Abstract
Background
Preschool age (3–5 years old) is a crucial period for children to acquire gross motor skills and develop executive functions (EFs). However, the association between the qualitative gross motor skills and EFs remains unknown in preschoolers, especially among overweight and obese children.
Methods
This was a cross-sectional, exploratory, and quantitative study carried out on 49 preschool children, divided into two subgroups according to their body mass index (overweight/obese: 24; eutrophic [normal weight]: 25). The mean age was 4.59 years. More than half of the sample were boys (55%) and most of the mothers had completed high school (67%) and were class C socioeconomic level (63%). Gross motor skills were assessed using the Test of Gross Motor Development-2, while EFs were evaluated using Semantic verbal fluency (SVF), Tower of Hanoi (TH), Day/Night Stroop, and Delayed Gratification tests. Multiple linear regression models adjusted for sex, age, maternal education, socioeconomic status, quality of the home environment, and quality of the school environment using the stepwise method were executed, considering the cognitive tasks as independent variables and gross motor skills as dependent variable.
Results
The overweight/obese preschoolers showed worse locomotor skills than their eutrophic peers and below average gross motor quotient (GMQ). Overweight/obese girls performed worse in OC skills than boys with excess weight. SVF (number of errors) and TH (rule breaks) explained 57.8% of the variance in object control (OC) skills and 40.5% of the variance in GMQ (p < .05) in the overweight/obese children. Surprisingly, there was no significant association between any of the EF tasks and gross motor skills in the eutrophic children.
Conclusion
A relationship between EF tasks (number of errors in SVF and rule breaks in TH) and gross motor skills (OC and GMQ) was demonstrated in the overweight/obese preschoolers, indicating that worse cognitive flexibility, working memory, planning, and problem solving are associated with worse gross motor skills in this population when compared to eutrophic children.
Keywords: Childhood obesity, Gross motor, Object control, Executive functions, Verbal fluency, Tower of Hanoi, Cognitive flexibility, Planning, Child development, Cognitive function
Introduction
Being overweight or obese, which is a global burden of disease risk, is defined as abnormal or excessive fat accumulation [1]. The presence of obesity in childhood exposes children to an increased risk of obesity in adulthood [2]. The preschool phase (3–5 years old) covers the “adiposity rebound”, in which the amount of fat mass is reduced to a minimum physiological value followed by subsequent rapid weight increase [3]. Furthermore, the preschool period is crucial for children to acquire gross motor skills and develop executive functions (EFs) [4, 5]. Excess weight at this stage in life has been shown to lead to earlier adiposity rebound, which is associated with worse performance of gross motor skills and EFs [3, 6–9].
Gross motor skills are simple movements in daily tasks such as walking, running, jumping, throwing a ball, and kicking a ball. These skills provide a basis for the future acquisition of complex motor skills used in performing activities related to physical fitness, health, and sports [10, 11]. Excess weight can hinder activities that involve the displacement of body mass due to a greater load against gravity, implying biomechanical limitations [12], and possible impairments of the musculoskeletal functions of obese children [13]. In addition, overweight/obese children that are unable to successfully engage in physical challenges may also resist participating in physical activities and overall learning opportunities. Therefore, children with excess adiposity may suffer slower increases in motor proficiency compared to healthy weight children [14].
EFs are higher-order cognitive skills and can be interpreted as central self-regulatory skills that orchestrate basic or domain-specific cognitive processes (e.g., language, attention, sensory input, motor output) to perform reasoning, planning, problem solving [15], and behavior according to the objective [16]. There is general agreement among researchers in the area that the core components of EFs are inhibitory control, working memory, and cognitive flexibility [17], but these core EF components are not yet completely differentiated during the preschool period [18, 19]. Although reasoning, planning and problem solving are developed from the core components of EFs and are therefore more complex EFs [17], preschoolers already show some degree of ability in reasoning, planning, and problem solving [20].
Initially, studies did not include cognitive flexibility in the analyses because preschool children were believed to lack the cognitive development necessary to perform this EF [21, 22]. This is because the development of cognitive flexibility is thought to be dependent on inhibition and working memory, which are still developing in the preschool period [23]. However, several studies have tested structural models of latent variables, including cognitive flexibility. Some studies [24, 25] found that a single latent EF factor provided a good fit to the data for preschoolers, although single-factor models did not outperform all other tested models and were favored for reasons of parsimony. Furthermore, two-factor models present a better fit than models with one or three factors in preschoolers [26]. One such model includes a working memory factor combined with an inhibition-flexibility factor [27–29], while another model includes an inhibition factor combined with a working memory-flexibility factor [26, 30–32]. Thus, all three EFs are present in preschool children, but it is important to consider the rudimentary aspect of EFs in preschoolers [17, 33], especially cognitive flexibility.
Regarding body weight, a previous study found that a greater linear increase in EFs in the preschool phase corresponds to a greater linear decline in body mass index (BMI) [34]. The hypothesis is that there is higher brain energy expenditure at this age, given the increased volume of cortical and subcortical structures. The higher energy requirement for brain development restricts the energy available for body growth, including fat deposition [35]. Thus, if other influences on BMI remain equal, the child with lower peak brain energy demand in the preschool period, or for whom brain energy demand peaks earlier or is of shorter duration, may experience an earlier adiposity rebound. This could lead to a higher lifelong obesity risk [34, 35]. In addition, children with higher general cognition (verbal skills and EFs) at age 4 had a lower likelihood of maintaining an unhealthy weight status between the ages of 4 years and 6 years, and of worsening weight status over time [9]. However, the cross-sectional association between worse EF performance and excess weight, compared to eutrophic (normal weight) children, has only been observed in children over 6 years of age [36, 37]. Before age 6, results may differ according to the cognitive function assessed, which and how many tasks are used to verify cognitive performance [38], and especially if these tasks were adequately adapted for the preschool phase [38, 39].
Mastery of motor tasks requires cognitive skills [40]. In addition to coactivation of the prefrontal cortex, cerebellum, and basal ganglia during various motor and cognitive tasks, motor and cognitive skills have several underlying processes in common, such as sequencing, monitoring, and planning [41]. Performance of complex motor tasks is extremely variable [42] and requires a higher level of EFs than the performance of simple motor tasks [43]. This variability determines whether, and to what extent, cognitive control processes are needed for successful task performance [43]. Overweight/obese preschoolers may have greater performance variability on gross motor tasks because of excess weight [44]. Thus, in overweight/obese preschoolers, motor tasks may be more challenging and present a stronger correlation with cognitive abilities than in normal weight preschoolers [45]. However, to the best of our knowledge, the relationship between EFs and gross motor skills has not been explored in overweight/obese preschoolers. Therefore, it is also not known whether EFs can predict gross motor skills in preschoolers with excess weight.
Studies that evaluated the EFs as predictors of motor performance, are scarce, especially in preschoolers [46–48]. Furthermore, these studies did not specifically focus on core components of EFs or on more complex EFs, but used some measures of EFs for assessment of general cognitive function. Thus, it may be possible to infer the prediction of EFs in preschoolers. The results of a study carried out by the research group of the present study showed that global cognitive function (orientation, attention and working memory, episodic memory, language, and constructional praxis) is an important predictor of gross motor skills in preschoolers, although no specific tests were used to assess the core components of EFs [46]. However, in a sample of children aged 5 to 6 years, Wassenberg et al. [47] found that global cognitive function (general verbal and non-verbal cognitive ability) was not related to motor performance, but rather to separate cognitive measures (visual motor integration, working memory, and number of correct words in the Semantic Verbal Fluency [SVF]), which were predictors of the global measure of motor performance (including fine and gross motor skills). On the other hand, Peyre et al. [48] found that attention and language skills at age 3 contribute to favorable changes in motor skills at age 6. The assessment of language skills also included the number of correct words in the SFV, but alone was not associated with motor skills. The SFV also assesses EFs [49], but the SVF scores needed for more detailed evaluation of EFs (i.e., amount of errors [49]), especially cognitive flexibility, were not evaluated in either study [47, 48]. Regarding planning, although it can also be related to gross motor skills [50], no previous study has exclusively evaluated this relationship in preschoolers. Therefore, a gap remains in relation to the possibility of an association between certain aspects of self-regulation and gross motor skills in the preschool period [43].
Most of the studies that evaluated the relationship between EFs and gross motor skills throughout childhood prioritized understanding the relationship in the opposite direction, that is, gross motor skills as predictors of each of the EFs separately [51–56]. However, in the preschool period, results in eutrophics are controversial [53, 54] as they depend on which EFs were analyzed and how they were evaluated, that is, whether or not cognitive flexibility was included [54–56], and the level of task difficulty [38, 39, 57]. Thus, as the association between EFs and gross motor skills in preschoolers is likely to depend on the level of performance of rudimentary EFs, models that predict EFs separately may find no association or have a smaller effect size [43, 54, 56]. Another critical point is that previous studies often quantitatively evaluated gross motor skills using whole body coordination tasks, involving strength, speed, or agility [43, 55, 58]. However, the qualitative gross motor skills seems more strongly related to EFs than quantitative measures [54]. In addition, studies usually did not control for important sources of stimuli for cognitive [59–61] and gross motor development [62–64] such as the child’s environment (i.e., home and school environment, maternal education and socioeconomic level), nor did they evaluate them for inclusion in statistical analysis as adjustment covariates [46–48, 54, 55].
Thus, for the present study, we considered that the early adiposity rebound is a result of excess adiposity in the critical period for cognitive and motor development. As such, the presence of excess weight may alter the relationship between EFs and gross motor skills. Therefore, the aim of this study was to investigate the association between EFs (including cognitive flexibility and planning) and qualitative gross motor skills in overweight/obese preschoolers. It also aimed to verify whether the possible associations are different in eutrophic preschoolers matched for age, sex, socioeconomic level, and maternal education using adjusted regression models including quality of the home environment and school environment. We hypothesized that executive control processes play a pivotal role for successful motor performance in overweight/obese preschool children, as most motor tasks are challenging in the presence of excess weight, and therefore require more cognitive control [42, 44, 65], reflecting compensatory dependencies between neurocognitive processes [45, 66].
Methods
Experimental design and participants
A cross-sectional, exploratory, and quantitative study was carried out with preschoolers aged 3 to 5 years from public schools in the city of Diamantina, MG, Brazil, in the second half of 2019. The university ethics committee granted ethical approval prior to beginning the project, under protocol number 2.355.943. The sample consisted of 49 children divided into two subgroups. The first group was the overweight/obese group, consisting of 24 children with a body mass index (BMI) ≥ 97th percentile (z-score > + 2). The second group included 25 eutrophic children with 3rd ≤ BMI < 85th percentile (− 2 < z-score < + 1) [67], matched for sex, age, socioeconomic level, maternal education, quality of the school environment, and quality of the home environment, which are confounding factors for motor skills and EFs [59–64]. As children from the overweight/obese group were recruited, eutrophic children of the same sex from the same classroom were also recruited so that the groups were composed of children of very similar ages and similar socio-environmental realities (later verified by comparing the socioeconomic level, maternal education, quality of the school environment, and quality of the home environment between the groups – Table 1). The exclusion criteria were children with low birth body weight; premature birth; complications during pregnancy or childbirth or any disease that may impair development; or having had an infectious process in the last 30 days prior to data collection.
Table 1.
