Abstract
Background:
Tuberculosis (TB) remains a major cause of morbidity and mortality in Ethiopia despite the increased availability of effective treatments. Trend analysis of issues and priorities affecting TB programs across different regions of the country is critical to ensure equitable and sustainable TB outcomes. We aimed to analyze the trends of TB in Bahir Dar, Northwest Ethiopia, over 5 years from 2015 to 2019.
Methods:
An institution-based, retrospective cross-sectional study was conducted in Bahir Dar, the capital city of the Amhara Region in Ethiopia. Five-year data and records of individual TB cases were reviewed from all public and private health-care facilities and health bureaus in Bahir Dar. Using a standard checklist adapted from the World Health Organization, data were abstracted relevant to sociodemographic characteristics of the patients, year and type of TB infection, and HIV status. SPSS version 20 software was used for data analysis.
Results:
Data of 4275 patients with TB were identified, of which 929 (21.7%) were smear-positive pulmonary TB, 1195 (28%) were smear-negative pulmonary TB, and 2151 (50.3%) were extrapulmonary TB patients. TB was more prevalent in the age group 15–34 years (51.2%), and females (55.5%). In the years from 2015 to 2019, the prevalence of all forms of TB was 922 (21.6%), 812 (19.0%), 843 (19.7%), 876 (20.5%), and 822 (19.2%), respectively, demonstrating a decreasing trend though inconsistent. The variables sex (adjusted odds ratio [AOR]: 1.734, 95% confidence interval [CI] [1.390–2.187]), HIV co-infection (AOR: 1.875, 95% CI [1.553–2.265]), and age <15 years (AOR: 1.372, 95% CI [1.121–1.680]) showed a significant association with TB infection.
Conclusions:
The prevalence of TB in Bahir Dar, Northwest Ethiopia, demonstrated a decreasing trend over the years from 2015 to 2019 but with inconsistencies. HIV co-infection significantly increased the risk of developing TB, and productive age groups and females were at the greater prevalence of TB, highlighting the importance of strengthening sustainable TB care and prevention interventions toward these groups of people.
Keywords: Ethiopia, TB-HIV co-infection, trend analysis, tuberculosis
Introduction
Tuberculosis (TB) remains one of the top ten causes of morbidity and mortality worldwide. According to the WHO 2020 report,[1] globally, an estimated 10.0 million people fell ill with TB in 2019, a number that has been declining very slowly in recent years. There were an estimated 1.2 million TB deaths among HIV-negative people in 2019 (a reduction from 1.7 million in 2000), and an additional 208,000 deaths among HIV-positive people (a reduction from 678,000 in 2000).[1] Efforts are underway to provide equitable access to quality and timely diagnosis, treatment, and prevention of TB, particularly in resource-constrained settings,[2–5] and TB incidence and deaths are falling.[1]
Ethiopia remains to be among the 30 countries reported with a high burden of TB, TB/HIV, and MDR-TB for 2015–2020.[1] Ethiopia already took steps to fight and end the TB epidemic by 2035. The country’s National TB Strategic Plan intends to reduce TB-related deaths by 95% and TB-related incidents by 90% in the years 2015–2035 and to ensure that no family is burdened with catastrophic expenses due to TB.[6] The national plan hopes to pave the way for robust TB case-finding strategies and diagnostic tools, and equitable and maximal access to TB-related services. These ambitions cannot be reached out without extensive efforts and investments in research and continuous evaluation of institutional performance.[7–10] Although there is a major decline in TB-associated mortality and morbidity, Ethiopia is still among the most affected countries with the highest number of TB cases. Even though the Ethiopian government has expanded TB diagnosis and treatment services to both public and private health-care facilities since 2004, TB is still a major public health concern in the country.
