Chan E., Nachev P. Multi-model mapping of phonemic fluency. Brain Communications. 2021;3 doi: 10.1093/BRAINCOMMS/FCAB232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo S., Caulo M., Pieri V., Falini A., Castellano A. Role of Functional Imaging Techniques to Assess Motor and Language Cortical Plasticity in Glioma Patients: A Systematic Review. Neural Plasticity. 2019;2019:1–16. doi: 10.1155/2019/4056436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochereau J., Lemaitre A.L., Wager M., Moritz-Gasser S., Duffau H., Herbet G. Network-behavior mapping of lasting executive impairments after low-grade glioma surgery. Brain Structure and Function. 2020;225:2415–2429. doi: 10.1007/s00429-020-02131-5. [DOI] [PubMed] [Google Scholar]
- Coello A.F., Moritz-Gasser S., Martino J., Martinoni M., Matsuda R., Duffau H. Selection of intraoperative tasks for awake mapping based on relationships between tumor location and functional networks. Journal of Neurosurgery. 2013;119(6):1380–1394. doi: 10.3171/2013.6.JNS122470. [DOI] [PubMed] [Google Scholar]
- Conway N., Wildschuetz N., Moser T., Bulubas L., Sollmann N., Tanigawa N., Meyer B., Krieg S.M. Cortical plasticity of motor-eloquent areas measured by navigated transcranial magnetic stimulation in patients with glioma. Journal of Neurosurgery. 2017;127:981–991. doi: 10.3171/2016.9.JNS161595. [DOI] [PubMed] [Google Scholar]
- Dallabona M., Sarubbo S., Merler S., Corsini F., Pulcrano G., Rozzanigo U., Barbareschi M., Chioffi F. Impact of mass effect, tumor location, age, and surgery on the cognitive outcome of patients with high-grade gliomas: A longitudinal study. Neuro-Oncology Practice. 2017;4:229–240. doi: 10.1093/nop/npw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedictis A., Duffau H., Paradiso B., Grandi E., Balbi S., Granieri E., Colarusso E., Chioffi F., Marras C.E., Sarubbo S. Anatomo-functional study of the temporo-parieto-occipital region: Dissection, tractographic and brain mapping evidence from a neurosurgical perspective. Journal of Anatomy. 2014;225:132–151. doi: 10.1111/joa.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De benedictis A., Marras C.E., Petit L., Sarubbo S. The inferior fronto-occipital fascicle: a century of controversies from anatomy theaters to operative neurosurgery. J Neurosurg Sci. 2021;65(6) doi: 10.23736/S0390-5616.21.05360-1. [DOI] [PubMed] [Google Scholar]
- Desmurget M., Bonnetblanc F., Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain. 2007;130:898–914. doi: 10.1093/BRAIN/AWL300. [DOI] [PubMed] [Google Scholar]
- Diao L., Yu H., Zheng J., Chen Z., Huang D., Yu L. Abnormalities of the uncinate fasciculus correlate with executive dysfunction in patients with left temporal lobe epilepsy. Magnetic Resonance Imaging. 2015;33:544–550. doi: 10.1016/j.mri.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Dick A.S., Tremblay P. Beyond the arcuate fasciculus: Consensus and controversy in the connectional anatomy of language. Brain. 2012;135(12):3529–3550. doi: 10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- Dragoy O., Zyryanov A., Bronov O., Gordeyeva E., Gronskaya N., Kryuchkova O., Klyuev E., Kopachev D., Medyanik I., Mishnyakova L., Pedyash N., Pronin I., Reutov A., Sitnikov A., Stupina E., Yashin K., Zhirnova V., Zuev A. Functional linguistic specificity of the left frontal aslant tract for spontaneous speech fluency: Evidence from intraoperative language mapping. Brain and Language. 2020;208 doi: 10.1016/j.bandl.2020.104836. [DOI] [PubMed] [Google Scholar]
- Duffau H. The death of localizationism: The concepts of functional connectome and neuroplasticity deciphered by awake mapping, and their implications for best care of brain-damaged patients. Revue Neurologique. 2021;177:1093–1103. doi: 10.1016/J.NEUROL.2021.07.016. [DOI] [PubMed] [Google Scholar]
- Duffau, H., 2020. Functional Mapping before and after Low-Grade Glioma Surgery: A New Way to Decipher Various Spatiotemporal Patterns of Individual Neuroplastic Potential in Brain Tumor Patients. Cancers 2020, Vol. 12, Page 2611 12, 2611. https://doi.org/10.3390/CANCERS12092611. [DOI] [PMC free article] [PubMed]
- Duffau H., Zalc B. Stimulation Mapping of Myelinated Tracts in Awake Patients. Brain Plasticity. 2016;2(1):99–113. doi: 10.3233/BPL-160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H. Stimulation mapping of white matter tracts to study brain functional connectivity. Nature Reviews. Neurology. 2015;11(5):255–265. doi: 10.1038/nrneurol.2015.51. [DOI] [PubMed] [Google Scholar]
