Abstract
Lipoxins are important drivers of inflammation resolution, suggesting a potential therapeutic benefit. Bicyclo[1.1.1]pentanes (BCPs) are potential isosteric replacements for arenes and/or alkyl groups within drug candidates. We carried out an asymmetric synthesis of four BCP-containing synthetic lipoxin A4 mimetics (BCP-sLXms) in which the key steps were a Suzuki coupling, an asymmetric ketone reduction, and a triethylborane-initiated radical bicyclopentylation. These mimetics were screened for their impact on inflammatory responses, and one imidazolo-BCP-sLXm (6a) was found to possess high anti-inflammatory activity.
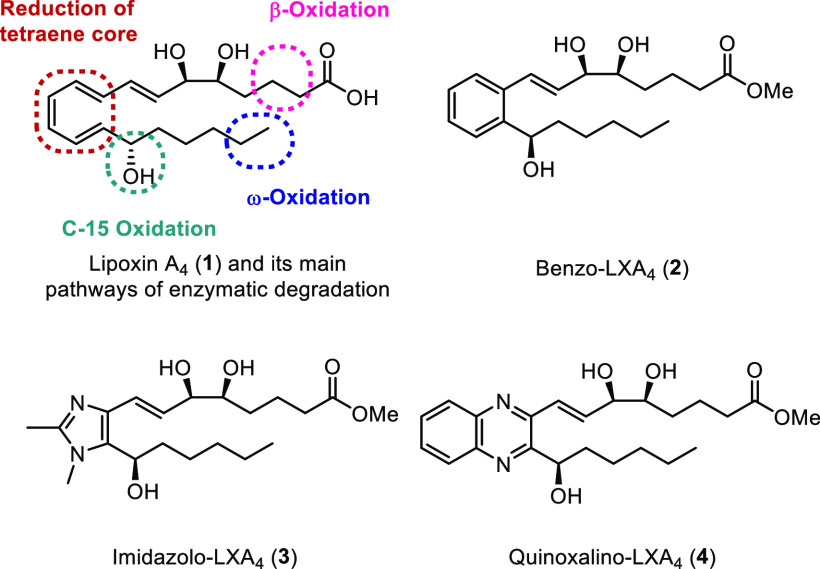
Inflammation is a critical response to infection and injury, and it is vital that the amplitude and duration of the inflammatory response be controlled in space and time. The precise regulation of the onset, duration, and resolution of inflammation reflects responses to distinct signaling molecules produced at specific times.1 Dysregulation of these processes underpins the pathology of numerous prevalent diseases.2 Lipoxins (LXs) make up a class of endogenously generated eicosanoids typically generated through transcellular metabolism at a site of inflammation.3 The generation of lipoxins marks the initiation of the resolution phase of inflammation. Additional lipid mediators have been identified, which promote the resolution of inflammation, and these are collectively described as specialized pro-resolving mediators (SPMs), which typically act on specific G protein-coupled receptors, including FPR2.4 LXA4 (1) and its aspirin-triggered C-15 epimer, AT-LXA4, have been shown to activate the FPR2 receptor and inhibit the recruitment of polymorphonuclear neutrophils (PMNs) to the site of inflammation, while promoting recruitment of monocytes and stimulating the nonphlogistic phagocytosis (efferocytosis) of apoptotic PMNs.5
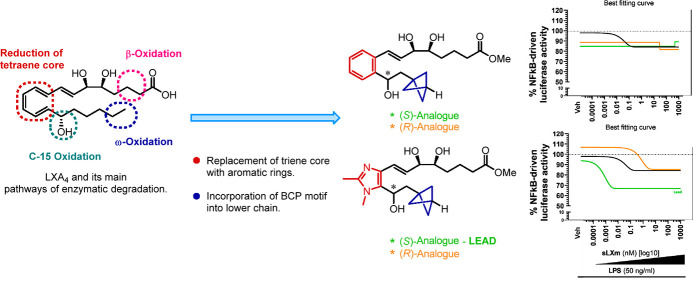
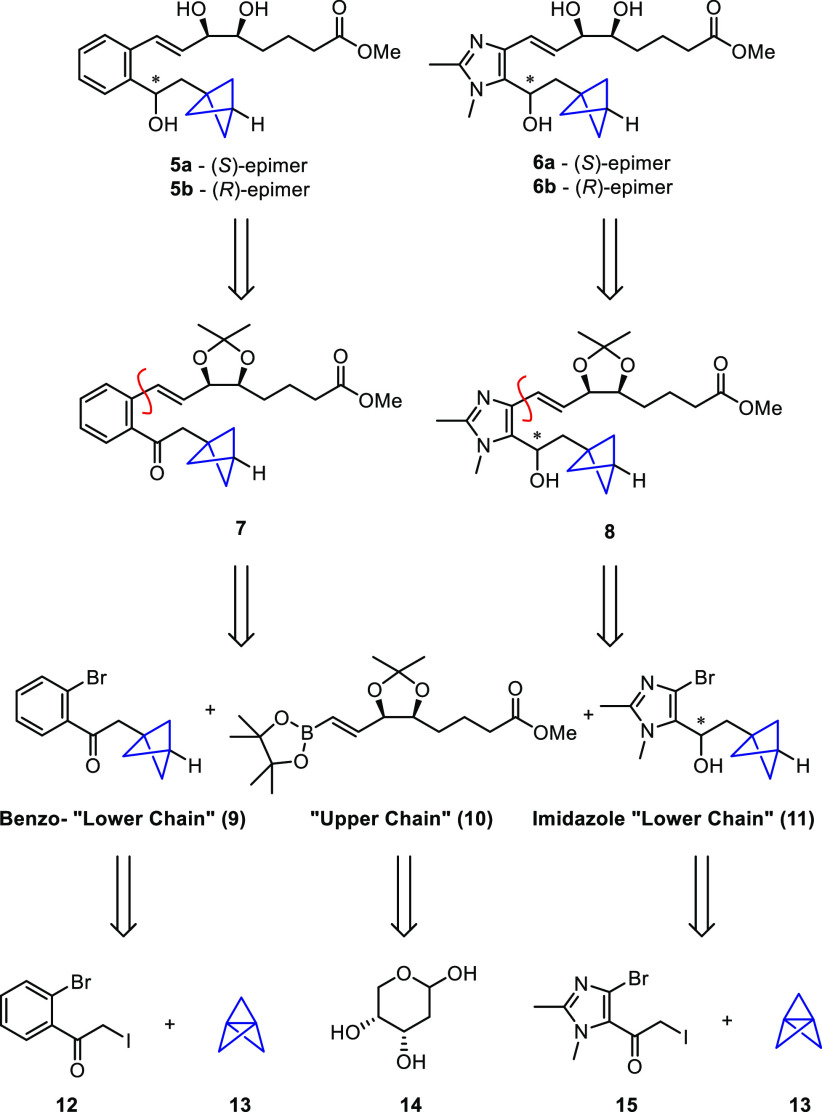
Although their potent anti-inflammatory properties have been well documented, their chemical and metabolic instability decreases the therapeutic exploitation of these actions. Metabolic instability is characterized by oxidation of the alcohol at C-15, reduction of the double bond between C-13 and C-14, and ω-oxidation at C-20 by P450 enzymes (Figure 