Abstract
Biochemical recurrence (BCR) is a cause of concern in advanced prostate cancer (PCa). Thus, novel diagnostic biomarkers are required to improve clinical care. However, research on PCa immunotherapy is also scarce. Hence, the present study aimed to explore promising BCR-related diagnostic biomarkers, and their expression pattern, prognostic value, immune response effects, biological functions, and possible molecular mechanisms were evaluated. GEO datasets (GSE46602, GSE70768, and GSE116918) were downloaded and merged as the training cohort, and differential expression analysis was performed. Lasso regression and SVM-RFE algorithm, as well as PPI analysis and MCODE algorithm, were then applied to filter BCR-related biomarker genes. The CIBERSORT and estimation of stromal and immune cells in malignant tumor tissues using expression data (ESTIMATE) methods were used to calculate the fractions of tumor-infiltrating immune cells. GO/DO enrichment analyses were used to identify the biological functions. The expression of latent transforming growth factor β-binding protein 2 (LTBP2) was determined by RT-qPCR and western blotting. The role of LTBP2 in PCa was determined by CCK-8, Transwell, and the potential mechanism was investigated by KEGG and GSEA and confirmed by western blotting. In total, 44 BCR-related differentially expressed genes (DEGs) in the training cohort were screened. LTBP2 was found to be a diagnostic biomarker of BCR in PCa and was associated with CD4+ T-cell infiltration and response to anti-PD-1/PD-L1 immunotherapy. Subsequently, using the ESTIMATE algorithm, it was identified that LTBP2 was associated with the tumor microenvironment and could be a predictor of the clinical benefit of immune checkpoint blockade. Finally, the expression and biological function of LTBP2 were evaluated via cellular experiments. The results showed that LTBP2 was downregulated in PCa cells and inhibited PCa proliferation and metastasis via the PI3K/AKT signaling pathway in vitro. In conclusion, LTBP2 was a promising diagnostic biomarker of BCR of PCa and had an important role in CD4+ T-cell recruitment. Moreover, it was associated with immunotherapy in patients with PCa who developed BCR, and it inhibited PCa proliferation and metastasis via the PI3K/AKT signaling pathway in vitro.
Keywords: prostate cancer, The Cancer Genome Atlas, Gene Expression Omnibus, tumor microenvironment, immunotherapy, LTBP2
Introduction
Prostate cancer (PCa) is the most pervasive tumor among solid male tumors, accounting for 26% of cases reported, and is also the second leading cause of tumor-associated deaths in men, accounting for 11% of cancer-specific deaths (1). Radical prostatectomy with androgen deprivation therapy (ADT) has become the basic treatment strategy for primary PCa (2). However, most primary PCa may locally relapse and develop into castration-resistant prostate cancer (CRPC) (3) or even metastatic PCa (4). Prostate-specific antigen (PSA) re-elevation following ADT, commonly known as biochemical recurrence (BCR), was the most prevalent technique to detect this problem (5). Nevertheless, PSA assays frequently failed to discover BCR or distant metastases in the first place, given its low sensitivity and specificity (6). Similarly, the clinical and pathological markers used to diagnose BCR of PCa (e.g., Gleason score, clinical and pathological stage) were still insufficient. Several diagnostic biomarkers for BCR of PCa have been described in the literature. For instance, Kim et al (7) reported that PSCA, COX-2, Ki67 were independent predictive biomarkers for BCR of PCa. Some studies have found that non-coding RNAs, such as lncRNAs play an essential role in the malignant progression and BCR of PCa (8,9). However, the exact mechanism of primary PCa progression remains unclear. Therefore, it is important to identify a stable and reliable biomarker for diagnosing BCR of PCa and to provide a guide for detecting the etiology of malignant progression in PCa and the mechanism of BCR.
Immune cells are an essential component of the tumor microenvironment (TME) and play a crucial role in tumorigenesis and progression, which has been investigated in numerous studies (10,11). Tumor immunotherapy, which activates the natural defense system of the body that is responsible for recognizing and removing bacteria, viruses, and tumor cells, is considered a promising cancer treatment modality for recurrent or metastatic cancers (12). Notably, immune checkpoint blockade (ICB) and T-cell therapy have made significant breakthroughs in improving the clinical prognosis of several types of solid tumors (13,14), demonstrating an effective response to immunotherapy for tumors. Literature (15,16) has revealed several biomarkers associated with immunity and prognosis, but few have been confirmed. Therefore, new biomarkers need to be identified as well as their association with the TME, and immunity and prognosis.
Given the aforementioned reasons, the present study aimed to identify potential diagnostic biomarkers of BCR in PCa and validate their correlation with immunity and prognosis. In this present study, latent transforming growth factor β-binding protein 2 (LTBP2) was screened and determined as a diagnostic biomarker gene associated with BCR of PCa by different algorithms, which could be confirmed by other external datasets. Next, the association between LTBP2 and immunity and prognosis was evaluated. The results revealed that the LTBP2 expression was associated with CD4+ T-cell recruitment. Moreover, the present study emphasized the important role of LTBP2 in inhibiting PCa invasion and metastasis in vitro and confirmed its exact molecular mechanism. Therefore, LTBP2 could be a novel diagnostic biomarker and potential immunotherapeutic target for BCR in PCa.
Materials and methods
Raw data sources, preparation, merging, and differential expression analysis
Available public transcriptome data for PCa from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) and The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/) were screened and downloaded based on the inclusion criteria, which were as follows: i) Sample size >30 days; ii) complete expression information of transcriptome data; and iii) sample information including the description of BCR. Relevant information is presented in Table SI. PCa samples from three datasets [GSE46602(17), GSE70768(18) and GSE116918(19)] were merged as the training cohort. The batch effect of non-biotechnical bias was eliminated using the ComBat algorithm (version 3.44.0) of the SVA package (20). Differential expression analysis was performed using the Bayesian algorithm (version 3.52.1) of the ‘limma’ package if the criteria adjusted P<0.05 was met (21). GSE70769(22) and TCGA-prostate adenocarcinoma (PRAD) dataset and corresponding clinical information were downloaded and used as validation cohorts.
Least absolute shrinkage and selection operator (Lasso) Cox regression analysis and support vector machine with recursive feature elimination (SVM-RFE) algorithm to obtain BCR-associated differential expression genes (DEGs)
Lasso regression analysis is a valuable method for identifying interpretable prediction rules in high-dimensional data, featuring a simultaneous selection of variables and elimination of high correlations among them to prevent overfitting (23). BCR-related differentially expressed genes (DEGs) were identified based on the best lambda values selected by 1,000 cross-validations using the glmnet package (version 4.1-4) in the R language (24). The SVM-RFE algorithm is essentially a backward elimination method for determining a subset of characteristics to optimize the performance of the classifier (25), which was initially designed to solve binary gene selection problems (26). The e1071 and kernlab packages in R software were used to implement SVM-RFE analysis to obtain BCR-related DEGs (27).
Gene Ontology (GO) biological function, The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotation, Disease Ontology (DO) enrichment analysis, and gene set enrichment analysis (GSEA)
GO (24) enrichment analysis of the 44 BCR-related DEGs was utilized for biological function enrichment studies involving molecular functions (MF), cellular components (CC), and biological processes (BP) using the ‘clusterProfiler (version 3.14.3) (28), enrichplot (29) and ggplot2 package (30)’ in R software (adjusted P<0.05). KEGG was widely employed to screen biological pathways (31). DO enrichment analysis was commonly used to identify large-scale disease enrichment research by clusterProfiler, GSEABase (version 1.58.0) (32), DOSE (version 3.22.0) (33), and enrichplot package in R language (adjusted P<0.05). GSEA analysis was applied to investigate potential differences in biological processes and signaling pathways in GEO merged dataset and TCGA-PRAD cohort by the ‘clusterProfiler and enrichplot package’ in R software. The gene set ‘c2.cp.kegg.v7.4.symbols.gmt’ was retrieved from the Molecular Signatures Database (MSigDB) (34), and the adjusted P-values <0.05 were regarded as statistically significant.
TME cell infiltration level and tumor-infiltrating immune cell profile, as well as correlation between immune infiltration and LTBP2 expression in PCa
Tumor cell TME infiltration level was estimated by immune score, stromal score and tumor purity for each sample using the estimation of stromal and immune cells in malignant tumor tissues using expression data (ESTIMATE) algorithm (35). The CIBERSORT algorithm was implemented to generate an estimate of the abundance distribution of each tumor cell in the tumor sample using the R package ‘e1071’ (36). Spearman correlation analysis was undertaken to determine the association between immune cell infiltration levels and the expression of LTBP2.
Gene expression and clinical benefits for ICB and TCGA pan-cancer analyses
To elucidate the interaction of LTBP2 on the ICB, the association between LTBP2 expression and three well-known immune checkpoint genes was explored using the Tumor Immune Estimation Resource (TIMER) database (37) and Gene Expression Profiling Interactive Analysis (GEPIA; http://gepia.cancer.pku.cn/) database. Transcriptomic data and corresponding clinicopathological features of TCGA pan-cancer were obtained from the UCSC Xena browser (https://xena.ucsc.edu/) and preprocessed as described above.