- Duffau H. The huge plastic potential of adult brain and the role of connectomics: New insights provided by serial mappings in glioma surgery. Cortex. 2014;58:325–337. doi: 10.1016/J.CORTEX.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Duffau H. Brain plasticity and tumors. Adv Tech Stand Neurosurg. 2008;33:3–33. doi: 10.1007/978-3-211-72283-1_1. [DOI] [PubMed] [Google Scholar]
- Duffau H. Brain plasticity: From pathophysiological mechanisms to therapeutic applications. Journal of Clinical Neuroscience. 2006;13:885–897. doi: 10.1016/J.JOCN.2005.11.045. [DOI] [PubMed] [Google Scholar]
- Duffau H., Capelle L., Denvil D., Sichez N., Gatignol P., Lopes M., Mitchell M.C., Sichez J.P., van Effenterre R. Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. Journal of Neurology, Neurosurgery & Psychiatry. 2003;74:901–907. doi: 10.1136/JNNP.74.7.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H., Herbet G., Moritz-Gasser S. Toward a pluri-component, multimodal, and dynamic organization of the ventral semantic stream in humans: lessons from stimulation mapping in awake patients. Frontiers in Systems Neuroscience. 2013;7:44. doi: 10.3389/fnsys.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau, H., Latini, F., Magge, R., Nakada, M., 2022. White Matter Tracts and Diffuse Lower-Grade Gliomas: The Pivotal Role of Myelin Plasticity in the Tumor Pathogenesis, Infiltration Patterns, Functional Consequences and Therapeutic Management. Frontiers in Oncology 12, 855587–855587. https://doi.org/10.3389/FONC.2022.855587. [DOI] [PMC free article] [PubMed]
- Fernández-Miranda J.C., Wang Y., Pathak S., Stefaneau L., Verstynen T., Yeh F.C. Asymmetry, connectivity, and segmentation of the arcuate fascicle in the human brain. Brain Structure and Function. 2015;220:1665–1680. doi: 10.1007/s00429-014-0751-7. [DOI] [PubMed] [Google Scholar]
- Findlater S.E., Desai J.A., Semrau J.A., Kenzie J.M., Rorden C., Herter T.M., Scott S.H., Dukelow S.P. Central perception of position sense involves a distributed neural network – Evidence from lesion-behavior analyses. Cortex. 2016;79:42–56. doi: 10.1016/J.CORTEX.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Forkel S.J., Thiebaut de Schotten M., Dell’Acqua F., Kalra L., Murphy D.G.M., Williams S.C.R., Catani M. Anatomical predictors of aphasia recovery: A tractography study of bilateral perisylvian language networks. Brain. 2014;137:2027–2039. doi: 10.1093/brain/awu113. [DOI] [PubMed] [Google Scholar]
- Foulon C., Cerliani L., Kinkingnéhun S., Levy R., Rosso C., Urbanski M., Volle E., Thiebaut de Schotten M. Advanced lesion symptom mapping analyses and implementation as BCBtoolkit. Gigascience. 2018 doi: 10.1093/gigascience/giy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A.D. White-matter pathways for speech and language processing. Handbook of Clinical Neurology. 2015;129:177–186. doi: 10.1016/B978-0-444-62630-1.00010-X. [DOI] [PubMed] [Google Scholar]
- Garyfallidis E., Brett M., Amirbekian B., Rokem A., van der Walt S., Descoteaux M., Nimmo-Smith I. Dipy, a library for the analysis of diffusion MRI data. Frontiers in Neuroinformatics. 2014;8:8. doi: 10.3389/FNINF.2014.00008/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanavati E., Salehinejad M.A., Nejati V., Nitsche M.A. Differential role of prefrontal, temporal and parietal cortices in verbal and figural fluency: Implications for the supramodal contribution of executive functions. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-40273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampiccolo, D., Duffau, H., 2022. Controversy over the temporal cortical terminations of the left arcuate fasciculus: a reappraisal. Brain. https://doi.org/10.1093/BRAIN/AWAC057. [DOI] [PubMed]
- Giovagnoli A.R., Del Pesce M., Mascheroni S., Simoncelli M., Laiacona M., Capitani E. Trail making test: normative values from 287 normal adult controls. The Italian Journal of Neurological Sciences. 1996;17(4):305–309. doi: 10.1007/BF01997792. [DOI] [PubMed] [Google Scholar]
- Glasser M.F., Rilling J.K. DTI tractography of the human brain’s language pathways. Cerebral Cortex. 2008;18:2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Han Z., Ma Y., Gong G., He Y., Caramazza A., Bi Y. White matter structural connectivity underlying semantic processing: Evidence from brain damaged patients. Brain. 2013;136:2952–2965. doi: 10.1093/brain/awt205. [DOI] [PubMed] [Google Scholar]