Characteristics of study participants and comparison between groups
| Variable | Eutrophic (n = 25) | Overweight/Obese (n = 24) | φ/φc/r | p |
|---|---|---|---|---|
| Sex, n (%) | 0.06 | .776 a | ||
| Male | 13.00 (52.00) | 14.00 (58.3) | ||
| Female | 12.00 (48.00) | 10.00 (41.7) | ||
| Age in years, median (min-max) | 5.00 (3.00–5.00) | 5.00 (3.00–5.00) | 0.09 | .534 b |
| Socioeconomic status, n (%) | 0.25 | .576 a | ||
| B | 4.00 (16.00) | 9.00 (37.50) | ||
| C | 18.00 (72.00) | 13.00 (54.10) | ||
| D-E | 3.00 (12.00) | 2.00 (8.30) | ||
| Maternal education, n (%) | 0.28 | .255 a | ||
| Elementary 1 | – | 2.00 (8.30) | ||
| Elementary 2 | 3.00 (12.0) | 3.00 (12.50) | ||
| High School | 18.00 (72.0) | 12.00 (50.00) | ||
| Graduated | 4.00 (16.0) | 7.00 (29.20) | ||
| ECERS in score, median (min-max) | 2.65 (1.90–2.90) | 2.71 (1.90–2.92) | 0.03 | .809 b |
| EC_HOME in score, median (min-max) | 37.00 (30.00–47.00) | 41.00 (30.00–50.00) | 0.25 | .077 b |
| BMI in kg/m2, median (min-max) | 15.40 (14.10–17.00) | 21.60 (19.00–30.10) | 0.86 | .000 b * |
| TGMD-2 in score, median (min-max) | ||||
| Locomotor | 9.00 (5.00–12.00) | 7.00 (4.00–14.00) | 0.39 | .007 b * |
| OC | 8.00 (4.00–15.00) | 8.00 (6.00–12.00) | 0.04 | .800 b |
| GMQ | 94.00 (76.00–118.00) | 85.00 (73.00–103.00) | 0.25 | .085 b |
| SVF in score, median (min-max) | ||||
| Word production | 41.00 (24.00–65.00) | 40.5 (26.00–57.00) | 0.02 | .904 b |
| Number of errors | 1.00 (0.00–4.00) | 0.00 (0.00–4.00) | 0.14 | .321 b |
| TH, median (min-max) | ||||
| Number of movements | 13.00 (6.00–15.00) | 13.00 (5.00–15.00) | 0.07 | .641 b |
| Rule breaks | 5.00 (2.00–8.00) | 5.00 (1.00–8.00) | 0.09 | .528 b |
| Delayed Gratification, median (min-max) | 15.00 (2.00–15.00) | 15.00 (1.00–15.00) | 0.09 | .524 b |
| Day/Night Stroop, median (min-max) | 16.00 (3.00–16.00) | 14.00 (8.00–16.00) | 0.15 | .296 b |
n: absolute value; %: percentage; Socioeconomic status: high income - A and B, average income - C; low income - D and E; Elementary 1: up to the fifth school year; Elementary 2: up to the ninth school year; High School: three years of intermediate/high school; Graduated: university education; ECERS Environment rating scales in early childhood education, EC_HOME Early Childhood Home Observation for Measurement of the Environment, BMI Body Mass Index, TGMD-2 Gross Motor Development Test 2, Locomotor Standard Score Locomotor, OC Standard Score Object Control, GMQ Gross Motor Quotient, SVF Semantic Verbal Fluency, TH Tower of Hanoi
aPearson Chi-Square
bMann Whitney
* Significant difference (p < .05)
BMI was calculated based on measurements of body weight and body length, using the World Health Organization (WHO) BMI curves as reference [67, 68]. WHO Anthro software version 3.2.2 (Geneva, Switzerland) was used to calculate BMI for age and sex, expressed in z-scores.
The sample size was estimated using GPower® (Franz Faul, Universitat Kiel, Germany), version 3.1.9.2. F tests were used for the multiple linear regression models. Gross Motor Quotient of The Gross Motor Development Test - second edition (TGMD-2) was considered a dependent variable, and the EFs (inhibition, working memory, and cognitive flexibility) as independent variables, using the correlation values between these variables for preschoolers obtained by Cook et al. [54]. As such, it was possible to calculate the squared multiple correlations (R2). The calculated R2 value was 0.35, from which we obtained an effect size f2 equal to 0.56. Thus, the sample size was estimated at 24 volunteers for each linear regression model, considering a power of 0.80, alpha error set to 5%.
Measures
Socioeconomic status
The Brazilian Economic Classification Criterion was used; this being a questionnaire based on the accumulation of material goods and educational materials. The general socioeconomic classification resulting from this criterion varies from A1 (indicating high economic class) to E (very low economic class) [69].
Quality of the home environment
The quality of the home environment where each child lives was assessed through the Home Observation for Measurement of the Environment in Early Childhood (EC_HOME) [70]. The instrument contains 55 items divided into 8 scales: learning materials, language stimulation, physical environment, responsiveness, academic stimulation, modeling, variety, and acceptance. Total score, obtained with the sum of the raw scores of the scales, was used for analysis. The instrument has already been used in a sample of preschoolers in the assessment of psychometric characteristics and demonstrated reliability and validity in a Brazilian sample of preschoolers [71].
Quality of the school environment
The quality of the school environment was assessed using the Early Childhood Environmental Rating Scale (ECERS), which consists of 43 items organized into 7 subscales: space and furnishings, personal care routines, language and literacy, learning activities, interactions, program structure, parents and staff. The final score of the scale is given by the average of the raw scores of the seven subscales, the quality interpretation of which is 1-inadequate, 3-minimal (basic), 5-good, and 7-excellent [72, 73]. Studies have shown that the ECERS has good reliability [74, 75]. The instrument has been translated into Portuguese and is widely used in studies with Brazilian preschoolers [73, 75].
Gross motor skills
The Test of Gross Motor Development - second version (TGMD-2) was used to evaluate gross motor skills development [76]. TGMD-2 is a standardized norm- and criterion-referenced test for the development of children between 3 and 10 years old, and an instrument with validity and reliability for Brazilian children (indices and values from 0.83 to 0.98) [77]. TGMD-2 is composed of 12 fundamental motor skills, which are subdivided into two subtests: six locomotor skills (run, gallop, hop, leap, horizontal jump, and slide) and six OC skills (striking a stationary ball, stationary dribble (bounce), kick, catch, overhand throw, and underhand roll). The test subject’s score for any skill is assessed as pass/fail (1 or 0) for each of 3 or 4 pattern criteria [76]. The sum of all criteria across all skills within a subtest produces the raw score for each subtest, according to gender and age. Using norm tables, the raw subtest score (Locomotor; OC) is converted to a standard score. Higher scores indicate better quality of movement patterns [76]. The subtest standard scores are combined and converted to an overall Gross Motor Quotient (GMQ) determining a child’s gross motor skills compared to the test’s standardized population. The most reliable score for the TGMD-2 is the GMQ as it is derived from adding the subtest standard scores and converting the sum to a quotient (i.e., a standard score with a mean of 100 and standard deviation of 15) [76]. Children with mean gross motor performance according to TGMD-2 achieve GMQ values between 90 and 110. Therefore, children with GMQ equal to or greater than 90 were considered within the expected range and those who reached up to 89 points were below expected. On the TGMD-2 subtests, children with mean standard scores reach values between 8 and 12. Thus, standardized scores below 8 were considered below expected and equal to or above 8 were within the expected range [76].
Assessment of executive functions
EFs were evaluated using the Semantic Verbal Fluency [49], Tower of Hanoi [78], Day/Night Stroop [79], and Delayed Gratification tests [80]. Semantic Verbal Fluency Tests (SVF) have been used to assess vocabulary and speed of mental processing [81, 82], working memory [83], inhibitory control [84], and cognitive flexibility [49]. Participants were asked to name items from five categories (Color, Food, Animals, Toys, and Body Parts) and their answers were orthographically transcribed in real time. The scores for the number of words produced and the number of wrong words in 60 seconds per category were calculated and used in the analyses. SVF has proved to be a valid assessment of both lexical semantic skills and EFs in children [85].
The Tower of Hanoi (TH) is a neuropsychological task used to assess planning and problem solving, in addition to evaluating working memory and inhibitory control [86, 87]. The standard version of the TH consists of three pegs and a pyramid of n-discs, decreasing in size from the bottom to the top. The disks start on one of the pegs, and the objective is to move the entire n-disk pyramid to another peg, subject to two restrictions: only one disk can be moved at a time, and at no point can a larger disk be placed on top of a smaller disk on any peg. Different configurations result in successively more difficult problems, increasing the number of moves needed to reproduce the final goal state configuration; where for each problem n-trials can be given [78]. TH showed high reliability through internal consistency in the study of Humes et al. [88] Based on the hypothesis that preschool children have problem-solving capacity, Klahr & Robinson [78] modified the TH so that it became sensitive to such a capacity. One such modification was the use of a story of monkeys jumping from tree to tree to encourage greater attention and improved grasp of the rules. In the present study, TH was adapted for preschoolers and administered using only two disks [89], with a single trial [90], and one more rule in the story of Klahr & Robinson [78], which was jumping from tree to tree without stopping to jump to the middle tree, making the minimum number of moves equal to 8. For the analysis we used the amount of moves to complete the tower and the number of rule breaks.
The Day/Night Stroop test [79] was used to assess the participants’ ability to inhibit prepotent responses. The Day/Night Stroop test has good internal reliability [91, 92] and good test-retest reliability [93]. The test has a pseudo-random sequence of 16 pictures - eight depicting the sun and the other eight depicting the moon - for which the child was asked to say “night” for the sun image, and “day” for the moon image. The raw score, the number of correct answers (range 0–16), was used in the analysis.
The Delayed Gratification task assesses inhibitory control and self-regulation [80]. Each child’s preference for candy or chocolate as a reward was checked before taking the test, then the children were asked to choose between an immediately available reward of a small amount and a delayed reward of a larger amount, if they waited alone for 15 minutes without ringing the bell. The gratification delay measure was the number of minutes waited [80]. Studies have attested to the construct validity of the task as a measure of delayed gratification in preschoolers [94, 95].
Procedures
The first session was carried out at the child’s home with the completion of the survey questionnaires to assess maternal education, socioeconomic data, quality of the home environment (EC-HOME), and anthropometric assessment. The second session was carried out in the school environment, where the daycare (school) environment assessment (ECERS) was applied. In the third session, the parents and the child were referred to the Exercise Physiology Laboratory (LAFIEX), on Campus 2 (UFVJM), to first evaluate the EFs and then the gross motor skills. All children were individually evaluated at the same places. The evaluation of each child lasted around 40 min.