1). There has been much interest in designing stable synthetic lipoxin mimetics,6 and we have previously described the synthesis of LXA4 mimetic 2 in which the triene of native LXA4 was replaced by a benzene ring.7 Since then, we have also described the synthesis and biological evaluation of a number of heteroaromatic LXA4 analogues containing different five- and six-membered heterocycles in place of the triene core. These have included pyridine, oxazole, imidazole (3), and quinoxaline (4) analogues that have shown favorable anti-inflammatory properties comparable or superior to those of native LXA4 (Figure 1).8−10 As part of our ongoing structure–activity relationship (SAR) studies, we sought to explore the effect of incorporating a bicyclo[1.1.1]pentane (BCP) moiety into the lower C-16–C-20 alkyl chain of our (hetero)aromatic LXA4 mimetics. In recent years, there has been significant interest in BCPs as sp3-rich surrogates within potentially bioactive molecules for para-substituted arenes as well as for tert-butyl groups and alkynes.11−14 We wanted to determine whether a BCP ring could also serve as a more rigid and metabolically resistant bioisostere for alkyl chains in fatty acid-derived molecules. Previous studies have shown that the incorporation of a phenoxy or p-fluorophenoxy substituent into the lower alkyl chain of LXA4 as a way of blocking ω-oxidation has beneficial effects on the compound’s metabolic stability and anti-inflammatory properties.15 A more recent study by Ishimura also demonstrated the potential benefits of incorporating small aliphatic rings into fatty acid derivatives as a way of increasing conformational rigidity.16 With this in mind, we selected four target BCP-containing analogues to be synthesized. These were benzo analogue 5a and imidazolo analogue 6a, as well as their C-15 epimers 5b and 6b, respectively, which were chosen so that their anti-inflammatory properties could be readily compared with those of native LXA4 as well as the current lead compound of our ongoing SAR studies, imidazole 3 and quinoxaline 4. Our retrosynthetic analysis (Scheme 1) proposes that all of the analogues could be synthesized via a Suzuki coupling between boronic ester “upper chain” 10 and a BCP-containing “lower chain” (9 or 11), followed by a stereoselective ketone reduction and acetonide deprotection.