- Hau J., Sarubbo S., Houde J.C., Corsini F., Girard G., Deledalle C., Crivello F., Zago L., Mellet E., Jobard G., Joliot M., Mazoyer B., Tzourio-Mazoyer N., Descoteaux M., Petit L. Revisiting the human uncinate fasciculus, its subcomponents and asymmetries with stem-based tractography and microdissection validation. Brain Structure and Function. 2017;222:1645–1662. doi: 10.1007/s00429-016-1298-6. [DOI] [PubMed] [Google Scholar]
- Hau J., Sarubbo S., Perchey G., Crivello F., Zago L., Mellet E., Jobard G., Joliot M., Mazoyer B.M., Tzourio-Mazoyer N., Petit L. Cortical Terminations of the Inferior Fronto-Occipital and Uncinate Fasciculi: Anatomical Stem-Based Virtual Dissection. Frontiers in Neuroanatomy. 2016;10:1–14. doi: 10.3389/fnana.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S., Eickhoff S.B., Amunts K. Specialisation in Broca’s region for semantic, phonological, and syntactic fluency? Neuroimage. 2008;40:1362–1368. doi: 10.1016/j.neuroimage.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Henry J.D., Crawford J.R. A meta-analytic review of verbal fluency performance in patients with traumatic brain injury. Neuropsychology. 2004;18:621–628. doi: 10.1037/0894-4105.18.4.621. [DOI] [PubMed] [Google Scholar]
- Herbet G., Lafargue G., Bonnetblanc F., Moritz-Gasser S., Menjot De Champfleur N., Duffau H. Inferring a dual-stream model of mentalizing from associative white matter fibres disconnection. Brain. 2014;137:944–959. doi: 10.1093/brain/awt370. [DOI] [PubMed] [Google Scholar]
- Herbet G., Lafargue G., Moritz-Gasser S., Bonnetblanc F., Duffau H. Interfering with the neural activity of mirror-related frontal areas impairs mentalistic inferences. Brain Structure and Function. 2015;220:2159–2169. doi: 10.1007/s00429-014-0777-x. [DOI] [PubMed] [Google Scholar]
- Herbet G., Maheu M., Costi E., Lafargue G., Duffau H. Mapping neuroplastic potential in brain-damaged patients. Brain. 2016;139:829–844. doi: 10.1093/BRAIN/AWV394. [DOI] [PubMed] [Google Scholar]
- Herbet G., Moritz-Gasser S., Boiseau M., Duvaux S., Cochereau J., Duffau H. Converging evidence for a cortico-subcortical network mediating lexical retrieval. Brain. 2016;139(11):3007–3021. doi: 10.1093/brain/aww220. [DOI] [PubMed] [Google Scholar]
- Herbet G., Moritz-Gasser S., Lemaitre A.L., Almairac F., Duffau H. Functional compensation of the left inferior longitudinal fasciculus for picture naming. Cognitive Neuropsychology. 2019;36:140–157. doi: 10.1080/02643294.2018.1477749. [DOI] [PubMed] [Google Scholar]
- Herbet G., Zemmoura I., Duffau H. Functional Anatomy of the Inferior Longitudinal Fasciculus: From Historical Reports to Current Hypotheses. Frontiers in Neuroanatomy. 2018 doi: 10.3389/fnana.2018.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hirshorn E.A., Thompson-Schill S.L. Role of the left inferior frontal gyrus in covert word retrieval: Neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44:2547–2557. doi: 10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Howells H., Puglisi G., Leonetti A., Vigano L., Fornia L., Simone L., Forkel S.J., Rossi M., Riva M., Cerri G., Bello L. The role of left fronto-parietal tracts in hand selection: Evidence from neurosurgery. Cortex. 2020;128:297–311. doi: 10.1016/j.cortex.2020.03.018. [DOI] [PubMed] [Google Scholar]
- Howells H., Thiebaut de Schotten M., Dell’Acqua F., Beyh A., Zappalà G., Leslie A., Simmons A., Murphy D.G., Catani M. Frontoparietal tracts linked to lateralized hand preference and manual specialization. Cerebral Cortex. 2018;28:2482. doi: 10.1093/cercor/bhy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hula W.D., Panesar S., Gravier M.L., Yeh F.C., Dresang H.C., Dickey M.W., Fernandez-Miranda J.C. Structural white matter connectometry of word production in aphasia: an observational study. Brain. 2020;143:2532–2544. doi: 10.1093/BRAIN/AWAA193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ius T., Angelini E., Thiebaut de Schotten M., Mandonnet E., Duffau H. Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: Towards a “minimal common brain”. Neuroimage. 2011;56:992–1000. doi: 10.1016/J.NEUROIMAGE.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. Neuroimage. 2002;17:825–841. doi: 10.1006/NIMG.2002.1132. [DOI] [PubMed] [Google Scholar]
- Jurado M.A., Mataro M., Verger K., Bartumeus F., Junque C. Phonemic and semantic fluencies in traumatic brain injury patients with focal frontal lesions. Brain Injury. 2000;14:789–795. doi: 10.1080/026990500421903. [DOI] [PubMed] [Google Scholar]
