Abstract
Fungal infections remain hardly treatable because of unstandardized diagnostic tests, limited antifungal armamentarium, and more specifically, potential toxic interactions between antifungals and immunosuppressants used during anti-inflammatory therapies, such as those set up in critically ill COVID-19 patients. Taking into account pre-existing difficulties in treating vulnerable COVID-19 patients, any co-occurrence of infectious diseases like fungal infections constitutes a double debacle for patients, healthcare experts, and the public economy. Since the first appearance of SARS-CoV-2, a significant rise in threatening fungal co-infections in COVID-19 patients has been testified in the scientific literature. Better management of fungal infections in COVID-19 patients is, therefore, a priority and requires highlighting common risk factors, relationships with immunosuppression, as well as challenges in fungal diagnosis and treatment. The present review attempts to highlight these aspects in the three most identified causative agents of fungal co-infections in COVID-19 patients: Aspergillus, Candida, and Mucorales species.
Keywords: Fungal infections, COVID-19, Aspergillus, Mucoromycetes, Pneumocystis, Candida
Viral respiratory infections are a major medical concern and increasingly recognized as leading causes of morbidities and mortality worldwide. In retrospect, four major viral outbreaks of respiratory illness have beset mankind over the last decades, including severe acute respiratory syndrome coronavirus (SARS-CoV) (2002–2004), H1N1 Influenza (2009–2010), Middle East respiratory syndrome coronavirus (MERS-CoV) (2012–2020), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (2019-present) [1]. These infections can be complicated by secondary infections that manifest during or following the initial infection, which often have more severe and sometimes fatal clinical outcomes [2]. Viral infections followed by the manifestation of a bacterial infection are typically referred to as superinfections. Whereas co-infections with multiple pathogens of bacterial, viral, or fungal origin are those occurring simultaneously, contributing to disease severity [3]. During the latter H1N1 pandemic, it was reported that 25% of the patients developed bacterial or fungal co-infections [4]. In addition, mortality rates were between 16% and 23% [5]. Interestingly, data regarding fungal respiratory infections in patients infected with SARS-CoV and MERS-CoV showed that infections by Aspergillus species, Mucorales species, and Candida species were the most common [6,7].
SARS-CoV-2 has emerged as the most devastating pandemic in modern history, stretching healthcare setting capacity all over the world. Besides the primary infection caused by SARS-CoV-2, a myriad of emerging complications and sequelae was reported to contribute remarkably to decreased survival of patients with coronavirus disease-2019 (COVID-19). Among these complications, co-infections represent a significant risk of morbidity and mortality, especially for patients in intensive care and undergoing mechanical ventilation, surpassing the death toll of the 2009 H1N1 pandemic and several influenza seasons [8]. These patients have been found to be prone to further fungal co-infections during the middle and late stages of the disease [9]. However, reports on the burden of fungal co-infections in COVID-19 patients are scarce, and the co-pathogens responsible for these infections remain unidentified, while only few recent studies reported 8% of co-infections in these patients [10]. It is still uncertain whether COVID-19 represents a significant risk factor that predisposes the patient to subsequent fungal infections. This lack of evidence can be explained by the fact that COVID-19 patients do not routinely undergo sputum culture for fungal isolation. Moreover, bacterial co-pathogens are commonly identified in viral respiratory tract infections, thus resulting in a delay in diagnosing fungal diseases that may be associated with considerable morbidity or mortality [11]. Furthermore, interventions for infection prevention and control measures are rigorously implemented to break chains of transmission and prevent secondary infections.
Superinfections and co-infections in COVID-19 patients can significantly increase the hospital length of stay and mortality rates [12]. Consequently, these conditions can worsen the clinical status and result in high public health costs and in shortage of available beds for new patients. Thus, prospective studies assessing the incidence of fungal co-infections are urgently needed to evaluate the risk of such co-infections in COVID-19 patients, as well as to optimize early identification in order to determine the right interventions for high-risk patients.
The purpose of this work is to feature the most recent studies in the context of fungal infections in COVID-19 patients as a means of highlighting the relation between immune dysregulation and susceptibility to fungal infections. In addition, research related to the incidence of the three most commonly reported fungal co-infections in hospitalized COVID-19 patients, their risk factors, current diagnostic approaches, and therapeutic strategies will be reviewed.
1. Immune dysregulation and fungal infections in COVID-19 patients
The co-pathogenesis of respiratory viral and fungal co-infections is characterized by complex interactions and involves a dynamic interplay between the virulence of co-infecting pathogens and the host immune defenses. Therefore, multiple host pathways are affected leading to the disruption of physical barriers, dysregulation of immune responses, and failure to return to homeostasis [13].
Several studies have pointed out that COVID-19 predisposes patients to a heightened risk of secondary infections. Otherwise, the immunopathogenesis of these infections in COVID-19 patients is poorly understood. Deciphering the complex pathogenesis of COVID-19-associated fungal infections necessitates a molecular and physiological understanding of the mechanisms whereby SARS-CoV-2 plays such facilitating roles in the onset of fungal infections. Multiple potential risk factors that have been identified to date include the viral infection itself, immunosuppressive/immunomodulatory therapies, previous antibiotic use, underlying chronic pulmonary disease, malignancy, mechanical ventilation, and uncontrolled diabetes [[14], [15], [16], [17]].
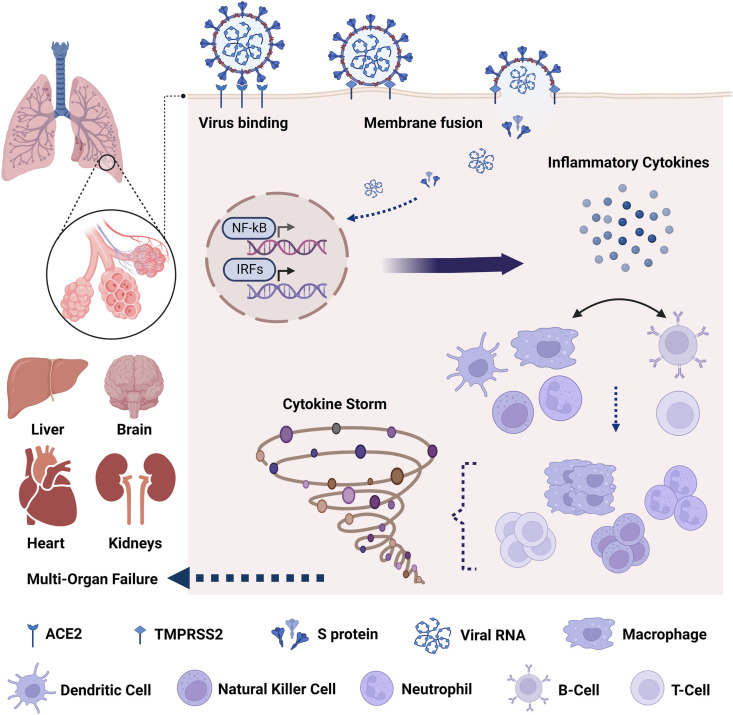
The respiratory tract is constantly exposed to diverse communities of microbes, and therefore, a multi-faceted response is triggered to respond to and clear these pathogenic threats. During viral infection, pathogen-associated molecular patterns (PAMPs), including viral proteins and nucleic acids, activate pattern recognition receptors (PRRs) of the host. In turn, PRR-mediated signaling pathways ultimately result in activation of the expression and release of proinflammatory mediators, including cytokines and type I and III interferons (IFNs) that recruit and activate more immune cells, allowing the activation of both innate and adaptive immune responses [18]. Recently, many studies have presented puzzling findings on the molecular mechanism of SARS-CoV-2 entry in the host and regulation by signaling pathways. Cell entry depends on the activity of TMPRSS2, a transmembrane protease serine 2 involved in the activation of Spike protein (S protein) which binds to the angiotensin-converting enzyme 2 (ACE2) receptors located on type II pneumocytes and alveolar macrophages [19]. Of note, it was recently highlighted that Omicron variant is able to efficiently enter lung cells via the endocytic pathway in a TMPRSS2-independent manner [20]. Upon recognition of S protein and viral RNA, Toll-like receptors (TLRs) trigger the downstream activation of the NF-κB and interferon regulatory factors (IRFs) pathways, and consequently, the production of IFNs and secretion of proinflammatory cytokines such as IL-6 and TNF-α (Fig. 1 ) [21,22]. Consequently, the viral infection leads to airway epithelial damage, loss of barrier function, decreased cilia movement, and functional defects [23]. This pathogenic mechanism may promote viral spread to the deeper lung parenchyma and generate a favorable environment for secondary infections in COVID-19 patients. Interestingly, damage of alveolar integrity can weaken antifungal immunological defenses and enable fungal invaders to reach blood vessels and surrounding tissues [24]. During the fungal invasion, excessive disruption of epithelial barrier at tight epithelial junctions is mediated by fungal proteases such as alkaline protease 1 from Aspergillus spp. Moreover, the release of fibrinous materials and the secretion of mucins lead to the obstruction of small airways creating a hypoxic microenvironment that can modulate various mechanisms of pathogen virulence and the physiology of immune cells of the host by dampening the oxygen-dependent antimicrobial activities of macrophages and neutrophils [25].
Fig. 1.
Immune functions and cytokine storm in COVID-19.
