Abstract
OBJECTIVE
We hypothesized that serum cannabidiol (CBD) concentrations would be higher in patients taking pharmaceutical- versus artisanal-CBD oil, and higher serum CBD concentrations would correlate with increased side effects and decreased seizure frequency.
METHODS
This was a retrospective chart review. We included patients with pharmacoresistant epilepsy, treated with artisanal-CBD or pharmaceutical-CBD (Epidiolex), and with quantitative serum CBD concentrations. We tracked epilepsy diagnosis, artisanal-CBD dosage, pharmaceutical-CBD dose, serum CBD concentration, clobazam concentration, N-desmethylclobazam concentration, seizure history (frequency of motor seizures), response to medication (percentage reduction in motor seizures), and side effects.
RESULTS
Forty-two patients met inclusion criteria. Mean serum CBD concentration was 51.1 ng/mL (artisanal group) and 124 ng/mL (pharmaceutical group) (p = 0.022). Patients receiving artisanal-CBD had no change in median overall seizures (IQR, −50% to 50%); the pharmaceutical-CBD group had median 50% reduction (IQR, −90% to no change) (p = 0.199).
CONCLUSIONS
Pharmaceutical-CBD achieves higher serum CBD concentrations than artisanal-CBD in pediatric patients with refractory epilepsy. These higher CBD concentrations are associated with increased reported adverse effects, but no detectable difference in seizure frequency.
Keywords: artisanal cannabidiol, cannabidiol, Dravet syndrome, Epidiolex, Lennox-Gastaut syndrome, pediatric epilepsy
Introduction
Cannabidiol (CBD) is a neuroactive Cannabis derivative with antiseizure properties. Purified, plant-based, pharmaceutical-CBD was US Food and Drug Administration (FDA) approved for the management of 2 treatment-refractory epilepsies (Lennox-Gastaut syndrome [LGS] and Dravet syndrome) in June 2018, with expanded indication for tuberous sclerosis in July 2020, but literature on its use for seizures dates to the 1970s.1,2 A 2014 Cochrane review of 4 randomized controlled trials concluded that these studies were “low quality” and “no reliable conclusions” could be made regarding the use of cannabinoids in the treatment of epilepsy.3 Patients with refractory epilepsy have been using non–FDA-approved, artisanal-CBD oil and other cannabis derivations as antiseizure medications (ASMs) for several years. These preparations may be manufactured without using standardized methods and may contain imprecise amounts of CBD and/or other substances including psychoactive compounds such as Δ-9-tetrahydrocannabinol (THC).4
In an open-label trial of 162 patients using pharmaceutical-CBD, 79% reported adverse events including: somnolence (25%), anorexia (19%), diarrhea (19%), and status epilepticus (6%).5 The data for artisanal-CBD use in epilepsy, especially in pediatrics, are more limited; specifically, there is a lack of serum CBD concentration monitoring recommendations available that relate to artisanal-CBD in pediatric epilepsy.6 There is no clear target therapeutic range, and data are limited and inconsistent when correlating serum CBD concentration with efficacy and/or side effects. A known drug-drug interaction between CBD and clobazam, in which CBD inhibits CYP 2C19 and CYP 3A4 isoenzymes that metabolize clobazam and its active metabolite, N-desmethylclobazam, leads to increased serum concentrations of both moieties and increased side effects, especially somnolence.7 A study of 81 patients taking CBD and other ASMs reported no change in serum valproic acid concentrations, but some patients reported increased somnolence on combination therapy.8 Driven by questions regarding the comparative safety and efficacy between artisanal- and pharmaceutical-CBD, we examined serum CBD concentrations, seizure control, and adverse effects in patients administered CBD. We hypothesized that serum CBD concentrations will be higher in patients taking pharmaceutical-CBD versus artisanal-CBD oil and that higher CBD concentrations will be correlated with increased side effects9 and decreased seizure frequency.