- Kamali A., Flanders A.E., Brody J., Hunter J.V., Hasan K.M. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Structure and Function. 2014;219:269–281. doi: 10.1007/s00429-012-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath H.O., Sperber C., Rorden C. Mapping human brain lesions and their functional consequences. Neuroimage. 2018;165:180–189. doi: 10.1016/J.NEUROIMAGE.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavé G., Heled E., Vakil E., Agranov E. Which verbal fluency measure is most useful in demonstrating executive deficits after traumatic brain injury? Journal of Clinical and Experimental Neuropsychology. 2011;33:358–365. doi: 10.1080/13803395.2010.518703. [DOI] [PubMed] [Google Scholar]
- Kemerdere R., de Champfleur N.M., Deverdun J., Cochereau J., Moritz-Gasser S., Herbet G., Duffau H. Role of the left frontal aslant tract in stuttering: a brain stimulation and tractographic study. Journal of Neurology. 2016;263:157–167. doi: 10.1007/s00415-015-7949-3. [DOI] [PubMed] [Google Scholar]
- Khan O.H., Herbet G., Moritz-Gasser S., Duffau H. The role of left inferior fronto-occipital fascicle in verbal perseveration: a brain electrostimulation mapping study. Brain Topogr. 2014;27(3):403–411. doi: 10.1007/s10548-013-0343-5. [DOI] [PubMed] [Google Scholar]
- Kinoshita M., de Champfleur N.M., Deverdun J., Moritz-Gasser S., Herbet G., Duffau H. Role of fronto-striatal tract and frontal aslant tract in movement and speech: an axonal mapping study. Brain Structure and Function. 2015;220:3399–3412. doi: 10.1007/s00429-014-0863-0. [DOI] [PubMed] [Google Scholar]
- Kinoshita M., Shinohara H., Hori O., Ozaki N., Ueda F., Nakada M., Hamada J.I., Hayashi Y. Association fibers connecting the Broca center and the lateral superior frontal gyrus: A microsurgical and tractographic anatomy. Clinical article. Journal of Neurosurgery. 2012;116:323–330. doi: 10.3171/2011.10.JNS11434. [DOI] [PubMed] [Google Scholar]
- Kuhnt D., Bauer M.H.A., Becker A., Merhof D., Zolal A., Richter M., Grummich P., Ganslandt O., Buchfelder M., Nimsky C. Intraoperative visualization of fiber tracking based reconstruction of language pathways in glioma surgery. Neurosurgery. 2012;70:911–919. doi: 10.1227/NEU.0b013e318237a807. [DOI] [PubMed] [Google Scholar]
- Langen C.D., Cremers L.G.M., de Groot M., White T., Ikram M.A., Niessen W.J., Vernooij M.W. Disconnection due to white matter hyperintensities is associated with lower cognitive scores. Neuroimage. 2018;183:745–756. doi: 10.1016/J.NEUROIMAGE.2018.08.037. [DOI] [PubMed] [Google Scholar]
- Lazar M., Alexander A.L., Thottakara P.J., Badie B., Field A.S. White Matter Reorganization After Surgical Resection of Brain Tumors and Vascular Malformations. AJNR. American Journal of Neuroradiology. 2006;27:1258. [PMC free article] [PubMed] [Google Scholar]
- Lezak M.D., Howieson D.B., Loring D.W., Fischer J.S. Oxford University Press; USA: 2004. Neuropsychological assessment. [Google Scholar]
- Li M., Zhang Y., Song L., Huang R., Ding J., Fang Y., Xu Y., Han Z. Structural connectivity subserving verbal fluency revealed by lesion-behavior mapping in stroke patients. Neuropsychologia. 2017;101:85–96. doi: 10.1016/j.neuropsychologia.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathologica. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- Mandonnet E., Capelle L., Duffau H. Extension of paralimbic low grade gliomas: Toward an anatomical classification based on white matter invasion patterns. Journal of Neuro-Oncology. 2006;78:179–185. doi: 10.1007/s11060-005-9084-y. [DOI] [PubMed] [Google Scholar]
- Mandonnet E., Nouet A., Gatignol P., Capelle L., Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130:623–629. doi: 10.1093/BRAIN/AWL361. [DOI] [PubMed] [Google Scholar]
- Mandonnet E., Sarubbo S., Duffau H. Proposal of an optimized strategy for intraoperative testing of speech and language during awake mapping. Neurosurgical Review. 2017;40(1):29–35. doi: 10.1007/s10143-016-0723-x. [DOI] [PubMed] [Google Scholar]
- Mandonnet E., Sarubbo S., Petit L. The nomenclature of human white matter association pathways: Proposal for a systematic taxonomic anatomical classification. Frontiers in Neuroanatomy. 2018;12:94. doi: 10.3389/FNANA.2018.00094/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang A., Schnabel J.A., Crum W.R., Modat M., Camara-Rey O., Palm C., Caseiras G.B., Jäger H.R., Ourselin S., Buzug T.M., Hawkes D.J. Consistency of parametric registration in serial MRI studies of brain tumor progression. Int J CARS. 2008;3(3-4):201–211. [Google Scholar]