Beyond this, emerging evidence supports that homing receptors expressed on T cells are crucial in their selective trafficking and migration to other organs and tissues during the course of COVID-19. This is followed by the aberrant release of more than 150 inflammatory cytokines and chemical mediators, which associates the generation of a cytokine storm characterized by rapid proliferation and hyperactivation of immune cells [26] (Fig. 1). This prolonged or overblown response, defined as cytokine storm, is a potentially fatal immune condition that can lead to subsequent immunopathogenic injuries to various organs and tissues, including lung epithelial and endothelial cell apoptosis, vascular leakage, alveolar edema, and hypoxia. With regard to severe cases of COVID-19, recent studies showed altered homeostasis of regulatory T cells (Tregs) subsets characterized by reduced naive Tregs and excessive memory Tregs production [27]. Recent findings also showed that cases of co-infections and secondary infections emerging in COVID-19 patients may result from immune dysregulation induced by the viral infection, especially CD4 T-helper cell (TH) depletion [28]. Besides, a serious disturbance in the internal environment is characterized by reduced lymphocyte count (especially T cells) and increased neutrophil-to-lymphocyte ratio (NLR), indicating another feature of COVID-19 disease severity. These features, in addition to imbalance in the TH subsets (TH1/TH2/TH17), contribute to exacerbated inflammatory responses and subsequently aggravated lung injury and may also influence the development of fungal co-infections [29]. Therefore, NLR and lymphocyte subset counts are useful markers in the early screening of critical illness in COVID-19 patients. In addition, high levels of proinflammatory cytokines (TNF-α, IL-1β, IL-2, and IL-6) and chemokines (IL-8) were detected in sera of many patients with severe COVID-19 [27]. TGF-β has also been suggested as a predictive factor of disease severity in patients with COVID-19 [30].
On the other side of the immunologic spectrum, the exuberant and unregulated release of danger-associated molecular patterns (DAMPs), corresponding to signal molecules released from damaged or dying cells due to severe COVID-19, promotes heightened inflammation and extensive damage of lung epithelial tissues [31]. This leads to higher vulnerability to invasive fungal infections [32]. In addition, DAMPs have been shown to regulate inflammation in fungal diseases, driving the transition from fungal colonization to pathogenicity [33]. Together these findings help to explain why critically ill COVID-19 patients (with hyperinflammatory responses) are more prone to fungal infections.
2. The paradox of immunosuppressants and COVID-19
Immunosuppressants may be used to attenuate the immunopathologic response in severe COVID-19 and improve the disease outcomes by targeting the cytokine storm. However, these drugs could be detrimental in the earlier stages of the disease [34]. Examples of immunosuppressive therapies targeting interleukins include tocilizumab, a humanized anti-IL-6 receptor monoclonal antibody that inhibits the downstream signal transduction of IL-6 and thereby perpetuates the inflammatory response [35], as well as anakinra, a recombinant interleukin IL-1 receptor antagonist [36]. Janus Kinase Inhibitors (baricitinib and fedratinib) have been shown to reduce the inflammatory effects of IL-6 via the JAK-STAT signaling pathway by inhibiting the upstream regulators of ACE2-mediated endocytosis of SARS-CoV-2 [37]. Moreover, the use of systemic corticosteroids has been advocated to alleviate inflammation by reducing the levels of circulating proinflammatory mediators, particularly in critically ill patients with acute respiratory distress syndrome (ARDS) [38,39]. In patients with COVID-19, corticosteroids such as dexamethasone, prednisone, methylprednisone, and hydrocortisone are often used to dampen inflammation. Since they are used for their anti-inflammatory properties, their administration should also be meticulously considered due to their immunosuppressive activities, particularly in individuals with underlying medical conditions [40]. In fact, the resulting immunosuppression aggravated with the exhaustion of the immune cells due to the viral infection can set the scene for other bacterial and fungal infections. Furthermore, the relationship between corticosteroid treatment and fungal infections (including candidemia, invasive aspergillosis, and mucormycosis) is well documented [41]. Some authors showed that severely ill COVID-19 patients hospitalized in intensive care units (ICUs) and receiving corticosteroid treatment are at high risk of invasive fungal infections such as pulmonary aspergillosis and candidemia [42]. However, a study conducted in a tertiary center in the United States and another Italian prospective cohort study did not confirm the role of immunosuppressants as one of the risk factors for candidemia in ICU patients with COVID-19 [43,44]. The findings of these studies delineated ICU length of stay and central venous catheter dwell time as risk factors for candidemia in COVID-19 patients.
Consequently, caution has been urged by the World Health Organization (WHO) in the use of corticosteroids for people with diabetes and other co-morbidities to reduce the risk of opportunistic secondary infections and the exacerbation of existing infectious diseases [45], especially since only dexamethasone, unlike other corticosteroids, has shown a preventive effect against severe forms of COVID-19 unless the patient is under the age of 70 [46,47].
3. Fungal co-infections in COVID-19 patients
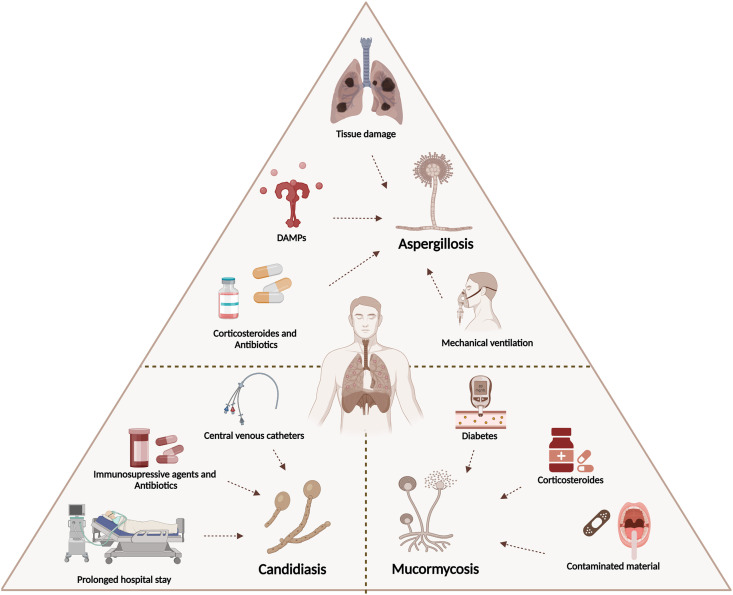
The inflammatory response caused by the immune dysregulation during COVID-19 is the main factor that drives the establishment of fungal infections. The most commonly reported fungal infections are due to three agents: Aspergillus, Candida, and Mucorales species. In the following sections, we spotlight the incidence, outcomes, risk factors and co-morbidities, diagnosis, and treatment of infections caused by these fungi in COVID-19 patients (Fig. 2 ).
Fig. 2.
Risk factors for fungal infections in COVID-19 patients.
It is worth mentioning that cases of pneumonia caused by Pneumocystis jirovecii have also been reported in COVID-19 patients, though the majority of patients suffer from acquired immunodeficiency syndrome (AIDS) [[48], [49], [50], [51], [52]]. However, whether to consider infection by SARS-CoV-2 as a risk factor for P. jirovecii is not clear-cut. This is because the fact that (i) the immune dysregulation in these territories is multi-factorial (AIDS and COVID-19), so any causative relationship with P. jirovecii pneumonia is difficult to state, and (ii) the possible bias in the diagnosis of P. jirovecii pneumonia related to shareable clinical features with COVID-19, which underestimates the eventual cases and renders hard the prediction of risk factors.
3.1. COVID-19-associated pulmonary aspergillosis (CAPA)
In the context of the current pandemic, COVID-19-associated pulmonary aspergillosis (CAPA) is defined as “invasive pulmonary aspergillosis in temporal proximity to a preceding SARS-CoV-2 infection” in patients requiring critical care [53]. Of note, patients with symptomatic COVID-19 confirmed by a positive RT-PCR test, and respiratory insufficiency requiring ICU admission, should be considered at high risk for CAPA. The European Confederation for Medical Mycology and the International Society for Human and Animal Mycology consensus criteria are used to categorize CAPA as “possible”, “probable”, and “proven” based on host factors, clinical factors, and mycological evidence [53,54]. “Proven” CAPA is defined as pulmonary or tracheobronchial infection based on histopathological or direct microscopic detection. “Probable” CAPA is defined as invasive aspergillus tracheobronchitis demonstrated by the observation of characteristic lesions through bronchoscopic analysis in conjunction with positive mycological evidence. A “possible” CAPA category was proposed to allow physicians to grade patients for whom assay validation has not been completed.
The average prevalence of CAPA is 10%, though it is widely variable among studies [55]. In a local Chinese hospital, 23.3% (60/243) of COVID-19 patients were reported to have Aspergillus co-infection [56]. In other retrospective studies conducted in Europe (Belgium, France, the Netherlands, and Germany) reporting cases of CAPA, Aspergillus fumigatus was the most common species isolated from COVID-19 patients, followed by Aspergillus flavus [53,[57], [58], [59]]. Several fatal cases of CAPA were likewise reported. A clinical case of CAPA and ARDS was described in a non-immunocompromised pregnant woman who died on the second day of admission [60]. Interestingly, a retrospective multicenter observational cohort showed that CAPA was the predominant infection (59.9%) in the ICUs during the first French pandemic wave [61]. Another national, multicentre, observational cohort study conducted in 18 French ICUs has reported a high prevalence of invasive pulmonary aspergillosis and candidaemia in mechanically ventilated patients with COVID-19 during the first wave [15]. Seven other fatal cases are summarized in Table 1 , including one rare case reporting an Aspergillus terreus infection [62]. Numerous studies have shown higher ICU mortality (>50%) in COVID-19 patients with proven/probable CAPA than critically ill COVID-19 patients without CAPA [63,64].
