Abstract
Several drugs and antibodies have been repurposed to treat COVID-19. Since the outcome of the drugs and antibodies clinical studies have been mostly inconclusive or with lesser effects, therefore the need for alternative treatments has become unavoidable. However, corticosteroids, which have a history of therapeutic efficacy against coronaviruses (SARS and MERS), might emerge into one of the pandemic's heroic characters. Corticosteroids serve an immunomodulatory function in the post-viral hyper-inflammatory condition (the cytokine storm, or release syndrome), suppressing the excessive immunological response and preventing multi-organ failure and death. Therefore, corticosteroids have been used to treat COVID-19 patients for more than last two years. According to recent clinical trials and the results of observational studies, corticosteroids can be administered to patients with severe and critical COVID-19 symptoms with a favorable risk–benefit ratio. Corticosteroids like Hydrocortisone, dexamethasone, Prednisolone and Methylprednisolone has been reported to be effective against SARS-CoV-2 virus in comparison to that of non-steroid drugs, by using non-genomic and genomic effects to prevent and reduce inflammation in tissues and the circulation. Clinical trials also show that inhaled budesonide (a synthetic corticosteroid) increases time to recovery and has the potential to reduce hospitalizations or fatalities in persons with COVID-19. There is also a brief overview of the industrial preparation of common glucocorticoids.
Abbreviations: 16-DPA, 16-dehydro pregnenolone acetate; ACE-2, Angiotensin-Converting Enzyme 2; AcOOH, Acetic peracid; COVID-19, Coronavirus Disease 2019; DC, Dendritic cells; DUSP1, Dual-specificity phosphatase; ELAM, Endothelial-leukocyte adhesion molecule; GILZ, Glucocorticoid-induced leucine zipper; GR, Glucocorticoid receptors; Hsp, Heat- shock proteins; ICAM, Intracellular adhesion molecule; IL, Interleukin; INCSs, Intranasal corticosteroids; MAP, Mitogen-activated protein; MERS, Middle East respiratory syndrome; NSAIDs, Non-steroidal anti-inflammatory drugs; RCTs, Randomized controlled trials; RdRp, RNA-dependent RNA polymerase; RNA, Ribonucleic acid; SARS, Severe acute respiratory syndrome; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; TGF, Transforming growth factor; USFDA, US Food and Drug Administration; VCAM, Vascular adhesion molecule; WHO, World Health Organization
Keywords: COVID-19, Corticosteroids, Glucocorticoids, Remdesivir, Repurposed drugs
1. Introduction
Due to emerging health conditions caused by COVID-19, WHO declared a pandemic on March 11, 2020 [1]. Since January 2020, physicians and researchers around the world are in continuous search of repurposed drugs for therapeutic treatment of COVID-19. As of 6th May 2022, about 513 million people were infected and over 6 million people suffered death due to COVID-19 [2]. As a result, the COVID-19 pandemic is being considered as the worst worldwide public health catastrophe since the influenza pandemic in 1918. COVID-19 disease passes through different stages: early infection, pulmonary and hyperinflammation phase, among those the hyperinflammation phase which is the most critical due to uncontrolled viral replication and cytokine storm, with hyperinflammation and immune suppression [3], [4]. The virus that causes this condition is known as SARS-CoV-2, which is a single-stranded RNA virus that belongs to the beta coronavirus family. Its spike subunit S1 is crucial for viral attaching to receptors on host cells such as the ACE-2, and S2 participates in virus-cell membrane fusion [5]. The virus normally penetrates into lung epithelial cells and cause a mild lung inflammation. But COVID-19 is characterized by systemic hyper-inflammation and multi-organ dysfunction when the endothelium is injured by direct viral invasion of endothelial cells or by the inflammasome [6]. As a result, altering the immunological host response to SARS-CoV-2 has swiftly become very essential for the researcher to against SARS-CoV-2. For more than last two years, many WHO-approved antivirals, antibiotics, NSAIDs, and other drugs were repurposed for therapeutic treatment against SARS-CoV-2 [7], [8], [9]. Many clinical trials are still ongoing with some non-conclusive findings, some of them were discontinued and the rest of them begin their trials recently [10], [11], [12]. Some new antiviral drugs against SARS-CoV-2 are now being developed but to hit the market successfully it will take few more years [13], [14], [15]. However, anti-inflammatory drugs like corticosteroids have been found to help in the hyperinflammatory stage of the disease. This fact is supported by pathological observations of pulmonary edema and hyaline membrane development in patients who died from COVID-19 [16]. In the past, corticosteroids have been used in conjunction with other antiviral medications to treat a variety of diseases and disorders, including herpes labialis, influenza, Bell's palsy etc., in every cases it was found that this combination therapy was more effective for patients than that of corticosteroid alone [17], [18], [19]. Therefore, using corticosteroids either alone or in conjunction with other antiviral medications may improve the results for COVID-19 patients. Herein we briefly discussed on clinical trial results of drugs like favipiravir [20], remdesivir [21], and Regeneron’s cocktail antibody REGN-COV2 [22] along with Tocilizumab (a monoclonal antibody) [23], [24], [25]. Corticosteroids are now commonly used to treat COVID-19 patients, and clinical trials with various corticosteroids are now underway in several countries [26], [27], [28], [29], [30]. In this article, we discuss about the industrial synthesis of four corticosteroids that are used as well as their mechanism of action, dosage regimen, briefly clinical trials data and also the possibility to use corticosteroids in combination with antiviral drugs in COVID-19.
2. Different therapeutic methods towards COVID-19 treatment and their clinical trial status
Many antiviral drugs/antibodies have been repurposed for the treatment of COVID-19, but in most of the cases their trial results are either not in favor of continuation of therapy or they showed little or no effect to severe COVID-19 patients. Among other drugs, antiviral drugs have been found to be beneficial in the early stages of the disease. In moderate COVID-19 patients, antiviral therapy did not provide supportive care in terms of clinical outcomes. Antivirals were more effective when given early in the course of the disease. Because viral respiratory illness mortality is exceptionally difficult to lower with antiviral drugs, no antiviral treatment has shown benefit in reducing COVID-19 mortality. Antiviral treatment is limited to severe instances in vulnerable populations for most respiratory viral infections due to a lack of effective therapies [31], [32]. However, antiviral medications are still important in the early stages of COVID-19. Some of the drugs recent clinical results are discussed below.
Favipiravir, a synthetic purine nucleoside analogue with broad-spectrum inhibitor of viral RdRp, was chosen because of its in vitro action against SARS-CoV-2, however, there is no indication of clinical efficacy [33], [34], [35]. Favipiravir did not significantly enhance viral clearance in a prospective, randomized, open-label trial of early vs late therapy in hospitalized COVID-19 patients. The updates for two clinical trials (only favipiravir (NCT04336904) and favipiravir/tocilizumab combination (NCT04310228)) to determine their efficacy and safety in the treatment of COVID-19, are still pending [36], [37].
Remdesivir is an adenosine nucleotide analogue prodrug, a potential RdRp inhibitor, which is required for SARS-CoV, MERS-CoV, and SARS-CoV-2 viral RNA production [38], [39]. This drug had been initially approved by different medical organization for the treatment of COVID-19 in the many countries of United States, Europe and Asia. Though later clinical trial results showed remdesivir had very little or no effect on mortality in RCTs, conclusive evidence to support its use is still lacking [37], [40], [41], [42].
Regeneron’s REGN-COV2, is a combination of casirivimab and imdevimab (two monoclonal antibodies) that target the SARS-COV-2 spike protein [22], [43]. In those COVID-19 patients, who are not admitted to hospital, the first descriptive data exhibited that REGN-COV2 can be an excellent alternative to reduce the time to symptom alleviation as well as viral load. REGN-COV2 has also shown potential in terms of decreasing medical visits. Different clinical trials like (NCT04381936, NCT04426695, and NCT04425629) or prevention (NCT04452318) for the treatment of COVID-19 are currently being conducted. On November 21, 2020, USFDA gave REGN-CoV-2 emergency approval for the treatment of mild or moderate COVID-19 symptoms in patients aged 12 and above (weighing at least 40 kg) and are at higher risk for developing severe COVID-19. This approval is based on the findings of a clinical trial that indicated a reduction in COVID-19-related hospitalization or emergency department visits in those at high risk of illness progression within 28 days of starting treatment [44], [45], [46].
Upregulation of the immune response, particularly the production of pro-inflammatory cytokines like IL-6, plays an important role in the pathogenesis of COVID-19 related severe illness. There has been a great interest in finding a targeted anti-inflammatory drug which lowers mortality in hospitalized COVID-19 patients.
Tocilizumab is a recombinant humanized monoclonal antibody and is approved for the treatment of a variety of disorders, including rheumatoid arthritis. Tocilizumab binds to soluble IL-6 receptors selectively, and thus blocks IL-6 signaling. This antibody has an extended elimination half-life and dose-dependent, nonlinear pharmacokinetics. Tocilizumab may be an appropriate and effective treatment for COVID-19 patients as it blocks the IL-6- receptor, which is a vital cytokine in the inflammatory storm that can lead to increased alveolar-capillary blood-gas exchange dysfunction, particularly decreased oxygen diffusion, followed by pulmonary fibrosis and finally organ failure. This antibody was tested in China to 20 patients having SARS-CoV-2 infection at the start of the pandemic to examine its efficacy [47], [48], [49].
