Abstract
Cardiovascular (CV) outcome trials (CVOTs) of type 2 diabetes mellitus (T2DM) therapies have mostly used randomized comparison with placebo to demonstrate non-inferiority to establish that the investigational drug does not increase CV risk. Recently, several glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium glucose cotransporter 2 inhibitors (SGLT-2i) demonstrated reduced CV risk. Consequently, future T2DM therapy trials could face new ethical and clinical challenges if CVOTs continue with the traditional, placebo-controlled design. To address this challenge, here we review the methodologic considerations in transitioning to active-controlled CVOTs and describe the statistical design of a CVOT to assess non-inferiority versus an active comparator and if non-inferiority is proven, using novel methods to assess for superiority versus an imputed placebo. Specifically, as an example of such methodology, we introduce the statistical considerations used for the design of the “Effect of Tirzepatide versus Dulaglutide on Major Adverse Cardiovascular Events (MACE) in Patients with Type 2 Diabetes” trial (SURPASS CVOT). It is the first active-controlled CVOT assessing antihyperglycemic therapy in patients with T2DM designed to demonstrate CV efficacy of the investigational drug, tirzepatide, a dual glucose-dependent insulinotropic polypeptide and GLP-1 RA, by establishing non-inferiority to an active comparator with proven CV efficacy, dulaglutide. To determine the efficacy margin for the hazard ratio, tirzepatide versus dulaglutide, for the composite CV outcome of death, myocardial infarction, or stroke (MACE-3), which is required to claim superiority versus an imputed placebo, the lower bound of efficacy of dulaglutide compared with placebo was estimated using a hierarchical Bayesian meta-analysis of placebo-controlled CVOTs of GLP-1 RAs. SURPASS CVOT was designed so that when the observed upper bound of the 95% confidence interval of the hazard ratio is less than the lower bound of efficacy of dulaglutide, it demonstrates non-inferiority to dulaglutide by preserving at least 50% of the CV benefit of dulaglutide as well as statistical superiority of tirzepatide to a theoretical placebo (imputed placebo analysis). The presented methods adding imputed placebo comparison for efficacy assessment may serve as a model for the statistical design of future active-controlled CVOTs.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01601-w.
Keywords: Type 2 diabetes mellitus, Antihyperglycemic, Cardiovascular, Non-inferiority trial, Imputed placebo
Introduction
In 2008, the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) issued guidance for the evaluation of cardiovascular (CV) safety of new antihyperglycemic therapies for type 2 diabetes mellitus (T2DM) [1, 2]. Based on this guidance, new investigational drugs for the treatment of T2DM were required to demonstrate no increased risk of major adverse CV events (MACE) compared with placebo. The standard for CV safety was statistically defined by achieving a relative risk compared with placebo, with an upper bound of 95% confidence interval (CI) of less than 1.8 for initial application and 1.3 for final approval.
Over the past decade, results from CV outcome trials (CVOTs) evaluating several new medication classes for T2DM such as dipeptidyl peptidase-4 inhibitors (DPP-4i), basal insulins, alpha-glucosidase inhibitors, glucagon-like peptide-1 receptor agonists (GLP-1 RA), and sodium glucose cotransporter-2 inhibitors (SGLT-2i) have been reported [3–9]. Only two of these trials [8, 9] compared the investigational medications with antihyperglycemic medications as active comparators, which were added to standard clinical care of patients with T2DM and established atherosclerotic cardiovascular disease (ASCVD) or multiple CV risk factors. In addition, a pragmatic open-label randomized active comparator TOSCA.IT trial evaluated CV outcomes of pioglitazone compared with sulfonylureas (glibenclamide, glimepiride or gliclazide) in patients with T2DM [10]. None of these active comparators (glimepiride, glibenclamide, gliclazide nor insulin glargine) had proven cardiovascular efficacy before their use in these CVOTs. However, numerous trials of SGLT-2i and GLP-1 RA medications have demonstrated reduction in MACE and/or fewer hospitalizations due to heart failure and/or reduction in risk for kidney disease progression [3–7, 11–14]. These results satisfied the regulatory requirements for evidence of CV safety and furthermore, their superior CV and kidney efficacy provided the basis for indications to use these agents to reduce CV and/or kidney disease risk.