Table 1.
Reported cases of fatal CAPA occurring in patients with COVID-19.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
|---|---|---|---|---|---|---|---|
| Gender, age | Male, 74 | Female, 42 | Male, 79 | Female, 66 | Male, 79 | Female, 56 | Male, 69 |
| Co-morbidities/past medical history |
|
|
|
None |
|
Diabetes | None |
| Initial presenting symptoms | Fever, cough, dyspnea, tachypnea, and hypoxemia | AML | Disorientation and hypoxia | Fever, myalgia, dry cough, and dyspnea | Fever, cough, and general fatigue | Severe pneumonia | Fever, chills, dry cough, and headache |
| Supportive care | Intubation, mechanical ventilation, and vasopressor support | Supplemental oxygen by nasal cannula (4 L/min) changed to non-invasive positive pressure ventilation (NIPPV) on day 9, rehydration with normal saline, endotracheal intubation, and mechanical ventilation | Intubation, invasive ventilation, hemodynamic support with norepinephrine, and continuous renal function replacement therapy | Supportive oxygen therapy with the nasal cannula (4 L/min) and intubation | Intubation and mechanical ventilation | N/A | Intubation and mechanical ventilation |
| Clinical features suggestive of COVID-19 | Acute respiratory failure, tachypnea, and hypoxemia | Fever, dry cough, dyspnea, and myalgia | Bilateral lung infiltrates | Diffuse bilateral ground-glass opacities | Fever, cough, general fatigue, and pulmonary bilateral ground-glass attenuations | Cavities in bilateral upper lobes containing soft tissue | Diffuse, ill-defined increased opacity in the peripheral portion of his right upper and both lower lung zones |
| Day of diagnosis of COVID-19 | Day 0 | Day 8 | Day 0 | Day 0 | One day before admission | Day 0 | Day 0 |
| Day of transfer to ICU | Day 0 | Day 10 | Day 0 | N/A | Day 4 | N/A | Day 7 |
| Day of diagnosis of aspergillosis | Day 4 | Day 9 | Day 14+ | Day 17+ | Day 24 | Day 32 | Day 36 |
| Laboratory investigations of aspergillosis |
|
|
|
|
- Papanicolaou stain of the tracheal aspirate indicative of A. fumigatus
|
|
|
| Other infections | Haemophilus influenza (at day 1 only) | None | Staphylococcus aureus | A. fumigatus (low count) | None | Stenotrophomonas maltophilia | None |
| Treatment for COVID-19 | Intravenous cefotaxime | Linezolid plus meropenem, lopinavir/ritonavir, and interferon β-1b | Hydrocortisone and meropenem | Interferon β-1a (subcutaneously), dexamethasone (intravenously), meropenem, and vancomycin | Inhaled ciclesonide, oral ivermectin, dexamethasone (substituted for methylprednisolone), meropenem, remdesivir, and heparin therapy | Intravenous methylprednisolone, dexamethasone, and prednisolone | Intravenous remdesivir, oral dexamethasone, and oral moxifloxacin |
| Treatment for aspergillosis | None | Intravenous liposomal amphotericin B | Anidulafungin and voriconazole next to vancomycin and ceftazidime | Voriconazole and caspofungin | Liposomal amphotericin B and recombinant human soluble thrombomodulin | Voriconazole | Voriconazole and liposomal amphotericin B |
| Other treatments | N/A |
|
Intravenous ceftriaxone and ciprofloxacin switched to flucloxacillin | N/A | N/A | N/A | N/A |
| Day and cause of death | Day 9 due to severe respiratory failure | Day 12 due to respiratory and hemodynamic instability | Day 17 due to severe diffuse alveolar damage | Day 29 due to respiratory failure | Day 28 due to multiple organ failures, including respiratory and renal failure | Day N/A, poor lung reserve, and other superimposed infections | Day 130 due to multiple organ failures, including respiratory, cardiogenic, and renal failure |
| Reference | [65] | [66] | [67] | [62] | [68] | [69] | [70] |
Of note, most patients were diagnosed with CAPA in a median of 8 days after ICU admission in contrast to influenza-associated acute pulmonary aspergillosis with a median onset of 3 days [[71], [72], [73]]. This can be explained by an intrahospital acquisition of CAPA and particularly in patients receiving systemic corticosteroids [61]. Nonetheless, to date, the data on the possibility of nosocomial transmission of CAPA and the effect of corticosteroid therapy as a risk factor is conflicting. Furthermore, the exuberant and unregulated release of DAMPs leads to ARDS that requires mechanical ventilation and predisposes patients with severe COVID-19 to pulmonary aspergillosis [74] (Fig. 2).
Pre-existing lung diseases may further contribute to an increased risk of COVID-19-associated aspergillosis. Therefore, differentiating aspergillosis as a potential complication of COVID-19 from a pre-existing infection in COVID-19 patients with underlying chronic pulmonary disease is very challenging. In this context, clinical assessment and findings of Aspergillus should be strongly considered in respiratory samples collected from critically ill patients. During the past few years, invasive aspergillosis in critically ill patients has been diagnosed using morphologic criteria based on histopathology-controlled cases [75]. Since then, diagnosis criteria have been improved and included the incorporation of tests for Aspergillus-specific antigens galactomannan (GM) and 1,3-β-D glucan (BDG), as well as fungus culture. Cultures of bronchoalveolar lavage (BAL) are performed without direct examination, and the GM index is recorded on BAL or serum [76]. Noteworthy, the level of circulating serum or plasma GM is relatively low and usually cannot be indicative of fungal burden in the early stages in all patients at risk of invasive pulmonary aspergillosis. Thus, detection of GM in BAL fluid samples can be used as a diagnostic method with improved accuracy [77]. The development of diagnostic tissue biomarkers would provide additional findings that guide personalized immunotherapy in CAPA patients. Moreover, nucleic acid amplification such as polymerase chain reaction (PCR)-based tests were included in the European Organization for the Research and Treatment of Cancer/Mycoses Study Group and Research Consortium (EORTC/MSGERC) criteria for proven invasive fungal disease in addition to the detection of the fungus by histopathologic/cytopathologic or direct microscopic examination, or culture of a specimen obtained by a sterile procedure [54]. An Aspergillus-specific PCR test is promising in enhancing the diagnosis of Aspergillus DNA in clinical specimens in conjunction with other diagnostic tests (e.g., antigen detection assays) and the patient's clinical context [78,79]. Pulmonary computed-tomography (CT)-scan may show radiological signs suggestive of invasive aspergillosis, such as nodules with surrounding ground-glass opacities (halo sign). However, radiologic features of COVID-19, especially at later stages, may indicate invasive pulmonary aspergillosis.
The second-generation antifungal voriconazole or isavuconazole is recommended as first-line therapy for possible, probable, and proven CAPA [80]. Further investigations will be required to develop a better understanding of voriconazole pharmacokinetics in these patients, especially changes in protein binding. The other triazole antifungal posaconazole, liposomal amphotericin B or echinocandins are alternative options in case of intolerance to voriconazole [80].
3.2. COVID-19-associated candidiasis (CAC)
COVID-19-associated candidiasis (CAC) has been less studied among COVID-19 patients during the first wave of the pandemic. However, the striking increase in the incidence of candidemia in patients admitted to the ICU has catapulted Candida infections to the forefront of fungal infections in COVID-19 patients [81]. Candida albicans is the most common yeast isolated in critically ill COVID-19 patients [82]. An epidemiological shift toward non-albicans Candida species has been recently reported in some geographical areas where Candida auris was predominant [83]. A two-to ten-fold increase in candidemia was reported during the pandemic period in patients with and without COVID-19 [42,84]. Nevertheless, the prevalence of CAC can vary significantly across different geographic regions and ranges from 0.4 to 23.5% [85]. Recent studies conducted in Europe reported incidences of 0.4%, 8%, and 12.6% in Spain, Italy, and the United Kingdom, respectively [[86], [87], [88]]. Other studies from Asia reported rates of 2.5%, 5%, and 23.5% in India, Iran, and China, respectively [83,89,90]. In addition, 37 patients out of 65 admitted to the COVID-19 unit at an acute care hospital in Florida were colonized with C. auris [91]. Another retrospective observational study conducted in New York showed that 5% of bloodstream infections in COVID-19 patients were caused by Candida spp [92]. Overall, mortality rates have varied between studies and have been reported to be between 40% and 70%, though differences in mortality rates between COVID-19 and non-COVID-19 patients were not found to be statistically significant [93]. Moreover, it was shown that candidemia in COVID-19 patients was not attributed to increased mortality.
