Objectives
This is a protocol for a Cochrane Review (intervention). The objectives are as follows:
To assess the effectiveness of school feeding programs for improving the physical and psychological health of children experiencing socioeconomic disadvantage across the world.
To compare the effectiveness of school feeding programs for children experiencing socioeconomic disadvantage and those experiencing socioeconomic advantage. This includes subgroups by income and gender.
To understand the process by which school feeding programs achieve (or fail to achieve) an impact on growth, cognitive development, and school performance.
To gather data that are useful for cost‐benefit analysis.
Background
Description of the condition
Although the world has made great advances in improved access to food and health care, good health is still unevenly and unfairly distributed according to socioeconomic position. Health and longevity are highest for the richest and decrease steadily with decreasing income (Baretto 2017; Wilkins 1983; Wilkinson 1996). These inequities are evident both within and between countries (Baretto 2017). Social gradients in health, or socioeconomic inequalities in health, are pervasive in all countries of the world (Diderichsen 2001) and are evident in most diseases, injuries, and health behaviours (Marchand 1998). Health inequalities have been defined as "the virtually universal phenomenon of variation in health indicators ... associated with socioeconomic status" (Last 1995). Inequalities may also be seen between different sexes or geographic groups. Health inequities "are unfair and remediable inequalities" (Peter 2001; Tan‐Torres Edejer 2001).
Socioeconomic differences in nutrition may be one of the most important factors causing disparities in health and mortality (Gwatkin 2007). In 2018, over 678 million people across the world were undernourished; many were children, and most lived in low‐ and middle‐income countries (LMIC; FAO/IFAD/UNICEF/WFP/WHO 2020). For example, over 250 million people in Africa were chronically undernourished in 2019 (World Vision 2021). Even in the USA, more than 2.7 million households with children experienced food insecurity in 2017 (USDA 2019). Overall, the incidence of malnutrition or micronutrient deficiencies (or both) is a health concern especially impacting LMIC.
Undernutrition and micronutrient deficiencies can adversely affect physical, mental, and social aspects of child health and development (Drake 2020; Kristjansson 2007). Effects on physical health may include being underweight, stunted growth, lowered immunity, and mortality. Early malnutrition or micronutrient deficiencies (or both) have been linked to poorer cognitive functioning (DiGirolamo, 2020; Mattei 2019). Overnight and morning fasting (e.g. skipping breakfast) can adversely affect performance on cognitive tasks, particularly for children who are nutritionally at risk (Pollitt 1995). Short‐term hunger also impacts classroom behaviour, as malnutrition can affect attention and interest, thus impairing knowledge retention (Afridi 2019; Levinger 1996; Read 1973). Moreover, extreme poverty and hunger often keep children, especially girls, out of school. "One of the reasons for this lag in progress towards universal primary education is the persistence of poverty, hunger, and malnutrition" (Jomaa 2011). Because these factors impair physical and cognitive development, food insecurity threatens the adequate development of children and youth who experience socioeconomic disadvantage around the world.
Description of the intervention
School feeding programs have been a longstanding global public health standard, focused on improving health and education outcomes for students experiencing socioeconomic disadvantage. These school feeding/meal programs exist in lower and middle‐income countries (LMIC), upper‐middle‐income, and high‐income countries (Wang 2020; WFP 2019. The goals of school feeding programs can include relieving short‐term hunger (Drake 2017; Drake 2020) and improving micronutrient status (Drake 2017), growth (Drake 2017), cognition (Cohen 2021), and academic performance (Anderson 2018; Cohen 2021) in both higher‐ and lower‐income countries. In LMIC, school feeding also aims to increase demand for schooling, enrolment, and attendance (Rutledge 2016).
School feeding programs involve full meals and/or snacks (including milk) for participating children aged 5 to 19 years at school; take‐home rations may also be provided as an additional initiative for families (Wang 2020). Take‐home rations can take the form of food provided to students to take home to their families after school hours or involve cash‐based transfers. Both forms reduce food‐related stresses on students and their families, contribute to a productive at‐home learning environment, and encourage student attendance (WFP 2020). They can be offered individually or within the same school feeding program.
Many LMICs are now running 'home‐grown' school feeding programs. These programs connect local smallholder farmers to the school meal programs and have several aims. They benefit school children by giving them access to fresh, local food. They can also benefit smallholder farmers and their communities by providing a stable market for the farmers (FAO and WFP 2018).
How the intervention might work
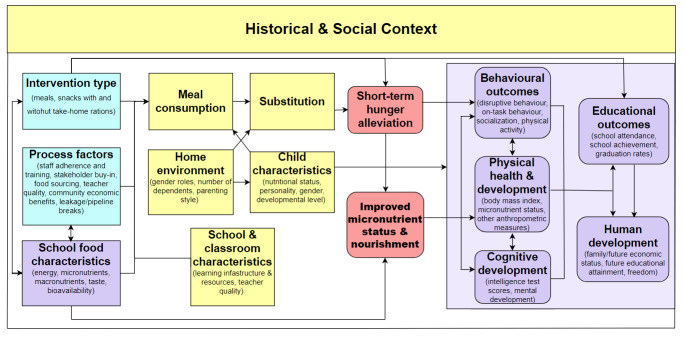
Our conceptual model of how the intervention might work is shown in Figure 1. This model is based on Kristjansson's previous school meals review and process evaluation (Greenhalgh 2007; Kristjansson 2007) and her review of feeding programs for children aged six months to five years (Kristjansson 2015). Our team and advisory board have also given valuable input. Additionally, we have gained insight from previous nonsystematic reviews. These include those by Adolphus 2013, Grantham‐McGregor 2005, Krishnaratne 2013, Levinger 1986, Levinger 1996, Papamandjaris, Pollitt 1978, Pollitt 1995, and Walker 1986.
1.
Conceptual model of the school meal program.
The model details how aspects of the intervention (shown in blue) relate to important contextual and process moderators (shown in yellow), intervention outputs (shown in red rounded rectangles), and intervention outcomes (shown in purple ovals).
School feeding is intended to supplement the food and nutrition given at home so children can eat more nutritious food. School meal design and delivery (e.g. the type of intervention (meals, snacks, or milk), meal palatability, the timing and frequency of feeding, nutritional quality, energy content, and factors contributing to study protocol adherence) can all influence school enrolment and attendance, food consumption, and substitution. Substitution refers to the fact that some children who receive food at school, especially in families who are experiencing extreme socioeconomic disadvantage, receive less or eat less food at home, allowing other family members to consume more. This reallocation may have mixed effects if the school child is malnourished; it can reduce the benefits of school feeding for that child, but it can improve the nutritional status of siblings (Gelli 2019). Child characteristics and contextual factors such as home environment, classroom quality, school quality, and the social context moderate the effectiveness of the intervention. School meals can also consist of income transfer to families which may boost the local economy (Jomaa 2011). Improved enrolment, attendance, attention, behaviour, nutritional status, and cognition can improve educational attainment.
Educational attainment is associated with benefits to adult health, gender equity, and economic outcomes. Viner 2017 found that secondary education was associated with more health benefits than primary education across low‐, middle‐, and high‐income countries. They also found that each additional year of education was associated with an 8% to 16% reduction in mortality (Viner 2017). Education is related to women's decision‐making authority in households across 70 developing countries (Le 2021). Furthermore, as measured by student achievement, educational attainment has been associated with economic outcomes and growth over time and across regions (Hanushek 2015). Given the link between educational attainment and adult health (Viner 2017), gender equity (Le 2021), and economic outcomes (Hanushek 2015), it is primarily through increased educational attainment that school meal programs are thought to have a wide societal impact.
Why it is important to do this review
In 2018, the World Food Program supported school feeding programs in 61 countries, covering 16.4 million school children (WFP 2019). A great deal of hope, time, and money is invested in these programs. It is therefore important to learn whether or not school meals are an effective and cost‐effective intervention for improving the health, nutritional status, cognitive functioning, school enrolment, and school performance of children who live in poor socioeconomic circumstances worldwide.
Although existing reviews provide valuable information, they fail to give us a comprehensive picture of the effectiveness of school feeding across the globe. All are limited in their scope: some to just a few countries, most to either developing or industrialised countries, others to one feeding time (e.g. morning), and others to just a few outcomes. Furthermore, none are systematic reviews. Thus, except for the Kristjansson 2007 review, systematic methods were not used; some lack details on search strategies, inclusion and exclusion criteria, and the number of studies found and considered; furthermore, for some, the quality of studies was not formally assessed.
The first systematic review on school feeding programs, by Kristjansson and colleagues, was published in 2007 and is now more than 14 years old (Kristjansson 2007). The review included 18 studies from across the world and found that school meals in LMIC can improve growth, attendance by four to six days a year, and math scores. This current review will not only update the previous review but will also involve a new and broader search of all the literature to ensure that we do not miss any relevant studies. This systematic review may contribute to the discourse surrounding the importance of school feeding programs in enhancing food security in LMIC and upper‐middle‐ and high‐income countries. Using this review, national authorities may be able to draw conclusions about the effectiveness of school meal programs for outcomes such as student nutrition, performance, and school attendance (Jomaa 2011; Kristjansson 2007; Wang 2020). Additionally, with our systematic analysis of the process of school feeding programs, the design and execution of these programs may be better understood and thus easier to apply. This review may inform many aspects of school meal implementation. This may allow for the development of context‐specific frameworks that meet community needs (WFP 2019). In the case that this review may result in further programs being introduced around the world, the hypothesized outcomes may involve reduced hunger, improved food and income security, and enhanced access to, and quality of, education (FAO and WFP 2018). Using the evidence‐based conclusions drawn from this review, limited national resources and funding may be effectively allocated to advance national nutrition standards — shaping the lives of future generations through reduced inequality (Kristjansson 2015).
Objectives
To assess the effectiveness of school feeding programs for improving the physical and psychological health of children experiencing socioeconomic disadvantage across the world.