The demonstration of beneficial effects of antihyperglycemic medications on CV risk in patients with T2DM has been widely recognized as a major advance, changing the clinical practice guidelines and consensus, recommendations of professional societies to emphasize prioritized use of SGLT-2i and GLP-1 RA in patients with or at high risk of CV disease [15–19]. This favorable new therapeutic landscape, on the other hand, presents significant challenges for the design, conduct, and interpretation of future CVOTs for antihyperglycemic medications. Key among these considerations include the use of a placebo control when antihyperglycemic medications with proven CV benefits are now available. For example, patients entering a placebo-controlled randomized trial who are randomized to placebo will not be allowed to receive GLP-1 RA during the trial, which may not be considered ethical nowadays. Moreover, the open-label use of other cardioprotective antihyperglycemic medications that mitigate CV risk in either trial arm as part of standard medical care could influence the primary outcome of the CVOT assessment. On the other hand, if members of new drug classes with unknown effects on CV outcomes are tested, placebo-controlled design (added to the best standard of care) may remain an important strategy to establish CV safety and efficacy. As T2DM CVOTs aim for similarly effective glycemic control in investigational and comparator groups, it is more likely that additional antihyperglycemic agents with CV efficacy will be added to patients receiving placebo. If the added medications are SGLT-2i and/or GLP-1 RA, both of which would be expected to decrease MACE, the intention-to-treat analysis of the safety and efficacy of the investigational medication would be confounded. Thus, the results may underestimate the potential benefit of the investigational therapy and may adversely influence interpretation of the safety and efficacy assessments.
An alternative to a placebo-controlled CVOT to demonstrate CV safety and/or benefit of a new diabetes medication could be direct randomized comparison of the investigational therapy with a medication that has established CV efficacy. As one of many examples, a similar approach has been used in trials to test new factor Xa inhibitor anticoagulant therapies versus the established anticoagulant therapy warfarin for stroke prevention [20–23]. Using an active comparator addresses the clinical and ethical concerns of a placebo-controlled trial as it provides high-risk participants in the control group with a proven treatment. The efficacy of anticoagulant therapies (with approximately 50%-70% reduction in risk of stroke) is however larger than the reduction in MACE risk by diabetes drugs with proven CV efficacy, which further complicates the statistical design of active-controlled diabetes CVOTs.
The evaluation of a superior treatment effect between the investigational versus diabetes drugs with proven CV efficacy, considering the relatively small but clinically meaningful risk reduction, would require larger sample size and/or longer trial duration to ascertain large number of events for adequate statistical power. For example, a diabetes CVOT designed with 90% power to demonstrate 10% greater relative risk reduction for an investigational therapy over an active comparator would require an accrual of 3786 primary outcomes. Assuming an annualized MACE event rate of 4%, this would approximate to 100,000 patient-years of exposure (e.g., 25,000 patients followed for an average of four years). A successful outcome in this setting would prove superiority of the investigational medication compared with active control, and by extension also in relation to placebo.
An alternative objective of an active-controlled diabetes CVOT could be to demonstrate superiority to an imputed placebo by demonstrating NI to a proven active comparator using sufficiently narrow NI margins. This approach has the advantage of a smaller sample size and/or shorter duration of the trial. Assuming that the comparator is proven to reduce CV relative risk by at least 5%, a NI margin of 1.05 could be justified. Using the same assumption for an annualized MACE event rate of 4% as above and accepting the NI margin of up to 1.05 to establish NI to the active comparator, the study with 1600 MACE events, less than half the size as the trial outlined above with direct superiority comparison with the active comparator would also establish CV efficacy versus an imputed placebo.
One example for the indirect demonstration of superiority versus imputed placebo is the International Joint Efficacy Comparison of Thrombolytics (INJECT) randomized, double-blind CVOT [24] that compared reteplase double-bolus administration with streptokinase in acute myocardial infarction (MI) and was designed to show imputed CV efficacy by demonstrating NI of reteplase to streptokinase for acute MI. While the trials comparing direct oral anticoagulants to warfarin for the treatment of atrial fibrillation did not use this method, the methodology has been applied posthoc across the family of trials to illustrate the methodology [25]. Such imputed placebo comparisons to date, to our knowledge, have not been used to support regulatory product labeling for indication of a medication as is the case with the SURPASS trial.
There is no precedent so far for the evaluation of an antihyperglycemic medication in T2DM for a CV risk reduction indication based on a CVOT designed to test NI versus an active comparator with proven CV efficacy due to the relatively recent demonstration of CV and kidney efficacy of SGLT-2is and GLP-1 RAs. In addition, the modest estimates of relative risk reduction of MACE by SGLT-2is and GLP-1 RAs also complicate such designs given the narrow NI margins required to underpin imputed placebo comparison.