Figure 1.
Lipoxin A4 (1) and examples of aromatic synthetic LXA4 mimetics (2–4).
Scheme 1. Retrosynthetic Analysis of Target BCP-Containing Aromatic LXA4 Mimetics 5 and 6.
Inspired by recent work reported by Anderson,17−19 we believed the key BCP moiety could be readily installed via a triethylborane-initiated atom transfer radical addition (ATRA) reaction between α-iodoketone 12 or 15 and [1.1.1]propellane (13).
The synthesis of boronic ester 10 was recently reported by us for the synthesis of our quinoxaline LXA4 analogues.10 The modular nature of our retrosynthetic strategy means that the same coupling partner can easily be used in a number of different Suzuki reactions to produce a wide array of different heteroaromatic LXA4 mimetics, including the target BCP-containing analogues. With boronic ester 10 in hand, we turned our attention to the synthesis of the BCP-containing lower chain unit 9 (Scheme 2). Iodoketone 12 was prepared from 2′-bromoacetophenone (16) via an α-bromination/Finkelstein sequence and then used as a substrate for the radical bicyclopentylation. Pleasingly, upon reaction with a solution of [1.1.1]propellane (13) in the presence of substoichiometric BEt3, complete conversion to iodo-BCP 17 was observed. However, the subsequent deiodination reaction, which was carried out immediately after the bicyclopentylation, proved to be somewhat problematic. To our surprise, tributyltin hydride in the presence of BEt3 resulted in no reaction, whereas switching the hydrogen atom source to tris(trimethylsilyl)silane (TTMSS) resulted in the successful formation of the desired ketone 9, albeit alongside a complex mixture of side products. Following column chromatography, ketone 9 was obtained as an inseparable mixture with debrominated product 18 in an approximately 2.5:1 ratio as determined by 1H NMR spectroscopic analysis. On the basis of this ratio, the yield of 9 was calculated to be approximately 28% over two steps. Despite the somewhat disappointing yield, the mixture of 9 and 18 was relatively easy to obtain and could be carried forward to the subsequent Suzuki coupling without any further attempts at purification. The desired coupled product 7 was obtained in 62% yield following a microwave-assisted reaction with 10 in the presence of Pd(PPh3)4 and aqueous K2CO3, after which the unreactive impurity 18 was readily removed via column chromatography. From this common intermediate, both C-15 epimers, 19a and 19b, were selectively formed via an asymmetric reduction of the ketone using either enantiomer of DIP chloride. (−)-DIP chloride afforded S-epimer 19a in 71% yield and a de of 98%, and (+)-DIP-chloride afforded R-epimer 19b in 51% yield and a de of 96%. Finally, the acetonide deprotection of both compounds was successfully carried out using camphorsulfonic acid (CSA), and target BCP-containing LXA4 analogues 5a and 5b were obtained in 45% and 24% yields, respectively.
Scheme 2. Asymmetric Synthesis of BCP-Containing Benzo-LXA4 Mimetics 5a and 5b.