The researchers underwent training to carry out the measurements of weight and height, and to administer the questionnaires, as well as the EF and gross motor tests. To ensure greater reliability, only one examiner for each test battery and session was used, ensuring internal control for the measurement of the outcomes in a sequential study.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 (Inc., USA). The Shapiro-Wilk test was performed to verify data normality and the Levene test to check for homoscedasticity. Regarding outliers, no variables were detected. Pearson’s chi-squared test was applied to compare the frequency of children in categorical variables (sex, socioeconomic status, and maternal education) between eutrophic and overweight/obese groups. The Mann-Whitney test was used to examine differences between groups. This is because the variables presented non-normal distribution and/or were heteroscedastic. Thus, the means were used only for the classification of gross motor performance according to the TGMD-2 [76]. For the effect size of the comparisons between the groups, φ and φc (for Pearson’s Chi-squared analyses with categorical or nominal variables, respectively), and r (Mann-Whitney) [96] were calculated. The effect size interpretation was carried out according to Cohen (cut points: small: .10; medium: .30; large: .50) [97]. Bivariate correlations between the variables of interest were explored using Pearson or Spearman correlations according to the normality of the data in each group (eutrophic, overweight/obese). With regard to the confounding variables used in the linear regression models, the results were presented only for age, sex, and maternal education showing a correlation with a cognitive test or the gross motor test. Variables that were significant at a level of p < .05 were included in the multiple linear regression analyses as were variables that were decided a priori regardless of significance level (confounding variables). A multiple linear regression was conducted using the forward stepwise method to determine whether EF tasks accounted for significant variance in gross motor skills (locomotor, OC, and GMQ), adjusting for sex, age, maternal education, socioeconomic status, quality of the home environment, and quality of the school environment. Forward stepwise regression was used because it is an appropriate analysis when you have many variables and are interested in identifying a useful subset of the predictors [98]. The F Statistic (probability of F) was used to determine whether a variable should be included in the model. Thus, in each step, the variable that had the smallest p-value below the specified limit (p < .05) was included in the model. The multiple linear regression assumptions (linearity, absence of multicollinearity, normal distribution, and homoscedasticity of residuals) were met. To assess the relative contribution of cognitive variables to the performance of gross motor skills, the variation partitioning technique was applied [99]. This analysis, performed using R, makes it possible to divide the total percentage of variation explained by shared and individual contributions from the set of predictor variables, in this case, the number of SVF errors and the rule breaks in the TH. The level of significance for analysis was set at 5%.
Results
The present study involved the participation of 49 children in the age group of 3 to 5 years, divided into two subgroups according to their BMI value (25 eutrophic, 24 overweight/obese). In the present study, the sample was composed mostly of boys; most of the mothers had completed high school and had average socioeconomic level (class C). The sample characteristics and the comparison between groups are reported in Table 1.
The results showed that overweight/obese preschoolers performed significantly lower on locomotor skills than their eutrophic peers with medium effect size, albeit not on object control skills or the overall test (GMQ) (Table 1). Regarding locomotor skills, the overweight/obese preschoolers presented below average scores (mean = 7.63 ± 2.08) according to the norms of the TGMD-2, whereas the eutrophic children presented average scores (mean = 9.08 ± 1.86). For OC skills, both the eutrophic (mean = 8.76 ± 2.69) and the overweight/obese children (mean = 8.33 ± 1.73) presented average performance. In addition, overweight/obese children showed below average gross motor skills (mean = 87.54 ± 9.09), whereas the eutrophic children presented average gross motor skills (mean = 93.20 ± 10.97). Regarding the confounding variables (sex, age, socioeconomic status, maternal education, school environment, and home environment) we did not find any difference between the groups (Table 1).
Bivariate correlations between all variables are presented in Table 2. The correlations varied according to BMI, whereby among the eutrophic preschoolers, age was significantly associated with word production and errors on SVF, and maternal education was significantly associated with movements on TH. Therefore, older children tend to have more word production and a greater number of errors in the SVF, and children who performed fewer movements in the TH tend to be children of mothers with higher education. However, in the presence of excess weight, these correlations were not significant. On the other hand, sex was significantly associated with OC skills only in the overweight/obese group. In this case, girls performed worse (OC skills median-girls = 7.50 ± 2.01; OC skills median-boys = 8.93 ± 1.26; U = 30, z = − 2.38, r = 0.49, p < .05) and below average (OC skills mean-girls = 7.50 ± 2.01; OC skills mean-boys = 8.93 ± 1.26) according to the norms of the TGDM-2. Overweight/obese girls also had a below average performance in OC skills, unlike eutrophic girls (OC skills mean = 8.58 ± 2.87) and eutrophic boys (OC skills mean = 8.92 ± 2.26), but the median did not show a significant difference between overweight/obese girls and eutrophic girls and boys (eutrophic girls: U = 45.5, z = − 0.98, r = 0.21, p = .32; eutrophic boys: U = 37, z = − 1.76, r = 0.37, p = .07).
Table 2.
Correlation between cognitive tests and gross motor skills in eutrophic and overweight/obesity preschoolers
| 1a | 2a | 3a | 4a | 5a | 6a | 7a | 8a | 9a | 10a | 11a | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Agea | 0.37 | 0.49 | −0.24 | −0.00 | − 0.11 | 0.31 | 0.18 | 0.35 | −0.18 | −0.19 | 0.15 | |
| 2. Sexa | 0.15 | 0.17 | −0.38 | −0.49 | − 0.28 | −0.26 | 0.05 | 0.27 | 0.26 | 0.16 | 0.27 | |
| 3. Maternal educationa | 0.20 | 0.22 | −0.10 | 0.06 | −0.03 | 0.09 | −0.12 | 0.26 | −0.16 | 0.06 | 0.03 | |
| 4. Locomotor | −0.27 | −0.25 | − 0.28 | 0.24 | 0.79 | −0.20 | −0.12 | − 0.24 | −0.17 | − 0.30 | −0.26 | |
| 5. OC | −0.24 | −0.16 | 0.01 | 0.38 | 0.71 | 0.18 | 0.15 | −0.22 | −0.67 | − 0.49 | −0.46 | |
| 6. GMQ | −0.29 | −0.20 | − 0.12 | 0.81 | 0.82 | −0.01 | −0.05 | − 0.32 | −0.53 | − 0.48 | −0.46 | |
| 7. Day/Night Stroopa | 0.22 | 0.09 | −0.27 | 0.13 | −0.14 | 0.00 | 0.23 | 0.06 | −0.20 | −0.38 | − 0.45 | |
| 8. Delayed gratificationa | 0.39 | −0.07 | −0.29 | − 0.12 | −0.35 | − 0.31 | 0.22 | 0.36 | −0.20 | −0.12 | 0.02 | |
| 9. Word production | 0.71 | 0.21 | 0,20 | −0.39 | −0.07 | − 0.32 | 0.03 | 0.34 | 0.27 | 0.11 | 0.06 | |
| 10. Errors of SVFa | 0.41 | 0.34 | 0.08 | −0.03 | −0.25 | −0.20 | 0.22 | 0.08 | 0.17 | 0.42 | 0.12 | |
| 11. Movements in THa | 0.13 | −0.19 | −0.43 | 0.06 | 0.02 | 0.03 | 0.14 | 0.06 | −0.18 | 0.08 | 0.43 | |
| 12. Rule breaks in TH | 0.28 | −0.05 | − 0.09 | −0.00 | 0.25 | 0.11 | −0.09 | 0.02 | 0.13 | −0.15 | 0.50 |
Bivariate correlations between EF tasks and gross motor skills in eutrophic (lower triangle) and overweight/obese (upper triangle) preschoolers
aVariables with non-normal distribution for which the Spearman correlation was performed
OC Standard Score Object Control, GMQ Gross Motor Quotient; SVF Semantic Verbal Fluency, TH Tower of Hanoi; Values in bold show correlation coefficients with a value of p < 0,05
As expected, GMQ was strongly correlated with each component score. Regarding EFs in the overweight/obese group, movements in TH were moderately correlated with errors on SVF. Furthermore, the measurements on EF tasks did not significantly correlate with gross motor skills in the group of eutrophic preschoolers or with locomotor skills in the overweight/obese preschoolers. Nevertheless, a significant moderate correlation was observed in the overweight/obese group between OC skills and the following EF tasks: number of wrong words in the SVF; movements that the child performed to complete TH; and number of rule breaks (TH). With the GMQ variable, significant moderate correlations were observed with the same EF tasks that correlated with OC skills. Although overweight girls had below-average performance in OC skills and a worse mean than overweight/obese boys, and poorer performance in OC skills than normal-weight girls, no correlation between sex and EFs was observed. This indicates that the association between EFs and OC skills may have occurred regardless of gender, which was later confirmed with the results of the multiple linear regression presented below.
In the multiple linear regressions, when the relationship between EFs and locomotor skills was analyzed, no variable remained in the model in either group (Table 3). Regarding OC skills and GMQ, the prediction of EFs was observed only in the overweight/obese group. For both OC skills and GMQ, the first variable inserted in the model was SVF (number of errors) and later TH (rule breaks). Thus, SVF (number of errors) and TH (rule breaks) explained 57.80% of the OC skills variance (p < .05) and 40.50% of the GMQ variance (p < .05). For each increase in the number of SVF errors, there was a value decrease of 0.59 for OC and 0.47 for GMQ. With respect to TH, for each increase in the number of rule breaks, there was a value decrease of 0.40 for OC and 0.36 for GMQ. Confounding variables of sex, age, maternal education, socioeconomic status, quality of the home environment, and quality of the school environment were included in the stepwise multiple linear regression analyses. These variables were not significant in any model in either group. Therefore, confounding variables were not added to the models (OC skills and GMQ of the overweight/obesity group) and did not affect the β and R2 values of the explanatory variables (errors on SVF and rule breaks on TH) (Table 3).
Table 3.
Multiple linear regression analysis (forward stepwise) between cognitive tests and gross motor skills for the overweight/obese group
| Predictors | OC | GMQ | ||||
|---|---|---|---|---|---|---|
| β | p-value | R2 | Β | p-value | R2 | |
| 0.578 | 0.405 | |||||
| TH (Number of movements) | −0.003 | .989 | −0.084 | .701 | ||
| TH (Rule breaks) | −0.400 | .011* | −0.364 | .044* | ||
| SVF (Number of errors) | −0.597 | .000* | −0.477 | .010* | ||
| Age in years | −0.007 | .964 | −0.111 | .533 | ||
| Sex | −0.133 | .402 | 0.014 | .941 | ||
| Maternal education | 0.048 | .747 | 0.052 | .765 | ||
| Socioeconomic status | 0.151 | .302 | 0.184 | .290 | ||
| ECERS | 0.054 | .718 | 0.075 | .671 | ||
| EC_HOME | −0.121 | .418 | 0.123 | .490 | ||
β: standardized regression coefficient, OC Standard Score Object Control, GMQ Gross Motor Quotient, TH Tower of Hanoi, SVF Semantic Verbal Fluency, ECERS Environment rating scales in early childhood education, EC_HOME Early Childhood Home Observation for Measurement of the Environment; * p < .05
The variation partitioning results showed a small overlap between number of SVF errors and number of rule breaks in TH (6.99 and 5.05%, in the explanatory model of OC and GMQ, respectively). The number of SVF errors was the most explanatory independent variable of the variance in OC (35.09%) (Fig. A.1) and GMQ (22.42%) in overweight/obese preschoolers (Fig. B.1).
Fig. 1.
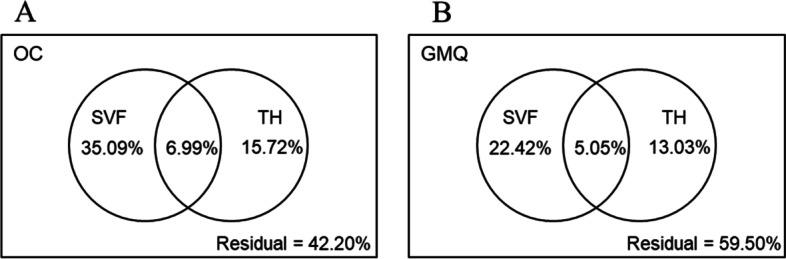
Variance partition analysis. OC: Standard Score Object Control; GMQ: Gross Motor Quotient; SVF: errors of Semantic Verbal Fluency; TH: rule breaks of Tower of Hanoi
Discussion
This study aimed to examine the association between EFs and gross motor skills in overweight/obese preschoolers and their eutrophic peers controlled for sex, age, maternal education, socioeconomic status, quality of the home environment, and quality of the school environment. Although these associations have been examined in eutrophic preschoolers, to the best of our knowledge, this is the first study to assess the association in overweight/obese individuals in this period of life. In addition, some EFs (cognitive flexibility and planning) in this age group remain poorly explored [38, 50, 54, 55] and most studies have verified the relationship between EFs and quantitatively assessed gross motor skills [43, 55, 58].