- Markov N.T., Ercsey-Ravasz M.M., Ribeiro Gomes A.R., Lamy C., Magrou L., Vezoli J., Misery P., Falchier A., Quilodran R., Gariel M.A., Sallet J., Gamanut R., Huissoud C., Clavagnier S., Giroud P., Sappey-Marinier D., Barone P., Dehay C., Toroczkai Z., Knoblauch K., van Essen D.C., Kennedy H. A Weighted and Directed Interareal Connectivity Matrix for Macaque Cerebral Cortex. Cerebral Cortex. 2014;24:17–36. doi: 10.1093/CERCOR/BHS270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Molina N., Siponkoski S.-T., Pitkäniemi A., Moisseinen N., Kuusela L., Pekkola J., Laitinen S., Särkämö E.-R., Melkas S., Kleber B., Schlaug G., Sihvonen A., Särkämö T. Neuroanatomical correlates of speech and singing production in chronic post-stroke aphasia. Brain Communications. 2022 doi: 10.1093/BRAINCOMMS/FCAC001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J., De P.C., Hamer W., Berger M.S., Lawton M.T., Arnold C.M., Marco De Lucas E., Martino J., De P.C., Berger M.S., Lawton Á.M.T., Arnold Á.C.M., De Lucas E.M., Duffau H. Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Struct Funct. 2013;218:105–121. doi: 10.1007/s00429-012-0386-5. [DOI] [PubMed] [Google Scholar]
- Moritz-Gasser S., Herbet G., Duffau H. Mapping the connectivity underlying multimodal (verbal and non-verbal) semantic processing: A brain electrostimulation study. Neuropsychologia. 2013;51:1814–1822. doi: 10.1016/j.neuropsychologia.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Cognitive Resources and Dual-Task Interference Effects at Retrieval in Normal People: The Role of the Frontal Lobes and Medial Temporal Cortex. Neuropsychology. 1994;8(4):524–534. [Google Scholar]
- Nakajima R., Kinoshita M., Okita H., Shinohara H., Nakada M. Disconnection of posterior part of the frontal aslant tract causes acute phase motor functional deficit. Brain and Cognition. 2021;151 doi: 10.1016/J.BANDC.2021.105752. [DOI] [PubMed] [Google Scholar]
- Nakajima R., Yordanova Y.N., Duffau H., Herbet G. Neuropsychological evidence for the crucial role of the right arcuate fasciculus in the face-based mentalizing network: A disconnection analysis. Neuropsychologia. 2018;115:179–187. doi: 10.1016/j.neuropsychologia.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Ng J.C.H., See A.A.Q., Ang T.Y., Tan L.Y.R., Ang B.T., King N.K.K. Effects of surgery on neurocognitive function in patients with glioma: a meta-analysis of immediate post- operative and long-term follow-up neurocognitive outcomes. J Neurooncol. 2018;141:167–182. doi: 10.1007/s11060-018-03023-9. [DOI] [PubMed] [Google Scholar]
- Ng S., Herbet G., Lemaitre A.L., Cochereau J., Moritz-Gasser S., Duffau H. Neuropsychological assessments before and after awake surgery for incidental low-grade gliomas. Journal of Neurosurgery. 2020;135:871–880. doi: 10.3171/2020.7.JNS201507. [DOI] [PubMed] [Google Scholar]
- Nimsky C., Ganslandt O., Hastreiter P., Wang R., Benner T., Sorensen A.G., Fahlbusch R. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2005;56:130–137. doi: 10.1227/01.NEU.0000144842.18771.30. [DOI] [PubMed] [Google Scholar]
- Novelli G., Papagno C., Capitani E., Laicona M., Vallar G., Cappa S.F. Tre test clinic di ricerca e produzione lessicale. Taratura su soggetti normali. Archivio di Psicologia Neurologia e psichiatria. 1986;4:477–506. [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Papagno C., Casarotti A., Comi A., Gallucci M., Riva M., Bello L. Measuring clinical outcomes in neuro-oncology. A battery to evaluate low-grade gliomas (LGG) Journal of Neuro-Oncology. 2012;108(2):269–275. doi: 10.1007/s11060-012-0824-5. [DOI] [PubMed] [Google Scholar]
- Papagno C., Casarotti A., Comi A., Pisoni A., Lucchelli F., Bizzi A., Riva M., Bello L. Long-term proper name anomia after removal of the uncinate fasciculus. Brain Structure and Function. 2016;221:687–694. doi: 10.1007/s00429-014-0920-8. [DOI] [PubMed] [Google Scholar]
- Papagno, C., Gallucci, M., Casarotti, A., Castellano, A., Falini, A., Fava, E., Giussani, C., Carrabba, G., Bello, L., Caramazza, A., 2011a. Connectivity constraints on cortical reorganization of neural circuits involved in object naming. Neuroimage 55, 1306–1313. https://doi.org/https://doi.org/10.1016/j.neuroimage.2011.01.005. [DOI] [PubMed]