Materials and Methods
We performed a retrospective chart review of CBD-treated children at Children's National Hospital from 2017 to 2021. Patients were identified by their treating neurologists and through an expanded access program using pharmaceutical-CBD (Epidiolex, GW Pharmaceuticals, Cambridge, UK) for pharmacoresistant epilepsy. We also retrieved comprehensive reports for the hospital system of all patients with quantitative CBD testing and included these patients. The expanded access program, which included patients 2 years of age to adult age who were not receiving any artisanal-CBD and with any type of refractory epilepsy, occurred prior to commercial availability of Epidiolex. We included patients with epilepsy treated with artisanal- or pharmaceutical-CBD who had quantitative serum CBD concentrations available. Artisanal-CBD was administered and dosed solely by caregivers.
We recorded demographics, epilepsy diagnosis, artisanal-CBD dosage/concentration (as reported by manufacturer on product bottle at visit), pharmaceutical-CBD dose, serum CBD concentration, seizure history, and patient or caregiver reported side effects as documented in the medical record. We also recorded if the patient was prescribed a ketogenic diet and for the purposes of this study, considered the ketogenic diet as an ASM.10 Blood was obtained for the determination of a random CBD concentration in serum that was quantitated with high performance liquid chromatography/tandem mass spectrometry (LC-MS/MS; NMS Labs, Horsham, PA). A patient blood sample was obtained at the clinic visit once the patient was estimated to be taking the stable dose for at least 2 weeks. Baseline total seizure frequency was recorded as of the last documented note prior to starting CBD. Duration of follow-up was defined as months between starting CBD and the date of last follow-up. We tracked overall percentage change in all seizures from baseline to the time of obtaining the first serum CBD concentration. Responder rate was defined as ≥50% reduction in overall seizures11,12 (all seizure types [motor, non-motor including absence and focal unaware seizures] were included in this analysis).
We also recorded the serum concentration for clobazam and its metabolite N-desmethylclobazam (if measured). The determination of parent and metabolite concentrations in serum was performed with LC-MS/MS (Mayo Clinic Laboratories, Rochester, MN). The number of ASMs was also tracked.
Statistical analysis was performed with Jamovi version 1.6.23 (The Jamovi Project, Sydney, Australia). For interval variables (CBD concentration, percentage change in seizures, clobazam concentration, N-desmethylclobazam concentration), we used 2-sided Welch t test of independent sample, assuming unequal variance and statistical significance if p < 0.05. For categorical variables (50% responder rate), we used the chi-squared test. Spearman rho was calculated for the relationship between CBD dose and the CBD concentration.
Anonymized data will be shared by reasonable request from any qualified investigator.
Results
Forty-two patients met inclusion criteria, 48% female, with mean age of 10.1 years (range, 3–20 years) (Table). Three patients in the artisanal group had incomplete documentation of seizure change and were excluded from this analysis. There were several products reported in the artisanal-CBD group. Four patients used Charlotte's Web, 1 used Palmetto Harmony, 2 used Haleigh's Hope, and 4 reported non-branded CBD oil use. There were 11 patients in the artisanal-CBD group whose charts were reviewed: LGS (n = 3), Dravet syndrome due to pathogenic SCN1A variant (n = 1), SCN2A developmental and epileptic encephalopathy (DEE) (n = 1), STXBP1 epileptic encephalopathy (n = 1), focal epilepsy due to structural etiology (n = 3), and genetic generalized epilepsy (n = 2). There were 31 patients in the pharmaceutical-CBD group—23 who were participating in a pharmaceutical-CBD (Epidiolex) expanded access program from September 2017 to March 2019, and 8 pharmacoresistant epilepsy patients—with the following diagnoses: LGS (n = 13), genetic generalized epilepsy (n = 9), DEE (n = 6), Dravet syndrome (n = 2), and focal unaware epilepsy of unknown etiology (n = 1).
Table.