Gross motor skills
A significant difference was observed between the groups for locomotor skills. The worse performance of the overweight/obese children when compared to the eutrophic children can be explained by the biomechanical restrictions: (1) less knee and hip flexion, indicating a more rigid posture during walking; (2) the increase in both the absolute amount of force applied to the joint and the muscular force needed to move the additional mass during ambulation; and (3) the increased compressive and shear forces at the capital femoral growth plate, which can alter the femoral angle in overweight children [100]. These restrictions make locomotor skills more challenging for overweight/obese children than for eutrophics [6]. This result may also be reflected in the below average standardized GMQ score for overweight/obese children, since the GMQ is a composite measure of the Locomotor and OC subscales. The results are in agreement with several studies showing that preschool children with excess weight perform worse in locomotor skills, as expected [101–103].
However, there was no difference in OC skills between eutrophic and overweight/obese preschoolers. OC skills demand high levels of functional coordination and control of objects with the hands, feet, or implements [104] and are important predictors for an active life with greater participation in sports in adolescence and adulthood [11]. It is typically from 6 to 7 years of age that the development of sport-related movement occurs with the motor instruction for OC activities becoming more extensive as children enter primary school [11]. Therefore, in the preschool phase, OC skills are less developed in games that normally require large displacements of the body such as ball games like basketball or soccer [105]. As such, the studies that found worse OC performance in the presence of excess weight were normally evaluating school-age children [106–108]. The execution/learning of OC skills in the preschool period does not require a complete displacement of body weight, but rather the spatial and temporal timing of limb movements [103]. Thus, worse performance in locomotor skills does not necessarily imply worse performance in object control. Although some OC skills on the TGMD-2 include criteria involving some form of body displacement [76], the worse performance in locomotor skills of overweight/obese children in the present study probably did not affect the performance of OC skills. Other studies have also observed the same performance in OC skills among overweight/obese and eutrophic preschoolers [109, 110], although there are also studies that have found worse OC performance in overweight preschoolers [111, 112]. However, these studies did not control for confounding variables.
Regarding gender, overweight/obese girls performed worse in OC skills than boys with excess weight. In addition, only overweight/obese girls performed below average for OC skills. Some studies have observed that girls perform worse in OC skills than boys [46, 113]. This difference can be attributed to different social expectations regarding gender and the nature of the activities offered, or to different innate psychological capacities related to spatial targeting according to gender [114]. However, in the present study, this difference was only observed in the presence of excess weight. Therefore, it is possible that overweight girls feel more inhibited in performing activities involving OC skills than other children [14].
EFs
Overweight/obese and eutrophic children did not differ in the performance of EF tasks. The impact of excess weight on EFs in the preschool period differs between studies [22, 115, 116]. However, a longitudinal study of eutrophic and obese preschoolers aged 4–6 years found that changes in EF scores associated with BMI occurred only at age 6. However, a higher overall EF score at age 4 was associated with reduced odds of being overweight at age 6 [9]. Therefore, the nature of the relationship between EFs and BMI may be longitudinal rather than cross-sectional in the preschool period [28]. On the other hand, studies show that there is a cross-sectional association between EFs and excess weight in schoolchildren and adolescents [9, 36, 37, 117–120].
Relationship between EFs and gross motor skills
In the present study, SVF (number of errors) and TH (number of rule breaks) explained 57.80% of the OC skills variance and 40.50% of the GMQ variance in the overweight/obese preschoolers. OC skills require greater cognitive demand than locomotor skills in the preschool period. Locomotor skills emerge first, and young children are therefore more familiar with activities involving locomotion, making EFs less necessary, regardless of BMI [42].
The errors category of the SVF assesses cognitive flexibility and working memory [49]. Thus, the negative association between SVF errors and OC skills in the present study enables the inference that a greater number of SVF errors implies less cognitive flexibility and worse working memory, which are associated with worse performance in OC skills in preschoolers with excess weight. Cognitive flexibility has traditionally been considered a function of the frontal lobe [8], but the prefrontal cortex has reciprocal connections with the cerebellum [121–123]. Thus, studies indicate that the cerebellum, in addition to its role in motor control, is important for language functions, being crucial for verbal fluency, express receptive grammatical processing, and the ability to identify and correct errors in language and writing [124]. In addition, the cerebellum is one of the regions most consistently associated with BMI and obesity [121]. Studies with children over 6 years old show that obese children have larger volumes of white matter in the left cerebellum [125] and decreased white matter organization at the level of the cerebellar peduncles, which is probably due to differences in myelination, axonal density, and/or fiber architecture [8]. These areas have been found to be involved in the frontal-subcortical network connections of the brain responsible for EFs, motor control, and coordination [121, 125]. Therefore, it is possible that excess weight during cognitive and gross motor development contributes to changes in the reciprocal connections between the prefrontal cortex and the cerebellum, favoring a greater interconnection between cognitive flexibility/working memory and OC skills. The relationship with GMQ is likely due to the observed relationship with OC skills. Therefore, less cognitive flexibility and worse working memory are associated with worse performance in OC skills and, consequently, worse GMQ in preschoolers with excess weight.
In eutrophic preschoolers, we found no relationship between SVF and gross motor skills. Two studies investigated the association between SVF word production and motor skills in preschoolers [47, 48], but did not assess BMI and did not assess SVF errors. One of the studies found no association between the variables [48], as observed in both groups in our sample. However, another study [47] found that SVF was a predictor of global motor skills, including gross motor skills. Therefore, the relationship may have been favored by the inclusion of fine motor skills in the total score.
In addition to SVF errors, number of rule breaks in TH also showed a negative association with OC skills and GMQ in the overweight/obese group. Rule breaks in TH lead to wrong moves that are counted in the sum of moves to complete the tower. This explains the observed correlation between rule breaks and movement in TH. Thus, rule breaking results in worse planning and problem solving, increasing the amount of moves needed to complete the tower [43, 126–129]. Therefore, according to the results of the present study, worse efficiency in action planning is associated with worse performance in OC skills and, consequently, worse GMQ in excess weight preschoolers. There are also studies that indicate that action planning is important for successful performance in games involving ball skills [130, 131]. As such, Westendorp et al. [129] carried out a ball skill intervention study with children with learning disorders (age 7–11 years old) and their results showed that ball skills improved with better planning and problem solving. Therefore, in this case, OC skills training was associated with better planning, that is, in the opposite direction to our results. However, it is possible that the relationship between OC skills and planning could be bidirectional and mediated by anticipatory planning. Anticipatory planning is an aspect of motor control necessary for reaching objects that takes into account the future states of the body during the sequence of motor actions while planning the intended maneuver, avoiding uncomfortable postures at the end of object manipulation [132]. A study with normal-weight children aged 5–6 years showed that planning performance in the Tower of London task, unlike inhibitory control and working memory, was a significant predictor of anticipatory planning performance [133]. Thus, if we consider that the quality of OC movement depends on anticipatory motor planning performance [134], it is possible that better planning favors better anticipatory motor planning performance, which in turn enables better performance in OC skills.
On the other hand, similar to that observed in our sample of eutrophic children, a study that evaluated normal-weight children between 3 and 10 years of age found no relationship between cognitive tasks, including TH performance with at least 3 disks (in this case, number of towers completed correctly) and performance on motor tasks that assessed motor planning [135]. In the presence of excess weight, one study found the relationship in school-age children. Obese children solved a lower number of problems, taking less time to plan the movement and more time to perform the movement, and with worse motor skills compared to eutrophic children. Within the total group, better general motor competence was significantly associated with better updating, inhibition control, and planning [136]. Therefore, being overweight can apparently alter the relationship between planning and gross motor skills. This is possible because obese children are normally impulsive [137, 138] and impulsiveness can interfere with planning ability, and, apparently, it may begin in preschool obesity [139].
Impulsivity has long been viewed as a multidimensional construct [140] which includes attentional impulsiveness (tendency to not focus on the task at hand, or thought insertions and racing thoughts), motor impulsiveness (tendency to act on the spur of the moment, or non-perseverance), and non-planning impulsiveness (tendency to not plan and think carefully, or avoid challenging mental tasks) [141]. Prefrontal volumes have been found to be inversely correlated with motor and non-planning impulsivity [142]. In obese children, prefrontal volumes are reduced compared with eutrophic children [143, 144], which, added to cerebellar changes [121, 125], can induce changes in prefrontal-cerebellar connectivity, possibly contributing to the development of impulsivity [145]. Furthermore, planning and problem-solving difficulties, in turn, are affected by other EFs, such as attention, working memory, cognitive flexibility, and inhibitory control, which can also be affected by excess weight [146].
In the present study, we observed a small explanatory percentage of the OC skills variance (6.90%) and the GMQ variance (5.05%) when considering the two tests together (SVF and TH). This was possible because both tests assess some similar EFs, such as working memory and inhibitory control. A study with preschool children observed that better working memory is associated with better performance during strength, speed, and manual dexterity tasks [42]. Other studies demonstrated the impact of obesity on working memory, since obese children performed worse on tasks involving working memory [147, 148]. Although there are also studies that show the association between inhibitory control and motor performance [149], and between inhibitory control and obesity [150], the joint covariance cannot be explained by inhibitory control, at least not alone, since no relationship was found with specific tests for inhibition (Day/Night Stroop and the Delayed Gratification task).
The Day/Night Stroop is considered adequate to be applied in children from 2.5 to 6 years old, although it should be considered that, unlike working memory/cognitive flexibility, the development of inhibitory control has heterotypic continuity [151]. Heterotypic continuity is defined as the “continuity of an inferred genotypic attribute presumed to underlie diverse phenotypic behaviors” [152] or as “the manifestation of the same underlying process through different behavioral presentations at different developmental periods” [153]. In other words, inhibitory control changes during development, from initial dependence on external sources of control to capacity for self-initiated internal forms of control [151]. These changes carry different manifestations of inhibitory control throughout child development and may affect task performance according to age and bio-psycho-social influences [151–153]. In this case, it can be difficult to detect a linear relationship with dependent variables. With respect to the Delayed Gratification task, a recent study demonstrated a new understanding that testing reflects social factors, meaning that children in supportive environments (i.e., warm, affectionate, and open communication in relationships with parents and teachers) can increasingly delay gratification to promote behavioral, social, and academic success throughout their development [154], probably independently of BMI. Thus, it is possible that the inhibitory control assessed by the Delayed Gratification task was impacted by the child’s belief in the expected behavior before the test, which may, consequently, have interfered in the assessment of the relationship between inhibitory control and gross motor skills. Future studies should consider controlling for social support variables, to compare eutrophic and overweight/obese preschoolers, and for evaluating the relationship with gross motor skills.