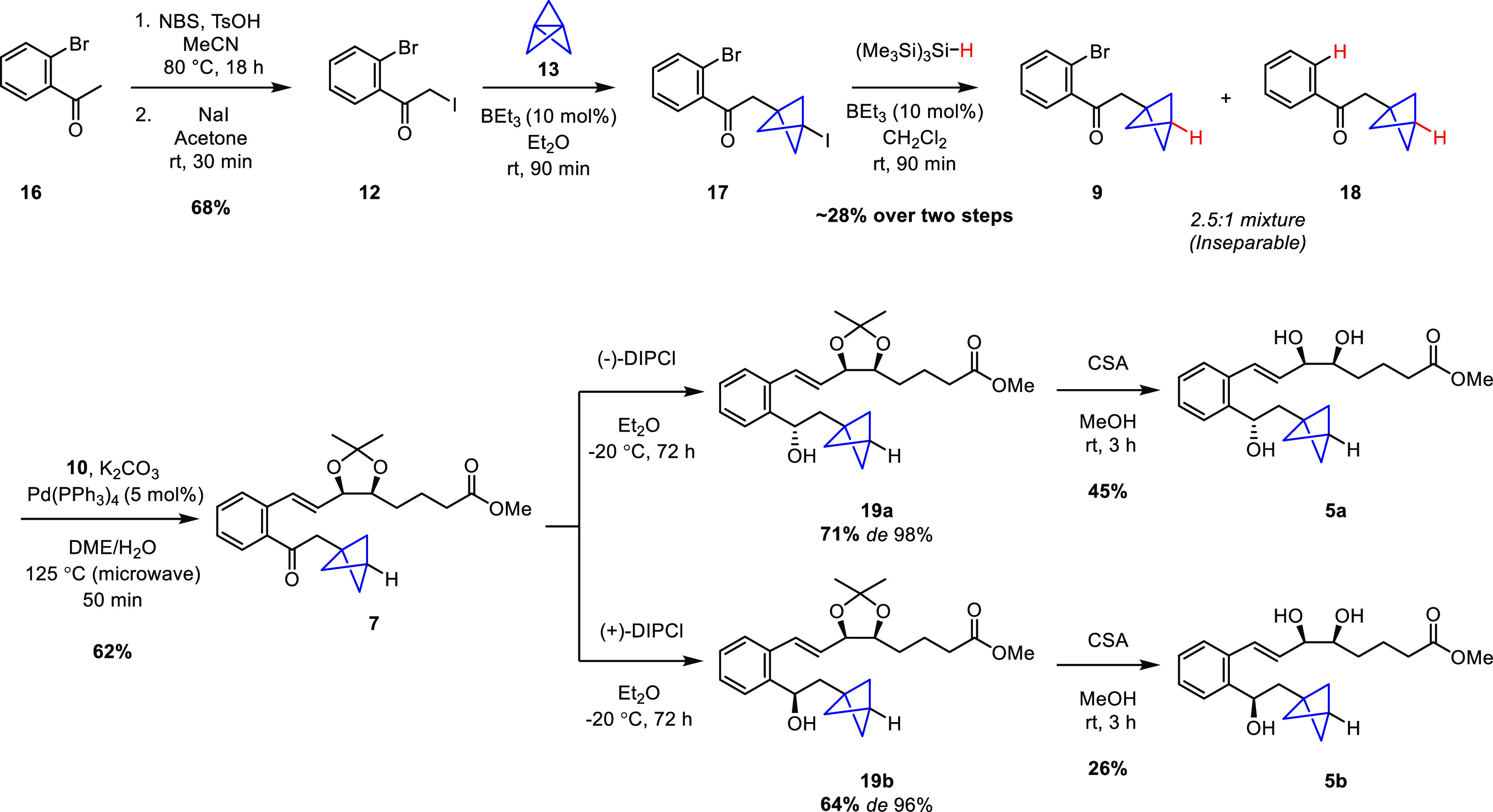
A very similar approach was used to synthesize the imidazole lower chain 11 (Scheme 3). 1,2-Dimethylimidazole (20) was first dibrominated with N-bromosuccinimide and then selectively acetylated using n-butyllithium and acetyl chloride to form methyl ketone 22. Once again, an α-bromination/Finkelstein sequence converted 22 to iodoketone 15, and a BEt3-initiated ATRA reaction with 13 followed by a TTMSS-mediated deiodination led to the formation of the desired BCP-containing ketone 24, which was successfully isolated in a somewhat low yield of 34% over two steps. On the basis of the synthesis of our previously reported imidazolo-LXA4 mimetics,9 we decided to carry out the asymmetric reduction of 24 before the Suzuki coupling by carrying out an asymmetric hydrogenation in the presence of Noyori’s catalyst, RuCl2[DM-BINAP][DAIPEN], under 20 bar of hydrogen gas. By using either enantiomer of the ruthenium catalyst, both enantiomers of 11 could be obtained in high enantiomeric excess following recrystallization from chloroform by vapor diffusion of pentane. The (R,R)-Ru catalyst afforded (S)-11 in 98% ee, while the (S,S)-Ru catalyst afforded (R)-11 in 99% ee. In each case, the absolute configuration was confirmed by X-ray crystallography.