3. Corticosteroids in COVID-19 treatment.
Clinical trials have recently revealed that antiviral therapy has little or no influence on the mortality of COVID-19 patients who are hospitalized. Additionally, several negative side effects such as chest discomfort, hyperuricemia, reduced neutrophils, vomiting, and others have also been reported [50], [51], [52]. A systemic inflammatory response had been developed in the majority of severe hospitalized COVID-19 patients, which in most cases resulted in lung damage and multisystem organ failure. Hence a new therapeutic methodology/ treatment for severe COVID-19 patients became the need of the hour. Corticosteroids' powerful anti-inflammatory properties have been found to prevent or lessen these negative effects. As a result, corticosteroids are commonly used to treat coronavirus infections. Corticosteroids are steroid hormones generated by the adrenal cortex that have been shown to be potent immunomodulators [53]. It has a role in a variety of physiological processes, including inflammatory control, immunological response, glucose metabolism, protein catabolism, stress, blood electrolyte levels and many others. Glucocorticoids (hydrocortisone, dexamethasone, prednisolone, methylprednisolone etc.) and mineralocorticoids (aldosterone, fludrocortisone, 11-deoxycorticosterone etc.) are the two main classes of corticosteroids. Glucocorticoids are endogenous molecules with anti-inflammatory, desensitizing, and antiallergy properties that affect carbohydrate, lipid, and protein metabolism. They are immunosuppressants that also have anti-shock and anti-toxic properties. Dexamethasone, Hydrocortisone, prednisolone, and methylprednisolone are the four major glucocorticoids used for the therapeutic treatment of COVID-19 patients (Table 1 ). Corticosteroids have genomic (in the case of low dosages) and nongenomic (in the case of high doses) mechanisms of action. The genomic approach takes longer time and is linked to more side effects than the non-genomic process, which shows major side effects. The main anti-inflammatory effect of corticosteroids is the inhibition of a pro-inflammatory gene that generates cytokines, chemokines, cell adhesion molecules (CAM), and the acute inflammatory response [54].
Table 1.
Comparison of structural and physical properties between the corticosteroids. Data collected from PubChem [55], [56], [57], [58].
| Parameters | Dexamethasone | Methylprednisolone | Prednisolone | Hydrocortisone |
|---|---|---|---|---|
| Molecular Weight | 392.5 | 374.5 | 360.4 | 362.5 |
| XLogP3 | 1.9 | 1.9 | 1.6 | 1.6 |
| LogP | 1.83 | 1.525 | 1.62 | 1.61 |
| LogS | −3.64 | −2.99 | −3.21 | −2.97 |
| Hydrogen Bond Donor Count | 3 | 3 | 3 | 3 |
| Hydrogen Bond Acceptor Count | 6 | 5 | 5 | 5 |
| Rotatable Bond Count | 2 | 2 | 2 | 2 |
| Topological Polar Surface Area | 94.8 Å2 | 94.8 Å2 | 94.8 Å2 | 94.8 Å2 |
| Heavy Atom Count | 28 | 27 | 26 | 26 |
| Formal Charge | 0 | 0 | 0 | 0 |
4. Synthesis of Glucocorticoids
Glucocorticoids are reported to be synthesized in number of ways either by microbiological treatment or industrial synthesis. In this situation it becomes inevitable for organic chemists to develop cost efficient methods for synthesis of pharmaceutically important corticosteroids. Herein we had summarized the industrial synthesis of prednisolone, dexamethasone, and hydrocortisone from natural product diosgenin [59]. While methyl prednisolone were reported to be synthesized from hydrocortisone [60].
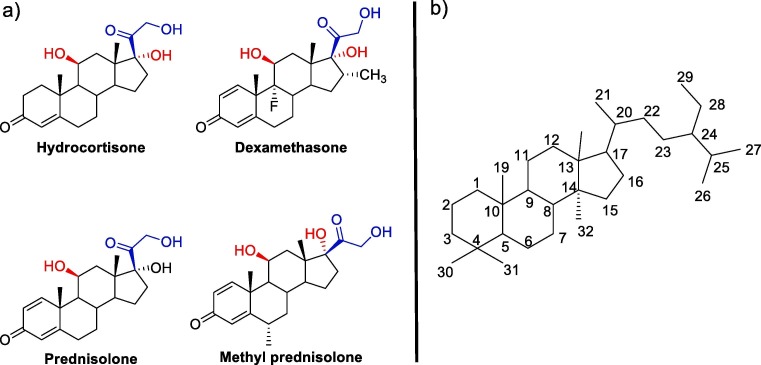
For the efficiency of glucocorticoid, the presence of hydroxyl groups (–OH) at β- (C-11) and α- (C-17) positions in the structure of the pregnane system is necessary. In general, glucocorticoid binding to receptor sites is expected to occur only when the β –OH and β –CO-CH2-OH at the C-11 (red colored) and C-17 (blue colored) respectively (Fig. 1 ) of the steroid system is present at the same time. In most cases, the presence of axially oriented additional bulky substituents in the steroids inhibits its binding to receptors, while no change observed for analogues with equatorial -substituents.
Fig. 1.
Common Steroids used for COVID-19 treatment: Skeleton for corticosteroids.
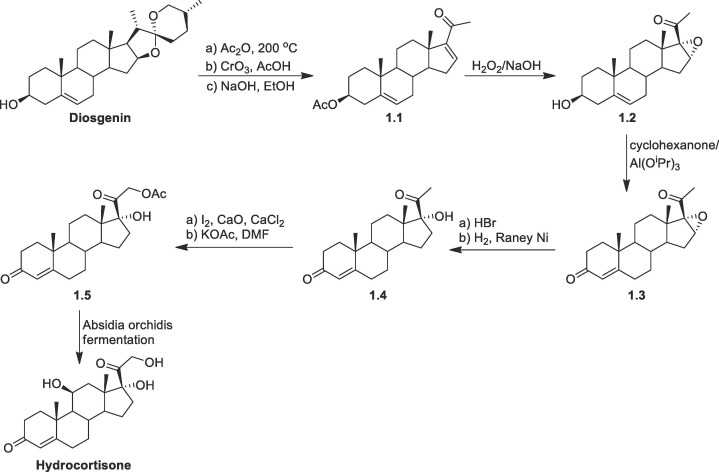
Hydrocortisone: The synthesis of hydrocortisone was reported via different routes by several researchers. Woodward and co-workers first reported the chemical synthesis of hydrocortisone in 1952 through many steps [61]. Later, Sarett et. al synthesized hydrocortisone from 3-ethoxypiperylene and quinone [62], [63], followed by other groups [64], [65]. They reported a modification of Woodward's method starting from methoxytoluquinone and methyl vinyl ketone while Velluz's synthesized, from 6-methoxytetralone and dichlorobutane [66]. In all of the cases the overall yields were very less (∼0.04 %) [67]. For the industrial synthesis of hydrocortisone, semi-synthetic pathways are followed starting from naturally occurring sterols. It also includes a multi-stage chemical method as well as a microbial bioconversion step.
One method starts from diosgenin (Scheme 1 ), which after successive reactions was converted to (16-DPA (1.1) [59]. Then compound 1.1 was converted to epoxide 1.2 by treatment of hydrogen peroxide/base followed by Oppenauer oxidation gives the 3-keto unsaturated intermediate 1.3. The epoxide 1.3 was converted to 17-hydroxy progesterone (1.4) by epoxide opening and then removal of HBr by H2/Raney Ni reduction. Following Ringold-Stork protocol compound 1.4 was converted to the corresponding acetate 1.5. In the final step the selective hydroxylation was achieved by the treatment of Absidia orchidis whereby both β- and α- (72: 17) hydroxyl isomers were formed. The major and the required isomer 11β- hydrocortisone was isolated by recrystallization.
Scheme 1.
Synthesis of hydrocortisone from diosgenin.
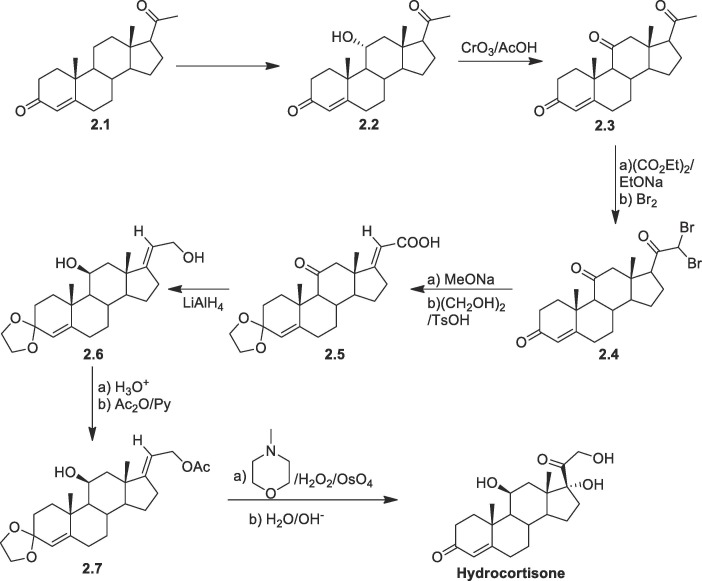
In another approach, the synthesis of hydrocortisone starting from progesterone was reported (Scheme 2 ). Here to avoid late-stage hydroxylation, progesterone was treated by microbial substance for hydroxylation and then by CrO3/CH3COOH for oxidation of alcohols to give compound 2.3. The triketone 2.3 thus formed was treated with diethyloxalate/base followed by bromine to form the product 2.4. Favorskii rearrangement of the dibromo product 2.4 leads to the formation of α,β-unsaturated acid followed by protection of enone by glycols to give the compound 2.5. Then the α,β-unsaturated acid moiety was reduced with LiAlH4 yields the compound 2.6. Conversion of compound 2.6 to hydrocortisone was performed by acetylation followed by dihydroxylation and then deprotection [59].
Scheme 2.
Synthesis of hydrocortisone from progesterone derivatives.
A novel biosynthesis for the preparation of hydrocortisone from carbon source by treatment with recombinant Saccharomyces cerevisiae strains was reported recently [68].
Dexamethasone: Another important corticosteroid is dexamethasone; it was first synthesized in 1957 and was approved for wide range of anti-inflammatory treatment in 1961 [69]. There are several routes for the synthesis of dexamethasone from the available steroids.