Finally, no relationship was found between EFs and gross motor skills in eutrophic children, possibly because the performance of gross motor skills in normal-weight children may also be linked to other aspects absent in obese individuals, such as greater body experimentation due to the absence of biomechanical impediments. The addition of these factors may have attenuated the influence of EFs, and, as such, some studies also did not find this relationship in the preschool period [53, 155]. However, some studies did find motor and cognitive skills to be related in eutrophic preschoolers [54, 55]. It is therefore possible that there is not a clear relationship between motor performance and EFs in young children due to the discontinuity in their typical development and the biological coping mechanism of diverting their energy to one specific emerging skill while ignoring others [156]. On the other hand, it has been suggested that stronger associations between developmental domains are expected in children with atypical development, reflecting abnormal dependencies between neurocognitive processes [66]. Nonetheless, in a cross-sectional study, it is not possible to verify if it is obesity that explains the association between EFs and gross motor skills, either because of a smaller linear increase in EFs throughout preschool age, making it difficult for the cognitive performance necessary to perform OC skills, or because obesity affects both cognitive and motor skills independently of each other at preschool age.
Strengths and limitations
The present study has several strengths. The analysis of EFs was performed with different and complementary instruments to access more than one aspect of EFs. In addition, we analyzed EFs scarcely explored in preschoolers (cognitive flexibility and planning). For this, we only used instruments adapted for preschool age, including a cognitive test with a lower degree of complexity to assess cognitive flexibility, as well as TH adaptations suggested by the literature that would enable us to verify the planning/problem solving capacity at preschool age. However, more studies are necessary to validate the EF measures for preschool age and to clarify the shared variance between the EFs in these measures to understand the impact of task impurities. In addition, we analyzed the quality of gross motor movements because they are more strongly related to EFs than quantitative measures [54]. Another strength is that the analyses were adjusted for important variables known to impact the development of gross motor skills and EFs. As such, it was possible to detect potential risks that may modify the development of cognitive and motor skills in the presence of excess weight.
Limitations include the absence of normative data from EF tasks for preschoolers. Thus, the evaluation of the EFs did not enable us to verify whether the performance was as expected for the age or not, or whether this could be a determinant for the relationship with gross motor skills [46, 47]. For the TGMD-2, United States norms [76] had to be used for the classification of gross motor performance in Brazil [157, 158], with validated use for Brazilian children [77]. However, cultural differences (i.e., typical United States sports such as baseball) may reflect on the performance of gross motor skills. Thus, it is possible to overestimate or underestimate the performance classification of gross motor skills of Brazilian children, although the associations observed in the present study were not analyzed according to the classification of gross motor performance. In addition, the forward stepwise regression results should be considered with caution, although the method was adequate to verify the most important predictor EFs in an exploratory study. Furthermore, our results limit the generalization of the findings because our sample was mostly composed of males, with mothers that had completed high school and were class C economic level.
Future directions
New studies should verify whether the relationship between EFs and gross motor skills in overweight/obese preschoolers is dependent on the degree and duration of obesity, and how this relationship progresses with adiposity rebound. In addition, further studies could also aim to verify if, and to what extent, various cognitive stimuli (i.e., playful activities for cognitive development with and without motor stimulation) collaborate with the development of OC skills in obese children during the preschool phase and if this would bring benefits to the execution of OC skills at later ages. Understanding these processes in the preschool phase can contribute to future measures to prevent childhood obesity and its consequences, such as the elaboration of educational guidelines for preschool. Furthermore, because preschooler development is multifactorial and influenced by both biology and the environment [61–66, 117], longitudinal studies are important to confirm whether these associations remain over time.
Conclusion
Overweight/obese preschoolers performed worse in locomotor skills than their eutrophic peers paired by sex, age, maternal education, and socioeconomic status, but demonstrated the same performance as their peers in OC and EF skills. We only found a relationship between EF tasks (number of SVF errors and TH rule breaks) and gross motor skills (OC and GMQ) in overweight children, indicating that worse cognitive flexibility, working memory, planning, and problem solving are associated with worse gross motor skills in this population compared to eutrophic children.
Acknowledgments
We thank the Universidade Federal dos Vales do Jequitinhonha e Mucuri for institutional support. We thank the CNPq (303539/2021-6), CAPES, and FAPEMIG (APQ-01898-18) for support and scholarships. The authors are grateful to the municipal education secretary and the directors of the public schools of Diamantina (MG).
Authors’ contributions
Amanda Cristina Fernandes: Formal analysis, Data Curation, Methodology. Ângela Alves Viegas: Formal analysis, Data Curation, Methodology, Writing Review & Editing – Original Draft. Ana Cristina Rodrigues Lacerda: Formal analysis, Data Curation, Methodology, Writing – Review & Editing. Juliana Nogueira Pontes Nobre: Data Curation, Writing – Review & Editing. Rosane Luzia De Souza Morais: Formal analysis, Data Curation, Writing – Review & Editing. Pedro Henrique Scheidt Figueiredo: Writing – Review & Editing. Henrique Silveira Costa: Writing – Review & Editing. Ana Cristina Resende Camargos: Writing – Review & Editing. Fernanda De Oliveira Ferreira: Writing – Review & Editing. Patrícia Martins de Freitas: Writing – Review & Editing. Thiago Santos: Writing – Review & Editing. Fidelis Antônio da Silva Júnior: Data Curation. Mário Bernardo-Filho: Writing – Review & Editing. Redha Taiar: Writing – Review & Editing. Alessandro Sartorio: Writing – Review & Editing. Vanessa Amaral Mendonça (corresponding author): Formal analysis, Data Curation, Methodology, Writing – Review & Editing. The author(s) read and approved the final manuscript.
Funding
CNPq (303539/2021–6), CAPES, and FAPEMIG (APQ-01898-18).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of Universidade Federal dos Vales do Jequitinhonha e Mucuri UFVJM (Protocol: 2.355.943), with written informed parental consent and participant assent. All methods were carried out in accordance with relevant guidelines and regulations in the manuscript.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare no financial interests/personal relationships which may be considered as potential competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (WHO) UNICEF-WHO-the World Bank: joint child malnutrition estimates - levels and trends. Geneva: WHO; 2019. Global database on child health and malnutrition. [Google Scholar]
- 2.Yi DY, Kim SC, Lee JH, Lee EH, Kim JY, Kim YJ, Yang HR. Clinical practice guideline for the diagnosis and treatment of pediatric obesity: recommendations from the committee on pediatric obesity of the Korean Society of Pediatric Gastroenterology Hepatology and Nutrition. Pediatr Gastroenterol Hepatol Nutr. 2019;22(1):1. doi: 10.3345/kjp.2018.07360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mo-suwan L, McNeil E, Sangsupawanich P, Chittchang U, Choprapawon C. Adiposity rebound from three to six years of age was associated with a higher insulin resistance risk at eight-and-a-half years in a birth cohort study. Acta Paediatr. 2017;106(1):128–134. doi: 10.1111/apa.13639. [DOI] [PubMed] [Google Scholar]
- 4.Stodden DF, Goodway JD, Langendorfer SJ, Roberton MA, Rudisill ME, Garcia C, Garcia LE. A developmental perspective on the role of motor skill competence in physical activity: an emergent relationship. Quest. 2008;60(2):290–306. doi: 10.1080/00336297.2008.10483582. [DOI] [Google Scholar]
- 5.Heineman KR, Schendelaar P, Van den Heuvel ER, Hadders-Algra M. Motor development in infancy is related to cognitive function at 4 years of age. Dev Med Child Neurol. 2018;60(11):1149–1155. doi: 10.1111/dmcn.13761. [DOI] [PubMed] [Google Scholar]
- 6.Kakebeeke TH, Lanzi S, Zysset AE, Arhab A, Messerli-Bürgy N, Stuelb K, Leeger-Aschmann CS, Schmutz EA, Meyer AH, Kriemler S, Munsch S, Jenni OG, Puder JJ. Association between body composition and motor performance in preschool children. Obes Facts. 2017;10(5):420–431. doi: 10.1159/000477406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rolland-Cachera MF, Deheeger M, Bellisle F, Sempe M, Guilloud-Bataille M, Patois E. Adiposity rebound in children: a simple indicator for predicting obesity. Am J Clin Nutr. 1984;39(1):129–135. doi: 10.1093/ajcn/39.1.129. [DOI] [PubMed] [Google Scholar]
- 8.Augustijn M, Deconinck F, D'Hondt E, Van Acker L, De Guchtenaere A, Lenoir M, Caeyenberghs K. Reduced motor competence in children with obesity is associated with structural differences in the cerebellar peduncles. Brain Imaging Behav. 2018;12(4):1000–1010. doi: 10.1007/s11682-017-9760-5. [DOI] [PubMed] [Google Scholar]
- 9.Guxens M, Mendez MA, Julvez J, Plana E, Forns J, Basagaña X, Torrent M, Sunyer J. Cognitive function and overweight in preschool children. Am J Epidemiol. 2009;170(4):438–446. doi: 10.1093/aje/kwp140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallahue DL, Ozmun JC, Goodway JD. Understanding motor development: infants, children, adolescents, adults. 7. New York: McGraw-Hill; 2013. [Google Scholar]
- 11.Bardid F, Huyben F, Lenoir M, Seghers J, De Martelaer K, Goodway JD, Deconinck FJ. Assessing fundamental motor skills in Belgian children aged 3–8 years highlights differences to US reference sample. Acta Paediatr. 2016;105(6):e281–e290.6. doi: 10.1111/apa.13380. [DOI] [PubMed] [Google Scholar]
- 12.Lima RA, Soares FC, Queiroz DR, Aguilar JA, Bezerra J, Barros MV. The importance of body weight status on motor competence development: from preschool to middle childhood. Scand J Med Sci Sports. 2021;31:15–22. doi: 10.1111/sms.13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castetbon K, Andreyeva T. Obesity and motor skills among 4 to 6-year-old children in the United States: nationally-representative surveys. BMC Pediatr. 2012;12(1):1–9. doi: 10.1186/1471-2431-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng J, East P, Blanco E, Kang Sim E, Castillo M, Lozoff B, Gahagan S. Obesity leads to declines in motor skills across childhood. Child Care Health Dev. 2016;42(3):343–350. doi: 10.1111/cch.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neisser U. Cognitive psychology: Classic edition. New York: Psychology press; 2014.