Previous studies that carried out trend analysis of TB in different regions of Ethiopia reported mainly a decreasing trend of the disease, while the result is not consistent across different settings. In the Tigray Region, the overall regional number of TB cases showed a decreasing trend, where the decrease for extrapulmonary TB was higher than pulmonary TB, while the annual average number of TB cases was much higher in the Western part of the region.[11] In the Northwestern Tigray, TB cases showed a cumulative declining trend over the years 2013–2018.[12] In the Bale Zone of Oromia Region, drug-resistant TB showed a decreasing trend over the period 2014–2018, although the trend did not reach statistical significance.[13] In the East Gojjam Zone of Amhara Region, the pulmonary TB case notification rate showed a decreasing trend over the period 2013–2019.[14] In Addis Ababa, the city capital of Ethiopia, TB and rifampicin-resistant TB showed a decreasing trend over the period 2016–2018.[15] In a specialized teaching hospital in Harari Region, the prevalence of TB showed a decreasing trend over the year 2015–2019; however, there were seasonal variations in TB incidence.[16] In Awi Zone, Northwest of Amhara Region, the magnitude of TB decreased over the period 2011–2016.[17] In 10 zones, five each from Amhara and Oromia Regions, TB showed seasonal variation, with a peak in April–June and a trough in October–December, and with the seasonal amplitude in 10% greater in Amhara than Oromia Region over the year 2010–2016.[18] In Bahir Dar, the city capital of the Amhara Region, TB trend analysis has not been made that would inform TB variability at different time points, performance of TB programs and strategies, and areas most amenable to improvement.
Trend analysis of issues and priorities affecting TB programs across different regions of Ethiopia is critical to ensure equitable and sustainable TB outcomes. In recent decades, analysis of health-care facility-level TB data has frequently been used in the analysis and interpretation of longitudinal data to inform policymaking. Thus, this study aimed to determine the trends of TB in Bahir Dar, Northwest Ethiopia, over 5 years from 2015 to 2019.
Methods
Study design
This was an institutional-based retrospective cross-sectional study.
Setting
The study was conducted in Bahir Dar, the capital city of the Amhara Region in Ethiopia. During the study period, there were 13 governmental health-care facilities – 3 hospitals and 10 health centers – and 24 private health-care facilities – 4 private hospitals and 20 private clinics – in the city.
Data source
Five-year data and records of individual cases screened for TB were reviewed and recorded from TB patient records at all government- and private-led health-care facilities that provide TB services, and TB case-based records and reports at health bureaus that monitor TB programs in Bahir Dar (n = 47). The facilities use sputum smear examination, clinical diagnosis, radiological examination, culture examinations, and Xpert MTB/Rif assay for the diagnosis of TB as available. Xpert MTB/Rif assay was employed for MDR-TB diagnosis. Both health facilities perform HIV screening and testing for TB suspects as well as TB screening for HIV suspects based on the national guidelines.
Data were collected with a data collection format adopted from the standard checklist of the World Health Organization. The checklist had four sections:
Patients’ sociodemographic characteristics: Age, sex, residence, HIV status, and unique identification number of all TB patients attending the health-care facilities
TB screening: WHO TB symptom screening results and year of diagnosis
Diagnosis: Sputum smear microscopy, Xpert MTB/RIF assay, TB liquid or solid culture, and drug susceptibility testing, and radiography as available
Treatment: Based on the national guideline treatment for TB, drug-resistant TB, or TB/HIV co-infection.
Trained data collectors collected the data. We categorized participants based on their age (<15, 15–24, 25–34, 35–44, 45–65, and 65+), sex (male/female), type of TB infection (PTB+, PTB−, and EPTB), HIV status (positive/negative), and year of TB diagnosis (2015, 2016, 2017, 2018, and 2019) as the major variables.
Statistical analysis
We checked and cleaned the collected data for their completeness, consistency, and clarity and double entered into SPSS software version 20. Descriptive analysis, frequencies, and figures were used to explain the findings. Bivariate logistic regression analysis was conducted primarily to check the association among variable. Multivariate logistic regression models were employed to analyze specific associations between variables. Odds ratio (OR) and 95% confidence intervals (CIs) were calculated using logistic regression model to measure the strength of an association. Mean and standard deviation was used to summarize continuous variables. P < 0.05 with corresponding 95% CI was considered statistically significant.
Ethical considerations
Ethical clearance for the conduct of this study was obtained from the Scientific and Ethics Review Committee of the Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia. Permission was sought from the Bahir Dar Health Office.