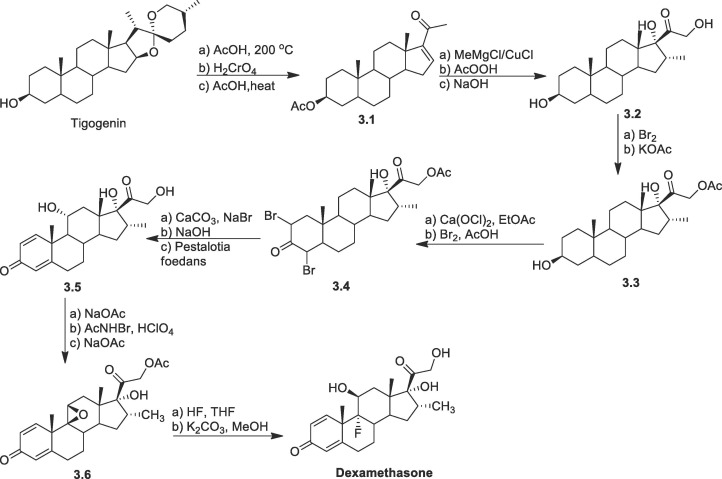
One of the important methods for the synthesis of dexamethasone is from readily available tigogenin (Scheme 3 ) [70], [71]. Initially tigogenin through multiple step reaction was converted to α, β-unsaturated ketone 3.1. Then enone 3.1 was treated with MeMgCl/CuCl to introduce the methyl moiety at C-16, followed by epoxidation with AcOOH and alkaline hydrolysis to get compound 3.2. Then, bromination followed by treatment with potassium acetate gives acetylation at C-21 gave compound 3.3. The oxidation at C-3 position was accomplished by oxidising with calcium hypochlorite and then on treatment Br2/AcOH in a dioxane-AcOH solvent generated 2,4-dibromoketone derivative 3.4. Under suitable conditions double dehydrobromination takes place following that, on treatment with the fungus Peslalotia foedans, yielded selective 11-hydroxy derivative 3.5. Between C-9 and C-11 in the C-ring, a double bond was also formed as a result of the sodium acetate treatment, then epoxidation with hypobromous acid (formed by mixing perchloric acid and N-bromo acetamide) followed by sodium acetate treatment resulted in 3.6. Dexamethasone was produced by treating epoxide 3.6 with HF in chloroform-THF to introduce fluorine at C-9. and finally, by hydrolysis of the acetyl unit.
Scheme 3.
Synthesis of dexamethasone from tigogenin.
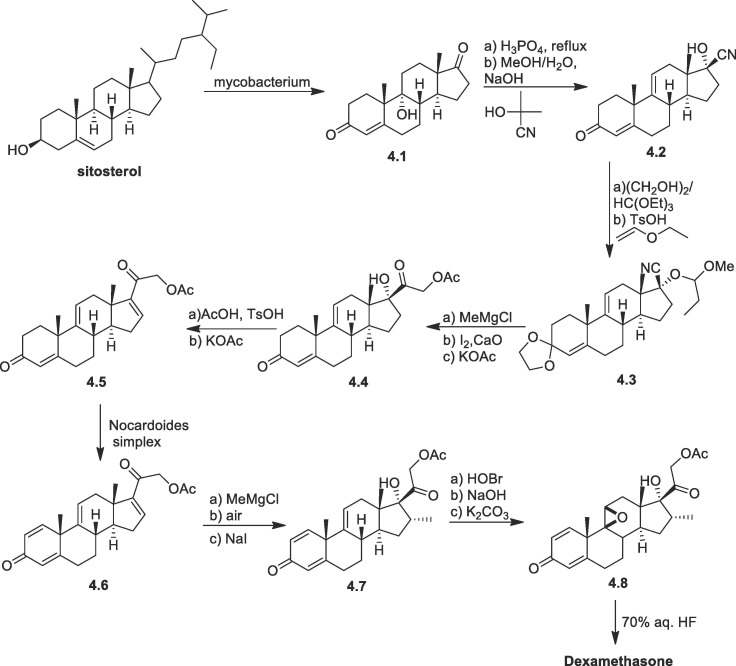
Upjohn and other companies developed an alternate method by converting sitosterol to 9α-hydroxyandrost-4-ene-3,17-dione (4.1) through a microbial fermentation process (Scheme 4 ) [72], [73]. Treatment of 4.1 with phosphoric acid followed by acetone cyanohydrin in alkaline MeOH/water yielded the cyano compound 4.2 [74]. Both enone at C-3 and hydroxy at C-17 were protected, using suitable reagents to form 4.3. After methylation of nitrile using MeMgCl hydrolysis were performed to generate acetoxy group. The acetoxy group at C21 was introduced via α-iodination and then 4.4 was produced by displacement with acetate. The hydroxyl group at C17 was converted to the double bond (between C16-C17) by esterification, followed by elimination by treatment with KOAc in DMSO at 80 °C yielding 4.5. Microbial fermentation of the compound 4.5 yielded C1-C2 double bond 4.6. In a single pot, the α methyl group at C-16 was introduced and the C-17 oxidation took place to synthesize compound 4.7. To synthesize dexamethasone, the C9 α-fluoro group was introduced via the conventional technique of epoxidation 4.8 followed by ring opening with HF.
Scheme 4.
Synthesis of dexamethasone from sitosterol.
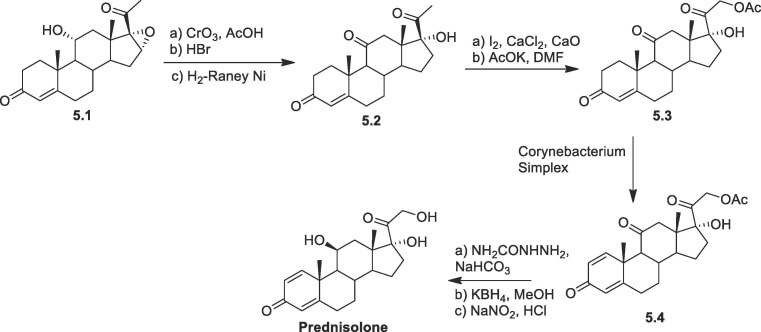
Prednisolone: Prednisolone are conveniently synthesized following a very efficient route starting with the oxidation of hydroxyl group at carbon-11 forming triketone derivative (Scheme 5 ), followed by epoxide opening with hydrobromic acid and subsequent H2/Raney-Ni reduction yielded 5.2 [75]. Next by following the Ringold-Stork method, the 21-methyl group is transformed to produce cortisone-21-acetate 5.3. Cortisone acetate 5.3 was later transformed into prednisone acetate 5.4 by the microbial treatment with Corynebacterium simplex. By following the reported protocol prednisone acetate 5.4 was be transformed into prednisolone. Prednisone acetate 5.4 was transformed to prednisolone by protection with semicarbazide then reduction with KBH4, followed by NaNO2 mediated deprotection.
Scheme 5.
Synthesis of prednisolone from triketone derivative.
The abovementioned procedure is being followed in most of the cases for the synthesis of prednisone and prednisolone across the world.
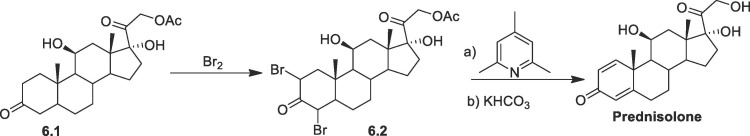
In another procedure (Scheme 6 ), 21-acetoxy-11β,17α-dihydroxy-5α-pregnan-3,20-dione (6.1), undergoes bromination at C-2 and C-4 by bromine in acetic acid gives dibromide 6.5. The product was further dehydrobrominated by heating in collidine followed by hydrolysis at C-21 produces prednisolone [60].
Scheme 6.
Synthesis of prednisolone from prednisone acetate.
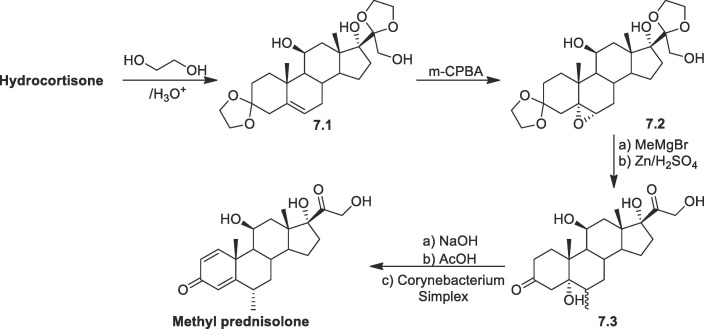
Methylprednisolone: Methylprednisolone, also known as 11,17,21-trihydroxy-6-methylpregna-1,4-dien-3,20-dione, varies from prednisolone by a methyl group at C-6 position of the steroid system. Therefore, a different strategy is required for introducing a methyl group at C-6 of the steroid skeleton. In one method starting from hydrocortisone (Scheme 7 ), carbonyl group is protected by ethylene glycol in the presence catalytic amount of acid, causing the movement of double bond from C-4–C-5 to C-5-C-6 position of the steroid to form 7.1. Then perbenzoic acid is used to convert the product to an epoxide 7.2. The resultant epoxide is then treated with MeMgBr, and the ketal protection is then removed by Zn/H2SO4 reduction, yielding the 5-hydroxy-6-methyl dihydrocortisone derivative 7.3. The resultant hydroxyketone 7.3 is dehydrated in alkaline condition, and then the product 6-methylcortisone is further microbiologically dehydrogenated at the C1-C2 position to get the required methylprednisolone [76], [77], [78].
Scheme 7.
Synthesis of methyl prednisolone from hydrocortisone.