To compare the effectiveness of school feeding programs for children experiencing socioeconomic disadvantage and those experiencing socioeconomic advantage. This includes subgroups by income and gender.
To understand the process by which school feeding programs achieve (or fail to achieve) an impact on growth, cognitive development, and school performance.
To gather data that are useful for cost‐benefit analysis.
Methods
Criteria for considering studies for this review
Types of studies
We will accept the following types of studies.
Experimental studies
Randomised controlled trials (RCTs) in which individuals are randomly allocated to a treatment arm and to a control arm. We will also accept cross‐over RCTs (Higgins 2019a).
Cluster‐randomised controlled trials (c‐RCTs) in which groups of people are allocated to different interventions by clusters such as school or area (Higgins 2019a). These may include stepped wedge RCTs. All c‐RCTs are required to have at least two intervention sites and two control sites.
Non‐randomised studies
We will also accept the following types of non‐randomised studies (NRS) because it is often impossible to randomise policy or program interventions.
Quasi‐randomised studies. These controlled trials use inappropriate methods for allocating people to interventions (Reeves 2019).
Controlled before‐after studies (CBAs) in which measurements are taken from both an experimental and a control group before and after the intervention and in which the researcher has control over which people are allotted to which group.
Interrupted time series (ITS). ITS studies may use data routinely collected for monitoring and surveillance in 'natural experiments' (Petticrew 2005) or data collected for a specific intervention. ITS studies must include at least three observations before the intervention and three observations after the intervention.
-
Controlled cohort studies. A cohort study is an observational study design where groups are assembled according to whether or not exposure to the intervention has occurred (Armijo‐Olivia 2012; Thomas 2004). Exposure to the intervention is not under the control of the investigators but under the control of others, such as decision‐makers. People may self‐select into the intervention. Controlled cohort studies can be either prospective or retrospective cohort studies.
Prospective cohort studies are those in which the intervention and control groups are assembled before the intervention and followed through time (Reeves 2019).
Retrospective cohort studies are those in which intervention and control groups are assembled based on past records.
To be included, all NRSs must be longitudinal and have at least two measurements of the outcome ‐ one near the start of the study and one just at the end of the intervention. We will not include NRSs that only have endpoint data.
Results for each type of study will be tabulated and analysed separately.
Types of participants
The participants for this review will comprise children and adolescents aged 5 to 19 who attend primary or secondary schools in any country. For our purposes, we will classify countries into two groups: low‐ and lower‐middle‐income countries (LMIC); and higher‐income countries (upper‐middle‐ and higher‐income countries). We will use the World Bank List of Country Incomes for the year of study inception (World Bank 2021). Studies must meet the following inclusion and exclusion criteria, which are somewhat different for these two groups.
Low‐ and lower‐middle‐income countries
Included
We will include studies in which children are classified as experiencing socioeconomic disadvantage by one or more criteria.
Children live in rural areas or villages.
Children live in an urban area and are described as experiencing socioeconomic disadvantage (e.g. poor or low‐income) or from lower‐income areas (e.g. slums).
Statistics show that 30% or more of the children in the sample suffer from undernutrition (nutritionist judgment).
The study is implicitly or explicitly aimed at children who live with socioeconomic disadvantage, and indicators of disadvantage are provided in the paper.
We will also include studies in which a proportion of children are advantaged, but results can be broken down by socioeconomic status (SES; or other measures of social disadvantage) or baseline nutritional status.
Excluded
We will exclude studies if children are from urban areas only if: a large proportion (more than 50%) of children are living in high SES circumstances, and results are not broken down by SES or other proxy variables; or where information is insufficient to allow us to judge the extent of disadvantage.
Higher‐income countries
Included
We will include studies in which a large percentage of children (60% or more) are classified as living in disadvantaged socioeconomic circumstances by one or more of the following criteria.
They are from areas described as economically marginalized or disadvantaged (e.g. low‐income areas, ghettos, social housing projects, lower‐income rural towns, or mining communities).
The children are from low SES backgrounds.
The children are from families that are described as experiencing socioeconomic disadvantage (including having unemployed parents).
The children are described as marginalized or 'at‐risk' due to social circumstances.
We will also include studies in which a significant proportion of children are advantaged, but results can be broken down by SES or baseline nutritional status.
Excluded
We will exclude studies if more than 40% of students are described as advantaged and results are not broken down by SES or other proxy variables.
Types of interventions
Included
We will include interventions that include providing meals (breakfast or lunch), snacks (including milk), and/or take‐home rations in a school setting.
To be included in our review, the meal/snack given must meet at least 10% of the daily energy requirement and at least 10% of the daily protein requirement for that age group.
If a multi‐armed study includes multiple independent comparisons, we will include all independent comparisons (Higgins 2019b). If a multi‐armed study includes several arms and one control group, we will choose the arm that is most relevant to our study question (Higgins 2019b). For example, if the study provides both in‐school feeding and take‐home rations, we will select the in‐school feeding arm. If the study has two intervention groups based on energy given, we will select the intervention group with the highest amount of energy given.
Excluded
We will exclude the following interventions: micronutrient supplementation or fortification of existing meals alone, stand‐alone nutrition education in schools or at home, obesity prevention programs, breastfeeding programs, food stamps, modifications to school meals to change nutrient content, community kitchens, food banks, and feeding centres.
Because of the increasing emphasis on reducing obesity, programs built around school meals increasingly aim to enhance the nutritional content of children's diets by increasing availability of, and access to, low‐fat choices, fruits, and vegetables (Coleman 2005; Luepker 1996). However, the focus of this review is not on changing the content of school meals but on the effect of the provision of food to children. Thus, we will not include these types of interventions in this review.
Comparators
Comparators can be either no treatment or placebo (low‐calorie drinks/snacks). The placebos must contain less than 3% of the daily energy requirement and less than 5% of the daily protein requirement.
Main Comparisons
We will include the following five comparisons.
Meals only versus control.
Snacks only versus control.
Take‐home rations only versus control.
Meals and snacks versus control.
Meals and take‐home rations versus control.
Types of outcome measures
Although we will prioritise the primary outcome measures, we will also consider secondary outcome measures.
Primary outcomes
Anthropometry (weight and height for age z‐scores and weight for height z‐scores).
Educational outcomes (reading, math, and science performance; school attendance; and enrolment).
Cognitive outcomes (verbal fluency, intelligence, reasoning, attendance, and memory assessed by a reliable and valid test).
Adverse outcomes (overweight/obesity and disruptive behaviour at school).
Secondary outcomes
Anthropometry (weight, height, and bone mineral density).
Physical health (nutritional status; vitamin A status; vitamin B12 status; zinc status; and haemoglobin and hematocrit as indices of anaemia, a condition in which the blood cannot carry enough oxygen, most often due to iron deficiency).
Behavioural outcomes (physical activity).
Adverse outcome (stigmatisation).
Consumption of healthier and unhealthier foods.
Timing of outcome measurement
We will consider both short‐term outcomes (less than three months) and longer‐term outcomes (three months or more) as some behavioural changes may be seen rapidly. Attention and behaviour are examples of short‐term outcomes. Longer‐term outcomes include growth, school achievement, and intelligence.
Search methods for identification of studies
This review focuses on studies that investigate feeding programs and initiatives implemented in elementary and secondary schools. The search strategy in the previous Cochrane review published in 2007 used outcomes such as growth, body mass, weight, and height to limit the search (Kristjansson 2007). This may have contributed to a narrower, more precise, and potentially biased strategy. We have updated the strategy to increase sensitivity. We also considered keywords, subject headings, and databases used in the strategy of a 2020 protocol on school feeding interventions in LMICs when designing the revised strategy (Wang 2020). We will conduct searches using both keywords and database‐specific controlled vocabulary in relevant databases, in addition to complementary searches to identify additional studies as well as pertinent grey literature.
Electronic searches
We will conduct searches in subject‐specific and multidisciplinary databases and registries to identify relevant published studies and clinical trials to include in this review. PL will execute searches in the following databases and registries.
Cochrane Central Register of Controlled Trials Ovid (current issue).
MEDLINE Ovid (1946 onward).
Embase Ovid (1947 onward).
Allied and Complementary Medicine Ovid (1985 onward).
APA PsycInfo Ovid (1806 onward).
CAB Abstracts CAB Direct (1973 onward).
CINAHL EBSCO (1981 onward).
Education Source EBSCO (1880 onward).
ERIC Ovid (1965 onward).
Food and Science Technology Abstracts EBSCO (1969 onward).
Global Health EBSCO (1973 onward).
Web of Science Core Collection Clarivate (1970 onwards; includes Science Citation Index Expanded, Social Sciences Citation Index, Arts & Humanities Citatio Index, Conference Proceedings Citation Index ‐ Science, Conference Proceedings Citation Index ‐ Social Science & Humanities, and Emerging Sources Citatation Index).
ProQuest Dissertations & Theses Global ProQuest (1637 onward).
ClinicalTrials.gov (clinicaltrials.gov/).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; trialsearch.who.int/).
Cochrane Database of Systematic Reviews (current issue) in the Cochrane Library.
Campbell Collaboration (www.campbellcollaboration.org/better-evidence.html).
Epistemonikos (www.epistemonikos.org/en/).
We will not use database limits or restrictions related to languages, dates, or publication types when searching the above resources.
The search strategy developed for MEDLINE Ovid was peer‐reviewed by another research librarian following the Peer Review of Electronic Search Strategies (PRESS) guideline for systematic reviews (McGowan 2016). This strategy is available in Appendix 1.
We will add included studies to Zotero, which integrates notifications from Retraction Watch, to determine if any of the studies have been retracted. We will also access each included study on its original publisher platform to verify whether any corrections or updates were made since the original text was published.