Tirzepatide is a novel, once-weekly, injectable, dual glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 RA, is being developed for the treatment of type 2 diabetes (T2D) and obesity [26] with CV safety demonstrated in the late phase clinical program [27, 28]. The ‘Effect of Tirzepatide versus Dulaglutide on Major Adverse Cardiovascular Events (MACE) in Patients with Type 2 Diabetes (SURPASS CVOT)’ trial is the first trial designed to evaluate the CV efficacy of a novel antihyperglycemic medication for T2DM compared with a blinded active comparator with proven, product labeled CV efficacy, dulaglutide. The development of the SURPASS CVOT statistical analysis plan is summarized here to review the methodology and analytic considerations of a trial designed to prove NI versus active comparator, with the novel addition of using imputed placebo method to assess superiority.
Defining the NI margin for a CVOT with an active comparator
For an active-controlled trial developed with a view of establishing CV efficacy, the determination of the NI margin is critical [29], and must be established a priori to ensure that the investigational therapy (a) would be superior to placebo if a placebo group had been included in the trial (referred to as margin 1, or “M1” in FDA guidance); and (b) is non-inferior to the active comparator (referred to as margin 2, or “M2” in FDA guidance). This latter, more stringent requirement ensures that trial success is declared only if an investigational therapy demonstrates both NI to the active comparator as well as superiority to an imputed placebo in integrated data analyses.
Establishing superiority of an investigational therapy to a putative placebo
Establishing whether an investigational therapy is superior to a putative placebo (i.e., hazard ratio (HR) [investigational therapy versus putative placebo] < 1.0), in an active comparator trial uses estimates of efficacy from (a) direct comparison of the efficacy of the investigational therapy and active comparator in the trial (HR of investigational therapy versus active comparator), and (b) comparison of the efficacy of the active comparator and placebo imputed from prior trials (HR of the active comparator versus placebo). One approach to estimate the HR (investigational therapy versus putative placebo) is simply multiplying the hazard ratios: HR (investigational therapy versus active comparator) × HR (active comparator versus placebo). Therefore, demonstrating that the HR of the investigational therapy versus an active comparator is less than the inverse of HR of the active comparator versus placebo with high probability ensures superiority to a putative placebo. This approach has been used previously in posthoc analyses of direct oral anticoagulants (DOAC) versus warfarin trials to assess the superiority of DOACs versus imputed placebo [25], and related statistical methods and limitations have been described, but to our knowledge not previously used to support regulatory medicinal product labeling considerations [30–32].
To account for variability in the efficacy of the active comparator in any new trial compared with previous placebo-controlled trials, FDA guidance recommends a more conservative approach for establishing superiority of the investigational therapy to a putative placebo. The standard approach to setting the M1 NI margin is to use the inverse of a conservative estimate of active comparator versus placebo, typically the upper bound of the 95% CI of efficacy of the active comparator (HR of active comparator versus placebo) estimated from pooled/meta-analyses of previous trials [29]. Stated differently, if the upper bound of the 95% CI for the HR (investigational therapy versus active comparator) is less than the inverse of upper bound of the 95% CI for the HR (active comparator versus placebo), the investigational therapy would be interpreted as superior relative to a putative placebo. When more than one placebo-controlled trial of the active comparator exists or several placebo-controlled trials of medications from the same pharmacological class have been conducted, a conservative estimate of the HR (active comparator versus placebo) can be derived using a meta-analysis of studies with similar design features [30, 33]. Study design features for consideration may include patient population, trial duration, event rates, and comparable inclusion or exclusion criteria. For example, the Stroke Prevention Using Oral Thrombin Inhibitor in Atrial Fibrillation V (SPORTIF V) CVOT evaluated NI of ximelagatran compared with warfarin on the occurrence of stroke in patients with non-valvular atrial fibrillation [34]. In this case, the NI margin was established by a meta-analysis of six trials comparing warfarin with placebo for stroke or embolic event risk. This analysis showed a benefit of warfarin versus placebo with a HR of 0.36 and 95% CI of 0.25–0.53 [29]. Therefore, in the SPORTIF V CVOT [34], an upper bound for the NI margin was set as 1.9 (1/0.53).