4.1. Mechanism of action for corticosteroids against SARS-CoV-2
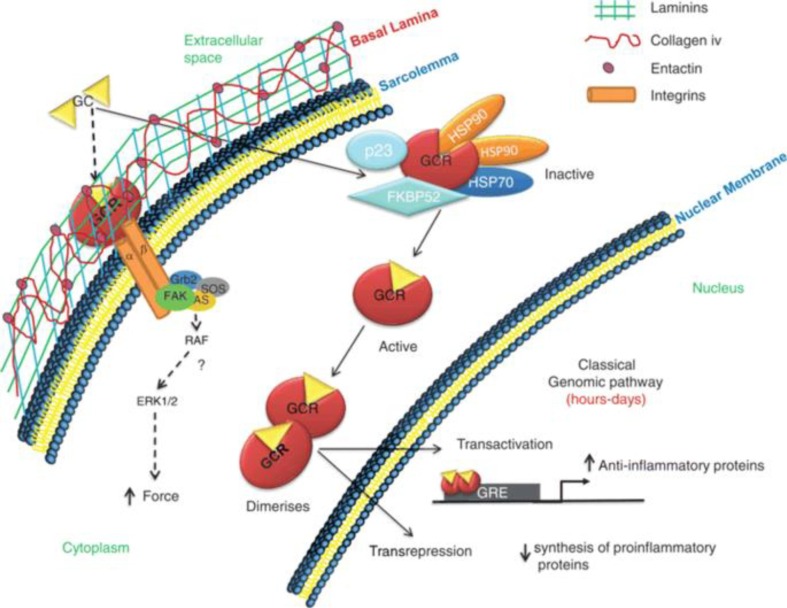
There have been several studies indicating that corticosteroids have been effective in the treatment of COVID-19. Corticosteroids act differently than other antiviral medicines. There are two types of mechanism that it follows namely non-genomic (applicable for high doses) and genomic (for low doses) (Fig. 2 ) [79], [80], [81]. In a nutshell, corticoids reduce inflammation by increasing the production and release of anti-inflammatory proteins while blocking the production and secretion of pro-inflammatory proteins. Because of their small size and lipophilic nature, corticosteroids can diffuse past the cell membrane and attach to GR in the cytoplasm and the GR dissociates from chaperone proteins Hsp70, Hsp90, and immunophilin [82]. The GR complex then trans locates into the nucleus for binding glucocorticoid response elements, as a result, the expression of several genes is managed [83], [84]. By concurrently activating histone deacetylases and inhibiting histone acetyltransferases, activated glucocorticoids reduce the expression of pro-inflammatory genes [85], [86]. Corticosteroids also inhibits immune cell extravasation, epithelial cell adhesion, chemotaxis, phagocytosis, and immune cell synthesis of antimicrobial effector chemicals, all of which are related with inflammation. Glucocorticoids exert their actions at the molecular level through trans repression and transactivation pathways, inducing anti-inflammatory genes like the DUSP1 and GILZ [87], an anti-inflammatory protein, MAP kinase phosphatase 1 and an inhibitor of NF-kB i.e., GILZ, that prevents nuclear translocation of GATA-3, which is involved in the generation of T helper (Th)2 cytokines, is also induced by glucocorticoids [87], [88]. They increase the formation of annexin 1, which reduces phospholipase A2 expression and enhances inflammation resolution and phagocytosis of apoptotic neutrophils by macrophages [26]. Glucocorticoids prevent leucocyte recruitment by reducing the synthesis of acute phase reactants and chemokines [89], [90]. The expression of VCAM-1, ELAM-1, and ICAM-1 are lowered to avoid leucocyte diapedesis [91], [92]. Glucocorticoids affect a wide range of cells. lymphocytes include Th1, Th2, and Th17 cells, CD8, as well as B and Treg cells; Myeloid cells include macrophages, tissue resident, monocytes, migratory, granulocytes and plasmacytoid DC [26], [79], [93]. In all subtype leucocyte maturation, differentiation, and proliferation are impaired by glucocorticoids. The number of monocytes/macrophages, DC, eosinophil and basophil granulocytes in the body is reduced. Glucocorticoids enhance the number of neutrophils generated by the bone marrow and their demarginating, as well as the TGF and anti-inflammatory cytokines IL-10 released by DCs. They suppress antigen presentation to T lymphocytes by lowering the expression of Fc receptors and MHC class II on the cell membrane [26], [94], [95].
Fig. 2.
Genomic (continuous arrows) and non-genomic (dashed arrows) mechanism of corticosteroids. (This image is taken from [54], an open access paper).
Corticosteroids have been demonstrated to upregulate anti-inflammatory molecules such as CD163 and IL-10 in macrophages and monocytes via the annexin A1 (also known as lipocortin 1)-mediated pathway [96], [97]. Additionally, corticosteroids like dexamethasone can induce the generation of host cytochrome P450-3A4, which can reduce the efficacy of other associated medications used for treatment [96], [98]. And also, Glucocorticoids inhibit lymphocytes and the production of pro-inflammatory cytokines such as interleukin IL-1, IL-2, IL-6, IL-8, VEGF, IFN-gamma, TNF, and prostaglandins. Among them five of these are significant and are linked to SARS-CoV-2 [54], [99].
4.2. Clinical trials related to corticosteroids and their outcomes
According to Paassen et al. among forty-four RCTs and observational studies, methyl prednisolone (n = 35) was prescribed in maximum number of studies [100]. Dexamethasone, hydrocortisone, and prednisolone were also been either as single or in multiple corticosteroids regimen. The dosing regimen was largely determined by the glucocorticoid half-life. Dexamethasone (6 mg once daily) has a lengthy half-life of 36–72 h, whereas prednisolone and methyl prednisolone (intermediate half-life 12–36 h) are given at 40 mg and 32 mg (e.g., 16 mg in every 12 h or 8 mg in every 6 h) daily, respectively. However, in the case of hydrocortisone (which has a short half-life of 8 to 12 h), 160 mg (50 mg every 8 h or 100 mg every 12 h) must be taken [101]. Some data related to corticosteroids in COVID-19 therapy are shown in Table 2 .
Table 2.
Comparison between the corticosteroids in COVID-19 treatment.
| Drugs | Clinical trial | Dosage | Stage | Reference |
|---|---|---|---|---|
| Dexamethasone | NCT04395105 | 16 mg/day IV OD from days 1 to 5 and 8 mg/day from days 6 to 10 | ARDS with confirmed respiratory infection due to SARS-CoV-2 | [107] |
| NCT04327401 | First 5 days 20 mg/day IV, followed by 10 mg IV 1/day for 5 days | Moderate and severe ARDS due to SARS-CoV-2 virus | [108] | |
| NCT04344730 | 10 dexamethasone 20 mg/ 5 ml, solution for injection in ampoule of 5 ml, from D1 to D10 for one patient. | Acute hypoxemic respiratory failure (AHRF) | [109] | |
| NCT04707534 | First 5 days 20 mg/day, for next 5 days 10 mg/day | Hospitalized patients with COVID-19 | [110] | |
| NCT04765371 | 6 mg/day for 10 days |
Patients with COVID-19 pneumonia requiring oxygen supplementation | [111] | |
| NCT04909918 | IV 8 mg/day given for 7 days | Patients with COVID-19 Disease Admitted to ICU | [112] | |
| Methylprednisolone | NCT04263402 | 1. < 40 mg/ day IV drip × 7 days 2. 40 to 80 mg/day IV drip × 7 days |
Early stage | [113] |
| IRCT20080901001165N52 | 500 mg IV infusion over 1 h. At days 2 and 3: 250 mg IV infusion over 1 h. At days 4 and 5: 100 mg IV infusion over 1 h | Moderate to severe pneumonia related to COVID-19 | [114] | |
| IRCT20200204046369N1 | 20 mg/day | Hospitalized patients with confirmed COVID-19 | [115] | |
| NCT04343729 | 0.5 mg/kg | Patients displaying severe acute respiratory syndrome indications | [116] | |
| NCT04909918 | For 7 days, 1 mg/kg/day in 2 divided doses per day | COVID-19 Diseased Patients Admitted in ICU | [112] | |
| NCT04438980 | 120 mg/day for 3 days | Patients with COVID-19 pneumonia with risen inflammatory biomarkers | [117] | |
| Hydrocortisone | NCT04348305 | 200 mg q 24 h bolus injections 50 mg (10 ml) every 6 h for 7 days | Patients with COVID-19 and severe hypoxia | [118] |
| NCT04359511 | 3.5 mg/kg/day continuous infusion for 10 days | COVID-19 Pneumonia | [119] | |
| Prednisolone | IRCT20080901001165N52 | Prior to discharge, use 25 mg PO once day. After that, taper off tab. prednisolone over the course of a month. | Patients with mild to severe COVID-19-related pneumonia | [114] |
| NCT04359511 | Prednisone 0.7 mg/kg/day PO OD for 10 days | COVID-19 Pneumonia | [119] | |
| NCT04765371 | 60 mg/day for 10 days | Patients with COVID-19 pneumonia requiring oxygen supplementation | [111] | |
| Budesonide | NCT04355637 | Inhaled budesonide | Hospitalized patients with COVID-19 | [120] |
| Ciclesonide | NCT04435795 | Ciclesonide 600mcg BID inhaled and intranasal ciclesonide 200 mcg DIE | Mild COVID-19 disease | [121] |
Budesonide is a synthetic corticosteroid that is inhaled and has high glucocorticoid and weak mineralocorticoid activity [102], [103], [104]. INCSs have been increasingly popular in recent years due to their efficacy. Bioavailability, intranasal environment, and variables that influence patient adherence should all be considered during the COVID-19 treatments. Many RCTs have recently begun to investigate the role of inhaled steroids in the treatment of COVID-19 in individuals who have been infected with SARS-CoV-2 (NCT04330586, NCT04355637, NCT04377711, NCT04416399, and NCT04331470) [105], [106]. The aforementioned trials have yet to produce any clear and conclusive data.
4.3. Corticosteroids in combination with other drugs
For the treatment of COVID-19, several combinations of corticosteroids and non-corticosteroids have been investigated. Only dexamethasone was given to hospitalized patients who did not require oxygen support; however, dexamethasone was administered in combination with antiviral drug remdesivir to critical patients who did require oxygen support and it was found that though corticosteroids – remdesivir therapy could not reduce the mortality rate of critical patients but was associated with a significant decrease in the incidence of Secondary Bacterial Infections in critically ill COVID-19 patients [122]. In another study Dexamethasone with other antiviral drug (remdesivir or favipiravir), it was observed that the use of steroids within ten days of beginning antiviral medications considerably reduces the probability of ICU hospitalization in the early stages of the disease [123]. Corticosteroid was also studied in combination with hydroxychloroquine, an antiviral drug, against hospitalized adults with COVID-19, and result exhibited that the combination of corticosteroid and antiviral drug was related to a reduced risk of clinical development, particularly in patients with pneumonia [124]. Corticosteroids-favipiravir combination treatment was also showed a better result in for improving the condition of COVID-19 patients with chronic obstructive pulmonary disease [125]. Dexamethasone was also used in conjunction with Tocilizumab (IV) or sarilumab in ICU patients (IV) [126]. In the clinical study Dexamethasone in combination with Tocilizumab, the risk of death in COVID‐19 patients was found to be lower than patients treated with tocilizumab alone [127]. Dexamethasone, Ivermectin, Aspirin 250, and Enoxaparin injections were administered to treat moderate to severe symptoms in certain circumstances [128].