Searching other resources
To identify relevant grey literature beyond theses and conference presentations that will be found through electronic searches, we will target the web sites and catalogues of the following organizations: UNESCO (via UNESDOC; unesdoc.unesco.org/), the World Food Programme (WFP; www.wfp.org/publications), and other United Nations publications (via the UN Digital Library; digitallibrary.un.org/), the WHO (www.who.int/publications), the World Bank (via its eLibrary; elibrary.worldbank.org/), and reports indexed on ReliefWeb’s site (reliefweb.int/updates?view=reports).
In addition, we will hand search reference lists from relevant knowledge syntheses (systematic and non‐systematic reviews) and all of those from included primary studies to see if other relevant studies should be considered. We will also search for citations of included articles. In addition, authors of conference presentations identified through the electronic searches will be contacted to see if their research has been published as articles.
Data collection and analysis
In this section, we describe our data extraction and the analyses that we will perform.
Selection of studies
We will use Covidence 2021 to manage studies identified in the search. Due to the breadth of the concepts and the diversity of sources to be searched, the initial search process will likely return more than 40,000 citations. Teams of two review authors each (EK, MO, AH, PCH, MD, AE, AN) will independently screen the titles and abstracts for inclusion. Review authors will classify each abstract as eligible, possibly eligible, or not eligible. We will retrieve full copies of all studies deemed eligible or possibly eligible by at least one review author for full‐text examination by two of the same seven review authors who will mark them as included or excluded. Where eligibility is unclear, we will mark the report as awaiting classification and contact the study authors for further information. Review authors will resolve disagreements by discussion. If necessary, we will consult with one of the other review authors.
We will provide details of studies that appear relevant but, after a thorough reading of the full text, should not be included, in the 'Characteristics of Excluded Studies' table (Lefevbre 2019). This table will also include well‐known studies that were excluded. We intend to review all studies found in hand searching that are not duplicates of the ones found in the formal search; EK will first double‐check the titles selected from hand‐searching for relevance. After the inclusion/exclusion process is finished, we will enter the articles that we identified in hand searching into a Microsoft Excel sheet (Microsoft Excel 2019) which we will check against studies found in the electronic search.
Data extraction and management
We will set up our data extraction form in Microsoft Excel (Microsoft Excel 2019) and pilot test it before full use. Pairs of review authors (MO, MD, AH, AN, PCH) will independently extract data in duplicate. If there are any discrepancies between the two authors in the data extraction, we will resolve these discrepancies by discussion.
We will extract author and publication details, context, and study design data, including the allocation level. We will also extract data on the description of the intervention, including duration, baseline nutritional status, and whether the population is described as experiencing adverse socioeconomic circumstances. We will also report all interventions in a multi‐armed study. We will use PROGRESS‐PLUS (O'Neill 2014; Welch 2019) as an organizing framework to assess social circumstances. Thus, we will extract data on place of residence, race/ethnicity, occupation, gender/sex, religion, education, SES, social capital, age, disability, and other participant characteristics. We will also extract data on outcome measures and tools, statistical analyses, and the fixed and marginal costs of the feeding program. Ideally, these would be separate from research costs. Finally, we will extract data on results, including intention to treat (ITT) analyses, treatment on the treated, standard error (SE), and standard deviation (SD).
In cases where two articles use the same data set, same waves, and the same outcomes, we will choose the most recent article.
If information is missing from the report, we will contact the study authors directly by email.
Process variables
To address Objective 3, several process elements will be extracted. Our list was chosen to represent factors that could impact the effectiveness of these programs and includes the following.
The intensity of the feeding (e.g. energy content of the meal/snack, percentage of average daily energy or protein requirement, and whether it is categorized as lower (interventions providing less than 15% of the average daily requirement for that age group) or higher (interventions providing 15% or more of the average daily requirement for that age group)).
Context (country and area).
Type of feeding (snack, meal, drink, and whether fortified or unfortified) and the time of day food was given.
Quality/acceptability of the food given.
The provider (who delivered the intervention).
Whether intake was monitored.
Child/youth adherence.
Substitution.
The cost of providing school meals.
Process evaluation
Contemporary research practice recommends process evaluation alongside empirical trials of complex interventions to identify how the intervention was implemented in practice, the mechanism by which it achieved its impact, and any local contextual issues that may have influenced outcomes (Calnan 2003; Campbell 2000). The process evaluation will be used to aid the interpretation of the findings.
Process evaluation can also be undertaken as part of a systematic review by extracting, analysing, and synthesising any data from the included studies that may help explain the mechanism(s) of action, heterogeneity of outcomes, or both. Thus, when a complex social intervention appears to have had a significant effect in one trial but no effect (or a negative effect) in another, an analysis of the link between the context, intervention, and outcome aims to generate further hypotheses about the circumstances in which this intervention might be more or less successful.
Study quality may also impact findings; studies of lower quality will often show higher effect sizes than those of higher quality. For example, biased outcome assessment is possible in situations where those who assess the outcome variables are not blinded to the study group (i.e. intervention or control).
MO and MD will tabulate the effects for each study in Excel (Microsoft Excel 2019) and sort them by the possible process elements to better understand the influence of process factors. We will begin with the study type, quality, and high versus low energy food. Energy content is an important process element, which will be assessed by the team's nutritionists (SL and JK). If possible, we will also estimate the recommended dietary intake (RDI) for other nutrients.
Rules used in calculating the energy content
When the total energy content or percent of average daily energy requirement is provided in the text of the study, this value will be used. When the energy content is not provided, but the descriptions of food are sufficient (quantity and type of food), the energy content of the meal/snack will be estimated using Food Data Central (USDA 2021).
When meals/snacks with different energy contents are provided on different days (e.g. Powell 1983), a weighted average will be taken (e.g. if a meal with 400 kcal was provided three days a week and a meal with 60 kcal was provided two days a week, then the weighted average of 264 kcal would be used).
When the number of days on which different meals/snacks are given is not specified (Agarwal 1989) or when the energy content is different in year one than year two of the study (Neumann 2003), a straight average will be used for the energy content.
Calculating the percent average daily requirement for energy
The percent of the average daily requirement for energy will be calculated by dividing the given or estimated average energy content of the meal/snack by the requirement for the age/sex‐specific target group in each study (FAO 2004). When the intervention group of a study is comprised of various age and sex groups, and outcomes are given for the entire group only, a weighted average for the average daily energy requirement will be used to calculate the percent average daily energy requirement. In addition, as a check, the percent average daily energy requirement will be calculated for each age and sex group for which there is a corresponding average daily energy requirement by dividing the total energy provided by the meal/snack by the age and sex‐specific average daily energy requirement. Energy intensity will be considered as a continuous variable. However, for purposes of helping to interpret the data, interventions will also be characterised as having two levels of energy content: lower (interventions providing less than 15% of the average daily energy requirement for that age group) and higher (interventions providing 15% or more of the average daily energy requirement for that age group).
After data extraction is completed, the 'Characteristics of included studies' table will be developed.
Assessment of risk of bias in included studies
We are interested in the effect of intervention and control group assignment on the study outcomes. We will use several tools; one for the RCTs, one for the NRSs except for ITS, and one for the ITS studies. At least two reviewers (EK, BS, MO, MD) will independently rate each aspect of risk of bias. Disagreements will be resolved by discussion with a third author. Two nutritionists (JK, SL) will assess the risk of bias for anthropometric measurements. A registered clinical psychologist (LJ) will assess the risk of bias for psychological measures.
RCTs
To rate the risk of bias for RCTs, we will use the Cochrane RoB 2 tool (ROB2 2021; Sterne 2019a; Sterne 2019b) as outlined in Chapter Eight of the Cochrane Handbook for Systematic Reviews of Interventions (hereafter referred to as the Cochrane Handbook; Higgins 2019c). We will also use the Crib Sheet (Higgins 2019d) and the Excel implementation sheet to guide us in our decision‐making (RoB Excel Tool 2022).
Bias arising from the randomisation process (allocation sequence generation and allocation concealment, baseline differences).
Bias due to deviations from intended interventions (non‐protocol interventions, the effect of assignment, blinding, and appropriate analyses).
Bias due to missing outcome data.
Bias due to measurement of the outcome.
Bias due to selection of the outcome.
For each of these domains, we will judge the result as low risk of bias, some concerns, or high risk of bias. To reach this judgment, we will follow signalling questions, which will help us to decide on these levels. These signalling questions have five response options: yes, probably yes, no, probably no, and no information.
Based on the judgments of the five domains, we will determine the overall risk of bias for each outcome within a study. This will be related to the highest risk in any domain; e.g. if all domains are low risk the overall ROB for that outcome will be low risk, but if any of the domains are judged to be of some concern the overall risk will be judged to be of some concern. If any of the domains are at high risk or if there are some concerns in multiple domains, the overall ROB for that outcome will be judged to be at high risk.
Cluster RCTs
For this, we will follow the criteria outlined in Chapter 23 of the Cochrane Handbook (Higgins 2019b). We will also as use the guidance document (Eldridge 2021), the Crib Sheet (Higgins 2019d), and the Excel Tool (RoB Excel Tool 2022).
The criteria are as follows.
Bias due to timing of identification or recruitment of participants (recruitment before randomisation, preventing knowledge of assignment to clusters, baseline imbalance).
Bias due to deviations from intended interventions (awareness of trial and their assigned intervention, caregiver's awareness, deviations from intended intervention, and appropriate analyses).
Bias due to missing outcome data (data for all clusters, number of missing in each cluster, missingness related to outcome variable).
Bias due to measurement of the outcome (measurement of appropriate, measurement different between groups, participants blinded to trial, outcome assessor aware of assignment, assessment influenced by knowledge of assignment).
Bias due to selection of reported results.
The overall risk of bias for a study/outcome is judged as low, moderate, serious, or critical using the criteria in Chapter 23 of the Cochrane Handbook (Higgins 2019b).
Please note that this tool for cluster‐RCTs can also be used with stepped wedge trials, realising that it does not cover the need for adjusting data for time trends (Higgins 2019b).
Crossover RCTs
To assess the ROB in a crossover trial, we will use the draft version of the ROB 2 tool for crossover trials (Higgins 2021). We will also use the Crib Sheet and Excel Tool. The criteria for crossover trials are as follows.