- 16.Isquith PK, Crawford JS, Espy KA, Gioia GA. Assessment of executive function in preschool-aged children. Ment Retard Dev Disabil Res Rev. 2005;11(3):209–215. doi: 10.1002/mrdd.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diamond A. Executive functions. Annu Rev Psychol Rev. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baum GL, Ciric R, Roalf DR, Betzel RF, Moore TM, Shinohara RT, Kahn AE, Vandekar SN, Rupert PE, Quarmley M, Cook PA, Elliott MA, Ruparel K, Gur RE, Gur RC, Bassett DS, Satterthwaite TD. Modular segregation of structural brain networks supports the development of executive function in youth. Curr Biol. 2017;27(11):1561–1572.e8. doi: 10.1016/j.cub.2017.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark SV, Semmel ES, Aleksonis HA, Steinberg SN, King TZ. Cerebellar-subcortical-cortical systems as modulators of cognitive functions. Neuropsychol Rev. 2021;31(3):422–446. doi: 10.1007/s11065-020-09465-1. [DOI] [PubMed] [Google Scholar]
- 20.Völter CJ, Call J. Younger apes and human children plan their moves in a maze task. Cognition. 2014;130(2):186–203. doi: 10.1016/j.cognition.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Chevalier N, Blaye A. Cognitive flexibility in preschoolers: the role of representation activation and maintenance. Dev Sci. 2008;11(3):339–353. doi: 10.1111/j.1467-7687.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- 22.Reinert KR, Po'e EK, Barkin SL. The relationship between executive function and obesity in children and adolescents: a systematic literature review. J Obes. 2013;2013:820956. doi: 10.1155/2013/820956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buttelmann F, Karbach J. Development and plasticity of cognitive flexibility in early and middle childhood. Front Psychol. 2017;8:1040. doi: 10.3389/fpsyg.2017.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiebe SA, Sheffield T, Nelson JM, Clark CA, Chevalier N, Espy KA. The structure of executive function in 3-year-olds. J Exp Child Psychol. 2011;108(3):436–452. doi: 10.1016/j.jecp.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes C, Ensor R, Wilson A, Graham A. Tracking executive function across the transition to school: a latent variable approach. Dev Neuropsychol. 2010;35(1):20–36. doi: 10.1080/87565640903325691. [DOI] [PubMed] [Google Scholar]
- 26.Miller MR, Giesbrecht GF, Müller U, McInerney RJ, Kerns KA. A latent variable approach to determining the structure of executive function in preschool children. J Cogn Dev. 2012;13(3):395–423. doi: 10.1080/15248372.2011.585478. [DOI] [Google Scholar]
- 27.Van der Ven SHG, Kroesbergen EH, Boom J, Leseman PPM. The structure of executive functions in children: a closer examination of inhibition, shifting, and updating. Br J Dev Psychol. 2013;31:70–87. doi: 10.1111/j.2044-835X.2012.02079.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee K, Bull R, Ho RMH. Developmental changes in executive functioning. Child Dev. 2013;84:1933–1953. doi: 10.1111/cdev.12096. [DOI] [PubMed] [Google Scholar]
- 29.Simanowski S, Krajewski K. Specific preschool executive functions predict unique aspects of mathematics development: a 3-year longitudinal study. Child Dev. 2019;90(2):544–561. doi: 10.1111/cdev.12909. [DOI] [PubMed] [Google Scholar]
- 30.Usai MC, Viterbori P, Traverso L, De Franchis V. Latent structure of executive function in five- and six-year-old children: a longitudinal study. Eur J Dev Psychol. 2013;11:447–462. doi: 10.1080/17405629.2013.840578. [DOI] [Google Scholar]
- 31.Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: four general conclusions. Curr Dir Psychol Sci. 2012;21(1):8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monette S, Bigras M, Lafrenière M-A. Structure of executive functions in typically developing kindergarteners. J Exp Child Psychol. 2015;140:120–139. doi: 10.1016/j.jecp.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Cristofori I, Cohen-Zimerman S, Grafman J. Executive functions. [S.l: s.n.] 2019. pp. 197–219. [DOI] [PubMed] [Google Scholar]
- 34.Kuzawa CW, Blair C. A hypothesis linking the energy demand of the brain to obesity risk. Proc Natl Acad Sci. 2019;116(27):13266–13275. doi: 10.1073/pnas.1816908116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blair C, Kuzawa CW, Willoughby MT. The development of executive function in early childhood is inversely related to change in body mass index: evidence for an energetic tradeoff? Dev Sci. 2020;23(1):e12860. doi: 10.1073/pnas.1816908116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz DH, Leonard G, Perron M, Richer L, Syme C, Veillette S, Pausova Z, Paus T. Visceral fat is associated with lower executive functioning in adolescents. Int J Obes (2005) 2013;37(10):1336–1343. doi: 10.1038/ijo.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirt T, Schreiber A, Kesztyüs D, Steinacker JM. Early life cognitive abilities and body weight: cross-sectional study of the association of inhibitory control, cognitive flexibility, and sustained attention with BMI percentiles in primary school children. J Obes. 2015;2015. 10.1155/2015/534651. [DOI] [PMC free article] [PubMed]
- 38.Morra S, Panesi S, Traverso L, Usai MC. Which tasks measure what? Reflections on executive function development and a commentary on Podjarny, Kamawar, and Andrews (2017) J Exp Child Psychol. 2018;167:246–258. doi: 10.1016/j.jecp.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Garon N, Bryson SE, Smith IM. Executive function in preschoolers: A review using an integrative framework. Psychol Bull. 2008;134(1):31–60. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- 40.Roebers CM, Kauer M. Motor and cognitive control in a normative sample of 7-year-olds. Dev Sci. 2009;12(1):175–181. doi: 10.1111/j.1467-7687.2008.00755.x. [DOI] [PubMed] [Google Scholar]
- 41.van der Fels IM, Te Wierike SC, Hartman E, Elferink-Gemser MT, Smith J, Visscher C. The relationship between motor skills and cognitive skills in 4-16 year old typically developing children: a systematic review. J Sci Med Sport. 2015;18(6):697–703. doi: 10.1016/j.jsams.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 42.Stuhr C, Hughes C, Stöckel T. Task-specific and variability-driven activation of cognitive control processes during motor performance. Sci Rep. 2018;8(1):10811. doi: 10.1038/s41598-018-29007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oberer N, Gashaj V, Roebers CM. Motor skills in kindergarten: internal structure, cognitive correlates and relationships to background variables. Hum Mov Sci. 2017;52:170–180. doi: 10.1016/j.humov.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Gill SV, Hung YC. Effects of overweight and obese body mass on motor planning and motor skills during obstacle crossing in children. Res Dev Disabil. 2014;35(1):46–53. doi: 10.1016/j.ridd.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 45.Krombholz H. The motor and cognitive development of overweight preschool children. Early Years. 2012;32(1):61–70. doi: 10.1080/09575146.2011.599795. [DOI] [Google Scholar]
- 46.Viegas ÂA, Mendonça VA, Pontes Nobre JN, Souza Morais RLD, Fernandes AC, Oliveira Ferreira FD, et al. Associations of physical activity and cognitive function with gross motor skills in preschoolers: cross-sectional study. J Mot Behav. 2021;1-16. 10.1080/00222895.2021.1897508. [DOI] [PubMed]
- 47.Wassenberg R, Feron FJ, Kessels AG, Hendriksen JG, Kalff AC, Kroes M, Vles JS. Relation between cognitive and motor performance in 5-to 6-year-old children: results from a large-scale cross-sectional study. Child Dev. 2005;76(5):1092–1103. doi: 10.1111/j.1467-8624.2005.00899.x. [DOI] [PubMed] [Google Scholar]
- 48.Peyre H, Albaret JM, Bernard JY, Hoertel N, Melchior M, Forhan A, Ramus F. Developmental trajectories of motor skills during the preschool period. Eur Child Adolesc Psychiatry. 2019;28(11):1461–1474. doi: 10.1007/s00787-019-01311-x. [DOI] [PubMed] [Google Scholar]
- 49.Amunts J, Camilleri JA, Eickhoff SB, Patil KR, Heim S, von Polier GG, Weis S. Comprehensive verbal fluency features predict executive function performance. Sci Rep. 2021;11(1):1–14. doi: 10.1038/s41598-021-85981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musculus L, Ruggeri A, Raab M. Movement matters! Understanding the developmental trajectory of embodied planning. Front Psychol. 2021;12:633100. doi: 10.3389/fpsyg.2021.633100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fathirezaie Z, Matos S, Khodadadeh E, Clemente FM, Badicu G, Silva AF, Zamani Sani SH, Nahravani S. The relationship between executive functions and gross motor skills in rural children aged 8-10 years. Healthcare (Basel, Switzerland) 2022;10(4):616. doi: 10.3390/healthcare10040616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geertsen SS, Thomas R, Larsen MN, Dahn IM, Andersen JN, Krause-Jensen M, Korup V, Nielsen CM, Wienecke J, Ritz C, Krustrup P, Lundbye-Jensen J. Motor skills and exercise capacity are associated with objective measures of cognitive functions and academic performance in preadolescent children. Plos One. 2016;11(8):e0161960. doi: 10.1371/journal.pone.0161960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cameron CE, Brock LL, Murrah WM, Bell LH, Worzalla SL, Grissmer D, Morrison FJ. Fine motor skills and executive function both contribute to kindergarten achievement. Child Dev. 2012;83(4):1229–1244. doi: 10.1111/j.1467-8624.2012.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cook CJ, Howard SJ, Scerif G, Twine R, Kahn K, Norris SA, Draper CE. Associations of physical activity and gross motor skills with executive function in preschool children from low-income South African settings. Dev Sci. 2019;22(5):e12820. doi: 10.1111/desc.12820. [DOI] [PubMed] [Google Scholar]
- 55.Stuhr C, Hughes C, Stöckel T. The role of executive functions for motor performance in preschool children as compared to young adults. Front Psychol. 2020;11:1552. doi: 10.3389/fpsyg.2020.01552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Houwen S, van der Veer G, Visser J, Cantell M. The relationship between motor performance and parent-rated executive functioning in 3- to 5-year-old children: what is the role of confounding variables? Hum Mov Sci. 2017;53:24–36. doi: 10.1016/j.humov.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Nigg JT. Annual research review: on the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. J Child Psychol Psychiatry. 2017;58(4):361–383. doi: 10.1111/jcpp.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jansen P, Scheer C, Zayed K. Motor ability and working memory in Omani and German primary school-aged children. Plos One. 2019;14(1):e0209848. doi: 10.1371/journal.pone.0209848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hackman DA, Gallop R, Evans GW, Farah MJ. Socioeconomic status and executive function: developmental trajectories and mediation. Dev Sci. 2015;18(5):686–702. doi: 10.1111/desc.12246. [DOI] [PubMed] [Google Scholar]
- 60.Campbell F, Conti G, Heckman JJ, Moon SH, Pinto R, Pungello E, Pan Y. Early childhood investments substantially boost adult health. Science (New York, NY) 2014;343(6178):1478–1485. doi: 10.1126/science.1248429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosen ML, Amso D, McLaughlin KA. The role of the visual association cortex in scaffolding prefrontal cortex development: a novel mechanism linking socioeconomic status and executive function. Dev Cogn Neurosci. 2019;39:100699. doi: 10.1016/j.dcn.2019.100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferreira L, Godinez I, Gabbard C, Vieira J, Caçola P. Motor development in school-age children is associated with the home environment including socioeconomic status. Child Care Health Dev. 2018;44(6):801–806. doi: 10.1111/cch.12606. [DOI] [PubMed] [Google Scholar]
- 63.True L, Pfeiffer KA, Dowda M, Williams HG, Brown WH, O'Neill JR, Pate RR. Motor competence and characteristics within the preschool environment. J Sci Med Sport. 2017;20(8):751–755. doi: 10.1016/j.jsams.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye A, Yan S, Huang K, Mao L, Ge X, Weng T, Zuo A, Tao X, Tao F. Maternal intelligence quotient and motor development in early childhood: the mediating role of mother's education. J Paediatr Child Health. 2019;55(1):87–94. doi: 10.1111/jpc.14123. [DOI] [PubMed] [Google Scholar]
- 65.Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71(1):44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- 66.Dyck MJ, Piek JP, Hay D, Smith L, Hallmayer J. Are abilities abnormally interdependent in children with autism? J Clin Child Adolesc Psychol. 2006;35:20–33. doi: 10.1207/s15374424jccp3501_3. [DOI] [PubMed] [Google Scholar]
- 67.WHO Multicentre Growth Reference Study Group. de Onis M. WHO child growth standards based on length/height, weight and age. Acta Paediatr. 2006;95:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 68.De Onis M, Lobstein T. Defining obesity risk status in the general childhood population: which cut-offs should we use? Int J Pediatr Obes. 2010;5(6):458–460. doi: 10.3109/17477161003615583. [DOI] [PubMed] [Google Scholar]
- 69.Associação Brasileira de Empresas de Pesquisa (ABEP). Critério de classificação econômica Brasil. São Paulo: ABEP; 2008.