- Papagno C., Miracapillo C., Casarotti A., Romero Lauro L.J., Castellano A., Falini A., Casaceli G., Fava E., Bello L. What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain. 2011;134:405–414. doi: 10.1093/brain/awq283. [DOI] [PubMed] [Google Scholar]
- Petrides M., Pandya D.N. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. Journal of Comparative Neurology. 1984;228:105–116. doi: 10.1002/CNE.902280110. [DOI] [PubMed] [Google Scholar]
- Porro-Muñoz D., Olivetti E., Sharmin N., Nguyen T.B., Garyfallidis E., Avesani P. Tractome: a visual data mining tool for brain connectivity analysis. Data Mining and Knowledge Discovery. 2015;29:1258–1279. doi: 10.1007/s10618-015-0408-z. [DOI] [Google Scholar]
- Puglisi G., Howells H., Sciortino T., Leonetti A., Rossi M., Conti Nibali M., Gabriel Gay L., Fornia L., Bellacicca A., Viganò L., Simone L., Catani M., Cerri G., Bello L. Frontal pathways in cognitive control: Direct evidence from intraoperative stimulation and diffusion tractography. Brain. 2019;142:2451–2465. doi: 10.1093/brain/awz178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi, G., Sciortino, T., Rossi, M., Leonetti, A., Fornia, L., Nibali, M.C., Casarotti, A., Pessina, F., Riva, M., Cerri, G., Bello, L., 2019b. Preserving executive functions in nondominant frontal lobe glioma surgery: An intraoperative tool, in: Journal of Neurosurgery. American Association of Neurological Surgeons, pp. 474–480. https://doi.org/10.3171/2018.4.JNS18393. [DOI] [PubMed]
- Raboutet C., Sauzéon H., Corsini M.M., Rodrigues J., Langevin S., N’Kaoua B. Performance on a semantic verbal fluency task across time: Dissociation between clustering, switching, and categorical exploitation processes. Journal of Clinical and Experimental Neuropsychology. 2010;32:268–280. doi: 10.1080/13803390902984464. [DOI] [PubMed] [Google Scholar]
- Reverberi C., Laiacona M., Capitani E. Qualitative features of semantic fluency performance in mesial and lateral frontal patients. Neuropsychologia. 2006;44:469–478. doi: 10.1016/J.NEUROPSYCHOLOGIA.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Rijnen S.J.M., Kaya G., Gehring K., Verheul J.B., Wallis O.C., Sitskoorn M.M., Rutten G.J.M. Cognitive functioning in patients with low-grade glioma: effects of hemispheric tumor location and surgical procedure. Journal of Neurosurgery. 2019;133:1671–1682. doi: 10.3171/2019.8.JNS191667. [DOI] [PubMed] [Google Scholar]
- Robinson G., Shallice T., Bozzali M., Cipolotti L. The differing roles of the frontal cortex in fluency tests. Brain. 2012;135:2202–2214. doi: 10.1093/brain/aws142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojkova K., Volle E., Urbanski M., Humbert F., Dell’Acqua F., Thiebaut de Schotten M. Atlasing the frontal lobe connections and their variability due to age and education: a spherical deconvolution tractography study. Brain Structure and Function. 2016;221:1751–1766. doi: 10.1007/s00429-015-1001-3. [DOI] [PubMed] [Google Scholar]
- Rosenthal, R., 1994. Parametric measures of effect size., in: The Handbook of Research Synthesis. pp. 231–244.
- Rudà R., Angileri F.F., Ius T., Silvani A., Sarubbo S., Solari A., Castellano A., Falini A., Pollo B., Del Basso De Caro M., Papagno C., Minniti G., De Paula U., Navarria P., Nicolato A., Salmaggi A., Pace A., Fabi A., Caffo M., Lombardi G., Carapella C.M., Spena G., Iacoangeli M., Fontanella M., Germanò A.F., Olivi A., Bello L., Esposito V., Skrap M., Soffietti R. italian consensus and recommendations on diagnosis and treatment of low-grade gliomas an intersociety (siNch/aiNo/siN) document. Journal of Neurosurgical Sciences. 2020;64(4) doi: 10.23736/S0390-5616.20.04982-6. [DOI] [PubMed] [Google Scholar]
- Sarubbo S., De Benedictis A., Maldonado I.L., Basso G., Duffau H. Frontal terminations for the inferior fronto-occipital fascicle: Anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Structure and Function. 2013;218:21–37. doi: 10.1007/s00429-011-0372-3. [DOI] [PubMed] [Google Scholar]
- Sarubbo S., De Benedictis A., Merler S., Mandonnet E., Balbi S., Granieri E., Duffau H. Towards a functional atlas of human white matter. Human Brain Mapping. 2015;36(8):3117–3136. doi: 10.1002/hbm.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbo S., De Benedictis A., Merler S., Mandonnet E., Barbareschi M., Dallabona M., Chioffi F., Duffau H. Structural and functional integration between dorsal and ventral language streams as revealed by blunt dissection and direct electrical stimulation. Human Brain Mapping. 2016;37(11):3858–3872. doi: 10.1002/hbm.23281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbo S., Petit L., De Benedictis A., Chioffi F., Ptito M., Dyrby T.B. Uncovering the inferior fronto-occipital fascicle and its topological organization in non-human primates: the missing connection for language evolution. Brain Structure and Function. 2019;224:1553–1567. doi: 10.1007/S00429-019-01856-2/FIGURES/6. [DOI] [PubMed] [Google Scholar]