Demonstrating non-inferiority of investigational therapy to the active comparator (Determination of the non-inferiority margin – M2)
The value of the M1 NI margin is set to ensure that the investigational therapy is superior to a putative placebo. However, if the M1 NI margin is too large, an investigational therapy may meet the mark for superiority to placebo without showing convincing NI to the active comparator. For example, due to the overwhelming efficacy of warfarin versus placebo, in the SPORTIF V CVOT [34], the M1 NI margin of 1.9 (i.e., the inverse of the efficacy of warfarin) sets a very wide margin for comparing ximelagatran and warfarin. If the M1 level was used alone, NI could be claimed for ximelagatran versus warfarin with an observed HR substantially > 1.0, raising the possibility that it had inferior efficacy for stroke risk compared with warfarin. To minimize the possibility of such an outcome, it is usually required that the investigational therapy preserves a minimal clinically acceptable difference in efficacy to the active comparator. The M2 NI margin or the minimal clinically acceptable difference in efficacy between the investigational therapy and active comparator relies in part on clinical judgment. FDA guidance suggests that determination of the M2 NI margin be guided by preservation of a pre-specified fraction of the efficacy of the active comparator compared to placebo. In general, it is assumed that preservation of at least 50% of the efficacy of the active comparator is appropriate to claim NI in CVOTs [29]. Accordingly, in the series of NI trials comparing the various DOACs with warfarin, M2 was chosen to be the square root of M1 [25], and for example in SPORTIF V CVOT [34], the M2 was chosen to be square root of 1.9, i.e., 1.378. This was to ensure ximelagatran preserves at least 50% of the efficacy of warfarin, the upper bound of 95% CI of ximelagatran versus warfarin needed to be less than 1.378 to demonstrate NI. In the SPORTIF V study, ximelagatran did not establish NI to warfarin since the upper bound of two-sided 95% CI was 2.21, exceeding the required threshold of 1.378 [29]. These methods have become standard and have been used routinely for registration trials of drugs to support claims of NI versus proven active comparators.
Regulatory guidance for NI [29] is generally presented in a frequentist point of view, where efficacy is viewed as a fixed value. Efficacy of the active comparator versus placebo may be viewed as a random variable as opposed to a fixed value. In this setting, the conservative estimate of the HR of active control versus placebo obtained from a Bayesian hierarchical meta-analysis of placebo-controlled trials of active control or from placebo-controlled trials from medications for the same class may be chosen as M1. Furthermore, a Bayesian approach can be used for combining distribution of efficacy of active control versus placebo together with the data from active controlled trial to assess the fraction of efficacy of the active control preserved by the investigational agent.
Statistical design of an active comparator CVOT to determine MACE efficacy for a new antihyperglycemic medication in patients with T2DM
To adapt to the current landscape of T2DM therapy, the SURPASS CVOT was designed to establish NI of tirzepatide to dulaglutide (a GLP-1 RA with proven CV efficacy), with the novel addition of a planned imputed placebo comparison to evaluate for superiority of CV efficacy. Dulaglutide was found superior to placebo in reducing MACE in The Researching Cardiovascular Events With a Weekly INcretin in Diabetes (REWIND) trial [3], a CVOT in patients with T2DM with or without established ASCVD; this result is now reflected in a product labeled indication. The REWIND trial evaluated CV outcomes of dulaglutide versus placebo, enrolling 9901 participants with or at high risk for atherosclerotic CV disease (mean age of 66.2 years, and a median follow-up of 5.4 years), demonstrating superiority of dulaglutide (HR 0.88; 95% CI 0.79–0.99) for the primary composite outcome of CV death, MI, or stroke. A subgroup analysis showed that the presence or absence of prevalent ASCVD did not modify the treatment efficacy of dulaglutide [3]. To account for possible variability in the trial-to-trial efficacy of dulaglutide, and variability in the medication-to-medication efficacy across the GLP-1 RA class, the distribution of CV risk reduction was generated from results of completed placebo-controlled GLP-1 RA trials (Table 1). As the SURPASS CVOT is conducted in patients with established ASCVD, the patient subgroups with prevalent ASCVD from REWIND and other comparable GLP-1 RA trials were used.
Table 1.