4.4. Quest for new corticosteroids in the treatment of COVID-19
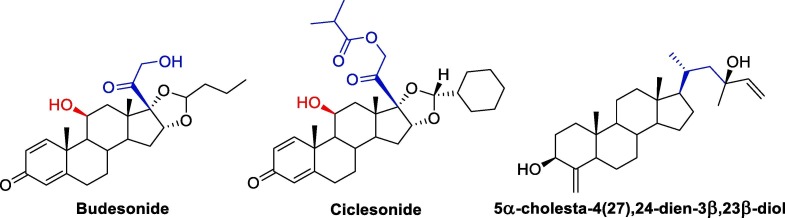
After the initial success of corticosteroids, now the researchers over the world are in search of natural/synthetic corticosteroids which will have a better efficacy with lesser side effects towards the COVID-19 treatment. Budesonide a synthetic glucocorticoid primarily known as nasal medication for asthma are now repurposed as a nasal medication against SARS-CoV-2. Ciclesonide is another inhaled corticosteroid which was tested in COVID-19 patients, but there are still insufficient evidences whether it is beneficial to COVID-19 patients or not [129]. Another new steroid 5α-cholesta-4(27),24-dien-3β,23β-diol was isolated from Ophiocoma dentata and studied against SARS-CoV-2 [130]. The in vitro result shows that the new isolated steroid can are effective in 95 % inhibition of the SARS-CoV-2 virus at 5 μg/ml concentration. Also, the IC50 value indicates that it is more potent than diclofenac. Therefore, these three drugs (Fig. 3 ) can be developed further into an effective drug against SARS-CoV-2 virus.
Fig. 3.
Skeleton of three new reported steroids.
Also, a new synthetic corticosteroid can be developed based on the fact that C-11 and C-17 should contain β- and α- hydroxy group respectively. In addition to hydroxyl group, β –CO-CH2-OH at C-17 position of the steroid system is also an essential in showing the activity towards SARS-CoV-2 virus as all the four corticosteroids (hydrocortisone, dexamethasone, prednisolone and methyl prednisolone) along with budesonide has this stereochemistry and functional groups common in their structure.
4.5. Adverse effects of corticosteroids in the treatment of COVID-19
Despite the risk of side effects such as transient hyperglycemia, steroids are generally safe in short-term use. Long-term use, on the other hand, has been related to cataracts, glaucoma, hypertension, fluid retention, psychological effects, weight gain, as well as an increased risk of infections and osteoporosis [15], [131].
5. Conclusion
In summary, in the battle against COVID-19, corticosteroids can be termed as double-edged sword and they should be used carefully as a short-course (e.g., up to 10 days) treatment agent for severe and critical hospitalized COVID-19 patients, considering the risk–benefit ratio [132]. There is no concrete clinical trial data to support the long-term administration of steroids in COVID-19 patients to avoid potentially harmful sequelae such as lung fibrosis diabetes mellitus, obesity, cardiovascular diseases and depression. More well-designed clinical studies are urgently needed to assess the safety and effectiveness of corticosteroids, as well as corticosteroids in combination with other drugs, in COVID-19 therapies. Finally, corticosteroids are a low-cost, readily available drug that should be regarded as standard of treatment in hospitalized COVID-19 patients who require respiratory support.
6. Consent for publication
Not applicable.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to the unknown reviewer for his/her thoughtful suggestion which elevates the quality of this review article.
References
- 1.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data, (n.d.). https://covid19.who.int/ (accessed May 8, 2022).
- 3.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J. Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.R.B. Polidoro, R.S. Hagan, R. de Santis Santiago, N.W. Schmidt, Overview: Systemic Inflammatory Response Derived From Lung Injury Caused by SARS-CoV-2 Infection Explains Severe Outcomes in COVID-19, Front. Immunol. 11 (2020) 1626. https://doi.org/10.3389/fimmu.2020.01626. [DOI] [PMC free article] [PubMed]
- 7.Costanzo M., De Giglio M.A.R., Roviello G.N. SARS-CoV-2: recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr. Med. Chem. 2020;27:4536–4541. doi: 10.2174/0929867327666200416131117. [DOI] [PubMed] [Google Scholar]
- 8.Paul S.S., Biswas G. Repurposed antiviral drugs for the treatment of COVID-19: syntheses, mechanism of infection and clinical trials. Mini-Rev. Med. Chem. 2021;21:1123–1143. doi: 10.2174/1389557521666201222145842. [DOI] [PubMed] [Google Scholar]
- 9.Russell B., Moss C., Rigg A., Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez M.A. Lack of effectiveness of repurposed drugs for COVID-19 treatment. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.635371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy E.K., Battula S., Anwar S., Sajith A.M. Drug re-purposing approach and potential therapeutic strategies to treat COVID-19. Mini-Rev. Med. Chem. 2021;21:704–723. doi: 10.2174/1389557520666201113105940. [DOI] [PubMed] [Google Scholar]
- 12.Repurposed Antiviral Drugs for Covid-19 — Interim WHO Solidarity Trial Results, N. Engl. J. Med. 384 (2021) 497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed]
- 13.Suryavanshi H., Chaudhari R.D., Patil V., Majumdar S., Debnath S., Biswas G. Design, synthesis and docking study of Vortioxetine derivatives as a SARS-CoV-2 main protease inhibitor. DARU J. Pharm. Sci. 2022;30(1):139–152. doi: 10.1007/s40199-022-00441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang R., Yu Z., Wang Y., Wang L., Huo S., Li Y., Liang R., Hao Q., Ying T., Gao Y., Yu F., Jiang S. Recent advances in developing small-molecule inhibitors against SARS-CoV-2. Acta Pharm. Sin. B. 2022;12:1591–1623. doi: 10.1016/j.apsb.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J., Yang W., Chen P., Guo J., Liu R., Wen P., Li K., Lu Y., Ma T., Li X., Qin S., Zhang Y., Wang Y., Pasin L. The proportion and effect of corticosteroid therapy in patients with COVID-19 infection: A systematic review and meta-analysis. PLOS ONE. 2021;16(4):e0249481. doi: 10.1371/journal.pone.0249481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.N. Arain, S.C. Paravastu, M.A. Arain, Effectiveness of topical corticosteroids in addition to antiviral therapy in the management of recurrent herpes labialis: a systematic review and meta-analysis, BMC Infect. Dis. 15 (2015) 82. doi: 10.1186/s12879-015-0824-0. [DOI] [PMC free article] [PubMed]
- 18.Numthavaj P., Thakkinstian A., Dejthevaporn C., Attia J. Corticosteroid and antiviral therapy for Bell’s palsy: a network meta-analysis. BMC Neurol. 2011;11:1. doi: 10.1186/1471-2377-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asuquo E., Martin A., Nzerem P. Evaluation of Cd(II) ion removal from aqueous solution by a low-cost adsorbent prepared from white yam (Dioscorea rotundata) waste using batch sorption. ChemEngineering. 2018;2:35. doi: 10.3390/chemengineering2030035. [DOI] [Google Scholar]
- 20.Hassanipour S., Arab-Zozani M., Amani B., Heidarzad F., Fathalipour M., Martinez-de-Hoyo R. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Sci. Rep. 2021;11:11022. doi: 10.1038/s41598-021-90551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bansal V., Mahapure K.S., Bhurwal A., Gupta I., Hassanain S., Makadia J., Madas N., Armaly P., Singh R., Mehra I., O’Horo J.C., Kashyap R. Mortality benefit of remdesivir in COVID-19: a systematic review and meta-analysis. Front. Med. 2021;7 doi: 10.3389/fmed.2020.606429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Musser B.J., Soo Y., Rofail D., Im J., Perry C., Pan C., Hosain R., Mahmood A., Davis J.D., Turner K.C., Hooper A.T., Hamilton J.D., Baum A., Kyratsous C.A., Kim Y., Cook A., Kampman W., Kohli A., Sachdeva Y., Graber X., Kowal B., DiCioccio T., Stahl N., Lipsich L., Braunstein N., Herman G., Yancopoulos G.D. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N. Engl. J. Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosas I.O., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., Skiest D., Aziz M.S., Cooper N., Douglas I.S., Savic S., Youngstein T., Del Sorbo L., Cubillo Gracian A., De La Zerda D.J., Ustianowski A., Bao M., Dimonaco S., Graham E., Matharu B., Spotswood H., Tsai L., Malhotra A. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N. Engl. J. Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang C., Zhao H. Tocilizumab in COVID-19 therapy: who benefits, and how? The Lancet. 2021;398:299. doi: 10.1016/S0140-6736(21)01380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubio-Rivas M., Mora-Luján José.M., Montero A., Aguilar García J.A., Méndez Bailón M., Fernández Cruz A., Oriol I., Teigell-Muñoz F.-J., Dendariena Borque B., De la Peña Fernández A., Fernández González R., Gil Sánchez R., Fernández Fernández J., Catalán M., Cortés-Rodríguez B., Mella Pérez C., Montero Rivas L., Suárez Fuentetaja R., Ternero Vega J.E., Ena J., Martin-Urda Díez-Canseco A., Pérez García C., Varona José.F., Casas-Rojo José.M., Millán Núñez-Cortés J. The use of corticosteroids or tocilizumab in COVID-19 based on inflammatory markers. J Gen Intern Med. 2022;37(1):168–175. doi: 10.1007/s11606-021-07146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Annane D. Corticosteroids for COVID-19. J. Intensive Med. 2021;1(1):14–25. doi: 10.1016/j.jointm.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohamed A.A., Mohamed N., Mohamoud S., Zahran F.E., Khattab R.A., El-Damasy D.A., Alsayed E., Abd-Elsalam S. SARS-CoV-2: the path of prevention and control. infect. Disord. – Drug Targets. 2021;21:358–362. doi: 10.2174/1871526520666200520112848. [DOI] [PubMed] [Google Scholar]