Results
Patient demographic characteristics
A total of 4275 participants’ data were analyzed for TB within the 5-year study period (2015–2019). The majority, 3770 (88.2%), of the participants’ data were collected from governmental health facilities. The detection rate of all forms of TB was 2804/4275 (6.6%), of which 2151 (50.3%) cases were extrapulmonary TB, 929 (21.7%) cases were bacteriologically confirmed pulmonary TB, and 1195 (28.0%) cases were clinically confirmed pulmonary TB. Female patients account for 55.5% of the total cases [Table 1].
Table 1:
Distribution of tuberculosis cases among governmental (public) and nongovernmental (private) health facilities in Bahir Dar, Northwest Ethiopian, from January 1, 2015, to December 30, 2019
| Year | Sex | TB status | |||||
|---|---|---|---|---|---|---|---|
| EPTB* | PTB−* | PTB+* | |||||
| Governmental* | Private* | Governmental* | Private* | Governmental* | Private* | ||
| 2015 | Female | 205 | 24 | 89 | 12 | 75 | 7 |
| Male | 226 | 28 | 116 | 21 | 103 | 16 | |
| 2016 | Females | 188 | 26 | 93 | 18 | 51 | 8 |
| Male | 183 | 25 | 108 | 27 | 71 | 14 | |
| 2017 | Female | 151 | 36 | 83 | 14 | 69 | 11 |
| Males | 178 | 35 | 110 | 29 | 117 | 10 | |
| 2018 | Female | 196 | 18 | 99 | 10 | 66 | 4 |
| Male | 204 | 15 | 121 | 10 | 125 | 8 | |
| 2019 | Female | 182 | 23 | 78 | 11 | 58 | 3 |
| Male | 188 | 20 | 131 | 15 | 106 | 7 | |
* EPTB: Extrapulmonary TB, *PTB−: Clinically confirmed TB, *PTB+: Bacteriologically confirmed TB, *Private, nongovernmental health facilities, TB: Tuberculosis, PTB: Pulmonary TB
Female accounts higher for both bacteriologically confirmed pulmonary TB cases, 577 (62.1%), and EPTB, 1098 (51%), within the 5 years from 2015 to 2019 [Table 2].
Table 2:
Prevalence of tuberculosis cases among sex in Bahir Dar, Northwest Ethiopia, from January 1, 2015, to December 30, 2019
| Variables | PTB− (%) | PTB+ (%) | EPTB (%) | Total (%) |
|---|---|---|---|---|
| Sex | ||||
| Male | 498 (41.7) | 352 (37.9) | 1053 (49.0) | 1903 (44.5) |
| Female | 697 (58.3) | 577 (62.1) | 1098 (51.0) | 2372 (55.5) |
| Total | 1195 (100) | 929 (100) | 2151 (110.9) | 4275 (100.00) |
PTB: Pulmonary tuberculosis, EPTB: Extra PTB
Patients from ages of 15–34 years were the most affected age groups (51.2%), and ages of 65 years were the least affected age group (7.5%). Table 3 summarizes the prevalence of TB among the different age groups.
Table 3:
Prevalence of tuberculosis among different age groups in Bahir Dar, Northwest Ethiopia, from January 1, 2015, to December 30, 2019
| Variables | PTB− (%) | PTB+ (%) | EPTB (%) | Total (%) |
|---|---|---|---|---|
| Age group | ||||
| <15 | 196 (16.4) | 17 (1.8) | 624 (29.0) | 837 (19.6) |
| 15–34 | 601 (50.3) | 634 (68.2) | 966 (44.9) | 2201 (51.5) |
| 35–44 | 162 (13.6) | 138 (14.9) | 111 (5.2) | 411 (9.6) |
| 45–65 | 181 (15.1) | 105 (11.3) | 220 (10.2) | 506 (11.8) |
| ≥65 | 55 (4.6) | 35 (3.8) | 230 (10.7) | 320 (7.5) |
| Total | 1195 (100) | 929 (100) | 2151 (100.0) | 4275 (100.00) |
PTB: Pulmonary tuberculosis, EPTB: Extra PTB
Trend of tuberculosis prevalence
In the years 2015–2019, the prevalence of all forms of TB was 922 (21.6%), 812 (19.0%), 843 (19.7%), 876 (20.5%), and 822 (19.2%), respectively. Within the 5-year study period, the highest prevalence rate of TB was 21.6% in the 2015 study period followed by 20.5% in 2019. Bacteriologically confirmed pulmonary TB was the lowest prevalence (21.7%) during our study period compared to clinically confirmed pulmonary TB (28%) and extrapulmonary TB (50.3%). The overall prevalence of TB prevalence over the 5 years had a declining trend but with inconsistencies [Figure 1].