Summary of completed GLP-1 RA cardiovascular outcome trials for the meta-analysis – primary outcomes and associated hazard ratio in patients with a history of atherosclerotic cardiovascular disease (ASCVD)
| Study | Medication | Treatment | Number of patients with MACE-3 | Hazard ratio (95% CI) |
|---|---|---|---|---|
| EXSCEL [36] | Exenatide | Active | 722 | 0.90 (0.82, 1.00) |
| Placebo | 786 | |||
| Total | 1508 | |||
| LEADER [6] | Liraglutide | Active | 536 | 0.83 (0.74, 0.93) |
| Placebo | 629 | |||
| Total | 1165 | |||
| HARMONY [37] | Albiglutide | Active | 338 | 0.78 (0.68, 0.90) |
| Placebo | 428 | |||
| Total | 766 | |||
| SUSTAIN-6 [5] | Semaglutide | Active | 98 | 0.72 (0.55, 0.93) |
| Placebo | 137 | |||
| Total | 235 | |||
| PIONEER-6 [4] | Semaglutide | Active | 57 | 0.83 (0.58, 1.17) |
| Placebo | 68 | |||
| Total | 125 | |||
| REWIND [3] | Dulaglutide | Active | 280 | 0.87 (0.74,1.02) |
| Placebo | 315 | |||
| Total | 595 |
CI confidence interval, EXSCEL Exenatide Study of Cardiovascular Event Lowering, GLP-1 RA glucagon-like peptide-1 receptor agonist, LEADER Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results, MACE major adverse cardiovascular events (a composite outcome comprising non-fatal myocardial infarction, non-fatal stroke, and death from cardiovascular causes), PIONEER Pre-operative wIndOw study of letrozole plus PR agonist (Megestrol Acetate) versus letrozole aloNE in post-menopausal patients with ER-positive breast cancer, REWIND Researching Cardiovascular Events With a Weekly Incretin in Diabetes, SUSTAIN Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes
In accordance with FDA guidance [27], for SURPASS CVOT, the NI margin was determined from an estimate of the HR (GLP-1 RA versus placebo) relative to MACE risk using a Bayesian hierarchical meta-analytic model (see supplemental information) for patients with established ASCVD. This analysis combined aggregate data from all available CVOTs described in Table 1. Exclusion of the ELIXA CVOT [36] was justified based on differences in the patient population from all other included trials and pharmacologic characteristics of lixisenatide compared with other GLP-1 RAs (see Fig. 1), as has also been done in published meta-analyses [37].
Fig. 1.
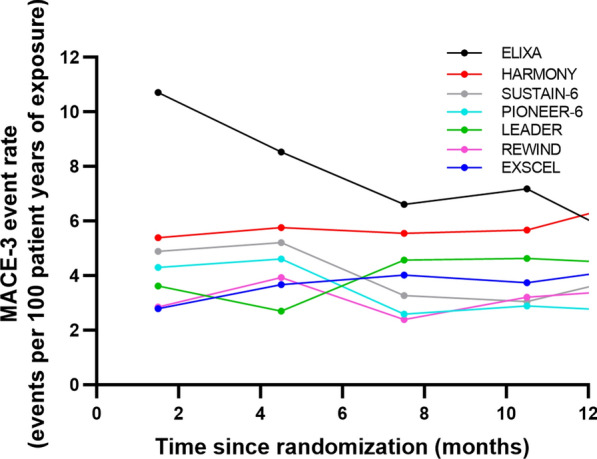
MACE outcome event rate over time in different cardiovascular outcome trials. ELIXA Evaluation of Lixisenatide in Acute Coronary Syndrome, EXSCEL Exenatide Study of Cardiovascular Event Lowering, GLP-1 RA glucagon-like peptide-1 receptor agonist, LEADER Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results, MACE major adverse cardiovascular events (a composite outcome comprising non-fatal myocardial infarction, non-fatal stroke, and death from cardiovascular causes), PIONEER Pre-operative wIndOw study of letrozole plus PR agonist (Megestrol Acetate) versus letrozole aloNE in post-menopausal patients with ER-positive breast cancer, REWIND Researching Cardiovascular Events With a Weekly Incretin in Diabetes, SUSTAIN Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes
The meta-analysis established the 50th percentile (median) of 0.85 and 97.5th percentile of 0.95 ensuring that the HR of dulaglutide versus placebo is less than 0.95 with a probability of 0.975. Accordingly, 1/0.95 = 1.05 was used as the pre-specified M1 NI margin in the SURPASS CVOT statistical analysis plan to demonstrate superiority of tirzepatide relative to a putative placebo.
The SURPASS CVOT trial was designed to accrue 1600 primary outcome events comprising the time to the first event of CV death, MI or stroke and provides approximately 90% power to establish superiority to a putative placebo with an assumed 10% lower MACE risk for tirzepatide compared with dulaglutide and a NI boundary of 1.05.