Bias that arises from the randomisation process; here, the randomisation is to sequence A or B of the cross‐over trial.
Bias due to deviations from intended interventions (non‐protocol interventions, the effect of assignment, blinding, and appropriate analyses).
Domain S: Bias arising from period and carryover effects.
Bias due to missing outcome data.
Bias in the measurement of the outcome.
Bias in the selection of the reported result.
Criteria for controlled cohort studies and CBAs
We will assess the risk of bias for this group of studies using the ROBINS‐I tool for non‐randomised studies (Reeves 2019; Sterne 2016). The domains are as follows.
Bias due to confounding.
Bias in the selection of participants for the study.
Bias in the classification of interventions.
Bias due to deviations from intended interventions.
Bias due to missing data.
Bias in the measurement of outcomes.
Bias in the selection of the reported result.
For each domain, there are five possible levels of bias: low, moderate, serious, critical, and no information. For each domain, signalling questions help the judgment of the level of bias. These signalling questions have five response options: yes, probably yes, no, probably no, and no information (Sterne 2016).
The judgment of the overall risk of bias will be the least favourable assessment across the domains. This judgment will be low risk of bias across all domains, some concerns in at least one domain but not at high risk of bias for any domain, or high risk of bias in at least one domain.
Criteria for ITSs
In assessing the methodological quality of ITS studies, we will use the Suggested risk of bias criteria for EPOC reviews (Cochrane EPOC 2022). The domains include the following.
Intervention is independent of other changes.
The shape of the intervention is prespecified.
Intervention unlikely to prevent data collection.
Knowledge of allocation.
Incomplete outcome data are adequately taken care of.
Selective outcome reporting.
Other risks of bias.
We will rate each domain as having low risk, unclear risk, or high risk of bias as per the criteria outlined within.
Measures of treatment effect
RCTs
Continuous outcomes
For continuous outcomes, the effect estimate of interest is the change or the mean difference between the experimental and control groups in the end‐of‐study outcome, adjusted for baseline outcome measure. Thus, we will enter the unstandardised regression coefficient that compares the change scores between experimental and control groups. We will use the model with adjustment for the highest number of covariates.
In cases where regression has not been performed, but when the baseline and end‐of‐study means and SDs are available (or the SD could be derived based on statistics provided e.g. 95% confidence interval (CI)), we will calculate the mean change for each of the two groups (end‐of‐study mean minus baseline mean). The SDs for change will be calculated using the formula: SQRT ((SD2 baseline + SD2 end‐of‐study) *2ρ) * (SDbaseline*SD end‐of‐study)). We will generally use 0.8 for the correlation between the baseline and the end of the study values, as recommended by our statistician. However, we will use 0.9 for the correlation between the weight, height, and BMI baseline and end‐of‐study measures; this was based on coefficients supplied by Du 2005 for the first Kristjansson school feeding review (Kristjansson 2015).
We will conduct sensitivity analyses for both growth and psychological outcomes with a P of 0.7.
Dichotomous outcomes
For dichotomous outcomes, the effect estimate of interest will be the risk ratio (RR) of the risk in the experimental group relative to the risk in the control group, or the odds ratio (OR) or the odds that a given outcome will occur. To get these statistics, we will enter the log of the OR or RR plus the SE. In cases where multiple coefficients are presented in the paper, we will select the one representing the analysis with the most control variables.
CBAs and controlled cohort studies
Continuous outcomes
For continuous outcomes, the effect estimate of interest is the change or the mean difference between the experimental and control groups in the end‐of‐study outcome, adjusted for baseline outcome measure. Thus, we will enter the unstandardised regression coefficient that compares the change scores between experimental and control groups. We will use the model with adjustment for the greater number of covariates.
In cases where regression has not been performed, but when the baseline and end‐of‐study means and SDs are available (or the SD could be derived based on statistics provided e.g. 95% CI), we will calculate the mean change for each of the two groups (end of‐study‐mean minus baseline mean). The SD for change will be calculated using the formula: SQRT ((SD2 baseline + SD2 end‐of‐study) *2ρ) * (SDbaseline*SD end‐of‐study)) where P is the correlation between baseline and end‐of‐study. We will typically use 0.8 for the correlation between the baseline and end‐of‐study values, as recommended by our statistician. However, we will use 0.9 for the correlation between weight, height, and BMI baseline and end‐of‐study measures; this was based on coefficients supplied by Du 2005 for the Kristjansson school feeding review (Kristjansson 2007).
We will conduct sensitivity analyses for both growth and psychological outcomes with a P of 0.7.
Dichotomous outcomes
For dichotomous outcomes, the effect estimate of interest will be the RR of the risk in the experimental group relative to the risk in the control group or the OR, representing the odds that a given outcome will occur. For these, we will enter the log of the OR or RR plus the SD. In cases where multiple coefficients are presented in the paper, we will select the one representing the analysis with the most control variables.
ITSs
For these studies, we will use estimates from time series regression. We will examine the trends/slopes and changes in the outcome level before and after the intervention and the mean change in the outcome level.
Unit of analysis issues
Cluster studies (cRCTs, CBAs, and ITSs)
For c‐RCTs and non‐randomised studies (CBAs and ITSs), we anticipate that the study authors will have presented their results after appropriately controlling for clustering effects (robust SEs or hierarchical linear models). We will document clustering and analysis methods used within the studies to control for this. We will correct the design effect using the variance inflation factor (VIF) if needed.
Calculating the VIF
First, we will calculate cluster size. When the numbers of participants in each analysis are provided, we will divide these numbers by the number of clusters to calculate the average cluster size. Otherwise, we will use the number of participants provided in the methods sections of the primary studies and divide that by the number of clusters.
Then, we will use appropriate intra‐cluster correlations (ICCs). For growth and biochemical outcomes, we will use the ICCs published in Du's 2005 letter to the editor (Du 2005). Thus, we will use ICCs of 0.025 and 0.016 for weight and height, respectively. We will conduct sensitivity analyses with ICCs of 0.01, 0.05, and 0.10 for weight and height. We will conduct further sensitivity analyses for each outcome to assess how large the ICC will need to be to change the results. For math, reading, science, attendance, and intelligence outcomes, we will use ICCs of 0.15, with sensitivity analyses at 0.10 and 0.20. This will be based on recommendations from the Schochet report for math and reading (Schochet 2008).
Then, for experimental and control groups separately, we will calculate the VIF as follows: (1+ (m‐1) multiplied by ICC), where m is the average cluster size (Ukoumunne 1999). We will then multiply the original SD by the square root of the VIF for experimental and control groups separately. We will then enter these adjusted SDs into Review Manager Web (RevMan Web 2022) data tables.
Dealing with missing data
As noted in the 'Data extraction and management' section, we will contact the authors for missing data. We will contact authors directly by email if there is information missing from the reports that would help us to conduct this review. We will ask for statistical data (e.g. SE) or information about their study design, or both.
We will consider missing participant data as well as missing statistics.
Did the authors do an ITT analysis taking into consideration loss to follow‐up in the study using imputation methods?
Did they provide us with the needed statistics (e.g. SD/SE) to conduct the meta‐analysis?
If SDs or SEs are not reported in the primary studies and we do not receive the data after contacting the authors, we will calculate such information from exact P values or from CIs using the RevMan Web 2022 online calculator (Drahota 2021).
Assessment of heterogeneity
We will use the following methods to assess clinical, methodological, and statistical heterogeneity.
First, we will visually examine forest plots for visible outliers and between‐study differences.
Second, we will use the I2 statistic to assess the level of heterogeneity. Specifically, we will use the rough interpretation guide presented in Deeks 2019 as a reference point (i.e. 0% to 40%: might not be important; 30% to 60%: moderate heterogeneity; 50% to 90%: substantial heterogeneity; 75% to 100%: considerable heterogeneity). When the I2 statistic is > 0.75, we will not perform a meta‐analysis with the studies but instead will look for the source of the heterogeneity.
Third, we will explore heterogeneity by conducting subgroup analyses, when possible. Sources of heterogeneity could include differences in participants, study type, intervention implementation, and context (e.g. rural versus urban).
Assessment of reporting biases
If more than ten studies are summarised in any one meta‐analysis, we will plot the possible trial effect against the SE and show it as a funnel plot (Higgins 2019a). Asymmetry could be caused by a relationship between effect and sample size or publication bias (Egger 1997). If we detect asymmetry, we will interpret the results in combination with a visual examination of the plots. This will allow for the exploration of small study effects and outliers. If we identify small study effects, we will conduct a sensitivity analysis to explore the impact on the meta‐analysis. We will apply this approach to the results of both RCTs and other types of studies.
Data synthesis
Quantitative meta‐analyses
We will conduct meta‐analyses when we have a minimum of two studies that can be combined (e.g. same intervention, outcome, and study design, I2 below 75). We will perform these meta‐analyses in RevMan Web (RevMan Web 2022).
We will perform meta‐analyses separately for each type of study design (RCTs, cluster RCTs, cross‐over RCTs, NRCTs, CBAs, controlled cohort, and ITSs). We will also complete separate meta‐analyses for higher‐income and lower‐income countries.
The generic inverse variance method will be used as the preferred method of meta‐analysis. Using this method, for continuous variables, we will enter unstandardised regression coefficients and SEs, which have been adjusted for relevant covariates. These statistics will be converted to the same units. For dichotomous outcomes, which may be adjusted for relevant covariates, we will enter the log of the RR and SEs, or the OR and SE.
For each meta‐analysis, we will provide an estimate with 95% CI; we will also generate a forest plot. In many instances, we will need to derive SEs from CIs using the RevMan calculator (Drahota 2021). We will use a random‐effects model for all analyses (Higgins 2019a), as clinical and methodological heterogeneity between studies is anticipated in terms of the type and implementation of interventions, participant groups, or outcomes measured. We will discuss the extent of the evidence against homogeneity in each analysis.