Scheme 3. Asymmetric Synthesis of BCP-Containing Imidazole Coupling Partners (S)-11 and (R)-11.
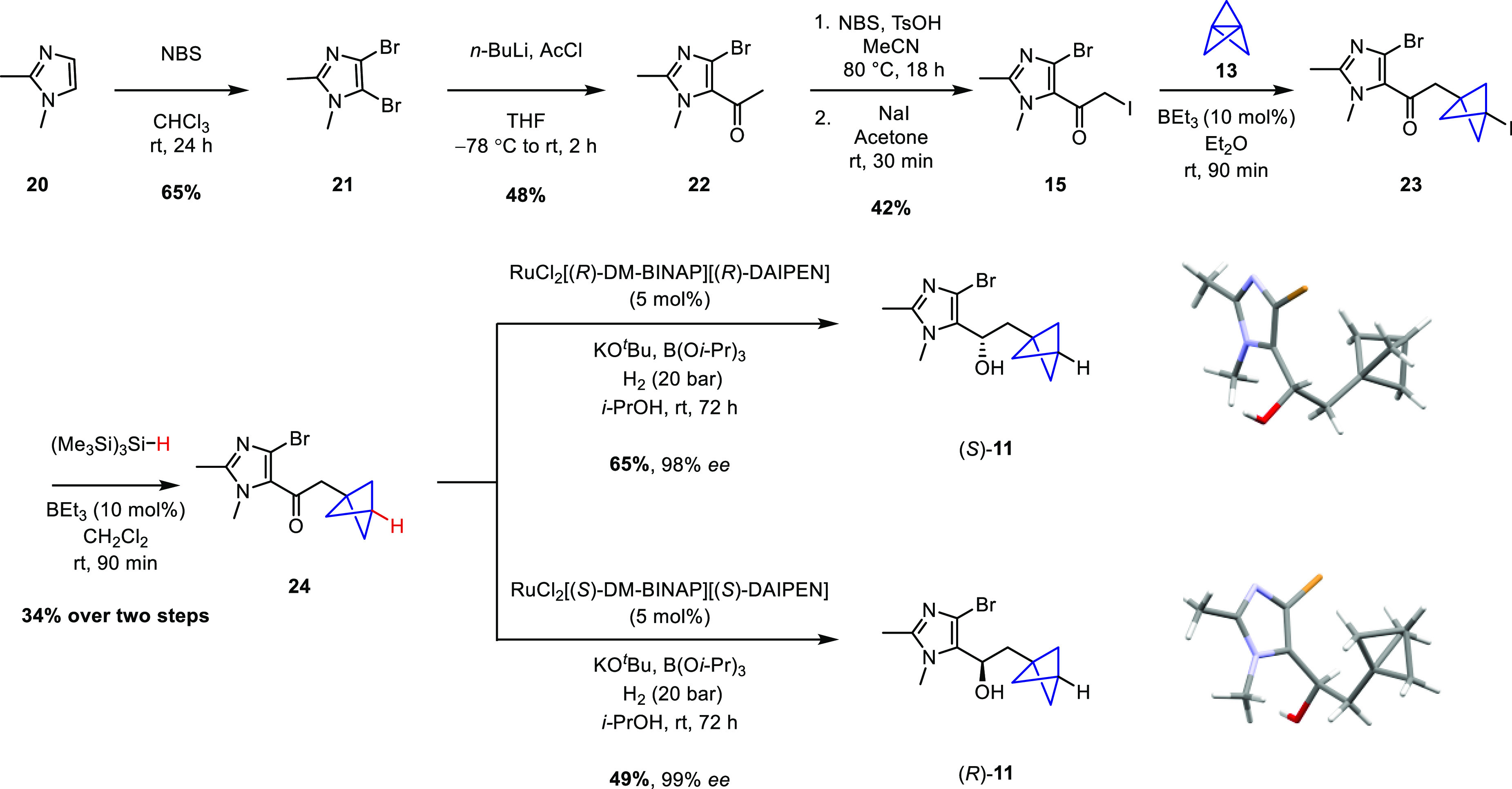
The Suzuki coupling between 10 and 11 was initially attempted using the same microwave-assisted conditions that were used to form 7, but no reaction was observed. However, after changing the catalyst to PdCl2(dppf) and refluxing the reaction mixture in toluene for 18 h, we obtained the desired coupled products 8a and 8b in 73% and 66% yields, respectively. Finally, the acetonide deprotection of both compounds was successfully carried out using ZrCl4, and two more target BCP-containing LXA4 analogues, 6a and 6b, were isolated in 71% and 77% yields, respectively (Scheme 4).
Scheme 4. Synthesis of BCP-Containing Imidazolo-LXA4 Mimetics 6a and 6b.
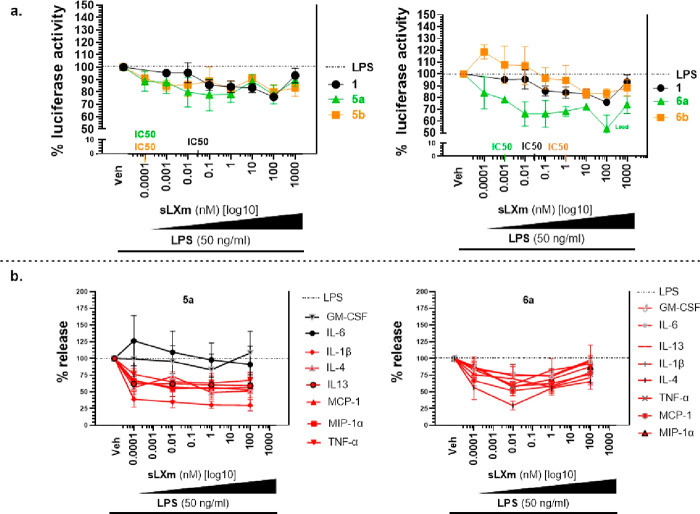
The four BCP-sLXms [derivatives of benzo-LXA47 (5a and 5b) or imidazolo-LXA4 (6a and 6b)] were screened for their impact on inflammatory responses, by measuring in vitro NFκB activity and the downstream release of pro-inflammatory cytokines from human monocyte cell lines stably transfected for a NFκB-driven luciferase reporter (THP-1-Lucia) (Figure 2a). Concentration–response studies (ranging from 1 fM to 1 mM) showed that BCP-sLXm 6a was the most efficacious and potent (IC50 in the picomolar range) anti-inflammatory compound, significantly attenuating lipopolysaccharide (LPS)-induced NFκB activity in monocytes by ∼50% and downregulating the LPS-triggered release of a series of pro-inflammatory cytokines [TNFα, MCP1, and MIP1α (see Table S15)].
Figure 2.
Effect of BCP-sLXms on (a) LPS-induced NFκB-driven luciferase activity in monocytes and (b) pro-inflammatory cytokine release.
In this study, the asymmetric synthesis of four novel BCP-containing sLXm analogues was successfully carried out via a modular approach relying on a Suzuki cross-coupling between a common “upper chain” and different BCP-containing “lower chains”. The data from biological evaluation clearly demonstrate the therapeutic potential of BCP-sLXms as novel inflammatory regulators.