Figure 2 illustrates the distribution of the HR (tirzepatide versus dulaglutide) × HR (dulaglutide versus placebo)(0.5) evaluated in a simulation study. In this simulation study, conducted to assess the probability that tirzepatide preserving 50% of the efficacy of dulaglutide, the distribution of efficacy of dulaglutide versus placebo is represented by the distribution of HR (GLP-1 RA versus placebo) obtained using the Bayesian hierarchical meta-analytic model described previously and the distribution of HR of tirzepatide versus dulaglutide is developed under the assumption of an observed HR of tirzepatide versus dulaglutide of 0.95, the maximum value needed to meet the requirement for tirzepatide to be superior to a putative placebo in SURPASS CVOT (see Supplemental Information).
Fig. 2.
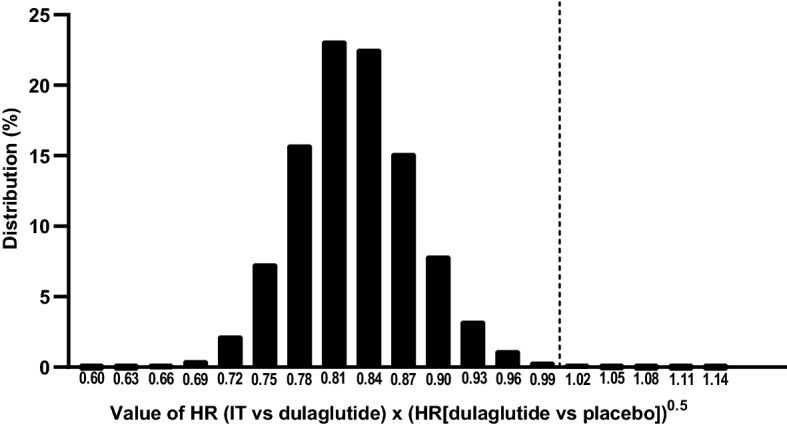
Demonstrating Preservation of 50% Efficacy of Dulaglutide. HR hazard ratio, IT investigational treatment. The simulation study showed a probability of 0.997 for tirzepatide to preserve 50% of dulaglutide efficacy. Therefore, due to the conservative choice of M1 margin and large sample size of SURPASS CVOT, a NI margin of 1.05 in the SURPASS CVOT will not only demonstrate NI of tirzepatide to dulaglutide, but also superiority of tirzepatide to a putative placebo
Discussion
Here the statistical assumptions and analytic methodology have been described that underlie the analytic approach used to design an active comparator trial, the SURPASS CVOT, designed not only to assess NI of tirzepatide versus the proven active comparator dulaglutide, but if NI is demonstrated, to also assess superiority of tirzepatide versus placebo, capitalizing on integrated data analyses to prove efficacy versus an imputed placebo. This methodology could serve as a model for future active-controlled CVOTs evaluating safety and efficacy of new antihyperglycemic medications for T2DM.
Demonstrating CV efficacy versus placebo for an investigational therapy in an active-controlled trial of a proven therapy minimally requires pre-specification of a NI margin based on integrated data analyses of existing data for the active comparator versus placebo to statistically demonstrate superiority against a putative placebo. In the statistical design of the SURPASS CVOT, such a threshold has been derived from a meta-analysis of placebo-controlled GLP-1 RA CVOTs. Bayesian methods were used to determine a NI margin that would establish NI of tirzepatide to dulaglutide and would also be adequate to establish the superiority of tirzepatide versus a putative placebo.
After reviewing the results of CVOTs of antihyperglycemic medications for T2DM in the 12 years since issuing their 2008 guidance, the FDA recently issued a new draft guidance removing pre- and post-marketing requirements for medications narrowly focused on excluding increased CV risk [30]. However, with the demonstration that some antihyperglycemic medications have CV benefits, evaluating therapies in outcomes trials of T2DM remains an important area of clinical investigation with major considerations with respect to the ethics of conducting placebo-controlled trials. It is now ethically indefensible to restrict background use of antihyperglycemic therapies that are proven to reduce CV for eligible patients, as these medications are now recommended as standard-of-care. Thus, present and future CVOTs of T2DM medications will need to compare investigational therapies directly with agents that have known benefits on the incidence of MACE, heart failure, and/or kidney disease progression.