- 28.Liu J., Zhang S., Dong X., Li Z., Xu Q., Feng H., Cai J., Huang S., Guo J., Zhang L., Chen Y., Zhu W., Du H., Liu Y., Wang T., Chen L., Wen Z., Annane D., Qu J., Chen D. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J. Clin. Invest. 2020;130:6417–6428. doi: 10.1172/JCI140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shuto H., Komiya K., Yamasue M., Uchida S., Ogura T., Mukae H., Tateda K., Hiramatsu K., Kadota J. A systematic review of corticosteroid treatment for noncritically ill patients with COVID-19. Sci. Rep. 2020;10:20935. doi: 10.1038/s41598-020-78054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner C., Griesel M., Mikolajewska A., Mueller A., Nothacker M., Kley K., Metzendorf M.-I., Fischer A.-L., Kopp M., Stegemann M., Skoetz N., Fichtner F. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst. Rev. 2021;2021 doi: 10.1002/14651858.CD014963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villamagna A.H., Gore S.J., Lewis J.S., Doggett J.S. The need for antiviral drugs for pandemic coronaviruses from a global health perspective. Front. Med. 2020;7 doi: 10.3389/fmed.2020.596587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vegivinti C.T.R., Evanson K.W., Lyons H., Akosman I., Barrett A., Hardy N., Kane B., Keesari P.R., Pulakurthi Y.S., Sheffels E., Balasubramanian P., Chibbar R., Chittajallu S., Cowie K., Karon J., Siegel L., Tarchand R., Zinn C., Gupta N., Kallmes K.M., Saravu K., Touchette J. Efficacy of antiviral therapies for COVID-19: a systematic review of randomized controlled trials. BMC Infect. Dis. 2022;22:107. doi: 10.1186/s12879-022-07068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelleni M.T. Tocilizumab, remdesivir, favipiravir, and dexamethasone repurposed for COVID-19: a comprehensive clinical and pharmacovigilant reassessment. SN Compr. Clin. Med. 2021;3:919–923. doi: 10.1007/s42399-021-00824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joshi S., Parkar J., Ansari A., Vora A., Talwar D., Tiwaskar M., Patil S., Barkate H. Role of favipiravir in the treatment of COVID-19. Int. J. Infect. Dis. 2021;102:501–508. doi: 10.1016/j.ijid.2020.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosaeed M., Alharbi A., Mahmoud E., Alrehily S., Bahlaq M., Gaifer Z., Alturkistani H., Alhagan K., Alshahrani S., Tolbah A., Musattat A., Alanazi M., Jaha R., Sultana K., Alqahtani H., Al Aamer K., Jaser S., Alsaedy A., Ahmad A., Abalkhail M., AlJohani S., Al Jeraisy M., Almaziad S., Albaalharith N., Alabdulkareem K., Alshowair A., Alharbi N.K., Alrabiah F., Alshamrani M., Aldibasi O., Alaskar A. Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial. Clin. Microbiol. Infect. 2022;28:602–608. doi: 10.1016/j.cmi.2021.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scavone C., Mascolo A., Rafaniello C., Sportiello L., Trama U., Zoccoli A., Bernardi F.F., Racagni G., Berrino L., Castaldo G., Coscioni E., Rossi F., Capuano A. Therapeutic strategies to fight COVID-19: Which is the status artis ? Br. J. Pharmacol. 2022;179(10):2128–2148. doi: 10.1111/bph.15452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abd-Elsalam S., Ahmed O.A., Mansour N.O., Abdelaziz D.H., Salama M., Fouad M.H.A., Soliman S., Naguib A.M., Hantera M.S., Ibrahim I.S., Torky M., Dabbous H.M., El Ghafar M.S.A., Abdul-Baki E.A., Elhendawy M. Remdesivir efficacy in COVID-19 treatment: a randomized controlled trial. Am. J. Trop. Med. Hyg. 2021 doi: 10.4269/ajtmh.21-0606. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Sternberg A., McKee D.L., Naujokat C. Novel drugs targeting the SARS-CoV-2/COVID-19 machinery. Curr. Top. Med. Chem. 2020;20:1423–1433. doi: 10.2174/1568026620999200517043137. [DOI] [PubMed] [Google Scholar]
- 39.Lee T.C., McDonald E.G., Butler-Laporte G., Harrison L.B., Cheng M.P., Brophy J.M. Remdesivir and systemic corticosteroids for the treatment of COVID-19: a Bayesian re-analysis. Int. J. Infect. Dis. 2021;104:671–676. doi: 10.1016/j.ijid.2021.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Indari O., Jakhmola S., Manivannan E., Jha H.C. An update on antiviral therapy against SARS-CoV-2: how far have we come? Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.632677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frediansyah A., Nainu F., Dhama K., Mudatsir M., Harapan H. Remdesivir and its antiviral activity against COVID-19: a systematic review. Clin. Epidemiol. Glob. Health. 2021;9:123–127. doi: 10.1016/j.cegh.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buckland M.S., Galloway J.B., Fhogartaigh C.N., Meredith L., Provine N.M., Bloor S., Ogbe A., Zelek W.M., Smielewska A., Yakovleva A., Mann T., Bergamaschi L., Turner L., Mescia F., Toonen E.J.M., Hackstein C.-P., Akther H.D., Vieira V.A., Ceron-Gutierrez L., Periselneris J., Kiani-Alikhan S., Grigoriadou S., Vaghela D., Lear S.E., Török M.Estée., Hamilton W.L., Stockton J., Quick J., Nelson P., Hunter M., Coulter T.I., Devlin L., Bradley J.R., Smith K.G.C., Ouwehand W.H., Estcourt L., Harvala H., Roberts D.J., Wilkinson I.B., Screaton N., Loman N., Doffinger R., Lyons P.A., Morgan B.P., Goodfellow I.G., Klenerman P., Lehner P.J., Matheson N.J., Thaventhiran J.E.D. Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-19761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Copin R., Baum A., Wloga E., Pascal K.E., Giordano S., Fulton B.O., Zhou A., Negron N., Lanza K., Chan N., Coppola A., Chiu J., Ni M., Wei Y., Atwal G.S., Hernandez A.R., Saotome K., Zhou Y., Franklin M.C., Hooper A.T., McCarthy S., Hamon S., Hamilton J.D., Staples H.M., Alfson K., Carrion R., Ali S., Norton T., Somersan-Karakaya S., Sivapalasingam S., Herman G.A., Weinreich D.M., Lipsich L., Stahl N., Murphy A.J., Yancopoulos G.D., Kyratsous C.A. The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies. Cell. 2021;184(15):3949–3961.e11. doi: 10.1016/j.cell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor P.C., Adams A.C., Hufford M.M., de la Torre I., Winthrop K., Gottlieb R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 2021;21:382–393. doi: 10.1038/s41577-021-00542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen H., Salkeld J., Agarwal S., Goodman A. Compassionate use of REGN-COV2 in the treatment of COVID-19 in a patient with impaired humoral immunity. Clin. Infect. Pract. 2021;12 doi: 10.1016/j.clinpr.2021.100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaki H., Miyata N., Yoshimura Y., Tachikawa N. High titer of antibody against the SARS-CoV-2 spike protein among patients receiving neutralizing antibody cocktail therapy with REGN-COV. Infection. 2022;50(3):771–774. doi: 10.1007/s15010-022-01779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarabia De Ardanaz L., Andreu-Ubero J.M., Navidad-Fuentes M., Ferrer-González M.Á., Ruíz del Valle V., Salcedo-Bellido I., Barrios-Rodríguez R., Cáliz-Cáliz R., Requena P. Tocilizumab in COVID-19: factors associated with mortality before and after treatment. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.620187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cortegiani A., Ippolito M., Greco M., Granone V., Protti A., Gregoretti C., Giarratano A., Einav S., Cecconi M. Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology. 2021;27:52–66. doi: 10.1016/j.pulmoe.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernández-Ruiz M., López-Medrano F., Aguado J.M. Tocilizumab for the treatment of COVID-19. Expert Opin. Biol. Ther. 2021;21:431–434. doi: 10.1080/14712598.2021.1880563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samaee H., Mohsenzadegan M., Ala S., Maroufi S.S., Moradimajd P. Tocilizumab for treatment patients with COVID-19: Recommended medication for novel disease. Int. Immunopharmacol. 2020;89 doi: 10.1016/j.intimp.2020.107018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Remdesivir Uses, Side Effects & Warnings - Drugs.com, (n.d.). https://www.drugs.com/mtm/remdesivir.html (accessed November 10, 2021).
- 52.Favipiravir (United States: Not commercially available; refer to Prescribing and Access Restrictions): Drug information – UpToDate, (n.d.). https://www.uptodate.com/contents/favipiravir-united-states-not-commercially-available-refer-to-prescribing-and-access-restrictions-drug-information/print (accessed November 10, 2021).
- 53.Casirivimab and Imdevimab, (n.d.). https://www.regeneron.com/medicines/casirivimab-imdevimab (accessed November 10, 2021).
- 54.Ahmed M.H., Hassan A. Dexamethasone for the Treatment of Coronavirus Disease (COVID-19): a Review. SN Compr. Clin. Med. 2020;2:2637–2646. doi: 10.1007/s42399-020-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dexamethasone | C22H29FO5 - PubChem, (n.d.). https://pubchem.ncbi.nlm.nih.gov/compound/5743 (accessed July 27, 2022).
- 56.Methylprednisolone | C22H30O5 - PubChem, (n.d.). https://pubchem.ncbi.nlm.nih.gov/compound/6741 (accessed July 27, 2022).
- 57.Prednisolone | C21H28O5 - PubChem, (n.d.). https://pubchem.ncbi.nlm.nih.gov/compound/5755 (accessed July 27, 2022).
- 58.Hydrocortisone | C21H30O5 - PubChem, (n.d.). https://pubchem.ncbi.nlm.nih.gov/compound/5754 (accessed July 27, 2022).