Acknowledgments
B.O. is grateful for the award of an Irish Research Council Enterprise Partnership Scheme Ph.D. Scholarship (EPSPG/2019/529) with Enterprise Partner SK Biotek Ireland. The authors thank Dr. Helge Müller-Bunz (University College Dublin) for X-ray crystal structure analysis, Dr. Yannick Ortin (University College Dublin) for help with NMR spectroscopy, and Dr. Jimmy Muldoon (University College Dublin) for mass spectrometric analysis [supported by a Science Foundation Ireland Infrastructure Award (18/RI/5702)]. M.d.G. was supported by an Irish Research Council (IRC) Government of Ireland Postdoctoral Fellowship (GOIPD/2017/1060) and also received funding from Science Foundation Ireland [PI Award to P.J.G. (11/PI/1206) and 15/IA/3152].
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c02345.
Experimental procedures, characterization data, and spectral data of all compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Cicchese J. M.; Evans S.; Hult C.; Joslyn L. R.; Wessler T.; Millar J. A.; Marino S.; Cilfone N. A.; Mattila J. T.; Linderman J. J.; Kirschner D. E. Dynamic Balance of Pro- and Anti-Inflammatory Signals Controls Disease and Limits Pathology. Immunol. Rev. 2018, 285 (1), 147–167. 10.1111/imr.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T.; Gilroy D. W. Chronic Inflammation: A Failure of Resolution?. Int. J. Exp. Pathol. 2007, 88 (2), 85–94. 10.1111/j.1365-2613.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara S. J.; Rodgers K.; Godson C. Lipoxins: Update and Impact of Endogenous pro-Resolution Lipid Mediators. Rev. Physiol., Biochem. Pharmacol. 2008, 47–70. 10.1007/112_2006_0606. [DOI] [PubMed] [Google Scholar]
- Corminboeuf O.; Leroy X. FPR2/ALXR Agonists and the Resolution of Inflammation. J. Med. Chem. 2015, 58 (2), 537–559. 10.1021/jm501051x. [DOI] [PubMed] [Google Scholar]
- Maderna P.; Cottell D. C.; Toivonen T.; Dufton N.; Dalli J.; Perretti M.; Godson C. FPR2/ALX Receptor Expression and Internalization Are Critical for Lipoxin A4 and Annexin-Derived Peptide-Stimulated Phagocytosis. FASEB J. 2010, 24 (11), 4240–4249. 10.1096/fj.10-159913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy C. D.; Guiry P. J. Recent Advances in the Chemistry and Biology of Stable Synthetic Lipoxin Analogues. MedChemComm 2010, 1 (4), 249. 10.1039/c0md00136h. [DOI] [Google Scholar]
- O’Sullivan T. P.; Vallin K. S. A.; Ali Shah S. T.; Fakhry J.; Maderna P.; Scannell M.; Sampaio A. L. F.; Perretti M.; Godson C.; Guiry P. J. Aromatic Lipoxin A 4 and Lipoxin B 4 Analogues Display Potent Biological Activities. J. Med. Chem. 2007, 50 (24), 5894–5902. 10.1021/jm060270d. [DOI] [PubMed] [Google Scholar]
- Duffy C. D.; Maderna P.; McCarthy C.; Loscher C. E.; Godson C.; Guiry P. J. Synthesis and Biological Evaluation of Pyridine-Containing Lipoxin A4 Analogues. ChemMedChem. 2010, 5 (4), 517–522. 10.1002/cmdc.200900533. [DOI] [PubMed] [Google Scholar]
- de Gaetano M.; Butler E.; Gahan K.; Zanetti A.; Marai M.; Chen J.; Cacace A.; Hams E.; Maingot C.; McLoughlin A.; Brennan E.; Leroy X.; Loscher C. E.; Fallon P.; Perretti M.; Godson C.; Guiry P. J. Asymmetric Synthesis and Biological Evaluation of Imidazole- and Oxazole-Containing Synthetic Lipoxin A4Mimetics (SLXms). Eur. J. Med. Chem. 2019, 162, 80–108. 10.1016/j.ejmech.2018.10.049. [DOI] [PubMed] [Google Scholar]