For cross‐over RCTs, we will use a paired t‐test with generic inverse variance (Higgins 2019b).
As noted above, we will determine whether the interventions, participants, or outcomes are similar or too different to combine. We will not combine different types of study designs. If the I2 for any meta‐analysis is > 75%, we will not combine all studies in the meta‐analysis.
Syntheses without meta‐analysis
If the effect sizes are given but do not have the variability estimate, we will summarise the data using medians and interquartile ranges.
We will also prepare an effect direction plot for studies that we could not use to conduct a quantitative meta‐analysis (Thompson 2013). We will narratively synthesise reports for studies based on effect direction as well, as suggested on page 327 in the Cochrane Handbook (Higgins 2019a). We will use the SWiM guidelines to ensure that we report our qualitative syntheses appropriately (Campbell 2020). These guidelines concern grouping studies together, the metric and transformation method used, the synthesis method used, priority setting, reporting of heterogeneity and certainty of the evidence, data presentation, and reporting results and limitations.
Drawing conclusions
When drawing our conclusions, we will consider the risk of bias, the certainty of the evidence, effect size, CI, statistical significance, and real‐world meaningfulness.
Avoiding dependency
To avoid dependency, we will be careful not to double‐count the same participants in the analyses. For multi‐arm studies, we will follow methods outlined in Chapter 23 in the Cochrane Handbook (Higgins 2019b).
Synthesizing data from process evaluations
We will follow a realist approach to synthesise the data collected from process evaluations. As recommended by the RAMESES methodological standards (Wong 2013), analysis and synthesis of findings will be an interpretive process, reached through reflection and discussion and requiring repeated reading and re‐reading of primary studies in light of the emerging synthesis. We will give particular attention to identifying and exploring the mechanisms by which study participants drew upon resources available to achieve the intended outcome.
This approach will result in a series of context‐mechanism‐outcome (CMO) configurations that represent hypotheses concerning which outcomes are generated through which mechanisms in which context (Rycroft‐Malone 2012). We will iteratively test theories and refined based on integrating evidence of effectiveness (i.e. what works) with evidence derived from process evaluations and implementation research (i.e. how and why it works). The effectiveness evidence will contribute to the outcome and mechanism components of a CMO, whereas the process/implementation evidence will contribute to the mechanism and context components of a CMO (Harden 2018). We will synthesise this data using the following steps.
Organization of extracted data into evidence tables. This may include the theory of change proposed by study authors, why they felt the intervention was needed and what they thought it would achieve, and authors’ conclusions about why the intervention had worked or not worked; differences in subgroups, and any explanations for these differences; and additional mechanisms proposed by authors.
Theming of context, mechanisms, and outcomes by individual reviewers.
Comparison of reviewers' themes for a specific article and formulation of chains of inference from the identified themes.
Linking of the chains of inference and tracking and linking of articles.
Hypothesis formulation (context, mechanism, and outcome configurations).
Integration of analysis of the underlying theories of how the feeding interventions are expected to bring about change, and ii) the findings of the trials and the process evaluations of how the theory worked or did not work in practice.
As an output of this synthesis, we will identify factors that enhance or reduce the effectiveness of school feeding programs.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses
We will conduct subgroup analysis across SES, age, and sex for the three primary outcomes: physical health, educational, and cognitive outcomes.
SES
To address Objective 2, we will perform subgroup analyses by SES or baseline nutritional status (as an indicator of socioeconomic disadvantage), or both, to determine whether school feeding works as well or better for children with socioeconomic disadvantage. This, in turn, would decrease socioeconomic inequities.
Furthermore, different treatment effects of school meals for children from various socioeconomic and nutritional statuses are expected. In high‐income societies where, for instance, obesity is a concern, school feeding aims to enhance access to fruit, vegetables, and low‐fat food (Coleman 2005; Luepker 1996), whereas, in lower‐income societies, the effects might include fighting against malnutrition. Studies also suggest that children from lower‐income countries are more likely to benefit from school meals.
Age and sex
Because growth rates and caloric needs may vary by age, we will conduct a subgroup analysis across two age groups for weight and height. These age groups are 5 to 11 years and 12 to 19 years. This age grouping was chosen because caloric needs vary by those ages. We also plan to do subgroup analyses by these two age groups for cognition, as it is possible that the effectiveness of school meals in changing cognitive outcomes may vary according to age. We also will conduct subgroup analyses by sex, as sex differences in development may occur. Furthermore, there may be cultural differences in how boys and girls are treated.
% RDI for energy given
In the earlier Kristjansson 2007 review, we found that effectiveness differed between studies that gave meals/snacks with high energy content and those that gave meals/snacks with low energy content. Therefore, we will run subgroup analyses by the level of energy given, dividing energy given into higher/lower groups using a median split.
The credibility of subgroup analyses
We will assess seven criteria to evaluate the credibility of subgroup analyses (Guyatt 2011).
Is the difference suggested by comparisons within rather than between studies?
Does the interaction test suggest a low probability that chance explains the apparent subgroup effect?
Were the subgroup analyses and their direction specified beforehand?
Is the subgroup analysis one of a few tested?
Is the magnitude of the subgroup effect large?
Was the same comparison with the same result done within a primary study?
Did the difference follow a common‐sense rationale?
Sensitivity analysis
If possible, we will perform sensitivity analysis to assess the impact of risk of bias and industry funding on the overall result of studies.
Risk of bias: we will re‐analyse the data, excluding studies at high risk of bias.
Bias due to industry funding: we will re‐analyse the data, excluding studies that are industry‐funded.
We will report the results of these sensitivity analyses for each source of bias.
Summary of findings and assessment of the certainty of the evidence
We will use GRADEpro GDT to create 'Summary of findings' tables for each of the following five comparisons.
Meals only versus control.
Snack only versus control.
Take‐home rations only versus control.
Meals and snacks versus control.
Meals and take‐home rations versus control.
In each table, we will present information on the populations and experimental and comparison interventions examined, as well as the magnitude of the effect of the interventions, the sum of available data on the outcomes (including the timing of the observations (whether short‐term or longer‐term)), and the certainty ratings of the evidence (Schünemann 2019). Where there is evidence for a particular outcome from both RCTs and non‐randomised studies, we will report the data from the RCTs and non‐randomised studies separately; we will report data from the RCTs first.
We will include the following outcomes in each table.
Change in height or height for age z‐score.
Change in reading performance.
Change in math performance.
Change in attendance.
Change in verbal fluency.
Adverse outcomes of overweight/obesity and behaviour problems.
Working independently, two review authors will use the GRADE approach to assess the certainty of the evidence for each outcome (Schünemann 2019); for narrative syntheses, we will follow Murad 2017. We will resolve differences in opinions by discussion with a third review author. We will judge the certainty of the evidence for each outcome as high, moderate, low, or very low (Schünemann 2019). We will base this rating on the presence of risk of bias, inconsistency, indirectness, imprecision, and publication bias, outlined in detail in Appendix 2. Non‐randomised studies assessed with ROBINS‐I will start as high certainty. All studies (RCTs and non‐randomised studies) can be downgraded to a lower level.
Acknowledgements
We would like to acknowledge the Cochrane Developmental, Psychosocial, and Learning Problems Review Group for their contributions and valuable feedback. We would also like to acknowledge the IMPACT Community of Practice with the WFP. We would also like to acknowledge the important contributions of the Cochrane Copy Editor, Carolyn Wayne.
We acknowledge the contribution of Allison Smith, Research Librarian at the University of Ottawa Library, who peer‐reviewed the MEDLINE search strategy using the Peer Review of Electronic Search Strategies (PRESS) guideline for systematic reviews (McGowan 2016).
The CRG Editorial Team are grateful to the following peer reviewers for their time and comments: Dr. Amanda Brand, Centre for Evidence‐Based Health Care, Stellenbosch University, South Africa, and Cochrane Nutrition; and Denny John, Adjunct Assistant Professor, Amrita Institute of Medical Sciences & Research Centre, Amrita Vishwa Vidyapeetham, Kochi, India.
Appendices
Appendix 1. MEDLINE Ovid search strategy
exp nutrition policy/
nutrition therapy/
exp meals/
food, fortified/
food services/
((feed* or food* or nutrition* or diet*) adj3 (program* or policy or policies or therap* or initiative* or intervention* or plan*)).ti,ab,kf.
((breakfast* or break‐fast* or break fast* or lunch* or dinner* or supper* or meal* or snack*) adj3 (program* or policy or policies or initiative* or intervention* or plan*)).ti,ab,kf
("food service*" or cater* or cafeteria* or canteen* or "tuck shop*" or "meal service*" or lunchroom*).ti,ab,kf
((beverage* or milk or meat* or egg* or fruit* or vegetable*) adj3 (program* or policy or policies or initiative* or intervention* or plan*)).ti,ab,kf.
((fortif* or enrich*) adj3 (food* or diet* or spread* or flour* or cereal*)).ti,ab,kf
((supplement* or complement*) adj3 (food* or feed* or diet* or nutrition* or nutrient* or micronutrient* or micro‐nutrient* or macronutrient* or macro‐nutrient*)).ti,ab,kf.
or/1‐11
schools/
(school*).ti,ab,kf.
or/13‐14
12 and 15
(school* adj3 (feed* or food* or breakfast* or break‐fast* or break fast* or lunch* or dinner* or supper* or meal* or snack*)).ti,kf.
16 or 17
Appendix 2. GRADE criteria for downgrading and upgrading the certainty of the evidence
Criteria for downgrading the certainty in the body of evidence: RCTs
Study limitations
We will base our judgments on the Cochrane RoB 2 across an outcome for different studies.