- Sarubbo, S., Tate, M., De Benedictis, A., Merler, S., Moritz-Gasser, S., Herbet, G., Duffau, H., 2020. Mapping critical cortical hubs and white matter pathways by direct electrical stimulation: an original functional atlas of the human brain. Neuroimage 205, 116237. [DOI] [PMC free article] [PubMed]
- Saviola F., Zigiotto L., Novello L., Zacà D., Annicchiarico L., Corsini F., Rozzanigo U., Papagno C., Jovicich J., Sarubbo S. The role of the default mode network in longitudinal functional brain reorganization of brain gliomas. Brain Structure and Function. 2022;1:1–15. doi: 10.1007/S00429-022-02490-1/FIGURES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J.D., Pandya D.N., Wang R., Dai G., D’Arceuil H.E., de Crespigny A.J., Wedeen V.J. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/BRAIN/AWL359. [DOI] [PubMed] [Google Scholar]
- Schmidt C.S.M., Nitschke K., Bormann T., Römer P., Kümmerer D., Martin M., Umarova R.M., Leonhart R., Egger K., Dressing A., Musso M., Willmes K., Weiller C., Kaller C.P. Dissociating frontal and temporal correlates of phonological and semantic fluency in a large sample of left hemisphere stroke patients. NeuroImage: Clinical. 2019;23 doi: 10.1016/J.NICL.2019.101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid H., Sebastian R., Schnur T.T., Hanayik T., Wright A., Tippett D.C., Fridriksson J., Rorden C., Hillis A.E. Important considerations in lesion-symptom mapping: Illustrations from studies of word comprehension. Human Brain Mapping. 2017;38:2990–3000. doi: 10.1002/HBM.23567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z., Janse E., Visser K., Meyer A.S. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Frontiers in Psychology. 2014;5:772. doi: 10.3389/fpsyg.2014.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoura N., Midorikawa A., Onodera T., Tsukada M., Yamada R., Tabei Y., Itoi C., Saito S., Yagi K. Damage to the left ventral, arcuate fasciculus and superior longitudinal fasciculus-related pathways induces deficits in object naming, phonological language function and writing, respectively. International Journal of Neuroscience. 2013;123:494–502. doi: 10.3109/00207454.2013.765420. [DOI] [PubMed] [Google Scholar]
- Sjöberg R.L., Stålnacke M., Andersson M., Eriksson J. The supplementary motor area syndrome and cognitive control. Neuropsychologia. 2019;129:141–145. doi: 10.1016/j.neuropsychologia.2019.03.013. [DOI] [PubMed] [Google Scholar]
- Smith D.V., Clithero J.A., Rorden C., Karnath H.-O. Decoding the anatomical network of spatial attention. Proceedings of the National Academy of Sciences. 2013;110(4):1518–1523. doi: 10.1073/pnas.1210126110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber C., Karnath H.O. Impact of correction factors in human brain lesion-behavior inference. Human Brain Mapping. 2017;38:1692–1701. doi: 10.1002/HBM.23490/FORMAT/PDF/OEBPS/PAGES/1.PAGE.XHTML. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefaniak J.D., Halai A.D., Lambon Ralph M.A. The neural and neurocomputational bases of recovery from post-stroke aphasia. Nat Rev Neurol. 2020;16(1):43–55. doi: 10.1038/s41582-019-0282-1. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M., Dell’Acqua F., Forkel S.J., Simmons A., Vergani F., Murphy D.G.M., Catani M. A lateralized brain network for visuospatial attention. Nature Neuroscience. 2011;14:1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- Toselli B., Tortora D., Severino M., Arnulfo G., Canessa A., Morana G., Rossi A., Fato M.M. Improvement in white matter tract reconstruction with constrained spherical deconvolution and track density mapping in low angular resolution data: A pediatric study and literature review. Frontiers in Pediatrics. 2017;5:182. doi: 10.3389/FPED.2017.00182/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier, J.D., Calamante, F., Connelly, A., 2010. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions, in: Proceedings of the International Society for Magnetic Resonance in Medicine. John Wiley & Sons, Inc. New Jersey, USA.