Figure 1:
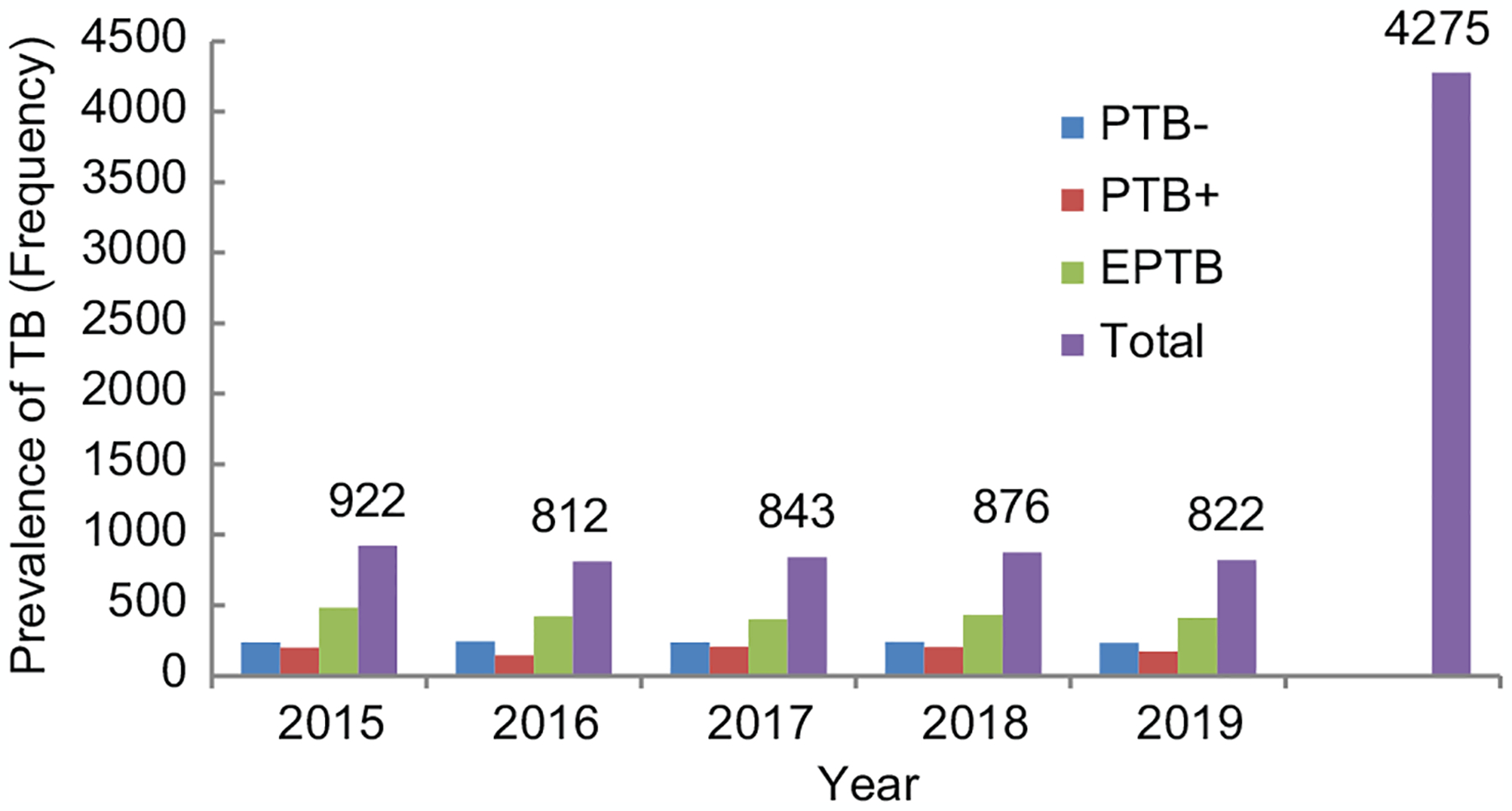
Graph showing the prevalence of tuberculosis in Bahir Dar, Northwest Ethiopia, from January 1, 2015, to December 30, 2019
Predictors of tuberculosis infection
The variables sex, HIV infection, and age <15 (adjusted OR [AOR]: 1.734, 1.875, and 1.372, CI: 1.390–2.187, 1.553–2.265, and 1.121–1.680; P < 0.001), respectively, were factors associated with TB infection [Table 4].