- 59.I. Herráiz, Chemical Pathways of Corticosteroids, Industrial Synthesis from Sapogenins, in: 2017: pp. 15–27. doi: 10.1007/978-1-4939-7183-1_2. [DOI] [PubMed]
- 60.R.S. Vardanyan, V.J. Hruby, Corticosteroids, in: Synth. Essent. Drugs, Elsevier, 2006: pp. 349–363. doi: 10.1016/B978-044452166-8/50027-3.
- 61.Woodward R.B., Sondheimer F., Taub D., Heusler K., McLamore W.M. The Total Synthesis of Steroids 1. J. Am. Chem. Soc. 1952;74:4223–4251. doi: 10.1021/ja01137a001. [DOI] [Google Scholar]
- 62.Sarett L.H., Arth G.E., Lukes R.M., Beyler R.E., Poos G.I., Johns W.F., Constantin J.M. Stereospecific total synthesis of cortisone. J. Am. Chem. Soc. 1952;74:4974–4976. doi: 10.1021/ja01139a532. [DOI] [Google Scholar]
- 63.Poos G.I., Arth G.E., Beyler R.E., Sarett L.H. Approaches to the total synthesis of adrenal steroids. 1 V. 4b-Methyl-7- ethylenedioxy-1,2,3,4,4aα,4b,5,6,7,8,10,10a β-dodecahydrophenanthrene-4 β-ol-1-one and related tricyclic derivatives. J. Am. Chem. Soc. 1953;75(2):422–429. [Google Scholar]
- 64.Barkley L.B., Farrar M.W., Knowles W.S., Raffelson H. A synthesis of dl-cortisone acetate. J. Am. Chem. Soc. 1953;75:4110–4111. doi: 10.1021/ja01112a535. [DOI] [Google Scholar]
- 65.Hems B.A. The chemistry of cortisone. J. Pharm. Pharmacol. 2011;5:409–437. doi: 10.1111/j.2042-7158.1953.tb14005.x. [DOI] [PubMed] [Google Scholar]
- 66.Velluz L., Nominé G., Mathieu J. Neuere Ergebnisse bei der Totalsynthese von Steroiden. Angew. Chem. 1960;72:725–730. doi: 10.1002/ange.19600721903. [DOI] [Google Scholar]
- 67.Torgov I.V. Progress in the total synthesis of steroids. Pure Appl. Chem. 1963;6:525–544. doi: 10.1351/pac196306040525. [DOI] [Google Scholar]
- 68.Szczebara F.M., Chandelier C., Villeret C., Masurel A., Bourot S., Duport C., Blanchard S., Groisillier A., Testet E., Costaglioli P., Cauet G., Degryse E., Balbuena D., Winter J., Achstetter T., Spagnoli R., Pompon D., Dumas B. Total biosynthesis of hydrocortisone from a simple carbon source in yeast. Nat. Biotechnol. 2003;21:143–149. doi: 10.1038/nbt775. [DOI] [PubMed] [Google Scholar]
- 69.Synthesis of Dexamethasone, Synfacts. 16 (2020) 1027. doi: 10.1055/s-0040-1705869.
- 70.Adhikari B., Sahu N. COVID-19 into chemical science perspective: chemical preventive measures and drug development. ChemistrySelect. 2021;6:2010–2028. doi: 10.1002/slct.202100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohta T., Zhang H., Torihara Y., Furukawa I. Improved synthetic route to dexamethasone acetate from tigogenin. Org. Process Res. Dev. 1997;1:420–424. doi: 10.1021/op9700338. [DOI] [Google Scholar]
- 72.Hogg J.A., Beal P.F., Nathan A.H., Lincoln F.H., Schneider W.P., Magerlein B.J., Hanze A.R., Jackson R.W. The adrenal hormones and related compounds. I. A “direct” synthesis of hydrocortisone acetate and cortisone acetate from 11α-hydroxyprogesterone. J. Am. Chem. Soc. 1955;77:4436–4438. doi: 10.1021/ja01621a092. [DOI] [Google Scholar]
- 73.Wovcha M.G., Antosz F.J., Knight J.C., Kominek L.A., Pyke T.R. Bioconversion of sitosterol to useful steroidal intermediates by mutants of Mycobacterium fortuitum. Biochim. Biophys. Acta BBA – Lipids Lipid Metab. 1978;531:308–321. doi: 10.1016/0005-2760(78)90213-8. [DOI] [PubMed] [Google Scholar]
- 74.Nitta I., Fujimori S., Ueno H. The syntheses of the corticoid side chain. I. An improved method for the preparation of 17α-hydroxyprogesterone from androst-4-ene-3,17-dione. Bull. Chem. Soc. Jpn. 1985;58:978–980. doi: 10.1246/bcsj.58.978. [DOI] [Google Scholar]
- 75.I. Herráiz, Chemical Pathways of Corticosteroids, Industrial Synthesis from Sapogenins, in: J.-L. Barredo, I. Herráiz (Eds.), Microb. Steroids, Springer New York, New York, NY, 2017: pp. 15–27. doi: 10.1007/978-1-4939-7183-1_2. [DOI] [PubMed]
- 76.Spero G.B., Thompson J.L., Magerlein B.J., Hanze A.R., Murray H.C., Sebek O.K., Hogg J.A. Adrenal hormones and related compounds. iv. 6-methyl steroids 1. J. Am. Chem. Soc. 1956;78:6213–6214. doi: 10.1021/ja01604a078. [DOI] [Google Scholar]
- 77.Spero G.B., Thompson J.L., Lincoln F.H., Schneider W.P., Hogg J.A. Adrenal hormones and related compounds. V. Fluorinated 6-methyl steroids. J. Am. Chem. Soc. 1957;79:1515–1516. doi: 10.1021/ja01563a075. [DOI] [Google Scholar]
- 78.Fried J.H., Arth G.E., Sarett L.H. Alkylated adrenal hormones. The synthesis of 6α-methyl cortical steroids. J. Am. Chem. Soc. 1959;81:1235–1239. doi: 10.1021/ja01514a055. [DOI] [Google Scholar]
- 79.Cain D.W., Cidlowski J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017;17:233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lecoq L., Vincent P., Lavoie-Lamoureux A., Lavoie J.-P. Genomic and non-genomic effects of dexamethasone on equine peripheral blood neutrophils. Vet. Immunol. Immunopathol. 2009;128:126–131. doi: 10.1016/j.vetimm.2008.10.303. [DOI] [PubMed] [Google Scholar]
- 81.Chikanza I.C. Mechanisms of Corticosteroid Resistance in Rheumatoid Arthritis. Ann. N. Y. Acad. Sci. 2002;966:39–48. doi: 10.1111/j.1749-6632.2002.tb04200.x. [DOI] [PubMed] [Google Scholar]
- 82.Picard D., Khursheed B., Garabedian M.J., Fortin M.G., Lindquist S., Yamamoto K.R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- 83.Nissen R.M., Yamamoto K.R. The glucocorticoid receptor inhibits NFκB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 2000;14:2314–2329. doi: 10.1101/gad.827900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luecke H.F., Yamamoto K.R. The glucocorticoid receptor blocks P-TEFb recruitment by NFκB to effect promoter-specific transcriptional repression. Genes Dev. 2005;19:1116–1127. doi: 10.1101/gad.1297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reily M.M., Pantoja C., Hu X., Chinenov Y., Rogatsky I. The GRIP1:IRF3 interaction as a target for glucocorticoid receptor-mediated immunosuppression. EMBO J. 2006;25:108–117. doi: 10.1038/sj.emboj.7600919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gopalaswamy R., Subbian S. Corticosteroids for COVID-19 therapy: potential implications on tuberculosis. Int. J. Mol. Sci. 2021;22:3773. doi: 10.3390/ijms22073773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berrebi D., Bruscoli S., Cohen N., Foussat A., Migliorati G., Bouchet-Delbos L., Maillot M.-C., Portier A., Couderc J., Galanaud P., Peuchmaur M., Riccardi C., Emilie D. Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: an anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10. Blood. 2003;101:729–738. doi: 10.1182/blood-2002-02-0538. [DOI] [PubMed] [Google Scholar]
- 88.Lasa M., Abraham S.M., Boucheron C., Saklatvala J., Clark A.R. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol. Cell. Biol. 2002;22:7802–7811. doi: 10.1128/MCB.22.22.7802-7811.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miyamasu M., Misaki Y., Izumi S., Takaishi T., Morita Y., Nakamura H., Matsushima K., Kasahara T., Hirai K. Glucocorticoids inhibit chemokine generation by human eosinophils. J. Allergy Clin. Immunol. 1998;101:75–83. doi: 10.1016/S0091-6749(98)70196-4. [DOI] [PubMed] [Google Scholar]
- 90.Pype J.L., Dupont L.J., Menten P., Van Coillie E., Opdenakker G., Van Damme J., Fan Chung K., Demedts M.G., Verleden G.M. Expression of monocyte chemotactic protein (MCP)-1, MCP-2, and MCP-3 by human airway smooth-muscle cells: modulation by corticosteroids and T-helper 2 cytokines. Am. J. Respir. Cell Mol. Biol. 1999;21:528–536. doi: 10.1165/ajrcmb.21.4.3660. [DOI] [PubMed] [Google Scholar]
- 91.Cronstein B.N., Kimmel S.C., Levin R.I., Martiniuk F., Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. 1992;89:9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stohlman S.A., Sakaguchi A.Y., Weiner L.P. Rescue of a positive stranded RNA virus from antigen negative neuroblastoma cells. Life Sci. 1979;24:1029–1035. doi: 10.1016/0024-3205(79)90323-0. [DOI] [PubMed] [Google Scholar]
- 93.Heming N., Sivanandamoorthy S., Meng P., Bounab R., Annane D. Immune effects of corticosteroids in sepsis. Front. Immunol. 2018;9:1736. doi: 10.3389/fimmu.2018.01736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bianchi M., Meng C., Ivashkiv L.B. Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids. Proc. Natl. Acad. Sci. 2000;97:9573–9578. doi: 10.1073/pnas.160099797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stary G., Klein I., Bauer W., Koszik F., Reininger B., Kohlhofer S., Gruber K., Skvara H., Jung T., Stingl G. Glucocorticosteroids modify langerhans cells to produce TGF-β and expand regulatory T cells. J. Immunol. 2011;186:103–112. doi: 10.4049/jimmunol.1002485. [DOI] [PubMed] [Google Scholar]
- 96.Mozo L., Suarez A., Gutierrez C. Glucocorticoids up-regulate constitutive interleukin-10 production by human monocytes. Clin. Htmlent Glyphamp Asciiamp Exp. Allergy. 2004;34:406–412. doi: 10.1111/j.1365-2222.2004.01824.x. [DOI] [PubMed] [Google Scholar]
- 97.Ehrchen J.M., Roth J., Barczyk-Kahlert K. More than suppression: glucocorticoid action on monocytes and macrophages. Front. Immunol. 2019;10:2028. doi: 10.3389/fimmu.2019.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eddleston J., Herschbach J., Wagelie-Steffen A.L., Christiansen S.C., Zuraw B.L. The anti-inflammatory effect of glucocorticoids is mediated by glucocorticoid-induced leucine zipper in epithelial cells. J. Allergy Clin. Immunol. 2007;119:115–122. doi: 10.1016/j.jaci.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 99.Zhong J., Tang J., Ye C., Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2:e428–e436. doi: 10.1016/S2665-9913(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Paassen J., Vos J.S., Hoekstra E.M., Neumann K.M.I., Boot P.C., Arbous S.M. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit. Care. 2020;24:696. doi: 10.1186/s13054-020-03400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Corticosteroids for COVID-19, (n.d.). https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 (accessed August 1, 2021).