Establishment of protein-protein interaction (PPI) network and molecular complex detection (MCODE) analysis to identify hub genes
The Search Tool for Retrieving Interacting Genes (STRING) database (https://cn.string-db.org/) (38,39) was applied to predict the PPI network for the 44 BCR-related DEGs, with a threshold of combined score >0.4. Moreover, the MCODE algorithm was used to identify hub genes (40).
Construction of lncRNA-miRNA-LTBP2 mRNA competing endogenous RNA (ceRNA) regulatory networks
The ceRNA regulatory network is a common upstream regulatory mechanism of target genes (41). LTBP2 was considered as the target gene, and the sponging miRNA was screened via the starBase database (https://starbase.sysu.edu.cn/) (42). Spearman correlation analysis was then used to identify miRNAs that were negatively correlated with LTBP2 expression. In addition, the sponging lncRNAs were identified by the starBase database and a negative correlation with miRNAs was confirmed by Spearman correlation analysis (cor >0.3; P-value <0.01). Finally, Cytoscape software (version 3.9.1) was utilized to construct the lncRNA-miRNA-LTBP2 ceRNA regulatory network (43).
Collection of gene expression data with immunotherapy response prediction
To evaluate the predictive value of LTBP2 in immunotherapeutic response, two immunotherapy cohorts, including clinical and transcriptomic data, were downloaded. The GSE78220 cohort (44), downloaded from the GEO database, is an anti-programmed cell death protein 1 (PD-1) immunotherapy cohort containing 27 samples with complete clinical information. The IMvigor210 cohort, obtained from http://research-pub.gene.com/IMvigor210CoreBiologies/, is an anti-PD-L1 immunotherapy cohort containing 298 samples with complete clinical data.
Cell culture and cell transfection
The human prostate cell (RWPE-1; cat. no. SCSP-5025) and human PCa cell lines (LNcap; cat. no. TCHu173), PC3 (cat. no. TCHu158) and DU145 cells (cat. no. TCHu222) were originally purchased from the Cell Bank of Shanghai Institute of Life Sciences, Chinese Academy of Sciences. RPMI-1640 medium (Procell Life Science & Technology Co., Ltd.) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.), penicillin (25 U/ml) and streptomycin (25 mg/ml; Gibco; Thermo Fisher Scientific, Inc.), were used to culture prostate cells and PCa cells at 37˚C in a humidified 5% CO2 environment. The sequence of LTBP2 was cloned in to a pcDNA3.1-vector to generate overexpression plasmid constructs by Shanghai GeneChem Co., Ltd. Lipofectamine 3000 reagent (Vazyme Biotech Co., Ltd.) was used for cell transfection according to the manufacturer's protocol (45). In short, the transfection reagent was added and samples were placed in a humidified 5% CO2 environment at 37˚C for 6 h, then changed to fresh medium and performed the subsequent experiments the next day.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated and extracted from cells and clinical tissues using E.Z.N.A.® Total RNA Kit I (50 preps) (Omega Bio-Tek, Inc.). Reverse transcription was then achieved with the HiScript II Q RT SuperMix reagent kit according to the manufacturer's protocol (cat. no. R223-01; Vazyme Biotech Co., Ltd.). PCR was implemented to measure Cq values using the SYBR Green PCR kit (Vazyme Biotech Co., Ltd.) according to the manufacturer's protocol (46). In short, qPCR was performed under the conditions: Holding at 50˚C for 2 min, 95.0˚C for 30 sec and 40 circles of 95.0˚C for 10 sec and 60˚C for 30 sec in an applied biosystems 7300 Realtime PCR instrument. The 2-ΔΔCq calculation method was employed to calculate the relative expression levels of LTBP2 (47-49). The primers for LTBP2 used in the present study were as follows: LTBP2 forward, 5'-AGCACCAACCACTGTATCAAAC-3' and reverse, 5'-CTCATCGGGAATGACCTCCTC-3'; GAPDH forward, 5'-ACCATCTTCCAGGAGCGAGAT-3' and reverse, 5'-GGGCAGAGATGATGACCCTTT-3'.
Cell Counting Kit-8 (CCK-8) cell proliferation assays
CCK-8 assay Kit (BioBIO EXCELLENCE) was used to perform the cell proliferation. In brief, the transfected LNcap and DU145 cells were seeded into 96-well plates at a density of 1,500 cells/well. Following seeding for 24, 48, 72 and 96 h, 10 µl CCK-8 reagent was added to each well and then incubated for another 3 h before detecting the optical density (OD) at 450 nm.
Transwell migration and Matrigel invasion assays
Cell migration and invasion assays were implemented in 8-µm pore size Transwell chambers, distinguishing that invasion assays required 0.5 mg/ml Matrigel pretreatment (37˚C for 1 h). Specifically, the transfected PCa cells (10x104) were resuspended in a serum-free medium and inoculated into the upper chamber, and 600 µl of medium containing 10% FBS was placed in the lower chamber and incubated at 37˚C for 8-20 h. Subsequently, migrating and invading cells were fixed in methanol (20 min at room temperature), stained with 0.1% crystal violet (20 min at room temperature), and photographed and counted using a light microscope at x10 magnification.
Western blot analysis
Western blotting was conducted using the same method previously reported in the literature (50). In short, cells were lysed with RIPA buffer (Beyotime Institute of Biotechnology), and protein was extracted. The protein concentration was then quantified utilizing a BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.). Subsequently, the protein (10 µg/well) was separated by 10% sodium dodecyl sulfate-polyacrylamide gels electrophoresis (SDS-PAGE) and electroblotted to polyvinylidene fluoride membranes (PVDF) (MilliporeSigma). Following blocking with 5% skim milk for 1 h at room temperature, the membrane was incubated with various specific primary antibodies overnight at 4˚C. Next, washing with TBST (Tween, 1:1,000) followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibody (Table SII) for 1 h at room temperature. Protein bands were treated with BeyoEcl Plus reagent (Beyotime Institute of Biotechnology) after washing with TBST and observed using an ECL system.
Statistical analysis
The statistical analysis was undertaken with R software (version 4.0.3) and GraphPad Prism 7 software (GraphPad Software, Inc.). The Perl programming language (version 5.30.2) was used for data processing. The Kaplan-Meier (K-M) survival analysis and log-rank tests were utilized to analyze the overall survival (OS), progression-free interval (PFI) and disease-specific survival (DSS). The associations between LTBP2 expression and various clinicopathological covariates were examined using a chi-square test. Data were obtained from at least three independent experiments in vitro and were expressed as mean ± SD. A P-value <0.05 was considered to indicate a statistically significant difference.
Results
Identification of 44 differentially expressed mRNAs between primary PCa and BCR of PCa based on the GEO merged dataset
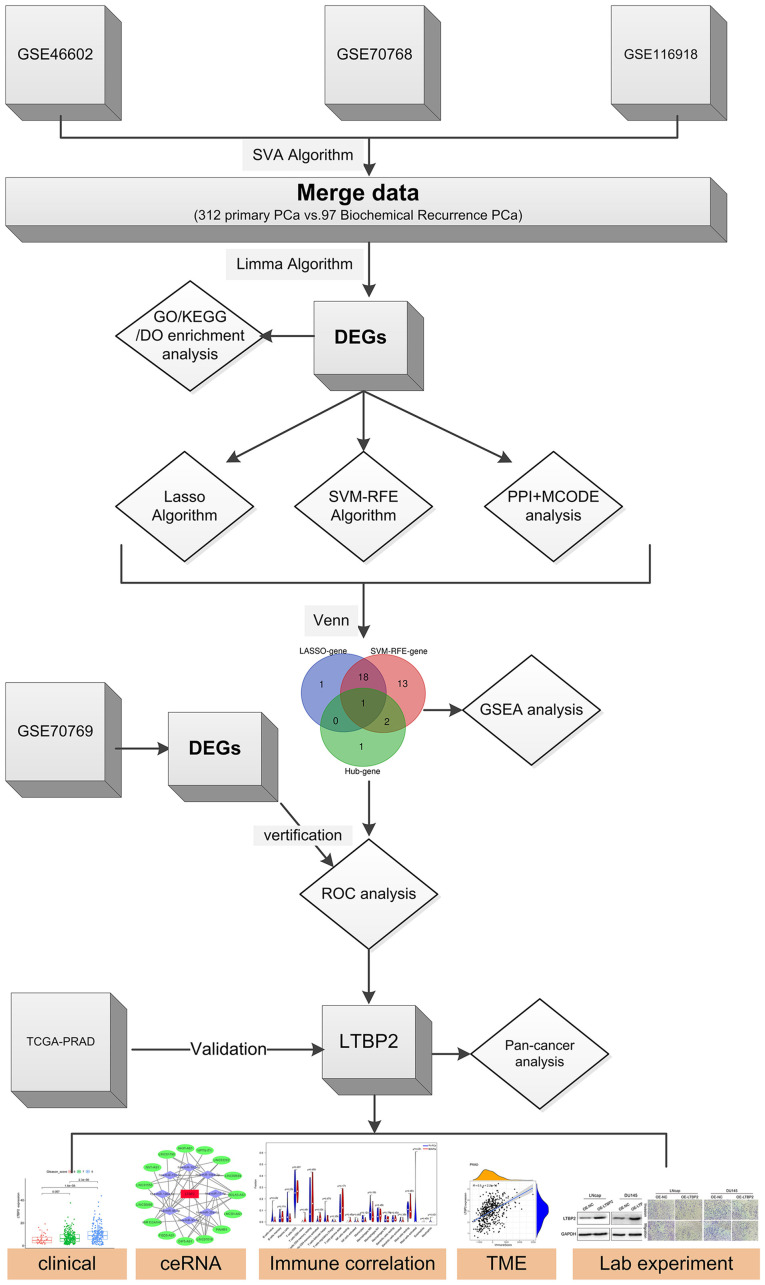
The flow chart of the present study is presented in Fig. 1. First, three GSE datasets (GSE46602, GSE70768, and GSE116918) were merged using the SVA algorithm to obtain 312 primary PCa and 97 BCR in PCa cases. The differential expression analysis of primary PCa and BCR in PCa was then performed using the R software Limma package based on the GEO merged datasets. A total of 44 BCR-related DEGs are presented in Fig. S1. Among them, 18 DEGs were in the downregulated subset and 26 in the upregulated subset.