Immune dysregulation in COVID-19 has not been linked to an increased susceptibility to candidiasis, but several interventions are considered important candidates for clinical risk factors. This includes prolonged hospital stays and mechanical ventilation predisposing pulmonary parenchyma to fungal proliferation and tissue invasion, the use of broad-spectrum antibiotics and immunosuppressive agents, in addition to the placement of central venous catheters [94,95]. Moreover, breaches of infection control that have occurred along with the over-occupancy of the ICUs might be additional factors [96]. Other factors include prolonged corticosteroid therapy and a history of chronic pulmonary disease [97] (Fig. 2).
Cases of CAC are difficult to diagnose due to the low concentration of microorganisms in blood or infected site and the culture of clinical samples on non-specific media [98,99]. Sterile culture is the gold-standard technique to diagnose candidiasis in clinical settings [100]. However, the sensitivity of blood cultures is approximately 50% when diagnosing invasive candidiasis due to the low numbers of fungal cells [98]. Furthermore, non-culture diagnostics, such as BDG and mannan antigen testing [using enzyme-linked immunosorbent assay (ELISA) commercial kits], in addition to molecular techniques such as PCR and T2 Candida panel (T2 Biosystems), are recommended to improve the detection of the fungus and shorten the detection period [98,101]. Otherwise, these techniques pose a significant challenge due to some limitations. A French retrospective study reported that BDG testing lacked sufficient sensitivity and specificity when performed on sera collected from patients with severe COVID-19 admitted to the ICU [102]. Serum BDG testing has been broadly used in COVID-19 patients for the detection of CAPA in developed countries, in contrast to not being available in developing countries [103]. Thus, the usefulness of other molecular techniques remains to be determined in the context of COVID-19 patients with ARDS. Although T2 Candida panel can detect and identify Candida species, its detection spectrum is limited to five species only (C. albicans, Candida glabrata, Candida tropicalis, Candida krusei, and Candida parapsilosis) with low sensitivity among populations with a low prevalence of invasive candidiasis [104]. Noteworthy, a combination of serological and molecular techniques is recommended in order to enhance the accuracy of the techniques.
Echinocandins (caspofungin or micafungin) are prescribed as first-line empirical treatment of CAC patients, with fluconazole as an accepted alternative for initial therapy. Still, two facts should be considered when selecting antifungal therapy. First is the emergence of resistance to antifungals, and second, the progressive shift of etiologic agents toward non-albicans species, notably C. glabrata and the recently emerged C. auris, both are characterized by a decreased susceptibility to most commonly used antifungal agents [105].
3.3. COVID-19-associated mucormycosis (CAM)
Before the COVID-19 pandemic, the prevalence of mucormycosis in India was estimated to be nearly 70 times higher as compared to the global data; however, the prevalence of the disease was low in Europe [[106], [107], [108]]. A dramatic 2.1-fold rise in mucormycosis was noted during the first wave of the COVID-19 pandemic when compared to the pre-COVID period in India. A wide array of case reports, case series, and systematic reviews described cases of COVID-19-associated mucormycosis (CAM) at unprecedented worldwide rates. Large case series were reported from India, including primarily rhino-orbital mucormycosis and rhino-orbital cerebral mucormycosis. In May 2021, the Indian government declared mucormycosis (black fungus) as a notifiable disease. Due to this detrimental situation, several Indian states have declared black fungus an epidemic. A multicenter retrospective study was conducted across India during the first wave [109]. It showed a 2.1-fold increase in mucormycosis cases during the study period when compared to corresponding months in the pre-COVID period. Notably, the prevalence of CAM was higher in ICU patients (1.6%) than that of patients managed in hospital wards (0.27%); this increase was tied to COVID uptick. Another nationwide retrospective study was conducted on CAM cases in France and reported a higher twelve-week mortality rate (88%) than the findings from the previous Indian study (45.7%) [110]. The cases described in high-income countries were mostly pulmonary or disseminated mucormycosis. A number of case reports are summarized in Table 2 .
Table 2.
Reported cases of mucormycosis occurring in patients with COVID-19 on the basis of clinical presentation.
| Clinical presentation of mucromycosis | Number of cases | Causative agents | References |
|---|---|---|---|
| Rhino-orbital | 10 |
|
[[111], [112], [113], [114], [115], [116], [117], [118], [119], [120]] |
| Rhino-cerebral | 1 | N/A | [121] |
| Rhino-orbital-cerebral | 2 |
|
[122,123] |
| Sino-orbital | 1 |
|
[124] |
| Paranasal | 1 | N/A | [125] |
| Pulmonary | 8 |
|
[[126], [127], [128], [129], [130], [131], [132], [133]] |
| Oral | 1 | N/A | [134] |
| Gastrointestinal | 1 | N/A | [135] |
| Cutaneous | 1 |
|
[136] |
| Disseminated | 1 | N/A | [137] |
Most of the case reports documented rhino-orbital mucormycosis and recommended surgical debridement to reduce the disease burden [[111], [112], [113], [114],138]. The only case of gastrointestinal mucormycosis was described in a COVID-19 patient in Brazil [135]. Interestingly, a very rare case of cutaneous mucormycosis was reported in India in a patient with severe COVID-19 pneumonia [136]. Moreover, pulmonary mucormycosis was diagnosed in an orthotopic heart transplant recipient with prior COVID-19 [133]. Arana et al. documented two kidney transplant patients on immunosuppressive treatment diagnosed with CAM [139]. In the latter cases, Rhizopus oryzae and Lichtheimia ramose were the causative agents of rhinosinusal and musculoskeletal mucormycosis, respectively. Mixed fungal infections with Aspergillus and Mucorales species were also described in some COVID-19 patients [117,126,129,133,140]. Literature has shown that mortality rates for CAM vastly depend on the site of infection; while COVID-19-associated rhino-orbital mucormycosis has an overall mortality rate in excess of 14% [141], pulmonary or disseminated mucormycosis mortality rates exceeded 80% [142].
Evidence of heightened incidence of mucormycosis has shed light on multiple predisposing factors and co-morbidities. Risk factors for mucormycosis vary considerably between developed and developing countries. While uncontrolled diabetes mellitus is designated as the major predisposing risk factor for mucormycosis in India, the disease is majorly linked to hematological malignancies and organ transplants in developed countries [109,143]. One cannot disregard that the poor hygiene and zinc deficiency in this country may be permissive for fungal infections in COVID-19 patients, although there are no supporting data so far. The rampant use of broad-spectrum antibacterial agents and steroids has increased the risk of mucormycosis in these patients or exacerbated pre-existing ones [129,144,145]. Mucormycosis was also associated with contaminated adhesive bandages, wooden tongue depressors, and contaminated air in hospitals [146] (Fig. 2).
Laboratory diagnosis of mucormycosis relies upon histopathological examination and direct microscopy, which must be confirmed by culture-based and PCR-based identification, matrix-assisted laser desorption ionization-time of flight (MALDI-TOF), and mass spectrometry [[147], [148], [149]]. BDG and GM are not shared by Mucorales species, they test negative in cases of mucormycosis [150]. Of note, cultures often produce no growth, so the clinical context of the patient remains crucial to establishing a diagnosis. Moreover, detection of circulating DNA in the blood by quantitative PCR is a useful approach [151,152].
The mainstay of management of CAM is based on the use of high-dose liposomal amphotericin B. Antifungal triazole (posaconazole or isavuconazole) may be used for salvage treatment. Few data support the use of combination antifungal therapy (posaconazole or isavuconazole with liposomal amphotericin B). Early complete surgical treatment is also recommended alongside antifungal therapy [153].
4. Conclusion
In the scenario of the COVID-19 pandemic, the dramatic surge in cases has led to the exclusion of treatable respiratory pathogens. Additional diagnostic testing for fungal infections in patients with severe COVID-19, particularly when other clinical features support co-infections, is important to prevent avoidable morbidity and mortality. Otherwise, judicious use of steroids is crucial to limit secondary fungal infections in hospital settings, along with stringent control of other risk factors. Further studies and recommendations are needed to prompt the diagnosis of fungal co-infections and to establish effective antifungal therapy that lacks noxious interactions with immunosuppressors, which may help improve outcomes in hospitalized COVID-19 patients.
Declaration of competing interest
The authors declare no conflict of interest.