Few limitations apply to the current study. The meta-analysis was conducted using published aggregate data. Meta-analyses of individual patient data are always preferred over trial summary level analyses and may improve precision of estimates and better account for differences in net exposure and duration of follow-up. However, ascertainment of patient-level data across trials is extremely complex and often not possible; and within the GLP-1 RA arena, summary level analyses have largely been consistent with individual trial results [37]. Additionally, an important assumption is that the efficacy of dulaglutide versus placebo demonstrated in the REWIND trial remains unchanged despite changing background care. The ‘best standard of care’ is evolving with time as numerous cardioprotective drug classes have been introduced to clinical practice in the last decade. The interaction of these drugs used as background care with any GLP-1 RA including dulaglutide on CV outcomes is not fully evaluated. Due to the different mechanism of actions (MOAs) of these drug classes when compared with GLP-1 RA, a meaningful interaction with the efficacy of GLP-1 RAs, including dulaglutide, is unlikely.
In summary, the rapidly evolving therapeutic environment for T2DM has provided new options for patients that can further mitigate risk for CV disease complications, which remains a major cause of mortality and morbidity in this population. However, these developments require new considerations for present and future CVOTs that will invariably necessitate the use active comparators with proven clinical efficacy to evaluate CV safety and incremental efficacy of novel agents. This approach requires careful consultation with regulatory agencies, but active comparator designs will become more refined and accepted as their utility increases.
Supplementary Information
Additional file 1. Bayesian hierarchical meta-analytic model. Posterior distribution of HR (tirzepatide versus dulaglutide) assuming SURPASS CVOT resulted in an observed HR (tirzepatide versus dulaglutide) of 0.95.
Acknowledgements
The authors thank Dr. Shirin Ghodke, Eli Lilly Services India Private Limited, for her writing and editorial contribution to this manuscript.
Author contributions
DD and JSR helped with conception, design, and interpretation of the data. SJN and SS helped with conception of the work and analysis/interpretation of data. SEN helped in interpretation of data. IP helped design the work, interpret the data, and draft the manuscript. CRH and JJK helped in conception and design of the work. DKM and GJW helped with conception and design of the work; acquisition, analysis, and interpretation of data; and drafted the manuscript. All authors have contributed to the critical revision of this manuscript and have given their approval to submit this version for publication. All authors read and approved the final manuscript.
Funding
This article is funded by Eli Lilly and Company with regards to data collection, analysis, interpretation, and writing of the manuscript.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed for the purpose of this publication.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
DKM reports honoraria, advisory board, and clinical trial executive committee fees from Eli Lilly; fees for consultancy and/or clinical trial executive committee from Boehringer Ingelheim, Sanofi, Novo Nordisk, AstraZeneca, Lexicon, Eisai Inc., Esperion, Metavant, Applied Therapeutics, and Afimmune; clinical trial data monitoring committee fees from Janssen Research and Development LLC, CSL Behring, and GlaxoSmithKline; advisory committee and global advisory board fees from Novo Nordisk and Merck Sharpe and Dohme; and clinical trial scientific advisory committee fees from Pfizer. DD reports research grants and advisory fees from Eli Lilly; research grants from Merck; consulting fees from Intarcia and Sun Pharmaceuticals; and editorial fees from American Diabetes Association journals. SJN reports research support, consulting and personal fees from Eli Lilly; grants and personal fees from AstraZeneca, Amgen, CSL Behring, Esperion, and Sanofi-Regeneron; grants from Anthera, Cerenis, Resverlogix, Novartis, and InfraReDx; and personal fees from Akcea, Boehringer Ingelheim, Kowa, Merck, Takeda, Pfizer, and Novo Nordisk. SEN reports grants from Eli Lilly, AstraZeneca, Esperion Therapeutics, Amgen, and Novo Nordisk. Although SEN works closely with pharmaceutical companies on the development of new therapies for cardiovascular disease, he maintains a longstanding policy of requiring companies to donate all related honoraria directly to charity so that he receives neither income, nor a tax deduction. IP, SS, CRH, JJK and GJW are employees and stockholders of Eli Lilly and Company. JSR was an employee of Eli Lilly and Company.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.U.S. Food and Drug Administration. Guidance for industry. Diabetes Mellitus - Evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. 2008. https://www.federalregister.gov/documents/2008/12/19/E8-30086/guidance-for-industry-on-diabetes-mellitus-evaluating-cardiovascular-risk-in-new-antidiabetic. Accessed 12 Feb 2022.