Figure 1.
Flow chart of the work on screening and identifying a key gene associated with biochemical recurrence of prostate cancer and validating some potential biological functions. PCa, prostate cancer; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; DO, Disease Ontology; DEGs, differentially expressed genes; Lasso, least absolute shrinkage and selection operator; SVM-RFE, support vector machine with recursive feature elimination; PPI, protein-protein interaction; MCODE, molecular complex detection; GSEA, gene set enrichment analysis; ROC, receiver operating characteristic; TCGA, The Cancer Genome ATLAS; PRAD, prostate adenocarcinoma; LTBP2, latent transforming growth factor β-binding protein 2; ceRNA, competitive endogenous RNA; TME, tumor microenvironment.
Genes are screened as BCR-associated key DEGs based on two different algorithms
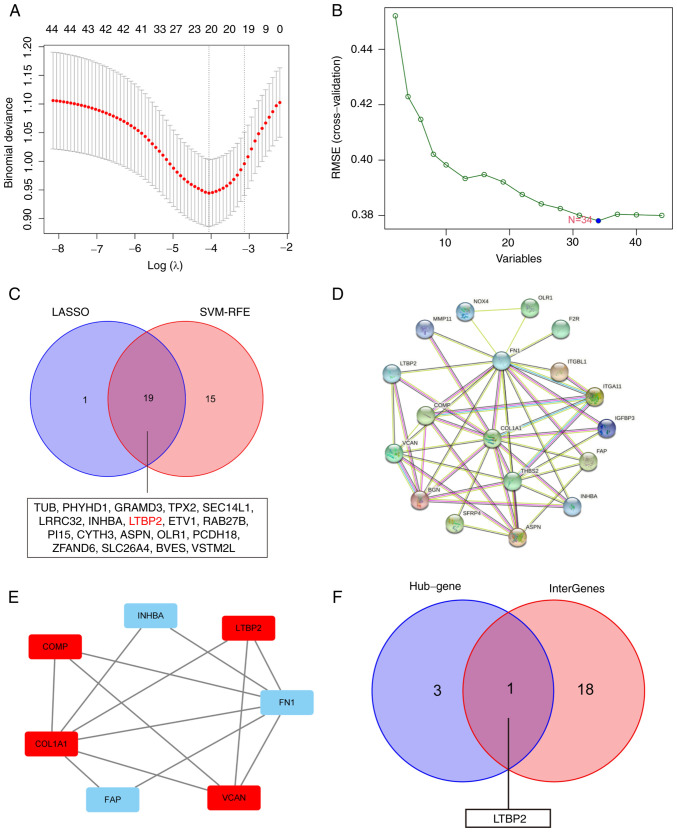
To obtain key BCR-associated DEGs, two distinct algorithms for screening were implemented. First, the Lasso Cox regression algorithm was applied, and 20 key genes were filtered out from 44 BCR-related DEGs (Fig. 2A). Similarly, the SVM-RFE algorithm was applied, and 34 key genes were screened out (Fig. 2B). Finally, 19 BCR-related DEGs were selected as candidates via overlapping (Fig. 2C).
Figure 2.
Screening and identification of LTBP2 as a key DEG associated with BCR of PCa. (A) Lasso Cox regression algorithm was applied to screen DEGs associated with BCR of PCa in GEO-merged datasets. (B) SVM-RFE algorithm was performed to identify DEGs associated with BCR of PCa in GEO-merged datasets. (C) Venn diagram showing the overlap of 19 BCR-based DEGs between the two different algorithms. (D) PPI analysis of BCR-related DEGs in PCa using STRING database. (E) The STRING outcomes were uploaded to Cytoscape software to identify hub genes using MCODE algorithm in the PPI network. (F) Venn diagram revealed that LTBP2 was the only BCR-associated hub gene. LTBP2, latent transforming growth factor β-binding protein 2; DEG, differentially expressed gene; BCR, biochemical recurrence; PCa, prostate cancer; Lasso, least absolute shrinkage and selection operator; GEO, Gene Expression Omnibus; SVM-RFE, support vector machine with recursive feature elimination; PPI, protein-protein interaction; STRING, The Search Tool for Retrieving Interacting Genes; MCODE, molecular complex detection.
Construction of a PPI network and MCODE analysis to obtain 4 hub genes
These 44 BCR-related DEGs were utilized to construct a PPI network using STRING software (Fig. 2D). The outcomes were uploaded to Cytoscape software and 4 hub genes were identified by MCODE algorithm (Fig. 2E). Ultimately, by overlapping the hub genes and key BCR-related DEGs, LTBP2 was found to be the only candidate diagnostic gene of BCR in PCa that should be investigated further (Fig. 2F).
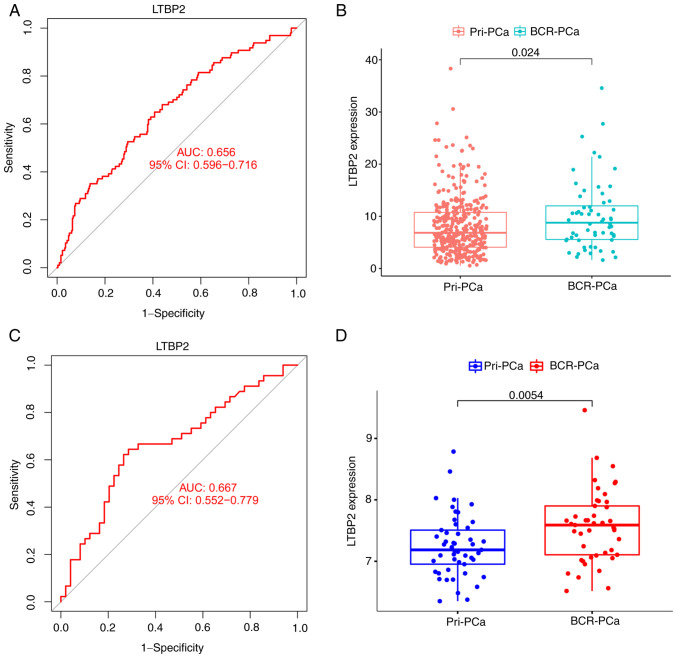
Subsequently, the ROC curve and its AUC value of GEO merged datasets (the training set) were calculated to assess the accuracy and sensitivity of LTBP2 as a BCR diagnostic gene for PCa. As indicated in Fig. 3A, LTBP2 had moderate accuracy and sensitivity, which was also consistent with the ROC curve results of the GEO validation cohort (GSE70769) (Fig. 3C). Additionally, the expression of LTBP2 in the primary PCa subgroup and the BCR of PCa subgroup was evaluated. The results demonstrated that the expression level of LTBP2 was statistically significantly higher in the BCR of PCa subgroup compared to the primary PCa subgroup in TCGA-PRAD (Fig. 3B) and GEO validation dataset (Fig. 3D).
Figure 3.
Evaluation of the accuracy and sensitivity of LTBP2 as a biomarker for BCR of PCa. (A and C) ROC curve and its AUC value in (A) GEO-merged datasets and (C) validation dataset (GSE70769). (B and D) The relative expression levels of LTBP2 in (B) TCGA-PRAD dataset and (D) a validation dataset (GSE70769). LTBP2, latent transforming growth factor β-binding protein 2; BCR, biochemical recurrence; PCa, prostate cancer; ROC, receiver operating characteristic; AUC, area under the curve; GEO, Gene Expression Omnibus; TCGA, The Cancer Genome ATLAS; PRAD, prostate adenocarcinoma; Pri-, primary.