- 70.Bradley R, Corwyn R. Caring for children around the world: a view from HOME. Int J Behav Dev. 2005;29(6):468–478. doi: 10.1080/01650250500146925. [DOI] [Google Scholar]
- 71.Dias NM, Mecca TP, Pontes JM, Bueno JODS, Martins GLL, Seabra AG. The family environment assessment: study of the use of the EC-HOME in a Brazilian sample. Trends Psychol. 2017;25:1897–1912. doi: 10.9788/TP2017.4-19. [DOI] [Google Scholar]
- 72.Harms T. The use of environment rating scales in early childhood education. Cad Pesqui. 2013;43(148):76–97. doi: 10.1590/S0100-15742013000100005. [DOI] [Google Scholar]
- 73.Harms T, Clifford RM, Cryer D. Escala de avaliação de ambientes de educação infantil (EcerS-revised) São Paulo: Mimeo; 2009. [Google Scholar]
- 74.Hadeed J. Reliability and validity of the early childhood environment rating scale, revised edition, ECERS-R in Arabic. Early Child Dev Care. 2014;184(6):819–842. doi: 10.1080/03004430.2013.818991. [DOI] [Google Scholar]
- 75.Mariano M, Caetano SC, Ribeiro da Silva A, Surkan PJ, Martins SS, Cogo-Moreira H. Psychometric properties of the ECERS-R among an epidemiological sample of preschools. Early Educ Dev. 2019;30(4):511–521. doi: 10.1080/10409289.2018.1554388. [DOI] [Google Scholar]
- 76.Ulrich D. The test of gross motor development. Austin: Prod-Ed; 2000. [Google Scholar]
- 77.Valentini NC. Validity and rebility of the TGMD-2 for Brazilian children. J Motor Behav. 2012;44:275–280. doi: 10.1080/00222895.2012.700967. [DOI] [PubMed] [Google Scholar]
- 78.Klahr D, Robinson M. Formal assessment of problem-solving and planning processes in preschool children. Cogn Psychol. 1981;13(1):113–148. doi: 10.1016/0010-0285(81)90006-2. [DOI] [Google Scholar]
- 79.Gerstadt CL, Hong YJ, Diamond A. The relationship between cognition and action: performance of children 31/2–7 years old on a stroop-like day-night test. Cognition. 1994;53(2):129–153. doi: 10.1016/0010-0277(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 80.Mischel W, Shoda Y, Peake PK. The nature of adolescent competencies predicted by preschool delay of gratification. J Pers Soc Psychol. 1988;54(4):687. doi: 10.1037//0022-3514.54.4.687. [DOI] [PubMed] [Google Scholar]
- 81.Kavé G, Sapir-Yogev S. Associations between memory and verbal fluency tasks. J Commun Disord. 2020;83:105968. 10.1016/j.jcomdis.2019.105968. [DOI] [PubMed]
- 82.Mitrushina M, Boone KB, Razani J, D'Elia LF. Handbook of normative data for neuropsychological assessment. Oxford: Oxford University Press; 2005.
- 83.Henry JD, Crawford JR. Verbal fluency deficits in Parkinson’s disease: a meta-analysis. J Int Neuropsychol Soc. 2004;10:608–622. doi: 10.1017/S1355617704104141. [DOI] [PubMed] [Google Scholar]
- 84.Hirshorn EA, Thompson-Schill SL. Role of the left inferior frontal gyrus in covert word retrieval: neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44:2547–2557. doi: 10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 85.Banerjee P, Grange DK, Steiner RD, White DA. Executive strategic processing during verbal fluency performance in children with phenylketonuria. Child Neuropsychol. 2011;17:105–117. doi: 10.1080/09297049.2010.525502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andeson JR, Albert MV, Fincham JM. Tracing problem solving in real time: fMRI analysis of the subject-paced tower of Hanoi. Massachusetts Institute Technol J Cogn Neurosci. 2005;17(8):1261–1274. doi: 10.1162/0898929055002427. [DOI] [PubMed] [Google Scholar]
- 87.Capovilla AGS, Assef ECS, Cozza HFP. Avaliação neuropsicológica das funções executivas e relação com desatenção e hiperatividade. Avaliação Psicológica. 2007;6(1):51–60. [Google Scholar]
- 88.Humes GE, Welsh MC, Retzlaff P, Cookson N. Towers of Hanoi and London: reliability and validity of two executive function tasks. Assessment. 1997;4(3):249–257. doi: 10.1177/107319119700400305. [DOI] [PubMed] [Google Scholar]
- 89.Harvey JM, O’Callaghan MJ, Mohay H. Executive function of children with extremely low birthweight: a case control study. Dev Med Child Neurol. 2007;41(5):292–297. doi: 10.1111/j.1469-8749.1999.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 90.Bull R, Espy KA, Senn TE. A comparison of performance on the towers of London and Hanoi in young children. J Child Psychol Psychiatry. 2004;45(4):743–754. doi: 10.1111/j.1469-7610.2004.00268.x. [DOI] [PubMed] [Google Scholar]
- 91.Rhoades BL, Greenberg MT, Domitrovich CE. The contribution of inhibitory control to preschoolers’ social–emotional competence. J Appl Dev Psychol. 2009;30(3):310–320. doi: 10.1016/j.appdev.2008.12.012. [DOI] [Google Scholar]
- 92.von Stauffenberg C, Campbell SB. Predicting the early developmental course of symptoms of attention deficit hyperactivity disorder. J Appl Dev Psychol. 2007;28(5–6):536–552. doi: 10.1016/j.appdev.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thorell LB, Wåhlstedt C. Executive functioning deficits in relation to symptoms of ADHD and/or ODD in preschool children. Infant Child Dev. 2006;15(5):503–518. doi: 10.1002/icd.475. [DOI] [Google Scholar]
- 94.Schwarz JC, Schrager JB, Lyons AE. Delay of gratification by preschoolers: evidence for the validity of the choice paradigm. Child Dev. 1983;54(3):620–625. doi: 10.2307/1130048. [DOI] [Google Scholar]
- 95.Mischel W, Ebbesen EB. Attention in delay of gratification. J Pers Soc Psychol. 1970;16(2):329–337. doi: 10.1037/h0029815. [DOI] [PubMed] [Google Scholar]
- 96.Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141(1):2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- 97.Cohen J. Statistical power analysis for the behavioral sciences. New York: Routledge; 2013.
- 98.Hocking RR. A biometrics invited paper. The analysis and selection of variables in linear regression. Biometrics. 1976;32(1):1–49. doi: 10.2307/2529336. [DOI] [Google Scholar]
- 99.Legendre P, Legendre L. Numerical Ecology. 853+XV. Amsterdam: Elsevier; 1998. [Google Scholar]
- 100.Shultz SP, Anner J, Hills AP. Paediatric obesity, physical activity and the musculoskeletal system. Obes Rev. 2009;10(5):576–582. doi: 10.1111/j.1467-789x.2009.00587.x. [DOI] [PubMed] [Google Scholar]
- 101.Graf C, Koch B, Kretschmann-Kandel E, Falkowski G, Christ H, Coburger S, Lehmacher W, Bjarnason-Wehrens B, Platen P, Tokarski W, Predel HG, Dordel S. Correlation between BMI, leisure habits and motor abilities in childhood (CHILT-project) Int J Obes Relat Metab Disord. 2004;28(1):22–26. doi: 10.1038/sj.ijo.0802428. [DOI] [PubMed] [Google Scholar]
- 102.Castetbon K, Andreyeva T. Obesity and motor skills among 4 to 6-year-old children in the United States: nationally-representative surveys. BMC Pediatr. 2012;12:28. doi: 10.1186/1471-2431-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Webster EK, Sur I, Stevens A, Robinson LE. Associations between body composition and fundamental motor skill competency in children. BMC Pediatr. 2021;21(1):444. doi: 10.1186/s12887-021-02912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barnett LM, Stodden D, Cohen KE, Smith JJ, Lubans DR, Lenoir M, Iivonen S, Miller AD, Laukkanen A, Dudley D, Lander NJ, Brown H, Morgan PJ. Fundamental movement skills: an important focus. J Teach Phys Educ. 2016;35(3):219–225. doi: 10.1123/jtpe.2014-0209. [DOI] [Google Scholar]
- 105.Houwen S, Visscher C, Hartman E, Lemmink KAPM. Gross motor skills and sports participation of children with visual impairments. Res Q Exerc Sport. 2007;78:16–23. doi: 10.1080/02701367.2007.10762235. [DOI] [PubMed] [Google Scholar]
- 106.Cliff DP, Okely AD, Morgan PJ, Jones RA, Steele JR, Baur LA. Proficiency deficiency: mastery of fundamental movement skills and skill components in overweight and obese children. Obesity (Silver Spring, Md.) 2012;20(5):1024–1033. doi: 10.1038/oby.2011.241. [DOI] [PubMed] [Google Scholar]
- 107.Hardy LL, Reinten-Reynolds T, Espinel P, Zask A, Okely AD. Prevalence and correlates of low fundamental movement skill competency in children. Pediatrics. 2012;130(2):e390–e398. doi: 10.1542/peds.2012-0345. [DOI] [PubMed] [Google Scholar]
- 108.Barros W, da Silva KG, Silva R, Souza A, da Silva A, Silva M, Fernandes M, de Souza SL, Souza V. Effects of overweight/obesity on motor performance in children: a systematic review. Front Endocrinol. 2022;12:759165. doi: 10.3389/fendo.2021.759165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saraiva L, Rodrigues LP, Cordovil R, Barreiros J. Influence of age, sex and somatic variables on the motor performance of pre-school children. Ann Hum Biol. 2013;40(5):444–450. doi: 10.3109/03014460.2013.802012. [DOI] [PubMed] [Google Scholar]
- 110.Hall C, Eyre E, Oxford SW, Duncan MJ. Relationships between motor competence, physical activity, and obesity in British preschool aged children. J Funct Morphol Kinesiol. 2018;3(4):57. doi: 10.3390/jfmk3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morano M, Colella D, Caroli M. Gross motor skill performance in a sample of overweight and non-overweight preschool children. Int J Pediatr Obes. 2011;6(sup2):42–46. doi: 10.3109/17477166.2011.613665. [DOI] [PubMed] [Google Scholar]
- 112.Vameghi R, Shams A, Shamsipour Dehkordi P. The effect of age, sex and obesity on fundamental motor skills among 4 to 6 years-old children. Pakistan J Med Sci. 2013;29(2):586–589. doi: 10.12669/pjms.292.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang SC, Lin SJ, Tsai CY. Effect of sex, age, and BMI on the development of locomotor skills and object control skills among preschool children. Percept Mot Skills. 2015;121(3):873–888. doi: 10.2466/10.PMS.121c29x0. [DOI] [PubMed] [Google Scholar]
- 114.Eather N, Bull A, Young MD, Barnes AT, Pollock ER, Morgan PJ. Fundamental movement skills: where do girls fall short? A novel investigation of object-control skill execution in primary-school aged girls. Prev Med Rep. 2018;18(11):191–195. doi: 10.1016/j.pmedr.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hughes SO, Power TG, O'Connor TM, Orlet Fisher J. Executive functioning, emotion regulation, eating self-regulation, and weight status in low-income preschool children: how do they relate? Appetite. 2015;89:1–9. doi: 10.1016/j.appet.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Granziera F, Guzzardi MA, Iozzo P, Granziera F, Guzzardi MA. Associations between the Mediterranean diet pattern and weight status and cognitive development in preschool children. Nutrients. 2021;13(11):3723. doi: 10.3390/nu13113723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perpiñá C, Segura M, Sánchez-Reales S. Cognitive flexibility and decision-making in eating disorders and obesity. Eat Weight Disord Stud Anorexia Bulimia Obes. 2017;22(3):435–444. doi: 10.1007/s40519-016-0331-3. [DOI] [PubMed] [Google Scholar]