Clinical and Demographic Data *
| Artisanal-CBD (n = 11) | Pharmaceutical-CBD (n = 31) | |
|---|---|---|
| Age, median (IQR), yr | 8 (5–13.3) | 9.5 (7–15.3) |
| F/U, median (IQR), mo | 7.8 (4.6–45) | 14 (4–21) |
| Sex, n (%) | ||
| Male | 4 (36) | 14 (45) |
| Female | 7 (64) | 17 (55) |
| Additional ASM | ||
| Median (IQR) | 2 (1–3) | 3 (2–3) |
| Using CLB, n (%) | 6 (55) | 6 (26) |
| Using VPA, n (%) | 2 (18) | 4 (17) |
| Using Keto, n (%) | 1 (9) | 2 (6) |
| Diagnosis, n (%) | ||
| LGS | 3 (27) | 13 (42) |
| Dravet | 1 (9) | 2 (5) |
| GGE | 2 (18) | 9 (29) |
| DEE | 2 (18) | 6 (19) |
| Focal | 3 (27) | 1 (3) |
| Seizure outcome | ||
| Overall, n (%) | 0 (−50 to 50) | −50 (−90 to 0) |
| 50% RR | 36 | 45 |
ASM, antiseizure medication; CBD, cannabidiol; CLB, clobazam; DEE, developmental and epileptic encephalopathy; F/U, follow-up; GGE, genetic generalized epilepsy; Keto, ketogenic diet; LGS, Lennox-Gastaut syndrome; VPA, valproic acid; 50% RR, 50% responder rate
* All values expressed as n (%) or median (IQR).
Median CBD dose was as follows: artisanal group, 4.3 mg/kg/day (IQR, 3.5–6.8 mg/kg/day); pharmaceutical group, 25 mg/kg/day (IQR, 20–25 mg/kg/day). Dose was not recorded for 2 patients in the artisanal group. Mean serum CBD concentration was as follows: artisanal group, 51.2 ng/mL; pharmaceutical group, 124 ng/mL (see Figure 1) (p = 0.022). There was a trend association between median CBD dose and CBD concentration (Spearman rho = 0.22; p = 0.089). At last follow-up (median follow-up, 7.8 months [IQR, 4.6–45 months] for artisanal group; 14.1 months [IQR, 4–20.7 months] for pharmaceutical group), patients treated with artisanal-CBD had no change in median overall seizures (IQR, −50% to 50%), whereas the pharmaceutical-CBD group had a median 50% reduction (IQR, −90% to no change; p = 0.199), as shown in Figure 2. The artisanal group had a 36% responder rate (n = 4/11) and the pharmaceutical group had a 45% responder rate (n = 14/31; p = 0.583). There were no reported side effects in the artisanal group; all reported side effects were in the pharmaceutical group. Thirteen patients reported adverse effects (somnolence, emesis, diarrhea, diminished appetite), 4 of whom ultimately discontinued CBD owing to side effects. Two patients in the pharmaceutical-CBD group discontinued CBD owing to lack of efficacy.
Figure 1.
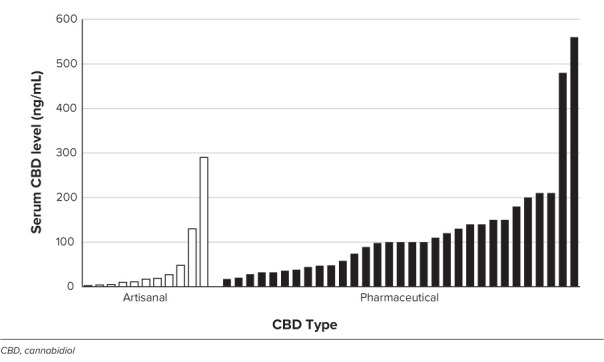
Serum cannabidiol concentration as a function of CBD type. Bars indicate individual patient serum CBD concentration. The mean CBD concentration is 51 ng/mL (artisanal group) versus 124 ng/mL (pharmaceutical group), p = 0.022.
Figure 2.
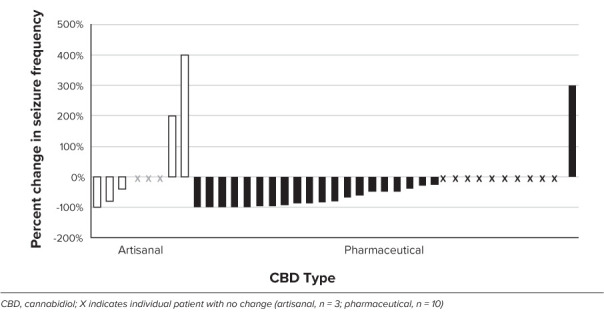
Percentage change in seizure frequency as a function of CBD type. Median no change (artisanal group), 50% reduction (pharmaceutical group).