Association of LTBP2 expression with clinicopathological features and overview of LTBP2 in human tumors in TCGA database
To assess the clinical value and application of LTBP2, the association between LTBP2 expression and clinicopathological traits and the impact on prognosis in TCGA-PRAD dataset were examined. The results revealed that LTBP2 was under-expressed in tumor tissues in the TCGA and Genotype-Tissue Expression (GTEx) database (Fig. 4A and B). Moreover, the ROC curve also clarified that the efficiency of LTBP2 expression levels was moderate in distinguishing PCa tissue from normal prostate tissue (AUC value=0.616) (Fig. 4F). Concurrently, a stratified analysis along with clinicopathological features was performed. The results highlighted in Fig. 4C-E and Table I indicated that LTBP2 expression was primarily upregulated with increased Gleason score and American Joint Committee on Cancer (AJCC) T stage (P<0.05). However, it was independent of increasing PSA value.
Figure 4.
Associations of LTBP2 expression with clinicopathological features and pan-cancer analysis. (A and B) Boxplots revealed that LTBP2 was under-expressed in tumor tissues compared with normal control tissues in (A) TCGA-PRAD dataset and (B) TCGA combined with GTEx dataset. (C-E) Boxplot indicating LTBP2 expression in different (C) Gleason-score, (D) AJCC T stage, (E) PSA-value of PCa samples from TCGA-PRAD dataset. (F) The ROC curves revealed the efficiency of LTBP2 expression levels to distinguish PCa tissues from normal prostate tissues. (G and H) Boxplots displaying the LTBP2 expression using pan-cancer analysis. (I and J) Boxplot indicating LTBP2 expression in various clinicopathological features using pan-cancer analysis. Subgroup comparison between different tumors in G-I were demonstrated using *P<0.05, **P<0.01 and ***P<0.001. LTBP2, latent transforming growth factor β-binding protein 2; TCGA, The Cancer Genome ATLAS; PRAD, prostate adenocarcinoma; GTEx, Genotype-Tissue Expression; AJCC, American Joint Committee on Cancer; PSA, prostate-specific antigen; PCa, prostate cancer; ROC, receiver operating characteristic.
Table I.
Comparison of clinical characteristics of prostate cancer patients in TCGA-PRAD database.
| Expression of LTBP2 | ||||
|---|---|---|---|---|
| Characteristics | Total | Low (%) | High (%) | P-value |
| Total samples, n | 449 | 249 | 250 | |
| Age, n (%) | 0.054 | |||
| ≤60 | 324 | 123 (24.6) | 101 (20.2) | |
| >60 | 275 | 126 (25.3) | 149 (29.9) | |
| T stage, n (%) | <0.001 | |||
| T2 | 189 | 117 (23.8) | 72 (14.6) | |
| T3 | 292 | 128(26) | 164 (33.3) | |
| T4 | 11 | 2 (0.4) | 9 (1.8) | |
| N stage, n (%) | 0.120 | |||
| N0 | 347 | 168 (39.4) | 179(42) | |
| N1 | 79 | 30(7) | 49 (11.5) | |
| M stage, n (%) | 0.99 | |||
| M0 | 455 | 228 (49.8) | 227 (49.6) | |
| M1 | 3 | 2 (0.4) | 1 (0.2) | |
| PSA (ng/ml), n (%) | 0.99 | |||
| <4 | 415 | 207 (46.8) | 208 (47.1) | |
| ≥4 | 27 | 14 (3.2) | 13 (2.9) | |
| Gleason score, n (%) | <0.001 | |||
| 6 | 46 | 33 (6.6) | 13 (2.6) | |
| 7 | 247 | 142 (28.5) | 105(21) | |
| 8 | 64 | 28 (5.6) | 36 (7.2) | |
| 9 | 138 | 45(9) | 93 (18.6) | |
| 10 | 4 | 1 (0.2) | 3 (0.6) | |
TCGA, The Cancer Genome ATLAS; PRAD, prostate adenocarcinoma; LTBP2, latent transforming growth factor β-binding protein 2; T, tumor, N, node; M, metastasis; PSA, prostate-specific antigen.
Furthermore, given the scarcity of LTBP2-associated cancer studies in solid tumors, as shown in Fig. S2, its expression was evaluated in 33 solid tumors. LTBP2 was differentially expressed in distinct cancer types and was under-expressed in numerous solid tumors compared to corresponding normal tissues (Fig. 4G and H). In addition, to describe the association between LTBP2 expression and clinicopathological features, LTBP2 expression was analyzed according to the different clinicopathological features in different solid tumors, such as the AJCC stage. Significant differences between LTBP2 expression and some clinical characteristics of different strata of certain tumors were identified (Fig. 4I and J). Moreover, a univariate Cox regression analysis was performed to assess the effect of LTBP2 on prognosis in different cancer types. The LTBP2 expression did not significantly affect OS (Fig. S3A), DSS (Fig. S3B), PFI (Fig. S3C) in PCa, paralleling the results obtained from the Kaplan-Meier survival analysis (Fig. S4).
Expression of LTBP2 is associated with CD4+ T-cell recruitment and linked to immunotherapeutic response
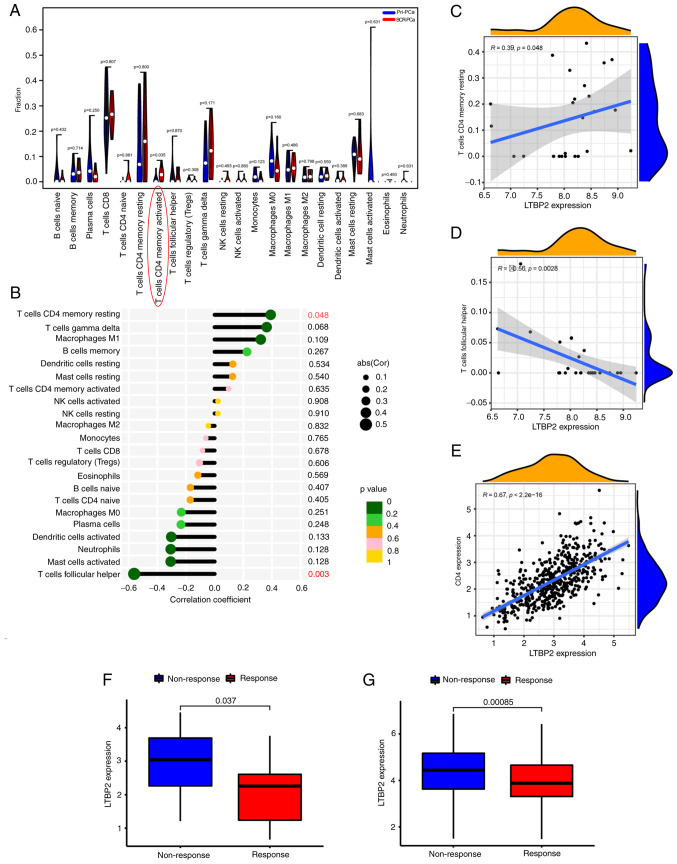
As reported in the literature, immunity plays an essential role in the development and treatment of tumors (51). Hence, the association between LTBP2 and immunity was investigated. The CIBERSORT algorithm was used to calculate the score of the tumor immune cells in pan-cancer. Spearman correlation analysis was performed and revealed that LTBP2 was associated with T-cell follicular helper and CD4+ T-cell memory resting recruitment as well as macrophage M2 polarization in TCGA-PRAD dataset in 33 solid tumors (Fig. S5). Subsequently, the correlation of LTBP2 with tumor immune infiltration cells in GEO merged datasets was validated. As revealed in Figs. S6A and 5A, there were significant differences in the proportion of immune cells between primary PCa and BCR of PCa, especially CD4 memory-activated T cells. Moreover, as revealed in Fig. 5B-D, LTBP2 expression was correlated with T-cell follicular helper and CD4+ T-cell memory resting in the GEO merged datasets, paralleling the results obtained from TCGA-PRAD dataset. Furthermore, the effect of LTBP2 expression on the correlation between different tumor-infiltrating immune cells in the GEO merged datasets was identified (Fig. S6B). Therefore, based on the aforementioned results, it was hypothesized that LTBP2 expression was associated with CD4+ T-cell recruitment, which was also verified in TCGA-PRAD dataset. As confirmed in Fig. 5E, LTBP2 expression revealed a significant positive correlation with CD4+ T-cell levels. However, whether LTBP2 had an effect on macrophage M2 polarization was not verified in the GEO merged cohort.