References
- 1.Bradley B.T., Bryan A. Emerging respiratory infections: the infectious disease pathology of SARS, MERS, pandemic influenza, and Legionella. Semin Diagn Pathol. 2019 May;36(3):152–159. doi: 10.1053/j.semdp.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris D.E., Cleary D.W., Clarke S.C. Secondary bacterial infections associated with influenza pandemics. Front Microbiol. 2017 Jun 23;8:1041. doi: 10.3389/fmicb.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brealey J.C., Sly P.D., Young P.R., Chappell K.J. Viral bacterial co-infection of the respiratory tract during early childhood. FEMS Microbiology Letters [Internet] 2015 May 1;362(10) doi: 10.1093/femsle/fnv062. https://academic.oup.com/femsle/article/doi/10.1093/femsle/fnv062/2096392 [cited 2022 Feb 27] Available from: [DOI] [PubMed] [Google Scholar]
- 4.MacIntyre C.R., Chughtai A.A., Barnes M., Ridda I., Seale H., Toms R., et al. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect Dis. 2018 Dec;18(1):637. doi: 10.1186/s12879-018-3548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verweij P.E., Rijnders B.J.A., Brüggemann R.J.M., Azoulay E., Bassetti M., Blot S., et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020 Aug;46(8):1524–1535. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007 Apr;170(4):1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milne-Price S., Miazgowicz K.L., Munster V.J. The emergence of the Middle East Respiratory Syndrome coronavirus. Pathogens Disease. 2014 Jul;71(2):121–136. doi: 10.1111/2049-632X.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cauley L.S., Vella A.T. Why is coinfection with influenza virus and bacteria so difficult to control? Discov Med. 2015 Jan;19(102):33–40. [PMC free article] [PubMed] [Google Scholar]
- 9.Gangneux J.P., Bougnoux M.E., Dannaoui E., Cornet M., Zahar J.R. Invasive fungal diseases during COVID-19: we should be prepared. J Mycolog Med. 2020 Jun;30(2) doi: 10.1016/j.mycmed.2020.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musuuza J.S., Watson L., Parmasad V., Putman-Buehler N., Christensen L., Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. Huber VC. PLoS One. 2021 May 6;16(5) doi: 10.1371/journal.pone.0251170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaturvedi V., Bouchara J.P., Hagen F., Alastruey-Izquierdo A., Badali H., Bocca A.L., et al. Eighty years of mycopathologia: a retrospective analysis of progress made in understanding human and animal fungal pathogens. Mycopathologia. 2018 Dec;183(6):859–877. doi: 10.1007/s11046-018-0306-1. [DOI] [PubMed] [Google Scholar]
- 12.Silva D.L., Lima C.M., Magalhães V.C.R., Baltazar L.M., Peres N.T.A., Caligiorne R.B., et al. Fungal and bacterial coinfections increase mortality of severely ill COVID-19 patients. J Hosp Infect. 2021 Jul;113:145–154. doi: 10.1016/j.jhin.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCullers J.A. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. 2014 Apr;12(4):252–262. doi: 10.1038/nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 14.Baddley J.W., Thompson G.R., Chen S.C.A., White P.L., Johnson M.D., Nguyen M.H., et al. Coronavirus disease 2019–associated invasive fungal infection. Open Forum Infect Dis. 2021 Dec 1;8(12) doi: 10.1093/ofid/ofab510. ofab510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangneux J.P., Dannaoui E., Fekkar A., Luyt C.E., Botterel F., De Prost N., et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French multicentre MYCOVID study. Lancet Respir Med. 2022 Feb;10(2):180–190. doi: 10.1016/S2213-2600(21)00442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Permpalung N., Chiang T.P.Y., Massie A.B., Zhang S.X., Avery R.K., Nematollahi S., et al. Coronavirus disease 2019–associated pulmonary aspergillosis in mechanically ventilated patients. Clin Infect Dis. 2022 Jan 7;74(1):83–91. doi: 10.1093/cid/ciab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costantini C., van de Veerdonk F.L., Romani L. Covid-19-Associated pulmonary aspergillosis: the other side of the coin. Vaccines. 2020 Dec 1;8(4):713. doi: 10.3390/vaccines8040713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tolle L.B., Standiford T.J. Danger-associated molecular patterns (DAMPs) in acute lung injury: DAMPs in ALI. J Pathol. 2013 Jan;229(2):145–156. doi: 10.1002/path.4124. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 Apr;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peacock T.P., Brown J.C., Zhou J., Thakur N., Newman J., Kugathasan R., et al. The SARS-CoV-2 variant, Omicron, shows rapid replication in human primary nasal epithelial cultures and efficiently uses the endosomal route of entry. Microbiology. 2022 Jan http://biorxiv.org/lookup/doi/10.1101/2021.12.31.474653 [Internet] Preprint. [cited 2022 Feb 27]. Available from: [Google Scholar]
- 21.Aboudounya M.M., Heads R.J. In: SARS-CoV-2 may bind and activate TLR4 to increase ACE2 expression, facilitating entry and causing hyperinflammation. Dozio E., editor. vol. 2021. 2021 Jan 14. COVID-19 and toll-like receptor 4 (TLR4) pp. 1–18. (Mediators of inflammation). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y., Kuang M., Li J., Zhu L., Jia Z., Guo X., et al. SARS-CoV-2 spike protein interacts with and activates TLR41. Cell Res. 2021 Jul;31(7):818–820. doi: 10.1038/s41422-021-00495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinot R., Hubert M., de Melo G.D., Lazarini F., Bruel T., Smith N., et al. SARS-CoV-2 infection induces the dedifferentiation of multiciliated cells and impairs mucociliary clearance. Nat Commun. 2021 Dec;12(1):4354. doi: 10.1038/s41467-021-24521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salazar F., Bignell E., Brown G.D., Cook P.C., Warris A. Pathogenesis of respiratory viral and fungal coinfections. Clin Microbiol Rev. 2022 Jan 19;35(1):e00094. doi: 10.1128/CMR.00094-21. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.André A.C., Laborde M., Marteyn B.S. The battle for oxygen during bacterial and fungal infections. Trends Microbiol. 2022 Jul;30(7):643–653. doi: 10.1016/j.tim.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Que Y., Hu C., Wan K., Hu P., Wang R., Luo J., et al. Cytokine release syndrome in COVID-19: a major mechanism of morbidity and mortality. Int Rev Immunol. 2022 Mar 4;41(2):217–230. doi: 10.1080/08830185.2021.1884248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in wuhan, China. Clin Infect Dis. 2020 Jul 28;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parrill A., Tsao T., Dong V., Huy N.T. SARS-CoV-2-induced immunodysregulation and the need for higher clinical suspicion for co-infection and secondary infection in COVID-19 patients. J Microbiol Immunol Infect. 2021 Feb;54(1):105–108. doi: 10.1016/j.jmii.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., et al. Targets of T Cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020 Jun;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghazavi A., Ganji A., Keshavarzian N., Rabiemajd S., Mosayebi G. Cytokine profile and disease severity in patients with COVID-19. Cytokine. 2021 Jan;137 doi: 10.1016/j.cyto.2020.155323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arastehfar A., Carvalho A., van de Veerdonk F.L., Jenks J.D., Koehler P., Krause R., et al. COVID-19 associated pulmonary aspergillosis (CAPA)—from immunology to treatment. JoF. 2020 Jun 24;6(2):91. doi: 10.3390/jof6020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tolle L.B., Standiford T.J. Danger-associated molecular patterns (DAMPs) in acute lung injury: DAMPs in ALI. J Pathol. 2013 Jan;229(2):145–156. doi: 10.1002/path.4124. [DOI] [PubMed] [Google Scholar]
- 33.Cunha C., Carvalho A., Esposito A., Bistoni F., Romani L. DAMP signaling in fungal infections and diseases. Front Immun [Internet] 2012:3. doi: 10.3389/fimmu.2012.00286. http://journal.frontiersin.org/article/10.3389/fimmu.2012.00286/abstract [cited 2022 Jul 30] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoot T.S., Kerckhoffs A.P.M., Hilbrands L.B., van Marum R.J. Immunosuppressive drugs and COVID-19: a review. Front Pharmacol. 2020 Aug 28;11:1333. doi: 10.3389/fphar.2020.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhaskar S., Sinha A., Banach M., Mittoo S., Weissert R., Kass J.S., et al. Cytokine storm in COVID-19—immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM Consortium position paper. Front Immunol. 2020 Jul 10;11:1648. doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. The Lancet Rheumatology. 2020 Jun;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stebbing J., Sánchez Nievas G., Falcone M., Youhanna S., Richardson P., Ottaviani S., et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021 Jan;7(1) doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu Meduri, Tolley E.A., Chrousos G.P., Stentz F. Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome: evidence for inadequate endogenous glucocorticoid secretion and inflammation-induced immune cell resistance to glucocorticoids. Am J Respir Crit Care Med. 2002 Apr;165(7):983–991. doi: 10.1164/ajrccm.165.7.2106014. [DOI] [PubMed] [Google Scholar]
- 39.Rocco P.R.M., Souza A.B., Faffe D.S., Pássaro C.P., Santos F.B., Negri E.M., et al. Effect of corticosteroid on lung parenchyma remodeling at an early phase of acute lung injury. Am J Respir Crit Care Med. 2003 Sep 15;168(6):677–684. doi: 10.1164/rccm.200302-256OC. [DOI] [PubMed] [Google Scholar]