- 118.Darcy AM, Fitzpatrick KK, Colborn D, Manasse S, Datta N, Aspen V, Shields CS, Le Grange D, Lock J. Set-shifting among adolescents with bulimic spectrum eating disorders. Psychosom Med. 2012;74(8):869–872. doi: 10.1097/PSY.0b013e31826af636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fitzpatrick KK, Darcy A, Colborn D, Gudorf C, Lock J. Set-shifting among adolescents with anorexia nervosa. Int J Eat Disord. 2012;45(7):909–912. doi: 10.1002/eat.22027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maayan L, Hoogendoorn C, Sweat V, Convit A. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity (Silver Spring, Md) 2011;19(7):1382–1387. doi: 10.1038/oby.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marron EM, Viejo-Sobera R, Cuatrecasas G, Redolar-Ripoll D, Lorda PG, Datta A, Bikson M, Magerowski G, Alonso-Alonso M. Prefronto-cerebellar neuromodulation affects appetite in obesity. Int J Obesity (2005) 2019;43(10):2119–2124. doi: 10.1038/s41366-018-0278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dickson PE, Cairns J, Goldowitz D, Mittleman G. Cerebellar contribution to higher and lower order rule learning and cognitive flexibility in mice. Neuroscience. 2017;345:99–109. doi: 10.1016/j.neuroscience.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kansal K, Yang Z, Fishman AM, Sair HI, Ying SH, Jedynak BM, Prince JL, Onyike CU. Structural cerebellar correlates of cognitive and motor dysfunctions in cerebellar degeneration. Brain J Neurol. 2017;140(3):707–720. doi: 10.1093/brain/aww327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Starowicz-Filip A, Chrobak AA, Moskała M, Krzyżewski RM, Kwinta B, Kwiatkowski S, Milczarek O, Rajtar-Zembaty A, Przewoźnik D. The role of the cerebellum in the regulation of language functions. Rola móżdżku w regulacji funkcji językowych. Psychiatr Pol. 2017;51(4):661–671. doi: 10.12740/PP/68547. [DOI] [PubMed] [Google Scholar]
- 125.Bauer CCC, Moreno B, González-Santos L, Concha L, Barquera S, Barrios FA. Child overweight and obesity are associated with reduced executive cognitive performance and brain alterations: a magnetic resonance imaging study in Mexican children. Pediatr Obes. 2014;10(3):196–204. doi: 10.1111/ijpo.241. [DOI] [PubMed] [Google Scholar]
- 126.Batista AX, Adda CC, Miotto EC, Lúcia MCSD, Scaff M. Torre de Londres e Torre de Hanói: contribuições distintas para avaliação do funcionamento executivo. Jornal Brasileiro de Psiquiatria. 2007;56(2):134–139. doi: 10.1590/S0047-20852007000200010. [DOI] [Google Scholar]
- 127.Alesi M, Bianco A, Luppina G, Palma A, Pepi A. Improving Children’s coordinative skills and executive functions: the effects of a football exercise program. Percept Mot Skills. 2016;122(1):27–46. doi: 10.1177/0031512515627527. [DOI] [PubMed] [Google Scholar]
- 128.Diamond A. Effects of physical exercise on executive functions: going beyond simply moving to moving with thought. Ann Sports Med Res. 2015;2(1):1011. [PMC free article] [PubMed] [Google Scholar]
- 129.Westendorp M, Houwen S, Hartman E, Mombarg R, Smith J, Visscher C. Effect of a ball skill intervention on children's ball skills and cognitive functions. Med Sci Sports Exerc. 2014;46(2):414–422. doi: 10.1249/MSS.0b013e3182a532b3. [DOI] [PubMed] [Google Scholar]
- 130.Best JR. Effects of physical activity on Children's executive function: contributions of experimental research on aerobic exercise. Dev Rev. 2010;30(4):331–551. doi: 10.1016/j.dr.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wall AT. The developmental skill-learning gap hypothesis: implications for children with movement difficulties. Adapt Phys Act Q. 2004;21(3):197–218. doi: 10.1123/apaq.21.3.197. [DOI] [Google Scholar]
- 132.Wunsch K, Henning A, Aschersleben G, Weigelt M. A systematic review of the end-state comfort effect in normally developing children and in children with developmental disorders. J Motor Learn Dev. 2013;1(3):59–76. doi: 10.1123/jmld.1.3.59. [DOI] [Google Scholar]
- 133.Stöckel T, Hughes CM. The relation between measures of cognitive and motor functioning in 5- to 6-year-old children. Psychol Res. 2016;80(4):543–554. doi: 10.1007/s00426-015-0662-0. [DOI] [PubMed] [Google Scholar]
- 134.Herbort O, Büschelberger J, Janczyk M. Preschool children adapt grasping movements to upcoming object manipulations: evidence from a dial rotation task. J Exp Child Psychol. 2018;167:62–77. doi: 10.1016/j.jecp.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 135.Wunsch K, Pfister R, Henning A, Aschersleben G, Weigelt M. No interrelation of motor planning and executive functions across young ages. Front Psychol. 2016;7:1031. doi: 10.3389/fpsyg.2016.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Augustijn M, DʼHondt E, Van Acker L, De Guchtenaere A, Lenoir M, Caeyenberghs K, Deconinck F. Role of motor competence and executive functioning in weight loss: A study in children with obesity. J Dev Behav Pediatr. 2018;39(8):642–651. doi: 10.1097/DBP.0000000000000589. [DOI] [PubMed] [Google Scholar]
- 137.Schmidt R, Sebert C, Kösling C, Grunwald M, Hilbert A, Hübner C, Schäfer L. Neuropsychological and neurophysiological indicators of general and food-specific impulsivity in children with overweight and obesity: a pilot study. Nutrients. 2018;10(12):1983. doi: 10.3390/nu10121983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nederkoorn C, Dassen FC, Franken L, Resch C, Houben K. Impulsivity and overeating in children in the absence and presence of hunger. Appetite. 2015;93:57–61. doi: 10.1016/j.appet.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 139.Meule A, Platte P. Facets of impulsivity interactively predict body fat and binge eating in young women. Appetite. 2015;87:352–357. doi: 10.1016/j.appet.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 140.Barratt ES, Patton JH. Impulsivity: cognitive, behavioral, and psychophysiological correlates. In: Zuck M, editor. Biological bases of sensation seeking, impulsivity, and anxiety. Hillsdale: Lawrence Erlbaum Associates; 1983. pp. 77–122. [Google Scholar]
- 141.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impusiveness scale. J Clin Psychol. 1995;51:768–74. 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1 [DOI] [PubMed]
- 142.Korponay C, Dentico D, Kral T, Ly M, Kruis A. Neurobiological correlates of impulsivity in healthy adults: lower prefrontal gray matter volume and spontaneous eye-blink rate but greater resting-state functional connectivity in basal ganglia-thalamo-cortical circuitry. NeuroImage. 2017;157:288–296. doi: 10.1016/j.neuroimage.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ronan L, Alexander-Bloch A, Fletcher PC. Childhood obesity, cortical structure, and executive function in healthy children. Cerebral Cortex (New York, N.Y).: 1991. 2020;30(4):2519–2528. doi: 10.1093/cercor/bhz257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dennis E, Manza P, Volkow ND. Socioeconomic status, BMI, and brain development in children. Transl Psychiatry. 2022;12(1):33. doi: 10.1038/s41398-022-01779-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Miquel M, Nicola SM, Gil-Miravet I, Guarque-Chabrera J, Sanchez-Hernandez A. A working hypothesis for the role of the cerebellum in impulsivity and compulsivity. Front Behav Neurosci. 2019;13:99. doi: 10.3389/fnbeh.2019.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rodrigues CL, Rocca C, Serafim A, Santos BD, Asbahr FR. Impairment in planning tasks of children and adolescents with anxiety disorders. Psychiatry Res. 2019;274:243–246. doi: 10.1016/j.psychres.2019.02.049. [DOI] [PubMed] [Google Scholar]
- 147.Willoughby MT, Wirth RJ, Blair CB, Family Life Project Investigators Executive function in early childhood: longitudinal measurement invariance and developmental change. Psychol Assess. 2012;24(2):418–431. doi: 10.1037/a0025779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 149.Ruddock S, Caeyenberghs K, Piek J, Sugden D, Hyde C, Morris S, Rigoli D, Steenbergen B, Wilson P. Coupling of online control and inhibitory systems in children with atypical motor development: a growth curve modelling study. Brain Cogn. 2016;109:84–95. doi: 10.1016/j.bandc.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 150.Adise S, White CN, Roberts NJ, Geier CF, Keller KL. Children's inhibitory control abilities in the presence of rewards are related to weight status and eating in the absence of hunger. Appetite. 2021;167:105610. doi: 10.1016/j.appet.2021.105610. [DOI] [PubMed] [Google Scholar]
- 151.Petersen IT, Hoyniak CP, McQuillan ME, Bates JE, Staples AD. Measuring the development of inhibitory control: the challenge of heterotypic continuity. Dev Rev. 2016;40:25–71. doi: 10.1016/j.dr.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Caspi A. Personality development. In: Eisenberg N, Damon W, Lerner RM, editors. Handbook of child psychology. 6. Hoboken: John Wiley Sons, Inc; 2006. [Google Scholar]
- 153.Cicchetti D, Rogosch FA. A developmental psychopathology perspective on adolescence. J Consult Clin Psychol. 2002;70(1):6. doi: 10.1037/0022-006X.70.1.6. [DOI] [PubMed] [Google Scholar]
- 154.Michaelson LE, Munakata Y. Same data set, different conclusions: preschool delay of gratification predicts later behavioral outcomes in a preregistered study. Psychol Sci. 2020;31(2):193–201. doi: 10.1177/0956797619896270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Livesey D, Keen J, Rouse J, White F. The relationship between measures of executive function, motor performance and externalising behaviour in 5-and 6-year-old children. Hum Mov Sci. 2006;25(1):50–64. doi: 10.1016/j.humov.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 156.Houwen S, Kamphorst E, van der Veer G, Cantell M. Identifying patterns of motor performance, executive functioning, and verbal ability in preschool children: a latent profile analysis. Res Dev Disabil. 2019;84:3–15. doi: 10.1016/j.ridd.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 157.Spessato BC, Gabbard C, Robinson L, Valentini NC. Body mass index, perceived and actual physical competence: the relationship among young children. Child Care Health Dev. 2013;39(6):845–850. doi: 10.1111/cch.12014. [DOI] [PubMed] [Google Scholar]
- 158.Nobre GC, Valentini NC, Nobre F. Fundamental motor skills, nutritional status, perceived competence, and school performance of Brazilian children in social vulnerability: gender comparison. Child Abuse Negl. 2018;80:335–345. doi: 10.1016/j.chiabu.2018.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