Figure 5.
mRNA expression of LTBP2 is correlated with the level of CD4+ T cells. (A) Violin plot displayed the fraction of tumor-infiltrating immune cells in primary PCa and BCR PCa in the GEO-merged dataset. (B) Lollipop chart revealed the correlation coefficient between LTBP2 expression and immune-infiltrating cells in the GEO-merged dataset. (C and D) The correlation scatter plot revealed that LTBP2 expression was (C) significantly positively correlated with T-cell CD4 memory resting, but (D) significantly negatively correlated with T-cell follicular helper in the GEO-merged dataset. (E) Correlation scatter plots showed a significant positive correlation between LTBP2 and CD4 T-cell expression validated in TCGA-PRAD dataset. (F and G) Boxplots revealed significant differences in LTBP2 expression between (F) different anti-PD-1 clinical response subgroups in the GSE78220 cohort and (G) other anti-PD-L1 clinical response groups in the IMvigor210 cohort. LTBP2, latent transforming growth factor β-binding protein 2; PCa, prostate cancer; BCR, biochemical recurrence; GEO, Gene Expression Omnibus; TCGA, The Cancer Genome ATLAS; PRAD, prostate adenocarcinoma; PD-1, programmed cell death protein 1.
Additionally, the predictive role of LTBP2 was investigated in the immunotherapeutic response against PD-1/PD-L1 based on two immunotherapy cohorts. As shown in Fig. 5F and G, patients with low LTBP2 expression had a significantly more robust immune response than those with high expression in the anti-PD-1 immunotherapy cohort (GSE78220) and anti-PD-L1 immunotherapy cohort (IMvigor210 cohort). Hence, these results confirmed the predictive role of LTBP2 on the immunotherapeutic benefit in PCa patients with BCR.
Expression of LTBP2 is associated with TME and can predict clinical benefit of ICB
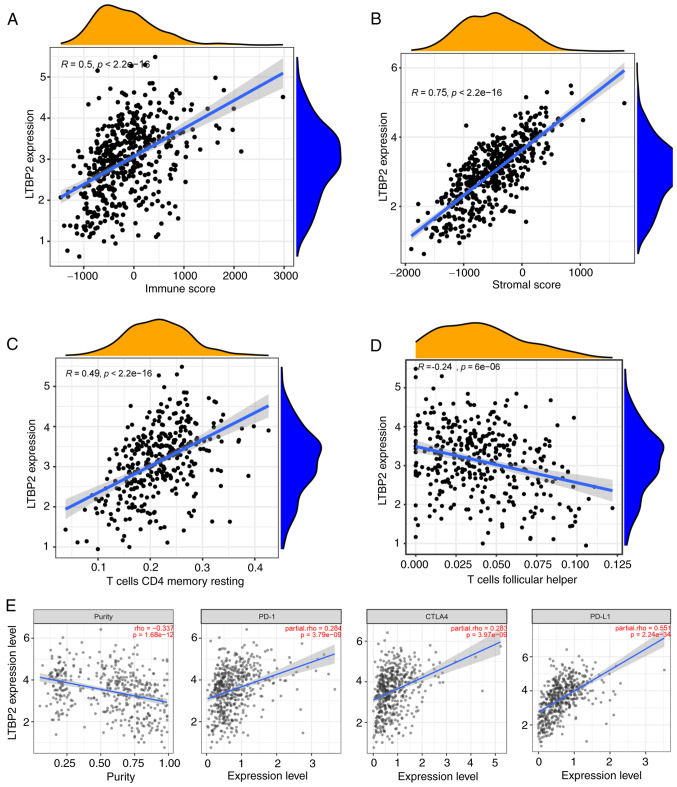
Given the aforementioned results, it was hypothesized that LTBP2 was also correlated with the TME. The ESTIMATE algorithm was used to calculate the tumor cell stromal score, immune score and tumor purity for each patient in TCGA-PRAD dataset. Spearman correlation analysis was performed and revealed that LTBP2 expression was positively correlated with immune score and stromal score in TCGA-PRAD dataset (Fig. 6A and B). In addition, LTBP2 expression was significantly positively correlated with T-cell CD4 memory resting and significantly negatively correlated with T-cell follicular helper in the TCGA-PRAD dataset, which validated the recruitment of LTBP2 to CD4+ T cells (Fig. 6C and D). LTBP2 expression was significantly associated with immune cell marker genes, except for CEACAM8 (Fig. S7; Table SIII). In addition, to further clarify the role of LTBP2 on ICB, the association between LTBP2 and several well-known immune checkpoint genes was explored. The results showed that the mRNA expression of LTBP2 was significantly positively correlated with the relative expression levels of PD-L1, CTLA4, and PD-1 (Fig. 6E), but negatively correlated with tumor purity in the TIMER database, as verified in the GEPIA database.
Figure 6.
mRNA expression of LTBP2 is correlated with the TME infiltration cell characteristics and immune checkpoint genes. (A and B) The correlation scatter plot revealed that LTBP2 expression was positively correlated with (A) immune score and (B) stromal score in TCGA-PRAD dataset. (C and D) The correlation scatter plot confirmed that LTBP2 expression was significantly positively correlated with T-cell CD4 memory resting, while it was significantly negatively correlated with T-cell follicular helper in TCGA-PRAD dataset. (E) The scatter plot showed that the mRNA expression of LTBP2 was significantly positively correlated with the relative expression of immune checkpoint genes, including PD-L1, CTLA4, PD-1, but negatively correlated with tumor purity in the TIMER database and GEPIA database (cor >0; P-value <0.001). LTBP2, latent transforming growth factor β-binding protein 2; TME, tumor microenvironment; TCGA, The Cancer Genome ATLAS; PRAD, prostate adenocarcinoma; PD-1, programmed cell death protein 1; TIMER, Tumor Immune Estimation Resource; GEPIA, Gene Expression Profiling Interactive Analysis.
GO/KEGG/DO functional enrichment analysis and GSEA analysis of the DEGs
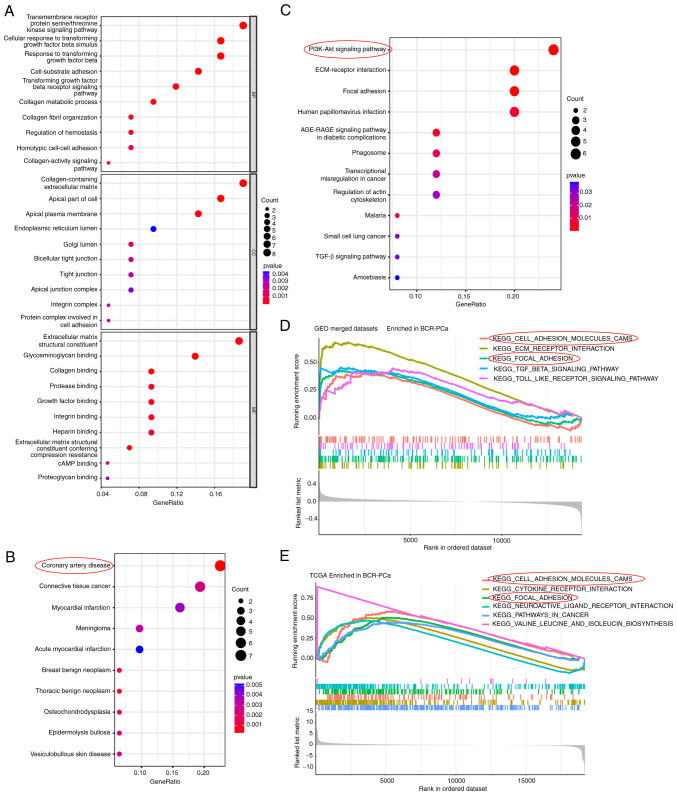
Subsequently, GO, KEGG, DO enrichment analyses and GSEA analysis was conducted to identify the biological functions and signaling pathways of LTBP2. These enrichment analyses were performed for the BCR-associated DEGs. The GO profiles revealed that these DEGs were integrally correlated with transforming growth factor-β (TGF-β) receptor and cellular metabolic processes. The top ten GO terms of MF, CC, and BP associated with 44 BCR-related are presented in Fig. 7A. Similarly, the DO enrichment analysis revealed that the top five DO terms had a close association with cardiovascular disease and connective tissue cancer (Fig. 7B). It was also observed that the top 5 signaling pathways based on KEGG analysis mainly participated in PI3K-AKT/ECM-receptor interaction/Focal adhesion/TGF-β signaling pathways (Fig. 7C). Finally, to systematically assess the potential biological functions and signaling pathways of these BCR-associated DEGs involved in molecular heterogeneity, the GSEA method was employed to identify and validate them in a GEO-merged BCR-related dataset (Fig. 7D) and TCGA-PRAD BCR-related dataset (Fig. 7E). The findings also demonstrated that the top 5 pathways were closely associated to cell adhesion molecules (CAMs) and focal adhesion.
Figure 7.