- Tournier J.D., Calamante F., Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. Neuroimage. 2007;35:1459–1472. doi: 10.1016/J.NEUROIMAGE.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Troyer A.K., Moscovitch M., Winocur G. Clustering and switching as two components of verbal fluency: Evidence from younger and older healthy adults. Neuropsychology. 1997;11:138–146. doi: 10.1037/0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- Troyer A.K., Moscovitch M., Winocur G., Alexander M.P., Stuss D. Clustering and switching on verbal fluency: The effects of focal frontal- and temporal-lobe lesions. Neuropsychologia. 1998;36:499–504. doi: 10.1016/S0028-3932(97)00152-8. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. Neuroimage. 2002;15:273–289. doi: 10.1006/NIMG.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ueno T., Saito S., Rogers T.T., Lambon Ralph M.A. Lichtheim 2: Synthesizing Aphasia and the Neural Basis of Language in a Neurocomputational Model of the Dual Dorsal-Ventral Language Pathways. Neuron. 2011;72:385–396. doi: 10.1016/J.NEURON.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Unsworth N., Spillers G.J., Brewer G.A. Variation in verbal fluency: A latent variable analysis of clustering, switching, and overall performance. Quarterly Journal of Experimental Psychology. 2011;64:447–466. doi: 10.1080/17470218.2010.505292. [DOI] [PubMed] [Google Scholar]
- Vavassori L., Sarubbo S., Petit L. Hodology of the superior longitudinal system of the human brain: a historical perspective, the current controversies, and a proposal. Brain Structure and Function. 2021;226(5):1363–1384. doi: 10.1007/s00429-021-02265-0. [DOI] [PubMed] [Google Scholar]
- Vergani F., Lacerda L., Martino J., Attems J., Morris C., Mitchell P., Thiebaut de Schotten M., Dell’Acqua F. White matter connections of the supplementary motor area in humans. Journal of Neurology, Neurosurgery and Psychiatry. 2014;85:1377–1385. doi: 10.1136/jnnp-2013-307492. [DOI] [PubMed] [Google Scholar]
- Viganò, L., Howells, H., Rossi, M., Rabuffetti, M., Puglisi, G., Leonetti, A., Bellacicca, A., Conti Nibali, M., Gay, L., Sciortino, T., Cerri, G., Bello, L., Fornia, L., 2021. Stimulation of frontal pathways disrupts hand muscle control during object manipulation. Brain. https://doi.org/10.1093/BRAIN/AWAB379. [DOI] [PMC free article] [PubMed]
- Vilasboas T., Herbet G., Duffau H. Challenging the Myth of Right Nondominant Hemisphere: Lessons from Corticosubcortical Stimulation Mapping in Awake Surgery and Surgical Implications. World Neurosurgery. 2017;103:449–456. doi: 10.1016/j.wneu.2017.04.021. [DOI] [PubMed] [Google Scholar]
- Visser M., Petr J., Müller D.M.J., Eijgelaar R.S., Hendriks E.J., Witte M., Barkhof F., van Herk M., Mutsaerts H.J.M.M., Vrenken H., de Munck J.C., de Witt Hamer P.C. Accurate MR Image Registration to Anatomical Reference Space for Diffuse Glioma. Frontiers in Neuroscience. 2020;14:585. doi: 10.3389/FNINS.2020.00585/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Sun D., Wang Y., Wang Y. Subcomponents and connectivity of the inferior fronto-occipital fasciculus revealed by diffusion spectrum imaging fiber tracking. Frontiers in Neuroanatomy. 2016;10:88. doi: 10.3389/FNANA.2016.00088/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagmurlu K., Middlebrooks E.H., Tanriover N., Rhoton A.L. Fiber tracts of the dorsal language stream in the human brain. Journal of Neurosurgery. 2016;124:1396–1405. doi: 10.3171/2015.5.JNS15455. [DOI] [PubMed] [Google Scholar]
- Zacà D., Corsini F., Rozzanigo U., Dallabona M., Avesani P., Annicchiarico L., Zigiotto L., Faraca G., Chioffi F., Jovicich J., Sarubbo S. Whole-brain network connectivity underlying the human speech articulation as emerged integrating direct electric stimulation, resting state FMRI and tractography. Frontiers in Human Neuroscience. 2018;12:405. doi: 10.3389/fnhum.2018.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarino B., Crespi M., Launi M., Casarotti A. A new standardization of semantic verbal fluency test. Neurological Sciences. 2014;35:1405–1411. doi: 10.1007/s10072-014-1729-1. [DOI] [PubMed] [Google Scholar]
- Zemmoura I., Burkhardt E., Herbet G. The inferior longitudinal fasciculus: anatomy, function and surgical considerations. J Neurosurg Sci. 2021;65(6) doi: 10.23736/S0390-5616.21.05391-1. [DOI] [PubMed] [Google Scholar]
- Zhang B., Chang J., Park J., Tan Z., Tang L., Lyu T., Han Y., Fan R., Gao Y., Kong J. Uncinate fasciculus and its cortical terminals in aphasia after subcortical stroke: A multi-modal MRI study. NeuroImage: Clinical. 2021;30 doi: 10.1016/J.NICL.2021.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigiotto L., Annicchiarico L., Corsini F., Vitali L., Falchi R., Dalpiaz C., Rozzanigo U., Barbareschi M., Avesani P., Papagno C., Duffau H., Chioffi F., Sarubbo S. Effects of supra-total resection in neurocognitive and oncological outcome of high-grade gliomas comparing asleep and awake surgery. Journal of Neuro-Oncology. 2020;148:97–108. doi: 10.1007/s11060-020-03494-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.