We shall downgrade by one if:
Most (70% or more) of the weight in the synthesis is given to studies with high risk of bias for allocation concealment; OR
Most (70% or more) of the weight in the synthesis is given to studies with low risk of bias for allocation concealment, but high risk of bias for deviations from intended interventions and high risk of bias due to missing outcome data; OR
Most of the weight (70% or more) in the synthesis is given to studies with 'some concerns' about allocation concealment, deviations from intended interventions, and incomplete outcome data; OR
Most of the weight (70% or more) in the synthesis is given to studies that have overall high risk of bias for that outcome.
We will downgrade by two levels if most of the weight in the synthesis is given to studies that have high risk of bias for allocation concealment, deviations from intended interventions, and incomplete outcome data.
Indirectness
If the papers do not directly address our questions, we will downgrade one level for indirectness.
Inconsistency
If there is high and unexplained heterogeneity (I2 = 75 or more) within the body of evidence, we will rate down one for inconsistency. If heterogeneity is explained by subgroup analysis, we will rate on the basis of number of participants required for an adequately powered individual study. However, it is important to note that we will not perform a meta‐analysis unless I2 is less than 75 and/or I 2 is more than 75 and subgroup analyses help to explain the source.
Imprecision
We will judge imprecision in the following manner.
If the 95% confidence interval (CI) excludes the null (e.g. a risk ratio (RR) of 1.0) and the total number of events or patients exceeds the optimal informations size (OIS) criterion, we will judge the precision to be adequate.
If the 95% CI includes appreciable benefit or harm (e.g. an RR of under 0.75 or over 1.25), we will downgrade for imprecision even if the OIS criterion is met.
If the OIS is not met, we will downgrade by one.
If both 1 and 2 are true (e.g. OIS not met, 95% CI included appreciable benefit or harm), we will downgrade by two levels.
In calculating the OIS, we will assume that we are trying to detect a small difference (D = 0.02). We will calculate the OIS using the appropriate program modules on the Boston School of Public Health web site for differences in proportion (LaMorte 2020), or Daniel Soper's sample size calculator (Soper 2021).
High probability of publication bias
We will judge this based on our funnel plots, if possible.
Criteria for downgrading the certainty in the body of evidence: controlled before and after, controlled cohort, or interrupted time series studies.
Study limitations
We will base our judgments of study limitations on the risk of bis from ROBINS‐I for each study outcome in the meta‐analysis. We shall downgrade by one if most (70% or more) weight in the synthesis was allotted to studies that were at overall serious risk of bias for that outcome.
We shall downgrade by two levels if most (70% or more) of the weight in the meta‐analysis was allotted to studies that were judged to have critical risk of bias for that outcome.
Indirectness
If the papers do not directly address our questions, we will downgrade one level for indirectness.
Inconsistency
If there is high and unexplained heterogeneity (I2 = 75 or more) within the body of evidence, we will rate down one for inconsistency. If heterogeneity is explained by subgroup analysis, we will rate on the basis of number of participants required for an adequately powered individual study. Please note that we will generally not perform a meta‐analysis if there is greater than 75% heterogeneity.
Imprecision
We will judge imprecision in the following manner.
If the 95% CI excludes the null (e.g. RR of 1.0) and the total number of events or patients exceeds the OIS criterion, we will judge precision to be adequate. It also will be judged to be adequate if the 95% CI does not include both appreciable benefit and appreciable harm.
If the 95% CI includes appreciable benefit or harm (e.g. an RR of under 0.75 or over 1.25), we will downgrade for imprecision even if the criterion is met.
If the OIS is not met, we will downgrade by one.
If both 2 and 3 are true (e.g. OIS is not met, 95% CI included very appreciable benefit or harm), we will downgrade by two levels.
In calculating the OIS, we will assume that we are trying to detect a small difference (D = 0.02). We will calculate the OIS using the appropriate program modules on the Boston School of Public Health website (LaMorte 2020) for differences in proportion or Daniel Soper's sample size calculator for linear regressions (Soper 2021).
High probability of publication bias
We will judge this based on our funnel plots, if possible.
Upgrading the evidence from non‐randomised studies
We will apply the following criteria for upgrading.
Large effect
We will consider "not only the point estimate but also the precision (width of the CI) around that effect: one should rarely and very cautiously rate up quality of evidence because of apparent large effects, if the CI overlaps substantially with effects smaller than the chosen threshold of clinical importance" (Chapter 5.3, Schünemann 2013).
We will follow the Cochrane Handbook for large effects of dichotomous data.
Large effect: RR > 2 or < 0.5.
Very large effect: RR > 5 or < 0.2.
For continuous variables, we will consider a Cohen's d of 0.5 as a large effect.
Dose‐response relationship
We will upgrade the evidence if there is clear evidence for a dose‐response relationship.
Plausible confounding
If plausible confounding in a rigorous non‐randomised study may have resulted in a underestimate of the effect, we will consider upgrading by one level.
Contributions of authors
Developed the concept, obtained the funding, and is the guarantor for the review: Elizabeth Kristjansson.
Wrote and edited the protocol: Elizabeth Kristjansson, Muna Osman, Michael Dignam, Julia Krasevec, Patrick Labelle, Andrea Huerta Galacia, Paige Cooke Hughes, Arghavan Nepton, Aganeta Enns, George Wells, Bev Shea, Selma Liberato, Jennifer Garner, and Vivian Welch.
Coordinated the development and revision of the protocol: Muna Osman.
Developed the conceptual mode with input from all authors and our advisory committee: Michael Dignam.
Developed the procedures for calculating energy content of the meals as well as for calculating the average daily requirement for energy: Julia Krasevec.
Developed the search strategy and wrote the literature search section: Patrick Labelle.
Wrote the majority of the analysis section: George Wells.
Provided input into the risk of bias section: Bev Shea.
Provided input into the cognitive measures: Laura Janzen.
Sources of support
Internal sources
-
University of Ottawa, Canada
Home institution of several review authors (BK, MO, MD, PL, GW, VW, AHG, AN, PCH)
External sources
-
World Food Programme, Italy
Provided funding for the Review. The WFP has no control over the design, planning or execution of this review; however, a few people from the WFP sit on the Advisory Board, to help develop the conceptual model and later disseminate the review's findings.
Declarations of interest
Elizabeth Kristjansson (EK) is a co‐chair and editor with the Campbell Collaboration Nutrition group and a member of the Advisory board of Cochrane Nutrition.
Muna Osman has declared that she has no conflicts of interest.
Michael Dignam has declared that he has no conflicts of interest.
Patrick R Labelle has declared that he has no conflicts of interest.
Olivia Magwood has declared that she has no conflicts of interest.
Andrea Huerta Galicia has declared that she has no conflicts of interest.
Paige Cooke‐Hughes has declared that she has no conflicts of interest.
George A Wells has declared that he has no conflicts of interest.
Julia Krasevec (JK) was a coauthor on the 2007 Cochrane Review for this topic while she worked at the Canadian International Development Agency (now Global Affairs Canada). JK currently works for UNICEF as a Statistics and Monitoring Specialist on Nutrition data. UNICEF has various publications that discuss school feeding; JK is acknowledged on the 2019 SOWC report (www.unicef.org/media/60806/file/SOWC-2019.pdf) and other UNICEF and interagency reports that mention school feeding.
Aganeta Enns has declared that she has no conflicts of interest.
Arghavan Nepton has declared that she has no conflicts of interest.
Laura Janzen has declared that she has no conflicts of interest.
Beverly Shea is an Editor with Cochrane Musculoskeletal.
Selma C Liberato has declared that she has no conflicts of interest.
Jennifer Garner has declared that she has no conflicts of interest.
Vivian Welch has declared that she has no conflicts of interest.
New
References
Additional references
Adolphus 2013
- Adolphus K, Lawton CL, Dye, L. The effects of breakfast on behavior and academic performance in children and adolescents. Frontiers in Human Neuroscience 2013;7:425. [DOI: 10.3389/fnhum.2013.00425] [PMCID: PMC3737458] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Afridi 2019
- Afridi F, Barooah B, Somanathan R. Hunger and performance in the classroom. Bonn (DE): IZA Institute of Labor Economics; 2019 September. IZA Discussion Paper No.: 12627 2019.
Agarwal 1989
- Agarwal DK, Agarwal KN, Upadhyay SK. Effect of mid-day meal programme on physical growth & mental function. Indian Journal of Medical Research 1989;90:163-74. [PMID: ] [PubMed] [Google Scholar]
Anderson 2018
- Anderson ML, Gallagher J, Ramirez Ritchie E. School meal quality and academic performance. Journal of Public Economics 2018;168:81-93. [DOI: 10.1016/j.jpubeco.2018.09.013] [DOI] [Google Scholar]
Armijo‐Olivia 2012
- Armijo-Olivo S, Stiles CR , Hagen NA , Biondo PD, Cummings GG J. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. Eval Clin Practice 2012;12(8):1111. [DOI] [PubMed] [Google Scholar]
Baretto 2017
- Baretto ML. Health inequalities: a global perspective. Ciência & Saude Coletiva 2017;22(7):2097–108. [DOI: 10.1590/1413-81232017227.02742017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Calnan 2003
- Calnan M, Ferlie E. Analysing process in healthcare: the methodological and theoretical challenges. Policy & Politics 2003;31(2):185-93. [DOI: 10.1332/030557303765371672] [DOI] [Google Scholar]
Campbell 2000
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321(7262):694-6. [DOI: 10.1136/bmj.321.7262.694] [PMCID: PMC1118564] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2020
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guide. BMJ 2020;368:l6890. [DOI: 10.1136/bmj.l6890] [PMCID: PMC7190266] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane EPOC 2022
- Cochrane Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/suggested_risk_of_bias_criteria_for_epoc_reviews.pdf (accessed 5 May 2022).