Table 4:
Predictors of TB infection in Bahir Dar, northwest, Ethiopian from January 1, 2015 – December 30, 2019
| Variables | TB status | P | 95% CI (COR) | P | 95% CI (AOR) | |
|---|---|---|---|---|---|---|
| PTB | EPTB | |||||
| HIV | ||||||
| Negative | 1166 | 1596 | 1 | 1 | ||
| Positive | 305 | 1201 | 0.01* | 2.9–2.5 (3.4) | 0.01** | 1.9–1.6 (2.3) |
| Sex | ||||||
| Female | 973 | 1499 | 1 | 1 | ||
| Male | 498 | 1305 | 0.01* | 1.7–1.5 (1.9) | 0.01** | 1.7–1.4 (2.2) |
| Age | ||||||
| <15 | 196 | 641 | 0.01* | 1.4–1.2 (1.7) | 0.01** | 1.4–1.1 (1.7) |
| 15–34 | 656 | 1545 | 1 | 1 | ||
| 35–44 | 162 | 249 | 0.01 | 0.7–0.5 (0.8) | 0.01 | 0.4–0.3 (0.5) |
| 45–65 | 192 | 314 | 0.01 | 0.7–0.6 (0.9) | 0.01 | 0.6–0.4 (0.8) |
| >65 | 265 | 55 | 0.01 | 0.09–0.11 (0.12) | 0.01 | 0.13–0.11 (0.22) |
significance AT P-value ≤0∙20;
significance at P-value <0∙05
TB: Tuberculosis, PTB: Pulmonary TB, EPTB: Extra PTB, CI: Confidence interval, COR: Crude odds ratio, AOR: Adjusted odds ratio
Discussion
In this study, 5-year data were collected to analyze the trends of TB in Bahir Dar, Northwest Ethiopia. A total of 4275 patient records were collected. The detection rate of all forms of TB was 6.6%, of which 50.3% were extrapulmonary TB, 21.7% were bacteriologically confirmed pulmonary TB, and 28.0% were clinically confirmed pulmonary TB. Female patients account for 55.5% of the total cases, and higher both for bacteriologically confirmed (62.1%) and extrapulmonary (51%) TB. The prevalence of TB burden was higher among people of productive age groups (15–34 years). In the years from 2015 to 2019, the prevalence of all forms of TB were 21.6%, 19.0%, 19.7%, 20.5%, and 19.2%, respectively. The variables sex, HIV infection, and age were shown to have a significant association with TB infection.
Previous TB trend analysis held in the Tigray Region,[11,12] Bale Zone of Oromia Region,[13] East Gojjam Zone of Amhara Region,[14] Addis Ababa,[15] Harari Region,[16] and Awi Zone of Northwest Amhara Region[17] reported a decreasing trend of TB over time. Our finding also shows a decreasing trend of the disease though it lacks consistency. Previous reports in the Harari Region[16] and some parts of the Amhara and Oromia Regions[18] reported seasonal variations in TB incidence.[16] Differences in comorbid diseases, the population’s knowledge about TB, institutional capacity for diagnosis of TB, and strategic approaches in the prevention of TB may contribute to variations in TB patterns and disease burden among these settings.