Biological function and pathway annotation enrichment analysis. (A) Bubble plot of GO enrichment analysis of the 44 BCR-associated DEGs revealing the enriched BP, CC and MF. (B) DO enrichment analysis of the 44 BCR-associated DEGs showing the enriched top 10 diseases. (C) KEGG pathway analysis revealed the enriched signaling pathways of the 44 BCR-associated DEGs. (D) GSEA showed the top five KEGG signaling pathways in BCR-PCa of the GEO-merged dataset. (E) GSEA enrichment analysis showed the top six KEGG signaling pathways in BCR-PCa in TCGA-PRAD dataset. GO, Gene Ontology; BCR, biochemical recurrence; DEGs, differentially expressed genes; BP, biological processes; CC, cellular components; MF, molecular functions; DO, Disease Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; GSEA, gene set enrichment analysis; PCa, prostate cancer; GEO, Gene Expression Omnibus.
Validation of LTBP2 expression and biological function in vitro and confirmation of the involvement of the PI3K/AKT signaling pathway in BCR of PCa
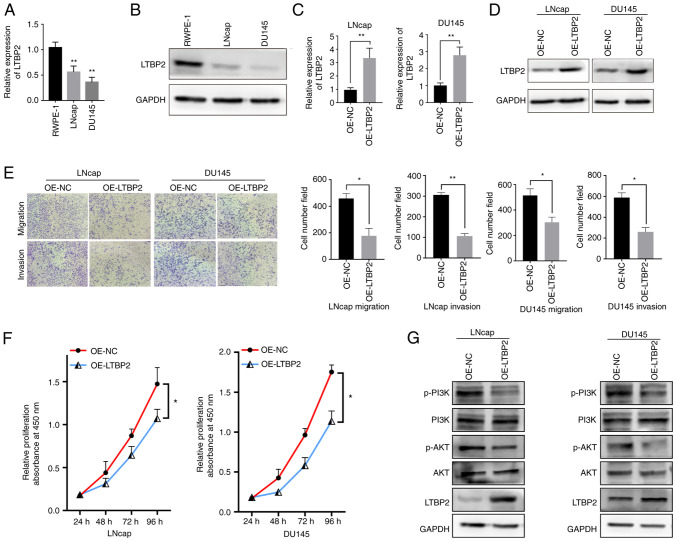
To better evaluate the expression and biological function of LTBP2, cellular experiments in vitro were carried out. RT-qPCR assays were performed to validate whether the LTBP2 expression was downregulated in TCGA-PRAD database. As revealed in Fig. 8A, LTBP2 was under-expressed in tumor cell lines, and similar results could be observed at the protein level via western blot analysis (Fig. 8B). To investigate the biological function of LTBP2 in PCa cells, an overexpression plasmid (OE-LTBP2) was constructed and transfected into LNcap and DU145 cells. RT-qPCR and western blot analysis revealed that OE- LTBP2 could upregulate the mRNA and protein expression levels of LTBP2 (Fig. 8C and D). Subsequently, Transwell assays revealed that overexpression of LTBP2 reduced cell migration and invasion abilities (Fig. 8E). Moreover, CCK-8 assays also showed that overexpression of LTBP2 significantly inhibited the proliferation of LNcap and DU145 cells (Fig. 8F)
Figure 8.
LTBP2 inhibits the proliferation, migration and invasion of PCa cells in vitro. (A) RT-qPCR assay showed the mRNA expression levels of LTBP2 in prostate cells (RWPE-1) and PCa cell lines (LNcap and DU145). (B) Western blot assay showed the protein expression levels of LTBP2 in prostate cells (REPW-1) and PCa cell lines (LNcap and DU145). (C) Relative expression of LTBP2 by RT-qPCR in PCa cells transfected with OE-LTBP2 and NC. (D) Relative expression of LTBP2 determined by western blotting in PCa cells transfected with OE-LTBP2 and NC. (E) Transwell assays showed the migration and invasive abilities of PCa cells transfected with OE-LTBP2 and NC. (F) CCK-8 assays showed the proliferation capacity of PCa cells transfected with OE-LTBP2 and NC. (G) Western blot assay displayed the protein levels changes in PCa cells transfected with OE-LTBP2 and NC. All experiments were repeated three times. *P<0.05, **P<0.01. LTBP2, latent transforming growth factor β-binding protein 2; PCa, prostate cancer; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; OE, overexpressed; NC, negative control; CCK-8, Cell Counting Kit-8.
Furthermore, the potential mechanisms by which LTBP2 inhibited PCa progression were further explored. Based on previous literature (52-54) and KEGG signaling pathway analysis as well as GSEA analysis, it was hypothesized that the downstream signaling pathway of LTBP2 may involve the PI3K/AKT pathway. Hence, the expression changes of proteins related to the PI3K/AKT signaling pathway were examined. As revealed in Fig. 8G, western blot results demonstrated that transfection with OE-LTBP2 resulted in a significant increase in LTBP2 expression levels, and a decrease in the protein levels of phosphorylated (p)-AKT and p-PI3K in LNcap and DU145 cells. Collectively, it was confirmed that LTBP2 may be involved in BCR of PCa progression and metastasis via the PI3K/AKT signaling pathway.
Discussion
Identification and characterization of the specific biomarkers for BCR of PCa may be important for the diagnosis and prognosis of prostate tumors. In the present study, it was demonstrated that LTBP2 could be a diagnostic biomarker for BCR of PCa, which is correlated with immune response. To identify diagnostic biomarkers associated with BCR of PCa, the screening algorithms, Lasso and SVM-RFE were used to screen 44 BCR-related DEGs and 19 differential genes associated with BCR were obtained via overlapping. PPI analysis and MCODE algorithm were also used to screen the 44 DEGs and 4 hub genes were identified. Finally, by overlapping the hub genes and key BCR-related DEGs, LTBP2 was revealed to be the only candidate diagnostic gene for BCR of PCa and was found to be associated with CD4+ T-cell recruitment and anti-PD-1/PD-L1 immunotherapy response. Subsequently, using the ESTIMATE algorithm, it was determined that LTBP2 was associated with the TME state and could predict the clinical benefit of ICB. Finally, the expression and biological function of LTBP2 were evaluated by cellular experiments. The results showed that LTBP2 was downregulated in PCa cells and inhibited PCa proliferation and metastasis via the PI3K/AKT signaling pathway. Collectively, the present study demonstrated that LTBP2 could be employed as a novel biomarker for diagnosing BCR in PCa and a potential immunotherapeutic tool, which could inhibit PCa proliferation and metastasis via the PI3K/AKT signaling pathway.
Several studies (7,15) have assessed the diagnostic biomarkers for BCR of PCa. However, the specific molecular mechanisms and their correlation with immunity and prognosis are still not well clarified. LTBP2, a member of the fibronectin or LTBP extracellular matrix (ECM) glycoprotein superfamily, which is characterized by repetitive domain structures, has an important influence on tumorigenesis development by regulating TGF-β activity, elastogenesis and maintenance of ECM structure (54,55). In the present study, LTBP2 was identified and validated as a novel diagnostic biomarker for BCR of PCa by public databases. Moreover, it was determined that LTBP2 was associated with immune response and TME. In addition, through cellular experiments, it was revealed that LTBP2 was under-expressed in PCa, which was consistent with the results obtained from the public databases. However, through pan-cancer analysis and several previous studies, it was observed that LTBP2 was upregulated in a variety of diseases, such as cervical adenocarcinoma (53), oral squamous cell carcinoma (54), lung myofibroblast (56), gastric cancer (52), colorectal cancer (57), glaucoma (58), which indicates that LTBP2 has diverse biological functions. For the first time, to the best of our knowledge, the present study reported the role of LTBP2 in PCa progression, especially in the diagnosis of BCR. In addition, the results of the present study indicated that LTBP2 was significantly positively correlated with CD4+ T-cell infiltration and TME score as well as ICB, suggesting that high expression of LTBP2 along with increased ICB and CD4+ T-cell recruitment could increase the clinical benefit of immunotherapy for PCa patients. Naturally, more studies are required to further confirm the aforementioned findings.
Undoubtedly, immunotherapy is a powerful treatment strategy for solid tumors, yet PCa appears to be excluded from the ongoing immunotherapy revolution. However, several studies have confirmed the value of immunotherapy in advanced PCa. Bilusic et al (59) reported that turning a ‘cold’ PCa TME into a ‘hot’ one by driving T cells into the tumor and combining it with ADT could be a new approach to PCa treatment. Gamat et al (60) found that treatment of CD4+ T cells with testosterone or DHT increased the level of the immunosuppressive cytokine IL-10, suggesting that androgens could have a direct negative effect on T-cell function. It was hypothesized that combining ADT with immunotherapy is a reasonable direction to improve the efficacy of PCa. Several studies have also reported that ICB plays a key role in the treatment of PCa. For example, Zhou et al and Zhang et al (61,62) found that WDR5 could combine with PD-L1 expression to influence the progression and chemosensitivity of PCa. The present study determined that LTBP2 was associated with CD4+ T cells and could predict clinical benefit from immunotherapy, particularly from ICB. This may provide new insights into the treatment for BCR of PCa.