Cohen 2021
- Cohen JF, Hecht AA, McLoughlin G, Turner L, Schwartz MB. Universal school meals and associations with student participation, attendance, academic performance, diet quality, food security, and body mass index: a systematic review. Nutrients 2021;13(3):911. [DOI: 10.3390/nu13030911] [PMCID: PMC8000006] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coleman 2005
- Coleman KJ, Tiller CL, Sanchez J, Heath EM, Sy O, Milliken G, et al. Prevention of the epidemic increase in child risk of overweight in low-income schools: the El Paso coordinated approach to child health. Archives of Pediatric & Adolescent Medicine 2005;159(3):217-24. [DOI: 10.1001/archpedi.159.3.217] [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence 2021 [Computer program]
- Veritas Health Innovation Covidence. Version accessed 12 May 2021. Melbourne, Australia .. registered in Australia (ACN 600 366 274, ABN 41 600 366 274). : Veritas Health Innovation, 2022. Available at covidence.org.
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:241-84. [DOI: 10.1002/9781119536604] [ISBN-13: 978-1119536628] [DOI] [Google Scholar]
Diderichsen 2001
- Diderichsen F, Evans T, Whitehead M. The social basis of disparities in health. In: Evans T, Whitehead M, Diderichsen F, Bhuiya A, Wirth M, editors(s). Challenging Inequities in Health: From Ethics to Action. Oxford (UK): Oxford University Health, 2001:12-23. [DOI: 10.1093/acprof:oso/9780195137408.001.0001] [ISBN-13: 978-0195137408] [DOI] [Google Scholar]
DiGirolamo, 2020
- DiGirolamo AM, Ochaeta L, Flores RMM. Early childhood nutrition and cognitive functioning in childhood and adolescence. Food and Nutrition Bulletin 2020;41(1 Suppl):S31-S40. [DOI: 10.1177/0379572120907763] [PMID: ] [DOI] [PubMed] [Google Scholar]
Drahota 2021
- Drahota A, Beller E. RevMan calculator. Available at training.cochrane.org/resource/revman-calculator (accessed 10 May 2021).
Drake 2017
- Drake L, Fernandes M, Aurino E, Kiamba J, Giyose B, Burbano C, et al. School feeding programs in middle childhood and adolescence. In: Bundy DA, De Silva N, Horton S, Jamison DT, Patton GC, editors(s). Child and Adolescent Health and Development. 3rd edition. Vol. 8. Washington (DC): International Bank for Reconstruction and Development/World Bank, 2017:147-64. [DOI: 10.1596/978-1-4648-0426-7] [ISBN-13: 978-1-4648-0423-6] [DOI] [PubMed] [Google Scholar]
Drake 2020
- Drake LJ, Lazrak N, Fernandes M, Chu K, Singh S, Ryckembusch D, et al. Establishing global school feeding programme targets: how many poor children globally should be prioritized, and what would be the cost of implementation? Frontiers in Public Health 2020;8:530176. [DOI: 10.3389/fpubh.2020.530176] [PMCID: 10.3389/fpubh.2020.530176] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Du 2005
- Du X, Zhu K, Trube A, Fraser DR, Greenfield H, Zhang Q, et al. Effects of school-milk intervention on growth and bone mineral accretion in Chinese girls aged 10-12 year: accounting for cluster randomisation. British Journal of Nutrition 2005;94(6):1038-9. [DOI: 10.1079/bjn20051584] [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder CE. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [PMCID: PMC2127453] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eldridge 2021
- Eldridge S, Campbell MK, Campbell MJ, Drahota AK, Giraudeau B, Reeves BC, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2): additional considerations for cluster-randomized trials . Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License 2021.
FAO/IFAD/UNICEF/WFP/WHO 2020
- Food and Agriculture Organisation (FAO), International Fund for Agriculture (IFAD), United Nations International Children's Emergency Fund (UNICEF), World Food Program (WFP), World Health Organization (WHO). The State of Food Security and Nutrition in the World 2020. Transforming Food Systems for Affordable Healthy Diets. Rome: FAO, 2020. [ISBN-13: 978-9251329016] [Google Scholar]
FAO 2004
- Food and Agriculture Organization (FAO). Human energy requirements. Report of a Joint FAO/WHO/UNU expert consultation; 2004. Available at www.fao.org/3/y5686e/y5686e.pdf. [ISBN-10: 9251052123]
FAO and WFP 2018
- Food and Agriculture Organisation (FAO), World Food Program (WFP). Home Grown School Feeding. Resource Framework. Synopsis; March 2018. Available at www.fao.org/3/i8724en/I8724EN.pdf.
Gelli 2019
- Gelli A, Aurino E, Folson G, Arhinful D, Adamba C, Osei-Akoto I, et al. A school meals program implemented at scale in Ghana increases height-for-age during midchildhood in girls and in children from poor households: a cluster randomized trial. Journal of Nutrition 2019;149 (8):1434-42. [DOI: 10.1093/jn/nxz079] [PMCID: PMC6686055] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 12 May 2021. Available at gradepro.org.
Grantham‐McGregor 2005
- Grantham-McGregor S. Can the provision of breakfast benefit school performance? Food and Nutrition Bulletin 2005;26(2 Suppl 2):S144-58. [DOI: 10.1177/15648265050262S204] [PMID: ] [DOI] [PubMed] [Google Scholar]
Greenhalgh 2007
- Greenhalgh, T Kristjansson, E and Robinson, V. Realist Review to Understand the Efficacy of School Feeding Programmes. BMJ 2007;335:858-861.http://dx.doi.org/10.1136/bmj.39359.525174.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfandf M, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294-302. [DOI: 10.1016/j.jclinepi.2011.03.017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gwatkin 2007
- Gwatkin DR, Rutstein S, Johnson K, Suliman E, Wagstaff A, Amouzou A. Socioeconomic differences in health, nutrition, and population within developing countries. An overview; September 2007. Available at tinyurl.com/3n3xwrnj. [PubMed]
Hanushek 2015
- Hanushek EA, Woessmann L. The Knowledge Capital of Nations: Education and the Economics of Growth. Cambridge (MA): MIT press, 2015. [ISBM: 9780262029179] [Google Scholar]
Harden 2018
- Harden A, Thomas J, Cargo M, Harris J, Pantoja T, Flemming K, et al. Cochrane Qualitative and Implementation Methods Group guidance series—paper 5: methods for integrating qualitative and implementation evidence within intervention effectiveness reviews. Journal of Clinical Epidemiology 2018;97:70-8. [DOI: 10.1016/j.jclinepi.2017.11.029] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2019a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [DOI: 10.1002/9781119536604] [ISBN-13: 978-1119536628] [DOI] [Google Scholar]
Higgins 2019b
- Higgins JP, Eldridge S, Li T, editor(s). Chaper 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:569-93. [DOI: 10.1002/9781119536604] [ISBN-13: 978-1119536628] [DOI] [Google Scholar]
Higgins 2019c
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:205-28. [DOI: 10.1002/9781119536604] [ISBN-13: 978-1119536628] [DOI] [Google Scholar]
Higgins 2019d
- Higgins JP, Savović J, Page MJ, Sterne JA, editor(s), on behalf of the Cochrane ROB2 Development Group. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) SHORT VERSION (CRIBSHEET); August 2019. Available at tinyurl.com/mt7uud2m.
Higgins 2021
- Higgins, JPT, Li, T and Sterne, J . Revised Cochrane risk of bias tool for randomized trials (RoB 2) Additional considerations for crossover trials Preliminary tool version, 18 March 2021. https://drive.google.com/file/d/11LFgCuDpWk5-BvBNbHtNzbJv5-qVpTWb/view 2021;Downloaded from website on July 11, 2022.
Jomaa 2011
- Jomaa LH, McDonnell E, Probart C. School feeding programs in developing countries: impacts on children's health and educational outcomes. Nutrition Reviews 2011;69(2):83-98. [DOI: 10.1111/j.1753-4887.2010.00369.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Krishnaratne 2013
- Krishnaratne S, White H, Carpenter E. Quality Education for All Children? What Works in Education in Developing Countries, Working Paper 20. New Delhi: International Initiative for Impact Evaluation (3ie), 2013. [WEB PAGE: tinyurl.com/sh7bc6bu] [Google Scholar]
Kristjansson 2015
- Kristjansson E, Francis DK, Liberato S, Benkhalti Jandu M, Welch VA, Batal M, et al. Food supplementation for improving the physical and psychosocial health of socio-economically disadvantaged children aged three months to five years. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD009924. [DOI: 10.1002/14651858.CD009924.pub2] [PMCID: PMC6885042] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
LaMorte 2020
- Sullivan L. Power and sample size determination. sphweb.bumc.bu.edu/otlt/MPH-Modules/BS/BS704_Power/ (accessed prior to 14 December 2021).
Last 1995
- Last JM, editor(s). A Dictionary of Epidemiology. 3rd edition. New York (NY): Oxford University Press, 1995. [ISBN-13: 978-0195096682] [Google Scholar]
Le 2021
- Le K, Nguyen M. How education empowers women in developing countries. The B.E. Journal of Economic Analysis & Policy 2021;21(2):511-36. [DOI: 10.1515/bejeap-2020-0046] [DOI] [Google Scholar]
Lefevbre 2019
- Lefevbre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al . Chapter 4: Searching for and selecting studies. . In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:67-107. [DOI: 10.1002/9781119536604] [978-1119536628] [DOI] [Google Scholar]
Levinger 1986
- Levinger B. School feeding programs in developing countries: an analysis of actual and potential impact. AID evaluation special study No.: 30; January 1986. Available at files.eric.ed.gov/fulltext/ED296785.pdf.