- 40.Gopalaswamy R., Subbian S. Corticosteroids for COVID-19 therapy: potential implications on tuberculosis. IJMS. 2021 Apr 6;22(7):3773. doi: 10.3390/ijms22073773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lionakis M.S., Kontoyiannis D.P. Glucocorticoids and invasive fungal infections. Lancet. 2003 Nov;362(9398):1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 42.Riche C.V.W., Cassol R., Pasqualotto A.C. Is the frequency of candidemia increasing in COVID-19 patients receiving corticosteroids? JoF. 2020 Nov 13;6(4):286. doi: 10.3390/jof6040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macauley P., Epelbaum O. Epidemiology and Mycology of Candidaemia in non-oncological medical intensive care unit patients in a tertiary center in the United States: overall analysis and comparison between non-COVID-19 and COVID-19 cases. Mycoses. 2021 Jun;64(6):634–640. doi: 10.1111/myc.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mastrangelo A., Germinario B.N., Ferrante M., Frangi C., Li Voti R., Muccini C., et al. Candidemia in coronavirus disease 2019 (COVID-19) patients: incidence and characteristics in a prospective cohort compared with historical non–COVID-19 controls. Clin Infect Dis. 2021 Nov 2;73(9):e2838–e2839. doi: 10.1093/cid/ciaa1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.WHO corticosteroids for COVID-19. https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 Available online:
- 46.Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., et al. The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020 Oct 6;324(13):1330. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021 Feb 25;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhat P., Noval M., Doub J.B., Heil E. Concurrent COVID-19 and Pneumocystis jirovecii pneumonia in a severely immunocompromised 25-year-old patient. Int J Infect Dis. 2020 Oct;99:119–121. doi: 10.1016/j.ijid.2020.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly S., Waters L., Cevik M., Collins S., Lewis J., Wu M.S., et al. Pneumocystis pneumonia, a COVID-19 mimic, reminds us of the importance of HIV testing in COVID-19. Clin Med. 2020 Nov;20(6):590–592. doi: 10.7861/clinmed.2020-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mang S., Kaddu-Mulindwa D., Metz C., Becker A., Seiler F., Smola S., et al. Pneumocystis jirovecii pneumonia and severe acute respiratory syndrome coronavirus 2 coinfection in a patient with newly diagnosed HIV-1 infection. Clin Infect Dis. 2021 Apr 26;72(8):1487–1489. doi: 10.1093/cid/ciaa906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broadhurst A.G.B., Lalla U., Taljaard J.J., Louw E.H., Koegelenberg C.F.N., Allwood B.W. The diagnostic challenge of pneumocystis pneumonia and COVID -19 co-infection in HIV. Respirology Case Reports [Internet] 2021 Apr;9(4) doi: 10.1002/rcr2.725. https://onlinelibrary.wiley.com/doi/10.1002/rcr2.725 [cited 2022 Aug 7] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alanio A., Dellière S., Voicu S., Bretagne S., Mégarbane B. The presence of Pneumocystis jirovecii in critically ill patients with COVID-19. J Infect. 2021 Apr;82(4):84–123. doi: 10.1016/j.jinf.2020.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koehler P., Bassetti M., Chakrabarti A., Chen S.C.A., Colombo A.L., Hoenigl M., et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021 Jun;21(6):e149–e162. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E., et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study Group education and research Consortium. Clin Infect Dis. 2020 Sep 12;71(6):1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kariyawasam R.M., Dingle T.C., Kula B.E., Vandermeer B., Sligl W.I., Schwartz I.S. Defining COVID-19 associated pulmonary aspergillosis: systematic review and meta-analysis. Clin Microbiol Infect. 2022 Jul;28(7):920–927. doi: 10.1016/j.cmi.2022.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu X., Ge Y., Wu T., Zhao K., Chen Y., Wu B., et al. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020 Aug;285 doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rutsaert L., Steinfort N., Van Hunsel T., Bomans P., Naesens R., Mertes H., et al. COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care. 2020 Dec;10(1):71. doi: 10.1186/s13613-020-00686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alanio A., Dellière S., Fodil S., Bretagne S., Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020 Jun;8(6):e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Arkel A.L.E., Rijpstra T.A., Belderbos H.N.A., van Wijngaarden P., Verweij P.E., Bentvelsen R.G. COVID-19–associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020 Jul 1;202(1):132–135. doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agrawal S., Phulware R.H. COVID-19 associated pulmonary aspergillosis (CAPA) in a pregnant woman. Autops Case Rep. 2021;11 doi: 10.4322/acr.2021.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bretagne S., Sitbon K., Botterel F., Dellière S., Letscher-Bru V., Chouaki T., et al. COVID-19-Associated pulmonary aspergillosis, fungemia, and pneumocystosis in the intensive care unit: a retrospective multicenter observational cohort during the first French pandemic wave. Cuomo CA. Microbiol Spectr. 2021 Oct 31;9(2):e01138. doi: 10.1128/Spectrum.01138-21. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abolghasemi S., Hakamifard A., Sharifynia S., Pourabdollah Toutkaboni M., Azhdari Tehrani H. Fatal invasive pulmonary aspergillosis in an immunocompetent patient with COVID-19 due to Aspergillus terreus : a case study. Clin Case Rep. 2021 Apr;9(4):2414–2418. doi: 10.1002/ccr3.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salmanton-García J., Sprute R., Stemler J., Bartoletti M., Dupont D., Valerio M., et al. COVID-19–Associated pulmonary aspergillosis, march–august 2020. Emerg Infect Dis. 2021;27(4):1077–1086. doi: 10.3201/eid2704.204895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ergün M., Brüggemann R.J.M., Alanio A., Dellière S., van Arkel A., Bentvelsen R.G., et al. Aspergillus test profiles and mortality in critically ill COVID-19 patients. Hanson KE, editor. J Clin Microbiol. 2021 Nov 18;59(12):e01229. doi: 10.1128/JCM.01229-21. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blaize M., Mayaux J., Nabet C., Lampros A., Marcelin A.G., Thellier M., et al. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis. 2020 Jul;26(7):1636–1637. doi: 10.3201/eid2607.201603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nasri E., Shoaei P., Vakili B., Mirhendi H., Sadeghi S., Hajiahmadi S., et al. Fatal invasive pulmonary aspergillosis in COVID-19 patient with acute myeloid leukemia in Iran. Mycopathologia. 2020 Dec;185(6):1077–1084. doi: 10.1007/s11046-020-00493-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Someren Gréve F., du Long R., Talwar R., Beurskens C.J.P., Voerman H.J., van Dijk K. Proven fatal invasive aspergillosis in a patient with COVID-19 and Staphylococcus aureus pneumonia. JoF. 2021 Mar 19;7(3):230. doi: 10.3390/jof7030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iwanaga Y., Kawanami T., Yamasaki K., Sakakibara H., Ikushima I., Ikegami H., et al. A fatal case of COVID-19-associated invasive pulmonary aspergillosis. J Infect Chemother. 2021 Jul;27(7):1102–1107. doi: 10.1016/j.jiac.2021.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim J.L., Khor I.S., Moh C.K., Chan Y.M., Lam Y.F., Lachmanan K.R. Two cases of COVID-19-associated pulmonary aspergillosis (CAPA) Respirology Case Reports [Internet] 2022 Apr;10(4) doi: 10.1002/rcr2.940. https://onlinelibrary.wiley.com/doi/10.1002/rcr2.940 [cited 2022 Aug 7] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim J.H., Kim M., Lim S., Park S.Y., Jegal Y., Lee T., et al. A fatal case report of invasive pulmonary aspergillosis and mucormycosis coinfection in an immunocompetent patient with coronavirus disease 2019 in Korea. Acute Crit Care. 2022 Jun 27 doi: 10.4266/acc.2021.01340. [Internet] Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prattes J., Wauters J., Giacobbe D.R., Salmanton-García J., Maertens J., Bourgeois M., et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients—a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect. 2022 Apr;28(4):580–587. doi: 10.1016/j.cmi.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Verweij P.E., Rijnders B.J.A., Brüggemann R.J.M., Azoulay E., Bassetti M., Blot S., et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020 Aug;46(8):1524–1535. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schauwvlieghe A.F.A.D., Rijnders B.J.A., Philips N., Verwijs R., Vanderbeke L., Van Tienen C., et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018 Oct;6(10):782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 74.Arastehfar A., Carvalho A., van de Veerdonk F.L., Jenks J.D., Koehler P., Krause R., et al. COVID-19 associated pulmonary aspergillosis (CAPA)—from immunology to treatment. JoF. 2020 Jun 24;6(2):91. doi: 10.3390/jof6020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mortensen K.L., Johansen H.K., Fuursted K., Knudsen J.D., Gahrn-Hansen B., Jensen R.H., et al. A prospective survey of Aspergillus spp. in respiratory tract samples: prevalence, clinical impact and antifungal susceptibility. Eur J Clin Microbiol Infect Dis. 2011 Nov;30(11):1355–1363. doi: 10.1007/s10096-011-1229-7. [DOI] [PubMed] [Google Scholar]
- 76.Fekkar A., Poignon C., Blaize M., Lampros A. Fungal infection during COVID-19: does Aspergillus mean secondary invasive aspergillosis? Am J Respir Crit Care Med. 2020 Sep 15;202(6):902–903. doi: 10.1164/rccm.202005-1945LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang S., Wang S., Wan Z., Li R., Yu J. The diagnosis of invasive and noninvasive pulmonary aspergillosis by serum and bronchoalveolar lavage fluid galactomannan assay. BioMed Res Int. 2015;2015:1–5. doi: 10.1155/2015/943691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patterson T.F., Thompson G.R., Denning D.W., Fishman J.A., Hadley S., Herbrecht R., et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barnes R.A., White P.L., Morton C.O., Rogers T.R., Cruciani M., Loeffler J., et al. Diagnosis of aspergillosis by PCR: clinical considerations and technical tips. Med Mycol. 2018 Apr 1;56(suppl_1):S60–S72. doi: 10.1093/mmy/myx091. [DOI] [PubMed] [Google Scholar]