All patients were co-administered another ASM. In both groups, patients were receiving on average 2 other ASMs, not including CBD. Six of the 11 patients in the artisanal group (55%) and 6 of the 31 patients in the pharmaceutical group (26%) were administered clobazam; 2 patients in the artisanal group (18%) and 4 patients in the pharmaceutical group (17%) used valproic acid. Other ASMs by drug (artisanal group number of patients, percentage of artisanal group/pharmaceutical group, percentage of pharmaceutical group) included levetiracetam (4, 36%/8, 26%); topiramate (2, 18%/4, 13%); lacosamide (1, 9%/4, 13%); zonisamide (1, 9%/4, 13%); lamotrigine (3, 27%/6, 19%); ketogenic diet (1, 9%/2, 6%); oxcarbazepine (2, 18%/3, 10%); phenobarbital (1, 9%/1, 3%); felbamate (1, 9%/4, 13%); vigabatrin (1, 9%/0, 0%); ethosuximide (0, 0%/5, 16%); rufinamide (0, 0%/6, 19%); clonazepam (0, 0%/4, 13%); tiagabine (0, 0%/1, 3%); gabapentin (0, 0%/1, 3%); and brivaracetam (0, 0%/1, 3%). The average serum clobazam concentration in the artisanal group was 408 ng/mL and the average serum clobazam concentration in the pharmaceutical group was 423 ng/mL (p = 0.938). The average serum N-desmethylclobazam concentration in the artisanal group was 8054 ng/mL, and 6780 ng/mL in the pharmaceutical group (p = 0.73). The reference range for serum clobazam is 30 to 300 ng/mL, and for N-desmethylclobazam, 300 to 3000 ng/mL.13
Discussion
We found that the use of pharmaceutical-CBD is associated with larger average CBD dosages and higher serum CBD concentrations than artisanal-CBD in pediatric patients with pharmacoresistant epilepsy. We also noted that the pharmaceutical-CBD group reported increased adverse effects. Given the wider acceptance of and expanding access to naturopathic treatments, we feel it is important to highlight the differences in CBD dosing and concentrations in a real-world cohort.
Given the typical recommended dosing of pharmaceutical-CBD at 10 to 20 mg/kg/day, our data suggest that the use of artisanal-CBD is associated with smaller CBD dosages, lower serum CBD concentrations, and may be associated with worse seizure control. In an Italian trial of pharmaceutical-grade, purified CBD oil in 29 refractory epilepsy patients (age, 1.9–16.3 years) using doses up to 25 mg/kg/day, 37.9% reported a 50% reduction in seizures, while 62.1% of the cohort had no reported change in seizure frequency. We found a similar responder rate in our pharmaceutical-CBD group (45%). Interestingly, serum CBD concentrations were not reported in this trial.14 A prospective trial of artisanal-CBD with 21 children (median age, 10.3 years; IQR, 6.8–12.6 years) at Colorado Children's Hospital found no correlation between serum CBD concentration and seizure control. The median reported serum CBD concentration in the Colorado study was 3.1 ng/mL (IQR, 1.9–8.1 ng/mL),15 considerably lower than what we found in our 2 cohorts. An open retrospective study of 37 mixed adult and pediatric patients with standardized CBD oil reported a 73% responder rate.16 Overall, our data suggest a wide range in reported seizure control but a possible trend for better seizure control in patients receiving pharmaceutical-CBD. However, there are conflicting data in the literature. A meta-analysis of 670 patients (age, 0.1–31 years) reported that 71% of patients using artisanal-CBD reported improvement, compared with only 46% using purified CBD.17 All patients in the artisanal group were from 6 retrospective studies (including 3 with survey-reported data), whereas all patients from the purified-CBD group were from prospective studies. Regardless, there was no difference between groups for “reduction of 50% or more in seizure frequency.”17 Our finding of no significant difference in responder rate is consistent with this finding.