Recently, LTBP2 was recently identified as an ECM glycoprotein, and its expression was associated with poor prognosis in several tumors. For example, Wang et al (52) found that LTBP2 promoted metastasis of gastric cancer cells and was associated with a poor prognosis. Turtoi et al (63) identified significant high expression of LTBP2 in pancreatic ductal adenocarcinoma tissue by 2D-nano-HPLC_MS/MS method and western blotting. By contrast, Chen et al (64) reported that LTBP2 was downregulated in nasopharyngeal carcinoma and it conferred a propensity to inhibit proliferation and metastasis in a favorable (growth factor-permitting) TME, which suggested that it had significant heterogeneity. However, the specific molecular mechanism that affected tumor progression has not been fully elucidated. In the present study, insight into the exact mechanism by which LTBP2 regulates PCa progression was provided. Based on KEGG pathway enrichment analysis and GSEA analysis and previous literature (50), it was confirmed that LTBP2 inhibited PCa proliferation and metastasis in vitro via the PI3K/AKT signaling pathway.
lncRNAs play an important regulatory role in the malignant progression and BCR of PCa (8,9). Therefore, the possible upstream molecular mechanisms were investigated. In fact, lncRNA-miRNA-LTBP2 ceRNA regulatory networks were constructed using the starBase database and Spearman correlation analysis, which contained 15 lncRNAs and 8 miRNAs (Fig. S8). Furthermore, it was confirmed that these lncRNAs were downregulated while the miRNAs were upregulated in PCa. Therefore, in the future LTBP2 will be further explored and its biological function will be further characterized from in vivo and in vitro experiments. Regretfully, it was determined that LTBP2 expression did not affect prognosis, including OS, DSS and PFI. In fact, clinical data related to BCR is being presently collected, to explore the association between LTBP2 expression and BCR of PCa. If LTBP2 is determined to be associated with prognosis related to BCR, this could be an important finding. Hence, the aforementioned results demonstrated that LTBP2 holds promise as a diagnostic biomarker for BCR of PCa and offered new perspectives for immunotherapy. In addition, a contradiction was found with regard to LTBP2 being lowly expressed in tumors, but its expression increased with TNM stage. This may require further validation in large clinical samples.
In conclusion, 44 BCR-related DEGs were screened using the GEO-merged datasets, and LTBP2 was then identified as a diagnostic biomarker for BCR of PCa based on Lasso, SVM-RFE algorithms, PPI analysis and MCODE algorithm. The stability and reliability of candidate genes were validated with the GEO validation dataset and TCGA-PRAD datasets. It was then determined that LTBP2 exerted a crucial role in CD4+ T-cell recruitment and TME state. Notably, LTBP2 expression enhanced the clinical benefit of immunotherapy for PCa patients with BCR. In addition, the upstream lncRNA-miRNA-LTBP2 ceRNA regulatory network was constructed by bioinformatics and the downstream signaling pathway and biological functions were validated by in vitro experiments based on KEGG enrichment analysis. It was determined that LTBP2 inhibited PCa progression and metastasis via the PI3K/AKT signaling pathway. In short, the present study provided novel insights into the role of LTBP2 in diagnosing BCR of PCa and facilitating personalized immunotherapy in patients with PCa.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by the National Natural Science Foundation of China (grant nos. 81872089, 81370849,81672551, and 81202034), and the Natural Science Foundation of Jiangsu Province (grant nos. BE2019751, BK20161434, and BK2012336), and the opening foundation (JSHD2021029).
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the TCGA and GEO repositories, https://portal.gdc.cancer.gov/repository and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE46602; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE70768; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE116918.
Authors' contributions
XZ and CT confirm the authenticity of all the raw data. All authors (XZ, CT, JC, WM, ML and MC) made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Nevedomskaya E, Baumgart SJ, Haendler B. Recent advances in prostate cancer treatment and drug discovery. Int J Mol Sci. 2018;19(1359) doi: 10.3390/ijms19051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundi D, Tosoian JJ, Nyame YA, Alam R, Achim M, Reichard CA, Li J, Wilkins L, Schwen Z, Han M, et al. Outcomes of very high-risk prostate cancer after radical prostatectomy: Validation study from 3 centers. Cancer. 2019;125:391–397. doi: 10.1002/cncr.31833. [DOI] [PubMed] [Google Scholar]
- 4.Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, Santis MD, Fanti S, Fossati N, Gandaglia G, Gillessen S, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: Treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79:263–282. doi: 10.1016/j.eururo.2020.09.046. [DOI] [PubMed] [Google Scholar]
- 5.Van den Broeck T, van den Bergh RCN, Briers E, Cornford P, Cumberbatch M, Tilki D, De Santis M, Fanti S, Fossati N, Gillessen S, et al. Biochemical recurrence in prostate cancer: The european association of urology prostate cancer guidelines panel recommendations. Eur Urol Focus. 2020;6:231–234. doi: 10.1016/j.euf.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Barry MJ, Simmons LH. Prevention of prostate cancer morbidity and mortality: Primary prevention and early detection. Med Clin North Am. 2017;101:787–806. doi: 10.1016/j.mcna.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Park WS, Park BR, Joo J, Joung JY, Seo HK, Chung J, Lee KH. Psca, cox-2, and ki-67 are independent, predictive markers of biochemical recurrence in clinically localized prostate cancer: A retrospective study. Asian J Androl. 2017;19:458–462. doi: 10.4103/1008-682X.180798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu P, Chen X, Xie R, Han J, Xie W, Wang B, Dong W, Chen C, Yang M, Jiang J, et al. LncRNA HOXD-AS1 regulates proliferation and chemo-resistance of castration-resistant prostate cancer via recruiting wdr5. Mol Ther. 2017;25:1959–1973. doi: 10.1016/j.ymthe.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu P, Chen X, Xie R, Xie W, Huang L, Dong W, Han J, Liu X, Shen J, Huang J, Lin T. A novel AR translational regulator lncrna lbcs inhibits castration resistance of prostate cancer. Mol Cancer. 2019;18(109) doi: 10.1186/s12943-019-1037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J, Li J, Li F, Tan HB. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126–133. doi: 10.1016/j.canlet.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: Removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27:1482–1492. doi: 10.1093/annonc/mdw168. [DOI] [PubMed] [Google Scholar]
- 12.Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomita Y, Ikeda T, Sakata S, Saruwatari K, Sato R, Iyama S, Jodai T, Akaike K, Ishizuka S, Saeki S, Sakagami T. Association of probiotic clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol Res. 2020;8:1236–1242. doi: 10.1158/2326-6066.CIR-20-0051. [DOI] [PubMed] [Google Scholar]
- 14.Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 15.Rui X, Shao S, Wang L, Leng J. Identification of recurrence marker associated with immune infiltration in prostate cancer with radical resection and build prognostic nomogram. BMC Cancer. 2019;19(1179) doi: 10.1186/s12885-019-6391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou Q, Bing ZT, Hu C, Li MY, Yang KH, Mo Z, Xie XW, Liao JL, Lu Y, Horie S, Lou MW. Rankprod combined with genetic algorithm optimized artificial neural network establishes a diagnostic and prognostic prediction model that revealed c1QTNF3 as a biomarker for prostate cancer. EBioMedicine. 2018;32:234–244. doi: 10.1016/j.ebiom.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mortensen MM, Høyer S, Lynnerup AS, Ørntoft TF, Sørensen KD, Borre M, Dyrskjøt L. Expression profiling of prostate cancer tissue delineates genes associated with recurrence after prostatectomy. Sci Rep. 2015;5(16018) doi: 10.1038/srep16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross-Adams H, Lamb AD, Dunning MJ, Halim S, Lindberg J, Massie CM, Egevad LA, Russell R, Ramos-Montoya A, Vowler SL, et al. Integration of copy number and transcriptomics provides risk stratification in prostate cancer: A discovery and validation cohort study. EBioMedicine. 2015;2:1133–1144. doi: 10.1016/j.ebiom.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain S, Lyons CA, Walker SM, McQuaid S, Hynes SO, Mitchell DM, Pang B, Logan GE, McCavigan AM, Rourke DO, et al. Validation of a metastatic assay using biopsies to improve risk stratification in patients with prostate cancer treated with radical radiation therapy. Ann Oncol. 2018;29:215–222. doi: 10.1093/annonc/mdx637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for rna-sequencing and microarray studies. Nucleic Acids Res. 2015;43(e47) doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engebretsen S, Bohlin J. Statistical predictions with glmnet. Clin Epigenetics. 2019;11(123) doi: 10.1186/s13148-019-0730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klosa J, Simon N, Westermark PO, Liebscher V, Wittenburg D. Seagull: Lasso, group lasso and sparse-group lasso regularization for linear regression models via proximal gradient descent. BMC Bioinformatics. 2020;21(407) doi: 10.1186/s12859-020-03725-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun S, Shen Y, Wang J, Li J, Cao J, Zhang J. Identification and validation of autophagy-related genes in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2021;16:67–78. doi: 10.2147/COPD.S288428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanz H, Valim C, Vegas E, Oller JM, Reverter F. SVM-RFE: Selection and visualization of the most relevant features through non-linear kernels. BMC Bioinformatics. 2018;19(432) doi: 10.1186/s12859-018-2451-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X, Tuck DP. MSVM-RFE: Extensions of SVM-RFE for multiclass gene selection on DNA microarray data. Bioinformatics. 2007;23:1106–1114. doi: 10.1093/bioinformatics/btm036. [DOI] [PubMed] [Google Scholar]