- 2.Gore MO, McGuire DK. Cardiovascular disease and type 2 diabetes mellitus: regulating glucose and regulating drugs. Curr Cardiol Rep. 2009;11:258–263. doi: 10.1007/s11886-009-0038-4. [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 4.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 5.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 6.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 8.Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322:1155–1166. doi: 10.1001/jama.2019.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pieber TR, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. NEJM. 2017;377:723–732. doi: 10.1056/NEJMoa1615692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaccaro O, Masulli M, Nicolucci A, Bonoro E, Del Prato S, Maggioni AP, et al. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial. Lancet Diabetes Endocrinol. 2017;5:887–897. doi: 10.1016/S2213-8587(17)30317-0. [DOI] [PubMed] [Google Scholar]
- 11.Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384:129–139. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 13.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 14.McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–158. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2020;63:221–228. doi: 10.1007/s00125-019-05039-w. [DOI] [PubMed] [Google Scholar]
- 16.Targets G. Standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S66–s76. doi: 10.2337/dc20-S006. [DOI] [PubMed] [Google Scholar]
- 17.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 18.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das SR, Everett BM, Birtcher KK, Brown JM, Januzzi JL, Jr, Kalyani RR, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the american college of cardiology solution set oversight committee. J Am Coll Cardiol. 2020;76:1117–1145. doi: 10.1016/j.jacc.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 21.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 22.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 23.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 24.Wilcox RG. Randomised, double-blind comparison of reteplase double-bolus administration with streptokinase in acute myocardial infarction (INJECT): trial to investigate equivalence. International Joint Efficacy Comparison of Thrombolytics. Lancet. 1995;346:329–336. doi: 10.1016/S0140-6736(95)92224-5. [DOI] [PubMed] [Google Scholar]
- 25.Verdecchia P, Angeli F, Lip GY, Reboldi G. Edoxaban in the evolving scenario of non vitamin K antagonist oral anticoagulants imputed placebo analysis and multiple treatment comparisons. PLoS ONE. 2014;9:e100478. doi: 10.1371/journal.pone.0100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karagiannis T, Avgerinos I, Liakos A, Del Prato S, Matthews DR, Tsapas A, et al. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia. 2022;65:1251–1261. doi: 10.1007/s00125-022-05715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomized, open-label, parallel-group, multi-centre, phase 3 trial. Lancet. 2021;398:1811–1824. doi: 10.1016/S0140-6736(21)02188-7. [DOI] [PubMed] [Google Scholar]
- 28.Sattar N, McGuire DK, Pavo I, Weerakkody GJ, Nishiyama H, et al. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med. 2022;28:591–598. doi: 10.1038/s41591-022-01707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Food and Drug Administration. Guidance for industry. Non-inferiority clinical trials to establish effectiveness. 2016. https://www.fda.gov/media/78504/download. Accessed 12 Jan 2022.
- 30.U.S. Food and Drug Administration. Draft guidance for industry. Type 2 diabetes mellitus: Evaluating the safety of new drugs for improving glycemic control. 2020. https://www.fda.gov/media/135936/download. Accessed 12 Jan 2022.
- 31.Hasselblad V, Kong DF. Statistical methods for comparison to placebo in active-control trials. Drug Inf J. 2001;35:435–449. doi: 10.1177/009286150103500212. [DOI] [Google Scholar]
- 32.Rothmann M, Li N, Chen G, Chi GY, Temple RT, Tsou HH. Non-inferiority methods for mortality trials in oncology. Stat Med. 2003;22:239–264. doi: 10.1002/sim.1400. [DOI] [PubMed] [Google Scholar]
- 33.James Hung HM, Wang SJ, Tsong Y, Lawrence J, O'Neil RT. Some fundamental issues with non-inferiority testing in active controlled trials. Stat Med. 2003;22:213–225. doi: 10.1002/sim.1315. [DOI] [PubMed] [Google Scholar]
- 34.U.S. Food & Drug Administration. Pharmacologic class. 2021. https://www.fda.gov/industry/structured-product-labeling-resources/pharmacologic-class. Accessed 01 Jan 2022.
- 35.Albers GW, Diener HC, Frison L, Grind M, Nevinson M, Partridge S, et al. Ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial. JAMA. 2005;293:690–698. doi: 10.1001/jama.293.6.690. [DOI] [PubMed] [Google Scholar]
- 36.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 37.Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomized trials. Lancet Diab Endocrinol. 2021;9:653–662. doi: 10.1016/S2213-8587(21)00203-5. [DOI] [PubMed] [Google Scholar]
- 38.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernandez AF, Green JB, Janmohamed S, D'Agostino RB, Sr, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (HARMONY Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Bayesian hierarchical meta-analytic model. Posterior distribution of HR (tirzepatide versus dulaglutide) assuming SURPASS CVOT resulted in an observed HR (tirzepatide versus dulaglutide) of 0.95.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed for the purpose of this publication.