- 102.Corticosteroids | COVID-19 Treatment Guidelines, (n.d.). https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids/ (accessed November 10, 2021).
- 103.Yu L.-M., Bafadhel M., Dorward J., Hayward G., Saville B.R., Gbinigie O., Van Hecke O., Ogburn E., Evans P.H., Thomas N.P.B., Patel M.G., Richards D., Berry N., Detry M.A., Saunders C., Fitzgerald M., Harris V., Shanyinde M., de Lusignan S., Andersson M.I., Barnes P.J., Russell R.E.K., Nicolau D.V., Ramakrishnan S., Hobbs F.D.R., Butler C.C., Yu L.-M., Bafadhel M., Dorward J., Hayward G., Saville B.R., Gbinigie O., van Hecke O., Ogburn E., Evans P.H., Thomas N.PB., Patel M.G., Richards D., Berry N., Detry M.A., Saunders C.T., Fitzgerald M., Harris V., Shanyinde M., de Lusignan S., Andersson M.I., Barnes P.J., Russell R.EK., Nicolau D.V., Ramakrishnan S., Hobbs FD.R., Butler C.C. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. The Lancet. 2021;398(10303):843–855. doi: 10.1016/S0140-6736(21)01744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramakrishnan S., Nicolau D.V., Langford B., Mahdi M., Jeffers H., Mwasuku C., Krassowska K., Fox R., Binnian I., Glover V., Bright S., Butler C., Cane J.L., Halner A., Matthews P.C., Donnelly L.E., Simpson J.L., Baker J.R., Fadai N.T., Peterson S., Bengtsson T., Barnes P.J., Russell R.E.K., Bafadhel M. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir. Med. 2021;9(7):763–772. doi: 10.1016/S2213-2600(21)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schultze A., Douglas I. COVID-19 and inhaled corticosteroids—another piece in an expanding puzzle. Lancet Respir. Med. 2021;9:674–675. doi: 10.1016/S2213-2600(21)00076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nicolau D.V., Bafadhel M. Inhaled corticosteroids in virus pandemics: a treatment for COVID-19? Lancet Respir. Med. 2020;8:846–847. doi: 10.1016/S2213-2600(20)30314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dexamethasone for COVID-19 Related ARDS: a Multicenter, Randomized Clinical Trial – Full Text View – ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04395105 (accessed July 27, 2022).
- 108.COVID-19-associated ARDS Treated With Dexamethasone: Alliance Covid-19 Brasil III – Full Text View – ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04327401 (accessed July 27, 2022).
- 109.Dexamethasone and Oxygen Support Strategies in ICU Patients With Covid-19 Pneumonia – Full Text View – ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04344730 (accessed July 27, 2022).
- 110.Dexamethasone for COVID-19 - Full Text View - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04707534 (accessed July 27, 2022).
- 111.Comparison Between Prednisolone and Dexamethasone on Mortality in Patients on Oxygen Therapy, With CoViD-19 – Full Text View – ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04765371 (accessed July 27, 2022).
- 112.Impact of Steroids on Inflammatory Response in Covid-19 – Full Text View – ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04909918 (accessed July 27, 2022).
- 113.The Efficacy of Different Hormone Doses in 2019-nCoV Severe Pneumonia – Full Text View – ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04263402 (accessed July 27, 2022).
- 114.Investigating the efficacy of high dose of glucocorticoid in patients with moderate to severe pneumonia related to COVID-19 | IRCT; 2020-05-05; TrialID: IRCT20080901001165N52 | ICTRP, (n.d.). https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/ictrp-IRCT20080901001165N52 (accessed July 27, 2022).
- 115.IRCT | Evaluation of Methylprednisolone Administration as a Therapeutic Option in the Coronavirus disease 2019 (COVID-19): A Randomized Controlled Study, (n.d.). https://www.irct.ir/trial/46776 (accessed July 27, 2022).
- 116.Methylprednisolone in the Treatment of Patients With Signs of Severe Acute Respiratory Syndrome in Covid-19 - Full Text View - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04343729 (accessed July 27, 2022).
- 117.Glucocorticoids in COVID-19 (CORTIVID) – Full Text View – ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04438980 (accessed July 27, 2022).
- 118.Hydrocortisone for COVID-19 and Severe Hypoxia – Full Text View – ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04348305 (accessed July 27, 2022).
- 119.Efficacy and Safety of Corticosteroids in Oxygen-dependent Patients With COVID-19 Pneumonia – Full Text View – ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04359511 (accessed July 27, 2022).
- 120.Inhaled Corticosteroid Treatment of COVID19 Patients With Pneumonia – Full Text View – ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04355637 (accessed July 27, 2022).
- 121.Inhaled Ciclesonide for Outpatients With COVID19 – Full Text View – ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04435795 (accessed July 27, 2022).
- 122.Mandadi S., Pulluru H., Annie F., Bhatt G.C. Comparative outcomes of combined corticosteroid and remdesivir therapy with corticosteroid monotherapy in ventilated COVID-19 patients. PLOS ONE. 2022;17(2):e0264301. doi: 10.1371/journal.pone.0264301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Y. Shionoya, T. Taniguchi, H. Kasai, N. Sakuma, S. Imai, K. Shikano, S. Takayanagi, M. Yahaba, T. Nakada, H. Igari, S. Sakao, T. Suzuki, Possibility of deterioration of respiratory status when steroids precede antiviral drugs in patients with COVID-19 pneumonia: a retrospective study, PLOS ONE. 16 (2021) e0256977. doi: 10.1371/journal.pone.0256977. [DOI] [PMC free article] [PubMed]
- 124.Ooi S.T., Parthasarathy P., Lin Y., Nallakaruppan V.D., Ng S., Tan T.C., Low S., Tang T. Antivirals with adjunctive corticosteroids prevent clinical progression of early coronavirus 2019 pneumonia: a retrospective cohort study. Open Forum Infect. Dis. 2020;7:ofaa486. doi: 10.1093/ofid/ofaa486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Inoue H., Jinno M., Ohta S., Kishino Y., Kawahara T., Mikuni H., Sato H., Yamamoto M., Sato Y., Onitsuka C., Goto Y., Ikeda H., Sato H., Uno T., Uchida Y., Kimura T., Miyata Y., Hirai K., Homma T., Watanabe Y., Kusumoto S., Suzuki S., Tokimatsu I., Tanaka A., Sagara H. Combination treatment of short-course systemic corticosteroid and favipiravir in a successfully treated case of critically ill COVID-19 pneumonia with COPD. Respir. Med. Case Rep. 2020;31 doi: 10.1016/j.rmcr.2020.101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hospitalized Adults: Therapeutic Management | COVID-19 Treatment Guidelines, (n.d.). https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management/ (accessed November 10, 2021).
- 127.Moosazadeh M., Mousavi T. Combination therapy of tocilizumab and steroid for COVID-19 patients: a meta-analysis. J. Med. Virol. 2022;94:1350–1356. doi: 10.1002/jmv.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ivermectin, Aspirin, Dexamethasone and Enoxaparin as Treatment of Covid 19 - Full Text View - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04425863 (accessed November 10, 2021).
- 129.Duvignaud A., Lhomme E., Onaisi R., Sitta R., Gelley A., Chastang J., Piroth L., Binquet C., Dupouy J., Makinson A., Lefèvre B., Naccache J.-M., Roussillon C., Landman R., Wallet C., Karcher S., Journot V., Nguyen D., Pistone T., Bouchet S., Lafon M.-E., Molimard M., Thiébaut R., de Lamballerie X., Joseph J.-P., Richert L., Saint-Lary O., Djabarouti S., Wittkop L., Anglaret X., Malvy D. Inhaled ciclesonide for outpatient treatment of COVID-19 in adults at risk of adverse outcomes: a randomised controlled trial (COVERAGE) Clin. Microbiol. Infect. 2022;28:1010–1016. doi: 10.1016/j.cmi.2022.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Abd El Hafez M.S.M., AbdEl-Wahab M.G., Seadawy M.G., El-Hosseny M.F., Beskales O., Saber Ali Abdel-Hamid A., El Demellawy M.A., Ghareeb D.A. Characterization, in-silico, and in-vitro study of a new steroid derivative from Ophiocoma dentata as a potential treatment for COVID-19. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-09809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coronavirus disease (COVID-19), (n.d.). https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=CjwKCAiA1aiMBhAUEiwACw25MWtzNki49mrpkfAg-tlmOgcimhu9z5xbyFk3kkAu1qwhObpKDjbwQhoCbyQQAvD_BwE (accessed November 10, 2021).
- 132.Therapeutics and COVID-19: living guideline, (n.d.). https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.2 (accessed August 1, 2021).