HIV incidence is decreasing in Ethiopia, and this may contribute to the reduction in TB as well. Our findings showed a significant association between HIV and the incidence of TB, and this is already a well-understood phenomenon. A higher prevalence of TB was reported among the productive age group of 15–34 years and the lowest among the age groups 65 and above, which was in line with the national report.[6] This could be explained by the fact that young people are prone to HIV comorbidity and they commonly develop infectious forms of TB.
The study showed a significantly higher prevalence of extrapulmonary TB as compared to smear-positive or smear-negative TB, which could be explained by the increased prevalence of extrapulmonary TB among TB-HIV co-infected individuals.[19] Ethiopia is estimated to miss 35% of incident TB cases annually,[6] and management of TB and drug-resistant TB face multiple challenges that would affect the attainment of the global End TB Strategy.[20–23]
This study has some limitations. Since this is retrospective data, we may encounter missed and incomplete data. Despite this, the study enabled a comprehensive insight into the trends of TB burden in Bahir Dar that could guide potential intervention strategies.
Conclusions
The prevalence of TB in Bahir Dar, Northwest Ethiopia, demonstrated a decreasing trend over the years from 2015 to 2019 but with inconsistencies. HIV co-infection significantly increased the risk of developing TB, and productive age groups and females were at the greater prevalence of TB, highlighting the importance of strengthening sustainable TB care and prevention interventions toward these groups of people.
Acknowledgment
The authors acknowledge the Center for Innovative Drug Development and Therapeutic Trials for Africa (CD-Africa), College of Health Sciences, Addis Ababa University, for supporting this study. The authors are thankful to Bahir Dar Health Office for their full cooperation in the data collection process.
Footnotes
For reprints contact: WKHLRPMedknow_reprints@wolterskluwer.com
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization (WHO). Global Tuberculosis Report 2020. Geneva, Switzerland; WHO; 2021. Available from: https://www.who.int/publications/i/item/9789240037021. [Last accessed on 2021 Oct 18] [Google Scholar]
- 2.Said B, Charlie L, Getachew E, Wanjiru CL, Abebe M, Manyazewal T. Molecular bacterial load assay versus culture for monitoring treatment response in adults with tuberculosis. SAGE Open Med 2021;9:20503121211033470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acharya B, Acharya A, Gautam S, Ghimire SP, Mishra G, Parajuli N, et al. Advances in diagnosis of tuberculosis: An update into molecular diagnosis of Mycobacterium tuberculosis. Mol Biol Rep 2020;47:4065–75. [DOI] [PubMed] [Google Scholar]
- 4.Getachew E, Adebeta T, Gebrie D, Charlie L, Said B, Assefa DG, et al. Pyrosequencing for diagnosis of multidrug and extensively drug-resistant tuberculosis: A systemic review and meta-analysis. J Clin Tuberc Other Mycobact Dis 2021;24:100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borah P, Deb PK, Venugopala KN, Al-Shar’i NA, Singh V, Deka S, et al. Tuberculosis: An update on pathophysiology, molecular mechanisms of drug resistance, Newer Anti-TB Drugs, Treatment Regimens and Host- Directed Therapies. Curr Top Med Chem 2021;21:547–70. [DOI] [PubMed] [Google Scholar]
- 6.Ethiopian Federal Ministry of Health (FMoH). National Comprehensive Tuberculosis, Leprosy and TB/HIV Training Manual for Health Care Workers. Addis Ababa: FMoH; 2016. [Google Scholar]