This study provides support that pharmaceutical-CBD is associated with higher serum CBD concentrations. An open-label single center study including 44 pediatric patients with refractory epilepsy found that there is a linear dose-response curve of CBD when using pharmaceutical-CBD oil. Larger CBD doses directly correlated to improved seizure control. Average serum CBD concentration in the pediatric subgroup was 115 ng/mL (IQR, 54–220 ng/mL) when using a dosage of 20 mg/kg/day (IQR, 12–25 mg/kg/day),18 similar to the 124 ng/mL found in the present study.
Some have argued that in patients receiving both clobazam and CBD, N-desmethylclobazam may be the potential cause of reported adverse effects such as somnolence.19 We did not find a correlation between these concentrations and reported side effects.
Our study has limitations. This is a real-world study in which artisanal-CBD dosing was solely controlled by the caregiver and given at significantly smaller (reported) dosages than for the pharmaceutical-CBD group. The purpose of this study was to highlight this difference in dosing due to the real-world scenario in the United States where physicians have limited data on which to base choice and dosing of artisanal-CBD; moreover, there are numerous, non-standardized, unregulated CBD formulations that caregivers are giving to their children. Future prospective, controlled studies are needed to better compare artisanal- and pharmaceutical-CBD. The retrospective nature of data collection and the small number of patients in the artisanal-CBD group limit our interpretation of efficacy, adverse effects, and serum concentrations. For example, the side effects are mostly by parental report; it is not possible to assign causality to CBD and/or state that this is clearly a dosing effect. Compliance was assumed and serum CBD concentrations were collected at timing determined by the treating physician and were not collected at standardized time points. Some patients had incomplete data owing to documentation variability, artisanal-CBD concentration, and medication changes that were not tracked. We did not track medication or other changes in ASMs, which could be a source of bias. Furthermore, we did not track other metabolic laboratory tests, such as liver function tests, or serum concentrations of other ASMs (because they are not checked frequently or were unavailable). We tracked compliance by confirming reported regular use of CBD in the medical record. This study is not randomized and/or controlled, and the sample size is small, so there may be other biases or variables at play. We note that our patient population is accessing and using non-standardized CBD preparations, which makes controlled study difficult. The dosing and administration of non–pharmaceutical-CBD was performed by caregivers.
Conclusion
Our data, which use a real-world population of pharmacoresistant epilepsy patients, suggest that pharmaceutical-CBD use is associated with larger CBD weight-based dosing and generates higher serum CBD concentrations, which may also lead to increased reported adverse effects. There was no difference in seizure frequency between artisanal- and pharmaceutical-CBD groups. Future studies under controlled settings, particularly focused on the effect of targeted serum CBD concentrations, could help to more fully elucidate these findings.
ABBREVIATIONS
- ASM
antiseizure medication
- CBD
cannabidiol
- DEE
developmental and epileptic encephalopathy
- FDA
US Food and Drug Administration
- LC-MS/MS
high performance liquid chromatography/tandem mass spectrometry
- LGS
Lennox-Gastaut syndrome
- THC
Δ-9-tetrahydrocannabinol
Footnotes
Disclosures. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study drug only was provided for patients enrolled in the Epidiolex expanded access program. No additional support was provided.
Ethical Approval and Informed Consent. Given the nature of this study, institutional review board/ethics committee review and informed consent were not required. This study was registered with the Children's National IRB under Protocol Pro00011858.