- 27.Li F, Zhao C, Xia Z, Wang Y, Zhou X, Li GZ. Computer-assisted lip diagnosis on traditional chinese medicine using multi-class support vector machines. BMC Complement Altern Med. 2012;12(127) doi: 10.1186/1472-6882-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu G, Wang LG, Han Y, He QY. ClusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Q, Xu H, Deng R, Wang Z, Li N, Qi Z, Zhao J, Huang W. Multi-omics analysis reveals prognostic value of tumor mutation burden in hepatocellular carcinoma. Cancer Cell Int. 2021;21(342) doi: 10.1186/s12935-021-02049-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu X, Sui Z, Zhang H, Wang Y, Yu Z. Integrated analysis of lncRNA-mediated ceRNA network in lung adenocarcinoma. Front Oncol. 2020;10(554759) doi: 10.3389/fonc.2020.554759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu P, Jiang W, Zhao J, Zhang H. Integrated analysis of genome-wide gene expression and DNA methylation microarray of diffuse large B-cell lymphoma with TET mutations. Mol Med Rep. 2017;16:3777–3782. doi: 10.3892/mmr.2017.7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gambardella A, Licata G, Sohrt A. Dose adjustment of biologic treatments for moderate-to-severe plaque psoriasis in the real world: A systematic review. Dermatol Ther (Heidelb) 2021;11:1141–1156. doi: 10.1007/s13555-021-00559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W, Treviño V, Shen H, Laird PW, Levine DA, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4(2612) doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang S, Zhang E, Long J, Hu Z, Peng J, Liu L, Tang F, Li L, Ouyang Y, Zeng Z. Immune infiltration in renal cell carcinoma. Cancer Sci. 2019;110:1564–1572. doi: 10.1111/cas.13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan JH, Zhou H, Cooper L, Huang JL, Zhu SB, Zhao XX, Ding H, Pan YL, Rong L. LAYN is a prognostic biomarker and correlated with immune infiltrates in gastric and colon cancers. Front Immunol. 2019;10(6) doi: 10.3389/fimmu.2019.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C, Jensen LJ. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–D815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al. The string database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bader GD, Hogue CW. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics. 2003;4(2) doi: 10.1186/1471-2105-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of cerna crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li JH, Liu S, Zhou H, Qu LH, Yang JH. Starbase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale clip-seq data. Nucleic Acids Res. 2014;42:D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shannon P, Markiel A, Ozier O, Baliga NC, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mancinelli S, Turcato A, Kisslinger A, Bongiovanni A, Zazzu V, Lanati A, Liguori GL. Design of transfections: Implementation of design of experiments for cell transfection fine tuning. Biotechnol Bioeng. 2021;118:4488–4502. doi: 10.1002/bit.27918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor SC, Nadeau K, Abbasi M, Lachance C, Nguyen M, Fenrich J. The ultimate qPCR experiment: Producing publication quality, reproducible data the first time. Trends Biotechnol. 2019;37:761–774. doi: 10.1016/j.tibtech.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Yu J, Mao W, Sun S, Hu Q, Wang C, Xu Z, Liu R, Chen S, Xu B, Chen M. Identification of an m6A-related lncRNA signature for predicting the prognosis in patients with kidney renal clear cell carcinoma. Front Oncol. 2021;11(663263) doi: 10.3389/fonc.2021.663263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen S, Wang L, Xu C, Chen H, Peng B, Xu Y, Yao X, Li L, Zheng J. Knockdown of regγ inhibits proliferation by inducing apoptosis and cell cycle arrest in prostate cancer. Am J Transl Res. 2017;9:3787–3795. [PMC free article] [PubMed] [Google Scholar]
- 49.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 50.Mao W, Wang K, Xu B, Zhang H, Sun S, Hu Q, Zhang L, Liu C, Chen S, Wu J, et al. CiRS-7 is a prognostic biomarker and potential gene therapy target for renal cell carcinoma. Mol Cancer. 2021;20(142) doi: 10.1186/s12943-021-01443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pulendran B, Davis MM. The science and medicine of human immunology. Science. 2020;369(eaay4014) doi: 10.1126/science.aay4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Liang WJ, Min GT, Wang HP, Chen W, Yao N. LTBP2 promotes the migration and invasion of gastric cancer cells and predicts poor outcome of patients with gastric cancer. Int J Oncol. 2018;52:1886–1898. doi: 10.3892/ijo.2018.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren Y, Lu H, Zhao D, Ou Y, Yu K, Gu J, Wang L, Jiang S, Chen M, Wang J, et al. LTPB2 acts as a prognostic factor and promotes progression of cervical adenocarcinoma. Am J Transl Res. 2015;7:1095–1105. [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Jiang C, Li N, Wang F, Xu Y, Shen Z, Yang L, Li Z, He C. The circEPSTI1/mir-942-5p/LTBP2 axis regulates the progression of oscc in the background of osf via emt and the PI3K/Akt/mTOR pathway. Cell Death Dis. 2020;11(682) doi: 10.1038/s41419-020-02851-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pang XF, Lin X, Du JJ, Zeng DY. LTBP2 knockdown by siRNA reverses myocardial oxidative stress injury, fibrosis and remodelling during dilated cardiomyopathy. Acta Physiol (Oxf) 2020;228(e13377) doi: 10.1111/apha.13377. [DOI] [PubMed] [Google Scholar]
- 56.Enomoto Y, Matsushima S, Shibata K, Aoshima Y, Yagi H, Meguro S, Kawasaki H, Kosugi I, Fujisawa T, Enomoto N, et al. LTBP2 is secreted from lung myofibroblasts and is a potential biomarker for idiopathic pulmonary fibrosis. Clin Sci (Lond) 2018;132:1565–1580. doi: 10.1042/CS20180435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang Y, Wang G, Zhao C, Geng R, Zhang S, Wang W, Chen J, Liu H, Wang X. High expression of LTBP2 contributes to poor prognosis in colorectal cancer patients and correlates with the mesenchymal colorectal cancer subtype. Dis Markers. 2019;2019(5231269) doi: 10.1155/2019/5231269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rauf B, Irum B, Khan SY, Kabir F, Naeem MA, Riazuddin S, Ayyagari R, Riazuddin SA. Novel mutations in LTBP2 identified in familial cases of primary congenital glaucoma. Mol Vis. 2020;26:14–25. [PMC free article] [PubMed] [Google Scholar]
- 59.Bilusic M, Madan RA, Gulley JL. Immunotherapy of prostate cancer: Facts and hopes. Clin Cancer Res. 2017;23:6764–6770. doi: 10.1158/1078-0432.CCR-17-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gamat M, McNeel DG. Androgen deprivation and immunotherapy for the treatment of prostate cancer. Endocr Relat Cancer. 2017;24:T297–T310. doi: 10.1530/ERC-17-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou Q, Chen X, He H, Peng S, Zhang Y, Zhang J, Cheng L, Liu S, Huang R, Xie R, et al. Wd repeat domain 5 promotes chemoresistance and programmed death-ligand 1 expression in prostate cancer. Theranostics. 2021;11:4809–4824. doi: 10.7150/thno.55814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J, Zhou Q, Xie K, Cheng L, Peng S, Xie R, Liu L, Zhang Y, Dong W, Han J, et al. Targeting WD repeat domain 5 enhances chemosensitivity and inhibits proliferation and programmed death-ligand 1 expression in bladder cancer. J Exp Clin Cancer Res. 2021;40(203) doi: 10.1186/s13046-021-01989-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turtoi A, Musmeci D, Wang Y, Dumont B, Somja J, Bevilacqua G, De Pauw E, Delvenne P, Castronovo V. Identification of novel accessible proteins bearing diagnostic and therapeutic potential in human pancreatic ductal adenocarcinoma. J Proteome Res. 2011;10:4302–4313. doi: 10.1021/pr200527z. [DOI] [PubMed] [Google Scholar]
- 64.Chen H, Ko JMY, Wong VCL, Hyytiainen M, Keski-Oja J, Chua D, Nicholls JM, Cheung FMF, Lee AWM, Kwong DLW, et al. LTBP-2 confers pleiotropic suppression and promotes dormancy in a growth factor permissive microenvironment in nasopharyngeal carcinoma. Cancer Lett. 2012;325:89–98. doi: 10.1016/j.canlet.2012.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the TCGA and GEO repositories, https://portal.gdc.cancer.gov/repository and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE46602; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE70768; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE116918.