- 7.Alene M, Assemie MA, Yismaw L, Gedif G, Ketema DB, Gietaneh W, et al. Patient delay in the diagnosis of tuberculosis in Ethiopia: A systematic review and meta-analysis. BMC Infect Dis 2020;20:797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manyazewal T, Woldeamanuel Y, Holland DP, Fekadu A, Blumberg HM, Marconi VC. Electronic pillbox-enabled self-administered therapy versus standard directly observed therapy for tuberculosis medication adherence and treatment outcomes in Ethiopia (SELFTB): Protocol for a multicenter randomized controlled trial. Trials 2020;21:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammed H, Oljira L, Roba KT, Yimer G, Fekadu A, Manyazewal T. Containment of COVID-19 in Ethiopia and implications for tuberculosis care and research. Infect Dis Poverty 2020;9:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manyazewal T, Marinucci F, Belay G, Tesfaye A, Kebede A, Tadesse Y, et al. Implementation and evaluation of a blended learning course on tuberculosis for front-line health care professionals. Am J Clin Pathol 2017;147:285–91. [DOI] [PubMed] [Google Scholar]
- 11.Menghistu HT, Mersha TT, Shumuye NA, Woldie BM, Redda YT, Hadush B, et al. Neglected tropical zoonotic diseases in Tigray region, northern Ethiopia: Spatial distribution and trend analysis of rabies, tuberculosis, schistosomiasis, and visceral leishmaniasis in humans. Zoonoses Public Health 2021;68:823–33. [doi: 10.1111/zph.12874]. [DOI] [PubMed] [Google Scholar]
- 12.Negash H, Legese H, Adhanom G, Mardu F, Tesfay K, Gebreslasie Gebremeskel S, et al. Six years trend analysis of tuberculosis in northwestern Tigrai, Ethiopia; 2019: A retrospective study. Infect Drug Resist 2020;13:643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedaso MH, Kalil FS. Trends of Drug Resistance Tuberculosis from 2014 to 2018, Bale Zone, Oromia Region, Ethiopia. Infect Drug Resist 2021;14:2073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asemahagn MA, Alene GD, Yimer SA. Spatial-temporal clustering of notified pulmonary tuberculosis and its predictors in East Gojjam Zone, Northwest Ethiopia. PLoS One 2021;16:e0245378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araya S, Negesso AE, Tamir Z. Rifampicin-resistant Mycobacterium tuberculosis among patients with presumptive tuberculosis in Addis Ababa, Ethiopia. Infect Drug Resist 2020;13:3451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodena D, Ataro Z, Tesfa T. Trend analysis and seasonality of tuberculosis among patients at the Hiwot Fana Specialized University Hospital, Eastern Ethiopia: A retrospective study. Risk Manag Healthc Policy 2019;12:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alemu T, Gutema H. Trend in magnitude of tuberculosis in Awi Zone, Northwest Ethiopia: A five-year tuberculosis surveillance data analysis. BMC Res Notes 2019;12:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gashu Z, Jerene D, Datiko DG, Hiruy N, Negash S, Melkieneh K, et al. Seasonal patterns of tuberculosis case notification in the tropics of Africa: A six-year trend analysis in Ethiopia. PLoS One 2018;13:e0207552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammed H, Oljira L, Roba KT, Ngadaya E, Ajeme T, Haile T, et al. Burden of tuberculosis and challenges related to screening and diagnosis in Ethiopia. J Clin Tuberc Other Mycobact Dis 2020;19:100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mussie KM, Yimer SA, Manyazewal T, Gradmann C. Exploring local realities: Perceptions and experiences of healthcare workers on the management and control of drug-resistant tuberculosis in Addis Ababa, Ethiopia. PLoS One 2019;14:e0224277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mussie KM, Gradmann C, Manyazewal T. Bridging the gap between policy and practice: A qualitative analysis of providers’ field experiences tinkering with directly observed therapy in patients with drug-resistant tuberculosis in Addis Ababa, Ethiopia. BMJ Open 2020;10:e035272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manyazewal T, Woldeamanuel Y, Blumberg HM, Fekadu A, Marconi VC. The fight to end tuberculosis must not be forgotten in the COVID-19 outbreak. Nat Med 2020;26:811–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Megerso A, Deyessa N, Jarso G, Tezera R, Worku A. Exploring community tuberculosis program in the pastoralist setting of Ethiopia: A qualitative study of community health workers’ perspectives in Borena Zone, Oromia Region. BMC Health Serv Res 2021;21:632. [DOI] [PMC free article] [PubMed] [Google Scholar]