References
- 1.Mechoulam R, Carlini EA. Toward drugs derived from cannabis. Die Naturwissenschaften . 1978;65(4):174–179. doi: 10.1007/BF00450585. [DOI] [PubMed] [Google Scholar]
- 2.Cunha JM, Carlini EA, Pereira AE et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology . 1980;21(3):175–185. doi: 10.1159/000137430. [DOI] [PubMed] [Google Scholar]
- 3.Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev . 2014;2014(3):Cd009270. doi: 10.1002/14651858.CD009270.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonn-Miller MO, Loflin MJE, Thomas BF et al. Labeling accuracy of cannabidiol extracts sold online. JAMA . 2017;318(17):1708–1709. doi: 10.1001/jama.2017.11909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devinsky O, Marsh E, Friedman D et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol . 2016;15(3):270–278. doi: 10.1016/S1474-4422(15)00379-8. [DOI] [PubMed] [Google Scholar]
- 6.Raucci U, Pietrafusa N, Paolino MC et al. Cannabidiol treatment for refractory epilepsies in pediatrics. Front Pharmacol . 2020;11:586110. doi: 10.3389/fphar.2020.586110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia . 2015;56(8):1246–1251. doi: 10.1111/epi.13060. [DOI] [PubMed] [Google Scholar]
- 8.Gaston TE, Bebin EM, Cutter GR et al. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia . 2017;58(9):1586–1592. doi: 10.1111/epi.13852. [DOI] [PubMed] [Google Scholar]
- 9.Devinsky O, Cross JH, Laux L et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med . 2017;376(21):2011–2020. doi: 10.1056/NEJMoa1611618. [DOI] [PubMed] [Google Scholar]
- 10.Winesett SP, Bessone SK, Kossoff EH. The ketogenic diet in pharmacoresistant childhood epilepsy. Expert Rev Neurother . 2015;15(6):621–628. doi: 10.1586/14737175.2015.1044982. [DOI] [PubMed] [Google Scholar]
- 11.Sperling MR, Abou-Khalil B, Aboumatar S et al. Efficacy of cenobamate for uncontrolled focal seizures: post hoc analysis of a Phase 3, multicenter, open-label study. Epilepsia . 2021;62(12):3005–3015. doi: 10.1111/epi.17091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barcs G, Walker EB, Elger CE et al. Oxcarbazepine placebo-controlled, dose-ranging trial in refractory partial epilepsy. Epilepsia . 2000;41(12):1597–1607. doi: 10.1111/j.1499-1654.2000.001597.x. [DOI] [PubMed] [Google Scholar]
- 13.Patsalos PN, Berry DJ, Bourgeois BF et al. Antiepileptic drugs--best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia . 2008;49(7):1239–1276. doi: 10.1111/j.1528-1167.2008.01561.x. [DOI] [PubMed] [Google Scholar]
- 14.Pietrafusa N, Ferretti A, Trivisano M et al. Purified cannabidiol for treatment of refractory epilepsies in pediatric patients with developmental and epileptic encephalopathy. Paediatr Drugs . 2019;21(4):283–290. doi: 10.1007/s40272-019-00341-x. [DOI] [PubMed] [Google Scholar]
- 15.Knupp KG, Rice JD, Helmkamp LJ et al. Prospective evaluation of oral cannabis extracts in children with epilepsy. Seizure . 2019;72:23–27. doi: 10.1016/j.seizure.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Marchese F, Vari MS, Balagura G et al. An open retrospective study of a standardized cannabidiol based-oil in treatment-resistant epilepsy. Cannabis Cannabinoid Res . 2022;7(2):199–206. doi: 10.1089/can.2019.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pamplona FA, da Silva LR, Coan AC. Potential clinical benefits of CBD-rich cannabis extracts over purified CBD in treatment-resistant epilepsy: observational data meta-analysis. Front Neurol . 2018;9:759. doi: 10.3389/fneur.2018.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szaflarski JP, Hernando K, Bebin EM et al. Higher cannabidiol plasma levels are associated with better seizure response following treatment with a pharmaceutical grade cannabidiol. Epilepsy Behav . 2019;95:131–136. doi: 10.1016/j.yebeh.2019.03.042. [DOI] [PubMed] [Google Scholar]
- 19.Klein P, Tolbert D, Gidal BE. Drug-drug interactions and pharmacodynamics of concomitant clobazam and cannabidiol or stiripentol in refractory seizures. Epilepsy Behav . 2019;99:106459. doi: 10.1016/j.yebeh.2019.106459. [DOI] [PubMed] [Google Scholar]