- de Gaetano M.; Tighe C.; Gahan K.; Zanetti A.; Chen J.; Newson J.; Cacace A.; Marai M.; Gaffney A.; Brennan E.; Kantharidis P.; Cooper M. E.; Leroy X.; Perretti M.; Gilroy D.; Godson C.; Guiry P. J. Asymmetric Synthesis and Biological Screening of Quinoxaline-Containing Synthetic Lipoxin A4Mimetics (QNX-SLXms). J. Med. Chem. 2021, 64 (13), 9193–9216. 10.1021/acs.jmedchem.1c00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykhailiuk P. K. Saturated Bioisosteres of Benzene: Where to Go Next?. Org. Biomol. Chem. 2019, 17 (11), 2839–2849. 10.1039/C8OB02812E. [DOI] [PubMed] [Google Scholar]
- Locke G. M.; Bernhard S. S. R.; Senge M. O. Nonconjugated Hydrocarbons as Rigid-Linear Motifs: Isosteres for Material Sciences and Bioorganic and Medicinal Chemistry. Chem. - Eur. J. 2019, 25 (18), 4590–4647. 10.1002/chem.201804225. [DOI] [PubMed] [Google Scholar]
- Measom N. D.; Down K. D.; Hirst D. J.; Jamieson C.; Manas E. S.; Patel V. K.; Somers D. O. Investigation of a Bicyclo[1.1.1]Pentane as a Phenyl Replacement within an LpPLA2 Inhibitor. ACS Med. Chem. Lett. 2017, 8 (1), 43–48. 10.1021/acsmedchemlett.6b00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov I. S.; Brocklehurst C. E.; Karaghiosoff K.; Koch G.; Knochel P. Synthesis of Bicyclo[1.1.1]Pentane Bioisosteres of Internal Alkynes and Para-Disubstituted Benzenes from [1.1.1]Propellane. Angew. Chem., Int. Ed. 2017, 56 (41), 12774–12777. 10.1002/anie.201706799. [DOI] [PubMed] [Google Scholar]
- Leonard M. O.; Hannan K.; Burne M. J.; Lappin D. W. P.; Doran P.; Coleman P.; Stenson C.; Taylor C. T.; Daniels F.; Godson C.; Petasis N. A.; Rabb H.; Brady H. R. 15-Epi-16-(Para-Fluorophenoxy)-Lipoxin A4-Methyl Ester, a Synthetic Analogue of 15-Epi-Lipoxin A4, Is Protective in Experimental Ischemic Acute Renal Failure. J. Am. Soc. Nephrol. 2002, 13 (6), 1657–1662. 10.1097/01.ASN.0000015795.74094.91. [DOI] [PubMed] [Google Scholar]
- Ishimura K.; Fukuda H.; Fujiwara K.; Muromoto R.; Hirashima K.; Murakami Y.; Watanabe M.; Ishihara J.; Matsuda T.; Shuto S. Synthesis of Resolvin E1 and Its Conformationally Restricted Cyclopropane Congeners with Potent Anti-Inflammatory Effect. ACS Med. Chem. Lett. 2021, 12 (2), 256–261. 10.1021/acsmedchemlett.0c00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo D. F. J.; Arroniz C.; Dürr A. B.; Mousseau J. J.; Stepan A. F.; Mansfield S. J.; Anderson E. A. Synthesis and Applications of Highly Functionalized 1-Halo-3-Substituted Bicyclo[1.1.1]Pentanes. Chem. Sci. 2018, 9 (23), 5295–5300. 10.1039/C8SC01355A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent J.; Arroniz C.; Shire B. R.; Sterling A. J.; Pickford H. D.; Wong M. L. J.; Mansfield S. J.; Caputo D. F. J.; Owen B.; Mousseau J. J.; Duarte F.; Anderson E. A. A General Route to Bicyclo[1.1.1]Pentanes through Photoredox Catalysis. ACS Catal. 2019, 9 (10), 9568–9574. 10.1021/acscatal.9b03190. [DOI] [Google Scholar]
- Pickford H. D.; Nugent J.; Owen B.; Mousseau J. J.; Smith R. C.; Anderson E. A. Twofold Radical-Based Synthesis of N,C-Difunctionalized Bicyclo[1.1.1]Pentanes. J. Am. Chem. Soc. 2021, 143, 9729–9736. 10.1021/jacs.1c04180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.