- 80.Koehler P., Bassetti M., Chakrabarti A., Chen S.C.A., Colombo A.L., Hoenigl M., et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021 Jun;21(6):e149–e162. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Routsi C., Meletiadis J., Charitidou E., Gkoufa A., Kokkoris S., Karageorgiou S., et al. Epidemiology of candidemia and fluconazole resistance in an ICU before and during the COVID-19 pandemic era. Antibiotics. 2022 Jun 4;11(6):771. doi: 10.3390/antibiotics11060771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seagle E.E., Jackson B.R., Lockhart S.R., Georgacopoulos O., Nunnally N.S., Roland J., et al. The landscape of candidemia during the coronavirus disease 2019 (COVID-19) pandemic. Clin Infect Dis. 2022 Mar 9;74(5):802–811. doi: 10.1093/cid/ciab562. [DOI] [PubMed] [Google Scholar]
- 83.Chowdhary A., Tarai B., Singh A., Sharma A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, april–july 2020. Emerg Infect Dis. 2020 Nov;26(11):2694–2696. doi: 10.3201/eid2611.203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kayaaslan B., Eser F., Kaya Kalem A., Bilgic Z., Asilturk D., Hasanoglu I., et al. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses. 2021 Sep;64(9):1083–1091. doi: 10.1111/myc.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arastehfar A., Carvalho A., Nguyen M.H., Hedayati M.T., Netea M.G., Perlin D.S., et al. COVID-19-Associated candidiasis (CAC): an underestimated complication in the absence of immunological predispositions? JoF. 2020 Oct 8;6(4):211. doi: 10.3390/jof6040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garcia-Vidal C., Sanjuan G., Moreno-García E., Puerta-Alcalde P., Garcia-Pouton N., Chumbita M., et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021 Jan;27(1):83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Antinori S., Bonazzetti C., Gubertini G., Capetti A., Pagani C., Morena V., et al. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia? Autoimmun Rev. 2020 Jul;19(7) doi: 10.1016/j.autrev.2020.102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.White P.L., Dhillon R., Cordey A., Hughes H., Faggian F., Soni S., et al. A national strategy to diagnose coronavirus disease 2019–associated invasive fungal disease in the intensive care unit. Clin Infect Dis. 2021 Oct 5;73(7):e1634–e1644. doi: 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salehi M., Ahmadikia K., Mahmoudi S., Kalantari S., Jamalimoghadamsiahkali S., Izadi A., et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: species identification and antifungal susceptibility pattern. Mycoses. 2020 Aug;63(8):771–778. doi: 10.1111/myc.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen J., Tian S., Han X., Chu Y., Wang Q., Zhou B., et al. Is the superbug fungus really so scary? A systematic review and meta-analysis of global epidemiology and mortality of Candida auris. BMC Infect Dis. 2020 Dec;20(1):827. doi: 10.1186/s12879-020-05543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prestel C., Anderson E., Forsberg K., Lyman M., de Perio M.A., Kuhar D., et al. Candida auris outbreak in a COVID-19 specialty care unit — Florida, july–august 2020. MMWR Morb Mortal Wkly Rep. 2021 Jan 15;70(2):56–57. doi: 10.15585/mmwr.mm7002e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nori P., Cowman K., Chen V., Bartash R., Szymczak W., Madaline T., et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021 Jan;42(1):84–88. doi: 10.1017/ice.2020.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Buil J.B., Schouten J.A., Wauters J., van de Hoeven H., Verweij P.E., CAC-SDD study group Absence of candidemia in critically ill patients with COVID-19 receiving selective digestive decontamination. Intensive Care Med. 2022 May;48(5):611–612. doi: 10.1007/s00134-022-06651-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nucci M., Barreiros G., Guimarães L.F., Deriquehem V.A.S., Castiñeiras A.C., Nouér S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses. 2021 Feb;64(2):152–156. doi: 10.1111/myc.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al-Hatmi A.M.S., Mohsin J., Al-Huraizi A., Khamis F. COVID-19 associated invasive candidiasis. J Infect. 2021 Feb;82(2):e45–e46. doi: 10.1016/j.jinf.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mulet Bayona J.V., Tormo Palop N., Salvador García C., Fuster Escrivá B., Chanzá Aviñó M., Ortega García P., et al. Impact of the SARS-CoV-2 pandemic in candidaemia, invasive aspergillosis and antifungal consumption in a tertiary hospital. JoF. 2021 May 31;7(6):440. doi: 10.3390/jof7060440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.White P.L., Price J.S., Backx M. Pneumocystis jirovecii pneumonia: epidemiology, clinical manifestation and diagnosis. Curr Fungal Infect Rep. 2019 Dec;13(4):260–273. [Google Scholar]
- 98.Clancy C.J., Nguyen M.H. Diagnosing invasive candidiasis. Kraft CS. J Clin Microbiol [Internet] 2018 May;56(5) doi: 10.1128/JCM.01909-17. https://journals.asm.org/doi/10.1128/JCM.01909-17 [cited 2022 Feb 25] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arastehfar A., Wickes B.L., Ilkit M., Pincus D.H., Daneshnia F., Pan W., et al. Identification of mycoses in developing countries. JoF. 2019 Sep 29;5(4):90. doi: 10.3390/jof5040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gonzalez-Lara M.F., Ostrosky-Zeichner L., Candidiasis Invasive. Semin Respir Crit Care Med. 2020 Feb;41(1):3–12. doi: 10.1055/s-0040-1701215. [DOI] [PubMed] [Google Scholar]
- 101.Tang D.L., Chen X., Zhu C.G., wei Li Z., Xia Y., Guo X.G. Pooled analysis of T2 Candida for rapid diagnosis of candidiasis. BMC Infect Dis. 2019 Dec;19(1):798. doi: 10.1186/s12879-019-4419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blaize M., Raoelina A., Kornblum D., Kamus L., Lampros A., Berger M., et al. Occurrence of candidemia in patients with COVID-19 admitted to five ICUs in France. JoF. 2022 Jun 28;8(7):678. doi: 10.3390/jof8070678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.White P.L., Dhillon R., Cordey A., Hughes H., Faggian F., Soni S., et al. A national strategy to diagnose coronavirus disease 2019–associated invasive fungal disease in the intensive care unit. Clin Infect Dis. 2021 Oct 5;73(7):e1634–e1644. doi: 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Monday L.M., Parraga Acosta T., Alangaden G. T2Candida for the diagnosis and management of invasive Candida infections. JoF. 2021 Mar 3;7(3):178. doi: 10.3390/jof7030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bassetti M., Righi E., Montravers P., Cornely O.A. What has changed in the treatment of invasive candidiasis? A look at the past 10 years and ahead. J Antimicrob Chemother. 2018 Jan 1;73(suppl_1):i14–i25. doi: 10.1093/jac/dkx445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Prakash H., Chakrabarti A. Global epidemiology of mucormycosis. JoF. 2019 Mar 21;5(1):26. doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Prakash H., Chakrabarti A. Epidemiology of mucormycosis in India. Microorganisms. 2021 Mar 4;9(3):523. doi: 10.3390/microorganisms9030523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scharmann U., Herbstreit F., Steckel N.K., Dedy J., Buer J., Rath P.M., et al. Prevalence of COVID-19 associated mucormycosis in a German tertiary care hospital. JoF. 2022 Mar 17;8(3):307. doi: 10.3390/jof8030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Patel A., Agarwal R., Rudramurthy S.M., Shevkani M., Xess I., Sharma R., et al. Multicenter epidemiologic study of coronavirus disease–associated mucormycosis, India. Emerg Infect Dis. 2021 Sep;27(9):2349–2359. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Danion F., Letscher-Bru V., Guitard J., Sitbon K., Dellière S., Angoulvant A., et al. Coronavirus disease 2019-associated mucormycosis in France: a rare but deadly complication. Open Forum Infect Dis. 2022 Feb 1;9(2):ofab566. doi: 10.1093/ofid/ofab566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mehta S., Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020 Sep 30;12(9) doi: 10.7759/cureus.10726. https://www.cureus.com/articles/40523-rhino-orbital-mucormycosis-associated-with-covid-19 [cited 2022 Feb 25]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2021 Apr;42:264.e5–264.e8. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Karimi-Galougahi M., Arastou S., Haseli S. Fulminant mucormycosis complicating coronavirus disease 2019 (COVID-19) Int Forum Allergy Rhinol. 2021 Jun;11(6):1029–1030. doi: 10.1002/alr.22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Waizel-Haiat S., Guerrero-Paz J.A., Sanchez-Hurtado L., Calleja-Alarcon S., Romero-Gutierrez L. A case of fatal rhino-orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID-19. Cureus. 2021 Feb 5;13(2) doi: 10.7759/cureus.13163. https://www.cureus.com/articles/51628-a-case-of-fatal-rhino-orbital-mucormycosis-associated-with-new-onset-diabetic-ketoacidosis-and-covid-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alamin M.A., Abdulgayoom M., Niraula S., Abdelmahmuod E., Ahmed A.O., Danjuma M.I. Rhino-orbital Mucormycosis as a complication of severe COVID-19 pneumonia. IDCases. 