Levinger 1996
- Levinger B. Nutrition, Health and Education for All. Newton (MA): Education Development Centre, 1996. [ISBN: 9780963704436] [Google Scholar]
Luepker 1996
- Luepker RV, Perry CL, McKinlay SM, Nader PR, Parcel GS, Stone EJ, et al. Outcomes of a field trial to improve children's dietary patterns and physical activity. The Child and Adolescent Trial for Cardiovascular Health (CATCH). JAMA 1996;275(10):768-76. [DOI: 10.1001/jama.1996.03530340032026] [PMID: ] [DOI] [PubMed] [Google Scholar]
Marchand 1998
- Marchand S, Wikler D, Landesman B. Class, health, and justice. Milbank Quarterly 1998;76(3):449-67. [DOI: 10.1111/1468-0009.00098] [PMCID: PMC2751091] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattei 2019
- Mattei D, Pietrobelli A. Micronutrients and brain development. Current Nutrition Reports 2019;8(2):99-107. [DOI: 10.1007/s13668-019-0268-z] [PMID: ] [DOI] [PubMed] [Google Scholar]
McGowan 2016
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. Journal of Clinical Epidemiology 2016;75:40-6. [DOI: 10.1016/j.jclinepi.2016.01.021] [PMID: ] [DOI] [PubMed] [Google Scholar]
Microsoft Excel 2019 [Computer program]
- Microsoft Excel. Microsoft Corporation, Version 16.0. Microsoft Corporation, 2019. Available at office.microsoft.com/excel.
Murad 2017
- Murad MH, Mustafa RA, Schünemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evidence Based Medicine 2017;22(3):85-7. [DOI: 10.1136/ebmed-2017-110668] [PMCID: PMC5502230] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neumann 2003
- Neumann CG, Bwibo NO, Murphy SP, Sigman M, Whaley S, Allen LH, et al. Animal source foods improve dietary quality, micronutrient status, growth and cognitive function in Kenyan school children: background, study design, and baseline findings. Journal of Nutrition 2003;133(11 Suppl 2):3941S-9S. [DOI: 10.1093/jn/133.11.3941S] [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Neill 2014
- O'Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. Journal of Clinical Epidemiology 2014;67(1):56-64. [DOI: 10.1016/j.jclinepi.2013.08.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Papamandjaris
- Papamandjaris, A. Breakfast and learning in children: a review of the effects of breakfast on scholastic performance. Breakfast for Learning. Canadian Living Foundation 2000.
Peter 2001
- Peter F, Evans T. Ethical dimensions of health equity. In: Evans T, Whitehead M, Diderichsen F, Bhuiya A, Wirth M, editors(s). Challenging Inequities in Health: From Ethics to Action. Oxford (UK): Oxford University Press, 2001:25-33. [DOI: 10.1093/acprof:oso/9780195137408.001.0001] [ISBN-13: 978-0195137408] [DOI] [Google Scholar]
Petticrew 2005
- Petticrew M, Cummins S, Ferrell C, et al. Natural experiments: an underused tool for public health? Public Health 2005;119(9):751-7. [DOI] [PubMed] [Google Scholar]
Pollitt 1978
- Pollitt E, Gersovitz M, Gargiulo M. Educational benefits of the United States school feeding program: a critical review of the literature. American Journal of Public Health 1978;68(5):477-81. [DOI: 10.2105/ajph.68.5.477] [PMCID: PMC1653885] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pollitt 1995
- Pollitt E. Does breakfast make a difference in school? Journal of the American Dietetic Association 1995;95(10):1134-9. [DOI: 10.1016/S0002-8223(95)00306-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Powell 1983
- Powell C, Grantham-McGregor S, Elston M. An evaluation of giving the Jamaican government school meal to a class of children. Human Nutrition. Clinical Nutrition 1983;37C(5):381-8. [PMID: ] [PubMed] [Google Scholar]
Read 1973
- Read MS. Malnutrition, hunger and behavior. II. Hunger, school feeding programs, and behavior. Journal of the American Dietetic Association 1973;63(4):386-91. [PMID: ] [PubMed] [Google Scholar]
Reeves 2019
- Reeves BC, Deeks JJ, Higgins JP, Shea B, Tugwell P, Wells GA. Chapter 24: Including non-randomized studies on intervention effects. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:595-620. [Google Scholar]
RevMan Web 2022 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). Version 4.6.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
ROB2 2021
- Revised Cochrane Risk-of-Bias tool for cluster-randomized trials. RoB 2 CRTSHORT VERSION (CRIBSHEET) . https://methods.cochrane.org/risk-bias-2 2021.
RoB Excel Tool 2022
- Excel tool to implement RoB 2. Available at drive.google.com/uc?export=download&id=1malyRF_b-DgvAGHssrdt4N9R7Yhljmt0.
Rutledge 2016
- Rutledge JG. Feeding the Future: School Lunch Programs as Global Social Policy. New Brunswick (NJ): Rutgers University Press, 2016. [DOI: 10.36019/9780813573342] [ISBN: 9780813573328] [DOI] [Google Scholar]
Rycroft‐Malone 2012
- Rycroft-Malone J, McCormack B, Hutchinson AM, DeCorby K, Bucknall TK, Kent B, et al. Realist synthesis: illustrating the method for implementation research. Implementation Science 2012;7:33. [DOI: 10.1186/1748-5908-7-33] [PMCID: PMC3514310] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schochet 2008
- Schochet P. Technical methods report: guidelines for multiple testing in impact evaluations of educational interventions. Washington (DC): Institute of Education Sciences, US Department of Education; 2008 May. Report No.: NCEE 20084018.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:375-402. [DOI: 10.1002/9781119536604] [ISBN-13: 9781119536628] [DOI] [Google Scholar]
Soper 2021 [Computer program]
- A-priori sample size calculator for structural equation models. Soper DS. Fullerton (CA): DS Soper, accessed 13 July 2021. Available at www.danielsoper.com/statcalc.
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI: 10.1136/bmj.i4919] [PMCID: PMC5062054] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2019a
- Sterne JA, Hernán MA, McLeenan A, Reeves BC, Higgins JP. Chapter 25: Assessing risk of bias in a non-randomized study. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch V, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:621-41. [DOI: 10.1002/9781119536604] [ISBN-13: 9781119536628] [DOI] [Google Scholar]
Sterne 2019b
- Stern, JAC, Savovic, J, Page MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al, . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366 4898 |:1-8. [DOI] [PubMed] [Google Scholar]
Tan‐Torres Edejer 2001
- Tan-Torres Edejer T. Health for some: health, poverty and equity at the beginning of the 21st century. In: Neufeld V, Johnson N, editors(s). Forging Links for Health Research. Perspectives from the Council on Health Research for Development. Ottawa (ON): International Development Research Centre, 2001:29-46. [ISBN-13: 978-0889369351] [Google Scholar]
Thomas 2004
- Thomas, B H, Ciliska, D, Dobbins, M & Micucci, S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews on Evidence-Based Nursing 2004;1(3):176-184. [DOI] [PubMed] [Google Scholar]
Thompson 2013
- Thomson HJ, Thomas S. The effect direction plot: visual display of non-standardised effects across multiple outcome domains. Research Synthesis Methods 2013;4(1):95-101. [DOI: 10.1002/jrsm.1060] [PMCID: PMC3688329] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. [PMID: ] [PubMed] [Google Scholar]
USDA 2019
- Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A, US Department of Agriculture, Economic Research Service. Household food security in the United States in 2018, ERR-270; September 2019. Available at www.ers.usda.gov/publications/pub-details/?pubid=94848.
USDA 2021
- Agricultural Research Service United States Department of Agriculture (USDA). FoodData Central (FDC). tinyurl.com/ts4prcjj (accessed 14 April 2021).
Viner 2017
- Viner RM, Hargreaves DS, Ward J, Bonell C, Mokdad AH, Patton G. The health benefits of secondary education in adolescents and young adults: an international analysis in 186 low-, middle- and high-income countries from 1990 to 2013. SSM - Population Health 2017;3:162-71. [DOI: 10.1016/j.ssmph.2016.12.004] [PMCID: PMC5742637] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 1986
- Walker, A, and Walker, B. School nutrition programmes: do they fulfill their purpose? Applied Nutrition 986;40A:125-135. [PubMed] [Google Scholar]
Wang 2020
- Wang D, Fawzi WW. Impacts of school feeding on educational and health outcomes of school-age children and adolescents in low- and middle-income countries: protocol for a systematic review and meta-analysis. Systematic Reviews 2020;9(1):55. [DOI: 10.1186/s13643-020-01317-6] [PMCID: PMC7075040] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Welch 2019
- Welch VA, Petkovic J, Jull J, Hartling L, Klassen T, Kristjansson E, et al. Chapter 16: Equity and special populations. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:433-49. [DOI: 10.1002/9781119536604] [ISBN-13: 978-1119536628] [DOI] [Google Scholar]
WFP 2019
- World Food Programme (WFP). Two minutes on school feeding; November 2019. Available at tinyurl.com/5bpy2yyy.
WFP 2020
- World Food Programme. State of school feeding worldwide 2020; February 2021. Available at www.wfp.org/publications/state-school-feeding-worldwide-2020.
Wilkins 1983
- Wilkins R, Adams O. Healthfulness of Life. Montreal (QC): Institute for Research on Public Policy [L'Institut de recerches politiques], 1983. [ISBN-10: 0-920380948] [Google Scholar]
Wilkinson 1996
- Wilkinson RG. Unhealthy Societies: The Afflictions of Inequality. London (UK): Routledge, 1996. [ISBN-13: 978-0415092357] [Google Scholar]
Wong 2013
- Wong G, Greenhalgh T, Westhorp G, Buckingham J, Pawson R. RAMESES publication standards: realist syntheses. BMC Medicine 2013;11:21. [DOI: 10.1186/1741-7015-11-21] [PMCID: PMC3558331] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
World Bank 2021
- World Bank. World Bank Country and Lending Groups. Available at datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed 7 February 2021).
World Vision 2021
- World Vision. Africa hunger, famine: facts, FAQs, and how to help. www.worldvision.org/hunger-news-stories/africa-hunger-famine-facts (accessed 21 May 2021).
References to other published versions of this review
Kristjansson 2007
- Kristjansson B, Petticrew M, MacDonald B, Krasevec J, Janzen L, Greenhalgh T, et al. School feeding for improving the physical and psychosocial health of disadvantaged students. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD004676. [DOI: 10.1002/14651858.CD004676.pub2] [DOI] [PubMed] [Google Scholar]