2021;26 doi: 10.1016/j.idcr.2021.e01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heydarifard Z., Safaei M., Zadheidar S., Ehsan S., Shafiei-Jandaghi N.Z. Mucormycosis infection in severe COVID-19 patient with multiple underlying health conditions. Clin Case Rep [Internet] 2021 Oct;9(10) doi: 10.1002/ccr3.5009. https://onlinelibrary.wiley.com/doi/10.1002/ccr3.5009 [cited 2022 Feb 25] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Palou E.Y., Ramos M.A., Cherenfant E., Duarte A., Fuentes-Barahona I.C., Zambrano L.I., et al. COVID-19 associated rhino-orbital mucormycosis complicated by gangrenous and bone necrosis—a case report from Honduras. Vaccines. 2021 Jul 27;9(8):826. doi: 10.3390/vaccines9080826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shakir M., Maan M.H.A., Waheed S. Mucormycosis in a patient with COVID-19 with uncontrolled diabetes. BMJ Case Rep. 2021 Jul;14(7) doi: 10.1136/bcr-2021-245343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mekonnen Z.K., Ashraf D.C., Jankowski T., Grob S.R., Vagefi M.R., Kersten R.C., et al. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plast Reconstr Surg. 2021 Mar;37(2):e40–e80. doi: 10.1097/IOP.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rao R., Shetty A., Nagesh C. Orbital infarction syndrome secondary to rhino-orbital mucormycosis in a case of COVID-19: clinico-radiological features. Indian J Ophthalmol. 2021;69(6):1627. doi: 10.4103/ijo.IJO_1053_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alekseyev K., Didenko L., Chaudhry B. Rhinocerebral mucormycosis and COVID-19 pneumonia. J Med Cases. 2021;12(3):85–89. doi: 10.14740/jmc3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ostovan V.R., Rezapanah S., Behzadi Z., Hosseini L., Jahangiri R., Anbardar M.H., et al. Coronavirus disease (COVID-19) complicated by rhino-orbital-cerebral mucormycosis presenting with neurovascular thrombosis: a case report and review of literature. J Neurovirol. 2021 Aug;27(4):644–649. doi: 10.1007/s13365-021-00996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Revannavar S.M., P S S, Samaga L., V K V COVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world? BMJ Case Rep. 2021 Apr;14(4) doi: 10.1136/bcr-2021-241663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maini A., Tomar G., Khanna D., Kini Y., Mehta H., Bhagyasree V. Sino-orbital mucormycosis in a COVID-19 patient: a case report. International Journal of Surgery Case Reports. 2021 May;82 doi: 10.1016/j.ijscr.2021.105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saldanha M., Reddy R., Vincent M.J. Title of the article: paranasal mucormycosis in COVID-19 patient. Indian J Otolaryngol Head Neck Surg. 2021 Apr 22 doi: 10.1007/s12070-021-02574-0. https://link.springer.com/10.1007/s12070-021-02574-0 [Internet] [cited 2022 Feb 25]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khan N., Gutierrez C.G., Martinez D.V., Proud K.C. A case report of COVID-19 associated pulmonary mucormycosis. Arch Clin Cases. 2020 Dec;7(3):46–51. doi: 10.22551/2020.28.0703.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Johnson A.K., Ghazarian Z., Cendrowski K.D., Persichino J.G. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Medical Mycology Case Reports. 2021 Jun;32:64–67. doi: 10.1016/j.mmcr.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kanwar A., Jordan A., Olewiler S., Wehberg K., Cortes M., Jackson B.R. A fatal case of Rhizopus azygosporus pneumonia following COVID-19. JoF. 2021 Feb 28;7(3):174. doi: 10.3390/jof7030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garg D., Muthu V., Sehgal I.S., Ramachandran R., Kaur H., Bhalla A., et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021 May;186(2):289–298. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zurl C., Hoenigl M., Schulz E., Hatzl S., Gorkiewicz G., Krause R., et al. Autopsy proven pulmonary mucormycosis due to Rhizopus microsporus in a critically ill COVID-19 patient with underlying hematological malignancy. JoF. 2021 Jan 27;7(2):88. doi: 10.3390/jof7020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pasero D., Sanna S., Liperi C., Piredda D., Branca G.P., Casadio L., et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2021 Oct;49(5):1055–1060. doi: 10.1007/s15010-020-01561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Placik D.A., Taylor W.L., Wnuk N.M. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiology Case Reports. 2020 Nov;15(11):2378–2381. doi: 10.1016/j.radcr.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Khatri A., Chang K.M., Berlinrut I., Wallach F. Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient – case report and review of literature. J Med Mycol. 2021 Jun;31(2) doi: 10.1016/j.mycmed.2021.101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pauli M.A., Pereira L. de M., Monteiro M.L., de Camargo A.R., Rabelo G.D. Painful palatal lesion in a patient with COVID-19. Oral surgery, oral medicine. Oral Pathology and Oral Radiology. 2021 Jun;131(6):620–625. doi: 10.1016/j.oooo.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Monte Junior E., Santos M., Ribeiro I.B., Luz G..O., Baba E.R., Hirsch B.S., et al. Rare and fatal gastrointestinal mucormycosis (zygomycosis) in a COVID-19 patient: a case report. Clin Endosc. 2020 Nov 30;53(6):746–749. doi: 10.5946/ce.2020.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Joshi A., Tambe R.R., Hinduja A., Sunil S., Varaiya A. Cutaneous mucormycosis in a patient of severe COVID-19 pneumonia: a rarer than rare case report. Indian J Crit Care Med. 2021 Nov 16;25(11):1318–1319. doi: 10.5005/jp-journals-10071-24026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hanley B., Naresh K.N., Roufosse C., Nicholson A.G., Weir J., Cooke G.S., et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. The Lancet Microbe. 2020 Oct;1(6):e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sarkar S., Gokhale T., Choudhury S., Deb A. COVID-19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69(4):1002. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arana C., Cuevas Ramírez R.E., Xipell M., Casals J., Moreno A., Herrera S., et al. Mucormycosis associated with COVID-19 in two kidney transplant patients. Transpl Infect Dis [Internet] 2021 Aug;23(4) doi: 10.1111/tid.13652. https://onlinelibrary.wiley.com/doi/10.1111/tid.13652 [cited 2022 Aug 8] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bellanger A.P., Navellou J.C., Lepiller Q., Brion A., Brunel A.S., Millon L., et al. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) patient. Infectious Diseases Now. 2021 Jan;51(7):633–635. doi: 10.1016/j.idnow.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sen M., Honavar S., Sengupta S., Rao R., Kim U., Sharma M., et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India - collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol. 2021;69(7):1670. doi: 10.4103/ijo.IJO_1565_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hoenigl M., Seidel D., Carvalho A., Rudramurthy S.M., Arastehfar A., Gangneux J.P., et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. The Lancet Microbe. 2022 Jul;3(7):e543–e552. doi: 10.1016/S2666-5247(21)00237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jeong W., Keighley C., Wolfe R., Lee W.L., Slavin M.A., Kong D.C.M., et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019 Jan;25(1):26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 144.Sharma S., Grover M., Bhargava S., Samdani S., Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021 May;135(5):442–447. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mulakavalupil B., Vaity C., Joshi S., Misra A., Pandit R.A. Absence of Case of Mucormycosis (March 2020–May 2021) under strict protocol driven management care in a COVID-19 specific tertiary care intensive care unit. Diabetes Metabol Syndr: Clin Res Rev. 2021 Jul;15(4) doi: 10.1016/j.dsx.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Walther G., Wagner L., Kurzai O. Outbreaks of Mucorales and the species involved. Mycopathologia. 2020 Oct;185(5):765–781. doi: 10.1007/s11046-019-00403-1. [Internet] [DOI] [PubMed] [Google Scholar]
- 147.Song G., Liang G., Liu W. Fungal Co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020 Aug;185(4):599–606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schrödl W., Heydel T., Schwartze V.U., Hoffmann K., Große-Herrenthey A., Walther G., et al. Direct analysis and identification of pathogenic Lichtheimia species by matrix-assisted laser desorption ionization–time of flight analyzer-mediated mass spectrometry. J Clin Microbiol. 2012 Feb;50(2):419–427. doi: 10.1128/JCM.01070-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ling H., Yuan Z., Shen J., Wang Z., Xu Y. Accuracy of matrix-assisted laser desorption ionization–time of flight mass spectrometry for identification of clinical pathogenic fungi: a meta-analysis. Warnock DW, editor. J Clin Microbiol. 2014 Jul;52(7):2573–2582. doi: 10.1128/JCM.00700-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yoshida M. Development of a method of measuring β-D-glucan and its use in preemptive therapy for invasive fungal infections. IJMS. 2021 Aug 27;22(17):9265. doi: 10.3390/ijms22179265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Millon L., Caillot D., Berceanu A., Bretagne S., Lanternier F., Morio F., et al. Evaluation of serum Mucorales polymerase chain reaction (PCR) for the diagnosis of mucormycoses: the MODIMUCOR prospective trial. Clin Infect Dis. 2022 Jan 5:ciab1066. doi: 10.1093/cid/ciab1066. [DOI] [PubMed] [Google Scholar]
- 152.Dannaoui E. Recent developments in the diagnosis of mucormycosis. JoF. 2022 Apr 28;8(5):457. doi: 10.3390/jof8050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cornely O.A., Alastruey-Izquierdo A., Arenz D., Chen S.C.A., Dannaoui E., Hochhegger B., et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical Mycology in cooperation with the mycoses study Group education and research Consortium. Lancet Infect Dis. 2019 Dec;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]